Abstract
Simple Summary
Currently, clinical studies exploring the impact of high body fat on toxicities after receiving immune checkpoint inhibitors (ICIs) among cancer patients are limited. Here, we analyze data from a health care system serving the mid-Atlantic geographic region to assess how body fat can affect the development of toxicities of ICIs. In our study, body mass index (BMI) was used as the measure of body fat, and the results suggested that cancer patients with a high BMI were more likely to have toxicities after receiving ICIs. Our study suggests that symptom management should be incorporated in the cancer care continuum of patients who receive ICIs, especially those with high BMI. In clinical settings, oncologists should inform cancer patients receiving ICIs with high BMI that their risk of post-treatment toxicities can be higher compared to their counterparts with lower BMI.
Abstract
Evidence regarding the association between body mass index (BMI) and immune-related adverse events (irAEs) among cancer patients receiving immune checkpoint inhibitors (ICIs) is limited. Here, we use cross-sectional hospital-based data to explore their relationship. Pre-treatment BMI was treated as an ordinal variable (<25, 25 to ≤30, ≥30 kg/m2). The outcome of interest was irAEs after ICI initiation. A multivariable logistic regression model estimated the adjusted odds ratio (aOR) and 95% confidence interval (CI) of BMI. A total of 684 patients with stage III or IV cancer were included in the study (lung: 269, melanoma: 204, other: 211). The mean age at the first dose of ICI was 64.1 years (SD = 13.5), 394 patients (57.6%) were male, and over one-third (N = 260, 38.0%) were non-White. Overall, 52.9% of patients had BMI ≥ 25 kg/m2 (25 to ≤30: 217, ≥30: 145) and 288 (42.1%) had irAEs after ICI treatment. Patients with higher BMI tended to have a higher rate of irAEs (<25: 35.7%, 25 to ≤30: 47.0%, ≥30: 49.0%). The multivariable logistic regression yielded consistent results (BMI ≥ 30 vs. BMI < 25: aOR = 1.47, 95% CI = 0.96–2.23; 25 ≤ BMI < 30 vs. BMI < 25: aOR = 1.46, 95% CI = 1.02–2.11, p-trend = 0.04). In conclusion, among patients with advanced cancer receiving ICIs, the rate of irAEs appears to be higher among those with higher BMI.
Keywords: immune checkpoint inhibitor, body mass index, immune-related adverse events, epidemiology
1. Introduction
Immune checkpoint inhibitors (ICIs), such as anti-cytotoxic T lymphocyte-associated antigen 4 (anti-CTLA-4), anti-programmed cell death 1 (anti-PD-1), and anti-programmed death-ligand 1 (anti-PD-L1), have revolutionized the management of malignant tumors, especially those at advanced stages [1,2,3]. By unleashing immune activity, ICIs may facilitate cancer regression and improve survival when administered as either first-line therapy or after other treatment modalities have failed [4,5,6]. However, as indications for ICI therapy have expanded to treat increasing numbers of patients, there are growing concerns regarding immune-related adverse events (irAEs), including potentially severe adverse events affecting major organ function and/or quality of life [7,8,9,10]. Some fatal outcomes related to irAEs have been reported as well [11,12]. In 2018, the American Society of Clinical Oncology and the National Comprehensive Cancer Network published guidelines regarding the management of adverse events associated with immunotherapy [13,14].
Body mass index (BMI)—an index that can be easily measured in clinical settings—has been widely used to assess body fat in human subjects [15]. Clinical evidence suggests that high body fat can increase the likelihood of developing treatment toxicities in cancer patients receiving systemic treatment by affecting multiple signaling pathways (e.g., inflammatory, metabolic) [16]. Thus, improving our understanding of the impact of BMI on the safety of ICIs is important for clinical practice, particularly in terms of identifying patient subgroups that may require additional monitoring during therapy. Notably, extant studies evaluating the relationship between BMI and irAEs in patients with cancer receiving ICIs are limited due to the relative novelty of this treatment modality versus other cancer therapies that have been used for decades. In addition, previous studies of associations between BMI and irAEs in patients receiving ICIs have methodological limitations [17,18,19], such as limited racial/ethnic diversity of the study population and small sample sizes with potentially large random errors, possibly compromising their robustness.
To elucidate the association between BMI and irAEs induced by ICIs and provide relevant evidence in the management of patients with advanced-stage cancer, we conducted a cross-sectional analysis of diverse patients with advanced-stage cancer treated within a health care system serving the mid-Atlantic geographic region.
2. Methods
2.1. Immuno-Oncology Database and Study Population
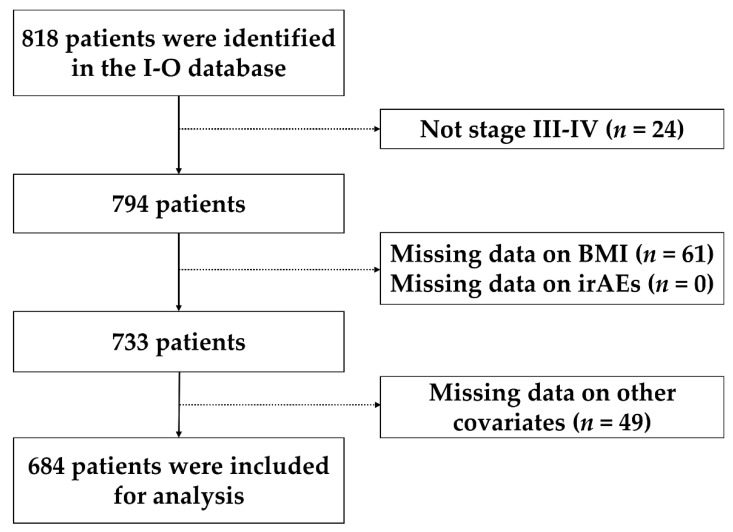
We used a centralized research data warehouse for immuno-oncology (I-O) database built for real-world data analysis over a 7-year period. The comprehensive I-O database at MedStar Health Hospitals was developed to track and assess all patients with advanced cancer treated with ICIs. Between January 2011 and April 2018, information was collected retrospectively on patients treated with ICIs at five MedStar Health hospitals (two DC area hospitals: MedStar Georgetown University Hospital, MedStar Washington Hospital Center; and three hospitals at Baltimore: MedStar Franklin Square Hospital, MedStar Good Samaritan Hospital, and MedStar Union Memorial Hospital). A total of 818 subjects treated with ICIs were identified in the I-O database, and they were included in the analysis if they had the following characteristics: (1) had stage III or IV cancer, (2) had pre-treatment BMI, (3) information on irAEs was not missing, and (4) had no missing values of other study covariates. This yielded a total of 684 (83.6%) participants for analysis (Figure 1).
