MULTISYSTEM INFLAMMATORY SYNDROME IN CHILDREN HISTORY, CASE DEFINITION AND INCIDENCE
A novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in Wuhan, China, in December 2019 causing a worldwide pandemic. Children were initially thought to largely be spared from severe disease, but in April 2020, cases of children with a severe inflammatory syndrome with some Kawasaki disease (KD)-like and/or toxic shock features emerged as a presumed postinfectious complication of SARS-CoV-2 infection from coronavirus disease 2019 (COVID-19) epicenters in Italy, the United Kingdom and New York. This new pediatric illness was named Pediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2 in the United Kingdom, and Multisystem Inflammatory Syndrome in Children (MIS-C) by the U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). This new syndrome was then observed worldwide, although there appear to be comparatively few reports of MIS-C or KD–like presentation from China and other Asian countries, despite being an early “hot spot” with a high incidence of SARS-CoV-2 infection.
Case definitions of MIS-C by the U.S. CDC and the WHO require the presence of fever in children with elevated inflammatory markers, multisystem organ involvement, evidence of recent SARS-CoV-2 infection or exposure and exclusion of alternative diagnoses. Differences between the 2 definitions include age (≤19 years for WHO vs. <21 years for CDC), duration of fever (at least 3 days for WHO vs. 1 day for CDC), requiring hospitalization for CDC and more extensive and specific laboratory criteria for the CDC definition.
As of late August 2021, over 4400 patients with MIS-C were reported to state public health departments in the United States (https://www.cdc.gov/mis-c/cases). Available estimates of cases worldwide are lacking, but a recent study was able to capture 614 children from 34 countries, including reported cases in low- and middle-income countries.1 The true disease burden is likely to be far greater as not every case was reported, and cases early in the pandemic may not have been recognized. It is challenging to estimate the incidence of MIS-C per child infected with SARS-CoV-2, because during acute infection these individuals are often asymptomatic and therefore may not get tested. The occurrence of MIS-C follows peaks of COVID-19 infection by median 4 weeks (range 2–5 weeks). Using state surveillance data for MIS-C across 7 US regions, and linking it to reported positive SARS-CoV-2 test results across the same regions, the incidence of MIS-C was estimated to be approximately 3 per 10,000 individuals <21 years of age infected with SARS-CoV-2.2 Limited, preprint data suggest an incidence rate of 0.045% (95% credible interval, 0.035%–0.068%) in England during the first wave, or 4.5 per 10,000 individuals infected with SARS-CoV-2. It is possible that this is an overestimate, given untested asymptomatic cases were modeled for inclusion, but all estimates to date have led to the conclusion that MIS-C is a relatively rare complication of SARS-CoV-2 infection in children. The incidence of MIS-C may change as more of the population is vaccinated and has been previously exposed to SARS-CoV-2.
CLINICAL CHARACTERISTICS AND PRESENTATION OF MIS-C PATIENTS
The median age of children diagnosed with MIS-C is 8–11 years, but ranges from 1 to 20 years.3 Rare cases with similar symptoms have been reported in adults, referred to as MIS in adults. The rates of MIS-C appear to vary by race and ethnicity, and Black and Hispanic children have been disproportionately affected by both acute COVID-19 and MIS-C.
A hallmark of MIS-C is systemic inflammation, as evidenced by fever and elevated inflammatory markers on laboratory testing, such as C-reactive protein. Abnormal blood cell counts (lymphocytopenia, neutrophilia, mild anemia, thrombocytopenia), mildly elevated liver enzymes, coagulopathy and elevated D-dimers are also very frequent. The extent of laboratory abnormalities appear to correlate with severity of illness.3
Patients with MIS-C most often present with fever, gastrointestinal, mucocutaneous, cardiorespiratory and/or neurocognitive symptoms. The gastrointestinal system is the most commonly involved organ system, reported in over 80% of patients across all age groups. Dermatologic or mucocutaneous symptoms occur in 85% of children 0–5 years of age, but only 60% of teenagers 13–20 years of age. More than half of patients present with hypotension and shock from systemic hyperinflammation/vasodilation or myocardial involvement, frequently requiring intensive care unit (ICU) admission. Cardiac and neurologic involvement are more common in teenagers than younger children. Respiratory symptoms are not a prominent feature in MIS-C, but some children may have respiratory symptoms due to shock or cardiogenic pulmonary edema, and less commonly direct pulmonary involvement. At this time, it remains unclear how SARS-CoV-2 variants will affect incidence and clinical presentation of MIS-C in children and adolescents.
The mechanisms underlying cardiovascular injury in MIS-C are not completely understood, but are hypothesized to result from direct viral cardiomyocyte toxicity, microvascular dysfunction and/or inflammation. Evidence of cardiovascular involvement is reported in 40%–80% of patients and includes elevated brain natriuretic peptide and troponin, ventricular dysfunction, pericardial effusion, coronary artery dilation or aneurysm and arrhythmias.3 Myocardial involvement is subclinical in many cases, with depressed left ventricular systolic dysfunction (defined as left ventricular ejection fraction ≤55%) in approximately a third of patients.4 Ventricular dysfunction is typically identified at the time of hospital admission, and appears to be transient with normalization of ventricular function in >90% of affected patients at 30 days and 99% at 90 days. However, cardiac magnetic resonance performed after discharge can identify ongoing involvement with higher sensitivity, and adequately powered follow-up studies are ongoing to determine long-term effects on the heart.
