Abstract
Class-specific enzyme-linked immunosorbent assays (ELISAs) with purified recombinant antigens of Borrelia burgdorferi sensu stricto and Western blot analyses with whole cells of this spirochete were used to test human sera to determine which antigens were diagnostically important. In analyses for immunoglobulin M (IgM) antibodies, 14 (82%) of 17 serum samples from persons who had erythema migrans reacted positively by an ELISA with one or more recombinant antigens. There was frequent antibody reactivity to protein 41-G (p41-G), outer surface protein C (OspC), and OspF antigens. In an ELISA for IgG antibodies, 13 (87%) of 15 serum samples had antibodies to recombinant antigens; reactivity to p22, p39, p41-G, OspC, and OspF antigens was frequent. By both ELISAs, serum specimens positive for OspB, OspE, and p37 were uncommon. Analyses of sera obtained from persons who were suspected of having human granulocytic ehrlichiosis (HGE) but who lacked antibodies to ehrlichiae revealed IgM antibodies to all recombinant antigens of B. burgdorferi except OspB and IgG antibodies to all antigens except OspE. Immunoblotting of sera from the study group of individuals suspected of having HGE reaffirmed antibody reactivity to multiple antigens of B. burgdorferi. There was minor cross-reactivity when sera from healthy subjects or persons who had syphilis, oral infections, or rheumatoid arthritis were tested by ELISAs with p37, p41-G, OspB, OspC, OspE, and OspF antigens. Although the results of class-specific ELISAs with recombinant antigens were comparable to those recorded for assays with whole-cell antigen and for individuals with confirmed clinical diagnoses of Lyme borreliosis, immunoblotting is still advised as an adjunct procedure, particularly when there are low antibody titers by an ELISA.
For the laboratory diagnosis of Lyme borreliosis, enzyme-linked immunosorbent assays (ELISAs) are relied on heavily for initial screening of sera (11, 14, 23). Immunoblotting methods also are used to detect antibodies to Borrelia burgdorferi (5, 6, 15, 20, 30, 31), but extensive application of these procedures can add considerable cost to diagnoses. Current ELISAs, which contain whole-cell B. burgdorferi sensu lato, can yield false-positive results (25). Cross-reactivity of treponemal antibodies with flagellin has been reported (2, 25), but nonspecific reactions also can occur when there are elevated concentrations of antibodies to other antigens shared among unrelated bacteria (14), such as Escherichia coli. Furthermore, antibodies to heat shock proteins, major antigens common to different bacteria (4), likewise can cause specificity problems. There is therefore a need to develop more specific ELISAs, without a loss of sensitivity, to evaluate these methods and to compare the results with those of immunoblot analyses.
The production and application of highly specific recombinant proteins (p) of B. burgdorferi, such as outer surface protein C (OspC), OspE, OspF, p22, p35 (47-kDa fibronectin-binding protein), and p39 have improved ELISA performance (2, 6, 10, 16, 23, 26, 27, 29). Evaluations of ELISAs with the VlsE antigen are also encouraging (17, 18). However, the immune responses of patients infected with B. burgdorferi vary greatly, and certain key antigens may not always be expressed in hosts or be recognized immunologically (2). A series of key immunologic markers of B. burgdorferi infections have been identified by performing Western blot analyses, and recombinant antigens have been produced for ELISAs. The purpose of the present study was to further evaluate the use of recombinant antigens of B. burgdorferi in class-specific ELISAs to determine which antigens are diagnostically most important.
MATERIALS AND METHODS
Study groups.
