Abstract
Aims
COVID‐19 pandemic caused by SARS‐CoV‐2 has become a public health crisis worldwide. In this study, we aimed at demonstrating the neutralizing potential of the IgY produced after immunizing chicken with a recombinant SARS‐CoV‐2 spike protein S1 subunit.
Methods and Results
E. coli BL21 carrying plasmid pET28a‐S1 was induced with IPTG for the expression of SARS‐CoV‐2 S1 protein. The recombinant His‐tagged S1 was purified and verified by SDS‐PAGE, Western blot and biolayer interferometry (BLI) assay. Then S1 protein emulsified with Freund's adjuvant was used to immunize layer chickens. Specific IgY against S1 (S1‐IgY) produced from egg yolks of these chickens exhibited a high titer (1:25,600) and a strong binding affinity to S1 (K D = 318 nmol L−1). The neutralizing ability of S1‐IgY was quantified by a SARS‐CoV‐2 pseudotyped virus‐based neutralization assay with an IC50 value of 0.99 mg ml−1. In addition, S1‐IgY exhibited a strong ability in blocking the binding of SARS‐CoV‐2 S1 to hACE2, and it could partially compete with hACE2 for the binding sites on S1 by BLI assays.
Conclusions
We demonstrated here that after immunization of chickens with our recombinant S1 protein, IgY neutralizing antibodies were generated against the SARS‐CoV‐2 spike protein S1 subunit; therefore, showing the potential use of IgY to block the entry of this virus.
Significance and Impact of the Study
IgY targeting S1 subunit of SARS‐CoV‐2 could be a promising candidate for pre‐ and post‐exposure prophylaxis or treatment of COVID‐19. Administration of IgY‐based oral preparation, oral or nasal spray may have profound implications for blocking SARS‐CoV‐2.
Keywords: antibody, COVID‐19, egg yolk immunoglobulin Y (IgY), hACE2, S1, SARS‐CoV‐2
INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has resulted in health, social and economic crises worldwide. As of now, there have been more than 237 million confirmed cases and over four million deaths. Various effective measures have been taken to control the COVID‐19 pandemic, and active immunization with vaccines has proved safe and efficacious (Keehner et al., 2021; Sadarangani et al., 2021). However, vaccines require a certain amount of time to trigger an immune response, and the safety and efficacy of COVID‐19 vaccines in medically complex patients remain unclear. On the contrary, one of the most effective options to control COVID‐19 can be based on therapeutic biological agents such as passive immunization with protective antibodies, which may provide immediate immunity to susceptible persons (Saghazadeh & Rezaei, 2020a). Antibody therapies have succeeded in the prevention and treatment of viral infection such as SARS‐CoV and MERS‐CoV (Saghazadeh & Rezaei, 2020b; Wu, Wang, Kuo, et al., 2020).
Egg yolk immunoglobulin (IgY), a counterpart to mammalian IgG, is the major serum antibody in avians. As a potential therapeutic antibody, IgY possesses many advantages compared with IgG due to its structural and immunological properties (Zhang, 2003). IgY can neither bind to Fc receptors nor activate complement components in mammalian (Grey, 1967); therefore, an exacerbation of COVID‐19 through antibody‐dependent enhancement (ADE) could be potentially avoided (Lee et al., 2020). In addition, its safety, effectiveness, and stability have been widely recognized (Krief et al., 2002; Perez de la Lastra et al., 2020). Numerous studies have demonstrated the remarkable neutralizing activity of IgY in in vitro and in vivo experiments against SARS‐CoV (Fu et al., 2006), influenza virus (Adachi et al., 2008), Ebola virus (Zhang et al., 2021), Zika virus (O’Donnell et al., 2019), Dengue virus (Fink et al., 2017), human norovirus (Zhu et al., 2019), etc. IgY can become an alternative for passive immunization (Abbas et al., 2019).
Like other coronaviruses, SARS‐CoV‐2 contains four structural proteins: spike (S), envelope, membrane, and nucleocapsid proteins. The S protein plays a key role in viral infection and pathogenesis, and it has been identified as a critical target for eliciting persistent neutralizing antibodies (Shanmugaraj et al., 2020; Yang & Du, 2021). Most of the neutralizing epitopes are located within the S1 subunit (Premkumar et al., 2020; Yuan et al., 2021). The S1 subunit is further divided into a receptor‐binding domain (RBD) and an N‐terminal domain (NTD), both of which have been identified as critical neutralizing epitopes (Chi et al., 2020; Huang et al., 2020; Zhou et al., 2019). We, therefore, considered that S1 could induce IgY antibodies against both RBD and NTD.
The aim of this study was to produce a recombinant SARS‐CoV‐2 spike protein S1 subunit in order to immunize chickens with it and to demonstrate the subsequent generation of neutralizing IgY antibodies.
MATERIALS AND METHODS
Prokaryotic expression, purification and identification of SARS‐CoV‐2 S1 protein
E. coli (BL21‐DE3‐pLysS) transformed with a SARS‐CoV‐2 S1 encoding plasmid (pET28a‐S1) and stably expressing SARS‐CoV‐2 S1 (containing a C‐terminal 6×His tag) was constructed as described previously (Xia et al., 2021) and kindly provided by Dr. Feng Cong (Guangdong Laboratory Animals Monitoring Institute, Guangzhou, China). The transformed E. coli was grown in LB medium containing 100 μg ml−1 kanamycin sulphate at 37°C with shaking at 200 rpm. At OD600 0.6, 1 mmol L−1 isopropyl‐β‐d‐thiogalactopyranoside (IPTG) was added to induce protein expression. Bacteria were collected, suspended and sonicated for 10 min in an ice bath. The His‐tagged S1 protein inclusion bodies were dissolved in 8 mol L−1 urea buffer and then the supernatant was loaded over nickel‐nitrilotriacetic acid (Ni‐NTA) resin (P2233, Beyotime) as described by the manufacturer. The protein was refolded via step‐wise dialysis, which removed the denaturant urea gradually. After dialysis, the insoluble substance was removed by centrifugation, and the protein solution was centrifuged with 10‐kDa cut‐off membranes (Millipore) for ultrafiltration.