Figure 1.
Flowchart for study participants selection. Abbreviations: BMI: body mass index, irAEs: immune-related adverse events.
2.2. Measures
The exposure of interest in our study was pre-treatment BMI. Specifically, patients’ pre-treatment height and body weight were extracted using SQL queries from electronic health records. BMI was calculated as body weight (kg) divided by height (m) squared and categorized as an ordinal variable (BMI < 25, 25 ≤ BMI < 30, and BMI ≥ 30) based on cutoffs suggested by the World Health Organization [20]. The outcome of interest was irAEs, which were verified for each patient by the investigators using Common Terminology Criteria for Adverse Events (CTCAE) v 4.03. The I-O database measures the following 15 adverse health events: colitis, hepatitis, skin rash, pruritus, other skin toxicities, pneumonitis, hypothyroidism, hyperthyroidism, hypophysitis, other endocrine toxicities, immune-related joint pain/arthritis, immune-related neurological toxicity, immune-related hematological toxicity, musculoskeletal toxicity, and other toxicities. For the current analysis, we treated irAEs as a binary variable (no irAEs vs. had any irAEs). Pharmacy records were used to identify the history of ICI receipt, which included detailed information regarding the utilization of anti-PD-1 pathway blockers (nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab), anti-CTLA-4 (ipilimumab), and combined modalities (ipilimumab plus nivolumab). Other relevant covariates regarding disease, patients’ demographic characteristics, and health-related behaviors were extracted from the electronic health records using SQL queries as well. Age at the first dose of ICI, sex, race, and smoking status at ICI initiation (never, current, former smoker) were obtained from patients’ self-reported information in health records. A total of 34 types of pre-existing comorbidities, which incorporated cardiovascular diseases, metabolic disorders, kidney disease, psychological disorders, prior cancer history, and infectious diseases, were considered for this current study. Patients’ functional status in terms of their ability to care for themselves, daily activity, and physical ability was reflected by their pre-treatment Eastern Cooperative Oncology Group (ECOG) Performance Status (PS); this index ranges from 0 to 5, with 0 suggesting no functional impairment and 5 indicating death [21].
2.3. Statistical Analysis
In the analysis, we first reported the number of observations and distribution of study covariates in the overall sample and by the number of irAEs (0, 1, and ≥2). A chi-squared test was used to examine if the distribution of study covariates differed by the number of irAEs; if the number of observations in any cell was smaller than 5, Fisher’s exact test was used. A descriptive summary of distribution of each individual type of irAE was conducted as well. The number of patients with irAEs and the rate of irAEs were summarized according to pre-treatment BMI categories, and we calculated point estimates as well as the 95% confidence interval of the irAE rate by BMI categories. An unadjusted and multivariable logistic regression model was used to estimate crude and adjusted odds ratios (ORs) for BMI, respectively; in these models, BMI < 25 kg/m2 was treated as the reference. The multivariable model adjusted for age (≤54, 55–64, 65–74, and ≥75 years), race (White, Black, and other), sex (male and female), smoking status (never, former, and current smoker), metastasis (yes vs. no), and lines of therapy (1 vs. 2+). The selection of confounders was based on a priori knowledge regarding the impacts of these covariates on BMI and treatment toxicities [22,23,24,25,26]. Specifically, smoking status was treated as an indicator of lifestyle behaviors that can affect BMI. Patients with metastatic cancer were more likely to have frailty that could affect body composition and risk of treatment toxicity [27], and patients receiving ICIs as the second (or more) line of therapy could have a differential physiological profile induced by prior therapies, which impacts metabolic homeostasis [28], suggesting that metastasis and line of therapy might be confounders between BMI and irAEs. A test for trend was conducted by treating BMI as a continuous variable in the model. As to the dose–response relationship between BMI and irAEs, we used a restricted cubic spline in the multivariable logistic regression model; specifically, we treated BMI = 21.8 kg/m2 as the reference in the dose–response curve because it was the mean value among patients with BMIs lower than 25 kg/m2. We assessed the non-linearity of the dose–response curve by comparing the model fit of the restricted cubic spline with a model fit assuming linearity for BMI via a likelihood ratio test [29]. A histogram was depicted along with the dose–response curve to summarize the distribution of pre-treatment BMI in our study population. We applied multiple imputations fit with 5 replicates of chained equations to assess if missing data impacted the association pattern of BMI. Subgroup analyses were conducted by age (<65 vs. ≥65 years), sex (male vs. female), race (White vs. non-White), burden of comorbidity (no multimorbidity vs. had multimorbidity), pre-treatment ECOG PS (<2 vs. ≥2), cancer type (lung vs. melanoma), dose of ICI (1–4 vs. ≥5), and ICI (nivolumab, pembrolizumab, and ipilumumab) using the same multivariable model. We chose multimorbidity and ECOG PS as an indicator for stratification because previous studies suggested that a higher burden of co-existing illnesses was associated with chronic inflammation [30,31] which could be a mediator between BMI and irAEs. We specifically compared association patterns between different cancer types, ICIs, and dose due to the potential biological heterogeneity across them. In subgroup analyses, BMI was treated as a binary variable (BMI ≥ 25 vs. BMI < 25) for sample size consideration, and we tested for interaction effects between BMI and the aforementioned factors used for subgroup analyses via a Wald test. In sensitivity analysis, we applied a multivariable linear regression model to investigate association between pre-treatment BMI and number of irAEs; in this linear regression, we adjusted for the same set of covariates as the primary logistic regression and estimated adjusted mean difference (aMD) and 95% CI for pre-treatment BMI. Two-sided p-values <0.05 were considered to be statistically significant. All statistical analyses were conducted in Stata version 15.0 (StataCorp, LLP, College Station, TX, USA).
