Abstract
Treatment resistant depression (TRD) is associated with poor outcomes, but a consensus is lacking in the literature regarding which compound represents the best pharmacological augmentation strategy to antidepressants (AD). In the present review, we identify the available literature regarding the pharmacological augmentation to AD in TRD. Research in the main psychiatric databases was performed (PubMed, ISI Web of Knowledge, PsychInfo). Only original articles in English with the main topic being pharmacological augmentation in TRD and presenting a precise definition of TRD were included. Aripiprazole and lithium were the most investigated molecules, and aripiprazole presented the strongest evidence of efficacy. Moreover, olanzapine, quetiapine, cariprazine, risperidone, and ziprasidone showed positive results but to a lesser extent. Brexpiprazole and intranasal esketamine need further study in real-world practice. Intravenous ketamine presented an evincible AD effect in the short-term. The efficacy of adjunctive ADs, antiepileptic drugs, psychostimulants, pramipexole, ropinirole, acetyl-salicylic acid, metyrapone, reserpine, testosterone, T3/T4, naltrexone, SAMe, and zinc cannot be precisely estimated in light of the limited available data. Studies on lamotrigine and pindolol reported negative results. According to our results, aripiprazole and lithium may be considered by clinicians as potential effective augmentative strategies in TRD, although the data regarding lithium are somewhat controversial. Reliable conclusions about the other molecules cannot be drawn. Further controlled comparative studies, standardized in terms of design, doses, and duration of the augmentative treatments, are needed to formulate definitive conclusions.
Keywords: augmentation, treatment-resistant depression, psychopharmacology, review
1. Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders and is associated with work/social dysfunction, accounting for a significant financial burden worldwide [1]. Among individuals receiving an adequate pharmacological treatment, only 30% reach a full symptom recovery. The remaining 70% of MDD patients will experience either a pharmacological response without remission or no response at all [2], thus configuring treatment resistant depression (TRD).
TRD is a complex phenomenon, influenced by different factors, including advanced age, comorbid psychiatric disorders, and medical illness [3]. There is no consensus on the definition of TRD. According to the European Medicine Agency, TRD patients are those who fail to show clinically meaningful improvement after treatment with at least two antidepressant (AD) agents of different classes, prescribed in adequate dosages for an appropriate period of time and with adequate affirmation of treatment adherence [4]. This definition is in agreement with the World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines [5] about unipolar depressive disorder. Of note, Thase and Rush [6] proposed a classification of TRD staging, which was cited in several studies and is currently accepted in the literature on this topic. However, a recent systematic review by Brown and collaborators [7] highlighted that only 48.4% of the published literature specified TRD as the failure of at least two treatments. Despite the assessment of TRD having improved over the last few years, the lack of consensus may limit the ability of researchers to generalize the findings on this topic.
In this regard, different strategies, including maximizing the initial treatment regimen, switching to another AD, or initiating a combination or augmentation therapy, were extensively utilized in order to enhance suboptimal responses to ADs [8]. However, there is a lack of agreement regarding the best option or the order that these different strategies ought to be used [9].
Despite the lack of consensus about the best treatment option, TRD is associated with social, occupational, and physical impairment, as well as high rates of mortality related to suicidal behaviors. Compared to non-resistant MDD, TRD is associated with a substantial increase in direct and indirect medical care costs, mainly due to recurrence and long hospitalizations [10]. In this sense, the identification of the most effective pharmacological strategies for TRD treatment may help the clinician prescribe an augmentative compound to AD early.
In light of these considerations, the purpose of the present review is to update the available data regarding the efficacy of different pharmacological strategies as augmentation in TRD in order to guide the clinician through the complex choice of the most adequate pharmacological treatments.
2. Materials and Methods
A search in the main psychiatric databases was performed (PubMed, ISI Web of Knowledge, PsychInfo). Original articles in English language from 1956 to 30 April 2020, with available abstract and full texts, were included.
The search was performed using the keywords “augmentation major depression”. At least two authors, who subsequently checked and extracted data from included articles, carried out the selection of appropriate papers. The first selection was performed considering the pertinence of the title and the information given in the abstract; the second selection was made after accurate reading of the research methods in the full-text.
Inclusion criteria were: (1) original articles; (2) mean age of patients over 18 years; (3) reported diagnosis of unipolar major depressive disorder [11] resistant to AD treatments; (4) clear definition of the treatment resistance using criteria widely accepted in literature (no response to one or two AD trials at adequate dosages and for adequate period of time; reduction of psychometric scores ≤ 50%); (5) mood improvement as primary outcome; (6) topic of the article focused on pharmacological augmentation in TRD.
Exclusion criteria were: (1) reviews, meta-analyses, commentaries, letters, case reports, pooled analyses, comments, case studies, study protocols; (2) mean age of the subjects under 18 years; (3) studies with combined treatments (augmentation is defined as the addition of a new compound to previous antidepressant); (4) mixed or not accurately described diagnoses (e.g., mixed group with bipolar and unipolar depression, anxiety), unless data were available for the subgroup of unipolar patients; (5) not accurately defined treatment resistance; (6) studies conducted on animals; (7) non-pharmacological augmentation (e.g., psychotherapies or neuromodulation techniques); (8) articles written not in English language. This section may be divided by subheadings.
3. Results
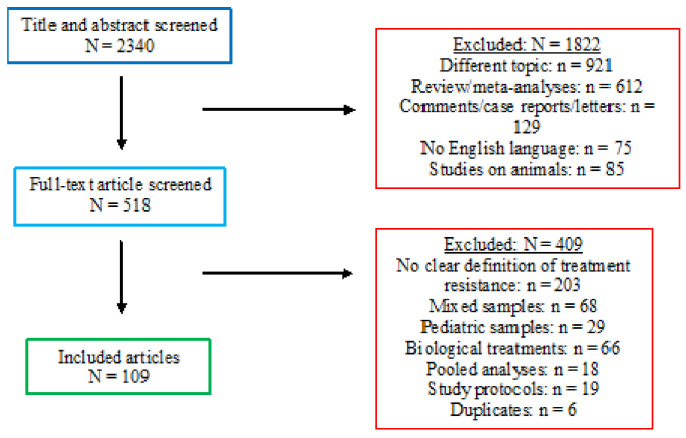
The search of all the databases provided a total of 1499 citations. Among these, 44 were identified as duplicates. Four-hundred-ninety studies were discarded because, after reviewing the abstracts, the papers dealt with another topic, and 52 studies were discarded because they were written in a different language. Reviews and meta-analyses (n = 433), as well as comments, case reports, and letters (n = 94), were excluded. Moreover, 31 papers were discarded because the studies were conducted on animals and 13 because a paediatric population was examined.
The full texts of the remaining 342 citations were examined in more detail, concluding that 235 studies did not meet the inclusion criteria because: one-hundred-twenty-four did not accurately define treatment resistance; forty-nine studies included mixed samples; forty-one articles were about neuromodulation; thirteen were pooled analyses; four were study protocols; four were duplicates.
One-hundred-seven studies met the inclusion criteria and were included in the present review (Figure 1).
Figure 1.
PRISMA flow-chart for systematic reviews.
3.1. Other Antidepressants and Buspirone
The pharmacological augmentation with other ADs, such as selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants (TCA), in TRD has been poorly investigated until now, additionally in light of the synergic effect and the concrete risk of adverse events [12]. Altamura and his group [13] published a randomized placebo-controlled study (RCT) demonstrating the efficacy of augmentative low-dose intravenous (IV) citalopram to oral SSRIs in 36 non-responders affected by MDD. Similarly, an open-label study reported the effectiveness of buspirone augmentation in patients with a poor response to SSRIs [14], and Taylor and Prather [15] demonstrated the effectiveness of augmentative nefazodone to ADs in a small group of 11 patients with TRD and presenting high levels of anxiety.
On the other hand, in a large double-blind RCT conducted by Licht and Qvitzau [16], the authors demonstrated that sertraline monotherapy at 100 mg/day has similar effects to a mianserin add-on, and, in a more recent RCT, mirtazapine did not show superiority over a placebo in improving the depressive symptoms in a large sample of TRD patients (n = 431) [17]. A description of the cited studies is reported in Table 1.
Table 1.
Summary of the studies about augmentation with other ADs and buspirone in TRD.
| Reference | n | Age (Years) | Design | Augmentation Molecule | Dosage | AD | Duration | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Altamura et al. [13] | 22 | 18–65 | Single-blind Comparison IV CIT/pcb | Citalopram | 10 mg/d (IV) | Paroxetine, Sertraline or Escitalopram | 5 days | HAM-D, MADRS | ↓ HAM-D: CIT > pcb (p < 0.01) ↓ MADRS: CIT > pcb (p < 0.05) |
| Licht and Qvitzau [16] | 253 | Mean (SD): 39 (±12) | Multicenter, double-blind Comparison SER100 + pcb/SER200 + pcb/SER100 + MIA | Mianserin | 30 mg/d | Sertraline | 5 weeks | HAM-D | ↓ HAM-D: - SER100 = SER100 + MIA (p = 0.85) - Both > SER200 (p < 0.05) |
| Kessler et al. [17] | 431 | 18–65 | Multicenter, double-blind Comparison mirtazapine/pcb | Mirtazapine | 30 mg/d | SSRIs or SNRIs | 1 year | BDI-II at week 12 | ↓ BDI-II: mirtazapine = pcb (p = 0.09) |
| Altamura et al. [18] | 54 | 18–65 | Head-to-head Single-blind Comparison IV CIT/CLO/pcb | Citalopram/clomipramine | CIT: 10 mg/d CLO: 25 mg/d | SSRIs | 5 days | HAM-D melancholy and anxiety-somatization scores | ↓ HAM-D: - CIT and CLO > pcb (p < 0.01) CIT = CLO on melancholy (p = 0.73) CIT > CLO on anxiety/somatization (p = 0.027) |
| Fava et al. [19] | 41 | Mean (SD): 39.6 (±9.9) | Head-to-head Double-blind Comparison FLU/FLU + Li/FLU + DES | Desipramine | FLU: 40–60 mg/d FLU 20 mg/d + Li: 300–600 mg/d FLU 20 mg/d + DES: 25–50 mg/d | Fluoxetine | 4 weeks | HAM-D | ↓ HAM-D: Whole sample: FLU > FLU + Li/FLU + DES (p = 0.05) Non-responders: FLU/FLU + Li > FLU + DES (p = 0.04) |
| Fava et al. [20] | 101 | Mean (SD): 41.6 (±10.6) | Head-to-head Double-blind Comparison FLU/FLU + Li/FLU + DES/pcb | Desipramine | ≈ | Fluoxetine | 4 weeks | HAM-D | FLU = FLU + Li = FLU + DES = pcb (p = 0.20) |
| Open studies | |||||||||
| Joffe and Schuller [14] | 25 | Range: 22–56 Mean: 40.2 | Open-label | Buspirone | Range: 20–50 mg/d Mean: 31.2 mg/d | Fluoxetine or Fluvoxamine | 3 weeks | CGI | Response rate: 68% Remission rate: 32% |
| Taylor and Prather [15] | 11 | Mean (SD): 53 (±10.5) | Open-label | Nefazodone | 50–300 mg/d Mean (SD): 200 (±134.2) mg/d | Various ADs | 9 months | Presence/absence anxiety/depression symptoms CGI | Depression remission (p < 0.005) Anxiety remission (p < 0.0005) |
| Navarro et al. [21] | 104 | Mean (SD): 47.9 (±8.4) | Head-to-head Open-label Comparison CIT/Li | Citalopram | CIT: 30 mg/d Li: 600 mg/d | Imipramine | 10 weeks | HAM-D | Remission: CIT > Li (p = 0.034) ↓ HAM-D: CIT > Li (p = 0.005) |
Key: ≈ = same as above; AD = antidepressant; BDI-II = Beck Depression Inventory—II; CGI = Clinical Global Impression; CIT = citalopram; CLO = clomipramine; d = day; DES = desipramine; FLU = fluoxetine; HAM-D = Hamilton Depression Rating Scale; IV = intravenous; Li = lithium; MADRS = Montgomery and Åsberg Depression Rating Scale; MIA = mianserin; pcb = placebo; RCT = randomized controlled trial; SD = standard deviation; SER = sertraline; SER100 = sertraline at 100 mg/d; SER200 = sertraline at 200 mg/d; SNRI = Serotonin and Norepinephrine Reuptake Inhibitor; SSRI = Selective Serotonin Reuptake Inhibitor.
Head-to-Head Studies
It has been demonstrated that short-term augmentation with low-dose IV citalopram or clomipramine is more effective than augmentation with a placebo in 54 patients with no response to SSRIs [18].
Three studies compared the efficacy of continuation with AD monotherapy versus augmentation with TCA/SSRIs or lithium. Fava and collaborators [19,20] published two double-blind studies including fluoxetine-resistant MDD individuals who were randomly assigned to higher dosages of fluoxetine, fluoxetine plus desipramine, or fluoxetine plus lithium. In the first study, including 41 subjects, high-dose fluoxetine was more efficacious than augmentative treatments. In contrast, the subsequent study (n = 101) showed no significant differences in the response rates across the three treatment groups. Finally, a recent randomized open-label study showed a higher percentage of response in favor of add-on citalopram with respect to add-on lithium in 104 MDD individuals treated unsuccessfully for 10 weeks with imipramine [21] (Table 1).
3.2. Second Generation Antipsychotics (SGAs)
3.2.1. Aripiprazole
Aripiprazole was the most investigated molecule among SGAs. A first retrospective chart review [22] showed the effectiveness of augmentative aripiprazole in 30 TRD patients. A more recent retrospective study reported significant improvements in both the Montgomery and Åsberg Depression Rating Scale (MADRS) and Young Mania Rating Scale (YMRS) total scores in 38 TRD patients, presenting a mixed specifier according to the Diagnostic and Statistical Manual of Mental Disorders—5th edition (DSM-5) [23].
Eight open-label studies reported positive results. The authors of two papers [24,25], involving a maximum of 15 TRD patients, demonstrated the effectiveness of aripiprazole in augmentation to AD treatment. These results were confirmed in the long-term in a large sample [26]. Fabrazzo and colleagues [27] demonstrated that a fixed dose of aripiprazole (5 mg/day) was effective in augmentation to TCA in 35 TRD patients. Adjunctive aripiprazole was equally effective when added to paroxetine or sertraline in 24 MDD patients [28], and low-dose augmentative aripiprazole also demonstrated its effectiveness in 9 TRD Taiwanese patients [29]. A 12-week prospective open-label multicenter study reported a significant amelioration of depressive symptoms with augmentative aripiprazole as shown by an endpoint response rate higher than 50% and a remission rate of about 40% [30]. Recently, Horikoshi and his group [31] published a randomized open-label study demonstrating the effectiveness of both low-dose (LD; 3 mg/day) and high-dose (HD; 12 mg/day) aripiprazole augmentation in TRD, with a statistically significant difference in terms of earliness of response in the HD versus LD group.
Three multicenter RCTs, conducted by the same research group [32,33,34], demonstrated the efficacy of the molecule in large samples (n = 362; n = 324; n = 349, respectively) of TRD patients. In contrast, an RCT [35] failed to find the superiority of augmentative low-dose aripiprazole (2–5 mg/day) versus a placebo. This latter sample (n = 221) was re-analyzed and the results were reported in two other papers. The first one [36] showed that a modest percentage of non-responders to 2 mg/day ameliorated with aripiprazole 5 mg/day, while the second [37] demonstrated a statistically significant improvement on the depression subscale of the Kellner Symptom Questionnaire but without statistically significant effects on the anxiety and hostility subscales. A further RCT was conducted on 181 patients suffering from late-life depression, and it demonstrated the efficacy of a maximum 15 mg/day augmentative dose of aripiprazole [38]. Finally, a Japanese research group carried out the aripiprazole depression multicenter efficacy (ADMIRE) study randomizing 540 patients to a placebo, fixed-dose (3 mg/d), or flexible-dose (3–15 mg/d) of aripiprazole. The groups with active treatment showed more improvement in depressive symptoms than the placebo [39], as also confirmed by a sub-analysis on the core depressive symptoms [40].
