Abstract
Defects in the nonhomologous end-joining (NHEJ) pathway of double-stranded DNA break repair severely impair V(D)J joining and selectively predispose mice to the development of lymphoid neoplasia. This connection was first noted in mice with the severe combined immune deficient (SCID) mutation in the DNA-dependent protein kinase (DNA-PK). SCID mice spontaneously develop thymic lymphoma with low incidence and long latency. However, we and others showed that low-dose irradiation of SCID mice dramatically increases the frequency and decreases the latency of thymic lymphomagenesis, but irradiation does not promote the development of other tumors. We have used this model to explore the mechanistic basis by which defects in NHEJ confer selective and profound susceptibility to lymphoid oncogenesis. Here, we show that radiation quantitatively and qualitatively improves V(D)J joining in SCID cells, in the absence of T-cell receptor-mediated cellular selection. Furthermore, we show that the lymphocyte-specific endonuclease encoded by the recombinase-activating genes (RAG-1 and RAG-2) is required for radiation-induced thymic lymphomagenesis in SCID mice. Collectively, these data suggest that irradiation induces a DNA-PK-independent NHEJ pathway that facilitates V(D)J joining, but also promotes oncogenic misjoining of RAG-1/2-induced breaks in SCID T-cell precursors.
Lymphocytes require the faithful execution of DNA repair processes to generate a highly diverse repertoire of antigen receptors. The variable region exon of each T-cell receptor (TCR) and B-cell antigen receptor (BCR) gene is assembled by site-specific cleavage and rejoining of variable (V), diversity (D), and joining (J) gene segments in developing T and B lymphocytes. Tandem genomic arrays of dozens to hundreds of V, D, or J gene segments allow combinatorial diversification of the available germline repertoire during lymphocyte development to create a large number of clonally distinct VDJ or VJ genes. In addition, terminal deoxynucleotidal transferase (TdT), a lymphocyte-specific polymerase, inserts non-germline-encoded nucleotides at V(D)J junctions, providing additional somatic diversification of the available germline repertoire (30, 44, 67). Thus, V(D)J recombination endows T and B lymphocytes with the capacity to specifically recognize and destroy an almost infinite array of pathogens. However, DNA breaks are highly recombinogenic and must be repaired efficiently to maintain genomic stability and minimize the risk of oncogenic transformation (31, 33, 43, 49, 90, 104). A high frequency of lymphoid tumors have chromosomal translocations involving antigen receptor genes (reviewed in reference 101), raising the question as to whether the V(D)J recombination process threatens genomic stability in lymphoid lineages.
The molecular mechanism of V(D)J cleavage has recently been elucidated by elegant biochemical studies (reviewed in references 93 and 106). This process is mediated by the lymphoid-specific RAG-1 and RAG-2 proteins, which bind recombination signal sequences flanking V(D)J gene segments and nick one DNA strand precisely between the coding segment and the recombination signal. The nick is rapidly converted to a double-stranded DNA break (DSB), generating two types of intermediates: hairpin-terminated V, D, or J coding ends and blunt, 5′ phosphorylated signal ends. The unusual hairpin structure of V(D)J coding ends is thought to reflect the evolutionary relationship of V(D)J recombination to DNA transposition (1, 56). Hairpin coding ends are short-lived intermediates that are not detectable in normal cells (102, 105), presumably because they are rapidly joined together. In contrast, signal ends are bound to RAG-1/2 proteins and persist (2, 55). In T-cell precursors, most signal ends are ligated together to form extrachomosomal circles that are lost during subsequent rounds of cell division (68, 81).
Repair of V(D)J breaks into signal joints and coding joints (CJ) occurs by nonhomologous end-joining (NHEJ), a major repair pathway in mammalian cells that can rejoin DSB originating from noncontiguous chromosomal segments (reviewed in reference 26). CJ formation is a specialized form of NHEJ requiring that the hairpin coding ends be opened prior to the end-joining reaction. The importance of NHEJ in lymphocyte-specific V(D)J recombination was first revealed by studies of SCID mice. These mice have a mutation in the NHEJ protein DNA-dependent protein kinase (DNA-PK) (6, 13, 28) that confers a global defect in DSB repair, causing hypersensitivity to ionizing radiation (9, 39, 53). The SCID mutation in DNA-PK also disrupts repair of RAG-1/2-induced DSB in lymphocyte precursors (105). This V(D)J joining defect severely impairs the generation of TCRβ-containing pre-TCR and immunoglobulin μ chain (Igμ)-containing pre-BCR, causing arrested lymphocyte development. These receptors transmit signals that select progenitor T (pro-T) and pro-B cells for clonal expansion and maturation (61, 119). Similar defects in V(D)J joining and general DSB repair are caused by loss-of-function mutations in other NHEJ proteins, such as KU70, KU80, XRCC4, and DNA ligase IV (37, 47, 74, 91, 92, 121).
Mutations in DNA-PK qualitatively and quantitatively affect V(D)J joining. The efficiency of CJ formation is reduced from 10- to 1,000-fold relative to that of wild-type cells (34, 77, 98, 116), whereas signal joint formation occurs with relatively normal efficiency but reduced fidelity (14, 42, 70, 77, 114, 115). The defect in CJ formation is manifested by the abnormal accumulation of hairpin coding end intermediates in lymphocyte precursors (42, 105, 121, 122). However, in cells from SCID mice (11, 34, 62, 69, 98) or DNA-PK null mice (42, 70, 114, 120), low levels of CJ formation can take place. Analyses of these rare CJ suggests that DNA-PK also influences the way in which coding ends are processed prior to joining. For example, SCID CJ typically display more extensive deletion of nucleotides from the 5′ and 3′ coding ends than their wild-type counterparts (35, 86, 108). In addition, SCID CJ frequently have long, palindromic (P) nucleotide additions (35, 63, 107) which are generated by asymmetric cleavage of the hairpin coding ends. In contrast, P additions are infrequently found in wild-type CJ, and they are rarely longer than three nucleotides. Finally, TdT-mediated addition of N-regions to TCR coding junctions appears normal in SCID thymocytes (35, 62). On the basis of these observations, it has been suggested that DNA-PK is required to recruit and/or activate factors that mediate hairpin cleavage (12, 122), a process which must precede the formation of CJ.
In addition to defective V(D)J joining and SCID, lymphocyte progenitors from mice harboring genetic defects in NHEJ proteins are also particularly susceptible to oncogenic transformation. This lymphoma-prone phenotype was first revealed by studies showing that in some mouse colonies, up to 15% of SCID mice spontaneously develop thymic lymphoma with long latency (16, 27). However, in our SCID colony, <5% of SCID mice developed lymphoma by 2 years of age. Strikingly, low-dose (100 to 175 cGy) irradiation of newborn SCID mice induces thymic lymphoma, but not other tumors, with very high frequency and short latency (29, 79, 88). Mice harboring other mutations in DNA-PK or in KU70 were subsequently shown to spontaneously develop thymic lymphoma, but other tumor types have not been reported (48, 59, 73).
The molecular basis by which defective NHEJ selectively promotes the development of lymphoid neoplasia remains unexplained. It has been suggested that the prevalence of chromosomal translocations involving antigen receptors in human lymphoid tumors results from the misjoining of RAG-1/2-induced breaks to chromosomal DSB in lymphocytes (reviewed in reference 101). While defects in NHEJ proteins may increase such misjoining events, the importance of RAG-1/2-induced breaks to lymphoid oncogenesis in NHEJ mutant mice has not been assessed. Moreover, it is not clear how potentially oncogenic misjoining of these breaks would occur in the context of the profound NHEJ defect conferred by mutations in DNA-PK or KU70/80.
We have used the irradiated SCID model of thymic lymphomagenesis to explore the mechanistic basis by which defects in NHEJ confer selective and profound susceptibility to lymphoid neoplasia. First, using extrachromosomal V(D)J recombination substrates and a transient-transfection approach, we show that V(D)J CJ formation is quantitatively and qualitatively improved by low-dose irradiation of SCID thymic lymphoma cell lines. Second, we use two different genetic strategies to show that V(D)J recombinase activity is required for radiation-induced lymphomagenesis in SCID mice, demonstrating that RAG-1/2-induced breaks are potentially oncogenic in the context of defective NHEJ.
MATERIALS AND METHODS
Cell lines and recombination assays.
The VL3-3M2, LK6.2, and LK8 cell lines have been described previously (28, 46). Cell lines (3 × 106 cells) were transiently transfected with 150 ng of pDR42κ/λ (deletional coding joints) (46) or pWTSJΔ (deletional signal joints) (72) using DEAE-dextran and osmotic shock as previously described (78). Two days later, plasmid DNA was harvested, digested with DpnI, and electroporated into Escherichia coli as previously described (46). Because the SCID thymic lymphoma cells displayed poor survival after the transfection procedure, they were stably infected with a Bcl-2-containing retrovirus (118). The SJ1 primer (5′-CTG GTC CGG TAA CGT GCT GAG-3′) was used to sequence the coding junctions of recombinant pDR42 clones by using an ABI 373 or ABI 377 automated sequencer. The origins and order (5′ to 3′) of recombination signal sequences on pDR42 are as follows: JκRSS(23), JλRSS(12), VκRSS(12), and VλRSS(23). All pDR42 recombinants shown had undergone recombination between JκRSS(23) and VκRSS(12).
Mice.
All mice were bred and housed in specific-pathogen-free conditions at the Hospital for Sick Children animal facility. RAG-2−/− C57BL/6 mice were obtained from GenPharm (Mountain View, Calif.) and were crossed with C.B-17 SCID (Prkdcscid/scid) mice in our animal facility to generate RAG-2+/− Prkdcscid/+ F1 mice. F1 progeny were then backcrossed with C.B-17 SCID mice to generate Prkdcscid/scid or Prkdcscid/+ mice, all of which have at least one copy of the wild-type RAG-2 allele. To identify Prkdcscid/scid progeny, peripheral blood was analyzed by flow cytometry for the absence of mature T or B cells with anti-TCRβ (FITC-H57-597) and anti-IgM (biotinylated R6-60.2) antibody. Alternatively, PCR amplification of tail DNA was used to identify the wild-type and scid alleles of DNA-PK as previously described (13). RAG-2 genotypes were determined by PCR amplification of tail DNA by using the following primers: RAG2-3 (anti-sense), GCCTGCTTATTGTCTCCTGGTATG; NEO-3′ (anti-sense), CCAACGCTATGTCCTGATAGCGGT; and RAG2-1 (sense), TTAATTCAACCAGGCTTCTCACTT. PCR amplification with the RAG2-1 and RAG2-3 primers detects the wild-type allele (973-bp amplicon), whereas amplification with the RAG2-3 and NEO-3′ primers detects the mutant allele (1,107-bp amplicon). RAG-2+/− Prkdcscid/scid animals were intercrossed to generate RAG-2−/− Prkdcscid/scid progeny (referred to as RAG-2−/− SCID).
