Abstract
The “normal” immune response to an insult triggers a highly regulated response determined by the interaction of various immunocompetent cells with pro- and anti-inflammatory cytokines. Under pathologic conditions, the massive elevation of cytokine levels (“cytokine storm”) could not be controlled until the recent development of hemoadsorption devices that are able to extract a variety of different DAMPs, PAMPs, and metabolic products from the blood. CytoSorb® has been approved for adjunctive sepsis therapy since 2011. This review aims to summarize theoretical knowledge, in vitro results, and clinical findings to provide the clinician with pragmatic guidance for daily practice. English-language and peer-reviewed literature identified by a selective literature search in PubMed and published between January 2016 and May 2021 was included. Hemoadsorption can be used successfully as adjunct to a complex therapeutic regimen for various conditions. To the contrary, this nonspecific intervention may potentially worsen patient outcomes in complex immunological processes. CytoSorb® therapy appears to be safe and useful in various diseases (e.g., rhabdomyolysis, liver failure, or intoxications) as well as in septic shock or cytokine release syndrome, although a conclusive assessment of treatment benefit is not possible and no survival benefit has yet been demonstrated in randomized controlled trials.
Keywords: hemoadsorption, CytoSorb®, amount of blood purified, COVID-19, immune system, cytokines, cytokine storm, sepsis, septic shock, hemophagocytic syndrome
1. Introduction to Hemoadsorption
Sepsis and septic shock are complex, life-threatening conditions with persistantly high [1] multiorgan failure-related mortality of up to 70%. Sepsis is the inglorious most common cause of death in critical care and one of the greatest challenges for healthcare systems worldwide [2,3,4,5].
Multiorgan failure in sepsis is predominately caused by dysfunctional microcirculation [6], induced and regulated by multiple humoral and cellular mechanisms. The different components of the immune system that are organized in highly complex, dynamic network-like structures are not well-understood [7]. The fact that sepsis and other inflammatory conditions are not uniform, but inter-individually distinct, causes clinically variable phenotypes of inflammatory states with alternating pro- and anti-inflammatory characteristics [8].
Conventional treatment includes early anti-infective use, volume resuscitation, and catecholamine therapy for hemodynamic stabilization and extracorporeal organ support, such as renal replacement therapy (RRT) [9]. Ideally, the dysregulated immune response is controlled and “immunologic homeostasis” is restored [10]. CytoSorb® is the most widely used hemoadsorption procedure at present [11] that specifically targets hyperinflammation by extracorporeal removal of pro-inflammatory substances, i.e., cytokines.
2. Methods
This scoping review was conducted in compliance with PRISMA-ScR [12]. Although less rigorous in methodology than a systematic review, the scoping review enjoys the benefit of answering important questions in the absence of highly graded evidence to the significance of hemoadsorption with CytoSorb®. We aimed to provide the clinician with an orientation to the state of research, to outline corresponding topic areas, and, without judging the methodological quality, to map the often-incomplete evidence. Our aim was to simplify the decision-making-process in practice for the intensive care physician at the bedside.
A selective literature search was performed in the PubMed database from January 2016 until 31 May 2021. The items used were: Basic of Immune System, CytoSorb, Hemoadsorption, Cytokine, Cytokine Storm, Sepsis, Septic Shock, and Hemophagocytic Syndrome. In addition, the database https://literature.cytosorb-therapy.com/ (accessed on 31 May 2021), which is freely available on the Internet, was included in the literature search to identify publications in sources not listed in PubMed.
English-language and peer-reviewed publications from January 2016 to May 2021 were included. In a few exceptions, seminal literature from before 2016 was also included. This was done after internal review if an important effect on overall understanding was expected.
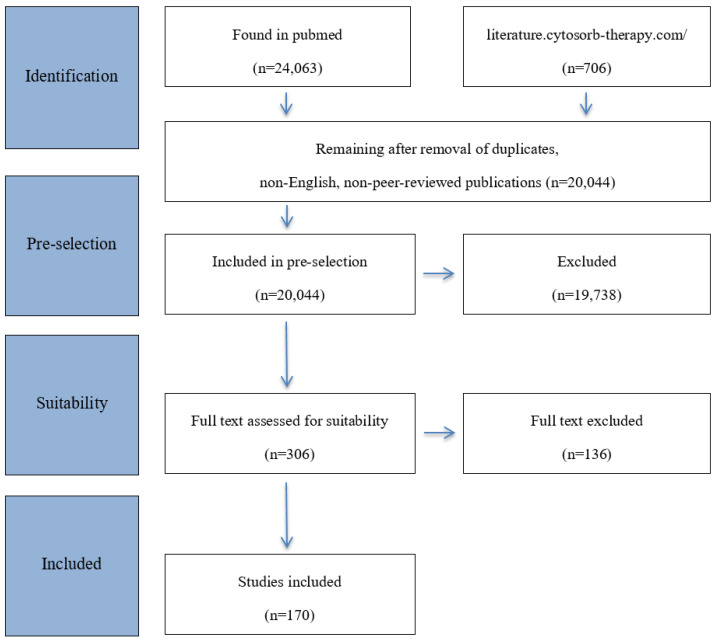
The primary publications found in the database search were then subjected to an internal multistep selection process. First, duplicates were removed. Selection was based on heading, keywords, and abstract, and weighted by publication date and context. Then, two by two experienced reviewers classified and included relevant publications (Figure 1) based on the following key criteria:
-
-
Infection/Host response
-
-
Regulation and dysregulation of the immune system
-
-
Cytokines and cytokine storm
-
-
Corona-Virus induced Disease 2019 (COVID-19)
-
-
Hemoadsorption with CytoSorb®—basic principles and function
-
-Hemoadsorption—Indications
-
○Systemic Inflammatory Response Syndrome (SIRS), sepsis and septic shock
-
○Trauma-induced inflammation (trauma, rhabdomyolysis)
-
○Liver failure, hyperbilirubinemia
-
○Acute respiratory distress syndrome (ARDS)
-
○Extracorporeal membrane oxygenation (ECMO)
-
○COVID-19-associated ARDS (CARDS)
-
○SIRS, perioperative use, cardiac surgery
-
○Intoxications
-
○Side effects
-
○Dosage of antibiotics
-
○
Figure 1.
Flowchart for study selection adapted from the PRISMA-ScR statement [12].
For these publications, the full text then assessed for its suitability with respect to the objective of the review and information was included accordingly. Each publication was evaluated according to the following criteria: (patho-)physiological basics, case reports, case series, letter, retrospective studies, prospective studies, and reviews. In order to provide an overview of the current state of clinical use, we deliberately refrained from a strict assessment of study quality and partially included publications of lower evidence (e.g., case reports).
The respective article assigned to the first appropriate group, so that, for example, the frequent formulation “Hemoadsorption with CytoSorb®” required a grouping in the chapter “Hemoadsorption”.
Finally, after extensive discussion among all authors, the texts assessed with respect to various factors (e.g., publication date, methodology, results, and impact) and summarized separately below for each topic.
3. Infection Response and Immune System Regulation
Injury or infection initially leads to local activation of humoral factors (e.g., complement factors) and activation of the innate immune system, e.g., via pattern recognition receptors (PRR’s). These are diverse sets of receptors located on the surfaces or internally of various cells of the immune system. They recognize both pathogen- and damage-associated molecular patterns (PAMPs/DAMPs). PAMPs (e.g., Staphylococcus aureus Toxic Shock syndrome Toxin and Clostridium perfringens toxin) are products formed by various microbes. DAMPs, released by damaged endogenous cells or formed during the processing of extracellular matrix, can trigger inflammatory reactions as alarmins via the induction of cytosolic multiprotein complexes, so-called inflammasomes with subsequent, e.g., gasdermin-D induced, proinflammatory cell death called pyroptosis [13,14,15,16].
3.1. Cytokines and the Cytokine Storm
Cytokines control cell proliferation and differentiation, the regulation of angiogenesis, and immune and inflammatory responses. Characteristic plasma cytokine profiles show TNFα, IL1, IL8, and MCP-1 peaks in the first minutes to hours after infection, followed by an IL6 increase [17,18]. IL10 is released later to control the inflammatory response [16,19].
Cytokines are key components to inflammation and their temporal and spatially fine-tuned interplay with, i.e., the cellular components of the immune system will most likely result in controlled inflammation with subsequent healing. However, severe injury and disease (Table 1) can cause overproduction of several cytokines that may cumulate in systemic inflammatory response syndrome (SIRS) and which is characterized by four criteria: tachypnea, tachycardia, leukopenia or leukocytosis (leucocyte count > 12,000 cells/μL or <4000/μL), and fever or hypothermia (body temperature > 38 °C or <36 °C). Although this syndrome presents itself inter-individually in very different degrees the SIRS criteria still seem useful to indicate a massive activation of the host defense triggered by, e.g., an infection [20].
Table 1.
External injuries and diseases that can trigger a SIRS potentially followed by Injury-associated Immunosuppression (IAI) or Sepsis Associated Immunosuppression (SAI) modified after [14].
| External Injuries | Disease |
|---|---|
| Polytrauma | Pancreatitis |
| Craniocerebral trauma | Liver insufficiency |
| Organ transplantation | Renal insufficiency |
| Burn | Stroke |
| Extensive surgery | Myocardial infarction |
| Cardio-pulmonary resuscitation | Heart failure |
| Cardiosurgical intervention | Sepsis and Septic Shock |
The term “cytokine storm” describes a very heterogeneous group of diseases characterized by excessive, life-threatening, potentially fatal hyperinflammation [21]. Once triggered, the massive release of proinflammatory cytokines as part of the early immune response causes shock and organ failure by microcirculatory disturbances and coagulopathy [9]. (Para-) clinical findings include: persistent fever, cytopenia, splenomegaly, hepatitis, or coagulopathies [22,23].
Infectious and non-infectious diseases such as active rheumatic diseases can cause a cytokine storm syndrome with massively elevated IL6 levels. [22]. In contrast, hemophagocytosis syndromes are characterized by high IFNγ and IL10 levels in combination with mildly elevated IL6 [24], an “anti-inflammatory cytokine storm with early immunosuppression”.
The pathophysiological response to the cytokine storm varies interindividually [17]. Elevated IL6 and resistin levels correlate with sepsis severity as well as end-organ damage and appear to be associated with increased mortality [25]. In general, high concentrations of proinflammatory cytokines are associated with increased mortality [26,27,28]. Elevated anti-inflammatory cytokines, such as IL10, are also predictive of sepsis severity and worsened outcome [28,29]. Relevant Cytokines are listed in Table 2.
Table 2.