3.2.2. Other SGAs
Promising results were reported for brexpiprazole, a molecule with some pharmacological similarities to aripiprazole. Two RCTs conducted on large samples by Thase and his group [41,42] demonstrated the superiority of brexpiprazole over a placebo at the highest dosages of 2 or 3 mg/day, but not at 1 mg/day. More recently, an open-label study with 51 TRD patients who had not responded to previous augmentative treatment demonstrated a significant reduction in the MADRS scores with brexpiprazole augmentation independently from the prior augmentative strategy [43]. One double-blind RCT confirmed that brexpiprazole was better than a placebo in reducing the MADRS scores among 393 TRD patients [44].
The results from a three-phase study, conducted on 489 patients, demonstrated that augmentative risperidone ameliorated depressive symptoms in the short period, but it was not more efficacious than a placebo in the long-term [45]. A sub-analysis of this latter sample showed the significant amelioration of depression with augmentative risperidone in 89 elderly patients who had not responded to citalopram [46]. A further RCT by Mahmoud and colleagues [47] reported the statistically significant improvement in the depressive symptoms in patients treated with risperidone compared to a placebo.
In one open-label study conducted on 20 TRD patients, Papakostas and his group [48] demonstrated the effectiveness of ziprasidone in augmentation to SSRIs.
Two open-label small sample studies suggested beneficial effects of quetiapine as augmentation treatment in TRD [49,50].
The augmentation of fluoxetine with olanzapine showed to be more efficacious than olanzapine or fluoxetine monotherapy in a double-blind small sample RCT [51]. These results were similar to those reported in an open-label study assessing the effectiveness of augmentative olanzapine in 11 TRD patients treated with milnacipran [52].
Finally, a recent RCT (n = 530) failed to prove the efficacy of augmentation with cariprazine in patients with MDD and inadequate previous response to antidepressants [53].
A description of the studies regarding SGA augmentation in TRD is reported in Table 2.
Table 2.
Summary of the studies about augmentation with SGAs in TRD.
| Reference | n | Age Mean (SD), y | Design | Augmentation Molecule | Dosage, mg/d | AD | Duration | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Berman et al. [32] | 362 | 46.5 (±10.6) | Multicenter, double-blind | Aripiprazole | 2–20 Mean: 11.8 | Escitalopram, fluoxetine, paroxetine, sertraline, or venlafaxine | 6 weeks | MADRS | ↓ MADRS: ARI > pcb (p < 0.001) |
| Berman et al. [34] | 349 | 45.4 (±10.9) | Multicenter, double-blind | Aripiprazole | 2–20 Mean: 10.7 | ≈ | 6 weeks | MADRS | ↓ MADRS: ARI > pcb (p < 0.001) |
| Fava et al. [35] | 221 | 45.4 (±10.3) | Multicenter, double-blind Phase I: ARI 2 mg/d/pcb Phase II: ARI 5 mg/d/pcb/ARI 2 mg/d | Aripiprazole | 2 or 5 | SSRIs or SNRIs | 12 weeks | MADRS | ↓ MADRS: ARI = pcb (p = 0.06) Response rate: ARI = pcb (p = 0.18) Remission rate: ARI = pcb (p = 0.50) |
| Mischoulon et al. [36] * | ≈ | ≈ | ≈ | Aripiprazole | ≈ | ≈ | ≈ | MADRS | ↓ MADRS: ARI 5 mg/d > ARI 2 mg/d (p < 0.0001) |
| Dording et al. [37] * | ≈ | ≈ | ≈ | Aripiprazole | ≈ | ≈ | ≈ | KSQ | ↓ KSQ depressive subscale: ARI > pcb (p = 0.03) ↓ KSQ anxiety, somatic, anger-hostility subscales: ARI = pcb (p > 0.05) |
| Kamijima et al. “ADMIRE” [39] | 540 | Fixed-ARI: 39.2 (±9.1) Flexi-ARI: 38.1 (±9.6) Pcb: 38.7 (±9.2) | Multicenter, double-blind Comparison fixed-ARI/flexi-ARI/pcb | Aripiprazole | Fixed-ARI: 3 Flexi-ARI: 3–15 (mean: 9.8) | Sertraline, fluvoxamine, paroxetine, milnacipran, duloxetine | 6 weeks | MADRS | ↓ MADRS: fixed-ARI = flexi-ARI > pcb (p < 0.01) Response and remission rates: fixed-ARI = flexi-ARI > pcb (p < 0.05) |
| Ozaki et al. [40] ** | ≈ | ≈ | Subgroup analysis of ADMIRE according to: sex, age, number of AD trials, MDD diagnosis, number of depressive episodes, duration of current episode, age at first episode, time since first episode, type of SSRI/SNRI, severity at the end of AD treatment | Aripiprazole | ≈ | ≈ | ≈ | MADRS in subgroups; MADRS and HAM-D single items | No interaction effects of treatment and subgroups; ↓ core depressive symptoms (p < 0.05) |
| Lenze et al. [38] | 181 | 66 27% of the total sample > 70 | Double-blind | Aripiprazole | 2–15 Mean remitters: 7 Mean non-remitters: 10 | Venlafaxine | 12 weeks | MADRS | Remission rate: ARI > pcb (p = 0.03) |
| Marcus et al. [33] | 324 | 44.6 (±11.0) | Multicenter, double-blind | Aripiprazole | Mean: 11 | Escitalopram, fluoxetine, paroxetine, sertraline, or venlafaxine | 6 weeks | MADRS | ↓ MADRS: ARI > pcb (p = 0.001) (d = 0.35) Remission rates: ARI > pcb (p = 0.016) Response rates: ARI > pcb (p < 0.001) |
| Hobart et al. [44] | 393 | BREX: 43.0 (±12.7) pcb: 42.7 (±12.5) | Double-blind | Brexpiprazole | 2 | SSRIs or SNRIs | 6 weeks | MADRS | ↓ MADRS: BREX > pcb (p = 0.007) |
| Hobart et al. | 443 | BREX: 43.6 (±11.5) QUE-XR: 44.6 (±11.6) pcb: 41.8 (±11.7) | Head-to-head Multicenter, double-blind Comparison BREX/QUE-XR/pcb | Brexpiprazole Quetiapine | BREX: Range: 2–3 Mean: 2.2 QUE-XR: Range: 150–300Mean: 198.5 | SSRIs or SNRIs | 6 weeks | MADRS | ↓ MADRS: BREX > pcb (p = 0.008) QUE-XR = pcb (p = 0.66) |
| Thase et al. [41] | 353 | BREX: 44.1 (±11.6) pcb: 45.2 (±11.3) | Multicenter, double-blind | Brexpiprazole | 2 | SSRIs or SNRIs | 6 weeks | MADRS | ↓ MADRS: BREX > pcb (p < 0.001) |
| Thase et al. [42] | 627 | BREX-1: 45.7 (±11.6) BREX-3: 44.5 (±11.2) pcb: 46.6 (±11.0) | Multicenter, double-blind Comparison BREX-1/BREX-3/pcb | Brexpiprazole | 1 or 3 | SSRIs or SNRIs | 6 weeks | MADRS | ↓ MADRS: BREX-3 > pcb (p = 0.008) BREX-1 = pcb (p = 0.07) |
| Earley et al. [53] | 435 | 44.2 (±11.6) | Double-blind | Cariprazine | 1.5–4.5 Mean: 2.97 | Various ADs | 8 weeks | MADRS | ↓ MADRS: CARI = pcb (p = 0.79) |
| Shelton et al. [51] | 28 | 42.0 (±11.0) | 6-week open-label fluoxetine in escalating doses; 8-week, double-blind, RCT: Comparison OLA + pcb/FLU + pcb/FLU + OLA | Olanzapine | Mean (SD) OLA + pcb: 12.5 (±5.3) Mean (SD) FLU + OLA: 13.5 (±4.1) | Fluoxetine | 8 weeks | MADRS, HAM-D | ↓ MADRS: FLU + OLA > OLA + pcb (p = 0.03) and FLU + pcb (p = 0.006) ↓ HAM-D: FLU + OLA > OLA + pcb (p = 0.03) FLU + OLA = FLU + pcb (p = 0.07) |
| Mahmoud et al. [47] | 268 | 45.9 (±10.1) | Multicenter, double-blind | Risperidone | 1–2 | Various ADs | 6 weeks | HAM-D | ↓ HAM-D: RIS > pcb (p < 0.001) Response rates: RIS > pcb (p = 0.004) Remission rates: RIS > pcb (p = 0.004) |
| Rapaport et al. [45] | Phase I: 445 Phase II: 348 Phase III: 241 | 46.3 (±12.6) | Phase I: 4–6 weeks open-label CIT monotherapy Phase II: 4–6 weeks open-label RIS augmentation Phase III: 24 weeks double-blind maintenance phase, comparison CIT + RIS/CIT + pcb |
Risperidone | Mean (SD): Phase II: 1.1 (±0.6) Phase III: 1.2 (±0.6) | Citalopram | (see Design) | Phases II: MADRS Phase III: time to relapse | ↓ MADRS (p < 0.001) Time to relapse: RIS > pcb (p = 0.05) Relapse rates: RIS < pcb (p = 0.05) |
| Alexopoulos et al. [46] *** | Phase I: 108 Phase II: 93 Phase III: 63 | 63.4 (±7.9) | ≈ | Risperidone | Mean (SD): Phase II: 0.7 (±0.3) Phase III: 0.8 (±0.3) | ≈ | ≈ | ≈ | ↓ MADRS (p < 0.001) Time to relapse: RIS > pcb (p = 0.07) |
| Open studies | |||||||||
| Barbee et al. [22] | 19 | 51.2 (±8.9) | Retrospective chart review | Aripiprazole | 2.5–7.5 | Various ADs | 6 weeks | CGI | (Very) Much improved: 52.6% Mildly improved: 26.3% Unchanged: 15.8% Minimally worse: 5.3% (p < 0.001) |
| Berman et al. [26] | 987 | 45.8 (±11.3) | Open-label | Aripiprazole | 2–30 Mean:10.1 | Various ADs | 52 weeks | CGI severity | CGI-S = 1 (not at all ill) or 2 (borderline ill): 69.7% |
| Chen et al. [29] | 9 | 38.3 (±12.2) | Open-label | Aripiprazole | Mean: 4.2 | Various ADs | 4 weeks | HAM-D | Response rate: 100% Remission rate 77.8% |
| Fabrazzo et al. [27] | 35 | 38.8 (±11.5) | Open-label | Aripiprazole | 5 | SSRIs, then CLO | 24 weeks | HAM-D | ↓ HAM-D (p < 0.0001) Response rate: 91.4% Remission rate: 34.3% |
| Han et al. [23] | 38 | 28.4 (±11.3) | Retrospective MDD with mixed specifier | Aripiprazole | Mean (SD): 4.0 (±0.8) | Various ADs | 8 weeks | MADRS | ↓ MADRS (p < 0.0001) Response rate: 32% Remission rate: 21% |
| Hellerstein et al. [25] | 14 | 46.1 (±13.0) | Open-label | Aripiprazole | Mean (SD): 22.5 (±9.9) | Sertraline, fluoxetine, duloxetine, venlafaxine | 12 weeks | HAM-D | ↓ HAM-D (p < 0.001) Response rate: 50% Remission rate: 28.6% |
| Horikoshi et al. [31] | 31 | LD group: 38.8 (±12.8) HD group: 44.2 (±13.9) | Open-label, R Comparison LD-ARI/HD-ARI | Aripiprazole | LD: 3 HD: 12 | Various ADs | 6 weeks | MADRS | ↓ MADRS: LD and HD (p < 0.001) Response rate: HD > LD (p = 0.015) |
| Jon et al. [30] | 86 | 45.6 (±13.7) | Multicenter, prospective, open-label | Aripiprazole | Max: 15 Mean: 6.9 | SSRIs or SNRIs | 6 weeks | MADRS | ↓ MADRS (p < 0.001) Response rate 52.3% Remission rate 39.8% |
| Patkar et al. [24] | 10 | 44.9 (±12.2) | Prospective, open-label | Aripiprazole | 10–30 Mean: 13.2 | Various ADs | 6 weeks | HAM-D | ↓ HAM-D (p < 0.001) Response rate: 70% Remission rate: 30% |
| Yoshimura et al. [28] | 24 | 39.0 (±12.0) | Open-label | Aripiprazole | Mean (SD) PAR group: 8.73 (±3.13) Mean (SD) SER group: 9.23 (±3.11) | Paroxetine or sertraline | 4 weeks | HAM-D | ↓ HAM-D (p < 0.0001) PAR + ARI = SER + ARI (p = 0.80) |
| Fava et al. [43] | 51 | 45.6 (±12.4) | Multicenter, open-label | Brexpiprazole | 2.25 (±0.74) | SSRIs or SNRIs | 6 weeks | MADRS | ↓ MADRS (p < 0.0001) |
| Boku et al. [52] | 7 | 53.2 (±24.0) | Open-label | Olanzapine | Mean (SD): 5.0 (±1.9) | Milnacipran | 8 weeks | HAM-D, CGI | ↓ HAM-D (p < 0.01) Response rate: 100% Remission rate: 100% ↓ CGI (p < 0.01) |
| Anderson et al. [50] | 18 | 46.3 | Open-label | Quetiapine | Max 300 mg twice Mean (SD): 245.0 (±68.0) | Various ADs | 8 weeks | MADRS | ↓ MADRS (p < 0.001) Response rate: 29% Remission rate: 17% |
| Sagud et al. [49] | 14 | 52.8 (±10.4) | Prospective, non-comparative, open-label | Quetiapine | Mean (SD): 315.0 (±109.0) | Various ADs | 20 weeks | HAM-D total score and insomnia/depressive mood/anxiety subscales | ↓ HAM-D total score (p < 0.001) ↓ HAM-D subscales (p < 0.001) |
| Papakostas et al. [48] | 13 | 41.9 (±10.1) | Open-label | Ziprasidone | Mean (SD): 82.1 (±48.9) | SSRIs | 6 weeks | HAM-D | Response rate: 61.5% Remission rate: 38.5% |
| Bauer et al. [54] | 557 | 18–65 | Head-to-head Open-label, R Comparison add-on QUE-XR/QUE-XR monotherapy/add-on LIT | Quetiapine | Mean (SD): add-on QUE-XR: 242.0 (±54.0) QUE-XR monotherapy: 238.0 (±60.0) add-on LIT: 882.0 (±212.0) | SSRIs or venlafaxine | 6 weeks | MADRS | ↓ MADRS: add-on QUE-XR = QUE-XR monotherapy = add-on LIT (p = 0.05) |
| Gobbi et al. | 86 | 49.9 (±13.3) | Head-to-head Naturalistic Comparison SGAs (ARI, OLA, RIS, QUE)/another AD | Aripiprazole Olanzapine Risperidone Quetiapine | Mean (SD): ARI 4.4 (±1.3) OLA 8.7 (±1.8) RIS 1.88 (±0.5) QUE 129.0 (±29.0) ADs at various dosages | SSRIs or SNRIs | 3 months | MADRS, HAM-D, QIDS, CGI | ↓ MADRS, HAM-D, QIDS, CGI scores from baseline to endpoint in both SGA and ADs groups (p < 0.001)↓ depressive symptoms MADRS and HAM-D: SGA > ADs (p < 0.05) |
| Mohamed et al. | 1137 | 54.4 (±12.2) | Head-to-head, R Comparison add-on ARI/add-on BUP/BUP switching | Aripiprazole | ARI max 10 BUP max 400 | Various ADs | 12 weeks | Remission at QIDS | Add-on ARI > BUP switching (p = 0.02) Add-on ARI = add-on BUP (p = 0.47) Add-on BUP = BUP switching (p = 0.09) |
Key: * = reanalysis on the sample recruited by Fava et al. [35]. ** = subgroup analysis of “ADMIRE” by Kamijima et al. [39]. *** = subgroup analysis on the patients over 55 of the total sample recruited by Rapaport et al. [45]. ≈ = same as above. AD = antidepressant; ARI = aripiprazole; BREX = brexpiprazole; BREX-1 = brexpiprazole 1 mg/d; BREX-3 = brexpiprazole 3 mg/d; BUP = bupropion; CARI = cariprazine; CGI = Clinical Global Impression; CIT = citalopram; CLO = clomipramine; FLU = fluoxetine; HAM-D = Hamilton Depression Rating Scale; HD = high-dose; KSQ = Kellner Symptom Questionnaire; LD = low-dose; LIT = lithium; MADRS = Montgomery and Asberg Depression Rating Scale; MDD = major depressive disorder; OLA = olanzapine; PAR = paroxetine; pcb = placebo; QUE (XR) = quetiapine (extended release); QIDS = Quick Inventory of Depressive Symptomatology; R = randomized; RCT = randomized controlled trial; RIS = risperidone; SER = sertraline; SGA = Second generation antipsychotic; SNRI = Serotonin and Norepinephrine Reuptake Inhibitor; SSRI = Selective Serotonin Reuptake Inhibitor; TCA = tricyclic antidepressants.
