Highlights
-
•
A compelling public health need based on evidence is the foundation for product innovation.
-
•
Reaching the unreached with product innovations will require substantial investment.
-
•
Aligned partnership and sustained resources accelerates innovation development.
-
•
Strategies that de-risk investment by developers incentivizes development and uptake.
-
•
Overcoming barriers to innovation needs integrated, strategic stakeholder collaboration.
Keywords: Immunization agenda 2030, Gavi 5.0, Zero-dose children, Vaccine Innovation Prioritisation Strategy (VIPS), Vaccine product innovations
Abstract
Vaccine-product innovations that address barriers to immunization are urgently needed to achieve equitable vaccine coverage, as articulated in the new Immunization Agenda 2030 and the Gavi 5.0 strategy. In 2020, the Vaccine Innovation Prioritisation Strategy (VIPS) prioritized three innovations, namely microarray patches (MAPs), heat-stable and controlled-temperature chain (CTC) enabled liquid vaccine formulations and barcodes on primary packaging. These innovations were prioritized based on the priority immunization barriers that they may help overcome in resource constrained contexts, as well as by considering their potential impact on health, coverage and equity, safety, economic costs and their technical readiness and commercial feasibility. VIPS is now working to accelerate the development and lay the foundation for future uptake of the three priority vaccine-product innovations, with the long term-goal to ensure equitable vaccine coverage and increased impact of vaccines in low- and middle- income countries.
To inform our strategic planning, we analyzed four commercially available vaccine product-innovations and conducted interviews with individuals from 17 immunization organizations, and/or independent immunization experts. The findings are synthesized into an ‘innovation conundrum’ that describes the challenges encountered in developing vaccine-product innovations and a vaccine-product innovation ‘theory of change’, which highlights actions that should be undertaken in parallel to product development to incentivize sustainable investment and prepare the pathway for uptake and impact.
1. Context
Despite impressive gains in immunization coverage over the past decade, including the development and deployment of effective new vaccines, significant gaps in coverage remain [1], [2]. Inequitable vaccine access persists, especially between high-income countries (HICs) and low-and-middle-income countries (LMICs), and within LMICs [3]. No vaccine exceeds 90% coverage at the global level and estimated coverage levels among several countries are either stagnating or in decline, ranging from 50–90% for some vaccines [4]. In 2019, an estimated 13.8 million children, approximately 1 out of 5 children, did not receive any vaccine [4]. The majority of these ‘zero-dose’ children live in remote, impoverished, or conflict settings with fragile health systems.
In 2018, the Vaccine Innovation Prioritisation Strategy (VIPS) comprised of the Gavi Secretariat, World Health Organization (WHO), Bill & Melinda Gates Foundation (BMGF), United Nations Children’s Fund (UNICEF) and PATH, was launched to identify priority vaccine-product innovations that best meet country needs, and that have the greatest potential to overcome barriers to immunization, [5], [6] thereby improving vaccine coverage and equity [7] Kristensen, 2021, published in this issue; Mvundura, 2021, published in this issue). The three prioritized innovations are: microarray patches (MAPs), heat-stable and controlled-temperature chain (CTC) enabled liquid vaccine formulations and barcodes on primary packaging. The VIPS process and collaboration, thus far, has established a common agenda on which vaccine-product innovations should be prioritized; however, this effort alone will not be sufficient to ensure their development for use in resource- constrained settings. Now, the goal of VIPS is to expedite the development and future uptake of these three priority vaccine-product innovations, with the long term-goal to ensure equitable access to, and improved effectiveness of vaccines in LMICs.
In 2021, Gavi the Vaccine Alliance enters its next strategic period (Gavi 5.0) with the vision to ‘leave no-one behind with immunization’ and a core focus on unreached and under-immunized children, with equity as the organizing principle [8] and innovation in immunization as a key strategic pillar. The new ten-year WHO Immunization Agenda 2030 (IA2030) [9] affirms the need for novel approaches and methods of delivery to achieve life-course immunization for all. Historically, a few licensed vaccine-product innovations, some specifically designed for use in low resource settings such as autodisable (AD) syringes and vaccine vial monitors (VVMs) have had significant programmatic impact, but the development took decades [5]. This can disincentivize future investment in innovations, and commercial commitment by the private sector, particularly if the products are intended mainly for LMIC markets, even though the public-health need and potential impact is usually greatest in these contexts.
The few vaccine-product innovations that have been licensed in recent years are aimed at solving relatively well-understood and widespread programmatic obstacles (e.g., shelf-life tracking, prevention of wastage due to temperature excursions, needle-reuse and reducing the risk of bloodborne disease). To date, only a limited number of innovations with the specific aim of overcoming the remaining coverage gaps to reach every 5th child with immunization, have reached the market. Such innovations have the disadvantage that the evidence base for impact is challenging to obtain, and their business case is often unattractive relative to other investment opportunities. This can impair investment of innovators, vaccine manufacturers, funders, countries and procurement organizations. These innovations, that in theory may have a huge impact, are thus not taken forward without substantial engagement by public health institutions, such as those involved in the VIPS initiative. Reaching every 5th child with vaccines by use of these innovations, that are likely to have a higher price than currently used vaccine presentations but could have a higher cost-effectiveness from a broader, societal impact, will require focused and significant investment.