Cytokine families and their functions with examples of pro- and anti-inflammatory cytokines; modified from [30,31,32].
| Family | Functions | Cytokine | Impact on Inflammation | Removal Rate on CytoSorb® (% at 120 min) [30]/[32] |
|---|---|---|---|---|
| Interferone (IFN) | Regulation of innate immunity; activation of antiviral effects; antiproliferative effects; pyrogenic effect. | IFNγ | Pro | 95.7/61 |
| Interleukine (IL) | Growth and differentiation of leukocytes. Proinflammatory effects: induction of cyclooxygenase II; expression of various adhesion molecules; NO synthase ↑; pyrogenic effect. |
IL1β IL2 IL6 IL7 |
Pro Pro/Anti Pro Pro |
97.2/n.d. 99.3/n.d. 99.6/78 n.d./n.d. |
| Antiinflammatory effects: inhibition of proinflammatory cytokine (e.g., IL1α, IL1β, TNF) and monocyte/macrophage, Promotion of Th2-lymphocytes | IL1RA IL4 IL10 IL11 IL13 |
Anti Anti Anti Anti Anti |
92.1/n.d. 99.9/n.d. 99.8/n.d. n.d./n.d. 94.2/n.d. |
|
| Chemokine | Control of chemotaxis; recruitment of leukocytes; predominantly proinflammatory activity. | IL8 MCP1 MIP-1α |
Pro Pro Pro |
100/n.d. 100/n.d. 97.3/97.4 |
| Colony-stimulating factors (CSF) | Stimulation of hematopoietic progenitor cell proliferation and -differentiation. | G-CSF | Pro | 99.4/n.d. |
| Transforming growth factors | Regulation of proliferation, differentiation, adhesion of cells. | TGFβ | Anti | n.d./n.d. |
| Tumor necrosis factor (TNF) | Proinflammatory; activates cytotoxic T-lymphocytes. | TNFα (Cachectin) |
Pro | 98.4/21.7 |
| Peptide hormone | Early-phase cytokine; uremic toxin; release from myeloid cells; neutrophil migration ↓; phagocytosis performance ↓. | Resistin | Pro | n.d./n.d. |
| Soluble Cytokine Receptors with Anti-inflammatory Activities | Inhibition of the natural ligands and thus suppression of the typical effect. | sIL-1RII sTNFRp55 |
Anti | n.d./n.d. |
n.d.: no data.
A severe, potentially fatal cytokine storm may possibly occur in association with COVID-19, leading to the excessive release of various pro- and anti-inflammatory cytokines (e.g., IL1, IL2, IL6 IL7, IL10, GCSF, IP10, MCP1, MIP1A, INFy, and TNFα) [33,34,35,36,37,38,39].
However, a recent, critically discussed rapid review [40] concluded that IL6, an important proinflammatory cytokine, synthesized in fibroblasts, monocytes, T-cells, and endothelial cells [41], is lower in patients with severe COVID-19 compared with other hyperinflammatory states associated with acute respiratory distress syndrome (ARDS), sepsis, or cytokine release syndrome (CRS) [42]. On the other hand, two randomized controlled trials (ReMAPCap and RECOVERY) showed that blockade of the IL6 pathway with tocilizumab improves the prognosis of COVID-19 patients [43,44]. In conclusion, the data remain controversial and it appears that the often-described hyperinflammation linked to COVID-19 is by no means the only pathomechanism [42].
3.2. Immunosuppression
To control inflammation, anti-inflammatory cytokines are increasingly released as the immune response progresses. Antigen presenting cell (APC) activation levels, expression of HLA-DR, and co-stimulatory molecules decrease. Antigen presentation, pro-inflammatory mediators, and phagocytosis are reduced. In consequence, an anti-inflammatory situation (IL10↑↑, APC↓) known as Sepsis Associated Immunosuppression (SAI) or Injury Associated Immunosuppression (IAI) may develop (Table 1). [14,45,46,47,48].
Major players in SAI may be myeloid-derived suppressor cells (MDSC). They mediate T-cell dysfunction, resulting in a higher incidence of secondary infections [48,49,50,51]. Another cellular entity contributing to SAI are regulatory T-cells (Tr1 cells), that, among other things, inhibit the activation of key inflammasomes (Nod-like receptor protein: pyrin domain containing 3 (NLRP3) inflammasome) through increased IL10 release [16,52]. Human leucocyte antigen (HLA-DR) plays an important role in T-cell activation as a key MHC class II molecule on monocytes/macrophages. HLA-DR establishes together with various co-factors (e.g., CD40-CD40L) the link between antigen-presenting cells and the T-cell receptor (TCR). The expression of HLA-DR is downregulated by the release of IL10. This may contribute to immunosuppression when IL10 levels are excessively high. As a clinical example, frequent immunosuppression with increase of infection (e.g., pneumonia) after CNS injury is (co-)caused by a reduced HLA-DR level (cut off: <8000 molecules/cell) longer than two days [14].
Based on a better understanding of the interplay of pro- and anti-inflammatory cytokines by overlapping, in part redundant, networks of cells and cytokines, new therapeutic approaches have been developed that, as with hemoadsorption, aim to modulate the amount of inflammatory cytokines as part of the host defense [34,35,38,53].
4. Hemoadsorption with CytoSorb®
4.1. Basics
To control a dysregulated immune response has long been the subject of various therapeutic efforts. The rational is to reduce the elevated concentrations of pro-and anti-inflammatory cytokines equally, with preserving their ratios, instead of intervening at a specific pathway in the complex immunological network [7,30]. Hemoadsorption is based on this therapeutic principle and aims to restore the immunologic balance [10] by reducing the plasma concentrations of pro- and anti-inflammatory mediators below a “toxic threshold” [54]. It is also possible that the change in the equilibrium of cytokines between the affected tissue and the blood plays an important role. As the concentration gradient increases, chemotaxis-mediated increased migration of immunocompetent cells into the affected region appears possible [55].
CytoSorb® (CytoSorbents Corporation, Monmouth Junction, NJ, USA), approved in Europe in 2011 for patients with excessive cytokine levels, is a high-tech polymer adsorbent with a total surface area of over 45,000 sqm and very high binding capacity that is applied for blood purification in an extracorporeal circuit. Highly porous polyvinylpyrrolidone-coated polystyrene-divinylbenzene beads (polymer beads with a size of: 300–800 µm) [41] bind through a combination of hydrophobic interactions, van der Waals’ forces, and charge-induced interactions a broad spectrum of molecules with a molecular weight <55 kDa. Various hydrophobic, pro-and anti-inflammatory mediators, immune response-triggering DAMPs or PAMPs, as well as endogenous metabolism-generated (e.g., bilirubin and myoglobin) or pharmacologic substances (e.g., anticoagulants and psychotropic drugs) are adsorbed [56,57,58,59,60,61,62]. Concentration is a major determinant of adsorption efficiency, meaning higher concentrations lead to faster adsorption [61,63].
The CytoSorb® adsorber is predominantly and in combination used with continuous renal replacement therapy. Operation as an additional hemoperfusion with any approved blood pump system is possible as well as integration into an extracorporeal membrane oxygenation (ECMO) circuit [64,65,66]. The required anticoagulation can be managed systemically with heparin or locoregionally with citrate [41,67,68].
4.2. Indications
Hemoadsorption with CytoSorb® may be used for the treatment in numerous indications (Table 3).
Table 3.
| Indications | Clinical Criteria | Paraclinical Criteria |
|---|---|---|
Rhabdomyolysis
|
Independent of renal function | Myoglobin > 1000 U/L (observe trend) |
Inflammation (SIRS) triggered by:
|
|
|
|
Liver failure/Hyperbilirubinemia bridging to transplant or to recovery |
Icterus |
|
|
Life-threatening bleeding under Direct Oral Anticoagulants (DOAC) |
Medical history (dose, last intake, extent of planned operation) Type of bleeding (major or minor bleeding) Availability of specific antidotes (andexanet alfa) |
Hemoadsorption with CytoSorb® requires an extracorporeal circuit, e.g., in the context of CRRT, ECMO or as a standalone procedure in the context of hemoperfusion. All these procedures have specific complications. In addition, the complications due to the intravascular access devices themselves must be considered.
If the CytoSorb® Adsorber is used as an adjunctive procedure, e.g., in septic shock or as part of ECMO treatment, these factors do not play a role. However, if primary CytoSorb® therapy is planned, the intensive care therapist must carefully consider the complications associated with the procedure when determining the indication. From the authors’ point of view, the use of CytoSorb® in the context of acute, time-critical treatment of intoxications (Table 4), life-threatening bleeding under DOAC [59], or acute rhabdomyolysis [60], e.g., in the context of malignant hyperthermia, is sufficiently well documented and should not be withheld from the patient, i.e., in these cases the establishment of an extracorporeal circuit, usually a CRRT, seems justified.
Table 4.
Drugs that can effectively be removed by CytoSorb® or in which a relevant decrease of serum concentrations must be expected (modified from [62]).
| Drug Group | Active Pharmaceutical Substances | References | |
|---|---|---|---|
| positive effect likely | Antiarrhythmics | Amitriptyline | [145] |
| Flecainide | [146] | ||
| Digoxin | [141] | ||
| Digitoxin | [147] | ||
| Antidepressant | Amitryptilin | [145,148] | |
| Anticonvulsants | Carbamazepine | [141] | |
| Valproic Acid | |||
| Phenytoin | |||
| Beta Blocker | Bisoprolol | [148] | |
| Calciumchannel blockers | Amlodipine | [148] | |
| Verapamil | [149] | ||
| Hypnotics and sedatives | Phenobarbital | [141] | |
| Psychotropic drugs | Quetiapine | [143] | |
| Venlafaxine | [150] | ||
| 3,4-Methylenedioxy-methamphetamine (MDMA, “Ecstasy”) | [151] | ||
| Toxins | Aflatoxine | [32,152] | |
| Toxic Shock Syndrome toxin-1 (TSST-1) | [32] | ||
| Viper Snake Venom | [153] | ||
|
positive or negative effect likely
(according to the indication) |
Anticoagulants | Dabigatran | [154] |
| Edoxaban | [155] | ||
| Rivaroxaban | [59,156] | ||
| Ticagrelor | [59,139,144,156] | ||
| Contrast agents | Iodixanol Iohexol |
[157,158] | |
| Immunosuppressives | Tacrolimus Cyclosporine |
[141] | |
| negative effect likely | Antibiotics | Amikacin, Vancomycin, Tobramycin, Gentamicin, Ciprofloxacin, Meropenem, Piperacillin, Flucloxacillin, Imipenem, Teicoplanin, Linezolid | [61,63,141,159,160] |
| Antimycotics | Fluconazole, Voriconazole | [63] |
In other situations (Table 3), we are very critical of the establishment of an extracorporeal circuit to perform hemoadsorption and consider the current data situation in this regard to be insufficient.
