Abstract
Pancreatic cancer (PC) is one of the most lethal forms of cancer, characterized by its aggressiveness and metastatic potential. Despite significant improvements in PC treatment and management, the complexity of the molecular pathways underlying its development has severely limited the available therapeutic opportunities. Toll-like receptors (TLRs) play a pivotal role in inflammation and immune response, as they are involved in pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Activation of TLRs initiates a signaling cascade, which in turn, leads to the transcription of several genes involved in inflammation and anti-microbial defense. TLRs are also deregulated in several cancers and can be used as prognostic markers and potential targets for cancer-targeted therapy. In this review we discuss the current knowledge about the role of TLRs in PC progression, focusing on the available TLRs-targeting compounds and their possible use in PC therapy.
Keywords: pancreatic cancer, toll-like receptor, inflammation, chemotherapy
1. Introduction
Pancreatic cancer (PC) is a lethal malignancy with a high mortality rate that is projected to become the second leading cause of cancer death in the next ten years [1]. Based on the GLOBOCAN 2020, it has been estimated that pancreatic cancer causes more than 466,000 deaths per year worldwide, ranking as the seventh cause of cancer death in males and females [2]. Because of the lack of early diagnosis, about 80% of patients show unresectable tumor or metastases with a 5-year survival rate of about 10%. This parameter can increase to 58% in a small percentage of patients in which tumor is detected at early stages [3]. The standard treatment for patients affected by PC is surgical resection followed by chemotherapy. This strategy, supported by different studies, results in the improvement of survival outcome. In particular, the CONKO-001 study shows that the addition of gemcitabine treatment after tumor resection results in an increased 5-years survival rate with a slight increase in the overall survival [4]. Other clinical trials are carried out in order to identify the best therapeutic regime that improves patient survival. For example, dual treatment with capecitabine and gemcitabine after tumor resection results in the amelioration of the median overall survival [5]. Another therapeutic approach that is often applied to PC patients is the administration of FOLFIRINOX (5FU, leucovorin, irinotecan and oxaliplatin). This latter strategy appears to lead to a higher overall survival, progression-free survival and response rate when compared to gemcitabine single treatment [6]. However, due to its toxicity, this therapeutic regime is not suitable for all patients making gemcitabine the standard drug used in the PC treatment [6]. Despite the advances in the understanding of pancreatic cancer pathogenesis, the causes of the insurgence of this neoplasia still remain unknown. Environmental factors, such as smoking, obesity, diabetes mellitus and chronic pancreatitis, represent a potential risk for the PC insurgence [7]. Moreover, several studies demonstrated that hereditary germline or somatic mutations are responsible for tumor progression. Particularly, mutations in genes that are associated with cell death and proliferation as well as mutations in genes associated with telomerase shortening result in PC insurgence and metastasis [8,9]. Apart from the alteration of tumor suppressor genes and of the ones involved in the cell cycle regulation, cytokines have been shown to have a role in the malignant transformation [10]. Chronic inflammation, indeed, can lead to the production of several cytokines that activate different signaling pathways. This cascade results in the upregulation of other proinflammatory cytokines, such as interleukin-6 (IL-6) which affect the progression of the pancreatic cancer [11]. Among these pathways, Toll-like receptors (TLRs) seem to be activated during pancreas inflammation in response to damage-associated molecular patterns (DAMPs) [12]. Upon activation, TLRs, through different pathways, lead to the transcriptional factor NF-κB which supports the inflammatory microenvironment [13]. Several studies demonstrated that TLRs are upregulated in different neoplasia such as breast, lung and colon cancer where they are associated with a favorable or with a poor prognosis [14]. Recent reports demonstrated that TLRs are highly expressed also in pancreatic cancer where they are involved in the regulation of cancer physiology and therefore, they may represent a novel target for the cancer therapy [15,16,17]. In this review, we report the current knowledge about the role of TLRs in PC progression and we describe the compounds that may be implied in = PC treatment.
2. Toll-like Receptors
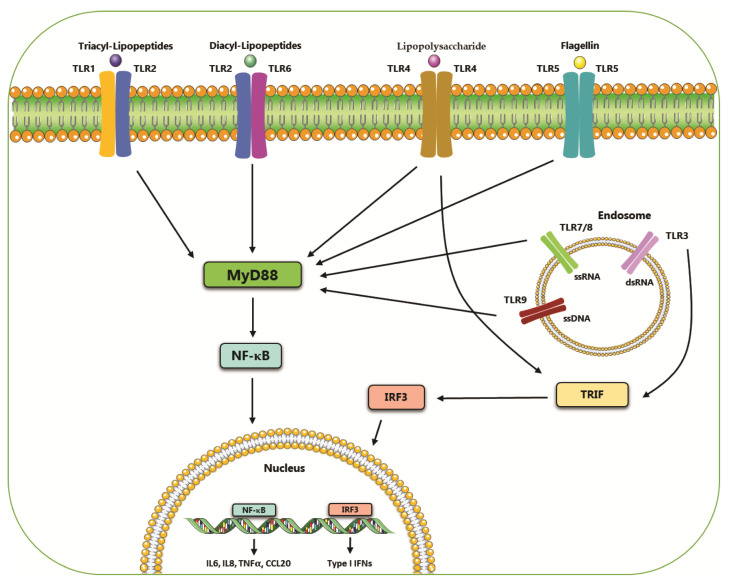
Toll-like receptors (TLRs) belong to the pattern recognition receptors (PRRs) family, which is involved in the activation of the innate immune response [18]. The PRRs family is able to recognize several pathogen-associated molecular patterns (PAMPs) deriving from pathogenic bacteria or fungi, viruses and protozoa [19]. TLRs consist of type I integral membrane glycoproteins with an extracellular N-terminal domain, that contains leucine-rich repeats (LRRs), and an intracellular C-terminal domain defined Toll/IL-1 receptor (TIR) domain [20,21]. TLRs family includes ten members: TLR1, TLR2, TLR4, TLR5, TLR6, TLR10, which are expressed extracellularly and TLR3, TLR7, TLR8, TLR9, that are expressed in the endosomes [22,23]. Furthermore, TLRs are classified according to the PAMPs that they are able to bind to: TLR1, TLR2, TLR4 and TLR6 recognize lipids, TLR5 and TLR10 detect proteins and TLR3, TLR7, TLR8 and TLR9 bind nucleic acids (Table 1) [24]. Upon stimulation, most of these receptors activate a signaling cascade that includes Myeloid differentiation primary response protein 88 (MyD88). This pathway, through the activation of several intermediates such as tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF-6), IL-1R-associated kinases (IRAK) and mitogen-activated kinases, leads to the activation of the transcriptional factor NF-κB [25], a pleiotropic factor involved in the activation of pro-inflammatory genes [26] (Figure 1). In addition, TLRs, in particular TLR3 and TLR4, are able to activate a non-MyD88-dependent pathway that involves TIR-domain-containing adapter-inducing interferon-β (TRIF) protein and is responsible for the synthesis of interferon (IFN) and/or NF-κB activation [27,28]. Defects in the activation of TLRs result in the alteration of immune homeostasis that is sustained by the upregulation of NF-κB and the production of pro-inflammatory cytokines. This contributes to the development and progression of several diseases including cancer, diabetes type 1 and autoimmune diseases [29,30,31] (Figure 1).
Table 1.
Toll-like receptors expressed in human pancreatic cancer cell lines.
| TLRs | Localization | PAMPs | Adaptor | Pancreatic Cancer Cell Line | Refs. |
|---|---|---|---|---|---|
| TLR1 | Plasma membrane | Triacyl lipopeptides | Myd88/TIRAP | Not reported | [19,32] |
| TLR2 | Plasma membrane | Lycolipids, lipoprotein, lipoteichoic acid, peptidoglycan, zymosan | Myd88/TIRAP | BxPC-3; MIA PaCa-2, MDA Panc-28, SU 8686, SW-1990, AsPC-1, Panc-1 | [19,33,34,35] |
| TLR3 | Endosome | Double-stranded RNA | TRIF | AsPC-1, Colo357, Panc-89, PancTu-1, Pt45P1 | [36,37,38] |
| TLR4 | Plasma membrane | Lipopolysaccharide (LPS), heat shock proteins | Myd88/TIRAP | AsPC-1, BxPC-3, CFPAC, MIA PaCa-2, MDA Panc-28, Panc-1, Sw-1990 | [34,35,39] |
| TLR5 | Plasma membrane | Flagellin | Myd88 | Not reported | [32] |
| TLR6 | Plasma membrane | Diacyl lipopeptides, lipoteichoic acid | Myd88/TIRAP | Not reported | [32] |
| TLR7 | Endosome | Single-stranded RNA | Myd88 | Colo357, MIA PaCa-2, MDA Panc-28, Panc-1, Sw-1990, Panc-89, PancTu-1, BxPC-3 | [38,40,41,42] |
| TLR8 | Endosome | Single-stranded RNA | Myd88 | Panc-1 | [41] |
| TLR9 | Endosome | DNA (CpG) | Myd88 | GER, MIA PaCa-2, MDA Panc-28, Panc-1, Sw-1990, T3M4 | [34,43,44] |
| TLR10 | Endosome | Unknown | Unknown | Not reported | [32] |
Figure 1.
Localization and ligands of toll-like receptors (TLRs). The surface-expressed TLRs recognize bacterial compounds while the intracellular receptors recognize virus-associated nucleic acids. Almost all these receptors activate a signaling pathway that, through Myd88, leads to the activation of the transcriptional factor NF-κB. TLR3 and TLR4 activate a Myd88-independent signaling which culminates in the activation of transcriptional factor IRF3.
3. Toll-like Receptor 1
TLR1 is expressed on the membrane of several lymphoid cell lines, including monocytes and lymphocytes and neuronal cells, such as CHP-212 and NT2-N [45,46]. This receptor is able to form a heterodimer with other TLRs acquiring the ability to recognize a broad range of antigens, such as bacterial proteins upon binding to TLR2 [47], and fungi upon binding to TLR6 [48]. Little is known about the role of this receptor in pancreatic cancer. Recently, a multivariate analysis has reported a positive correlation between the higher TLR1 expression and a better prognosis in pancreatic cancer patients who had received no post-operative adjuvant chemotherapy [49].
4. Toll-like Receptor 2
TLR2 expression profile varies among cell types with higher expression levels found on the plasma membrane of immune cells. Besides its role in inflammatory diseases, TLR2 plays, also, an antitumor activity that is exerted by several mechanisms such as enhancement of T-cell immunity, induction of apoptosis in TLR2-positive tumors and enhancement of the innate immunity [50]. In pancreatic cancer, increased TLR2 expression has a controversial role in the regulation of the pathophysiology of this neoplasia (Table 2). In particular, in pancreatic cancer cells, upon the binding of HMGB1 (High mobility group box1) to TLR2, the PI3K/pAKT pathway is activated with subsequential induction of the epithelial-mesenchymal transition necessary for the metastatic phenotype [33,34]. Furthermore, previous reports also described a role of TLR2 in the maintenance of stemness in ovarian and breast cancer cells Lately, a recent study demonstrated that, in pancreatic cancer cells, the interaction HMGB1 with TLR2 leads to the activation of Wnt/β-catenin in CD133+ cancer cells and it is responsible for the activation of stem cell genes, such as NANOG, OCT4 and SOX2 [35]. Leppanen et al. further discussed TLR2 expression in the pancreatic intraepithelial neoplasia (PanIN), a precursor of pancreatic cancer. Particularly, TLR2 expression varies among the different grades of severity of these lesions with lower expression in PanIN1 and higher TLR2 expression in PanN3 [51].
