Abstract
Syphilis, caused by the spirochete Treponema pallidum subspecies pallidum, continues to be a major public health problem worldwide. Recent increases in the number of syphilis cases, in addition to the lack of an efficient vaccine against T. pallidum for humans, highlights an urgent need for the design and development of an efficacious syphilis vaccine. Here, we assess the vaccine potential of the adhesion protein Tp0136 and the outer membrane protein Tp0663. Rabbits were subcutaneously immunized with recombinant proteins Tp0136, Tp0663, or control PBS. Immunization with Tp0136 or Tp0663 generated a strong humoral immune response with high titers of IgG, as assessed by ELISA. Moreover, animals immunized with Tp0136 or Tp0663 exhibited attenuated lesion development, increased cellular infiltration at the lesion sites, and inhibition of treponemal dissemination to distant organs compared to the unimmunized animals. These findings indicate that Tp0136 and Tp0663 are promising syphilis vaccine candidates. Furthermore, these results provide novel and important information for not only understanding the pathogenic mechanisms of spirochetes, but also the development of spirochete-specific subunit vaccines.
Keywords: Treponema pallidum, Tp0136, Tp0663, vaccine candidate, dissemination
Introduction
Syphilis, caused by the spirochetal bacterium Treponema pallidum subsp. pallidum (T. pallidum), continues to be a globally prevalent disease, with an estimated burden of 36 million cases worldwide (1). In recent years, the rate of infectious syphilis has been sharply rising among men who have sex with other men (2–7). The incidence of congenital syphilis infections is also on the rise, with an estimated 1.36 million pregnant women infected worldwide each year, and approximately 520,000 of these pregnancies result in adverse outcomes (8). Moreover, syphilis also increases the risk of HIV transmission and acquisition (9). Despite the continued sensitivity of T. pallidum to treatment with penicillin, there is an urgent need for the development of an efficacious syphilis vaccine to complement the traditional screening and treatment approaches for the global elimination of syphilis, especially considering the increasing prevalence of syphilis worldwide.
Tp0136, a putative T. pallidum outer surface lipoprotein, has been implicated in treponemal dissemination (10). Tp0136 has been demonstrated to promote the migration of HMEC-1 cells and fibroblasts, contributing to the mechanism of chancre self-healing in syphilis (11, 12). Furthermore, Tp0136 is located on the T. pallidum surface, and as such, facilitates direct interaction with the host environment (10). Since T. pallidum Tp0136 has been shown to mediate attachment to plasma Fibronectin (Fn) and human cellular Fn (13), an abundant and ubiquitous ECM protein important for interactions between cells and the surrounding matrix, both of which are in close proximity to the vascular endothelium, interaction of Tp0136 with these molecules may contribute to facilitating T. pallidum dissemination via the bloodstream. Tp0663, a putative outer membrane protein that has surface-exposed epitopes (14), reacts strongly with serum antibodies from syphilis patients and T. pallidum-infected rabbits (15, 16), implying that it may have the potential to be a candidate vaccine.
To date, the development of a syphilis vaccine has achieved varying degrees of success (17, 18). Complete protection against T. pallidum challenge has been demonstrated only by immunizing rabbits with γ-irradiated whole cell T. pallidum preparations, which is considered an impractical immunization regimen (19). Partial protection, based upon attenuated lesion development, has been achieved in rabbits immunized with Tp0136 (10), TprI (20), TprK (21), Tp92 (BamA) (22), 4D (23), Gpd (24), TprF (25), endoflagella (26), and FlaB3 (27). Recent investigations have demonstrated that the immunization of rabbits with the laminin-binding adhesion protein Tp0751 protected against the dissemination of T. pallidum following intradermal challenge (28). Although these immunization regimens are impractical for use in humans, these studies indicate the importance of T. pallidum surface antigens and adhesins in conferring protection and significantly promote the development of a syphilis vaccine.
Here, we evaluated the immunoprotective capacity of Tp0663 and Tp0136 in a rabbit model of experimental syphilis. We showed that rabbits immunized with Tp0663 or Tp0136 displayed attenuated lesion development and exhibited a reduced treponemal burden in multiple distant organs when compared with the unimmunized rabbits. These findings demonstrated that Tp0663 or Tp0136 immunization inhibits treponemal dissemination to distant organ sites. Our results provide evidence that Tp0663 and Tp0136 may represent promising vaccine candidates for syphilis.
Materials and Methods
T. pallidum Propagation
Propagation of T. pallidum (Nichols strain) was performed as previously described (29). The bacteria were harvested from the treponemal suspensions by low-speed centrifugation to remove rabbit testicular debris.
Recombinant Protein Expression and Purification
The Tp0136 and Tp0663 genes, with an N-terminal hexahistidine tag, were separately cloned into the expression vector pET28a. The purification of recombinant Tp0136 and Tp0663 proteins was performed as previously described (10, 30), and then treated with Detoxi-Gel™ Endotoxin Removing Gel. Limulus amebocyte lysate (Chinese Horseshoe Crab Reagent Manufactory, Ltd., Xiamen, China) was used to detect the endotoxin in the Purified proteins, which were found to be less than 0.03 endotoxin unit (EU)/mL.
Immunization Procedure
A cohort of nine male specific-pathogen free New Zealand White rabbits (2.5 – 3.0 kg, 13 – 15 weeks of age; The Animal Department of University of South China, Hengyang, China) with negative VDRL and FTA-Abs serology were selected for the immunization experiments. Rabbits were randomly divided into three groups (PBS control group, Tp0136-immunized group, and Tp0663-immunized group), with three animals in each group. Each animal was injected three times at two week intervals (0, 2, and 4 weeks) with 150 μg of test antigen or PBS emulsified in a 1:1 mixture with complete Freund’s adjuvant system (complete adjuvant for the first challenge and incomplete for the subsequent challenge; Sigma-Aldrich, St. Louis, MO, USA) at each immunization. The immunizations were divided equally among four subcutaneous and two intramuscular injection sites. The antiserum was collected to estimate the serum antibody titers.
