Conflict of interest
None.
Funding source
None.
Sir,
As a response to the coronavirus disease (COVID‐19) pandemic, several vaccines have been developed globally and are being rolled out at a fast pace for public immunization. Drug controller general of India has approved use of Covaxin™ (BBV152, manufactured by Bharat Biotech, Hyderabad, India) and Covishield™ (Oxford–AstraZeneca COVID‐19 vaccine, manufactured by Serum Institute of India, Pune, India) for restricted use in emergency situation. 1 We describe three cases of Covishield™ induced minor cutaneous adverse effects.
First case was a 25‐year‐old woman with complaints of low‐grade fever, joint pains and red, and painful nodules over legs and forearm of three days duration. History suggestive of any infection preceding the eruption was denied. She gave history of receiving Covishield 7 days prior to the episode. Cutaneous examination revealed multiple erythematous tender nodules over bilateral shins and forearms (Fig. 1a). Laboratory evaluation revealed elevated inflammatory markers. SARS‐Cov‐2 was not detected by reverse transcription (RT)‐PCR. An extensive workup to rule out underlying aetiologies of erythema nodosum (EN) was negative. Skin biopsy revealed infiltration of dermal and subcutaneous tissue with lymphomononuclear cells and neutrophils, consistent with erythema nodosum (Fig. 1b). Patient was treated with topical mometasone cream and oral paracetamol. Dermatological symptoms had resolved completely at follow‐up visit after 2 weeks. No recurrence was observed on receiving second dose after 4 weeks. Infections are the most common cause of EN, and this eruption has been described in association with COVID‐19 infection as well. 2 , 3 EN associated with other vaccines has been rarely reported 4 ; however, this is the first report of any COVID‐19 vaccine‐induced EN to the best of our knowledge.
Figure 1.
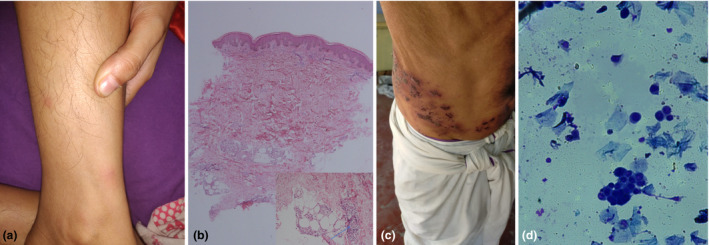
(a) Multiple erythematous nodules over the leg. (b) Histopathology of lesion revealing lymphoplasmacytic infiltrate around deep dermal vessels and infiltration of subcutaneous fat by lymphomononuclear cells and a few neutrophils (arrow). (c) Closely grouped vesicles coalescing to form bullae and erosions with haemorrhagic crusting in the right T10 dermatome in Case 2. (d) Tzanck smear demonstrating presence of acantholytic cells and multinucleate giant cells (May‐Grünwald Giemsa stain; original magnification: 1000×).
Second case was a 55‐year‐old man who presented with first episode of herpes zoster of both T10 dermatomes after COVID‐19 vaccination (Fig. 1C). Tzanck smear from the base of blister revealed acantholytic cells and multinucleate giant cells (Fig. 1D). He gave previous history of varicella and had not been vaccinated against herpes zoster. He reported receiving first dose of Covishield 3 days prior to cutaneous eruption. He denied any history of previous unusual infections or family history of any immune defects. Fasting blood sugar level was 95 mg/dL, and HIV test and COVID‐19 RT‐PCR were negative. He was prescribed oral valcyclovir 1 g thrice a day for 1 week. Resolution of symptoms was noted on follow‐up visit after 2 weeks. Immunomodulation by various vaccines has been reported to result in herpes zoster reactivation previously. 5 A recent observational study reported six cases of herpes zoster reactivation in patients with autoimmune rheumatic diseases. 6 Herpes zoster duplex bilateralis has been reported rarely in immunocompetent adults. 7
Third case was a 24‐year‐old man with complaints of asymptomatic cutaneous eruption from three days. Examination revealed several well‐defined round to oval salmon‐coloured papules and plaques up to 3 cm in diameter covered by fine white scales over his trunk, back and axillae (Fig. 2a,b). Patient reported receiving Covishield 24 h prior to onset of cutaneous lesions. Laboratory evaluation was normal, and COVID‐19 RT‐PCR was negative. Patient did not consent for skin biopsy. In view of characteristic morphology and supportive dermatoscopic findings (Fig. 2c), a diagnosis of pityriasis rosea (PR) was made and he was prescribed topical mometasone cream. Further progression of rash could not be monitored as the patient was lost to follow‐up. PR‐like rash has been reported previously with COVID‐19 infection 8 and other COVID‐19 mRNA vaccines. 9 A possible explanation is diversion of T cells towards vaccine resulting in reduced control on infectious agents such as HHV‐6/7 and their consequent reactivation. 10
Figure 2.