3. Results
Table 1 presents the characteristics of our study population by number of irAEs. A total of 288 (42.1%) participants had at least 1 irAE (164 had 1 and 124 had ≥2). The study population had a mean age at ICI initiation of 64.1 years (SD = 13.5); 57.6% of them were male, and 62.0% and 26.6% self-reported as White or Black, respectively. Over half of patients had a smoking history (former: 48.3%, current: 9.6%), 12.0% were living without comorbidities, and over three-fourths (78.5%) of patients had ECOG PS < 2. Among these patients, 39.3% and 29.8% had lung cancer or melanoma, respectively, and 82.5% had documented metastases. Over one-third of patients (38.3%) received ICI as their first-line therapy. Over half (54.4%) of patients received less than 5 doses of ICIs. Nivolumab (38.5%), pembrolizumab (27.9%), and ipilimumab (11.8%) were the most commonly used ICIs, and about ten percent (10.4%) of patients used nivolumab plus ipilumumab. Patients with a higher number of irAEs were more likely to be White, have fewer baseline comorbidities or lower ECOG PS scores, have melanoma, use ICI as the first-line therapy, receive a higher number of doses of ICIs, and have non-pembrolizumab ICIs (ps < 0.05). Rates of colitis (10.1%), hepatitis (9.8%), skin rash (16.4%), pruritus (5.0%), and hypothyroidism (7.9%) were relatively higher compared to other irAEs (Table S1).
Table 1.
Summary of study characteristics.
| Variables | Overall (N = 684) | irAEs | p-Value * | ||
|---|---|---|---|---|---|
| 0 (N = 396) | 1 (N = 164) | ≥2 (N = 124) | |||
| N (%) | N (%) | N (%) | N (%) | ||
| Age at first dose (years) | |||||
| ≤54 | 147 (21.5) | 77 (19.4) | 36 (21.9) | 34 (27.4) | 0.50 |
| 55–64 | 175 (25.6) | 98 (24.8) | 44 (26.8) | 33 (26.6) | |
| 65–74 | 207 (30.3) | 124 (31.3) | 48 (29.3) | 35 (28.2) | |
| ≥75 | 155 (22.7) | 97 (24.5) | 36 (21.9) | 22 (17.7) | |
| Sex | |||||
| Male | 394 (57.6) | 224 (56.6) | 88 (53.7) | 82 (66.1) | 0.09 |
| Female | 290 (42.4) | 172 (43.4) | 76 (46.3) | 42 (33.9) | |
| Race | |||||
| White | 424 (62.0) | 220 (55.6) | 107 (65.2) | 97 (78.2) | <0.01 |
| Black | 182 (26.6) | 130 (32.8) | 39 (23.8) | 13 (10.5) | |
| Other | 78 (11.4) | 46 (11.6) | 18 (11.0) | 14 (11.3) | |
| Smoking status | |||||
| Never | 288 (42.1) | 156 (39.4) | 69 (42.1) | 63 (50.8) | 0.27 |
| Former | 330 (48.3) | 201 (50.8) | 78 (47.6) | 51 (41.1) | |
| Current | 66 (9.6) | 39 (9.8) | 17 (10.4) | 10 (8.1) | |
| Comorbidities | |||||
| 0 | 82 (12.0) | 40 (10.1) | 18 (11.0) | 24 (19.4) | 0.04 |
| 1 | 131 (19.2) | 80 (20.2) | 30 (18.3) | 21 (16.9) | |
| 2 | 157 (22.9) | 88 (22.2) | 34 (20.7) | 35 (28.2) | |
| ≥3 | 314 (45.9) | 188 (47.5) | 82 (50.0) | 44 (35.5) | |
| ECOG PS at first dose | |||||
| 0 | 189 (27.6) | 75 (18.9) | 52 (31.7) | 62 (50.0) | <0.01 |
| 1 | 348 (50.9) | 203 (51.3) | 87 (53.1) | 58 (46.8) | |
| ≥2 | 147 (21.5) | 118 (29.8) | 25 (15.2) | 4 (3.2) | |
| Cancer type | |||||
| Lung | 269 (39.3) | 184 (46.5) | 59 (36.0) | 26 (21.0) | <0.01 |
| Melanoma | 204 (29.8) | 75 (18.9) | 53 (32.3) | 76 (61.3) | |
| Other | 211 (30.9) | 137 (34.6) | 52 (31.7) | 22 (17.7) | |
| Metastasis | |||||
| No | 120 (17.5) | 62 (15.7) | 35 (21.3) | 23 (18.6) | 0.26 |