The response to the COVID-19 pandemic has emphasized the urgent need for vaccine products with attributes that can address context-specific barriers to immunization [5], [6] and provides an opportunity to leverage unprecedented investment, momentum and partnership. Significant disruptions to existing immunization programs caused by the pandemic have reaffirmed the need for innovative vaccine products to facilitate routine immunization, and catch-up and recovery campaigns [10]. All of these factors could advance novel vaccine-product innovations, such as new delivery devices or presentations including MAPs, that have the potential to transform the development of, and access to, other non-COVID-19 vaccines. However, the rapid development and deployment of diverse COVID-19 vaccines has, in some instances, resulted in vaccine products that are not optimal for use in LMICs. This, in addition to the diversity of vaccine product options, presents new challenges to already over-burdened health systems with limited resources [11].
To inform the 5-year action plans to drive development and/or uptake of the 3 vaccine-product innovations prioritized by the VIPS process, we analyzed 4 vaccine-product-innovation case studies and conducted interviews with individuals from 17 immunization partner stakeholders and/or independent immunization experts, all of whom have experience with vaccine-product innovation. All case-study synopses were reviewed by at least 2 independent experts. The intent was to assess the broader vaccine-product innovation environment, beyond the technical and regulatory obstacles of product development, with the aim to identify key challenges, and drivers that enabled success. The findings are synthesized into an ‘innovation conundrum’ that describes the challenges facing vaccine-product innovation development and a vaccine-product innovation ‘theory of change’ (ToC), which highlights actions that should be undertaken in parallel to product development, to incentivize sustainable investment and prepare the pathway for uptake and impact. This analysis was undertaken in the context of the VIPS strategic planning for its priority innovations, but the interviews were innovation agnostic and the ToC identifies recommendations relevant for the broader vaccine-product innovation pipeline.
2. The retrospective innovation environment: Four vaccine-product innovation case studies
Historically, for parenteral vaccines used in LMICs, the standard presentation has been glass, multi-dose vial presentations, requiring delivery through an end-to-end cold chain, usually with reconstitution and administration with a needle and syringe (N&S). While this presentation is low-cost to manufacture, simple to deliver and often highly cost-effective, it can have drawbacks that limit coverage and equity in immunization such as vaccine wastage, or reluctance to open a vial leading to missed opportunities for vaccination [12], [13], [14]. In addition, the use of needles and syringes for administration was associated with the potential for needle re-use. Over past decades, there have been efforts to develop and implement new vaccine-product innovations to overcome some of these delivery and administration barriers and four are described in these case studies (Box 1, Box 2, Box 3, Box 4, [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [17], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [15], [16], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]).
Box 1.
Product development to uptake of Auto-disable (AD) syringes.
 |
Box 2.
Product development to uptake of UnijectTM compact prefilled AD device (cPAD).
 |
Box 3.
Product development to uptake of isposable syringe jet injectors (DSJIs).
 |
Box 4.
Product development to uptake of Vaccine vial monitors (VVMs).
 |
For vaccine-product innovations, the steps on the pathway from conceptualization to introduction and uptake in LMICs will vary, depending on their complexity. Following licensure, they generally require WHO PQ, potentially pre-determined by a WHO policy recommendation. WHO PQ is a pre-requisite for Gavi financing and UNICEF procurement for introduction in LMICs. The indicative timelines for this end-to-end process are shown in Fig. 1, using five example products.
Fig. 1.
Indicative timelines from product development to implementation for four vaccine-product innovations. Explanatory notes: ‘Need identified’ is from the perspective of global health organizations; ‘Licensure’ is by any stringent national regulatory agency; LMIC introduction refers to first use in LMIC immunization; WHO PQ: this process was not available for some products until 1987; WHO policy recommendation may be needed for vaccine-product innovations as a prerequisite for LMIC introduction[77].
3. Reflections on the case studies: Common characteristics that are needed to support vaccine innovation
The pathway and timeline to uptake in LMICs of the four vaccine-product innovation types described in the case studies suggests that some fundamental environmental characteristics are needed to facilitate development and uptake:
-
-
Articulation of a clear public health need is required to catalyze interest. Strong evidence of the public-health need for and benefit of the innovation, in the context of either existing or emerging alternative solutions is required in an environment where price is the fundamental driver for decision making. A well-articulated rationale will also serve to encourage stakeholders across all levels to agree on a common agenda and to advocate for solutions to be developed. However, this assessment of public health need must occur throughout the lengthy pathway of product development since public health priorities and the environment in which they are intended to be used in may change over time, with an impact on the perceived value of the innovation.
-
-
Simple solutions are compelling for investment. Key characteristics include whether the vaccine-product innovation could be developed independently of a vaccine manufacturer, or the vaccine development process; if it simplifies logistics; and if it needs extensive training to introduce.
-
-
Financing at all stages of the continuum is crucial. In addition to push-funding for research and development (R&D) to translate concepts into candidates, it is also important to align and co-ordinate global policy setting, financing mechanisms and procurement agencies early on. Creation of novel financing and procurement mechanisms, such as the Injection Safety Support mechanism for AD syringes can create a market ‘pull’, which de-risks product development. Other novel strategies include providing the innovations at zero cost to enable assessment of effectiveness through implementation or pilot studies. These approaches can help to develop evidence to drive policy and procurement mechanisms.
