Abstract
Differentiation between Mycobacterium tuberculosis and M. avium is essential for the treatment of mycobacterial infections. We have developed an easy and rapid detection assay for the diagnosis of mycobacterial diseases. This is a PCR-hybridization assay based on selective amplification of a 16S rRNA gene sequence using pan-Mycobacterium primers followed by hybridization of the amplification products to biotinylated M. tuberculosis and M. avium-specific probes. A total of 55 mycobacterial isolates were tested. For all isolates, results concordant with those of conventional identification methods were obtained. Moreover, we developed a method for extraction of DNA from Ziehl-Neelsen-positive smears which allows the recovery of intact target DNA in our PCR-hybridization assay. Our method was able to confirm all culture results for 59 Ziehl-Neelsen-positive smears from clinical specimens (35 sputum, 11 lymph node biopsy, 6 stool, 4 pus, 2 urine, and 1 pericardial fluid specimens). These data suggest that our PCR-hybridization assay, which is simple to perform and less expensive than commercial probe methods, may be suitable for the identification of M. tuberculosis and M. avium. It could become a valuable alternative approach for the diagnosis of mycobacterial infections when applied directly to DNA extracted from Ziehl-Neelsen-positive smears as well.
Since the mid-1980s, mycobacterial infections have become increasingly widespread for a number of biological and social reasons, in particular, the human immunodeficiency virus epidemic. Together with the increasing incidence of tuberculosis, the incidences of Mycobacterium avium diseases and other nontuberculous mycobacterial infections have also increased (12, 20, 21). Rapid discrimination between M. tuberculosis and M. avium is of primary importance for the initiation of a correct chemotherapeutic regimen, because the two infections require different types of therapy and management (12, 13, 20).
The advent of PCR has been a breakthrough in the diagnosis of mycobacterial infections. A number of M. tuberculosis-specific sequences can now be amplified (3, 5, 10, 11, 18), and different PCR, restriction enzyme analysis, and hybridization assays have been developed for the diagnosis of M. avium complex infection (2, 4, 6–8, 16, 17, 22, 24–27, 30, 31). However, PCR methods are complicated, limited to few mycobacterial species, and restricted to research laboratories; in addition, the high cost of the commercial methods somewhat hampers the practical use of amplification diagnosis in routine analysis, mainly for different clinical samples.
The aim of this study was to develop a PCR-hybridization technique based on the amplification of a 16S rRNA gene sequence using pan-Mycobacterium primers followed by hybridization of the amplification products to M. tuberculosis- and M. avium-specific probes; this technique may be an alternative in-house method for species differentiation. Moreover, we evaluated the possible use of this method in routine mycobacteriosis diagnosis of Ziehl-Neelsen-positive smears obtained from clinical specimens.
MATERIALS AND METHODS
Strains and clinical specimens.
The mycobacterial strains (11 American Type Culture Collection [ATCC] strains and 44 clinical isolates) and the nonmycobacterial strains (4 ATCC strains and 9 clinical isolates) listed in Table 1 were used to determine the coverage of the pan-Mycobacterium amplification and the specificity of the M. tuberculosis- and M. avium-specific primers. Hence, 38 acid-fast-bacillus (AFB)-positive smears from different clinical specimens (18 sputum, 9 lymph node biopsy, 6 stool, 2 pus, 2 urine, and 1 pericardial fluid specimens) with culture-confirmed infection by M. tuberculosis or M. avium were subsequently investigated by PCR-hybridization. Thirty AFB-negative smears (13 sputum, 10 lymph node biopsy, 4 pus, and 3 stool specimens) and 21 AFB-positive smears (17 sputum, 2 lymph node biopsy, and 2 pus specimens) obtained from patients affected by mycobacterioses other than those caused by M. tuberculosis and M. avium were also tested as negative controls (Table 2). Sample decontamination, Ziehl-Neelsen staining, cultural isolation, and identification were performed by standard methods (14, 15, 29).
TABLE 1.