Human sera that had been stored at −60°C at the Connecticut Agricultural Experiment Station and used in earlier investigations (21–25) were reanalyzed by class-specific ELISAs (21, 23). The first group consisted of 17 serum samples from 17 patients who had physician-diagnosed erythema migrans and antibodies to B. burgdorferi whole cells, as determined by a polyvalent ELISA. These persons, all Connecticut residents, sought medical attention and gave blood samples prior to antibiotic therapy between 1 and 5 weeks after the onset of illness. There was no clinical or serologic evidence of granulocytic ehrlichial infections in these patients. An additional 17 serum samples from 17 persons who had antibodies to both B. burgdorferi and the human granulocytic ehrlichiosis (HGE) agent in polyvalent assays (24) were included in the study to determine reactivities to specific B. burgdorferi antigens. These patients, also from Connecticut, had thrombocytopenia or leukopenia and elevated antibody titers to the NCH-1 strain of the HGE agent. A third study group consisted of 18 serum samples from 18 subjects who were suspected of having HGE (24) on the basis of thrombocytopenia or leukopenia and who had immunoglobulins to B. burgdorferi but who lacked antibodies to granulocytic ehrlichiae. Sera from this group were selected for immunoblotting to confirm previous ELISA results (24) and to compare banding patterns with reactions to recombinant antigens of B. burgdorferi in ELISAs. To further assess the specificities of class-specific ELISAs with recombinant B. burgdorferi antigens, sera from individuals with the following were selected: syphilis (n = 24 serum specimens), acute necrotizing ulcerative gingivitis or periodontitis (n = 6), and rheumatoid arthritis (n = 7). The syphilitic sera had antibodies to Treponema pallidum at concentrations of 1:1,024 or greater, as determined by a standardized fluorescent-treponemal antibody-absorption test (25). The final group consisted of 29 serum samples from healthy subjects (negative controls) who lived in urban or suburban areas of Connecticut where this disease and ticks are uncommon. Details on the clinical findings, sources of sera, and results of serologic testing for antibodies to the HGE agent have been reported elsewhere (21–25).
Antigens.
B. burgdorferi (strain 2591) whole-cell antigen and the following recombinant antigens were tested by class-specific ELISAs: p22, p37, p39, p41-G, OspB, OspC, OspE, and OspF. Strain 2591 is very closely related to the B31 and N40 strains of B. burgdorferi; all three of these strains were isolated in the northeastern United States. Strains from the United States, the former Soviet Union, and Japan share key immunodominant proteins (22), and when used as antigens in ELISAs, serologic test results for human sera were similar. As described earlier (8, 16, 23, 26), all recombinant antigens were cloned and were expressed as fusion proteins in E. coli at Yale University (p22, p37, p41-G, OspB, OspE, and OspF) or the University of Connecticut (p39 and OspC). The p39 antigen was produced from the DNA of strain 2591 after amplification by PCR methods with primers (upstream primer, 5′-TAGTGGTAAAGGTACTCTT-3′; downstream primer, 5′-TTAAATAAATTCTTTAAGAAAC-3′) whose sequences were based on a previously published sequence (GenBank accession no. L24194) (28). The purified glutathione S-transferase fusion proteins were produced from either strain 2591 or strain N40 of B. burgdorferi, isolates of spirochetes from Connecticut and New York State, respectively. Affinity-purified glutathione transferase was tested in negative controls with sera, buffers, and other reagents to check for false-positive reactions in ELISAs.
ELISAs.
Class-specific assays, developed before (21, 23), were used to detect immunoglobulin M (IgM) or IgG antibodies. The reagents used in tests with whole-cell, p41-G, OspB, OspC, OspE, and OspF antigens and net optical density (OD) values for determination of cutoff values for positive serum specimens have been reported previously (21, 23). In the evaluation of p22, p37, and p39 antigens, 23 to 29 serum specimens from healthy subjects were analyzed by the ELISAs to determine critical regions for positive results. For serum dilutions of 1:160, 1:320, and 1:640 or greater, net OD values were highest for the p39 antigen (0.18, 0.16, and 0.11, respectively) compared with those computed for the p22 (0.08, 0.04, and 0.4, respectively) and the p37 (0.04, 0.04, and 0.04, respectively) antigens in analyses for IgM antibodies. Cutoff values for detection of IgG antibodies were the same as those calculated for the p22 antigen in the ELISA for IgM antibody but were higher for the p39 (0.51, 0.33, and 0.22, respectively) and the p37 (0.23, 0.17, and 0.12, respectively) antigens. In each case, cutoff values were determined by performing statistical analyses (three standard deviations plus the mean) with the net absorbance values for the respective data sets. Antigen concentrations, determined by a commercially available protein assay (Bio-Rad, Richmond, Calif.), of 5 μg of protein per ml were most suitable for retrieval of optimal reactivity with positive human sera.