Table 2.
Association between TLRs expression and pancreatic cancer prognosis.
| TLRs | Expression | Pancreatic Cancer Prognosis | Refs. |
|---|---|---|---|
| TLR1 | High | Favorable | [49] |
| TLR2 | High | Favorable | [15] |
| Unfavorable | [33,34,35,51] | ||
| TLR3 | High | Unfavorable | [36] |
| TLR4 | High | Favorable | [15] |
| Unfavorable | [39,52,53,54] | ||
| TLR5 | High | Unfavorable | [55,56] |
| TLR7 | High | Unfavorable | [16,41,57] |
| TLR8 | High | Unfavorable | [41,57] |
| TLR9 | High | Favorable | [43,58,59,60] |
| Unfavorable | [44,61] |
5. Toll-like Receptor 3
TLR3 is an endosomal receptor expressed in monocytes and dendritic cells with the ability to recognize double-stranded RNAs. Upon stimulation, this receptor activates a signaling pathway that ends up either in the activation of NF-κB or in the interferon-beta (IFNβ) production upon IRF3 activation. Previous evidence demonstrated the interplay between TLR3 and Wnt5a signaling in pancreatic cancer (Table 2). Particularly, PC cells show high expression levels of TLR3 associated with increased cancer cell proliferation and with constitutive activation of the Wnt5a signaling [36]. However, despite TLR3 expression in PC cells, it is still unclear which role it plays in pancreatic cancer pathophysiology.
6. Toll-like Receptor 4
TLR4 is a surface receptor, expressed either as homodimer or heterodimer together with TLR6 on the membrane of many immune cells, that recognizes the lipopolysaccharide (LPS), the major component of Gram-negative bacteria. Upon stimulation, TLR4 activates a downstream cascade which involves several adaptor molecules and culminates in the activation of the transcription factor NF-κB [62,63,64]. TLR4 expression is linked to several diseases. It has been reported that high TLR4 activation, upon LPS stimulation, is involved in the alteration of cytosolic Ca2+ and in cell death promotion, thus contributing to Alzheimer’s disease pathogenesis [65,66]. Moreover, PAMPs-induced TLR4 activation plays also a crucial role in inflammatory skin diseases [67,68]. Particularly, TLR4 stimulation activates a signaling that, through the recruitment of members of the CBM complex, leads to the activation of NF-κB-induced genes necessary for the maintenance of the inflammatory state [69,70]. Furthermore, TLR4 activation is involved in the promotion of several cancers, such as cervical [71], colorectal [72], and prostate cancer [73]. TLR4 upregulation has been found also in pancreatic cancer where it plays a central role in tumor progression. It has been demonstrated that stromal leukocytes from pancreatic cancer patients show high TLR4 expression levels. These data were confirmed by in vivo experiments in which KRAS mutated mice show upregulated levels of TLR4 both in stromal and epithelial cells while, on the other hand, TLR4−/− mice had a reduction in tumor growth. Moreover, the high expression of TLR4 results in the activation of several NF-κB-induced genes, such as matrix metalloproteinases 2 and 9 (MMP2 and MMP9). Previous evidence reported that the proteolytic activity of these metalloproteinases is increased in pancreatic cancer cells co-cultured with M2-polarized macrophages in which the epithelial-mesenchymal transition (EMT) program is activated by TLR4/IL10 signaling pathway [52].
TLR4 upregulation is also involved in pancreatic cancer angiogenesis. It is well known that hypoxia upregulates different pro-angiogenic pathways that promote vessel growth [53]. Among these, the induction of TLR4 receptor by hypoxia-inducible transcription factor 1 alpha (HIF-1α) may facilitate pancreatic cancer growth as demonstrated in vitro by the exposure of PANC1 cells to hypoxic stress. Moreover, the regulation of TLR4 mediated by HIF-1α has been confirmed by knockdown experiments in which HIF-1α depletion is associated with inhibition of hypoxia-induced TLR4 overexpression and to pancreatic cancer regression [39].
Recently, a new role for TLR4 in pancreatic cancer progression has been reported. Specifically, the stimulation of TLR4 and CAP1 receptors with Resistin, a hormone released by macrophages in the cancer microenvironment, activates the STAT3 pathway that confers to pancreatic cancer cells the ability to resist cancer therapy [54]. Lately, Lanki et al. described a positive correlation for TLR2 and TLR4 in pancreatic cancer regression. In particular, they showed that the expression of these TLRs correlates with a favorable prognosis in patients with small tumor size and lymph-node-negative disease [15].
However, further investigation should be performed to shed light on the role of these receptors in pancreatic cancer pathogenesis.
7. Toll-like Receptor 5
TLR5 is expressed on the surface of several cell lines, such as adipocytes, leukocytes, intestinal and lung epithelial cells and in some tumor cells. Upon stimulation with flagellin, from mobile bacteria, TLR5 activates a signaling pathway that regulates several processes including insulin resistance, maintenance of lung and intestinal homeostasis and cancer [74]. Little is known about the role of TLR5 in pancreatic cancer. However, it has recently been shown that ligands within the gut microbiome of pancreatic cancer patients are recognized by TLR5, which, upon interaction with TLR2, activates a signaling cascade that leads to the cancer growth enhancement and to the suppression of innate and adaptive immune response [55,56].
Furthermore, in other cancers, polymorphisms in TLR5 receptor drive a differential cancer-promoting inflammation that is responsible for different clinical outcomes of cancer patients. Indeed, in breast cancer, deficiency in TLR5 activity is associated with an increased cancer progression while, on the other hand, TLR5 upregulation in ovarian cancer has a negative effect on long-term survival [75].
8. Toll-like Receptor 7
TLR7 is an endosomal receptor whose activation leads to an immune response upon the recognition of viral ssRNA. This sensor is predominantly expressed in specific immune cells such as plasmacytoid dendritic cells (pDCs) and B cells and in lower levels in keratinocytes, hepatocytes and epithelial cells [76]. The role of TLR7 and its agonists in cancer insurgence and progression is currently still controversial and further investigation is required to shed light on TLR7 function in cancer pathogenesis. Indeed, in renal and bladder cancer, upon activation with selective agonists, TLR7 shows anti-proliferative and apoptosis-inducing effects that result in reduced tumorigenesis. On the other hand, an opposite role has been described for TLR7 agonists in cell chronic lymphocytic leukemia cells where TLR7 stimulation acts as a pro-survival factor [40].
In pancreatic cancer, TLR7 expression was markedly increased in the progression from PanINs to metastatic cancer both in humans and mice. In particular, stimulation of TLR7 receptor increases cancer progression through the downregulation of cell cycle members such as cyclin D1 and p16, the downregulation of phosphatase and tensin homolog deleted on chromosome (PTEN) and the upregulation of p27, p53, p21, cyclin B1, PPARγ and TGF-β [16]. Furthermore, in epithelial cells, TLR7 stimulation leads to multiple signaling activation, such as STAT3, Notch, MAP kinase and NF-κB in epithelial cells, confirming that pancreatic cancer is driven by stromal inflammation [16].
Recent studies described a stage-dependent expression of TLR7 and TLR8 in the ductal pancreatic cancer [41]. A functional analysis performed in pancreatic cancer cells, PANC1, showed that increased chronic inflammation due to higher NF-κB activation and COX-2 expression is responsible for the increased cancer cell proliferation and chemoresistance [41]. Furthermore, it has been demonstrated that TLR7 and TLR8 activation is linked to Notch-2 receptor stimulation which results in the insurgence of chemoresistance against 5-fluorouracil in PANC-1 cells 10 [57].
9. Toll-like Receptor 9
Like TLR3 and TLR7, TLR9 is an endosomal receptor that recognizes unmethylated CpG-DNA and viral DNA [77,78]. This receptor shows a different expression profile with high expression in immune cells, such as plasmacytoid dendritic cells (pDCs), monocytes/macrophages, T and B cells and in non-immune cells, such as respiratory epithelial cells and keratinocytes [79,80].
Furthermore, TLR9 expression is associated with unfavorable prognosis in several cancers including squamous cell carcinoma of the tongue, esophageal adenocarcinoma and prostate cancer [81,82,83] and with a favorable prognosis in renal cell carcinoma and in triple-negative breast cancer [84,85]. It has been reported that, in pancreatic cancer, high TLR9 expression is associated with the increase of patient survival up to 15 months [58]. In particular, the stimulation of TLR9 plays an inhibitory role in pancreatic cancer cell proliferation. This effect was confirmed by in vivo studies in which the stimulation of TLR9 receptor with a synthetic agonist leads to pancreatic cancer regression through the inhibition of cancer cells growth and the enhancement of the immune response [43,59,60]. On the other hand, the activation of TLR9 is responsible for the fibrotic phenotype of pancreatic stellate cells and for the promotion of epithelial cell proliferation, and this confers to TLR9 a cancer-promoting role in the pancreatic cancer [44]. Recently, it has been reported that TLR9 expression is associated with microenvironmental pathogens. Indeed, during the pancreatic transformation, the gut microbiome may act as a source of TLR9 ligands which are responsible for the increased expression of TLR9 [61]. Nevertheless, further evidence is required to better define the association between TLR9 prognostic effect and microenvironment pathogens.
10. TLRs Agonists
TLR agonists play a fundamental role in activating innate and adaptive immune responses, and are, therefore, considered to be potent immunomodulators. For this reason, their use is being explored in cancer treatment both as monotherapy or in combined therapeutic strategies [86]. It has been suggested that TLR agonists could enhance the sensitivity of cancer cells to chemotherapy, radiation, and immunotherapy as well as improve the immunogenicity of ex vivo dendritic cells (DC) vaccines [87]. While the use of TLR agonists for the prevention and/or treatment of several disorders is a promising approach, many of these compounds were withdrawn from further studies due to limited efficacy or for the presence of side effects [88,89]. In this section, we will discuss the use of TLR agonists in PC treatment, while a more comprehensive list of the main TLR agonists is present in Table 3.
Table 3.
