Abstract
Introduction:
Albuterol can trigger supraventricular tachycardia (SVT). The clinical characteristics, incidence, and risk factors of SVT after inhaled SABA treatment in children are currently unknown. Through review of regional care delivery, we will describe cases of SVT during asthma treatment in hospital-based settings, define the incidence of SVT in our population, and evaluate risk factors of SABA-induced SVT.
Methods:
We identified hospital-based care episodes of children 0–18 years old between 2006 and 2015 recorded in the Intermountain Healthcare EDW with either 1) diagnosis codes for both asthma and SVT or 2) both SABA and adenosine listed as billed medications. Controls were matched with cases by age and sex to determine risk factors for SVT after SABA using conditional logistic regression.
Results:
Of 93 care episodes meeting criteria, we found 7 cases of SVT after SABA treatment in 6 patients over 10 years. In our population, the incidence of SVT is 3.9 per 10,000 episodes of SABA treatment, and 5.1 per 10,000 children with asthma receiving hospital-based asthma care. Two episodes of SVT followed treatment with only levalbuterol, three after only albuterol, and two after both albuterol and levalbuterol treatment. Five cases of SVT were converted to sinus rhythm with adenosine, one converted with synchronized electrical cardioversion, and one resolved spontaneously. No cases of SVT led to death. No examined variables were associated with SABA-induced SVT.
Conclusions:
SVT is rare during hospital-based treatment for acute asthma using inhaled SABAs and has low morbidity and mortality.
Keywords: Case reports, control/management
Introduction
Acute asthma exacerbations in children are common. It is estimated that acute asthma leads to approximately 640 000 emergency department (ED) visits and 157 000 hospitalizations each year in the United States (1). Inhaled selective short-acting beta-2 agonists (SABA) such as albuterol and levalbuterol are recommended in national guidelines for all children with acute asthma (2). Over 85% of children who present to EDs with acute asthma are treated with albuterol (3).
Higher doses of inhaled SABA treatment may reduce hospitalization for children with acute asthma, especially for children with severe acute asthma (4). While SABAs are typically well tolerated with minimal cardiovascular side effects, concern persists that high dosing of inhaled SABA may increase the incidence of arrhythmias such as supraventricular tachycardia (SVT) (5). SVT can be reversed using maneuvers that increase vagal tone, through intravenous infusion of adenosine, or through synchronized cardioversion (6). In the process of implementing a nurse-initiated protocol to improve the timing and increase the intensity of SABA treatment at Primary Children’s Hospital (PCH), concern about possibly increasing the risk of SVT prompted this investigation.
The incidence of SVT after SABA treatment in children is currently unknown. Risk factors for developing SVT after SABA use are also currently unknown. Our aim was to improve the general understanding of SVT induced by SABA treatment in children with acute asthma.
Objectives
Through review of regional data, we will determine the incidence and risk factors of SABA-induced SVT and describe cases of SVT induced by albuterol.
Methods
This investigation is a retrospective case series and retrospective cohort study. We screened for episodes of SVT associated with SABA treatment for asthma in children 0–18 years of age during hospital-based episodes (ED visit or hospitalization) at any Intermountain Healthcare hospital between 2006 and 2015. Intermountain Healthcare is the non-profit organization that provides the majority of health care in the Intermountain West, operating 22 hospitals in the region, and the largest provider of ED care for children in the state of Utah, accounting for 77% of all acute asthma visits in EDs in 2013. We identified episodes of SVT electronically using the Intermountain Healthcare Electronic Data Warehouse (EDW), an electronic database that links clinical, financial, and administrative data across the Intermountain Healthcare System, including PCH. We reviewed all cases that met either of two criteria:
a care episode with diagnosis codes for both asthma (ICD9 492.xx or ICD10 J45.xx) and SVT (ICD9 427.0 or 427.61 or ICD10 I47.1) OR
a care episode with both an inhaled SABA (albuterol or levalbuterol) and adenosine listed as billed medications.
To determine which of these episodes were truly SVT due to a beta-agonist, we reviewed all paper and electronic charts of all episodes. We defined a case of SVT due to beta-agonist a priori as SVT stated as a diagnosis in physician documentation and confirmed by electrocardiogram or cardiorespiratory monitoring, following treatment with a SABA during the same ED visit or at a referring clinical site. All episodes were reviewed by two abstractors (Woodward and Johnson) following practice extraction on a small set of example records. Cases were determined only with unanimous agreement between the two abstractors. To determine incidence, we regarded the numerator as the number of cases identified as outlined above and the denominator as the number of patients receiving hospital-based asthma care over the same time period, defined as a primary diagnosis of asthma and albuterol billed in the same encounter in the EDW. We calculated incidence as defined below:
To account for the possibility that some children may have multiple episodes of SVT or multiple acute asthma episodes, we report incidence both as the number of episodes of SVT divided by the number of episodes of acute asthma treatment, and as the number of children who experienced SVT divided by the number of children who received acute asthma treatment.