Intradermal Challenge
Twenty-one days after the final immunization, rabbits were sedated and intradermally challenged at eight sites along their shaved backs with 0.8 mL of 1×107 freshly isolated T. pallidum (Nichols strain). Challenge sites were examined daily and photographed every three days to document lesion development. Dark-field microscopic (Nikon Canada, Mississauga, ON, Canada) was used to evaluate the presence of motile spirochetes in the lesion aspirates 21 days post-challenge.
Analysis of a Specific Antibody Response to Recombinant Proteins
To evaluate the specific antibody response, 1 mL of blood was collected from rabbit ear veins in each group (n = 3, 3, 3) at 0, 2, 4 and 6 weeks following the first immunization. The blood was then centrifuged in order to separate the serum. The specific antibody levels were determined by indirect ELISA. Immulon microtiter 96-well plates (Thermo Labsystems, Franklin, MA) were coated at 4°C overnight with 10 μg purified recombinant protein. Excess antigen was washed off with PBS, and 200 μL of blocking buffer was added. After removal of blocking buffer, 100 μL of anti-serum was applied to the wells, starting with a 1/1000 dilution and further diluting the serum two-fold for each row of the plate. After 2 hours of incubation at 37°C, the wells were washed three times with wash buffer. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000) was subsequently added to the wells and incubated at 37°C for 1 hour. The plates were rinsed three times with wash buffer, and 100 μL of TMB peroxidase substrate was added per well and incubated at 37°C for 15 minutes. Then 100 μL of 1 M sulfuric acid was added to stop the color reaction. Each experiment was repeated three times, and the absorbance was measured at a wavelength of 450 nm (BioTek Instruments, Winooski, VT, USA).
Detection of Rabbit Interferon γ
The IFN-γ concentration in the serum collected from the rabbit ear veins after the last immunization were evaluated according to the manufacturer’s instructions by using an IFN-γ ELISA kit (Cusabio Biotech Co., Ltd., Wuhan, China).
Extraction of T. pallidum DNA From Tissues and qPCR
The genomic DNA (gDNA) was extracted from T. pallidum-challenged rabbit tissues, including lesion biopsies at the local infection site, as well as liver, blood, spleen, and testicles, by using a DNeasy Blood and Tissue Kit (Qiagen, Shanghai, China) according to the protocol recommended by the manufacturer. Three tissue samples from each rabbit organ were analyzed for reproducibility. The gDNA was used as the template in the qPCR mixture according to the manufacturer’s standard protocol for QuantiFast SYBR one-step qPCR (Qiagen, Shanghai, China) and performed using a LightCycle 96 apparatus (Roche, Basel, Switzerland). Quantification of T. pallidum gDNA was determined using primers for the endoflagellar sheath protein (flaA) gene of T. pallidum and the collagenase-1 precursor (MMP-1) gene of rabbits ( Table 1 ). A standard curve was created for flaA using a 10-fold serial dilution range from 107 to 101 copies of T. pallidum gDNA with an efficiency of 99.2% and an R2 value of 0.990. A standard curve was created for MMP-1 using a two-fold serial dilution from 100 to 0.0488 ng/mL of rabbit gDNA with an efficiency of 99.1% and an R2 value of 0.995. The original gDNA concentration of rabbit tissue used to create the standard curve was obtained from spectrophotometric measurements. PCR conditions for flaA and MMP-1 were as follows: initial denaturation at 95°C for 7 minutes, followed by 40 cycles of 95°C for 10 seconds, 56°C for 20 seconds, and 72°C for 20 seconds, A melt-curve analysis was performed with the following program: 95°C for 10 seconds, 65°C for 260 seconds, and 97°C for 1 second. Each assay was performed in triplicate. In addition, each assay was run with a no-template control.
Table 1.
Primer sequences used in qPCR.
| Gene | Organism | Sequence (5’ to 3’) | Annealing Temp (°C) |
|---|---|---|---|
| flaA | T. pallidum | F: AACGCAAACGCAATGATAAA | 56 |
| R: CCAGGAGTCGAACAGGAGATAC | |||
| MMP-1 | Rabbit | F: TTGCTTCTTCACACCAGAATGCTGT | 56 |
| R: TTGCTTCTTCACACCAGAATGCTGT |
Histopathology
Tissues taken from skin lesions and testicles of each rabbit were fixed in formalin and embedded in paraffin, The tissues were then stained with H&E to analyze the abundance of immune cells, including lymphocytes, macrophages, and plasma cells, via microscopy.
Statistical Analysis
Results are reported as means ± SD. All comparisons of differences between the test and control groups were assessed via the Students t test using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA). P < 0.05 indicated a significant difference.
Results
Antibody Response of Rabbits to Immunization With Recombinant T. pallidum Proteins
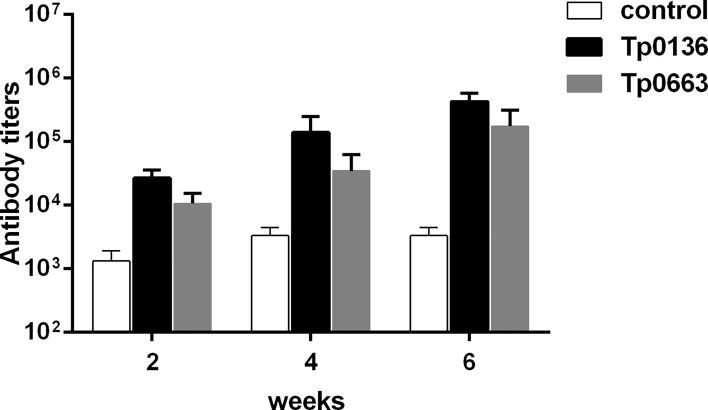
To determine whether T. pallidum membrane proteins Tp0136 and Tp0663 were able to induce specific antibody response in rabbits, we performed serial two-fold dilutions of serum to detect specific antibody titers. As shown in Figure 1 , Tp0136 and Tp0663 induced high titers of anti-Tp0136 and anti-Tp0663 antibodies starting at week 2 compared with PBS-immunized rabbits. Moreover, antibody levels after the first and second immunizations were higher in animals immunized with Tp0136 than in animals immunized with Tp0663. These results indicate that T. pallidum membrane proteins Tp0136 and Tp0663 are able to induce specific antibodies.
Figure 1.
