Abstract
Pseudomonas aeruginosa, an important opportunistic pathogen, is capable of producing various virulence factors and forming biofilm that are regulated by quorum sensing (QS). It is known that targeting virulence factor production and biofilm formation instead of exerting selective pressure on growth such as conventional antibiotics can reduce multidrug resistance in bacteria. Therefore, many quorum-sensing inhibitors (QSIs) have been developed to prevent or treat this bacterial infection. In this study, wogonin, as an active ingredient from Agrimonia pilosa, was found to be able to inhibit QS system of P. aeruginosa PAO1. Wogonin downregulated the expression of QS-related genes and reduced the production of many virulence factors, such as elastase, pyocyanin, and proteolytic enzyme. In addition, wogonin decreased the extracellular polysaccharide synthesis and inhibited twitching, swimming, and swarming motilities and biofilm formation. The attenuation of pathogenicity in P. aeruginosa PAO1 by wogonin application was further validated in vivo by cabbage infection and fruit fly and nematode survival experiments. Further molecular docking analysis, pathogenicity examination of various QS-related mutants, and PQS signal molecule detection revealed that wogonin could interfere with PQS signal molecular synthesis by affecting pqsA and pqsR. Taken together, the results indicated that wogonin might be used as an anti-QS candidate drug to attenuate the infection caused by P. aeruginosa.
Keywords: wogonin, P. aeruginosa, quorum sensing, virulence factors, biofilm, molecular docking
1. Introduction
It has been known that the mass use of antibiotics increases bacterial resistance [1] and the development of new drugs has obviously slowed down, leading to a decrease in the cure rate and an increase in the mortality rate for bacterial infectious diseases. Novel therapy strategies have been urgently needed for bacterial infection treatment, especially for the antibiotic-resistance pathogens.
Pseudomonas aeruginosa, a Gram-negative opportunistic pathogen, is frequently found in the department of respiratory and critical care medicine and can cause acute and chronic infections in immunocompromised or burned patients. During acute infection, P. aeruginosa synthesizes adhesion substances such as extracellular polysaccharides for adherence, secretes protease to destroy the structure of host cells for entrance, and produces some virulence factors such as pyocyanin to defend itself against the immune system of host [2]. To initiate chronic infection, P. aeruginosa often forms a biofilm, which relates to extracellular polysaccharide and bacterial motility [3]. Biofilm formation is capable of increasing bacterial drug resistance hundreds of times [4].
Virulence factor production and biofilm formation of P. aeruginosa are crucial for its pathogenicity. Most of the virulence factor production and biofilm formation of this bacterium are regulated by quorum-sensing (QS) systems [5,6,7]. The QS system is a cell-to-cell communication system firstly discovered in Gram-negative bacteria. Bacteria produce and detect signal molecules to regulate their behavior in a cell density-dependent manner through QS system [8,9]. It is reported that three classic QS systems including las, rhl, and pqs systems are present in P. aeruginosa, although iqs was previously reported, recent studies showed that this system is still controversial [10]. The QS system of P. aeruginosa is a complex hierarchic circuitry. The las system consists of LasI/LasR. LasI synthesizes N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-AHL), and this molecule is detected by transcription regulator LasR. LasR/3-oxo-C12-AHL complex activates lasI and some other genes such as rhlI/rhlR, the second QS system, when a threshold concentration is reached. RhlI produces N-Butanoyl-L-homoserine lactone (C4-HSL), which is detected by transcription regulator RhlR [11]. RhlR/C4-HSL complex activates rhlI and other genes such as rhlAB for rhamnolipid production [12]. In addition, P. aeruginosa has the third QS system, pqs, which produces signaling molecule 2-heptyl-3-hydroxy-4-quinolone (PQS) by operon pqsABCDE and transcription regulator PqsR [13]. pqs system regulates the expression of some virulence-related genes by PqsR such as pyocyanin and biofilm formation [14]. Considering the key roles of the QS systems in virulence factor production and biofilm formation of P. aeruginosa, QS systems are considered to be ideal targets for screening drugs to treat bacterial infections [15]. Many quorum sensing inhibitors (QSIs) have been developed for the treatment of bacterial infection, such as hordenine [16], 5-hydroxymethylfurfural [17], rhubarb [18], trans-cinnamaldehyde [19], and salicylic acid [19].
In China, traditional Chinese medicinal herbs have been used to treat diseases for thousands of years. Among them, those with the function of “Qing Re Jie Du” are the most frequently used, and “Qing Re Jie Du” means anti-bacteria and anti-inflammation. Therefore, these medicinal herbs are believed to be safe and promising to screen QSIs [20,21,22]. In this study, in order to identify the active substance with QS inhibitory function for the treatment of P. aeruginosa infection, we first examined 18 types of traditional Chinese medicinal herbs with the function of “Qing Re Jie Du”. Agrimonia pilosa was found to exhibit the optimal QS inhibitory effects. Further investigation suggested that the active ingredient in A. pilosa was wogonin, which showed anti-virulence and anti-biofilm effects. Wogonin reduced the production of the virulence factors like pyocyanin, elastase, and Psl exopolysaccharides and inhibited motility and biofilm formation. In addition, it was also found that wogonin attenuated the rot degree of Chinese cabbage (Brassica pekinensis) and improved the survival rate in the animal models (Drosophila melanogaster and Caenorhabditis elegans) after P. aeruginosa infection. Molecular docking analysis showed that wogonin had strong ability to bind PqsR protein and the thin-layer chromatography (TLC) results displayed that wogonin reduced the production of PQS. In summary, wogonin could target pqs QS system to weaken the pathogenicity of P. aeruginosa, indicating that wogonin might be used as an anti-QS candidate drug to attenuate the infection caused by P. aeruginosa.
2. Results
2.1. Inhibition of P. aeruginosa Virulence and Pathogenicity by the Crude Extracts of A. pilosa
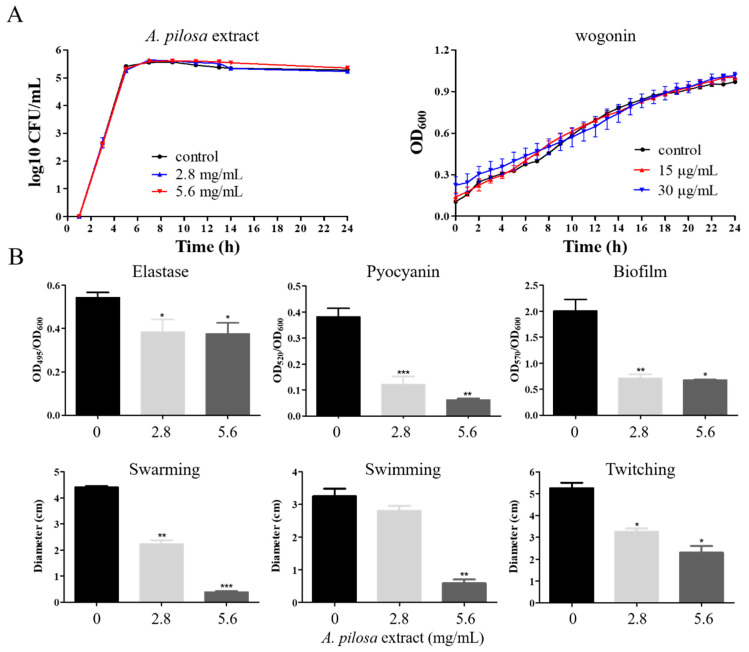
In order to screen potential QSIs of P. aeruginosa, 18 kinds of Chinese medicinal herbs with the function of “Qing Re Jie Du” were selected. The active ingredients of them were, respectively, extracted by deionized water and 70% ethanol, and the effects of the crude extracts on the expression of QS-related genes were examined. The results showed that the extracts of A. pilosa with deionized water or 70% ethanol both inhibited the most QS-related genes (Table 1) but did not impact the growth of P. aeruginosa PAO1 (Figure 1A). Note that the deionized water extracts increased the expression of one QS-related gene, pqsH, and inhibited that of the others. However, the 70% ethanol extracts inhibited that of all the tested genes. Therefore, in the following study, 70% ethanol extracts of A. pilosa were used. Sd the QS system regulates most of the virulence factors and pathogenicity [7,23], we further examined the effects of the extracts from A. pilosa on the virulence phenotypes of PAO1.The virulence-related phenotypes, including motility (twitching, swimming, and swarming), elastase and pyocyanin production, and biofilm formation, were reduced when different concentrations of the 70% ethanol extracts of A. pilosa were used (Figure 1B). The pathogenicity detection by the Chinese cabbage model showed that the decay area gradually became small with the increase of the extract concentrations of A. pilosa (Figure S1). Therefore, some important component(s) from the extracts of A. pilosa would be selected for further study.