PCR amplification of different mycobacterial and nonmycobacterial species using the pan-Mycobacterium primers
| Speciesa | No. of isolates | Pan-Mycobacterium amplification resultc |
|---|---|---|
| Mycobacterium | ||
| M. asiaticum ATCC 25276 | 1 | + |
| M. avium ATCC 15769 | 1 | + |
| M. fortuitum ATCC 6841 | 1 | + |
| M. gordonae ATCC 14470 | 1 | + |
| M. haemophilum ATCC 43160 | 1 | + |
| M. intracellulare ATCC 13950 | 1 | + |
| M. marinum ATCC 11565 | 1 | + |
| M. phlei ATCC 11758 | 1 | + |
| M. scrofulaceum ATCC 19981 | 1 | + |
| M. smegmatis ATCC 19420 | 1 | + |
| M. tuberculosis ATCC 27294 | 1 | + |
| M. aviumb | 20 | + |
| M. bovisb | 1 | + |
| M. chelonaeb | 1 | + |
| M. kansasiib | 1 | + |
| M. tuberculosisb | 20 | + |
| M. xenopib | 1 | + |
| Other bacteria and fungusa | ||
| Acinetobacter calcoaceticus ATCC 23055 | 1 | − |
| Citrobacter freundii ATCC 8090 | 1 | − |
| Enterobacter cloacae ATCC 13047 | 1 | − |
| Neisseria meningitidis ATCC 13102 | 1 | − |
| Candida albicansb | 1 | − |
| Escherichia colib | 1 | − |
| Haemophilus influenzaeb | 1 | − |
| Klebsiella pneumoniaeb | 1 | − |
| Proteus mirabilisb | 1 | − |
| Pseudomonas aeruginosab | 1 | − |
| Staphylococcus aureusb | 1 | − |
| Staphylococcus epidermidisb | 1 | − |
| Streptococcus pneumoniaeb | 1 | − |
Mycobacterial and bacterial species and a fungus used to determine the coverage of primers M-OU-1 and M-OL-2.
Clinical isolates from the University of Milan, Milan, Italy.
Amplification results were determinated by gel electrophoresis. Symbols: +, presence of amplification products; −, absence of amplification products.
TABLE 2.
Comparison of PCR-hybridization assay results with culture results for the detection of M. tuberculosis and M. avium from Ziehl-Neelsen-positive smears
| Specimen origin | Ziehl- Neelsen resulta | No. of specimens | Identification by culture | PCR-hybridization assay result obtained with a probe specific for:
|
|
|---|---|---|---|---|---|
| M. tuberculosis | M. avium | ||||
| Sputum | 1+ | 5 | M. tuberculosis | Positive | Negative |
| 2+ | 1 | M. tuberculosis | Positive | Negative | |
| 3+ | 1 | M. tuberculosis | Positive | Negative | |
| 4+ | 4 | M. tuberculosis | Positive | Negative | |
| 1+ | 5 | M. avium | Negative | Positive | |
| 2+ | 1 | M. avium | Negative | Positive | |
| 4+ | 1 | M. avium | Negative | Positive | |
| 1+ | 1 | M. kansasii | Negative | Negative | |
| 2+ | 1 | M. kansasii | Negative | Negative | |
| 1+ | 3 | M. gordonae | Negative | Negative | |
| 2+ | 4 | M. gordonae | Negative | Negative | |
| 1+ | 4 | M. xenopi | Negative | Negative | |
| 2+ | 2 | M. xenopi | Negative | Negative | |
| 1+ | 1 | M. asiaticum | Negative | Negative | |
| 1+ | 1 | M. fortuitum | Negative | Negative | |
| − | 13 | None | Negative | Negative | |
| Lymph node | 1+ | 5 | M. tuberculosis | Positive | Negative |
| 4+ | 1 | M. tuberculosis | Positive | Negative | |
| 1+ | 3 | M. avium | Negative | Positive | |
| 2+ | 1 | M. scrofulaceum | Negative | Negative | |
| 1+ | 1 | M. chelonae | Negative | Negative | |
| − | 10 | None | Negative | Negative | |
| Stool | 2+ | 2 | M. tuberculosis | Positive | Negative |
| 2+ | 3 | M. avium | Negative | Positive | |
| 4+ | 1 | M. avium | Negative | Positive | |
| − | 3 | None | Negative | Negative | |
| Pus | 2+ | 2 | M. tuberculosis | Positive | Negative |
| 1+ | 1 | M. chelonae | Negative | Negative | |
| 1+ | 1 | M. fortuitum | Negative | Negative | |
| − | 4 | None | Negative | Negative | |
| Urine | 2+ | 2 | M. tuberculosis | Positive | Negative |
| Pericardial fluid | 3+ | 1 | M. tuberculosis | Positive | Negative |
Clinical specimens were examined by microscopy after Ziehl-Neelsen staining, and the bacillary index was scored according to the scale of the American Thoracic Society (29).
