Abstract
Aims
Long-term clinical outcome data for second-generation drug-eluting stents (DES) are critical for the assessment of safety and efficacy. Five-year results from the RESOLUTE China Registry are presented in this report.
Methods and results
The RESOLUTE China Registry is a prospective, multicentre, observational study for all-comers requiring coronary stent implantation. The primary endpoint was target lesion failure (TLF) at one year, and the main secondary endpoint was definite or probable stent thrombosis at one year. Additional secondary endpoints assessed up to 5 years include rates of all deaths, target vessel myocardial infarction (TVMI) and target lesion revascularisation (TLR). A total of 1,800 patients were enrolled from December 2010 to March 2012 at 30 sites in China and implanted with Resolute DES. At 5 years, TLF was 9.8%, TVMI 3.2%, TLR 4.6% and very late stent thrombosis 0.5%. Results of pre-specified subgroup analyses show 5-year TLF rates of 14.3% for diabetics and 13.4% for patients with chronic total occlusions.
Conclusions
The RESOLUTE China Registry is the largest study of Asian patients treated with second-generation Resolute DES. Clinical outcomes illustrate a robust safety and efficacy profile of Resolute DES in a real-word Asian population, including favourable performance in complex patient subsets.
Introduction
The first drug-eluting stents (DES) were introduced in 2003 as an alternative to bare metal stents (BMS) and have demonstrated reduced risk of restenosis in percutaneous coronary interventions (PCI) compared to BMS, balloon angioplasty and vascular brachytherapy 1 2. Design iterations have produced “second-generation” DES to improve deliverability and biocompatibility and thus lessen the inflammatory response after intervention. Several studies have reported an improved safety profile with second-generation DES compared to first-generation DES and BMS 3 4. The second-generation Resolute™ and Resolute Integrity™ DES (Medtronic, Minneapolis, MN, USA) have been studied in multiple prospective clinical studies with over 7,600 patients enrolled worldwide in the RESOLUTE Global Clinical Program. Long-term follow-up data on DES patients are important to illustrate the continued safety and efficacy of the intervention. Five-year clinical outcomes have been published from several studies in the RESOLUTE Global Clinical Program 5 6, but more long-term outcome data are needed specifically for the Asian population. The current report presents 5-year clinical outcomes for the RESOLUTE China Registry (R-China Registry).
Methods
STUDY DESIGN
The study design of the R-China Registry has been reported previously 7 8. Briefly, the R-China Registry is a prospective, multicentre, observational study in an all-comers Chinese population. Patients were 18 years or older and acceptable candidates for PCI, with minimal exclusion criteria. After implantation with Resolute DES, patients were followed at 30 days, 6 months, and annually to 5 years by office visit or phone call. At each follow-up, data on adverse events and dual antiplatelet therapy (DAPT) usage were collected. The trial complies with the Declaration of Helsinki, local ethics committees approved the clinical protocol at each enrolling centre, and informed consent was obtained from all patients.
Study oversight was provided by the steering committee comprised of physician investigators and sponsor representatives. Safety oversight was provided by an independent data safety monitoring board. Outcomes were adjudicated by a clinical events committee of cardiologists not participating in the clinical study. Monitoring by R&G Pharma Studies (Shanghai, China) was conducted at all study centres to verify source data from at least half of all enrolled patients, and the source was verified for all serious adverse events.
STATISTICAL ANALYSIS
The primary endpoint was one-year target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (MI; Q-wave and non-Q-wave) or clinically driven target lesion revascularisation (TLR). The main secondary endpoint was overall stent thrombosis at one year, defined as definite or probable stent thrombosis, according to the Academic Research Consortium (ARC) definition. The following secondary endpoints were assessed annually up to 5 years: TLF, all deaths, stent thrombosis rates, MI, TLR, clinically driven target vessel revascularisation (TVR), significant bleeding complication, and target vessel failure (TVF). TVF is a composite endpoint of cardiac death, target vessel MI (TVMI), or TVR. Significant bleeding complication is defined as bleeding that led to an interruption of antiplatelet medication, required transfusion, resulted in substantial haemodynamic compromise requiring treatment, or intracerebral bleeding. Deaths were considered cardiac unless an unequivocal non-cardiac cause could be established. All MI, including TVMI, were adjudicated according to the extended historical definition 9.
Clinical outcomes at 5 years were assessed for the following pre-specified subgroups: treatment of long lesions (≥18 mm length), small vessels (≤2.75 mm diameter), multiple vessels, chronic total occlusions (CTO), overlapping stents and bifurcations, as well as treatment in patients with acute myocardial infarction (AMI) within 72 hours or a history of diabetes mellitus. Clinical outcomes at 5 years in complex patients were also analysed, where complex patients were defined as having a bifurcation, bypass grafts, in-stent restenosis, AMI within 72 hours, left ventricular ejection fraction <30%, unprotected left main coronary artery, more than two vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, more than one lesion per vessel, lesion with thrombus or total occlusion (pre-procedure Thrombolysis In Myocardial Infarction [TIMI]=0).
All analyses were conducted based on the intention to treat; no data imputation for missing values was performed. Nominal variables are presented as percentages and continuous variables as mean±standard deviation. The counts and percentages of clinical events are presented in table format and the incidence of clinical events is presented using the Kaplan-Meier method in figure format. Statistical analyses were performed using SAS software, version 9.1 or later (SAS Institute, Cary, NC, USA).
