Abstract
Background
The COVID‐19 pandemic delayed diagnosis and care for some acute conditions and reduced monitoring for some chronic conditions. It is unclear whether new diagnoses of chronic conditions such as dementia were also affected. We compared the pattern of incident Alzheimer's disease and related dementia (ADRD) diagnosis codes from 2017 to 2019 through 2020, the first pandemic year.
Methods
Retrospective cohort design, leveraging 2015–2020 data on all members 65 years and older with no prior ADRD diagnosis, enrolled in a large integrated healthcare system for at least 2 years. Incident ADRD was defined as the first ICD‐10 code at any encounter, including outpatient (face‐to‐face, video, or phone), hospital (emergency department, observation, or inpatient), or continuing care (home, skilled nursing facility, and long‐term care). We also examined incident ADRD codes and use of telehealth by age, sex, race/ethnicity, and spoken language.
Results
Compared to overall annual incidence rates for ADRD codes in 2017–2019, 2020 incidence was slightly lower (1.30% vs. 1.40%), partially compensating later in the year for reduced rates during the early months of the pandemic. No racial or ethnic group differences were identified. Telehealth ADRD codes increased fourfold, making up for a 39% drop from face‐to‐face outpatient encounters. Older age (85+) was associated with higher odds of receiving telecare versus face‐to‐face care in 2020 (OR:1.50, 95%CI: 1.25–1.80) and a slightly lower incidence of new codes; no racial/ethnic, sex, or language differences were identified in the mode of care.
Conclusions
Rates of incident ADRD codes dropped early in the first pandemic year but rose again to near pre‐pandemic rates for the year as a whole, as clinicians rapidly pivoted to telehealth. With refinement of protocols for remote dementia detection and diagnosis, health systems could improve access to equitable detection and diagnosis of ADRD going forward.
Keywords: dementia diagnosis, incident ADRD codes, telehealth
Key Points
The COVID‐19 pandemic was associated with a small decrease in the annual incidence rate of new ADRD diagnoses from 1.40% (2017–2019) to 1.30% in 2020.
A fourfold increase in diagnoses made via telehealth compensated for the drop in diagnoses in face‐to‐face encounters.
Why Does this Paper Matter?
A sustained increase in telehealth utilization could be leveraged to facilitate ADRD diagnosis in health systems working toward value‐based, dementia‐capable care.
BACKGROUND
The COVID‐19 pandemic has been a transformative stressor on healthcare systems worldwide, causing an overnight shift from in‐person to telehealth care and raising concern about delayed diagnosis and treatment of non‐COVID conditions such as myocardial infarction and stroke 1 , 2 , 3 and reduced monitoring of chronic conditions such as diabetes. 4 It is less clear whether the diagnosis of long‐term chronic conditions such as dementia might also be affected. The well‐known underdiagnosis of Alzheimer's disease and related dementias (ADRDs) could be further exacerbated by shifts in clinical focus and health system operations necessary to manage the pandemic. Alternatively, the broad adoption of telehealth might unexpectedly facilitate diagnosis, propelling the United States toward earlier, more timely detection and diagnosis of ADRD 5 needed for proactive, population‐based care management. 6 We examined the pattern of incident ADRD diagnoses using diagnostic codes from the pre‐pandemic years, 2017–2019, through the height of the pandemic in 2020, within a large integrated healthcare system that serves a diverse patient population.
METHODS
Data for this retrospective cohort study were drawn from Kaiser Permanente Southern California (KPSC). Members 65 years and older were considered at risk for ADRD (527,727 in 2017 to 610,892 in 2020) and included if enrolled in the health plan for at least 2 years before January 1, 2017. Incident diagnoses were defined as the first ADRD diagnosis code in the electronic medical record between January 1, 2017 and December 31, 2020. All 12 months of the 2020 pandemic year were included in the analyses. The study was approved by the KPSC (#12565) Institutional Review Board.
ADRD diagnoses were defined by the International Classification of Diseases Clinical Modification codes obtained from electronic health records (EHRs), and administrative and claims data: ICD‐10‐CM (F01.50, F01.51, F02.80, F02.81, F03.90, F03.91, G30.0, G30.1, G30.8, G30.9, G31.01, G31.09, and G31.83). While the use of two repeated diagnosis codes can improve the identification of new cases, 7 , 8 our aim was to assess how the COVID‐19 pandemic may have affected incident ADRD diagnosis coding patterns within a health system. Delaying by a year to ascertain ADRD cases more accurately could miss opportunities to learn from this natural experiment.