3.2.3. Head-to-Head Studies
No significant differences in terms of effectiveness were found between quetiapine extended release (XR) at 300 mg/day and lithium as add-on therapies [54] in 557 TRD patients. Furthermore, brexpiprazole, but not quetiapine, resulted to be superior to a placebo in a further double-blind RCT study [55].
Gobbi and collaborators [56] reported that both the augmentation with another AD or with a SGA (olanzapine, risperidone, quetiapine, aripiprazole) improved depressive symptoms over time; hovewer, SGAs performed better than Ads (Table 2).
3.2.4. Switch versus Augmentation
In a randomized study by Mohamed and co-authors [57], aripiprazole augmentation was significantly more effective than switching to bupropion monotherapy in terms of remission rates, while the difference regarding the augmentation strategy for both the compounds did not result to be statistically significant (Table 2).
3.3. Mood Stabilizers
3.3.1. Lithium
Among the treatment strategies for patients with TRD, the add-on of lithium to an ongoing AD therapy is one of the most widely studied treatment options.
A first double-blind RCT with 34 tricyclic-resistant depressed patients [58] reported that higher range doses of lithium (750 mg/day), but not low ones (250 mg/day), may have a significant AD effect. In contrast, a subsequent study on 92 TRD subjects found no significant difference between lithium and placebo augmentation in 35 subjects resistant to nortriptyline treatment [59].
One RCT, evaluating the efficacy of lithium augmentation in the 4-month continuation treatment of 27 TRD patients, showed that relapses occurred only in the placebo group, thus suggesting that patients responding to lithium augmentation should continue the treatment for a minimum of 6 months or even longer [60]. Two years later, Bschor and collaborators [61] studied 22 individuals from the same sample, finding no significant differences between lithium and placebo in terms of 1-year recurrence rates and concluding that lithium should be maintained for at least 1 year after reaching response to lithium augmentation in TRD patients.
Most of the open-label studies reported promising results for lithium augmentation in TRD subjects. Thase and collaborators [62] demonstrated its effectiveness in 20 non-responders to imipramine and psychotherapy. A subsequent open-label study reported a significant improvement in 13 out of 20 patients suffering from MDD resistant to desipramine [63], and, in the same year, Fontaine and colleagues [64] showed the effectiveness of lithium augmentation in 60 outpatients with MDD resistant to desipramine or fluoxetine. Subsequently, an open-label dose-response study with 11 sertraline-resistant MDD subjects demonstrated that most patients responded within 1 week, but the degree of response was not associated with lithium plasma levels [65]. Some years later, an open-label study with 22 depressed outpatients not responding to venlafaxine showed that eight of them responded and two reached remission [66]. Similarly, another open-label study, carried out on 13 MDD patients not responding to venlafaxine, reported that 38.5% of the total sample achieved a response with lithium augmentation [67].
Two open-label studies [68,69] failed to find a significant improvement in the depressive symptoms of, respectively, 13 and 21 elderly TRD patients. In contrast, a recent multicenter cohort study reported a greater clinical amelioration with lithium add-on therapy in geriatric TRD patients versus non-geriatric ones [70].
A description of the studies regarding lithium augmentation in TRD is reported in Table 3.
Table 3.
Summary of the studies about lithium augmentation in TRD.
| Reference | n | Age Mean (SD), Years | Design | Dosage (mg/d)/Plasma Levels (mmol/L) | AD | Duration | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||
| Bauer et al. [60] | 27 | 47.4 (±16.9) | I: open, acute treatment phase II: RC continuation phase | Mean (SD): 980.0 (±295.6)/0.65 (±0.14) | Various ADs | 4 months | Relapse in phase II (HAM-D) | Relapse rate: Li < pcb (p = 0.02) |
| Bschor et al. [61] * | 22 | 46.4 (±15.7) | Double-blind Follow-up maintenance phase study | N.A. | Various ADs | 1 year | Recurrence (HAM-D) | Recurrence rate: Li = pcb (p = 0.49) |
| Joffe et al. | 50 | 37.4 (±11.2) | Head-to-head Double-blind Comparison Li/T3/pcb | Mean: Li: 935.3/0.68 T3: 37.5 mcg |
Desipramine, imipramine | 2 weeks | HAM-D | ↓ HAM-D: Li > pcb (p = 0.04) T3 > pcb (p = 0.02) Li = T3 (p > 0.05) |
| Kok et al. | 32 | 71.9 (±7.8) | Augmentation vs. switch Double-blind comparison VFX/NOR Not remitted patients → Open-label comparison Li augmentation/switch to PHE/switch to TCA/switch to ECT | Mean (SD): Li: 586.0 (±86.0)/0.82 (±0.15) PHE: 53.0 (±8.0) | Venlafaxine, nortriptyline | 12 weeks | MADRS | ↓ MADRS: Li (p < 0.001) TCA (p < 0.01) PHE (p > 0.05) ECT (p > 0.05) |
| Nierenberg et al. [59] | 35 | 37.2 (±8.3) | Double-blind | N.A./0.61 (range: 0.6–0.9) | Nortriptyline | 6 weeks | HAM-D, CGI | ↓ HAM-D and ↓ CGI: Li = pcb (p > 0.05) |
| Stein and Bernadt, [58] | 34 | 47.2 (±19.5) | Double-blind Experimental group: Li 250 3 weeks → 750 6 weeks Controls: pcb 3 weeks → Li 250 3 weeks → Li 750 3 weeks | Dose/mean (SD) Experimental group: 250/0.25 (±0.12) 750/0.78 (±0.35) Controls: 250/0.25 (±0.15) 750/0.65 (±0.21) | TCAs | 9 weeks | MADRS | ↓ MADRS: Li 250 = pcb (p = 0.81) Li 750 > Li 250 (p = 0.009) |
| Yoshimura et al. | 30 | Li: 39.0 (±8.0) OLA: 42.0 (±7.0) ARI: 40.0 (±10.0) | Head-to-head Comparison Li/OLA/ARI | Mean (SD): Li: 458.0 (±103.0)/N.A. OLA: 7.0 (±5.0) ARI: 9.0 (±6.0) | Paroxetine | 4 weeks | HAM-D | ↓ HAM-D: Li = OLA = ARI (p < 0.001) Response—remission rates: Li 40–20% OLA 30–10% ARI 40–20% |
| Open studies | ||||||||
| Bertschy et al. [67] | 13 | 45 | Open-label | Mean (SD): N.A./0.75 (±0.12) | Venlafaxine | 4 weeks | MADRS | ↓ MADRS: Li = pcb (p = 0.20) |
| Buspavanich et al. [70] | Tot: 167 Geriatric: 22 Non-geriatric: 145 | Tot: 48.3 (±13.9) Geriatric: 71.9 (±5.6) Non-geriatric: 44.8 (±11.0) | Prospective multicenter cohort study Comparison geriatric/non-geriatric patients | Mean (SD): 150.0 (±89.9)/0.68 (±0.2) | Various ADs | 4 weeks | HAM-D | Response rate: geriatric > non-geriatric patients (p = 0.04) |
| Dallal et al. [63] | 20 | 27–63 42.0 (±10.3) | Open-label | Range: 150–300/0.5–1.2 | Desipramine | 6 weeks | CGI | ↓ CGI (p < 0.01) |
| Dinan [65] | 11 | 37–59 | Open-label | Dose/mean (SD): 400/0.26 (±0.1) or 800/0.6 (±0.1) | Sertraline | 1 week | HAM-D | ↓ HAM-D (p < 0.01) Response not related to Li plasma levels |
| Doree et al. | 20 | QUE: 52.3 (±8.1) Li: 49.3 (±9.4) | Head-to-head Open-label Comparison QUE/Li | Mean: QUE: 430 (range: 300–700) Li: N.A./0.78 | Various ADs | 8 weeks | HAM-D MADRS | ↓ HAM-D both QUE and Li: p < 0.0001 QUE > Li (p < 0.05) ↓ MADRS: both QUE and Li: p < 0.0001 QUE > Li (p < 0.05) |
| Flint and Rifat [69] | 21 | 64–88 75.6 (±7.1) | Prospective, open-label | Mean (SD): N.A./0.67 (±0.17) | Nortriptyline, fluoxetine, phenelzine | 2 weeks | HAM-D, HAD | Response rate: 24% |
| Fontaine et al. [64] | 60 | 24–55 FLU+Li: 42.6 (±8.6) DES + Li: 41.0 (±8.8) | Open-label Comparison FLU + Li/DES + Li | Mean (SD): FLU + Li: 570.0 (±120.8)/N.A. DES + Li: 660.0 (±165.3)/N.A. | Fluoxetine, Desipramine | 6 weeks | CGI | ↓ CGI: FLU + Li = DES + Li Response rate FLU + Li: 60% Response rate DES + Li: 56.6% Rapid response (1 week): FLU + Li > DES + Li (N.S.) |
| Gervasoni et al. | 10 | N.A. | Head-to-head Observational Comparison Li-s/Li-s + T3 | Li-s: 1320/median: 0.52 (range: 0.38–1.10)T3: 37.5 mcg | Clomipramine | 2 months | MADRS | Remission rates: Li-s = 10% Li-s + T3 = 0 |
| Hoencamp et al. [66] | 22 | 43.0 (±13.0) | Open-label | Dose/mean (SD): 600/0.66 (±0.19) | Venlafaxine | 7 weeks | HAM-D MADRS CGI |
↓ HAM-D (p = 0.001) Response rate: 34.8% Remission rate: 8.7% ↓ MADRS (p = 0.005) ↓ CGI (p = 0.001) |
| Ivkovic et al. | 88 | LAM: 54.2 (±13.7) Li: 49.3 (±12.3) | Head-to-head Open-label | Mean (SD): LAM: 117.7 (±54.3) Li: 900/N.A. | Various ADs | 8 weeks | HAM-D, CGI | LAM = Li ↓ HAM-D (p = 0.83) ↓ CGI (p = 0.92) Within 2nd week: LAM > Li ↓ HAM-D (p = 0.01) ↓ CGI (p = 0.02) |
| Kok et al. [71] | 28 | Li: 73.6 (±7.3) PHE: 72.6 (±7.7) | Head-to-head Open-label, R Comparison Li augmentation/switch to PHE | Mean (SD) Li: 527.0 (±96.0)/0.71 (±0.17) PHE: 46.0 (±9.0) | TCA or venlafaxine | 6 weeks | MADRS remission | ↓ MADRS Li (p < 0.001) PHE (p = 0.85) Remission rates: Li > PHE (p = 0.04) Response rates: Li > PHE (p = 0.03) |
| Schindler and Anghelescu | 34 | Li: 50.3 (±13.6) LAM: 45.1 (±13.4) |
Head-to-head Open-label, R Comparison Li/LAM | Mean: Li: N.A./0.71 LAM: 152.9 | Various ADs | 8 weeks | HAM-D | ↓ HAM-D: Li = LAM (p = 0.11) |
| Schüle et al. | 46 | 50.78 (±12.27) | Head-to-head Open-label Comparison MIR monotherapy/Li augmentation/CAR augmentation | Mean (SD): Li: 917.0 (±144.1)/0.71 (±0.13) CAR: 370.0 (±67.5)/32.4 (±8.16) | Mirtazapine | 3 weeks | HAM-D | Response rates: MIR: 21.7% Li 53.8% CAR 20.0% Li > MIR (p = 0.05) CAR = MIR (p = 0.91) |
| Thase et al. [62] | 20 | 40.4 (±9.9) | Open-label | Responders: N.A./0.56 (±0.22) Non-responders: N.A./0.83 (±0.19) | Imipramine | 6 weeks | HAM-D | ↓ HAM-D (p < 0.001) Response rate: 65% |
| Whyte et al. | Augmentation group: 53 Switch group: 12 | Augmentation group: 76.2 (±5.7) Switch group: 78.8 (±7.2) | Augmentation vs. switch Open-label Comparison Li/NOR/BUP/switch to VFX | Max, median (range): Li: 300 (225–300)/0.5–0.7 NOR: 35 (10–50) BUP: 200 (50–400) VFX: 244 (150–300) | Paroxetine | Median (range), weeks: Li: 7 (1–22.3) NOR: 17 (0.2–28.7) BUP: 5.6 (0.3–30) VFX: 12 (8.7–12) |
HAM-D | Response rates: Li: 43% NOR: 31% BUP: 45% VFX: 41.7% |
| Zimmer et al. [68] | 13 | 74.1 (±8.2) | Open-label | Range/mean (SD): 300–450/0.65 (±0.20) | Nortriptyline | 3 weeks | HAM-D | ↓ HAM-D (p < 0.001) |
Key: * = study including subjects without relapse during the 4-months continuation phase reported by Bauer et al. [60]. AD = antidepressant; ARI = aripiprazole; BUP = bupropion; CAR = carbamazepine; CGI = Clinical Global Impression; DES = desipramine; ECT = Electro-Convulsive Therapy; FLU = fluoxetine; HAD = Hospital Anxiety and Depression; HAM-D = Hamilton Depression Rating Scale; IMAO = Monoamine Oxidase Inhibitor; LAM = lamotrigine; Li = lithium; Li 250 = lithium at 250 mg/d; Li 750 = lithium at 750 mg/d; Li-s = lithium sulfate; MADRS = Montgomery and Asberg Depression Rating Scale; MIR = mirtazapine; N.A. = not available; N.S. = not statistically significant; NOR = nortriptyline; OLA = olanzapine; pcb = placebo; PHE = phenelzine; QUE = quetiapine; R = randomized; RC = randomized controlled; RCT = randomized controlled trial; SD = standard deviation; T3: triiodothyronine; TCA = tricyclic antidepressant; VFX = venlafaxine.
3.3.2. Head-to-Head Studies: Lithium
One open trial, including 28 elderly TRD inpatients, showed that the response and remission rates were higher in the lithium augmentation group than in the phenelzine one [71].
Two studies compared lithium with SGA as an add-on therapy in TRD. The first open-label study randomized 20 TRD patients to either lithium or quetiapine add-on. The scores of the Hamilton Rating Scale for Depression (HAM-D) significantly improved from the baseline in both groups, with a greater improvement in the quetiapine than the lithium group after the first 4 weeks of augmentation [72]. The second study was an RCT conducted on a sample of 30 TRD patients who did not show a significant difference in clinical amelioration with lithium, olanzapine, or aripiprazole augmentation [73].
Three studies compared lithium versus anticonvulsant augmentation.
In the first randomized, open-label study, including 34 TRD patients, no significant differences were observed in the HAM-D scores between lamotrigine and lithium treatment groups, both at baseline and after 8 weeks [74]. In contrast, an open-label trial, including 88 TRD patients, reported significant clinical improvement within the second week in the lamotrigine group compared to the lithium group, while no differences were observed at the study endpoint [75]. The third open-label study, conducted on 46 TRD inpatients, found that lithium, but not carbamazepine, significantly augmented the AD effect of mirtazapine [76].
Finally, two studies investigated the efficacy of lithium compared to triiodothyronine (T3) augmentation in TRD. Joffe and collaborators [77] carried out an RCT study including 50 depressed subjects resistant to TCA treatment, and they found that both T3 and lithium were similarly more effective than a placebo in decreasing HAM-D scores. In a subsequent observational study, lithium-sulfate augmentation was found to lead to higher remission rates than lithium-sulfate plus T3 augmentation [78] (Table 3).
3.3.3. Lithium Augmentation versus Switching Strategies
Two studies with elderly subjects compared the effect of augmentation versus switch strategies. In the first one (n = 65), the authors observed similar rates and speed of response with bupropion, nortriptyline, or lithium augmentation compared to switching to venlafaxine [79]. In the second one (n = 32), both lithium augmentation and switching to TCA were reported to be effective strategies, differently from switching to phenelzine or to electro-convulsive therapy (ECT) [80] (Table 3).
