Abstract
We studied 198 macrolide-resistant S. pneumoniae isolates obtained from adults and children to evaluate whether 2-μg clindamycin disks can distinguish between isolates manifesting ermB- versus mefE-mediated resistance to clarithromycin and to determine the relative frequency with which each resistance mechanism occurred in these populations. The mefE gene was predominant among 109 isolates from children, occurring in 73.4% versus 50.6% of 89 isolates from adults. Three isolates (1.5%) did not amplify either gene. Among 125 mefE+ isolates, the MIC of clarithromycin at which 90% of the isolates tested were inhibited, determined by Etest, was 32 μg/ml versus >256 μg/ml in 70 ermB+ isolates. All ermB+ isolates were highly resistant to clindamycin (MICs >256 μg/ml), whereas all mefE+ isolates were susceptible to clindamycin using the 2-μg disk. Testing S. pneumoniae from the respiratory tract for susceptibility to clindamycin by agar disk diffusion is an easy and inexpensive method to estimate the frequency of resistance mediated by ermB in specific patient populations. Macrolide resistance mediated by ermB is usually of greater magnitude than that due to mefE. Clinical studies are needed to determine the significance of high- versus low-level macrolide resistance in S. pneumoniae.
Macrolide resistance in Streptococcus pneumoniae has increased during the 1990s to the extent that over 30% of clinical isolates are now resistant in some communities (16–18). Formerly, it was believed that all macrolide resistance in S. pneumoniae was due to target modification by ermB methylase carried on the conjugative transposon Tn1545, which dimethylates a specific adenine residue in the peptidyl transferase center of 23s rRNA, simultaneously conferring high-level resistance to lincosamides and streptogramin B (MLSB phenotype) (1, 3, 4, 7, 8, 10, 16). More recently, it has been observed that many isolates of S. pneumoniae are susceptible to clindamycin but are resistant to macrolides, including erythromycin and clarithromycin (M phenotype). These isolates have been shown to exhibit a noninducible, macrolide-specific efflux mechanism, encoded by mefE (5, 10–16; K. Y. Gay, D. Miller, M. Farlay, F. Tenover, and D. Stephens, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemotherapy, abstr. C-33, 1998).
We studied 198 macrolide-resistant S. pneumoniae isolates obtained from clinical specimens from adults and children to evaluate whether 2-μg clindamycin disks can accurately distinguish between isolates manifesting ermB- versus mefE-mediated resistance and to determine the relative frequency with which each resistance mechanism occurred in these populations.
MATERIALS AND METHODS
Microorganisms.
One hundred ninety-eight nonduplicate clinical isolates of S. pneumoniae obtained primarily from upper and lower respiratory tract specimens from 109 children in Birmingham, Ala., Greenville-Spartanburg, S.C., and Nashville, Tenn. between 1990 and 1999 were evaluated. Eighty-nine additional isolates obtained from adults in Birmingham, Ala. between 1995 and 1999 were studied for comparative purposes. Isolates were chosen from the culture collections of the investigators based solely on resistance to erythromycin as determined by prior screening on blood agar plates containing erythromycin (1 μg/ml). Isolates were stored frozen at −70°C and then thawed and passaged twice on Trypticase soy agar with 5% sheep blood (Remel, Inc., Lenexa, Kans.). A single colony of each isolate was cloned for susceptibility testing and PCR analysis.
Susceptibility tests.
Clindamycin disks (2 μg; Remel) and Etest strips (AB BIODISK, Piscataway, N.J.) for clindamycin and clarithromycin were used to determine susceptibilities for lincosamide and macrolide antibiotic classes. Testing was performed by the standard agar disk diffusion methodology published by the National Committee for Clinical Laboratory Standards (NCCLS) (9) and according to the manufacturer's instructions for Etest strips, using Mueller-Hinton agar with 5% sheep blood (Remel). Agar plates were incubated for 24 h in an atmosphere supplemented with 5% CO2. NCCLS criteria (9) were used for interpretation of disk diffusion inhibitory zones and Etest MICs. S. pneumoniae ATCC 49619 was used as a control.
Genetic analysis by PCR.