List of agonists of Toll-like receptors.
| Drug Name | Target | Drug Class | Refs. |
|---|---|---|---|
| Pam3CSK4 | TLR1/2 | Synthetic triacylated lipopeptide | [119] |
| MALP-2 | TLR2/6 | synthetic lipopeptide | [90,91] |
| PSK | TLR2 | Protein-bound polysaccharide | [96] |
| PAUF | TLR2/4 | Peptide | [103,120] |
| SMP-105 | TLR2 | Components of cell wall skeleton isolated from Mycobacterium Bovis | [121] |
| CBLB612 | TLR2/6 | Synthetic derivative of mycoplasma lipopeptide | [122] |
| Phenylmethimazole (C10) | TLR3 | Methimazole derivative | [36] |
| Poly I:C | TLR3 | Synthetic analog of viral dsRNA (polyinosinic-polycytidylic acid) | [123,124] |
| PolyICLC | TLR3 | Polyinosinic-polycytidylic acid mixed with the stabilizers carboxymethylcellulose and polylysine | [125] |
| Poly-IC12U | TLR3 | Poly I:C derivative with shorter half life and less toxicity | [126] |
| IPH 3102 | TLR3 | Synthetic dsRNA agent | [127] |
| ARNAX | TLR3 | Synthetic DNA/RNA hybrid molecule | [128] |
| MPLA | TLR4 | Lipid A derivative | [129] |
| OK-432 | TLR4 | Lyophilized mixture of group A Streptococcus pyogenes | [130] |
| AS04 | TLR4 | Combination of MPLA and aluminum salt | [131] |
| GLA-SE (G100) | TLR4 | Glucopyranosyl lipid-A oil-in-water emulsion | [132] |
| CBLB502 | TLR5 | derivative of Salmonella flagellin | [133] |
| M-VM3 (Mobilan) | TLR5 | Recombinant non-replicating adenovirus that directs expression of human Toll-like receptor 5 and of a flagellin derivative that acts as a selective agonist of TLR5 | [134] |
| ssRNA40 | TLR7 | 20-mer phosphorothioate protected single-stranded RNA oligonucleotide containing a GU-rich sequence | [16] |
| Gardiquimod | TLR7 | Imidazoquinoline compound | [42] |
| Resiquimod (R848) | TLR7 | Imidazoquinoline compound | [110] |
| Bistriazolyl | TLR7 | Small Molecule | [135] |
| VTX1463 | TLR8 | Small Molecule | [136] |
| CpG-1826 | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [122] |
| CpG-7909 | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [137] |
| IMO-2155 | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [138] |
| MGN1703 | TLR9 | Covalently closed natural DNA molecule | [139] |
| dSLIM | TLR9 | MGN1703 derivative | [140] |
| SD-101 | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [141] |
| KSK-CpG | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [142] |
| ODN2216 | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [143] |
| ODN M362 | TLR9 | Oligodeoxynucleotide containing immunostimulatory CpG motifs | [144] |
Macrophage activating lipopeptide-2 (MALP-2) is a synthetic lipopeptide capable of inducing immune responses through TLR2 and TLR6 activation [90,91]. It has been shown that MALP-2 can reduce tumor growth, prolong survival and increase the efficacy of gemcitabine treatments in murine in vivo models of pancreatic adenocarcinoma (PDAC) [92].
Since the cell line (Panc-2) used to generate those data do not express TLR2, the authors hypothesized that the MALP-2 effect could be through CD8+ lymphocytes and NK cells. Indeed, it has been shown that MALP-2 can activate DC through TLR2/TLR6 [93], even if, a later study described that this lipopeptide is unable to induce DC-TLR2 mediated NK cell activation [94]. However, results of a phase I/II trial that include MALP-2 treatment were encouraging [95]. In particular, ten patients who underwent laparotomy with incomplete or no resection of pancreatic adenocarcinoma were treated intraoperatively with MALP-2. The trial showed that the drug was well tolerated with a median survival of 9.3 months and mean survival of 17.1 months. An increase in the expression of co-stimulatory molecules on lymphocytes, and cytotoxic T and NK cells infiltrating the tumor was observed, leading to the hypothesis that MALP-2 could increase the activation of both the innate and the adaptive immune system. Currently, no other clinical trials were reported.
Another TLR2 agonist is protein-bound polysaccharide-K (PSK) that has been shown to enhance apoptosis and inhibit tumor growth in human PDAC cell lines [96]. More recently, evidences showed that PSK also inhibits hedgehog signaling by downregulating the expression of mastermind-like 3 (MAML3) and recombination signal binding protein for the immunoglobulin-kappa-J region (RBPJ) under hypoxia, inhibiting Smoothened (SMO) transcription and, thus, suppressing the malignant phenotype of PDAC cells [97,98].
Pancreatic adenocarcinoma upregulated factor (PAUF) is a protein overexpressed in the human PDAC [99] and other cancers [100,101,102]. PAUF promotes metastasis by regulating the TLR/CXCR4 activation [103].
PAUF has been shown to be an endogenous ligand for TLR2 and TLR4, it activates the canonical signaling pathways of TLR2-tumor progression locus 2 (TPL2)/mitogen-activated ERK kinase (MEK)/extracellular signal-regulated kinase (ERK), but it fails to mediate TLR2-induced NF-κB activation [103]. Recently, it has been demonstrated that PAUF can enhance the antigen-specific CD8+ T cell antitumor immunity of DC vaccines [104].
Polycytidylic acid (Poly I:C) is a TLR3 agonist capable of enhancing the cytotoxic activity and granzyme A/B production of γδ T cells in vitro [38]. Cell lines from pancreatic adenocarcinomas, squamous cell carcinomas of head and neck and lung carcinomas expressing TLR3 and TLR7 were pretreated with Poly I: C before co-culture with γδ T cells. The authors hypothesized that the observed effect on cytotoxicity was a consequence of the upregulation of CD54 on the tumor cells, and its consequent interaction with CD11a/CD18 expressed on γδ T cells.
Although it was also reported that Poly I:C can accelerate pancreatic carcinogenesis in KRAS-mutated mice [16] more recent evidence still supports its possible use as an adjuvant [37,105,106].
Metzger et al. provided evidence that Poly I:C can functionally reprogram myeloid-derived suppressor cells (MDSC) in orthotopically implanted KrasG12D p53fl/R172H Ptf1a-Cre (KPC) pancreatic tumors. Whole transcriptomic analysis of MDSC populations showed an IFN pathway-enriched gene signature together with a shift from an M2/G2- towards an M1/G1-polarized phenotype. The authors showed that the suppressive phenotype is promoted by IFN receptor 1 (IFNAR1), however, it is not clear if the effect of Poly I:C is mainly through TLR3 or RIG-I-like helicases (RLH) [107].
The effect of Poly I:C on macrophage polarization could make this compound attractive in combination with hypomethylating agents and immunotherapy.
It has been recently shown, using the KPC mouse model (KrasLSL.G12D/+; P53LSL.R172H/+; Pdx1-Cretg/+), that low dose treatment with the hypomethylating drug decitabine (DAC) can increase the efficacy of immune checkpoint inhibitors (ICI) therapy. However, the authors also reported an increase of M2 macrophages, following DAC treatment, that are predicted to antagonize ICI antitumor effects [108]. Therefore, it is tempting to speculate that adding Poly I:C to the sequential therapy DAC+ICI could further improve the efficacy of this combination by promoting the M1 polarization of TAMs.
Finally, administration of a formulation of Poly I:C with polyethylenimine ([Poly I:C]PEI) induced apoptosis in PDAC cells but not in normal pancreatic epithelial cells [109]. Specifically, [Poly I:C]PEI, on one hand, repressed XIAP and survivin expression and, on the other hand, it is responsible for the immune response activation through MDA-5, RIG-I and NOXA induction and inhibition of AKT phosphorylation. In vivo administration of [Poly I:C]PEI inhibited tumor growth via AKT-mediated XIAP degradation in both subcutaneous and quasi-orthotopic models of PDAC [107].
Phenylmethimazole (C10) is a derivative of methimazole with anti-inflammatory properties. Specifically, its inhibitory effect on TLR3 leads to suppression of the dsRNA induced, TLR3-mediated IRF3/IFN-pathway. The administration of C10 was effective in inhibiting growth and migration of cancer cell lines, as well as in inhibiting tumor growth in vivo in nude or severe combined immunodeficient mice both for human pancreatic cancer and malignant melanoma [36]. Several studies described a negative role of the TLR7 agonist, gardiquimod, in pancreatic cancer progression. In particular, the stimulation with this agonist inhibits cell proliferation and activates the apoptotic program through the downregulation of B-cell lymphoma 2 (BCL2), cyclin B1 and cyclin E and through the upregulation of B-cell-associated X protein. Furthermore, upon stimulation with gardiquimod, TLR7 induces the expression of anticancer genes such as PTEN and tissue inhibitor of metalloproteinase 1 (TIMP-1) and it downregulates the expression of VEGF in BxPC-3 pancreatic cancer cells [42]. Another reported TLR7/8 agonist is Resiquimod (R848), which plays a role in the remodeling of pancreatic cancer immune microenvironment and is responsible for the activation of an anti-tumor response. In particular, R848 exerts an anti-cancer activity through the increasing of CD8+ T-cell infiltration, the formation of tertiary lymphoid structures and decreased Treg concentration, all events associated with a better prognosis in the human neoplasia [110].
Recently, a polysaccharide isolated from Strongylocentrotus nudus eggs (SEP) has been shown to inhibit pancreatic cancer growth through TLR4/MAPKs/NF-κB pathway signals and activate NK cells in vitro and in vivo. Moreover, the same compound also increased gemcitabine anti-tumor activity by up-regulating NKG2D/MICA while reducing its side effects through the suppression of ROS release in vitro and in vivo [111]. Interestingly, these findings seem to contradict previous reports suggesting that TLR4 inhibition by triptolide can enhance the sensitivity of pancreatic cancer cells to gemcitabine by inhibiting the TLR4/NF-κB signaling [112]. These discrepancies although not totally unexpected in pre-clinical models, highlight the complexity of successfully translating TLR-targeting compounds to the clinic.
Similar to TLR3 and TLR7, TLR9 is expressed on endosomal membranes of several immune cells [113], and it has been linked to acute pancreatitis and cancer [12,114].
Synthetic TLR9 agonists are oligodeoxynucleotides containing immunostimulatory CpG motifs (CpGODNs) and are used as vaccine adjuvants or as antiallergic agents [115].
The addition of low-dose CpGODNs targeting TLR9 to a vaccine based on immune stimulatory complexes (ISCOM) inhibited the tumor immune evasion in an orthotopic model of pancreatic carcinoma inducing an effective CTL-mediated tumor cell killing and prolonging mice survival [59]. Moreover, CpG-ODNs treatment showed synergy in combination with gemcitabine in an orthotopic human pancreatic carcinoma xenograft mouse model, reducing metastasis and overall survival compared with monotherapy alone [60]. Immunomodulatory nucleotides (IMO) are second-generation CpG-ODNs with higher metabolic stability. It has been shown that IMO administration can synergize with the anti-EGFR monoclonal antibody cetuximab by interfering with EGFR-dependent signaling both in vitro and in vivo systems of pancreatic carcinoma [116,117].
Another CpG-ODN (ODN2216) has been reported to be able to reduce proliferation and migration ability in PANC-1 cells in vitro [43]. Additionally, more recently, intratumoral injection of IMO-2155 has been shown to trigger a potent immune reaction, especially in combination with systemic anti-PD1 therapy in pancreatic cancer orthotopic model [118]. The authors hypothesize that IMO-2125 local treatment recruited antigen-presenting cells (APCs) into the tumor thus leading to a more effective immune response [118].
Currently, for pancreatic cancer, combination therapy including TLR9-activating CpGs is being evaluated in two clinical trials (Clinicaltrials.gov accessed on 28 October 2021: # NCT04612530 and # NCT04050085). At this time both studies are still recruiting and no result has been posted. However, they will help to shed light on whether TLR9 agonists could represent a valid strategy to potentiate immunotherapy as well as chemotherapy in PC.
11. TLRs Antagonists
TLR antagonists are compounds able to reduce or inhibit activation of TLRs signaling, therefore acting as modulators of the native immunity.
They can generally be divided into two families: direct and indirect TLR antagonists.