3.3.4. Antiepileptic Drugs
In a first double-blind RCT, conducted on 23 TRD patients, lamotrigine failed to significantly reduce both HAM-D and MADRS scores with respect to a placebo [81]. Similarly, another double-blind RCT showed no differences between lamotrigine and placebo treatment groups of 34 TRD subjects [82]. On the other hand, three retrospective studies reported some possible benefits of lamotrigine augmentation to an AD regimen in TRD individuals. In the first one, by Barbee and Jamhour [83], 48.4% of the 31 included patients were rated much or very much improved on the Clinical Global Impression (CGI) scale after 6 weeks of treatment. No differences were found in the doses of lamotrigine administered to responders and non-responders. A subsequent paper reported an improvement in 76% of the 25 TRD enrolled patients [84]. Finally, 34 TRD subjects treated with augmentative lamotrigine showed an early and statistically significant improvement of some target symptoms, such as depressed mood, loss of interest, cognitive impairment, irritability, and anergy, while sleep disturbance did not ameliorate [85].
A small double-blind RCT (n = 20) found no differences in the overall response rates between phenytoin augmentation and a placebo in MDD subjects resistant to SSRIs [86].
One open-label study, conducted on 20 elderly subjects suffering from TRD and generalized anxiety disorder, demonstrated a statistically significant reduction in depression scores after 4-week pregabalin augmentation, with a further improvement between the 8th and the 12th week; also, a significant improvement of anxiety was noted [87]. Similarly, another study conducted with an open-label design on 14 TRD subjects demonstrated the effectiveness of valproate as an augmentation treatment to ADs, lasting 7 months [88].
As some studies reported the beneficial effects of topiramate on the depressive phase of bipolar disorder, the efficacy of this drug as an add-on therapy in TRD was assessed. Mowla and Kardeh [89], in a double-blind RCT (n = 53), demonstrated the superiority of topiramate over a placebo in reducing HAM-D total scores; in particular, depressed mood, insomnia, agitation, anxiety symptoms, and suicidality significantly improved in the topiramate group.
Finally, zonisamide was evaluated as a possible augmentation strategy in the treatment of TRD subjects, in consideration of partial pharmacodynamic overlap with lamotrigine, as well as the possible effect on the serotonergic system; the authors concluded that zonisamide might be a potential augmentation option for MDD patients not responding to duloxetine [90].
A description of the studies regarding augmentation with antiepileptic drugs in TRD is reported in Table 4.
Table 4.
Summary of the studies about augmentation with antiepileptic drugs in TRD.
| Reference | n | Age Mean (SD), y | Design | Augmentation Molecule | Dosage | AD | Duration | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Barbosa et al. [81] | 15 | 30.2 (±8.4) | Double-blind | Lamotrigine | Max: 100 mg/d | Fluoxetine | 6 weeks | HAM-D MADRS CGI | ↓ HAM-D: LAM = pcb (p = 0.21) ↓ MADRS: LAM = pcb (p = 0.46) ↓ CGI: LAM > pcb (p = 0.03) |
| Santos et al. [82] | 27 | 38.2 (±8.7) | Double-blind | Lamotrigine | Max: 200 mg/d | Various ADs | 8 weeks | CGI MADRS | ↓ CGI: LAM = pcb (p = 0.45) ↓ MADRS: LAM = pcb (p = 0.45; p-adj = 0.88) Response rates: LAM = pcb (p = 0.60) |
| Shapira et al. [86] | 20 | 47.5 (±14.1) | Double-blind | Phenytoin | N.A. | Fluvoxamine, fluoxetine, paroxetine | 4 weeks | HAM-D | ↓ HAM-D: PHE = pcb (p = 0.30) |
| Mowla and Kardeh [89] | 42 | 36.2 | Double-blind | Topiramate | Range: 100–200 mg/d Mean: 173.15 mg/d | Fluoxetine, citalopram, sertraline | 8 weeks | HAM-D CGI | ↓ HAM-D TOP: p < 0.001 ↓ HAM-D and ↓ CGI: TOP > pcb (p < 0.001) |
| Fang et al. [91] | 193 | Range: 18–65 | Head-to-head Multicenter, double-blind Comparison RIS/VAL/BUS/TRZ/T3 | Valproate | RIS: 2 mg/d VAL: 600 mg/d BUS: 30 mg/d TRZ: 100 mg/d T3: 80 mg/d | Paroxetine | 8 weeks | Remission at HAM-D | Remission rates: overall 37.3% RIS: 26.7% VAL: 48.7% BUS: 32.6% TRZ: 42.6% T3: 37.5% RIS = VAL = BUS = TRZ = T3 (p = 0.25) |
| Open studies | |||||||||
| Barbee and Jamhour [83] | 31 | 50.2 (±11.2) | Retrospective | Lamotrigine | Mean: 112.9 mg/d | Various ADs | Mean 41.8 weeks (at least 6 weeks) | CGI | (Very) much improved: 48.4% Mildly improved: 22.6% Unchanged: 29.0% |
| Gutierrez et al. [85] | 34 | 48.0 (±7.4) | Retrospective | Lamotrigine | Mean: 113.3 mg/d | Various ADs | 1 year | Medication Visit by MD | ↓ scores in target symptoms: - cognitive impairment, depressed mood, irritability, loss of interest (p < 0.01) energy (p < 0.001) sleep disturbance (p > 0.05) |
| Rocha and Hara [84] | 25 | Range: 18–65 | Retrospective | Lamotrigine | Mean (SD): 155.0 (±64.5) mg/d | Various ADs | 4 weeks | CGI | Response rate: 76% |
| Karaiskos et al. [87] | 20 | 72.6 (±6.3) | Open-label | Pregabalin | Mean (SD): 106.0 (±78.0) mg/d | Various ADs | 12 weeks | HAM-D HAM-A |
↓ HAM-D: p < 0.01 ↓ HAM-A: p < 0.05 |
| Ghabrash et al. [88] 2015 | 14 | Range: 19–59 | Comparison of psychometric scores at T0 (pre-treatment) with scores at: T1 (1 month) T4 (4 months) T7 (7 months) | Valproate | 375–1000 mg/d | Various ADs | 7 months | MADRS CGI |
↓ MADRS: T0 vs. T1 (p < 0.001) T4 (p < 0.001) T7 (p < 0.001) ↓ CGI: T0 vs. T1 (p = 0.03) T4 (p < 0.001) T7 (p < 0.001) |
| Fornaro et al. [90] | 24 | 50.7 (±0.2) | Open-label | Zonisamide | 75 mg/d | Duloxetine | 12 weeks | HAM-D | Response rate: 58.3% |
Key: AD = antidepressant; BUS = buspirone; CGI = Clinical Global Impression; GAF = Global Assessment of Functioning; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; LAM = lamotrigine; MADRS = Montgomery–Åsberg Depression Rating Scale; MD = medical doctor; pcb = placebo; PHE = phenytoin; RCT = randomized controlled trial; RIS = risperidone; SD = standard deviation; SSRI = Selective Serotonin Reuptake Inhibitor; TOP = topiramate; TRZ = trazodone; VAL = valproate.
3.3.5. Head-To-Head Studies: Antiepileptics
One retrospective study compared different molecules, including valproate, as an add-on therapy to paroxetine in 225 TRD patients. The remission rates were 48.7% for valproate, 26.7% for risperidone, 32.6% for buspirone, 42.6% for trazodone, and 37.5% for thyroid hormone, but the difference between the adjunctive treatments did not result to be statistically significant [91] (Table 4).
3.4. Ketamine and Es-Ketamine
Ketamine is a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist used as an anesthetic in clinical practice. Two open-label small-sample studies [92,93] reported a significant reduction in depressive symptoms when intravenous ketamine was administered as an add-on therapy.
Five double-blind RCTs presented contrasting results. Fava and colleagues [94] demonstrated that 0.5 and 1.0 mg/kg of IV ketamine, but not 0.1 and 0.2 mg/kg, were better than a placebo in reducing the HAM-D6 total score in 86 TRD subjects 24 h after the infusion, not at day 3. In contrast, Ionescu and colleagues [95] enrolled 26 TRD outpatients, showing that IV ketamine at 0.5 mg/kg did not outperform a placebo in terms of short- or long-term efficacy. Some authors reanalyzed the sample enrolled by Fava and his group [94], searching for more specific differences in terms of gender, time to relapse, or suicidal ideation. The analyses on varying ketamine doses, administered IV, on 50 men and 49 women with TRD, did not lead to significant differences between the sexes in treatment response [96]. More recently, Salloum and colleagues [97] reanalyzed a subgroup of 56 patients and demonstrated that time to relapse after varying doses (0.1-0.5-1.0 mg/kg) of a single administration of IV ketamine was dose-related. Moreover, a single infusion of IV ketamine significantly reduced suicidal ideation after one month, but not early after the administration in a subgroup of 56 TRD patients [98].
The nasal-spray formulation of esketamine, the S-enantiomer of ketamine, has been recently approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as augmentation treatment in TRD. Two double-blind RCTs regarding the efficacy of esketamine in the short-term [99,100] and one regarding time to relapse in the long-term [101] supported the approval of this compound for TRD. In particular, the short-term studies reported the superiority of esketamine over a placebo as augmentation in TRD patients over a 4-week period, although this difference was not statistically significant when esketamine was administered at 84 mg twice weekly in the study by Fedgchin et al. [99]. The RTC by Daly and colleagues [101] demonstrated the superiority of esketamine over a placebo in preventing the relapse of 297 TRD patients who achieved a stable response or remission after 16 weeks of esketamine add-on therapy to their current AD treatment.
More recently, Ochs-Ross and collaborators [102] reported no differences between esketamine and a placebo in reducing the MADRS scores in a sample of elderly TRD patients, although there was a significant reduction in 65–74 years-old patients, different from subjects with an age ≥ 75 years. Promising results were also found by Wajs and colleagues [103], who reported that improvements in depressive symptoms after esketamine augmentation remained in the long-term.
A description of the studies regarding ketamine and esketamine augmentation in TRD is reported in Table 5.
Table 5.
Summary of the studies about augmentation with IV ketamine, esketamine nasal spray, and psychostimulant drugs in TRD.
| Reference | n | Age Mean (SD), y | Design | Augmentation Molecule | Dosage | AD | Duration | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Daly et al. [101] * | 297 | 46.3 (±11.1) | Multicenter, double-blind TRD patients → 16 weeks ESK augmentation → R maintenance phase: comparison ESK/pcb | Esketamine | Remitters: 56 mg or 84 mg/2 weeks Responders: 56 mg or 84 mg once weekly | SSRIs or SNRIs | Median among remitters: 17.7 weeks Median among responders: 19.4 weeks | Time to relapse at MADRS | Relapse among remitters: pcb > ESK (p = 0.003) Relapse among responders: pcb > ESK (p < 0.001) |
| Fedgchin et al. [99] | 315 | 46.3 (±11.2) | Multicenter, double-blind Comparison ESK56/ESK84/pcb | Esketamine | 56 mg or 84 mg twice weekly | Escitalopram, Sertraline, Venlafaxine, Duloxetine | 4 weeks | MADRS | ↓ MADRS: ESK56 > pcb (p = 0.03) ESK84 = pcb (p = 0.09) |
| Ochs-Ross et al. [102] | 122 | 70.0 (±4.52) | Double-blind | Esketamine | 28 mg, 56 mg or 84 mg twice weekly | ≈ | ≈ | ≈ | ↓ MADRS: ESK = pcb (p = 0.06) 65–74 years old: ESK > pcb (p = 0.02) ≥ 75 years old: ESK = pcb (p = 0.93) |
| Popova et al. [100] | 197 | ESK: 44.9 (±12.6) pcb: 46.4 (±11.1) | Multicenter, double-blind | Esketamine | 56 mg or 84 mg twice weekly | ≈ | ≈ | ≈ | ↓ MADRS: ESK > pcb (p = 0.02) |
| Fava et al., 2020 [94] | 86 | KETA 0.1: 43.1 (±11.9) KETA 0.2: 45.5 (±14.6) KETA 0.5: 48.6 (±12.9) KETA 1.0: 47.4 (±10.1) | Double-blind Comparison KETA 0.1-0.2-0.5-1.0 mg/kg/pcb | IV Ketamine | 0.1-0.2-0.5-1.0 mg/kg | Various ADs | 30 days | HAM-D6 at day 1 and 3 | ↓ HAM-D6: KETA > pcb (p = 0.03) KETA 0.1-0.2-0.5-1.0 mg/kg > pcb (p = 0.04) ↓ HAM-D6 day 1: KETA 0.1–0.2 mg/kg = pcb (p-adj = 0.14 and 0.79) KETA 0.5 mg/kg > pcb (p-adj < 0.001) KETA 1.0 mg/kg > pcb (p-adj = 0.04) ↓ HAM-D6 day 3: KETA 0.1-0.2-0.5-1.0 mg/kg = pcb (p > 0.05) |
| Freeman et al. [96] ** | 99 | 18–70 Males: 47.5 (±12.5) Females: 44.8 (±12.7) |
≈ | IV Ketamine | ≈ | ≈ | ≈ | ≈ | ↓ HAM-D6: males = females (groupxgender p = 0.69) |
| Feeney et al. [98] ** | 56 | 45.7 (±12.3) | ≈ | IV Ketamine | 0.1-0.5-1.0 mg/kg | ≈ | ≈ | MADRS suicide item | ↓ MADRS suicide item at day 30: KETA > pcb (p = 0.03) |
| Ionescu et al. [95] | 26 | 45.4 (±12.4) | Double-blind | IV Ketamine | 0.5 mg/kg | Various ADs | 3 weeks | HAM-D | ↓ HAM-D: KETA = pcb (p = 0.47) |
| Salloum et al. [97] ** | 56 | KETA 0.1: 47.0 (±8.1) KETA 0.5: 45.5 (±11.9) KETA 1.0: 45.3 (±9.6) | Double-blind Comparison KETA 0.1/0.5/1.0 mg/kg | IV Ketamine | 0.1-0.5-1.0 mg/kg | Various ADs | 30 days | MADRS | At day 3: Response rate: 48% Remission rate: 34% At day 30: Remission rate: 21% |
| Price et al. [104] | 15 | 50.0 (±12.0) | Pcb substitution Comparison FEN/pcb | Fenfluramine (Amphetamine) | 89.0 (±26.0) mg/d | Desipramine | Mean (SD): 16.4 (±5.0) d | SCRS HAM-D |
↓ SCRS and ↓ HAM-D: FEN = pcb (p > 0.05) |
| Richards et al. [105] | Study 1: pcb = 201, LDX = 201; Study 2: pcb = 213, LDX = 211 | Study 1 LDX: 42.2 (±12.3) Study 2 LDX: 42.0 (±11.6) | Multicenter, double-blind Comparison LDX/pcb | Lisdexamfetamine dimesylate (Amphetamine) | Study 1: 46.5 (±13.7) mg/d Study 2: 43.4 (±14.3) mg/d | Various ADs | 16 weeks | MADRS | ↓ MADRS Study 1: LDX = pcb (p = 0.88) ↓ MADRS Study 2: LDX = pcb (p = 0.58) |
| Patkar et al. [106] | 50 | 48.5 | Double-blind | Metilphenidate | 34.2 (±6.3) mg/d | Various ADs | 4 weeks | HAM-D | ↓ HAM-D: MPH = pcb (p = 0.22) Response rates: MPH > pcb (p = 0.12) |
| Ravindran et al. [107] | 134 | 43.8 (±11.0) | Multicenter, double-blind | Metilphenidate | 36.4 (±9.1) mg/d | Various ADs | 5 weeks | MADRS | ↓ MADRS: MPH = pcb (p = 0.74) |
| Open studies | |||||||||
| Wajs et al. [103] *** | 150 | 52.2 (±13.7) | Multicenter, open-label | Esketamine | Flexible 14–84 mg once weekly or every-other-week | ≈ | 1 year | ≈ | Response rate: 76.5% Remission rate: 58.2% |
| Cusin et al. [93] | 12 | 48.9 | Open-label 6 infusions (2/week): infusion 1–3: 0.50 mg/kg (IV); infusion 4–6: 0.75 mg/kg (IV) | IV Ketamine | Mean (SD): infusion 1–3: 29.0 (±16.2) mg infusion 4–6: 43.5 (±24.3) mg | Various ADs | 3 weeks | HAM-D | ↓ HAM-D: p < 0.001 Response rate: 41.7% Remission rate: 16.7% |
| Schiroma et al. [92] | 14 | 54.0 | Open-label | IV Ketamine | 0.5 mg/kg | Various ADs | 12 days | MADRS | ↓ MADRS (p < 0.001) |
| Nasr et al. [108] | 78 | 44.0 | Retrospective | Modafinil | 249.0 (±122.0) mg/d | Various ADs | 9 months | CDRS | ↓ CDRS: p < 0.01 |
Key: * = analysis in the long-term of patients who achieved response in the studies by Fedgchin et al. [99] and Popova et al. [100]. ** = reanalysis on a subgroup of patients recruited by Fava et al. [94]. *** = analysis in the long-term, including, in the total sample, patients who completed the study by Ochs-Ross et al. [102]. ≈ = same as above; AD = antidepressant; CDRS = Carroll Depression Rating Scale; ESK = esketamine nasal spray; ESK56 = esketamine at 56 mg/d; ESK84 = esketamine at 84 mg/d; FEN = fenfluramine; HAM-D = Hamilton Depression Rating Scale; HAM-D6 = Hamilton Depression Rating Scale—6 items; IV = intravenous; KETA = ketamine; LDX = lisdexamfetamine dimesylate; MADRS = Montgomery and Asberg Depression Rating Scale; MPH = metilphenidate; p-adj = p value adjusted for multiple comparisons; pcb = placebo; RCT = randomized, controlled trial; SCRS = Short Clinical Rating Scale; SD = standard deviation.