To evaluate possible risk factors for SVT, we matched each case with six age- and gender-matched controls obtained from the EDW. Through chart review we collected possible risk factors for SVT from electronic and paper medical records of cases and controls including:
weight – as recorded electronically at ED arrival or hospital admission
BMI – calculated from electronically recorded values
Race – as stated by patient or parent on standard hospital survey
number of days of illness – from text of treating physicians’ notes
documented history of asthma, arrhythmia (ICD9 427.xx or ICD10 J45.xx), or congenital heart disease – from electronic ICD codes or from treating physicians’ notes
number of chronic asthma medications – from text of treating physicians’ notes
home use of albuterol or levalbuterol – from text of treating physicians’ notes
family history of arrhythmia or asthma – from text of treating physicians’ notes
temperature, heart rate, and respiratory rate on ED arrival – as recorded electronically
total number of doses of SABA while in hospital (limited to number of doses prior to SVT in cases) – as recorded in paper and electronic nurse administration records
total dose of SABA over time while in hospital, defined as total number of doses divided by total hospital length of stay (limited to number of doses prior to SVT in cases)
method of SABA administration (nebulized or inhaler) – as recorded in paper and electronic nurse administration records
To characterize cases and controls, we also collected patient disposition, length of ED stay, and total length of hospital stay. All data was collected on a standardized spreadsheet. Reviewers were not blinded to case or control assignment due to the obvious nature of SVT diagnosis in the medical record but were blinded to case association with specific controls. Missing data was marked as such and not included in analysis. For analysis, a single dose of SABA was determined as either 2.5 mg of nebulized albuterol, 2 or more puffs of albuterol by inhaler, 0.63 mg of nebulized levalbuterol, or 2 or more puffs of levalbuterol by inhaler. To evaluate the likelihood of developing SVT after SABA use, the possible risk factors were evaluated by a conditional logistic regression to determine the confidence interval and p values. A p values <0.05 was considered to be statistically significant. The institutional review boards of the University of Utah and PCH Privacy Board approved this study with a waiver of informed consent.
Results
Our initial search resulted in 93 episodes meeting either of the above screening criteria: 53 episodes flagged for diagnosis codes and 47 episodes flagged for medication billing, with 7 episodes flagged for both. Of the flagged episodes, 82 episodes were determined to not be SVT and 18 were found to be SVT. Of the 18 episodes that were SVT, 11 episodes were not following SABA treatment. Of the remaining 7 episodes of SVT after SABA treatment for acute asthma over 10 years, 2 occurred in the same patient (Figure 1). In our population, the incidence of SVT is 3.9 cases per 10 000 episodes of hospital-based asthma care, and 5.1 children per 10 000 children with asthma receiving hospital-based asthma care.
Figure 1.
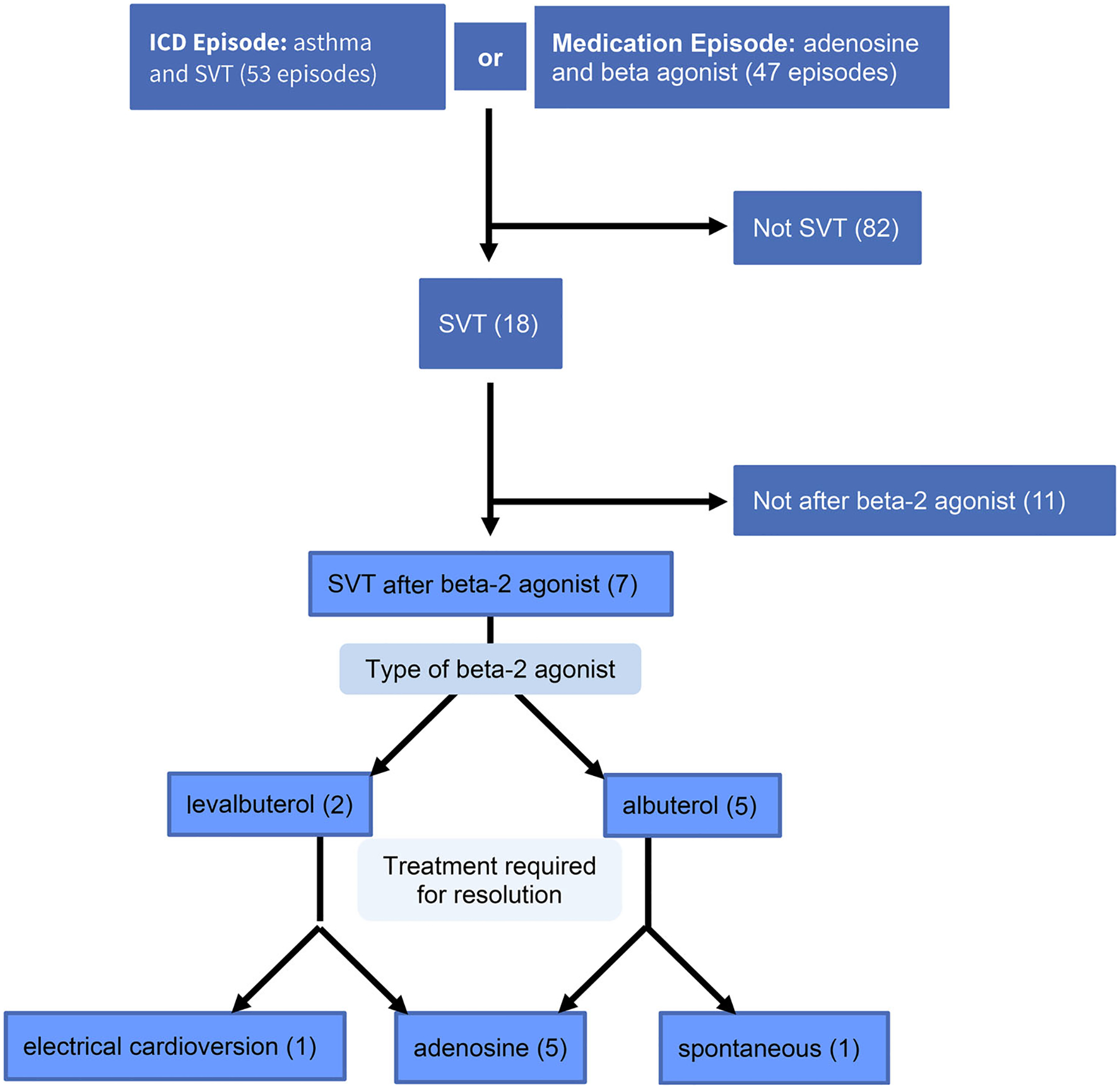
Patients selected for evaluation.