Extraction of DNA from cultures and Ziehl-Neelsen-positive smears.
Mycobacterial DNA was extracted from cultures in accordance with the method described by van Embden et al. (28). Stained microscopic preparations were washed in xylol and absolute ethanol, scraped with a sterile blade, and collected in a microcentrifuge tube containing phosphate buffer solution. The samples were centrifuged for 10 min at 13,000 rpm, and pellets were resuspended in 100 μl of lysis buffer (Tris-HCl, 10 mM; KCl, 50 mM; MgCl2, 2.5 mM; Tween 20, 0.45%; Nonidet P-40, 0.45%; proteinase K, 10 mg/ml) and incubated for 3 h at 56°C or overnight at 37°C. The samples were then incubated for 15 min at 95°C and centrifuged for 15 min at 15,000 × g, and the supernatants were transferred to a new microcentrifuge tube and directly used for PCR.
PCR assay.
The primers and probes were designed on the basis of the published 16S rRNA fragment sequence (2, 22). The first step of the PCR-hybridization assay was DNA amplification using a pair of pan-Mycobacterium primers able to amplify all mycobacterial species: M-OU-1 (5′-ATAAGCCTGGGAAACTGGGT-3′ [positions 142 to 162]) and M-OL-2 (5′-CACGCTCACAGTTAAGCCGT-3′ [positions 614 to 633]) (Genset, Paris, France). The amplification was performed in a total volume of 50 μl. The reaction mixture consisted of 5 pmol of primers, 200 μM deoxynucleosides triphosphates (dATP, dCTP, and dGTP), 190 μM dTTP, 2.5 U of Taq polymerase Gold (Perkin-Elmer, Norwalk, Conn.), 5 μl of 10× Taq polymerase buffer, 1.5 mM MgCl2, 1 μg of target DNA (from either cultures or smears), and 10 μM digoxigenin (DIG)-11-dUTP (Boehringer GmbH, Mannheim, Germany). The cycling parameters were as follows: 10 cycles of annealing at 58°C for 1 min, elongation at 72°C for 90 s, and denaturation at 94°C for 1 min; 40 cycles of annealing at 58°C for 30 s, elongation at 72°C for 40 s, and denaturation at 94°C for 20 s; and a final annealing (58°C for 1 min) and elongation (72°C for 5 min) step.
Hybridization assay.
After amplification, the DIG-labeled amplified product was hybridized with two biotinylated probes designed to differentiate between M. tuberculosis (5′-ACCACAAGACATGCATCCCG-3′ [positions 182 to 201]) and M. avium (5′-ACCAGAAGACATGCGTCTTG-3′ [positions 182 to 201]).
Briefly, streptavidin-coated plates (Labsystems Oy, Helsinki, Finland) were incubated with a probe at a concentration of 0.1 ng/ml overnight at 4°C. Then, 10 μl of the amplification product was denatured at 98°C for 10 min, diluted in 100 μl of hybridization solution (1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 2× Denhardt's solution, 1 mM EDTA [pH 8.0], 10 mM Tris [pH 7.5]), and incubated in the wells for 1 h at 50°C. After five washes with 6.7 mM phosphate buffer (pH 6.4) containing 130 mM NaCl and 0.1% Tween 20, a horseradish peroxidase-conjugated anti-DIG monoclonal antibody (150 mU/ml) (Boehringer) was added, and the plates were incubated for 1 h at room temperature. Finally, after five washes, the chromogenic substrate (3,3′,5,5′-tetramethylbenzidine) was added. The colorimetric reaction was read at 450 nm using a spectrophotometer. Optical density values lower than 0.4 were considered negative. This cutoff value was the mean of negative controls plus 3 standard deviations.