Incident ADRD codes were identified in encounters from outpatient (face‐to‐face, phone, video), hospital‐based (emergency department, observation stays, and inpatient), and continuing care (home, skilled nursing, long‐term care) settings. Sociodemographics (age, sex, race/ethnicity, and non‐English spoken language requiring an interpreter), results from cognitive [MMSE, 9 MoCA, 10 SLUMS 11 ], and functional [Functional Assessment Staging Tool (FAST) 12 ] assessments were extracted from administrative, membership, and clinical records from the encounter at which the incident ADRD code was identified. MoCA, SLUMS, and FAST scores were obtained from flowsheets and MMSE scores from keyword searches of provider notes. All were anchored to the incident code encounter date. Logistic regression models (SAS version 9.4 for Windows (SAS Institute, Cary, NC) were used to examine the association of sociodemographic characteristics (age, sex, race/ethnicity, and spoken language) with encounter types resulting in an incident ADRD code, using a 2‐sided p‐value <0.05 as the a priori threshold for statistical significance.
RESULTS
Compared to 2017–2019, the incidence rate for ADRD diagnostic codes in patients 65 years and older decreased slightly in 2020 below the mean annual rate for the prior 3 years (1.40% vs. 1.30%) (Table 1). Fewer new ADRD diagnoses were coded in March–May 2020, immediately following widespread adoption of physical (social) distancing policies but a sustained increase over 2019 rates followed from June–December 2020 (Figure S1). For the 65–74 age cohort, incidence rates held steady across race/ethnic groups across the 4 years (Figure 1). For the 75–84 age cohort, incidence rates showed a decreasing trend overall with the exception of Blacks. For the 85+ age cohort, incidence rates showed a decreasing trend for Whites but not other race/ethnic groups.
TABLE 1.
Characteristics of patients with incident Alzheimer's disease and related dementia (ADRD) codes in 2020 compared to 2017–2019
| 2017 | 2018 | 2019 | 2020 | 2020 | |||
|---|---|---|---|---|---|---|---|
| At risk members aged 65 years and older | 527,727 | 558,252 | 585,980 | 610,892 | Face‐to‐face | Phone | Video |
| Incident ADRD codes 95%CI |
7568 (1.43%) 1.40–1.47% |
7872 (1.41%) 1.38–1.44% |
7976 (1.36%) 1.33–1.39% |
7950 (1.30%) 1.27–1.33% |
(n = 2298) | (n = 1130) | (n = 472) |
| Age | 82.2 (7.60) | 82.2 (7.59) | 82.3 (7.71) | 82.2 (7.69) | 80.9 (7.11) | 82.2 (7.85) | 82.5 (7.40) |
| 65–74 | 1344 (17.8%) | 1375 (17.5%) | 1414 (17.7%) | 1434 (18%) | 477 (20.8%) | 201 (17.8%) | 78 (16.5%) |
| 75–84 | 3186 (42.1%) | 3343 (42.5%) | 3341 (41.9%) | 3280 (41.3%) | 1057 (46%) | 464 (41.1%) | 198 (41.9%) |
| ≥85 | 3038 (40.1%) | 3154 (40.1%) | 3221 (40.4%) | 3236 (40.7%) | 764 (33.2%) | 465 (41.2%) | 196 (41.5%) |
| Female | 4373 (57.8%) | 4524 (57.5%) | 4681 (58.7%) | 4465 (56.2%) | 1312 (57.1%) | 661 (58.5%) | 307 (65%) |
| Race/ethnicity | |||||||