The six children who experienced seven episodes of SVT following SABA treatment ranged in age from one month to six years. Three children were male and three were female. Three children had a history of asthma and two had a history of prior SVT. One patient had congenital heart disease with heterotaxy, atrial septal defect, and left ventricular dominance. One child had a family history of myocardial infarction and arrhythmia. One patient was diagnosed with Wolf-Parkinson-White Syndrome after the episode of SVT (Table 1).
Table 1.
Characteristics of SVT cases.
| Patient | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Age | 1 month | 15 months | 2 years | 3 years | 3 years | 3 years | 6 years |
| Sex | M | M | F | F | M | F | F |
| History of asthma | no | no | yes | yes | yes | yes | no |
| History of SVT | no | no | yes | yes | yes | no | no |
| History of heart disease | yes | no | no | no | no | no | no |
| Family history of arrhythmia | no | no | no | no | yes | no | no |
| Number of days of illness | 1 | 3 | 1 | 1 | 2 | 1 | 2 |
| Pre-hospital beta-2 agonist | N/A | 1 dose albuterol | 2 doses albuterol | levalbuterol every 4 hours for 1 day | 2 doses albuterol | 2 doses levalbuterol | 4 doses albuterol |
| Beta-2 agonist before SVT | 6 doses of albuterol 2.5 mg over 12 hours with decreasing interval (15 mg total) | 5 doses of albuterol 2.5 mg over 48 hours (12.5 mg total) | 2 doses of levalbuterol 0.63 mg over 1 hour (1.26 mg total) | Arrived to ED in SVT after 2.5 mg albuterol at PCP office | Arrived to ED in SVT after 1.26 mg levalbuterol at PCP office | albuterol 10 mg/hr for 4 hours | albuterol 20 mg/hr for 5 hours |
| Patient location at onset of SVT | ICU | Hospital Floor | ED | PCP | PCP | ICU | ICU |
| Conversion | adenosine 0.4mg (0.1 mg/kg) | spontaneous | 2 doses adenosine 1.3 mg (0.1 mg/kg) | adenosine 1.4 mg (0.1 mg/kg) then 2 doses 2.8 mg (0.2 mg/kg) | 3 doses adenosine 0.6 mg (0.1 mg/kg) then electrical cardioversion | adenosine 1.5 mg (0.1 mg/kg) | adenosine 12 mg (0.6 mg/kg) |
| Disposition Physician-stated cause for SVT | ICU albuterol | ICU mediastinal mass | ICU levalbuterol | floor albuterol | floor levalbuterol | ICU undiagnosed WPW | ICU albuterol |
Two episodes of SVT followed treatment with levalbuterol, three followed treatment with albuterol, and two followed treatment with both levalbuterol and albuterol. Five cases of SVT were converted to sinus rhythm with adenosine, three of these requiring more than one dose of adenosine for conversion. One case converted with synchronized electrical cardioversion at an outside hospital following three doses of adenosine. One case resolved spontaneously. No cases of SVT led to death. All SVT cases occurred within the first 3 days of illness. Most patients used SABA at home prior to seeking physician care (5/7). All cases received multiple doses of SABA prior to developing SVT, either at home, in their primary care physician office, or in the hospital. Three children were noted to be in SVT in the intensive care unit (ICU), two patients developed SVT at an outpatient clinic, one child developed SVT in the ED, and one was noted to be in SVT on the general pediatric hospital ward. Most episodes of SVT were monitored in the ICU after SVT (5/7), though in all these episodes ICU admission was deemed necessary for respiratory distress independent of SVT; four episodes of severe asthma and one for elective intubation in the setting of a mediastinal mass affecting breathing. Two patients were monitored on the general hospital ward, and both of these children had a prior history of SABA-induced SVT. The physician stated cause for SVT for most patients was inhaled SABA (5/7), with the stated cause for SVT in the remaining two children being a mediastinal mass and undiagnosed Wolf-Parkinson-White syndrome.
We evaluated the association of possible risk factors (Tables 2 and 3) with development of SVT using conditional logistic regression (Table 4). However, the model was not converged due to insufficient sample size for race, levalbuterol use at home, history of SVT, and family history of arrhythmia. A high SABA dose over time was close to statistical association with SVT (p = 0.053). The following factors were not associated with SVT: weight; BMI; race; documented history of asthma or congenital heart disease; home use of albuterol; number of chronic asthma medications; family history of arrhythmia or asthma; number of days of illness; temperature, heart rate, and respiratory rate on ED arrival; and total dose of SABA while in hospital. The confidence intervals of the unadjusted point estimate for the odds of association of each factor with SVT all contained 1 (Table 5).
Table 2.