In analyses of test sera, polystyrene plates contained negative and positive control sera and checks for glutathione transferase, phosphate-buffered saline (PBS) solution, and horseradish peroxidase-labeled goat anti-human antibodies. Positive control sera, obtained from subjects who had erythema migrans, were confirmed as having antibodies to B. burgdorferi by Western blot analyses. The working dilution of both conjugates, which were commercially prepared (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), was 1:10,000. Murine monoclonal antibodies used earlier (23) and an antiserum available for the p39 recombinant antigen were included in addition to the immunoblotting-positive human control sera to verify the reactivities of recombinant antigens in the ELISAs.
Immunoblots.
In general, procedures used previously (16) were applied to perform Western blot analyses of human sera. Briefly, B. burgdorferi (strain N40) proteins, resolved in 12% gels by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, were transferred to nitrocellulose membranes. Human sera were diluted to 1:100 in PBS solution before testing. The blocking reagent was PBS solution containing 5% nonfat dry milk. The secondary antibody (a 1:1,000 dilution of alkaline phosphatase-conjugated goat anti-human immunoglobulin) and substrates used in color development were purchased (Kirkegaard & Perry Laboratories).
RESULTS
In analyses for IgM antibodies, 14 (82%) serum samples from the patients who had erythema migrans and antibodies to whole-cell B. burgdorferi in an ELISA also tested positive with one or more recombinant antigens (Table 1). The numbers of serum specimens positive for p41-G, OspC, and OspF antigens were lower than those recorded positive by an ELISA with whole-cell antigen, but these recombinant proteins were recognized more frequently than the p22, p37, p39, OspB, and OspE antigens. All serum samples from 18 subjects who were diagnosed with HGE and who lacked antibodies to ehrlichiae were positive for IgM antibody to one or more recombinant antigens; the reactivities of the samples for p22, p37, p39, p41-G, OspC, OspE, and OspF antigens were notably more frequent than the reactivities of the samples from the erythema migrans group. A similar pattern of IgM antibody reactivity (except for reactivity to OspE) was noted when sera from patients who had antibodies to the HGE agent and B. burgdorferi were tested. In tests of reproducibility (i.e., different days of analyses), antibody titers for 30 (77%) of 39 serum samples varied twofold or less, whereas titers for seven and two serum samples differed by fourfold and eightfold, respectively.
TABLE 1.
Reactivity of human sera to whole cells and recombinant antigens of B. burgdorferi in class-specific ELISA for IgM antibodies
| Study group | Total no. of serum samples tested | No. of serum samples positive for reactivity to:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole cellsa | p22 | p37 | p39 | p41-Ga | Outer surface proteinsa
|
|||||
| B | C | E | F | |||||||
| EMb | 17 | 17 | 1 | 3 | 2 | 9 | 0 | 7 | 4 | 5 |
| HGEc | 18 | 18 | 6 | 11 | 8 | 13 | 0 | 16 | 5 | 12 |
| HGE and LBd | 17 | 17 | 5 | 5 | 7 | 12 | 0 | 12 | 3 | 7 |
| Syphilise | 24 | 20 | 2 | 0 | 3 | 4 | 0 | 0 | 0 | 4 |
| Oral infectionsf | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| RAg | 7 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 0 |
| Normalh | 29 | 0 | 5 | 1 | 4 | 2 | 0 | 1 | 0 | 0 |
In reanalyses of sera (excluding sera from the HGE groups), some results were the same as those published earlier (23, 24) and are listed here for comparison.
EM, erythema migrans; no clinical or serologic evidence of HGE.
HGE, suspected HGE with antibodies to B. burgdorferi (24) but lacking antibodies to ehrlichiae.
Antibodies to the organisms that cause both HGE and Lyme borreliosis (LB).
Secondary or latent syphilis with high titers.
Gingivitis or periodontitis infections with treponemal antibodies (24).
RA, rheumatoid arthritis; no clinical signs of Lyme borreliosis.
Healthy subjects with no clinical evidence of spirochetoses.
