Abstract
Background & Aims:
Hepatocellular carcinoma (HCC) surveillance rates are suboptimal in clinical practice. We aimed to elicit providers’ opinions on the following aspects of HCC surveillance: preferred strategies, barriers and facilitators, and the impact of a patient’s HCC risk on the choice of surveillance modality.
Methods:
We conducted a web-based survey among gastroenterology and hepatology providers (40% faculty physicians, 21% advanced practice providers, 39% fellow-trainees) from 26 U.S. medical centers in 17 states.
Results:
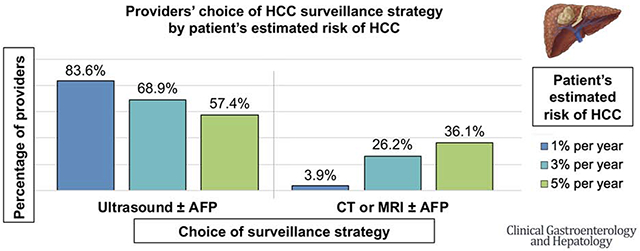
Of 654 eligible providers, 305 (47%) completed the survey. Nearly all (98.4%) of the providers endorsed semi-annual HCC surveillance in patients with cirrhosis, with 84.2% recommending ultrasound ± alpha fetoprotein (AFP) and 15.4% recommending computed tomography (CT) or magnetic resonance imaging (MRI). Barriers to surveillance included limited HCC treatment options, screening test effectiveness to reduce mortality, access to transportation, and high out-of-pocket costs. Facilitators of surveillance included professional society guidelines. Most providers (72.1%) would perform surveillance even if HCC risk was low (≤0.5% per year), while 98.7% would perform surveillance if HCC risk was ≥1% per year. As a patient’s HCC risk increased from 1% to 3% to 5% per year, providers reported they would be less likely to order ultrasound ± AFP (83.6% to 68.9% to 57.4%; p<0.001) and more likely to order CT or MRI ± AFP (3.9% to 26.2% to 36.1%; p<0.001).
Conclusion:
Providers recommend HCC surveillance even when HCC risk is much lower than the threshold suggested by professional societies. Many appear receptive to risk-based HCC surveillance strategies that depend on patients’ estimated HCC risk, instead of our current “one-size-fits all” strategy.
Keywords: Liver cancer, screening, ultrasound, computed tomography, magnetic resonance imaging
Graphical Abstract
Introduction
Professional societies recommend hepatocellular carcinoma (HCC) surveillance in patients with cirrhosis using semi-annual abdominal ultrasound with or without serum alpha fetoprotein (AFP).1–3 Successful implementation of HCC surveillance programs, however, has been challenging with only 24% of eligible patients receiving timely HCC surveillance.4 Various healthcare system, provider,5 and patient-level factors,6 as well as the perceived effectiveness of existing HCC surveillance strategies, have been identified as potential barriers to improving HCC surveillance rates.
Guidelines currently recommend a “one-size-fits-all” HCC surveillance strategy, whereby the same tests (ultrasound ± AFP) are recommended for all patients with cirrhosis irrespective of their underlying HCC risk, so long as the annual risk exceeds 1.5%.7 Many studies, however, demonstrated that HCC risk in individual patients varies widely from <1% to >5% per year. Though various risk scores and prediction models have been developed to estimate HCC risk in patients with cirrhosis, there has yet to be a single model incorporated into societal guidelines or routine clinical practice.8–11 It is possible that improvements in HCC surveillance rates could be achieved if patients are first stratified according to HCC risk, then offered individualized risk-appropriate surveillance. For example, more effective but expensive surveillance strategies using computed tomography (CT) or magnetic resonance imaging (MRI) could be cost-effective if utilized in higher risk groups.12
Although surveillance rates are higher in subspecialty clinics than in primary care practices,4, 13 few studies have focused on gastroenterology and hepatology providers’ approach to HCC surveillance. Thus, our aim was to assess existing HCC surveillance practices, and investigate whether gastroenterology and hepatology providers’ decisions to screen patients for HCC and their choice of surveillance test are influenced by patient-specific HCC risk.
Methods
Data source and provider population
We conducted an anonymous web-based survey (Supplemental Table 1) using a convenience sample of self-identified gastroenterology and hepatology providers at 26 academic institutions, safety-net health systems, private hospitals, and Veteran Affairs medical centers from 17 states in the U.S. The survey was designed using a conceptual model (Supplemental Figure 1) based on the Theory of Reasoned Action and Theory of Planned Behavior, which have been used to study healthcare providers’ behaviors.5, 14, 15 Questions were adapted from prior surveys16, 17 and pre-tested by 10 providers including gastroenterology fellows, primary care providers, gastroenterologists, and hepatologists via cognitive interviewing until saturation of feedback was achieved. The final survey had 29 questions, took 10 minutes to complete, and was organized into 4 sections:
Hepatocellular carcinoma surveillance practices (3 questions)
Factors that may influence hepatocellular carcinoma surveillance practices (4 questions)
Clinical scenarios designed to assess how hepatocellular carcinoma risk might affect the choice of surveillance strategy (9 questions)
Provider and institutional information (13 questions)
The survey was executed on the REDCap platform. A web link to the survey was distributed via email to eligible providers between October 28 and December 8, 2019. A single provider at each site distributed the survey to all eligible providers (faculty physicians, advanced practice providers (APP), and gastroenterology or hepatology fellow-trainees caring for patients with cirrhosis over the age of 18) at his or her institution. A reminder email to complete the survey was sent 2 weeks after the initial request. There was no incentive to participate in this study. Incomplete surveys (n=80) and surveys completed by non-gastroenterology and hepatology providers (n=2) were excluded from the final analysis. This study was approved by the Institutional Review Board at the University of Washington.
Outcome measures
The survey first assessed provider and institution characteristics, and providers’ current HCC surveillance practices. It then asked providers to rank the importance of patient-, provider-, and system-level factors on ordering HCC surveillance using a 5-point Likert scale ranging from “not at all important” to “extremely important”. Finally, the survey presented case scenarios to evaluate whether the availability of patient-specific annual risk of HCC influences providers’ decisions to order HCC surveillance. Specifically, providers were asked about case scenarios where the annual risk of HCC for each patient varied from “low risk” (<1.5% per year) to “high risk” (≥1.5% per year). A subgroup analysis by provider type (faculty vs. APP vs. trainee) was performed to assess for variations in surveillance-related practices and attitudes toward risk-based stratification.