Results
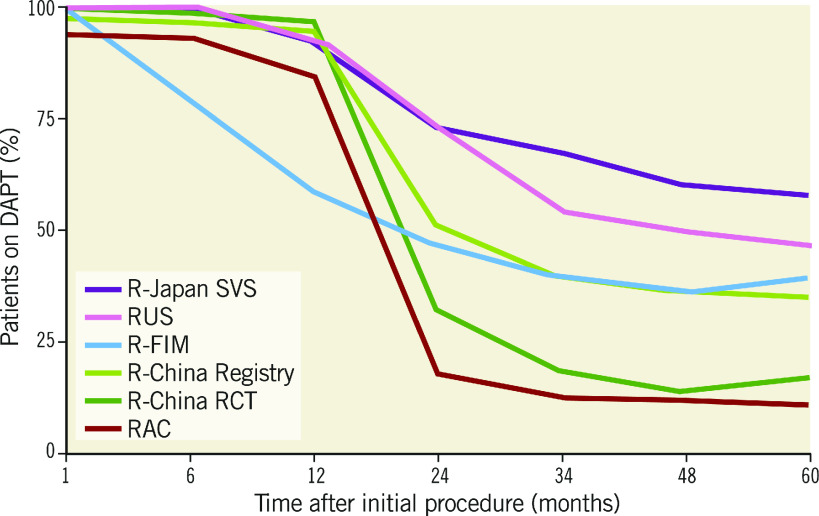
From December 2010 to March 2012, 1,800 patients were enrolled in the R-China Registry at 30 clinical study centres in China. Baseline and procedural characteristics have been reported previously and are shown in Table 1 and Table 2 7 8. Percentages of patients with various comorbidities or lesion/procedural complexity are included in Table 3. There were 61% complex patients, 29% of patients with a history of diabetes, 68% with long lesions and 43% with small vessels. DAPT usage for the R-China Registry was 39%, 36% and 35% at 3, 4, and 5 years post procedure, respectively (Figure 1). The rate of reported significant bleeding events at 5 years was 2.2%. The follow-up visit compliance rate for 5-year follow-up was 88.1%.
Table 1. Baseline demographics and clinical characteristics.
| Patient characteristic | N=1,800 | |
| Age, years | 61.3±10.9 | |
| Male | 75.6% (1,361/1,800) | |
| Current smoker | 35.9% (646/1,800) | |
| Diabetes mellitus | 29.4% (530/1,800) | |
| Hyperlipidaemia | 40.8% (734/1,800) | |
| Hypertension | 64.1% (1,153/1,800) | |
| Previous MI | 35.3% (631/1,788) | |
| Previous PCI | 12.8% (230/1,800) | |
| Previous CABG | 1.2% (21/1,800) | |
| Reason for revascularisation | Silent angina | 2.5% (44/1,783) |
| Stable angina | 7.5% (134/1,783) | |
| Unstable angina | 58.6% (1,045/1,783) | |
| (Acute) MI | 31.4% (560/1,783) | |
| Left ventricular ejection fraction <30% | 0.6% (8/1,330) | |
| Serum creatinine, µmol/l | 80.43±31.80 | |
| Data presented as mean±SD or % (n/N). CABG: coronary artery bypass graft; MI: myocardial infarction; PCI: percutaneous coronary intervention | ||
Table 2. Procedural characteristics.
| <p>N=1,800 patients</p> <p>N=2,327 lesions</p> | ||
| Vessel location (per patient) | Left anterior descending | 62.4% (1,123) |
| Left circumflex | 23.3% (419) | |
| Right coronary artery | 32.1% (578) | |
| Left main | 2.2% (40) | |
| Bypass graft | 0.7% (12) | |
| Number of lesions with predilatation | 82.8% (1,927) | |
| ACC/AHA lesion class B2/C | 67.6% (1,574) | |
| Multiple lesions | 34.0% (612) | |
| Multiple vessels | 28.3% (510) | |
| Number of patients with staged procedure planned | 7.7% (139) | |
| Lesion length, mm | 24.90±13.71 | |
| Reference vessel diameter, mm | 3.02±0.50 | |
| Total stent length, mm | Per patient | 42.4±28.3 |
| Per lesion | 29.5±15.4 | |
| Number of stents | Per patient | 1.8±1.1 |
| Per lesion | 1.3±0.5 | |
| Diameter stenosis, % | Pre-procedure | 86.18±12.77 |
| Post-procedure | 0.30±2.42 | |
| Data presented as mean±SD or % (N). ACC/AHA: American College of Cardiology/American Heart Association | ||
Table 3. Percent of enrolled patients with diabetes and/or complex lesion/procedural characteristics.
| Characteristic | Percent of total enrolled patients (N=1,800) |
| History of diabetes mellitus | 29% |
| Long lesions (≥18 mm) | 68% |
| Small vessels (≤2.75 mm) | 43% |
| Bifurcation | 18% |
| Overlapping stents | 20% |
| Chronic total occlusion | 9% |
| AMI within 72 hours | 10% |
| Complex patient* | 61% |
| *Complex patient defined as having a bifurcation, bypass grafts, in-stent restenosis, AMI <72 hours, left ventricular ejection fraction <30%, unprotected left main coronary artery, more than 2 vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, more than 1 lesion per vessel, lesion with thrombus or total occlusion (pre-procedure TIMI=0). AMI: acute myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction | |
Figure 1.