The PCR used in this study, performed with primers complementary to conserved regions in the erm genes, allows detection of MLSB and M phenotype resistance in S. pneumoniae (1, 12–16; Gay et al., 38th ICAAC). Genomic DNA from all clinical isolates was obtained by removing a 1-cm2 area of bacteria grown overnight on Trypticase soy agar with 5% sheep blood (Remel), resuspending it in 50 μl of Millipore water, and boiling it for 5 min. Samples were placed on ice and centrifuged to remove cellular debris. PCRs were prepared using 5 μl of this lysate plus oligonucleotide primers designed to amplify ermB (0.6 kb) and mefE (1.7 kb) at a concentration of 4 μM for each primer (15, 17). Reactions were carried out using Ready-to-Go PCR Beads (Pharmacia Biotech, Piscataway, N.J.) and were subjected to 35 cycles of denaturation at 95°C for 1 min, annealing at 58°C for 2 min, and extension at 72°C for 2 min in a Perkin-Elmer 480 DNA Thermal Cycler (Perkin-Elmer, Norwalk, Conn.). Ten microliters of each reaction mixture was subjected to 1% agarose gel electrophoresis for 1.5 h, and the gel was then stained with ethidium bromide. Positive controls included primers for RR142, a conserved S. pneumoniae sequence of 0.4 kb, to demonstrate the presence of amplifiable DNA and the absence of inhibitors. Negative controls were carried out using primers for both genes with an erythromycin-susceptible isolate.
RESULTS
Table 1 summarizes clarithromycin and clindamycin MICs and clindamycin susceptibility according to agar disk diffusion in relation to the presence of ermB or mefE. The distribution of individual MICs is shown graphically in Fig. 1. Clindamycin susceptibility and resistance results determined by Etest and disk diffusion were 100% concordant, using NCCLS criteria for interpretation (9).
TABLE 1.
Susceptibilities of S. pneumoniae by disk diffusion and Etest according to mechanism of macrolide resistance
| Resistance mechanism | Etest MICs (μg/ml) of:
|
Clindamycin 2-μg disk inhibitory zone range (mm) | |||||
|---|---|---|---|---|---|---|---|
| Clarithromycin
|
Clindamycin
|
||||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | ||
| mefE+(n = 125) | 2–128 | 4 | 32 | ≤0.25 | ≤0.25 | ≤0.25 | 21–31 |
| ermB+(n = 70) | 2–>256a | >256 | >256 | >256 | >256 | >256 | 6b |
| Neither (n = 3) | 4–>256 | — | — | 0.25–64 | — | — | 6b–22 |
Two ermB+ isolates had high-level resistance to clindamycin (>256 μg/ml) but clarithromycin MICs were 2 μg/ml.
The width of the disk is 6 mm. There was no evidence of inhibition surrounding it.
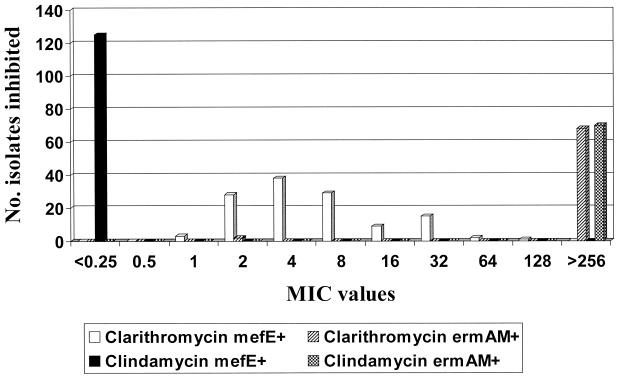
FIG. 1.
Clindamycin and clarithromycin MIC distribution for 195 S. pneumoniae isolates in which either mefE or ermB was amplified.
High-level clindamycin resistance (MICs >256 μg/ml) was observed in all 70 isolates in which ermB was amplified. There were two isolates that expressed the MLSB phenotype (clindamycin MICs, 8 and 64 μg/ml; clarithromycin MICs, >256 μg/ml) that could not be shown to contain either mefE or ermB despite repeated attempts by PCR or by DNA-DNA hybridization. A third isolate that expressed the M phenotype (clindamycin MIC, ≤0.25 μg/ml; clarithromycin MIC, 4 μg/ml) also could not be shown to possess either genetic marker. Among 125 clarithromycin-resistant isolates in which mefE was amplified, all were clindamycin susceptible (M phenotype), and clarithromycin MICs were generally lower than those for the ermB+ isolates. The clarithromycin MIC at which 90% of the isolates tested were inhibited (MIC90) for mefE+ isolates was at least fourfold less than that for the ermB+ isolates (32 and >256 μg/ml, respectively). There were only 2 of 70 (2.9%) ermB+ isolates for which high-level clarithromycin resistance (>256 μg/ml) was not present. In both cases the clindamycin MIC was >256 μg/ml, whereas the clarithromycin MIC was only 2 μg/ml. MIC tests were repeated a second time by Etest and again by broth microdilution, yielding the same results, within 1 dilution.