Coronary artery aneurysms affect 8%–13% of patients with MIS-C, and most (93%) of these are relatively small (Z score of the right coronary or left anterior descending artery <5). Arrhythmias are a relatively rare complication of MIS-C and occur in 12% of patients.
Neurologic involvement is common in patients with MIS-C, and includes altered consciousness, headache, loss of smell or taste, seizures or motor impairment.5 Neurologic involvement is transient in most patients, but severe involvement and fatal cases have been reported. Thrombotic complications are more common in MIS-C than in children hospitalized for acute COVID-19 (6.5% vs. 2.1%, respectively).6
DIFFERENTIAL DIAGNOSIS OF MIS-C
Infections With Bacteria or Other Pathogens
Features of toxic shock syndrome can be prominent in patients suspected of having MIS-C, particularly hypotension, rash and multiorgan involvement. Due to similarities with septic shock, broad-spectrum antibiotics should be initiated for most critically ill patients until culture results are available. Infection with toxin-producing bacteria, however, including Staphylococcus aureus and Streptococcus pyogenes, excludes MIS-C, as an alternative explanation has been identified. In some cases with concomitant infection, especially those that are not usually characterized by marked inflammation (urinary tract infection, acute otitis media) and when systemic illness may seem out of proportion, MIS-C sometimes remains in the differential diagnosis for treatment purposes.
Acute COVID-19 Infection
There are overlapping features between acute severe COVID-19 and MIS-C patients; however, demographics and clinical presentation can aid in differentiation.4 In the United States, patients with MIS-C (vs. acute severe COVID-19) are more likely to be between 6 and 12 years of age (vs. 0–5 or 13–20 years) and less likely to have underlying medical conditions, and more likely to have cardiovascular and/or mucocutaneous involvement. Patients with MIS-C also are more likely to have high C-reactive protein values and thrombocytopenia compared with those with acute COVID-19. Social vulnerability has led to higher frequency of SARS-CoV-2 exposure in racial and ethnic minority groups, but using white non-Hispanic patients as a baseline, a U.S. public health surveillance registry found Black non-Hispanic patients to be at higher risk of MIS-C than acute COVID-19.4
Kawasaki Disease
Up to half of the patients with MIS-C meet criteria for complete or incomplete KD. Due to the overlap in symptoms, SARS-CoV-2 testing and exposure history are often useful to distinguish MIS-C from KD. However, testing will be less of a distinguishing feature over time as seroprevalence increases from either wild-type infection or vaccination. Patients with MIS-C are typically older than those with KD. Gastrointestinal symptoms and myocardial dysfunction/shock are also more frequent in MIS-C, although may occur in KD, especially in the approximately 5% of patients that develop KD shock syndrome. Importantly, the risk of coronary artery complications in MIS-C is not limited to patients with KD criteria, as they have been described in all patients with MIS-C.
TREATMENT FOR MIS-C
Early in the pandemic, treatment for MIS-C was extrapolated from KD and toxic shock syndrome with widespread use of intravenous immunoglobulin (IVIG). As the highly inflammatory nature of MIS-C became more evident and concerns regarding potentiation of viral replication decreased, glucocorticoids were also frequently used, especially in patients who failed to improve with IVIG. Other treatments used for initial MIS-C treatment, and not infrequently for rescue treatment in refractory cases, included biologic agents such as anakinra, an interleukin 1 receptor antagonist, anti-interleukin 6 therapies and tumor necrosis factor inhibitors. However, exclusion of infection as the cause of symptoms before using more aggressive immunosuppression is strongly recommended.