3.5. Psychostimulants
3.5.1. Flenfluramine
Flenfluramine, a sympathomimetic amine that can activate the serotoninergic pathways in the brain, was studied by Price and co-authors [104] in 15 TRD patients, demonstrating no statistically significant evidence of either transient or maintained clinical improvement during the 2 weeks of fenfluramine augmentation to desipramine (Table 5).
3.5.2. Lisdexamfetamine Dimesylate
In the research paper by Richards and colleagues [105], the authors reported the results of two multicenter double-blind RCTs for a total of 826 TRD patients, showing that lisdexamfetamine dimesylate did not provide benefits over a placebo (Table 5).
3.5.3. Methylphenidate
Methylphenidate (MPH), a central nervous system (CNS) stimulant, was studied as a potential augmentation therapy for patients with refractory depression, but the preliminary findings appear unpromising.
A first double-blind RCT [106] showed no statistically significant differences between the extended-release MPH and placebo in 50 TRD patients. Similarly, another double-blind RCT [107] reported no statistically significant differences between osmotic-release oral system MPH and placebo at endpoint on the MADRS total scores, although MPH was superior to the placebo in improving apathy and fatigue as measured by the multidimensional assessment of fatigue (MAF) scale and the apathy evaluation scale (AES) (Table 5).
3.5.4. Modafinil
A retrospective chart review conducted by Nasr [108] on 78 TRD outpatients reported preliminary but promising findings about augmentation with modafinil, a wake-promoting agent. In particular, 11 depressed subjects achieved remission and most patients improved in depressive symptoms, particularly sleepiness, fatigue, and anergy (Table 5).
3.6. Non-Psychopharmacological Agents
3.6.1. Acetylsalicylic Acid (ASA)
Mendlewicz and colleagues [109] reported that ASA augmentation to SSRIs in 17 TRD patients significantly reduced the HAM-D total scores, and it was associated with a response rate of 52.4% and remission rate of 43%. Moreover, 82% of responders achieved remission (Table 6).
Table 6.
Summary of the studies about augmentation with non-psychopharmacological agents, other molecules and supplements in TRD.
| Reference | n | Age (Years) | Design | Augmentation Molecule | Dosage | AD | Duration | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| McAllister-Williams et al. [110] | 165 | Range: 18–65 | Double-blind | Metyrapone | 500 mg/bid | Various ADs | 3 weeks | MADRS | ↓ MADRS: MET = pcb (p = 0.74) |
| Nettis et al. [112] | 39 | MIN (n = 18): 47.0 (±10.0) pcb (n = 21): 43.7 (±10.7) | Double-blind Comparisons: MIN vs. pcb CRP+/MIN vs. CRP-/MIN vs. CRP+/pcb vs. CRP-/pcb | Minocycline | 200 mg/d | ≈ | 4 weeks | HAM-D | ↓ HAM-D: - MIN = pcb (p = 0.13) - CRP+/MIN > other subgroups (p < 0.001) |
| Mischoulon et al. | 12 | Range: 18–65 | Double-blind | Naltrexone | 1 mg/bid | Dopaminergic agents | 3 weeks | ≈ | ↓ HAM-D: LNT = pcb (p = 0.30) |
| Moreno et al. [113] | 10 | Mean (SD): 43.0 (±13.0) | Double-blind, crossover | Pindolol | 2.5 mg/tid | Desipramine, fluoxetine, bupropion | 2 weeks | ≈ | ↓ HAM-D: PIN = pcb (p = 0.72) |
| Perez et al. | 78 | Mean (SD): 47.1 (±10.1) | Double-blind | Pindolol | 2.5 mg/tid | Clomipramine, fluoxetine, fluvoxamine, paroxetine | 10 days | ≈ | ↓ HAM-D: PIN = pcb (p = 0.22) Remission rates: PIN = pcb (p > 0.05) |
| Sokolski et al. [114] | 9 | N.A. | Double-blind | Pindolol | 7.5 mg | Paroxetine | 4 weeks | ≈ | ↓ HAM-D: PIN > pcb (p = 0.001) |
| Perry et al. | 34 | Mean (SD): PIN: 49.0 (±13.0) pcb: 43.0 (±11.0) | Double-blind, hemi-crossover | Pindolol | 2.5 mg/bid | Fluoxetine, paroxetine, sertraline | 6 weeks | ≈ | ↓ HAM-D: PIN = pcb (p = 0.93) ↓ HAM-D core mood item: PIN = pcb (p = 0.50) |
| Price et al. | 8 | Mean (SD): 50.5 (±13.2) | Double-blind | Reserpine | 5 mg/bid (IM) | Desipramine | 12 days | SCRS, HAM-D | ↓ SCRS and ↓ HAM-D: RES = pcb (p > 0.05) |
| Targum et al. | 234 | Range: 21–69 Mean (SD): 47.2 (±10.78) | Multicenter, double-blind | SAME | 800 mg/d | Various ADs | 6 weeks | HAM-D, MADRS, IDS-SR30 | SAME = pcb: ↓HAM-D (p = 0.83) ↓MADRS (p = 0.42) ↓IDS-SR30 (p = 0.70) |
| Dichtel et al. | 87 women | Range: 21–70 Mean (SD): 47.0 (±14.0) | Double-blind | Testosterone | Mean (SD): 12.2 mg/d (±5.6) | SSRIs or SNRIs | 8 weeks | MADRS | ↓ MADRS: TXT = pcb (p = 0.91) |
| Siwek et al. | 21 | Range: 18–55 Mean (SD): 46.2 (±5.8) | Double-blind | Zinc | 25 mg/d | Imipramine | 12 weeks | HAM-D, MADRS, BDI, CGI | Zinc > pcb: ↓ HAM-D (p < 0.02) ↓ MADRS (p < 0.01) ↓ BDI (p < 0.02) ↓ CGI (p < 0.01) |
| Open studies | |||||||||
| Mendlewicz et al. [109] | 17 | Range: 29–62 Mean (SD): 46.1 (±9.7) | Open-label | ASA | 160 mg/d | SSRIs | 4 weeks | HAM-D | ↓ HAM-D (p < 0.0001) Response rate: 52.4% Remission rate: 43% |
| Avari et al. [111] | 13 | 73.1 (±11.2) | ≈ | Minocycline | 100 mg twice/d | Various ADs | 8 weeks | MADRS | ↓ MADRS Remission rate: 31% |
| Miller et al. | 9 women | Range: 25–59 Mean (SD): 48.1 (±12.2) | ≈ | Testosterone | 300 mcg/d (transdermal) | SSRIs or SNRIs | ≈ | ≈ | ↓ MADRS (p = 0.004) |
| Rudas et al. | 9 | Range: 21–68 | ≈ | T3/T4 | Range: 150–300 mcg/d Mean (SD): 235.0 (±58.0) mcg/d | Various ADs | ≈ | HAM-D | ↓ HAM-D (p < 0.01) |
| Hori and Kunugi | 12 | 18–64 | ≈ | Pramipexole | 0.25–3 mg/d | ≈ | 12 weeks | HAM-D, CGI | ↓ HAM-D (p < 0.0001) ↓ CGI (p = 0.003) |
| Cassano et al. | 7 | 18–74 | Prospective, open-label | Ropinirole | 0.25–1.5 mg/d | ≈ | 16 weeks | MADRS, CGI | ↓MADRS (p < 0.02) Response rate: 40% Remission rate: 40% |
| De Berardis et al. | 25 | Mean (SD): 32.0 (±5.1) | Open- label, single-blind | SAME | 800 mg/d | ≈ | 8 weeks | HAM-D | ↓ HAM-D (p < 0.001) Response rate: 62.5% Remission rate: 37.5% |
Key: ≈ = same as above; AD = antidepressant; ASA = acetylsalicylic acid; BDI = Beck Depression Inventory; bid = bis in die; CGI = Clinical Global Impression; d = day; HAM-D = Hamilton Depression Rating Scale; IDS-SR30 = Self-rated Inventory of Depressive Symptomatology; IM = intramuscular; LNT = low-dose naltrexone; MADRS = Montgomery and Asberg Depression Rating Scale; MET = metyrapone; MIN = minocycline; pcb = placebo; PIN = pindolol; RCT = randomized controlled trial; RES = reserpine; SAME = S-AdenosylMethionine; SCRS = Short Clinical Rating Scale for Depression; SD = standard deviation; SNRI = Serotonin-Norepinephrine Reuptake Inhibitor; SSRI = Selective Serotonin Reuptake Inhibitor; tid = ter in die; TXT = testosterone.
3.6.2. Metyrapone
Metyrapone, a blocker of cortisol synthesis, was investigated in a double-blind RCT on 165 patients, showing no significant amelioration of depressive symptoms compared to a placebo [110] (Table 6).
3.6.3. Minocycline
One open pilot trial conducted by Avari and colleagues [111] on a small sample of older adults affected by TRD reported that, after 8 weeks of augmentation with minocycline, the remitters represented 31% of the sample. A year later, one RCT was conducted on 39 TRD subjects who took minocycline (200 mg/day) as an adjunctive treatment to the AD one. The authors did not demonstrate a significant superiority of minocycline with respect to a placebo in improving the HAM-D scores at week 4 [112]. The difference became significant when the patients were stratified for the baseline C-reactive protein (CRP) plasma levels, showing a greater improvement in the CRP+ group (CRP ≥ 3 mg/L) treated with minocycline than all the other groups (Table 6).
3.6.4. Pindolol
Two double-blind RCTs were conducted on small samples of TRD patients (n = 10 and n = 9, respectively). In the first one, by Moreno and co-authors [113], pindolol did not show a statistically significant difference with respect to a placebo at the endpoint in terms of the amelioration of depressive symptoms. In contrast, in the second one, the patients exhibited significant improvement on HAM-D total scores; the authors argued that a single high dose of pindolol (7.5 mg) is a more effective augmentation strategy in SSRI-refractory patients compared with the same total dose given at 2.5 mg t.i.d. [114] (Table 6).
Two further double-blind RCTs on larger samples failed to demonstrate the efficacy of pindolol as an augmentative agent in TRD. Perez and colleagues [115] enrolled 80 TRD outpatients and showed that the HAM-D scores and remission rates were not significantly different in patients taking a placebo or pindolol (2.5 mg t.i.d.). Moreover, Perry and collaborators [116], using a hemi-crossover design, showed that there were no significant differences in AD response between the subjects receiving a placebo or pindolol as add-on therapy to SSRI monotherapy (Table 6).
3.6.5. Reserpine
Price and colleagues [117] reported the results of eight patients affected by melancholic depression and who were treated with reserpine in augmentation to desipramine. Only one patient had a resolution of the depressive and psychotic symptoms within 48 h, but he relapsed within 2 weeks; the depressive symptoms did not significantly ameliorate in the total sample (Table 6).
3.6.6. Testosterone
Miller and collaborators [118] tested low-dose transdermal testosterone in a group of nine women with TRD using an open-label pilot protocol. The authors reported a statistically significant improvement in the mean MADRS scores beginning from week 2 and maintained during the 8-week follow-up period. Two-thirds of the subjects achieved a response and one-third remitted. Differently, a double-blind RTC recently conducted in a larger sample of women (n = 101) did not demonstrate a significant superiority of low-dose testosterone to a placebo after 8 weeks of augmentation in improving depressive symptoms, fatigue, and sexual function [119] (Table 6).
3.6.7. T3/T4
Rudas and his group [120] conducted an open study with nine TRD patients, showing a significant reduction in the HAM-D total scores; in particular, four of them reached a good response to high-dose T4 (Table 6).
3.7. Other Molecules and Supplements
3.7.1. Anti-Parkinson/Dementia Agents
One open trial reported a significant reduction in the psychometric scores in 12 TRD subjects receiving pramipexole as an augmentative treatment [121]. Similarly, Cassano and his group [122], adding ropinirole to TCA or SSRIs in a prospective open trial, obtained a significant decrease in the MADRS total score at the endpoint in seven TRD patients (Table 6).
3.7.2. Naltrexone
In a pilot double-blind RCT of low-dose naltrexone (LDN) (1 mg b.i.d.) versus placebo augmentation conducted on 12 TRD patients, the authors documented that neither the main outcome (HAM-D scores) or global outcome (CGI scores) measures presented a significant improvement for the LDN group over a placebo, with only the MADRS scores (secondary outcome) attaining a significant difference between the LDN and placebo (Mischoulon et al. 2017) [123].
3.7.3. S-adenosyl Methionine (SAMe)
De Berardis and his group [124] conducted an open-label, single-blind study: 33 patients with stage II TRD, according to Thase and Rush classification [6], received SAMe in addition to the existing treatment, showing a significant reduction in the HAM-D total scores. In contrast, in a double-blind RCT carried out by Targum and collaborators [125] on 234 TRD subjects, no statistically significant difference was found between the SAMe and placebo groups (Table 6).
3.7.4. Supplements
Siwek and co-authors [126], in a double-blind RCT, investigated the effect of adjunctive zinc in 21 patients resistant to imipramine, demonstrating a significant decrease in the depression scores of zinc in comparison to a placebo (Table 6).
4. Discussion and Expert Opinion
Despite the fact that the evidence regarding the comparative effectiveness of the common treatments for MDD is evolving [127], to date, the heterogeneous characteristics of depression complicate the treatment options. Particularly, TRD is associated with a failure to respond to different treatment trials, reflecting the complexity of this multifactorial disorder. However, several strategies have been proposed, such as dose optimization, the switch to another therapeutic class, or augmentation. Although augmentation allows the limitation of the transition period between one antidepressant to another, the evidence comparing augmentation versus other strategies is limited, sparse, and equivocal. Moreover, previous meta-analyses have not generated clear conclusions with regard to the efficacy of the available augmentation agents since they have been restricted by small sample size and a paucity of direct comparators between the treatments [128,129]. Nevertheless, the poly-therapy related to augmentation strategies may lead to side effect burden and complicate adherence. However, some evidence-based considerations should be made about the effectiveness of augmentation agents in adult TRD patients.
According to the present review, lithium and aripiprazole represent the most studied agents in relation to this topic. Lithium add-on is one of the oldest and most established augmentation strategies. At present, augmentative lithium did not show more efficacy than a placebo in three RCTs for a total of 91 patients both in acute treatment [58,59] and in prevention of relapses [61], with the exception of one 4-month follow-up study [60]. On the other hand, eight independent open-label studies, for a total of 334 patients, reported promising findings regarding augmentation with lithium in TRD patients [62,63,64,65,66,68], including elderly subjects [69,70]. Only one small-sample open-label trial did not demonstrate the superiority of lithium over a placebo [67]. Furthermore, the head-to-head studies predominantly confirmed that lithium add-on was as effective as adjunctive antidepressants, SGAs, antiepileptics, and T3. Taken as a whole, the studies on lithium suggest a potential role of this compound as an augmentative treatment in TRD. However, most of these studies involved augmenting tricyclic antidepressants. Therefore, evidence on the use of lithium as an add-on therapy to current antidepressants (e.g., SSRIs and SNRIs) is limited. In addition, whilst lithium augmentation may decrease suicide risk [130], the multiple drug–drug interactions, together with its risks of toxicity, require the frequent monitoring of the lithium serum concentration.
