Dear Editor,
On 11th March 2020, the World Health Organization declared the coronavirus disease 2019 (COVID‐19) outbreak a global pandemic. In view of the rapidly spreading and highly fatal novel virus, the Pfizer‐BioNTech (BNT162b2) and the Moderna (mRNA‐1273) COVID‐19 vaccines were authorized for emergency use. Most common adverse events were immediate local injection site reactions, urticaria and morbilliform eruption. In addition, the Moderna trial described delayed injection site reactions in 1.2% of participants eight or more days following the vaccine. 1 , 2 , 3 Wei reported four cases of cutaneous manifestations several days after vaccination, which he denominated ‘COVID arm’. 4 Recently, two further scientific reports were published on this subject. 5 , 6 Consistent with these findings, we also observed delayed skin reactions in four patients, one of which was a Pfizer–BioNTech vaccine recipient.
Case 1: A 73‐year‐old woman presented with erythematous papules coalescing to a red plaque with scaling, around the injection site of the first dose of the Moderna vaccine. She reported that few hours after the injection, she noticed mild erythema and tenderness, but her symptoms resolved after two days. Nine days later, a pruritic plaque appeared and gradually spread to 7cm. The rash resolved with topical methylprednisolone–aceponate 0.1% cream and desloratadine within four days.
Case 2: A 74‐year‐old lady presented with an erythematous plaque in her left arm with no associated symptoms (Fig. 1). The rash appeared at the site of injection eight days following the second dose of the Pfizer–BioNTech vaccine. She had no side effects after the first dose of the vaccine. Her past medical history included chronic obstructive pulmonary disease (COPD) and pulmonary hypertension and had no allergies. The rash resolved with methylprednisolone‐aceponate 0.1% cream after three days.
Figure 1.
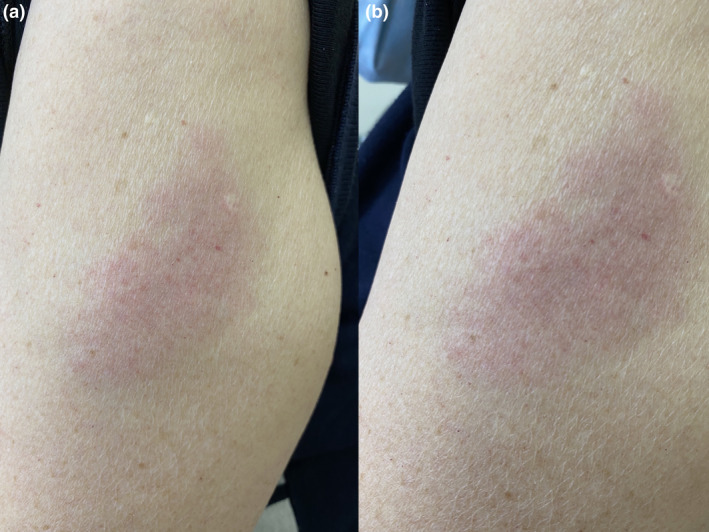
(a) Erythematous plaque at the injection site 8 days following the second dose of the Pfizer–BioNTech vaccination. (b) Swelling is evident.
Case 3: A 51‐year‐old woman with no past medical history and no known allergies developed an erythematous patch on her left upper arm nine days after the first dose of the Moderna vaccine. She initially felt that the skin surrounding the injection site was warm and gradually became erythematous. There was no associated pain or pruritus. She applied methylprednisolone–aceponate 0.1% cream, and the erythema started decreasing the following day and completely resolved within three days.
Case 4: Α 53‐year‐old woman presented with an erythematous indurated plaque on her left arm 9 days after the Moderna vaccine (Fig. 2). She reported a mild initial local reaction at the injection site that resolved 48 hours later. The indurated plaque presented eleven days after vaccination, gradually spreading to 8cm diameter and resolved after topical mometasone furoate 0.1% cream application five days later.
Figure 2.