| Yes | 564 (82.5) | 334 (84.3) | 129 (78.7) | 101 (81.4) | |
| Lines of ICI therapy | |||||
| 1 | 262 (38.3) | 126 (31.8) | 63 (38.4) | 73 (58.9) | <0.01 |
| 2 | 290 (42.4) | 177 (44.7) | 73 (44.5) | 40 (32.3) | |
| ≥3 | 132 (19.3) | 93 (23.5) | 28 (17.1) | 11 (8.9) | |
| ICI dose | |||||
| 1–2 | 186 (27.2) | 140 (35.4) | 29 (17.7) | 17 (13.7) | <0.01 |
| 3–4 | 186 (27.2) | 98 (24.7) | 52 (31.7) | 36 (29.0) | |
| 5–10 | 156 (22.8) | 83 (21.0) | 45 (27.4) | 28 (22.6) | |
| ≥11 | 156 (22.8) | 75 (18.9) | 38 (23.2) | 43 (34.7) | |
| ICI modalities | |||||
| Nivolumab | 263 (38.5) | 181 (45.7) | 57 (34.8) | 25 (20.2) | <0.01 |
| Pembrolizumab | 191 (27.9) | 125 (31.6) | 41 (25.0) | 25 (20.2) | |
| Ipilimumab | 81 (11.8) | 32 (8.1) | 27 (16.5) | 22 (17.7) | |
| Nivolumab + Ipilumumab | 71 (10.4) | 13 (3.3) | 18 (11.0) | 40 (32.3) | |
| Other | 78 (11.4) | 45 (11.4) | 21 (12.8) | 12 (9.7) | |
Abbreviations: ECOG PS: Eastern Cooperative Oncology Group Performance Status, ICI: immune checkpoint inhibitor, irAEs: immune-related adverse events. * A chi-square test was used to calculate the p-value, and Fisher’s exact test was applied if the number in the cell was smaller than 5.
The mean BMI was 26.1 kg/m2 (SD = 5.9); over half (52.9%) of the patients had BMI ≥ 25 kg/m2 (25 to ≤30: 217, ≥30: 145). The rate of irAEs became higher as BMI increased, although the point estimates were similar between overweight and obese patients (BMI < 25: 35.7%, 25 ≤ BMI < 30: 47.0%, BMI ≥ 30: 49.0%). The unadjusted model suggested positive associations between BMI and irAEs, and this pattern did not change in the multivariable model (BMI ≥ 30 vs. BMI < 25: aOR = 1.47, 95% CI = 0.96–2.23, 25 ≤ BMI < 30 vs. BMI < 25: aOR = 1.46, 95% CI = 1.02–2.11, p-trend = 0.04) (Table 2). Effect measures of other covariates in the multivariable logistic regression model are present in Table S2.
Table 2.
Association between pre-treatment BMI with irAEs (N = 684).
| Pre-treatment BMI (kg/m2) | Had irAEs/Total | Rate (%) of irAEs and 95% CI | cOR and 95% CI | aOR and 95% CI |
|---|---|---|---|---|
| <25 | 115/322 | 35.7 (30.7, 41.1) | REF | REF |
| 25 to <30 | 102/217 | 47.0 (40.0, 53.7) | 1.60 (1.12, 2.27) | 1.46 (1.02, 2.11) |
| ≥30 | 71/145 | 49.0 (40.9, 57.0) | 1.73 (1.16, 2.57) | 1.47 (0.96, 2.23) |
| p-trend < 0.01 | p-trend = 0.04 |
Abbreviations: aOR: adjusted odds ratio, BMI: body mass index, CI: confidence interval, cOR: crude odds ratio, irAEs: immune-related adverse events. The multivariable model adjusted for age, race, sex, smoking status, metastasis, and lines of therapy.
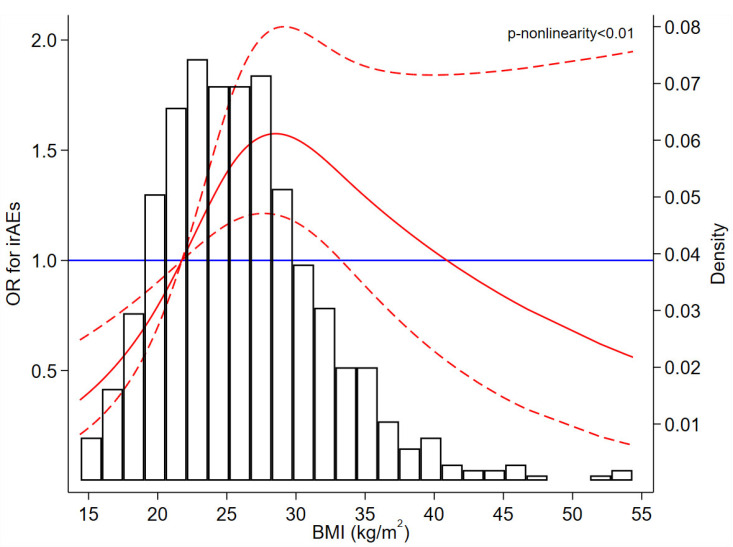
The dose–response curve showed a non-linear relationship between BMI and irAEs, and the curve was statistically significant when BMI was lower than approximately 34 kg/m2; specifically, the odds of irAEs reached the highest value when BMI was about 29 kg/m2 (Figure 2). The histogram shows that only a small fraction of participants had extremely high pre-treatment BMI (Figure 2). For example, only 47 (6.9%) patients had pre-treatment BMI ≥ 35 kg/m2 and 17 (2.5%) had pre-treatment BMI ≥ 40 kg/m2. The results obtained after multiple imputations were largely unchanged (Table S3).