-
-
Broad applicability. If a vaccine-product innovation can be implemented across multiple vaccines, it could achieve lower production costs through increased volume across multiple vaccine applications (economies of scale) and will be more compelling from the perspective of all stakeholders.
-
-
Lower cost products are less risky. In addition to R&D costs, it is useful to have an early understanding of the likely cost of manufacturing and the acceptable price point in LMIC markets. Economic analysis that compare the innovation with alternative interventions can help sustain long-term investment through to the point of introduction.
4. The innovation environment: Lessons learned and the Innovation Conundrum
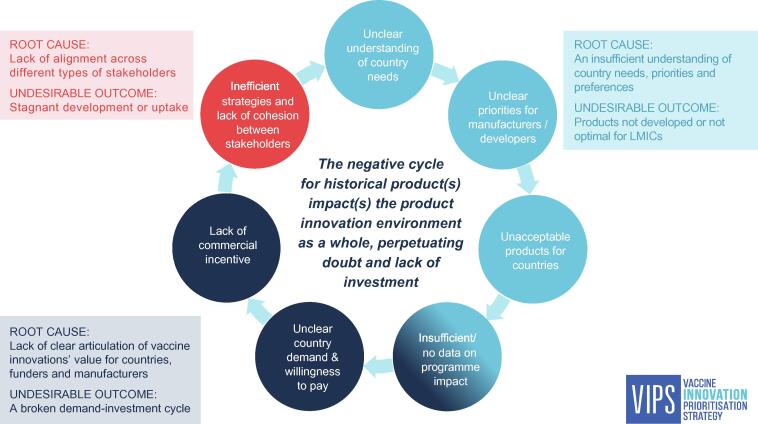
The analysis of the case studies (Boxes 1–4) together with the inputs gathered through the interviews with global immunization stakeholders reveal that the common characteristics listed above are not always in place. Furthermore, the challenges facing vaccine product development are largely interconnected, leading to a cycle of stagnation, referred to as the ‘Innovation Conundrum’ (Fig. 2). Three overarching root causes to the challenges and their undesirable outcomes can be identified:
-
i)
An insufficient understanding of country needs, including priorities and preferences leads to products not being developed that could address country priorities, or to development of new products that may not address a country need resulting in low country uptake. The basis for this could be insufficient data on barriers to immunization. Such data provide the evidence and rationale for developing innovative solutions, but must be monitored over the course of innovation development to ensure the innovation is addressing a relevant need at the time of introduction.
-
ii)
Lack of alignment across different types of stakeholders leads to a lack of coherence between investment in product development and country demand, which is particularly detrimental for innovations that may have a higher price than existing vaccines, and/or protracted timelines. Such an alignment between different types of stakeholders (e.g., push funders, developers, regulators, policy makers and financing agencies/procurers) is important for priority product innovations or attributes, including alignment on how their public-health value will be measured and how and where to deploy resources.
-
iii)
The lack of a clear articulation of value for product-innovations leads to limited investment by product developers, vaccine manufacturers, and funders. Compounding this, historical market failures for products to reach sufficient uptake or realize return on investment leads to further hesitancy. This potentially creates a ‘broken’ demand-investment cycle, resulting in slow development and/or low market uptake.
Fig. 2.
The Innovation Conundrum for vaccine-product innovation: The Innovation Conundrum describes discrete yet interlinked challenges (circles) to effective vaccine-product innovation. The source for stagnation in innovation can originate at any part of this cycle (and may be impacted by other challenges such as technical obstacles); not all elements need to be in place or are linearly causal.
The authors acknowledge that the challenges to vaccine-product innovation are numerous, interdependent and complex, and that the conundrum represents a highly simplified version of a multiplexed problem. These challenges will need to be addressed holistically and in an integrated manner to advance vaccine-product innovation and achieve impact.
5. Creating an enabling environment for vaccine-product innovation
In considering how immunization partners can best drive vaccine-product innovation in the current innovation environment, and in the context of the challenges identified in the Innovation Conundrum, we present three over-arching, desirable outcomes, that if achieved, could transform the vaccine-product innovation environment:
Desired outcome 1:Clarity on country perspectives and priorities serve as the foundation for vaccine-product innovation: To position vaccine-product innovations for uptake, new products must be driven by a clear public-health need and use case. Product characteristics must be aligned with country context/intended use case to realize public-health impact. To achieve this, country and regional engagement need to be central to the entire product-development-to-country-uptake continuum, in the context of barriers to immunization and the drive to achieve coverage and equity at the subnational level, to inform product innovation prioritization, development, and assessment of potential demand and willingness-to-pay.
Desired outcome 2:An aligned partnership and sustained resources to drive accelerateddevelopment and uptake of vaccine-product innovations: Stakeholder alignment through the entire product-development-to-country-uptake continuum must be established from the outset to ensure engagement and coordination through the entire product lifecycle. This will require integrated end-to-end strategies across immunization partners and an iterative feedback loop across all stakeholders, i.e., vaccine manufacturers, innovation developers, regulators, policy makers, funders, procurers and implementation partners.