Direct TLR antagonists are mostly compounds that competitively bind to the TLRs but fail to induce the conformational change necessary for the signal propagation. On the other hand, indirect TLR antagonists block TLR-associated signaling without competing with their ligands [145,146].
The potential use of TLRs inhibitors in the treatment of human cancers has been evaluated in both pre-clinical and clinical studies.
Given the importance of the TLR4 pathway in cancer progression, it’s not surprising that currently most of the inhibitors developed are directed against TLR4 (Table 4).
Table 4.
List of antagonists of TLRs.
| Drug Name | Target | Drug Class | Refs. |
|---|---|---|---|
| TAK-242 (Resatorvid) | TLR4 | Small molecule inhibitor | [150] |
| CRX-526 | TLR4 | Synthetic lipopolysaccharide | [163] |
| CX-01 | TLR4 | heparin-derived olysaccharide | [164] |
| CXC195 | TLR4 | tetramethylpyrazine analogue | [165] |
| Eritoran (E5564) | TLR4 | Synthetic lipopolysaccharide | [168] |
| Atractylenolide-1 | TLR4 | sesquiterpene compound | [169] |
| Triptolide | TLR4 | diterpenoid epoxide | [170] |
| Paeonol | TLR4 | Small molecule inhibitor | [171] |
| NI-0101 | TLR4 | Monoclonal antibody | [172] |
| Nalmefene (JKB-121) | TLR4 | Small molecule inhibitor | [173] |
| Ibudilast (AV-411, N-166) | TLR4 | Small molecule inhibitor | [174] |
| Polymyxin B (PMB) | TLR4 | Cyclic polypeptide antibiotic | [175] |
| OPN305 | TLR2 | Monoclonal antibody | [176] |
| Hydroxychloroquine | TLR7,9 | Quinolone | [177] |
E5564 (also known as Eritoran) is a structural analog of the lipid A portion of LPS which competitively binds to TLR4/MD-2, thus resulting in inhibition of LPS-induced inflammatory responses. Although originally developed for the treatment of severe sepsis [147] there is evidence supporting its use in the cancer therapy [147]. In 2016, in fact, Deguchi et al. found that Eritoran inhibited lung cancer progression in vivo, likely due to reduced tumor angiogenesis, lower levels of TAMs and CD11b+Ly6C++Ly6G– myeloid-derived cells infiltration with a consequent increase in CD8+ T-cell tumor infiltration [148]. Moreover, Kuo et al. showed that treatment with Eritoran in murine models of colorectal carcinoma was able to inhibit the progression of bacterial LPS-induced colon cancer through induction of CD14/Src/PKCζ-mediated apoptosis and the blockade of TLR4-dependent proliferation [149].
TAK-242 (also known as Resatorvid) is a small molecule inhibitor developed by Takeda. Its inhibitory effect is a consequence of the selective binding to TLR4 TIR-domain, therefore preventing its interaction TRAM or TIRAP [150].
Reports from different groups show this compound as negatively affecting proliferation and invasiveness of different cancer cell lines while enhancing the anti-cancer effects of chemotherapeutic agents [151,152,153,154]. Moreover, topical TAK-242 administration was shown to suppress solar UV-induced skin tumorigenesis in SHK-1 mice antagonizing chronic UV-induced inflammatory signaling [155].
Surfactant protein A-derived (SPA4) is a peptide inhibitor derived from the TLR4-interacting region of the SP-A [156]. It has been shown that it can inhibit LPS-stimulated inflammatory responses, migration and invasion of colon cancer SW480 [157]. Similar results were also reported in the lung cancer [158,159].
CRX526 (also known as an aminoalkyl-glucosaminide-phosphate) is a synthetic molecule that mimics the active component of LPS that binds to TLR4 (lipid A) [160,161]. CRX-526 was reported to significantly reduce the tumor volume of colon cancer xenograft mice models likely through the suppression of the TLR4/NF-κB p65 axis [162].
CX-01 is a heparin-derived polysaccharide with a low anticoagulant activity that targets the interaction of TLR4 and its ligand high mobility group 1 protein (HMGB1) [163].
The use of this TLR4 inhibitor has been evaluated in clinical trials for the treatment of refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Remarkably, it has shown promise in combination with chemotherapy or with epigenetic therapy (Azacitidine) [163,164].
CXC195 is an indirect TLR4 antagonist that inhibits the interactions of TLR4, MyD88 and NF-κB. It also inhibits the translocation of NF-κB to the nucleus, as well as its DNA binding activity. It has been shown that CXC195 can induce apoptosis and inhibits proliferation in cellular models of hepatocellular carcinoma cells [165,166] and bladder carcinoma [167].
12. Conclusions
Despite the progress made in translational research and therapy, pancreatic cancer still remains a lethal disease. Emerging evidence demonstrates that inflammation and pancreatic cancer are strongly associated. In this scenario, TLRs play a crucial role by activating the pro-inflammatory pathways responsible for the production of cytokines ad chemokines necessary to create a favorable microenvironment for tumor growth. Since these receptors play a dual role, as they activate pathways that lead to immunosuppressive cytokines production, the employments of TLR agonists and antagonists in cancer therapy may represent a potential strategy to improve the survival rate of patients with pancreatic cancer. Furthermore, a better comprehension of the molecular adaptors and their role in the TLRs signal transduction may help for the modulation of TLRs response in therapeutic treatments. Here we summarized the current knowledge about the implication of TLRs in PC, but despite the encouraging results, further studies are needed to better comprehend the molecular signature of pancreatic cancer and successfully employ TLRs modulation in a clinical setting.
Author Contributions
Conceptualization A.O. and P.M.; writing the original draft A.O. and P.M.; writing-review and editing A.O. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Kaur S., Baine M.J., Jain M., Sasson A.R., Batra S.K. Early diagnosis of pancreatic cancer: Challenges and new developments. Biomarkers Med. 2012;6:597–612. doi: 10.2217/bmm.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert A., Schwarz L., Borbath I., Henry A., Van Laethem J.-L., Malka D., Ducreux M., Conroy T. An update on treatment options for pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2019;11:1758835919875568. doi: 10.1177/1758835919875568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teague A., Lim K.-H., Wang-Gillam A. Advanced pancreatic adenocarcinoma: A review of current treatment strategies and developing therapies. Ther. Adv. Med. Oncol. 2015;7:68–84. doi: 10.1177/1758834014564775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.-L., Choné L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 7.Tsai H.-J., Chang J.S. Environmental Risk Factors of Pancreatic Cancer. J. Clin. Med. 2019;8:1427. doi: 10.3390/jcm8091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goral V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac. J. Cancer Prev. 2015;16:5619–5624. doi: 10.7314/APJCP.2015.16.14.5619. [DOI] [PubMed] [Google Scholar]
- 9.Chen C., Fang Y., Yao Q., Chen Z., Xiang J., William F.E., Gibbs R.A. Genetic and molecular alterations in pancreatic cancer: Implications for personalized medicine. Med. Sci. Monit. 2013;19:916–926. doi: 10.12659/MSM.889636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friess H., Guo X.-Z., Nan B.-C., Kleeff J., Buchler M.W. Growth Factors and Cytokines in Pancreatic Carcinogenesis. Ann. N. Y. Acad. Sci. 1999;880:110–121. doi: 10.1111/j.1749-6632.1999.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Duijneveldt G., Griffin M.D.W., Putoczki T.L. Emerging roles for the IL-6 family of cytokines in pancreatic cancer. Clin. Sci. 2020;134:2091–2115. doi: 10.1042/CS20191211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaz J., Akbarshahi H., Andersson R. Controversial role of toll-like receptors in acute pancreatitis. World J. Gastroenterol. 2013;19:616–630. doi: 10.3748/wjg.v19.i5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shadhu K., Xi C. Inflammation and pancreatic cancer: An updated review. Saudi J. Gastroenterol. 2019;25:3–13. doi: 10.4103/sjg.SJG_390_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato Y., Goto Y., Narita N., Hoon D.S. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009;2((Suppl. S1)):205–214. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanki M.A., Seppänen H.E., Mustonen H.K., Böckelman C., Juuti A.T., Hagström J.K., Haglund C.H. Toll-like receptor 2 and Toll-like receptor 4 predict favorable prognosis in local pancreatic cancer. Tumor Biol. 2018;40:1010428318801188. doi: 10.1177/1010428318801188. [DOI] [PubMed] [Google Scholar]
- 16.Ochi A., Graffeo C.S., Zambirinis C.P., Rehman A., Hackman M., Fallon N., Barilla R., Henning J.R., Jamal M., Rao R., et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012;122:4118–4129. doi: 10.1172/JCI63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaz J., Andersson R. Intervention on toll-like receptors in pancreatic cancer. World J. Gastroenterol. 2014;20:5808–5817. doi: 10.3748/wjg.v20.i19.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogensen T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthoney N., Foldi I., Hidalgo A. Toll and Toll-like receptor signalling in development. Development. 2018;145:dev156018. doi: 10.1242/dev.156018. [DOI] [PubMed] [Google Scholar]
- 21.Botos I., Segal D.M., Davies D.R. The Structural Biology of Toll-like Receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Zhu C., Wu D., Jiang X. Possible Role of Toll-Like Receptor 4 in Acute Pancreatitis. Pancreas. 2010;39:819–824. doi: 10.1097/MPA.0b013e3181ca065c. [DOI] [PubMed] [Google Scholar]
- 23.Muzio M., Polentarutti N., Bosisio D., Manoj Kumar P.P., Mantovani A. Toll-like receptor family and signalling pathway. Biochem. Soc. Trans. 2000;28:563–566. doi: 10.1042/bst0280563. [DOI] [PubMed] [Google Scholar]