Table 1.
Crude extract concentrations of different Chinese medicinal herbs and the effects on QS-related genes.
| Chinese Medicinal Herbs | Extraction Solvent a | Concentration (mg/mL) |
lasR b | lasI | rhlR | rhlI | pqsH | phzM | phzA1 | phzA2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Agrimonia pilosa | water | 56 | ↓ | ↓ | ↓ | - | ↑ | - | ↓ | ↓ |
| ethanol | 27 | ↓ | ↓ | ↓ | - | - | ↓ | - | ↓ | |
| Sargentodoxa cuneata | water | 88 | - | - | ↓ | - | - | ↓ | - | - |
| ethanol | 34 | - | - | ↑ | - | ↓ | ↓ | ↓ | - | |
| Ash bark | water | 60 | - | - | ↓ | - | ↓ | ↑ | ↓ | - |
| ethanol | 13.4 | - | - | - | - | ↓ | - | - | - | |
| Rhizoma paridis | water | 78 | ↑ | ↑ | ↑ | - | - | ↑ | - | - |
| ethanol | 47 | - | - | - | - | - | ↓ | - | - | |
| Herba asari | water | 182 | - | - | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ethanol | 17.5 | - | - | - | - | ↓ | - | - | - | |
| Stephania tetrandra | water | 40 | - | - | - | - | - | - | - | - |
| ethanol | 14 | - | - | - | - | - | - | - | - | |
| Lonicerae japonicae | water | 108 | - | ↑ | ↑ | - | - | ↑ | - | ↑ |
| ethanol | 36 | - | - | - | - | - | - | ↓ | - | |
| Tussilago farfart | water | 202 | - | - | - | - | - | - | ↓ | - |
| ethanol | 44 | - | - | - | - | - | - | ↓ | - | |
| Isatidids folium | water | 350 | - | - | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ethanol | 34 | - | - | - | - | - | - | - | - | |
| Balloon flower | water | 330 | - | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ethanol | 52 | - | - | - | - | - | - | - | ↓ | |
| Radix arnebiae | water | 10 | ↓ | - | - | ↓ | - | ↓ | - | - |
| ethanol | 10 | - | - | - | - | - | - | - | - | |
| Hedyotis diffusa | water | 104 | - | - | - | - | - | - | - | - |
| ethanol | 12.5 | - | - | - | - | - | - | - | - | |
| Herba lobeliae | water | 186 | ↑ | ↑ | ↑ | - | ↑ | - | - | - |
| ethanol | 18 | - | - | - | - | - | - | - | ↓ | |
| Dwarf lilyturf | water | 396 | - | - | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ethanol | 50 | - | - | - | - | - | ↓ | - | - | |
| Pulsa tilia radix | water | 68 | - | - | - | ↓ | ↓ | ↓ | - | - |
| ethanol | 40 | - | - | - | - | - | - | - | - | |
| Subprostrate sophra | water | 106 | - | ↑ | ↑ | - | ↑ | - | - | - |
| ethanol | 35 | - | - | - | - | - | ↓ | ↓ | - | |
| Asteris rasix | water | 288 | ↑ | ↑ | - | - | ↓ | ↓ | - | - |
| ethanol | 73 | - | ↓ | - | - | - | - | ↓ | ↓ | |
| Cortex dictamni | water | 170 | - | - | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ethanol | 34 | - | - | ↑ | - | ↓ | - | - | - |
a Chinese medicinal herbs were extracted by deionized water and 70% ethanol, respectively. b up arrows mean increased expression; down arrows mean reduced expression; transverse lines mean no change.
Figure 1.
The effects of the crude extracts from A. pilosa and wogonin on P. aeruginosa PAO1 growth and virulence factors. (A) When PAO1 was cultured, various concentrations of the crude extracts from A. pilosa and wogonin were used. To examine the effects of crude extracts from A. pilosa on growth, colony-forming units (CFUs) were examined every 2 h for 24 h to avoid the interference of the crude extract color. Regarding wogonin, OD600 of the culture was measured every 2 h for 24 h. (B) The concentrations of the crude extracts from A. pilosa used were 0, 2.8, and 5.6 mg/mL, respectively. The change of the phenotypes related to QS systems were examined, including elastase, pyocyanin, biofilm, swarming, swimming, and twitching. The error bars represent standard errors. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
2.2. Wogonin Inhibited QS-Regulated Genes in P. aeruginosa PAO1
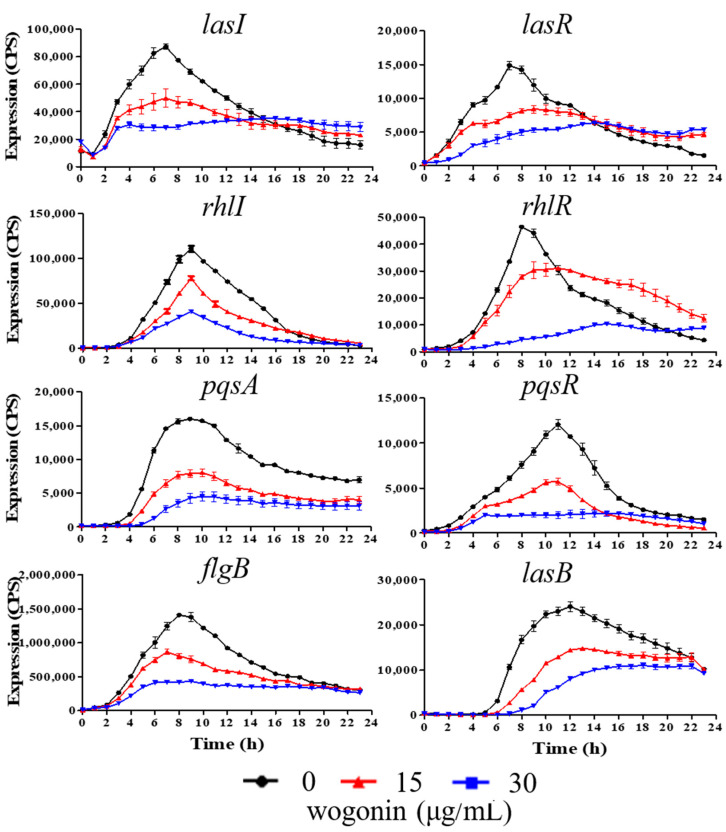
Flavonoids are the most main components of A. pilosa, and wogonin as one family of flavonoids has been used to suppress inflammatory responses [24]. We speculated that wogonin might be involved in the anti-QS activity of A. pilosa. To examine our speculation, the effects of wogonin on the expression of QS-related genes were first detected. The results showed that 15 μg/mL and 30 μg/mL wogonin inhibited the expression of five genes including rhlI, pqsA, pqsR, flgG, and lasB during a 24-h period and reduced the expression of three genes lasI, lasR, and rhlR before ~12 h but not after 12 h (Figure 2). In addition, 15 μg/mL and 30 μg/mL wogonin had no significant effects on PAO1 growth (Figure 1A). The results were similar to those of the crude extracts of A. pilosa, indicating that the active ingredient observed in the above-mentioned phenomena might be wogonin.
Figure 2.
The transcriptional profiles of the genes related to QS systems under the conditions of different concentrations of wogonin. Black lines, no wogonin; red lines, 15 μg/mL wogonin; blue lines, 30 μg/mL wogonin. The promoter activities were measured using a lux-reporter system. The relative expression values of various genes were presented as CPS (Count Per Second) normalized to OD600. The error bars represent standard errors.
