Abstract
Objectives: To describe clinical characteristics and management of intensive care units (ICU) patients with laboratory-confirmed COVID-19 and to determine 90-day mortality after ICU admission and associated risk factors. Methods: This observational retrospective study was conducted in six intensive care units (ICUs) in three university hospitals in Marseille, France. Between 10 March and 10 May 2020, all adult patients admitted in ICU with laboratory-confirmed SARS-CoV-2 and respiratory failure were eligible for inclusion. The statistical analysis was focused on the mechanically ventilated patients. The primary outcome was the 90-day mortality after ICU admission. Results: Included in the study were 172 patients with COVID-19 related respiratory failure, 117 of whom (67%) received invasive mechanical ventilation. 90-day mortality of the invasively ventilated patients was 27.4%. Median duration of ventilation and median length of stay in ICU for these patients were 20 (9–33) days and 29 (17–46) days. Mortality increased with the severity of ARDS at ICU admission. After multivariable analysis was carried out, risk factors associated with 90-day mortality were age, elevated Charlson comorbidity index, chronic statins intake and occurrence of an arterial thrombosis. Conclusion: In this cohort, age and number of comorbidities were the main predictors of mortality in invasively ventilated patients. The only modifiable factor associated with mortality in multivariate analysis was arterial thrombosis.
Keywords: Covid-19, SARS-CoV-2, intensive care unit, acute respiratory distress syndrome, mechanical ventilation, prognostic factors
1. Introduction
Coronavirus disease (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide from China. It was declared pandemic by World Health Organization in March 2020 [1]. Despite all efforts its propagation still remains difficult to manage in Western countries, placing constant pressure on healthcare systems by an increasing need for intensive care units (ICUs) beds and prolonged hospitalisations. As of 1 June 2021, 5,677,172 confirmed cases and 109,662 related-deaths were reported in France [2]. To date, several publications [3,4,5,6,7,8] have given a consistent description of the critically ill patients with COVID-19. Most have reported a 28-day mortality ranging between 28 and 44% with patients still receiving ICU support at the point of publication. To our knowledge, only two European publications reported a 3-months mortality. The first is the French prospective study COVID-ICU [9], which described a cohort of 4244 patients admitted in ICU with a 90-day mortality of 31%. The second is the Dutch retrospective study ProVENT-COVID [10], which reported a 43% mortality rate at day 90 in a cohort of 533 patients who all received invasive mechanical ventilation (IMV). The aim of our multicentre study was to describe the characteristics and management of the 172 critically ill patients admitted in our institution (Assistance Publique—Hôpitaux de Marseille, AP-HM, Marseille, France) and to evaluate risk factors associated with 90-day mortality. Particular attention was given to the group of invasively ventilated patients to allow comparison with other works.
2. Materials and Methods
2.1. Study Design
In this observational retrospective study data were collected from all consecutive adult patients with SARS-CoV-2 admitted to six ICUs of three university hospitals in Marseille (Hôpital de la Timone, Hôpital Nord de Marseille, Hôpital de la Conception, Marseille, France) in a single institution (Assistance Publique des Hôpitaux de Marseille, AP-HM—Aix Marseille University, Marseille, France) during the first wave of the outbreak from 10 March 2020 until 10 May 2020. The SARS-CoV-2 positive diagnosis was defined as a positive result of real-time reverse transcriptase-polymerase chain reaction (PCR) assay of nasal or pharyngeal swabs. Some patients were excluded from analysis, including patients with unconfirmed positive PCR, patients without respiratory symptom and those with viral confirmation concomitant to another organ failure.
2.2. Data Collection
Data were obtained retrospectively for each patient from their electronic charts. We collected the baseline characteristics including age, sex, body mass index (BMI) > 25 kg/m2, comorbidities and long-term medications. Metabolic syndrome was confirmed using the International Diabetes Federation definition [11]. ABO blood group was reported using electronic records of our institutional blood bank. To assess the severity, several scoring systems were used based on the data collected during the first 24 h after ICU admission: SAPS II (Simplified Acute Physiology Score II), SOFA (Sequential Organ Failure Assessment), APACHE II (Acute Physiology and Chronic Health Evaluation II), Charlson comorbidity index, NEWS and modified NEWS scores [12], MuLBSTA score, Murray score, CURB-65 score and ROX index. When patients underwent non-invasive ventilation, an oxygen flow—FiO2 conversion table was used to estimate the PaO2:FiO2 ratio (see Figure S1). Regarding the laboratory results, only worst values within first 48 h after ICU admission were considered for statistical analysis. We reported analysis of the first chest computed tomography (CT) scan performed after hospital admission, if one exists, based on automated volumetry. Severity of disease based on CT scan was then graded as normal, minimal, intermediate or severe by our radiologists, based on the number of affected pulmonary segments (See Appendix A).
