Abstract
Cancers arising from gastrointestinal epithelial cells are common, aggressive, and difficult to treat. Progress in this area resulted from recognizing that the biological behavior of these cancers is highly dependent on bioactive molecules released by neurocrine, paracrine, and autocrine mechanisms within the tumor microenvironment. For many decades after its discovery as a neurotransmitter, acetylcholine was thought to be synthesized and released uniquely from neurons and considered the sole physiological ligand for muscarinic receptor subtypes, which were believed to have similar or redundant actions. In the intervening years, we learned this former dogma is not tenable. (1) Acetylcholine is not produced and released only by neurons. The cellular machinery required to synthesize and release acetylcholine is present in immune, cancer, and other cells, as well as in lower organisms (e.g., bacteria) that inhabit the gut. (2) Acetylcholine is not the sole physiological activator of muscarinic receptors. For example, selected bile acids can modulate muscarinic receptor function. (3) Muscarinic receptor subtypes anticipated to have overlapping functions based on similar G protein coupling and downstream signaling may have unexpectedly diverse actions. Here, we review the relevant research findings supporting these conclusions and discuss how the complexity of muscarinic receptor biology impacts health and disease, focusing on their role in the initiation and progression of gastric, pancreatic, and colon cancers.
Keywords: muscarinic receptors, gastric cancer, pancreatic cancer, colorectal cancer, acetylcholine
1. Introduction
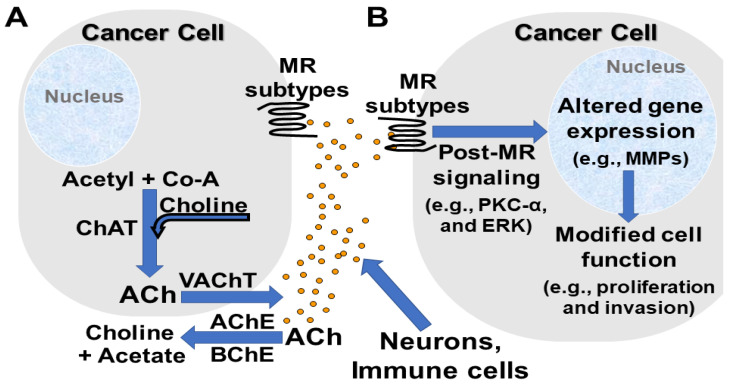
The role of neurotransmitters in modulating cancer cell behavior has emerged as a major focus of investigation and a promising avenue for developing novel therapeutics [1,2,3]. This takes on particular importance for cancers of the organs in the gastrointestinal (GI) tract that are innervated by both the central and enteric nervous systems [1,4]. The complexity of this field is highlighted by evidence that so-called neurotransmitters (e.g., acetylcholine, ACh) can be produced and released by non-neural cells (e.g., immunocytes, stromal cells, and cancer cells themselves) (Figure 1). The actions of these agents are further modulated by the differential distribution and regulation of their receptors and the enzymes required for their hydrolysis (e.g., acetyl- and butyrylcholinesterases) [5] (Figure 1). Understanding the dynamics of these interactions and their underlying mechanisms is likely to open the door to developing new biomarkers and therapeutic modalities.
Figure 1.
Capability of neurons, immunocytes, and cancer cells to produce and release acetylcholine (ACh) that promotes cancer progression. (A). Neurons, immunocytes, and cancer cells express enzymes (e.g., choline acetyltransferase, ChAT) and transporters (e.g., vesicular acetylcholine transporter, VAChT) necessary to produce and release ACh. Acetyl- (AChE) and butyrylcholinesterases (BChE) in the extracellular space rapidly hydrolyze ACh to acetate and choline. (B). ACh activates muscarinic receptor (MR) subtypes expressed by adjacent cancer cells. Post-muscarinic receptor signaling activates several protein kinases (e.g., protein kinase C-α, PKC-α), and transcription factors (e.g., extracellular signal-regulated protein kinase 1/2, ERK1/2), thereby altering the expression of genes that encode for proteins that modify cell function and promote cancer cell proliferation, survival, migration, invasion, and metastasis (e.g., matrix metalloproteinases [MMPs] like MMP1, MMP7, and MMP10).
Here, because there is an abundance of novel information and the incidence of tumors in these organs is increasing in younger populations [6,7], we focus on the differential actions of activating muscarinic receptor subtypes on the evolution and progression of cancers of the stomach, pancreas, and colon. Innovative therapies for these cancers are urgently needed since effective treatment options are limited once adenocarcinomas arising from these organs progress to surgically unresectable, advanced, and invasive stages [8,9]. Notably, for these GI cancers, even novel immunomodulators, like PD-1 checkpoint inhibitors, appear to hold little promise for successful treatment as their effects tend to be modest and lack durability [10,11]. Thus, both the gaps in current knowledge and the need for new therapeutic approaches are highlighted in this review.
To provide a comprehensive overview of the role muscarinic receptor subtypes and their ligands play in the progression of these GI cancers, we divided this review into discrete sections. Focusing on gastric, pancreatic, and colon cancer, we review information regarding the distribution of neural and non-neural sources of ACh and other agents reported to modulate muscarinic receptor function by direct or allosteric interactions, the distribution and expression of the five muscarinic receptor subtypes (M1R–M5R, encoded by CHRM1–CHRM5), and their differential roles in modulating cancer cell behavior. Nonetheless, it may be difficult to compartmentalize these highly integrated actions. To unravel their complexity, a systems biology approach may be helpful and, based on the implications for cancer therapeutics, appears warranted.
2. Muscarinic Receptor Ligands
2.1. Overview
Whereas ACh was commonly thought to be synthesized and released from neurons only, emerging evidence from several laboratories indicates that the cellular machinery required to synthesize and release ACh is also present in immune, cancer, and other cell types (Table 1), as well as in lower organisms (e.g., bacteria) that comprise the gut microbiome. In this section, we review the challenges faced by investigators attempting to measure tissue concentrations of ACh and their changes over time and the evidence that, by allosteric and other mechanisms, cholesterol, and its metabolic by-products (e.g., bile acids), interact with and alter the function of G protein-coupled receptors, including muscarinic receptors.
Table 1.
Neuronal and non-neuronal cells reported to produce and release acetylcholine.
| Sources of Acetylcholine | Refs. | |
|---|---|---|
| Neurons | ||
| Autonomic nervous system | Preganglionic sympathetic/parasympathetic neurons | [23] |
| Peripheral nervous system | Terminal ends of axons at neuromuscular junctions | [23] |
| Central nervous system | Primarily interneurons | [23,24] |
| Non-Neuronal Cells | ||
| Immunocytes | CD4 + T cells; B cells; NK cells | [25,26,27] |
| Placental trophoblast | [28] | |
| Keratinocytes | [29] | |
| Cardiomyocytes | [30] | |
| Airway epithelial cells | [31] | |
| Vascular endothelial cells | [32] | |
| Urothelial cells | [33,34] | |
| Cancer cells | Colon, stomach, lung, and others | [12,35,36] |
2.2. Neuronal and Non-Neuronal Acetylcholine
Although initially considered solely a neurotransmitter, abundant evidence obtained over the past 20 years indicates that many non-neuronal cells possess the capacity to synthesize and release ACh [12,13,14,15,16,17,18] (Table 1). This is suggested by identifying acetylcholine, as well as its derivatives in tissues; typically, these molecules are identified using either radiolabeled choline, a precursor to ACh, or an electrochemical detection method such as high-performance liquid chromatography with electrochemical detection (HPLC-ED) [12]. Radiolabeled choline was detected in keratinocytes [19] and, using similar methods, choline and glycerophosphocholine were detected in human [20] and chicken cardiomyocytes [21]. In fact, it is conceivable that non-neuronal ACh plays a larger and more important role in modulating the behavior and aggressiveness of GI cancer cells than does ACh derived from classical neuronal release. For example, increased radiolabeled choline uptake in human urothelial cancers [22] suggests neoplastic epithelial cells may have an augmented need for choline to produce ACh. Likewise, investigators used HPLC-ED to detect ACh production and release from H508 and Caco-2 human colon cancer cell lines [12].