The detection limit of the PCR-hybridization assay, as estimated from serial dilutions of standard amounts of DNA from M. tuberculosis H37Ra and M. avium ATCC 15769, varied from 1 pg to 1 fg of mycobacterial DNA and human DNA. DNA extracted from Ziehl-Neelsen-positive smears was amplified with β-globin primers PC03 (5′-ACACAACTGTGTTCACTACC-3′) and PC04 (5′-GGTGAACGTGGATGAAGTTG-3′) as described previously (23) to assess the potential presence of PCR inhibitors and the integrity of the template DNA. All the experiments were performed in triplicate in order to evaluate reproducibility. Each PCR-hybridization assay was performed using positive (M. tuberculosis H37Ra and M. avium ATCC 15769) and negative (human DNA and distilled water) controls and sterile procedures, following contamination-free guidelines to prevent false-positive results (15). The chance of PCR contamination was minimized by physical separation of the amplified products from the starting materials, and all pre-PCR processing of materials took place in a room separate from the PCR site (which had a circulation-free, sterile bench and UV lighting) (19).
RESULTS
The PCR-hybridization assay was performed on 55 strains (11 ATCC strains and 44 clinical isolates) of different cultured mycobacteria (21 M. tuberculosis, 21 M. avium, and 13 other mycobacterial strains).
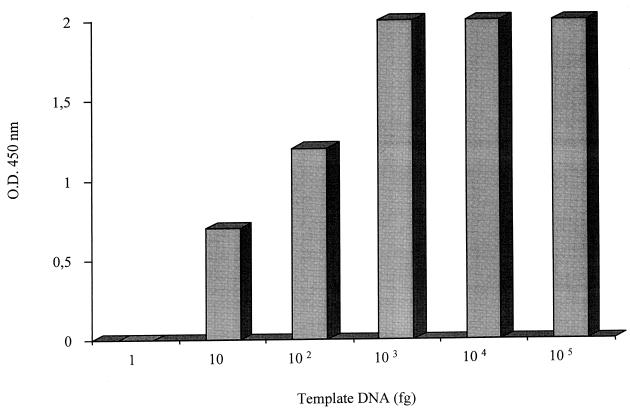
Amplification of the 484-bp fragment by pan-Mycobacterium primers was successful in all 55 mycobacterial isolates. No amplification of human DNA or DNA from 13 bacterial species other than mycobacteria was ever observed under these conditions, indicating the mycobacterial specificity of the primers. The M-OU-1 and M-OL-2 primers efficiently amplified DNA from all mycobacterial isolates, confirming their ability in the identification of all the Mycobacterium species (Table 1). The limit of detection of this method was approximately 100 fg of mycobacterial DNA (Fig. 1). Biotinylated M-TB and M-AV oligonucleotides, specific for M. tuberculosis and M. avium, respectively, were used as probes with 10 μl of mycobacterial DNA amplified with the M-OU-1 and M-OL-2 primers. Twenty-one M. tuberculosis strains were found to be positive when hybridized with the M. tuberculosis-specific probe (M-TB) and negative when hybridized with the M. avium-specific probe (M-AV). All 21 M. avium strains generated a positive signal with the M-AV probe and no signal with the M-TB probe. There was no cross-hybridization with any of the other mycobacterial strains tested, except for M. bovis. No cross-hybridization with 13 other bacterial species or human chromosomal DNA was observed (Table 3).
FIG. 1.
Detection limit for the PCR-hybridization assay, as estimated from serial dilutions of standard amounts of human DNA and mycobacterial DNA (M. tuberculosis H37Ra and M. avium ATCC 15769). O.D., optical density.
TABLE 3.