Statistical analysis
Descriptive analysis including frequency (%) for categorical variables and mean (SD) for continuous variables was used to summarize provider and institution characteristics, HCC surveillance practices, and the perceived importance of factors on ordering HCC surveillance. Chi-square test was used for categorical variables when comparing outcomes by provider type. Statistical significance was defined as p<0.05 (two-sided). Analyses were done using Stata version 15 (Stata Corp LP, College Station, TX).
Results
Provider characteristics
Among 654 eligible providers, 305 providers completed the survey for a response rate of 47%. Provider characteristics are summarized in Table 1. Approximately 51% of providers were female and 53% were non-Hispanic white; there was good representation of all provider types, including 40% faculty physicians, 21% APPs, and 39% trainees. Most providers (53%) saw >100 unique patients with cirrhosis annually and >80% worked in an academic setting.
Table 1.
Characteristics of 305 gastroenterology and hepatology providers who completed a web-based survey regarding their attitudes toward HCC surveillance
| Characteristics | N (%) | Characteristics | N (%) |
|---|---|---|---|
|
| |||
| Provider | Institution | ||
|
| |||
| Male | 150 (49.2) | ||
|
| |||
| Race/ethnicity | Region of U.S. | ||
| White, non-Hispanic | 160 (52.5) | Northeast | 44 (14.3) |
| Black, non-Hispanic | 9 (2.95) | Midwest | 89 (29.0) |
| Hispanic | 18 (5.90) | South | 60 (19.5) |
| American Indian/Alaska | 1 (0.33) | West | 86 (28.0) |
| Native | 99 (32.5) | Unknown | 28 (9.12) |
| Asian/Pacific Islander | 18 (5.90) | ||
| Other | |||
|
| |||
| Provider type | Type of clinical practice | ||
| Faculty physician | 122 (40.0) | Academic | 252 (82.6) |
| Advanced practice provider | 64 (21.0) | Veteran Affairs | 25 (8.20) |
| Trainee | 119 (39.0) | Safety-net/public | 14 (4.59) |
| Gastroenterology | 104 (34.1) | Private | 14 (4.59) |
| Advanced/Transplant | 15 (4.92) | ||
| Hepatology | |||
|
| |||
| Provider specialty | Liver transplant center | 266 (87.2) | |
| Gastroenterology | 124 (40.7) | ||
| Hepatology | 181 (59.3) | ||
|
| |||
| Years at current clinical site | |||
| <5 years | 181 (59.3) | ||
| 5-10 years | 71 (23.3) | ||
| 11-15 years | 24 (7.87) | ||
| 16-20 years | 13 (4.26) | ||
| >20 years | 16 (5.25) | ||
|
| |||
| Years total in practice | |||
| <5 years | 158 (51.8) | ||
| 5-10 years | 61 (20.0) | ||
| 11-15 years | 30 (9.84) | ||
| 16-20 years | 17 (5.57) | ||
| >20 years | 39 (12.8) | ||
|
| |||
| Time spent on patient care | |||
| <25% | 16 (5.25) | ||
| 25-50% | 28 (9.18) | ||
| 51-75% | 63 (20.7) | ||
| >75% | 198 (64.9) | ||
|
| |||
| Number of unique patients with cirrhosis seen in clinic in 1 year | |||
| None | 2 (0.66) | ||
| 1-25 | 31 (10.2) | ||
| 26-50 | 37 (12.1) | ||
| 51-75 | 38 (12.5) | ||
| 76-100 | 36 (11.8) | ||
| >100 | 161 (52.8) | ||
Current HCC surveillance practices
Nearly all providers (98.4%) endorsed semi-annual HCC surveillance, most commonly with abdominal ultrasound ± AFP (84.2%; 71.8% ultrasound with AFP, 12.4% ultrasound only) (Table 2). However, 15.4% of providers endorsed CT or MRI-based HCC surveillance. Nearly half of the providers (51%) thought HCC surveillance was the responsibility of both the primary care provider and subspecialist. Trainees were more likely than faculty or APPs to order ultrasound alone (20% vs. 10% vs. 3%, respectively) whereas faculty (75%) and APPs (75%) were more likely to order ultrasound with AFP (vs. 67% trainees). APPs were more likely to order CT or MRI with AFP than trainees or faculty (16% vs. 10% vs. 8.4%, respectively, p=0.024, Table 2).
Table 2.
Current HCC surveillance practices reported by gastroenterology and hepatology providers, stratified by provider type
| Overall (N=305) | Faculty physician (N=122) | Advanced practice provider (N=64) | Trainee (N=119) | P-value* | |
|---|---|---|---|---|---|
|
| |||||
| Recommended interval for HCC surveillance | |||||
| Never | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.068 |
| Annually | 3 (0.98) | 2 (1.64) | 0 (0.00) | 1 (0.84) | |
| Every 6 months | 300 (98.4) | 120 (98.4) | 62 (96.9) | 118 | |
| Every 3 months | 2 (0.66) | 0 (0.00) | 2 (3.12) | (99.2) | |
| Other | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| 0 (0.00) | |||||
|
| |||||
| Most commonly recommended test for HCC surveillance | |||||
| AFP only | 1 (0.33) | 0 (0.00) | 1 (1.56) | 0 (0.00) | 0.024 |
| Ultrasound only | 38 (12.4) | 12 (9.84) | 2 (3.12) | 24 (20.2) | |
| Ultrasound + AFP | 219 (71.8) | 91 (74.6) | 48 (75.0) | 80 (67.2) | |
| CT or MRI ± AFP | 32 (10.5) | 12 (9.84) | 10 (15.6) | 10 (8.40) | |
| Other | 15 (4.92) | 7 (5.74) | 3 (4.69) | 5 (4.20) | |
| Alternate (Ultrasound vs. CT) + AFP | 4 (26.7) | 1 (14.3) | 2 (66.7) | 1 (20.0) | 0.166 |
| Alternate Ultrasound vs. MRI | 5 (33.3) | 1 (14.3) | 0 (0.00) | 4 (80.0) | |
| Alternate (Ultrasound vs. MRI) + AFP | 4 (26.7) | 3 (42.9) | 1 (33.3) | 0 (0.00) | |
| Alternate Ultrasound vs. CT or MRI | 1 (6.67) | 1 (14.3) | 0 (0.00) | 0 (0.00) | |
| Alternate (Ultrasound vs. CT or MRI) + AFP | 1 (6.67) | 1 (14.3) | 0 (0.00) | 0 (0.00) | |
|
| |||||
| Provider who should be responsible for HCC surveillance | |||||
| Primary care provider | 10 (3.28) | 5 (4.10) | 0 (0.00) | 5 (4.20) | 0.115 |
| Gastroenterologist or Hepatologist | 139 (45.6) | 59 (48.4) | 35 (54.7) | 45 (37.8) | |
| Both primary care and Gastroenterologist or Hepatologist | 156 (51.2) | 58 (47.5) | 29 (45.3) | 69 (58.0) | |
P-value considered significant if <0.05
AFP: Alpha fetoprotein. CT: Computed tomography. HCC: Hepatocellular carcinoma. MRI: Magnetic resonance imaging.