Nonetheless, relevant evidence for non-neuronal ACh production and/or release must be examined thoughtfully and critically. By itself, the presence of an enzyme important for ACh synthesis, e.g., choline acetyltransferase (ChAT), does not provide sufficient evidence that ACh is produced and released at concentrations that will effectively alter the function of neighboring cells by autocrine or paracrine mechanisms (Figure 1). Tissue diffusion rapidly dissipates released ACh, thereby making it difficult to measure tissue concentrations of ACh reliably. Unlike other neurotransmitters, no technique can fix ACh to tissue to facilitate immunohistochemical quantification [18]. Moreover, enzymes that efficiently hydrolyze ACh, e.g., acetylcholinesterase (AChE), limit the duration that bioactive concentrations are attained and maintained. Hence, it is important to measure tissue levels of ACh over time-this remains technically challenging [12].
2.3. Bile Acids as Physiological Bioactive Muscarinic Receptor Ligands
Bile acids comprise a large family of cholesterol derivatives that can be further modified in the gut by bacterial hydroxylases and in the liver by conjugation, primarily with glycine and taurine, and sulfation. The major effect of these structural modifications is to alter the amphipathic nature of these agents, which for decades were considered only to function in the digestion and absorption of lipids. Over the past 22 years, evidence has emerged from several laboratories indicating that selected bile acids are, in fact, signaling molecules that, amongst other actions, interact with muscarinic receptors and modulate post-receptor signaling [37,38,39]. Although the molecular determinants that mediate bile acid interactions with muscarinic receptors remain obscure, modeling has suggested the possibility that molecular mimicry between bile acid and ACh structures may explain competition for muscarinic receptor binding sites [40]. It is also conceivable, if not more likely, that bile acids exert their effects by allosteric modulation of ligand-muscarinic receptor interactions. Not surprisingly, cholesterol, the parent molecule for bile acid synthesis, shares structural similarity with bile acids. Notably, cholesterol was shown to modulate the behavior of Class A G protein-coupled receptors, including M1R, allosterically [41].
Bile acids, released from the liver and gallbladder into the common bile duct, can access gastric, pancreatic, and colonic adenocarcinomas via the GI lumen, respectively, by refluxing into the stomach and pancreatic duct, and spilling into the colon (Figure 2) [42,43]. Under certain circumstances, including prior cholecystectomy resulting in loss of bile storage in the gallbladder, intestinal surgery rerouting bile flow, and deficient intestinal release of fibroblast growth factor-19 (FGF19) resulting in unrestrained bile acid production by the liver, sustained elevation of bile acid levels in the pancreatic duct, stomach, and colon have the potential to alter the function of both normal and neoplastic epithelial cells by muscarinic mechanisms [44].
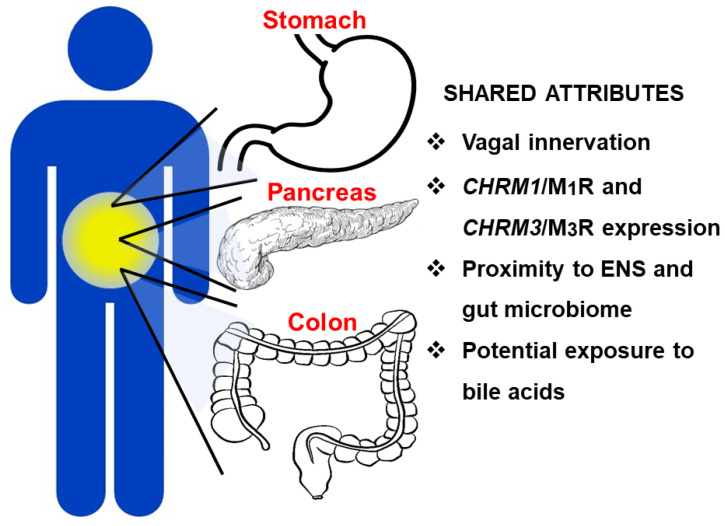
Figure 2.
Key attributes shared by the stomach, pancreas, and colon that facilitate and promote cancer progression and metastasis. These anatomically proximate GI organs share vagal innervation, expression of CHRM1/M1 and CHRM3/M3 subtype muscarinic receptors, and exposure to luminal bile acids and the bacteria, viruses, and fungi that comprise the gut microbiome; these shared attributes can promote the development and progression of adenocarcinomas. ENS, enteric nervous system.
In several studies employing colon cancer cell lines in vitro, deoxycholic and lithocholic acids, and their glycine and taurine conjugates, modulate muscarinic receptor activation [37,40,44]. This line of investigation was initiated by the surprising observation that a bile acid, lithocholyltaurine, competed with a cholinergic agonist, carbamylcholine, for binding to the same muscarinic receptors on gastric mucosal cells [45]. Subsequently, the functional interaction of bile acids with muscarinic receptors was confirmed independently by investigators studying bile acid-induced cardiac arrhythmias [46]. Although the biological implications of the functional interaction of bile acids with muscarinic receptors are broad [44], perhaps the most important clinical impact is on gastrointestinal neoplasia. In human H508 colon cancer cells that express high levels of M3R, lithocholyltaurine at concentrations achievable in the human colon [47] caused a dose-dependent increase in cell proliferation [37], effects not observed in human SNU-C4 colon cancer cells that lack M3R expression [37]. Using stool obtained from the cecum of deceased humans, Hamilton et al., showed that bile acids in the proximal colon achieve concentrations in the range of 10–100 µM [47]. Bile acid concentrations are likely higher in living persons and in those following cholecystectomy or with ileal disease or resection that disrupts the enterohepatic circulation of bile acids. Notably, these ‘physiological’ concentrations of fecal bile acids were shown to stimulate colon cancer cell proliferation in vitro [47].
ACh and taurine and glycine conjugates of lithocholic and deoxycholic acids induce cell proliferation by post-receptor signaling involving transactivation of epidermal growth factor receptors (EGFR) and post-EGFR p44/42 mitogen activated protein kinase (MAPK) signaling [38]. EGFR transactivation is mediated by matrix metalloproteinase-7 (MMP7)-catalyzed release of heparin binding-EGF-like growth factor (HB-EGF), an EGFR ligand [48]. Post-M3R signaling also involves the activation of protein kinase C-α and p38 MAPK, with evidence that potentiating crosstalk between post-receptor signaling pathways augments cell proliferation, migration, and invasion [49].
3. Muscarinic Receptor Subtypes, Signaling, and Anatomic and Cellular Distribution
3.1. Overview
Since muscarinic receptors represent an ancient form of signaling, it is not surprising that the genetic lineage for the ACh receptors family in general, and muscarinic receptors (mAChR) in particular, can be traced to the earliest life forms with >80% receptor homology preserved amongst vertebrates [50]. Although muscarinic receptor subtypes are expressed in all vertebrates examined, there are important differences. For example, M1R is not expressed in teleosts or chickens and other birds [50]. This finding suggests that the actions regulated by M1R are not critical for the life and health of these organisms, that redundant actions among the muscarinic receptors compensate for the absence of M1R, or that M1R functions are assumed by other signaling pathways.
In the following sections, we consider the tissue distribution and actions of each of the five muscarinic receptor subtypes (Table 2). By way of overview, it is important that this family of receptors is segregated into two subfamilies, M1R, M3R, and M5R (sometimes referred to as MRodd), and M2R and M4R (MReven). The former, coupled to Gq/11, signal initially via changes in cellular phospholipids and calcium. The latter, coupled to Gi/o, signal via changes in cellular cAMP. All five muscarinic receptor subtypes are expressed in the brain, where M1R, M2R, and M4R predominate. M3R appears to play a larger role outside the brain, including throughout the GI tract where it appears to play a major role in regulating normal physiological processes, like fluid and electrolyte secretion [50].
Table 2.