- 24.Yu L., Wang L., Chen S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010;14:2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deguine J., Barton G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 27.Mlcochova P., Winstone H., Zuliani-Alvarez L., Gupta R.K. TLR4-Mediated Pathway Triggers Interferon-Independent G0 Arrest and Antiviral SAMHD1 Activity in Macrophages. Cell Rep. 2020;30:3972–3980.e5. doi: 10.1016/j.celrep.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzone P., Congestri M., Scudiero I., Polvere I., Voccola S., Zerillo L., Telesio G., Vito P., Stilo R., Zotti T. UBAC1/KPC2 Regulates TLR3 Signaling in Human Keratinocytes through Functional Interaction with the CARD14/CARMA2sh-TANK Complex. Int. J. Mol. Sci. 2020;21:9365. doi: 10.3390/ijms21249365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jialal I., Kaur H., Devaraj S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J. Clin. Endocrinol. Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian S., Tus K., Li Q.-Z., Wang A., Tian X.-H., Zhou J., Liang C., Bartov G., McDaniel L.D., Zhou X.J., et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.So E.Y., Ouchi T. The application of Toll like receptors for cancer therapy. Int. J. Biol. Sci. 2010;6:675–681. doi: 10.7150/ijbs.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoni M., Andrikou K., Sotte V., Bittoni A., Lanese A., Pellei C., Piva F., Conti A., Nabissi M., Santoni G., et al. Toll like receptors and pancreatic diseases: From a pathogenetic mechanism to a therapeutic target. Cancer Treat. Rev. 2015;41:569–576. doi: 10.1016/j.ctrv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Chen X., Zhang L., Jiang Y., Song L., Liu Y., Cheng F., Fan X., Cao X., Gong A., Wang D., et al. Radiotherapy-induced cell death activates paracrine HMGB1-TLR2 signaling and accelerates pancreatic carcinoma metastasis. J. Exp. Clin. Cancer Res. 2018;37:77. doi: 10.1186/s13046-018-0726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimmig T., Moench R., Kreckel J., Haack S., Rueckert F., Rehder R., Tripathi S., Ribas C., Chandraker A., Germer C.T., et al. Toll Like Receptor 2, 4, and 9 Signaling Promotes Autoregulative Tumor Cell Growth and VEGF/PDGF Expression in Human Pancreatic Cancer. Int. J. Mol. Sci. 2016;17:2060. doi: 10.3390/ijms17122060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X., Cheng F., Liu Y., Zhang L., Song L., Cai X., You T., Fan X., Wang D., Gong A., et al. Toll-like receptor 2 and Toll-like receptor 4 exhibit distinct regulation of cancer cell stemness mediated by cell death-induced high-mobility group box 1. EBioMedicine. 2019;40:135–150. doi: 10.1016/j.ebiom.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz A.L., Malgor R., Dickerson E., Weeraratna A.T., Slominski A., Wortsman J., Harii N., Kohn A.D., Moon R., Schwartz F.L., et al. Phenylmethimazole Decreases Toll-Like Receptor 3 and Noncanonical Wnt5a Expression in Pancreatic Cancer and Melanoma Together with Tumor Cell Growth and Migration. Clin. Cancer Res. 2009;15:4114–4122. doi: 10.1158/1078-0432.CCR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujisawa M., Kanda T., Shibata T., Sasaki R., Masuzaki R., Matsumoto N., Nirei K., Imazu H., Kuroda K., Sugitani M., et al. Involvement of the Interferon Signaling Pathways in Pancreatic Cancer Cells. Anticancer Res. 2020;40:4445–4455. doi: 10.21873/anticanres.14449. [DOI] [PubMed] [Google Scholar]
- 38.Shojaei H., Oberg H.H., Juricke M., Marischen L., Kunz M., Mundhenke C., Gieseler F., Kabelitz D., Wesch D. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res. 2009;69:8710–8717. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- 39.Fan P., Zhang J.-J., Wang B., Wu H.-Q., Zhou S.-X., Wang C.-Y., Zhang J.-H., Tian Y., Wu H.-S. Hypoxia-inducible factor-1 up-regulates the expression of Toll-like receptor 4 in pancreatic cancer cells under hypoxic conditions. Pancreatology. 2012;12:170–178. doi: 10.1016/j.pan.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Zou B.-B., Wang F., Li L., Cheng F.-W., Jin R., Luo X., Zhu L.-X., Geng X., Zhang S.-Q. Activation of Toll-like receptor 7 inhibits the proliferation and migration, and induces the apoptosis of pancreatic cancer cells. Mol. Med. Rep. 2015;12:6079–6085. doi: 10.3892/mmr.2015.4130. [DOI] [PubMed] [Google Scholar]
- 41.Grimmig T., Matthes N., Höland K., Tripathi S., Chandraker A., Grimm M., Moench R., Moll E.-M., Friess H., Tsaur I., et al. TLR7 and TLR8 expression increases tumor cell proliferation and promotes chemoresistance in human pancreatic cancer. Int. J. Oncol. 2015;47:857–866. doi: 10.3892/ijo.2015.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F., Jin R., Zou B.B., Li L., Cheng F.W., Luo X., Geng X., Zhang S.Q. Activation of Toll-like receptor 7 regulates the expression of IFN-lambda1, p53, PTEN, VEGF, TIMP-1 and MMP-9 in pancreatic cancer cells. Mol. Med. Rep. 2016;13:1807–1812. doi: 10.3892/mmr.2015.4730. [DOI] [PubMed] [Google Scholar]
- 43.Wu H.-Q., Wang B., Zhu S.-K., Tian Y., Zhang J.-H., Wu H.-S. Effects of CPG ODN on biological behavior of PANC-1 and expression of TLR9 in pancreatic cancer. World J. Gastroenterol. 2011;17:996–1003. doi: 10.3748/wjg.v17.i8.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zambirinis C.P., Levie E., Nguy S., Avanzi A., Barilla R., Xu Y., Seifert L., Daley D., Greco S.H., Deutsch M., et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015;212:2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letiembre M., Liu Y., Walter S., Hao W., Pfander T., Wrede A., Schulz-Schaeffer W., Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: A similar pattern. Neurobiol. Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Miranda-Hernandez S., Baxter A.G. Role of toll-like receptors in multiple sclerosis. Am. J. Clin. Exp. Immunol. 2013;2:75–93. [PMC free article] [PubMed] [Google Scholar]
- 47.Jin M.S., Kim S.E., Heo J.Y., Lee M.E., Kim H.M., Paik S.-G., Lee H., Lee J.-O. Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Netea M.G., Van De Veerdonk F., Verschueren I., Van Der Meer J.W., Kullberg B.J. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol. Med. Microbiol. 2008;52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- 49.Lanki M., Seppänen H., Mustonen H., Hagström J., Haglund C. Toll-like receptor 1 predicts favorable prognosis in pancreatic cancer. PLoS ONE. 2019;14:e0219245. doi: 10.1371/journal.pone.0219245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huynh A.S., Chung W.J., Cho H.-I., Moberg V.E., Celis E., Morse D.L., Vagner J. Novel Toll-like Receptor 2 Ligands for Targeted Pancreatic Cancer Imaging and Immunotherapy. J. Med. Chem. 2012;55:9751–9762. doi: 10.1021/jm301002f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leppänen J., Helminen O., Huhta H., Kauppila J.H., Isohookana J., Haapasaari K.-M., Karihtala P., Parkkila S., Saarnio J., Lehenkari P.P., et al. Toll-like receptors 2, 4 and 9 and hypoxia markers HIF-1alpha and CAIX in pancreatic intraepithelial neoplasia. APMIS. 2018;126:852–863. doi: 10.1111/apm.12894. [DOI] [PubMed] [Google Scholar]
- 52.Liu C.Y., Xu J.Y., Shi X.Y., Huang W., Ruan T.Y., Xie P., Ding J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 53.Krock B.L., Skuli N., Simon M.C. Hypoxia-Induced Angiogenesis: Good and Evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M., Yan L., Wang G., Jin R. Resistin effects on pancreatic cancer progression and chemoresistance are mediated through its receptors CAP1 and TLR4. J. Cell. Physiol. 2019;234:9457–9466. doi: 10.1002/jcp.27631. [DOI] [PubMed] [Google Scholar]
- 55.Pushalkar S., Hundeyin M., Daley D., Zambirinis C.P., Kurz E., Mishra A., Mohan N., Aykut B., Usyk M., Torres L.E., et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8:403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sethi V., Vitiello G., Saxena D., Miller G., Dudeja V. The Role of the Microbiome in Immunologic Development and its Implication For Pancreatic Cancer Immunotherapy. Gastroenterology. 2019;156:2097–2115.e2. doi: 10.1053/j.gastro.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 57.Güngör C., Zander H., Effenberger K.E., Vashist Y.K., Kalinina T., Izbicki J.R., Yekebas E., Bockhorn M. Notch Signaling Activated by Replication Stress–Induced Expression of Midkine Drives Epithelial–Mesenchymal Transition and Chemoresistance in Pancreatic Cancer. Cancer Res. 2011;71:5009–5019. doi: 10.1158/0008-5472.CAN-11-0036. [DOI] [PubMed] [Google Scholar]
- 58.Leppänen J., Helminen O., Huhta H., Kauppila J.H., Isohookana J., Haapasaari K.-M., Lehenkari P., Saarnio J., Karttunen T.J. High toll-like receptor (TLR) 9 expression is associated with better prognosis in surgically treated pancreatic cancer patients. Virchows Arch. 2017;470:401–410. doi: 10.1007/s00428-017-2087-1. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs C., Duewell P., Heckelsmiller K., Wei J., Bauernfeind F., Ellermeier J., Kisser U., Bauer C.A., Dauer M., Eigler A., et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int. J. Cancer. 2011;128:897–907. doi: 10.1002/ijc.25399. [DOI] [PubMed] [Google Scholar]
- 60.Pratesi G., Petrangolini G., Tortoreto M., Addis A., Belluco S., Rossini A., Selleri S., Rumio C., Menard S. Balsari, A. Therapeutic synergism of gemcitabine and CpG-oligodeoxynucleotides in an orthotopic human pancreatic carcinoma xenograft. Cancer Res. 2005;65:6388–6393. doi: 10.1158/0008-5472.CAN-05-0602. [DOI] [PubMed] [Google Scholar]