Provider attitudes on barriers and facilitators of HCC surveillance
Providers rated several factors as being very or extremely important to ordering HCC surveillance, including society guideline recommendations (96.1%), test effectiveness for early detection of HCC (88.2%), and patient’s individual risk for developing HCC (85.3%) (Supplemental Table 2). They also rated several factors as being very or extremely important to not ordering HCC surveillance, including limited treatment options if diagnosed with HCC (28.5%), limited test effectiveness to reduce mortality (24.3%), and insufficient time during a clinic visit (22.6%). Additional factors, including system and patient-level factors, are listed in Supplemental Table 2 by order of importance.
HCC risk influences provider surveillance practice patterns
We presented providers with several different patient scenarios. First, when providers were asked to consider a scenario comparing patients with HCC risk of 0.1% and 0.5% per year, 61% reported they would order HCC surveillance for both patients, while 10.5% reported they would order surveillance only for the patient with a risk of 0.5% per year (Figure 1). APPs were the most likely to order HCC surveillance for both patients (89% vs. 57% trainees vs. 49% faculty) while faculty were the least likely to order HCC surveillance for either patient (39% vs. 8% APPs vs. 27% trainees) (p<0.001, Figure 2A). When given a scenario comparing patients with HCC risk of 1% and 3% per year, 86% of providers reported they would order HCC surveillance for both patients, while 13% reported they would order surveillance only for the patient with a risk of 3% per year. There was no significant difference in response by provider type (p=0.203, Figure 2B). Lastly, when given a scenario comparing patients with HCC risk of 3% and 5% per year, 99.7% of providers reported they would order HCC surveillance for both patients. There was no significant difference in response by provider type (p=0.457, Figure 2C).
Figure 1. Proportion of providers recommending HCC surveillance based on a patient’s estimated annual risk of HCC.
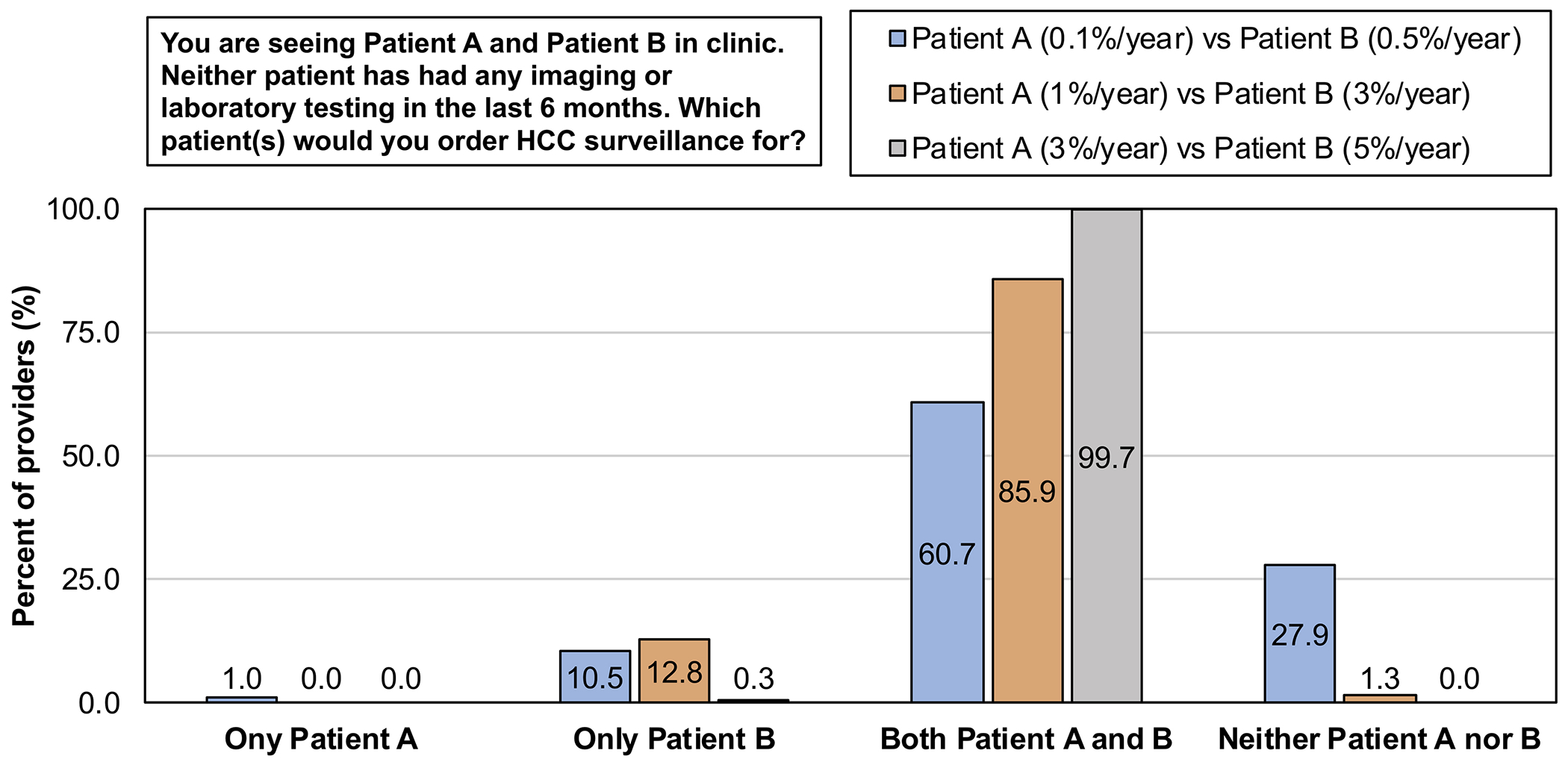
HCC: Hepatocellular carcinoma.