G-protein coupling, physiological ligands, tissue distribution, and effects of muscarinic acetylcholine receptor subtype activation in health and disease.
| MR Subtype (Gene) |
G-Protein | Ligands | Tissue Distribution | Physiological Actions | Actions in GI Cancers | Refs. |
|---|---|---|---|---|---|---|
| M1R (CHRM1) |
Gq/11 | ACh, BA, cholesterol (allosteric) | Brain, gastric mucosa, respiratory epithelium, skin, melanocytes, immunocytes | Mediates gastric pepsinogen secretion | Protects against PDAC and colon neoplasia | [34,40,41,50,51,52,53] |
| M2R (CHRM2) |
Gi/o | ACh | Brain, heart, ENS, gastric mucosa, skin, bladder, melanocytes, smooth muscle, immunocytes | Modulates cardiac rhythm, GI motility | None reported | [34,50,53,54,55,56] |
| M3R (CHRM3) |
Gq/11 | ACh, BA | Gastric chief and parietal cells, colon epithelial cells, smooth muscle, ENS, brain, skin, melanocytes, immunocytes | Mediates gastric acid and pepsinogen secretion; GI motility | Promotes gastric and colon cancer cell proliferation and PDAC severity | [37,38,50,52,57,58,59,60,61,62] |
| M4R (CHRM4) |
Gi/o | ACh | Brain, gastric mucosa, small intestine, skin, melanocytes, immunocytes | Enhances gastric acid secretion; regulates striatal dopamine release | None reported | [34,50,57,63,64] |
| M5R (CHRM5) |
Gq/11 | ACh | Brain, cerebral vasculature, ENS; mRNA expressed in testes, placenta, thyroid, small intestine, immunocytes | Enhances gastric acid secretion; regulates striatal dopamine release; mediates SNc excitability | None reported | [34,50,53,61,63,65,66] |
Refs, references; MR, muscarinic receptor; ACh, acetylcholine; BA, bile acids; ENS, enteric nervous system; GI, gastrointestinal; PDAC, pancreatic ductal adenocarcinoma; SNc, substantia nigra pars compacta.
3.2. CHRM1/M1R
CHRM1/M1R was the first muscarinic receptor subtype successfully knocked out globally in mice. Consequently, the impacts of both global M1R deficiency and, more recently, conditional M1R deficiency in a number of organs, have permitted elucidation of M1R actions in considerable detail [67]. M1R is the dominant muscarinic receptor subtype in the central nervous system; it is also expressed in the respiratory epithelium and skin, and widely in the GI tract where it contributes to the regulation of salivary, gastric, and pancreatic secretion.
Located largely in forebrain areas such as the neocortex, hippocampus, and striatum, M1Rs are the primary muscarinic receptors implicated in higher-level cognitive functions, including learning and memory [68,69]. M1R inactivation results in hyperactive mice with elevated dopamine levels, which potentially explains impaired cognitive processing. These findings underly the utility of M1Rs as potential therapeutic targets for Parkinson’s disease and psychiatric disorders (e.g., schizophrenia) [70].
Although not the functionally dominant muscarinic receptor subtype in the heart, M1R plays a role in catecholamine-mediated cardiac activity and may be upregulated in certain conditions, e.g., chronic atrial fibrillation [71,72]. M1R, expressed by the normal prostate [73,74], is upregulated in animal and in vitro models of prostate cancer [75,76]. M1R expression by salivary and pancreatic tissue is important for the regulation of fluid and electrolyte secretion from sublingual and submandibular glands [77,78] and amylase secretion from pancreatic acini [79], respectively. As discussed in greater detail below, the relationship between M1Rs and GI cancers appears protective; M1Rs act as tumor suppressors for pancreatic ductal and colon adenocarcinoma [51,80].
3.3. CHRM2/M2R
CHRM2/M2R is prominently expressed in the brain, heart, skin, bladder, smooth muscle, and GI tract [34]. In the brain, the highest levels of M2R expression are in the cerebral cortex, forebrain, caudate and putamen, and thalamus, although M2R is also expressed in the brainstem and cervical spinal cord [55]. M2R is the predominant muscarinic receptor subtype expressed in the heart, playing key regulatory roles in cardiovascular electrophysiology, e.g., modulating heart rate and contractility by altering the activity of inwardly rectifying potassium channels and inducing vascular dilation via nitric oxide release, respectively [65]. In the lungs, M2R plays a role in airway responsiveness; M2R dysregulation augments irritant- and inflammation-induced bronchoconstriction [81,82].
Within the GI tract, M2R is the primary muscarinic receptor expressed by smooth muscle cells and interstitial cells of Cajal in the enteric nervous system and modulates GI motility [34,54]. Additionally, knockout mouse models were used to show that M2R and M4R expression plays a role in regulating the autoinhibition of ileal ACh release [56]. Although potential roles for M2R expression and activation are reported for brain (glioblastoma) and small cell lung cancers [34], to date, M2R has not been reported to play a role in modulating the behavior of any GI tract cancer.
3.4. CHRM3/M3R
The M3 receptor has a wide anatomic distribution, notably in the GI tract, bladder, eye, central and peripheral vasculature, and exocrine and endocrine glands. Though not the proportionally dominant muscarinic receptor within the GI tract, M3Rs are functionally important, inducing smooth muscle contraction and affecting motility in the stomach, ileum, and colon [83,84]. M3R similarly regulates smooth muscle contraction within the bladder, lung, and eye and, in these locations, is likely the primary mediator of muscarinic receptor-induced contraction [85,86,87,88]. In the vasculature, M3Rs are localized within the endothelium and smooth muscle and regulate endothelial-dependent relaxation and dilation of blood vessels, mediated by nitric oxide [89,90].
M3R has secretory functions throughout the body. In concert with M1R and M5R, respectively, M3R contributes to pepsinogen and gastric acid secretion [52,53]. M3R plays an important role in endocrine and exocrine pancreatic secretion, with many potential clinical applications [79,91]. Hyperactivation of M3Rs induces pancreatitis in mice, and allosteric modulation of M3R activity can normalize glucose homeostasis in obese mice [92,93]. The contribution of M3Rs to exocrine gland secretion, and, in particular, to salivary gland secretion, helps explain the common side effect of dry mouth (xerostomia) associated with the use of muscarinic receptor antagonists [94] and the finding of anti-M3R antibodies in Sjogren’s syndrome [95,96]. As discussed below, regarding its oncologic role, M3R activation enhances gastric, pancreatic, and colon cancer cell proliferation, and is a biomarker for metastases and a poor prognostic for these cancers [60,61,97].
3.5. CHRM4/M4R
Like M2R, M4R is a Gi protein-coupled receptor that reduces cellular levels of cAMP. M4R is expressed primarily in the stomach, duodenum, small intestine, and brain, especially in the striatum, however relatively high expression of CHRM4 mRNA is also reported in the spleen [63,65,98]. M4R is strongly expressed in the stomach, where it was recently discovered to play a role in gastric acid secretion, a process primarily driven by M3R activation. Although the underlying mechanism and its relevance to human biology remains unclear, it is hypothesized that M4R activation inhibits the release of somatostatin, an inhibitory bioactive peptide, from gastric and duodenal D cells, thereby disinhibiting M3R-mediated gastric acid secretion from parietal cells [57].
In recent years, there has been more interest regarding the role of M4R in the brain. M4R activation negatively regulates dopamine release from the striatum via signaling dependent on expression of CB2 cannabinoid receptors; induced depression of dopamine release is sustained ex vivo after removal of M4R agonists and is accompanied in vivo by anti-psychotic-like behavior in mice [64,98]. Imbalances in cholinergic transmission contribute to the development of many neuropsychiatric disorders including Alzheimer’s disease and schizophrenia, therefore, M4R is under active investigation as a potential drug target. Notably, an M1R and M4R agonist, xanomeline, is now in phase III clinical trials for psychiatric disorders [99].