Antibody titers in rabbits immunized with recombinant Tp0136 or Tp0663 protein. New Zealand rabbits were immunized with 150 μg of recombinant protein Tp0136 or Tp0663 emulsified in complete Freund’s adjuvant system. The control rabbits were immunized with PBS emulsified in complete Freund’s adjuvant system. Each animal was injected three times at two week intervals (0, 2, and 4 weeks). Blood was drawn from the rabbit ear veins at 2, 4, and 6 week, and the IgG antibody levels were determined by indirect ELISA. Each set of data is based on measurements derived from three rabbits. To determine antibody concentrations (titers), two-fold serial dilutions of serum were made and the endpoint titer was considered to be the last serum dilution with readings higher than the mean + 3SD of the negative controls.
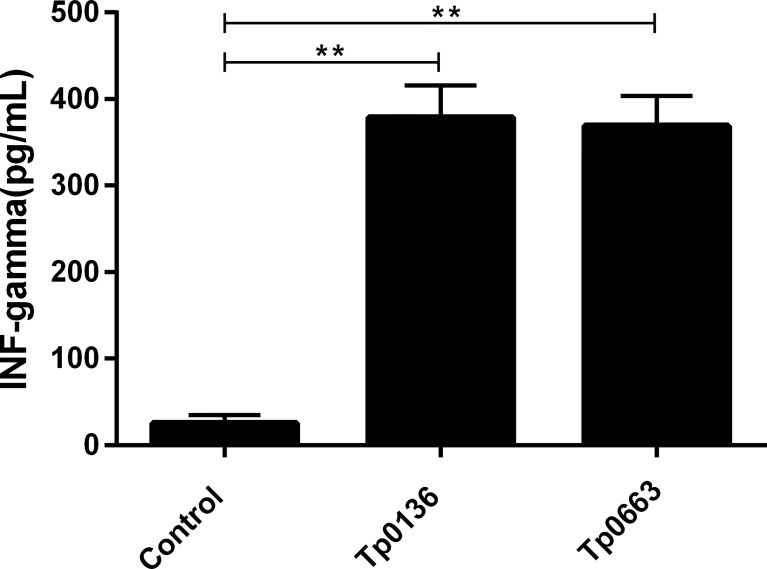
Tp0136 or Tp0663 Immunization Induced IFN-γ Secretion
Since Th1-related cytokines are critical to the efficient clearance of T. pallidum from lesion sites (31), it is important to assess the immunoprotective effect of a potential syphilis vaccine. In this study, serum was collected from Tp0136- or Tp0663-immunized rabbits following the final immunization. The levels of IFN-γ were then measured by ELISA ( Figure 2 ). Compared to that of rabbits immunized with PBS alone, the production of IFN-γ was significantly increased in the rabbits immunized with Tp0136 or Tp0663.
Figure 2.
Production of IFN-γ in rabbits immunized with recombinant Tp0136 or Tp0663 protein. Following the final immunization, blood was collected from each rabbits’ ear vein. The IFN-γ level in the blood was evaluated by ELISA according to the manufacturer’s instructions. The results are expressed as the mean ± SD from three individual rabbits in each group. Each experiment was performed in three independent experiments (**P < 0.01).
Tp0136 or Tp0663 Immunization Attenuated Lesion Development
Three weeks after the final immunization, all of the rabbits were sedated and intradermally challenged at eight sites along their shaved back with 0.8 × 107 of freshly isolated T. pallidum (total of 0.8× 107 T. pallidum/rabbit). During the subsequent 21 days, the appearance of lesions at the site of challenge were monitored, since chancre are unique to syphilis and represent the first clinical sign of infection (32). Lesions were monitored every three days for diameter and ulceration. Obvious lesions were present on all of the rabbits by day 11; however, Tp0136- or Tp0663-immunized animals presented delayed lesion development and smaller-size swellings compared to the control group ( Figure 3A ). Moreover, 21 lesion indurations (total of 24 sites) presented on control animals on day 4-6, and 24 lesion indurations (total of 24 sites) presented on Tp0136-immunized animals by day 8. However, only 16 lesion indurations (total of 24 sites) presented on Tp0663-immunized animals by day 8 ( Table 2 ). The lesion diameter peak observed in control animals and Tp0136 immunized animals appeared at day 18, while in the Tp0663-immunized group the lesion diameter peak appeared at day 15 ( Figure 3A and Table 2 ). The lesion diameter measured in the control group was larger than that in the vaccine groups, especially in the Tp0663-immunized group over the 21-day measurement period ( Figure 3A and Table 2 ). Ulceration in the control group was apparent, and 85.7% of the lesions were ulcerated on day 21 ( Figure 3B and Table 2 ). However, animals immunized with Tp0136 or Tp0663 presented only three ulcerations or no ulcerations, respectively, as compared to the control animals on day 21 ( Figure 3B and Table 2 ). At day 21 post-challenge, dark-field microscopy was performed to evaluate the presence of viable T. pallidum in every lesion. As shown in Table 2 , the ratio of lesions positive for motile T. pallidum in the control group was significantly higher than those in the two immunized groups.
Figure 3.