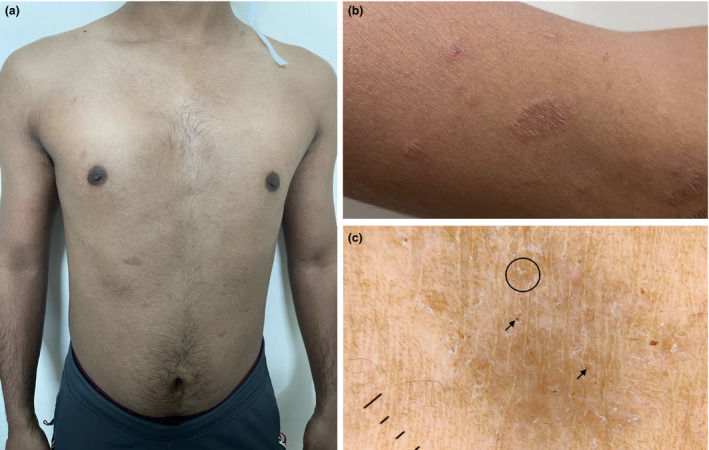
(a) Well‐defined erythematous papulosquamous eruption involving trunk. (b) Close‐up view of the lesions over proximal arm. (c) Dermatoscopy of the lesions revealing irregularly distributed fine white scaling, peripheral patchy distribution of dotted vessels (circle) and brown globules (arrows) over a diffuse reddish‐brown background (Dermlite II, hybrid M, 10×, polarized).
Cutaneous adverse effects of Moderna and Pfizer vaccines have been described recently, 6 but similar reports with Covishield are lacking in literature. We report three adverse events temporally related to Oxford–AstraZeneca COVID‐19 vaccine. While reactivation of herpes and pityriasis rosea have been described with COVID‐19 vaccination before, erythema nodosum has not been reported previously. As these adverse effects were mild and resolved without sequelae, the general population should feel assured about its safety and should be encouraged to adopt vaccination at the earliest.
Acknowledgements
An informed consent was given by the patients for publication of the image and clinical details after reading the manuscript.
Data availability statement
Data are available on request.
References
- 1. Research ICoM . COVID‐19 vaccines under trials in India, 2021. URL https://vaccine.icmr.org.in/covid‐19‐vaccine (last accessed: 29 April 2021).
- 2. Suter P, Mooser B, Pham Huu Thien HP. Erythema nodosum as a cutaneous manifestation of COVID‐19 infection. BMJ Case Rep 2020; 13: e236613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conforti C, Dianzani C, Agozzino M et al. Cutaneous manifestations in confirmed COVID‐19 patients: a systematic review. Biology 2020; 9: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen PR. Combined reduced‐antigen content tetanus, diphtheria, and acellular pertussis (tdap) vaccine‐related erythema nodosum: case report and review of vaccine‐associated erythema nodosum. Dermatol Ther 2013; 3: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet 1999; 353: 810. [DOI] [PubMed] [Google Scholar]
- 6. Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series [published online ahead of print]. Rheumatology (Oxford) 2021; keab345. 10.1093/rheumatology/keab345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vijay A, Dalela G. Herpes zoster duplex bilateralis in immuno‐competent patients: report of two cases. J Clin Diagn Res 2015; 9: WR01–WR03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: e436‐e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine‐induced pityriasis rosea and pityriasis rosea‐like eruptions: a review of the literature. J Eur Acad Dermatol Venereol 2016; 30: 544–545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.