The protein concentration was quantified using the BCA Protein Assay kit (Beyotime). Purification was determined by SDS‐PAGE and analysed with Image J software. The rabbit anti‐S primary antibody (1:3000; 40589‐T62, Sino Biological) and horseradish peroxidase (HRP)‐conjugated goat anti‐rabbit IgG secondary antibody (1:5000; S0001, Affinity) were used for Western blot analysis to detect the purified S1 protein.
To detect the functional activity of the purified His‐tagged S1 protein, we used biolayer interferometry (BLI) to study the binding kinetics and affinity of S1 to human angiotensin‐converting enzyme 2 (hACE2; 10108‐H02H, Sino Biological) on an Octet K2 instrument (FortéBio; Wang et al., 2020; Yang et al., 2020). First, the Ni‐NTA biosensors were loaded with 25 μg ml−1 of the His‐tagged S1 protein, and then dipped into PBST shaking for 300 s. After that, the S1 immobilized sensors were incubated with twofold serial diluted hACE2 for 300 s. Finally, the dissociation step was performed in PBST for 600 s. Binding kinetics were evaluated using a 1:1 Langmuir binding model (ForteBio Data Analysis 9.0 software).
Chicken immunization
The project was approved by the Ethics Committee of West China Hospital of Stomatology (WCHSIRB‐D‐2020‐394). Eight Lohmann‐Brown layer chickens (5‐month‐old) purchased from a local company were randomly divided into two groups, the S1 group (n = 5) and the control group (n = 3). Chickens in the S1 group were immunized by subcutaneous injections with S1 protein (500 μg/dose/animal). Booster immunizations were administered with the same dose at the end of the 2nd and 4th week after the primary immunization. For the first injection, the antigen was emulsified at a 1:1 ratio with the complete Freund's adjuvant and for two subsequent boosters with the incomplete adjuvant. The control group was injected with a mixture of PBS and adjuvant. Chickens were housed in a clean, ventilated environment. Eggs were pooled by group and by week of immunization. All chickens were euthanized to measure serum biochemical parameters, histopathological examination, and lymphocyte subset (CD3+, CD3+CD4+ and CD3+CD8+) analysis, at the end of the 7th week (see Figures [Link], [Link], [Link]). The schematic illustration of the immunization procedure is shown in Figure 1a.
FIGURE 1.
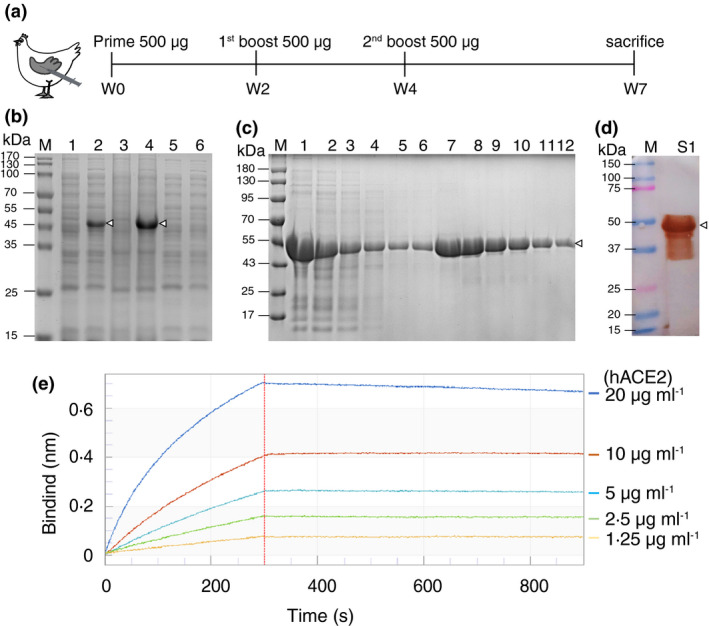
SARS‐CoV‐2 S1 protein preparation and the immunization strategy of chickens. (a) Schematic diagram of the immunization strategy to generate IgY against SARS‐CoV‐2 S1 protein. Booster immunizations of the same dose were performed at both the end of the 2nd and 4th week after the primary immunization (500‐μg protein/dose/animal) (S1 group, n = 5). The control chickens (n = 3) were similarly immunized and boosted with the mixture of PBS and adjuvant. (b) SDS‐PAGE analysis of proteins distributed between soluble and insoluble cell fraction after bacterial cell disruption. Lane 1 and 2, the total proteins of the transformed E. coli before and after induction. Lane 3 and 4, the total insoluble inclusion body proteins before and after induction; Lane 5 and 6, the total soluble protein before and after induction. (c) Analysis of SARS‐CoV‐2 S1 protein in different purification steps by SDS‐PAGE. Lane 1, inclusion body proteins solubilized in 8 mol L−1 Urea; Lane 2, supernatant after passing through Ni‐NTA resin; Lane 3–5, the wash of Ni‐NTA resin; Lane 6–12, elution from Ni‐NTA resin. (d) The functional protein (folded form) was analysed by Western blotting using anti‐S antibody and developed using DAB. M, molecular weight ladder. The triangle denotes the band corresponding to the SARS‐CoV‐2 S1 protein. (e) Binding profiles of SARS‐CoV‐2 S1 to hACE2 measured by BLI in Octet K2. Kinetic constants were calculated using a minimum of five dilutions of hACE2
Isolation and purification of IgY
IgY antibodies were obtained by water dilution, ammonium sulphate precipitation and ultrafiltration (Akita & Nakai, 1992; Fishman & Berg, 2018; Gao et al., 2019; Hatta et al., 1990; Liu et al., 2017; Sudjarwo et al., 2017; Xiao et al., 2019). More specifically, three to five eggs per week were randomly selected from each replicate for IgY purification at the end of the 7‐week trial. Eggs from the same group were pooled together for IgY purification. The egg yolk was separated from the egg white and mixed with nine volumes of water, then the pH was adjusted to 5.0 to 5.2 with 2 mol L−1 HCl. After incubating at 4°C for 12 h, the water‐soluble fraction (WSF) was separated by centrifugation (10,000 g at 4°C for 30 min). The saturated ammonium sulphate solution was mixed with two volumes of IgY‐rich WSF and incubated at 4°C for another 12 h to precipitate the IgY. The concentrated antibody precipitate was resuspended in PBS, and ultrafiltration was used to finally remove ammonium sulphate and to replace the storing buffer with PBS. Protein concentration was measured by BCA assay. The yield and purity of IgY were determined by SDS‐PAGE and analysed with Image J software.