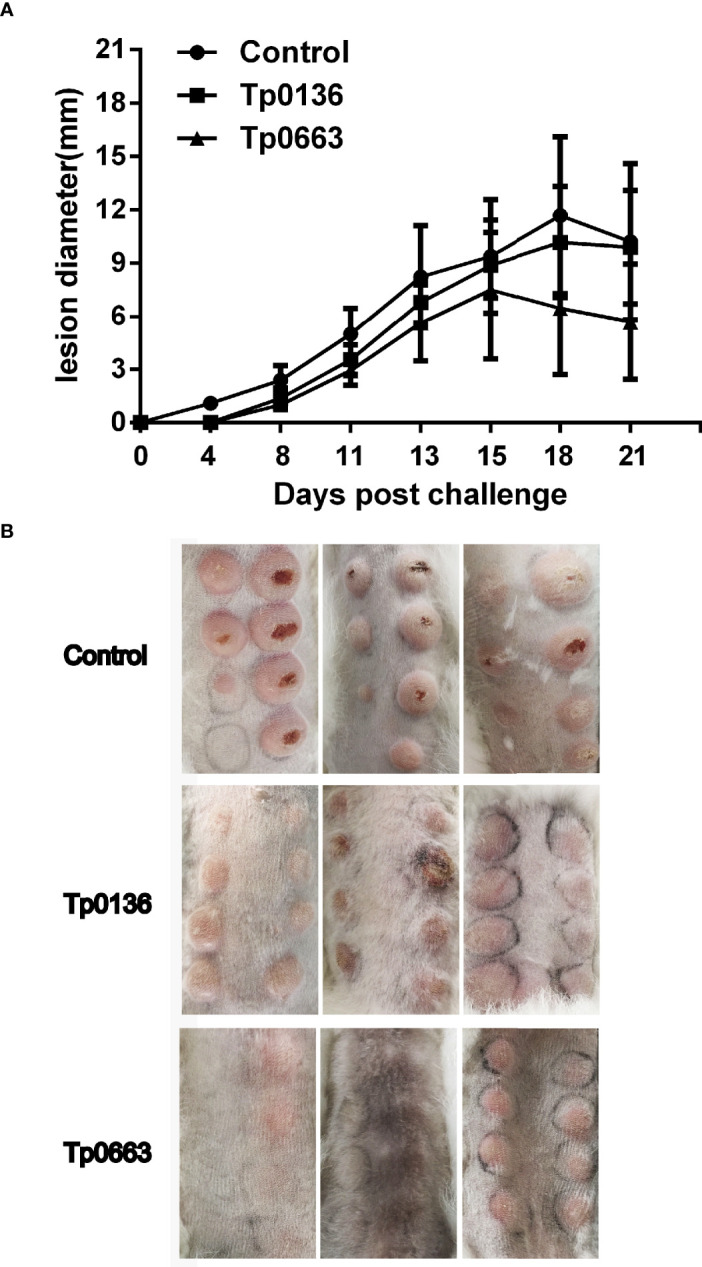
Immunization with recombinant Tp0136 or Tp0663 protein attenuated lesion development. (A) Lesion diameters were monitored over 21 days in the control, Tp0136-immunized or Tp0663-immunized animals following T. pallidum challenge. (B) Indurated lesions from eight locations on the backs of the rabbits were taken 21 days post-intradermal challenge with T. pallidum (Nichols strain).
Table 2.
Lesion data for control and immunized animals.
| Immunogen | Number of lesions/number of sites (%) a | Number of DF-positive lesions/total lesions (%) b | Number of Ulcerative lesions/total lesions (%) c | Lesion status | |
|---|---|---|---|---|---|
| Median day d | Diameter (mm) e | ||||
| Tp0136 | 24/24 (100) | 8/24 (33.3) | 3/24 (12.5) | 11 (4-18) | 10.17 ± 3.12 |
| Tp0663 | 16/24 (66.7) | 4/16 (25) | 0/16 (0) | 11.5 (8-15) | 7.5 ± 3.92 |
| Control | 21/24 (87.5) | 17/21 (81) | 18/21 (85.7) | 13 (8-18) | 11.67 ± 4.42 |
a The denominator indicates the total number of challenge sites.
b The denominator indicates the total number of lesions examined by dark-field. microscopy. DF, dark-field.
c The denominator indicates the total number of lesions in the treatment group.
d The median day of lesion development with the range in parentheses.
e The maximal lesion size of lesions (Mean ± SD) in the treatment group.
Tp0136 or Tp0663 Immunizations Inhibited T. pallidum Dissemination
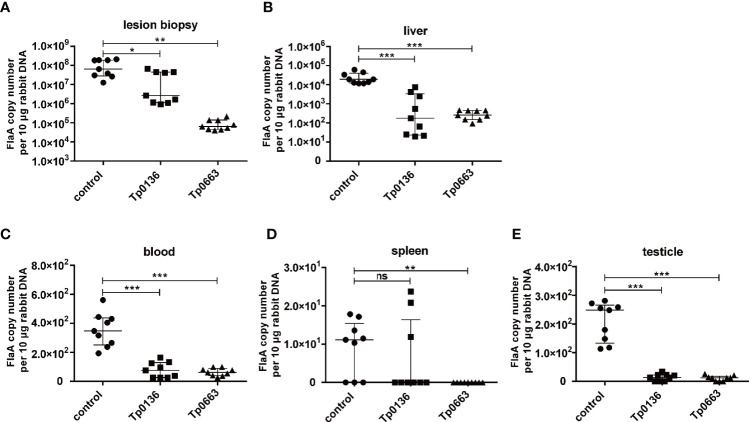
To determine whether Tp0136 or Tp0663 immunization protects against T. pallidum dissemination, all of the rabbits were killed at day 21 post-challenge. We then used real-time quantitative PCR (qPCR) to assess the burden of T. pallidum in biopsies from both primary and distal lesion sites, including blood, spleen, liver, and testicles. Analysis of the treponemal burden of the primary lesion sites revealed that T. pallidum DNA concentration in the three control animals was slightly increased compared with the Tp0136-immunized animals, but was substantially higher than Tp0663-immunized animals ( Figure 4A ). Moreover, we tested the treponemal burden in the blood of the rabbits, since T. pallidum is spread through blood. As shown in Figure 4C , we found that the treponemal burden in the blood was lower in the immunized animals than in the non-immunized controls. Owing to the propensity for T. pallidum to disseminate to distant organ sites, such as the spleen, liver, and testicles (33), we also analyzed the levels of T. pallidum in distant organ sites. Our results showed the capacity of T. pallidum to disseminate from primary lesion sites to distant organ sites ( Figures 4B, D, E ), with the liver and testicle extracts exhibiting a similar trend towards lower treponemal burden in all of the rabbits immunized with recombinant proteins. The most striking decrease was observed for the treponemal burden in the testicles of the vaccinated animals relative to the control animals ( Figure 4E ). Importantly, minimal treponemal DNA in spleen was detected in the Tp0663-immunized animals. Taken together, these results indicate that immunization with Tp0136 or Tp0663 inhibits T. pallidum dissemination to distant organ sites.
Figure 4.