Patterns of serum reactivity to whole-cell and recombinant antigens of B. burgdorferi in ELISAs for IgG antibodies were similar to those observed in analyses for IgM antibodies. Thirteen (87%) of 15 serum samples from persons who had erythema migrans and antibodies to whole-cell antigens also reacted to one or more recombinant antigens. The numbers of serum samples with reactivity to the p22, p37, p41-G, OspE, and OspF antigens for the erythema migrans study group were comparable to those recorded for both HGE groups (Table 2). However, reactivity to p37 and OspE remained infrequent. Aside from the results for the p22, OspB, and OspE antigens, the numbers of serum specimens with reactivity to the remaining recombinant antigens for the HGE study groups were lower in analyses for IgG antibodies than in tests for IgM antibodies. In both class-specific assays, OspC, OspF, and p41-G were frequently recognized antigens for subjects with B. burgdorferi infections. Tests on the reproducibility of antibody titers for 31 serum samples analyzed on different days revealed changes of twofold or less (78%), fourfold (10%), or eightfold (12%).
TABLE 2.
Reactivity of human sera to whole cells and recombinant antigens of B. burgdorferi in class-specific ELISA for IgG antibodies
| Study groupa | Total no. of serum samples tested | No. of serum samples positive for reactivity to:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole cellsb | p22 | p37 | p39 | p41-Gb | Outer surface proteinsb
|
|||||
| B | C | E | F | |||||||
| EMc | 15 | 15 | 5 | 2 | 8 | 6 | 0 | 6 | 1 | 6 |
| HGE | 18 | 18 | 4 | 3 | 2 | 7 | 2 | 11 | 0 | 7 |
| HGE and LB | 17 | 17 | 6 | 2 | 0 | 8 | 9 | 5 | 0 | 7 |
| Syphilis | 24 | 23 | 2 | 0 | 4 | 3 | 0 | 1 | 0 | 4 |
| Oral infections | 6 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 |
| RA | 7 | 1 | 2 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| Normal | 28 | 0 | 4 | 0 | 4 | 1 | 0 | 3 | 0 | 2 |
See Table 1, footnotes b to h, for explanations of the study groups.
In reanalyses of sera (excluding sera from the HGE groups), some results were the same as those published earlier (23, 24) and are listed here for comparison.
All sera were positive in ELISAs for IgG antibodies to whole-cell B. burgdorferi antigen (23).
Analyses were performed to assess specificity. Syphilitic sera with high concentrations of T. pallidum antibodies (1:1024 or greater) reacted frequently with B. burgdorferi whole-cell antigen in ELISAs for the detection of IgM (Table 1) and IgG antibodies (Table 2). Aside from this study group, the prevalence of false-positive reactions with whole-cell antigen was low when sera from persons who had oral infections or rheumatoid arthritis were tested. The sera from healthy subjects were negative by both class-specific ELISAs with whole-cell antigen. The overall frequency of nonspecific results for the syphilitic sera was low when recombinant antigens were tested. Similarly, infrequent false-positive reactions were noted by both class-specific assays when sera from persons who had oral infections or rheumatoid arthritis were tested with p22, p37, p41-G, OspC, OspE, and OspF antigens. The reactivities to the p22 and p39 antigens of the sera from the rheumatoid arthritis study group paralleled those recorded when sera from healthy subjects were tested by both class-specific ELISAs. Of the total of 27 serum specimens from healthy subjects with false-positive reactions by screening with recombinant antigens in both class-specific assays, 9 (33%) had a titer of 1:160. The antibody titers for the remaining 18 serum specimens ranged from 1:320 (n = 11) to 1:640 (n = 7). In analyses for IgM antibodies, a titer of 1:160 was recorded for three and two serum specimens tested with OspC and p41-G antigens, respectively. There were no false-positive reactions in analyses with OspB, OspE, and OspF antigens. The prevalence of nonspecific reactions was 2 specimens or less when 29 serum specimens from healthy subjects were tested with p37, p41-G, and OspC antigens. In tests for IgG antibodies, the numbers of false-positive specimens were likewise low when p37, OspB, OspC, OspE, and OspF antigens were screened with 28 serum specimens from healthy subjects; an antibody titer of 1:160 was recorded for each reaction. Controls for glutathione transferase, PBS buffers, and enzyme were negative by all tests.