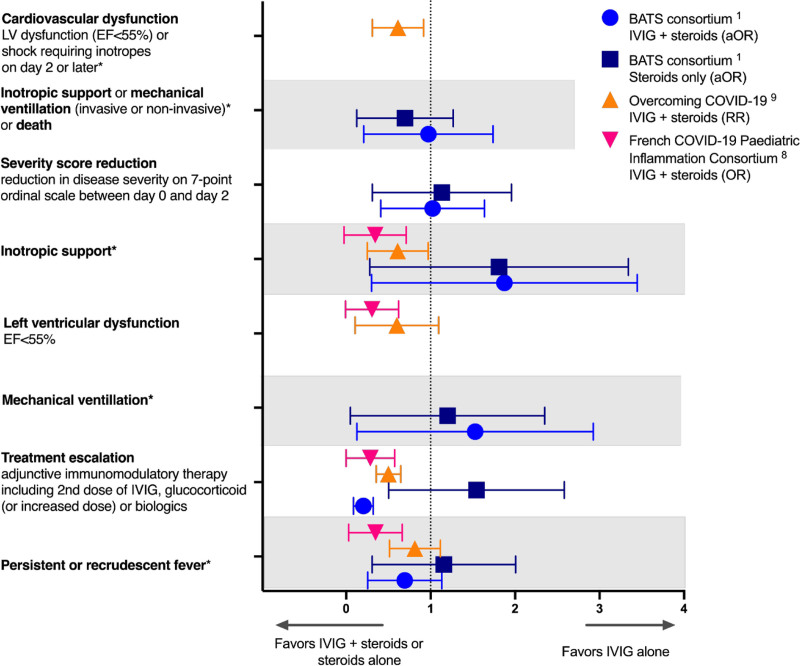
No randomized trials evaluating therapies for MIS-C have been published to date. An early, small study by Belhadjer et al7 indicated that combination therapy with IVIG and glucocorticoids, compared with IVIG alone, led to shorter time to normalization of cardiac function. A subsequent retrospective study utilizing a propensity score analysis demonstrated that IVIG and glucocorticoids (vs. IVIG alone) was associated with less fever as the primary outcome, with decreased need for second-line therapy, hemodynamic support, left ventricular dysfunction and ICU length of stay as secondary outcomes.8 Larger propensity analyses evaluating the benefit of early, combined treatment with IVIG plus glucocorticoids as treatment for MIS-C in the US-based Overcoming COVID-19 Network and the international Best Available Treatment Study (BATS) demonstrated conflicting findings (Fig. 1).1,9 Patients in the Overcoming COVID-19 cohort treated with IVIG plus glucocorticoids, as compared with those treated with IVIG alone, had improved short-term cardiovascular outcomes with improved left ventricular ejection fraction and less vasopressor requirement measured at 2 or more days after initial MIS-C treatment.9 In contrast, there were no significant differences in a composite outcome of inotropic support, mechanical ventilation or death in the BATS cohort across treatment groups.1 The BATS study revealed possible benefit for those patients who met WHO criteria for MIS-C and who were treated with glucocorticoids, as compared with IVIG alone, as the composite outcome had an adjusted odds ratio of 0.3 (95% confidence intervals, 0.1–0.85). This may be useful to consider in critically ill patients in resource-limited settings where IVIG may not be available. Likely reasons for the discrepant findings between the studies include differences in cohort definitions (CDC vs. WHO vs. broader definitions of MIS-C) and in markedly greater illness severity in the Overcoming COVID-19 cohort.
FIGURE 1.
Comparison of clinical outcomes in MIS-C based on immunomodulatory treatment in 3 large studies. *Two or more days following initial treatment with immunomodulatory therapy. EF indicates ejection fraction; LV, left ventricular.
OUTCOMES OF MIS-C
Children with MIS-C can be critically ill. In a systematic review including reports from North America, Europe and Asia, approximately two-third of patients require admission to the ICU for a median of 5 days (interquartile range, 4–8 days), 40% inotropic support, 15% mechanical ventilation and 2% extracorporeal membrane oxygenation. The majority of children recovered with reported mortality rate around 2%.10
Longitudinal follow-up of patients with MIS-C remains limited. In a small cohort of patients, most organ-specific sequelae had resolved by 6 months.11 However, almost half of the patients had poor exercise tolerance on 6-minute walk test and 20% reported severe emotional difficulties. Large, longitudinal studies are underway to better quantitate long-term outcomes of MIS-C, and how they differ from outcomes after COVID-19 infection and “long COVID.”
CONCLUSIONS
MIS-C is a severe postinfectious complication of SARS-CoV-2 infection mainly affecting children and adolescents. Despite the life-threatening presentation of MIS-C in some patients, overall mortality is low with relatively rapid resolution of organ dysfunction. Clarifying the pathogenesis, identifying genetic and other risk factors for MIS-C, determining the optimal treatment strategies tailored to severity of illness, and identifying the effects of MIS-C on long-term health are priorities for study through multidisciplinary, international collaboration. Whether COVID-19 vaccination will have an impact on the risk of MIS-C remains to be determined.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.McArdle AJ, Vito O, Patel H, et al. ; BATS Consortium. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; Centers for Disease Control and Prevention COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of us children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaRovere KL, Riggs BJ, Poussaint TY, et al. ; Overcoming COVID-19 Investigators. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitworth H, Sartain SE, Kumar R, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z, Auriau J, Méot M, et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. 2020;142:2282–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouldali N, Toubiana J, Antona D, et al. ; French COVID-19 Paediatric Inflammation Consortium. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son MBF, Murray N, Friedman K, et al. ; Overcoming COVID-19 Investigators. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med. 2021;385:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushik A, Gupta S, Sood M, et al. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020;39:e340–e346. [DOI] [PubMed] [Google Scholar]
- 11.Penner J, Abdel-Mannan O, Grant K, et al. ; GOSH PIMS-TS MDT Group. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health. 2021;5:473–482. [DOI] [PubMed] [Google Scholar]