Abstract
Objective:
To compare gait biomechanics 6 months following anterior cruciate ligament (ACL) reconstruction (ACLR) between patients with the highest and lowest concentrations of synovial fluid (SF) interleukin-6 (IL-6) and matrix metalloproteinase-3 (MMP-3), as well as compared to uninjured controls.
Design:
SF concentrations of IL-6 and MMP-3 were collected 7 ± 4 days post injury in 38 ACL injured patients (55% female, 21±4yrs, 25.3 ± 5.2BMI). ACL injured individuals were stratified into the lowest and highest quartiles based on IL-6 (IL-6Lowest and IL-6Highest) and MMP-3 (MMP-3Lowest and MMP-3Highest) concentrations. Gait biomechanics were collected on the injured limb 6 months post-ACLR and in 38 uninjured controls (50% female, 21±3yrs, 23.8 ± 2.8BMI). Functional analyses of variance were used to compare vertical ground reaction force (vGRF), knee flexion angle (KFA), and internal knee extension moment (KEM) waveforms throughout stance phase of gait to determine the proportions of stance differing between limbs and groups.
Results:
Compared to uninjured controls, IL-6High and MMP-3High ACL subgroups demonstrated lesser vGRF (largest differences: IL-6, 7.88%BW; MMP-3, 11.05%BW) during early-stance and greater vGRF (largest differences: IL-6, 6.21%BW; MMP-3, 5.85%BW) in mid-stance, lesser KFA (largest differences: IL-6, 3.11°; MMP-3, 3.72°) and lesser KEM (largest differences: IL-6, 0.96%BW•m; MMP-3, 1.07%BW•m) in early-stance, as well as greater KFA in mid-stance (largest differences: IL-6, 1.5°; MMP-3, 2.95°).
Conclusions:
High SF concentrations of a proinflammatory cytokine and a degradative enzyme early post-ACL injury are associated with aberrant gait biomechanics in the injured limb at 6 months post-ACLR (i.e., lesser vGRF, KFA and KEM) linked to posttraumatic osteoarthritis development
Keywords: Biomarkers, Inflammation, ACL, posttraumatic osteoarthritis, knee
1. Introduction
Anterior cruciate ligament (ACL) injuries are one of the most common traumatic knee injuries which occur most frequently in young physically active individuals1. Over the past two decades, there has been an overall increase in the number of ACL reconstructions (ACLR) performed in the United States2. Unfortunately, individuals who sustain an ACL injury are at high risk of developing post-traumatic osteoarthritis (PTOA)3. Specifically, one in three individuals with an ACL injury will develop radiographic PTOA within the first decade post-injury4. The pathogenesis of PTOA is multifaceted, as altered joint biochemistry and aberrant biomechanics caused by the injury are hypothesized to interact and influence the onset and progression of joint tissue breakdown5,6. The interaction between these factors has been demonstrated in cross-sectional studies reporting associations between aberrant gait biomechanics and deleterious concentrations of biomarkers related to joint tissue breakdown in individuals with an ACLR7–9.
There is a lack of longitudinal research to comprehensively determine all of the early aberrant gait biomechanics that are associated with radiographic PTOA at later time points following ACLR. Previous work has demonstrated that gait strategies that elicit lesser tibiofemoral contact forces 6 months post-ACLR are associated with an increased likelihood of developing PTOA at 5 years post-ACLR10. It is possible that the development of aberrant gait biomechanics, that are hypothesized to perpetuate the ongoing breakdown of joint tissues following ACL injury5,6, may be triggered by the initial biochemical response occurring in the first several days following ACL injury. Identifying the association between the initial synovial fluid (SF) biochemical response following ACL injury and later gait biomechanical waveforms may lead to the development of novel early intervention strategies to more effectively modify the cascade of biochemical and biomechanical changes that lead to PTOA onset.
Individuals with ACLR exhibit underloading and a stiffened-knee gait strategy compared to uninjured controls 6 months following ACLR. Underloading can be described as a flattening of the vertical ground reaction force (vGRF) waveform exhibiting lower peaks during early and late stance, and greater vGRF during midstance11. A stiffened-knee gait strategy is characterized by lesser knee flexion angles (KFA) during early stance and lesser total knee flexion excursion between early and midstance (i.e., total knee range of motion between heel strike and midstance), as well as a lesser internal knee sagittal moments during early and late stance12. Underloading and a stiffened-knee gait strategy have been associated with outcomes related to early PTOA development. Increased serum concentrations of biomarkers of type-II collagen turnover (i.e. C2C:CPII ratios)8 and cartilage breakdown (i.e. cartilage oligomeric matrix protein)9, as well as deleterious changes in magnetic resonance imaging markers of cartilage composition13 and worse patient-reported function14. Additionally, the components of the stiffened gait strategy (i.e., lesser peak KFA during early stance, lesser sagittal excursion during middle stance, and lesser internal sagittal moments during early and late stance) have been implicated in the onset, severity, and progression of radiographic knee osteoarthritis15–20. Therefore, vGRF, KFA, and internal knee sagittal moments are important gait biomechanics outcomes related to PTOA onset.
Pro-inflammatory cytokines such as Interleukin-6 (IL-6) and degradative enzymes such as Matrix Metalloproteinase-3 (MMP-3) are biomarkers linked to joint cartilage breakdown21,22 that are dramatically upregulated immediately following ACL injury23,24. Serum concentrations of both IL-6 and MMP-3 have been associated with aberrant gait biomechanics 6 months following ACLR7. Specifically, IL-6 is a pro-inflammatory cytokine associated with joint space narrowing and is used as predictor of knee cartilage loss22. IL-6 is known to promote cartilage degeneration via down regulation of type II collagen synthesis and upregulation of MMPs responsible for driving cartilage extracellular matrix breakdown25. MMP-3 not only degrades cartilage extracellular matrix (e.g., proteoglycans, link protein, and type II collagen)21, but also increases the activity of other cartilage degrading MMPs26. MMP-3 concentrations are elevated after the first year following ACL injury compared to control participants24 and are positively correlated to osteoarthritis severity21. While IL-6 and MMP-3 are important biomarkers related to joint tissue breakdown known to be upregulated following ACL injury, it remains unknown if concentrations of these SF biochemical markers prior to ACLR relate to the development of aberrant biomechanics throughout the stance phase of walking gait 6 months following ACLR.
Therefore, the primary purpose of this study was to compare gait biomechanics associated with PTOA development (i.e., vGRF, KFA, and internal KEM) throughout the stance phase 6 months following ACLR in individuals with high vs. low synovial fluid (SF) concentrations of IL-6 and MMP-3 measured in the first 15 days following ACL injury. Our secondary purpose was to separately compare biomechanics throughout the stance phase 6 months post-ACLR from individuals with high and low SF concentrations of IL-6 and MMP-3 to the gait biomechanics from an uninjured control cohort. We hypothesized that individuals with the highest concentrations of SF IL-6 and SF MMP-3 in the first 15 days following ACL injury would exhibit greater underloading and utilization of the stiffened-knee gait strategy compared to the individuals with the lowest concentrations of SF IL-6 and SF MMP-3 following ACL injury.