The patient management, including supportive measures and pharmacologic agents, was performed at the discretion of the treating physicians, including decision of tracheal intubation. Respiratory support devices, mechanical ventilation settings and pressure levels in the first 24 h were recorded. For the patients undergoing IMV, Day-1 PaO2:FiO2 ratio and its corresponding acute respiratory distress syndrome (ADRS) severity based on the Berlin definition were reported [13]. Mechanical power (J/min) was calculated using following formula: 0.098 × tidal volume × respiratory rate × (peak pressure—0.5 × driving pressure) [14]. Driving pressure was defined as plateau pressure minus positive end-expiratory pressure (PEEP).
Significative events and complications during ICU stay such as thrombosis or nosocomial infections were reported. Ventilator-associated pneumonia was only considered if clinical suspicion was associated with microbiological documentation. Otherwise, all clinical and radiological criteria were required [15]. Sepsis-3 criteria were used for sepsis shock authentication [16]. Withholding an invasive ventilation for ethical reasons formally documented in the patient chart was reported. Number of daily available ICU beds and occupation rates were obtained by the administration of our local institution.
Finally, we considered date of symptoms onset, date of admission to hospital and ICU, date of death or hospital discharge and vital status at hospital discharge and after 3 months for evaluation. For this last information, we contacted hospitals and rehabilitation centres by phone if the patients had already left our institution.
2.3. Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or as median with interquartile range (Q1, Q3), and categorical variables are reported as count and percentages. Comparisons of means values between two groups were performed using student t-test or Mann–Whitney U. Comparisons of percentages were performed using Chi-square test or (Fisher’s exact test, as appropriate). The overall survival (OS) was defined as the time from the date of ICU admission to date of death. In order to identify predictive factors of death, univariate and multivariate survival analyses were performed using the Cox proportional-hazards model. Multivariate analysis included variables that were statistically significant in the univariate analysis and takes into account multiple comparisons with an FDR analysis. The results are reported as hazard-ratios with 95% confidence intervals. All statistical tests were two-sided and the threshold for statistical significance was p < 0.05. Statistical analysis was performed using PASW Statistics version 17.02 (IBM SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Enrolled Patients and Characteristics
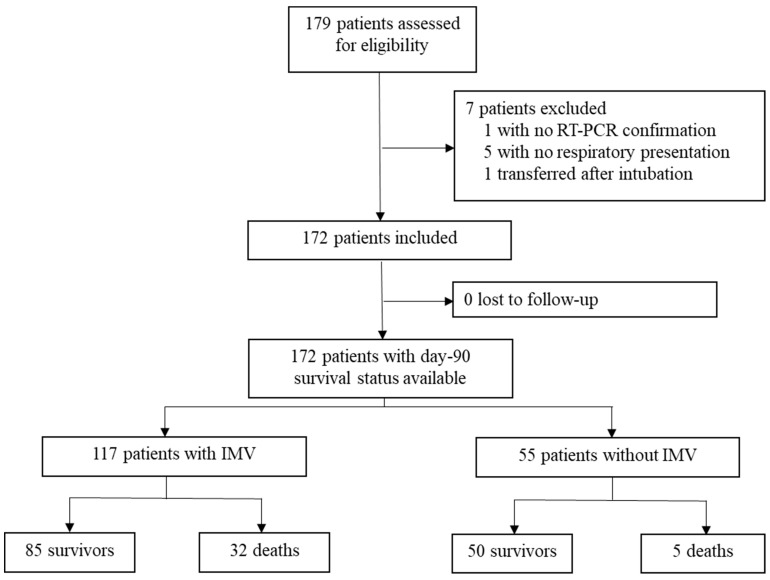
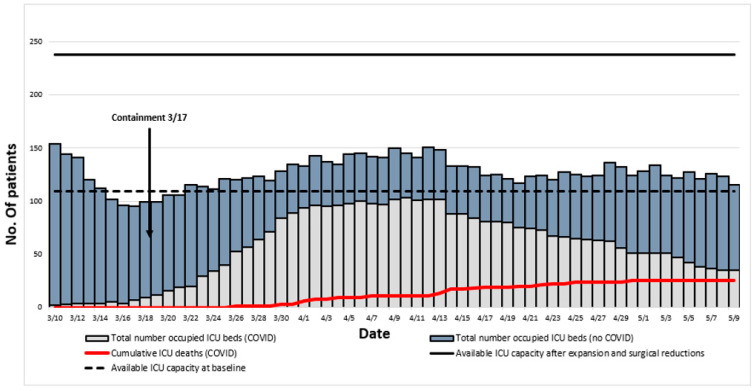
Between 10 March 2020 and 10 May 2020, 172 patients were admitted in ICU for a pneumonia with laboratory-confirmed SARS-CoV-2 infection. The flow chart of the study inclusions is reported in Figure 1. Their complete characteristics and outcomes are reported in Supplementary Tables S1 and S2. Evolution of daily inpatient prevalence and available ICU capacity during this period is reported in Figure 2.