DAPT usage at 5 years in the RESOLUTE Global Clinical Program. DAPT: dual antiplatelet therapy; FIM: first in man; RAC: RESOLUTE-III All-comers Trial; RCT: randomised clinical trial; SVS: small vessel substudy
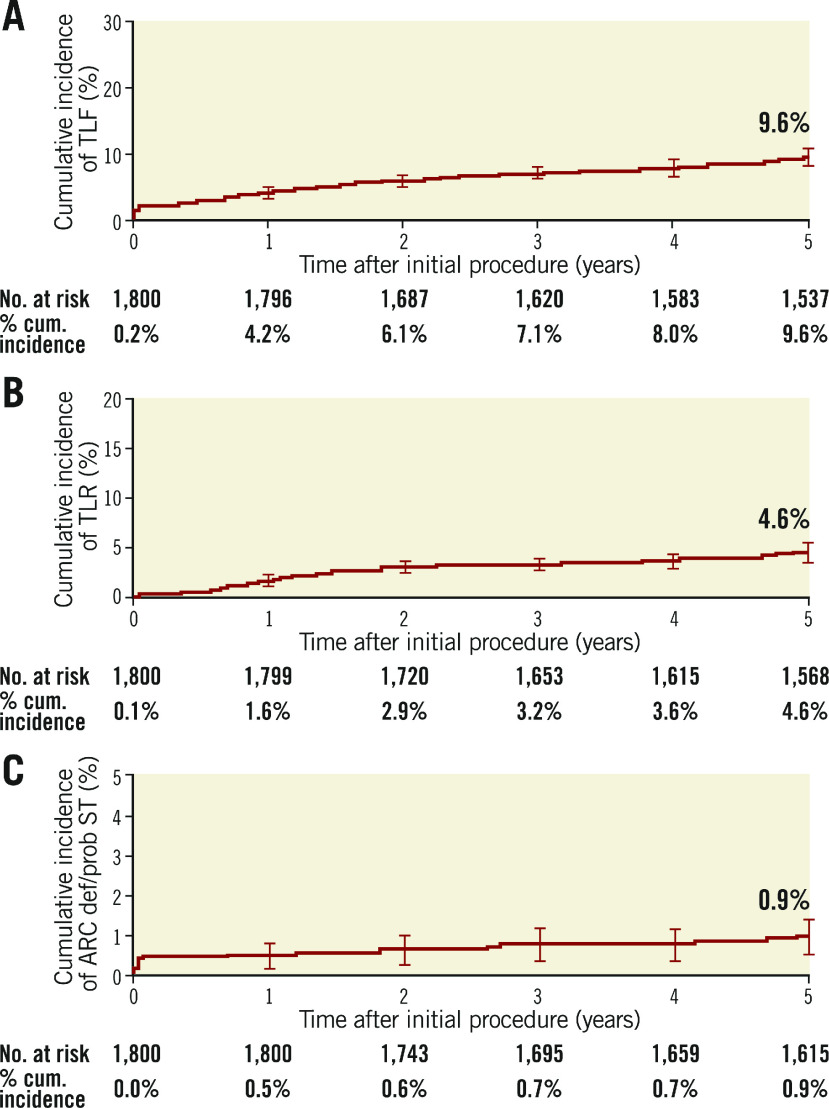
Clinical outcomes and rates of stent thrombosis at 5 years are shown in Table 4. At 5 years, TLF was 9.8%, cardiac death 3.5%, TVMI 3.2% and TLR 4.6%. The rate of very late stent thrombosis at 5 years was 0.5%. The cumulative incidence of events using Kaplan-Meier analysis is shown for TLF, TLR and stent thrombosis for 0 to 5 years in Figure 2. Rates of TLF, cardiac death/TVMI, TLR and stent thrombosis at 5 years were analysed for pre-specified subgroups (Figure 3). The rates of TLF across multiple all-comer clinical studies in the RESOLUTE Global Clinical Program are compared from 0 to 5 years (Figure 4).
Table 4. Clinical and safety outcomes at 5 years.
| Secondary endpoint | Cumulative incidence (N=1,701) |
| TLF | 9.8% (167) |
| Cardiac death | 3.5% (59) |
| Non-cardiac death | 3.1% (52) |
| TVMI | 3.2% (54) |
| Cardiac death or TVMI | 6.1% (103) |
| TLR | 4.6% (78) |
| TVR | 5.8% (98) |
| Very late stent thrombosis (definite/probable)* | 0.5% (9) |
| Significant bleeding complications | 2.2% (38) |
| Data presented as % (n). *Academic Research Consortium (ARC) definition. TLF: target lesion failure; TLR: target lesion revascularisation; TVMI: target vessel myocardial infarction; TVR: target vessel revascularisation | |
Figure 2.
Cumulative incidence of events using Kaplan-Meier analysis to 5 years. A) TLF. B) TLR. C) Stent thrombosis. cum.: cumulative; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation
Figure 3.