Figure 2. The impact of patients’ estimated annual HCC risk on providers’ decision to perform or not perform HCC surveillance.
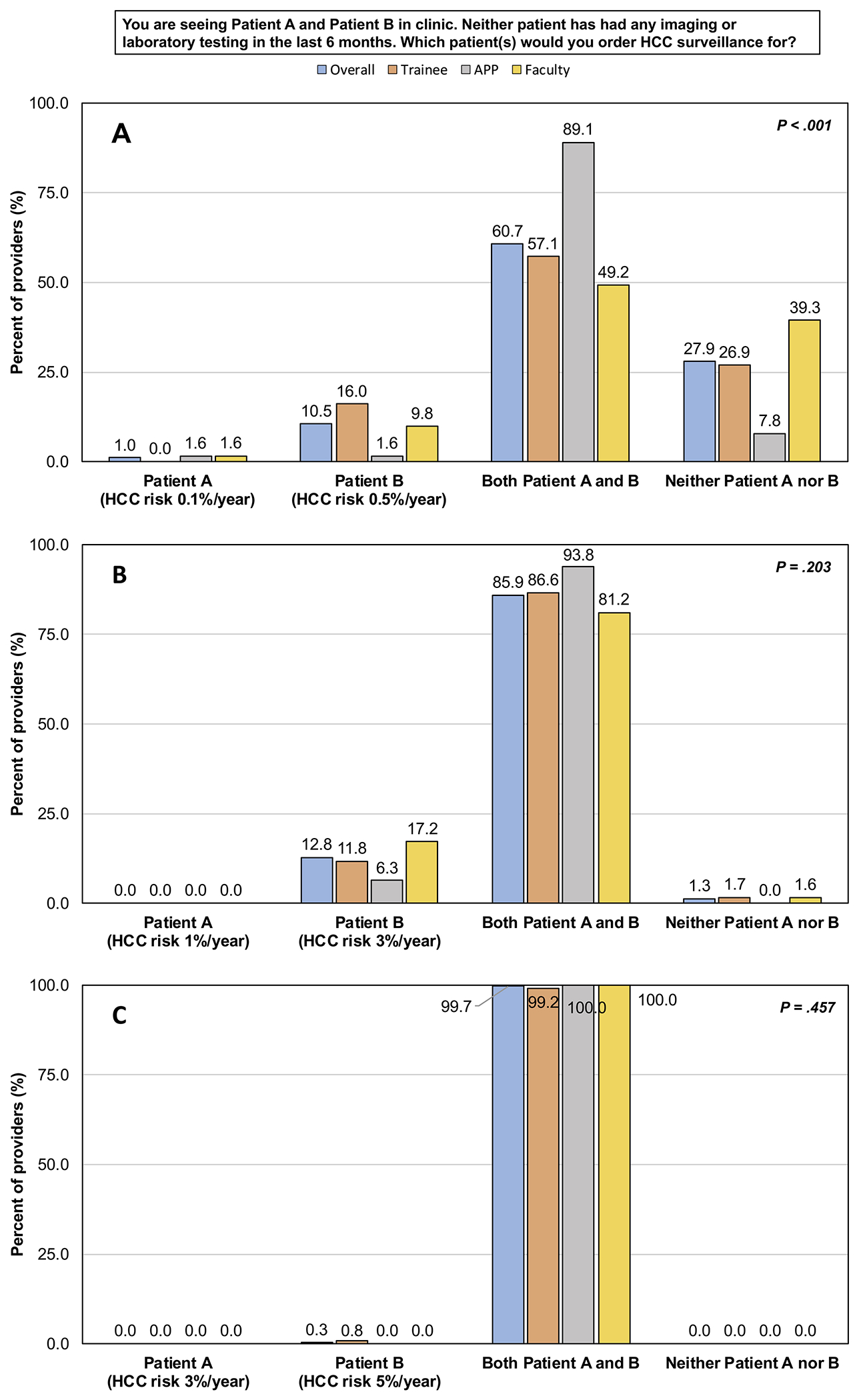
(A) Scenario 1: HCC risk of 0.1%/year vs. 0.5%/year.
(B) Scenario 2: HCC risk of 1%/year vs. 3%/year.
(C) Scenario 3: HCC risk of 3%/year vs. 5%/year.
APP: Advanced practice provider. HCC: Hepatocellular carcinoma.
Next, when providers were presented with a scenario where surveillance capacity was unlimited (i.e. all eligible patients can receive surveillance), providers chose a median annual HCC risk of >0.5% per year (range >0%-3%) as the threshold above which they would begin surveillance (Figure 3A), consistent with the prior scenario comparing patients with HCC risk of 0.1% and 0.5% per year (Figure 1). When surveillance capacity was limited (i.e. not all eligible patients can receive surveillance), providers chose a median annual HCC risk of >1% per year (range >0%-4%) as the threshold above which they would begin surveillance (Figure 3B).
Figure 3. Providers’ attitude on estimated annual HCC risk above which surveillance would be recommended by provider type.