4.2.1. SIRS, Sepsis and Septic Shock
The adjunctive therapy of SIRS, sepsis, or septic shock with CytoSorb® should help to reduce, downregulate, or prevent an excessive immune response (“cytokine storm”). Capillary leakage and vasoplegia should be alleviated and the microcirculation improved [70]. Since no direct measurements of efficacy are available, a decrease in IL6-concentration and vasopressor requirement, or an increase in lactate clearance have been suggested as surrogate parameters [71]. A decrease in lactate of >2 mmol/L and vasopressor requirements, i.e., to below 20% of the initial dose [70], are supposed to indicate therapeutic success [65,71] whereby the duration of treatment should depend on the individual patient’s response [41]. Several in vitro studies exhibited effective adsorption of a broad spectrum of PAMPs and DAMPs or various cytokines by CytoSorb®. Procalcitonin is equally adsorbed, which must be taken into account in the interpretation of lab test results [30,32]. However, the IL6 concentration, which is frequently used to indicate start and efficacy of hemoadsorption therapy and for the assessment of the effect of hemoadsorption, has recently been questioned for its usefulness since the levels are balanced by the endogenous turn-over and the extracorporeal elimination [72,73,74]. CytoSorb® therapy could be useful in septic shock [75,76], but indication, indication thresholds and duration of therapy are controversially discussed. With explicit attention to the limitations of data from animal studies, especially the conditional applicability to humans, Peng et al. demonstrated prolonged survival in the rat model under hemoadsorption [77]. A randomized controlled trial demonstrated no mortality reduction for hemoadsorption in ARDS patients with low case severity (APACHE II: 23) [78]. Methodologically, the study was criticized for its short, discontinuous CytoSorb® use (6 h/d for 7 d) which did not significantly reduce IL6 levels. In contrast, data from the CytoSorb® registry reported a mean CytoSorb® application duration of 50 h [79]. Brouwer et al. reported a significant reduction of mortality in patients with septic shock if continuous CytoSorb® therapy was applied for 56 h [65]. However, the statistical method of “stabilized inverse probability of treatment weigths (sIPTW)” has been scrutinized [80]. Probably, the application of a higher “dose” (amount of blood purified (ABP), unit: l blood/kg bw), higher blood flow, in combination with a longer treatment (ca. 85 h), is associated with lower mortality [72]. More retrospective studies demonstrated a mortality benefit for hemoadsorption [81], especially if CytoSorb® therapy was started early (within 12 h) [82]. On the other hand, several recently published studies have failed to demonstrate any effect of hemoadsorption on mortality [74,83].
The optimization of application duration, adsorber exchange modalities, and blood flow rate may be crucial for success and is subject of intensive research.
However, clear evidence of a survival benefit for CytoSorb® therapy in the form of a randomized, prospective study (RCT) is still missing.
4.2.2. Trauma Induced Inflammation, Injury Associated Immunosuppression and Rhabdomyolysis
Various injuries ((poly-) trauma, burns, and major surgery) can lead to massive SIRS with, e.g., increased DAMPs-induced expression of pro- and anti-inflammatory mediators, occurring especially within the first 24 h [84,85]. Individually different, a phase of immune paralysis, the IAI follows. CytoSorb® may offer a therapeutic approach here, via control of the massive release of various DAMPs (e.g., high mobility group box-1 protein (HMGB1) or extracellular histones) [86]. Trauma is often aggravated by muscle injury and reperfusion syndrome with subsequent rhabdomyolysis. This may also be caused by reperfusion syndrome following occlusion of large vessels. Non-traumatic diseases (malignant hyperthermia, autoimmune diseases, and intoxications) may also be causative [87]. Myoglobin precipitation (haemoprotein MG17.8 kDa) in the renal tubules (crush kidney), combined with elevated concentrations of free oxygen radicals [88], can damage the kidneys to the point of acute failure (ARF) [89]. Important therapeutic measures include early aggressive volume therapy, consistent surgical therapy for compartmental syndromes [90], and, if necessary, RRT [91]. However, RRT cannot prevent ARF [90]. CytoSorb® rapidly lowers myoglobin serum levels due to its high myoglobin extraction rate and thus can be recommended as part of a multimodal treatment approach [87,88,92]. It remains unclear whether CytoSorb® can prevent ARF in rhabdomyolysis.
4.2.3. Liver Failure and Hyperbilirubinemia
In up to 40% of critically ill patients, hyperbilirubinemia is present with an increased risk of death [93]. Clinical and paraclinical findings typically include varying degrees of icterus, lactic acidosis, coagulation disorders, hepatic encephalopathy, and circulatory insufficiency. ARF is an additional complication. Acute or acute-on-chronic liver failure (ALF/ACLF) can be primary (e.g., viral) or secondary (e.g., cholestasis-, hypoxemia-, or shock-related). It is based on a complex immunopathology involving the release of various PAMPs and DAMPs, as well as the release of a variety of cytokines and the activation of various immune cells such as monocytes and macrophages. Frequently, localized hepatic inflammation leads to generalized vasopathy and multiorgan failure via SIRS [94,95].
In addition to water-soluble ammonia, hydrophilic direct bilirubin and bile acids, the accumulation of indirect, albumin-bound, i.e., hydrophobic bilirubin plays an important role in the pathogenesis of acute liver failure [57,96]. Progression to ALF or ACLF may be perpetuated by “hepatic SIRS” [97]. CytoSorb® adsorbs indirect bilirubin and bile acids and modulates the concentrations of involved cytokines [57,98] while RRT controls serum concentrations of ammonia and direct bilirubin [99,100]. With CytoSorb®, bilirubin is released from its strong albumin binding and gets adsorbed while albumin concentrations remain virtually unchanged [56,57], resulting in an overall improvement in liver function [41]. This is true even when used for several weeks [62]. CytoSorb® therapy may be a simple, user-friendly alternative to bridge to functional recovery or orthotopic liver transplantation [93,101]. Initial clinical trial results appear positive [69].
4.2.4. Acute Respiratory Distress Syndrome (ARDS) and Extracorporeal Membrane Oxygenation (ECMO)
Hallmarks of ARDS, one of the most common diagnoses in intensive care units [102], are acute inflammation with increased pulmonary vascular permeability, increased lung weight, and loss of aerated lung tissue [103]. The main symptom is rapidly progressive respiratory distress coupled with a “cytokine storm” that develops with the typical course of pro- and anti-inflammatory cytokine peaks [17,104].
Specific therapeutic procedures do not yet exist. Rather, evidence-based treatment is characterized by supportive therapeutic procedures. These include, for example, lung-protective ventilation [105], adequately high PEEP levels [106], prone positioning [107,108], or the establishment of extracorporeal membrane oxygenation (ECMO) [109,110].
In addition to the underlying disease, the foreign surface of the ECMO circuit itself, rapidly amplifies hyperinflammation by complement, endothelial, and leukocyte activation of varying degrees [66,111,112], making CytoSorb® therapy a rational option. Several studies have reported a decrease in extravascular lung water (normalization of pulmonary vascular permeability), vasopressor requirements [104,113], and inflammatory parameters, associated with rapid clinical stabilization [114]. The high ECMO blood flow rates have a dose-increasing, effect-enhancing, and thus, if high enough (ABP > 13 L/kg), possibly mortality-lowering effect [72].
4.2.5. COVID-19-Associated ARDS (CARDS)
In COVID-19, a potentially massive cytokine storm may cause lethal destruction of the lung with attenuated pulmonary vasoregulation, ventilation-perfusion mismatch, and high risk of thrombosis and impairment of other organ functions via microvascular damage (endothelitis) as well [33,35,36,37,53,115]. This may explain the phenotypic differences between “typical” ARDS and CARDS characterized by hypoxemia, normal compliance, and altered ventilation-perfusion ratio [116,117]. Based on pathophysiological considerations, some authors recommend to use [36,118,119,120] or consider [121] CytoSorb® in COVID-19. Since initial experience was positive and suggested a therapeutic effect [122], CytoSorb® was temporarily approved in April 2020 by the US Food and Drug Administration (FDA) for emergency use in patients with CRS under certain conditions [123].
Supady and colleagues studied the effect of CytoSorb® therapy in 34 patients with severe COVID-19 pneumonia who received ECMO [124] in a randomized controlled trial. The authors interpreted their data to mean that early initiation of CytoSorb® therapy had a negative effect on survival. Although seemingly well balanced, the study was critizised for incomplete data on treatment details and a survival rate in the control group with ECMO only [73,125] that was much higher than in any other ECMO study. Since no previous study had reported any detrimental effects, hemoadsorption for COVID-19 patients should be used with caution and only in the context of clinical trials.
4.2.6. Post-Pump Syndrome and Perioperative Use in Cardiac Surgery
Cardiopulmonary bypass (CPB) is by principle similar to ECMO, is routinely used in cardiac surgery, and is known to cause a complex inflammatory response with mediator release (C3a, C5a, histamine, IL6, IL8, and TNFα) immediately after blood contact with the foreign surface. Foremost is the activation of the complement and coagulation systems [58,112,126,127]. The SIRS in CPB, termed “post-pump syndrome”, is prognostically relevant [128,129,130] and clinically characterized by increased vascular permeability, decreased peripheral vascular resistance, hypotension and tachycardia, and an increased risk of thrombosis. This suggests that hemoadsorption is reasonable [127,131] and a high ABP may be favourable [72]. Various case series and retrospective studies demonstrated that CytoSorb® application resulted in normalization of cytokine levels, hemodynamic stabilization, reduced vasopressor requirements, less renal replacement therapy, and normalization of serum lactate concentrations [126,132,133,134,135,136].
Two randomized pilot studies on intraoperative CytoSorb® use under CPB failed to demonstrate any advantage for hemoadsorption, which may be due to the small patient numbers, low cytokine levels, and short CytoSorb® application duration [127,130].
In contrast, the multicenter study REFRESH I [58] showed a significant reduction in free hemoglobin, an independent predictor of mortality, on ECMO [137]. Following investigations could not confirm these findings [138].
4.2.7. Intoxications
Due to its binding properties, CytoSorb® is an option in the acute treatment of overdoses or intoxications with various drugs [98] (Table 4). These molecules often contain central hydrophobic structures (e.g., benzene rings) that are adsorbed on the polymer beads even in case of high plasma protein binding [139]. As example, therapeutic levels of rivaroxaban or ticagrelor can be eliminated before emergency surgery [59,140]. Maximization of blood flow rapidly leads to high clearance in time-critical situations. The duration of therapy, blood flow rates, and changing intervals considering the adsorber saturation kinetics should be individually adjusted to achieve optimal clearance [127,141,142,143,144].