Other augmentation strategies with the most evidence-based support include atypical antipsychotics and, more recently, ketamine and esketamine. While the exact mechanism of augmentation is not entirely known, it is hypothesized that SGAs can act as 5-HT2A receptor antagonists, alpha 2 adrenergic antagonists, 5-HT1A agonists, and monoamine reuptake inhibitors [131].
Among the SGAs, aripiprazole has been the most studied agent. According to our review, we included 19 studies with nine RCTs involving a total of 1977 patients [32,33,34,35,36,37,38,39,40]. All the RCTs, with the exception of one [35], showed the superiority of aripiprazole over a placebo in improving the depressive symptoms of the TRD subjects, both at fixed and flexible medium-low doses [39]. The effectiveness of the augmentation with aripiprazole was also confirmed by open-label trials for a total of 1253 patients [22,23,24,25,26,27,28,29,30,31]. One of these studies reported that medium doses (12 mg/day) were more effective than low doses (3 mg/day) [31]. Moreover, olanzapine and quetiapine showed promising results, although drawn from studies with very limited samples [49,50,51,52]. To date, the FDA has approved aripiprazole, as well as quetiapine and olanzapine plus fluoxetine, as therapeutic options for major depression or specifically for TRD. Although risperidone has not been FDA-approved for the treatment of TRD, it should be used clinically as an augmentation strategy. Indeed, risperidone showed superiority in ameliorating acute symptoms [47] and preventing relapses compared to a placebo in three RCTs for a total of 616 subjects [45,46]. On the other hand, the data concerning ziprasidone are both limited and mixed [48]. Apart from its relatively favorable side effect profile, it is not presently recommended as an augmentation strategy. Finally, the serotonin–dopamine activity modulator—brexpiprazole—is the first “third generation” antipsychotic receiving FDA approval for augmentation in TRD. So far, evidence about its efficacy is limited to the clinical trials that led to this approval [43,44]. Thus, real-world data about the efficacy and safety of brexpiprazole are particularly urged.
Evidence of glutamatercic system impairments in MDD has emerged from clinical studies reporting that the sub-anesthetic (0.5 mg/kg) IV administration of ketamine is related to a rapid antidepressant response and reduction of suicidal ideation [132]. The results regarding IV ketamine augmentation in TRD seem promising. According to our review in which three out of the five studies are RCTs, including a total of 112 patients [94,95,97], it has been reported that low doses of the molecule have a rapid and significant AD effect. However, no studies indicate that the antidepressant acute effects of ketamine are maintained in the long-term. Nevertheless, adverse events have been reported in different studies, and the IV formulation of ketamine may hamper its administration in several clinical settings [133]. In this framework, esketamine, the S-enantiomer of ketamine, has about a four-fold greater affinity for the glutamate receptor than ketamine, thus allowing the use of much lower doses and reducing the risk of dose-dependent dissociative symptoms associated with ketamine administration. Although the nasal-spray formulation of esketamine was approved by the FDA after showing its efficacy in TRD patients, the clinical relevance of esketamine use after the induction phase is not fully understood. Although many studies focused on the short-term efficacy of esketamine [99,100,102], namely the 4-week induction phase, our knowledge about the long-term effect of this compound is poor, both in patients who discontinued the treatment after the induction phase and those who continued the AD. Continued esketamine treatment following the induction phase may be associated with stable efficacy in relapse prevention among TRD patients [101,103]. However, the long-term antidepressant and anti-suicidal effects of esketamine after discontinuation might be inconsistent [134]. Indeed, TRD has a chronic course, poor clinical stabilization, and high suicidal risk, and, therefore, to date, it is difficult to know whether clinicians should continue to use esketamine after the acute episode, for how long, at which dose, and in which patients. Moreover, another relevant question is how esketamine should be discontinued. The potential abuse and effects on cognition should be taken into account, especially in the case of prolonged administration [135,136]. Therefore, other controlled studies, more adherent to the real-world clinical practice, are needed to evaluate their efficacy and safety in the long-term, also in light of the potential risk of abuse of intranasal esketamine [127,128]. Moreover, to date, this molecule cannot be administered at home, thus limiting an easy use in clinical practice.
Finally, the augmentation of an AD with another one has limited evidence, and add-on therapy with antiepileptic drugs, pramipexole, ropinirole ASA, metyrapone, reserpine, testosterone, T3/T4, naltrexone, SAMe, modafinil, amphetamines, or zinc cannot be recommended on the basis of the current evidence.
Despite the limitations of this review, including the heterogeneity of the included studies in terms of the duration and doses of the augmentative treatment, setting of care (inpatients/outpatients), class of augmented AD and design, the focus on efficacy and not on safety and tolerability data, as well as the strict and specific inclusion criteria that may have limited the number of eligible studies, we suggest that future studies should take into account the following observations:
-
–
use of appropriate TRD definition, more homogeneous populations, as well as rating scales for the evaluation of severity of depression in order to allow pooled analyses and meta-analytical approaches of large samples of patients [129];
-
–
more clarity in defining whether certain symptoms are part of depression or the result of comorbid psychiatric conditions [137];
-
–
larger samples are required to obtain more reliable data;
-
–
combined treatments might be associated with potential dangerous side effects, so future studies should quantify the risk/benefit ratio of different augmentation strategies;
-
–
pharmacokinetic interactions should be monitored in the long-term, measuring drug plasma levels regularly.
-
–
Furthermore, the directions of future research regarding augmentation agents for TRD treatment could be the following:
-
–
understanding the biological nature of treatment response, thus increasing biological insights into the pathophysiology of TRD;
-
–
focus on searching for other molecules with more potent and longer-lasting antidepressant effects than esketamine;
-
–
identification of more robust biomarkers for clinical practice, allowing early interventions and, ultimately, improving remission outcomes.
5. Conclusions
Our search identified different pharmacological augmentation treatment options for TRD. Taken as a whole, the common prescription of SGAs as an add-on therapy to AD in TRD patients seems to be supported by some evidence of efficacy, in particular for aripiprazole. Among the other molecules, lithium might represent a valid therapeutic option, but more studies are needed to draw definitive conclusions and, given the relatively high burden of potential acute and chronic side effects, lithium augmentation should be considered a second-line choice. Esketamine has shown some data of efficacy in TRD patients, but more evidence from the real world of clinical practice is needed to implement its use in the long-term. Overall, clinicians should interpret these findings cautiously in light of the evidence of potential treatment-related adverse effects.
Author Contributions
A.C., data collection, analysis, and writing; E.C., data analysis and writing; M.C. (Martina Capellazzi), data collection and editing assistance; I.T., data collection and editing assistance; F.M., data collection and editing assistance; M.M., editing assistance; F.C., editing assistance and supervision; M.C. (Massimo Clerici), editing assistance and supervision; M.B., data analysis, editing assistance, and supervision; A.D., editing assistance and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by a grant (2019-3396—title: “shedding light on treatment-resistant depression and unraveling ketamine’s fast-acting antidepressant mechanisms of action through patient-derived induced pluripotent stem cells”) from the Italian Cariplo Foundation, which has no involvement in any stage of research and dissemination of results.
Conflicts of Interest
M.M. served as speaker for Janssen. All other authors declare they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessler R.C. The costs of depression. Psychiatr. Clin. N. Am. 2012;35:1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi M.H., Fava M., Wisniewski S.R., Thase M.E., Quitkin F., Warden D., Ritz L., Nierenberg A.A., Lebowitz B.D., Biggs M.M., et al. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 3.Gronemann F.H., Jorgensen M.B., Nordentoft M., Andersen P.K., Osler M. Socio-demographic and clinical risk factors of treatment-resistant depression: A Danish population-based cohort study. J. Affect. Disord. 2020;261:221–229. doi: 10.1016/j.jad.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Committee for Medicinal Products for Human Use (CHM) Guideline on Clinical Investigation of Medicinal Products in the Treatment of Depression. European Medicines Agency; Amsterdam, The Netherlands: 2013. EMA/CHMP/185423/2010 Rev. 2 previously (CPMP/EWP/518/97, Rev. 1) [Google Scholar]
- 5.Bauer M., Pfennig A., Severus E., Whybrow P.C., Angst J., Möller H.J., World Federation of Societies of Biological Psychiatry World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry. 2013;14:334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- 6.Thase M.E., Rush A.J. Treatment-Resistant Depression. In: Bloom F.E., Kupfer D.J., editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York, NY, USA: 1995. pp. 1081–1097. [Google Scholar]
- 7.Brown S., Rittenbach K., Cheung S., McKean G., MacMaster F.P., Clement F. Current and Common Definitions of Treatment-Resistant Depression: Findings from a Systematic Review and Qualitative Interviews. Can. J. Psychiatry. 2019;64:380–387. doi: 10.1177/0706743719828965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papakostas G.I. Managing partial response or nonresponse: Switching, augmentation, and combination strategies for major depressive disorder. J. Clin. Psychiatry. 2009;70:16–25. doi: 10.4088/JCP.8133su1c.03. [DOI] [PubMed] [Google Scholar]
- 9.Trevino K., McClintock S.M., McDonald Fischer N., Vora A., Husain M.M. Defining treatment-resistant depression: A comprehensive review of the literature. Ann. Clin. Psychiatry. 2014;26:222–232. [PubMed] [Google Scholar]
- 10.Gibson T.B., Jing Y., Smith Carls G., Kim E., Bagalman J.E., Burton W.N., Tran Q.V., Pikalov A., Goetzel R.Z. Cost burden of treatment resistance in patients with depression. Am. J. Manag. Care. 2010;16:370–377. doi: 10.1016/S1098-3015(10)73947-4. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Publishing; Washington, DC, USA: 2013. [Google Scholar]
- 12.Thomas S.J., Shin M., McInnis M.G., Bostwick J.R. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: Strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35:433–449. doi: 10.1002/phar.1576. [DOI] [PubMed] [Google Scholar]
- 13.Altamura A.C., Dell’Osso B., Buoli M., Bosi M., Mundo E. Short-term intravenous citalopram augmentation in partial/nonresponders with major depression: A randomized placebo-controlled study. Int. Clin. Psychopharmacol. 2008;23:198–202. doi: 10.1097/YIC.0b013e3282fe9d54. [DOI] [PubMed] [Google Scholar]
- 14.Joffe R.T., Schuller D.R. An open study of buspirone augmentation of serotonin reuptake inhibitors in refractory depression. J. Clin. Psychiatry. 1993;54:269–271. [PubMed] [Google Scholar]
- 15.Taylor F.B., Prather M.R. The efficacy of nefazodone augmentation for treatment-resistant depression with anxiety symptoms or anxiety disorder. Depress. Anxiety. 2003;18:83–88. doi: 10.1002/da.10115. [DOI] [PubMed] [Google Scholar]
- 16.Licht R.W., Qvitzau S. Treatment strategies in patients with major depression not responding to first-line sertraline treatment. A randomised study of extended duration of treatment, dose increase or mianserin augmentation. Psychopharmacology. 2002;161:143–151. doi: 10.1007/s00213-002-0999-0. [DOI] [PubMed] [Google Scholar]
- 17.Kessler D., Burns A., Tallon D., Lewis G., MacNeill S., Round J., Hollingworth W., Chew-Graham C., Anderson I., Campbell J., et al. Combining mirtazapine with SSRIs or SNRIs for treatment-resistant depression: The MIR RCT. Health Technol. Assess. 2018;22:1–136. doi: 10.3310/hta22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altamura A.C., Dell’Osso B., Buoli M., Zanoni S., Mundo E. Intravenous augmentative citalopram versus clomipramine in partial/nonresponder depressed patients: A short-term, low dose, randomized, placebo-controlled study. J. Clin. Psychopharmacol. 2008;28:406–410. doi: 10.1097/JCP.0b013e31817d5931. [DOI] [PubMed] [Google Scholar]
- 19.Fava M., Rosenbaum J.F., McGrath P.J., Stewart J.W., Amsterdam J.D., Quitkin F.M. Lithium and tricyclic augmentation of fluoxetine treatment for resistant major depression: A double-blind, controlled study. Am. J. Psychiatry. 1994;151:1372–1374. doi: 10.1176/ajp.151.9.1372. [DOI] [PubMed] [Google Scholar]
- 20.Fava M., Alpert J., Nierenberg A., Lagomasino I., Sonawalla S., Tedlow J., Worthington J., Baer L., Rosenbaum J.F. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial responders and nonresponders to fluoxetine. J. Clin. Psychopharmacol. 2002;22:379–387. doi: 10.1097/00004714-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Navarro V., Boulahfa I., Obach A., Jerez D., Diaz-Ricart M., Gastó C., Guarch J. Lithium augmentation versus citalopram combination in imipramine-resistant Major Depression: A 10-week randomized open-label study. J. Clin. Psychopharmacol. 2019;39:254–257. doi: 10.1097/JCP.0000000000001024. [DOI] [PubMed] [Google Scholar]
- 22.Barbee J.G., Conrad E.J., Jamhour N.J. Aripiprazole augmentation in treatment-resistant depression. Ann. Clin. Psychiatry. 2004;16:189–194. doi: 10.1080/10401230490521954. [DOI] [PubMed] [Google Scholar]
- 23.Han C., Wang S.M., Bahk W.M., Lee S.J., Patkar A.A., Masand P.S., Pae C.U. The potential utility of aripiprazole augmentation for Major Depressive Disorder with Mixed Features Specifier: A retrospective study. Clin. Psychopharmacol. Neurosci. 2019;17:495–502. doi: 10.9758/cpn.2019.17.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patkar A.A., Peindl K., Mago R., Mannelli P., Masand P.S. An open-label, rater-blinded, augmentation study of aripiprazole in treatment-resistant depression. Prim. Care Companion J. Clin. Psychiatry. 2006;8:82–87. doi: 10.4088/PCC.v08n0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellerstein D.J., Batchelder S., Hyler S., Arnaout B., Corpuz V., Coram L., Weiss G. Aripiprazole as an adjunctive treatment for refractory unipolar depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:744–750. doi: 10.1016/j.pnpbp.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Berman R.M., Thase M.E., Trivedi M.H., Hazel J.A., Marler S.V., McQuade R.D., Carson W., Baker R.A., Marcus R.N. Long-term safety and tolerability of open-label aripiprazole augmentation of antidepressant therapy in major depressive disorder. Neuropsychiatr. Dis. Treat. 2011;7:303–312. doi: 10.2147/NDT.S18333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabrazzo M., Perris F., Monteleone P., Esposito G., Catapano F., Maj M. Aripiprazole augmentation strategy in clomipramine-resistant depressive patients: An open preliminary study. Eur. Neuropsychopharmacol. 2012;22:132–136. doi: 10.1016/j.euroneuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura R., Kishi T., Hori H., Ikenouchi-Sugita A., Katsuki A., Umene-Nakano W., Iwata N., Nakamura J. Comparison of the efficacy between paroxetine and sertraline augmented with aripiprazole in patients with refractory major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39:355–357. doi: 10.1016/j.pnpbp.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Chen S.J., Hsiao Y.L., Shen T.W., Chen S.T. The effectiveness and safety of adjunctive aripiprazole in Taiwanese patients with antidepressant-refractory major depressive disorder: A prospective, open-label trial. J. Clin. Psychopharmacol. 2012;32:56–60. doi: 10.1097/JCP.0b013e31823f6c7f. [DOI] [PubMed] [Google Scholar]