Figure 1.
Flow Chart of the population study.
Figure 2.
Evolution of daily inpatient prevalence and available ICU capacity.
117 patients (67%) received IMV. Baseline characteristics are reported in Table 1. At ICU admission median age of the ventilated patients was 63 (56–72) years, with 88 men (75.2%) and 29 women (24.8%). The most frequent reported comorbidities were hypertension 68/117 (58.1%), BMI > 25 kg/m2 73/117 (62.4%), diabetes 24/117 (20.5%) and immunodeficiency 24/117 (20.5%). The average Charlson comorbidity index was 3 (2–4). Their median SAPS II and SOFA score in the first 24h after ICU admission were 34 (27–40) and 5 (3–7), respectively. A chest computed tomography in the first days after hospitalisation was available for 84/117 patients (72%). Based on automated volumetry, the median volume of lung lesions was 31.8% (15.6–46.6) of the parenchyma, mostly ground glass. Lymphopenia was commonly observed, with a median value of 0.69 (0.5–0.95) × 109/L. The inflammatory syndrome was characterised by elevated values for CRP and ferritin with respective medians of 201 mg/L (126–302) and 1418 ng/mL (968–2321).
Table 1.
Baseline characteristics of the 117 patients on invasive mechanical ventilation according to their 90-day survival status.
| Total (N = 117) |
Survivors (N = 85) |
Non-Survivors (N = 32) |
p-Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age | 63 (56–72) | 61 (54–67) | 71.5 (62.75–77) | <0.001 |
| Male | 88 (75.2) | 64 (75.3) | 24 (75.0) | 0.974 |
| ABO blood group | ||||
| A | 42 (35.9) | 32 (37.6) | 10 (31.2) | 0.605 |
| B | 17 (14.5) | 13 (15.3) | 4 (12.5) | 0.419 |
| O | 41 (35) | 30 (35.3) | 11 (34.4) | 0.366 |
| AB | 2 (1.7) | 1 (1.2) | 1 (3.1) | 0.218 |
| BMI > 25 kg·m2 | 73 (62.4) | 59 (69.4) | 14 (43.8) | 0.011 |
| Hypertension | 68 (58.1) | 48 (56.5) | 20 (62.5) | 0.556 |
| Diabetes | 24 (20.5) | 15 (17.6) | 9 (28.1) | 0.211 |
| Metabolic syndrome | 34 (29.1) | 29 (34.1) | 5 (15.6) | 0.05 |
| Chronic respiratory disease | 22 (18.8) | 15 (17.6) | 7 (21.9) | 0.602 |
| Chronic kidney disease | 11 (9.4) | 8 (9.4) | 3 (9.4) | 0.995 |
| Immunodeficiency | 24 (20.5) | 15 (17.6) | 9 (28.1) | 0.211 |
| Statins intake | 23 (19.7) | 12 (14.1) | 11 (34.4) | 0.014 |
| Systemic steroids intake | 9 (7.7) | 6 (7.1) | 3 (9.4) | 0.675 |
| Charlson index | 3 (2–4) | 2 (1–3) | 4 (2–6) | <0.001 |
| SAPS II score | 34 (27–40) | 30.5 (25–38.25) | 38 (35–45) | 0.02 |
| SOFA score | 5 (3–7) | 4 (3–7) | 5 (3–6) | 0.966 |
| Total lungs volume on CT (cm3) | 3257 (2447–4016) | 3142 (2285–3804) | 3742 (3275–4158) | 0.01 |
| Lesions/lungs ratio on CT (%) | ||||
| Ground glass/lungs ratio | 24.8 (12.6–34.4) | 26.9 (13.6–36.6) | 21.5 (7.1–28.2) | 0.862 |
| Condensations/lungs ratio | 5.1 (2.1–12.4) | 6.6 (3.1–16) | 2.1 (0.3–8.2) | 0.495 |
| All lesions/lungs ratio | 31.8 (15.6–46.6) | 34.1 (19.9–49.7) | 26.8 (9.2–36.3) | 0.034 |
| Days from symptoms onset to intubation |
9 (6–11.25) | 8.5 (6–11) | 9 (6–12) | 0.3 |
| Days from ICU admission to intubation |
0 (0–1) | 1 (0–1) | 0 (0–1) | 0.364 |
| Biology (worst value during first 48H after ICU admission) | ||||
| Lymphocyte count (×109/L) | 0.69 (0.5–0.95) | 0.7 (0.53–1) | 0.61 (0.44–0.78) | 0.382 |
| Neutrophil to Lymphocyte Ratio | 11.1 (8.3–15.5) | 10.6 (7.5–15.3) | 12.6 (10.5–19.8) | 0.005 |
| D-Dimers (mg/L) | 3.44 (1.64–5) | 3.34 (1.52–5) | 4 (1.84–5) | 0.9 |
| Fibrinogen (g/L) | 8.1 (6.9–9.4) | 8.1 (7.1–9.5) | 8 (6.9–9.4) | 0.995 |
| CRP (mg/L) | 201(126–302) | 179 (120–248) | 283 (162–324) | 0.29 |
| Creatinine (µmol/L) | 85 (67–138) | 84 (65–127) | 98 (78–153) | 0.048 |