3.6. CHRM5/M5R
Patterns of M5R expression remain poorly understood due to the paucity of currently available M5R-selective agonists and antagonists. M5R has been detected in the myenteric plexus of the enteric nervous system (ENS), in midbrain dopaminergic neurons, where it is the predominant muscarinic receptor, and in the cerebral vasculature [61,65,66,98]. In the midbrain, M5R activation is essential for neuronal excitability in the substantia nigra pars compacta, and its activation in the striatum modulates dopamine release, suggesting a role for M5R in maintaining a balance between the central cholinergic and dopaminergic systems [66]. M5R mRNA (CHRM5) has been detected in many human tissues, most notably the testes, placenta, thyroid gland, and small intestine, although the significance of its expression in those tissues is uncertain [63,65].
Experimental evidence indicates that M5R plays a role in enhancing gastric acid secretion via promotion of histamine secretion from enterochromaffin-like (ECL) cells. Compared to control mice, following treatment with the muscarinic receptor agonist carbamylcholine, M5R-deficient mice exhibited significantly reduced gastric acid and histamine secretion in vivo [53]. Chrm5 was detected in whole stomach samples removed from wild-type mice, but not in samples of fundic or antral gastric mucosa, suggesting M5R is expressed in the underlying ENS [53]. Based on these findings, it is plausible that enteric neurons directly or indirectly modulate paracrine release of neuropeptides, which can then stimulate ECL cells to release histamine that, in turn, stimulates acid secretion from gastric parietal cells. Given the recent discovery that glial cells in the myenteric plexus of the ENS express M3R and M5R, further research is needed to determine the precise role of the ENS in the cholinergic regulation of gastric acid secretion [61].
4. Differential Role of Muscarinic Receptor Subtype Activation in GI Cancers
4.1. Overview
As discussed above, in terms of physiological and pathophysiological GI epithelial cell function, M1R and M3R expression and activation have been studied to the relative exclusion of the other muscarinic receptor subtypes. To some extent, this was driven by the initial availability of animal models, e.g., Chrm1 and Chrm3 knockout mice with deficient expression alone or in combination of M1R and M3R [80]. Our understanding of the relative roles played by each of the five muscarinic receptor subtypes in GI neoplasia is likely to be extended now that knockout and transgenic mice with varying expression of M2R, M4R, and M5R are also available. Below, we consider the similarities and differences between M1R and M3R expression and activation in gastric, pancreatic, and colon cancer (Figure 2).
4.2. Gastric Adenocarcinoma
ACh and muscarinic receptor subtypes play prominent roles in the initiation and progression of gastric cancer; differential effects depend on the predominant muscarinic receptor subtype activated. Recent work indicates a likely oncogenic role for the ACh-M3R interaction; human gastric adenocarcinoma cells overexpress M3R, and expression levels correlate with cancer stage and the presence of lymph node metastases [100]. Conversely, M3R deficiency inhibits human gastric adenocarcinoma cell proliferation and induces apoptosis in vitro and in vivo [100]. Even in the absence of stimulation by exogenous ACh, M3R antagonism inhibits cancer cell proliferation, suggesting constitutive M3R activity or M3R activation by autocrine production and release of ACh is sufficient to drive gastric neoplasia [100]; like other GI cancers considered in this review, gastric adenocarcinoma cells can produce and release ACh [58,59]. M3R activation results in transactivation of EGFR and downstream activation of MAPK/ERK signaling [35]. In addition, release of ACh from a subset of tuft cells also appears to contribute to pro-proliferative signaling, presumably through a similar mechanism. Unlike in the case of M3R antagonism, the use of selective antagonists of M1R, M2R, and M4R failed to attenuate the pro-proliferative effects of ACh on gastric cancer cells [100]. Likewise, others found that gastric stem cells, identified by their expression of the marker Lgr5, co-express CHRM3, the gene encoding M3R, but not CHRM1, CHRM2, CHRM4, or CHRM5 [58].
4.3. Pancreatic Adenocarcinoma
In pancreatic ductal adenocarcinoma (PDAC), prominent differential actions of muscarinic receptor subtypes are reported, i.e., M1R activation protects against neoplasia whereas M3R activation promotes pancreatic cancer progression. Renz et al. found that cholinergic signaling through M1R suppressed both PDAC development and the cancer stem cell compartment (CSC) [51]. By expanding the CSC, murine subdiaphragmatic vagotomy accelerated PDAC development, reversed by treatment with a muscarinic receptor agonist [51]. Treating mice with a non-selective muscarinic receptor agonist, bethanechol, hindered PDAC progression and prolonged animal survival. The tumor suppressive effects were mediated partially via M1R and post-muscarinic receptor MAPK/EGFR and P13K/AKT signaling. In contrast, M3R expression correlates with worse clinical outcomes. Zhang et al. detected cytoplasmic M3R overexpression in all 58 human PDAC specimens they tested [60]. M3R intensity correlated positively with higher PDAC grades, lymph node metastasis, and shorter overall survival. Interestingly, PDAC cells with high levels of M3R expression were detected at the invasive tumor front and in metastatic lymph nodes and parasympathetic fibers.
4.4. Colon Adenocarcinoma
M3R activation and post-receptor signaling is instrumental to colon cancer initiation, invasion, and metastasis. M3R is overexpressed in colon cancer, a finding that correlates with poor prognostic features including increased tumor burden, invasion, and metastasis [62,101,102]. M3R signal transduction, via the EGFR/ERK and PKC/p38 MAPK pathways, results in the induction and release of selected matrix metalloproteinases (MMP1, MMP7, and MMP10), collagenases that facilitate cell invasion by breaking down the extracellular matrix [103,104]. Additional studies showed that ACh-induced MMP1 expression and colon cancer cell invasion can be abolished by pre-treatment with inhibitors of muscarinic receptor or MMP1 activation [12,105].
Colon cancer initiation is associated with altered functioning of immunocytes in the tumor microenvironment; these immunocytes also express muscarinic receptors (Table 1) [1]. Human T cells release ACh for autocrine and paracrine signaling, a process promoted by activation of M3R, T cell activation by immunomodulators (e.g., lipopolysaccharide), and activation of adhesion molecules on the T cell surface; production and secretion of ACh was also identified in human B cells, macrophages, and dendritic cells [1,106]. Given the immune cell-rich environment of the GI tract, particularly the colon, it is plausible that regulation of the immune system via M3R also modulates carcinogenesis, for example by producing cytokines that facilitate perineural invasion or otherwise enhancing the tumor microenvironment [107].
A detailed mechanistic understanding of how luminal bile acids, long associated with colon cancer risk, promote colon neoplasia remains elusive. Several lines of investigation identify a prominent role for the functional interaction of selected bile acids with muscarinic receptors, primarily M3R expressed by normal colon epithelial cells and overexpressed by colon cancer cells [37,40,44]. Recent work suggests that, via post-M3R and Wnt/β-catenin signaling, bile acids may induce the transformation of normal human colonic epithelial cells into colon cancer stem cells [108]. Amongst other evidence, this conclusion is supported by bile acid exposure-induced expression of stem cell and epithelial-mesenchymal transition markers [108]. Suppressing M3R expression using RNA interference attenuated bile acid-induced expression of markers of colon cancer stem cells. It appears plausible that M3R activation by exposure to luminal bile acids may explain, at least in part, the increased incidence of colon cancer in individuals who consume diets high in saturated fats that are known to increase levels of secondary bile acids in the stool [42,103,109,110].
Although M3R deficiency attenuates carcinogen-induced colon neoplasia in mice, M1R-deficient mice and those deficient in both M1R and M3R failed to exhibit decreased tumor formation and proliferation [80]. These findings suggest that M1R expression/activation suppresses colon cancer formation and may suppress the pro-neoplastic effects of M3R expression/activation. Notably, this may open the door to a therapeutic strategy that blocks M3R expression/activation while enhancing M1R expression/activation to prevent or treat colon neoplasia. In this respect, initial findings in an orthotopic xenograft mouse model suggest that darifenacin, a selective M3R antagonist approved in the United States, Canada, and the European Union to treat urinary incontinence, could be repurposed to attenuate the growth of M3R-expressing colon cancers [62]. In vitro, darifenacin also appeared to suppress ACh-stimulated colon cancer cell invasion, with a corresponding suppression of MMP1 mRNA expression, presumably via inhibition of p38, ERK1/2, and Akt signaling [62]. Whether this therapeutic approach prevents or attenuates metastatic disease in mice remains to be determined; current studies were underpowered to answer these questions [62]. Nonetheless, the results of these exploratory preclinical studies appear promising and suggest that clinical trials of FDA-approved M3R-selective antagonists are warranted. Although the development of highly subtype-selective muscarinic antagonists is difficult, in part due to the highly conserved amino acid sequence at the ligand-binding site, the recent discovery of several structural features unique to the M3R subtype may facilitate drug development [111].