Immunization with Tp0136 or Tp0663 inhibited T. pallidum dissemination. Spirochete numbers were evaluated in control, Tp0136-immunized, or Tp0663-immunized animals by using qPCR to measure flaA DNA concentrations in lesion biopsies. The bacterial burdens in lesion biopsies are shown (A) at the local infection site, as well as in the disseminated infection sites of the (B) liver, (C) blood, (D) spleen, and (E) testicles at day 21 post-infection. Results were normalized within each tissue type based on the concentration of rabbit genomic DNA and presented as median ± interquartile range. Significance was assessed using Students t test (ns, not significant; P > 0.05,*P < 0.05, **P < 0.01, ***P < 0.001). Points correspond to three separately extracted tissue samples from each rabbit organ (nine total points in each group) were analyzed for reproducibility.
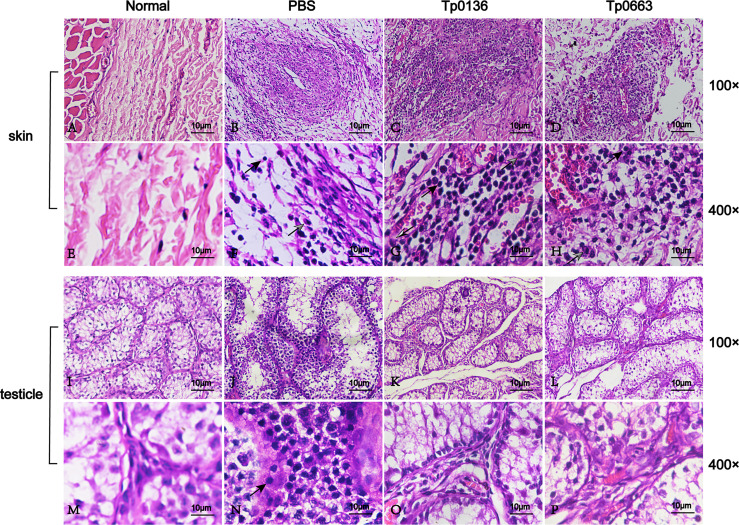
Immunization With Tp0136 or Tp0663 Promoted Inflammatory Infiltration
Since T. pallidum is thought to be cleared by macrophages via antibody-mediated opsonophagocytosis (34–36), hematoxylin and eosin (H&E) staining of biopsy samples obtained from incipient lesion sites of all of the animals was used to analyze the cellular infiltration, which was used to associate the decrease in the pathogen burden within primary lesion sites of vaccinated rabbits to immune cells ( Figures 5A – H ). Tp0136- or Tp0663-immunized rabbits had increased levels of immune cell infiltrate, including lymphocytes, macrophages, and plasma cells, relative to PBS-immunized rabbits ( Figures 5A – H ). Furthermore, analysis of the H&E-stained biopsy samples obtained from distant testicles revealed that inflammatory infiltrations were notably reduced in vaccinated animals compared to PBS-immunized animals ( Figures 5I – P ).
Figure 5.
Histopathological changes of skin and testicle tissue after T. pallidum infection in rabbits. Skin and testicle tissues from normal tissue control (A, E, I, M), PBS- (B, F, J, N), Tp0136- (C, G, K, O), or Tp0663-immunized rabbits (D, H, L, P, respectively) were sectioned and stained with H&E 21 days after infection with T. pallidum. Rabbits immunized with PBS and uninfected rabbits were used as controls. Inflammatory infiltrates in the skin and testicle tissue of rabbits were localized and predominantly lymphohistiocytic (black arrow = lymphocyte; black and white arrow = macrophage; gray arrow = neutrophil).
Discussion
Although development of T. pallidum vaccines has achieved varying degrees of success (17) and long-term in vitro culture of T. pallidum has progressed greatly (37), no effective syphilis vaccine is currently available. One reason is that the molecular mechanisms underlying T. pallidum pathogenesis are poorly understood. Current research regarding the development of a syphilis vaccine focuses primarily on outer membrane proteins and adhesins. In this study, we investigated if immunization with the putative outer membrane protein Tp0663 and adhesion protein Tp0136 were able to induce a sufficiently strong and specific immune response in rabbits, thereby offering protection against T. pallidum infection. The results provide clear evidence that compared to unimmunized rabbits, rabbits immunized with Tp0136 or Tp0663 presented increased specific antibody titers, attenuated lesion development, increased cellular infiltration at the lesion sites, and inhibition of treponemal dissemination to distant organs.
During syphilis infection, primary lesions in human or in rabbits contain mostly macrophages, CD4+ T cells, CD8+ T cells, and natural killer (NK) cells (38, 39). A strong delayed-type hypersensitivity reaction, mediated by local type 1 (Th1) cytokines produced predominantly by CD4+ T cells, is critical to the efficient clearance of T. pallidum from primary lesions (40). The subsequent Th1 cytokine-mediated activation of resident macrophages promotes opsonophagocytosis of treponemes (31, 39, 41, 42), which is thought to be the major mechanism of clearance. The histological analysis of primary lesion sites in our study revealed an increase in the cellular infiltration in Tp0663-immunized and Tp0136-immunized animals at day 21 post-challenge. Moreover, our results showed that Tp0663 and Tp0136 achieved high levels of serum antibodies against each protein after immunization. These antibodies possibly facilitated the retention of T. pallidum at the primary lesion sites by two potential mechanisms. One mechanism could be that the antibodies inhibited treponemal dissemination via blocking Tp0136-mediated invasion, which would be consistent with a previous study that showed Tp0136-specific serum inhibited the attachment of T. pallidum to both plasma and cellular Fn (13). The other mechanism could be that the antibodies opsonized T. pallidum, which promoted its clearance through opsonophagocytosis since Tp0663 and Tp0136 are both surface-exposed outer membrane proteins (10, 14). It is likely that both of these mechanisms may contribute to the function of Tp0663 and Tp0136 in protecting against T. pallidum dissemination. However, the specific mechanism remains to be further studied. Furthermore, the Tp0663-immunized or Tp0136-immunized animals showed a higher level of Th1 cytokine IFN-γ when compared to control animals, which was in accordance with the fact that the IFN-γ-dominated Th1 response facilitates the elimination of T. pallidum in the early infection stage (31).