4.3. Side Effects
CytoSorb® is a CE-approved bio- and hemocompatible medical device. Cellular components (e.g., leukocytes/platelets) are minimally extracted. In principle, adhesion of blood cells to the polymer beads is detectable by electron microscopy [138], but the induction of hemolysis or other clinically significant side effects have not been reported [161]. Similarly, there is no evidence for a “CytoSorb®-induced” inflammatory response or coagulopathy [138]. CytoSorb® therapy is considered to be safe [72,133]. However, it is to be expected that in patients receiving relevant long-term medication, e.g., anticonvulsants and immunosuppressives, the drug concentrations drop significantly during hemoadsorption [127].
Interaction with the CytoSorb® adsorber appears possible from a pharmacokinetic point of view for a variety of the drugs used in intensive care, but requires further investigation. As an example, the adsorption of triiodothyronine [127,162] can be considered. The function as an iodine donor for various immunological pathways is limited with it; e.g., neutrophil granulocyte function is compromised and mortality is increased in patients with low fT3 and fT4 levels in septic shock [163]. Whether hemoadsorption possibly enhances such a pathomechanism has not been investigated until now but cannot be ruled out pathophysiologically.
4.4. Dosage of Antibiotics
Antimicrobial chemotherapy is elementary in sepsis therapy and should start within the first hour of diagnosis [9,164]. Anti-infective dosing is very complex and depends on various determinants including hyperdynamic/hypodynamic circulation, volume of distribution, hepatic/renal clearance, albumin concentration, and extracorporeal organ support (ECMO, RRT) [165]. There is an increased risk of mis- or underdosing with hemoadsorption [166,167,168] and dosing needs to be adjusted even under CRRT [169].
CytoSorb® may aggravate this problem by an “anti-antibiotic effect”. Relevant adsorption phenomena (mainly in-vitro data) with high extraction rates [63] have been described for various anti-infectives (Table 4). According to animal data (hemoperfusion over 6h, without adsorber change), the influence of CytoSorb® on the total clearance of the investigated 17 various anti-infectives seems negligible. An additional dose has been recommended for fluconazole and linezolid only [160]. First clinical data for linezolid suggest similar behavior in humans [159]. Due to the initial high volume of distribution and the decrease in clearance within a few hours after CytoSorb® installation, various authors recommend an additional anti-infective dose per adsorber for a variety of antibiotics [127,141,159,170]. Drug monitoring under hemoadsorption therefore seems to be essential [160].
5. Perspectives
The interaction of various pro- or anti-inflammatory components of the immune system with different organ systems (cytokine storm) is causative for SIRS, sepsis, and septic shock, the most frequent indications for CytoSorb® use. Modulation of the host defense by “immunomodulation” with a “theoretical” reduction in the plasma concentration of various cytokines and good biocompatibility may potentially help to control shock and prevent multiorgan failure. However, potentially deleterious effects due to the nonspecific interference with complex immunologic processes should be considered. Therefore, hemoadsorption with CytoSorb® as adjunctive therapy in severe cytokine dysregulation states can currently only be recommended in the context of clinical trials. In septic shock, a decrease in mortality under hemoadsorption with CytoSorb® has not yet been clearly demonstrated. Whether a postulated “anti-antibiotic effect” has undesired effects remains speculative. On the other hand, it remains undetermined which patients, if any, could potentially benefit from such a therapy. Possibly, a recently presented dynamic scoring system may be useful to help identifying the right patients [82]. None of these have been scrutinized by adequately powered randomized controlled trials. It is possible that the lack of clear data to date is due to the heterogeneity of the studies published to date. This relates to differences in study type, statistical models, patient populations studied, study design, or primary or secondary outcome. For illustration, some studies on the use of CytoSorb® in septic shock are summarized in Table 5.
Table 5.
A selection of current studies that have investigated the use of CytoSorb® therapy in sepsis and septic shock. Note the heterogeneity of the studies in terms of study design, patient populations, and outcome parameters. n.d. no data, CS—CytoSorb®, RCT—randomized controlled trail, SOFA—Sepsis Organ Failure Assessment Score, APACHE II—Acute Physiology and chronic Health Evaluation Score.
| Author | Indication | Study Design | Number of Patients | APACHE II | SOFA (pre) | Procedure | Blood Flow (mL/min) | Adsorber Useful Life (h) | No of Adsorber/Patient | Change Interval (h) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scharf et al. Ann. Intensive Care. 2021 [74] |
Septic shock (“cytokine storm”) | Propensity score matching analysis; retrospective | 38 with CS. 105 without CS. |
n.d. | n.d. | ECMO, RRT, Hemo-perfusion | n.d. | 7–12 (Median 9) | 1 | n.d. | No difference between groups |
| Schultz et al. Journal of Critical Care. 2021 [72] |
Septic shock | Retrospective cohort study | 70 with CS. | 30.2 | 13.8 | CVVHD | 100–200 | 26.75 | 3.2 | 24 | With high dose 28-d mortality ↓ |
| Supady et al. Lancet Respir. Med. 2021 [124] |
Severe COVID 19-pneumonia with ECMO | Single centre, open-label RCT | 17 with CS. 17 without CS. |
n.d. | 9.0 9.0 |
ECMO | 100–700 | 24 | 3 | 24 | 28 d mortality ↑ |
| Rugg et al. Biomedicines. 2020 [124] |
Septic shock | Retrospective study; “genetic” matched analysis | 42 with CS. 42 without CS. |
n.d. | 13.0 12.0 |
CRRT | n.d. | 24 (38 Patients had only 1 CS) |
1 | 24 | 28 d and in hospital Mortality ↓ |
| Kogelmann et al. Journal of the Intensive Care Society. 2020 [104] |
septic shock (Pneumonia + ARDS + ECMO) | case series | 7 | 28–56 | 11–16 | CVVHD | 100–150 | (12)/24 | 4.14 | (12)/24 | Observed mortality ↓ vs. predicted mortality |
| Schitteck et al. Ann. Intensive Care. 2020 [83] |
septic shock | Retrospective and prospective cohort study | 43 with CS. 33 without CS. |
39 35 |
n.d. | CVVHDF | n.d. | n.d. | n.d. | changed with the CRRT |
No difference between groups in mortality LOS ICU ↓ |
| Brouwer et al. Crit. Care. 2019 [65] |
septic shock | propensity score weighted retrospective | 67 with CS. 49 without CS. |
n.d. | 13.8 12.8 |
CRRT | 250–400 | 24 | n.d. | 24 | 28 d mortality ↓ |
| Schädler et al. PlosOne. 2017 [78] |
severe sepsis, septic shock + ALI | multicenter RCT | 47 with CS. 50 without CS. |
24.6 23.8 |
n.d. | Hemo-perfusion | 200–250 | 6 (for 7 d) | 7 | 24 | no effect |
| Kogelmann et al. Crit. Care. 2017 [70] |
septic shock | case series | 26 | 27–48 | 8–20 | CVVHD | 100–150 | (12)/24 | 2.61 | (12)/24 | Observed mortality ↓ vs. predicted mortality |
| Friesecke et al. J. Artif. Organs. 2017 [71] |
septic shock | Prospective interventional study | 20 | n.d. | 14.3 | CVVH/CVVHD | 189 142 |
~24 | n.d. | 24 | Lactat ↓, Vasopressor ↓ Interleukin 6 ↓ |
Therefore, prospective studies of single indications for clearly defined patient groups, e.g., severe burn patients with cytokine storm and myoglobinemia, under more precisely definable “experimental” conditions would be useful to generate evidence for the respective efficacy or inefficacy of hemoadsorption on the basis of possibly also smaller study cohorts.
Due to its special physicochemical properties, the range of indications for CytoSorb® has been significantly expanded in recent years. Hemoadsorption can be part of complex treatment regimens in liver failure, rhabdomyolysis, or intoxication.
Further high-quality randomized controlled trials of hemoadsorption are urgently needed and should consider factors such as blood flow, dose (amount of blood purified (ABP)), average adsorber use time, and total duration of hemoadsorption (Table 5). Potential side effects or interactions, in addition to a patient population clearly defined by indication and disease severity, should be equally investigated. Based on the findings, relevant contraindications should be formulated. Getting answers to these questions would be a prerequisite for adopting CytoSorb® therapy into routine intensive care medicine.
Acknowledgments
The authors would like to thank all employees of the clinics and institutes mentioned above who were involved in the clinical treatment.
Abbreviations
| ABP | Amount of blood purified [L/kg] |
| ACLF | Acute-on-chronic liver failure |
| ALF | Acute liver failure |
| ARF | Acute renal failure |
| APC | Antigen presenting cell |
| ARDS | Acute respiratory distress syndrome |
| COVID-19 | Corona-Virus induced Disease 2019 |
| CRS | Cytokine release syndrome |
| DAMPs | damage-associated molecular patterns |
| HMGB1 | high mobility group box-1 protein |
| IAI | Injury Associated Immunosuppression |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDSC | myeloid-derived suppressor cells |
| NLRP3 | Nod-like receptor protein: pyrin domain containing 3 |
| IL | interleukin |
| RRT | renal replacement therapy |
| PAMPs | pathogen-associated molecular patterns |
| PRR’s | Pattern recognition receptors |
| SAI | Sepsis Associated immunosuppression |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Corona-Virus 2 |
| SIRS | Systemic Inflammatory Response Syndrome |
| sIL-1RII | Soluble IL-1 receptor type 2 |
| sTNFRp55 | Solube TNF receptor p55 |
| TSST-1 | Toxic Shock syndrome Toxin-1 |
Author Contributions
T.K. has conducted the literature search, reviewed, assigned, and evaluated and ex- or included the literature according to the methodological guidelines, and was a major contributor in writing of the manuscript; E.S. was involved in the literature search, conceived the methodology and participated in writing of the manuscript; J.P. has accompanied the literature research, has assigned the literature (preselection), was responsible for organizational aspects and has contributed to the manuscript; C.K. has evaluated and ex-/included literature according to the methodological guidelines, and has contributed the manuscript; D.H. has revised the manuscript; C.E. has evaluated and ex-/included literature according to the methodological guidelines, and helped to revise the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived because this is a review of the currently available literature and thus a presentation of the current state of medical science to place hemoadsorption with CytoSorb® in the clinical context.
Informed Consent Statement
Obtaining informed consent from patients was not required because reference was made only to previously published studies.