2.3. Inhibition of P. aeruginosa Virulence and Pathogenicity by Wogonin
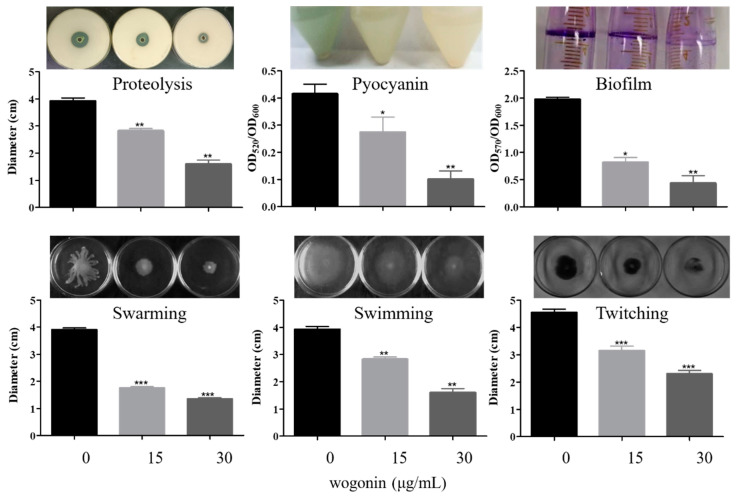
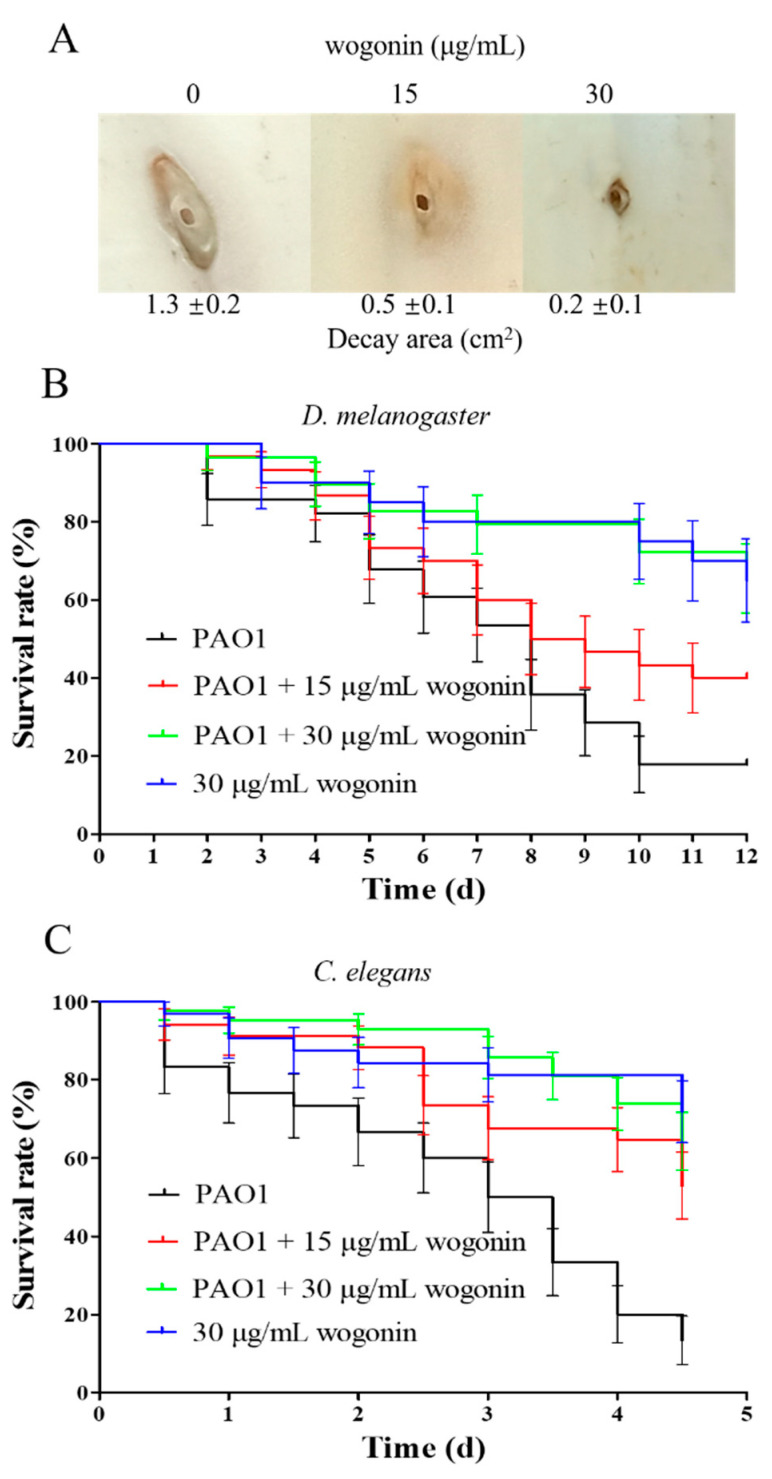
The effects of wogonin on virulence and pathogenicity of P. aeruginosa PAO1 were further investigated. The results showed that 15 μg/mL and 30 μg/mL wogonin inhibited motility including swarming, swimming, and twitching, reduced the production of Psl polysaccharide, pyocyanin, and elastase, and impacted the ability of proteolysis and biofilm formation (Figure 3). Interestingly, the concentration of wogonin at 15 μg/mL almost eradicated the 10-h biofilm (Figure S2). Three models in vivo—Chinese cabbage, fruit fly, and nematode—were used to examine the effects of wogonin on the pathogenicity of PAO1. The results showed that wogonin could weaken the rot degree of the stem in the Chinese cabbage infection model and improved the survival rates in the infection models of fruit fly and nematode (Figure 4). The results were also comparable with those of the crude extracts of A. pilosa.
Figure 3.
The effects of wogonin on P. aeruginosa PAO1 virulence factors, including proteolysis, pyocyanin, biofilm, swarming, swimming, and twitching. The wogonin concentrations used were 0, 15, and 30 μg/mL, respectively. The error bars represent standard errors. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Figure 4.
The effects of wogonin on P. aeruginosa PAO1 pathogenicity. After infection, the decay area of cabbage (A) and the survival rates of D. melanogaster (B) and C. elegans (C) were, respectively, measured with or without wogonin addition. Dark lines, only PAO1 infection; red lines, PAO1 infection and 15 μg/mL wogonin addition; green lines, PAO1 infection and 30 μg/mL wogonin addition; blue, only 30 μg/mL wogonin addition. The error bars represent standard errors.
2.4. Wogonin Mainly Affected the pqs System of P. aeruginosa PAO1
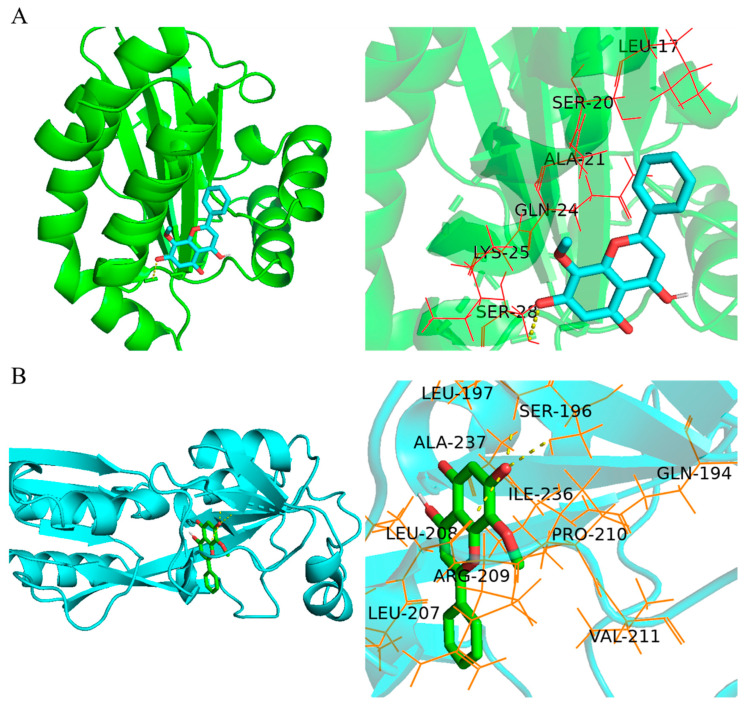
In order to investigate the molecular mechanisms of wogonin against the QS systems of P. aeruginosa PAO1, molecular docking analysis in silico was performed to predict the targets of wogonin. The results showed that wogonin could bind LasR protein mainly with Ala, Ser, Gln, Leu, and Lys amino acids, and it also bound Ser by a hydrogen bond (Figure 5A and Figure S3A). The whole energy binding with LasR was −2.44 kcal/mol. Wogonin could bind PqsR protein mainly with Ala, Arg, Ile, Pro, Val, and Gln amino acids, and it also bound Ser and Leu by three hydrogen bonds (Figure 5B and Figure S3B). The whole energy binding with PqsR was −4.49 kcal/mol. As the 3D structure of RhlR was not available in the PDB database, the molecular docking analysis of RhlR was not performed. Taken together, it was concluded that wogonin could bind LasR and PqsR, and thereafter weakened the downstream virulence and pathogenic factors.