Descriptive statistics of participant characteristics.
| Variables | Control (n = 42) | Case (n = 7) | Total (n = 49) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Nmiss | Mean(±SD) | min-max | n | Nmiss | Mean(±SD) | min-max | n | Nmiss | Mean(SD) | min-max | |
| Age in months | 42 | 0 | 34.4(± 19.9) | 7, 72 | 7 | 0 | 31,4(± 22.2) | 1, 72 | 49 | 0 | 34(± 20.1) | 1, 72 |
| Height (cm) | 39 | 3 | 93.7(± 17.8) | 50, 125 | 7 | 0 | 93.1(± 21.3) | 55, 114 | 46 | 3 | 93.6(± 18.1) | 50, 125 |
| Weight (kg) | 42 | 0 | 15.5(± 7.2) | 7.6, 49.8 | 7 | 0 | 13.8(± 5.3) | 4.4, 21 | 49 | 0 | 15.3(± 6.9) | 4.4, 49.8 |
| BSA | 39 | 3 | 0.6(± 0.2) | 0.3, 1.3 | 7 | 0 | 0.6(± 0.2) | 0.3, 0.8 | 46 | 3 | 0.6(± 0.2) | 0.3, 1.3 |
| BMI | 39 | 3 | 17(± 3.9) | 11.1, 32.1 | 7 | 0 | 15.6(± 2.7) | 11.3, 20.3 | 46 | 3 | 16.7(± 3.8) | 11.1, 32.1 |
| Temperature in arrival (C) | 42 | 0 | 37.6(± 0.8) | 36, 39.8 | 7 | 0 | 37.1(± 0.5) | 36.2, 37.8 | 49 | 0 | 37.5(± 0.8) | 36, 39.8 |
| Respiratory rate | 42 | 0 | 44.8(± 11.8) | 24, 76 | 7 | 0 | 45(± 4.7) | 40, 53 | 49 | 0 | 44.8(± 11) | 24, 76 |
| Heart rate | 42 | 0 | 150.5(± 18.6) | 109, 185 | 7 | 0 | 191(± 42.8) | 153, 253 | 49 | 0 | 156.2(± 27) | 109, 253 |
| Total dose levalbuterol given in ED and hospital (mg) | 42 | 0 | 0.1(± 0.5) | 0, 3.1 | 7 | 0 | 0.8(± 1.7) | 0, 4.4 | 49 | 0 | 0.2(± 0.8) | 0, 4.4 |
| Levalbuterol doses | 42 | 0 | 0.1(± 0.8) | 0, 5 | 7 | 0 | 1.3(± 2.6) | 0, 7 | 49 | 0 | 0.3(± 1.2) | 0, 7 |
| Total dose of albuterol in ED and hospital (mg) | 42 | 0 | 47.1(± 125.7) | 0, 707.5 | 7 | 0 | 25.7(± 39.7) | 0, 110 | 49 | 0 | 44(± 117.2) | 0, 707.5 |
| Albuterol doses | 42 | 0 | 18.8(± 50.3) | 0, 283 | 7 | 0 | 10.3(± 15.9) | 0, 44 | 49 | 0 | 17.6(± 46.9) | 0, 283 |
| Levalbuterol plus albuterol doses | 42 | 0 | 18.9(± 50.2) | 0, 283 | 7 | 0 | 11.6(± 16.3) | 0, 44 | 49 | 0 | 17.9(± 46.9) | 0, 283 |
| Beta agonist doses/time (hours) | 39 | 3 | 0.7(± 1.1) | 0, 4.6 | 7 | 0 | 1.8(± 2.3) | 0, 6.5 | 46 | 3 | 0.9(± 1.3) | 0, 6.5 |
| Number of chronic asthma control medication | 42 | 0 | 1(± 1) | 0, 3 | 7 | 0 | 0.9(± 0.7) | 0, 2 | 49 | 0 | 0.9(± 0.9) | 0, 3 |
| Number of days of illness prior to presentation | 42 | 0 | 2.9(± 2.6) | 1, 14 | 7 | 0 | 1.6(± 0.8) | 1, 3 | 49 | 0 | 2.7(± 2.4) | 1, 14 |
Table 3.
Demographic characteristics of participants.
| Variables | Level | Control (n = 42) %(n) |
Case (n = 7) %(n) |
Total (n = 49) %(n) |
|---|---|---|---|---|
| Gender | Female | 57.14 (24) | 57.14 (4) | 57.14 (28) |
| Male | 42.86 (18) | 42.86 (3) | 42.86 (21) | |
| Race | Other | 52.38 (22) | 0 | 44.9 (22) |
| White | 47.62 (20) | 100 (7) | 55.1 (27) | |
| History of CHD | No | 90.48 (38) | 85.71 (6) | 89.8 (44) |
| Yes | 9.52 (4) | 14.29 (1) | 10.2 (5) | |
| Family history of arrhythmia | No | 100 (42) | 85.71 (6) | 97.96 (48) |
| Yes | 0 | 14.29 (1) | 2.04 (1) | |
| History of diagnosed asthma | No | 30.95 (13) | 42.86 (3) | 32.65 (16) |
| Yes | 69.05 (29) | 57.14 (4) | 67.35 (33) | |
| History of documented SVT | No | 100 (42) | 57.14 (4) | 93.88 (46) |
| Yes | 0 | 42.86 (3) | 6.12 (3) | |
| Use levalbuterol at home | No | 97.62 (41) | 42.86 (3) | 89.8 (44) |
| Yes | 2.38 (1) | 57.14 (4) | 10.2 (5) | |
| Use albuterol at home | No | 45.24 (19) | 85.71 (6) | 51.02 (25) |
| Yes | 54.76 (23) | 14.29 (1) | 48.98 (24) |
Table 4.