To generate TCRβ transgenic Prkdcscid/scid (TCRβ-SCID) mice, Prkdcscid/+ mice expressing a Vβ8.2 TCRβ transgene were obtained from E. W. Shores and A. Singer (111) and backcrossed with C.B-17 SCID mice. Prkdcscid/scid progeny were identified as described above. TCRβ transgenic animals were identified by PCR amplification of tail DNA by using a Vβ8 sense primer (TAAGCGGCCGCGAGGCTGCAGTCACCCAAA) and a Dβ2Jβ2.3 antisense primer (CAGCGTTTCTGCACTGTTATCACC). Prkdcscid/scid mice segregating the TCRβ transgene were obtained from the progeny of TCRβ+/− Prkdcscid/scid animals backcrossed with C.B-17 Prkdcscid/scid mice. The incidence of radiation-induced thymic lymphoma was evaluated after three and seven backcross generations, with similar results. Prkdcscid/scid mice were γ-irradiated (100 cGy from a GammaCell 40 137Cs source, dose rate = 1.25 Gy/min) within 1 to 3 days of birth as previously described (29), and RAG-2−/− mice were irradiated as newborns (400 cGy) or as adults (750 cGy), as previously described (50).
Flow cytometry.
Phenotypic analyses of thymocytes for surface expression of lineage and developmental markers were performed as previously described (29) using a FACScan flow cytometer with Lysis II software or a FACSCalibur flow cytometer with CellQuest software (Becton Dickinson & Co., Mountain View, Calif.). The following monoclonal antibodies were affinity purified from hybridoma culture supernatants by using protein A- or protein G-Sepharose (Pharmacia, Baie d'Urfe, Quebec, Canada): CD4 (YTS 191.1), CD8 (YTS 169.4), and TCRβ (H57-597). Purified antibodies were conjugated to fluorescein isothiocyanate (FITC) or biotin using standard techniques. FITC-CD25 (7D4) and biotinylated mouse anti-IgM (R6-60.2) were purchased from Pharmingen (San Diego, Calif.). Streptavidin-phycoerythrin (Av-PE) was purchased from Caltag (South San Francisco, Calif.) and used as a second-stage reagent with biotinylated primary antibodies. Propidium iodide staining and flow cytometric evaluation of cell cycle analysis was performed as previously described (49).
RT-PCR.
Reverse transcriptase (RT)-coupled PCR amplifications were performed as previously described (29). Briefly, total RNA was isolated from the thymuses of individual animals, and 2 μg of RNA was reverse transcribed into cDNA at 42°C in a reaction mixture containing 50 mM Tris (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 0.1 mg of bovine serum albumin/ml, 0.5 mM concentrations of each deoxynucleotide triphosphate (dATP, dCTP, dGTP, dTTP; Promega, Madison, Wis.), 2.5 μg of oligo(dT) primer/ml, 25 U of ribonuclease inhibitor (Promega), and 2.5 U of RT (AMV-RT; Promega). cDNA was amplified (31 cycles, 55°C annealing) with a panel of eight TCR Vβ-specific primers (Vβ1, Vβ3, Vβ5, Vβ7, Vβ9, Vβ11, Vβ14, and Vβ17) coupled with a TCR Cβ1/2 antisense primer in separate reactions (36). PCR products were resolved by agarose gel electrophoresis, Southern blotted, and hybridized to a radiolabeled [α-32P]dCTP-labeled TCR-Cβ probe. Autoradiography was performed using a PhosphorImager with ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.).
Detection of TCRδ coding ends.
High-molecular-weight DNA was prepared from homogenized kidney or single-cell thymocyte suspensions and was digested with restriction endonucleases, electrophoresed through 0.8% agarose, and transferred to a Gene Screen Plus nylon membrane (NEN DuPont, Boston, Mass.). Hybridization was carried out using random hexamer-primed [α-32P]dCTP-labeled Jδ1 probe no. 4 (82). Autoradiography was performed using a PhosphorImager with ImageQuant 3.0 software (Molecular Dynamics).
RESULTS
Irradiation improves V(D)J CJ formation in SCID cells.
Previously, we have shown that low-dose irradiation of SCID mice partially relieves the developmental arrest of CD4-CD8 double-negative (DN) pro-T cells and promotes their expansion and maturation to the CD4-CD8 double-positive (DP) pre-T-cell stage (29). Low-dose irradiation also promotes the appearance of thymocytes with functional rearrangements at the TCRβ, TCRγ, and TCRδ loci (15, 29, 82). Analyses of these CJ sequences showed that they resembled those from normal thymocytes in several respects, suggesting that NHEJ activity is qualitatively or quantitatively enhanced during the cellular response to DNA damage. However, normal CJ can be made at very low frequencies in SCID cells (19, 21, 34, 54, 69, 98). Furthermore, there is strong selection in vivo for precursors that have made functional TCRβ, TCRγ, and TCRδ rearrangements (60, 83, 85, 95). Thus, it is equally plausible that irradiation selects for rare SCID pro-T cells that have managed to repair their V(D)J breaks using the inefficient NHEJ machinery.
To distinguish between these two possibilities, we examined the effect of irradiation on CJ formation in wild-type versus SCID cell lines that were transiently transfected with an extrachromosomal recombination substrate plasmid, pDR42. In RAG-1/2-expressing cells, this plasmid undergoes deletional CJ formation (103). This cell culture strategy, which has been widely used to examine the efficiency and fidelity of CJ formation in SCID versus wild-type cells (reviewed in reference 71), allowed us to directly measure the effect of irradiation on CJ formation in the absence of TCR-mediated cellular selection events that occur in vivo. For these experiments we have used thymic lymphoma cell lines derived from wild-type or SCID mice because they have a pre-T-cell phenotype, exemplified by expression of CD4 and CD8, as well as RAG-1, RAG-2, and TdT (data not shown and reference 46). These cell lines express wild-type (VL3-3M2) or SCID mutant (LK6.2, LK3C, and LK8) DNA-PK (28).
To determine if irradiation increases the efficiency of CJ formation, cells were treated with 0 or 100 cGy of ionizing irradiation at different times before or after transfection with pDR42. Three to six independent experiments were performed with each cell line, and data from three representative experiments are shown in Table 1. As expected, CJ formation in the untreated SCID thymic lymphoma cell lines is <10% of that seen in VL3-3M2 in a given experiment. Since RAG-1/2 protein levels are similar among these cell lines (data not shown), the defect in CJ formation in LK6.2, LK3C, and LK8 is attributable to the SCID mutation in DNA-PK. Although irradiation had no reproducible effects on the efficiency of CJ formation in VL3-3M2, it improved the efficiency of CJ formation in the SCID cell lines by up to 17-fold, but more typically by three- to fourfold (Table 1). To determine if irradiation could also improve the repair of non-hairpin blunt DSB, we transfected cells with pWTSJΔ, a plasmid that undergoes deletional signal joint formation. As documented for other SCID cell lines (14, 77, 99, 116), LK6.2 and LK3C exhibit a normal frequency of signal joint formation but reduced fidelity, since <50% of the signal joints are precise (Table 2). The low precision of signal joint formation in SCID cells reflects deletion or N additions at the signal ends prior to joining (77). In contrast to CJ formation, irradiation did not increase the frequency or the fidelity of signal joint formation in LK6.2 or LK3C cells (Table 2), suggesting that irradiation specifically improves repair of DSB with hairpin ends.
TABLE 1.
Effect of irradiation on CJ formation in wild-type and SCID thymic lymphoma cellsa
| Cell line | Expt no. | Irradiation | No. of Ampr | No. of Camr | %R | IR/C |
|---|---|---|---|---|---|---|
| VL3-3M2 | 1 | − | 2,610,000 | 124,000 | 4.75 | |
| 0.5 h (A) | 2,740,000 | 248,000 | 9.05 | 1.91 | ||
| LK6.2 | 1 | − | 154,000 | 615 | 0.40 | |
| 0.5 h (B) | 106,000 | 2,260 | 2.13 | 5.34 | ||
| 0.5 h (A) | 247,000 | 4,350 | 1.76 | 4.41 | ||
| LK3C | 1 | − | 69,300 | 142 | 0.20 | |
| 0.5 h (B) | 12,300 | 77 | 0.63 | 3.06 | ||
| VL3-3M2 | 2 | − | 398,000 | 29,600 | 7.4 | |
| 0.5 h (B) | 192,000 | 13,800 | 7.2 | 0.97 | ||
| LK6.2 | 2 | − | 439,000 | 2,480 | 0.6 | |
| 0.5 h (B) | 9,600 | 225 | 2.3 | 4.15 | ||
| VL3-3M2 | 3 | − | 1,260,000 | 8,140 | 0.65 | |
| 0.5 h (B) | 2,510,000 | 50,000 | 1.99 | 3.08 | ||
| LK6.2 | 3 | − | 929,000 | 429 | 0.05 | |
| 0.5 h (B) | 121,000 | 964 | 0.80 | 17.25 | ||
| LK8 | 3 | − | 1,870,000 | 694 | 0.04 | |
| 0.5 h (B) | 379,000 | 411 | 0.11 | 2.92 |
The efficiency of CJ formation was measured as percent recombination (%R) for each cell line as described in Materials and Methods. Briefly, 40 to 48 h after transient transfection with pDR42, plasmid DNA harvested from each cell line was electroporated into E. coli. The percent recombination (%R) was calculated as the ratio (×100) between the number of colonies resistant to ampicillin (Ampr) and those resistant to ampicillin plus chloramphenicol (Camr). Cell lines were treated with 100 cGy of γ-irradiation at the indicated times before (B) or after (A) transfection with pDR42. The number of cells recovered after 48 h did not vary more than twofold within each group. The effect of irradiation on the efficiency of CJ formation was calculated as the ratio of recombination frequencies of irradiated cultures to those of control cultures (IR/C). −, no irradiation.
TABLE 2.