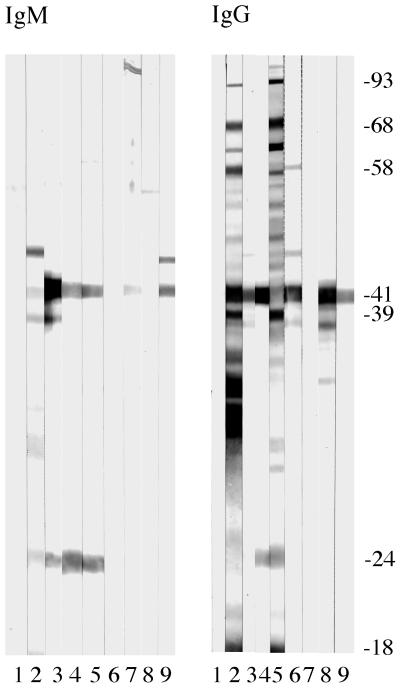
Western blot analyses of 18 serum specimens from 18 subjects who were suspected of having HGE but who lacked antibodies to granulocytic ehrlichiae frequently revealed specific antibodies to two or more key immunodominant proteins of B. burgdorferi (Fig. 1). The immune responses of the patients varied. In analyses for IgM antibodies, 14 serum specimens had antibodies to proteins with molecular masses of about 24 kDa (OspC), 39 kDa, and 41 kDa. Five of these samples also contained antibodies to a 93-kDa peptide. One serum sample had antibodies to the 39- and 41-kDa proteins, while an immunoblot of another serum specimen showed distinct bands at about 19 kDa (OspE), 24 kDa, and 39 kDa. Two serum specimens had antibodies only to flagellin (41 kDa). In analyses for IgG antibodies, immunoblots showed frequent reactivities to multiple proteins of B. burgdorferi. Thirteen serum specimens had antibodies to OspC and to the 39- and 41-kDa proteins. Other peptides often recognized immunologically had molecular masses of about 68 kDa (n = 10 serum specimens) and 37 kDa (n = 5). Less frequent antibody reactivity was noted for 34-kDa (n = 3) and 93-kDa (n = 2) proteins.
FIG. 1.
Representative immunoblots of individual human sera, obtained from patients who had suspected granulocytic ehrlichiosis in Connecticut during 1995 and 1996, showing IgM or IgG antibodies to lysates of B. burgdorferi whole-cell antigen. Molecular masses are indicated in kilodaltons. Lanes 3 to 5 show positive reactivities of test sera for IgM antibody, lanes 6 to 9 were judged to be negative, and lanes 1 and 2 contained negative and positive control sera, respectively. In analyses of the same sera for IgG antibodies, lanes 5 and 8 were positive, lanes 3, 4, 6, 7, and 9 were graded as negative, and lanes 1 and 2 contained negative and positive control sera, respectively.
The results of the ELISAs and immunoblotting were compared to determine if reactions to key recombinant antigens in the former tests agreed with those in the latter test with whole-cell antigen. Class-specific analyses were conducted with 18 serum specimens from HGE patients who lacked antibodies to ehrlichiae but who had immunoglobulins to B. burgdorferi at titers of 1:320 to 1:40,960. In tests for IgM antibodies, results of both assays were in agreement when the OspC (n = 8 serum specimens), p39 (n = 2), or p41-G (n = 7) antigen was used separately in an ELISA. When the findings were discordant, three serum specimens were positive by an ELISA with OspC antigen and negative by immunoblotting, whereas seven other serum specimens were negative by an ELISA and positive by Western blot analysis (i.e., distinct bands for a 24-kDa protein were visible). Immunoblotting methods were usually more sensitive than ELISAs when the reactivities to several antigens were evaluated. For example, 16 and 11 serum samples which were negative for IgM antibody by an ELISA with p39 and p41-G antigens, respectively, were positive by immunoblotting. In analyses for IgG antibodies, the findings obtained by both assays were in agreement when an ELISA contained the OspC (n = 10 serum specimens), p39 (n = 2), or p41-G (n = 8) antigen. Two serum specimens were positive by an ELISA with OspC but were negative by immunoblotting, whereas six serum specimens were negative by an ELISA with OspC but were positive by immunoblotting. In addition, 16 and 10 serum specimens were negative when they were tested by an ELISA with the p39 or the p41-G antigen, respectively, but distinct bands were observed for these proteins in immunoblots.