4.5. Role of Muscarinic Receptor Activation within the Enteric Nervous System in GI Neoplasia
The enteric nervous system (ENS), the GI tract’s intrinsic nervous system, consists of neurons and glial cells organized into two concentric plexuses around the lumen. ENS neurons and glia participate in muscarinic signaling, and the ENS’s location in the GI milieu uniquely positions it to influence GI cancers [1,112,113]. For example, nerves underlying the gastric mucosa provide much of that region’s ACh, which activates M3R to trigger the release of additional neurotrophic factors [112]. In the context of gastric adenocarcinoma, this results in tropism of ENS neurons toward cancer stem cell foci and promotes carcinogenesis, in part by activating downstream pro-proliferative effectors (e.g., Yes-Associated Protein) and optimizing paracrine nerve–cancer interactions in the tumor microenvironment [59,112,113].
In recent years, attention focused on potential roles for the ENS and its muscarinic components in colon carcinogenesis. In addition to stimulating cancer stem cell growth, promoting colon cancer invasion, and even serving as routes for the physical migration and dissemination of colon cancer cells, the ENS promotes colon carcinogenesis via muscarinic interactions with cells in the tumor microenvironment [114,115].
5. Conclusions and Future Directions
An enhanced understanding of the biology of muscarinic receptor subtypes and the role their expression and activation plays in GI neoplasia is likely to identify several novel therapeutic approaches. Both orthosteric agents, which compete for binding at the active ligand binding site, and allosteric agents, which bind elsewhere on muscarinic receptors and alter the conformation of the ligand binding site, can be used to modulate muscarinic receptor activity. Existing FDA-approved, orthosteric muscarinic receptor agonists (e.g., bethanechol) or antagonists (e.g., darifenacin) can be repurposed for testing in therapeutic trials for gastric, pancreatic, and colon cancer. However, due to the nearly identical amino acid sequence at the ligand-binding site, the hurdles to developing agents with true muscarinic receptor subtype selectivity may prove insurmountable. Instead, developing agents targeting less conserved allosteric binding sites is likely to offer a more promising path to developing effective therapeutics [93].
Areas for further exploration include a more granular exploration of cell-type specific muscarinic receptor subtype expression (e.g., employing single cell RNAseq) [116], gaining insights into how muscarinic receptor subtypes currently thought to signal by the same pathways have different, sometimes opposing, effects (e.g., M1R and M3R) [80], and understanding the mechanisms that underly promiscuous coupling of muscarinic receptor subtypes to different G proteins that may alter their downstream signaling and actions [34]. The biological basis for such alterations is unknown and worthy of exploration since the results may apply to GPCRs in general. In this context, it will be important to overcome key experimental limitations, like the difficulty obtaining GPCR-specific antibodies for immunoblotting [117]. Although mRNA expression provides useful information, for many reasons, including post-transcriptional regulation of gene expression, it may not correlate precisely with protein expression, the ultimate functional determinant. Filling these gaps in knowledge and overcoming these technical limitations will allow us to harness the therapeutic potential of targeting muscarinic receptors subtypes to treat GI cancer. From a clinical perspective, advances in this area of investigation are most likely to be impactful by offering novel treatment modalities targeting muscarinic receptor subtypes for cancers resistant to other forms of therapy.
Author Contributions
Writing–original draft preparation, A.S., M.H.S., M.A., S.H., G.X. and J.-P.R.; writing–review and editing, A.S., M.H.S., M.A., S.H., G.X. and J.-P.R.; supervision, J.-P.R.; funding acquisition, G.X. and J.-P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Program, VA Merit Award grant numbers BX002777 and BX004890. Margaret H. Sundel and Madeline Alizadeh were supported by the U.S. National Institutes of Health, grant number T32 DK067872-17. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schledwitz A., Xie G., Raufman J.P. Exploiting unique features of the gut-brain interface to combat gastrointestinal cancer. J. Clin. Investig. 2021;131:e143776. doi: 10.1172/JCI143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faulkner S., Jobling P., March B., Jiang C.C., Hondermarck H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019;9:702–710. doi: 10.1158/2159-8290.CD-18-1398. [DOI] [PubMed] [Google Scholar]
- 3.Monje M., Borniger J.C., D’Silva N.J., Deneen B., Dirks P.B., Fattahi F., Frenette P.S., Garzia L., Gutmann D.H., Hanahan D., et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell. 2020;181:219–222. doi: 10.1016/j.cell.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furness J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 5.Soreq H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015;38:448–458. doi: 10.1016/j.tins.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R.L., Torre L.A., Soerjomataram I., Hayes R.B., Bray F., Weber T.K., Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A., Center M.M., DeSantis C., Ward E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 10.Andre T., Shiu K.K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., Smith D., Garcia-Carbonero R., Benavides M., Gibbs P., et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 11.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng K., Samimi R., Xie G., Shant J., Drachenberg C., Wade M., Davis R.J., Nomikos G., Raufman J.P. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G591–G597. doi: 10.1152/ajpgi.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawashima K., Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: Overview of non-neuronal cholinergic systems and their biological significance. J. Pharmacol. Sci. 2008;106:167–173. doi: 10.1254/jphs.FM0070073. [DOI] [PubMed] [Google Scholar]
- 14.Beckmann J., Lips K.S. The non-neuronal cholinergic system in health and disease. Pharmacology. 2013;92:286–302. doi: 10.1159/000355835. [DOI] [PubMed] [Google Scholar]
- 15.Wessler I., Kirkpatrick C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah N., Khurana S., Cheng K., Raufman J.P. Muscarinic receptors and ligands in cancer. Am. J. Physiol. Cell Physiol. 2009;296:C221–C232. doi: 10.1152/ajpcell.00514.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips P.A., Yang L., Shulkes A., Vonlaufen A., Poljak A., Bustamante S., Warren A., Xu Z., Guilhaus M., Pirola R., et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii T., Mashimo M., Moriwaki Y., Misawa H., Ono S., Horiguchi K., Kawashima K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017;8:1085. doi: 10.3389/fimmu.2017.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann K., Grafe F., Wohlrab W., Neubert R.H., Brandsch M. Functional characterization of a high-affinity choline transport system in human keratinocytes. J. Investig. Dermatol. 2002;119:118–121. doi: 10.1046/j.1523-1747.2002.01801.x. [DOI] [PubMed] [Google Scholar]
- 20.Gramatyka M., Skorupa A., Sokol M. Nuclear magnetic resonance spectroscopy reveals metabolic changes in living cardiomyocytes after low doses of ionizing radiation. Acta Biochim. Pol. 2018;65:309–318. doi: 10.18388/abp.2018_2568. [DOI] [PubMed] [Google Scholar]
- 21.Rabkin S.W., Cheng K.M. A genetic abnormality of cardiac myocytes from the blind mutant (RC) chick heart: Abnormalities of cardiac structure and choline transport. Basic Res. Cardiol. 1992;87:610–617. doi: 10.1007/BF00788671. [DOI] [PubMed] [Google Scholar]
