Authors contributions
All authors contributed equally to the conceptualizing, drafting and revising of the submitted text.
Conflict of interest
None of the authors have any relevant conflicts or funding to disclose.
Dear Editor,
A healthy 68‐year‐old female presented with an erythematous nodule on the outer aspect of her left arm (Fig. 1a,b). The location of the nodule coincided with the injection site of the second dose of the Pfizer‐BioNTech (Pfizer, Inc., New York City, NY, USA) SARS‐CoV‐2 mRNA vaccine, administered three months before. The nodule was preceded by a pruritic macule which emerged a week after inoculation, and which steadily evolved to the lesion with which the patient presented. The patient had not experienced any side effects related to the administration of the first vaccine dose, which she had received 3 weeks before in the ipsilateral arm. Dermoscopic evaluation of the nodule revealed dotted vessels and shiny white lines on an erythematous background.
Figure 1.
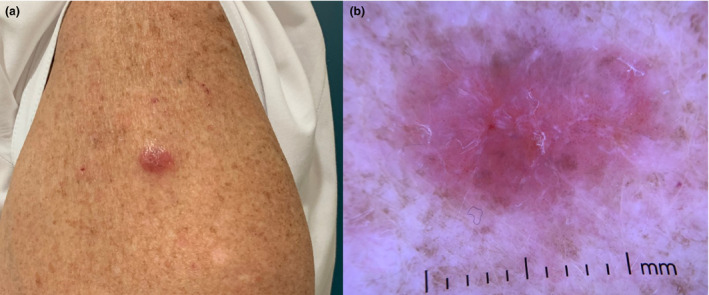
(a) Erythematous nodule on the patient’s left arm as observed clinically. (b) Dermoscopic evaluation of the nodule revealed dotted vessels on an erythematous background and shiny white lines.
Histological examination of the excised nodule revealed a wedge‐shaped, nodular infiltrate of small mature lymphocytes and rare blasts, extending from the superficial dermis to the subcutis, being denser in the superficial dermis (Fig. 2a). Occasional lymphocytes extended into the basal layers of the epidermis with associated basal cell vacuolar damage, Civatte body formation and spongiosis, in keeping with an interface (Fig. 2a,b). Immunohistochemistry revealed a mixture of B and T cells (Fig. 2c,d) with a predominance of T cells and with a low Ki67 index. The T cells expressed all T cell antigens. No light chain restriction was identified in the B‐cells.
Figure 2.
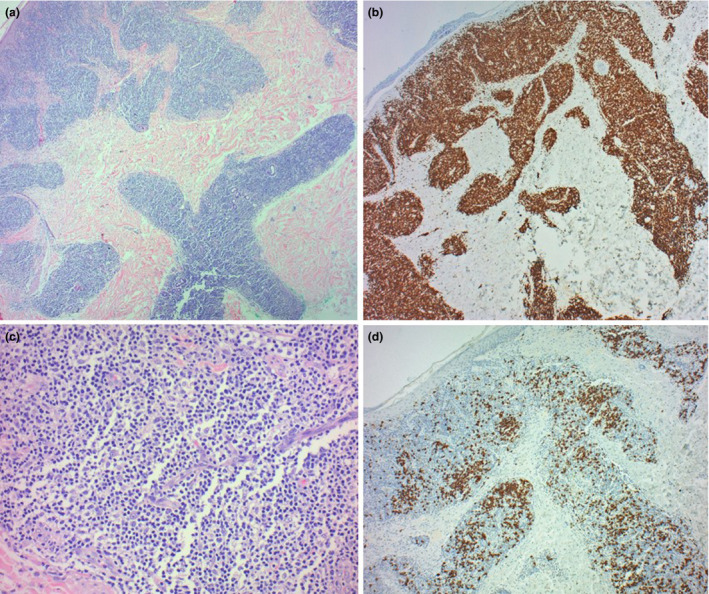
(a) Nodular lymphoid infiltrate extending from the superficial dermis to the deep dermis, with a focal interface at the basal layers of the epidermis. (H&E, x100). (b) Polymorphous infiltrate with a predominance of small mature lymphocytes. (H&E, x 200). Immunohistochemistry shows a mixture of B and T cells, highlighted by CD3 (c) and CD20 (d) respectively, with a predominance of T cells (x100).
Based on clinical and histological features, a diagnosis of cutaneous pseudolymphoma was made.
Vaccination site‐associated pseudolymphoma (also referred to as lymphoid hyperplasia and lymphocytoma cutis) is an exceedingly rare – albeit documented – phenomenon. 1 Reactions at the mRNA SARS‐CoV‐2 injection site have been reported in up to 84.2% of vaccinated patients, with erythema being the commonest. 2 Development of pseudolymphoma at the SARS‐CoV‐2 injection site is however previously undescribed. The acute or delayed occurrence of cutaneous pseudolymphoma has been reported at the injection site of Hepatitis A, Hepatitis B, 1 quadrivalent human papilloma virus, 3 early summer time meningoencephalitis and tetanus vaccination. 4 Subcutaneous papules, nodules and erythematous patches can be presenting signs of vaccine‐associated cutaneous pseudolymphoma. 4 The patient in this case had received various vaccinations throughout her lifetime, including the influenza vaccine on a yearly basis but had not experienced any localized or systemic side effects. It has been proposed that cutaneous pseudolymphoma may represent a reaction to vaccine adjuvants such as aluminium hydroxide. 1 This adjuvant is not found in the Pfizer‐BioNtech SARS‐CoV‐2 mRNA vaccine. 5
By flagging this unique adverse drug reaction, we hope to broaden physician’s repertoire of differential diagnoses when presented with SARS‐CoV‐2‐related injection site reactions.
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of their case details.
Data availability statement
The data that support the finds of this manuscript are available from the corresponding author, DM, upon reasonable request.
References
- 1. Maubec E, Pinquier L, Viguier M et al. Vaccination‐induced cutaneous pseudolymphoma. J Am Acad Dermatol 2005; 52: 623–629. [DOI] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos Pinheiro R, Duarte B, João A, Lencastre A. Cutaneous pseudolymphoma following quadrivalent human papillomavirus vaccination ‐ a rare adverse event. J Dtsch Dermatol Ges 2018; 16: 465–467. [DOI] [PubMed] [Google Scholar]
- 4. Cerroni L, Borroni RG, Massone C, Chott A, Kerl H. Cutaneous B‐cell pseudolymphoma at the site of vaccination. Am J Dermatopathol 2007; 29: 538–542. [DOI] [PubMed] [Google Scholar]
- 5. Pfizer, Inc . Vaccine information fact sheet for recipients and caregivers about comirnaty (COVID‐19 vaccine, mRNA) and Pfizer‐BioNTech COVID‐19 vaccine to prevent coronavirus disease 2019 (COVID‐19). 2021. https://www.fda.gov/media/144414/download.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finds of this manuscript are available from the corresponding author, DM, upon reasonable request.