2.2. Participants
Thirty-eight ACL injured and 38 Uninjured Controls between the ages of 16 and 35 years were enrolled in the study. All ACL injured and Uninjured Controls met the following inclusion criteria: (1) a BMI between 18–35 kg/m2, (2) no history of neurological disorder, (3) no lower extremity joint injury in the previous 6 months (other than the initial ACL injury), (4) no previous diagnosis of any diseases that affect joints, (5) no history of osteoarthritis, and (6) not currently pregnant. We excluded individuals from the ACLR cohort who sustained an ACL injury more than 15 days prior to initial presentation in the orthopaedic clinic as well as individuals who were not planning to undergo ACLR following or sustained a knee injury that required a multi-ligament surgical reconstruction. All ACL injured individuals underwent arthroscopically assisted bone-patellar tendon-bone autograft ACLR from one of three participating orthopeadic surgeons as previously reported7. All patients were prescribed physical therapy and were provided a standardized timeline for rehabilitation goals adapted from a review of best practice guidelines27. Individuals enrolled in the Uninjured Control group were physically active and had no history of: (1) lower or upper extremity joint surgery, (2) ligamentous knee injury, (3) concussion or head injury in the previous 6 months, (4) chronic ankle instability or balance disorders, and (5) cardiac condition or stroke.
A previous study28 demonstrated a moderate effect (0.06BW; d = 0.60) for the largest magnitude differences in vGRF waveforms between symptomatic and asymptomatic ACLR individuals throughout stance using the functional waveform gait analysis. We utilized parameters consistent with previously published literature29 to define the calculation of mean differences between the highest and lowest quartiles and variability estimates across the waveform using 5 gait trials from each participant. Therefore, we estimated that quartiles with 9 individuals (with 5 gait trials) would be capable of detecting a statistically significant moderate mean difference between waveforms, assuming similar inter-trial variability as previously reported (two tailed alpha= 0.05; 1-ß = 0.8; G*Power Statistical power Analysis Software v3.1)30. Therefore, we enrolled a large enough initial cohort (ACLR cohort; n=37) to allow for quartiles to include an estimated 9 individuals needed to detect a difference.
2.3. Procedures
As much SF as possible was aspirated from the knee joint without lavage using a superior-lateral suprapatellar approach. SF samples were stored on ice until centrifuged at 3000 × g for 10 min at 4°C. The supernatants were aliquoted into 1.0 mL cryovials and stored at −80 °C until analysis. SF was assessed for MMP-3 (1:2,000 dilution) and IL-6 (1:10 dilution) concentrations using commercially available enzyme linked immunosorbent assays (ELISA) (R & D Systems, Minneapolis, Minnesota, United States) according to the manufacture’s protocols. The optical density for the ELISA was determined within 30 minutes of assay completion, using a microplate reader (SpectraMax M2e; Molecular Devices, San Jose, California, United States) set at 450nm with a correction reading set at 540 nm. All assays were performed in duplicate for both standards and unknowns and demonstrated inter-assay and intra-assay variability less than 10%.
Demographics and biomechanics were collected on the second visit. We used the five subscales of the Knee Injury and Osteoarthritis Outcome Score (KOOS) to assess pain (KOOS Pain), symptoms (KOOS Symptoms), function in activities of daily living (KOOS ADL), function in activities of sport and recreation (KOOS Sport), and knee-related quality of life (KOOS QOL) at both visits (i.e., pre-ACLR and 6 months post-ACLR).31 All participants were outfitted with one rigid cluster of three retroreflective markers placed over the sacrum and 26 additional retroflective markers placed on palpable anatomical landmarks32. A static trial was then collected and used to create the segment-linkage model. To determine self-selected walking speed, participants were instructed to walk over a 6-meter distance that included 2 staggered and embedded force-plates (40 × 60 cm, FP406010, Bertec Corporation, Columbus, Ohio, United States), at the pace they would “comfortably walk over a sidewalk”. After participants felt comfortable walking in the laboratory, the speed of five walking trials measured by two sets of infrared timing gates (TF100, Trac Tronix, Lenexa, Kansas, United States) was averaged in order to maximize consistency in subsequent testing trials. Data from five error-free walking trials were then collected. Errors were considered as failing to: (1) individually strike an individual force plate with each foot, (2) maintain a walking speed within ±5% of the pre-determined self-selected walking speed, and (3) not undergo any visible alterations to gait during the trial (e.g., trip or stutter step). Marker positions were collected at 120 Hz using a 10-camera motion capture system (Vicon, Nexus, Denver, Colorado, United States) and force data was sampled at 1200 Hz. All data were low-pass filtered at 10 Hz (4th order recursive Butterworth). We evaluated kinematic and kinetic outcomes from the injured limb of the ACLR cohort. Approximately, 76% of the ACL injured cohort injured their dominant limb; therefore, we assessed the dominant limb for 76% of the Uninjured Control cohort via random assignment, which has been found to be an adequate means of matching limbs for Uninjured Controls33. Dominant limb was defined as the limb chosen to kick a ball.
2.4. Gait Biomechanics Processing
Biomechanical outcomes during the stance phase of walking were analyzed on a global coordinate system using Visual3D software (C-Motion, Germantown, Maryland, United States). Hip joint centers were estimated using the Bell and Brand hip joint CODA coordinate system34. Knee and ankle joint centers were identified using a radius half the distance between the medial and lateral epicondyles and malleoli, respectively. Knee kinematics were calculated using the angle of the shank relative to the thigh using Euler angles (sagittal/frontal/transverse sequence). We defined knee flexion as positive values. Internal joint moments were calculated using anthropometrics, synchronized kinematics and ground reaction force data, and a standard inverse dynamics approach on Visual3D software (C-Motion, Germantown, MD). We multiplied KEM by −1 as to present internal KEM as positive values in the current analysis. vGRF was normalized to body weights (BW) in newtons (N). Internal moments were normalized to the product of BW (N) and height (m). For our primary analyses, we choose to express vGRF and internal moments as BW and BW ×Height, respectively, in order to evaluate differences in how individuals were loading their limb and knee joint irrespective of absolute differences in body mass. We also provided supplemental data for absolute vGRF (Supplemental Figure 3) and internal KEM (Supplemental Figure 4). vGRF, KFA, and internal KEM data during stance were time normalized to 101 data points prior to analysis.
2.5. Statistical Analyses
For the purpose of this study, we retrospectively organized the ACL cohort into two sets of quartiles based on concentrations of SF IL-6 and SF MMP-3 within the first 15 days following ACL injury. While our primary analyses evaluated differences between individuals with the lowest (1st quartile; IL-6Lowest and MMP-3Lowest) the highest (4th quartile; IL-6Highest and MMP-3Highest) and the biomarker concentrations at the time of ACL injury, comparisons between the highest and high (3rd quartile; IL-6High and MMP-3High) quartiles, high and low (2nd quartile; IL-6Low and MMP-3Low) quartiles, as well as low and lowest quartiles were also evaluated (Supplemental Table 1). Additionally, we evaluated differences in absolute vGRF and internal KEM between Uninjured Controls and each lowest and highest IL-6 and MMP-3 quartile (Supplemental Table 2). Descriptive statistics were reported for the demographic factors of each quartile based on concentrations of each SF IL-6 and SF MMP-3.
Next, we performed separate functional waveform gait analyses29 for each biomechanical outcome (vGRF, KFA, and internal KEM) to evaluate planned comparisons between (1) Lowest and Highest Quartiles, (2) Uninjured Controls and Lowest Quartiles, and (3) Uninjured Controls and Highest Quartiles for each biomarker (IL-6 and MMP-3). The functional waveform gait analysis facilitates comparison of biomechanical outcomes at each percentile of the stance phase rather than only at certain discrete time points during stance. The functional waveform gait analysis allows for the detection of the portions of stance and magnitudes by which those portions of stance differ between groups for biomechanical outcomes29. Functional waveform gait analyses were performed as previously reported28 using the functional data analysis package in R statistical computing software (version 2.2.6). Specifically, functional models were fit to the finite waveforms using cubic b-splines after which residual curves were plotted to verify the fit of the models. 95% confidence intervals for each biomechanical waveform were then calculated for each group. Comparisons between waveforms were considered different at any percentile of the stance phase where mean differences and corresponding 95% confidence intervals did not cross zero29. We reported the largest difference between the ensemble curves and corresponding between-group effect sizes (Cohen’s d) within the proportions of stance demonstrating differences for each comparison as described above.