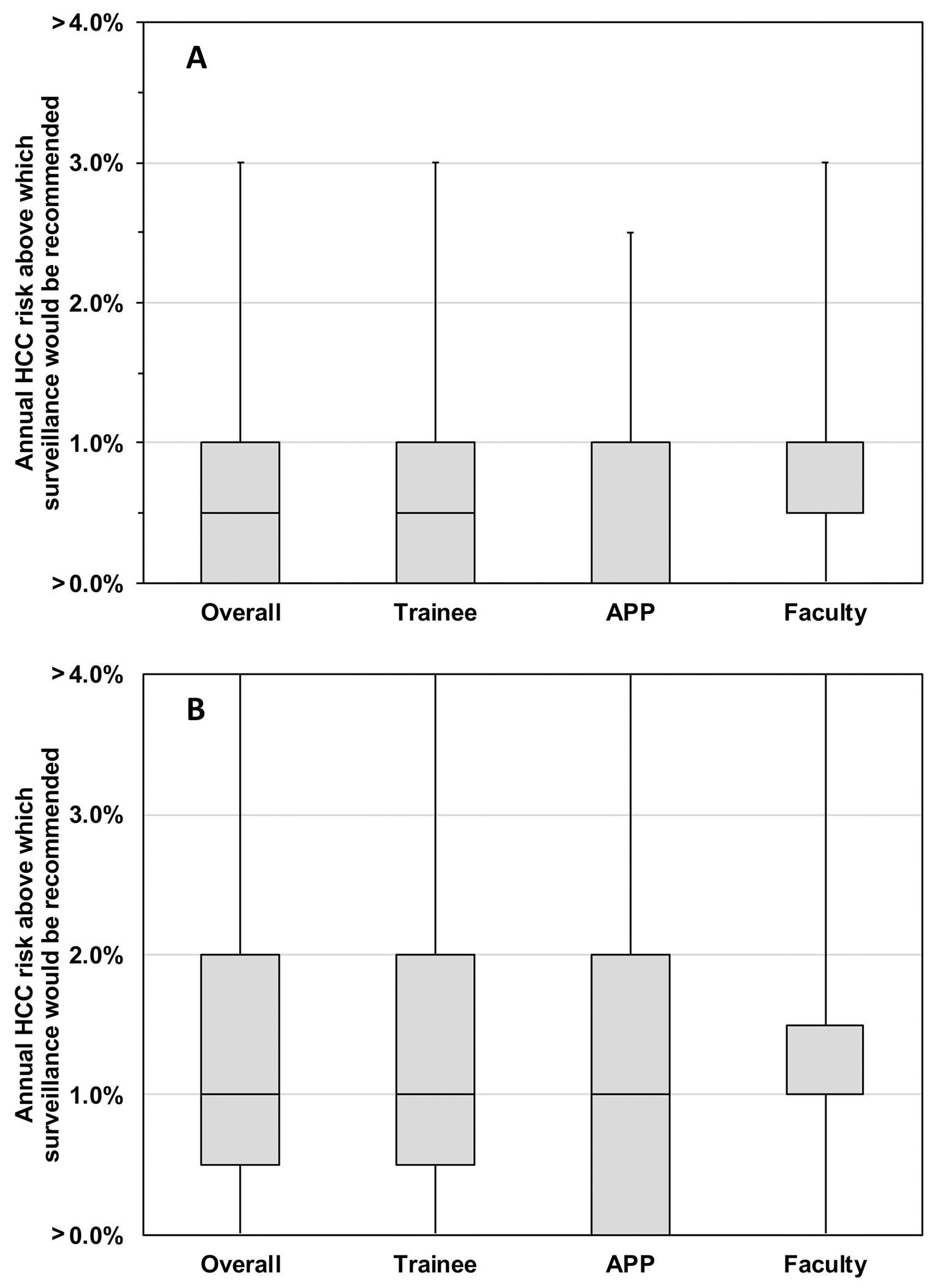
(A) Scenario when surveillance capacity is unlimited.
(B) Scenario when surveillance capacity is limited.
APP: Advanced practice provider. HCC: Hepatocellular carcinoma.
As HCC risk incrementally increased from 1% to 3% to 5% per year, providers were increasingly less likely to select ultrasound ± AFP (83.6% to 68.9% to 57.4%) and more likely to select CT or MRI ± AFP (3.9% to 26.2% to 36.1%) as the surveillance strategy (p<0.001, Figure 4A). There was a statistically significant difference in the choice of surveillance test between 1% and 3% per year across all provider types (Figure 4B–D).
Figure 4. The impact of patients’ estimated annual HCC risk on providers’ choice of HCC surveillance strategy.