Hybridization to various bacterial species of M. tuberculosis- and M. avium-specific oligonucleotide probes
| Bacterial species (n) | No. of specimens with the indicated result obtained with a probe specific for:
|
|||
|---|---|---|---|---|
|
M. avium
|
M. tuberculosis
|
|||
| Positive | Negative | Positive | Negative | |
| M. tuberculosis (21) | 0 | 21 | 21 | 0 |
| M. avium (21) | 21 | 0 | 0 | 21 |
| Other mycobacteria (12) | 0 | 12 | 0 | 12 |
| M. bovis (1) | 0 | 1 | 1 | 0 |
| Other bacteria (13) | 0 | 13 | 0 | 13 |
The detection limit of the hybridization assay was 10 fg of mycobacterial DNA (Fig. 1).
To test the possible use in clinical practice of the PCR-hybridization assay, 59 Ziehl-Neelsen-positive and 30 Ziehl-Neelsen-negative smears obtained from clinical samples were tested in parallel with conventional culturing. Amplification of the β-globin gene segment was achieved in all 89 samples. PCR amplification with the M-OU-1 and M-OL-2 primers was successful in all of the 59 Ziehl-Neelsen-positive smears. The amplified products were hybridized with the DIG-labeled M-TB and M-AV oligonucleotides. Consistent with the results obtained from culture samples, the hybridization method applied to clinical specimens was positive exclusively with the corresponding species (specificity, 100%). The same was true for both microscopically strongly positive and paucibacillary specimens (sensitivity, 100%). The probes did not hybridize to the amplification products of any other mycobacterial species or mycobacterial culture-negative specimens. The results are summarized in Table 2.
DISCUSSION
Differentiation between M. tuberculosis and M. avium is essential for the diagnosis and treatment of mycobacterial infections in AIDS patients. Cultural isolation and identification of mycobacterial species usually are performed by time-consuming biochemical tests or by genetic probes, which are quite expensive. Several molecular genetic methods have also been recently reported. These include amplification of species-specific sequences, PCR amplification, restriction enzyme analysis, hybridization with species-specific oligonucleotide probes, and nucleic acid sequence determination. Thus, PCR methods could potentially provide very sensitive, specific, and rapid tests for the detection of mycobacteria. Over the years, several studies have been published proposing different amplification protocols directly feasible for clinical specimens, such as sputum, bronchial washings, and tissue biopsies, mostly for the identification of M. tuberculosis (1, 3, 9). However, no easy method is currently available for the detection and identification of M. tuberculosis and of M. avium. The direct identification of mycobacterial species is hampered by the poor performance of the methods and is often possible only in a few reference centers. Moreover, the wide use of commercial PCR methods still remains quite expensive for routine analysis, rendering impractical direct application to all samples based only on clinical suspicion.
In this study, we propose a simple, in-house nucleic acid amplification protocol that allows direct detection and species identification of M. avium and M. tuberculosis (the most relevant mycobacteria in the clinical setting) with DNA extracted from Ziehl-Neelsen-positive smears.
The good performance of the method that we developed for the extraction of DNA from smears is evidenced by the maintenance of intact template DNA and by the absence of PCR inhibitors, as shown by the results for the β-globin internal control.
Our results show that the PCR-hybridization assay using M-TB and M-AV as probes specific for M. tuberculosis and M. avium, respectively, was highly sensitive and specific; correct identification of M. tuberculosis and M. avium organisms was achieved in all samples. In fact, 10 fg of nucleic acids, corresponding to approximately 3 organisms, yielded a clear-cut positive result. In parallel, the results for Ziehl-Neelsen-positive smears obtained from clinical specimens (sputum, lymph node, stool, pus, urine, and pericardial fluid) proved that our PCR-hybridization assay also allows direct identification of M. tuberculosis and M. avium even in paucibacillary specimens. The applicability of the method directly to DNA extracted from smears could become a valuable alternative approach for the routine diagnosis of mycobacterial infections when used with Ziehl-Neelsen-positive smears.
The PCR-hybridization method with Ziehl-Neelsen-positive smears coupled the advantage of the amplification techniques for rapid diagnosis and the concomitant identification of the different mycobacterial species, allowing the early establishment of the appropriate therapeutic regimens and the prompt isolation of infected patients.