| LDH (UI/L) | 447 (368–535) | 435 (347–491) | 539 (443–635) | 0.005 |
| Ferritin (ng/mL) | 1418 (968–2321) | 1175 (950–1778) | 2728 (2342–6049) | 0.021 |
| Invasive ventilation parameters during first 24 h | ||||
| PaO2/FiO2 | 130 (100–180) | 140 (100–180) | 120 (100–160) | 0.457 |
| PEEP (cmH2O) | 12 (10.3–14) | 12 (12–14) | 12 (10–15) | 0.483 |
| Plateau pressure (cm H2O) | 25 (21.7–28.3) | 23.5 (21.0–28.2) | 26.0 (23.6–28.8) | 0.353 |
| Respiratory compliance (mL/cmH2O) | 33 (28.2–45) | 40 (29–46) | 31 (27–40) | 0.018 |
| Mechanical power (J/min) | 15.6 (13.3–19.6) | 15.5 (13.5–19.1) | 15.9 (12–19.8) | 0.836 |
Results are expressed as n (%) or median (25th–75th percentile). Statistical significance in bold.
3.2. Severity of ARDS and Respiratory Support
High flow oxygen and non-invasive ventilation before intubation were used for 69/117 (59%) and 16/117 (13.7%) patients, respectively. In these invasively ventilated patients, 19/117 (16.2%) suffered from mild, 60/117 (51.3%) from moderate and 24/117 (20.5%) from severe ARDS. Veno-venous Extra Corporeal Membrane Oxygenation (ECMO) was provided to 25/117 (19.7%) patients with a median duration of 13.5 (10–21.5) days.
3.3. Complications and Outcomes
Complications and outcomes according to the day-90 survival status are presented in Table 2. Ventilator-associated pneumonia occurred in 71/117 (60.7%). Venous thrombosis (including patients suffering from pulmonary embolism) were diagnosed in 35/117 (29.9%) while arterial thrombosis were diagnosed in 10/117 (8.5%). Of note, a severe bleeding event was reported in 25 patients (21.5%).
Table 2.
Management, complications and outcomes of the 117 patients on invasive mechanical ventilation according to their 90-day survival status.
| Total (N = 117) |
Survivors (N = 85) |
Non-Survivors (N = 32) |
p-Value | |
|---|---|---|---|---|
| Management in ICU | ||||
| Use of non-invasive ventilation before intubation |
16 (13.7) | 12 (14.1) | 4 (12.5) | 0.693 |
| Use of high-flow oxygen before intubation |
69 (59) | 51 (60) | 18 (56.2) | 0.589 |
| Neuromuscular blockade | 113 (96.6) | 82 (96.5) | 31 (96.9) | 0.923 |
| Prone positioning | 99 (84.6) | 72 (84.7) | 27 (84.4) | 0.965 |
| ECMO | 23 (19.7) | 18 (21.2) | 5 (15.6) | 0.501 |
| Vasopressors | 105 (89.7) | 74 (87.1) | 31 (96.9) | 0.122 |
| Renal replacement therapy | 20 (17.1) | 12 (14.1) | 8 (25) | 0.167 |
| Corticosteroids a | 23 (19.7) | 14 (12.0) | 9 (7.7) | 0.157 |
| Hydroxychloroquine (10 days) with azithromycin (5 days) |
45 (38.5%) | 36(42.4) | 9 (28.1) | 0.159 |
| Remdesivir | 0 (0) | 0(0) | 0 (0) | - |
| Lopinavir-ritonavir | 18 (15.4) | 13(15.3) | 5 (15.6) | 0.965 |
| Complications | ||||
| Ventilator associated pneumonia | 71 (60.7) | 53 (62.4) | 18 (56.2) | 0.547 |
| Septic shock | 47 (40.2) | 32 (37.6) | 15 (46.9) | 0.364 |
| Venous thrombosis or pulmonary embolism |
35 (29.9) | 29 (34.1) | 6 (18.8) | 0.106 |
| Arterial thrombosis | 10 (8.5) | 4 (4.7) | 6 (18.8) | 0.015 |
| Severe bleeding event | 25 (21.4) | 17 (20) | 8 (25) | 0.556 |
| Outcomes | ||||
| Duration of ventilation (days) | 20 (9–33) | 21 (11–34) | 18 (6.75–25.25) | 0.06 |
| Ventilator-free days at d28 (days) | 2 (1–7) | 4 (1–7) | 0 (0–1) | <0.001 |
| Length of stay in ICU (days) | 29 (17–46) | 33 (19–53) | 21 (6.75–31.75) | 0.02 |
| Length of in hospital (days) | 37 (24–53) | 42 (29–57) | 25 (8.75–38.25) | <0.001 |
| 28-day mortality | 21 (17.9) | 0 (0) | 21 (60) | - |
Results are expressed as n (%) or median (25th–75th percentile). Statistical significance in bold. a Irrespective of the dose and the timing.