Figure 5.
In silico molecular docking analysis. (A) The active sites of LasR CBD (PDBID:6D6A) docked with wogonin, including Ala, Ser, Gln, Leu, Lys, and Ser, and the binding energy is −2.44 kcal/mol. (B) The active sites of PqsR CBD (PDBID: 4JVI) docked with wogonin, including Ala, Arg, Ile, Pro, Val, and Gln, and the binding energy is −4.49 kcal/mol. 3D and 3D partial enlarged graphics were exhibited from left to right. Molecular docking was performed using Autodock Vina v.1.1.2, and graphics were generated with PyMOL v2.4.1 software.
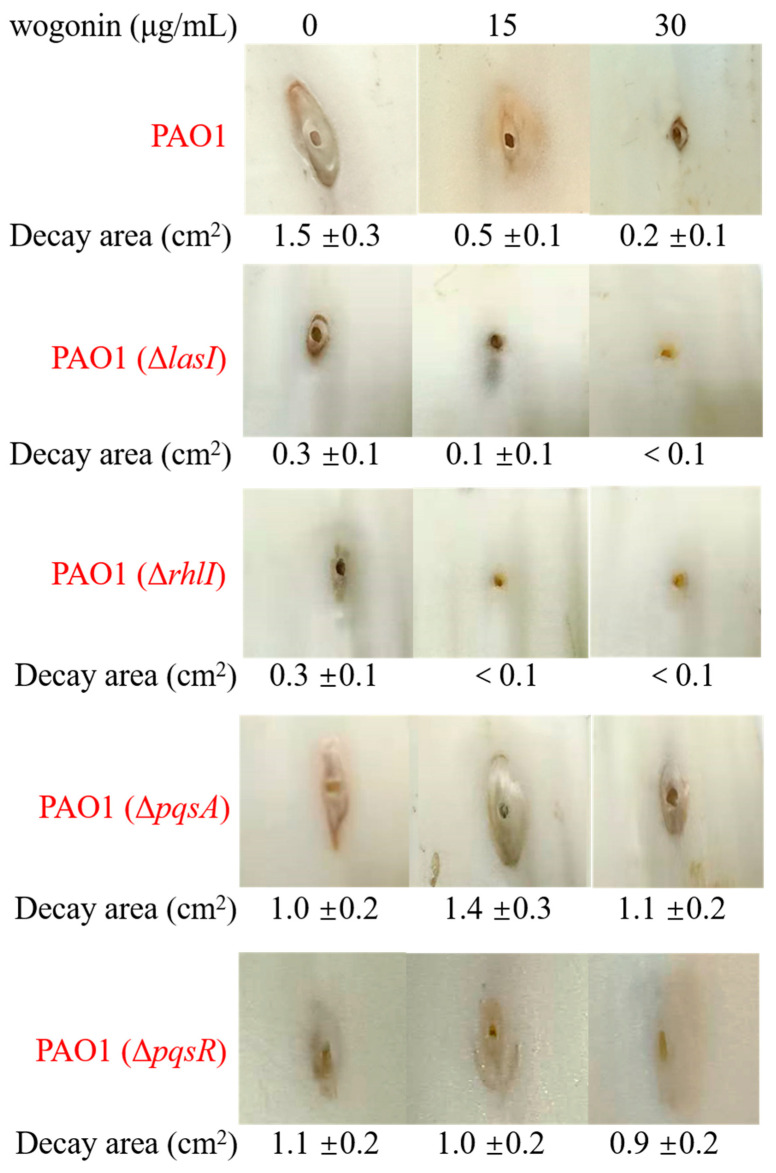
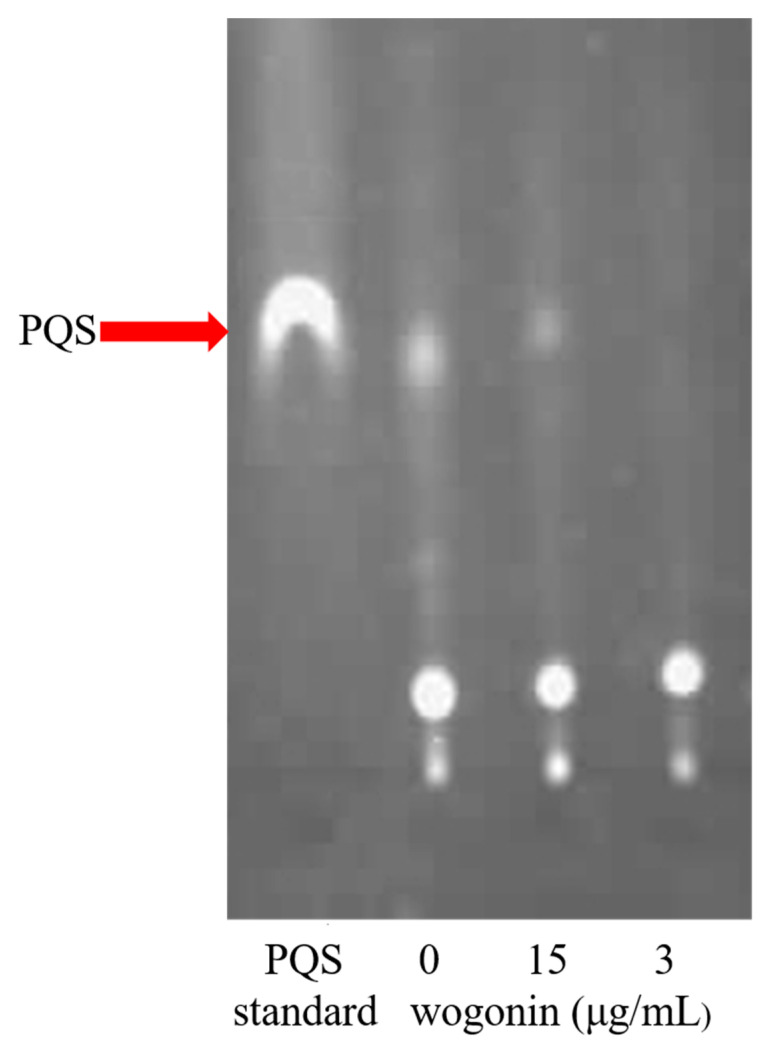
The molecular docking results showed that wogonin could bind LasR and PqsR, but it was not clear which one took a dominant role. In order to identify the main target, we further examined the effects of wogonin on the pathogenicity of three QS-deleted mutants by the cabbage infection model. The results showed that wogonin attenuated the pathogenicity of the wild type strain, lasI deletion mutant, and rhlI deletion mutant, but not pqsA or pqsR single-gene deletion mutants, indicating that the pqs system played the dominant role during wogonin treatment (Figure 6). Furthermore, the TLC analysis for PQS production showed that wogonin at 15 μg/mL reduced the production of PQS signal molecules, and 30 μg/mL wogonin blocked the synthesis of PQS signal molecules (Figure 7). Taken together, all the results from the molecular docking and the pathogenicity analysis indicated that wogonin might affect PQS signal molecule production by interfering with pqsA or pqsR.
Figure 6.
The decay area of cabbage after infection by various QS deletion mutants without or with 15 and 30 μg/mL wogonin, including PAO1 wild type strain, PAO1(ΔlasI), PAO1(ΔrhlI), PAO1(ΔpqsA), and PAO1(ΔpqsR). The errors represent standard errors.
Figure 7.
Wogonin inhibited PQS molecule production by TLC analysis. After being grown for 24 h without or with 15 and 30 μg/mL wogonin application, the PQS molecule from 5 mL culture of PAO1 was extracted and examined by TLC plate and photographed with long-wave UV light. Lane 1, PQS molecule standard; lane 2, no wogonin addition; lane 3, 15 μg/mL wogonin; lane 4, 30 μg/mL wogonin. The photograph was representative of three individual experiments.