Our method is relatively simple, rapid, and widely applicable in experienced clinical laboratories, and the fact that it is “home brew” means that it is relatively inexpensive. Moreover, limiting the use of the PCR-hybridization assay only to AFB-positive smears could reduce both the costs and the probability of false-positive PCR results. Our assay can be easily performed on stored specimens and therefore can be used in retrospective studies. The use of different specific probes allows direct etiological diagnosis rather than diagnosis by exclusion, as with the M. tuberculosis probe. In this work, we designed the two specific probes for the most clinically important mycobacterial species but, by using available software (GenBank), it is also possible to design specific probes within the 16S rRNA of almost all mycobacterial species and to adapt the screening panel to the species prevalence in each geographical area. However, no differentiation between members of the M. tuberculosis complex can be made, because of the 16S rRNA homology within these sequences.
Further studies will help to establish whether the PCR-hybridization technique can also be used to detect and identify M. tuberculosis and M. avium from particular biological samples (formalin-fixed, paraffin-embedded tissues) and especially from blood specimens at an early stage of growth, given the occurrence of bacteremia observed in immunocompromised populations, such as human immunodeficiency virus-infected subjects.
ACKNOWLEDGMENTS
We are grateful to A. Riva for critical reading of the manuscript and valuable advice.
This work was supported by a grant from the Italian National Institute of Health 2nd National Tuberculosis Project.
REFERENCES
- 1.Beige J, Lokies J, Schaberg T, Finckh U, Mauch M, Lode H, Kohler B, Rolfs A. Clinical evaluation of Mycobacterium tuberculosis PCR assay. J Clin Microbiol. 1995;33:90–95. doi: 10.1128/jcm.33.1.90-95.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böddinghaus B, Rogall T, Flohr T, Blöcker H, Böttger E C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990;28:1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brisson-Noël A, Gicquel B, Lecossier D, Lévy-Frébault V, Nassif X, Hance A J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989;ii:1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z H, Butler W R, Baumstark B R, Ahearn D G. Identification and differentiation of Mycobacterium avium and M. intracellulare by PCR. J Clin Microbiol. 1996;34:1267–1269. doi: 10.1128/jcm.34.5.1267-1269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarridge J E, III, Shawar R M, Shinnick T M, Plikaytis B B. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine mycobacteriology laboratory. J Clin Microbiol. 1993;31:2049–2056. doi: 10.1128/jcm.31.8.2049-2056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousins D, Francis B, Dawson D. Multiplex PCR provides a low-cost alternative to DNA probe methods for rapid identification of Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1996;34:2331–2333. doi: 10.1128/jcm.34.9.2331-2333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Beenhouwer H, Liang Z, De Rijk P, Van Eekeren C, Portaels F. Detection and identification of mycobacteria by DNA amplification and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1995;33:2994–2998. doi: 10.1128/jcm.33.11.2994-2998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devallois A, Picardeau M, Goh K S, Sola C, Vincent V, Rastogi N. Comparative evaluation of PCR and commercial DNA probes for detection and identification to species level of Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1996;34:2756–2759. doi: 10.1128/jcm.34.11.2756-2759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Wit D, Steyn L, Shoemaker S, Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990;28:2437–2441. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenach K D, Cave M D, Bates J H, Crawford J T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990;161:977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- 11.Herrera E A, Segovia M. Evaluation of mtp40 genomic fragment amplification for specific detection of Mycobacterium tuberculosis in clinical specimens. J Clin Microbiol. 1996;34:1108–1113. doi: 10.1128/jcm.34.5.1108-1113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 13.Hoy J, Mijch A, Sandland M, Grayson L, Lucas R, Dwyer B. Quadruple-drug therapy for Mycobacterium avium-intracellulare bacteremia in AIDS. J Infect Dis. 1990;161:801–805. doi: 10.1093/infdis/161.4.801. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins P A, Pattyn S R, Portels F. Diagnostic bacteriology. In: Ratledge C, Stanford J, editors. The biology of the mycobacteria. London, England: Academic Press Ltd.; 1982. pp. 441–476. [Google Scholar]