| White | 4241 (56%) | 4257 (54.1%) | 4320 (54.2%) | 4142 (52.1%) | 1161 (50.5%) | 576 (51%) | 207 (43.9%) |
| Asian/Pacific Islander | 608 (8%) | 679 (8.6%) | 696 (8.7%) | 671 (8.4%) | 209 (9.1%) | 90 (8%) | 54 (11.4%) |
| Black | 967 (12.8%) | 1000 (12.7%) | 1000 (12.5%) | 1097 (13.8%) | 286 (12.4%) | 151 (13.4%) | 70 (14.8%) |
| Hispanic | 1685 (22.3%) | 1836 (23.3%) | 1858 (23.3%) | 1950 (24.5%) | 612 (26.6%) | 294 (26%) | 138 (29.2%) |
| Multiple/Other/Unknown | 67 (0.9%) | 100 (1.3%) | 102 (1.3%) | 90 (1.1%) | 30 (1.3% | 19 (1.7%) | 3 (0.6%) |
| Non‐English speaking, requires an interpreter | 777 (10.3%) | 822 (10.4%) | 848 (10.6%) | 915 (11.5%) | 317 (13.8%) | 142 (12.6%) | 66 (14%) |
| Encounter type | |||||||
| Internal encounters | 6315 (83.4%) | 6469 (82.2%) | 6436 (80.7%) | 6184 (77.8%) | |||
| Face‐to‐face | 3597 (57%) | 3655 (56.5%) | 3760 (58.4%) | 2298 (37.2%) | ‐ | ‐ | ‐ |
| Phone | 234 (3.7%) | 336 (5.2%) | 410 (6.4%) | 1130 (18.3%) | ‐ | ‐ | ‐ |
| Video | 3 (0%) | 3 (0%) | 5 (0.1%) | 472 (7.6%) | ‐ | ‐ | ‐ |
| Geriatrics/Neurology/Psychiatry a | 2457 (64.1%) | 2506 (62.7%) | 2604 (62.4%) | 2205 (56.5%) | 1409 (61.3%) | 450 (39.8%) | 346 (73.3%) |
| Primary care a | 1290 (33.6%) | 1377 (34.5%) | 1468 (35.2%) | 1588 (40.7%) | 846 (36.8%) | 625 (55.3%) | 117 (24.8%) |
| Other specialties a | 87 (2.3%) | 111 (2.8%) | 103 (2.5%) | 107 (2.7%) | 43 (1.9%) | 55 (4.9%) | 9 (1.9%) |
| Hospital‐based (ED/Obs/Inpatient) | 2253 (35.7%) | 2404 (37.2%) | 2225 (34.6%) | 2246 (36.3%) | ‐ | ‐ | ‐ |
| Continuing care: Home/SNF/LTC | 228 (3.6%) | 71 (1.1%) | 36 (0.6%) | 38 (0.6%) | ‐ | ‐ | ‐ |
| External encounters b | 1253 (16.6%) | 1403 (17.8%) | 1540 (19.3%) | 1766 (22.2%) | ‐ | ‐ | ‐ |
| Any cognitive assessment c | 1384 (34.1%) | 1433 (35.3%) | 1632 (38.8%) | 1296 (32.9%) | 1008 (43.9%) | 130 (11.5%) | 156 (33.1%) |
| MOCA, n% | 515 (12.7%) | 578 (14.2%) | 680 (16.1%) | 562 (14.3%) | 461 (20.1%) | 59 (5.2%) | 41 (8.7%) |
| mean (SD) | 15.7 (5.23) | 15.4 (5.55) | 15.6 (6.08) | 16.1 (6.38) | 15.9 (6.44) | 16.6 (5.76) | 17.1 (6.55) |
| SLUMS, n% | 112 (2.8%) | 171 (4.2%) | 288 (6.8%) | 272 (6.9%) | 233 (10.1%) | 6 (0.5%) | 33 (7%) |
| mean (SD) | 14.1 (5.95) | 13.0 (4.94) | 12.8 (5.53) | 12.8 (5.21) | 12.9 (5.03) | 12.7 (7.74) | 12.5 (6.05) |
| MMSE, n% | 834 (20.5%) | 796 (19.6%) | 770 (18.3%) | 595 (15.1%) | 413 (18%) | 77 (6.8%) | 104 (22%) |
| mean (SD) | 20.2 (5.74) | 20.1 (5.77) | 19.5 (6.07) | 19.2 (6.59) | 19.2 (6.39) | 18.8 (6.98) | 19.2 (6.95) |
| FAST staging, n% d | 3 (0.1%) | 74 (1.8%) | 818 (19.4%) | 701 (17.8%) | 484 (21.1%) | 66 (5.8%) | 150 (31.8%) |
| mean (SD) | 4.3 (0.58) | 4.8 (1.13) | 4.5 (0.82) | 4.4 (0.79) | 4.3 (0.70) | 4.6 (0.92) | 4.6 (0.92) |
Note: Data are presented as n(%) or mean(SD).
Abbreviations: ED, emergency department; LTC, long‐term care; MMSE, Mini‐Mental Status Exam; MoCA, Montreal Cognitive Assessment; Obs, observation stay; SNF, skilled nursing facility; SLUMS, St Louis University Mental Status Exam.