Desired outcome 3:De-risked investments for vaccine manufacturers and innovation developers incentivize development and uptake of products with clear public-health impact: LMIC markets can be defined to a large extent as markets with low price-points for a product to be regarded as affordable. Without a solid evidence base to demonstrate eventual impact of product innovation, it will be difficult to make policy recommendations, justify potential greater public spending, funding, or generate country demand. Compounding this, health systems differ widely between and within countries. The generation of evidence on the programmatic and public-health impact of a given vaccine-product innovation across potential use cases through in-country implementation research, modelling studies and/or demonstration pilots can help to provide the rationale for broad procurement and policy recommendations, support country decision-making and inform introduction. Early indicators on country thresholds for decision-making can in turn guide the product-development strategy, helping to ensure that innovations are fit for purpose and meet the expectations of end users.
In addition to evidence on programmatic impact, the full value that a vaccine-product innovation could offer in terms of broader public-health and socio-economic gains (i.e., individual and population-based benefits) needs to be demonstrated as a justification for potentially increased price from the perspectives of countries and funders (Hutubessy et al, submitted). This full value assessment includes evaluation of impact of the innovation on e.g., vaccine coverage gaps, health and socioeconomic inequity, reaching zero dose children, improving healthcare system utilization, reducing antimicrobial resistance, environmental impact, etc. and should be used to determine the potential demand, willingness-to-adopt and willingness-to-pay, to inform decision making.
Ultimately, evidence on eventual impact and articulation of the full value of an innovation can help rationalize sustained and consistent demand and generate a ‘pull’ for innovative vaccine products and support the dialogue on public-health value versus willingness-to-pay. They can then inform the need for new and integrated policy, procurement/financing and introduction mechanisms that may be needed to de-risk investment and bridge between the potential price premium of an innovation and willingness-to-pay. This work should take place in parallel to product development to provide greater clarity and certainty to manufacturers and developers on the pathway to country uptake.
The actions to create a robust, enabling environment for product innovation and to achieve the desired outcomes described above are summarized in Fig. 3.
Fig. 3.
Vaccine-product innovation theory of change: recommendations to transform the vaccine-product innovation environment: This proposed theory of change summarizes recommendations (italic text) to address challenges to effective vaccine-product innovation identified in the Innovation Conundrum (Fig. 2, central circles). These recommendations create enabling outputs (peripheral circles) or intermediate outcomes that contribute to the desired final outcomes (outer boxes) that serve to transform the vaccine product innovation environment. Notes: 1manufacturers / developers, funders/donors, policy makers, regulators; PPCs: Preferred Product Characteristics;TPPs: Target Product Profiles.
6. The impact of the COVID-19 pandemic on vaccine-product innovation
Creation of an enabling environment could be transformative for vaccine-product innovation but will require an aligned vision as well as close collaboration of all global-health partners at national, regional and global levels. There is unparalleled precedence for this in the era of the COVID-19 response, with new mechanisms such as COVAX, led by the Coalition for Epidemic Preparedness Innovations (CEPI), Gavi and the WHO, to accelerate the development of and equitable access to COVID-19 vaccines.
The necessity for a global response to address the COVID-19 pandemic has reinforced the need for innovative vaccine products that can improve access to vaccines. Innovations are needed to reduce cold-chain requirements and reliance on vials, needles and syringes, to enhance compliance and increase equitable coverage by easing administration, and to stretch vaccine supplies by using less antigen.
There have also been significant disruptions of existing immunization activities in the wake of the pandemic, increasing coverage and equity gaps for EPI vaccines. As programs recover, they will need to prioritize implementation of catch-up campaigns for missed populations [78] and new modes of delivery other than N&S will be needed, ideally in the short term. The need to mitigate the risk of vaccine shortages and diversify delivery strategies has highlighted the potential benefits of two of the four vaccine product innovations featured in the case studies [57], [79], [80], [81] as well as other novel delivery approaches. While innovative presentations such as MAPs won’t be available for COVID-19 vaccine deployment in LMICs, possibly for some years, there is interest in the development of innovations such as these for future pandemic preparedness and response [82], [83]. Some of these innovations could facilitate equitable coverage, not only for pandemic vaccines, but could also help to address potential future significant disruptions of regular immunization activities and accelerate catch up and recovery [84], [85].
7. Conclusion
Transforming the vaccine-product innovation environment is critical to achieve the IA2030 and Gavi 5.0 goals to ‘leave no one behind in immunization’ and to overcome the persistent gaps in coverage and equity, including reaching zero-dose children. Achieving these goals can be accelerated by vaccine product innovation, but this will require considerable financial incentives to overcome the associated investment risk. As such, a paradigm shift in traditional ways of working is needed to rationalize and accelerate the most compelling vaccine-product innovations. Examination of historical case studies and consultation with diverse stakeholders in the immunization community has underscored the importance of close collaboration of all stakeholders across the entire product lifecycle continuum from the outset, to derive and implement holistic strategies founded on immunization needs of countries and to de-risk investments by improving clarity in innovation priorities, demonstrating potential socio-economic value and increasing certainty on the demand and potential return on investment.
The catalytic impact of such an aligned and coordinated approach around a common goal is exemplified by the unprecedented speed and spirit of the global response to develop COVID-19 vaccines. The COVID-19 pandemic is also a reminder of the need for the world to be better prepared for the next pandemic, in which new vaccine-product innovations could ease delivery of both the pandemic as well as routine vaccines.
The time is now to transform the vaccine-product innovation environment by leveraging the new ecosystem of accelerators for COVID-19 vaccine, to deliver to the global coverage and equity agenda, and ensure better preparedness for future pandemics.