The 90-day mortality rate was 27.4% for the patients requiring IMV. Among the patients who received invasive or non-invasive ventilation on the day of ICU admission, the day-90 mortality rate increased with the severity of ARDS at ICU admission (13.5%, 23.5% and 28.6% for mild, moderate and severe ARDS, respectively). Median duration of ventilation and median length of stay in ICU for intubated patients were 20 (9–33) days and 29 (17–46) days, respectively. Multivariable analysis is shown in Table 3. Age, Charlson index, chronic statins treatment and arterial thrombosis were associated with increased risk of 90-day mortality in the group of mechanically ventilated patients (analysis of survival according to age categories is reported in Figure S2).
Table 3.
Univariate and multivariate Cox regression analysis (N = 117).
| Associated Factors |
Univariate HR (95% CI) |
p-Value | Multivariate 1 HR (95% CI) |
p-Value | Multivariate 2 HR (95% CI) |
p-Value |
|---|---|---|---|---|---|---|
| Age ≥ 65 years | 3.27 (1.51–7.08) | 0.007 | 4.17 (1.48–11.73) | 0.010 | - | - |
| Male | 0.86 (0.39–1.92) | 0.718 | - | - | - | - |
| Charlson Index ≥ 3 |
5.58 (1.96–15.90) | 0.005 | - | - | 3.72 (1.07–12.92) | 0.05 |
| Arterial thrombosis |
2.22 (0.91–5.42) | 0.098 | 3.79 (1.22–11.80) | 0.022 | 2.86 (1.00–8.20) | 0.05 |
| Statins intake |
2.55 (1.23–5.31) | 0.020 | 3.78 (1.51–9.43) | 0.010 | 3.59 (1.44–8.93) | 0.186 |
HR (95% CI): Hazard Ratio (95% Confidence Interval). Due to collinearity between age and Charlson Comorbidity index, we performed two different models with the same included variables, but in model 1 with age and model 2 with Charlson Comorbidity index. Statistical significance in bold with an FDR analysis.
4. Discussion
4.1. Mortality
In this cohort of 172 critically ill patients with COVID-19, overall 28-day and 90-day mortality were 15.7% and 21.5%, respectively. This is noticeably lower than in previous reports [3,4,5,6,7,8,9,10,17]. As a major risk factor for mortality, the various proportion of patients undergoing IMV in each of these cohorts seems to be the main determinant of these heterogeneous outcomes. Indeed, an observational study in Vancouver [8] with a similar number of intubated patients reported a comparable mortality in ICU. If we focus on the day-90 outcome, our observation is consistent with the mortality of 31% reported in the European prospective study COVID-ICU [9] where 80% of patients had undergone IMV. Nevertheless, even the number of deaths in the subgroups of invasively ventilated patients varies widely between these studies, ranging from 30% to 96.8%. In our cohort, corresponding 28-day and 90-day mortality was 18.8% and 27.4%, respectively. Lower mortality and later deaths led in our cohort to longer lengths of ICU and hospital stays with 29 (17–46) and 37 (24–53) days for ventilated patients respectively. Median duration of IMV was also longer than in other studies and that traditionally observed in non-COVID ARDS [18]. This can create a vicious circle in overwhelmed hospitals: if surviving from COVID-19 needs time, physicians may be faced with difficult limitations to advance life support in patients with long invasive ventilation.
4.2. Risk Factors
Baseline characteristics of the mechanically ventilated patients in our cohort are similar to those reported elsewhere. Age, number of comorbidities and dyslipidaemia authenticated by statins intake were associated with 90-day mortality in multivariate analysis. As previously reported, male gender, hypertension, diabetes or metabolic syndrome are overrepresented in these critically ill patients compared to the general population, but are consistent variables in a multivariate model to predict death. More surprisingly, overweight stands in this cohort as a protective factor for mortality. This result has to be taken with caution due to the absence of stratification on BMI and the potential coexistence of confusion factors. This may be due to an empirical decision for early ICU admission management, showing a greater concern of practitioners for these patients. As well, studies with more complete data for this variable found no effect of obesity on final mortality in ICU [9,10,19,20]. Looking now at initial results of paraclinical exams, we did not find any unexpected parameter associated with mortality; most of them attest to the severity, but are unable to predict good or bad evolution in severe cases. Regarding ABO blood group distribution, we did not find any difference with general population, as it was mentioned is some other publications [21,22,23]. Finally, it has been reported that severity of the extent on initial chest CT could be predictive of a bad evolution [24]. In our cohort, we found an association between damage volumes assessed by automated volumetry or by visual scoring and 90-day mortality only in univariate analysis. Statistical difference on the criteria of total lungs volume shall be ignored, as a quick analysis showed that age was an obvious confusion factor. It is important to note that due to the pathophysiology of the COVID-19 disease and the extensive lesions that occur over time, the time to perform chest CT is probably an important variable to handle. If we examine the prognostic scores, usual critical care score, such as SAPS II, still remained the most accurate to predict final outcome. Respiratory scores calculated on initial parameters were not relevant to estimate probability of death. Lastly, number of arterial thrombotic events was significantly higher in non-survivors despite similar anticoagulation therapy, but it occurred mostly in already severe cases, including, for example, patients on ECMO, representing 5/10 (50%) cases of arterial thrombosis.