Only outpatient (face‐to‐face, phone, video) encounters within Kaiser Permanente.
A majority (81%) of the encounters external to Kaiser Permanente were for hospital‐based care.
Cognitive assessments completed during non‐ED/hospital encounters.
FAST was officially adopted by geriatrics in 2019.
FIGURE 1.
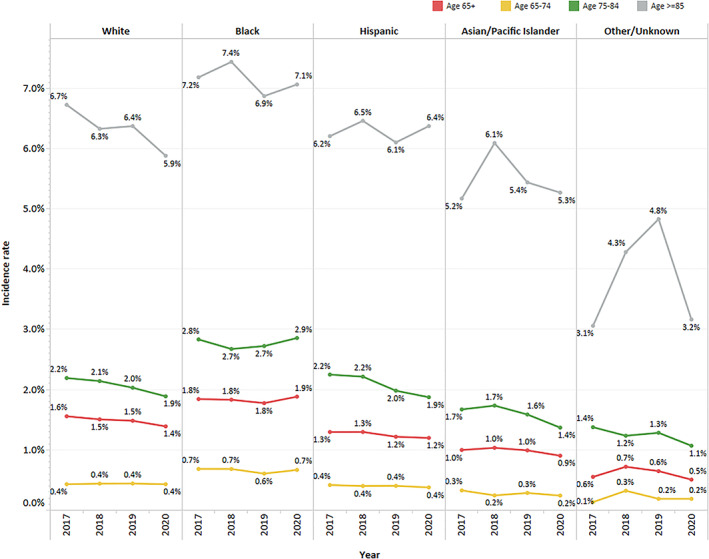
Incident Alzheimer's disease and related dementia (ADRD) codes by race/ethnicity and age cohorts. The sample for the “other/unknown” cohort was relatively small across the study years (n: 67–102)
Telehealth‐based new ADRD codes increased fourfold in 2020 (phone and video visits contributing 29% and 12%, respectively), whereas the proportion from outpatient face‐to‐face encounters dropped by 39% (Figure 2). The proportion of codes entered by primary care physicians increased by 5% (likely attributable to temporary closure of specialty clinics as staff were redeployed elsewhere to address urgent COVID‐19 care needs). There was a slight decrease between 2019 and 2020 (39% vs. 33%) in the percentage of ADRD incident codes supported by a structured cognitive assessment (MOCA, SLUMS, or MMSE) (Table 1), likely due to the completion of fewer cognitive assessments in video (33%) and phone (12%) than face‐to‐face (44%) encounters. However, mean cognitive assessment scores in 2020 were similar to those in prior years (suggesting at least moderate dementia severity). Stage at diagnosis (FAST, formally adopted in 2019 and only by the geriatric service line) also showed no appreciable difference across years. The proportion of new hospital‐based (emergency, observation, and inpatient) ADRD codes remained stable during the pandemic year with very low use (3.4%) of structured cognitive assessment in any year.
FIGURE 2.
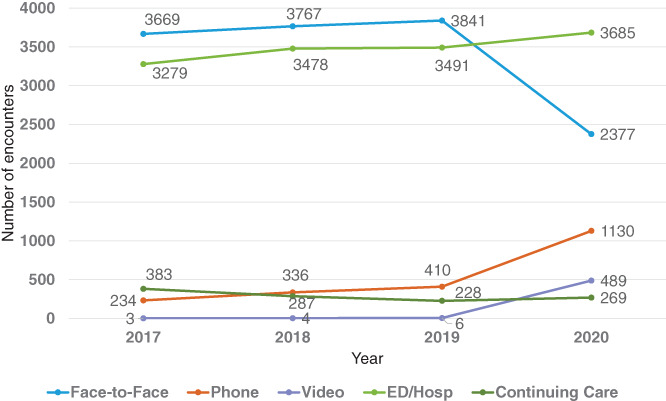
Incident ADRD codes by all encounter types, 2017–2020. Includes Kaiser Permanente and claims encounters. Outpatient (face‐to‐face, phone, video); hospital (emergency department, observation stay, inpatient admission); continuing Care (home/skilled nursing. facility/long‐term care)
There were no substantive differences in incident ADRD codes by age, sex, race/ethnicity, or English language proficiency in 2020 compared to the pre‐pandemic years (Table 1). For 2020 outpatient encounters with a new ADRD code, patients receiving telecare (phone or video) versus face‐to‐face care were older and more often women; after adjusting for all other sociodemographic factors, patients aged 85+ years were more likely to have an incident ADRD code in a telehealth encounter than patients aged 65–74 years (OR: 1.50, 95%CI: 1.25–1.80, p < 0.001) (Table S1). Video visits were more often performed by dementia specialists (geriatricians, neurologists, and psychiatrists) than primary care providers, who used more phone visits, and specialists performed nearly all the cognitive assessments that were completed during video (98%) and phone (85%) visits.