Table 2 shows the relative frequencies of ermB and mefE in the three pediatric populations and the single adult population studied. Among pediatric isolates, mefE was clearly predominant, accounting for 63.5 to 87% of all macrolide-resistant S. pneumoniae isolates studied, with an average of 73.4%. However, in adult isolates, the frequencies of ermB and mefE were very similar, at 49.9 and 50.6%, respectively. There were no isolates shown to possess both genetic markers.
TABLE 2.
Frequency of mefE and ermB in macrolide-resistant S. pneumoniae isolates from children versus those from adults
| Group | No. (%) of isolates that were:
|
||
|---|---|---|---|
| mefE+ | ermB+ | Neither | |
| Alabama adults (n = 89) | 45 (50.6) | 44 (49.4) | 0 |
| Alabama children (n = 52) | 33 (63.5) | 17 (32.7) | 2 (3.8) |
| South Carolina children (n = 46) | 40 (87) | 5 (10.9) | 1 (2.2) |
| Tennessee children (n = 11) | 7 (63.6) | 4 (36.4) | 0 |
| Total pediatric isolates (n = 109) | 80 (73.4) | 26 (23.9) | 3 (2.2) |
| Total isolates (n = 198) | 125 (63.1) | 70 (35.4) | 3 (1.5) |
DISCUSSION
Disk diffusion has been recommended for determining susceptibilities of S. pneumoniae to erythromycin and clindamycin (3). Erythromycin disks (15 μg) also predict clarithromycin susceptibilities (9), even though actual MICs of the latter are likely to be somewhat lower. We have shown that the 2-μg clindamycin disk can be used to distinguish macrolide resistance mediated by ermB versus mefE in clinical isolates of S. pneumoniae. Isolates containing ermB were highly resistant to clindamycin in every instance and had no detectable inhibitory zones surrounding the disks. In contrast, isolates containing mefE were uniformly susceptible to clindamycin using either MICs or disk inhibitory zone data (9). Although clindamycin resistance was present in every instance in which the ermB gene could be demonstrated in macrolide-resistant S. pneumoniae, a small percentage of macrolide-resistant isolates, 1.5%, do not amplify ermB or mefE genes. Thus, a different mechanism of resistance is operative, possibly related to a mutation in ribosomal RNA, and clindamycin activity can be variable (4, 14).
Susceptibility or resistance to clindamycin in macrolide-resistant S. pneumoniae also gives clues to the magnitude of macrolide resistance in most instances. Among 125 mefE+ isolates, clarithromycin MICs were ≤32 μg/ml in 122 (97.6%) isolates versus >256 μg/ml in 68 of 70 (97.1%) ermAM+ isolates. The MIC90 for mefE+ isolates (32 μg/ml) was identical to that reported by Johnston et al. (4) and eightfold higher than the findings of Shortridge et al. (13). However, both of these studies were performed using broth microdilution cultures incubated in ambient air as opposed to agar-gradient diffusion with Etests incubated in 5% CO2 where MICs of macrolides for S. pneumoniae macrolides may be elevated by 1 to 2 dilutions. Despite increasing macrolide resistance in S. pneumoniae amidst widespread usage of these drugs as empiric therapy for respiratory infections, reports of macrolide treatment failures are relatively uncommon. This observation may be related to the predominance of low-level resistance mediated by mefE in some populations and to the favorable bronchopulmonary pharmacokinetics of drugs such as clarithromycin. Concentrations of clarithromycin in epithelial lining fluid are substantially higher than the MICs for many mefE+ isolates (11–13). Outcome-based clinical studies with microbiologic data will be necessary to fully understand the clinical significance of low-level macrolide resistance in mefE+ isolates of S. pneumoniae.
Clindamycin MIC breakpoints and inhibitory zone diameters for S. pneumoniae have been included in NCCLS documents (9), but no recommendations have been made for routine testing of clindamycin against this organism, apparently because of the belief that macrolide resistance is predominantly of the MLSB type, and possibly because macrolide resistance has become more widespread in the United States only within the past few years. However, clindamycin is often the only oral agent available for treating children with pneumococcal infections caused by beta-lactam- and/or macrolide-resistant organisms since fluoroquinolones are not approved for general use in this population, and it may become more widely used in pediatrics when a pneumococcal etiology of an infection is known or strongly suspected. Clinical laboratories should routinely test and report the drug, and the NCCLS should consider adding it to their list of suggested agents for testing against this organism.