- 22.Sassa N., Kato K., Abe S., Iwano S., Ito S., Ikeda M., Shimamoto K., Yamamoto S., Yamamoto T., Gotoh M., et al. Evaluation of 11C-choline PET/CT for primary diagnosis and staging of urothelial carcinoma of the upper urinary tract: A pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:2232–2241. doi: 10.1007/s00259-014-2871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macintosh F.C. The distribution of acetylcholine in the peripheral and the central nervous system. J. Physiol. 1941;99:436–442. doi: 10.1113/jphysiol.1941.sp003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolf N.J., Butcher L.L. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res. Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 25.Rosas-Ballina M., Olofsson P.S., Ochani M., Valdes-Ferrer S.I., Levine Y.A., Reardon C., Tusche M.W., Pavlov V.A., Andersson U., Chavan S., et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reardon C., Duncan G.S., Brustle A., Brenner D., Tusche M.W., Olofsson P.S., Rosas-Ballina M., Tracey K.J., Mak T.W. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc. Natl. Acad. Sci. USA. 2013;110:1410–1415. doi: 10.1073/pnas.1221655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W., Li D., Han R., Zhang C., Jin W.N., Wood K., Liu Q., Shi F.D., Hao J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl. Acad. Sci. USA. 2017;114:E6202–E6211. doi: 10.1073/pnas.1705491114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sastry B.V. Human placental cholinergic system. Biochem. Pharmacol. 1997;53:1577–1586. doi: 10.1016/S0006-2952(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 29.Grando S.A., Kist D.A., Qi M., Dahl M.V. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J. Investig. Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- 30.Roy A., Fields W.C., Rocha-Resende C., Resende R.R., Guatimosim S., Prado V.F., Gros R., Prado M.A. Cardiomyocyte-secreted acetylcholine is required for maintenance of homeostasis in the heart. FASEB J. 2013;27:5072–5082. doi: 10.1096/fj.13-238279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proskocil B.J., Sekhon H.S., Jia Y., Savchenko V., Blakely R.D., Lindstrom J., Spindel E.R. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology. 2004;145:2498–2506. doi: 10.1210/en.2003-1728. [DOI] [PubMed] [Google Scholar]
- 32.Kirkpatrick C.J., Bittinger F., Nozadze K., Wessler I. Expression and function of the non-neuronal cholinergic system in endothelial cells. Life Sci. 2003;72:2111–2116. doi: 10.1016/S0024-3205(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 33.Hanna-Mitchell A.T., Beckel J.M., Barbadora S., Kanai A.J., de Groat W.C., Birder L.A. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–2302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ockenga W., Kuhne S., Bocksberger S., Banning A., Tikkanen R. Non-neuronal functions of the m2 muscarinic acetylcholine receptor. Genes. 2013;4:171–197. doi: 10.3390/genes4020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H., Xia H., Tang Q., Xu H., Wei G., Chen Y., Dai X., Gong Q., Bi F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 2017;7:40802. doi: 10.1038/srep40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song P., Sekhon H.S., Jia Y., Keller J.A., Blusztajn J.K., Mark G.P., Spindel E.R. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- 37.Cheng K., Chen Y., Zimniak P., Raufman J.P., Xiao Y., Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim. Biophys. Acta. 2002;1588:48–55. doi: 10.1016/S0925-4439(02)00115-1. [DOI] [PubMed] [Google Scholar]
- 38.Cheng K., Raufman J.P. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem. Pharmacol. 2005;70:1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Shant J., Cheng K., Marasa B.S., Wang J.Y., Raufman J.P. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp. Cell Res. 2009;315:432–450. doi: 10.1016/j.yexcr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raufman J.P., Chen Y., Cheng K., Compadre C., Compadre L., Zimniak P. Selective interaction of bile acids with muscarinic receptors: A case of molecular mimicry. Eur. J. Pharmacol. 2002;457:77–84. doi: 10.1016/S0014-2999(02)02690-0. [DOI] [PubMed] [Google Scholar]
- 41.Jakubik J., El-Fakahany E.E. Allosteric Modulation of GPCRs of Class A by Cholesterol. Int. J. Mol. Sci. 2021;22:1953. doi: 10.3390/ijms22041953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raufman J.P., Dawson P.A., Rao A., Drachenberg C.B., Heath J., Shang A.C., Hu S., Zhan M., Polli J.E., Cheng K. Slc10a2-null mice uncover colon cancer-promoting actions of endogenous fecal bile acids. Carcinogenesis. 2015;36:1193–1200. doi: 10.1093/carcin/bgv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng K., Metry M., Felton J., Shang A.C., Drachenberg C.B., Xu S., Zhan M., Schumacher J., Guo G.L., Polli J.E., et al. Diminished gallbladder filling, increased fecal bile acids, and promotion of colon epithelial cell proliferation and neoplasia in fibroblast growth factor 15-deficient mice. Oncotarget. 2018;9:25572–25585. doi: 10.18632/oncotarget.25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raufman J.P., Cheng K., Zimniak P. Activation of muscarinic receptor signaling by bile acids: Physiological and medical implications. Dig. Dis. Sci. 2003;48:1431–1444. doi: 10.1023/A:1024733500950. [DOI] [PubMed] [Google Scholar]
- 45.Raufman J.P., Zimniak P., Bartoszko-Malik A. Lithocholyltaurine interacts with cholinergic receptors on dispersed chief cells from guinea pig stomach. Am. J. Physiol. 1998;274:G997–G1004. doi: 10.1152/ajpgi.1998.274.6.G997. [DOI] [PubMed] [Google Scholar]
- 46.Sheikh Abdul Kadir S.H., Miragoli M., Abu-Hayyeh S., Moshkov A.V., Xie Q., Keitel V., Nikolaev V.O., Williamson C., Gorelik J. Bile acid-induced arrhythmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes. PLoS ONE. 2010;5:e9689. doi: 10.1371/journal.pone.0009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton J.P., Xie G., Raufman J.P., Hogan S., Griffin T.L., Packard C.A., Chatfield D.A., Hagey L.R., Steinbach J.H., Hofmann A.F. Human cecal bile acids: Concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G256–G263. doi: 10.1152/ajpgi.00027.2007. [DOI] [PubMed] [Google Scholar]
- 48.Cheng K., Xie G., Raufman J.P. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem. Pharmacol. 2007;73:1001–1012. doi: 10.1016/j.bcp.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Said A.H., Hu S., Abutaleb A., Watkins T., Cheng K., Chahdi A., Kuppusamy P., Saxena N., Xie G., Raufman J.P. Interacting post-muscarinic receptor signaling pathways potentiate matrix metalloproteinase-1 expression and invasion of human colon cancer cells. Biochem. J. 2017;474:647–665. doi: 10.1042/BCJ20160704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen J.E., Bergqvist C.A., Larhammar D. Evolution of the Muscarinic Acetylcholine Receptors in Vertebrates. eNeuro. 2018;5 doi: 10.1523/ENEURO.0340-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renz B.W., Tanaka T., Sunagawa M., Takahashi R., Jiang Z., Macchini M., Dantes Z., Valenti G., White R.A., Middelhoff M.A., et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018;8:1458–1473. doi: 10.1158/2159-8290.CD-18-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie G., Drachenberg C., Yamada M., Wess J., Raufman J.P. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G521–G529. doi: 10.1152/ajpgi.00105.2004. [DOI] [PubMed] [Google Scholar]
- 53.Aihara T., Nakamura Y., Taketo M.M., Matsui M., Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M(3) and M(5) but not M(1) muscarinic acetylcholine receptors in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1199–G1207. doi: 10.1152/ajpgi.00514.2004. [DOI] [PubMed] [Google Scholar]