Determination of IgY binding titer
Enzyme‐linked immunosorbent assay (ELISA) was used to detect the antigen‐binding activity of egg yolk IgY and serum samples as described previously (Palaniyappan et al., 2012; Zhang et al., 2016). Plates were coated with S1 protein at 50 ng per well in carbonate‐bicarbonate buffer (0.05 mol L−1, pH 9.6) overnight at 4°C. The plates were then washed thrice with 0.05% PBST and blocked with blocking buffer (2% BSA in PBS) at 37°C for 2 h. Serial diluted IgY WSF (from 400‐ to 102,400‐fold) and pooled chicken serum (from 1600‐ to 204,800‐fold) were added (100 μl per well), respectively, and incubated for 1 h at 37°C, followed by incubation with HRP‐conjugated rabbit anti‐chicken IgY secondary antibody (1:6000, 100 μl per well) for 1 h at 37°C. The plates were washed four times with PBST, and developed using 3,3’,5,5'‐tetramethylbiphenyldiamine (TMB) substrate (PR1200; Solarbio), following 2 mol L−1 H2SO4 addition to stop the reaction. The optical density (OD) was measured at 450 nm with a microplate reader (SpectraMax iD5). The egg yolk IgY and the serum samples of the control group were used as negative controls. The S1‐specific IgY antibody (S1‐IgY) binding titer was defined as the largest dilution at which the OD450 nm (S1 group)/OD450 nm (Control group) ratio ≥2.1 (Wen et al., 2012).
SARS‐CoV‐2 S pseudotyped virus‐based neutralization assay
The pooled purified egg yolk IgY and the pooled serum samples were used for neutralization assay. The SARS‐CoV‐2 S pseudotyped virus was kindly granted by Professor Weijin Huang from the National Institutes for Food and Drug Control. The virus neutralization assay was conducted as previously described (Nie et al., 2020; Zhang et al., 2021). Briefly, 50 μl of pseudoviruses (~5 × 104 relative light units, RLU) were incubated with IgY or serum serial dilutions for 1 h at 37°C. Then, 50 μl of ACE2‐transfected HEK293T cells were added into the plates (2 × 104 cells per well), followed by incubation at 37°C in a humidified atmosphere with 5% CO2. Wells with no IgY or serum added served as virus controls, and wells with only cells and culture medium added served as the cell controls. The RLU of infected cells were detected using the Luciferase Assay Kit (E2610; Promega) 24‐h post‐infection. The Reed‐Muench method (Matumoto, 1949; Nie et al., 2020) was used to calculate the virus neutralization titer for the IgY and the serum samples.
The blocking capacity and competition assay of S1‐IgY
The binding affinity between S1‐IgY and S1 protein was determined by BLI as previously described (Wang et al., 2020; Yang et al., 2020). Moreover, to verify the ability of IgY to block the binding of hACE2 to S1 and the binding competition between S1‐IgY and hACE2 to the S1 protein, we conducted two sets of BLI assays by exposing S1 first to S1‐IgY and then to hACE2 or vice versa (Tan et al., 2017; Wu, Wang, Shen, et al., 2020). All experiments were performed at room temperature, and the biosensors were loaded with S1 protein (25 μg ml−1) for 600 s to a response level of ~8 nm.
For the blocking experiment, C‐IgY and N‐IgY (the specific IgY against the conservative nucleocapsid protein of SARS‐CoV‐2; Lyu et al., 2021) were used as negative control and nonspecific control. After S1 protein was immobilized to Ni‐NTA biosensors, the biosensors were first dipped into (i) 1500 μg ml−1 S1‐IgY, (ii) 375 μg ml−1 S1‐IgY, (iii) 1500 μg ml−1 N‐IgY and (iv) 1500 μg ml−1 C‐IgY shaking for 600s, respectively. The biosensors were then dipped into PBST for dissociation for 600 s, moved into 20 μg ml−1 hACE2 shaking for another 600 s, followed by another dissociation for 600 s. To determine the competitive characteristics, the biosensors immobilized with S1 protein were dipped into wells containing 20 μg ml−1 hACE2 for 300 s to get saturation. Subsequently, the biosensors were moved into (i) 1500 μg ml−1 S1‐IgY, (ii) a mixture of 20 μg ml−1 hACE2 and 1500 μg ml−1 S1‐IgY, (iii) 20 μg ml−1 hACE2 and (iv) PBST for another 300 s. Finally, the dissociation step was performed in PBST for 600 s. The responses of the S1‐IgY and hACE2 binding to S1 were compared, and competitive/noncompetitive response was determined.
RESULTS
The recombinant purified SARS‐CoV‐2 S1 protein showed binding activity to hACE2
The solubility of the His‐tagged S1 fusion protein was identified and the result indicated that, after induced by IPTG, the transformed E. coli BL21 expressed S1 proteins efficiently in the form of inclusion body (Figure 1b). The purity reached 95% after NTA affinity chromatography (Figure 1c). Approximately, 300 μg of the purified protein was obtained from 50 ml of the culture medium. Western blot analysis on the renatured protein revealed an approximately 45‐kDa mass band corresponding to the expressed fusion protein (Figure 1d). According to kinetics analysis, the affinity of SARS‐CoV‐2 S1 binding to hACE2 was very high (K D: 1.98 nmol L−1), with an association rate constant (k on) of 3.51 × 104 (mol L−1)−1 s−1 and a dissociation rate constant (k off) of 6.94 × 10−5 s−1 (Figure 1e).