Tp0136 was previously hypothesized to be a syphilis vaccine candidate (10). Since Tp0136 reacts strongly with serum antibodies from T. pallidum-infected rabbits and syphilis patients and can bind to host Fn and laminin (13), we preceded with rabbit vaccination experiments. Our findings were in agreement with the previous phenomenon that a significant delay in ulceration was observed in Tp0136-immunized rabbits (10). In addition, we further found that the recombinant Tp0136 protein was able to protect against T. pallidum dissemination from the primary site of infection to distant organs, indicating that Tp0136 may be a promising syphilis vaccine candidate. Owing to the fact that Tp0136 gene is extremely variable, it is possible that further work with the regions that are completely conserved among strains may generate better immune protection. Moreover, it is a good strategy to formulate a multi-component vaccine consisting of Tp0136 and other protective antigens. Although the surface-exposed outer membrane proteins are thought to be the most promising syphilis vaccine candidates at present, the identification of outer membrane proteins are elusive (22, 43). Brinkman et al. first showed that Tp0136 was surface localized (10), but then Cox et al. found that Tp0136 was periplasmic (44). Thus, it is necessary to determine the position of Tp0136 in T. pallidum, which may contribute to clarifying the protective mechanism of the recombinant Tp0136 protein against T. pallidum.
Although Tp0663 has not been well studied, some researchers have suggested that it has surface-exposed epitopes and could be reactive with sera from syphilis patients and New Zealand rabbits infected with the T. pallidum (14–16), suggesting that Tp0663 is readily expressed by T. pallidum. Although several individual antigens have previously been selected for immunization and tested for their protective capacity in rabbit models, only a few outer membrane proteins were proven to prevent chancre development. In this study, rabbits immunized with Tp0663 showed significantly delayed chancre development. In addition, unlike other outer membrane proteins, the chancre presented in Tp0663-immunized rabbits healed quickly, even compared to Tp0136-immunized rabbits and unimmunized rabbits. Similarly, qPCR and dark-field microscopy analysis suggested that the treponemal burden at the primary lesion sites of Tp0663-immunized rabbits were far less than that found in the primary lesion sites of Tp0136-immunized and unimmunized rabbits. This is probably because the anti-Tp0663 antibodies may have aided the macrophages in removing the majority of T. pallidum. However, the concrete mechanism needs further study.
Although this study exhibited promising results regarding the efficacy of Tp0136 and Tp0663 as syphilis vaccine candidates, it should not be ignored that the statistical power was limited because of the very small sample size of 3 rabbits per group as well as the fact that the rabbits were outbred. However, this pilot study can provide parameters for future investigations. Another limitation of this study was the use of an extremely high challenge dose. Challenge with a lower dose would likely have revealed more significant differences in lesion development. In addition, non-living vaccine antigens, such as single recombinant antigens, are often poorly immunogenic and require an adjuvant to help stimulate protective immunity based on antibodies and effector T cell functions. Although complete Freund’s adjuvant is unacceptable for human use due to undesirable side effects attributed to its use (45), Freund’s adjuvant is effective at activating innate immunity and developing antigen-specific T and B lymphocytes (46). Moreover, complete Freund’s adjuvant is also effective in stimulating a cellular immune response and may potentiate the production of IgG and IgA (47). Complete Freund’s adjuvant was used to enhance the immune response and preliminarily assess the vaccine potential of Tp0136 and Tp0663 in this study. However, clinically used and tested adjuvants should be used in further experiments.
In summary, the results presented in our study indicate that the T. pallidum adhesion protein Tp0136 and the outer membrane protein Tp0663 are promising vaccine candidates. Immunization with these proteins significantly attenuated lesion development and reduced treponemal dissemination within the host, resulting in protection similar to that with Tp0751 (28). Preventing the dissemination of T. pallidum is a vital requirement for a syphilis vaccine, as it would prevent the development of all stages of syphilis and the transmission of congenital syphilis. These results provide novel and important information for the understanding T. pallidum pathogenesis. Furthermore, these studies also provide insights into the development of spirochetal subunit vaccines, which may offer a timely solution to confront public health-targeted syphilis elimination.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
All of the animal studies were approved by the Ethics Committee of the University of South China.
Author Contributions
The experiment was designed by YX, MX, and YW, and completed by MX, HL, and KZ. The statistics of the experimental data were done by MT, FZ, and TZ together. All authors contributed to this article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 82002182, 81702046, and 81471576), Natural Science Foundation of Hunan Province (grant numbers 2021JJ40479 and 2019JJ50535), Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study (grant number 2015-351), and Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control Foundation (grant number 2014-5).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to extend our sincere thanks to Jian Yu in Department of Experimental Zoology, Hengyang Medical School of University of South China, Hunan, China, who has provided animals to us.