DISCUSSION
Class-specific ELISAs with recombinant antigens can be used to confirm clinical diagnoses of Lyme borreliosis during early stages of infection. However, recombinant antigen-based ELISAs have their limitations. Of the recombinant antigens evaluated in analyses for IgM antibodies, OspC, OspF, and p41-G were diagnostically the most important. This supports earlier work (6, 9, 10, 23, 26, 30, 31), which included analyses of recombinant antigens derived from different genospecies of B. burgdorferi. The reactivities of IgM antibody in serum with p22, p37, p39, and OspE, however, were infrequent and of less help in confirming past or current B. burgdorferi infections. Other investigators have found p37 (12) and p39 (7, 29) to be important markers. With a subsequent expansion of the immune response and production of IgG antibodies during later stages of illness, p22, p39, and OspF antigens became more useful in laboratory diagnoses. Antibody reactivities to the p22 and p39 antigens have been found to be important indicators of B. burgdorferi infections (1, 2, 6, 7, 16, 20, 29). Other antigens, such as p35 (47-kDa fibronectin-binding protein) and p93, likewise have been shown to be important markers for early and late Lyme borreliosis, respectively (2, 5–8, 11, 19, 27). Although highly specific for B. burgdorferi infections, OspA and OspB antigens are usually recognized during late Lyme borreliosis (2), if at all. In tick transmission studies, mice produced antibodies to p39 but not to OspA (13). Furthermore, there is considerable variation in antibody responses in humans infected with B. burgdorferi. These findings are probably due to differences in the variable expression of proteins by spirochetes (2, 6) and host immune responses. Therefore, a panel of key recombinant antigens should be tested separately by ELISAs as the first method, and immunoblotting with whole-cell antigen should be used as an adjunct method. Future studies should include a thorough evaluation of ELISAs and immunoblots with mixtures of key recombinant antigens to determine if the sensitivities and specificities of the assay results are comparable to or better than those obtained when recombinant antigens are tested separately.
Analyses of 18 serum specimens from patients who had HGE (but who lacked antibodies to granulocytic ehrlichiae) revealed antibodies to B. burgdorferi by class-specific ELISAs with recombinant antigens and in Western blot analyses with whole-cell antigens. In general, the patterns of reactivity in ELISAs paralleled those noted for sera from persons who had erythema migrans. The numbers of positive reactions, however, to p22, p37, p39, p41-G, OspC, OspE, and OspF in tests for IgM antibody were more frequent for both HGE groups than for the erythema migrans group. With the exception of results for p39, OspB, and OspC, the numbers of serum specimens positive for IgG antibodies differed very little among the HGE and erythema migrans study groups. It is possible that a patient with a current HGE infection and previous exposure to B. burgdorferi may produce antibodies to both agents (i.e., polyclonal immune restimulation). Alternatively, there may be nonspecific polyclonal activation following a single infection and consequent cross-reactivity in serologic tests due to immunologic responses to common heat shock proteins or other antigens shared by these unrelated bacteria. In our experience, cross-reactivity is minimal when homologous and heterologous antigens of the HGE agent and B. burgdorferi are tested with corresponding antisera (24). We suspect that persons who live in tick-infested areas and who experience multiple tick bites are probably exposed to different pathogens over several weeks or months. Therefore, when HGE, Lyme borreliosis, or human babesiosis is suspected, tests for the other tick-associated illnesses should be conducted.