- 54.Iino S., Nojyo Y. Muscarinic M(2) acetylcholine receptor distribution in the guinea-pig gastrointestinal tract. Neuroscience. 2006;138:549–559. doi: 10.1016/j.neuroscience.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Spencer D.G., Jr., Horvath E., Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: Relation to cholinergic nuclei and projections. Brain Res. 1986;380:59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi T., Fujinami K., Goto H., Fujita A., Taketo M.M., Manabe T., Matsui M., Hata F. Roles of M2 and M4 muscarinic receptors in regulating acetylcholine release from myenteric neurons of mouse ileum. J. Neurophysiol. 2005;93:2841–2848. doi: 10.1152/jn.00986.2004. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi K., Endoh T., Hayashi S., Aihara T. Activation of Muscarinic Acetylcholine Receptor Subtype 4 Is Essential for Cholinergic Stimulation of Gastric Acid Secretion: Relation to D Cell/Somatostatin. Front. Pharmacol. 2016;7:278. doi: 10.3389/fphar.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao C.M., Hayakawa Y., Kodama Y., Muthupalani S., Westphalen C.B., Andersen G.T., Flatberg A., Johannessen H., Friedman R.A., Renz B.W., et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Xu J., Xia Y., Yin K., Li Z., Li B., Wang W., Xu H., Yang L., Xu Z. Muscarinic acetylcholine receptor 3 mediates vagus nerve-induced gastric cancer. Oncogenesis. 2018;7:88. doi: 10.1038/s41389-018-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Xiu D., Zhan J., He X., Guo L., Wang J., Tao M., Fu W., Zhang H. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Onco. Targets Ther. 2016;9:6719–6726. doi: 10.2147/OTT.S111382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delvalle N.M., Fried D.E., Rivera-Lopez G., Gaudette L., Gulbransen B.D. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G473–G483. doi: 10.1152/ajpgi.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hering N.A., Liu V., Kim R., Weixler B., Droeser R.A., Arndt M., Pozios I., Beyer K., Kreis M.E., Seeliger H. Blockage of Cholinergic Signaling via Muscarinic Acetylcholine Receptor 3 Inhibits Tumor Growth in Human Colorectal Adenocarcinoma. Cancers. 2021;13:3220. doi: 10.3390/cancers13133220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 64.Foster D.J., Wilson J.M., Remke D.H., Mahmood M.S., Uddin M.J., Wess J., Patel S., Marnett L.J., Niswender C.M., Jones C.K., et al. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron. 2016;91:1244–1252. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saternos H.C., Almarghalani D.A., Gibson H.M., Meqdad M.A., Antypas R.B., Lingireddy A., AbouAlaiwi W.A. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol. Genom. 2018;50:1–9. doi: 10.1152/physiolgenomics.00062.2017. [DOI] [PubMed] [Google Scholar]
- 66.Foster D.J., Gentry P.R., Lizardi-Ortiz J.E., Bridges T.M., Wood M.R., Niswender C.M., Sulzer D., Lindsley C.W., Xiang Z., Conn P.J. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J. Neurosci. 2014;34:3253–3262. doi: 10.1523/JNEUROSCI.4896-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamilton S.E., Loose M.D., Qi M., Levey A.I., Hille B., McKnight G.S., Idzerda R.L., Nathanson N.M. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc. Natl. Acad. Sci. USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abrams P., Andersson K.E., Buccafusco J.J., Chapple C., de Groat W.C., Fryer A.D., Kay G., Laties A., Nathanson N.M., Pasricha P.J., et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anagnostaras S.G., Murphy G.G., Hamilton S.E., Mitchell S.L., Rahnama N.P., Nathanson N.M., Silva A.J. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 70.Gerber D.J., Sotnikova T.D., Gainetdinov R.R., Huang S.Y., Caron M.G., Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hardouin S.N., Richmond K.N., Zimmerman A., Hamilton S.E., Feigl E.O., Nathanson N.M. Altered cardiovascular responses in mice lacking the M(1) muscarinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 2002;301:129–137. doi: 10.1124/jpet.301.1.129. [DOI] [PubMed] [Google Scholar]
- 72.Heijman J., Kirchner D., Kunze F., Chretien E.M., Michel-Reher M.B., Voigt N., Knaut M., Michel M.C., Ravens U., Dobrev D. Muscarinic type-1 receptors contribute to IK,ACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation. Int. J. Cardiol. 2018;255:61–68. doi: 10.1016/j.ijcard.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anisuzzaman A.S., Morishima S., Suzuki F., Tanaka T., Yoshiki H., Sathi Z.S., Akino H., Yokoyama O., Muramatsu I. Assessment of muscarinic receptor subtypes in human and rat lower urinary tract by tissue segment binding assay. J. Pharmacol. Sci. 2008;106:271–279. doi: 10.1254/jphs.FP0071435. [DOI] [PubMed] [Google Scholar]
- 74.Witte L.P., Chapple C.R., de la Rosette J.J., Michel M.C. Cholinergic innervation and muscarinic receptors in the human prostate. Eur. Urol. 2008;54:326–334. doi: 10.1016/j.eururo.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 75.Yin Q.Q., Xu L.H., Zhang M., Xu C. Muscarinic acetylcholine receptor M1 mediates prostate cancer cell migration and invasion through hedgehog signaling. Asian J. Androl. 2018;20:608–614. doi: 10.4103/aja.aja_55_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mannan Baig A., Khan N.A., Effendi V., Rana Z., Ahmad H.R., Abbas F. Differential receptor dependencies: Expression and significance of muscarinic M1 receptors in the biology of prostate cancer. Anticancer Drugs. 2017;28:75–87. doi: 10.1097/CAD.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 77.Gautam D., Duttaroy A., Cui Y., Han S.J., Deng C., Seeger T., Alzheimer C., Wess J. M1-M3 muscarinic acetylcholine receptor-deficient mice: Novel phenotypes. J. Mol. Neurosci. 2006;30:157–160. doi: 10.1385/JMN:30:1:157. [DOI] [PubMed] [Google Scholar]
- 78.Ryberg A.T., Warfvinge G., Axelsson L., Soukup O., Gotrick B., Tobin G. Expression of muscarinic receptor subtypes in salivary glands of rats, sheep and man. Arch. Oral Biol. 2008;53:66–74. doi: 10.1016/j.archoralbio.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 79.Gautam D., Han S.J., Heard T.S., Cui Y., Miller G., Bloodworth L., Wess J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J. Pharmacol. Exp. Ther. 2005;313:995–1002. doi: 10.1124/jpet.105.084855. [DOI] [PubMed] [Google Scholar]
- 80.Cheng K., Xie G., Khurana S., Heath J., Drachenberg C.B., Timmons J., Shah N., Raufman J.P. Divergent effects of muscarinic receptor subtype gene ablation on murine colon tumorigenesis reveals association of M3R and zinc finger protein 277 expression in colon neoplasia. Mol. Cancer. 2014;13:77. doi: 10.1186/1476-4598-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castro J.M., Resende R.R., Mirotti L., Florsheim E., Albuquerque L.L., Lino-dos-Santos-Franco A., Gomes E., de Lima W.T., de Franco M., Ribeiro O.G., et al. Role of m2 muscarinic receptor in the airway response to methacholine of mice selected for minimal or maximal acute inflammatory response. Biomed. Res. Int. 2013;2013:805627. doi: 10.1155/2013/805627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlenz H., Kummer W., Jositsch G., Wess J., Krasteva G. Muscarinic receptor-mediated bronchoconstriction is coupled to caveolae in murine airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;298:L626–L636. doi: 10.1152/ajplung.00261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiba T., Bharucha A.E., Thomforde G.M., Kost L.J., Phillips S.F. Model of rapid gastrointestinal transit in dogs: Effects of muscarinic antagonists and a nitric oxide synthase inhibitor. Neurogastroenterol. Motil. 2002;14:535–541. doi: 10.1046/j.1365-2982.2002.00357.x. [DOI] [PubMed] [Google Scholar]
- 84.Eglen R.M., Harris G.C. Selective inactivation of muscarinic M2 and M3 receptors in guinea-pig ileum and atria in vitro. Br. J. Pharmacol. 1993;109:946–952. doi: 10.1111/j.1476-5381.1993.tb13712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P., Luthin G.R., Ruggieri M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- 86.Choppin A., Eglen R.M., Hegde S.S. Pharmacological characterization of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br. J. Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gil D.W., Krauss H.A., Bogardus A.M., WoldeMussie E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Investig. Ophthalmol. Vis. Sci. 1997;38:1434–1442. [PubMed] [Google Scholar]