3. Discussion
P. aeruginosa is among the top three major human nosocomial pathogens to cause morbidity and mortality in immunocompromised patients. It often causes severe hospital-acquired infections, leading to bacteremia, pneumonia, and so on [3,25]. Virulence factors play important roles in the acute infection of P. aeruginosa. It is believed that anti-virulence therapy leads lower occurrence of drug resistance as it does not affect growth and exerts low selective pressure [26,27]. This bacterium can also form biofilms to cause chronic infection and increase bacterial drug resistance. Preventing biofilm formation or dispersing old biofilms is helpful in eradicating the infecting bacteria [5]. Most of the virulence factor production and biofilm formation are regulated by QS systems of P. aeruginosa [7]. Therefore, targeting QS systems may represent an adjunctive treatment approach for alleviating P. aeruginosa infection.
Many QSIs were developed to treat bacterial infections. Several natural extracts from food with plant origins have been found to display inhibition function for P. aeruginosa QS systems. For example, sulforaphane from broccoli and gingerol from ginger could inhibit the las system [27], vanillin impaired the pqs system [28], hordenine from sprouting barley downregulated three QS systems [29], leaf extracts of mango suppressed virulence factors and biofilm formation in the tested bacteria with unknown mechanisms [30], and pyranoanthocyanins from red wine interfered with QS system at distinct stages in a strain-specific manner [31]. Diallyl sulfide from garlic inhibited QS systems of P. aeruginosa and enhanced biosynthesis of three B vitamins through its thioether group [32].
Due to the abundance and pharmacological effects of traditional Chinese medicinal herbs, searching QSIs from traditional Chinese medicinal herbs may be promising. Among them, some have been found to show anti-QS function. For example, the extracts from the mixture of Artemisiae argyi folium, the root bark of Cortex dictamni, and the root of Solanum melongena could completely inhibit pqs system of P. aeruginosa [21]. Paeonol from peony root interfered with las system, reduced virulence production, and inhibited biofilm formation [23]. Coumarin, a Chinese medicinal herb-derived phenolic compound, reduced protease and pyocyanin production and inhibited biofilm formation of different P. aeruginosa strains by impairing QS systems and changing cyclic diguanylate (c-di-GMP) metabolism [33]. Okanin in Coreopsis tinctoria Nutt interfered with QS system of Chromobacterium violaceum by the downregulation of vio operon [22].
In this study, wogonin, the active ingredient from A. pilosa, was found to target the pqs QS system of P. aeruginosa to inhibit PQS molecule synthesis. Consequently, wogonin reduced the production of many virulence factors and weakened the pathogenicity of the bacterium. It could effectively protect plant stem from this bacterial infection and increase the survival rates of fruit fly and nematode after infection. Taken together, wogonin attenuated the pathogenicity of P. aeruginosa both in acute and chronic infections, indicating that wogonin could be an alternative method to inhibit human opportunistic pathogen P. aeruginosa. However, in order to broaden its application scope, it is worthy of further investigation to verify its function for treating infections due to other human pathogens. It is also interesting to use antibiotics together with QSIs to synergistically treat bacterial infections in the future.
It is worth mentioning that although traditional Chinese medicinal herbs with the function of “Qing Re Jie Du” have been used to treat many common diseases in China for thousands of years, some underlying mechanisms remain unknown. A. pilosa has been found to have the effects of anti-inflammation, anti-oxidation, and anti-tumor, and is widely used for the treatment of diabetes, tumor, Meniere’s syndrome, trichomonas vaginitis, and other diseases [34]. It was reported that the active components of A. pilosa contain flavonoids, triterpenoids, tannins, phenols, and other chemical substances. Wogonin, an ingredient of A. pilosa, belongs to flavonoids and has been used as an inhibitor of inflammatory mediators produced by macrophages, lymphocytes, microglia, and endothelial cells [24]. The molecular mechanisms of inhibition involve several signaling pathways, such as ER stress-mediated apoptosis and autophagy, mitogen-activated protein kinase (MAPK), and transcription factor inhibition of NF-κB [35]. Here, it was found that the crude extracts of A. pilosa and an ingredient wogonin both could inhibit most of virulence factors and biofilm formation to attenuate pathogenicity of P. aeruginosa PAO1 by targeting pqs system. These results help to explain the antibacterial activity of traditional Chinese medicinal herbs with the function of “Qing Re Jie Du”. It also provides a research direction to examine the effects of other traditional Chinese medicinal herbs with similar function.
4. Materials and Methods
4.1. Bacterial Strains, Plasmids and Culture Conditions
Bacterial strains used in the study are shown in Table 2. LB (Luria–Bertani) broth and agar were used for bacterial culture at 37 °C. When necessary, the media were supplemented with kanamycin (Kan, 50 μg/mL) or trimethoprim (Tmp, 300 μg/mL) purchased from Amresco (Solon, PA, USA).
Table 2.
Bacterial strains used in this study.
| Strains/Plasmids | Description | Source or Reference |
|---|---|---|
| Strains | ||
| E. coli DH10B | F– mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZ ΔM15 ΔlacX74 recA1 araΔ139 Δ(ara leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
| PAO1 | P. aeruginosa wild type strain | This lab |
| PAO1 (ΔlasI) | lasI deletion mutant of PAO1 | [36] |
| PAO1 (ΔrhlI) | rhlI deletion mutant of PAO1 | [37] |
| PAO1 (ΔpqsA) | pqsA deletion mutant of PAO1 | This study |
| PAO1 (ΔpqsR) | pqsR deletion mutant of PAO1 | [38] |
| ΔpqsA/CTX-pqsA | CTX-pqsA integrated into PAO1(ΔpqsA) chromosome; Tcr | This study |
| ΔpqsA/pAK-pqsA/CTX-pqsA | ΔpqsA/CTX-pqsA complemented by plasmid pAK-pqsA; Cbr | This study |
| ΔpqsR/CTX-pqsA | CTX-pqsA integrated into PAO1(ΔpqsR) chromosome; Tcr | This study |
| ΔpqsR/pAK-pqsR/CTX-pqsA | ΔpqsR/CTX-pqsA complemented by plasmid pAK-pqsR; Cbr | This study |
| Plasmids | ||
| pMS402 | Expression reporter vector carrying the promoterless luxCDABE; Kanr, Tmpr | [39] |
| pEX18-Tc | Broad host range gene replacement vector; sacB, Tcr | [40] |
| pRK2013 | Broad host range helper vector; Traþ, Kanr | [41] |
| pKD-lasI | pMS402-containing lasI promoter region; Kanr, Tmpr | [42] |
| pKD-lasR | pMS402-containing lasR promoter region; Kanr, Tmpr | [42] |
| pKD-lasB | pMS402-containing lasB promoter region; Kanr, Tmpr | This lab |
| pKD-rhlI | pMS402-containing rhlI promoter region; Kanr, Tmpr | [42] |
| pKD-rhlR | pMS402-containing rhlR promoter region; Kanr, Tmpr | [42] |
| pKD-rhlA | pMS402-containing rhlA promoter region; Kanr, Tmpr | This lab |
| pKD-pqsA | pMS402-containing pqsA promoter region; Kanr, Tmpr | [43] |
| pKD-pqsR | pMS402-containing pqsR promoter region; Kanr, Tmpr | [43] |
| pKD-flgB | pMS402-containing flgB promoter region; Kanr, Tmpr | This lab |
| mini-CTX-lacZ | Integration plasmid-containing attP site for integration at chromosomal attB site; Tcr | [44] |
| CTX-pqsA | mini-CTX-lacZ-containing pqsA gene region | This study |
| pAK1900 | E. coli-P. aeruginosa shuttle cloning vector carrying plac upstream of MCS; Ampr, Cbr | [45] |
| pAK-pqsA | pAK1900-containing pqsA gene region | This study |
| pAK-pqsR | pAK1900-containing pqsR gene region | This study |
4.2. Active Substance Extraction of Traditional Chinese Medicinal Herbs
Eighteen kinds of traditional Chinese medicinal herbs with the function of “Qing Re Jie Du” were purchased from the local pharmacy (Tongrentang, Beijing, China). For extraction of active substances, the grounded herb was first immersed in deionized water or 70% ethanol, respectively. For deionized water extraction, the herb was boiled for 2 h twice and the extracted liquids were merged. Regarding the 70% ethanol extraction, the heat reflux method was used. Then, the two kinds of extracts were separately filtered by Buchi funnel, concentrated by a rotary evaporator, freeze-dried by a vacuum freeze dryer, and kept at −20 °C. Before the examination, extracts were, respectively, dissolved in deionized water (it was designed as the water extracts) or 70% ethanol (it was designed as the ethanol extracts) and sterilized using a 0.22 μm filter. Wogonin standard was purchased from Shanghai Yuanye Bio-Technology Co. Ltd. (Shanghai, China).