3. Results
Participant demographics, biomarker concentration, injury, and time data associated with the Uninjured Controls and ACLR individuals organized into the IL-6 Quartiles and MMP-3 Quartiles are listed in Table I and Table II, respectively. All percentages of stance phase demonstrating between quartile differences and the corresponding effect sizes for vGRF, KFA, and internal KEM are listed in Table III. Functional gait waveform analyses between IL-6 and MMP-3 Lowest and Low, Low and High, and High and Highest quartiles are in Supplemental Figures 1–2. Individual trajectories of all individuals from the ACL Cohort and Uninjured Controls, separated by IL-6 and MMP-3 quartile, are shown in Supplemental Figure 5.
Table I. Participant Demographics – IL-6 Quartiles.
Table I shows the demographical, injury, and time data associated with the Uninjured Controls and ACLR individuals organized into the IL-6 Quartiles
| Uninjured Control Group | Entire ACLR Cohort | IL-6 Lowest Quartile | IL-6 Low Quartile | IL-6 High Quartile | IL-6 Highest Quartile | |
|---|---|---|---|---|---|---|
| n | 38 | 38 | 10 | 9 | 9 | 10 |
| Sex (Female) | 19 | 21 | 8 | 4 | 5 | 4 |
| Age (yrs) | 21.26 ±3.06 | 20.95 ± 3.52 | 20.70 ± 4.97 | 20.56 ± 3.20 | 19.89 ± 1.54 | 22.5 ±3.31 |
| Height (m) | 1.74 ±0.08 | 1.76 ±0.13 | 1.67 ±0.10 | 1.77 ±0.10 | 1.79 ±0.14 | 1.80 ±0.13 |
| Mass (kg) | 72.25 ± 12.29 | 74.29 ± 13.81 | 63.73 ± 10.39 | 79.44 ± 12.98 | 86.35 ± 10.98 | 77.61 ± 16.02 |
| Body Mass Index (kg/m2) | 23.89 ± 2.80 | 24.64 ± 3.83 | 23.71 ±4.12 | 24.77 ± 3.44 | 26.30 ± 4.41 | 23.97 ± 3.38 |
| 6 Month Gait Speed (m/s) | 1.27 ±0.12 | 1.23 ±0.12 | 1.24 ±0.13 | 1.18 ±0.13 | 1.22 ±0.13 | 1.24 ±0.15 |
| KOOS Symptoms Score (Pre-ACLR) | NA | 44.27 ± 17.33 | 40.89 ± 15.84 | 53.33 ± 23.99 | 40.00 ± 15.18 | 43.00 ± 12.19 |
| KOOS Pain Score (Pre-ACLR) | NA | 56.19 ± 19.18 | 54.00 ± 12.20 | 56.89 ± 26.94 | 55.44 ± 17.06 | 58.20 ± 9.04 |
| KOOS Activities of Daily Living Score (Pre-ACLR) | NA | 61.11 ± 18.89 | 62.75 ± 9.45 | 61.56 ±30.28 | 61.56 ± 13.46 | 59.00 ± 3.54 |
| KOOS Sports Score (Pre-ACLR) | NA | 20.95 ± 32.81 | 19.44 ±31.77 | 24.44 ± 28.99 | 26.11 ±41.29 | 14.50 ± 14.53 |
| KOOS Quality of Life Score (Pre-ACLR) | NA | 23.62 ± 18.44 | 26.56 ± 9.75 | 30.00 ± 29.63 | 19.67 ± 14.85 | 18.80 ± 19.03 |
| KOOS Symptoms Score (6 Month) | NA | 74.58 ± 15.52 | 69.78 ±21.37 | 79.50 ± 11.46 | 74.22 ± 13.08 | 75.3 ± 15.23 |
| KOOS Pain Score (6 Month) | NA | 84.58 ±11.91 | 79.00 ± 19.16 | 87.12 ±8.25 | 87.11 ±7.22 | 85.30 ± 9.04 |
| KOOS Activities of Daily Living Score (6 Month) | NA | 94.61 ± 10.42 | 90.00 ± 19.86 | 98.50 ± 2.45 | 94.33 ± 4.53 | 95.90 ± 3.54 |
| KOOS Sports Score (6 Month) | NA | 65.89 ± 17.22 | 62.44 ±25.91 | 69.38 ± 16.13 | 63.33 ± 10.90 | 68.50 ± 14.53 |
| KOOS Quality of Life Score (6 Month) | NA | 52.69 ± 17.37 | 52.78 ± 14.97 | 54.00 ± 13.84 | 49.56 ± 22.50 | 54.40 ± 19.03 |
| Previous Contralateral ACL Reconstruction | NA | 4 | 1.20 ±0.42 | 1.11 ±0.33 | 1 | 0 |
| Time Between Injury and Synovial Fluid Aspiration (Days) | NA | 6.29 ± 3.98 | 7.43 ± 3.46 | 7.29 ± 3.25 | 7.17 ±4.36 | 3.75 ± 4.23 |
| Time Between Injury and ACL Reconstruction (Days) | NA | 31.74 ± 15.06 | 29.75 ± 14.76 | 28.38 ± 12.41 | 40.50 ±8.17 | 30.67 ± |
| Time Between Surgery and 6mo Biomechanics (Days) | NA | 202.35 ±31.12 | 211.60 ±40.75 | 202.25 ± 17.47 | 205.78 ±31.63 | 190.1 ±28.52 |
| Chondral Injury | NA | 12 | 5 | 3 | 2 | 2 |
| Lateral Meniscus Tear | NA | 29 | 7 | 5 | 8 | 9 |
| Medial Meniscus Tear | NA | 8 | 2 | 3 | 1 | 2 |
| Any Concomitant Injury | NA | 33 | 9 | 6 | 9 | 9 |
| Synovial Fluid IL-6 Concentraron (ng/ml) | NA | 7.876.84 ±9.194.76 | 654.60 ± 649.71 | 3,570.44 ± 547.54 | 6,637.22 ± 1,822.52 | 20,090.5 ± 10,007.03 |
| Synovial Fluid MMP-3 Concentraron (ng/ml) | NA | 6,725.09 ± 11,230.91 | 4,517.40 ±3,115.5 | 15,392.33 ±20,988.15 | 5,555.33 ± 3,490.77 | 2,185.05 ± 1,281.58 |
Data presented as mean ± standard deviation.
Table II. Participant Demographics – MMP-3 Quartiles.