- 88.Roffel A.F., Elzinga C.R., Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm. Pharmacol. 1990;3:47–51. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 89.Beny J.L., Nguyen M.N., Marino M., Matsui M. Muscarinic receptor knockout mice confirm involvement of M3 receptor in endothelium-dependent vasodilatation in mouse arteries. J. Cardiovasc. Pharmacol. 2008;51:505–512. doi: 10.1097/FJC.0b013e31816d5f2f. [DOI] [PubMed] [Google Scholar]
- 90.Khurana S., Chacon I., Xie G., Yamada M., Wess J., Raufman J.P., Kennedy R.H. Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R-/- mice. Eur. J. Pharmacol. 2004;493:127–132. doi: 10.1016/j.ejphar.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 91.Ehrenkaufer R.L., Klam S., Makoroff K., Giandinoto S., Morton T., Moroney D., Nowak P. Internal-surface reversed-phase chromatography for plasma metabolite analysis of radiopharmaceuticals. Int. J. Rad. Appl. Instrum. B. 1992;19:651–657. doi: 10.1016/0883-2897(92)90099-K. [DOI] [PubMed] [Google Scholar]
- 92.Wan J., Wang J., Wagner Ii L.E., Wang O.H., Gui F., Chen J., Zhu X., Haddock A.N., Edenfield B.H., Haight B., et al. Pancreatic specific CHRM3 activation causes pancreatitis in mice. JCI Insight. 2021;6:e132585. doi: 10.1172/jci.insight.132585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu L., Rossi M., Cohen A., Pham J., Zheng H., Dattaroy D., Mukaibo T., Melvin J.E., Langel J.L., Hattar S., et al. Allosteric modulation of beta-cell M3 muscarinic acetylcholine receptors greatly improves glucose homeostasis in lean and obese mice. Proc. Natl. Acad. Sci. USA. 2019;116:18684–18690. doi: 10.1073/pnas.1904943116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scully C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003;9:165–176. doi: 10.1034/j.1601-0825.2003.03967.x. [DOI] [PubMed] [Google Scholar]
- 95.Lee B.H., Gauna A.E., Perez G., Park Y.J., Pauley K.M., Kawai T., Cha S. Autoantibodies against muscarinic type 3 receptor in Sjogren’s syndrome inhibit aquaporin 5 trafficking. PLoS ONE. 2013;8:e53113. doi: 10.1371/journal.pone.0053113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koo N.Y., Li J., Hwang S.M., Choi S.Y., Lee S.J., Oh S.B., Kim J.S., Lee E.B., Song Y.W., Park K. Functional epitope of muscarinic type 3 receptor which interacts with autoantibodies from Sjogren’s syndrome patients. Rheumatology. 2008;47:828–833. doi: 10.1093/rheumatology/ken064. [DOI] [PubMed] [Google Scholar]
- 97.Frucht H., Jensen R.T., Dexter D., Yang W.L., Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin. Cancer Res. 1999;5:2532–2539. [PubMed] [Google Scholar]
- 98.Van der Westhuizen E.T., Choy K.H.C., Valant C., McKenzie-Nickson S., Bradley S.J., Tobin A.B., Sexton P.M., Christopoulos A. Fine Tuning Muscarinic Acetylcholine Receptor Signaling Through Allostery and Bias. Front. Pharmacol. 2021;11:2217. doi: 10.3389/fphar.2020.606656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brannan S.K., Sawchak S., Miller A.C., Lieberman J.A., Paul S.M., Breier A. Muscarinic Cholinergic Receptor Agonist and Peripheral Antagonist for Schizophrenia. N. Engl. J. Med. 2021;384:717–726. doi: 10.1056/NEJMoa2017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L., Zhi X., Zhang Q., Wei S., Li Z., Zhou J., Jiang J., Zhu Y., Yang L., Xu H., et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour Biol. 2016;37:2105–2117. doi: 10.1007/s13277-015-4011-0. [DOI] [PubMed] [Google Scholar]
- 101.Peng Z., Heath J., Drachenberg C., Raufman J.P., Xie G. Cholinergic muscarinic receptor activation augments murine intestinal epithelial cell proliferation and tumorigenesis. BMC Cancer. 2013;13:204. doi: 10.1186/1471-2407-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng K., Shang A.C., Drachenberg C.B., Zhan M., Raufman J.P. Differential expression of M3 muscarinic receptors in progressive colon neoplasia and metastasis. Oncotarget. 2017;8:21106–21114. doi: 10.18632/oncotarget.15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Felton J., Cheng K., Shang A.C., Hu S., Larabee S.M., Drachenberg C.B., Raufman J.P. Two sides to colon cancer: Mice mimic human anatomical region disparity in colon cancer development and progression. J. Cancer Metastasis Treat. 2018;4:51. doi: 10.20517/2394-4722.2018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie G., Cheng K., Shant J., Raufman J.P. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G755–G763. doi: 10.1152/ajpgi.90519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raufman J.P., Shant J., Xie G., Cheng K., Gao X.M., Shiu B., Shah N., Drachenberg C.B., Heath J., Wess J., et al. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis. 2011;32:1396–1402. doi: 10.1093/carcin/bgr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujii T., Ushiyama N., Hosonuma K., Suenaga A., Kawashima K. Effects of human antithymocyte globulin on acetylcholine synthesis, its release and choline acetyltransferase transcription in a human leukemic T-cell line. J. Neuroimmunol. 2002;128:1–8. doi: 10.1016/S0165-5728(02)00111-X. [DOI] [PubMed] [Google Scholar]
- 107.Amit M., Na’ara S., Gil Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer. 2016;16:399–408. doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 108.Farhana L., Nangia-Makker P., Arbit E., Shango K., Sarkar S., Mahmud H., Hadden T., Yu Y., Majumdar A.P. Bile acid: A potential inducer of colon cancer stem cells. Stem Cell Res. Ther. 2016;7:181. doi: 10.1186/s13287-016-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hodge A.M., Williamson E.J., Bassett J.K., MacInnis R.J., Giles G.G., English D.R. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int. J. Cancer. 2015;137:1224–1234. doi: 10.1002/ijc.29479. [DOI] [PubMed] [Google Scholar]
- 110.O’Keefe S.J., Li J.V., Lahti L., Ou J., Carbonero F., Mohammed K., Posma J.M., Kinross J., Wahl E., Ruder E., et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kruse A.C., Li J., Hu J., Kobilka B.K., Wess J. Novel insights into M3 muscarinic acetylcholine receptor physiology and structure. J. Mol. Neurosci. 2014;53:316–323. doi: 10.1007/s12031-013-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayakawa Y., Sakitani K., Konishi M., Asfaha S., Niikura R., Tomita H., Renz B.W., Tailor Y., Macchini M., Middelhoff M., et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan X., Sivakumar S., Bednarsch J., Wiltberger G., Kather J.N., Niehues J., de Vos-Geelen J., Valkenburg-van Iersel L., Kintsler S., Roeth A., et al. Nerve fibers in the tumor microenvironment in neurotropic cancer-pancreatic cancer and cholangiocarcinoma. Oncogene. 2021;40:899–908. doi: 10.1038/s41388-020-01578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duchalais E., Guilluy C., Nedellec S., Touvron M., Bessard A., Touchefeu Y., Bossard C., Boudin H., Louarn G., Neunlist M., et al. Colorectal Cancer Cells Adhere to and Migrate Along the Neurons of the Enteric Nervous System. Cell Mol. Gastroenterol. Hepatol. 2018;5:31–49. doi: 10.1016/j.jcmgh.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vales S., Bacola G., Biraud M., Touvron M., Bessard A., Geraldo F., Dougherty K.A., Lashani S., Bossard C., Flamant M., et al. Tumor cells hijack enteric glia to activate colon cancer stem cells and stimulate tumorigenesis. EBioMedicine. 2019;49:172–188. doi: 10.1016/j.ebiom.2019.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sriram K., Wiley S.Z., Moyung K., Gorr M.W., Salmeron C., Marucut J., French R.P., Lowy A.M., Insel P.A. Detection and Quantification of GPCR mRNA: An Assessment and Implications of Data from High-Content Methods. ACS Omega. 2019;4:17048–17059. doi: 10.1021/acsomega.9b02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michel M.C., Wieland T., Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch. Pharm. 2009;379:385–388. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.