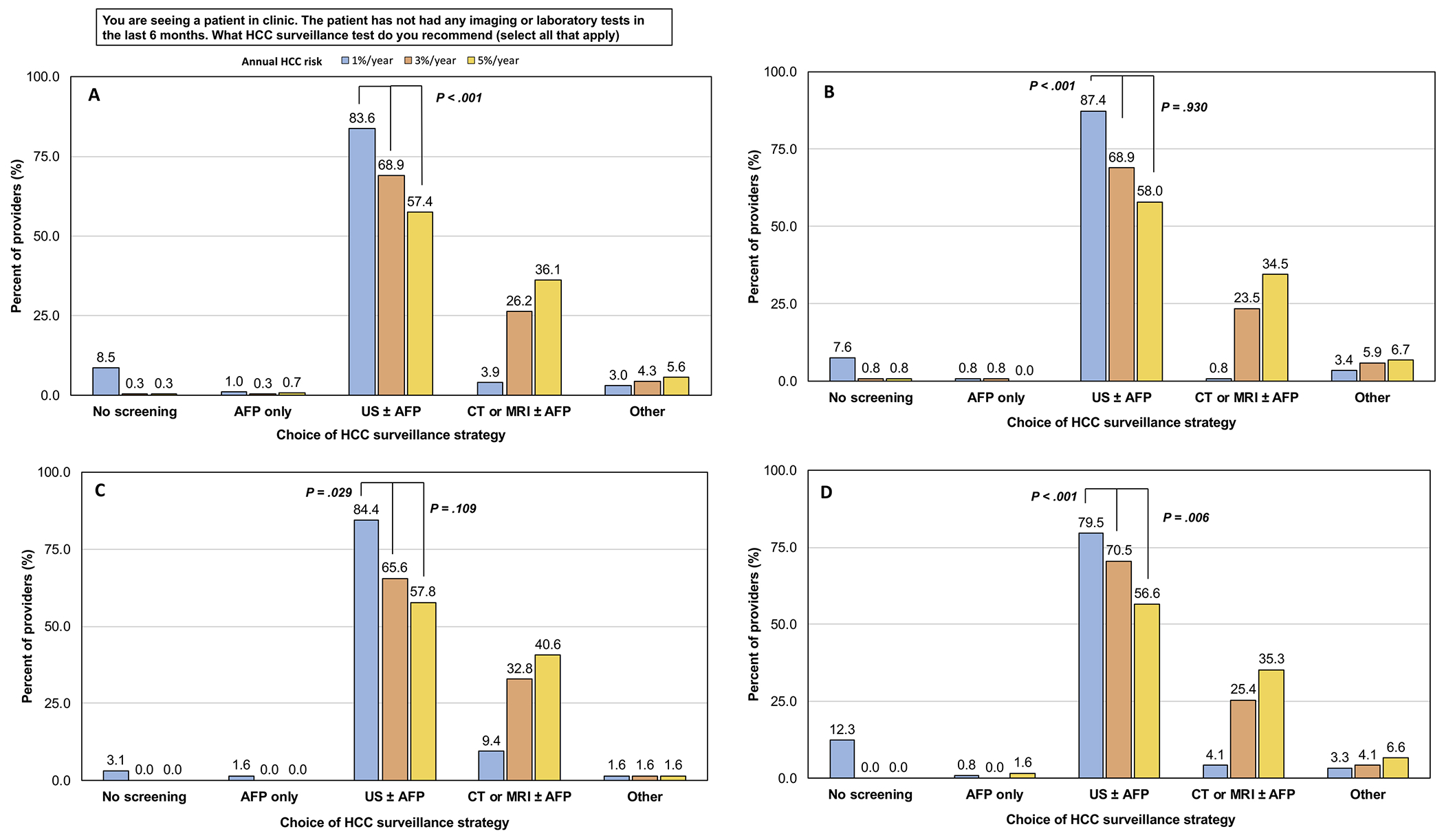
(A) All providers. (B) Trainee. (C) Advanced practice provider. (D) Faculty.
AFP: Alpha fetoprotein. CT: Computed tomography. HCC: Hepatocellular carcinoma. MRI: Magnetic resonance imaging. US: Ultrasound.
Discussion
Despite the many benefits of HCC surveillance in patients with cirrhosis including early detection of HCC and possible reduction in mortality, HCC surveillance remains underutilized in clinical practice.4, 18 We found that nearly all surveyed gastroenterology and hepatology providers endorsed semi-annual HCC surveillance using ultrasound ± AFP, but a substantial proportion of providers also recommended CT or MRI-based surveillance. Most providers endorsed screening even when the patients’ risk of HCC was much lower than the threshold recommended by professional societies. We also showed that the higher the patients’ HCC risk, the more likely providers were to order CT or MRI over ultrasound for surveillance. This suggests that providers are receptive to the concept of personalizing HCC surveillance based on a patient’s category of HCC risk. Implementation of a risk-based surveillance strategy rather than the current “one-size-fits-all” strategy may improve overall HCC surveillance rates and outcomes.
Most providers recommend ultrasound with AFP for HCC surveillance
Nearly all (84%) of our surveyed providers recommended abdominal ultrasound ± AFP for HCC surveillance, in accordance with professional society guidelines. However, a much larger proportion recommended ultrasound with AFP (72%) over ultrasound alone (12%). Compared to a prior survey of gastroenterology and hepatology providers, which reported that 36% and 25% of providers ordered ultrasound only and ultrasound with AFP for surveillance, respectively19, the rates of using ultrasound with AFP has increased. This may be explained by the multiple studies that have since shown that ultrasound with AFP is more effective than either alone for detecting HCC. A retrospective cohort study showed that the sensitivity and specificity for detecting HCC using AFP alone (threshold of 20 ng/mL) was 52.9% and 93.3%, but improved to 99.2% and 68.3% when combined with ultrasound.20 A subsequent meta-analysis also found that ultrasound alone detected fewer cases of HCC than ultrasound with AFP (relative risk 0.88).21 However, surveillance using ultrasound with AFP still has its limitations (Supplemental Figure 2); its pooled sensitivity and specificity for detecting early-stage HCC is only 63% and 45% respectively21 and test characteristics can be further limited by a patient’s body habitus and steatosis, which remain concerns in patients at higher risk of developing HCC.
More providers are using cross-sectional imaging for HCC surveillance
Studies focused on using CT, MRI, or abbreviated MRI for HCC surveillance have been on the rise.22, 23 In two prior studies, 2% of gastroenterology and hepatology providers19 and 12% of primary care providers16 reported using CT or MRI-based HCC surveillance, with cross-sectional imaging more common at liver transplant centers.19 We found that 15% of our surveyed providers recommended using CT or MRI for surveillance. While this may be related to the proportion of our surveyed providers practicing at liver transplant centers, it also suggests that providers are adopting cross-sectional imaging in clinical practice despite the lack of endorsement in guidelines, and the absence of high-quality data to support its use for surveillance. A prior randomized controlled trial among U.S. veterans with cirrhosis found that semi-annual ultrasound was more sensitive (71.4% vs. 66.7%) than annual CT for HCC surveillance.23 However, more recent data suggests that in select patient groups, such as patients with obesity,24 nonalcoholic steatohepatitis cirrhosis,24 an annual HCC risk >5%,25 and HBV cirrhosis,26 CT or MRI may perform better than ultrasound in detecting HCC. Similarly, in the PRIUS study, contrast-MRI had greater sensitivity (84.8% vs. 27.3%) for detecting very early-stage HCC than ultrasound27 and semi-annual MRI was considered cost-effective when the annual HCC risk exceeded 1.81%.28 Only the PRIUS study was prospective in design, but providers appear to be increasingly receptive to the use of cross-sectional imaging for surveillance.