Table II shows the demographical, injury, and time data associated with the Uninjured Controls and ACLR individuals organized into the MMP-3 Quartiles
| Uninjured Control Group | Entire ACLR Cohort | MMP-3 Lowest Quartile | MMP-3 Low Quartile | MMP-3 High Quartile | MMP-3 Highest Quartile | |
|---|---|---|---|---|---|---|
| n | 38 | 38 | 10 | 9 | 9 | 10 |
| Sex (Female) | 19 | 21 | 8 | 3 | 6 | 4 |
| Age (yrs) | 21.26 ±3.06 | 20.95 ± 3.52 | 20.70 ± 1.64 | 22.22 ± 4.58 | 21.67 ±4.36 | 19.40 ±0.10 |
| Height (m) | 1.74 ± 0.08 | 1.76 ±0.13 | 1.72 ±0.09 | 1.77 ±0.17 | 1.77 ±0.15 | 1.76 ±0.14 |
| Mass (kg) | 72.25 ± 12.29 | 77.62 ± 16.35 | 66.91 ± 8.53 | 77.47 ± 18.02 | 82.05 ±11.88 | 71.83 ± 12.63 |
| Body Mass Index (kg/m2) | 23.89 ± 2.80 | 24.64 ± 3.83 | 22.69 ± 2.94 | 24.92 ± 2.85 | 27.75 ± 5.24 | 23.55 ± 2.20 |
| 6 Month Gait Speed (m/s) | 1.27 ±0.12 | 1.23 ±0.12 | 1.32 ±0.10 | 1.26 ±0.10 | 1.19 ±0.14 | 1.12 ± 13.96 |
| KOOS Symptoms Score (Pre-ACLR) | NA | 44.27 ± 17.33 | 48.5 ± 13.90 | 32.56 ± 15.74 | 44.63 ± 12.23 | 50.30 ±21.78 |
| KOOS Pain Score (Pre-ACLR) | NA | 56.19 ± 19.18 | 59.00 ± 18.66 | 50.67 ± 17.73 | 58.88 ± 23.84 | 56.20 ± 18.88 |
| KOOS Activities of Daily Living Score (Pre-ACLR) | NA | 61.11 ± 18.89 | 65.90 ± 20.07 | 54.11 ± 17.61 | 61.50 ±22.81 | 62.44 ± 16.01 |
| KOOS Sports Score (Pre-ACLR) | NA | 20.95 ±32.81 | 34.50 ± 38.62 | 14.44 ± 30.66 | 19.38 ± 34.89 | 14.50 ±27.13 |
| KOOS Quality of Life Score (Pre-ACLR) | NA | 23.62 ± 18.44 | 23.20 ± 12.78 | 13.22 ±9.67 | 30.75 ±11.81 | 27.70 ± 28.87 |
| KOOS Symptoms Score (6 Month) | NA | 74.58 ± 15.52 | 75.70 ± 12.07 | 71.00 ± 13.36 | 72.89 ± 22.39 | 79.13 ± 5.63 |
| KOOS Pain Score (6 Month) | NA | 84.58 ±11.91 | 84.20 ± 8.08 | 83.22 ± 8.57 | 81.56 ±20.09 | 90.00 ± 3.96 |
| KOOS Activities of Daily Living Score (6 Month) | NA | 94.61 ± 10.42 | 95.70 ± 4.27 | 95.67 ± 4.39 | 89.89 ± 19.66 | 97.38 ± 17.41 |
| KOOS Sports Score (6 Month) | NA | 65.89 ± 17.22 | 68.00 ± 12.29 | 70.78 ± 13.01 | 58.89 ± 24.59 | 65.63 ± 17.41 |
| KOOS Quality of Life Score (6 Month) | NA | 52.69 ± 17.37 | 58.80 ± 15.96 | 51.33 ± 16.20 | 47.44 ± 22.48 | 52.50 ± 14.59 |
| Previous Contralateral ACL Reconstruction | NA | 4 | 0 | 2 | 1 | 1 |
| Time Between Injury and Synovial Fluid Aspiration (Days) | NA | 6.29 ± 3.98 | 3.57 ± 2.23 | 4.56 ± 2.74 | 9.00 ± 4.36 | 9.29 ± 3.73 |
| Time Between Injury and ACL Reconstruction (Days) | NA | 31.74 ± 15.06 | 30.63 ± 19.34 | 33.00 ± 15.49 | 31.83 ± 15.16 | 31.38 ± 12.51 |
| Time Between Surgery and 6mo Biomechanics (Days) | NA | 202.35 ± 31.12 | 192.33 ±32.63 | 217.56 ±42.90 | 189.44 ± 10.48 | 209.30 ± 25.35 |
| Chondral Injury | NA | 12 | 1 | 2 | 7 | 2 |
| Lateral Meniscus Tear | NA | 29 | 6 | 8 | 8 | 7 |
| Medial Meniscus Tear | NA | 8 | 1 | 2 | 1 | 4 |
| Any Concomitant Injury | NA | 33 | 7 | 9 | 9 | 8 |
| Synovial Fluid IL-6 Concentraron (ng/ml) | NA | 7,876.84 ±9,194.76 | 11,702.24 ± 12,025.98 | 9,667.50 ± 11,783.23 | 6,228.53 ± 6,082.87 | 3,923.33 ±2,662.10 |
| Synovial Fluid MMP-3 Concentraron (ng/ml) | NA | 6,725.09 ± 11,230.91 | 1,392.95 ±446.13 | 2,852.11 ±419.77 | 4,421.44 ±519.87 | 17,616.20 ± 18,282.74 |
Data presented as mean ± standard deviation.
Table 3. Portions of Stance Demonstrating Relevant Mean Differences.
Table 3 shows the Mean Differences of vertical ground reaction force, knee flexion angle, and internal knee extension moment between the Uninjured Control group and each of the Lowest and Highest IL-6 and MMP-3 ACLR Quartiles and between the Lowest and Highest IL-6 ACLR Quartiles and the Lowest and Highest MMP-3 ACLR Quartiles.
| Vertical Ground Reaction Force (%BW) | Knee Flexion Angle (°) | Internal Knee Extension Moment (%BW×Height) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Portions of Stance with Differences between Ensemble Curves (%) | Largest Difference Between Ensemble Curves± (Corresponding % of stance) | Effect Size (95% Confidence Interval) of Largest Difference Between Ensemble Curves | Portions of Stance with Differences between Ensemble Curves (%) | Largest Difference Between Ensemble Curves± (Corresponding % of stance) | Effect Size (95% Confidence Interval) of Largest Difference Between Ensemble Curves | Portions of Stance with Differences between Ensemble Curves (%) | Largest Difference Between Ensemble Curves± (Corresponding % of stance) | Effect Size (95% Confidence Interval) of Largest Difference Between Ensemble Curves | ||