Data Availability Statement
This article contains only previously published data that have been validated through a peer-review process. No new data were generated or analyzed in this study. Data sharing is not relevant to this article.
Conflicts of Interest
The authors declare that they have no competing interests. TK has received lecture fees from Cytosorbent Europe. The Department of Anesthesiology, Surgical Intensive Care, Emergency and Pain Medicine, Ruhr University Bochum, Klinikum Herford, 32120 Herford, Germany has received an unrestricted research grant from Cytosorbent Europe in 2018.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luhr R., Cao Y., Söderquist B., Cajander S. Trends in sepsis mortality over time in randomised sepsis trials: A systematic literature review and meta-analysis of mortality in the control arm, 2002–2016. Crit. Care. 2019;23:241. doi: 10.1186/s13054-019-2528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torio C.M., Andrews R.M. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); Rockville, MD, USA: 2013. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160. [PubMed] [Google Scholar]
- 3.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K. Global burden of sepsis: A systematic review. Crit. Care. 2015;19:P21. doi: 10.1186/cc14101. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C., Thomas-Rueddel D.O., Hartmann M., Hartog C.S., Welte T., Heublein S., Dennler U., Reinhart K. Hospital Incidence and Mortality Rates of Sepsis. Dtsch. Arztebl. Int. 2016;113:159–166. doi: 10.3238/arztebl.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SepNet Critical Care Trials Group Incidence of severe sepsis and septic shock in German intensive care units: The prospective, multicentre INSEP study. Intensive Care Med. 2016;42:1980–1989. doi: 10.1007/s00134-016-4504-3. [DOI] [PubMed] [Google Scholar]
- 6.Henzler D., Scheffler M., Westheider A., Köhler T. Microcirculation measurements: Barriers for use in clinical routine. Clin. Hemorheol. Microcirc. 2017;67:505–509. doi: 10.3233/CH-179229. [DOI] [PubMed] [Google Scholar]
- 7.Rieckmann J.C., Geiger R., Hornburg D., Wolf T., Kveler K., Jarrossay D., Sallusto F., Shen-Orr S.S., Lanzavecchia A., Mann M., et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat. Immunol. 2017;18:583–593. doi: 10.1038/ni.3693. [DOI] [PubMed] [Google Scholar]
- 8.Morrell E.D., Kellum J.A., Pastor-Soler N.M., Hallows K.R. Septic acute kidney injury: Molecular mechanisms and the importance of stratification and targeting therapy. Crit. Care. 2014;18:501. doi: 10.1186/s13054-014-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., Kumar A., Sevransky J.E., Sprung C.L., Nunnally M.E., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 10.Honore P.M., Hoste E., Molnár Z., Jacobs R., Joannes-Boyau O., Malbrain M.L.N.G., Forni L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care. 2019;9:56. doi: 10.1186/s13613-019-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krenn C.G., Steltzer H. Hemoadsorption for blood purification-incomparability of clinically available procedures. Med. Klin. Intensivmed. Notf. 2021;116:449–453. doi: 10.1007/s00063-020-00702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 13.Diamond C.E., Khameneh H.J., Brough D., Mortellaro A. Novel perspectives on non-canonical inflammasome activation. Immunotargets Ther. 2015;4:131–141. doi: 10.2147/ITT.S57976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfortmueller C.A., Meisel C., Fux M., Schefold J.C. Assessment of immune organ dysfunction in critical illness: Utility of innate immune response markers. Intensive Care Med. Exp. 2017;5:49. doi: 10.1186/s40635-017-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D., Han Z., Oppenheim J.J. Alarmins and immunity. Immunol. Rev. 2017;280:41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V. Inflammasomes: Pandora’s box for sepsis. J. Inflamm. Res. 2018;11:477–502. doi: 10.2147/JIR.S178084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonavia A., Miller L., Kellum J.A., Singbartl K. Hemoadsorption corrects hyperresistinemia and restores anti-bacterial neutrophil function. Intensive Care Med. Exp. 2017;5:36. doi: 10.1186/s40635-017-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fajgenbaum D.C., June C.H. Cytokine Storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supady A., Bode C., Duerschmied D. Cytokine Storm. N. Engl. J. Med. 2021;384:e59. doi: 10.1056/NEJMc2036236. [DOI] [PubMed] [Google Scholar]
- 22.Weaver L.K., Behrens E.M. Weathering the storm: Improving therapeutic interventions for cytokine storm syndromes by targeting disease pathogenesis. Curr. Treat. Opt. Rheumatol. 2017;3:33–48. doi: 10.1007/s40674-017-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonavia A., Groff A., Karamchandani K., Singbartl K. Clinical Utility of Extracorporeal Cytokine Hemoadsorption Therapy: A Literature Review. Blood Purif. 2018;46:337–349. doi: 10.1159/000492379. [DOI] [PubMed] [Google Scholar]
- 24.Tothova Z., Berliner N. Hemophagocytic Syndrome and Critical Illness: New Insights into Diagnosis and Management. J. Intensive Care Med. 2015;30:401–412. doi: 10.1177/0885066613517076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singbartl K., Miller L., Ruiz-Velasco V., Kellum J.A. Reversal of Acute Kidney Injury-Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit. Care Med. 2016;44:e492–e501. doi: 10.1097/CCM.0000000000001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murugan R., Wen X., Shah N., Lee M., Kong L., Pike F., Keener C., Unruh M., Finkel K., Vijayan A., et al. Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol. Dial. Transpl. 2014;29:1854–1864. doi: 10.1093/ndt/gfu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinto B.M., Iizuka I.J., Monte J.C., Santos B.F., Pereira V., Durão M.S., Dalboni M.A., Cendoroglo M., Santos O.F., Batista M.C. TNF-α depuration is a predictor of mortality in critically ill patients under continuous veno-venous hemodiafiltration treatment. Cytokine. 2015;71:255–260. doi: 10.1016/j.cyto.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Frencken J.F., van Vught L.A., Peelen L.M., Ong D.S.Y., Klein Klouwenberg P.M.C., Horn J., Bonten M.J.M., van der Poll T., Cremer O.L., Consortium M. An Unbalanced Inflammatory Cytokine Response Is Not Associated With Mortality Following Sepsis: A Prospective Cohort Study. Crit. Care Med. 2017;45:e493–e499. doi: 10.1097/CCM.0000000000002292. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhry H., Zhou J., Zhong Y., Ali M.M., McGuire F., Nagarkatti P.S., Nagarkatti M. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669–684. [PMC free article] [PubMed] [Google Scholar]
- 30.Malard B., Lambert C., Kellum J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018;6:12. doi: 10.1186/s40635-018-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy K., Weaver C. Janeway Immunologie. 9th ed. Springer Spektrum; Berlin/Heidelberg, Germany: 2018. [Google Scholar]
- 32.Gruda M.C., Ruggeberg K.G., O’Sullivan P., Guliashvili T., Scheirer A.R., Golobish T.D., Capponi V.J., Chan P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE. 2018;13:e0191676. doi: 10.1371/journal.pone.0191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Napp L.C., Bauersachs J. Extracorporeal Hemoadsorption: An Option for COVID-19-Associated Cytokine Storm Syndrome. Shock. 2020;54:700–701. doi: 10.1097/SHK.0000000000001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J., et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Assessing the importance of interleukin-6 in COVID-19. Lancet Respir. Med. 2021;9:e13. doi: 10.1016/S2213-2600(20)30600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houschyar K.S., Pyles M.N., Rein S., Nietzschmann I., Duscher D., Maan Z.N., Weissenberg K., Philipps H.M., Strauss C., Reichelt B., et al. Continuous hemoadsorption with a cytokine adsorber during sepsis—A review of the literature. Int. J. Artif. Organs. 2017;40:205–211. doi: 10.5301/ijao.5000591. [DOI] [PubMed] [Google Scholar]
- 42.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O., et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., Annane D., Beane A., van Bentum-Puijk W., Berry L.R., et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boomer J.S., To K., Chang K.C., Takasu O., Osborne D.F., Walton A.H., Bricker T.L., Jarman S.D., Kreisel D., Krupnick A.S., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venet F., Lukaszewicz A.C., Payen D., Hotchkiss R., Monneret G. Monitoring the immune response in sepsis: A rational approach to administration of immunoadjuvant therapies. Curr. Opin. Immunol. 2013;25:477–483. doi: 10.1016/j.coi.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfortmueller C.A., Meisel C., Schefold J.C. Interpreting Immune Mediator Dysbalance in Sepsis. Crit. Care Med. 2017;45:e1094–e1095. doi: 10.1097/CCM.0000000000002547. [DOI] [PubMed] [Google Scholar]
- 48.Uhel F., Azzaoui I., Grégoire M., Pangault C., Dulong J., Tadié J.M., Gacouin A., Camus C., Cynober L., Fest T., et al. Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. Am. J. Respir. Crit. Care Med. 2017;196:315–327. doi: 10.1164/rccm.201606-1143OC. [DOI] [PubMed] [Google Scholar]
- 49.Darcy C.J., Minigo G., Piera K.A., Davis J.S., McNeil Y.R., Chen Y., Volkheimer A.D., Weinberg J.B., Anstey N.M., Woodberry T. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit. Care. 2014;18:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janols H., Bergenfelz C., Allaoui R., Larsson A.M., Rydén L., Björnsson S., Janciauskiene S., Wullt M., Bredberg A., Leandersson K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J. Leukoc. Biol. 2014;96:685–693. doi: 10.1189/jlb.5HI0214-074R. [DOI] [PubMed] [Google Scholar]