Effect of irradiation on signal joint formation in wild-type and SCID thymic lymphomasa
| Cell line | Irradiation | %R | IR/C | % Fidelity (n) |
|---|---|---|---|---|
| VL3-3M2 | − | 1.87 | 76 (30) | |
| + | 3.56 | 1.90 | 85 (20) | |
| LK6.2 | − | 14.51 | 35 (34) | |
| + | 12.23 | 0.84 | 23 (34) | |
| LK3C | − | 4.01 | 47 (15) | |
| + | 1.51 | 0.38 | 33 (15) |
Experiments to measure the efficiency and fidelity of signal joint formation were performed as described in Table 1, except that cell lines were transfected with the deletional signal joint substrate pWTSJΔ 30 min before irradiation. To assess the fidelity of signal joint formation, recombinant plasmids were purified from chloramphenicol-resistant colonies and digested with ApaLI. Shown are the percentages of colonies that could be digested with ApaLI (%R), indicating that they had precise signal joints. The number (n) of colonies tested in each group is shown. IR/C, ratio of recombination frequencies of irradiated cultures to those of control cultures.
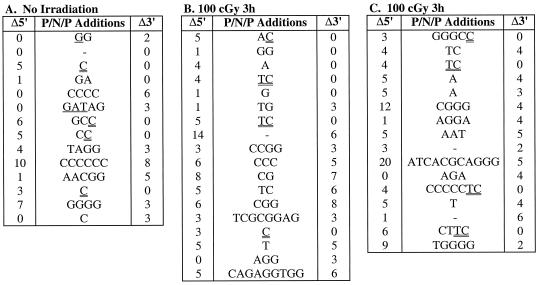
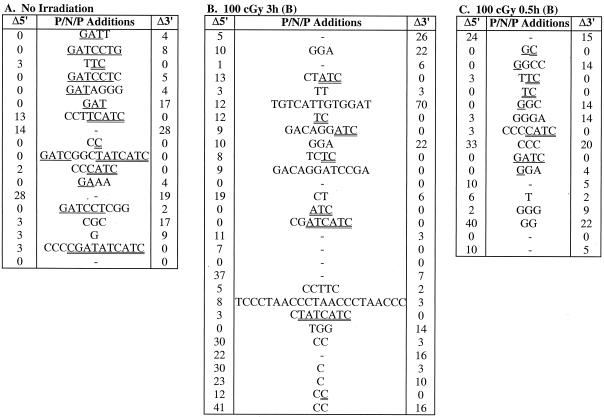
Having observed that irradiation improved the efficiency of CJ formation in SCID cells, we sequenced the coding junctions of multiple recombinant pDR42 clones to determine if this improvement was also evident at the level of coding end processing. Coding junction sequences from independent recombinants are shown in Fig. 1 and 2, and the distribution of nucleotide deletions and P nucleotide additions is summarized in Tables 3 and 4. The coding junctions of recombinants derived from wild-type and SCID cells that were not irradiated showed the expected characteristics. For example, no coding ends from wild-type recombinants had deletions of ≥10 nucleotides, whereas 20 to 23% of those from LK6.2 and LK8 recombinants had large deletions (Table 3 and data not shown). The average length of coding end deletions was also considerably greater in the SCID cell lines (Table 3). The intact coding ends from the SCID recombinants also displayed substantially more P additions than those from VL3-3M2 recombinants (Table 4). Most notable was the high frequency (29 to 47%) of long (>3) P additions in LK6.2 and LK8 recombinants (Table 4 and data not shown). Finally, the length of P additions was significantly greater in the SCID recombinants. TdT-mediated addition of N nucleotides was similar in all cell lines (Fig. 1 and 2). These data confirm that V(D)J joining in LK6.2 and LK8 cells displays anomalies typical of SCID cells, consistent with the DNA-PK mutation in these cell lines (28).
FIG. 1.
Effect of irradiation on coding end processing in wild-type thymic lymphoma cells. VL3-3M2 cells were transfected with pDR42, at various times before (B) or after (C) treatment with 0 (A) or 100 cGy of γ-irradiation, as indicated. After 48 h of culture, plasmid DNA was recovered and used to transform E. coli. pDR42 recombinant plasmids were purified from chloramphenicol-resistant colonies, and the coding junctions were sequenced. Shown are the 5′ P (single underline), N, and 3′ P (double underline) additions (P/N/P) at the CJ for each recombinant pDR42 clone. The number of nucleotides deleted from the 5′ (Δ5′) and 3′ (Δ3′) coding flanks are also indicated. P nucleotides were identified based on palindromy with the 3′ end of the 5′ coding flank (5′-ACAGGAAACAGGATC-3′) or the 5′ end of the 3′ coding flank (5′-GATGATATCGTCGAC-3′) sequences. For junctions where nucleotides could be assigned either to the coding flank or as P additions, the assignment was made to minimize the degree of P additions. The data represent independent clones derived from two independent transfections. The frequencies of independent recombinants sequenced were 93% (A), 100% (B), and 100% (C).
FIG. 2.
Effect of irradiation on coding end processing in SCID thymic lymphoma cells. LK6.2 cells were transfected with pDR42 3 h (B) or 0.5 h (C) before treatment with 0 (A) or 100 cGy of γ-irradiation, as indicated. Recombinant pDR42 clones were isolated and sequenced as described for Fig. 1. The frequency of independent recombinants sequenced was 64% (A), 83% (B), and 65% (C). B, before treatment.
TABLE 3.
Effect of irradiation on coding end deletions in wild-type and SCID cellsa
| Cell line | No. of deletions | Irradiation
|
|
|---|---|---|---|
| None [no. (%)] | 100 cGy [no. (%)] | ||
| VL3-3M2 | 0 | 11 (39) | 13 (19) |
| 1–5 | 12 (43) | 41 (61) | |
| 6–10 | 5 (18) | 11 (16) | |
| 11–20 | 0 (0) | 3 (4) | |
| 21–30 | 0 (0) | 0 (0) | |
| >30 | 0 (0) | 0 (0) | |
| Avg, SD | 4.4, 2.5 | 4.9, 3.2 | |
| LK6.2 | 0 | 17 (47) | 30 (29) |
| 1–5 | 10 (27) | 19 (19) | |
| 6–10 | 2 (6) | 15 (19) | |
| 11–20 | 5 (14) | 14 (16) | |
| 21–30 | 2 (6) | 9 (12) | |
| >30 | 0 (0) | 5 (5) | |
| Avg, SD | 10.2, 8.9 | 13.4, 12.4 | |
The number and percent of coding ends with the indicated numbers of nucleotides deleted are shown for each group. Shown is a summary of data from Fig. 1 and 2. Since the time of irradiation (relative to transfection) did not alter the distribution of coding end deletions, all the data for irradiated cells from Fig. 1 and 2 have been pooled.
TABLE 4.
Effect of irradiation on P additions in wild-type and SCID cellsa
| Cell line | No. of P added | Irradiation
|
|
|---|---|---|---|
| None [no. (%)] | 100 cGy [no. (%)] | ||
| VL3-3M2 | 0 | 5 (45) | 5 (38) |
| 1–3 | 6 (55) | 8 (62) | |
| 4–11 | 0 (0) | 0 (0) | |
| Avg, SD | 1.33, 0.82 | 1.63, 0.52 | |
| LK6.2 | 0 | 3 (18) | 13 (43) |
| 1–3 | 6 (35) | 13 (43) | |
| 4–11 | 8 (47) | 4 (14) | |
| Avg, SD | 4.50, 2.47 | 2.59, 1.80 | |
The number and percent of intact coding ends that have the indicated numbers of P nucleotides added are shown for each group. Shown is a summary of data from Fig. 1 and 2. Since the time of irradiation (relative to transfection) did not alter the distribution of P nucleotides, all the data for irradiated cells from Fig. 1 and 2 have been pooled. The calculations are restricted to intact coding ends because P additions are not observed when there is deletion from the coding end. The difference between the lengths of P additions in untreated VL3-3M2 and LK6.2 cells is statistically significant (Mann-Whitney U test, U = 7.0; two-tailed P value, 0.003). The difference between the lengths of P additions in untreated and irradiated LK6.2 cells is also statistically significant (Mann-Whitney U test, U = 60.5; two-tailed P value, 0.018).
Irradiation did not significantly affect nucleotide deletion or P nucleotide addition at coding ends in VL3-3M2 cells (Tables 3 and 4). In marked contrast, irradiation had striking effects on the processing of coding ends in LK6.2 cells. Most notably, irradiation increased the frequency of intact coding ends lacking P additions from 18 to 43%, and it decreased the frequency of coding ends with long P nucleotides from 47 to 14% (Table 4). The average length of P additions in LK6.2 was also significantly decreased after irradiation. In contrast to the striking effect of irradiation on P additions, it did not ameliorate the extensive deletion from coding ends in SCID cells (Table 3). Collectively, these data suggest that low-dose irradiation selectively improves repair of CJ in SCID cells by facilitating the opening of hairpin coding ends.
RAG-2 deficiency protects SCID mice from radiation-induced thymic lymphoma.
The above data demonstrate that irradiation partially restores V(D)J joining in SCID T-cell precursors, likely explaining why irradiation of neonatal SCID mice promotes some degree of normal T-cell development (29). However, most irradiated newborn SCID mice subsequently succumb to thymic lymphoma, suggesting that irradiation also promotes the development of neoplastic T-cell precursors. Given that the SCID mutation in DNA-PK impairs NHEJ, the role of irradiation in inducing lymphomagenesis in SCID mice could be to generate large numbers of DSB, some of which are mis-repaired to generate oncogenic chromosomal aberrations in T-cell precursors. However, we sought to determine whether there could be a mechanistic connection between radiation-induced CJ formation and tumorigenesis in T-cell precursors. To test the hypothesis that irradiation could induce oncogenic misjoining of RAG-1/2-mediated breaks that contribute to radiation-induced lymphomagenesis in SCID mice, we bred RAG-2-deficient SCID mice. Both the SCID and RAG-2 mutations block T-lymphocyte development at the DN pro-T-cell stage, due to failure to generate TCRβ-containing pre-TCR complexes (87, 109). RAG-2 SCID double-mutant mice maintain the global SCID NHEJ defect but fail to create DSB adjacent to recombination signal sequences in lymphocyte progenitors. Thus, this strategy allowed us to determine whether RAG-induced breaks are essential for radiation-induced lymphomagenesis in SCID mice.