Figure 2.
Plot depicting the distribution of pre-treatment BMI and the dose–response relationship between pre-treatment BMI and irAEs. BMI = 21.8 was treated as a reference in the dose-response curve. The solid red line is the fitted line, red dash lines are 95% confidence intervals of OR, and the blue solid line is the reference line. Abbreviations: BMI: body mass index, irAEs: immune-related adverse events, OR: odds ratio.
Results of subgroup analyses are presented in Table 3. Significant interactions were identified for age and multimorbidity. The association between overweight (BMI ≥ 25 vs. BMI < 25) and irAEs was positively significant for patients younger than 65 years, whereas the association for older participants (≥65 years) was almost null (<65: aOR = 2.18, 95% CI = 1.36–3.51, ≥65: aOR = 1.08, 95% CI = 0.69–1.69, p-interaction = 0.02). Among patients without multimorbidity, people with higher BMI had a 3.20-fold relative increase in odds of having irAEs (BMI ≥ 25 vs. BMI < 25: aOR = 4.20, 95% CI = 2.11–8.37), but the association was null for those with multimorbidity (BMI ≥ 25 vs. BMI < 25: aOR = 1.03, 95% CI = 0.67–1.52, p-interaction < 0.01). In addition, the Wald test did not suggest a significant interaction between ICI type and overweight in relation to irAEs (for nivolumab: aOR[BMI ≥ 25 vs. BMI < 25] = 0.93, 95% CI = 0.54–1.60; for pembrolizumab: aOR[BMI ≥ 25 vs. BMI < 25] = 1.92, 95% CI = 1.00–3.71; for ipilumumab: aOR[BMI ≥ 25 vs. BMI < 25] = 1.21, 95% CI = 0.55–2.65; p-interaction = 0.21), although the point estimates were largely different.
Table 3.
Association of pre-treatment BMI (≥25 vs. <25 kg/m2) with irAEs in subgroups.
| Subgroup | Had irAEs/Total (%) | aOR and 95% CI | p-Interaction |
|---|---|---|---|
| Age at first dose of ICI (years) | |||
| <65 | 147/322 (45.7) | 2.18 (1.36, 3.51) | 0.02 |
| ≥65 | 141/362 (39.0) | 1.08 (0.69, 1.69) | |
| Sex | |||
| Male | 170/394 (43.1) | 1.31 (0.84, 2.02) | 0.29 |
| Female | 118/290 (40.7) | 1.73 (1.04, 2.89) | |
| Race | |||
| White | 204/424 (48.1) | 1.72 (1.13, 2.61) | 0.18 |
| Non-white | 84/260 (32.3) | 1.16 (0.67, 1.99) | |
| Multimorbidity | |||
| No | 93/213 (43.7) | 4.20 (2.11, 8.37) | <0.01 |
| Yes | 195/471 (41.4) | 1.03 (0.69, 1.52) | |
| Pre-treatment ECOG | |||
| <2 | 259/537 (48.2) | 1.48 (1.03, 2.13) | 0.50 |
| ≥2 | 29/147 (19.7) | 1.01 (0.41, 2.48) | |
| Cancer type | |||
| Lung | 85/269 (31.6) | 1.34 (0.78, 2.33) | 0.99 |
| Melanoma | 129/204 (63.2) | 1.32 (0.69, 2.51) | |
| ICI dosage | |||
| 1–4 | 134/372 (36.1) | 1.29 (0.81, 2.05) | 0.92 |
| ≥5 | 154/312 (49.4) | 1.42 (0.87, 2.30) | |
| ICI type | |||
| Nivolumab † | 82/263 (31.2) | 0.93 (0.54, 1.60) | 0.21 |
| Pembrolizumab ‡ | 66/191 (34.6) | 1.92 (1.00, 3.71) | |
| Ipilumumab § | 107/152 (70.4) | 1.21 (0.55, 2.65) |
Abbreviations: aOR: adjusted odds ratio, BMI: body mass index, CI: confidence interval, ECOG PS: Eastern Cooperative Oncology Group Performance Status, ICI: immune checkpoint inhibitor, irAEs: immune-related adverse events. The multivariable model was adjusted for the same set of covariates as the primary analysis except the variable used for stratification. † Among these patients, 133 (50.6%) had lung cancer, 22 (8.4%) had melanoma, and 108 (41.0%) had other types of cancer. ‡ Among these patients, 78 (40.8%) had lung cancer, 39 (20.4%) had melanoma, and 74 (38.8%) had other types of cancer. § Among these patients, 71 (46.7%) used ipilumumab plus nivolumab. A total of 5 (3.3%) patients had lung cancer, 138 (90.8%) had melanoma, and 9 (5.9%) had other types of cancer.
Although point estimates obtained from multivariable linear regression were non-significant (Table S4), the p-trend (0.045) suggested statistical significance and indicated a potential positive dose–response relationship between BMI and the severity of irAEs.