S1‐IgY exhibited high titer and strong binding affinity
High antigen‐binding specificity of anti‐SARS‐CoV‐2 S1‐IgY could be obtained from immunized chickens. The titer of S1‐IgY in egg yolk attained a peak level (1:25,600) at week 6 (Figure 2a). The titer of serum antibodies attained the peak (1:102,400) both at week 5 and week 7, with a lower titer (1:51,200) at week 6 (Figure 2b). As Figure 2c showed, IgY was composed of heavy chains (about 65 kDa) and light chains (about 27 kDa). The crude IgY yield determined by BCA assay was about 31.15 ± 1.55 mg per millilitre of egg yolk, with a purity of nearly 60%. The purity of IgY increased to more than 90% after further purification, and the purified IgY yield was 9.98 ± 0.23 mg per millilitre of egg yolk. Figure 2d displays the time course of binding and dissociation of S1‐IgY WSF at various concentrations to S1. S1‐IgY WSF was found to bind to S1 with nanomolar affinity. It displayed rapid binding kinetics, with a k on of 8.00 × 102 (mol L−1)−1 s−1 and a k off of 2.54 × 10−4 s−1, resulting in a K D of 318 nmol L−1.
FIGURE 2.
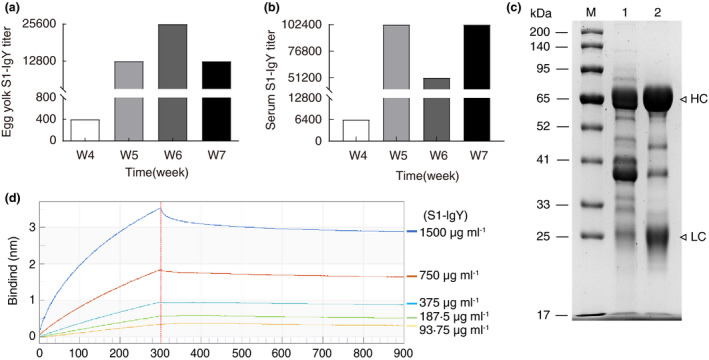
The strong immunization response to SARS‐CoV‐2 S1 protein in chickens. The change of S1‐specific antibody binding titers in the egg yolk (a) and the serum (b). The level of S1‐specific antibody binding titers was measured by ELISA using the purified recombinant SARS‐CoV‐2 S1 protein (OD at 450 nm) as an antigen and expressed as an ELISA value. (c) The SDS‐PAGE profile of IgY. Two IgY chains appeared on the SDS‐PAGE gel. HC, heavy chain ≈ 65 kDa; LC, light chain ≈ 27 kDa. M, molecular weight ladder; Lane 1, water‐soluble fraction (WSF); Lane 2, purified IgY. (d) Binding profiles of SARS‐CoV‐2 S1 to S1‐IgY. Binding kinetics were measured for five concentrations of S1‐IgY at twofold serial dilution ranging from 1500 to 93.75 μg ml−1
Organs, including lung, liver, and kidney of the chickens from the S1 group were grossly and histologically normal at the end of this study. Evaluation of the biochemical parameters showed all the indexes were normal except for several slight changes. Although there were no significant differences for the lymphocyte subset percentage in spleen and peripheral blood, there was a tendency for an increase (see Figures [Link], [Link], [Link]).
S1‐IgY demonstrated high neutralizing activity against SARS‐CoV‐2 pseudovirus infection
The neutralization assay showed that SARS‐CoV‐2 was neutralized by S1‐IgY and the IC50 value (the concentration causing 50% inhibition of SARS‐CoV‐2 pseudovirus infection) was 0.99 mg ml−1 (Figure 3a). C‐IgY did not demonstrate any inhibitory effect in this assay (Figure 3a). Serum from the S1 group demonstrated neutralizing activity against pseudovirus infection with an IC50 of 1:18, the control serum showed no neutralizing activity (Figure 3b). Eighteen‐fold diluted serum of immunized chickens achieved the same level of inhibitory efficiency as 0.99 mg ml−1 S1‐IgY did.
FIGURE 3.
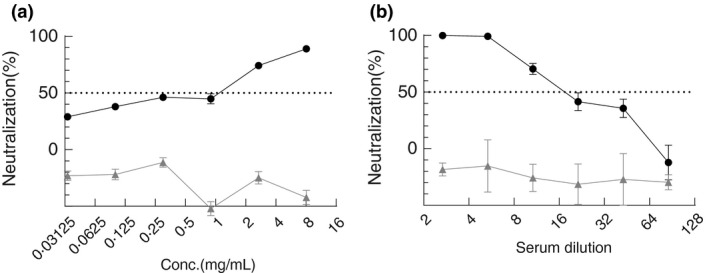
Characteristics of the neutralization activity of IgY antibodies against pseudotyped SARS‐CoV‐2. Serial dilutions of the pooled egg yolk IgY or the pooled serum preparations were pre‐incubated with the pseudotyped SARS‐CoV‐2 at 37°C for 1 h before they were added to 293T‐hACE2 cells. Luciferase activity was measured 24 h later to calculate IC50 of the antibody. Pseudovirus neutralization curves of IgY (a) and serum (b) from the control group (▲) and the S1 group (●) were shown. Data were expressed as mean ± SD from 3 to 5 replicates
S1‐IgY displayed strong ability in blocking the binding of SARS‐CoV‐2 S1 to hACE2 and partial competition with hACE2 for the binding sites on S1
The attachment of S1 to hACE2 was completely blocked by the pretreatment of S1 with a high concentration (1500 μg ml−1) of S1‐IgY, and partially blocked by a lower concentration (375 μg ml−1) of S1‐IgY. No blocking by 1500 μg ml−1 C‐IgY and N‐IgY was detected (Figure 4a). As shown in Figure 4b, after S1 was almost saturated with hACE2, S1‐IgY could bind to S1 in the presence of 20 μg ml−1 soluble hACE2 or without hACE2. The mixture of S1‐IgY and hACE2 exhibited a lower response level than S1‐IgY alone.
FIGURE 4.