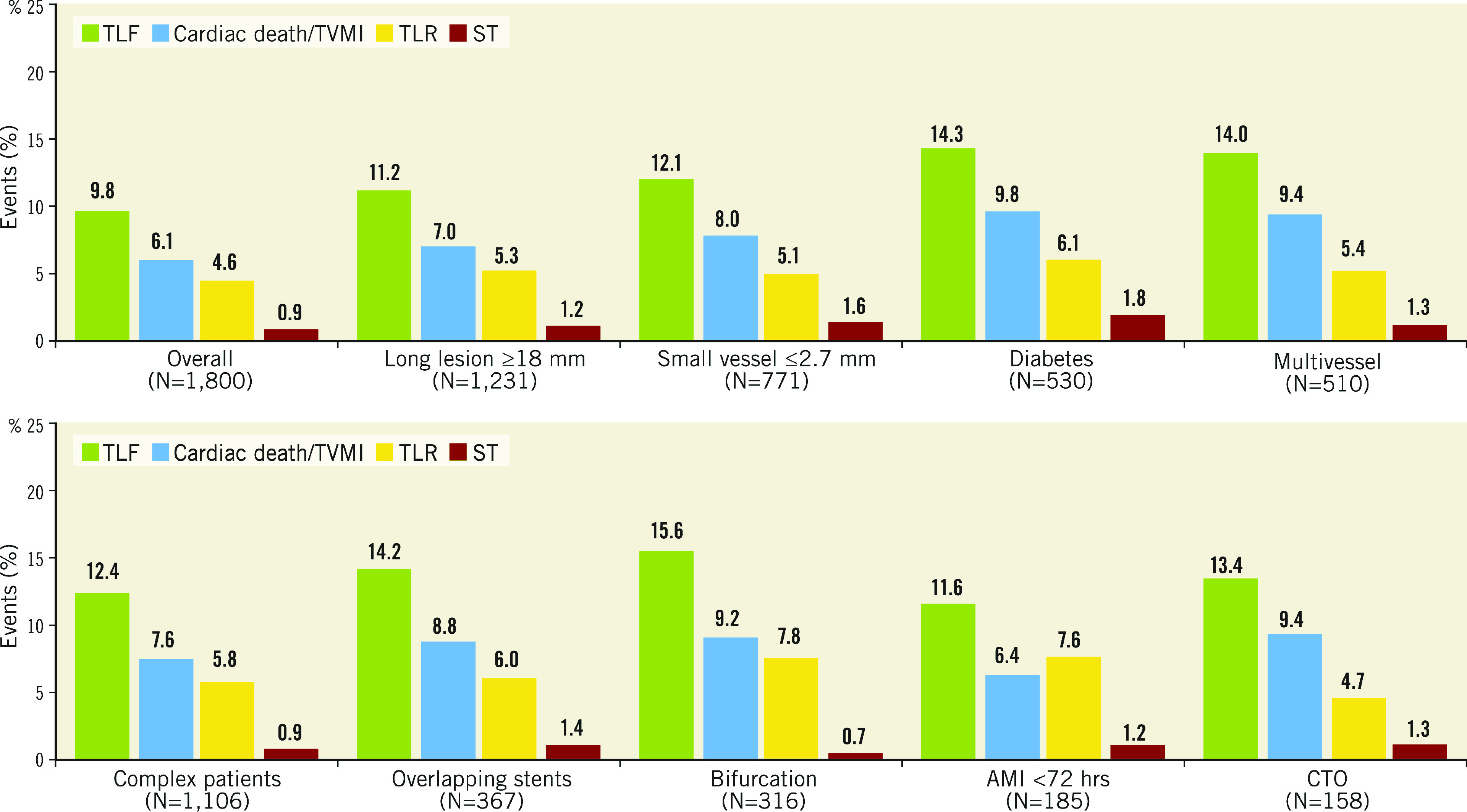
Subgroup analyses: cumulative incidence of secondary endpoints at 5 years. AMI: acute myocardial infarction; CTO: chronic total occlusion; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation; TVMI: target vessel myocardial infarction
Figure 4.