Conditional logistic regression: univariate analysis.
| Unadjusted point estimatea | |||
|---|---|---|---|
| Covariates | Point estimate | 95% CI | P valuea |
| Race(ref = other) | NA | NA | NAa |
| Height (cm) | 0.98 | 0.88,1.10 | 0.74 |
| Weight (kg) | 0.88 | 0.68,1.15 | 0.36 |
| BSA | NA | NA | NA a |
| BMI | 0.86 | 0.63,1.18 | 0.34 |
| Temperature in arrival (C) | 0.43 | 0.12,1.49 | 0.18 |
| Respiratory rate | 1.002 | 0.93,1.08 | 0.96 |
| Heart rate | 1.07 | 0.99,1.14 | 0.08 |
| Total dose levalbuterol given in ED and hospital (mg) | 2.001 | 0.901,4.44 | 0.09 |
| Levalbuterol doses | 1.55 | 0.94,2.56 | 0.09 |
| Total dose of albuterol in ED and hospital (mg) | 0.997 | 0.99,1.01 | 0.66 |
| Albuterol doses | 0.994 | 0.97,1.02 | 0.66 |
| Levalbuterol plus albuterol doses | 0.995 | 0.97,1.02 | 0.70 |
| Beta agonist doses/time | 2.38 | 0.99,5.75 | 0.053 |
| Number of chronic asthma control medications | 0.91 | 0.40,2.07 | 0.81 |
| Number of days of illness prior to presentation | 0.47 | 0.18,1.25 | 0.13 |
| Use albuterol at home(ref = no) | 0.15 | 0.017,1.31 | 0.08 |
| Use levalbuterol at home(ref = no) | NA | NA | NAa |
| History of diagnosed asthma(ref = no) | 0.59 | 0.11,3.15 | 0.54 |
| History of documented SVT(ref = no) | NA | NA | NAa |
| Family history of arrhythmia(ref = no) | NA | NA | NAa |
| History of CHD(ref = no) | 1.62 | 0.14,18.63 | 0.70 |
Note: conditional logistic regression is conducted to evaluate the likelihood of having SVT.
The model was not converged due to insufficient sample size of case.
Cases
1: A one-month-old 4.4 kg Caucasian male with heterotaxy and unrepaired atrioventricular septal defect on aspirin, furosemide, and heparin was hospitalized at PCH since birth and receiving intermittent albuterol for increased work of breathing. He developed SVT in the ICU 24 h after the albuterol interval was shortened to every two hours, receiving 15 mg of albuterol total in 24 h. A heart rate in the 220 s on telemetry monitoring was confirmed on electrocardiogram (EKG) to be SVT. Following unsuccessful vagal maneuvers he converted to normal sinus rhythm with 0.4 mg (0.1 mg/kg) adenosine. Albuterol was changed to levalbuterol at this time with doses scheduled every 4 h. He had no further episodes of SVT during hospitalization.
2: A 15-month-old previously healthy 11 kg Caucasian male with tracheomalacia presented to his primary care physician’s office after 3 days of coughing and one day of respiratory distress. After no improvement with nebulized budesonide and albuterol, he was sent to a local general ED where respiratory distress was treated with IV methylprednisolone, inhaled racemic epinephrine, and 2.5 mg of inhaled albuterol. He was given IV ceftriaxone to treat pneumonia, suggested by a large left-sided opacity on chest radiography. He was admitted to the general hospital ward at the general hospital and continued on intermittent nebulized albuterol and racemic epinephrine, receiving five doses over 48 h without improvement. On the day after admission tachycardia at 262 bpm was diagnosed as SVT by EKG. This resolved spontaneously after IV fluid bolus; no vagal maneuvers or adenosine were used. He was transferred to the ICU at PCH where he received further evaluation and treatment for what was determined to be a mediastinal lymphatic malformation, treated with flovent to reduce airway inflammation, sclerotherapy, and continued intermittent doses of levalbuterol and albuterol. He had a second episode of SVT in the hospital two weeks later unrelated to any dosing of SABA which resolved with adenosine 1.1 mg. With no ventricular pre-excitation on EKG, he was then started on oral digoxin, and had another episode of SVT three weeks later during a crying episode which resolved without intervention. He stopped taking digoxin after two years, and had no subsequent episodes of SVT. He was never diagnosed with asthma.
3: A two-year-old 13 kg Caucasian female with asthma and an episode of albuterol-induced SVT nine months prior presented to the PCH ED with one day of rhinorrhea and difficulty breathing that did not improve with two doses of levalbuterol at home. On presentation an EKG showed a narrow-complex tachycardia with a rate of 175 bpm consistent with slow SVT. After two doses of 0.63 mg levalbuterol, a second EKG showed sinus tachycardia with a rate of 181 bpm, though shortly after this her heart rate was noted to be 280 bpm on cardiorespiratory monitoring. Conversion was briefly successful with 1.3 mg (0.1 mg/kg) adenosine IV, though quickly followed by reversion to SVT. Within 5 min she was given another dose of adenosine 1.3 mg with successful conversion to normal sinus rhythm at 178 bpm. After a third dose of levalbuterol and 8 mg of dexamethasone she was admitted to the ICU where treatment continued with nebulized levalbuterol and ipratropium, IV methylprednisolone, and oral flecainide. She was later discharged without further complications or SVT in the hospital. Family history was notable for a father with asthma, but negative for arrhythmias.