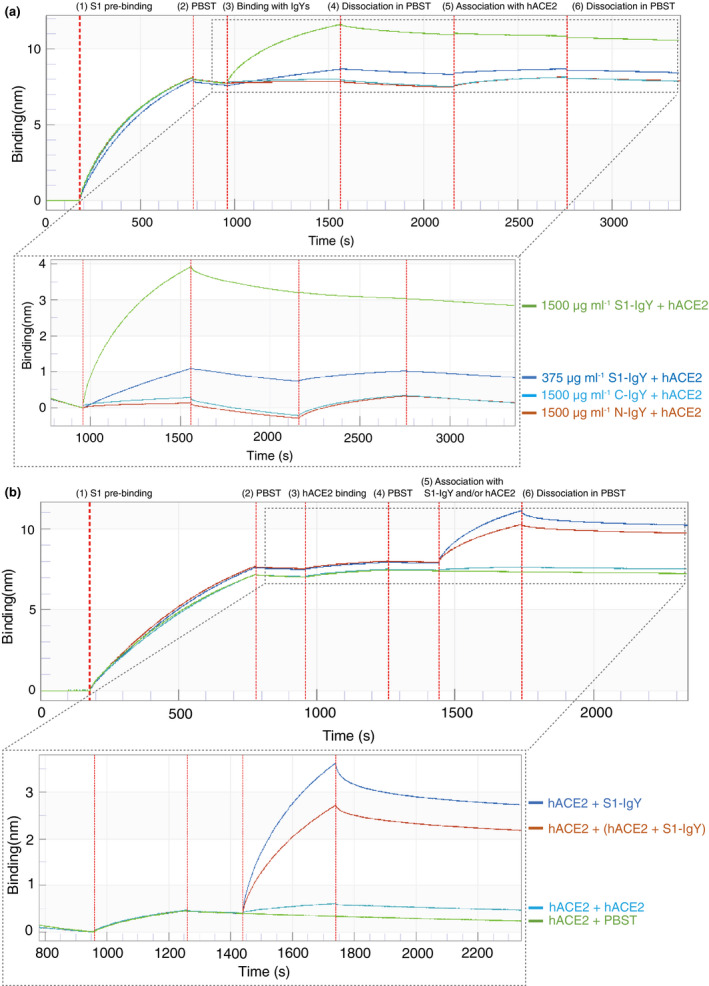
Competition binding to SARS‐CoV‐2 S1 between S1‐IgY and hACE2. (a) The interaction between SARS‐CoV‐2 S1 protein and hACE2 was inhibited by S1‐IgY. SARS‐CoV‐2 S1 was sequentially bound by S1‐IgY, C‐IgY or N‐IgY at the indicated concentration followed by the hACE2 receptor. The legend lists the immobilized SARS‐CoV‐2 S1, IgY and receptors that correspond to each curve. (b) S1‐IgY exhibited partial competition with hACE2 for the binding sites on S1. After loading with SARS‐CoV‐2 S1, the sensors were pre‐incubated with hACE2 for 300 s and then dipped into S1‐IgY, hACE2 or the mixture of the two for 600 s. PBST served as a nonbinding control. The graph displays the time course of binding patterns. The second binding and dissociation steps revealed the competition of S1‐IgY and hACE2 with SARS‐CoV‐2 S1 (enlarged box)
DISCUSSION
There is an urgent need of prophylactic and therapeutic interventions for the ongoing COVID‐19 pandemic worldwide. IgY can be extracted from eggs and thus is beneficial in terms of animal welfare. IgY has been described as a safe option since it is unable to activate the human complement (Larsson et al., 1992) or bind to the rheumatoid factor (Larsson et al., 1991) due to its structural features. Moreover, the phylogenetic distance between humans and chickens leads to a stronger immune response of IgY to human antigens (Gassmann et al., 1990).
Till now, several studies have been carried out on the potential anti‐SARS‐CoV‐2 effect of IgY (Lu et al., 2020; Shen et al., 2021; Wei et al., 2021). IgY targeting the full‐length S protein, S1 subunit or RBD were produced, and all studies showed a high titer and variable neutralizing activity against SARS‐CoV‐2. Our results align with the above findings. However, various factors like the different immunization procedures, test animals, or the time of antibody collection may contribute to the difference between research groups. Compared with the previously reported monoclonal antibodies (IC50 ranged from 0.03 to 50 μg ml−1; Brouwer et al., 2020; Chi et al., 2020; Ju et al., 2020), the IC50 value of S1‐IgY in our study was relatively high (IC50: 0.99 mg ml−1), which may be attributed to the fact that the specific IgY only accounts for 1%–10% of the total IgY antibodies (Michael et al., 2010). Interestingly, IgY targeting the S1 protein has been previously found to be able to competitively bind to six SARS‐CoV‐2 spike protein mutants (Wei et al., 2021). Moreover, epitope mapping revealed that two of five linear epitopes of IgY‐S (IgY targeting S protein) in SARS‐CoV‐2 S were cross‐reactive with SARS‐CoV S (Lu et al., 2020). This suggests that IgY may provide reliable protection against SARS‐CoV‐2, including the variants. Taken together, these findings and the work on IgY against SARS‐CoV‐2 presented here strengthen the potential application of IgY antibodies for the control of COVID‐19.
Our study differs from those described above in that the antigen we used was obtained from an inducible prokaryotic expression system. This expression system has been well explored and widely used for recombinant protein production (Jia & Jeon, 2016). It is the most economical way to produce large amounts of recombinant proteins in a short time, which makes it more favourable for the large‐scale production of antibodies. In addition, we observed a lower K D value (1.98 nmol L−1) than the other studies (8.02–145.7 nmol L−1; Wang et al., 2020; Yang et al., 2020), and this could indicate that the recombinant S1 we used seems to be much more biologically active (Zhou et al., 2020). Interestingly, a study reported that SARS‐CoV‐2 was more efficiently neutralized by the serum derived from mice immunized with S1 when compared with that of mice immunized with RBD (Wang et al., 2021). Moreover, another study showed that the S1 IgG titers were a better predictor of protection than the RBD IgG titers (Kurup et al., 2021). Considering that the NTD and RBD account for major portions of the SARS‐CoV‐2 S1 subunit (Huang et al., 2020), IgY antibodies induced by S1 likely bind to both RBD and NTD. The former could block viral entry by disrupting RBD binding to the hACE2 receptor, whilst the latter could do it by restraining the conformational changes of the S protein that are required for S2‐mediated membrane fusion (Kurup et al., 2021; Yang & Du, 2021).