Minor cross-reactivity was noted in analyses when sera from healthy subjects were tested with recombinant antigens of B. burgdorferi by ELISAs, but no false-positive reactions were recorded when whole-cell antigen was used. The latter was probably due to an overall blocking effect associated with the presence of numerous antigens, including diagnostically irrelevant components of B. burgdorferi, in plate wells. The infrequent false-positive reactions associated with the p39, OspC, OspE, and OspF antigens have been reported previously (5, 6, 23), but many of these reactions occurred at a low serum dilution (1:160). Although tests on reproducibility show little or no change in antibody titers for the majority of serum specimens reanalyzed, fourfold or greater differences in titration endpoints can sometimes occur (20). Moreover, cross-reactivity with treponemal antibodies is often associated with shared flagellar antigens (25), but these infections can be separated from Lyme borreliosis by performing the Venereal Disease Research Laboratory or fluorescent-treponemal antibody-absorption test. It is also possible that a small number of persons in the healthy control group may have had a prior unknown exposure to B. burgdorferi with no clinical evidence of infection. Thus, it is suggested that Western blot analysis continue to be performed with sera, particularly those that at low dilutions react with recombinant antigens in ELISAs. High-titer antibody reactions (1:640 or greater) to p22, p37, p41-G, OspC, OspE, and OspF antigens in ELISAs are likely to be highly specific.
In general, immunoblotting methods appeared to be more sensitive than ELISAs. Results of blotting confirmed the ELISA findings reported earlier (24), and the banding patterns were similar to those described previously (5, 6). An increased sensitivity of immunoblotting for IgG antibodies has been reported (6), but in other laboratories, ELISA results have had sensitivity comparable to or greater than that of immunoblotting (5). In the present study, there was agreement in results of both procedures for some sera, but there were also discordant findings. Moreover, our immunoblotting results were inconclusive for some sera obtained from persons during early Lyme borreliosis, when current criteria (3, 6) for a laboratory diagnosis of Lyme borreliosis were followed. With slow rises in IgM antibody production, banding patterns can be weak or lacking. Therefore, whenever possible, multiple serum samples obtained at intervals of at least 6 weeks or greater should be tested by immunoblotting and ELISAs to verify seroconversions or to document a fourfold or greater increase in antibody concentrations.
ACKNOWLEDGMENTS
We thank Tia Blevins, Manchuan Chen, and Hong Tao for technical assistance.
This work was supported, in part, by grants from the Centers for Disease Control and Prevention and Emerging Infections Program (grants CCU-111188-02, CCU-106581, and HR8/CCH113382-01), the National Institutes of Health (grants PO-1-AI-30548 and AI-49387), the Mathers Foundation, the Arthritis Foundation, the American Heart Association, federal Hatch funds administered by the U.S. Department of Agriculture, and funds from the state of Connecticut (Charles Goodyear Award). We also acknowledge a private contribution from Phyllis E. Mazik. Jacob W. IJdo is supported by a fellowship from the L. P. Markey Charitable Trust, and Erol Fikrig is a recipient of a clinical scientist award in translational research from the Burroughs Wellcome Fund. Richard A. Flavell is an investigator at the Howard Hughes Medical Institute.