4.3. Gene Expression Assay
The expression levels of the QS-related genes including lasI, lasR, rhlI, rhlR, lasB, and rhlA were analyzed using the lux-reporter system as our previous study [46]. In brief, the promoter regions of these target genes were, respectively, fused with the luxCDABE genes in the vector pMS402. The strains containing the different promoters-luxCDABE fusion reporters were inoculated in LB broth supplemented with 300 μg/mL Tmp overnight. Then, the culture was transferred to the new LB broth at a ratio of 1:100 and incubated for ~3 h until the log-growth phase. Five microliters of log-phase culture was inoculated into parallel wells on a 96-well black plate containing 95 μL of LB broth with the water or ethanol extracts of different Chinese medicinal herbs and wogonin, respectively. To prevent evaporation, 60 μL of mineral oil was added into each well. Promoter activity and bacterial growth were measured every 30 min for 24 h in a Victor multilabel plate reader (Wallac model 1450, Perkin-Elmer, MA, USA).
4.4. Measurement of Virulence Factors
4.4.1. Pyocyanin
Pyocyanin was analyzed according to the previous report [47]. Briefly, after growing in Pseudomonas broth medium (0.14%NaCl, 1%K2SO4, and 2% tryptone) with different concentrations of the extract of A. pilosa or wogonin at 37 °C for 24 h, the culture was centrifuged to obtain supernatant. After 3 mL of chloroform was added to 5 mL of supernatant and vigorously vortexed for 2 min, the mixture was centrifuged at 8000 rpm for 10 min. Then, the chloroform layer was transferred to a fresh tube and mixed with 1 mL of 0.2 M HCl. After centrifugation again, the top layer was removed and OD520 was measured. The amount of pyocyanin (μg/mL) was obtained using the formula: OD520/OD600 × 17.072.
4.4.2. Biofilm Formation
The biofilm formation of wild type strain PAO1 with or without the extracts of A. pilosa or wogonin was tested according to the previous method [48]. The strain with different concentrations of the extracts of A. pilosa or wogonin was added in sterile test tubes and incubated at 37 °C until the biofilm was formed. Followed by removal of non-adherent cells from the tubes and gentle rinsing three times with sterile PBS, biofilm biomass was evaluated by crystal violet staining. The attached crystal violet was washed with anhydrous ethanol and its absorbance was measured at OD570 and analyzed.
In order to determine the effects of wogonin on the formed biofilm, after PAO1 was grown in a 96-well polyvinyl chloride plate (Corning/Costar, Corning, NY, USA) at 37 °C for 10 h, the culture was removed and new LB broth with 15 or 30 μg/mL wogonin was added and incubated at 37 °C overnight. The biofilm biomass was evaluated by crystal violet staining as the above-mentioned. Each samples had 12 replicates.
4.4.3. Proteolytic Enzyme
After diluting overnight culture to OD600 = 0.2, 2 μL of aliquots were spotted onto LB agar with 5% skim milk with or without wogonin addition. After incubation at 37 °C overnight, the hydrolysis ring diameters were determined.
4.4.4. Motility
The motility was determined in three kinds of media as previously reported [49]. The medium used for swarming motility assay consisted of 0.6% agar, 0.8% nutrient broth, and 0.5% glucose with or without the extracts of A. pilosa or wogonin. The medium for swimming motility assay consisted of 0.3% agar, 0.5% NaCl, and 1% peptone with or without the extracts of A. pilosa or wogonin. The medium used for twitching motility assay was thin LB agar plates with or without the extracts of A. pilosa or wogonin. All the plates were dried at room temperature overnight before use. Two microliters of diluted overnight culture (OD600 = 0.5) was spotted on swarming or swimming agar plates. After the samples were incubated at 37 °C for 24 h, the swarming and swimming motility diameters were measured. The twitching motility was observed by stab-inoculating bacteria through the thin LB medium. After incubated at 37 °C for 48 h, the twitching bacterial cells were stained with crystal violet and washed with PBS to determine diameters.
4.4.5. Elastase
Elastase assay was performed as previously described [50]. In brief, PAO1 was grown in LB broth with the addition of different concentrations of the extracts of A. pilosa. Then, 1 mL of overnight culture was centrifuged, and the supernatant was kept. After the supernatant was added into a new tube with 10 mg/mL Elastin-Congo Red (Sigma, Saint Louis, MO, USA) in 2 mL of reaction buffer (1 mM CaCl2, 0.1 M Tris-HCl buffer, pH 7.0) and kept at 37 °C for 3 h, the mixture was centrifuged at 3000× g for 10 min. OD495 of the supernatant was used to estimate elastase activity.
4.4.6. Exopolysaccharide Production Assay
Psl polysaccharide production assay was carried out according to the previous method [51]. In brief, the diluted overnight of PAO1 (OD600 = 0.005) was spotted onto Congo Red plates (1% tryptone, 1% agar, 4% Congo Red, and 2% Coomassie blue) with or without wogonin. The colony morphology and color were observed after 3 d incubation at 37 °C.
4.4.7. Rhamnolipid Measurement
The rhamnolipid was measured as previously described [49]. In brief, after being grown in LB broth with or without the extracts of A. pilosa for 2 d, the culture was centrifuged at 12,000 rpm for 5 min. The supernatant was mixed with an equal volume of ethyl acetate and vortexed vigorously. After centrifugation, the organic phase was collected and dissolved in 4 mL of chloroform and added in 200 μL of freshly prepared 1 g/L methylene blue solution (pH 8.6). After vortexing for 4 min and standing for 15 min, OD638 of the solution was measured, and the values were calculated to obtain rhamnolipid concentration according to a calibration curve.
4.5. In Vivo Pathogenicity Assay
The pathogenicity of PAO1 with or without wogonin application was investigated using Chinese cabbage (B. pekinensis), fruit fly (D. melanogaster Canton S), and nematode (C. elegans N2) infection models as reported previously [52,53,54].
For the Chinese cabbage infection assay, the overnight culture was centrifuged and resuspended to OD600 = 2.0 in 10 mM MgSO4. After the surface of Chinese cabbage was disinfected with 0.1% H2O2, ten microliters of bacterial cells with or without the extracts of A. pilosa or wogonin was inoculated on the stem of Chinese cabbage for 6 d at 30 °C and the rot area of Chinese cabbage was observed and analyzed.
For the fruit fly infection assay, the cells from 1.0 mL of the overnight culture were collected by centrifugation and resuspended in 5% sucrose with an OD600 of 2.0. Then, 100 μL of the resuspended cells with or without wogonin was spotted onto a sterile filter that was placed on the surface of 2 mL of solidified agar with 5% sucrose in a 20 mL glass tube. Before adding to each tube, all the fruit flies were starved for 3 h. The cold shock method was used to anesthetize fruit flies during sorting and transferring. The tubes with fruit flies were stored at 25 °C in a humidity-controlled environment. The number of live fruit flies was counted and documented at 24 h intervals for 12 d.
For the nematode infection examination, NGM plates (0.3% NaCl, 0.25% tryptone, 1 mM MgSO4, 1 mM CaCl2, 5 μg/mL cholesterol, 100 μg/mL FUDR, and 2% agar) with or without wogonin was used. The nematodes in the L4 phase by synchronization treatment were transferred to the NGM plates, and then the survival rate of nematodes was recorded every 12 h for 5 d.
4.6. Molecular Dynamics Simulation
Molecular dynamics simulation was carried out according to the previous report [55]. In brief, wogonin was used to dock the transcriptional regulators of QS circuits of P. aeruginosa PAO1, LasR, and PqsR. Due to the lack of crystal structure, RhlR was not analyzed. The 3D structure of the two proteins—LasR (PDB ID: 6D6A) and PqsR (PDB ID: 4JVI)—was downloaded from Protein Data Bank (http://www.pdb.org, accessed on 30 September 2021). The 3D-crystal structure of wogonin was docked to the binding sites of LasR and PqsR using Auto Dock Vina. The optimal conformation was confirmed based on the number of hydrogen bonds, hydrophobic interaction force, and binding energy. The 2D-crystal structures and 3D-crystal structures were, respectively, obtained by Discovery Studio2016 and PyMOL v2.4.1 software.