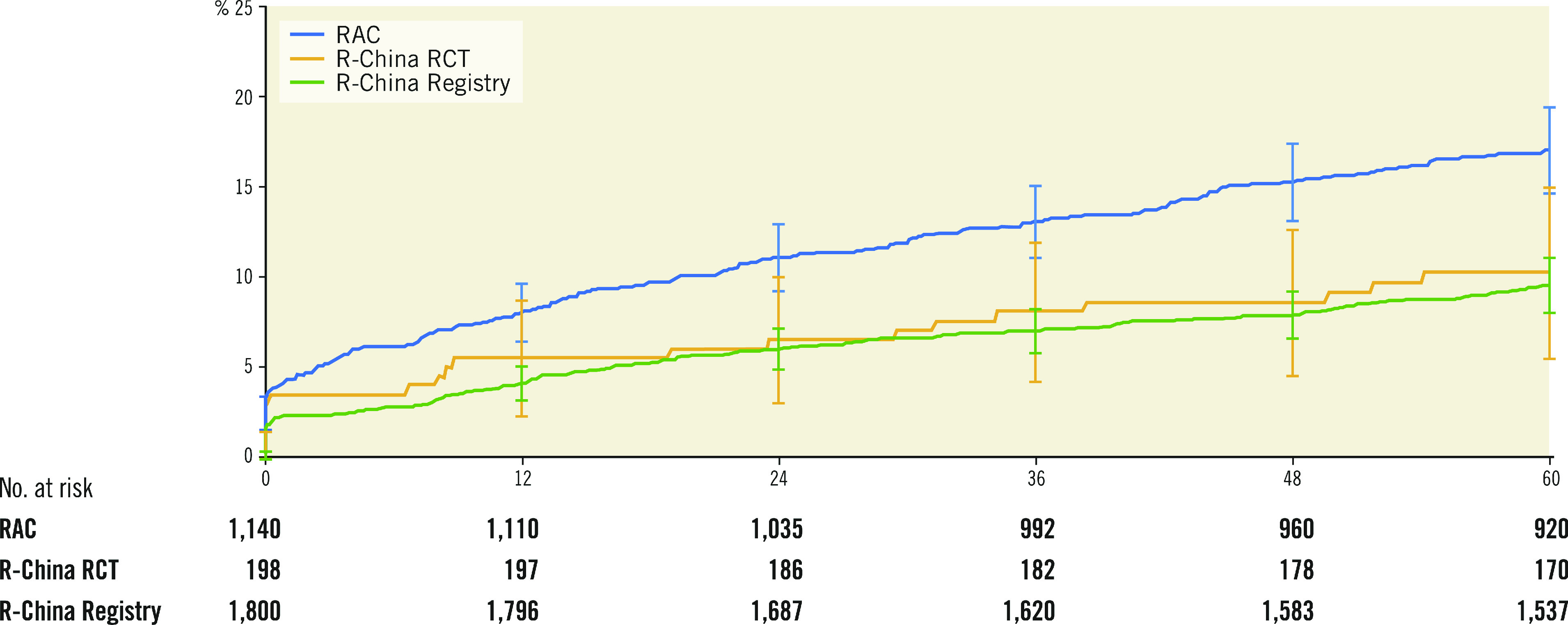
Cumulative incidence up to 5 years after Resolute DES implantation of TLF in RESOLUTE-III All-comers, RESOLUTE China Randomized Controlled Trial and RESOLUTE China Registry.
Discussion
Results at 5 years in the R-China Registry include low rates of TLF and other secondary endpoints in a trial where 61% of patients were complex, illustrating the robust safety and efficacy profile of Resolute DES in this all-comers Chinese population.
Other DES trials to report 5-year clinical outcomes include COMPARE II 10, SPIRIT III 11 and TWENTE 12. The rates of 5-year TLF were 13.4% (biolimus-eluting stents [BES]) vs 11.5% (everolimus-eluting stents [EES]) for COMPARE II, 12.7% (EES) vs 19.0% (paclitaxel-eluting stents [PES]) for SPIRIT III and 15.0% (Resolute DES) vs 16.2% (EES) for TWENTE 10 11 12. These 5-year TLF rates are all higher than the TLF rate in the R-China Registry (9.8%), driven by a low TLR rate of 4.6%. As the R-China Registry was an all-comers registry, patient selection bias and lack of mandated angiographic follow-up could have influenced the TLR rate; however, long-term outcome data after PCI in the Chinese population are limited, so this is speculative.
The TLF rate for the R-China Registry compared to other Resolute clinical studies with 5-year follow-up was similar to the TLF rate of 10.3% for Resolute patients in the R-China randomised controlled trial (RCT) but less than the rate of 17.1% for Resolute patients in the RESOLUTE-III All-comers (RAC) trial (Figure 4). There was a higher proportion of complex patients in RAC compared to the R-China Registry (67% vs 61%, respectively), which could have contributed to the difference in TLF rates. The TLF rate reported for pooled Resolute trial data was midway between these rates at 13.4% 6. The TLF rate for RESOLUTE Japan, included in the pooled analysis of Resolute studies, was also low with a rate of 5.6% at 4 years 13. Reports of differences in thrombotic and thrombolytic status for Asian patients versus patients in Western countries have been postulated as reasons for differences in the underlying causes of thrombotic events 14 15.
Patients with small vessels (reference vessel diameter [RVD] <2.75 mm) experienced a TLF rate of 12.1% and a TLR rate of 5.1% at 5 years, higher than for the overall cohort in the R-China Registry. Limited data exist on long-term follow-up of patients with small vessel disease after DES implantation. The PERSEUS Small Vessel trial reported a high TLF rate of 20.1% and a TLR rate of 13.6% at 5 years for lesions of RVD between 2.25 and 2.75 mm 16. Historically, PCI in small vessels has resulted in an increase in adverse events 17. However, several recent studies have reported no differences in clinical outcomes or angiographic measurements for small (RVD ≤2.5 mm) compared to non-small vessels after DES implantation, including no association of increased major adverse cardiac events (MACE) or TVR at 1 year 18, no difference in early or late restenosis at 8 months 19, and no difference in TLR at 2 years 20 for treatment in small vessels. Notably, favourable short-term outcomes were reported for a subsequent iteration of the R-ZES, the Resolute Onyx™ ZES (Medtronic), for patients with very small (RVD <2.25 mm) lesions: 5.0% TLF and 2.0% TLR at 1 year 21, suggesting that advances in stent technology may ameliorate the performance of DES in small vessels after PCI.