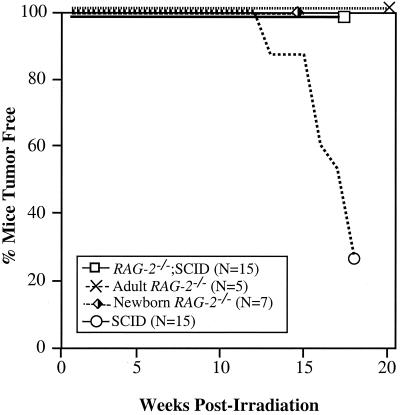
Mice were irradiated within 48 h of birth (100 cGy) and evaluated for the development of lymphoid tumors. Consistent with our previous studies (29), 73% of irradiated SCID mice (RAG-2+/+ or RAG-2+/−) developed thymic lymphoma between 13 and 18 weeks, as evidenced by invasive thymic masses that compromised respiration (Fig. 3). In some cases, dissemination of tumor to peripheral lymphoid organs was evident (data not shown). Ten tumors were assessed by flow cytometry: all had a DP pre-T-cell phenotype, and 4 out of 10 were TCRβ and CD3 positive. That the tumor cells had a more mature phenotype than thymocytes from untreated SCID mice is consistent with our previous studies showing that low-dose irradiation promotes the development of TCRβ+ DP thymocytes from DN progenitors in SCID mice (29). Strikingly, all irradiated RAG-2−/− SCID and irradiated RAG-2−/− mice were healthy and showed no evidence of thymic lymphoma at the time of sacrifice at 16 to 20 weeks postirradiation (Fig. 3). These data demonstrate a mechanistic dependence on RAG function for the radiation-induced development of thymic lymphoma in SCID mice.
FIG. 3.
Effect of RAG-2 function on thymic lymphomagenesis in irradiated SCID mice. SCID (RAG-2+/+ or RAG-2+/−) and RAG-2−/− SCID mice were irradiated (100 cGy) within 48 h of birth. RAG-2−/− mice were irradiated as newborns (400 cGy) or adults (750 cGy) as previously described (50). Depicted is the percentage of tumor-free mice of each genotype up to 20 weeks postirradiation. Mice showing no signs of morbidity were sacrificed at 16 weeks (newborn RAG-2−/−), 18 weeks (SCID and RAG-2−/− SCID), or 20 weeks (adult RAG-2−/−) and showed no evidence of lymphoma by gross pathology. N, number of mice analyzed in each group.
TCRβ-transgenic SCID mice are protected from radiation-induced thymic lymphoma.
V(D)J recombination occurs at multiple different stages of T-cell development, but targeted disruption of the RAG-1 or RAG-2 genes universally ablates V(D)J recombinase activity. We sought to determine if SCID mice could be protected from radiation-induced thymic lymphoma by ablating V(D)J recombinase activity only at a particular developmental stage. The TCRβ, -δ, and -γ loci are all accessible to the V(D)J recombinase machinery in immature DN thymocytes, but recent data suggest that these loci rearrange at different developmental stages. In normal thymocytes, Vγ-to-Jγ rearrangements, Vδ-to-DJδ rearrangements, and Dβ-to-Jβ rearrangements are all evident at the CD44+CD25+ DN stage, whereas Vβ to DJβ rearrangements predominantly occur at the later CD44−CD25+ DN stage (18, 45). In contrast to successful rearrangements of TCRδ or TCRγ, in-frame Vβ-DJβ rearrangements promote a large burst of proliferation (57, 95). Expression of a functionally rearranged TCRβ transgene allows the formation of a pre-TCR complex which specifically prevents the V-DJ step of endogenous TCRβ rearrangement (4, 117). Therefore, we bred TCRβ-transgenic SCID (TCRβ-SCID) mice to determine whether inhibiting V(D)J recombinase activity specifically at the highly proliferative CD44−CD25+ DN stage was sufficient to protect against radiation-induced thymic lymphoma in SCID mice.
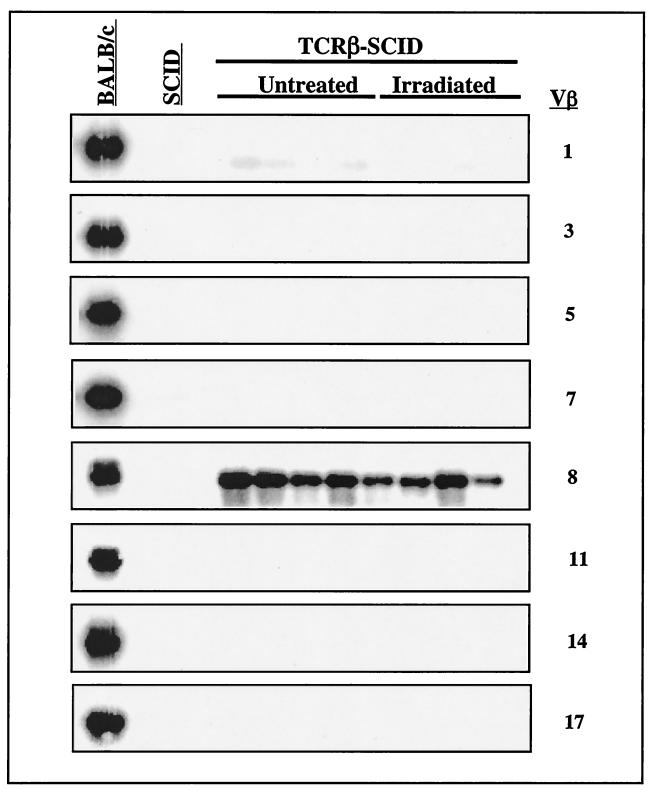
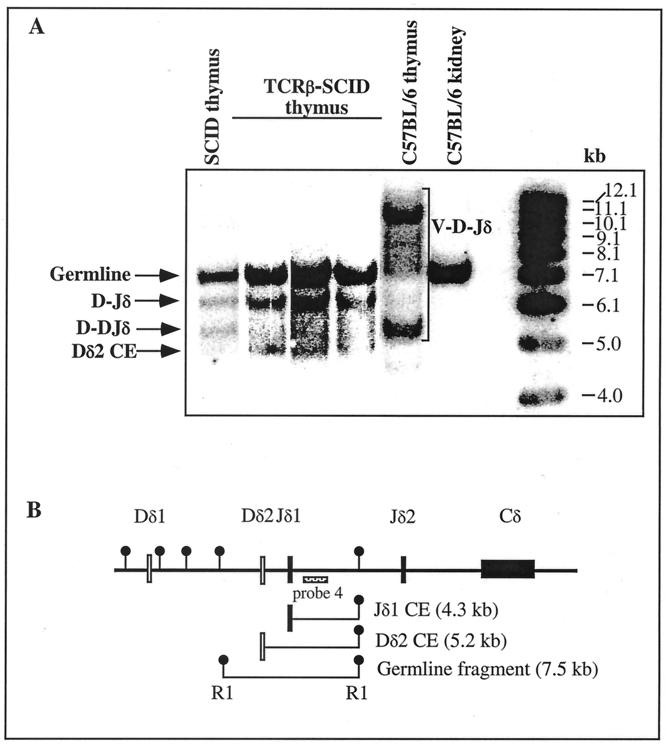
Transcripts of endogenous VDJβ rearrangements involving 8 Vβ and all 12 possible Jβ gene segments were not detectable in TCRβ-SCID thymocytes (untreated or irradiated) by RT-PCR (Fig. 4). These data agree with previous studies showing that transgenic expression of TCRβ prevents Vβ to DJβ rearrangements in thymocytes (3, 4). This effect was specific for the TCRβ locus, since Southern blot analysis showed that TCRβ coding end breaks were present in TCRβ-SCID thymocytes (Fig. 5). Partial (D-Jδ or D-DJδ) rearrangements, which have been well-described for SCID thymocytes (20, 105), were also abundant in TCRβ-SCID thymocytes (Fig. 5). As expected from previous studies (66, 111), we observed that the TCRδ transgene bypasses the SCID defect in pre-TCR generation and promotes the development of DP thymocytes (Fig. 6). However, the number of CD25+ DN thymocytes, presumably the initial targets of the radiation-induced neoplastic process, was similar in newborn SCID and TCRβ-SCID mice (Fig. 6). In wild-type mice, the DN-DP transition is accompanied by the onset of recombination at the TCRα locus, and we previously showed that TCRα coding end breaks are readily detectable in TCRβ-SCID thymocytes (82). Finally, TCRβ-SCID mice had similar frequencies of proliferating thymocytes as their transgene-negative littermates, and this frequency was not affected by irradiation (Table 5). Collectively, these observations confirm that the TCRβ transgene selectively prevents Vβ to DJβ rearrangements in CD44−CD25+ DN thymocytes, but does not decrease proliferation or prevent the generation of TCRδ or of TCRα coding end breaks in SCID thymocytes at earlier or later stages of T-cell development, respectively.
FIG. 4.
Endogenous TCRβ expression in TCRβ-SCID mice. Thymus RNA from individual 2-week-old untreated or irradiated TCRβ-SCID mice was reverse transcribed into cDNA. RT-PCR was performed using eight different Vβ-specific sense primers coupled with a TCR-Cβ anti-sense primer. The products were Southern blotted and hybridized with a TCR-Cβ probe. Note that, as expected, Vβ8 transcripts were detectable in all thymus samples from SCID mice expressing the Vβ8.2 transgene. Thymocyte RNA obtained from 4- to 6-week-old nontransgenic SCID and BALB/c mice were included as negative and positive controls, respectively, for the presence of TCRβ transcripts.
FIG. 5.
Southern blot detection of TCRδ coding end breaks and rearrangements in TCRβ-SCID thymocytes. (A) Genomic DNA was extracted from thymocyte cell suspensions prepared from 4 individual 6-week-old TCRβ-SCID mice and a nontransgenic littermate, as well as from the thymus and kidney of a 4-week-old wild-type C57BL/6 animal. The DNA was digested with EcoRI, electrophoresed, and Southern blotted. The membrane was hybridized with probe no. 4, which detects a single germline fragment between Jδ1 and Jδ2 and allows detection of rearrangement to Jδ1 (82). The positions of germline fragments, Dδ2 coding end breaks (CE), DJδ or D-DJδ partial rearrangements, and complete V-D-Jδ rearrangements are indicated. Molecular size standards are included in the far right lane, with sizes in kilobases. (B) Partial TCRδ locus map. The position of probe no. 4 within the TCRδ locus is displayed (adapted from reference 82). EcoRI sites are indicated, as are the CE breaks and germline fragment detected in this assay.
FIG. 6.