Although the great potential of chicken IgY for COVID‐19 has been proposed by several labs (Lu et al., 2020; Shen et al., 2021; Wei et al., 2021), to our knowledge, the affinity of IgY to antigens has not been reported yet. Most current anti‐SARS‐CoV‐2 antibodies neutralize viruses by binding to epitopes on the S protein of SARS‐CoV‐2 (Tai et al., 2020; Tian et al., 2020). In this work, we characterized the binding kinetics of IgY and found that S1‐IgY showed nanomolar binding affinity to S1 protein. Consistent with the results of the pseudotyped virus‐based neutralization assays, BLI assay confirmed that the pretreatment of S1‐IgY effectively prevented the combination of SARS‐CoV‐2 S1 and hACE2 at the molecular level. Moreover, the competition analysis revealed that S1‐IgY displayed partial competition with hACE2 for the binding to SARS‐CoV‐2 S1. This suggests that S1‐IgY and hACE2 may recognize different epitopes on SARS‐CoV‐2 S1 with partial overlap. The S1‐IgY‐mediated blocking effect may be related to conformational changes or steric clashes, as reported elsewhere (Lv et al., 2020).
The mucosal immunity is defective in controlling viral infections (Yewdell, 2021). Hence IgY‐based therapies like oral preparations, oral sprays or nasal sprays could help in strengthening the barrier function of oral, nasal and gastrointestinal mucosa, as previously demonstrated (Rahman et al., 2013; Somasundaram et al., 2020). As the main entrance of viruses to the body, oral and nasal cavities are also high‐risk sites for SARS‐CoV‐2 infection (Huang et al., 2021). Consistent with what we originally envisioned, a recent study found that IgY was maintained at detectable concentrations in the nasal and oral cavities for a matter of hours after administration (Shen et al., 2021). Therefore, an IgY‐based oral preparation, oral spray or nasal spray could have favourable clinical implications for the current COVID‐19 epidemic.
In conclusion, we demonstrated that after immunization of chickens with the recombinant SARS‐CoV‐2 S1 protein produced by an inducible prokaryotic expression system, S1‐IgY antibodies with neutralizing activity against pseudotyped SARS‐CoV‐2 were generated. Moreover, S1‐IgY showed nanomolar binding affinity to S1 protein and displayed partial competition with hACE2 for the binding to S1. Therefore, IgY targeting S1 subunit of SARS‐CoV‐2 could be a promising candidate for pre‐ and post‐exposure prophylaxis or treatment of COVID‐19.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Fig S1
Fig S2
Fig S3
ACKNOWLEDGEMENTS
This study was supported by grants from the Special Funds for Prevention and Control of COVID‐19 of Sichuan University (2020scunCoV10009), Independent Research Project of State Key Laboratory of Oral Diseases (SKLOD202004) and Key Projects of Sichuan Provincial Department of Science and Technology (2020YFSY0008).
Bao, L. , Zhang, C. , Lyu, J. , Yi, P. , Shen, X. , Tang, B. , et al. (2022) Egg yolk immunoglobulin (IgY) targeting SARS‐CoV‐2 S1 as potential virus entry blocker. Journal of Applied Microbiology, 132, 2421–2430. 10.1111/jam.15340
REFERENCES
- Abbas, A.T. , El‐Kafrawy, S.A. , Sohrab, S.S. & Azhar, E.I.A. (2019) IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Human Vaccines & Immunotherapeutics, 15, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi, K. , Handharyani, E. , Sari, D.K. , Takama, K. , Fukuda, K. , Endo, I. et al. (2008) Development of neutralization antibodies against highly pathogenic H5N1 avian influenza virus using ostrich (Struthio camelus) yolk. Molecular Medicine Reports, 1, 203–209. [PubMed] [Google Scholar]
- Akita, E.M. & Nakai, S. (1992) Immunoglobulins from egg yolk: isolation and purification. Journal of Food Science, 57(3), 629–634. [Google Scholar]
- Brouwer, P.J.M. , Caniels, T.G. , van der Straten, K. , Snitselaar, J.L. , Aldon, Y. , Bangaru, S. et al. (2020) Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science, 369, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, X. , Yan, R. , Zhang, J. , Zhang, G. , Zhang, Y. , Hao, M. et al. (2020) A neutralizing human antibody binds to the N‐terminal domain of the Spike protein of SARS‐CoV‐2. Science, 369, 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, A.L. , Williams, K.L. , Harris, E. , Alvine, T.D. , Henderson, T. , Schiltz, J. et al. (2017) Dengue virus specific IgY provides protection following lethal dengue virus challenge and is neutralizing in the absence of inducing antibody dependent enhancement. PLoS Neglected Tropical Diseases, 11, e0005721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman, J.B. & Berg, E.A. (2018) Isolation of IgY from chicken eggs. Cold Spring Harbor Protocols, 2018, pdb.prot099150. [DOI] [PubMed] [Google Scholar]
- Fu, C.Y. , Huang, H. , Wang, X.M. , Liu, Y.G. , Wang, Z.G. , Cui, S.J. et al. (2006) Preparation and evaluation of anti‐SARS coronavirus IgY from yolks of immunized SPF chickens. Journal of Virological Methods, 133, 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, E. , Wu, S. , Xu, Q. , Zeng, Y. , Tan, N. , He, S. et al. (2019) Enterovirus type 71‐immunized chicken egg yolk immunoglobulin has cross antiviral activity against coxsackievirus A16 in vitro. Experimental and Therapeutic Medicine, 18, 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, M. , Thömmes, P. , Weiser, T. & Hübscher, U. (1990) Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. The FASEB Journal, 4, 2528–2532. [DOI] [PubMed] [Google Scholar]
- Grey, H.M. (1967) Duck immunoglobulins: II. Biologic and immunochemical studies. Journal of Immunology, 98, 820–826. [PubMed] [Google Scholar]
- Hatta, H. , Kim, M. & Yamamoto, T. (1990) A novel isolation method for hen egg yolk antibody, "IgY". Agricultural and Biological Chemistry, 54, 2531–2535. [PubMed] [Google Scholar]