Funding
WHO’s work was supported by the Bill & Melinda Gates Foundation, Seattle, WA [grant INV-003658]. PATH’s work was supported by the Bill & Melinda Gates Foundation, Seattle, WA [grant OPP1200373].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Acknowledgements
The authors sincerely thank the following individuals for their consultation or review of sections of this manuscript: Carsten Mantel (MMGH Consulting), Heather Deehan (UNICEF), Michael Free (independent), Roderick Hausser (independent), Julian Hickling (Working-in-Tandem), Rebecca Jones (Working in Tandem), Mark Kane (independent), Umit Kartoglu (Extensio et Progressio), Melissa Malhame (MMGH Consulting), Lisa Menning (WHO), Vivian Hsu (Bill & Melinda Gates Foundation and VIPS working group member), Ted Prusik (Zebra Technologies), Michael Royals (Thrivant Health), Jim Robinson (independent), Soleine Scotney & Alex Bowles (Clinton Health Access Initiative).
References
- 1.MacDonald N., Mohsni E., Al-Mazrou Y., Kim Andrus J., Arora N., Elden S., et al. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine. 2020;38(33):5364–5371. doi: 10.1016/j.vaccine.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Who U. IMMUNIZATION COVERAGE: ARE WE LOSING GROUND? 2020; Available from: https://data.unicef.org/wp-content/uploads/2020/07/WUENIC-Immunization-coverage-are-we-losing-ground-brochure-2020.pdf
- 3.Utazi C.E., et al. Mapping vaccination coverage to explore the effects of delivery mechanisms and inform vaccination strategies. Nat Commun. 2019;10(1):1633. doi: 10.1038/s41467-019-09611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO-UNICEF estimates of DTP3 coverage. 2020; Available from: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html.
- 5.Botwright S., et al. How can we evaluate the potential of innovative vaccine products and technologies in resource constrained settings? A total systems effectiveness (TSE) approach to decision-making. Vaccine X. 2020;6 doi: 10.1016/j.jvacx.2020.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haidari Leila A., Wahl Brian, Brown Shawn T., Privor-Dumm Lois, Wallman-Stokes Cecily, Gorham Katie, et al. One size does not fit all: The impact of primary vaccine container size on vaccine distribution and delivery. Vaccine. 2015;33(28):3242–3247. doi: 10.1016/j.vaccine.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavi. The Vaccine Innovation Prioritisation Strategy (VIPS). 2020; Available from: https://www.gavi.org/our-alliance/market-shaping/vaccine-innovation-prioritisation-strategy.
- 8.Gavi. Gavi Phase V (2021-2025) 2021; Available from: https://www.gavi.org/our-alliance/strategy/phase-5-2021-2025.
- 9.IMMUNIZATION AGENDA 2030. 2020; Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030.
- 10.Abbas K., et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health. 2020;8(10):e1264–e1272. doi: 10.1016/S2214-109X(20)30308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub Rebecca L., Subramanian Laura, Karlage Ami, Ahmad Iman, Rosenberg Julie. COVID-19 Vaccine To Vaccination: Why Leaders Must Invest In Delivery Strategies Now. Health Aff (Millwood) 2021;40(1):33–41. doi: 10.1377/hlthaff.2020.01523. [DOI] [PubMed] [Google Scholar]
- 12.Organization, W.H. WHO policy statement: multi-dose vial policy (MDVP): handling of multi-dose vaccine vials after opening. 2014; Available from: https://apps.who.int/iris/handle/10665/135972.
- 13.Krudwig Kirstin, Knittel Barbara, Karim Ali, Kanagat Natasha, Prosser Wendy, Phiri Guissimon, et al. The effects of switching from 10 to 5-dose vials of MR vaccine on vaccination coverage and wastage: A mixed-method study in Zambia. Vaccine. 2020;38(37):5905–5913. doi: 10.1016/j.vaccine.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikechukwu Udo Ogbuanu 1, A.J.L., Blanche-Philomene Melanga Anya 3, Mbaihol Tamadji 4, Geoffrey Chirwa 5, Kwame W Chiwaya 6, Mohamed El-Hafiz Djalal 7, Dah Cheikh 8, Zorodzai Machekanyanga 9, Joseph Okeibunor 3, Colin Sanderson 10, Richard Mihigo 3, Can vaccination coverage be improved by reducing missed opportunities for vaccination? Findings from assessments in Chad and Malawi using the new WHO methodology. PLoS One, 2019. 14: p. e0210648. [DOI] [PMC free article] [PubMed]
- 15.Hauri Anja M, Armstrong Gregory L, Hutin Yvan J F. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15(1):7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen L., et al. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77(10):789–800. [PMC free article] [PubMed] [Google Scholar]
- 17.Aylward B., et al. Model-based estimates of the risk of human immunodeficiency virus and hepatitis B virus transmission through unsafe injections. Int J Epidemiol. 1995;24(2):446–452. doi: 10.1093/ije/24.2.446. [DOI] [PubMed] [Google Scholar]
- 18.PATH, Technology Solutions for Global Health: SoloShot™. 2013.
- 19.Organization, W.H. Evaluation of Injection Technologies. 1987; Available from: https://apps.who.int/iris/bitstream/handle/10665/62530/WHO_EPI_CCIS_87.2.pdf;jsessionid=C07060E4333E0F4B2667D38DFEEFE3B7?sequence=1.