|
| ||||||||||
| IL-6 | Lowest Quartile minus Highest Quartile | 1–11 | 3.72 (11%) | d=0.37 (−0.04,0.77) | 1–7 | 2.51(1%) | d=0.58 (0.17,0.99) | 2–21 | −0.61 (16%) | d=−0.65 (−1.06,−0.23) |
| 13–21 | 4.65 (18%) | d=0.43 (0.02,0.84) | ||||||||
| 32–44 | −3.83 (38%) | d=−0.69 (−1.11,−0.28) | ||||||||
| 91–100 | 2.80 (93%) | d=0.46 (0.05,0.87) | ||||||||
|
| ||||||||||
| Uninjured Controls minus Lowest Quartile | 38–56 | −3.47 (48%) | d=−0.70 (−1.11,−0.28) | 13–35 | 2.44 (26%) | d=0.64 (0.22,1.05) | 24–28 | −0.43 (24%) | d=−0.47 (−0.88,−0.06) | |
| 87–100 | −5.60 (93%) | d=−1.15 (−1.60,−0.72) | 54–77 | −2.20 (65%) | d=−0.84 (−1.26,−0.42) | 50–82 | 0.58 (70%) | d=1.06 (0.63,1.49) | ||
| 94–100 | 3.26 (100%) | d=0.79 (−0.37,1.21) | ||||||||
|
| ||||||||||
| Uninjured Controls minus Highest Quartile | 2–25 | 7.88 (19%) | d=0.94 (0.52,1.35) | 5–34 | 3.11 (17%) | d=0.82 (0.42,1.23) | 2–26 | −0.96 (18%) | d=−1.13 (1.35,2.07) | |
| 32–59 | −6.21 (40%) | d=−1.40 (−1.84,−0.96) | 58–71 | −1.50 (65%) | d=−0.57 (−0.97,−0.17) | 51–87 | 0.64 (69%) | d=1.20 (−1.68,−0.98) | ||
| 92–100 | −2.80 (93%) | d=−0.58 (−0.98,−0.18) | ||||||||
|
| ||||||||||
| MMP-3 | Lowest Quartile minus Highest Quartile | 3–35 | 1.23 (13%) | d=1.17 (0.73,1.57) | 16–25 | 2.29 (20%) | d=0.48 (0.07,0.89) | 1–10 | 0.43 (1%) | d=0.94 (0.52,1.37) |
| 42–60 | −5.03 (52%) | d=−0.82 (−1.17,−0.40) | 49–100 | −4.07 (100%) | d=−0.88 (−1.30,−0.46) | 74–100 | −0.59 (85%) | d=−0.81 (−1.23,−0.39) | ||
| 70–86 | 3.33 (82%) | d=0.33 (0.02,0.83) | ||||||||
| 96–100 | 1.37 (96%) | d=0.01 (0.01,0.83) | ||||||||
|
| ||||||||||
| Uninjured Controls minus Lowest Quartile | 30–43 | −3.33 (34%) | d=−0.71 (−1.13,−0.30) | 25–32 | 1.96 (28%) | d=0.51 (0.10,0.92) | 1–29 | −0.69 (18%) | d=−0.86 (−1.28,−0.44) | |
| 89–100 | −4.91 (93%) | d=−1.03 (−1.46,−0.60) | 89–100 | 3.59 (100%) | d=0.97 (0.54,1.40) | 97–98 | 0.11 (97%) | d=−0.58 (0.17,0.99) | ||
|
| ||||||||||
| Uninjured Controls minus Highest Quartile | 1–29 | 11.05 (17%) | d=1.31 (0.87,1.74) | 10–38 | 3.72 (22%) | d=0.99 (0.57,1.41) | 1–3 | 0.22 (1%) | d=0.62 (0.22,1.02) | |
| 37–60 | −5.85 (49%) | d=−1.20 (−1.62,−0.77) | 51–90 | −2.95 (68%) | d=−1.15 (−1.57,−0.72) | 11–35 | −1.07 (20%) | d=−1.24 (−1.67,−0.81) | ||
| 73–82 | 3.19 (80%) | d=0.53 (0.13,0.93) | 81–100 | −0.45 (87%) | d=−0.82 (−1.23,−0.41) | |||||
| 92–100 | −3.24 (94%) | d=−0.79 (−1.20,−0.38) | ||||||||
BW = Body Weight
Indicates greatest absolute differences between two ensemble curves within a given range
d=Cohen’s between group effect size (95% confidence interval).
3.1. vGRF Differences Between Groups
IL-6:
Compared to IL-6Lowest, vGRF was lesser in the IL-6Highest group between 1–11%, 13–21%, and 91–100% of stance and greater between 32–44% of stance (Figures 1A.1 and 1A.2). Compared to Uninjured Controls, vGRF in the IL-6Lowest group was greater between 38–56% and 87–100% of stance (Figure 1A.3). Compared to Uninjured Controls, IL-6Highest vGRF was lesser between 2–25% of stance and greater between 32–59% and 92–100% of stance (Figure 1A.4).
Figure 1. Vertical Ground Reaction Forces (.1) and Mean Difference Curves (.2-.4) of Uninjured Controls and Highest and Lowest IL-6 (A) and MMP-3 (B) ACL Cohort Quartiles.
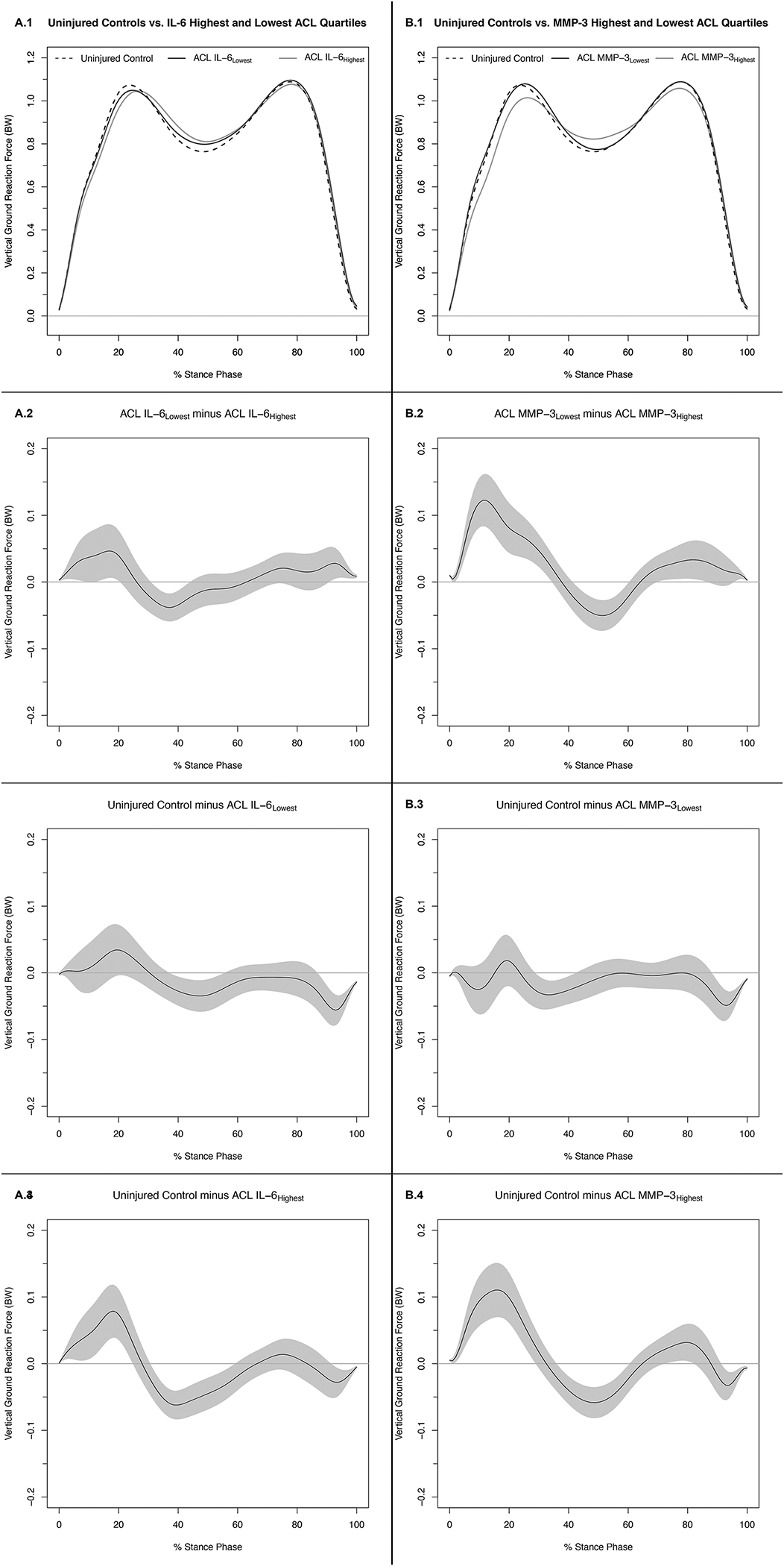
Figures 1A.1 and 1B.1 depict mean ensemble waveforms plotted over the stance phase of walking, for mean vertical ground reaction force (vGRF), normalized to body weight (BW) for the Uninjured Controls and Highest and Lowest IL-6 (A) and MMP-3 (B) ACL Cohort Quartiles. Figures 1A.2–4 and 1B.2–4 depict corresponding pairwise comparison functions, and associated 95% confidence intervals (grey bands), indicating the mean differences between the Uninjured Controls and Lowest (.3), Uninjured Controls and Highest (.4), and Lowest and Highest (.2) IL-6 (A) and MMP-3 (B) Quartiles. Differences between groups existed whenever the 95% confidence intervals did not overlap zero.