- Huang, N.I. , Pérez, P. , Kato, T. , Mikami, Y.U. , Okuda, K. , Gilmore, R.C. et al. (2021) SARS‐CoV‐2 infection of the oral cavity and saliva. Nature Medicine, 27, 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Yang, C. , Xu, X.‐F. , Xu, W. & Liu, S.‐W. (2020) Structural and functional properties of SARS‐CoV‐2 spike protein: potential antivirus drug development for COVID‐19. Acta Pharmacologica Sinica, 41, 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, B. & Jeon, C.O. (2016) High‐throughput recombinant protein expression in Escherichia coli: current status and future perspectives. Open Biology, 6, 160196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju, B. , Zhang, Q. , Ge, J. , Wang, R. , Sun, J. , Ge, X. et al. (2020) Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature, 584, 115–119. [DOI] [PubMed] [Google Scholar]
- Keehner, J. , Horton, L.E. , Pfeffer, M.A. , Longhurst, C.A. , Schooley, R.T. , Currier, J.S. et al. (2021) SARS‐CoV‐2 infection after vaccination in health care workers in California. New England Journal of Medicine, 384, 1774–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief, A. , Letesson, J.‐J. & Billen, D. (2002) Comparison between ‘IgY technology’ from chickens and ‘IgG technology’ from mice for production of tailor‐made antibodies. Tetrahedron Letters, 43(10), 1843–1846. [Google Scholar]
- Kurup, D. , Malherbe, D.C. , Wirblich, C. , Lambert, R. , Ronk, A.J. , Zabihi Diba, L. et al. (2021) Inactivated rabies virus vectored SARS‐CoV‐2 vaccine prevents disease in a Syrian hamster model. PLoS Path, 17, e1009383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, A. , Karlsson‐Parra, A. & Sjöquist, J. (1991) Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clinical Chemistry, 37, 411–414. [PubMed] [Google Scholar]
- Larsson, A. , Wejåker, P.‐E. , Forsberg, P.‐O. & Lindahl, T. (1992) Chicken antibodies: a tool to avoid interference by complement activation in ELISA. Journal of Immunological Methods, 156, 79–83. [DOI] [PubMed] [Google Scholar]
- Lee, W.S. , Wheatley, A.K. , Kent, S.J. & DeKosky, B.J. (2020) Antibody‐dependent enhancement and SARS‐CoV‐2 vaccines and therapies. Nature Microbiology, 5, 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , He, Q. , Wang, W. , Zhou, B. , Li, B. , Zhang, Y. et al. (2017) Preparation and neutralization efficacy of IgY antibodies raised against Deinagkistrodon acutus venom. Journal of Venomous Animals and Toxins including Tropical Diseases, 23, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Wang, Y. , Zhang, Z. , Huang, J. , Yao, M. , Huang, G. et al. (2020) Generation of chicken IgY against SARS‐COV‐2 spike protein and epitope mapping. Journal of Immunology Research, 2020, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, Z. , Deng, Y.Q. , Ye, Q. , Cao, L. , Sun, C.Y. , Fan, C. et al. (2020) Structural basis for neutralization of SARS‐CoV‐2 and SARS‐CoV by a potent therapeutic antibody. Science, 369, 1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, J. , Bao, L. , Shen, X. , Yan, C. , Zhang, C. , Wei, W. et al. (2021) The preparation of N‐IgY targeting SARS‐CoV‐2 and its immunomodulation to IFN‐γ production in vitro. International Immunopharmacology, 96, 107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matumoto, M. (1949) A note on some points of calculation method of LD50 by Reed and Muench. The Japanese Journal of Experimental Medicine, 20, 175–179. [PubMed] [Google Scholar]
- Michael, A. , Meenatchisundaram, S. , Parameswari, G. , Subbraj, T. , Selvakumaran, R. & Ramalingam, S. (2010) Chicken egg yolk antibodies (IgY) as an alternative to mammalian antibodies. Indian Journal of Science and Technology, 3(4), 468–474. [Google Scholar]
- Nie, J. , Li, Q. , Wu, J. , Zhao, C. , Hao, H. , Liu, H. et al. (2020) Quantification of SARS‐CoV‐2 neutralizing antibody by a pseudotyped virus‐based assay. Nature Protocols, 15, 3699–3715. [DOI] [PubMed] [Google Scholar]
- O’Donnell, K.L. , Meberg, B. , Schiltz, J. , Nilles, M.L. & Bradley, D.S. (2019) Zika virus‐specific IgY results are therapeutic following a lethal zika virus challenge without inducing antibody‐dependent enhancement. Viruses, 11, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan, A. , Das, D. , Kammila, S. , Suresh, M.R. & Sunwoo, H.H. (2012) Diagnostics of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) nucleocapsid antigen using chicken immunoglobulin Y. Poultry Science, 91, 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de la Lastra, J.M. , Baca‐Gonzalez, V. , Asensio‐Calavia, P. , Gonzalez‐Acosta, S. & Morales‐delaNuez, A. (2020) Can immunization of hens provide oral‐based therapeutics against COVID‐19? Vaccines, 8, 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar, L. , Segovia‐Chumbez, B. , Jadi, R. , Martinez, D.R. , Raut, R. , Markmann, A.J. et al. (2020) The receptor‐binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Science Immunology, 5, eabc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, S. , Van Nguyen, S. , Icatlo, F.C. Jr , Umeda, K. & Kodama, Y. (2013) Oral passive IgY‐based immunotherapeutics. Human Vaccines & Immunotherapeutics, 9, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani, M. , Marchant, A. & Kollmann, T.R. (2021) Immunological mechanisms of vaccine‐induced protection against COVID‐19 in humans. Nature Reviews Immunology, 21, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh, A. & Rezaei, N. (2020a) Immune‐epidemiological parameters of the novel coronavirus—a perspective. Expert Review of Clinical Immunology, 16(5), 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh, A. & Rezaei, N. (2020b) Towards treatment planning of COVID‐19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti‐antibodies, immunoglobulins, and corticosteroids. International Immunopharmacology, 84, 106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugaraj, B. , Malla, A. & Phoolcharoen, W. (2020) Emergence