4.3. Management
We will now formulate several hypotheses to explain the lower mortality observed in our cohort. First, median values of the different severity of illness scores, including SAPS II, APACHE II or SOFA, are slightly but invariably lower than in the other studies. As our patients were comparable in terms of age distribution and comorbidities, it would mean that we had encountered less severe cases. However, this difference was not found in the group of invasively ventilated patients when regarding PaO2/FiO2 ratio, ventilation pressure levels or lung compliances recorded after intubation. In our opinion, the main point to highlight is the late date of our first admissions in ICU compared to the rest of France and Europe. Indeed, our first critically ill patient was admitted on 10 March when general containment began in France only seven days later on 17 March. If this measure did have an efficiency, then it certainly benefited us by reducing the surge of patients, maybe allowing admission of less severe cases. Our study confirms that a major increase in ICU beds may be organised without any significant increase in mortality. After reorganisation our institution included up to 238 ICU beds, against 109 ICU beds before the outbreak. Finally, the highest number of COVID inpatients in ICU at the same time was 104, allowed us to admit them all in standard ICU beds (see Figure 2). In addition, early stop of nonurgent surgeries contributed to release the pressure on beds, but also enabled allocation to ICUs of qualified professionals, such as nurses with previous experience in critical care and anaesthesiologists, who are also trained in intensive care medicine in France. Lastly, special efforts were made by the different private hospitals in Marseille, which admitted a large number of patients with COVID-19 in all degrees of severity. As we truly believe that there is a strong correlation between workload in ICUs and global mortality [25], we are convinced that all mentioned measures contributed to the observed outcomes.
The second great advantage of a late epidemy was the possibility we had to learn from the experience formed in the first plagued regions. It consisted mostly of two salient points. First of them was the rapid emerging evidence of an endothelial dysfunction [26,27], with noticeably high number of thrombotic events. On 3 April 2020, the French Society of Anaesthesia and Intensive Care Medecine published guidelines assuming the necessity to treat severe cases with curative anticoagulation [28]. At this time, only 16% of our patients had already left ICU or were deceased, thus the others probably benefitted from this therapy although it is not still clearly established by strong evidence. The second point concerns respiratory support devices. Initially, early intubation was widely practiced in Europe [29], what could have led to a misconception of COVID-19 as a respiratory disease with two profiles [30]. However, this was not reported in later studies [9,10,31]. Observing that the use of high-flow nasal canula had not led to an increase of contaminations in the units that had tried it, we provided therefore this support to almost all the patients who did not request immediate invasive ventilation. We held back intubation until it seems unavoidable, what explains the lower rate of invasively ventilated patients in our cohort. The trend over time observed in the study COVID-ICU [9] confirmed that this rate did not only depend on the ARDS severity but also on the choice of an early or delayed intubation strategy.
The last hypothesis to consider is the strategy of massive testing that was adopted by our institution [32]. This has been reported elsewhere as a protective factor [33]. Early recognition and hospitalisation of severe cases might have prevented some additional admissions of exhausted patients who would have waited too long at home, until the moment where invasive ventilation had become inevitable. Notably, no specific antiviral treatment (hydroxychloroquine, lopinavir/ritonavir, etc.) was associated with lower mortality in our cohort.
4.4. Strengths and Limitations
The major strengths of this study are the exhaustive description of our cohort, including original data rarely reported in similar publications up to this day like prognostic scores or chest tomography volumetry, and the 3-months follow-up. Our study included all consecutive patients with COVID-19 related acute respiratory failure. As we were able to maintain usual standard in our critical care practices, due to a contained pressure on our ICUs, we believe that outcomes presented here are much closer to the true clinical course of the disease.
This study has also several limitations. Our cohort is relatively small. Data were collected retrospectively from electronic charts which lead to a significative proportion of missing data or the absence of stratification for some variables. In particular, data concerning mechanical parameters were scarce. Of course, because of the retrospective nature of the study, any relationship between engaged therapies and final vital status should be interpretated with caution. This study was conducted before the results of the RECOVERY trial [34] and then the wide use of dexamethasone in severe COVID-19; therefore, we cannot analyse its effects. Finally, all inclusions were made in the same geographical area, which could limit an extension of the conclusions to other areas.