- 51.Mathias B., Delmas A.L., Ozrazgat-Baslanti T., Vanzant E.L., Szpila B.E., Mohr A.M., Moore F.A., Brakenridge S.C., Brumback B.A., Moldawer L.L., et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Ann. Surg. 2017;265:827–834. doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singhal T. A Review of Coronavirus Disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronco C., Tetta C., Mariano F., Wratten M.L., Bonello M., Bordoni V., Cardona X., Inguaggiato P., Pilotto L., d’Intini V., et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif. Organs. 2003;27:792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 55.Girardot T., Schneider A., Rimmelé T. Blood Purification Techniques for Sepsis and Septic AKI. Semin. Nephrol. 2019;39:505–514. doi: 10.1016/j.semnephrol.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Calabrò M.G., Febres D., Recca G., Lembo R., Fominskiy E., Scandroglio A.M., Zangrillo A., Pappalardo F. Blood Purification with CytoSorb in Critically Ill Patients: Single-Center Preliminary Experience. Artif. Organs. 2019;43:189–194. doi: 10.1111/aor.13327. [DOI] [PubMed] [Google Scholar]
- 57.Gemelli C., Cuoghi A., Magnani S., Atti M., Ricci D., Siniscalchi A., Mancini E., Faenza S. Removal of Bilirubin with a New Adsorbent System: In Vitro Kinetics. Blood Purif. 2019;47:10–15. doi: 10.1159/000492378. [DOI] [PubMed] [Google Scholar]
- 58.Gleason T.G., Argenziano M., Bavaria J.E., Kane L.C., Coselli J.S., Engelman R.M., Tanaka K.A., Awad A., Sekela M.E., Zwischenberger J.B. Hemoadsorption to Reduce Plasma Free Hemoglobin during Cardiac Surgery: Results of REFRESH I Pilot Study. Semin. Thorac. Cardiovasc. Surg. 2019;31:783–793. doi: 10.1053/j.semtcvs.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Hassan K., Kannmacher J., Wohlmuth P., Budde U., Schmoeckel M., Geidel S. Cytosorb Adsorption During Emergency Cardiac Operations in Patients at High Risk of Bleeding. Ann. Thorac. Surg. 2019;108:45–51. doi: 10.1016/j.athoracsur.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Dilken O., Ince C., van der Hoven B., Thijsse S., Ormskerk P., de Geus H.R.H. Successful Reduction of Creatine Kinase and Myoglobin Levels in Severe Rhabdomyolysis Using Extracorporeal Blood Purification (CytoSorb®) Blood Purif. 2020;49:1–5. doi: 10.1159/000509898. [DOI] [PubMed] [Google Scholar]
- 61.Dimski T., Brandenburger T., MacKenzie C., Kindgen-Milles D. Elimination of glycopeptide antibiotics by cytokine hemoadsorption in patients with septic shock: A study of three cases. Int. J. Artif. Organs. 2020;43:753–757. doi: 10.1177/0391398820917151. [DOI] [PubMed] [Google Scholar]
- 62.Köhler T., Pletz M.W., Altmann S., Kirchner C., Schwier E., Henzler D., Winde G., Eickmeyer C. Pericarditis Caused by Enterococcus faecium with acute liver failure treated by a multifaceted approach including antimicrobials and hemoadsorption. Case Rep. Crit. Care. 2021;2021:8824050. doi: 10.1155/2021/8824050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.König C., Röhr A.C., Frey O.R., Brinkmann A., Roberts J.A., Wichmann D., Braune S., Kluge S., Nierhaus A. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs. 2019;42:57–64. doi: 10.1177/0391398818812601. [DOI] [PubMed] [Google Scholar]
- 64.Datzmann T., Träger K. Extracorporeal membrane oxygenation and cytokine adsorption. J. Thorac. Dis. 2018;10:S653–S660. doi: 10.21037/jtd.2017.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brouwer W.P., Duran S., Kuijper M., Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care. 2019;23:317. doi: 10.1186/s13054-019-2588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Napp L.C., Ziegeler S., Kindgen-Milles D. Rationale of Hemoadsorption during Extracorporeal Membrane Oxygenation Support. Blood Purif. 2019;48:203–214. doi: 10.1159/000500015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maynar J., Martínez-Sagasti F., Herrera-Gutiérrez M., Martí F., Candel F.J., Belda J., Castaño S., Sanchez-Izquierdo J. Direct hemoperfusion with polymyxin B-immobilized cartridge in severe sepsis due to intestinal perforation: Hemodynamic findings and clinical considerations in anticoagulation therapy. Rev. Esp. Quim. 2013;26:151–158. [PubMed] [Google Scholar]
- 68.Dimski T., Brandenburger T., Slowinski T., Kindgen-Milles D. Feasibility and safety of combined cytokine adsorption and continuous veno-venous hemodialysis with regional citrate anticoagulation in patients with septic shock. Int. J. Artif. Organs. 2020;43:10–16. doi: 10.1177/0391398819866459. [DOI] [PubMed] [Google Scholar]
- 69.Scharf C., Liebchen U., Paal M., Becker-Pennrich A., Irlbeck M., Zoller M., Schroeder I. Successful elimination of bilirubin in critically ill patients with acute liver dysfunction using a cytokine adsorber and albumin dialysis: A pilot study. Sci. Rep. 2021;11:10190. doi: 10.1038/s41598-021-89712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kogelmann K., Jarczak D., Scheller M., Drüner M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care. 2017;21:74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friesecke S., Stecher S.S., Gross S., Felix S.B., Nierhaus A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs. 2017;20:252–259. doi: 10.1007/s10047-017-0967-4. [DOI] [PubMed] [Google Scholar]
- 72.Schultz P., Schwier E., Eickmeyer C., Henzler D., Köhler T. High-dose CytoSorb hemoadsorption is associated with improved survival in patients with septic shock: A retrospective cohort study. J. Crit. Care. 2021;64:184–192. doi: 10.1016/j.jcrc.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Koehler T., Schwier E., Henzler D., Eickmeyer C. Does adjunctive hemoadsorption with CytoSorb affect survival of COVID-19 patients on ECMO? A critical statement. J. Crit. Care. 2021;66:187–188. doi: 10.1016/j.jcrc.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scharf C., Schroeder I., Paal M., Winkels M., Irlbeck M., Zoller M., Liebchen U. Can the cytokine adsorber CytoSorb® help to mitigate cytokine storm and reduce mortality in critically ill patients? A propensity score matching analysis. Ann. Intensive Care. 2021;11:115. doi: 10.1186/s13613-021-00905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh Y.P., Chhabra S.C., Lashkari K., Taneja A., Garg A., Chandra A., Chhabra M., Singh G.P., Jain S. Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb®) in the management of septic shock: A retrospective observational study. Int. J. Artif. Organs. 2020;43:372–378. doi: 10.1177/0391398819891739. [DOI] [PubMed] [Google Scholar]
- 76.Paul R., Sathe P., Kumar S., Prasad S., Aleem M., Sakhalvalkar P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device CytoSorb. World J. Crit. Care Med. 2021;10:22–34. doi: 10.5492/wjccm.v10.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng Z.Y., Carter M.J., Kellum J.A. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit. Care Med. 2008;36:1573–1577. doi: 10.1097/CCM.0b013e318170b9a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schädler D., Pausch C., Heise D., Meier-Hellmann A., Brederlau J., Weiler N., Marx G., Putensen C., Spies C., Jörres A., et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE. 2017;12:e0187015. doi: 10.1371/journal.pone.0187015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brunkhorst F., Schein M., Braune A., Schumacher U. 9th International Congress “Sepsis and Multiorgan Dysfunction”—International Registry on the use of the CytoSorb-adsorber in ICU Patients ( NCT02312024)—Results from the 8th interim analysis. Infection. 2019;45:S1–S67. doi: 10.1007/s15010-019-01341-2. [DOI] [Google Scholar]
- 80.Nadeem R., Obaida Z.A., Kamat S. Hemoadsorption of cytokines by CytoSorb filter: A simulation study without human factor-pilot is the difference. Crit. Care. 2020;24:13. doi: 10.1186/s13054-019-2716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rugg C., Klose R., Hornung R., Innerhofer N., Bachler M., Schmid S., Fries D., Ströhle M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective ‘Genetic’ Matched Analysis. Biomedicines. 2020;8:539. doi: 10.3390/biomedicines8120539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kogelmann K., Hübner T., Schwameis F., Drüner M., Scheller M., Jarczak D. First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock. J. Clin. Med. 2021;10:2939. doi: 10.3390/jcm10132939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schittek G.A., Zoidl P., Eichinger M., Orlob S., Simonis H., Rief M., Metnitz P., Fellinger T., Soukup J. Adsorption therapy in critically ill with septic shock and acute kidney injury: A retrospective and prospective cohort study. Ann. Intensive Care. 2020;10:154. doi: 10.1186/s13613-020-00772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finnerty C.C., Jeschke M.G., Herndon D.N., Gamelli R., Gibran N., Klein M., Silver G., Arnoldo B., Remick D., Tompkins R.G., et al. Temporal cytokine profiles in severely burned patients: A comparison of adults and children. Mol. Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neher M.D., Weckbach S., Flierl M.A., Huber-Lang M.S., Stahel P.F. Molecular mechanisms of inflammation and tissue injury after major trauma—Is complement the “bad guy“? J. Biomed. Sci. 2011;18:90. doi: 10.1186/1423-0127-18-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber B., Lackner I., Baur M., Fois G., Gebhard F., Marzi I., Schrezenmeier H., Relja B., Kalbitz M. Effects of Circulating HMGB-1 and Histones on Cardiomyocytes-Hemadsorption of These DAMPs as Therapeutic Strategy after Multiple Trauma. J. Clin. Med. 2020;9:1421. doi: 10.3390/jcm9051421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scharf C., Liebchen U., Paal M., Irlbeck M., Zoller M., Schroeder I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit. Care. 2021;25:41. doi: 10.1186/s13054-021-03468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linden K., Scaravilli V., Kreyer S.F., Belenkiy S.M., Stewart I.J., Chung K.K., Cancio L.C., Batchinsky A.I. Evaluation of the Cytosorb™ Hemoadsorptive Column in a Pig Model of Severe Smoke and Burn Injury. Shock. 2015;44:487–495. doi: 10.1097/SHK.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 89.Coban Y.K. Rhabdomyolysis, compartment syndrome and thermal injury. World J. Crit. Care Med. 2014;3:1–7. doi: 10.5492/wjccm.v3.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michelsen J., Cordtz J., Liboriussen L., Behzadi M.T., Ibsen M., Damholt M.B., Møller M.H., Wiis J. Prevention of rhabdomyolysis-induced acute kidney injury—A DASAIM/DSIT clinical practice guideline. Acta Anaesthesiol. Scand. 2019;63:576–586. doi: 10.1111/aas.13308. [DOI] [PubMed] [Google Scholar]