- 20.WHO-UNICEF-UNFPA joint statement* on the use of auto-disable syringes in immunization services. 2003; Available from: https://apps.who.int/iris/bitstream/handle/10665/63650/WHO_VB_99.25_eng.pdf?sequence=1&isAllowed=y.
- 21.Promoting the exclusive use of injection safety devices for all immunization activities. 2019; Available from: https://www.who.int/immunization/documents/policies/RUP_JointStatement.pdf?ua=1#:~:text=In%201999%2C%20the%20release%20of,with%20the%20goal%20of%20phasing.
- 22.Levin A., et al. A global health partnership's use of time-limited support to catalyze health practice change: the case of GAVI's Injection Safety Support. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pépin J, A.C.C., Pépin E, Nault V. , Evolution of the global use of unsafe medical injections, 2000-2010. 4;8(12):e80948. doi: 10.1371/journal.pone.0080948. PMID: 24324650; PMCID: PMC3851995. PLoS One, 2013 8(12): p. e80948. [DOI] [PMC free article] [PubMed]
- 24.Hoekstra E.J., et al. Measles supplementary immunization activities and GAVI funds as catalysts for improving injection safety in Africa. J Infect Dis. 2011;204(Suppl 1):S190–S197. doi: 10.1093/infdis/jir073. [DOI] [PubMed] [Google Scholar]
- 25.UNICEF. Safe injection equipment: supply and demand update. 2018; Available from: https://www.unicef.org/supply/media/451/file/Safe%20injection%20equipment:%20supply%20and%20demand%20update.pdf.
- 26.UNICEF. The right choice of a syringe. 2021; Available from: https://www.unicef.org/supply/stories/right-choice-syringe.
- 27.PATH. Global COVID-19 Vaccine Syringe Supply Assessment. 2020; Available from: https://path.azureedge.net/media/documents/PATH_Global_COVID_syringe_supply_assessment_path.org_3.8.21.pdf.
- 28.Pre-qualification of single-use injection devices under the PQS system: Guidelines for manufacturers. 2008; Available from: file:///C:/Users/giersingb/Downloads/PQS_E008_E013_manufacturer_guide1.4.pdf.
- 29.Steinglass R, B.D., Grabowsky M, Laghari AG, Khan MA, Qavi A, Evans P. , Safety, effectiveness and ease of use of a non-reusable syringe in a developing country immunization programme. . Bull World Health Organ. , 1995. 73(1): p. 57-63. [PMC free article] [PubMed]
- 30.WHO guideline on the use of safety-engineered syringes for intramuscular, intradermal and subcutaneous injections in health-care settings. 2015; Available from: http://www.safetymedproducts.be/wp-content/uploads/2015/08/9.-WHO-SAFETY-DEVICES-GUIDELINES-20152.pdf. [PubMed]
- 31.website, B. BD uniject™ auto-disable pre-fillable injection system. Available from: https://drugdeliverysystems.bd.com/products-and-services/products/prefillable-syringe-systems/vaccine-syringes/uniject-auto-disable-pre-fillable-injection-system.
- 32.PATH. A HealthTech historical profile: The uniject device. 2005; Available from: https://path.azureedge.net/media/documents/TS_hthp_uniject.pdf.
- 33.Nogier Cyril, Hanlon Patrick, Wiedenmayer Karin, Maire Nicolas. Can a Compact Pre-Filled Auto-Disable Injection System (cPAD) Save Costs for DTP-HepB-Hib Vaccine as Compared with Single-Dose (SDV) and Multi-Dose Vials (MDV)? Evidence from Cambodia, Ghana, and Peru. Drugs Real World Outcomes. 2015;2(1):43–52. doi: 10.1007/s40801-015-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UNICEF. Reaching women with tetanus vaccine could save thousands of lives. 2004; Available from: https://www.unicef.org/media/media_19215.html.
- 35.Schmid D.A., Macura-Biegun A., Rauscher M. Development and introduction of a ready-to-use pediatric pentavalent vaccine to meet and sustain the needs of developing countries–Quinvaxem(R): the first 5 years. Vaccine. 2012;30(44):6241–6248. doi: 10.1016/j.vaccine.2012.07.088. [DOI] [PubMed] [Google Scholar]
- 36.DTwP-HepB-hib vaccine available in a compact, pre-filled auto-disable injection technology (cPAD). . 2015; Available from: https://www.who.int/immunization/programmes_systems/service_delivery/InfoBulletin_Uniject_March2015_FINAL_ENG.pdf. [DOI] [PMC free article] [PubMed]
- 37.PATH. The uniject injection system: Multi-country experience and evidence. briefing summary. PATH. 2011. 2011; Available from: https://path.azureedge.net/media/documents/RH_depo_subq_experience.pdf.
- 38.Cole Kimberly, Saad Abdulmumin. The Coming-of-Age of Subcutaneous Injectable Contraception. Glob Health Sci Pract. 2018;6(1):1–5. doi: 10.9745/GHSP-D-18-00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedita Jeff, Perrella Stefanie, Morio Matt, Berbari Michael, Hsu Jui-Shan, Saxon Eugene, et al. Cost of goods sold and total cost of delivery for oral and parenteral vaccine packaging formats. Vaccine. 2018;36(12):1700–1709. doi: 10.1016/j.vaccine.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UNICEF Supply pricing data. 2021; Available from: https://www.unicef.org/supply/pricing-data.