Adverse event rates were higher for R-China Registry patients in pre-specified subgroups compared to the overall cohort. Specifically, TLF was 14.3% and TLR 6.1% in diabetic patients at 5 years (Figure 3). To our knowledge, this is the only 5-year clinical study follow-up reported for Chinese diabetic patients post DES implantation, although short-term outcomes have been published. A large single-centre non-randomised Chinese study comparing outcomes in diabetic versus non-diabetic patients found that diabetic patients had worse outcomes including death, MI, revascularisation, and MACE at 2 years 22. However, despite the increase in adverse events, diabetic status was not an independent predictor of mortality after propensity score matching 22. The multicentre RESOLUTE-CHINA DIABETES study demonstrated favourable outcomes in diabetic patients implanted with Resolute DES 23.
DAPT usage has varied widely across Resolute clinical studies (Figure 1), ranging from 11% to 62.5% at 5 years. DAPT prescription rates were generally higher in the US and Japanese trials (R-38, R-Japan SVS and RUS), whereas, in the European-based RAC trial, DAPT prescription was lowest despite relatively high lesion and patient complexity. DAPT usage in the R-China Registry was 35% at 5 years and bleeding events were rare (2.2%). While European Society of Cardiology and American College of Cardiology/American Heart Association guidelines recommend at least 6 months DAPT for newer-generation DES in stable patients without high bleeding risk, there is limited consensus for the optimal duration of DAPT in patients with high bleeding risk after PCI 24 25. Recent and ongoing studies are being conducted to evaluate the potential of shortened (1-month) DAPT in high bleeding risk patients 26 27 28. The rate of definite/probable stent thrombosis in the R-China Registry was 0.5% at 5 years and similar to rates in other published studies 5 6 10 11 12.
Limitations
As in other registries, there was no control group in this trial. Intravascular ultrasound and optical coherence tomography data were not collected since these imaging procedures were not in common practice in China when the study was enrolling patients. Although not all patient data were monitored in the R-China Registry, an analysis at one year found no difference in outcomes between monitored and unmonitored patients 7. Subgroup analyses were pre-specified, but randomised trials are needed to confirm the findings. Results from this study may be specific to China and may not necessarily be indicative of outcomes in other Asian or Western countries.
Conclusions
The RESOLUTE China Registry is the largest study of Asian patients treated with second-generation Resolute DES. Long-term follow-up to 5 years shows overall low clinical event rates. At 5 years, 35% of patients remained on DAPT. Clinical outcomes illustrate a robust safety and efficacy profile of Resolute DES in a real-word Asian population, including favourable performance in complex patient subsets.
Impact on daily practice
Long-term outcomes after percutaneous coronary intervention are needed to demonstrate the safety and efficacy of drug-eluting stents. The results of the R-China Registry show the continued safety and efficacy of the Resolute drug-eluting stent at 5 years in a Chinese population.
Acknowledgements
Lisa Bousquette, MS, and Linda Zhi provided expert study management (both employees of Medtronic).
Funding
The RESOLUTE China Registry (ClinicalTrials.gov NCT01243749) was funded by Medtronic.
Conflict of interest statement
B. Ferri and M. Liu are employees of Medtronic. The other authors have no conflicts of interest to declare.
Abbreviations
- DES
drug-eluting stent(s)
- PCI
percutaneous coronary intervention
- TLF
target lesion failure
- TLR
target lesion revascularisation
- TVMI
target vessel myocardial infarction
Contributor Information
Shubin Qiao, Department of Cardiology, Fu Wai Hospital, National Center for Cardiovascular Diseases of China, Beijing, China.
Lianglong Chen, Department of Cardiology, Union Hospital, Fujian Medical University, Fujian, China.
Shao-Liang Chen, Department of Cardiology, Nanjing First Hospital, Jiangshu, China.
Weimin Wang, Department of Cardiology, Peking University People’s Hospital, Beijing, China.
Beth Ferri, Division of Coronary and Structural Heart, Medtronic PLC, Santa Rosa, CA, USA.
Minglei Liu, Division of Coronary and Structural Heart, Medtronic PLC, Santa Rosa, CA, USA.
Guoying Zhu, Department of Cardiology, Wuhan Asia Heart Hospital, Hubei, China.