5. Conclusions
In this case series of 172 critically ill patients with COVID-19 in Marseille, France, the 90-day mortality was 21.5% in the whole cohort and 27.4% in the group of mechanically ventilated patients. This is lower than in many previous similar publications and it may reinforce the idea of a correlation between collective capacity to contain the overwhelming aspect of ICUs and final outcomes. This study allowed us to learn lessons for future COVID-19 outbreaks. Age and comorbidities had a major impact on outcome. The only modifiable factor associated with mortality in multivariate analysis was arterial thrombosis. The prevalence of thrombotic events was very high in our cohort. This encourages an empirical strategy of systematic anticoagulation despite no clear evidence of its benefit in randomised trials. It shows also that a safe increase in ICU beds may be anticipated during pandemics with the help of anaesthesiologists and nurses who are not practising ICU care on a regular basis but have an experience in intensive care, supervised by ICU specialists. These observations may also encourage some empirical strategies that have been now widely adopted, such as systematic anticoagulation or delayed intubation, even though randomised studies are needed.
Acknowledgments
We thank J. Albanese, N. Bruder, P. Chanez, M. Gainnier, J.R. Harle, L. Papazian, J.M. Forel, C. Guidon, V. Veit, L. Zieleskiewicz for collecting cases. We gratefully acknowledge all the health care workers on the front line and all the patients involved in the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10235650/s1, Figure S1: FiO2 estimation based on O2 flow (L/min); Figure S2: Kaplan–Meier analysis of survival during the 90 days following ICU admission, according to age categories (<65 or ≥65 years); Table S1: Complete data of the 172 patients according to their 90-day survival status; Table S2: Complete data of the 117 ventilated patients according to their 90-day survival status.
Appendix A
Methodology of the Chest Computed Tomography Analysis
We reported analysis of the first chest computed tomography (CT) realized after hospital admission. All patients underwent low-dose chest computed tomography (LDCT) on the same system (Revolution EVO -GE Healthcare, WI, USA). All LDCT were unenhanced scans, in profound and maximal inspiration, with following parameters: detector collimation: 0.625 mm; field of view: 500 mm; matrix: 512 × 512; pitch: 1.375; gantry speed: 0.35 s; 100–120 KV; 45 mAs; and reconstructed slice thickness: 1.2 mm. All imaging data were reconstructed using high resolution and standard algorithms. Pre-established top anatomic border was the lower part of the neck. Bottom boundary was location of the adrenal gland.
The extent of the lesions (ground glass opacities, crazy paving or areas of consolidation) was visually classified into 4 different types for each lung segment: no lesion was defined as a strictly normal pattern and was rated 0, minimal involvement was defined as less than 25% of segment involvement and was rated 1, intermediate involvement was defined for a segment with more than 25% and less than 50% involvement and severe involvement more than 50% of a segment and rated 10. The final score was obtained by summing the score of each segment. It was ranked between 0 and 200 that allow classifying 4 groups: normal (score = 0), minimal (1–19), intermediate (20–49), severe (50–200).
Another analysis consisted in complete deep learning (DL) pipeline that allows a fully automated segmentation of COVID-19 pulmonary lesions on LDCT and computation of lesions volumes and extent.
Author Contributions
M.V. and D.T. contributed to the conception and design of the study, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. D.T., M.V., Y.B., J.B., H.M., J.C., M.B., N.P., A.B., D.L., S.B., A.J., V.B. and B.P. contributed to the acquisition of data. J.B., D.T., G.A., P.S., M.L., L.V., N.B. and M.V. contributed to the analysis and interpretation of the data. A.L. and L.B. contributed to the statistical analyses. All authors participated in manuscript writing, revision and approval for final submission. M.V. and D.T. contributed equally and shared first authorship. All authors have read and agreed to the published version of the manuscript.
Funding
The authors receive no funding for this work.
Institutional Review Board Statement
This study was approved by the local Ethics Commission (2020-53) and the French Society of Anaesthesia and Intensive Care Medecine (00010254-2020-06).
Informed Consent Statement
According to French law, a written informed consent from patients was not necessary, owing to the retrospective nature of the study, but the patients had the possibility to withdraw their health data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author: max-ime.volff@laposte.net.
Conflicts of Interest
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Mar 11, 2020. [(accessed on 5 August 2021)]. Available online: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 2.Santé Publique France—COVID-19, Point Épidémiologique Hebdomadaire du 3 Juin 2021. [(accessed on 5 August 2021)]. Available online: www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-3-juin-2021.