- 91.Bagley W.H., Yang H., Shah K.H. Rhabdomyolysis. Intern. Emerg. Med. 2007;2:210–218. doi: 10.1007/s11739-007-0060-8. [DOI] [PubMed] [Google Scholar]
- 92.Daum H.C., Schmidt B.M.W., Napp L.C. Effects of Hemoadsorption with CytoSorb during Severe Rhabdomyolysis. Blood Purif. 2020;50:273–274. doi: 10.1159/000508277. [DOI] [PubMed] [Google Scholar]
- 93.Piwowarczyk P., Kutnik P., Potręć-Studzińska B., Sysiak-Sławecka J., Rypulak E., Borys M., Czczuwar M. Hemoadsorption in isolated conjugated hyperbilirubinemia after extracorporeal membrane oxygenation support. Cholestasis of sepsis: A case report and review of the literature on differential causes of jaundice in ICU patient. Int. J. Artif. Organs. 2019;42:263–268. doi: 10.1177/0391398819834012. [DOI] [PubMed] [Google Scholar]
- 94.Triantafyllou E., Woollard K.J., McPhail M.J.W., Antoniades C.G., Possamai L.A. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front. Immunol. 2018;9:2948. doi: 10.3389/fimmu.2018.02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bajaj J.S., Kamath P.S., Reddy K.R. The Evolving Challenge of Infections in Cirrhosis. N. Engl. J. Med. 2021;384:2317–2330. doi: 10.1056/NEJMra2021808. [DOI] [PubMed] [Google Scholar]
- 96.Jalan R. Acute liver failure: Current management and future prospects. J. Hepatol. 2005;42((Suppl. S1)):S115–S123. doi: 10.1016/j.jhep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 97.Clària J., Stauber R.E., Coenraad M.J., Moreau R., Jalan R., Pavesi M., Amorós À., Titos E., Alcaraz-Quiles J., Oettl K., et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–1264. doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 98.Riva I., Broletti V., Soffia S., Napolitano G., Freddi C., Amboni P., Marchesi G., Fabretti F. Comparative Efficacy Between Two In Vivo Techniques for Bilirubin and Bile Acids Removal. Blood Purif. 2019;47:3–37. doi: 10.1159/000500179. [DOI] [Google Scholar]
- 99.Acar U., Gökkaya Z., Akbulut A., Ferah O., Yenidünya Ö., Açık M.E., Tokat Y., Yentür E. Impact of Cytokine Adsorption Treatment in Liver Failure. Transpl. Proc. 2019;51:2420–2424. doi: 10.1016/j.transproceed.2019.01.167. [DOI] [PubMed] [Google Scholar]
- 100.Guarneri M., Calandra L., Di Bella R., Riccobene R., Vccaro F., Mulè G., Vicari E., Vella D., Montalbano K., Tranchida V., et al. Successful Treatment of Bilirubin Nephropathy by CytoSorb Hemodialysis. Blood Purif. 2019;47:P28. doi: 10.1159/000500179. [DOI] [Google Scholar]
- 101.Dhokia V.D., Madhavan D., Austin A., Morris C.G. Novel use of Cytosorb™ haemadsorption to provide biochemical control in liver impairment. J. Intensive Care Soc. 2019;20:174–181. doi: 10.1177/1751143718772789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 103.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S., Force A.D.T. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 104.Kogelmann K., Scheller M., Drüner M., Jarczak D. Use of hemoadsorption in sepsis-associated ECMO-dependent severe ARDS: A case series. J. Intensive Care Soc. 2020;21:183–190. doi: 10.1177/1751143718818992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A., Network A.R.D.S. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 106.Briel M., Meade M., Mercat A., Brower R.G., Talmor D., Walter S.D., Slutsky A.S., Pullenayegum E., Zhou Q., Cook D., et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 107.Sud S., Friedrich J.O., Taccone P., Polli F., Adhikari N.K., Latini R., Pesenti A., Guérin C., Mancebo J., Curley M.A., et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: Systematic review and meta-analysis. Intensive Care Med. 2010;36:585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 108.Guérin C. Prone positioning acute respiratory distress syndrome patients. Ann. Transl. Med. 2017;5:289. doi: 10.21037/atm.2017.06.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C., Da Silva D., Zafrani L., Tirot P., Veber B., et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 110.Combes A., Schmidt M., Hodgson C.L., Fan E., Ferguson N.D., Fraser J.F., Jaber S., Pesenti A., Ranieri M., Rowan K., et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020;46:2464–2476. doi: 10.1007/s00134-020-06290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McILwain R.B., Timpa J.G., Kurundkar A.R., Holt D.W., Kelly D.R., Hartman Y.E., Neel M.L., Karnatak R.K., Schelonka R.L., Anantharamaiah G.M., et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab. Investig. 2010;90:128–139. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Millar J.E., Fanning J.P., McDonald C.I., McAuley D.F., Fraser J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care. 2016;20:387. doi: 10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruenger F., Kizner L., Weile J., Morshuis M., Gummert J.F. First successful combination of ECMO with cytokine removal therapy in cardiogenic septic shock: A case report. Int. J. Artif. Organs. 2015;38:113–116. doi: 10.5301/ijao.5000382. [DOI] [PubMed] [Google Scholar]
- 114.Träger K., Schütz C., Fischer G., Schröder J., Skrabal C., Liebold A., Reinelt H. Cytokine Reduction in the Setting of an ARDS-Associated Inflammatory Response with Multiple Organ Failure. Case Rep. Crit. Care. 2016;2016:9852073. doi: 10.1155/2016/9852073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kommoss F.K.F., Schwab C., Tavernar L., Schreck J., Wagner W.L., Merle U., Jonigk D., Schirmacher P., Longerich T. The Pathology of Severe COVID-19-Related Lung Damage. Dtsch. Arztebl. Int. 2020;117:500–506. doi: 10.3238/arztebl.2020.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berhés M., Fábián Á., László I., Végh T., Molnár C., Fülesdi B., Koszta G. Organ replacement therapy and life-supporting treatment modalities in critically ill COVID-19 patients. Orv. Hetil. 2020;161:704–709. doi: 10.1556/650.2020.31813. [DOI] [PubMed] [Google Scholar]
- 119.Ronco C., Bagshaw S.M., Bellomo R., Clark W.R., Husain-Syed F., Kellum J.A., Ricci Z., Rimmelé T., Reis T., Ostermann M. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif. 2020;50:17–27. doi: 10.1159/000508125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Badulak J., Antonini M.V., Stead C.M., Shekerdemian L., Raman L., Paden M.L., Agerstrand C., Bartlett R.H., Barrett N., Combes A., et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iannaccone G., Scacciavillani R., Del Buono M.G., Camilli M., Ronco C., Lavie C.J., Abbate A., Crea F., Massetti M., Aspromonte N. Weathering the Cytokine Storm in COVID-19: Therapeutic Implications. Cardiorenal. Med. 2020;10:277–287. doi: 10.1159/000509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rampino T., Gregorini M., Perotti L., Ferrari F., Pattonieri E.F., Grignano M.A., Valente M., Garrone A., Islam T., Libetta C., et al. Hemoperfusion with CytoSorb as Adjuvant Therapy in Critically Ill Patients with SARS-CoV2 Pneumonia. Blood Purif. 2020;50:566–571. doi: 10.1159/000511725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.U.S. Food and Drug Administration Emergency Use Authorizations for Medical Devices. [(accessed on 17 June 2020)]; Available online: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations-medical-devices.
- 124.Supady A., Weber E., Rieder M., Lother A., Niklaus T., Zahn T., Frech F., Müller S., Kuhl M., Benk C., et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): A single centre, open-label, randomised, controlled trial. Lancet Respir. Med. 2021;9:755–762. doi: 10.1016/S2213-2600(21)00177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Putzu A., Schorer R. Hemoadsorption in critically ill patients with or without COVID-19: A word of caution. J. Crit. Care. 2021 doi: 10.1016/j.jcrc.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Träger K., Fritzler D., Fischer G., Schröder J., Skrabal C., Liebold A., Reinelt H. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: A case series. Int. J. Artif. Organs. 2016;39:141–146. doi: 10.5301/ijao.5000492. [DOI] [PubMed] [Google Scholar]
- 127.Poli E.C., Rimmelé T., Schneider A.G. Hemoadsorption with CytoSorb. Intensive Care Med. 2019;45:236–239. doi: 10.1007/s00134-018-5464-6. [DOI] [PubMed] [Google Scholar]
- 128.Harig F., Feyrer R., Mahmoud F.O., Blum U., von der Emde J. Reducing the post-pump syndrome by using heparin-coated circuits, steroids, or aprotinin. Thorac. Cardiovasc. Surg. 1999;47:111–118. doi: 10.1055/s-2007-1013121. [DOI] [PubMed] [Google Scholar]
- 129.Halter J., Steinberg J., Fink G., Lutz C., Picone A., Maybury R., Fedors N., DiRocco J., Lee H.M., Nieman G. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J. Extra Corpor. Technol. 2005;37:272–277. [PMC free article] [PubMed] [Google Scholar]
- 130.Bernardi M.H., Rinoesl H., Dragosits K., Ristl R., Hoffelner F., Opfermann P., Lamm C., Preißing F., Wiedemann D., Hiesmayr M.J., et al. Effect of hemoadsorption during cardiopulmonary bypass surgery—A blinded, randomized, controlled pilot study using a novel adsorbent. Crit. Care. 2016;20:96. doi: 10.1186/s13054-016-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang W.R., Garg A.X., Coca S.G., Devereaux P.J., Eikelboom J., Kavsak P., McArthur E., Thiessen-Philbrook H., Shortt C., Shlipak M., et al. Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J. Am. Soc. Nephrol. 2015;26:3123–3132. doi: 10.1681/ASN.2014080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Born F., Pichlmaier M., Peterß S., Khaladj N., Hagl C. Systemic Inflammatory Response Syndrome in Heart Surgery: New possibilities for treatment through the use of a cytokine adsorber during ECC? Kardiotechnik. 2014;2:1–10. [Google Scholar]
- 133.Träger K., Skrabal C., Fischer G., Datzmann T., Schroeder J., Fritzler D., Hartmann J., Liebold A., Reinelt H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass—A case series. Int. J. Artif. Organs. 2017;40:240–249. doi: 10.5301/ijao.5000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Träger K., Skrabal C., Fischer G., Schroeder J., Marenski L., Liebold A., Reinelt H., Datzmann T. Hemoadsorption treatment with CytoSorb in patients with extracorporeal life support therapy: A case series. Int. J. Artif. Organs. 2019;43:422–429. doi: 10.1177/0391398819895287. [DOI] [PubMed] [Google Scholar]