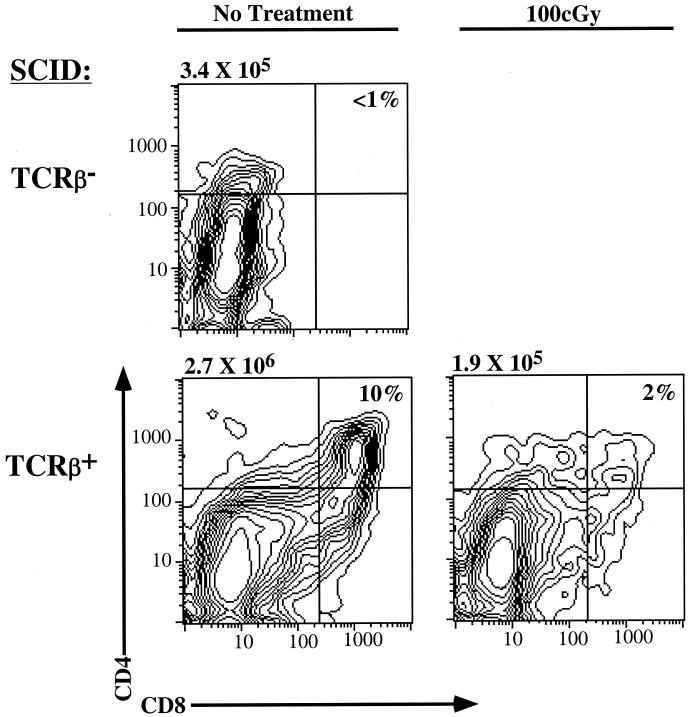
Impact of TCRβ transgene on T-cell development in SCID mice. Thymocytes from two 4-day-old TCRβ-SCID mice and a nontransgenic littermate were evaluated for CD4 and CD8 expression by flow cytometry. One TCRβ-SCID mouse treated with 100 cGy of γ-irradiation 24 h prior to this analysis is also shown for comparison. The cellularity of each thymus is indicated. The absolute number of CD25+ DN cells in each sample did not vary by more than twofold.
TABLE 5.
Effect of TCRβ transgene on proliferation of SCID thymocytesa
| Treatment group | Age (weeks) | Tg | n | % (± SD) S/G2/M |
|---|---|---|---|---|
| Not irradiated | 2 | − | 3 | 24.7 ± 5.6 |
| + | 7 | 32.6 ± 1.0 | ||
| Irradiated | 6 | − | 3 | 43.7 ± 5.4 |
| + | 4 | 46.3 ± 4.5 | ||
| Irradiated | 10 | − | 3 | 22.9 ± 4.0 |
| + | 3 | 29.4 ± 2.2 |
Effect of the TCRβ transgene (Tg) on the proliferative status of SCID thymocytes. Three litters from a TCRβ-SCID × SCID mating were irradiated with 100 cGy within 48 h after birth or were left untreated. Mice were sacrificed at 2 to 10 weeks of age, and the percentage of cells in the S, G2, and M phases of the cell cycle was quantitated by flow cytometric analysis of propidium iodide-stained thymocytes.
To measure the impact of the TCRβ transgene on radiation-induced lymphomagenesis in SCID mice, newborn litters from SCID × TCRβ-SCID matings were left untreated or were irradiated and examined 16 to 24 weeks later (Table 6). DP thymocytes are extremely radiosensitive in wild-type as well as in SCID mice (25, 84), and few remained 24 h after irradiation of TCRβ-SCID mice (Fig. 6). Consistent with our previous findings (29), four out of six (60%) of irradiated nontransgenic SCID littermates were moribund 16 to 24 weeks after irradiation and, upon necropsy, were found to have large thymic masses composed primarily of DP lymphoblasts (Table 6). In marked contrast, all irradiated TCRβ-SCID mice appeared healthy at 16 to 24 weeks. Given our relatively small sample size, it is possible that we could have missed low-frequency long-latency tumors in irradiated TCRβ-SCID mice. However, we believe this is unlikely because the cellularity of their thymuses was no different than that of age-matched control (untreated) TCRβ-SCID mice (Table 6). Thus, we could find no evidence of thymic hyperplasia that might be indicative of a preneoplastic thymus (Table 6). In addition, given that 70 to 90% of nontransgenic SCID mice develop thymic lymphoma by 24 weeks of age (Table 6 and reference 29), the complete absence of thymic hyperplasia and morbidity in irradiated TCRβ-SCID mice at this time point is striking. These data suggest that preventing Vβ to DJβ rearrangement in SCID pro-T cells confers complete protection from radiation-induced lymphomagenesis in SCID mice.
TABLE 6.
Effect of TCRβ transgene on irradiation-induced thymic lymphomagenesis in SCID micea
| Strain | Irradiation | Cellularity (10−6) | No. with thymic lymphoma/total |
|---|---|---|---|
| TCRβ-SCID | − | 4.3 ± 3.9 | 0/7 |
| SCID | + | 122.5 ± 67.0 | 4/6 |
| TCRβ-SCID | + | 5.8 ± 3.0 | 0/12 |
Effect of a TCRβ transgene on radiation-induced thymic lymphomagenesis in SCID mice. TCRβ-SCID mice and their nontransgenic SCID littermates were irradiated with 100 cGy within 48 h after birth or were left untreated and evaluated at 16 to 24 weeks of age for the presence of thymic lymphoma. All irradiated TCRβ-SCID mice appeared to be healthy and showed no evidence of lymphoma at the time of sacrifice. In contrast, their irradiated nontransgenic littermates developed thymic lymphoma, as evidenced by invasive thymic masses that compromised respiration.
DISCUSSION
In this paper we demonstrate that low-dose irradiation quantitatively and qualitatively improves CJ formation in SCID cells, in the absence of TCR-mediated cellular selection. We also show that V(D)J recombinase activity is required for radiation-induced thymic lymphomagenesis in SCID mice. Together, these findings suggest that the radiation-induced NHEJ activity also results in the oncogenic misjoining of RAG-1/2-induced breaks in T-cell precursors. Below, we discuss the implications of these observations on mechanisms of V(D)J joining as well as mechanisms by which defective NHEJ promotes lymphoid neoplasia.
Irradiation transiently improves V(D)J joining in SCID lymphocyte progenitors.
The SCID defect in CJ formation results in the accumulation of hairpin coding ends in lymphocyte precursors (105, 122). Thus, it has been suggested that DNA-PK is required to recruit and/or activate factors that mediate hairpin cleavage (12, 122), a process which must precede the formation of CJ. Although CJ can be formed inefficiently in SCID cells, they frequently have long P nucleotide additions (35, 63, 107), possibly reflecting a role for DNA-PK in directing the site of hairpin cleavage to within a few nucleotides of the tip. Data presented in Table 1 show that irradiation improves the efficiency of CJ formation in SCID cells. More strikingly, few of these junctions displayed long P nucleotide additions, which are hallmarks of inefficient CJ formation in untreated SCID cells (Table 4). Binnie et al. have recently reported that irradiation of different SCID thymic lymphoma cell lines had no effect on the efficiency of CJ formation (10). In addition, Binnie et al. showed that irradiation decreased the frequency of coding ends with P additions slightly (by 18%), but it had no effect on the length of P additions. These authors included caffeine (which has undefined effects on recombination) in some of their assays. Moreover, the cell lines they used did not display the excessive coding end deletion characteristic of SCID cells. Thus, differences in assay conditions or cell line characteristics may explain why we observed a more pronounced effect of irradiation on CJ formation in SCID cell lines.
Our observations suggest that irradiation has induced an activity that facilitates cleavage of hairpin coding ends, increasing the efficiency of CJ formation in SCID cells and decreasing the frequency of coding ends with long P additions from 47 to 14% (Table 4). Proteins shown to have hairpin endonuclease activity include RAG-1/2 (8, 110) and MRE11 (96, 97). However, the protein(s) responsible for hairpin opening in vivo has not been identified, so it is difficult to speculate on how irradiation might affect its expression and/or functions in cells harboring the SCID mutation in DNA-PK.
The rare coding junctions that form in SCID cells typically display excessive deletion (35, 86, 108), suggesting that the processing of opened hairpin ends is also regulated by DNA-PK. However, in contrast to hairpin cleavage, irradiation did not ameliorate the extensive deletion from coding ends in SCID cells (Table 3). Similarly, irradiation did not improve signal joint formation in SCID cells, and most signal joints remained imprecise (Table 2). Although we (29, 82) and others (120) did not report extensive deletions at TCR coding junctions in thymocytes from irradiated DNA-PK mutant mice, this likely reflected strong selection in vivo for precursors that could form pre-TCR. Thus, the absence of cellular pre-TCR-mediated cellular selection in the experiments we report here revealed a differential effect of irradiation on hairpin opening versus exonucleolytic processing of coding ends, suggesting that the two events are mediated by separate biochemical entities.
How does irradiation improve CJ formation in SCID cells?
The hypothesis that irradiation increases the activity of mutant DNA-PK present in SCID cells seems unlikely for several reasons. First, SCID mutant DNA-PK is truncated (6, 13, 28), and protein levels are reduced 10- to 50-fold relative to those of wild-type cells (28). Second, irradiation promotes caspase-mediated cleavage and inactivation of DNA-PK kinase activity (22, 23, 52, 112, 113). Finally, cells from mice with null mutations in DNA-PK show virtually identical defects in NHEJ and V(D)J recombination to those seen in SCID cells, suggesting that the SCID mutation in DNA-PK is effectively null (42, 70, 114, 120). These studies suggest that a DNA-PK-independent NHEJ pathway can inefficiently effect CJ formation (120), and we favor the notion that irradiation improves the efficiency and fidelity of this pathway in SCID cells. Such a pathway may be analogous to DNA damage inducible DNA repair activities in bacteria (38) and yeast (32).
The role of V(D)J recombinase activity in radiation-induced lymphomagenesis in SCID mice.
We have shown that V(D)J recombinase activity plays an essential role in radiation-induced lymphomagenesis in SCID mice. The role of V(D)J recombinase activity could simply be to promote precursor proliferation through the production of pre-TCR. Given the NHEJ defect conferred by the SCID mutation, the probability of a cell sustaining oncogenic mutations could be greatly enhanced by the repeated cell division that is induced by pre-TCR signals. However, introduction of a TCRβ transgene promotes the development and proliferation of DP thymocytes in SCID mice (Fig. 6 and reference 66) but does not promote the development of thymic lymphoma (Table 5). These findings demonstrate that a proliferative stimulus, per se, is not sufficient to induce neoplastic transformation of SCID thymocytes.