- 41.Levin C.E., et al. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Organ. 2005;83(6):456–461. [PMC free article] [PubMed] [Google Scholar]
- 42.UNICEF. Supply of children’s five-in-one vaccine secured at lowest-ever price. 2016; Available from: https://www.unicef.org/media/media_92936.html.
- 43.Horn H, O.B., Schau G, Investigations into the risk of infection by the use of jet injectors. Health Soc Serv J, 1975. 85: p. 2396-2397.
- 44.Canter J., et al. An outbreak of hepatitis B associated with jet injections in a weight reduction clinic. Arch Intern Med. 1990;150(9):1923–1927. [PubMed] [Google Scholar]
- 45.Bruce G. Weniger, M.J.P., Alternative vaccine delivery methods. 6th Edition ed. Vaccies. 2013.
- 46.Okayasu H., et al. Affordable inactivated poliovirus vaccine: strategies and progress. J Infect Dis. 2014;210(Suppl 1):S459–S464. doi: 10.1093/infdis/jiu128. [DOI] [PubMed] [Google Scholar]
- 47.Ekwueme D.U., Weniger B.G., Chen R.T. Model-based estimates of risks of disease transmission and economic costs of seven injection devices in sub-Saharan Africa. Bull World Health Organ. 2002;80(11):859–870. [PMC free article] [PubMed] [Google Scholar]
- 48.Daly Catherine, Molodecky Natalia A., Sreevatsava Meghana, Belayneh Asalif D., Chandio Shoukat A., Partridge Jeff, et al. Needle-free injectors for mass administration of fractional dose inactivated poliovirus vaccine in Karachi, Pakistan: A survey of caregiver and vaccinator acceptability. Vaccine. 2020;38(8):1893–1898. doi: 10.1016/j.vaccine.2019.12.059. [DOI] [PubMed] [Google Scholar]
- 49.Bullo U.F., et al. An experience of mass administration of fractional dose inactivated polio vaccine through intradermal needle-free injectors in Karachi, Sindh, Pakistan. BMC Public Health. 2021;21(1):44. doi: 10.1186/s12889-020-10041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaudinski Martin R, Houser Katherine V, Morabito Kaitlyn M, Hu Zonghui, Yamshchikov Galina, Rothwell Ro Shauna, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. 2018;391(10120):552–562. doi: 10.1016/S0140-6736(17)33105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.PATH. Disposable-cartridge jet injectors: changing the landscape of vaccination and immunization delivery programs worldwide. 2008; Available from: https://www.path.org/media-center/disposable-cartridge-jet-injectors-changing-the-landscape-of-vaccination-and-immunization-delivery-programs-worldwide/.
- 52.Inovio acquires needle-free jet injector tech from Bioject for $5.5M. 2016; Available from: https://www.fiercepharma.com/drug-delivery/inovio-acquires-needle-free-jet-injector-tech-from-bioject-for-5-5m.
- 53.Flu Vaccination by Jet Injector. Available from: https://www.cdc.gov/flu/prevent/jet-injector.htm.
- 54.Review, E.P. PharmaJet partners with Serum to launch needle-free MMR vaccine. 2017; Available from: https://www.europeanpharmaceuticalreview.com/news/49494/pharmajet-partners-serum-launch-needle-free-mmr-vaccine/.
- 55.Okayasu H., et al. Intradermal Administration of Fractional Doses of Inactivated Poliovirus Vaccine: A Dose-Sparing Option for Polio Immunization. J Infect Dis. 2017;216(suppl_1):S161–S167. doi: 10.1093/infdis/jix038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anand Abhijeet, Molodecky Natalie A., Pallansch Mark A., Sutter Roland W. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule. Vaccine. 2017;35(22):2993–2998. doi: 10.1016/j.vaccine.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.PharmaJet. PharmaJet’s Needle-free System to be used in Australian Clinical COVID-19 Trial. 2020; Available from: https://pharmajet.com/pharmajets-needle-free-system-to-be-used-in-australian-clinical-covid-19-trial/.
- 58.PharmaJet. PharmaJet and Diomics partner on SARS-CoV-2 immune response monitoring. 2020; Available from: https://pharmajet.com/pharmajet-and-diomics-partner-on-sars-cov-2-immune-response-monitoring/.
- 59.Wire, B. Global NGO expands use of PharmaJet for Polio Eradication Campaigns. 2021; Available from: https://www.businesswire.com/news/home/20210309005036/en/Global-NGO-expands-use-of-PharmaJet-for-Polio-Eradication-Campaigns.
- 60.Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products 2018; Available from: https://www.fda.gov/media/76403/download.
- 61.FDA Updated Communication on Use of Jet Injectors with Inactivated Influenza Vaccines. 2014; Available from: https://www.fda.gov/vaccines-blood-biologics/vaccines/fda-updated-communication-use-jet-injectors-inactivated-influenza-vaccines.
- 62.Papania MJ, Z.D., Jarrahian C. 1320–1353.e17. doi: 10.1016/B978-0-323-35761-6.00068-7. Epub 2017 Jul 17. PMCID: PMC7152424., Technologies to Improve Immunization. Plotkin's Vaccines. . 2018:: p. 1320–1353.