4: The child described in case 3 above presented again at 3 years old and 14.1 kg to her primary care pediatrician’s office with 1 day of rhinorrhea and cough that failed to respond to levalbuterol every four hours at home. She was on inhaled fluticasone inhaled twice a day and levalbuterol as needed. She had been treated with flecainide to prevent SVT, but this was discontinued 2 months prior to presentation when the prescription ran out. At the pediatrician’s office, she was given 2.5 mg albuterol and then noted to have a heart rate in the 270 s. She was transferred to the PCH ED and given a 1.4 mg (0.1 mg/kg) dose of adenosine with conversion to a normal heartrate but return of SVT within 5 s. She was then given a second dose of 0.2 mg/kg adenosine with a similar result. She was given a third dose of 0.2 mg/kg adenosine, which resulted in sustained normal rate and rhythm. She was admitted to the general hospital ward and treated with oral flecainide, inhaled levalbuterol every 2 h, and oral prednisolone. She was discharged without further episodes of SVT in the hospital. She had several more episodes of SVT over the next year while taking flecainide, none requiring adenosine or hospitalization, and underwent successful ablation 16 months after this admission.
5: A three-year-old 18.1 kg Caucasian male with a history of asthma and a prior episode of albuterol-induced SVT three months prior presented to a primary care clinic after two doses of levalbuterol at home for increasing work of breathing resulting in lethargy and a rapid heart rate. Vagal maneuvers at home initially seemed successful. At the clinic, his heart rate was 250 bpm so he was transferred to a local general ED where he was started on 1.5 L oxygen by nasal cannula and vagal maneuvers were attempted without success. He was given adenosine three times without relief of symptoms. He was then successfully electrically cardioverted. The exact doses of adenosine and the presence of hemodynamic instability were not available in our record review. He was transferred to PCH and admitted directly to the general hospital ward where he was closely monitored with no further episodes of SVT or complications. He was started on oral diltiazem 9 mg three times daily after his EKG showed no pre-excitation, and also received inhaled beclomethasone and a five day course of oral prednisolone. He did not have further follow-up with cardiology or further admissions to PCH for SVT.
6: A three-year-old 15.1 kg Caucasian female with asthma presented to the PCH ED with one day of difficulty breathing and rhinorrhea, receiving two doses of inhaled levalbuterol and one dose of inhaled budesonide at home prior to presentation. In the ED, she was started on 0.5 L oxygen by nasal cannula and given two doses of inhaled levalbuterol (0.63 mg each) and ipratropium (0.5 mg each) along with 10 mg oral dexamethasone. She was admitted to the general hospital ward and received 0.63 mg inhaled levalbuterol every 2 h with occasional doses every hour, 44 mcg inhaled fluticasone twice daily, and 15 mg oral prednisolone daily. On the first day of hospitalization, she required levalbuterol every hour. In response to widened pulse pressures (90 s/20s), capillary blood gas 7.34/34/57/19, and lactate 4.15, she was given a 20 ml/kg saline bolus and SABA dose increased to 1.25 mg levalbuterol every hour. Her diastolic blood pressures remained near 40 and her O2 requirement increased to 2 L, so she was started on methylprednisolone 1 mg/kg IV q6 h. Eight hours after admission, she received a second NS bolus of 20 ml/kg due to low blood pressures and was transferred to the ICU. She was started on 10 mg/hour of continuous nebulized albuterol and placed on telemetry monitoring. After approximately 4 h of treatment, ICU staff noted a heart rate in the 270 s. Vagal maneuvers were attempted without success, though the specific maneuver attempted was not documented. She was given magnesium sulfate and IV methylprednisolone just prior to 0.1 mg adenosine administration, which resulted in successful conversion to normal rhythm. EKG after conversion showed delta waves, leading to a diagnosis of Wolf-Parkinson-White. She was discharged without further episodes of SVT or complications. She had 1 further episode of possible SVT after levalbuterol at an outside hospital that resolved with vagal maneuvers. Her WPW resolved with ablation 13 months after hospitalization without other complications.
7: A six-year-old 21 kg otherwise healthy Caucasian female presented to a general ED after 2 days of increased cough, rhinorrhea, and respiratory distress. She had been seen the day before at a different ED and prescribed cough medicine and azithromycin. At this presentation, she was started on 3 L O2 by nasal cannula and given IV methylprednisolone and 5 mg inhaled albuterol, then transferred to the PCH ED due lack of improvement. She received 5 mg inhaled albuterol in transit. In the PCH ED she was given 10 mg nebulized albuterol and maintenance IV fluids, then admitted to the ICU for continuous nebulized albuterol treatment at 20 mg/hr, magnesium sulfate at 50 mg/kg once, and IV methylprednisolone 1 mg/kg twice daily. After approximately 5 h of treatment, she was noted on monitoring to be in SVT with a rate in the 250 s. Albuterol was discontinued. Vagal maneuvers were attempted without success. She converted to normal sinus rhythm with a single dose of 12 mg IV adenosine. At this time, she was changed to levalbuterol. She had no further episodes and was discharged without further complication.
Discussion
We present our description of the incidence of SVT in children following inhaled SABA. To our knowledge, this has not been previously defined. We found SVT after SABA use to be rare in this population and to not lead to substantial morbidity or mortality. For patients receiving hospital-based treatment for asthma, no studied factors were found to be associated with SVT, though the number of SABA doses over time was near association (p = 0.053). However, the 95% confidence interval of the odds of this association overlapped 1, limiting our ability to conclude that this is a true association.
Our finding that SVT after inhaled SABA is very rare is consistent with the scarcity of published case reports. Prior case reports of SVT in children with asthma are limited to a 4 year-old boy with severe asthma and multiple hospitalizations treated with adenosine (7), a 19-month-old boy who experienced SVT after a mix of oral and inhaled SABA and resolved with vagal maneuvers (8), an 8-year-old child with recurrent SVT (9), and 2-year-old and 6-year-old boys whose SVT resolved with adenosine (10).