A more likely mechanism to explain the essential function of V(D)J recombinase activity for thymic lymphomagenesis in irradiated SCID mice is that oncogenic mutations are generated by the radiation-induced misjoining of RAG-1/2-mediated breaks to random DSB. This notion is consistent with our demonstration that irradiation specifically improves the joining of RAG-1/2-mediated hairpin coding ends. V(D)J misjoining events are thought to give rise to the chromosomal translocations between antigen receptor genes and proto-oncogenes that are found in many lymphocytic leukemias and lymphomas (101). We used fluorescent in situ hybridization probes specific for chromosome 6 (TCRβ) or chromosome 14 (TCRα/δ) to look for translocations in four thymic lymphomas from irradiated SCID mice, but none were found (D. Vesprini, C. J. Guidos, and J. S. Danska, unpublished data). However, this technique is not sufficiently sensitive to detect deletions, insertions, or small translocations.
V(D)J recombinase-dependent genetic alterations could also give rise to oncogenic mutations that do not involve antigen receptor genes. The recent demonstration that RAG-1/2 functions as a transposase provides one possible mechanism by which RAG-induced DSB could be generated outside antigen receptor loci (1, 56). Indeed, RAG-1/2-mediated cleavage of “cryptic” or nonconsensus recombination signal sequences has been shown to occur at non-Ig or non-TCR loci (72). Cryptic site recombination appears to underlie several different oncogenic deletions, not detected by standard karyotype analyses, found in patients with T-cell acute lymphoblastic leukemia (5, 17, 24). Moreover, recurring site-specific deletions between closely linked cryptic recombination signal sequences in the hypoxanthine-guanine phosphoribosyltransferase gene are frequently found in the peripheral T cells of healthy individuals (40, 41). It has been estimated that there are millions of potential cryptic sites in the human genome, and several of these were shown to function as joining signals in recombination substrate plasmids (72). Indeed, a recent study has shown that two DSB on different chromosomes are sufficient to promote frequent translocations (104). Thus, cryptic site recombination may be an important type of RAG-1/2-induced genetic alteration contributing to transformation of T-cell precursors in irradiated SCID mice.
We have previously shown that irradiation increases a different kind of misjoining event in SCID thymocytes, known as trans-rearrangement, by 50- to 100-fold (80). In trans-rearrangement, V(D)J coding ends originating from antigen receptor loci on different chromosomes are joined together. The ratio of cis-to-trans rearrangements is 500 to 1,000:1 in normal cells (7, 80). Similarly, trans-joining of coding ends is severely impaired relative to cis-joining in normal cells simultaneously transfected with two different recombination substrate plasmids, and this bias is enforced at the joining, rather than the cleavage, step of V(D)J recombination (51). However, in thymocytes from irradiated SCID mice, the ratio of cis-to-trans rearrangements is approximately 5:1 (80), suggesting that the absence of functional DNA-PK greatly promotes interlocus misjoining events. Thus, an important function of DNA-PK may be to minimize trans-joining and other misjoining events by tethering coding ends within the post-cleavage complex, which contains the cleaved coding and signal ends, RAG-1/2 proteins, and DNA-PK (2, 55, 64). Although trans-joining events are not pathogenic per se, they serve as a good marker for genomic instability and the risk of oncogenic misjoining events in lymphocytes (reviewed in reference 65).
Surprisingly, we found that a TCRβ transgene was as effective as the RAG-2 null mutation in protecting SCID mice from radiation-induced thymic lymphomas. In contrast to the RAG-2 mutation, which ablates all V(D)J recombinase activity, the TCRβ transgene specifically inhibits recombinase activity only for the Vβ to DJβ rearrangement step in CD44−CD25+ DN thymocytes. It is possible that this particular rearrangement event is more prone to generate oncogenic translocations than other TCR rearrangements. However, we favor the view that it is the developmental stage specificity of this rearrangement event that is crucial, rather than the nature of the rearrangement. In contrast to most rearrangements of TCRγ, TCRδ, and TCRα, Vβ-to-DJβ rearrangement takes place in CD44−CD25+ DN thymocytes that have a high proliferative potential (18, 45). Thus, we suggest that misjoining of RAG-1/2-induced breaks has greater oncogenic potential at this highly proliferative stage of T-cell development.
Differential impact of NHEJ and DNA damage checkpoint defects on mechanisms of lymphomagenesis.
The NHEJ defect imparted by KU70 deficiency promotes the spontaneous development of thymic lymphomas (48, 73). In contrast to the SCID mutation in DNA-PK, KU70 disruption permits limited T-cell development to occur, and this is accompanied by the generation of some normal TCRβ rearrangements (48, 94). In contrast, no complete IgH rearrangements occurred, and B-cell development was completely ablated in KU70−/− mice. Thus, the development of thymic lymphoma is correlated with a limited degree of normal TCR gene rearrangement and T-cell development in both irradiated SCID mice and in KU70-deficient mice. These parallels lead us to suggest that defective NHEJ activity, though capable of mediating V(D)J joining with low efficiency, confers a high risk of sustaining oncogenic V(D)J misjoining events in T-cell precursors. In agreement with this notion, Alt and colleagues have recently shown that the absence of the NHEJ protein ligase 4 causes frequent nonreciprocal translocations in fibroblasts (33).
Mutations in p53 and ATM, which disrupt DNA damage checkpoints, also confer a high risk of developing thymic lymphomas in mice. It was suggested that disruption of the checkpoint could promote survival or proliferation of thymocytes carrying oncogenic chromosomal translocations involving antigen receptor genes. Surprisingly, however, RAG-1/2 deficiency had little impact on lymphomagenesis in p53-deficient mice (76, 89), but protected (75) or significantly reduced the frequency and latency (100) of thymic lymphomagenesis in ATM-deficient mice. Interestingly, the tumors that developed with short latency in the RAG-2+ ATM-deficient mice had cytogenetic abnormalities within the TCRα/δ locus, suggesting a role for aberrant V(D)J recombination in these malignancies (100). Although ATM is primarily thought of as a checkpoint protein, several lines of evidence suggest that ATM-deficient cells also have DSB repair defects, though more subtle than the SCID NHEJ defect (reviewed in reference 58). Based on the differing extents to which V(D)J recombinase activity contributes to thymic lymphoma risk in ATM, p53, and SCID mutant mice, we suggest that there are at least two lymphomagenesis pathways in thymocytes: a pathway promoted by defective DSB repair (in SCID and ATM mice) that is dependent on V(D)J recombinase activity and a second pathway promoted by defective DNA damage checkpoints (in p53 and ATM mutant mice) that is independent of V(D)J recombinase activity.
ACKNOWLEDGMENTS
We thank David Schatz, Ferenc Livak, and Stephen Meyn for critical reading of the manuscript. We also thank David Schatz and Ferenc Livak for the TCRδ probe, Susanna Lewis for the pWTSJΔ plasmid and advice on the use of extrachromosomal recombination substrate plasmids, Gill Wu for pDR42, Al Singer and Wendy Shores for TCRβ-transgenic mice, and Andrew Paterson for statistical calculations.
C.W. was supported by a RESTRACOMP studentship from the Hospital for Sick Children. J.D. and C.G. hold Scientist Awards from the National Cancer Institute of Canada and the Medical Research Council of Canada, respectively. This work was supported by the National Cancer Institute of Canada (with funds from the Canadian Cancer Society).