References
- Dibra A, Kastrati A, Alfonso F, Seyfarth M, Pérez-Vizcayno MJ, Mehilli J, Schomig A. Effectiveness of drug-eluting stents in patients with bare-metal in-stent restenosis: Meta-analysis of randomized trials. J Am Coll Cardiol. 2007;49:616–23. doi: 10.1016/j.jacc.2006.10.049. [DOI] [PubMed] [Google Scholar]
- Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Ochala A, Kellock A, Parise H, Mehran R and Investigators H-AT. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946–59. doi: 10.1056/NEJMoa0810116. [DOI] [PubMed] [Google Scholar]
- Navarese EP, Finn AV, von Birgelen C, Di Pasquale G, Aprami TM, Suryapranata H, Kubica J, Kim HS, Meredith IT, Tandjung K, Mauri L, Waksman R, Kereiakes DJ, Kandzari DE, Kowalewski M, Andreotti F, Claessen B, Kedhi E. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ. 2013;347:f6530. doi: 10.1136/bmj.f6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabate M, Baz JA, Brugaletta S, Backx B, Silvestro A, Masotti M, Tebaldi M, Bordes P, Alfonso F, van Geuns RJ, Martin-Yuste V, Gomez-Hospital JA, Cequier A, Vazquez N, Bethencourt A, den Heijer P, Tespili M, Valgimigli M, Mainar V, Hernandez-Antolin R, Serra A, Iñiguez A, van Es GA, Serruys PW. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–90. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Serruys PW, Silber S, Kelbaek H, Richardt G, Morel MA, Negoita M, Buszman PE, Windecker S. Comparison of zotarolimus-and everolimus-eluting coronary stents: Final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. doi: 10.1161/CIRCINTERVENTIONS.114.002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh RW, Silber S, Chen L, Chen S, Hiremath S, Neumann FJ, Qiao S, Saito S, Xu B, Yang Y, Mauri L. 5-Year Safety and Efficacy of Resolute Zotarolimus-Eluting Stent: The RESOLUTE Global Clinical Trial Program. JACC Cardiovasc Interv. 2017;10:247–54. doi: 10.1016/j.jcin.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Qiao S, Chen L, Chen S, Wang W, Zhu G. One-year outcomes from an all-comers Chinese population of patients implanted with the Resolute zotarolimus-eluting stent. Am J Cardiol. 2014;113:613–20. doi: 10.1016/j.amjcard.2013.10.042. [DOI] [PubMed] [Google Scholar]
- Qiao S, Chen L, Chen S, Wang W, Zhu G. Three-year outcomes from an all-comers Chinese population treated with the Resolute zotarolimus-eluting stent: RESOLUTE China Registry. AsiaIntervention. 2016;2:101–7. [Google Scholar]
- Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, Serruys PW. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871–4. doi: 10.4244/EIJV5I7A146. [DOI] [PubMed] [Google Scholar]
- Vlachojannis GJ, Smits PC, Hofma SH, Togni M, Vazquez N, Valdes M, Voudris V, Slagboom T, Goy JJ, den Heijer P, van der Ent M. Biodegradable Polymer Biolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents in Patients With Coronary Artery Disease: Final 5-Year Report From the COMPARE II Trial (Abluminal Biodegradable Polymer Biolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent). JACC Cardiovasc Interv. 2017;10:1215–21. doi: 10.1016/j.jcin.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Gada H, Kirtane AJ, Newman W, Sanz M, Hermiller JB, Mahaffey KW, Cutlip DE, Sudhir K, Hou L, Koo K, Stone GW. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6:1263–6. doi: 10.1016/j.jcin.2013.07.009. [DOI] [PubMed] [Google Scholar]
- von Birgelen C, van der Heijden LC, Basalus MW, Kok MM, Sen H, Louwerenburg HW, van Houwelingen KG, Stoel MG, de Man FH, Linssen GC, Tandjung K, Doggen CJ, van der Palen J, Löwik MM. Five-Year Outcome After Implantation of Zotarolimus- and Everolimus-Eluting Stents in Randomized Trial Participants and Nonenrolled Eligible Patients: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2017;2:268–76. doi: 10.1001/jamacardio.2016.5190. [DOI] [PubMed] [Google Scholar]
- Saito S, Maehara A, Vlachojannis GJ, Parise H, Mehran R RESOLUTE Japan Investigators. Clinical and angiographic evaluation of the resolute zotarolimus-eluting coronary stent in Japanese patients – long-term outcome in the RESOLUTE Japan and RESOLUTE Japan small vessel study. Circ J. 2015;79:96–103. doi: 10.1253/circj.CJ-14-0836. [DOI] [PubMed] [Google Scholar]
- Gorog DA, Yamamoto J, Saraf S, Ishii H, Ijiri Y, Ikarugi H, Wellsted DM, Mori M, Yamori Y. First direct comparison of platelet reactivity and thrombolytic status between Japanese and Western volunteers: possible relationship to the “Japanese paradox”. Int J Cardiol. 2011;152:43–8. doi: 10.1016/j.ijcard.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Han WX, Gao C, Feng J, Chen ZF, Zhang J, Luo CM, Pan JY. Comparison of clinical outcomes after drug-eluting stent implantation in diabetic versus nondiabetic patients in China: A retrospective study. Medicine (Baltimore) 2017;96:e6647. doi: 10.1097/MD.0000000000006647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kereiakes DJ, Cannon LA, Dauber I, Ball M, Bertolet B, Foster M, Nersesov AY, Underwood PL, Allocco DJ, Dawkins KD. Long-term follow-up of the platinum chromium TAXUS Element (ION) stent: The PERSEUS Workhorse and Small Vessel trial five-year results. Catheter Cardiovasc Interv. 2015;86:994–1001. doi: 10.1002/ccd.25877. [DOI] [PubMed] [Google Scholar]