DISCUSSION
Prior (2014) estimates from Medicare fee‐for‐service claims found annual incident ADRD diagnosis rates of 3.1%–3.6% for beneficiaries 65 years and older. 8 In this large integrated healthcare system serving a racially diverse, Medicare Advantage (MA) patient population, incidence rates were lower (1.30%) but consistent across time, including the peak COVID‐19 pandemic year. A dramatic increase in codes recorded with telephone visits, combined with a smaller increase in video visits, compensated for a decrease in face‐to‐face encounter diagnoses. The shift to more remote care in 2020 had no overall differential effect across demographic cohorts, compared to the pre‐pandemic years, although rates in the oldest group were slightly lower. These findings together suggest that the COVID‐19 stay‐at‐home orders and rapid pivoting to telehealth did not greatly alter patterns of ADRD diagnosis coding and may mark an inflection point for greater future use of remote care to improve access and diagnosis of ADRD.
This report provides first‐time insights into the effects of the COVID‐19 pandemic on the pattern of incident ADRD codes within a large integrated health system, but some limitations are worth noting. We relied on the first‐occurring ADRD code, which increases diagnostic sensitivity at an expected cost in specificity and accuracy. However, this approach follows the standard practice of health systems and payers in identifying clinical populations for payment and performance improvement programs, and unpublished data (under review) showed that most (89%) individuals with an incident face‐to‐face encounter code for ADRD did have a second within a year. In exploring how the pandemic‐induced “natural experiment” might affect clinicians' coding patterns, the same single‐code approach provides early insights. Future efforts will examine whether individuals with an incident ADRD code via telehealth are as likely to have a repeat code as those first diagnosed face‐to‐face, and whether patterns of care following a first diagnosis vary with the type of visit at which it was recorded. This integrated health system had been encouraging telephone visits across primary and specialty care for several years before the pandemic, so the transition to telehealth may have been less disruptive for both providers and patients/families than might be expected under fee‐for‐service Medicare and in other healthcare systems and settings.
The rate of incident ADRD diagnoses we observed was low in this MA plan relative to incident case rates found in fee‐for‐service Medicare claims 8 and cohort studies that use formal criterion‐based diagnoses. 13 , 14 Although selective enrollment of healthier, lower cost beneficiaries was a broad policy concern for MA plans in the 1990s, changes to MA plan administration and payments as part of the 2003 Medicare Modernization Act were successful in decreasing favorable risk selection. 15 , 16 Differential disenrollment of individuals who have, or are developing as‐yet undiagnosed ADRD, could result in low, yet potentially accurate incidence rates, but does not explain the lower observed rate in this study. One‐year KPSC disenrollment rates are similar for members with (12%) and without (11%) ADRD, contrasting with aggregated nationwide MA plan data (2014–2015), where disenrollment rates for beneficiaries with an ADRD diagnosis were double those without (9.0% vs. 4.2%) and combined disenrollment and plan switching for both groups were much higher (28.7% in ADRD vs 27.0%) 17 than in KPSC. It is important to also note that the prevalence of ADRD (5.5%) in this health plan is comparable to published rates from other MA plans. 18 , 19
Fee‐for‐service health systems have generally been slow to take the advantage of new Centers for Medicare and Medicaid Services (CMS) benefits that could enable population‐level improvements in dementia detection (e.g., annual wellness visit), subsequent care (e.g., cognitive assessment and care planning and management), and quality measurement (e.g., the merit‐based incentive payment system for dementia). For MA plans, CMS added a new dementia hierarchical condition category coding modifier in 2020, reflecting the higher cost of dementia care 20 ; it remains to be seen whether higher risk adjusted payments will promote increased detection and diagnosis.