4.7. PQS Signal Molecule Production Assay
PQS production was analyzed as previously described [56]. Briefly, after growth for 24 h, a 5 mL culture of PAO1 was collected and extracted three times with acidified ethyl acetate. The extract was dried and resuspended in the mixture with ethyl acetate and acetonitrile (v/v, 1:1). One microliter extract was loaded onto a TLC plate. Dichloromethane and methanol (v/v, 95:5) were used as the developing solvent. After the components of the samples were separated on TLC plates, the plates were photographed with long-wave UV light.
4.8. Construction and Complementation of In-Frame Deletion Mutants
The in-frame deletion mutation was performed by a sacB-based method [40]. In brief, the upstream and downstream fragments of the gene pqsA were amplified by the primers sets pqsA-up1, pqsA-down1, pqsA-up2, and pqsA-down2 (Table 3). After the fragments were ligated with the vector pEX18Tc by overlapping sequences, the plasmid pEX18Tc-pqsA was consequently obtained. The pqsA gene deletion was carried out by triparental mating as previously described [57]. Briefly, PAO1 and E. coli DH10B strains containing pEX18Tc-pqsA or pRK2013 were, respectively, grown overnight, collected, washed with PBS, and suspended in SOC medium. The bacterial mixture with a ratio of 1:1:1 was spotted onto a LB agar plate. After growth at 37 °C for 12 h, the bacterial cells were resuspended in 500 µL of LB liquid medium and plated onto PIA plates with 300 μg/mL tetracycline addition. The obtained mutant was verified by PCR.
Table 3.
Primers used in this study.
| Primers | Sequence (5′→3′) | Restriction Sites | Function |
|---|---|---|---|
| pKD-pqsA-up | CATCTCGAGCCAGGGTCAGTGTCC | Xho I | Luminous reporter construction |
| pKD-pqsA-down | TGTGGATCCCAGAACCTCGGTCAG | BamH I | Luminous reporter construction |
| pqsA-up1 | CAGGAAACAGCTATGACCATGATTACGAAGTACCTTGGAAGTCGAGC | - | pqsA knockout |
| pqsA-down1 | CAACATGCCCGTTCCTCGGAACAGAACCTCGGTC | - | pqsA knockout |
| pqsA-up 2 | GACCGAGGTTCTGTTCCGAGGAACGGGCATGTTG | - | pqsA knockout |
| pqsA-down 2 | CGACGGCCAGTGCCAAGCTTCGAACGGATCGAAGCTG | - | pqsA knockout |
| pAK-pqsA-up | TATGAGCTCCCGTGGTTCTTCTCC | Sac I | pqsA complement |
| pAK-pqsA-down | CGTTCTAGAGCATCAGCATCTCGTC | Xba I | pqsA complement |
| pAK-pqsR-up | TATTTAGGTGACACTATAGAATACTCAAGCTTGAATAAGGGATGCCTATTC | Hind III | pqsR complement |
| pAK-pqsR-down | GAATTGTAATACGACTCACTATAGGGCGAATTCGAGCTCCCACGGCCAGCGTC | EcoR I | pqsR complement |
To complement pqsA deletion, after the gene pqsA was amplified using the primer sets pAK-pqsA-up and pAK-pqsA-down (Table 3) and ligated into pAK1900 vector, the vector was transferred into E. coli DH10B by electroporation. Integrants were selected on PIA plates containing 250 μg/mL Cb.
4.9. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.0.1. All the experiments were repeated at least three times. One-way analysis of variance (ANOVA) was performed to determine significant differences (p < 0.05).
5. Conclusions
The anti-QS activity from the extracts of 18 traditional Chinese medicinal herbs with the function of “Qing Re Jie Du” was examined. Among them, the crude extracts of A. pilosa showed the optimal inhibitory effects against QS systems. The extracts weakened most of virulence factors and pathogenicity of P. aeruginosa PAO1, inhibited biofilm formation, but did not influence this bacterial growth under the condition with the tested concentrations. Further investigation showed that wogonin, one of the active ingredients of A. pilosa, exhibited similar phenotypes with the crude extracts of A. pilosa. It downregulated the QS-related gene expression and attenuated most of the virulence factor production and bacterial pathogenicity in cabbage infection and fruit fly and nematode survival experiments. In silico molecular docking analysis, the pathogenicity observation of various QS-related mutants and PQS signal molecule examination confirmed that wogonin could mainly interfere with PQS signal molecular synthesis by affecting pqsA and pqsR. These results are helpful for understanding the function of traditional Chinese medicinal herbs named “Qing Re Jie Du” and providing an alternative approach for bacterial infections.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222312699/s1.
Author Contributions
S.W. and L.S. conceived and designed research. Y.F., X.H. and L.Y. conducted experiments. X.C. and C.L. contributed new reagents or analytical tools. S.W., L.S. and Y.F. analyzed data. S.W. and L.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by grants from the Provincial Natural Science Foundation of Shaanxi Province (2018ZDXM-SF-004), National Natural Science Foundation of China (31770152 and 31400094), National Key R&D Program of China (2021YFC1808902), and Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province (ARRLKF21-04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lau G.W., Hassett D.J., Ran H., Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Usher L.R., Lawson R.A., Geary I., Taylor C.J., Bingle C.D., Taylor G.W., Taylor G., Whyte M.K.B. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: A potential mechanism of persistent infection. J. Immunol. 2002;168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 3.Moradali M.F., Ghods S., Rehm B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall C.W., Mah T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017;41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 5.Turkina M.V., Vikstrom E. Bacteria-Host Crosstalk: Sensing of the Quorum in the Context of Pseudomonas aeruginosa Infections. J. Innate Immun. 2019;11:263–279. doi: 10.1159/000494069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flemming H.C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 7.Pena R.T., Blasco L., Ambroa A., Gonzalez-Pedrajo B., Fernandez-Garcia L., Lopez M., Bleriot I., Bou G., García-Contreras R., Wood T.K., et al. Relationship Between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019;10:1100. doi: 10.3389/fmicb.2019.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papenfort K., Bassler B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moura-Alves P., Puyskens A., Stinn A., Klemm M., Guhlich-Bornhof U., Dorhoi A., Furkert J., Kreuchwig A., Protze J., Lozza L., et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science. 2019;366:eaaw1629. doi: 10.1126/science.aaw1629. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis P. Putting an end to the Pseudomonas aeruginosa IQS controversy. MicrobiologyOpen. 2020;9:e962. doi: 10.1002/mbo3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster M., Greenberg E.P. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006;296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 2006;30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 13.Pesci E.C., Milbank J.B.J., Pearson J.P., McKnight S., Kende A.S., Greenberg E., Iglewski B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diggle S.P., Matthijs S., Wright V.J., Fletcher M.P., Chhabra S.R., Lamont I., Kong X., Hider R., Cornelis P., Cámara M., et al. The Pseudomonas aeruginosa 4-Quinolone Signal Molecules HHQ and PQS Play Multifunctional Roles in Quorum Sensing and Iron Entrapment. Chem. Biol. 2007;14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen T.B., Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006;296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Beury-Cirou A., Tannières M., Minard C., Soulère L., Rasamiravaka T., Dodd R.H., Queneau Y., Dessaux Y., Guillou C., Vandeputte O.M., et al. At a supra-physiological concentration, human sexual hormones act as quorum-sensing inhibitors. PLoS ONE. 2013;8:e83564. doi: 10.1371/journal.pone.0083564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkumari J., Borkotoky S., Reddy D., Mohanty S.K., Kumavath R., Murali A., Suchiang K., Busi S. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: Insights from in vitro, in vivo and in silico studies. Microbiol. Res. 2019;226:19–26. doi: 10.1016/j.micres.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Chu W., Zhou S., Jiang Y., Zhu W., Zhuang X., Fu J. Effect of Traditional Chinese Herbal Medicine with Antiquorum Sensing Activity on Pseudomonas aeruginosa. Evid. Based Complementary Altern. Med. 2013;2013:648257. doi: 10.1155/2013/648257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S., Rudden M., Smyth T.J., Dooley J.S.G., Marchant R., Banat I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019;103:3521–3535. doi: 10.1007/s00253-019-09618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priya K., Yin W.F., Chan K.G. Anti-quorum sensing activity of the traditional Chinese herb, Phyllanthus amarus. Sensors. 2013;13:14558–14569. doi: 10.3390/s131114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Q., Bhasme P., Wang Z., Wang L., Wang S., Zeng Y., Wang Y., Ma L.Z., Li Y. Chinese medicinal herb extract inhibits PQS-mediated quorum sensing system in Pseudomonas aeruginosa. J. Ethnopharmacol. 2020;248:112272. doi: 10.1016/j.jep.2019.112272. [DOI] [PubMed] [Google Scholar]