The final observations from this study relate to epidemiologic data for various patient populations. Although there have been limited reports of the frequency of mefE in macrolide-resistant S. pneumoniae isolates, varying from 54 to 85% (4, 13, 16; Gay et al., 38th ICAAC), this is the first study to describe the frequency of these genes specifically in pneumococcal isolates from pediatric patients from different geographic locations and to compare the frequencies of these genes in isolates from children and adults in the same community. Based on our preliminary findings, mefE accounted for the majority of macrolide resistance in pneumococci from pediatric populations in three southeastern states, ranging from 63.5 to 87%, in contrast to isolates from adults from Alabama in whom rates of mefE and ermB occurrence were similar, each accounting for approximately 50% of the macrolide-resistant isolates. S. pneumoniae isolates obtained from children are often different from those found in adults. Therefore, it is not surprising that we observed different frequencies of mefE in children and adults from the same geographic area.
In summary, we suggest that testing clindamycin susceptibility routinely in respiratory isolates of S. pneumoniae is desirable in view of the fact that this drug is often the most active oral agent, other than quinolones, against this organism. Testing S. pneumoniae with clindamycin and erythromycin disks is an easy and inexpensive method to estimate the frequency of MLSB resistance. Since treatment of pneumococcal respiratory infections is usually empiric and bacterial isolates are often unavailable for testing, this information can be valuable for patient care.
ACKNOWLEDGMENTS
The technical assistance of Eneida Brookings and Shawn Banks is gratefully acknowledged.
REFERENCES
- 1.Arthur M, Molinas C, Mabilat C, Courvalin P. Detection of erythromycin resistance by the polymerase chain reaction using primers in conserved regions of erm rRNA methylase genes. Antimicrob Agents Chemother. 1990;34:2024–2026. doi: 10.1128/aac.34.10.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark C, Jacobs M R, Appelbaum P C. Antipneumococcal activities of levofloxacin and clarithromycin as determined by agar dilution, microdilution, E-test, and disk diffusion methodologies. J Clin Microbiol. 1998;36:3579–3584. doi: 10.1128/jcm.36.12.3579-3584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasola E L, Bajksouzian S, Appelbaum P C, Jacobs M R. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston N J, de Azavedo J C, Kelner J D, Low D E. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2424–2426. doi: 10.1128/aac.42.9.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones R N, Cormican M C, Wanger A. Clindamycin resistance among erythromycin-resistant Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 1996;25:201–204. doi: 10.1016/s0732-8893(96)00100-9. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen J H, Swenson J M, Tenover F C, Barry A, Ferraro M J, Murray P R, Reller L B. Development of interpretive criteria and quality control limits for macrolide and clindamycin susceptibility testing of Streptococcus pneumoniae. J Clin Microbiol. 1996;34:2679–2684. doi: 10.1128/jcm.34.11.2679-2684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeClercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeClercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: 9th informational supplement, M100-S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 10.Nishijima T, Saito Y, Aoki A, Toriya M, Toyonaga Y, Fujii R. Distribution of mefE and ermB genes in macrolide-resistant strains of Streptococcus pneumoniae and their variable susceptibility to various antibiotics. J Antimicrob Chemother. 1999;43:637–643. doi: 10.1093/jac/43.5.637. [DOI] [PubMed] [Google Scholar]
- 11.Patel K, Xuan D, Tessier P R, Nightingale C, Russomanno J, Quintiliani R. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob Agents Chemother. 1996;40:2375–2379. doi: 10.1128/aac.40.10.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodvold K A, Gottfried M H, Dansiger L H, Servi R J. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob Agents Chemother. 1997;41:1399–1402. doi: 10.1128/aac.41.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shortridge V D, Doern G, Bruggemann A, Beyer J, Flamm R K. Prevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995. Clin Infect Dis. 1999;29:1186–1188. doi: 10.1086/313452. [DOI] [PubMed] [Google Scholar]
- 14.Shortidge V D, Flamm R K, Ramer N, Beyer J, Tanaka S K. Novel mechanism of macrolide resistance in Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 1996;26:73–78. doi: 10.1016/s0732-8893(96)00183-6. [DOI] [PubMed] [Google Scholar]
- 15.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waites K B, Swiatlo E, Gray B, Brookings E. In vitro activities of oral antimicrobial agents against penicillin-resistant Streptococcus pneumoniae: implications for outpatient treatment. S Med J. 1997;90:621–626. doi: 10.1097/00007611-199706000-00008. [DOI] [PubMed] [Google Scholar]