Providers recommend HCC surveillance even when annual risk of HCC is low
We also found that the majority of our surveyed providers recommended HCC surveillance even for patients at low risk of developing HCC. Nearly 62%, 71%, and 86% of our surveyed providers recommended surveillance in patients with a 0.1%, 0.5%, and 1% per year risk of developing HCC, respectively. Providers also reported that their threshold to start HCC surveillance would begin at 0.5% per year and increase to 1% per year if they faced a scenario with limited resources. These findings are especially interesting as it suggests that gastroenterology and hepatology providers are unlikely to forego HCC surveillance for patients even when annual HCC risk is <1.5% per year, contrary to current recommendations.7
Providers would vary HCC surveillance strategies based on patients’ risk of developing HCC
Our most novel finding was that our surveyed providers’ choice of HCC surveillance strategy varied based on patients’ category of annual risk of HCC. As the annual risk of HCC increased, providers were more likely to order CT or MRI over ultrasound for surveillance. The proportion of providers who would order an ultrasound ± AFP decreased from 84% to 57%, while those who would order CT or MRI ± AFP increased from 4% to 36%, as the patients’ annual HCC risk increased from 1% to 5% per year. This trend persisted across provider types. We believe these findings illustrate the importance of considering implementation of risk-based HCC surveillance to not only improve, but also standardize HCC surveillance practices. Multiple studies have already described risk scores and prediction models to estimate HCC risk in patients with cirrhosis.8–11 Though further studies are needed to validate these existing tools in additional patient populations, risk-based surveillance may present an exciting opportunity to improve HCC-related outcomes in patients with cirrhosis and rationalize resource utilization.
HCC surveillance strategies vary by provider type
We also found significant differences in HCC surveillance recommendations by provider type. Trainees were most likely to order ultrasound alone, while APPs were most likely to order cross-sectional imaging and screening for patients with annual HCC risks of 0.1% and 0.5%. Reasons for these findings may include incomplete awareness of guidelines, trainees modeling after the practices of their faculty physicians, and APPs being more concerned about missing HCC, prompting more aggressive screening. In fact, a recent study also found that while patients under the care of APPs received more consistent HCC screening, they also had higher healthcare expenditures, partly driven by cross-sectional imaging.29 Additional studies are needed to explore the reasons behind specific preferences for HCC surveillance and how providers understand HCC risk, but these findings suggest that better education for trainees and APPs may also help standardize screening strategies.
Various factors influence HCC surveillance completion
Similar to prior studies, we also found that barriers to ordering and obtaining HCC surveillance included limited clinical time and patients’ difficulties with cost of care, transportation, and scheduling, whereas the presence of HCC-related guidelines was an important facilitator of surveillance.5, 6, 16 Several studies have already shown that tailored interventions such as electronic reminders for providers and mailed invitations to patients can improve HCC surveillance rates.30, 31 Our findings support the ongoing need to address health system and patient-level challenges to improve access and adherence to HCC surveillance even after the order is placed.
Strengths and limitations
Our study has several strengths. We evaluated the attitudes of a nationwide sample of gastroenterology and hepatology providers on risk-based HCC surveillance, and assessed for variations in HCC surveillance practices by provider type. Our findings may help inform future HCC surveillance practices and guidelines. Despite these strengths, we acknowledge several limitations. First, the generalizability of our findings may be limited as a large proportion of our providers were affiliated with academic institutions and liver transplant centers. Second, our findings are representative of a convenience sample of U.S. providers; it is possible that providers more involved in HCC-related care selectively completed the survey resulting in non-response bias. Response bias inherent to survey design also cannot be excluded. Lastly, we assessed providers’ attitudes about risk-based surveillance using clinical scenarios, which may or may not correlate with real-life practices should providers have access to their patients’ estimated annual risk of HCC. To the best of our knowledge, we attempted to gather insight into providers’ potential behaviors, but additional studies assessing the impact of a risk-based surveillance strategy in the real-world, especially in non-academic and primary care settings, would be helpful in confirming our findings.
In summary, in a national survey of U.S. gastroenterology and hepatology providers, most recommended semi-annual HCC surveillance in accordance with societal guidelines; however, when presented with individual patient scenarios, the perceived annual risk of HCC impacted provider recommendations for surveillance and choice of modality. Many providers recommended continuing surveillance for patients even when the risk of developing HCC was <1.5% per year, and the use of CT or MRI-based surveillance was more likely when providers perceived patients to have a higher annual risk of HCC. HCC surveillance strategies also varied by provider type, highlighting the importance of educating providers about existing guidelines to standardize practices. Overall, providers appear receptive to utilizing a risk-based HCC surveillance strategy, rather than the current “one-size-fits-all” approach, to personalize HCC surveillance based on patients’ category of HCC risk. This approach, which may require more objective risk stratification measures such as biomarker testing, in addition to minimizing multilevel barriers, may improve HCC surveillance rates in clinical practice.
Supplementary Material
What You Need to Know.
Background:
Rates of hepatocellular carcinoma (HCC) surveillance in patients with cirrhosis continue to be suboptimal. Gastroenterology and hepatology providers’ attitudes on risk-based HCC surveillance are unknown.
Findings:
Providers would recommend HCC surveillance even when annual risk of HCC is low. Providers are more likely to order cross-sectional imaging over ultrasound as a patient’s risk of HCC increases.
Implications for patient care:
Many providers appear receptive to implementing a risk-based HCC surveillance strategy over our current “one-size-fits all” strategy for HCC surveillance.
Grant Support
This study was supported by NIH grant number T32DK007742 to Nicole J. Kim, NIH/NCI grant number R01CA196692 and VA CSR&D grant number I01CX001156 to George N. Ioannou, NIH/NIAAA grant number K24AA022523 to Mandana Khalili, NIH grant number T32DK007534 to Andrew M. Moon, NIH grant number T32DK060414 to Jin Ge, and NCATS/NIH grant numbers UL1TR002319, KL2TR002317, and TL1TR002318 to the Institute of Translational Health Sciences at the University of Washington.