MMP-3:
Compared to MMP-3Lowest, MMP-3Highest vGRF was lesser between 3–35%, 70–86, and 92–100% of stance and greater between 42–60% of stance (Figures 1B.1 and 1B.2). Compared to Uninjured Controls, MMP-3Lowest vGRF was greater between 30–43% and 89–100% of stance (Figure 1B.3). Compared to Uninjured Controls, MMP-3Highest vGRF was lesser between 1–29% and 73–82% of stance and greater between 37–60% and 92–100% of stance (Figure 1B.4). Fifty percent (3/6) of the comparisons conducted in the supplemental analyses that evaluated absolute vGRF (i.e., not normalizing to participant BW) demonstrated markedly different trends for mean differences upon visual inspection compared to the primary analyses. Specifically, comparisons between Uninjured Controls and IL-6Highest (Supplemental Figure 3 B.4) demonstrate greater vGRF in IL-6Highest compared to Uninjured Controls throughout the majority of stance phase. Compared to both MMP-3Lowest (Supplemental Figure 3 D.4) and Uninjured Controls (Supplemental Figure 3 D.4), MMP-3Highest similarly demonstrates greater vGRF throughout the majority of stance phase.
3.2. KFA Differences Between Groups
IL-6:
Compared to IL-6Lowest, IL-6Highest KFA was lesser between 1–7% of stance (Figures 2A.1 and 2A.2). Compared to Uninjured Controls, IL-6Lowest KFA was lesser between 13–35% and 94–100% of stance and greater between 54–77% of stance (Figure 2A.3). Compared to Uninjured Controls, IL-6Highest KFA was lesser between 5–34% and greater between 58–71% of stance (Figure 2A.3).
Figure 2. Knee Flexion Angles (.1) and Mean Difference Curves (.2-.4) of Uninjured Controls and Highest and Lowest IL-6 (A) and MMP-3 (B) ACL Cohort Quartiles.
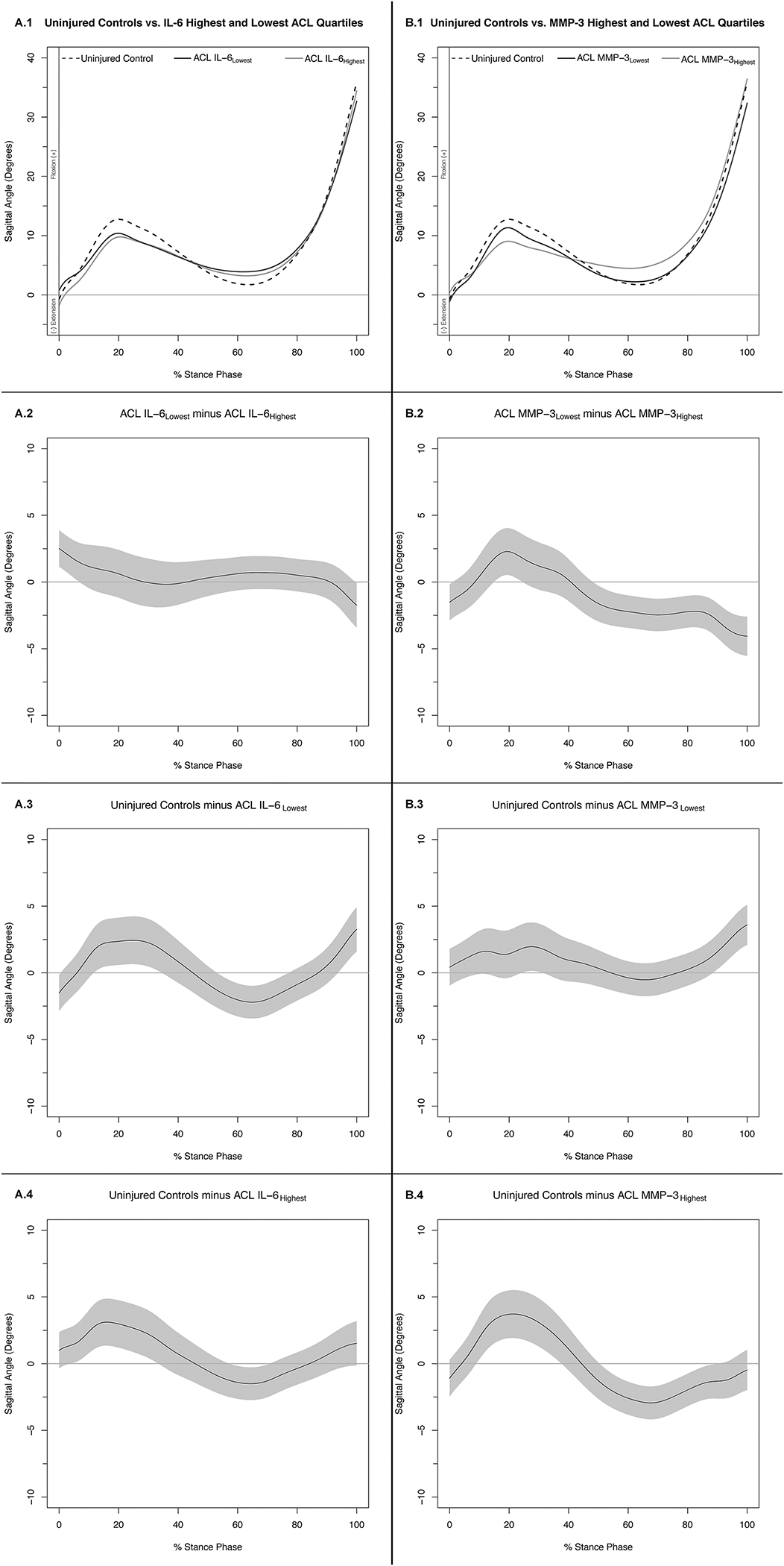
Figures 1A.1 and 1B.1 depict mean ensemble waveforms plotted over the stance phase of walking, for mean knee flexion angles for the Uninjured Controls and Highest and Lowest IL-6 (A) and MMP-3 (B) ACL Cohort Quartiles. Figures 1A.2–4 and 1B.2–4 depict corresponding pairwise comparison functions, and associated 95% confidence intervals (grey bands), indicating the mean differences between the Uninjured Controls and Lowest (.3), Uninjured Controls and Highest (.4), and Lowest and Highest (.2) IL-6 (A) and MMP-3 (B) Quartiles. Differences between groups existed whenever the 95% confidence intervals did not overlap zero.
MMP-3:
Compared to MMP-3Lowest, MMP-3Highest KFA was lesser between 16–25% of stance and greater between 49–100% of stance (Figures 2B.1 and 2B.2). Compared to Uninjured Controls, MMP-3Lowest KFA was lesser between 25–32% and 89–100% of stance. Compared to Uninjured Controls, MMP-3Highest KFA was lesser between 10–38% of stance and greater between 51–90% of stance (Figure 2B.3).