- 30.Jon D.I., Kim D.H., Seo H.J., Kwon Y.J., Kim M.D., Yang J.C., Suh H.S., Min K.J., Pae C.U., Bahk W.M. Augmentation of aripiprazole for depressed patients with an inadequate response to antidepressant treatment: A 6-week prospective, open-label, multicenter study. Clin. Neuropharmacol. 2013;36:157–161. doi: 10.1097/WNF.0b013e3182a31f3d. [DOI] [PubMed] [Google Scholar]
- 31.Horikoshi S., Miura I., Ichinose M., Yamamoto S., Ito M., Watanabe K., Kanno-Nozaki K., Kaneko H., Yabe H. Low- and high-dose aripiprazole augmentation and plasma levels of homovanillic acid in major depressive disorder: A randomized, open-label study. Hum. Psychopharmacol. 2019;34:e2696. doi: 10.1002/hup.2696. [DOI] [PubMed] [Google Scholar]
- 32.Berman R.M., Marcus R.N., Swanink R., McQuade R.D., Carson W.H., Corey-Lisle P.K., Khan A. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A multicenter, randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry. 2007;68:843–853. doi: 10.4088/JCP.v68n0604. [DOI] [PubMed] [Google Scholar]
- 33.Marcus R.N., McQuade R.D., Carson W.H., Hennicken D., Fava M., Simon J.S., Trivedi M.H., Thase M.E., Berman R.M. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A second multicenter, randomized, double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 2008;28:156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- 34.Berman R.M., Fava M., Thase M.E., Trivedi M.H., Swanink R., McQuade R.D., Carson W.H., Adson D., Taylor L., Hazel J., et al. Aripiprazole augmentation in major depressive disorder: A double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197–206. doi: 10.1017/S1092852900020216. [DOI] [PubMed] [Google Scholar]
- 35.Fava M., Mischoulon D., Iosifescu D., Witte J., Pencina M., Flynn M., Harper L., Levy M., Rickels K., Pollack M. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study) Psychother. Psychosom. 2012;81:87–97. doi: 10.1159/000332050. [DOI] [PubMed] [Google Scholar]
- 36.Mischoulon D., Witte J., Levy M., Papakostas G.I., Pet L.R., Hsieh W.H., Pencina M.J., Ward S., Pollack M.H., Fava M. Efficacy of dose increase among nonresponders to low-dose aripiprazole augmentation in patients with inadequate response to antidepressant treatment: A randomized, double-blind, placebo-controlled, efficacy trial. J. Clin. Psychiatry. 2012;73:353–357. doi: 10.4088/JCP.10m06541. [DOI] [PubMed] [Google Scholar]
- 37.Dording C., Cassiello C., King F., 4th, Pencina M., Fava M., Mischoulon D. The effects of aripiprazole on the subscales of the Kellner Symptom Questionnaire in treatment resistant depression. Int. Clin. Psychopharmacol. 2013;28:238–244. doi: 10.1097/YIC.0b013e32836220df. [DOI] [PubMed] [Google Scholar]
- 38.Lenze E.J., Mulsant B.H., Blumberger D.M., Karp J.F., Newcomer J.W., Anderson S., Dew M.A., A Butters M., A Stack J., E Begley A., et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–2412. doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamijima K., Higuchi T., Ishigooka J., Ohmori T., Ozaki N., Kanba S., Kinoshita T., Koyama T., ADMIRE Study Group Aripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: A randomized, double-blind, placebo-controlled study (ADMIRE study) J. Affect. Disord. 2013;151:899–905. doi: 10.1016/j.jad.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki N., Otsubo T., Kato M., Higuchi T., Ono H., Kamijima K., ADMIRE Study Group Efficacy of aripiprazole augmentation in Japanese patients with major depressive disorder: A subgroup analysis and Montgomery-Åsberg Depression Rating Scale and Hamilton Rating Scale for Depression item analyses of the Aripiprazole Depression Multicenter Efficacy study. Psychiatry Clin. Neurosci. 2015;69:34–42. doi: 10.1111/pcn.12214. [DOI] [PubMed] [Google Scholar]
- 41.Thase M.E., Youakim J.M., Skuban A., Hobart M., Augustine C., Zhang P., McQuade R.D., Carson W.H., Nyilas M., Sanchez R., et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: A phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J. Clin. Psychiatry. 2015;76:1224–1231. doi: 10.4088/JCP.14m09688. [DOI] [PubMed] [Google Scholar]
- 42.Thase M.E., Youakim J.M., Skuban A., Hobart M., Zhang P., McQuade R.D., Nyilas M., Carson W.H., Sanchez R., Eriksson H. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: A phase 3, randomized, double-blind study. J. Clin. Psychiatry. 2015;76:1232–1240. doi: 10.4088/JCP.14m09689. [DOI] [PubMed] [Google Scholar]
- 43.Fava M., Okame T., Matsushima Y., Perry P., Weiller E., Baker R.A. Switching from inadequate adjunctive or combination treatment options to brexpiprazole adjunctive to antidepressant: An open-label study on the effects on depressive symptoms and cognitive and physical functioning. Int. J. Neuropsychopharmacol. 2017;20:22–30. doi: 10.1093/ijnp/pyw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobart M., Skuban A., Zhang P., Augustine C., Brewer C., Hefting N., Sanchez R., McQuade R.D. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with Major Depressive Disorder. J. Clin. Psychiatry. 2018;79:17m12058. doi: 10.4088/JCP.17m12058. [DOI] [PubMed] [Google Scholar]
- 45.Rapaport M.H., Gharabawi G.M., Canuso C.M., A Mahmoud R., Keller M.B., A Bossie C., Turkoz I., A Lasser R., Loescher A., Bouhours P., et al. Effects of risperidone augmentation in patients with treatment-resistant depression: Results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology. 2006;31:2505–2513. doi: 10.1038/sj.npp.1301113. [DOI] [PubMed] [Google Scholar]
- 46.Alexopoulos G.S., Canuso C.M., Gharabawi G.M., Bossie C.A., Greenspan A., Turkoz I., Reynolds C., 3rd Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am. J. Geriatr. Psychiatry. 2008;16:21–30. doi: 10.1097/JGP.0b013e31813546f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmoud R.A., Pandina G.J., Turkoz I., Kosik-Gonzalez C., Canuso C.M., Kujawa M.J., Gharabawi-Garibaldi G.M. Risperidone for treatment-refractory major depressive disorder: A randomized trial. Ann. Intern. Med. 2007;147:593–602. doi: 10.7326/0003-4819-147-9-200711060-00003. [DOI] [PubMed] [Google Scholar]
- 48.Papakostas G.I., Petersen T.J., Nierenberg A.A., Murakami J.L., Alpert J.E., Rosenbaum J.F., Fava M. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J. Clin. Psychiatry. 2004;65:217–221. doi: 10.4088/JCP.v65n0212. [DOI] [PubMed] [Google Scholar]
- 49.Sagud M., Mihaljević-Peles A., Mück-Seler D., Jakovljević M., Pivac N. Quetiapine augmentation in treatment-resistant depression: A naturalistic study. Psychopharmacology. 2006;187:511–514. doi: 10.1007/s00213-006-0452-x. [DOI] [PubMed] [Google Scholar]
- 50.Anderson I.M., Sarsfield A., Haddad P.M. Efficacy, safety and tolerability of quetiapine augmentation in treatment resistant depression: An open-label, pilot study. J. Affect. Disord. 2009;117:116–119. doi: 10.1016/j.jad.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Shelton R.C., Tollefson G.D., Tohen M., Stahl S., Gannon K.S., Jacobs T.G., Buras W.R., Bymaster F.P., Zhang W., Spencer K.A., et al. A novel augmentation strategy for treating resistant major depression. Am. J. Psychiatry. 2001;158:131–134. doi: 10.1176/appi.ajp.158.1.131. [DOI] [PubMed] [Google Scholar]
- 52.Boku S., Inoue T., Honma H., Honma H., Nakagawa S., Koyama T. Olanzapine augmentation of milnacipran for stage 2 treatment-resistant major depression: An open study. Hum. Psychopharmacol. 2011;26:237–241. doi: 10.1002/hup.1197. [DOI] [PubMed] [Google Scholar]
- 53.Earley W.R., Guo H., Németh G., Harsányi J., Thase M.E. Cariprazine augmentation to antidepressant therapy in Major Depressive Disorder: Results of a randomized, double-blind, placebo-controlled trial. Psychopharmacol. Bull. 2018;48:62–80. doi: 10.64719/pb.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer M., Dell’osso L., Kasper S., Pitchot W., Dencker Vansvik E., Köhler J., Jørgensen L., Montgomery S.A. Extended-release quetiapine fumarate (quetiapine XR) monotherapy and quetiapine XR or lithium as add-on to antidepressants in patients with treatment-resistant major depressive disorder. J. Affect. Disord. 2013;151:209–219. doi: 10.1016/j.jad.2013.05.079. [DOI] [PubMed] [Google Scholar]
- 55.Hobart M., Skuban A., Zhang P., Josiassen M.K., Hefting N., Augustine C., Brewer C., Sanchez R., McQuade R.D. Efficacy and safety of flexibly dosed brexpiprazole for the adjunctive treatment of major depressive disorder: A randomized, active-referenced, placebo-controlled study. Curr. Med. Res. Opin. 2018;34:633–642. doi: 10.1080/03007995.2018.1430220. [DOI] [PubMed] [Google Scholar]
- 56.Gobbi G., Ghabrash M.F., Nuñez N., Tabaka J., Di Sante J., Saint-Laurent M., Vida S., Kolivakis T., Low N., Cervantes P., et al. Antidepressant combination versus antidepressants plus second-generation antipsychotic augmentation in treatment-resistant unipolar depression. Int. Clin. Psychopharmacol. 2018;33:34–43. doi: 10.1097/YIC.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 57.Mohamed S., Johnson G.R., Chen P., Hicks P.B., Davis L.L., Yoon J., Gleason T.C., Vertrees J.E., Weingart K., Sheetal M., et al. Effect of antidepressant switching vs augmentation on remission among patients with Major Depressive Disorder unresponsive to antidepressant treatment: The VAST-D Randomized Clinical Trial. JAMA. 2017;318:132–145. doi: 10.1001/jama.2017.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein G., Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br. J. Psychiatry. 1993;162:634–640. doi: 10.1192/bjp.162.5.634. [DOI] [PubMed] [Google Scholar]
- 59.Nierenberg A.A., Papakostas G.I., Petersen T., Montoya H.D., Worthington J.J., Tedlow J., Alpert J.E., Fava M. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J. Clin. Psychopharmacol. 2003;23:92–95. doi: 10.1097/00004714-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Bauer M., Bschor T., Kunz D., Berghöfer A., Ströhle A., Müller-Oerlinghausen B. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am. J. Psychiatry. 2000;157:1429–1435. doi: 10.1176/appi.ajp.157.9.1429. [DOI] [PubMed] [Google Scholar]
- 61.Bschor T., Berghöfer A., Ströhle A., Kunz D., Adli M., Müller-Oerlinghausen B., Bauer M. How long should the lithium augmentation strategy be maintained? A 1-year follow-up of a placebo-controlled study in unipolar refractory major depression. J. Clin. Psychopharmacol. 2002;22:427–430. doi: 10.1097/00004714-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Thase M.E., Kupfer D.J., Frank E., Jarrett D.B. Treatment of imipramine-resistant recurrent depression: II. An open clinical trial of lithium augmentation. J. Clin. Psychiatry. 1989;50:413–417. [PubMed] [Google Scholar]
- 63.Dallal A., Fontaine R., Ontiveros A., Elie R. Lithium carbonate augmentation of desipramine in refractory depression. Can. J. Psychiatry. 1990;35:608–611. doi: 10.1177/070674379003500709. [DOI] [PubMed] [Google Scholar]
- 64.Fontaine R., Ontiveros A., Elie R., Vézina M. Lithium carbonate augmentation of desipramine and fluoxetine in refractory depression. Biol. Psychiatry. 1991;29:946–948. doi: 10.1016/0006-3223(91)90062-Q. [DOI] [PubMed] [Google Scholar]
- 65.Dinan T.G. Lithium augmentation in sertraline-resistant depression: A preliminary dose-response study. Acta Psychiatr. Scand. 1993;88:300–301. doi: 10.1111/j.1600-0447.1993.tb03461.x. [DOI] [PubMed] [Google Scholar]
- 66.Hoencamp E., Haffmans J., Dijken W.A., Huijbrechts I.P. Lithium augmentation of venlafaxine: An open-label trial. J. Clin. Psychopharmacol. 2000;20:538–543. doi: 10.1097/00004714-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Bertschy G., Ragama-Pardos E., Aït-Ameur A., Muscionico M., Favre S., Roth L. Lithium augmentation in venlafaxine non-responders: An open study. Eur. Psychiatry. 2003;18:314–317. doi: 10.1016/j.eurpsy.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer B., Rosen J., Thornton J.E., Perel J.M., Reynolds C.F. Adjunctive lithium carbonate in nortriptyline-resistant elderly depressed patients. J. Clin. Psychopharmacol. 1991;11:254–256. doi: 10.1097/00004714-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Flint A.J., Rifat S.L. A prospective study of lithium augmentation in antidepressant-resistant geriatric depression. J. Clin. Psychopharmacol. 1994;14:353–356. doi: 10.1097/00004714-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Buspavanich P., Behr J., Stamm T., Schlattmann P., Bschor T., Richter C., Hellweg R., Heinz A., Berger M., Hindinger C., et al. Treatment response of lithium augmentation in geriatric compared to non-geriatric patients with treatment-resistant depression. J. Affect. Disord. 2019;251:136–140. doi: 10.1016/j.jad.2019.03.057. [DOI] [PubMed] [Google Scholar]
- 71.Kok R.M., Vink D., Heeren T.J., Nolen W.A. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: An open, randomized, controlled trial. J. Clin. Psychiatry. 2007;68:1177–1185. doi: 10.4088/JCP.v68n0803. [DOI] [PubMed] [Google Scholar]
- 72.Dorée J.P., Des Rosiers J., Lew V., Gendron A., Elie R., Stip E., Tourjman S.V. Quetiapine augmentation of treatment-resistant depression: A comparison with lithium. Curr. Med. Res. Opin. 2007;23:333–341. doi: 10.1185/030079906X162809. [DOI] [PubMed] [Google Scholar]
- 73.Yoshimura R., Hori H., Umene-Nakano W., Ikenouchi-Sugita A., Katsuki A., Atake K., Nakamura J. Comparison of lithium, aripiprazole and olanzapine as augmentation to paroxetine for inpatients with major depressive disorder. Ther. Adv. Psychopharmacol. 2014;4:123–129. doi: 10.1177/2045125313514767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schindler F., Anghelescu I.G. Lithium versus lamotrigine augmentation in treatment resistant unipolar depression: A randomized, open-label study. Int. Clin. Psychopharmacol. 2007;22:179–182. doi: 10.1097/YIC.0b013e328014823d. [DOI] [PubMed] [Google Scholar]
- 75.Ivković M., Damjanović A., Jovanović A., Cvetić T., Jasović-Gasić M. Lamotrigine versus lithium augmentation of antidepressant therapy in treatment-resistant depression: Efficacy and tolerability. Psychiatr. Danub. 2009;21:187–193. [PubMed] [Google Scholar]
- 76.Schüle C., Baghai T.C., Eser D., Nothdurfter C., Rupprecht R. Lithium but not carbamazepine augments antidepressant efficacy of mirtazapine in unipolar depression: An open-label study. World J. Biol. Psychiatry. 2009;10:390–399. doi: 10.1080/15622970701849978. [DOI] [PubMed] [Google Scholar]
- 77.Joffe R.T., Singer W., Levitt A.J., MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch. Gen. Psychiatry. 1993;50:387–393. doi: 10.1001/archpsyc.1993.01820170065008. [DOI] [PubMed] [Google Scholar]
- 78.Gervasoni N., Aubry J.M., Gex-Fabry M., Bertschy G., Bondolfi G. Is there a place for tricyclic antidepressants and subsequent augmentation strategies in obtaining remission for patients with treatment resistant depression? Pharmacol. Res. 2009;59:202–206. doi: 10.1016/j.phrs.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Whyte E.M., Basinski J., Farhi P., Dew M.A., Begley A., Mulsant B.H., Reynolds C.F. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J. Clin. Psychiatry. 2004;65:1634–1641. doi: 10.4088/JCP.v65n1208. [DOI] [PubMed] [Google Scholar]
- 80.Kok R.M., Nolen W.A., Heeren T.J. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr. Scand. 2009;119:274–281. doi: 10.1111/j.1600-0447.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- 81.Barbosa L., Berk M., Vorster M. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J. Clin. Psychiatry. 2003;64:403–407. doi: 10.4088/JCP.v64n0407. [DOI] [PubMed] [Google Scholar]