4. Discussion
Overall, our research suggests that about 40% of patients with advanced-stage cancer receiving ICIs had irAEs. In the multivariable model, patients with a higher BMI at ICI initiation had a higher rate of irAEs compared to patients with BMI under 25 kg/m2. Although the dose–response curve becomes non-significant when BMI is higher than about 34 kg/m2, the significant part of the curve suggests that patients who are overweight or obese are at higher odds of irAEs than those with lower BMI. We speculate that the wide 95% CI and inverse association observed in the right tail of the dose–response curve are caused by sparse data of observations with high BMI. The subgroup analyses of age and multimorbidity indicate that the impact of BMI on irAEs is much stronger among younger and healthier patients. One potential reason is that people with younger age and lower burden of comorbidities have more favorable homeostasis and are less likely to have immunosenescence [32,33]; this suggests that younger and healthier patients tend to have a higher likelihood of treatment-induced immune reactivity, which can be an upstream event of irAEs [34,35]. Thus, high BMI [36,37] synergistically interacts with treatment-induced immunity and increases the likelihood of irAEs among these younger and healthier patients with cancer, whereas these synergistic effects are not observed in older and more vulnerable patients because they may not have a strong response to ICIs due to immune senescence. The impact of BMI appears to be more substantial in patients receiving pembrolizumab than nivolumab and ipilumumab, although there is no significant interaction. However, the distributions of cancer type for each individual ICI treatment modality were quite different; thus, we cannot determine if the magnitude of interaction between BMI and ICI is affected by the heterogeneity of cancer type.
To our knowledge, there are two meta-analyses exploring the association between BMI and irAEs among patients with cancer receiving ICIs, and a total of 10 effect measures of BMI from published studies or abstracts were included for quantitative synthesis in these meta-analyses [38,39]. Although both of them concluded that high BMI was associated with a higher rate of irAEs, methodological limitations in the meta-analyses and included studies should be considered. Particularly, only 3 [17,18,19] of these effect measures included for synthesis were from published original studies that adjusted for potential confounders, indicating that the results of these meta-analyses could be biased to some extent. Among the studies included in the aforementioned meta-analyses, Daly et al. analyzed 84 patients with stage IV melanoma treated with ipilimumab and found that overweight (BMI ≥ 25 kg/m2) was associated with a 3-fold increase in odds of irAEs (OR = 4.01, 95% CI = 1.03–15.69) [18]. Another study conducted in France investigated 92 patients with stage IV cancer (lung, kidney, or melanoma) and found a positive association between BMI and irAEs (BMI ≥ 25 vs. <25: OR = 5.94, 95% CI = 1.25–28.29) [19]. However, the wide 95% CIs in these studies indicate that statistical imprecision may be induced by their small sample sizes. Our study used a larger sample size and investigated the relationship in different subgroups; this induces less random error and provides evidence for oncologists so that they can better identify patients whose risk of irAEs is more likely to be affected by high BMI.
The underlying mechanisms regarding the effects of BMI on irAEs remain to be determined. One speculation is that pharmacokinetic changes induced by obesity can impact the absorption, distribution, metabolism, and excretion of ICIs, and such change has the potential to affect the risk of irAEs following the utilization of ICIs [18]. Another explanation is that the ICI dosage was based on patients’ weight before 2018 [40]; thus, our patients with higher BMI (who were mainly treated before 2018) were more likely to have received a higher dose of ICI, which could ultimately increase the likelihood of irAEs. However, a flat dose has been widely used in ICI treatment modalities after 2018, indicating that the “BMI-dose–irAEs” relationship may be diminished to some extent for cancer patients receiving ICIs currently.
Our study has several methodological strengths. Health information was obtained directly from medical records, which is more valid than self-reports. The large sample size of our dataset ensured good power and precision in effect estimates. As compared to previously published studies investigating a similar topic, we examined the interaction between BMI and other covariates in relation to irAEs, which allowed us to explore factors inducing heterogeneity in the association between BMI and irAEs in cancer patients. The application of restricted cubic spline allowed us to assess the impact of BMI on irAEs from a non-linear dose–response perspective. However, several limitations should be noted when interpreting our results. First, this is a cross-sectional analysis without follow-up; thus, we are unable to conduct a time-to-event analysis to assess how soon these irAEs occurred. Second, to ensure better statistical power, we pooled cases of all types of cancer in the sample, which might have introduced some clinical heterogeneity because the underlying pathogenesis characteristics of various types of cancer can be different. Similarly, we summed all types of irAEs in logistic regressions since the number of each individual irAE was small. Although all of these study participants received ICIs, the specific type of ICI can be different. In clinical practice, nivolumab is more likely to be used for lung cancer, whereas ipilimumab is usually prescribed for melanoma; furthermore, 152 patients in our study received ipilumumab, but about half (n = 71) of them received nivolumab simultaneously, which made it hard for us to explore the effects of BMI on toxicities induced by each type of ICI among this subgroup. All these heterogeneities suggest that future studies with larger samples of irAEs and more homogeneous populations are needed to further disentangle the relationship between BMI and irAEs. Lastly, since both ICI utilization and the pre-existing burden of comorbidities can contribute to the development of adverse events in our study population, we could not determine if these adverse events were caused by ICIs or the synergistic effects between ICIs and comorbidities.
Our study has some health implications. In clinical settings, patients with advanced-stage cancer and a high BMI should be informed of the potential higher likelihood of irAEs before receiving ICI treatment. Oncologists should inform cancer patients with high BMI that their risk of irAEs can be higher compared to their counterparts with lower BMI. Symptom management should be incorporated in the cancer care continuum of patients who receive ICIs for treatment, especially those with high BMI. Currently, some ongoing randomized controlled trials (RCTs) are investigating interventional strategies that can reduce the risk of irAEs in cancer patients receiving ICIs as well as the underlying biological mechanisms. However, to date, no completed RCTs regarding irAEs management have been published [41,42]. Our results may provide a new target for intervention in future RCTs aimed at controlling irAEs in cancer patients.
5. Conclusions
In conclusion, our study suggests that higher pre-treatment BMI is associated with a higher rate of irAEs among patients with advanced-stage cancer receiving ICIs, especially those with younger age or a low burden of comorbidities. Future prospective cohort studies with clear temporality will be needed to verify our results from a causal perspective.