3.2. Internal KEM Differences between Conditions
IL-6:
Compared to IL-6Lowest IL-6Highest internal KEM was greater between 2–21% of stance (Figures 3A.1 and 3A.2). Compared to Uninjured Controls, IL-6Lowest internal KEM was greater between 24–28% of stance and lesser between 50–82% of stance (Figure 3A.3). Compared to Uninjured Controls, IL-6Highest internal KEM was greater between 2–26% of stance and lesser between 51–87% of stance (Figure 3A.3).
Figure 3. Internal Knee Extension Moments (.1) and Mean Difference Curves (.2-.4) of Uninjured Controls and Highest and Lowest IL-6 (A) and MMP-3 (B) ACL Cohort Quartiles.
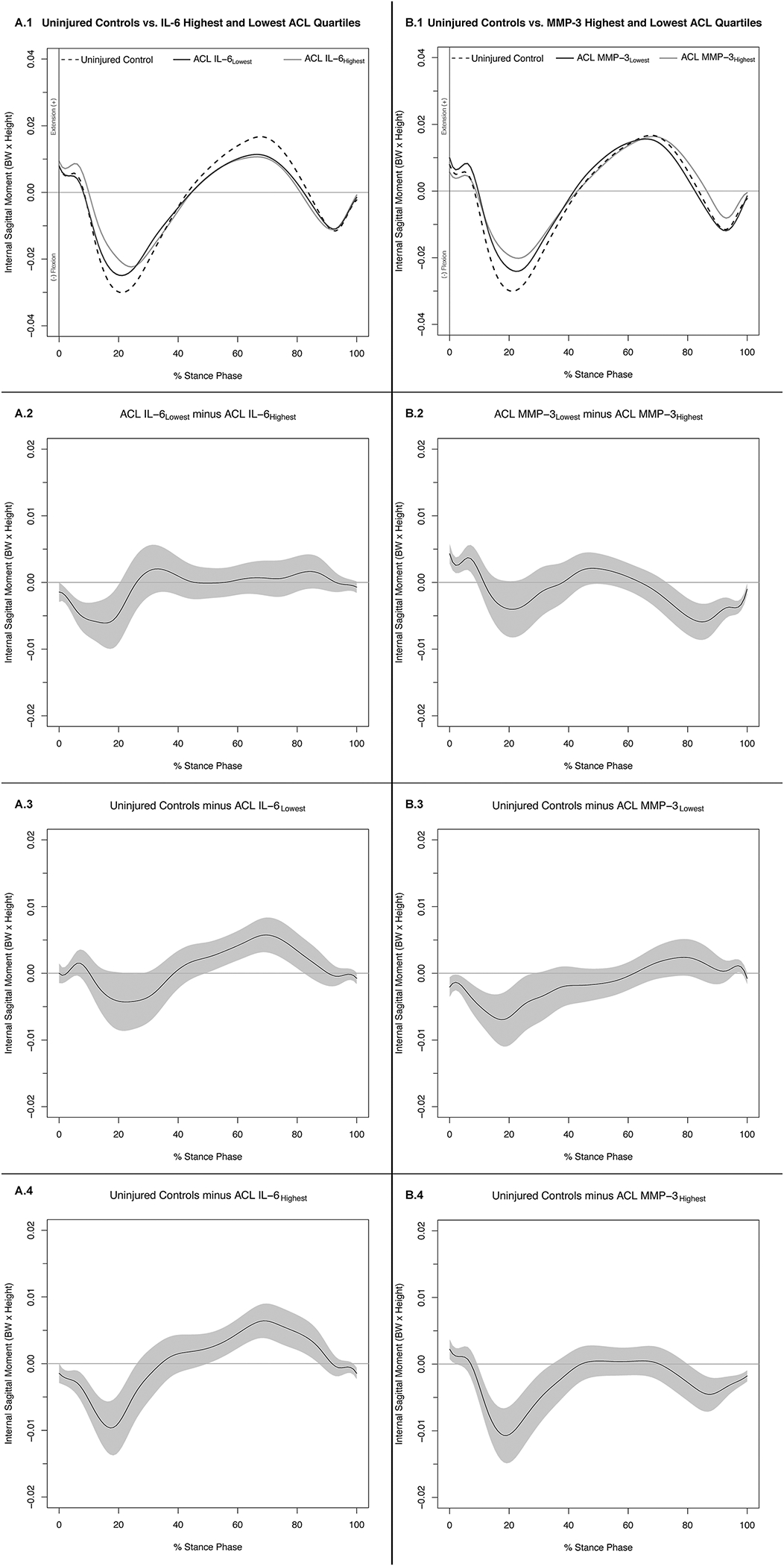
Figures 1A.1 and 1B.1 depict mean ensemble waveforms plotted over the stance phase of walking, for mean internal knee extension moments normalized to body weight (BW × Height), for the Uninjured Controls and Highest and Lowest IL-6 (A) and MMP-3 (B) ACL Cohort Quartiles. Figures 1A.2–4 and 1B.2–4 depict corresponding pairwise comparison functions, and associated 95% confidence intervals (grey bands), indicating the mean differences between the Uninjured Controls and Lowest (.3), Uninjured Controls and Highest (.4), and Lowest and Highest (.2) IL-6 (A) and MMP-3 (B) Quartiles. Differences between groups existed whenever the 95% confidence intervals did not overlap zero.
MMP-3:
Compared to MMP-3Lowest, the MMP-3Highest internal KEM was lesser between 1–10% and greater between 74–100% of stance (Figures 3B.1 and 3B.2). Compared to Uninjured Controls, MMP-3Lowest internal KEM was greater between 1–29% of stance and lesser between 97–98% of stance (Figure 3B.3). Compared to Uninjured Controls, MMP-3Highest internal KEM was lesser between 1–3% and greater between 11–35% and 81–100% of stance (Figure 3B.4). While the specific percentiles of stance indicating regions of significance differed (Supplemental Table 2), supplemental analysis using absolute KEM demonstrated similar trends for mean differences upon visual inspection compared to the primary analyses for all IL-6 and MMP-3 comparisons (Supplemental Figure 4).
4. Discussion
Overall, our primary results indicate that individuals with the highest SF concentrations of IL-6 and MMP-3 early following ACL injury are more likely to demonstrate a greater degree of underloading and a propensity to utilize the stiffened-knee gait strategy during gait at 6 months following ACLR compared to individuals with the lowest concentrations of IL-6 and MMP-3 and Uninjured Controls. Additionally, our primary results demonstrate that individuals with the lowest SF concentrations of IL-6 and MMP-3 early following ACL injury are more likely to match gait patterns of Uninjured Controls 6 months following ACLR compared to individuals with the highest concentrations of IL-6 and MMP-3. This is the first study to demonstrate that higher SF concentrations of pro-inflammatory biomarkers in the first 15 days following an ACL injury are associated with aberrant gait biomechanics 6 months following ACLR. These data suggest that the early joint tissue biochemical response to ACL injury may be a target for mitigating future aberrant gait biomechanics that contribute to joint tissue breakdown following knee injury.
We expected individuals with the highest concentrations of IL-6 and MMP-3 to demonstrate gait patterns consistent with underloading of the ACLR limb. It is hypothesized that underloading disrupts the normal cyclical loading and unloading of joint tissues needed for continued optimal knee joint tissue health12,28,35,36. Overall individuals in IL-6Highest and MMP-3Highest demonstrated these characteristics of underloading compared to individuals in IL-6Lowest and MMP-3Lowest as well as Uninjured Controls. Specifically, individuals in MMP-3Highest demonstrated patterns of underloading with lesser vGRF peaks during early and late stance, and greater vGRF during midstance compared to MMP-3Lowest and Uninjured Controls. Similarly, individuals in IL-6Highest demonstrated lesser vGRF during early stance and greater vGRF during midstance compared to individuals in IL-6Lowest and Uninjured Controls. Conversely, compared to Uninjured Controls, individuals in IL-6Lowest and MMP-3Lowest exhibited differences only during midstance (i.e., greater vGRF in IL-6Lowest and MMP-3Lowest) and had no differences in peak vGRF during early or late stance. Additionally, we found evidence that underloading of the ACLR limb became incrementally more pronounced between all four MMP-3 Quartiles (MMP-3Lowest, MMP-3Low, MMP-3High, MMP-3Highest) suggesting a dose effect between greater SF concentrations of MMP-3 and more pronounced underloading profiles for vGRF (Supplemental Figure 1).