REFERENCES
- 1.Agrawal A, Eastman Q M, Schatz D G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A, Schatz D G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S J, Abraham K M, Nakayama T, Singer A, Perlmutter R M. Inhibition of T-cell receptor β chain rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson S J, Levin S D, Perlmutter R M. Protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor β-chain genes. Nature. 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- 5.Aplan P D, Lombardi D P, Ginsberg A M, Cossman J, Bertness V L, Kirsch I R. Disruption of the human SCL locus by “illegitimate” V-(D)-J recombinase activity. Science. 1990;250:1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- 6.Araki R, Fujimori A, Hamatani K, Mita K, Saito T, Mori M, Fukumura R, Morimyo M, Muto M, Itoh M, Tatsumi K, Abe M. Nonsense mutation at Tyr-4046 in the DNA-dependent protein kinase catalytic subunit of severe combined immune deficiency mice. Proc Natl Acad Sci USA. 1997;94:2438–2443. doi: 10.1073/pnas.94.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey S N, Rosenberg N. Assessing the pathogenic potential of the V(D)J recombinase by interlocus immunoglobulin light-chain gene rearrangement. Mol Cell Biol. 1997;17:887–894. doi: 10.1128/mcb.17.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besmer E, Mansilla-Soto J, Cassard S, Sawchuk D J, Brown G, Sadofsky M, Lewis S M, Nussenzweig M C, Cortes P. Hairpin coding end opening is mediated by RAG1 and RAG2 proteins. Mol Cell. 1998;2:817–828. doi: 10.1016/s1097-2765(00)80296-8. [DOI] [PubMed] [Google Scholar]
- 9.Biedermann K A, Sun J, Giaccia A J, Tosto L M, Brown J M. Scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci USA. 1991;88:1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binnie A, Olson S, Wu G E, Lewis S M. Gamma-irradiation directly affects the formation of coding joints in SCID cell lines. J Immunol. 1999;163:5418–5426. [PubMed] [Google Scholar]
- 11.Blackwell T K, Malynn B A, Pollock R R, Ferrier P, Covey L R, Fulop G M, Phillips R A, Yancopoulos G D, Alt F W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal but abnormal coding joins. EMBO J. 1989;8:735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blunt T, Finnie N J, Taccioli G E, Smith G C M, Demengeot J, Gottlieb T M, Mizuta R, Varghese A J, Alt F W, Jeggo P A, Jackson S P. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 13.Blunt T, Gell D, Fox M, Taccioli G E, Lehmann A R, Jackson S P, Jeggo P A. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA. 1996;93:10285–10290. doi: 10.1073/pnas.93.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogue M A, Jhappan C, Roth D B. Analysis of variable (diversity) joining recombination in DNA-dependent protein kinase (DNA-PK)-deficient mice reveals DNA-PK-independent pathways for both signal and coding joint formation. Proc Natl Acad Sci USA. 1998;95:15559–15564. doi: 10.1073/pnas.95.26.15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogue M A, Zhu C, Aguilar-Cordova E, Donehower L A, Roth D B. p53 is required for both radiation-induced differentiation and rescue of V(D)J rearrangement in scid mouse thymocytes. Genes Dev. 1996;10:553–565. doi: 10.1101/gad.10.5.553. [DOI] [PubMed] [Google Scholar]
- 16.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 17.Brown L, Cheng J T, Chen Q, Siciliano M J, Crist W, Buchanan G, Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990;9:3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capone M, Hockett R D, Jr, Zlotnik A. Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes. Proc Natl Acad Sci USA. 1998;95:12522–12527. doi: 10.1073/pnas.95.21.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll A M, Bosma M J. Detection and characterization of functional T cells in mice with severe combined immune deficiency. Eur J Immunol. 1988;18:1965–1971. doi: 10.1002/eji.1830181215. [DOI] [PubMed] [Google Scholar]
- 20.Carroll A M, Bosma M J. T-lymphocyte development in scid mice is arrested shortly after the initiation of T-cell receptor δ chain recombination. Genes Dev. 1991;5:1357–1366. doi: 10.1101/gad.5.8.1357. [DOI] [PubMed] [Google Scholar]
- 21.Carroll A M, Hardy R R, Bosma M J. Occurrence of mature B (Ig+ and B220+) and T (CD3+) lymphocytes in scid mice. J Immunol. 1989;17:1467–1471. [PubMed] [Google Scholar]
- 22.Casciola-Rosen L, Nicholoson D W, Chong T, Rowan K R, Thornberry N A, Miller D K, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic cell death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casciola-Rosen L A, Anhalt G J, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625–1634. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cayuela J M, Gardie B, Sigaux F. Disruption of the multiple tumor suppressor gene MTS1/p16(INK4a)/CDKN2 by illegitimate V(D)J recombinase activity in T-cell acute lymphoblastic leukemias. Blood. 1997;90:3720–3726. [PubMed] [Google Scholar]
- 25.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 26.Critchlow S E, Jackson S P. DNA end-joining: from yeast to man. Trends Biochem Sci. 1998;23:394–398. doi: 10.1016/s0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 27.Custer R P, Bosma G C, Bosma M J. Severe combined immunodeficiency (scid) in the mouse. Am J Pathol. 1985;120:464–477. [PMC free article] [PubMed] [Google Scholar]
- 28.Danska J S, Holland D P, Mariathasan S, Williams K W, Guidos C J. Biochemical and genetic defects in the DNA-dependent protein kinase in murine scid lymphocytes. Mol Cell Biol. 1996;16:5507–5517. doi: 10.1128/mcb.16.10.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danska J S, Pflumio F, Williams C, Huner O, Dick J E, Guidos C J. Rescue of T cell-specific V(D)J recombination in SCID mice by DNA-damaging agents. Science. 1994;266:450–455. doi: 10.1126/science.7524150. [DOI] [PubMed] [Google Scholar]
- 30.Davis M M, Bjorkman P J. T-cell antigen receptor and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 31.Difilippantonio M J, Zhu J, Chen H T, Meffre E, Nussenzweig M C, Max E E, Ried T, Nussenzweig A. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature. 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson D O, Sekiguchi J M, Chang S, Frank K M, Gao Y, DePinho R A, Alt F W. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci USA. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrier P, Covey L R, Li S C, Suh H, Malynn B A, Blackwell T K, Morrow M A, Alt F W. Normal recombination substrate Vh to DJh rearrangements in pre-B cell lines from scid mice. J Exp Med. 1990;171:1909–1918. doi: 10.1084/jem.171.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fish S M, Bosma M J. Abnormal deletions in the T-cell receptor delta locus of mouse thymocytes. Mol Cell Biol. 1994;14:4455–4464. doi: 10.1128/mcb.14.7.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox C J, Danska J S. Molecular analysis of mouse T cell receptor expression using PCR. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 2. New York, N.Y: John Wiley and Sons, Inc.; 1997. pp. 10.27.1–10.27.20. [DOI] [PubMed] [Google Scholar]
- 37.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H L, Cheng L, Davidson L, Kangaloo L, Alt F W. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 38.Friedberg E C, Walker G C, Siede W. SOS responses and DNA damage tolerance in prokaryotes. In: Friedberg E C, editor. DNA repair and mutagenesis. Washington, D. C.: ASM Press; 1995. pp. 407–464. [Google Scholar]
- 39.Fulop G, Phillips R. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 40.Fuscoe J C, Zimmerman L J, Harrington-Brock K, Burnette L, Moore M M, Nicklas J A, O'Neill J P, Albertini R J. V(D)J recombinase-mediated deletion of the hprt gene in T-lymphocytes from adult humans. Mutat Res. 1992;283:13–20. doi: 10.1016/0165-7992(92)90116-y. [DOI] [PubMed] [Google Scholar]
- 41.Fuscoe J C, Zimmerman L J, Lippert M J, Nicklas J A, O'Neill J P, Albertini R J. V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res. 1991;51:6001–6005. [PubMed] [Google Scholar]
- 42.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver D T, Alt F W. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y, Ferguson D O, Xie W, Manis J P, Sekiguchi J, Frank K M, Chaudhuri J, Horner J, DePinho R A, Alt F W. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature. 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 44.Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- 45.Godfrey D I, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8-thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 46.Groves T, Katis P, Madden Z, Manickam K, Ramsden D, Wu G E, Guidos C J. In vitro maturation of clonal CD4+CD8+ cell lines in response to TCR engagement. J Immunol. 1995;154:5011–5022. [PubMed] [Google Scholar]
- 47.Gu Y, Jin S, Gao Y, Weaver D T, Alt F W. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Y, Seidl K J, Rathbun G A, Zhu C, Manis J P, van der Stoep N, Davidons L, Cheng H-L, Sekiguchi J M, Frank K, Stanhope-Baker P, Schlissel M S, Roth D B, Alt F W. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 49.Guidos C J, Williams C J, Grandal I, Knowles G, Huang M, Danska J S. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphoycte precursors. Genes Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 50.Guidos C J, Williams C J, Wu G E, Paige C J, Danska J S. Development of CD4+CD8+ thymocytes in RAG-deficient mice through a T cell receptor β chain-independent pathway. J Exp Med. 1995;181:1187–1195. doi: 10.1084/jem.181.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han J O, Steen S B, Roth D B. Intermolecular V(D)J recombination is prohibited specifically at the joining step. Mol Cell. 1999;3:331–338. doi: 10.1016/s1097-2765(00)80460-8. [DOI] [PubMed] [Google Scholar]
- 52.Han Z, Malik N, Carter T, Reeves W H, Wyche J H, Hendrickson E A. DNA-dependent protein kinase is a target for a CPP32-like apoptotic protease. J Biol Chem. 1996;271:25035–25040. doi: 10.1074/jbc.271.40.25035. [DOI] [PubMed] [Google Scholar]
- 53.Hendrickson E A, Qin X-Q, Bump E A, Schatz D G, Oettinger M, Weaver D T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci USA. 1991;88:4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendrickson E A, Schissel M S, Weaver D T. Wild-type V(D)J recombination in scid pre-B cells. Mol Cell Biol. 1990;10:5397–5407. doi: 10.1128/mcb.10.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiom K, Gellert M. Assembly of a 12/23 paired signal complex: a critical control point in V(D)J recombination. Mol Cell. 1998;1:1011–1019. doi: 10.1016/s1097-2765(00)80101-x. [DOI] [PubMed] [Google Scholar]
- 56.Hiom K, Melek M, Gellert M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman E S, Passoni L, Crompton T, Leu T M, Schatz D G, Koff A, Owen M J, Hayday A C. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 58.Jeggo P A, Carr A M, Lehmann A R. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 1998;14:312–316. doi: 10.1016/s0168-9525(98)01511-x. [DOI] [PubMed] [Google Scholar]
- 59.Jhappan C, Morse H C, Fleischman R D, Gottesman M M, Merlino G. DNA-PKcs: a T-cell tumour suppressor encoded at the mouse scid locus. Nat Genet. 1997;17:483–485. doi: 10.1038/ng1297-483. [DOI] [PubMed] [Google Scholar]
- 60.Kang J, Coles M, Cado D, Raulet D H. The developmental fate of T cells is critically influenced by TCRgammadelta expression. Immunity. 1998;8:427–438. doi: 10.1016/s1074-7613(00)80548-8. [DOI] [PubMed] [Google Scholar]
- 61.Karasuyama H, Rolink A, Melchers F. Surrogate light chain in B cell development. Adv Immunol. 1996;63:1–41. doi: 10.1016/s0065-2776(08)60853-6. [DOI] [PubMed] [Google Scholar]
- 62.Kienker L J, Kuziel W A, Garni-Wagner B A, Kumar V, Tucker P W. T cell receptor gamma and delta gene rearrangements in scid thymocytes. Similarity to those in normal thymocytes. J Immunol. 1991;147:4351–4359. [PubMed] [Google Scholar]