Acknowledgments
We appreciate the data scientists at the Innovation Center for Biomedical Informatics (ICBI) of Georgetown University, who contributed to data gathering and preparation for the I-O database. We also appreciate Drs. Subha Madhavan and Anas Belouali of Georgetown University for their work relevant to the data management of the I-O database.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/23/6109/s1, Table S1: Summary of each individual irAE. Table S2: Effect measures of other covariates in the primary multivariable logistic regression. Table S3: Effect measures of BMI obtained via multiple imputation. Table S4: Association between pre-treatment BMI and number of irAEs.
Author Contributions
Conceptualization, D.Z. and D.B.; methodology, D.Z.; software, D.Z.; validation, D.Z., N.J.S., S.A., A.L.P., M.B.A. and D.B.; formal analysis, D.Z.; investigation, D.Z. and N.J.S.; resources, S.M., A.B. and M.B.A.; data curation, S.M., A.B., N.J.S. and M.B.A.; writing—original draft preparation, D.Z.; writing—review and editing, D.Z., N.J.S., M.C., M.B., M.T.S., S.A., A.L.P., S.M., A.B., M.B.A. and D.B.; visualization, D.Z.; supervision, M.B.A. and D.B.; funding acquisition, S.M. and M.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded internally by the Georgetown Lombardi Comprehensive Cancer Center.
Institutional Review Board Statement
This is a secondary data analysis of de-identified dataset. Due to the de-identified nature of the data analyzed, this study was exempt from review by the Georgetown University Institutional Review Board.
Informed Consent Statement
Patient consent was waived because this is a secondary data analysis of a de-identified dataset.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
M.B.A. has served as an advisory board member for BMS, Merck, Novartis, Arrowhead, Eisai, Pfizer, Werewolf, Fathom, Pneuma, Leads, Pyxis Oncology, PACT, Elpis, and Takeda. M.B.A. has served as a consultant for BMS, Merck, Novartis, Pfizer, Genentech-Roche, Exelixis, ImmunoCore, Iovance, Surface, COTA, Idera, Agenus, Apexigen, Ascher Bio, Neoleuken, Adagene, Sanofi, SeaGen, and AstraZeneca. M.B.A. has received research grants (to institution) from BMS, Merck, Pfizer, and Genentech and owns stock in Werewolf, Pyxis Oncology, and Elpis. All remaining authors have declared no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Antonia S.J., Borghaei H., Ramalingam S.S., Horn L., De Castro Carpeno J., Pluzanski A., Burgio M.A., Garassino M., Chow L.Q.M., Gettinger S., et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet Oncol. 2019;20:1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F., et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D., Tailor T.D., Kim C., Atkins M.B., Braithwaite D., Akinyemiju T. Immunotherapy Utilization Among Patients With Metastatic NSCLC: Impact of Comorbidities. J. Immunother. 2021;44:198–203. doi: 10.1097/CJI.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 7.Asnani A. Cardiotoxicity of Immunotherapy: Incidence, Diagnosis, and Management. Curr. Oncol. Rep. 2018;20:44. doi: 10.1007/s11912-018-0690-1. [DOI] [PubMed] [Google Scholar]
- 8.Dong J., Chen H. Cardiotoxicity of Anticancer Therapeutics. Front. Cardiovasc. Med. 2018;5:9. doi: 10.3389/fcvm.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escudier M., Cautela J., Malissen N., Ancedy Y., Orabona M., Pinto J., Monestier S., Grob J.J., Scemama U., Jacquier A., et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 10.Jain V., Mohebtash M., Rodrigo M.E., Ruiz G., Atkins M.B., Barac A. Autoimmune Myocarditis Caused by Immune Checkpoint Inhibitors Treated with Antithymocyte Globulin. J. Immunother. 2018;41:332–335. doi: 10.1097/CJI.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 11.Cheng F., Loscalzo J. Autoimmune Cardiotoxicity of Cancer Immunotherapy. Trends Immunol. 2017;38:77–78. doi: 10.1016/j.it.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Johnson D.B., Balko J.M., Compton M.L., Chalkias S., Gorham J., Xu Y., Hicks M., Puzanov I., Alexander M.R., Bloomer T.L., et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer J.R., Lacchetti C., Thompson J.A. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pr. 2018;14:247–249. doi: 10.1200/JOP.18.00005. [DOI] [PubMed] [Google Scholar]
- 14.Thompson J.A., Schneider B.J., Brahmer J., Andrews S., Armand P., Bhatia S., Budde L.E., Costa L., Davies M., Dunnington D., et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 15.Deurenberg P., Yap M., van Staveren W.A. Body mass index and percent body fat: A meta analysis among different ethnic groups. Int. J. Obes. Relat. Metab. Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 16.Georgiadis M.S., Steinberg S.M., Hankins L.A., Ihde D.C., Johnson B.E. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J. Natl. Cancer Inst. 1995;87:361–366. doi: 10.1093/jnci/87.5.361. [DOI] [PubMed] [Google Scholar]