of novel coronavirus 2019‐nCoV: need for rapid vaccine and biologics development. Pathogens, 9, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, H. , Cai, Y. , Zhang, H. , Wu, J. , Ye, L. , Yang, P. et al. (2021) Anti‐SARS‐CoV‐2 IgY isolated from egg yolks of hens immunized with inactivated SARS‐CoV‐2 for immunoprophylaxis of COVID‐19. Virologica Sinica, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram, R. , Choraria, A. & Antonysamy, M. (2020) An approach towards development of monoclonal IgY antibodies against SARS CoV‐2 spike protein (S) using phage display method: a review. International Immunopharmacology, 85, 106654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudjarwo, S.A. , Eraiko, K. , Sudjarwo, G.W. & Koerniasari. (2017) The potency of chicken egg yolk immunoglobulin (IgY) specific as immunotherapy to Mycobacterium tuberculosis infection. Journal of Advanced Pharmaceutical Technology & Research, 8, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, W. , He, L. , Zhang, X. , Pu, J. , Voronin, D. , Jiang, S. et al. (2020) Characterization of the receptor‐binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cellular & Molecular Immunology, 17, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. , Zhang, H. , Chai, Y. , Song, H. , Tong, Z. , Wang, Q. et al. (2017) An unexpected N‐terminal loop in PD‐1 dominates binding by nivolumab. Nature Communications, 8, 14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, X. , Li, C. , Huang, A. , Xia, S. , Lu, S. , Shi, Z. et al. (2020) Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus‐specific human monoclonal antibody. Emerging Microbes & Infections, 9, 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Wang, S. , Li, D. , Wei, D.‐Q. , Zhao, J. & Wang, J. (2020) Human intestinal defensin 5 inhibits SARS‐CoV‐2 invasion by cloaking ACE2. Gastroenterology, 159, 1145–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Wang, L. , Cao, H. & Liu, C. (2021) SARS‐CoV‐2 S1 is superior to the RBD as a COVID‐19 subunit vaccine antigen. Journal of Medical Virology, 93, 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, S. , Duan, S. , Liu, X. , Wang, H. , Ding, S. , Chen, Y. et al. (2021) Chicken Egg Yolk Antibodies (IgYs) block the binding of multiple SARS‐CoV‐2 spike protein variants to human ACE2. International Immunopharmacology, 90, 107172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, J. , Zhao, S. , He, D. , Yang, Y. , Li, Y. & Zhu, S. (2012) Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus. Antiviral Research, 93, 154–159. [DOI] [PubMed] [Google Scholar]
- Wu, R. , Wang, L. , Kuo, H.D. , Shannar, A. , Peter, R. , Chou, P.J. et al. (2020) An update on current therapeutic drugs treating COVID‐19. Current Pharmacology Reports, 6(3), 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Wang, F. , Shen, C. , Peng, W. , Li, D. , Zhao, C. et al. (2020) A noncompeting pair of human neutralizing antibodies block COVID‐19 virus binding to its receptor ACE2. Science, 368(6496), 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, B. , Shen, X. , He, Y. , Pan, X. , Liu, F.‐L. , Wang, Y. et al. (2021) SARS‐CoV‐2 envelope protein causes acute respiratory distress syndrome (ARDS)‐like pathological damages and constitutes an antiviral target. Cell Research, 31, 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Hu, Q. , Jiao, L. , Cui, X. , Wu, P. , He, P. et al. (2019) Production of anti‐Trichophyton rubrum egg yolk immunoglobulin and its therapeutic potential for treating dermatophytosis. Microbial Pathogenesis, 137, 103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Petitjean, S.J.L. , Koehler, M. , Zhang, Q. , Dumitru, A.C. , Chen, W. et al. (2020) Molecular interaction and inhibition of SARS‐CoV‐2 binding to the ACE2 receptor. Nature Communications, 11, 4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. & Du, L. (2021) SARS‐CoV‐2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduction and Targeted Therapy, 6, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell, J.W. (2021) Individuals cannot rely on COVID‐19 herd immunity: durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS Path, 17, e1009509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, M. , Liu, H. , Wu, N.C. & Wilson, I.A. (2021) Recognition of the SARS‐CoV‐2 receptor binding domain by neutralizing antibodies. Biochemical and Biophysical Research Communications, 538, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W.W. (2003) The use of gene‐specific IgY antibodies for drug target discovery. Drug Discovery Today, 8, 364–371. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Diraviyam, T. , Li, X. , Yao, G. & Michael, A. (2016) Preparation of chicken IgY against recombinant E2 protein of bovine viral diarrhea virus (BVDV) and development of ELISA and ICA for BVDV detection. Bioscience, Biotechnology, and Biochemistry, 80, 2467–2472. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wei, Y. , Li, Y. , Wang, X. , Liu, Y. , Tian, D. et al. (2021) IgY antibodies against Ebola virus possess post‐exposure protection in a murine pseudovirus challenge model and excellent thermostability. PLoS Neglected Tropical Diseases, 15, e0008403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Chen, Y. , Zhang, S. , Niu, P. , Qin, K. , Jia, W. et al. (2019) Structural definition of a neutralization epitope on the N‐terminal domain of MERS‐CoV spike glycoprotein. Nature Communications, 10, 3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Ma, Y. , Lu, M. , Zhang, Y. , Li, A. , Liang, X. et al. (2019) Efficient production of human norovirus‐specific IgY in egg yolks by vaccination of hens with a recombinant vesicular stomatitis virus expressing VP1 protein. Viruses, 11, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3