Tachycardia can trigger SVT in adults and children even in the absence of asthma, likely due to tachycardia being a side effect of adrenergic stimulation of the AV node. Other important triggers of SVT include electrolyte abnormalities, direct mechanical stimulation of the AV node by central lines, and thyroid disease. SABA medications used in asthma are selective for the beta-2 receptor, producing bronchial smooth muscle relaxation (11) and subsequent symptomatic relief. Albuterol is typically well tolerated, with the most common side effects being tachycardia due to reflex cardiac stimulation from peripheral vasodilation, direct stimulation of atrial beta-2 receptors, and possibly from myocardial beta-1 receptors with large doses of SABA. In addition to tachycardia, increased potassium entry into skeletal muscle from SABA can lead to hypokalemia (12), and vasodilation in previously constricted pulmonary blood vessels can produce temporary ventilation-perfusion mismatch (13), all pro-arrhythmic conditions in addition to the direct beta-1 stimulation of the myocardium by SABA.
SVT is the most common arrhythmia in infancy and childhood, with estimates suggesting an incidence of up to 1 in 250 individuals (14, 15). SVT is characterized by a rapid regular heart rate, usually greater than 200 beats per minute in children, absent P waves and narrow QRS complexes on EKG. Most patients in this study were noted to be in SVT on cardiac monitoring and treated prior to evaluation by EKG. All EKGs performed after conversion showed tachycardia with resolution of the SVT, and no EKGs noted pre-excitation. In this case series, SVT was generally treated using adenosine after vagal maneuvers were unsuccessful, and was treated by electrical cardioversion in only one case in 10 years.
Albuterol is a racemic mixture of both enantiomers of the albuterol molecule, and levalbuterol was developed as an isolated levantiomer of albuterol to produce bronchodilation without tachycardia. In our study, two episodes of SVT occurred after only levalbuterol, three after only albuterol, and two after levalbuterol followed by albuterol. In the two patients who developed SVT after levalbuterol only, SVT occurred during or immediately after treatment with levalbuterol. The proximity of treatment to SVT onset increases the likelihood that the levalbuterol was the trigger for SVT. However, our study was not designed to determine any difference in risk of SVT between levalbuterol and albuterol. More study is needed to further clarify any possible difference in risk of SVT between albuterol and levalbuterol, though prior research suggests both produce equivalent degrees of tachycardia (16).
Vagal maneuvers, such as a placing a bag of ice on the face, are the first line of treatment for SVT in hemodynamically stable patients, followed by adenosine. Adenosine transiently blocks conduction through the AV node, which often terminates the SVT, although sometimes multiple doses are required. In our study, one patient converted to a normal sinus rhythm spontaneously and the 6 remaining cases required 1 or more doses of adenosine, with only one patient failing this therapy and requiring electrical cardioversion. To our knowledge, none of the patients in this study were hemodynamically unstable, though we could not directly review outside records of the one child who underwent electrical cardioversion. Of the five patients who achieved stable normal sinus rhythm after adenosine, two required only 1 dose for resolution of SVT. Prior retrospective studies of adenosine in pediatric SVT suggest that infants are less likely to successfully respond to adenosine (6). The only patient to require cardioversion in our study was three years old.
We found the number of SABA doses over time to be the only factor to approach statistical significance as a risk factor for developing SVT. If confirmed in subsequent studies, this may suggest that the risk of SVT is dependent on the concentration of SABA presenting to cardiac receptors responsible for tachyarrhythmia and SVT. Two of the seven episodes of SVT in our series occurred after continuous SABA; after 4 h of 10 mg/hr in a 3-year-old child and 5 h at 20 mg/hr in a 6 year-old child. Though our study cannot reach firm conclusion regarding the safety of continuous SABA, patients on continuous albuterol may benefit from more intensive monitoring because of their sustained large SABA exposure. In a survey of SABA use in New Zealand and Australia, all cases of witnessed SVT occurred with use of intravenous SABA (17), which likely delivers much higher concentrations of SABA to the heart than inhaled SABA. Surprisingly, we did not identify any cases of SVT after intravenous SABA in our population, likely due to our screening methodology as well as large variation between sites in the use of second-line asthma therapies, with intravenous SABA used rarely in our region (18).
Though prior studies suggest that congenital heart disease, prior arrhythmias, and fevers are predisposing factors for SVT in children (9), we could not evaluate these associations in this study because of limitations in retrospective data and relatively small number of patients with prior arrhythmias and congenital heart disease. Future exploration of predisposing factors for SVT will require the examination of larger numbers of cases of SVT, likely in larger populations.
Limitations
Most limitations of this study arise from the small number of children that developed SVT after SABA use over this 10-year period. This limits our ability to detect associations between patient factors and risk for SVT, and increases uncertainty around our prevalence estimates of SVT after SABA.
Cases were identified using billing and coding information. This introduces the likelihood that we missed cases not billed or coded according to our explicit criteria, possibly falsely deflating our estimate of incidence. However, this approach could reasonably be applied to other populations given that billing and coding information is generally available. These criteria did result in our inclusion of two patients who experienced SVT after albuterol but did not have a diagnosis of asthma. Both these children received albuterol due to respiratory symptoms, and both had other significant medical conditions known to be associated with arrhythmia. The inclusion of these patients may falsely inflate the incidence of SVT among children with asthma.
Potentially, we could have missed patients who received albuterol at one location, developed SVT, and were then transferred to another location where they received adenosine. In this case, albuterol and adenosine would not be billed in the same encounter, though we would expect that many of these patients should have diagnosis codes for both asthma and SVT.