- 63.Mvundura M., et al. Evaluating the cost per child vaccinated with full versus fractional-dose inactivated poliovirus vaccine. Vaccine X. 2019;2 doi: 10.1016/j.jvacx.2019.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bavdekar A., et al. Clinical study of safety and immunogenicity of pentavalent DTP-HB-Hib vaccine administered by disposable-syringe jet injector in India. Contemp Clin Trials Commun. 2019;14 doi: 10.1016/j.conctc.2019.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization Fractional-dose inactivated poliovirus vaccination campaign, Telangana state, India, June 2016. Wkly Epidemiol Rec. 2016;91(34):397–403. [PubMed] [Google Scholar]
- 66.Haldar Pradeep, Agrawal Pankaj, Bhatnagar Pankaj, Tandon Rajiv, McGray Sarah, Zehrung Darin, et al. Fractional-dose inactivated poliovirus vaccine. India. Bull World Health Organ. 2019;97(5):328–334. doi: 10.2471/BLT.18.218370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gamage D., Ginige S., Palihawadana P. National introduction of fractional-dose inactivated polio vaccine in Sri Lanka following the global “switch”. WHO South East Asia J Public Health. 2018;7(2):79–83. doi: 10.4103/2224-3151.239418. [DOI] [PubMed] [Google Scholar]
- 68.UNICEF, W.H.O. WHO-UNICEF policy statement on the use of vaccine vial monitors in immunization services. 1999; Available from: https://apps.who.int/iris/bitstream/handle/10665/67808/WHO-V%26B-99.18-eng.pdf?sequence=1&isAllowed=y.
- 69.WHO-UNICEF, WHO-UNICEF policy statement on the use of vaccine vial monitors in immuntzatton servtces 1999.
- 70.Kartoglu, U. The Book of VVM. Available from: http://kartoglu.ch/vvm/?fbclid=IwAR0ZkXylUe8IneVZ9bcg6O9ep8AwK6bmgjudTlCSX1gDhddrAx6IGYix6Gs.
- 71.WHO-UNICEF, WHO-UNICEF policy statement on the implementation of vaccine vial monitors : the role of vaccine vial monitors in improving access to immunization. 2007.
- 72.Organization, W.H., Assessing programmatic suitability of vaccine candidates for WHO prequalification. 2014.
- 73.PATH, A HealthTech Historical Profile: Vaccine Vial Monitors. 2005.
- 74.Pär Eriksson B.D.G., Jaillard Philippe, Morgan Christopher. Jean Bernard Le Gargasson, Vaccine vial monitor availability and use in low- and middle-income countries: A systematic review. Vaccine. 2017;35(17):2155–2161. doi: 10.1016/j.vaccine.2016.11.102. [DOI] [PubMed] [Google Scholar]
- 75.Bruce Aylward J.L. Key elements for improving supplementary immunization activities for polio eradication. Bulletin of the World Health Organisation. 2005;83:268–273. [Google Scholar]
- 76.Corporation, Z.T., 2021 HEATmarker® VVM Worldwide Public Donor Market Price Guide. 2021.
- 77.From Vaccine Development to Policy: A Brief Review of WHO Vaccine-Related Activities and Advisory Processes 2017; Available from: https://www.who.int/immunization/policy/WHO_vaccine_development_policy.pdf?ua=1.
- 78.Organization, W.H. Immunization as an essential health service: guiding principles for immunization activities during the COVID-19 pandemic and other times of severe disruption. 2020; Available from: https://www.who.int/publications/i/item/immunization-as-an-essential-health-service-guiding-principles-for-immunization-activities-during-the-covid-19-pandemic-and-other-times-of-severe-disruption.
- 79.PharmaJet partners with Immunomic Therapeutics and EpiVax to develop and deliver COVID-19 Vaccine. 2020; Available from: https://pharmajet.com/pharmajet-partners-with-immunomic-therapeutics-and-epivax-to-develop-and-deliver-covid-19-vaccine/.
- 80.PharmaJet and Abnova partner to develop and deliver COVID-19 mRNA Vaccine using Needle-free Injection technology. 2020; Available from: https://pharmajet.com/pharmajet-and-abnova-partner-to-develop-and-deliver-covid-19-mrna-vaccine-using-needle-free-injection-technology/.
- 81.Apiject, When COVID-19 vaccine and injectable therapeutics become available, the RAPID Consortium can ease a U.S. supply chain problem by filling and finishing 300+ million prefilled syringes per month. 2021.
- 82.countermeasures-gov, M. BARDA establishes four new partnerships to explore innovative vaccine delivery technologies. 2020; Available from: https://www.medicalcountermeasures.gov/newsroom/2020/barda-new-partnerships/.
- 83.Innovation, C.f.E.P. Calls for proposals. 2021; Available from: https://cepi.net/get_involved/cfps/.
- 84.Olorunsaiye Comfort Z., Yusuf Korede K., Reinhart Kylie, Salihu Hamisu M. COVID-19 and Child Vaccination: A Systematic Approach to Closing the Immunization Gap. Int J MCH AIDS. 2020;9(3):381–385. doi: 10.21106/ijma.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Organization, W.H. Closing Immunization Gaps Caused by COVID-19. 2020; Available from: https://www.who.int/immunization/programmes_systems/policies_strategies/Closing_Immunization_Gaps_caused_by_COVID-19_v11.pdf?ua=1.