- 63.Kienker L J, Kuziel W A, Tucker P W. T cell receptor gamma and delta gene junctional sequences in SCID mice: excessive P nucleotide insertion. J Exp Med. 1991;174:769–773. doi: 10.1084/jem.174.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim D R, Oettinger M A. Functional analysis of coordinated cleavage in V(D)J recombination. Mol Cell Biol. 1998;18:4679–4688. doi: 10.1128/mcb.18.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirsch I R, Lista F. Lymphocyte-specific genomic instability and risk of lymphoid malignancy. Semin Immunol. 1997;9:207–215. doi: 10.1006/smim.1997.0071. [DOI] [PubMed] [Google Scholar]
- 66.Kishi H, Borgulya P, Scott B, Karjalainen K, Traunecker A, Kaufman J, Von Boehmer H. Surface expression of the β T cell receptor (TCR) chain in the absence of other TCR or CD3 proteins on immature T cells. EMBO J. 1991;10:93–100. doi: 10.1002/j.1460-2075.1991.tb07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komori T, Okada A, Stewart V, Alt F W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- 68.Kong F K, Chen C L, Six A, Hockett R D, Cooper M D. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci USA. 1999;96:1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotloff D B, Bosma M J, Ruetsch N R. Scid mouse pre-B cells with intracellular μ chains: analysis of recombinase activity and IgH gene rearrangements. Int Immunol. 1993;5:383–391. doi: 10.1093/intimm/5.4.383. [DOI] [PubMed] [Google Scholar]
- 70.Kurimasa A, Ouyang H, Dong L-J, Wang S, Li X, Cordon-Cardo C, Chen D J, Li G C. Catalytic subunit of DNA-dependent protein kinase: impact of lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:1403–1408. doi: 10.1073/pnas.96.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis S M. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv Immunol. 1994;56:27–149. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- 72.Lewis S M, Agard E, Suh S, Czyzyk L. Cryptic signals and the fidelity of V(D)J joining. Mol Cell Biol. 1997;17:3125–3136. doi: 10.1128/mcb.17.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li G C, Ouyang H, Li X, Nagasawa H, Little J B, Chen D J, Ling C C, Fuks Z, Cordon-Cardo C. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 74.Li Z, Otevrel T, Gao Y, Cheng H L, Seed B, Stamato T D, Taccioli G E, Alt F W. The XRCC4 gene encodes a novel protein involved in DNA double-strand break repair and V(D)J recombination. Cell. 1995;83:1079–1089. doi: 10.1016/0092-8674(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 75.Liao M J, Van Dyke T. Critical role for Atm in suppressing V(D)J recombination-driven thymic lymphoma. Genes Dev. 1999;13:1246–1250. doi: 10.1101/gad.13.10.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao M J, Zhang X X, Hill R, Gao J, Qumsiyeh M B, Nichols W, Van Dyke T. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol Cell Biol. 1998;18:3495–3501. doi: 10.1128/mcb.18.6.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lieber M R, Hesse J E, Lewis S, Bosma G C, Rosenberg N, Mizuuchi K, Bosma M J, Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 78.Lieber M R, Hesse J E, Mizuuchi K, Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987;1:751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- 79.Lieberman M, Hansteen G A, Waller E K, Weissman I L, Sen-Majumdar A. Unexpected effects of the severe combined immunodeficiency mutation on murine lymphomagenesis. J Exp Med. 1992;176:399–405. doi: 10.1084/jem.176.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lista F, Bertness V, Guidos C J, Danska J S, Kirsch I R. The absolute number of trans-rearrangements between the TCRγ and TCRβ loci is predictive of lymphoma risk: a severe combined immune deficiency (SCID) murine model. Cancer Res. 1997;57:4408–4413. [PubMed] [Google Scholar]
- 81.Livak F, Schatz D G. T cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol Cell Biol. 1996;16:609–618. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Livak F, Welsh S, Guidos C J, Crispe I N, Danska J S, Schatz D G. Transient restoration of gene rearrangement at multiple T cell receptor loci in γ-irradiated scid mice. J Exp Med. 1996;184:419–428. doi: 10.1084/jem.184.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Livak F, Wilson A, MacDonald H R, Schatz D G. Alpha beta lineage-committed thymocytes can be rescued by the gamma delta T cell receptor (TCR) in the absence of TCR beta chain. Eur J Immunol. 1997;27:2948–2958. doi: 10.1002/eji.1830271130. [DOI] [PubMed] [Google Scholar]
- 84.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 85.Mallick C, Dudley E, Viney J, Owen M, Hayday A. Rearrangement and diversity of T cell receptor β chain genes in thymocytes: a critical role for the β chain in development. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 86.Malynn B A, Blackwell T K, Fulop G M, Rathbun G A, Furley A J W, Ferrier P, Heinke L B, Phillips R A, Yancopolous G D, Alt F W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988;54:453. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- 87.Mombaerts P, Iacomini J, Johnson R, Herrup K, Tonegawa S, Papaioannou V. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 88.Murphy W J, Durum S K, Anver M R, Ferris D K, McVicar D W, O'Shea J J, Ruscetti S K, Smith M R, Young H A, Longo D L. Induction of T cell differentiation and lymphomagenesis in the thymus of mice with severe combined immune deficiency (SCID) J Immunol. 1994;153:1004–1014. [PubMed] [Google Scholar]
- 89.Nacht M, Jacks T. V(D)J recombination is not required for the development of lymphoma in p53-deficient mice. Cell Growth Differ. 1998;9:131–138. [PubMed] [Google Scholar]
- 90.Nacht M, Strasser A, Chan Y R, Harris A W, Schlissel M, Bronson R T, Jacks T. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev. 1996;10:2055–2066. doi: 10.1101/gad.10.16.2055. [DOI] [PubMed] [Google Scholar]
- 91.Nussenzweig A, Chen C, Costa Soares V, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 92.Nussenzweig A, Sokol K, Burgman P, Li L, Li G C. Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: the effects of ionizing radiation on growth, survival, and development. Proc Natl Acad Sci USA. 1997;94:13588–13593. doi: 10.1073/pnas.94.25.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oettinger M A. V(D)J recombination: on the cutting edge. Curr Opin Cell Biol. 1999;11:325–329. doi: 10.1016/S0955-0674(99)80044-1. [DOI] [PubMed] [Google Scholar]
- 94.Ouyang H, Nussenzweig A, Kurimasa A, da Costa Soares V, Li X, Cordon-Cardo C, Li W-H, Cheong N, Nussenzweig M, Iliakis G, Chen D J, Li G C. Ku70 is required for DNA repair but not for T cell antigen receptor recombination in vivo. J Exp Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Passoni L, Hoffman E S, Kim S, Crompton T, Pao W, Dong M Q, Owen M J, Hayday A C. Intrathymic delta selection events in gammadelta cell development. Immunity. 1997;7:83–95. doi: 10.1016/s1074-7613(00)80512-9. [DOI] [PubMed] [Google Scholar]
- 96.Paull T T, Gellert M. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- 97.Paull T T, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pennycook J L, Chang Y, Celler J, Phillips R A, Wu G E. High frequency of normal DJH joints in B cell progenitors in severe combined immune deficiency mice. J Exp Med. 1993;178:1007–1016. doi: 10.1084/jem.178.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pergola F, Zdzienicka M Z, Lieber M R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993;13:3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petiniot L K, Weaver Z, Barlow C, Shen R, Eckhaus M, Steinberg S M, Ried T, Wynshaw-Boris A, Hodes R J. Recombinase-activating gene (RAG) 2-mediated V(D)J recombination is not essential for tumorigenesis in Atm-deficient mice. Proc Natl Acad Sci USA. 2000;97:6664–6669. doi: 10.1073/pnas.97.12.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rabbitts T H. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 102.Ramsden D A, Gellert M. Formation and resolution of double-strand break intermediates in V(D)J rearrangement. Genes Dev. 1995;9:2409–2420. doi: 10.1101/gad.9.19.2409. [DOI] [PubMed] [Google Scholar]
- 103.Ramsden D A, Wu G E. Mouse κ light chain recombination signal sequences mediate recombination more frequently than do those of λ light chain. Proc Natl Acad Sci USA. 1991;88:10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697–700. doi: 10.1038/35015097. [DOI] [PubMed] [Google Scholar]
- 105.Roth D, Menetski J, Nakajima P, Bosma M, Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992;70:983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- 106.Schatz D G. V(D)J recombination moves in vitro. Semin Immunol. 1997;9:149–159. doi: 10.1006/smim.1997.0068. [DOI] [PubMed] [Google Scholar]
- 107.Schuler W, Ruetsch N R, Amster M, Bosma M J. Coding joint formation of endogenous T cell receptor genes in lymphoid cells from scid mice: unusual P-nucleotide additions in VJ-coding joints. Eur J Immunol. 1991;21:589–596. doi: 10.1002/eji.1830210309. [DOI] [PubMed] [Google Scholar]
- 108.Schuler W, Weiler I J, Schuler A, Phillips R A, Rosenberg N, Mak T W, Kearney J F, Perry R P, Bosma M J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986;46:963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- 109.Shinkai Y, Rathbun G, Lam K P, Oltz E, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A, Alt F. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 110.Shockett P E, Schatz D G. DNA hairpin opening mediated by the RAG1 and RAG2 proteins. Mol Cell Biol. 1999;19:4159–4166. doi: 10.1128/mcb.19.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shores E, Nakayama T, Wiest D, Takahama Y, Sharrow S, Singer A. Structurally distinct T cell receptor complexes on developmentally distinct T cell populations in severe combined immunodeficiency mice expressing a TCRβ transgene. J Immunol. 1993;150:1263–1275. [PubMed] [Google Scholar]
- 112.Song Q, Burrows S R, Smith G, Lees-Miller S P, Kumar S, Chan D W, Trapani J A, Alnemri E, Litwack G, Lu H, Moss D J, Jackson S, Lavin M F. Interleukin-1 beta-converting enzyme-like protease cleaves DNA-dependent protein kinase in cytotoxic T cell killing. J Exp Med. 1996;184:619–626. doi: 10.1084/jem.184.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song Q, Lees-Miller S P, Kumar S, Zhang Z, Chan D W, Smith G C, Jackson S P, Alnemri E S, Litwack G, Khanna K K, Lavin M F. DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 1996;15:3238–3246. [PMC free article] [PubMed] [Google Scholar]
- 114.Taccioli G E, Amatucci A G, Beamish H J, Gell D, Xiang X H, Torres Arzayus M I, Priestley A, Jackson S P, Marshak Rothstein A, Jeggo P A, Herrera V L. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity. 1998;9:355–366. doi: 10.1016/s1074-7613(00)80618-4. [DOI] [PubMed] [Google Scholar]
- 115.Taccioli G E, Cheng H-L, Varghese A J, Whitmore G, Alt F W. A DNA repair defect in Chinese hamster ovary cells affects V(D)J recombination similarly to the murine scid mutation. J Biol Chem. 1994;269:7439–7442. [PubMed] [Google Scholar]
- 116.Taccioli G E, Rathbun G, Oltz E, Starnato T, Jeggo P A, Alt F W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- 117.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 118.Vaux D L, Cory S, Adams J M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 119.von Boehmer H, Fehling H J. Structure and function of the pre-T cell receptor. Annu Rev Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 120.Wang C, Bogue M A, Nguyen A P, Roth D B. Irradiation-induced rescue of thymocyte differentiation and V(D)J recombination in mice lacking the catalytic subunit of DNA-dependent protein kinase. J Immunol. 1999;163:6065–6071. [PubMed] [Google Scholar]
- 121.Zhu C, Bogue M A, Lim D, Hasty P, Roth D B. Ku86-deficient mice exhibit severe combined immune deficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 122.Zhu C, Roth D B. Characterization of coding ends in thymocytes of scid mice: implications for the mechanism of V(D)J recombination. Immunity. 1995;2:101–112. doi: 10.1016/1074-7613(95)90082-9. [DOI] [PubMed] [Google Scholar]