- 22.Mu Y., Zeng H., Chen W. Okanin in Coreopsis tinctoria Nutt is a major quorum-sensing inhibitor against Chromobacterium violaceum. J. Ethnopharmacol. 2020;260:113017. doi: 10.1016/j.jep.2020.113017. [DOI] [PubMed] [Google Scholar]
- 23.Yang D., Hao S., Zhao L., Shi F., Ye G., Zou Y., Song X., Li L., Yin Z., He X., et al. Paeonol Attenuates Quorum-Sensing Regulated Virulence and Biofilm Formation in Pseudomonas aeruginosa. Front. Microbiol. 2021;12:692474. doi: 10.3389/fmicb.2021.692474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao W., Yin M., Wu K., Lu G., Deng B., Zhang Y., Qian L., Jia X., Ding Y., Gong W., et al. High-dose wogonin exacerbates DSS-induced colitis by up-regulating effector T cell function and inhibiting Treg cell. J. Cell. Mol. Med. 2017;21:286–298. doi: 10.1111/jcmm.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Sully E.K., Malachowa N., Elmore B.O., Alexander S.M., Femling J.K., Gray B.M., DeLeo F., Otto M., Cheung A.L., Edwards B.S., et al. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014;10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quave C.L., Lyles J.T., Kavanaugh J.S., Nelson K., Parlet C.P., Crosby H.A., Heilmann K.P., Horswill A.R. Castanea sativa (European Chestnut) Leaf Extracts Rich in Ursene and Oleanene Derivatives Block Staphylococcus aureus Virulence and Pathogenesis without Detectable Resistance. PLoS ONE. 2015;10:e0136486. doi: 10.1371/journal.pone.0136486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mok N., Chan S.Y., Liu S.Y., Chua S.L. Vanillin inhibits PqsR-mediated virulence in Pseudomonas aeruginosa. Food Funct. 2020;11:6496–6508. doi: 10.1039/D0FO00046A. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J.W., Luo H.Z., Jiang H., Jian T.K., Chen Z.Q., Jia A.Q. Hordenine: A Novel Quorum Sensing Inhibitor and Antibiofilm Agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018;66:1620–1628. doi: 10.1021/acs.jafc.7b05035. [DOI] [PubMed] [Google Scholar]
- 30.Husain F.M., Ahmad I., Al-Thubiani A.S., Abulreesh H.H., AlHazza I.M., Aqil F. Leaf Extracts of Mangifera indica L. Inhibit Quorum Sensing—Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front. Microbiol. 2017;8:727. doi: 10.3389/fmicb.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coelho P., Oliveira J., Fernandes I., Araújo P., Pereira A., Gameiro P., Bessa L. Pyranoanthocyanins Interfering with the Quorum Sensing of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Mol. Sci. 2021;22:8559. doi: 10.3390/ijms22168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W.R., Zeng T.H., Yao J.W., Zhu L.P., Zhang Z.Q., Xie X.B., Shi Q.S. Diallyl sulfide from garlic suppresses quorum-sensing systems of Pseudomonas aeruginosa and enhances biosynthesis of three B vitamins through its thioether group. Microb. Biotechnol. 2021;14:677–691. doi: 10.1111/1751-7915.13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Sass A., Van Acker H., Wille J., Verhasselt B., Van Nieuwerburgh F., Kaever V., Crabbé A., Coenye T. Coumarin Reduces Virulence and Biofilm Formation in Pseudomonas aeruginosa by Affecting Quorum Sensing, Type III Secretion and C-di-GMP Levels. Front. Microbiol. 2018;9:1952. doi: 10.3389/fmicb.2018.01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C.Y., Yu Q.M., Kong H.J., Lee J.Y., Yang K.M., Seo J.S. Antioxidant and Anti-Inflammatory Activities of Agrimonia pilosa Ledeb. Extract. Evid. Based Complementary Altern. Med. 2020;2020:8571207. doi: 10.1155/2020/8571207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong G., Wang H., Kong X., Duan R., Dong T.T.X., Tsim K.W.K. Flavonoids are identified from the extract of Scutellariae Radix to suppress inflammatory-induced angiogenic responses in cultured RAW 264.7 macrophages. Sci. Rep. 2018;8:17412. doi: 10.1038/s41598-018-35817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson J.P., Pesci E.C., Iglewski B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brint J.M., Ohman D.E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang H., Li L., Kong W., Shen L., Duan K. Identification of a novel regulator of the quorum-sensing systems in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2009;293:196–204. doi: 10.1111/j.1574-6968.2009.01544.x. [DOI] [PubMed] [Google Scholar]
- 39.Duan K., Dammel C., Stein J., Rabin H., Surette M.G. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 2003;50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 40.Hoang T.T., Karkhoff-Schweizer R.R., Kutchma A.J., Schweizer H.P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 41.Ditta G., Stanfield S., Corbin D., Helinski D.R. Broad host range DNA cloning system for gram-negative bacteria: Construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan K., Surette M.G. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol. 2007;189:4827–4836. doi: 10.1128/JB.00043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H., Li L., Dong Z., Surette M.G., Duan K. The YebC family protein PA0964 negatively regulates the Pseudomonas aeruginosa quinolone signal system and pyocyanin production. J. Bacteriol. 2008;190:6217–6227. doi: 10.1128/JB.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoang T.T., Kutchma A.J., Becher A., Schweizer H.P. Integration-proficient plasmids for Pseudomonas aeruginosa: Site-specific integration and use for engineering of reporter and expression strains. Plasmid. 2000;43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 45.Poole K., Neshat S., Krebes K., Heinrichs D.E. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 1993;175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becher A., Schweizer H.P. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques. 2000;29:948–950. doi: 10.2144/00295bm04. [DOI] [PubMed] [Google Scholar]
- 47.Essar D.W., Eberly L., Hadero A., Crawford I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He X., Hwang H.M., Aker W.G., Wang P., Lin Y., Jiang X., He X. Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiol. Res. 2014;169:759–767. doi: 10.1016/j.micres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Wang S., Yu S., Zhang Z., Wei Q., Yan L., Ai G., Liu H., Ma L.Z. Coordination of swarming motility, biosurfactant synthesis, and biofilm matrix exopolysaccharide production in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2014;80:6724–6732. doi: 10.1128/AEM.01237-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohman D.E., Cryz S.J., Iglewski B.H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 1980;142:836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liberto M.C., Matera G., Quirino A., Lamberti A.G., Capicotto R., Puccio R., Barreca G.S., Focà E., Cascio A., Focà A. Phenotypic and genotypic evaluation of slime production by conventional and molecular microbiological techniques. Microbiol. Res. 2009;164:522–528. doi: 10.1016/j.micres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Fito-Boncompte L., Chapalain A., Bouffartigues E., Chaker H., Lesouhaitier O., Gicquel G., Bazire A., Madi A., Connil N., Véron W., et al. Full Virulence of Pseudomonas aeruginosa Requires OprF. Infect. Immun. 2011;79:1176–1186. doi: 10.1128/IAI.00850-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chugani S.A., Whiteley M., Lee K.M., D’Argenio D., Manoil C., Greenberg E.P. QscR, A modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2001;98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarabhai S., Sharma P., Capalash N. Ellagic acid derivatives from Terminalia chebula Retz. downregulate the expression of quorum sensing genes to attenuate Pseudomonas aeruginosa PAO1 virulence. PLoS ONE. 2013;8:e53441. doi: 10.1371/journal.pone.0053441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajkumari J., Borkotoky S., Murali A., Suchiang K., Mohanty S.K., Busi S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb. Pathog. 2018;118:48–60. doi: 10.1016/j.micpath.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Calfee M.W., Coleman J.P., Pesci E.C. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2001;98:11633–11637. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L., Wang W., Sun W., Surette M., Duan K. Characterization of a cryptic plasmid from Pseudomonas sp. and utilization of its temperature-sensitive derivatives for genetic manipulation. Plasmid. 2010;64:110–117. doi: 10.1016/j.plasmid.2010.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.