REFERENCES
- 1.Aguero-Rosenfeld M E, Nowakowski J, McKenna D F, Carbonaro C A, Wormser G P. Serodiagnosis in early Lyme disease. J Clin Microbiol. 1993;31:3090–3095. doi: 10.1128/jcm.31.12.3090-3095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akin E, McHugh G L, Flavell R A, Fikrig E, Steere A C. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to p35 correlates with mild and brief arthritis. Infect Immun. 1999;67:173–181. doi: 10.1128/iai.67.1.173-181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association of State and Territorial Public Health Laboratory Directors and the Centers for Disease Control and Prevention. Proceedings of the Second National Conference on Serologic Diagnosis of Lyme Disease. Washington, D.C.: Association of State and Territorial Public Health Laboratory Directors; 1995. Recommendations; pp. 1–7. [Google Scholar]
- 4.Carreiro M M, Laux D C, Nelson D R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990;58:2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 6.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawcett P T, Rose C, Gibney K M, Chase C A, Kiehl B, Doughty R A. Detection of antibodies to the recombinant p39 protein of Borrelia burgdorferi using enzyme immunoassay and immunoblotting. J Rheum. 1993;20:734–738. [PubMed] [Google Scholar]
- 8.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi p35 and p37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 9.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber M A, Shapiro E D, Bell G L, Sampieri A, Padula S J. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J Infect Dis. 1995;171:724–727. doi: 10.1093/infdis/171.3.724. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore R D, Jr, Kappel K J, Johnson B J B. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore R D, Jr, Murphree R L, James A M, Sullivan S A, Johnson B J B. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J Clin Microbiol. 1999;37:548–552. doi: 10.1128/jcm.37.3.548-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golde W T, Kappel K J, Dequesne G, Feron C, Plainchamp D, Capiau C, Lobet Y. Tick transmission of Borrelia burgdorferi to inbred strains of mice induces an antibody response to P39 but not to outer surface protein A. Infect Immun. 1994;62:2625–2627. doi: 10.1128/iai.62.6.2625-2627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen K, Hindersson P, Pedersen N S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988;26:338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson M, Mollegard I, Steirnstedt G, Henriksson M A, Wretlind B. Characterization of antibody response in patients with Borrelia meningitis. Serodiagn Immunother Infect Dis. 1988;2:375–386. [Google Scholar]
- 16.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrenz M B, Hardham J M, Owens R T, Nowakowski J, Steere A C, Wormser G P, Norris S J. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol. 1999;37:3997–4004. doi: 10.1128/jcm.37.12.3997-4004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang F T, Steere A C, Marques A R, Johnson B J B, Miller J N, Philipp M T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol. 1999;37:3990–3996. doi: 10.1128/jcm.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luft B J, Mudri S, Jiang W, Dattwyler R J, Gorevic P D, Fisher T, Munoz P, Dunn J J, Schubach W H. The 93-kilodalton protein of Borrelia burgdorferi: an immunodominant protoplasmic cylinder antigen. Infect Immun. 1992;60:4309–4321. doi: 10.1128/iai.60.10.4309-4321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma B, Christen B, Leung D, Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992;30:370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnarelli L A, Anderson J F. Enzyme-linked immunosorbent assays for the detection of class-specific immunoglobulins to Borrelia burgdorferi. Am J Epidemiol. 1988;127:818–825. doi: 10.1093/oxfordjournals.aje.a114864. [DOI] [PubMed] [Google Scholar]
- 22.Magnarelli L A, Anderson J F, Johnson R C, Nadelman R B, Wormser G P. Comparison of different strains of Borrelia burgdorferi sensu lato used as antigens in enzyme-linked immunosorbent assays. J Clin Microbiol. 1994;32:1154–1158. doi: 10.1128/jcm.32.5.1154-1158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnarelli L A, Fikrig E, Padula S J, Anderson J F, Flavell R A. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J Clin Microbiol. 1996;34:237–240. doi: 10.1128/jcm.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnarelli L A, IJdo J W, Anderson J F, Padula S J, Flavell R A, Fikrig E. Human exposure to a granulocytic Ehrlichia and other tick-borne agents in Connecticut. J Clin Microbiol. 1998;36:2823–2827. doi: 10.1128/jcm.36.10.2823-2827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnarelli L A, Miller J N, Anderson J F, Riviere G R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990;28:1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padula S J, Dias F, Sampieri A, Craven R B, Ryan R W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol. 1994;32:1733–1738. doi: 10.1128/jcm.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Probert W S, Johnson B J B. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 28.Simpson W J, Cieplak W, Schrumpf M E, Barbour A G, Schwan T G. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol Lett. 1994;119:381–388. doi: 10.1111/j.1574-6968.1994.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 29.Simpson W J, Schrumpf M E, Schwan T G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilske B, Fingerle V, Preac-Mursic V, Jouris-Heipke S, Hofmann A, Loy H, Pfister H-W, Rossler D, Soutschek E. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med Microbiol Immunol. 1994;183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]
- 31.Wilske B, Schierz G, Preac-Mursic V, Von Busch K, Kuhbeck R, Pfister H-W, Einhaupl K. Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth's syndrome) J Infect Dis. 1986;153:304–314. doi: 10.1093/infdis/153.2.304. [DOI] [PubMed] [Google Scholar]