- 15.Kent P T, Kubica G. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control; 1985. [Google Scholar]
- 16.Kulski J K, Khinsoe C, Pryce T, Christiansen K. Use of multiplex PCR to detect and identify Mycobacterium avium and Mycobacterium intracellulare in blood culture fluids of AIDS patients. J Clin Microbiol. 1995;33:668–674. doi: 10.1128/jcm.33.3.668-674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lappayawichit P, Rienthong S, Rienthong D, Chuchottaworn C, Chaiprasert A, Panbangred W, Saringcarinkul H, Palittapongarnpim P. Differentiation of Mycobacterium species by restriction enzyme analysis of amplified 16S-23S ribosomal DNA spacer sequences. Tuber Lung Dis. 1996;77:257–263. doi: 10.1016/s0962-8479(96)90010-6. [DOI] [PubMed] [Google Scholar]
- 18.Manjunath N, Shankar P, Rajan L, Bhargava A, Saluja S, Shriniwas Evaluation of a polymerase chain reaction for the diagnosis of tuberculosis. Tubercle. 1991;72:21–27. doi: 10.1016/0041-3879(91)90020-s. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti G, Gori A, Catozzi L, Vago L, Nebuloni M, Rossi M C, Degli Esposti A, Bandera A, Franzetti F. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512–1517. doi: 10.1128/jcm.36.6.1512-1517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masur H the Public Health Task Force on Prophylaxis and Therapy for Mycobacterium avium. Special report. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immunodeficiency virus. N Engl J Med. 1993;329:898–905. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- 21.Nightingale S D, Byrd L T, Southern P M, Jockusch J D, Cal S X, Winne B A. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 22.Oggioni M R, Fattorini L, Li B, De Milito A, Zazzi M, Pozzi G, Orefici G, Valensin P E. Identification of Mycobacterium tuberculosis complex, Mycobacterium avium and Mycobacterium intracellulare by selective nested polymerase chain reaction. Mol Cell Probes. 1995;9:321–326. doi: 10.1016/s0890-8508(95)91604-0. [DOI] [PubMed] [Google Scholar]
- 23.Saiki R K, Scharf S, Faloona F, Mullis K B, Horn G T, Erlich H A, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis of sickle cell anemia. Science. 1985;230:1350–1357. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 24.Sansila A, Hongmanee P, Chuchottaworn C, Rienthong S, Rienthong D, Palittapongarnpim P. Differentiation between Mycobacterium tuberculosis and Mycobacterium avium by amplification of the 16S-23S ribosomal DNA spacer. J Clin Microbiol. 1998;36:2399–2403. doi: 10.1128/jcm.36.9.2399-2403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stauffer F, Haber H, Rieger A, Mutschlechner R, Hasenberger P, Tevere V, Young K K Y. Genus level identification of mycobacteria from clinical specimens by using an easy-to-handle Mycobacterium-specific PCR assay. J Clin Microbiol. 1998;36:614–617. doi: 10.1128/jcm.36.3.614-617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telenti A, Marchesi F, Balz M, Bally F, Bottger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weg J G, Farar L S, Kaplan A I, Matthews J H, Sbarbaro J A, Bates J H, et al. Diagnostic standards and classification of tuberculosis and other mycobacterial diseases. Am Rev Respir Dis. 1981;123:343–358. doi: 10.1164/arrd.1981.123.3.343. [DOI] [PubMed] [Google Scholar]
- 30.Wilton S, Cousins D. Detection and identification of multiple mycobacterial pathogens by DNA amplification in a single tube. PCR Methods Appl. 1992;1:269–273. doi: 10.1101/gr.1.4.269. [DOI] [PubMed] [Google Scholar]
- 31.Zhonghing L I, Bai G H, Fordham C, Marino P, Brennan M J, Gine N, Morris S L. Rapid detection of Mycobacterium avium in stool samples from AIDS patients by immunomagnetic PCR. J Clin Microbiol. 1996;34:1903–1907. doi: 10.1128/jcm.34.8.1903-1907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]