Disclosures
Patricia P. Bloom serves as a consultant for Synlogic Inc. Shaun Chandna has served on an advisory board for Dova Pharmaceuticals and Targeted Oncology, has served as a speaker for the Chronic Liver Disease Foundation/Focus Medical Communications, has received sponsored travel for research support from Genfit/Covance and Arrowhead Pharmaceuticals, and is expected to receive research funding from Arrowhead Pharmaceuticals. Catherine Frenette served as a consultant for Wako/Fujifilm Diagnostics. Michael Fuchs has received grant support to the McGuire Research Institute from H3B, Exact Sciences, Bayer, and BMS. Mandana Khalili is a recipient of research grant (to her institution) from Gilead Sciences Inc and Intercept Pharmaceuticals, and she has served as consultant for Gilead Sciences Inc. Yuval A. Patel serves as a consultant for Intercept. Anjana Pillai is on the Speaker’s Bureau for Simply Speaking Hepatitis and Eisai Inc, and Medical Advisory Board for Exelixis and Genentech. Amit G. Singal serves as consultant for Wako/Fujifilm Diagnostics, Exact Sciences, Glycotest, GRAIL, Roche, and Bayer, and is an Associate Editor of Clinical Gastroenterology and Hepatology. Ju Dong Yang serves as consultant for Exact Sciences. All other authors have nothing to disclose as potential conflicts.
Abbreviations
- AFP
Alpha fetoprotein
- APP
Advanced practice provider
- CT
Computed tomography
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- MRI
Magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preprint Server: Not applicable.
Writing Assistance: None.
References
- 1.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–80. Epub 2017/01/29. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–70. Epub 2017/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf E, Rich NE, Marrero JA, et al. Utilization of hepatocellular carcinoma surveillance in patients with cirrhosis: A systematic review and meta-analysis. Hepatology. 2020. Epub 2020/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton-Fitzgerald E, Tiro J, Kandunoori P, et al. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(4):791–8 e1. Epub 2014/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65(3):875–84. Epub 2016/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50. Epub 2018/04/07. [DOI] [PubMed] [Google Scholar]
- 8.Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12(6):568–74. Epub 2011/04/19. [DOI] [PubMed] [Google Scholar]
- 9.loannou GN, Green PK, Beste LA, et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69(5):1088–98. Epub 2018/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou GN, Green P, Kerr KF, et al. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71(3):523–33. Epub 2019/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun HS, Kim BK, Park JY, et al. Design and validation of risk prediction model for hepatocellular carcinoma development after sustained virological response in patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2020;32(3):378–85. Epub 2020/02/06. [DOI] [PubMed] [Google Scholar]
- 12.Goossens N, Singal AG, King LY, et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8(6):e101. Epub 2017/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg DS, Taddei TH, Serper M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65(3):864–74. Epub 2016/08/18. [DOI] [PubMed] [Google Scholar]
- 14.Millstein SG. Utility of the theories of reasoned action and planned behavior for predicting physician behavior: a prospective analysis. Health Psychol. 1996;15(5):398–402. Epub 1996/09/01. [DOI] [PubMed] [Google Scholar]
- 15.Roberto AJ, Shafer MS, Marmo J. Predicting substance-abuse treatment providers’ communication with clients about medication assisted treatment: a test of the theories of reasoned action and planned behavior. J Subst Abuse Treat. 2014;47(5):307–13. Epub 2014/08/06. [DOI] [PubMed] [Google Scholar]
- 16.Simmons OL, Feng Y, Parikh ND, et al. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol. 2019;17(4):766–73. Epub 2018/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich NE, Yang JD, Perumalswami PV, et al. Provider Attitudes and Practice Patterns for Direct-Acting Antiviral Therapy for Patients With Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019. Epub 2019/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim NJ, Magee C, Cummings C, et al. Liver Disease Monitoring Practices After Hepatitis C Cure in the Underserved Population. Hepatol Commun. 2018;2(10):1274–83. Epub 2018/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi K, Mendler M, Gish R, et al. Hepatocellular carcinoma surveillance: a national survey of current practices in the USA. Dig Dis Sci. 2014;59(12):3073–7. Epub 2014/07/17. [DOI] [PubMed] [Google Scholar]
- 20.Chang TS, Wu YC, Tung SY, et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Am J Gastroenterol. 2015;110(6):836–44; quiz 45. Epub 2015/04/15. [DOI] [PubMed] [Google Scholar]
- 21.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154(6):1706–18 e1. Epub 2018/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan MV, McDonald SJ, Ong YY, et al. HCC screening: assessment of an abbreviated non-contrast MRI protocol. Eur Radiol Exp. 2019;3(1):49. Epub 2019/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pocha C, Dieperink E, McMaken KA, et al. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38(3):303–12. Epub 2013/06/12. [DOI] [PubMed] [Google Scholar]
- 24.Samoylova ML, Mehta N, Roberts JP, et al. Predictors of Ultrasound Failure to Detect Hepatocellular Carcinoma. Liver Transpl. 2018;24(9):1171–7. Epub 2018/05/22. [DOI] [PubMed] [Google Scholar]
- 25.Park HJ, Jang HY, Kim SY, et al. Non-enhanced Magnetic Resonance Imaging as a Surveillance Tool for Hepatocellular Carcinoma: Comparison with Ultrasound. J Hepatol. 2019. Epub 2019/12/15. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Kang SH, Lee M, et al. Improved Detection of Hepatocellular Carcinoma by Dynamic CT in Cirrhotic Patients With Chronic Hepatitis B: A Multi-Center Study. J Gastroenterol Hepatol. 2020. Epub 2020/03/30. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3(4):456–63. Epub 2016/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HL, An J, Park JA, et al. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology. 2019;69(4):1599–613. Epub 2018/10/27. [DOI] [PubMed] [Google Scholar]
- 29.Tapper EB, Hao S, Lin M, et al. The Quality and Outcomes of Care Provided to Patients with Cirrhosis by Advanced Practice Providers. Hepatology. 2020;71(1):225–34. Epub 2019/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beste LA, Ioannou GN, Yang Y, et al. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13(1):172–9. Epub 2014/05/13. [DOI] [PubMed] [Google Scholar]
- 31.Singal AG, Tiro JA, Marrero JA, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology. 2017;152(3):608–15 e4. Epub 2016/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