- 61.Ciernikova S., Novisedlakova M., Cholujova D., Stevurkova V., Mego M. The Emerging Role of Microbiota and Microbiome in Pancreatic Ductal Adenocarcinoma. Biomedicines. 2020;8:565. doi: 10.3390/biomedicines8120565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y.-C., Yeh W.-C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Bhattacharyya S., Gill R., Chen M.L., Zhang F., Linhardt R.J., Dudeja P.K., Tobacman J.K. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J. Biol. Chem. 2008;283:10550–10558. doi: 10.1074/jbc.M708833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazzone P., Scudiero I., Ferravante A., Paolucci M., D’Andrea L.E., Varricchio E., Telesio G., De Maio C., Pizzulo M., Zotti T., et al. Functional Characterization of Zebrafish (Danio rerio) Bcl10. PLoS ONE. 2015;10:e0122365. doi: 10.1371/journal.pone.0122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calvo-Rodriguez M., García-Rodríguez C., Villalobos C., Núñez L. Role of Toll Like Receptor 4 in Alzheimer’s Disease. Front. Immunol. 2020;11:1588. doi: 10.3389/fimmu.2020.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balducci C., Frasca A., Zotti M., La Vitola P., Mhillaj E., Grigoli E., Iacobellis M., Grandi F., Messa M., Colombo L., et al. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by beta-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav. Immun. 2017;60:188–197. doi: 10.1016/j.bbi.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Smith R.L., Hebert H., Massey J., Bowes J., Marzo-Ortega H., Ho P., McHugh N.J., Worthington J., Barton A., Griffiths C., et al. Association of Toll-like receptor 4 (TLR4) with chronic plaque type psoriasis and psoriatic arthritis. Arch. Dermatol. Res. 2016;308:201–205. doi: 10.1007/s00403-016-1620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun L., Liu W., Zhang L.-J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019;2019:1824624. doi: 10.1155/2019/1824624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scudiero I., Mazzone P., D’Andrea L.E., Ferravante A., Zotti T., Telesio G., De Rubis G., Reale C., Pizzulo M., Muralitharan S., et al. CARMA2sh and ULK2 control pathogen-associated molecular patterns recognition in human keratinocytes: Psoriasis-linked CARMA2sh mutants escape ULK2 censorship. Cell Death Dis. 2017;8:e2627. doi: 10.1038/cddis.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Telesio G., Scudiero I., Pizzulo M., Mazzone P., Zotti T., Voccola S., Polvere I., Vito P., Stilo R. The E3 Ubiquitin Ligase RNF7 Negatively Regulates CARD14/CARMA2sh Signaling. Int. J. Mol. Sci. 2017;18:2581. doi: 10.3390/ijms18122581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang N., Xie F., Chen L., Chen F., Sui L. The effect of TLR4 on the growth and local inflammatory microenvironment of HPV-related cervical cancer in vivo. Infect. Agents Cancer. 2020;15:12. doi: 10.1186/s13027-020-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang X., Zhu Y. TLR4 Signaling Promotes Immune Escape of Human Colon Cancer Cells by Inducing Immunosuppressive Cytokines and Apoptosis Resistance. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2012;20:15–24. doi: 10.3727/096504012X13425470196092. [DOI] [PubMed] [Google Scholar]
- 73.Ou T., Lilly M., Jiang W. The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer. Front. Immunol. 2018;9:1188. doi: 10.3389/fimmu.2018.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caballero I., Boyd J., Almiñana C., Sanchez-Lopez J.A., Basatvat S., Montazeri M., Lay N.M., Elliott S., Spiller D.G., White M.R.H., et al. Understanding the dynamics of Toll-like Receptor 5 response to flagellin and its regulation by estradiol. Sci. Rep. 2017;7:40981. doi: 10.1038/srep40981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rutkowski M.R., Stephen T.L., Svoronos N., Allegrezza M.J., Tesone A.J., Perales-Puchalt A., Brencicova E., Escovar-Fadul X., Nguyen J.M., Cadungog M.G., et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petes C., Odoardi N., Gee K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front. Immunol. 2017;8:1075. doi: 10.3389/fimmu.2017.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Müller T., Hamm S., Bauer S. TLR9-Mediated Recognition of DNA. Handb. Exp. Pharmacol. 2008;183:51–70. doi: 10.1007/978-3-540-72167-3_3. [DOI] [PubMed] [Google Scholar]
- 78.Kumagai Y., Takeuchi O., Akira S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008;60:795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Chuang Y.C., Tseng J.C., Huang L.R., Huang C.M., Huang C.F., Chuang T.H. Adjuvant Effect of Toll-Like Receptor 9 Activation on Cancer Immunotherapy Using Checkpoint Blockade. Front. Immunol. 2020;11:1075. doi: 10.3389/fimmu.2020.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patra M., Shah M., Choi S. Toll-like receptor-induced cytokines as immunotherapeutic targets in cancers and autoimmune diseases. Semin. Cancer Biol. 2020;64:61–82. doi: 10.1016/j.semcancer.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Kauppila J.H., Korvala J., Siirilä K., Manni M., Mäkinen L.K., Hagström J., Atula T.S., Haglund C., Selander K.S., Saarnio J., et al. Toll-like receptor 9 mediates invasion and predicts prognosis in squamous cell carcinoma of the mobile tongue. J. Oral Pathol. Med. 2015;44:571–577. doi: 10.1111/jop.12272. [DOI] [PubMed] [Google Scholar]
- 82.Kauppila J.H., Takala H., Selander K.S., Lehenkari P.P., Saarnio J., Karttunen T.J. Increased Toll-like receptor 9 expression indicates adverse prognosis in oesophageal adenocarcinoma. Histopathology. 2011;59:643–649. doi: 10.1111/j.1365-2559.2011.03991.x. [DOI] [PubMed] [Google Scholar]
- 83.Väisänen M.-R., Jukkola-Vuorinen A., Vuopala K.S., Selander K.S., Vaarala M.H. Expression of Toll-like receptor-9 is associated with poor progression-free survival in prostate cancer. Oncol. Lett. 2013;5:1659–1663. doi: 10.3892/ol.2013.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronkainen H., Hirvikoski P., Kauppila S., Vuopala K.S., Paavonen T.K., Selander K.S., Vaarala M.H. Absent Toll-like receptor-9 expression predicts poor prognosis in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2011;30:84. doi: 10.1186/1756-9966-30-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuomela J., Sandholm J., Karihtala P., Ilvesaro J., Vuopala K.S., Kauppila J.H., Kauppila S., Chen D., Pressey C., Härkönen P., et al. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res. Treat. 2012;135:481–493. doi: 10.1007/s10549-012-2181-7. [DOI] [PubMed] [Google Scholar]
- 86.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iribarren K., Bloy N., Buqué A., Cremer I., Eggermont A., Fridman W.H., Fucikova J., Galon J., Špíšek R., Zitvogel L., et al. Trial Watch: Immunostimulation with Toll-like receptor agonists in cancer therapy. OncoImmunology. 2016;5:e1088631. doi: 10.1080/2162402X.2015.1088631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hussein W.M., Liu T.-Y., Skwarczynski M., Toth I. Toll-like receptor agonists: A patent review (2011–2013) Expert Opin. Ther. Pat. 2014;24:453–470. doi: 10.1517/13543776.2014.880691. [DOI] [PubMed] [Google Scholar]
- 89.Basith S., Manavalan B., Yoo T.H., Kim S.G., Choi S. Roles of toll-like receptors in Cancer: A double-edged sword for defense and offense. Arch. Pharmacal Res. 2012;35:1297–1316. doi: 10.1007/s12272-012-0802-7. [DOI] [PubMed] [Google Scholar]
- 90.Morr M., Takeuchi O., Akira S., Simon M.M., Muhlradt P.F. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur. J. Immunol. 2002;32:3337–3347. doi: 10.1002/1521-4141(2002012)32:12<3337::AID-IMMU3337>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 91.Takeuchi O., Kaufmann A., Grote K., Kawai T., Hoshino K., Morr M., Mühlradt P.F., Akira S. Cutting Edge: Preferentially theR-Stereoisomer of the Mycoplasmal Lipopeptide Macrophage-Activating Lipopeptide-2 Activates Immune Cells Through a Toll-Like Receptor 2- and MyD88-Dependent Signaling Pathway. J. Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 92.Schneider C., Schmidt T., Ziske C., Tiemann K., Lee K.-M., Uhlinsky V., Behrens P., Sauerbruch T., Schmidt-Wolf I.G.H., Mühlradt P.F., et al. Tumour suppression induced by the macrophage activating lipopeptide MALP-2 in an ultrasound guided pancreatic carcinoma mouse model. Gut. 2004;53:355–361. doi: 10.1136/gut.2003.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weigt H., Muhlradt P.F., Emmendorffer A., Krug N., Braun A. Synthetic mycoplasma-derived lipopeptide MALP-2 induces maturation and function of dendritic cells. Immunobiology. 2003;207:223–233. doi: 10.1078/0171-2985-00234. [DOI] [PubMed] [Google Scholar]
- 94.Sawahata R., Shime H., Yamazaki S., Inoue N., Akazawa T., Fujimoto Y., Fukase K., Matsumoto M., Seya T. Failure of mycoplasma lipoprotein MALP-2 to induce NK cell activation through dendritic cell TLR2. Microbes Infect. 2011;13:350–358. doi: 10.1016/j.micinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt J., Welsch T., Jager D., Muhlradt P.F., Buchler M.W., Marten A. Intratumoural injection of the toll-like receptor-2/6 agonist ‘macrophage-activating lipopeptide-2’ in patients with pancreatic carcinoma: A phase I/II trial. Br. J. Cancer. 2007;97:598–604. doi: 10.1038/sj.bjc.6603903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosendahl A.H., Sun C., Wu D., Andersson R. Polysaccharide-K (PSK) increases p21WAF/Cip1 and promotes apoptosis in pancreatic cancer cells. Pancreatology. 2012;12:467–474. doi: 10.1016/j.pan.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Yamasaki A., Onishi H., Imaizumi A., Kawamoto M., Fujimura A., Oyama Y., Katano M. Protein-bound Polysaccharide-K Inhibits Hedgehog Signaling Through Down-regulation of MAML3 and RBPJ Transcription Under Hypoxia, Suppressing the Malignant Phenotype in Pancreatic Cancer. Anticancer Res. 2016;36:3945–3952. [PubMed] [Google Scholar]
- 98.Onishi H., Morisaki T., Nakao F., Odate S., Morisaki T., Katano M. Protein-bound polysaccharide decreases invasiveness and proliferation in pancreatic cancer by inhibition of hedgehog signaling and HIF-1α pathways under hypoxia. Cancer Lett. 2013;335:289–298. doi: 10.1016/j.canlet.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 99.Kim S.A., Lee Y., Jung D.E., Park K.H., Park J.Y., Gang J., Jeon S.B., Park E.C., Kim Y.-G., Lee B., et al. Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci. 2009;100:828–836. doi: 10.1111/j.1349-7006.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi C.H., Kang T.H., Song J.S., Kim Y.S., Chung E.J., Ylaya K., Kim S., Koh S.S., Chung J.-Y., Kim J.-H., et al. Elevated expression of pancreatic adenocarcinoma upregulated factor (PAUF) is associated with poor prognosis and chemoresistance in epithelial ovarian cancer. Sci. Rep. 2018;8:12161. doi: 10.1038/s41598-018-30582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J., Chung J.-Y., Kim T.-J., Lee J.-W., Kim B.-G., Bae D.-S., Choi C.H., Hewitt S.M. Genomic Network-Based Analysis Reveals Pancreatic Adenocarcinoma Up-Regulating Factor-Related Prognostic Markers in Cervical Carcinoma. Front. Oncol. 2018;8:465. doi: 10.3389/fonc.2018.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Escudero-Paniagua B., Bartolome R.A., Rodriguez S., De Los Rios V., Pintado L., Jaen M., Lafarga M., Fernandez-Acenero M.J., Casal J.I. PAUF/ZG16B promotes colorectal cancer progression through alterations of the mitotic functions and the Wnt/beta-catenin pathway. Carcinogenesis. 2020;41:203–213. doi: 10.1093/carcin/bgz093. [DOI] [PubMed] [Google Scholar]
- 103.Park H.D., Lee Y., Oh Y.K., Jung J.G., Park Y.W., Myung K., Kim K.-H., Koh S.S., Lim D.-S. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene. 2010;30:201–211. doi: 10.1038/onc.2010.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang T.H., Kim Y.S., Kim S., Yang B., Lee J.-J., Lee H.-J., Lee J., Jung I.D., Han H.D., Lee S.-H., et al. Pancreatic adenocarcinoma upregulated factor serves as adjuvant by activating dendritic cells through stimulation of TLR4. Oncotarget. 2015;6:27751–27762. doi: 10.18632/oncotarget.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Silva A., Mount A., Krstevska K., Pejoski D., Hardy M.P., Owczarek C., Scotney P., Maraskovsky E., Morelli A.B. The Combination of ISCOMATRIX Adjuvant and TLR Agonists Induces Regression of Established Solid Tumors In Vivo. J. Immunol. 2015;194:2199–2207. doi: 10.4049/jimmunol.1402228. [DOI] [PubMed] [Google Scholar]