- van der Heijden LC, Kok MM, Danse PW, Schramm AR, Hartmann M, Löwik MM, Linssen GC, Stoel MG, Doggen CJ, von Birgelen C. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: Insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J. 2016;176:28–35. doi: 10.1016/j.ahj.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Guedeney P, Singh S, Meredith IT, Allocco D, Underwood P, Sorrentino S, Horwitz PA, Giugliano G, Lopez M, Haghighat A, Claessen BE, Gigliotti OS, Othman I, Wang JC, Tamis L, Davis S, Aquino M, Kandzari DE, Mehran R, Batchelor W. Small-vessel PCI outcomes in men, women, and minorities following platinum chromium everolimus-eluting stents: Insights from the pooled PLATINUM Diversity and PROMUS Element Plus Post-Approval studies. Catheter Cardiovasc Interv. 2019;94:82–90. doi: 10.1002/ccd.28071. [DOI] [PubMed] [Google Scholar]
- Tama N, Uzui H, Horita Y, Namura M, Tada H. Initial and late efficacy of everolimus-eluting stents for small and non-small coronary lesions from evaluating delayed late loss study. Heart Vessels. 2017;32:1415–23. doi: 10.1007/s00380-017-1018-z. [DOI] [PubMed] [Google Scholar]
- Caputo R, Leon M, Serruys P, Neumann FJ, Yeung A, Windecker S, Belardi JA, Silber S, Meredith I, Widimský P, Saito S, Mauri L. Performance of the resolute zotarolimus-eluting stent in small vessels. Catheter Cardiovasc Interv. 2014;84:17–23. doi: 10.1002/ccd.25485. [DOI] [PubMed] [Google Scholar]
- Price MJ, Saito S, Shlofmitz RA, Spriggs DJ, Attubato M, McLaurin B, Popma Almonacid A, Brar S, Liu M, Moe E, Mehran R. First Report of the Resolute Onyx 2.0-mm Zotarolimus-Eluting Stent for the Treatment of Coronary Lesions With Very Small Reference Vessel Diameter. JACC Cardiovasc Interv. 2017;10:1381–8. doi: 10.1016/j.jcin.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Song L, Xu B, Gao R, Yang Y, Qiao S, Zhao X, Zhang Y, Gao L, Gao Z, Chen J, Jiang L, Jiang P, Xu J, Tang X, Song Y, Yuan J. Impact of Diabetes Mellitus on Percutaneous Coronary Intervention in Chinese Patients: A Large Single-Center Data. Angiology. 2018;69:540–7. doi: 10.1177/0003319717735226. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Chen Y, Liu X, Xu P, Tang L, Feng L, Zhang D, Wang B, Zheng H, Xia Y, Chen S, Zhang J, Guo J, Lu C, Jiang L, Fang W, Tang Q, Wu Y, Zhou Y, Liu Y, Li H, Li X, He S, Wang L, Jiang J, Jiang T, Li B, Su X, Wang Y, Li Y, Xu Y, Shen Z, Zhang R RESOLUTE-DIABETES CHINA Investigators. Safety and efficacy of zotarolimus-eluting stents in the treatment of diabetic coronary lesions in Chinese patients: The RESOLUTE-DIABETES CHINA Study. J Diabetes. 2019;11:204–13. doi: 10.1111/1753-0407.12832. [DOI] [PubMed] [Google Scholar]
- Levine GN, Mauri L, Smith PK, Sabatine MS, O’Gara PT, Newby LK, Mukherjee D, Mehran R, Mack MJ, Bates ER, Lange RA, Granger CB, Fleisher LA, Fihn SD, Brindis RG, Bittl JA, Smith SC Jr. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–55. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Montalescot G, Zamorano JL, Windecker S, Steg PG, Roffi M, Petricevic M, Neumann FJ, Mauri L, Bueno H, Kolh P, Kastrati A, Jüni P, Jeppsson A, Costa F, Collet JP, Byrne RA, Levine GN ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–60. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- Kedhi E, Latib A, Abizaid A, Kandzari D, Kirtane AJ, Mehran R, Price MJ, Simon D, Worthley S, Zaman A, Brar S, Liu M, Stone GW, Windecker S. Rationale and design of the Onyx ONE global randomized trial: A randomized controlled trial of high-bleeding risk patients after stent placement with 1 month of dual antiplatelet therapy. Am Heart J. 2019;214:134–41. doi: 10.1016/j.ahj.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Mauri L, Kirtane AJ, Windecker S, Yeh RW, Dauerman HL, Price MJ, Christen T, Allocco DJ, Meredith IT, Kereiakes DJ. Rationale and design of the EVOLVE Short DAPT Study to assess 3-month dual antiplatelet therapy in subjects at high risk for bleeding undergoing percutaneous coronary intervention. Am Heart J. 2018;205:110–7. doi: 10.1016/j.ahj.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Urban P, Talwar S, Stoll HP, Greene S, Gregson J, Zambahari R, Oldroyd K, Eberli F, Abdellaoui M, Berland J, Garot P, Meredith IT, Valdes-Chavarri M, Brunel P, Iñiguez A, Richardt G, Lipiecki J, Naber C, Carrié D, Pocock SJ, Abizaid A, Morice MC LEADERS FREE Investigators. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015;373:2038–47. doi: 10.1056/NEJMoa1503943. [DOI] [PubMed] [Google Scholar]