References
- 1. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PloS One (2015) 10:e0143304. doi: 10.1371/journal.pone.0143304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Voux A, Kidd S, Grey JA, Rosenberg ES, Gift TL, Weinstock H, et al. State-Specific Rates of Primary and Secondary Syphilis Among Men Who Have Sex With Men - United States, 2015. MMWR Morb Mortal Wkly Rep (2017) 66:349–54. doi: 10.15585/mmwr.mm6613a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jansen K, Schmidt AJ, Drewes J, Bremer V, Marcus U. Increased Incidence of Syphilis in Men Who Have Sex With Men and Risk Management Strategies, Germany, 2015. Euro Surveill (2016) 21:30382. doi: 10.2807/1560-7917.ES.2016.21.43.30382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Botham SJ, Ressler KA, Maywood P, Hope KG, Bourne CP, Conaty SJ, et al. Men Who Have Sex With Men, Infectious Syphilis and HIV Coinfection in Inner Sydney: Results of Enhanced Surveillance. Sex Health (2013) 10:291–8. doi: 10.1071/SH12142 [DOI] [PubMed] [Google Scholar]
- 5. Burchell AN, Allen VG, Gardner SL, Moravan V, Tan DH, Grewal R, et al. High Incidence of Diagnosis With Syphilis Co-Infection Among Men Who Have Sex With Men in an HIV Cohort in Ontario, Canada. BMC Infect Dis (2015) 15:356. doi: 10.1186/s12879-015-1098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen J, Gibin M, Sile B, Simms I. Identifying and Interpreting Spatiotemporal Variation in Diagnoses of Infectious Syphilis Among Men, England: 2009 to 2013. Sex Transm Infect (2016) 92:380–6. doi: 10.1136/sextrans-2015-052306 [DOI] [PubMed] [Google Scholar]
- 7. Chen G, Cao Y, Yao Y, Li M, Tang W, Li J, et al. Syphilis Incidence Among Men Who Have Sex With Men in China: Results From a Meta-Analysis. Int J STD AIDS (2017) 28:170–8. doi: 10.1177/0956462416638224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newman L, Kamb M, Hawkes S, Gomez G, Say L, Seuc A, et al. Global Estimates of Syphilis in Pregnancy and Associated Adverse Outcomes: Analysis of Multinational Antenatal Surveillance Data. PloS Med (2013) 10:e1001396. doi: 10.1371/journal.pmed.1001396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidd S, Torrone E, Su J, Weinstock H. Reported Primary and Secondary Syphilis Cases in the United States: Implications for HIV Infection. Sex Transm Dis (2018) 45:S42–7. doi: 10.1097/OLQ.0000000000000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brinkman MB, Mcgill MA, Pettersson J, Rogers A, Matejkova P, Smajs D, et al. A Novel Treponema Pallidum Antigen, TP0136, Is an Outer Membrane Protein That Binds Human Fibronectin. Infect Immun (2008) 76:1848–57. doi: 10.1128/IAI.01424-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X, Gao ZX, Lin SW, Tong ML, Liu LL, Lin LR, et al. Recombinant Treponema Pallidum Protein Tp0136 Promotes Fibroblast Migration by Modulating MCP-1/CCR2 Through TLR4. J Eur Acad Dermatol Venereol (2020) 34:862–72. doi: 10.1111/jdv.16162 [DOI] [PubMed] [Google Scholar]
- 12. Luo X, Lin SW, Xu QY, Ke WJ, Gao ZX, Tong ML, et al. Tp0136 Targets Fibronectin (RGD)/Integrin Beta1 Interactions Promoting Human Microvascular Endothelial Cell Migration. Exp Cell Res (2020) 396:112289. doi: 10.1016/j.yexcr.2020.112289 [DOI] [PubMed] [Google Scholar]
- 13. Ke W, Molini BJ, Lukehart SA, Giacani L. Treponema Pallidum Subsp. Pallidum TP0136 Protein Is Heterogeneous Among Isolates and Binds Cellular and Plasma Fibronectin via Its NH2-Terminal End. PloS Negl Trop Dis (2015) 9:e0003662. doi: 10.1371/journal.pntd.0003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Champion CI, Blanco DR, Exner MM, Erdjument-Bromage H, Hancock RE, Tempst P, et al. Sequence Analysis and Recombinant Expression of a 28-Kilodalton Treponema Pallidum Subsp. Pallidum Rare Outer Membrane Protein (Tromp2). J Bacteriol (1997) 179:1230–8. doi: 10.1128/jb.179.4.1230-1238.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mckevitt M, Brinkman MB, Mcloughlin M, Perez C, Howell JK, Weinstock GM, et al. Genome Scale Identification of Treponema Pallidum Antigens. Infect Immun (2005) 73:4445–50. doi: 10.1128/IAI.73.7.4445-4450.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brinkman MB, Mckevitt M, Mcloughlin M, Perez C, Howell J, Weinstock GM, et al. Reactivity of Antibodies From Syphilis Patients to a Protein Array Representing the Treponema Pallidum Proteome. J Clin Microbiol (2006) 44:888–91. doi: 10.1128/JCM.44.3.888-891.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lithgow KV, Cameron CE. Vaccine Development for Syphilis. Expert Rev Vaccines (2017) 16:37–44. doi: 10.1080/14760584.2016.1203262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cullen PA, Cameron CE. Progress Towards an Effective Syphilis Vaccine: The Past, Present and Future. Expert Rev Vaccines (2006) 5:67–80. doi: 10.1586/14760584.5.1.67 [DOI] [PubMed] [Google Scholar]
- 19. Miller JN. Immunity in Experimental Syphilis. VI. Successful Vaccination of Rabbits With Treponema Pallidum, Nichols Strain, Attenuated by -Irradiation. J Immunol (1973) 110:1206–15. [PubMed] [Google Scholar]
- 20. Giacani L, Sambri V, Marangoni A, Cavrini F, Storni E, Donati M, et al. Immunological Evaluation and Cellular Location Analysis of the Tpri Antigen of Treponema Pallidum Subsp. Pallidum. Infect Immun (2005) 73:3817–22. doi: 10.1128/IAI.73.6.3817-3822.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgan CA, Lukehart SA, Van Voorhis WC. Immunization With the N-Terminal Portion of Treponema Pallidum Repeat Protein K Attenuates Syphilitic Lesion Development in the Rabbit Model. Infect Immun (2002) 70:6811–6. doi: 10.1128/IAI.70.12.6811-6816.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic Potential, Protective Capacity, and Sequence Conservation of the Treponema Pallidum Subspecies Pallidum Tp92. J Infect Dis (2000) 181:1401–13. doi: 10.1086/315399 [DOI] [PubMed] [Google Scholar]
- 23. Borenstein LA, Radolf JD, Fehniger TE, Blanco DR, Miller JN, Lovett MA. Immunization of Rabbits With Recombinant Treponema Pallidum Surface Antigen 4D Alters the Course of Experimental Syphilis. J Immunol (1988) 140:2415–21. [PubMed] [Google Scholar]