A diagnosis of dementia depends on the presence of at least some functional dependency that is due to cognitive impairment (relative to expectations for an individual's demographic niche). While the use of validated tools to identify and stage cognitive and functional impairment is recommended as part of the diagnostic process, 21 , 22 our data show that primary care and inpatient care clinicians make relatively little use of formal assessments and that where data are available, initial diagnoses occur on average around mid‐stage ADRD, when the symptoms of dementia are often obvious in conversation and family and others may have already been experiencing strain for some time. A cognitive assessment tied to a new diagnosis was documented in 62% of those made by geriatricians, 33% by neurologists and psychiatrists, and 14% by primary care providers. While there has been limited effort to date to achieve diagnostic harmonization across clinical disciplines, other system factors could play a role in the underuse or inaccurate ascertainment of assessment tool use. Our automated keyword data extraction method could miss some, and scores from paper tools might not be referenced in electronic notes. Lack of clinician knowledge, training, support, and incentives to use the structured assessments that are retrievable from the EMR (MoCA, SLUMS, FAST) would be expected to lead to underutilization. In addition, when cognitive impairment is obvious, providers may see no value in using an assessment tool.
Whether or not to detect and diagnose dementia continues to be debated. It is generally accepted that the use of screening tools can improve the detection of clinically relevant cognitive impairment, but detection without defined pathways for care has limited value. 23 We observed a small increase in COVID‐era dementia diagnoses in primary care; our results suggest that specialists—geriatricians, neurologists, and psychiatrists—could help boost detection and diagnosis by developing streamlined, telehealth‐friendly protocols that can be used by primary care providers as the first step to a future care pathway. 24 Systematic dementia care management that engages patients, caregivers, and clinicians can improve the lives of people living with ADRD and their caregivers, 23 , 24 , 25 , 26 , 27 , 28 and, at least to some extent, health system metrics. 29 , 30 , 31 Specialists have a role to play in assuring that health system leaders understand the growing evidence supporting structured dementia care management as well as evolving evidence on biomedical and psychosocial interventions.
CONCLUSIONS
The COVID‐19 pandemic stay‐at‐home orders and rapid pivot to telehealth did not greatly alter the rate of incident ADRD codes for adults aged 65 years and older in 2020 compared to the 3 years before the pandemic in a large integrated healthcare system. The natural experiment created by the pandemic could serve as the starting point for facilitating earlier ADRD diagnosis and new care pathways going forward.
CONFLICT OF INTEREST
The authors have no conflicts to report.
AUTHOR CONTRIBUTIONS
Drs. Nguyen and Borson and Ms. Aiyu Chen designed the study. Ms. Chen completed the data extraction and summaries. All authors assisted with data interpretation, contributed substantially to manuscript preparation, and approved the submitted manuscript.
SPONSOR'S ROLE
No sponsor had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Data S1. Supporting Information.
Borson S, Chen A, Wang SE, Nguyen HQ. Patterns of incident dementia codes during the COVID‐19 pandemic at an integrated healthcare system. J Am Geriatr Soc. 2021;69(12):3389-3396. doi: 10.1111/jgs.17527
Funding information Funding was provided by the Kaiser Permanente Southern California Care Improvement Research Team.
REFERENCES
- 1. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID‐19 and other causes, march‐April 2020. JAMA. 2020;324(5):510‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teo KC, Leung WCY, Wong YK, et al. Delays in stroke onset to hospital arrival time during COVID‐19. Stroke. 2020;51(7):2228‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan PS, Girotra S, Tang Y, Al‐Araji R, Nallamothu BK, McNally B. Outcomes for out‐of‐hospital cardiac arrest in the United States during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2021;6(3):296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright A, Salazar A, Mirica M, Volk LA, Schiff GD. The invisible epidemic: neglected chronic disease management during COVID‐19. J Gen Intern Med. 2020;35(9):2816‐2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. BOLD Public Health Centers of Excellence. 2020, https://www.cdc.gov/aging/funding/phc/index.html.