- 106.Mehla K., Tremayne J., Grunkemeyer J.A., O’Connell K.A., Steele M.M., Caffrey T.C., Zhu X., Yu F., Singh P.K., Schultes B.C., et al. Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors. Cancer Immunol. Immunother. 2018;67:445–457. doi: 10.1007/s00262-017-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Metzger P., Kirchleitner S.V., Kluge M., Koenig L.M., Horth C., Rambuscheck C.A., Bohmer D., Ahlfeld J., Kobold S., Friedel C.C., et al. Immunostimulatory RNA leads to functional reprogramming of myeloid-derived suppressor cells in pancreatic cancer. J Immunother. Cancer. 2019;7:288. doi: 10.1186/s40425-019-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gonda T.A., Fang J., Salas M., Do C., Hsu E., Zhukovskaya A., Siegel A., Takahashi R., Lopez-Bujanda Z.A., Drake C.G., et al. A DNA Hypomethylating Drug Alters the Tumor Microenvironment and Improves the Effectiveness of Immune Checkpoint Inhibitors in a Mouse Model of Pancreatic Cancer. Cancer Res. 2020;80:4754–4767. doi: 10.1158/0008-5472.CAN-20-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhoopathi P., Quinn B.A., Gui Q., Shen X.-N., Grossman S.R., Das S.K., Sarkar D., Fisher P.B., Emdad L. Pancreatic Cancer–Specific Cell Death Induced In Vivo by Cytoplasmic-Delivered Polyinosine–Polycytidylic Acid. Cancer Res. 2014;74:6224–6235. doi: 10.1158/0008-5472.CAN-14-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Michaelis K.A., Norgard M.A., Zhu X., Levasseur P.R., Sivagnanam S., Liudahl S.M., Burfeind K.G., Olson B., Pelz K.R., Angeles Ramos D.M., et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019;10:4682. doi: 10.1038/s41467-019-12657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie X.M.L., Zhou Y., Shen W., Xu D., Dou J., Shen B., Zhou C. Polysaccharide enhanced NK cell cytotoxicity against pancreatic cancer via TLR4/MAPKs/NF-κB pathway in vitro/vivo. Carbohydr. Polym. 2019;225:115223. doi: 10.1016/j.carbpol.2019.115223. [DOI] [PubMed] [Google Scholar]
- 112.Ma J.X., Sun Y.L., Yu Y., Zhang J., Wu H.Y., Yu X.F. Triptolide enhances the sensitivity of pancreatic cancer PANC-1 cells to gemcitabine by inhibiting TLR4/NF-kappaB signaling. Am. J. Transl. Res. 2019;11:3750–3760. [PMC free article] [PubMed] [Google Scholar]
- 113.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 114.Noack J., Jordi M., Zauner L., Alessi D., Burch A., Tinguely M., Hersberger M., Bernasconi M., Nadal D. TLR9 agonists induced cell death in Burkitt’s lymphoma cells is variable and influenced by TLR9 polymorphism. Cell Death Dis. 2012;3:e323. doi: 10.1038/cddis.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krieg A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 116.Rosa R., Melisi D., Damiano V., Bianco R., Garofalo S., Gelardi T., Agrawal S., Di Nicolantonio F., Scarpa A., Bardelli A., et al. Toll-like Receptor 9 Agonist IMO Cooperates with Cetuximab in K-Ras Mutant Colorectal and Pancreatic Cancers. Clin. Cancer Res. 2011;17:6531–6541. doi: 10.1158/1078-0432.CCR-10-3376. [DOI] [PubMed] [Google Scholar]
- 117.Damiano V., Caputo R., Bianco R., D’Armiento F.P., Leonardi A., De Placido S., Bianco A.R., Agrawal S., Ciardiello F., Tortora G. Novel Toll-Like Receptor 9 Agonist Induces Epidermal Growth Factor Receptor (EGFR) Inhibition and Synergistic Antitumor Activity with EGFR Inhibitors. Clin. Cancer Res. 2006;12:577–583. doi: 10.1158/1078-0432.CCR-05-1943. [DOI] [PubMed] [Google Scholar]
- 118.Carbone C., Piro G., Agostini A., Delfino P., De Sanctis F., Nasca V., Spallotta F., Sette C., Martini M., Ugel S., et al. Intratumoral injection of TLR9 agonist promotes an immunopermissive microenvironment transition and causes cooperative antitumor activity in combination with anti-PD1 in pancreatic cancer. J. Immunother. Cancer. 2021;9:e002876. doi: 10.1136/jitc-2021-002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dowling J.K., Dellacasagrande J. Toll-Like Receptors: Ligands, Cell-Based Models, and Readouts for Receptor Action. Methods Mol. Biol. 2016;1390:3–27. doi: 10.1007/978-1-4939-3335-8_1. [DOI] [PubMed] [Google Scholar]
- 120.Lee Y., Kim S.J., Park H.D., Park E.H., Huang S.M., Jeon S.B., Kim J.-M., Lim D.-S., Koh S.S. PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression. Oncogene. 2010;29:56–67. doi: 10.1038/onc.2009.298. [DOI] [PubMed] [Google Scholar]
- 121.Murata M. Activation of Toll-like receptor 2 by a novel preparation of cell wall skeleton from Mycobacterium bovis BCG Tokyo (SMP-105) sufficiently enhances immune responses against tumors. Cancer Sci. 2008;99:1435–1440. doi: 10.1111/j.1349-7006.2008.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J.-K., Balic J.J., Yu L., Jenkins B. TLR Agonists as Adjuvants for Cancer Vaccines. Adv. Exp. Med. Biol. 2017;1024:195–212. doi: 10.1007/978-981-10-5987-2_9. [DOI] [PubMed] [Google Scholar]
- 123.Schulz O., Diebold S.S., Chen M., Näslund T.I., Nolte M.A., Alexopoulou L., Azuma Y.-T., Flavell R.A., Liljestrom P., Sousa C.R.E. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 124.Kodama S., Nomi N., Suzuki M., Kodama N. Toll-like receptor 3 signaling induces apoptosis in human head and neck cancer via survivin associated pathway. Oncol. Rep. 2010;24:225–231. doi: 10.3892/or_00000850. [DOI] [PubMed] [Google Scholar]
- 125.Saxena M., Sabado R.L., La Mar M., Mohri H., Salazar A.M., Dong H., Da Rosa J.C., Markowitz M., Bhardwaj N., Miller E. Poly-ICLC, a TLR3 Agonist, Induces Transient Innate Immune Responses in Patients With Treated HIV-Infection: A Randomized Double-Blinded Placebo Controlled Trial. Front. Immunol. 2019;10:725. doi: 10.3389/fimmu.2019.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martins K.A.O., Bavari S., Salazar A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev. Vaccines. 2015;14:447–459. doi: 10.1586/14760584.2015.966085. [DOI] [PubMed] [Google Scholar]
- 127.Wang X., Smith C., Yin H. Targeting Toll-like receptors with small molecule agents. Chem. Soc. Rev. 2013;42:4859–4866. doi: 10.1039/c3cs60039d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seya T., Takeda Y., Matsumoto M. A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv. Drug Deliv. Rev. 2019;147:37–43. doi: 10.1016/j.addr.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 129.Fensterheim B.A., Young J.D., Luan L., Kleinbard R.R., Stothers C.L., Patil N.K., McAtee-Pereira A.G., Guo Y., Trenary I., Hernandez A., et al. The TLR4 Agonist Monophosphoryl Lipid A Drives Broad Resistance to Infection via Dynamic Reprogramming of Macrophage Metabolism. J. Immunol. 2018;200:3777–3789. doi: 10.4049/jimmunol.1800085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rebuffini E., Zuccarino L., Grecchi E., Carinci F., Merulla V.E. Picibanil (OK-432) in the treatment of head and neck lymphangiomas in children. Dent. Res. J. 2012;9((Suppl. S2)):S192–S196. doi: 10.4103/1735-3327.109752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Didierlaurent A.M., Morel S., Lockman L., Giannini S.L., Bisteau M., Carlsen H., Kielland A., Vosters O., Vanderheyde N., Schiavetti F., et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 132.Lu H., Betancur A., Chen M., Ter Meulen J.H. Toll-Like Receptor 4 Expression on Lymphoma Cells Is Critical for Therapeutic Activity of Intratumoral Therapy With Synthetic TLR4 Agonist Glucopyranosyl Lipid A. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bai H., Sun F., Yang G., Wang L., Zhang Q., Zhang Q., Zhan Y., Chen J., Yu M., Li C., et al. CBLB502, a Toll-like receptor 5 agonist, offers protection against radiation-induced male reproductive system damage in mice †. Biol. Reprod. 2019;100:281–291. doi: 10.1093/biolre/ioy173. [DOI] [PubMed] [Google Scholar]
- 134.Eremina N.V., Kazey V.I., Mishugin S.V., Leonenkov R.V., Pushkar D.Y., Mett V.L., Gudkov A.V. First-in-human study of anticancer immunotherapy drug candidate mobilan: Safety, pharmacokinetics and pharmacodynamics in prostate cancer patients. Oncotarget. 2020;11:1273–1288. doi: 10.18632/oncotarget.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xia Y., Wang M., DeMaria O., Tang J., Rocchi P., Qu F., Iovanna J.L., Alexopoulou L., Peng L. A Novel Bitriazolyl Acyclonucleoside Endowed with Dual Antiproliferative and Immunomodulatory Activity. J. Med. Chem. 2012;55:5642–5646. doi: 10.1021/jm300534u. [DOI] [PubMed] [Google Scholar]
- 136.Royer C.M., Rudolph K., Dietsch G.N., Hershberg R.M., Barrett E.G. VTX-1463, a novel TLR-8 agonist, attenuates nasal congestion after ragweed challenge in sensitized beagle dogs. Immun. Inflamm. Dis. 2016;4:45–51. doi: 10.1002/iid3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cooper C.L., Davis H.L., Morris M.L., Efler S.M., Adhami M.A., Krieg A.M., Cameron D.W., Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: A double-blind phase I/II study. J. Clin. Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 138.Agrawal S., Kandimalla E.R. Synthetic agonists of Toll-like receptors 7, 8 and 9. Biochem. Soc. Trans. 2007;35:1461–1467. doi: 10.1042/BST0351461. [DOI] [PubMed] [Google Scholar]