- 17.Cortellini A., Bersanelli M., Santini D., Buti S., Tiseo M., Cannita K., Perrone F., Giusti R., De Tursi M., Zoratto F., et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur. J. Cancer. 2020;128:17–26. doi: 10.1016/j.ejca.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Daly L.E., Power D.G., O’Reilly A., Donnellan P., Cushen S.J., O’Sullivan K., Twomey M., Woodlock D.P., Redmond H.P., Ryan A.M. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer. 2017;116:310–317. doi: 10.1038/bjc.2016.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch L., Bellesoeur A., Boudou-Rouquette P., Arrondeau J., Thomas-Schoemann A., Kirchgesner J., Gervais C., Jouinot A., Chapron J., Giraud F., et al. The impact of body composition parameters on severe toxicity of nivolumab. Eur. J. Cancer. 2020;124:170–177. doi: 10.1016/j.ejca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Body Mass Index—BMI. 2021. [(accessed on 10 September 2021)]. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 21.Blagden S.P., Charman S.C., Sharples L.D., Magee L.R., Gilligan D. Performance status score: Do patients and their oncologists agree? Br. J. Cancer. 2003;89:1022–1027. doi: 10.1038/sj.bjc.6601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baser S., Shannon V.R., Eapen G.A., Jimenez C.A., Onn A., Lin E., Morice R.C. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest. 2006;130:1784–1790. doi: 10.1016/S0012-3692(15)50902-1. [DOI] [PubMed] [Google Scholar]
- 23.Gajra A., Marr A.S., Ganti A.K. Management of patients with lung cancer and poor performance status. J. Natl. Compr. Cancer Netw. 2014;12:1015–1025. doi: 10.6004/jnccn.2014.0098. [DOI] [PubMed] [Google Scholar]
- 24.West H.J., Jin J.O. JAMA Oncology Patient Page. Performance Status in Patients With Cancer. JAMA Oncol. 2015;1:998. doi: 10.1001/jamaoncol.2015.3113. [DOI] [PubMed] [Google Scholar]
- 25.Ottaiano A., De Divitiis C., Capozzi M., Avallone A., Pisano C., Pignata S., Tafuto S. Obesity and Cancer: Biological Links and Treatment Implications. Curr. Cancer Drug Targets. 2018;18:231–238. doi: 10.2174/1568009617666170330125619. [DOI] [PubMed] [Google Scholar]
- 26.Sarfati D., Koczwara B., Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 2016;66:337–350. doi: 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 27.Ethun C.G., Bilen M.A., Jani A.B., Maithel S.K., Ogan K., Master V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017;67:362–377. doi: 10.3322/caac.21406. [DOI] [PubMed] [Google Scholar]
- 28.Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review) Int. J. Oncol. 2019;54:407–419. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd B.E., Rebeiro P.F., Caribbean, Central and South America Network for HIV Epidemiology (CCASAnet) Brief Report: Assessing and Interpreting the Association Between Continuous Covariates and Outcomes in Observational Studies of HIV Using Splines. J. Acquir. Immune Defic. Syndr. 2017;74:e60–e63. doi: 10.1097/QAI.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim S.Y., Lee J.H., Gide T.N., Menzies A.M., Guminski A., Carlino M.S., Breen E.J., Yang J.Y.H., Ghazanfar S., Kefford R.F., et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019;25:1557–1563. doi: 10.1158/1078-0432.CCR-18-2795. [DOI] [PubMed] [Google Scholar]
- 31.Figaro M.K., Kritchevsky S.B., Resnick H.E., Shorr R.I., Butler J., Shintani A., Penninx B.W., Simonsick E.M., Goodpaster B.H., Newman A.B., et al. Diabetes, inflammation, and functional decline in older adults: Findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29:2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- 32.Pomatto L.C.D., Davies K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017;595:7275–7309. doi: 10.1113/JP275072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W., Wong G., Hwang Y.Y., Larbi A. The untwining of immunosenescence and aging. Semin. Immunopathol. 2020;42:559–572. doi: 10.1007/s00281-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luoma A.M., Suo S., Williams H.L., Sharova T., Sullivan K., Manos M., Bowling P., Hodi F.S., Rahma O., Sullivan R.J., et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell. 2020;182:655–671.e622. doi: 10.1016/j.cell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isik B., Alexander M.P., Manohar S., Vaughan L., Kottschade L., Markovic S., Lieske J., Kukla A., Leung N., Herrmann S.M. Biomarkers, Clinical Features, and Rechallenge for Immune Checkpoint Inhibitor Renal Immune-Related Adverse Events. Kidney Int. Rep. 2021;6:1022–1031. doi: 10.1016/j.ekir.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zatterale F., Longo M., Naderi J., Raciti G.A., Desiderio A., Miele C., Beguinot F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 38.Guzman-Prado Y., Ben Shimol J., Samson O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2021;70:89–100. doi: 10.1007/s00262-020-02663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You Y., Jiang C., Peng K., He W., Wang L., Jin Y., Xia L. The predictive value of body mass index on prognosis and adverse events of cancers treated with immunotherapy: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2021;70:2323–2335. doi: 10.1007/s00262-021-02858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monirul S., Rigal M., Chouahnia K., Le Jouan M., Apparuit M., Paix A., Jacolot A., Zelek L., Duchemann B. Budget Impact Analysis of Fixed Dose Versus Weight-Based Dosing Regimen of Nivolumab and Pembrolizumab in the Treatment of Non-Small Cell Lung Cancer. Vaccines. 2020;8:730. doi: 10.3390/vaccines8040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brahmer J.R., Abu-Sbeih H., Ascierto P.A., Brufsky J., Cappelli L.C., Cortazar F.B., Gerber D.E., Hamad L., Hansen E., Johnson D.B., et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer. 2021;9:e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauer C., Krauss J., Jager D., Zschabitz S., Haag G.M., Walle T., Sauer S., Kiermeier S., Friederich H.C., Maatouk I. eHealth intervention to manage symptoms for patients with cancer on immunotherapy (SOFIA): A study protocol for a randomised controlled external pilot trial. BMJ Open. 2021;11:e047277. doi: 10.1136/bmjopen-2020-047277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.