To define the denominator for incidence, we included only patients with both a primary diagnosis of asthma and billed albuterol. Some cases may have been missed by this method if a diagnosis of asthma was not in the primary billing position. A more broad definition that included a diagnosis of asthma in other billing positions would likely increase the denominator, reducing the estimate of SVT prevalence.
All patients who developed SVT after SABA use in our population were Caucasian and lived in a large but limited geographic area, limiting extrapolation of these results to other populations.
Conclusions
SVT is rare during SABA treatment with low morbidity and no measured mortality. Wolf-Parkinson White Syndrome (and the presence of an antegrade-conducting accessory pathway) was identified in only 1 of 6 children with SVT. A high SABA dose over time might be a risk factor for developing beta-agonist induced SVT, but further research is needed.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Roemer M. Health care expenditures for the five most common children’s conditions, 2008: estimates for U.S. Civilian Noninstitutionalized Children, Ages 0–17; Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 2.National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart Lung and Blood Institue, National Institutes of Health, U.S. Department of Health and Human Services; 2007. [Google Scholar]
- 3.Sills MR, Ginde AA, Clark S, Camargo CA. Multicenter analysis of quality indicators for children treated in the emergency department for asthma. Pediatrics. 2012;129(2):e325–32. doi: 10.1542/peds.2010-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo CA, Spooner C, Rowe BH. Continuous versus intermittent beta-agonists for acute asthma. Cochrane Database Syst Rev. 2011;4:CD001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzola E, Bozzola M, Barberi S, Cutrera R, Villani A. Safety and potential side effects of beta 2-agonists: a still debated question. Int J Pediatr Child Health. 2013;1(1):4–10. doi: 10.12974/2311-8687.2013.01.01.2. [DOI] [Google Scholar]
- 6.Lewis J, Arora G, Tudorascu DL, Hickey RW, Saladino RA, Manole MD. Acute management of refractory and unstable pediatric supraventricular tachycardia. J Pediatr. 2016:95–98. [DOI] [PubMed] [Google Scholar]
- 7.Cook P, Scarfone RJ, Cook RT. Adenosine in the termination of albuterol-induced supraventricular tachycardia. Ann Emerg Med. 1994;24(2):316–319. doi: 10.1016/S0196-0644(94)70146-6. [DOI] [PubMed] [Google Scholar]
- 8.Keller KA, Bhisitkul DM. Supraventricular tachycardia: a complication of nebulized albuterol. Pediatr Emerg Care. 1995;11(2):98–99. doi: 10.1097/00006565-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Duane M, Chandran L, Morelli P. Recurrent supraventricular tachycardia as a complication of nebulized albuterol treatment. Clin Pediatr (Phila)). 2000;39(11): 673–677. doi: 10.1177/000992280003901109. [DOI] [PubMed] [Google Scholar]
- 10.Trachsel D, Newth CJL, Hammer J. Adenosine for salbutamol-induced supraventricular tachycardia. Intensive Care Med. 2007;33(9):1676–1676. doi: 10.1007/s00134-007-0673-4. [DOI] [PubMed] [Google Scholar]
- 11.Hilal-Dandan R, Brunton LL. Pulmonary pharmacology In: Goodman and Gilman’s manual of pharmacology and therapeutics, 2e. New York (NY): McGraw-Hill Education; 2016. [Google Scholar]
- 12.Del Río-Navarro B, Gazca-Aguilar A, Quibrera Matienzo JA, Rodríguez Galván Y, Sienra-Monge JJ. Metabolic and electrocardiographic effects of albuterol in pediatric asthmatic patients treated in an emergency room setting. Allergol Immunopathol (Madr)). 2015;27(1):18–23. doi: 10.1157/13057765. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto LG, Wiebe RA, Rosen LM, Ringwood JW, Uechi CM, Miller NC, Beardsly ES, Toshi AS, Sugimoto SP, MacPherson KA, et al. Oxygen saturation changes during the pediatric emergency department treatment of wheezing. Am J Emerg Med. 1992; 10(4):274–284. doi: 10.1016/0735-6757(92)90002-F. [DOI] [PubMed] [Google Scholar]
- 14.Moak JP. Supraventricular tachycardia in the neonate and infant. Prog Pediatr Cardiol. 2000;11(1):25–38. doi: 10.1016/S1058-9813(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 15.Schlechte EA, Boramanand N, Funk M. Supraventricular tachycardia in the pediatric primary care setting: age-related presentation, diagnosis, and management. J Pediatr Heal Care. 2008;22(5): 289–299. doi: 10.1016/j.pedhc.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Kelly A, Kennedy A, John BM, Duane B, Lemanowicz J, Little J. A comparison of heart rate changes associated with levalbuterol and racemic albuterol in pediatric cardiology patients. Ann Pharmacother. 2013; 47(5):644–650. doi: 10.1345/aph.1S003. [DOI] [PubMed] [Google Scholar]
- 17.Habashy D, Lam LT, Browne GJ. The administration of beta2-agonists for paediatric asthma and its adverse reaction in Australian and New Zealand emergency departments: a cross-sectional survey. Eur J Emerg Med. 2003;10(3):219–224. doi: 10.1097/00063110-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Bratton SL, Newth CJL, Zuppa AF, Moler FW, Meert KL, Berg RA, Berger J, Wessel D, Pollack M, Harrison R, et al. Critical care for pediatric asthma: wide care variability and challenges for study. Pediatr Crit Care Med. 2012;13(4):407–414. doi: 10.1097/PCC.0b013e318238b428. [DOI] [PMC free article] [PubMed] [Google Scholar]