- 139.Wittig B., Schmidt M., Scheithauer W., Schmoll H.-J. MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: From bench to bedside. Crit. Rev. Oncol. 2015;94:31–44. doi: 10.1016/j.critrevonc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 140.Kapp K., Kleuss C., Schroff M., Wittig B. Genuine Immunomodulation With dSLIM. Mol. Ther.—Nucleic Acids. 2014;3:e170. doi: 10.1038/mtna.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith M., García-Martínez E., Pitter M.R., Fucikova J., Spisek R., Zitvogel L., Kroemer G., Galluzzi L. Trial Watch: Toll-like receptor agonists in cancer immunotherapy. OncoImmunology. 2018;7:e1526250. doi: 10.1080/2162402X.2018.1526250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qi X.-F., Zheng L., Kim C.-S., Lee K.-J., Kim D.-H., Cai D.-Q., Qin J.-W., Yu Y.-H., Wu Z., Kim S.-K. CpG oligodeoxynucleotide induces apoptosis and cell cycle arrest in A20 lymphoma cells via TLR9-mediated pathways. Mol. Immunol. 2013;54:327–337. doi: 10.1016/j.molimm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 143.Jiang W., Lederman M.M., Salkowitz J.R., Rodriguez B., Harding C.V., Sieg S.F. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. J. Virol. 2005;79:4109–4119. doi: 10.1128/JVI.79.7.4109-4119.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simons M.P., O’Donnell M.A., Griffith T.S. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2008;26:341–345. doi: 10.1016/j.urolonc.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kanzler H., Barrat F.J., Hessel E.M., Coffman R.L. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 146.Rezaei N. Therapeutic targeting of pattern-recognition receptors. Int. Immunopharmacol. 2006;6:863–869. doi: 10.1016/j.intimp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Raja S.G., Dreyfus G.D. Eritoran: The evidence of its therapeutic potential in sepsis. Core Évid. 2008;2:199–207. doi: 10.2147/CE.S7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deguchi A., Tomita T., Ohto U., Takemura K., Kitao A., Akashi-Takamura S., Miyake K., Maru Y. Eritoran inhibits S100A8-mediated TLR4/MD-2 activation and tumor growth by changing the immune microenvironment. Oncogene. 2016;35:1445–1456. doi: 10.1038/onc.2015.211. [DOI] [PubMed] [Google Scholar]
- 149.Kuo W.-T., Lee T.-C., Yu L.C.-H. Eritoran Suppresses Colon Cancer by Altering a Functional Balance in Toll-like Receptors That Bind Lipopolysaccharide. Cancer Res. 2016;76:4684–4695. doi: 10.1158/0008-5472.CAN-16-0172. [DOI] [PubMed] [Google Scholar]
- 150.Ii M., Matsunaga N., Hazeki K., Nakamura K., Takashima K., Seya T., Hazeki O., Kitazaki T., Iizawa Y. A Novel Cyclohexene Derivative, Ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), Selectively Inhibits Toll-Like Receptor 4-Mediated Cytokine Production through Suppression of Intracellular Signaling. Mol. Pharmacol. 2006;69:1288–1295. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 151.Kashani B., Zandi Z., Bashash D., Zaghal A., Momeny M., Poursani E.M., Pourbagheri-Sigaroodi A., Mousavi S.A., Ghaffari S.H. Small molecule inhibitor of TLR4 inhibits ovarian cancer cell proliferation: New insight into the anticancer effect of TAK-242 (Resatorvid) Cancer Chemother. Pharmacol. 2020;85:47–59. doi: 10.1007/s00280-019-03988-y. [DOI] [PubMed] [Google Scholar]
- 152.Zandi Z., Kashani B., Bashash D., Poursani E.M., Mousavi S.A., Chahardoli B., Ghaffari S.H. The anticancer effect of the TLR4 inhibition using TAK-242 (resatorvid) either as a single agent or in combination with chemotherapy: A novel therapeutic potential for breast cancer. J. Cell. Biochem. 2019;121:1623–1634. doi: 10.1002/jcb.29397. [DOI] [PubMed] [Google Scholar]
- 153.Zandi Z., Kashani B., Poursani E.M., Bashash D., Kabuli M., Momeny M., Mousavi-Pak S.H., Sheikhsaran F., Alimoghaddam K., Mousavi S.A., et al. TLR4 blockade using TAK-242 suppresses ovarian and breast cancer cells invasion through the inhibition of extracellular matrix degradation and epithelial-mesenchymal transition. Eur. J. Pharmacol. 2019;853:256–263. doi: 10.1016/j.ejphar.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 154.Kashani B., Zandi Z., Karimzadeh M.R., Bashash D., Nasrollahzadeh A., Ghaffari S.H. Blockade of TLR4 using TAK-242 (resatorvid) enhances anti-cancer effects of chemotherapeutic agents: A novel synergistic approach for breast and ovarian cancers. Immunol. Res. 2019;67:505–516. doi: 10.1007/s12026-019-09113-8. [DOI] [PubMed] [Google Scholar]
- 155.Blohm-Mangone K., Burkett N.B., Tahsin S., Myrdal P.B., Aodah A., Ho B., Janda J., McComas M., Saboda K., Roe D.J., et al. Pharmacological TLR4 Antagonism Using Topical Resatorvid Blocks Solar UV-Induced Skin Tumorigenesis in SKH-1 Mice. Cancer Prev. Res. 2018;11:265–278. doi: 10.1158/1940-6207.CAPR-17-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Awasthi S., Brown K., King C., Awasthi V., Bondugula R. A Toll-Like Receptor-4-Interacting Surfactant Protein-A-Derived Peptide Suppresses Tumor Necrosis Factor-α Release from Mouse JAWS II Dendritic Cells. J. Pharmacol. Exp. Ther. 2011;336:672–681. doi: 10.1124/jpet.110.173765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rakhesh M., Cate M., Vijay R., Shrikant A., Shanjana A. A TLR4-interacting peptide inhibits lipopolysaccharide-stimulated inflammatory responses, migration and invasion of colon cancer SW480 cells. OncoImmunology. 2012;1:1495–1506. doi: 10.4161/onci.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Awasthi S., Singh B., Ramani V., Xie J., Kosanke S. TLR4-interacting SPA4 peptide improves host defense and alleviates tissue injury in a mouse model of Pseudomonas aeruginosa lung infection. PLoS ONE. 2019;14:e0210979. doi: 10.1371/journal.pone.0210979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Awasthi S., Rahman N., Rui B., Kumar G., Awasthi V., Breshears M., Kosanke S. Lung and general health effects of Toll-like receptor-4 (TLR4)-interacting SPA4 peptide. BMC Pulm. Med. 2020;20:179. doi: 10.1186/s12890-020-01187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baker P.J., Hraba T., Taylor C.E., Myers K.R., Takayama K., Qureshi N., Stuetz P., Kusumoto S., Hasegawa A. Structural features that influence the ability of lipid A and its analogs to abolish expression of suppressor T cell activity. Infect. Immun. 1992;60:2694–2701. doi: 10.1128/iai.60.7.2694-2701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stöver A.G., Correia J.D.S., Evans J., Cluff C.W., Elliott M.W., Jeffery E.W., Johnson D.A., Lacy M.J., Baldridge J.R., Probst P., et al. Structure-Activity Relationship of Synthetic Toll-like Receptor 4 Agonists. J. Biol. Chem. 2004;279:4440–4449. doi: 10.1074/jbc.M310760200. [DOI] [PubMed] [Google Scholar]
- 162.Huang H.Y., Zhang Z.J., Cao C.B., Wang N., Liu F.F., Peng J.Q., Ren X.J., Qian J. The TLR4/NF-kappaB signaling pathway mediates the growth of colon cancer. Eur. Rev. Med. Pharmacol. Sci. 2014;18:3834–3843. [PubMed] [Google Scholar]
- 163.Huselton E.C.A., DiPersio J.F., Jacoby M., Pusic I., Romee R., Schroeder M., Uy G.L., Westervelt P. Updated Study Results of CX-01, an Inhibitor of CXCL12/CXCR4, and Azacitidine for the Treatment of Hypomethylating Agent Refractory AML and MDS. Blood. 2019;134:3915. doi: 10.1182/blood-2019-132065. [DOI] [Google Scholar]
- 164.Kovacsovics T.J., Mims A., Salama M.E., Pantin J., Rao N., Kosak K.M., Ahorukomeye P., Glenn M.J., Deininger M.W.N., Boucher K.M., et al. Combination of the low anticoagulant heparin CX-01 with chemotherapy for the treatment of acute myeloid leukemia. Blood Adv. 2018;2:381–389. doi: 10.1182/bloodadvances.2017013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y., Tu Q., Yan W., Xiao D., Zeng Z., Ouyang Y., Huang L., Cai J., Zeng X., Chen Y.J., et al. CXC195 suppresses proliferation and inflammatory response in LPS-induced human hepatocellular carcinoma cells via regulating TLR4-MyD88-TAK1-mediated NF-kappaB and MAPK pathway. Biochem. Biophys. Res. Commun. 2015;456:373–379. doi: 10.1016/j.bbrc.2014.11.090. [DOI] [PubMed] [Google Scholar]
- 166.Chen X.-L., Fu J.-P., Shi J., Wan P., Cao H., Tang Z.-M. CXC195 induces apoptosis and endoplastic reticulum stress in human hepatocellular carcinoma cells by inhibiting the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 2015;12:8229–8236. doi: 10.3892/mmr.2015.4479. [DOI] [PubMed] [Google Scholar]
- 167.Zeng T., Peng L., Chao C., Fu B., Wang G., Wang Y., Zhu X. WITHDRAWN: IRE1α-TRAF2-ASK1 complex-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to CXC195-induced apoptosis in human bladder carcinoma T24 cells. Biochem. Biophys. Res. Commun. 2015;460:530–536. doi: 10.1016/j.bbrc.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 168.Czeslick E., Struppert A., Simm A., Sablotzki A. E5564 (Eritoran) inhibits lipopolysaccharide-induced cytokine production in human blood monocytes. Inflamm. Res. 2006;55:511–515. doi: 10.1007/s00011-006-6057-3. [DOI] [PubMed] [Google Scholar]
- 169.Liu H., Zhang G., Huang J., Ma S., Mi K., Cheng J., Zhu Y., Zha X., Huang W. Atractylenolide I modulates ovarian cancer cell-mediated immunosuppression by blocking MD-2/TLR4 complex-mediated MyD88/NF-kappaB signaling in vitro. J. Transl. Med. 2016;14:104. doi: 10.1186/s12967-016-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Premkumar V., Dey M., Dorn R., Raskin I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem. Biol. 2010;10:3. doi: 10.1186/1472-6769-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou J., Liu Q., Qian R., Liu S., Hu W., Liu Z. Paeonol antagonizes oncogenesis of osteosarcoma by inhibiting the function of TLR4/MAPK/NF-kappaB pathway. Acta Histochem. 2020;122:151455. doi: 10.1016/j.acthis.2019.151455. [DOI] [PubMed] [Google Scholar]
- 172.Monnet E., Lapeyre G., Van Poelgeest E., Jacqmin P., De Graaf K., Reijers J., Moerland M., Burggraaf J., De Min C. Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin. Pharmacol. Ther. 2017;101:200–208. doi: 10.1002/cpt.522. [DOI] [PubMed] [Google Scholar]
- 173.Zhang N.-L., Wang H.-Q., Yang J., Yang P., Kang P., Zhao T. Effects of nalmefene hydrochloride on TLR4 signaling pathway in rats with lung ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2020;24:461–468. doi: 10.26355/eurrev_202001_19947. [DOI] [PubMed] [Google Scholar]
- 174.Li X., Zou Y., Fu Y.Y., Xing J., Wang K.Y., Wan P.Z., Wang M., Zhai X.Y. Ibudilast Attenuates Folic Acid-Induced Acute Kidney Injury by Blocking Pyroptosis Through TLR4-Mediated NF-kappaB and MAPK Signaling Pathways. Front. Pharmacol. 2021;12:650283. doi: 10.3389/fphar.2021.650283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gowayed M.A., El Achy S., Kamel M.A., El-Tahan R.A. Polymyxin B prevents the development of adjuvant arthritis via modulation of TLR/Cox-2 signaling pathway. Life Sci. 2020;259:118250. doi: 10.1016/j.lfs.2020.118250. [DOI] [PubMed] [Google Scholar]
- 176.Arslan F., Houtgraaf J.H., Keogh B., Kazemi K., de Jong R., McCormack W.J., O’Neill L.A., McGuirk P., Timmers L., Smeets M.B., et al. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ. Cardiovasc. Interv. 2012;5:279–287. doi: 10.1161/CIRCINTERVENTIONS.111.967596. [DOI] [PubMed] [Google Scholar]
- 177.Lamphier M., Zheng W., Latz E., Spyvee M., Hansen H., Rose J., Genest M., Yang H., Shaffer C., Zhao Y., et al. Novel Small Molecule Inhibitors of TLR7 and TLR9: Mechanism of Action and Efficacy In Vivo. Mol. Pharmacol. 2014;85:429–440. doi: 10.1124/mol.113.089821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.