- 24. Cameron CE, Castro C, Lukehart SA, Van Voorhis WC. Function and Protective Capacity of Treponema Pallidum Subsp. Pallidum Glycerophosphodiester Phosphodiesterase. Infect Immun (1998) 66:5763–70. doi: 10.1128/IAI.66.12.5763-5770.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun ES, Molini BJ, Barrett LK, Centurion-Lara A, Lukehart SA, Van Voorhis WC. Subfamily I Treponema Pallidum Repeat Protein Family: Sequence Variation and Immunity. Microbes Infect (2004) 6:725–37. doi: 10.1016/j.micinf.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 26. Champion CI, Miller JN, Borenstein LA, Lovett MA, Blanco DR. Immunization With Treponema Pallidum Endoflagella Alters the Course of Experimental Rabbit Syphilis. Infect Immun (1990) 58:3158–61. doi: 10.1128/iai.58.9.3158-3161.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng K, Xu M, Xiao Y, Luo H, Xie Y, Yu J, et al. Immunogenicity and Protective Efficacy Against Treponema Pallidum in New Zealand Rabbits Immunized With Plasmid DNA Encoding Flagellin. Emerg Microbes Infect (2018) 7:177. doi: 10.1038/s41426-018-0176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lithgow KV, Hof R, Wetherell C, Phillips D, Houston S, Cameron CE. A Defined Syphilis Vaccine Candidate Inhibits Dissemination of Treponema Pallidum Subspecies Pallidum. Nat Commun (2017) 8:14273. doi: 10.1038/ncomms14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukehart SA, Marra CM. Isolation and Laboratory Maintenance of Treponema Pallidum. Curr Protoc Microbiol (2007)Chapter 12:Unit 12A 11. doi: 10.1002/9780471729259.mc12a01s7 [DOI] [PubMed] [Google Scholar]
- 30. Xu M, Xie Y, Jiang C, Xiao Y, Kuang X, Zhao F, et al. A Novel ELISA Using a Recombinant Outer Membrane Protein, Rtp0663, as the Antigen for Serological Diagnosis of Syphilis. Int J Infect Dis (2016) 43:51–7. doi: 10.1016/j.ijid.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 31. Leader BT, Godornes C, Vanvoorhis WC, Lukehart SA. CD4+ Lymphocytes and Gamma Interferon Predominate in Local Immune Responses in Early Experimental Syphilis. Infect Immun (2007) 75:3021–6. doi: 10.1128/IAI.01973-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lafond RE, Lukehart SA. Biological Basis for Syphilis. Clin Microbiol Rev (2006) 19:29–49. doi: 10.1128/CMR.19.1.29-49.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salazar JC, Rathi A, Michael NL, Radolf JD, Jagodzinski LL. Assessment of the Kinetics of Treponema Pallidum Dissemination Into Blood and Tissues in Experimental Syphilis by Real-Time Quantitative PCR. Infect Immun (2007) 75:2954–8. doi: 10.1128/IAI.00090-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker-Zander SA, Lukehart SA. Macrophage-Mediated Killing of Opsonized Treponema Pallidum. J Infect Dis (1992) 165:69–74. doi: 10.1093/infdis/165.1.69 [DOI] [PubMed] [Google Scholar]
- 35. Baker-Zander SA, Shaffer JM, Lukehart SA. Characterization of the Serum Requirement for Macrophage-Mediated Killing of Treponema Pallidum Ssp. Pallidum: Relationship to the Development of Opsonizing Antibodies. FEMS Immunol Med Microbiol (1993) 6:273–9. doi: 10.1111/j.1574-695X.1993.tb00339.x [DOI] [PubMed] [Google Scholar]
- 36. Shaffer JM, Baker-Zander SA, Lukehart SA. Opsonization of Treponema Pallidum Is Mediated by Immunoglobulin G Antibodies Induced Only by Pathogenic Treponemes. Infect Immun (1993) 61:781–4. doi: 10.1128/iai.61.2.781-784.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edmondson DG, Hu B, Norris SJ. Long-Term In Vitro Culture of the Syphilis Spirochete Treponema Pallidum Subsp. Pallidum. MBio (2018) 9:e01153-18. doi: 10.1128/mBio.01153-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Voorhis WC, Barrett LK, Nasio JM, Plummer FA, Lukehart SA. Lesions of Primary and Secondary Syphilis Contain Activated Cytolytic T Cells. Infect Immun (1996) 64:1048–50. doi: 10.1128/iai.64.3.1048-1050.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cruz AR, Ramirez LG, Zuluaga AV, Pillay A, Abreu C, Valencia CA, et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PloS Negl Trop Dis (2012) 6:e1717. doi: 10.1371/journal.pntd.0001717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carlson JA, Dabiri G, Cribier B, Sell S. The Immunopathobiology of Syphilis: The Manifestations and Course of Syphilis Are Determined by the Level of Delayed-Type Hypersensitivity. Am J Dermatopathol (2011) 33:433–60. doi: 10.1097/DAD.0b013e3181e8b587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore MW, Cruz AR, Lavake CJ, Marzo AL, Eggers CH, Salazar JC, et al. Phagocytosis of Borrelia Burgdorferi and Treponema Pallidum Potentiates Innate Immune Activation and Induces Gamma Interferon Production. Infect Immun (2007) 75:2046–62. doi: 10.1128/IAI.01666-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hawley KL, Cruz AR, Benjamin SJ, La Vake CJ, Cervantes JL, Ledoyt M, et al. Ifngamma Enhances CD64-Potentiated Phagocytosis of Treponema Pallidum Opsonized With Human Syphilitic Serum by Human Macrophages. Front Immunol (2017) 8:1227. doi: 10.3389/fimmu.2017.01227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radolf JD. Treponema Pallidum and the Quest for Outer Membrane Proteins. Mol Microbiol (1995) 16:1067–73. doi: 10.1111/j.1365-2958.1995.tb02332.x [DOI] [PubMed] [Google Scholar]
- 44. Cox DL, Luthra A, Dunham-Ems S, Desrosiers DC, Salazar JC, Caimano MJ, et al. Surface Immunolabeling and Consensus Computational Framework to Identify Candidate Rare Outer Membrane Proteins of Treponema Pallidum. Infect Immun (2010) 78:5178–94. doi: 10.1128/IAI.00834-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sivakumar SM, Safhi MM, Kannadasan M, Sukumaran N. Vaccine Adjuvants - Current Status and Prospects on Controlled Release Adjuvancity. Saudi Pharm J (2011) 19:197–206. doi: 10.1016/j.jsps.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity (2010) 33:492–503. doi: 10.1016/j.immuni.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Billiau A, Matthys P. Modes of Action of Freund’s Adjuvants in Experimental Models of Autoimmune Diseases. J Leukoc Biol (2001) 70:849–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.