- 3.Armstrong R.A., Kane A.D., Cook T.M. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 4.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., et al. Risk Factors Associated with Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quah P., Li A., Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: A systematic review of the emerging literature. Crit. Care. 2020;24:285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra A., Fergusson N.A., Lloyd-Smith E., Wormsbecker A., Foster D., Karpov A., Crowe S., Haljan G., Chittock D.R., Kanji H.D., et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: A case series. CMAJ. 2020;192:E694–E701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2020;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botta M., Tsonas A.M., Pillay J., Boers L.S., Algera A.G., Bos L.D., Dongelmans D.A., Hollmann M.W., Horn J., Vlaar A.P., et al. Ventilation management and clinical outcome in invasively ventilated COVID-19 patients (PRoVENT-COVID)—A national, multicentre, observational cohort study. Lancet Respir. Med. 2020;9:139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti K.G.M.M., Zimmet P., Shaw J., IDF Epidemiology Task Force Consensus Group The metabolic syndrome—A new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 12.Liao X., Wang B., Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: Preparing intensive care units—The experience in Sichuan Province, China. Intensive Care Med. 2020;46:357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L., Tonetti T., Cressoni M., Cadringher P., Herrmann P., Moerer O., Protti A., Gotti M., Chiurazzi C., Carlesso E., et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016;42:1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 15.Leone M., Bouadma L., Bouhemad B., Brissaud O., Dauger S., Gibot S., Hraiech S., Jung B., Kipnis E., Launey Y., et al. Hospital-acquired pneumonia in ICU. Anaesth. Crit. Care Pain Med. 2018;37:83–98. doi: 10.1016/j.accpm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L., Xu J., Wu Y., Huang C., Ouyang Y., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., Van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020;180:1436–1446. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kooistra E.J., de Nooijer A.H., Claassen W.J., Grondman I., Janssen N.A.F., Netea M.G., van de Veerdonk F.L., van der Hoeven J.G., Kox M., Pickkers P. A higher BMI is not associated with a different immune response and disease course in critically ill COVID-19 patients. Int. J. Obes. 2021;45:687–694. doi: 10.1038/s41366-021-00747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoiland R.L., Fergusson N.A., Mitra A.R., Griesdale D.E.G., Devine D.V., Stukas S., Cooper J., Thiara S., Foster D., Chen L.Y.C., et al. The association of ABO blood group with indices of disease severity and multiorgan dysfunction in COVID-19. Blood Adv. 2020;4:4981–4989. doi: 10.1182/bloodadvances.2020002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y., Cheng Y., Cheng G., Chui C.H., Lau F.Y., Chan P.K.S., Ng M.H.L., Sung J.J.Y., Wong R.S.M. ABO Blood Group and Susceptibility to Severe Acute Respiratory Syndrome. JAMA. 2005;293:1447–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 23.Latz C.A., Decarlo C., Boitano L., Png C.Y.M., Patell R., Conrad M.F., Eagleton M., Dua A. Blood type and outcomes in patients with COVID-19. Ann. Hematol. 2020;99:2113–2118. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombi D., Bodini F.C., Petrini M., Maffi G., Morelli N., Milanese G., Silva M., Sverzellati N., Michieletti E. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology. 2020;296:E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fergusson N.A., Ahkioon S., Nagarajan M., Park E., Ding Y., Ayas N., Dhingra V.K., Chittock D.R., Griesdale D.E.G. Association of intensive care unit occupancy during admission and inpatient mortality: A retrospective cohort study. Can. J. Anaesth. 2019;67:213–224. doi: 10.1007/s12630-019-01476-8. [DOI] [PubMed] [Google Scholar]
- 26.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Gandet F.F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susen S., Tacquard C.A., Godon A., Mansour A., Garrigue D., Nguyen P., Godier A., Testa S., Albaladejo P., Gruel Y. Traitement Anticoagulant pour la Prévention du Risque Thrombotique Chez Un Patient Hospitalisé avec COVID-19 et Surveillance de L’hémostase. Propositions du GIHP et du GFHT. [(accessed on 10 September 2021)]. Available online: https://sfar.org/download/traitement-anticoagulant-pour-la-prevention-du-risque-thrombotique-chez-un-patient-hospitalise-avec-covid-19-et-surveillance-de-lhemostase.
- 29.Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrando C., Suarez-Sipmann F., Mellado-Artigas R., Hernández M., Gea A., Arruti E., Aldecoa C., Martínez-Pallí G., Martínez-González M.A., Slutsky A.S., et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IHU Méditerranée Infection—Statistics of Testing and Hospitalization for SARS-CoV-2 Infection in the Area of Marseille, France. [(accessed on 14 September 2021)]. Available online: https://www.mediterranee-infection.com/
- 33.Liang L.-L., Tseng C.-H., Ho H.J., Wu C.-Y. Covid-19 mortality is negatively associated with test number and government effectiveness. Sci. Rep. 2020;10:12567. doi: 10.1038/s41598-020-68862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent J.-L., Rello J., Marshall J.K., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author: max-ime.volff@laposte.net.