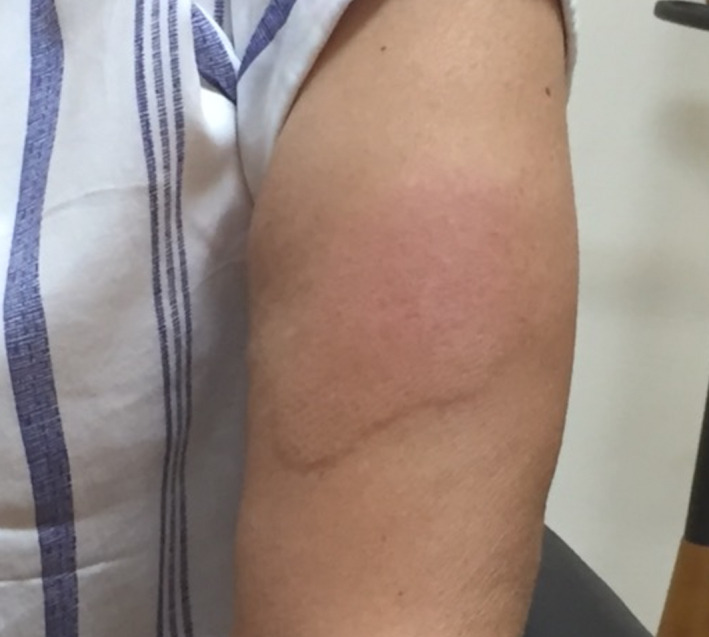
Erythematous indurated plaque 9 days after the first dose of the Moderna vaccine.
Although all previously reported reactions were after the Moderna vaccination, the similarities in both mRNA vaccines could predict that a similar reaction with the Pfizer vaccine should be expected. This is a delayed reaction that should not be confused with the local inflammation observed during the first hours following the injection. We agree with the hypothesized mechanism of a T‐cell‐mediated delayed hypersensitivity reaction supported by the histopathologic findings, as previously reported. 5 No hypothesis on predisposing factors can be made as all patients were Caucasians, vaccination policy with the mRNA vaccines was age group‐targeted and the cases reported are too limited to assess possible comorbidities.
Even though both vaccines are administered intramuscularly, one cannot rule out the possibility that some final solution particles could be inoculated intradermally in the process of needle insertion. In such cases, a delayed hypersensitivity reaction might be more easily initiated. However, assessment of such a hypothesis on a prospective model is difficult to investigate. 7 , 8 Differences in the biodistribution and pharmacokinetics of m‐RNAs, as well as the properties of the different lipid nanoparticles, could also have a role in the pathogenesis of this late‐onset reaction; however, pharmacokinetic data of both vaccines in humans are scarce.
We believe that the term ‘COVID vaccine arm’ would be more appropriate than ‘COVID arm’ to describe the phenomenon, and it should not be considered a contraindication to subsequent vaccine doses. 5
Funding
The authors declare no funding sources.
Disclosures
Prof Gregoriou has no conflicts of interest to declare. Dr Kleidona has no conflicts of interest to declare. Dr Tsimpidakis has no conflicts of interest to declare. Prof Nicolaidou has no conflicts of interest to declare. Prof Stratigos has no conflicts of interest to declare. Prof Rigopoulos has no conflicts of interest to declare.
The patients in this manuscript have given written informed consent for publication of their case details.
References
- 1. Polack FP, Thomas SJ, Kitchin N et al. Safety and Efficacy of the BNT162b2 mRNA Covid‐19 Vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B et al. Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. “COVID arm”: A reaction to the Moderna vaccine. JAAD case reports 2021; 10: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localised hypersensitivity reactions to the moderna COVID‐19 vaccine: A case series. JAMA Dermatol 2021; 157: 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blumenthal KG, Freeman EE, Saff RR et al. Delayed Large Local Reactions to mRNA‐1273 Vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gyldenløve M, Skov L, Hansen CB, Garred P. Recurrent injection‐site reactions after incorrect subcutaneous administration of COVID‐19 vaccine. J Euro Acad Dermatol Venereol 2021; 35: e545–e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng JY. Inadvertent subcutaneous injection of COVID‐19 vaccine. Postgrad Med J 2021; 97: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]