- 135.Nemeth E., Szigeti S., Varga T., Daroczi L., Barati Z., Merkely B., Gal J. Continuous cytokine haemoadsorption incorporated into a venoarterial ECMO circuit for the management of postcardiotomy cardiogenic and septic shock—A case report. Perfusion. 2018;33:593–596. doi: 10.1177/0267659118777442. [DOI] [PubMed] [Google Scholar]
- 136.Nemeth E., Kovacs E., Racz K., Soltesz A., Szigeti S., Kiss N., Csikos G., Koritsanszky K.B., Berzsenyi V., Trembickij G., et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin. Transpl. 2018;32:e13211. doi: 10.1111/ctr.13211. [DOI] [PubMed] [Google Scholar]
- 137.Omar H.R., Mirsaeidi M., Socias S., Sprenker C., Caldeira C., Camporesi E.M., Mangar D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE. 2015;10:e0124034. doi: 10.1371/journal.pone.0124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wisgrill L., Lamm C., Hell L., Thaler J., Berger A., Weiss R., Weber V., Rinoesl H., Hiesmayr M.J., Spittler A., et al. Influence of hemoadsorption during cardiopulmonary bypass on blood vesicle count and function. J. Transl. Med. 2020;18:202. doi: 10.1186/s12967-020-02369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Angheloiu G.O., Gugiu G.B., Ruse C., Pandey R., Dasari R.R., Whatling C. Ticagrelor Removal from Human Blood. JACC Basic Transl. Sci. 2017;2:135–145. doi: 10.1016/j.jacbts.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Javanbakht M., Trevor M., Rezaei Hemami M., Rahimi K., Branagan-Harris M., Degener F., Adam D., Preissing F., Scheier J., Cook S.F., et al. Ticagrelor Removal by CytoSorb. Pharm. Open. 2020;4:307–319. doi: 10.1007/s41669-019-00183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reiter K., Bordoni V., Dall’Olio G., Ricatti M.G., Soli M., Ruperti S., Soffiati G., Galloni E., D’Intini V., Bellomo R., et al. In vitro removal of therapeutic drugs with a novel adsorbent system. Blood Purif. 2002;20:380–388. doi: 10.1159/000063108. [DOI] [PubMed] [Google Scholar]
- 142.Koertge A., Wasserkort R., Wild T., Mitzner S. Extracorporeal Hemoperfusion as a Potential Therapeutic Option for Critical Accumulation of Rivaroxaban. Blood Purif. 2018;45:126–128. doi: 10.1159/000484923. [DOI] [PubMed] [Google Scholar]
- 143.Giuntoli L., Dalmastri V., Cilloni N., Orsi C., Stalteri L., Demelas V., Giuliani G., Gordini G., De Ponti F., La Manna G. Severe quetiapine voluntary overdose successfully treated with a new hemoperfusion sorbent. Int. J. Artif. Organs. 2019;42:516–520. doi: 10.1177/0391398819837686. [DOI] [PubMed] [Google Scholar]
- 144.Sibilio S., Moneta A., Di Mauro A., Marotta A., Malara G., Barberini L., Fattuoni C., Cirri S., Montisci A., Donatelli F. Use of Cytosorb during Cardiopulmonary Bypass in a Patient Underwent Emergent CABG after a Loading Dose of Ticagrelor. Blood Purif. 2019;47:3–37. [Google Scholar]
- 145.Paland M. Use of CytoSorb in cases of acute amitriptyline intoxication. J. Clin. Pharm. Ther. 2021;46:1476–1479. doi: 10.1111/jcpt.13373. [DOI] [PubMed] [Google Scholar]
- 146.De Schryver N., Hantson P., Haufroid V., Dechamps M. Cardiogenic Shock in a Hemodialyzed Patient on Flecainide: Treatment with Intravenous Fat Emulsion, Extracorporeal Cardiac Life Support, and CytoSorb(R) Hemoadsorption. Case Rep. Cardiol. 2019;2019:1905871. doi: 10.1155/2019/1905871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Breuer T.G.K., Quast D.R., Wiciok S., Labedi A., Ellrichmann G. Successful Treatment of Severe Digitoxin Intoxication with CytoSorb® Hemoadsorption. Blood Purif. 2021;50:137–140. doi: 10.1159/000510292. [DOI] [PubMed] [Google Scholar]
- 148.Zickler D., Nee J., Arnold T., Schröder T., Slowinski T., Eckardt K.U., Körner R., Kruse J.M. Use of Hemoadsorption in Patients with Severe Intoxication Requiring Extracorporeal Cardiopulmonary Support-A Case Series. ASAIO J. 2021;67:e186–e190. doi: 10.1097/MAT.0000000000001362. [DOI] [PubMed] [Google Scholar]
- 149.Capponi V.J., Young W.T., Chan P.P., Lavonas E.J. A novel adsorbent system rapidly clears Verapamil from human blood; Proceedings of the 2nd Annual International Conference on advances in Critical Care Nephrology AKI & CRRT Meeting; San Diego, CA, USA. 7–10 March 2017. [Google Scholar]
- 150.Schroeder I., Zoller M., Angstwurm M., Kur F., Frey L. Venlafaxine intoxication with development of takotsubo cardiomyopathy: Successful use of extracorporeal life support, intravenous lipid emulsion and CytoSorb®. Int. J. Artif. Organs. 2017;40:358–360. doi: 10.5301/ijao.5000595. [DOI] [PubMed] [Google Scholar]
- 151.Lang C.N., Sommer M.J., Neukamm M.A., Staudacher D.L., Supady A., Bode C., Duerschmied D., Lother A. Use of the CytoSorb adsorption device in MDMA intoxication: A first-in-man application and in vitro study. Intensive Care Med. Exp. 2020;8:21. doi: 10.1186/s40635-020-00313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruggeberg K.G., O’Sullivan P., Kovacs T.J., Dawson K., Capponi V.J., Chan P.P., Golobish T.D., Gruda M.C. Hemoadsorption Improves Survival of Rats Exposed to an Acutely Lethal Dose of Aflatoxin B. Sci. Rep. 2020;10:799. doi: 10.1038/s41598-020-57727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paul R., Jha B., Shetty V. A case of Viper Snake Bite Presenting with Gangrene and Sepsis associated Multiorgan Failure, Successfully treated with CytoSorb® as an adjunct therapy—A clinical experience. J. Evid. Based Med. Healthc. 2018;5:559–561. doi: 10.18410/jebmh/2018/114. [DOI] [Google Scholar]
- 154.Angheloiu A.A., Angheloiu G.O. Removal of dabigatran using sorbent hemadsorption. Int. J. Cardiol. 2019;293:73–75. doi: 10.1016/j.ijcard.2019.06.078. [DOI] [PubMed] [Google Scholar]
- 155.Angheloiu A.A., Tan Y., Ruse C., Shaffer S.A., Angheloiu G.O. In-Vitro Sorbent-Mediated Removal of Edoxaban from Human Plasma and Albumin Solution. Drugs R D. 2020;20:217–223. doi: 10.1007/s40268-020-00308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mair H., Jilek C., Haas B., Lamm P. Ticagrelor and Rivaroxaban Elimination with CytoSorb Adsorber Before Urgent Off-Pump Coronary Bypass. Ann. Thorac. Surg. 2020;110:e369–e370. doi: 10.1016/j.athoracsur.2020.03.108. [DOI] [PubMed] [Google Scholar]
- 157.Angheloiu G.O., Hanscheid H., Wen X., Capponi V., Anderson W.D., Kellum J.A. Experimental first-pass method for testing and comparing sorbent polymers used in the clearance of iodine contrast materials. Blood Purif. 2012;34:34–39. doi: 10.1159/000339816. [DOI] [PubMed] [Google Scholar]
- 158.Angheloiu G.O., Hänscheid H., Reiners C., Anderson W.D., Kellum J.A. In vitro catheter and sorbent-based method for clearance of radiocontrast material during cerebral interventions. Cardiovasc. Revasc. Med. 2013;14:207–212. doi: 10.1016/j.carrev.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 159.Köhler T., Schwier E., Kirchner C., Winde G., Henzler D., Eickmeyer C. Hemoadsorption with CytoSorb and the early course of linezolid plasma concentration during septic shock. J. Artif. Organs. 2021:1–5. doi: 10.1007/s10047-021-01274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schneider A.G., André P., Scheier J., Schmidt M., Ziervogel H., Buclin T., Kindgen-Milles D. Pharmacokinetics of anti-infective agents during CytoSorb hemoadsorption. Sci. Rep. 2021;11:10493. doi: 10.1038/s41598-021-89965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Datzmann T., Träger K., Reinelt H., von Freyberg P. Elimination Rates of Electrolytes, Vitamins, and Trace Elements during Continuous Renal Replacement Therapy with Citrate Continuous Veno-Venous Hemodialysis: Influence of Filter Lifetime. Blood Purif. 2017;44:210–216. doi: 10.1159/000477454. [DOI] [PubMed] [Google Scholar]
- 162.Kellum J.A., Venkataraman R., Powner D., Elder M., Hergenroeder G., Carter M. Feasibility study of cytokine removal by hemoadsorption in brain-dead humans. Crit. Care Med. 2008;36:268–272. doi: 10.1097/01.CCM.0000291646.34815.BB. [DOI] [PubMed] [Google Scholar]
- 163.Luo B., Yu Z., Li Y. Thyroid hormone disorders and sepsis. Biomed. Mater. Eng. 2017;28:S237–S241. doi: 10.3233/BME-171646. [DOI] [PubMed] [Google Scholar]
- 164.Levy M.M., Evans L.E., Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 165.Roberts J.A., Roberts D.M. Antibiotic dosing in critically ill patients with septic shock and on continuous renal replacement therapy: Can we resolve this problem with pharmacokinetic studies and dosing guidelines? Crit. Care. 2014;18:156. doi: 10.1186/cc13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luyt C.E., Bréchot N., Trouillet J.L., Chastre J. Antibiotic stewardship in the intensive care unit. Crit. Care. 2014;18:480. doi: 10.1186/s13054-014-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roberts D.M., Liu X., Roberts J.A., Nair P., Cole L., Roberts M.S., Lipman J., Bellomo R., RENAL Replacement Therapy Study Investigators A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit. Care. 2015;19:84. doi: 10.1186/s13054-015-0818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kirchner C., Sibai J., Schwier E., Henzler D., Eickmeyer C., Winde G., Köhler T. Dosing of Antimycotic Treatment in Sepsis-Induced Liver Dysfunction by Functional Liver Testing with LiMAx®. Case Rep. Crit. Care. 2019;2019:5362514. doi: 10.1155/2019/5362514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li A.M., Gomersall C.D., Choi G., Tian Q., Joynt G.M., Lipman J. A systematic review of antibiotic dosing regimens for septic patients receiving continuous renal replacement therapy: Do current studies supply sufficient data? J. Antimicrob. Chemother. 2009;64:929–937. doi: 10.1093/jac/dkp302. [DOI] [PubMed] [Google Scholar]
- 170.Ankawi G., Xie Y., Yang B., Xie P., Ronco C. What Have We Learned about the Use of Cytosorb Adsorption Columns? Blood Purif. 2019;48:196–202. doi: 10.1159/000500013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article contains only previously published data that have been validated through a peer-review process. No new data were generated or analyzed in this study. Data sharing is not relevant to this article.