- 82.Santos M.A., Rocha F.L., Hara C. Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: A randomized, placebo-controlled, double-blind study. Prim. Care Compan. J. Clin. Psychiatry. 2008;10:187–190. doi: 10.4088/PCC.v10n0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barbee J.G., Jamhour N.J. Lamotrigine as an augmentation agent in treatment-resistant depression. J. Clin. Psychiatry. 2002;63:737–741. doi: 10.4088/JCP.v63n0813. [DOI] [PubMed] [Google Scholar]
- 84.Rocha F.L., Hara C. Lamotrigine augmentation in unipolar depression. Int. Clin. Psychopharmacol. 2003;18:97–99. doi: 10.1097/00004850-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Gutierrez R.L., McKercher R.M., Galea J., Jamison K.L. Lamotrigine augmentation strategy for patients with treatment-resistant depression. CNS Spectr. 2005;10:800–805. doi: 10.1017/S1092852900010324. [DOI] [PubMed] [Google Scholar]
- 86.Shapira B., Nemets B., Trachtenberg A., Belmaker R.H. Phenytoin as an augmentation for SSRI failures: A small controlled study. J. Affect. Disord. 2006;96:123–126. doi: 10.1016/j.jad.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Karaiskos D., Pappa D., Tzavellas E., Siarkos K., Katirtzoglou E., Papadimitriou G.N., Politis A. Pregabalin augmentation of antidepressants in older patients with comorbid depression and generalized anxiety disorder-an open-label study. Int. J. Geriatr. Psychiatry. 2013;28:100–105. doi: 10.1002/gps.3800. [DOI] [PubMed] [Google Scholar]
- 88.Ghabrash M.F., Comai S., Tabaka J., Saint-Laurent M., Booij L., Gobbi G. Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression. World J. Biol. Psychiatry. 2016;17:165–170. doi: 10.3109/15622975.2015.1073856. [DOI] [PubMed] [Google Scholar]
- 89.Mowla A., Kardeh E. Topiramate augmentation in patients with resistant major depressive disorder: A double-blind placebo-controlled clinical trial. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:970–973. doi: 10.1016/j.pnpbp.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 90.Fornaro M., Martino M., Dalmasso B., Colicchio S., Benvenuti M., Rocchi G., Escelsior A., Perugi G. An open pilot study of zonisamide augmentation in major depressive patients not responding to a low dose trial with duloxetine: Preliminary results on tolerability and clinical effects. Ann. Gen. Psychiatry. 2011;10:23. doi: 10.1186/1744-859X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang Y., Yuan C., Xu Y., Chen J., Wu Z., Cao L., Yi Z., Hong W., Wang Y., Jiang K., et al. A pilot study of the efficacy and safety of paroxetine augmented with risperidone, valproate, buspirone, trazodone, or thyroid hormone in adult Chinese patients with treatment-resistant major depression. J. Clin. Psychopharmacol. 2011;31:638–642. doi: 10.1097/JCP.0b013e31822bb1d9. [DOI] [PubMed] [Google Scholar]
- 92.Shiroma P.R., Johns B., Kuskowski M., Wels J., Thuras P., Albott C.S., Lim K.O. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J. Affect. Disord. 2014;155:123–129. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 93.Cusin C., Ionescu D.F., Pavone K.J., Akeju O., Cassano P., Taylor N., Eikermann M., Durham K., Swee M., Chang T., et al. Ketamine augmentation for outpatients with treatment-resistant depression: Preliminary evidence for two-step intravenous dose escalation. Aust. N. Z. J. Psychiatry. 2017;51:55–64. doi: 10.1177/0004867416631828. [DOI] [PubMed] [Google Scholar]
- 94.Fava M., Freeman M.P., Flynn M., Judge H., Hoeppner B.B., Cusin C., Ionescu D.F., Mathew S.J., Chang L.C., Iosifescu D.V., et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) Mol. Psychiatry. 2020;25:1592–1603. doi: 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ionescu D.F., Bentley K.H., Eikermann M., Taylor N., Akeju O., Swee M.B., Pavone K.J., Petrie S.R., Dording C., Mischoulon D., et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. J. Affect. Disord. 2019;243:516–524. doi: 10.1016/j.jad.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 96.Freeman M.P., Papakostas G.I., Hoeppner B., Mazzone E., Judge H., Cusin C., Mathew S., Sanacora G., Iosifescu D., DeBattista C., et al. Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J. Psychiatr. Res. 2019;110:166–171. doi: 10.1016/j.jpsychires.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salloum N.C., Fava M., Hock R.S., Freeman M.P., Flynn M., Hoeppner B., Cusin C., Iosifescu D.V., Trivedi M.H., Sanacora G., et al. Time to relapse after a single administration of intravenous ketamine augmentation in unipolar treatment-resistant depression. J. Affect. Disord. 2020;260:131–139. doi: 10.1016/j.jad.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feeney A., Hock R.S., Freeman M.P., Flynn M., Hoeppner B., Iosifescu D.V., Trivedi M.H., Sanacora G., Mathew S.J., Debattista C., et al. The effect of single administration of intravenous ketamine augmentation on suicidal ideation in treatment-resistant unipolar depression: Results from a randomized double-blind study. Eur. Neuropsychopharmacol. 2021;49:122–132. doi: 10.1016/j.euroneuro.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fedgchin M., Trivedi M., Daly E.J., Melkote R., Lane R., Lim P., Vitagliano D., Blier P., Fava M., Liebowitz M., et al. Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1) Int. J. Neuropsychopharmacol. 2019;22:616–630. doi: 10.1093/ijnp/pyz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Popova V., Daly E.J., Trivedi M., Cooper K., Lane R., Lim P., Mazzucco C., Hough D., Thase M.E., Shelton R.C., et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined with a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry. 2019;176:428–438. doi: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- 101.Daly E.J., Trivedi M.H., Janik A., Li H., Zhang Y., Li X., Lane R., Lim P., Duca A.R., Hough D., et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with Treatment-Resistant Depression: A randomized clinical trial. JAMA Psychiatry. 2019;76:893–903. doi: 10.1001/jamapsychiatry.2019.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ochs-Ross R., Daly E.J., Zhang Y., Lane R., Lim P., Morrison R.L., Hough D., Manji H., Drevets W.C., Sanacora G., et al. Efficacy and Safety of Esketamine Nasal Spray Plus an Oral Antidepressant in Elderly Patients With Treatment-Resistant Depression-TRANSFORM-3. Am. J. Geriatr. Psychiatry. 2020;28:121–141. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 103.Wajs E., Aluisio L., Holder R., Daly E.J., Lane R., Lim P., George J.E., Morrison R.L., Sanacora G., Young A., et al. Esketamine nasal spray plus oral antidepressant in patients with Treatment-Resistant Depression: Assessment of long-term safety in a Phase 3, open-label study (SUSTAIN-2) J. Clin. Psychiatry. 2020;81:19m12891. doi: 10.4088/JCP.19m12891. [DOI] [PubMed] [Google Scholar]
- 104.Price L.H., Charney D.S., Delgado P.L., Heninger G.R. Fenfluramine augmentation in tricyclic-refractory depression. J. Clin. Psychopharmacol. 1990;10:312–317. doi: 10.1097/00004714-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Richards C., McIntyre R.S., Weisler R., Sambunaris A., Brawman-Mintzer O., Gao J., Geibel B., Dauphin M., Madhoo M. Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: Results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J. Affect. Disord. 2016;206:151–160. doi: 10.1016/j.jad.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Patkar A.A., Masand P.S., Pae C.U., Peindl K., Hooper-Wood C., Mannelli P., Ciccone P. A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. Clin. Psychopharmacol. 2006;26:653–656. doi: 10.1097/01.jcp.0000246212.03530.fd. [DOI] [PubMed] [Google Scholar]
- 107.Ravindran A.V., Kennedy S.H., O'Donovan M.C., Fallu A., Camacho F., Binder C.E. Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: Results of a double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry. 2008;69:87–94. doi: 10.4088/JCP.v69n0112. [DOI] [PubMed] [Google Scholar]
- 108.Nasr S. Modafinil as adjunctive therapy in depressed outpatients. Ann. Clin. Psychiatry. 2004;16:133–138. doi: 10.1080/10401230490486954. [DOI] [PubMed] [Google Scholar]
- 109.Mendlewicz J., Kriwin P., Oswald P., Souery D., Alboni S., Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: A pilot open-label study. Int. Clin. Psychopharmacol. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 110.McAllister-Williams R.H., Anderson I.M., Finkelmeyer A., Gallagher P., Grunze H.C., Haddad P.M., Hughes T., Lloyd A.J., Mamasoula C., McColl E., et al. Antidepressant augmentation with metyrapone for treatment-resistant depression (the ADD study): A double-blind, randomised, placebo-controlled trial. Lancet Psychiatry. 2016;3:117–127. doi: 10.1016/S2215-0366(15)00436-8. [DOI] [PubMed] [Google Scholar]
- 111.Avari J.N., Kanellopoulos D., Solomonov N., Oberlin L., Alexopoulos G.S. Minocycline augmentation in older adults with persistent depression: An open label proof of concept study. Int. Psychogeriatr. 2020;32:881–884. doi: 10.1017/S1041610220001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nettis M.A., Lombardo G., Hastings C., Zajkowska Z., Mariani N., Nikkheslat N., Worrell C., Enache D., McLaughlin A., Kose M., et al. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: Results from a double-blind randomised clinical trial. Neuropsychopharmacology. 2021;46:939–948. doi: 10.1038/s41386-020-00948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moreno F.A., Gelenberg A.J., Bachar K., Delgado P.L. Pindolol augmentation of treatment-resistant depressed patients. J. Clin. Psychiatry. 1997;58:437–439. doi: 10.4088/JCP.v58n1005. [DOI] [PubMed] [Google Scholar]
- 114.Sokolski K.N., Conney J.C., Brown B.J., DeMet E.M. Once-daily high-dose pindolol for SSRI-refractory depression. Psychiatry Res. 2004;125:81–86. doi: 10.1016/j.psychres.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Pérez V., Soler J., Puigdemont D., Alvarez E., Artigas F. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Grup de Recerca en Trastorns Afectius. Arch. Gen. Psychiatry. 1999;56:375–379. doi: 10.1001/archpsyc.56.4.375. [DOI] [PubMed] [Google Scholar]
- 116.Perry E.B., Berman R.M., Sanacora G., Anand A., Lynch-Colonese K., Charney D.S. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: A double-blind, randomized, controlled trial. J. Clin. Psychiatry. 2004;65:238–243. doi: 10.4088/JCP.v65n0215. [DOI] [PubMed] [Google Scholar]
- 117.Price L.H., Charney D.S., Heninger G.R. Reserpine augmentation of desipramine in refractory depression: Clinical and neurobiological effects. Psychopharmacology. 1987;92:431–437. doi: 10.1007/BF00176473. [DOI] [PubMed] [Google Scholar]
- 118.Miller K.K., Perlis R.H., Papakostas G.I., Mischoulon D., Losifescu D.V., Brick D.J., Fava M. Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS Spectr. 2009;14:688–694. doi: 10.1017/S1092852900023944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dichtel L.E., Carpenter L.L., Nyer M., Mischoulon D., Kimball A., Deckersbach T., Dougherty D.D., Schoenfeld D.A., Fisher L., Cusin C., et al. Low-Dose Testosterone Augmentation for Antidepressant-Resistant Major Depressive Disorder in Women: An 8-Week Randomized Placebo-Controlled Study. Am. J. Psychiatry. 2020;177:965–973. doi: 10.1176/appi.ajp.2020.19080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rudas S., Schmitz M., Pichler P., Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol. Psychiatry. 1999;45:229–233. doi: 10.1016/S0006-3223(98)00033-X. [DOI] [PubMed] [Google Scholar]
- 121.Hori H., Kunugi H. The efficacy of pramipexole, a dopamine receptor agonist, as an adjunctive treatment in treatment-resistant depression: An open-label trial. Sci. World J. 2012;2012:372474. doi: 10.1100/2012/372474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cassano P., Lattanzi L., Fava M., Navari S., Battistini G., Abelli M., Cassano G.B. Ropinirole in Treatment-Resistant Depression: A 16-week pilot study. Can. J. Psychiatry. 2005;50:357–360. doi: 10.1177/070674370505000612. [DOI] [PubMed] [Google Scholar]
- 123.Mischoulon D., Hylek L., Yeung A.S., Clain A.J., Baer L., Cusin C., Ionescu D.F., Alpert J.E., Soskin D.P., Fava M. Randomized, proof-of-concept trial of low dose naltrexone for patients with breakthrough symptoms of major depressive disorder on antidepressants. J. Affect. Disord. 2017;208:6–14. doi: 10.1016/j.jad.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 124.De Berardis D., Marini S., Serroni N., Rapini G., Iasevoli F., Valchera A., Signorelli M., Aguglia E., Perna G., Salone A., et al. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: An open label, fixed dose, single-blind study. Sci. World J. 2013;2013:204649. doi: 10.1155/2013/204649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Targum S.D., Cameron B.R., Ferreira L., MacDonald I.D. An augmentation study of MSI-195 (S-adenosylmethionine) in Major Depressive Disorder. J. Psychiatr. Res. 2018;107:86–96. doi: 10.1016/j.jpsychires.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 126.Siwek M., Dudek D., Paul I.A., Sowa-Kućma M., Zieba A., Popik P., Pilc A., Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009;118:187–195. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 127.Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., Leucht S., Ruhe H.G., Turner E.H., Higgins J.P.T., et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults With Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus. 2018;16:420–429. doi: 10.1176/appi.focus.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou X., Ravindran A.V., Qin B., Del Giovane C., Li Q., Bauer M., Liu Y., Fang Y., Da Silva T., Zhang Y., et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: Systematic review and network meta-analysis. J. Clin. Psychiatry. 2015;76:e487–e498. doi: 10.4088/JCP.14r09204. [DOI] [PubMed] [Google Scholar]
- 129.Strawbridge R., Carter B., Marwood L., Bandelow B., Tsapekos D., Nikolova V.L., Taylor R., Mantingh T., De Angel V., Patrick F., et al. Augmentation therapies for treatment-resistant depression: Systematic review and meta-analysis. Br. J. Psychiatry. 2019;214:42–51. doi: 10.1192/bjp.2018.233. [DOI] [PubMed] [Google Scholar]
- 130.Girlanda F., Cipriani A., Agrimi E., Appino M.G., Barichello A., Beneduce R., Bighelli I., Bisoffi G., Bisogno A., Bortolaso P., et al. Effectiveness of lithium in subjects with treatment-resistant depression and suicide risk: Results and lessons of an underpowered randomised clinical trial. BMC Res. Notes. 2014;7:731. doi: 10.1186/1756-0500-7-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blier P., Szabo S.T. Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J. Clin. Psychiatry. 2005;66:30–40. [PubMed] [Google Scholar]
- 132.Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry Clin. Neurosci. 2019;73:613–627. doi: 10.1111/pcn.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith-Apeldoorn S.Y., Veraart J., Kamphuis J., van Asselt A., Touw D.J., Aan Het Rot M., Schoevers R.A. Oral esketamine for treatment-resistant depression: Rationale and design of a randomized controlled trial. BMC Psychiatry. 2019;19:375. doi: 10.1186/s12888-019-2359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Capuzzi E., Caldiroli A., Capellazzi M., Tagliabue I., Marcatili M., Colmegna F., Clerici M., Buoli M., Dakanalis A. Long-Term Efficacy of Intranasal Esketamine in Treatment-Resistant Major Depression: A Systematic Review. Int. J. Mol. Sci. 2021;22:9338. doi: 10.3390/ijms22179338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fornaro M., Fusco A., Anastasia A., Cattaneo C.I., De Berardis D. Brexpiprazole for treatment-resistant major depressive disorder. Expert Opin. Pharmacother. 2019;20:1925–1933. doi: 10.1080/14656566.2019.1654457. [DOI] [PubMed] [Google Scholar]
- 136.Kryst J., Kawalec P., Pilc A. Efficacy and safety of intranasal esketamine for the treatment of major depressive disorder. Expert Opin. Pharmacother. 2020;21:9–20. doi: 10.1080/14656566.2019.1683161. [DOI] [PubMed] [Google Scholar]
- 137.Huang S.S., Chen H.H., Wang J., Chen W.J., Chen H.C., Kuo P.H. Investigation of early and lifetime clinical features and comorbidities for the risk of developing treatment-resistant depression in a 13-year nationwide cohort study. BMC Psychiatry. 2020;20:541. doi: 10.1186/s12888-020-02935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]