- 6. Gupta R, Roh L, Lee C, et al. The population health value framework: creating value by reducing costs of Care for Patient Subpopulations with Chronic Conditions. Acad Med. 2019;94(9):1337‐1342. [DOI] [PubMed] [Google Scholar]
- 7. Harding BN, Floyd JS, Scherrer JF, et al. Methods to identify dementia in the electronic health record: comparing cognitive test scores with dementia algorithms. Healthcare (Amsterdam, Netherlands). 2020;8(2):100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thunell J, Ferido P, Zissimopoulos J. Measuring Alzheimer's disease and other dementias in diverse populations using Medicare claims data. J Alzheimer Dis. 2019;72(1):29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 10. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 11. Tariq SH, Tumosa N, Chibnall JT, Perry MH 3rd, Morley JE. Comparison of the Saint Louis university mental status examination and the mini‐mental state examination for detecting dementia and mild neurocognitive disorder‐a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900‐910. [DOI] [PubMed] [Google Scholar]
- 12. Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull. 1988;24(4):653‐659. [PubMed] [Google Scholar]
- 13. Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737‐1746. [DOI] [PubMed] [Google Scholar]
- 14. Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer's disease dementia from 1994 to 2012 in a population study. Alzheimer Dement. 2019;15(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McWilliams JM, Hsu J, Newhouse JP. New risk‐adjustment system was associated with reduced favorable selection in medicare advantage. Health Aff (Millwood). 2012;31(12):2630‐2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newhouse JP, Price M, Huang J, McWilliams JM, Hsu J. Steps to reduce favorable risk selection in medicare advantage largely succeeded, boding well for health insurance exchanges. Health Aff (Millwood). 2012;31(12):2618‐2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyers DJ, Rahman M, Rivera‐Hernandez M, Trivedi AN, Mor V. Plan switching among Medicare advantage beneficiaries with Alzheimer's disease and other dementias. Alzheimer Dementia (New York, NY). 2021;7(1):e12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powers BW, Antol DD, Zhao Y, et al. Association between primary care payment model and telemedicine use for Medicare advantage enrollees during the COVID‐19 pandemic. JAMA Health Forum. 2021;2(7):e211597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jutkowitz E, Bynum JPW, Mitchell SL, et al. Diagnosed prevalence of Alzheimer's disease and related dementias in Medicare advantage plans. Alzheimer Dementia (Amsterdam, Netherlands). 2020;12(1):e12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pyenson BS, Steffens C. Including Dementia in the Part C Medicare Risk Adjuster: Health Services Issues. 2019; https://www.milliman.com/en/insight/including‐dementia‐in‐the‐part‐c‐medicare‐risk‐adjuster‐health‐services‐issues, 2021.
- 21. What Is Dementia? Symptoms, Types, and Diagnosis. National Institute on Aging. https://www.nia.nih.gov/health/what‐dementia‐symptoms‐types‐and‐diagnosis. [Google Scholar]
- 22. Diagnostic criteria for dementia. Dementia Australia. https://www.dementia.org.au/information/for‐health‐professionals/clinical‐resources/diagnostic‐criteria‐for‐dementia. [Google Scholar]
- 23. Owens DK, Davidson KW, et al. Screening for cognitive impairment in older adults: US preventive services task force recommendation statement. JAMA. 2020;323(8):757‐763. [DOI] [PubMed] [Google Scholar]
- 24. Owens AP, Ballard C, Beigi M, et al. Implementing remote memory clinics to enhance clinical care during and after COVID‐19. Front Psych. 2020;11:579934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148‐2157. [DOI] [PubMed] [Google Scholar]
- 26. Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713‐726. [DOI] [PubMed] [Google Scholar]
- 27. Possin KL, Merrilees JJ, Dulaney S, et al. Effect of collaborative dementia care via telephone and internet on quality of life, caregiver well‐being, and health care use: the care ecosystem randomized clinical trial. JAMA Intern Med. 2019;179:1658‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Academies of Sciences E, Medicine . Meeting the Challenge of Caring for Persons Living with Dementia and their Care Partners and Caregivers: A Way Forward. The National Academies Press; 2021. [PubMed] [Google Scholar]
- 29. Jennings LA, Laffan AM, Schlissel AC, et al. Health care utilization and cost outcomes of a comprehensive dementia care program for Medicare beneficiaries. JAMA Intern Med. 2019;179(2):161‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jennings LA, Hollands S, Keeler E, Wenger NS, Reuben DB. The effects of dementia care co‐management on acute care, hospice, and long‐term care utilization. J Am Geriatr Soc. 2020;68(11):2500‐2507. [DOI] [PubMed] [Google Scholar]
- 31. French DD, LaMantia MA, Livin LR, Herceg D, Alder CA, Boustani MA. Healthy aging brain center improved care coordination and produced net savings. Health Aff (Millwood). 2014;33(4):613‐618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