The stiffened-knee gait strategy, commonly reported following ACLR12,37 and in individuals with osteoarthritis38, may inhibit optimal energy attenuation in lower extremity joints. This gait pattern has been associated with increased severity of knee osteoarthritis and decreased femoral cartilage thickness in otherwise healthy individuals38–40. Overall, individuals in both Highest and Lowest IL-6 and MMP-3 quartiles demonstrated greater use of the stiffened-knee gait strategy compared to Uninjured Controls. Specifically, individuals from the ACL cohort demonstrated lesser internal knee extension moments during early stance, lesser KEM during late stance, and lesser KFA and knee flexion excursion in early to mid-stance. Of note however, only IL-6 Highest and Lowest quartiles demonstrated lesser peak internal KEM in late stance compared to Uninjured Controls, which is a gait pattern that has previously been identified in asymptomatic individuals with MRI evidence of cartilage loss and high concentrations of a pro-inflammatory cytokine (i.e. tumor necrosis factor-α)20. Further, compared to individuals in IL-6Highest and MMP-3Highest individuals in IL-6Lowest and MMP-3Lowest generally demonstrated a less pronounced stiffened-knee gait strategy. Compared to IL-6Highest, IL-6Lowest demonstrated greater internal knee flexion moments in early stance, while compared to MMP-3Highest, MMP-3Lowest demonstrated greater KFA during early stance and KFA excursion between early and middle stance. Overall, our findings provide evidence that those with the highest concentrations of SF IL-6 and MMP-3 within the first 15 days of ACL injury demonstrate more aberrant gait biomechanics 6 months post-ACLR.
It is commonly hypothesized that aberrant joint loading triggers harmful biological joint tissue changes9,38–40. However, there is a lack of longitudinal data to determine if changes in gait biomechanics influence changes in joint tissue biochemistry and subsequent metabolism. Previous studies in humans41,42 have demonstrated that an inflammatory response occurs immediately after injury and again soon after ACLR, each of which may disrupt normal gait biomechanics. This disruption of gait biomechanics may cause a cyclical breakdown in joint tissues as aberrant joint tissue loading triggers and perpetuates deleterious changes in joint tissue biochemistry that could lead to PTOA onset. Our study suggests the existence of a more complex relationship between biomechanics and biochemistry than previously assumed whereby deleterious joint tissue biochemistry may precede the onset of aberrant biomechanics. While our study cannot confirm causality due to potential confounding variables, we can hypothesize from our results that early joint tissue biochemistry following ACL injury may influence gait differences 6 months post-ACLR. Experimental human knee effusion models have demonstrated that in the absence of pain or disease, increased acute joint effusion via experimental saline injection causes aberrant biomechanics associated with PTOA development, including exhibiting the stiffened-knee gait patterns and underloading in the experimentally effused limb43–45. Thereby, greater early joint effusion in ACL injured patients caused by a greater inflammatory response may influence the development of aberrant gait biomechanics. While our study did not seek to evaluate sex differences in outcomes, there were more males in and IL-6Highest (n=6) and MMP-3Highest (n=6) compared to IL-6Lowest (n=2) and MMP-3Lowest (n=2). Future studies should determine the effect of sex on early joint biochemistry following ACL injury. Additionally, none of the individuals from MMP-3Highest overlapped with individuals from IL-6Highest and only 20% of the individuals from MMP-3Lowest overlapped with individuals from IL-6Lowest. This finding suggests that high concentrations of IL-6 and MMP-3 may uniquely associate with aberrant gait biomechanics. Furthermore, our results demonstrate that the magnitude of differences between MMP-3Highest and MMP-3Lowest was greater than the magnitude of differences between IL-6Highest and IL-6Lowest. Moreover, the gait patterns of individuals in MMP-3Lowest closely match the gait patterns of Uninjured Controls (Figures 1–3) suggesting that those who present with the lowest MMP-3 concentrations acutely following ACL injury may not exhibit aberrant gait biomechanics 6 months post-ACLR. Conversely, individuals with lowest IL-6 concentrations acutely following injury demonstrated several differences in gait patterns compared to the Uninjured Controls (Figures 1–3) suggesting that low concentrations of IL-6 early following ACL injury may not be as strongly linked to normalized gait patterns at 6 months post-ACLR.
Further research is needed to elucidate whether the relationship between gait biomechanics and joint tissue biochemistry following ACL injury reflects a causal effect as the current study did not account for potential confounding variables. Such a study is important as it could allow for the development of novel therapeutic targets to delay or prevent the development of aberrant gait biomechanics associated with PTOA development. Previous efforts46,47 have attempted to inhibit the proinflammatory cytokine IL-1 for the purpose of altering the deleterious cascade of biochemical changes caused by joint inflammation following ACL injury that may contribute to PTOA onset. Our results suggest that treating the early biochemical response to ACL injury may benefit the development of optimal gait biomechanics that decrease the risk of future joint tissue degradation.
We conducted supplemental analyses in order to present between-quartile differences in absolute values of vGRF and KEM not normalized to the mass of each individual. Overall, supplemental analyses comparing the highest and lowest IL-6 and MMP-3 quartiles for absolute KEM demonstrated waveform trends across stance that were similar to the primary analyses for internal KEM expressed as BW×Height (Supplemental Figure 4). Conversely, supplemental analyses comparing highest and lowest IL-6 and MMP-3 quartiles for absolute vGRF demonstrated distinctly different results from our primary analyses. Specifically, the highest IL-6 and MMP-3 quartiles demonstrated greater absolute vGRF throughout the majority of stance phase compared to the lowest IL-6 and MMP-3 quartiles and Uninjured Controls (Supplemental Figure 3 B.2, B.4, D.2, and D.4). Interpreting the between-quartile differences across stance phase for absolute vGRF is limited as the differences in vGRF may be impacted by moderate to strong effect sizes exhibiting greater baseline body mass for both the highest IL-6 (Effect Size: Cohen’s d=1.03) and MMP-3 quartiles (Effect Size: Cohen’s d =0.46) compared to the lowest quartiles. Therefore, it is possible, that absolute forces exerted on the lower extremity may be critical to understanding the complex relationship between joint tissue biochemistry and gait biomechanics. As such, future studies may seek to compare the relationships between joint biochemistry and both the absolute forces and relative forces (i.e. normalized to BW) exerted across the limb or to specific lower extremity joints during gait.
Our study was the first to evaluate how initial knee joint biochemistry early following ACL injury associates with walking gait biomechanics 6 months post-ACLR; yet, there are limitations that should be addressed and inform future research. While the focus of the study was to associate concentrations of SF IL-6 and MMP-3, other biomarkers of inflammation (e.g., granulocyte-macrophage colony-stimulating factor), cartilage turnover (e.g., cartilage oligomeric matrix protein) and matrix degradation (e.g., MMP-1) associated with PTOA could further elucidate the associations of early joint biochemistry and subsequent gait biomechanics following ACLR. Additionally, other biomechanical variables of interest (e.g., knee adduction moment) have been associated with idiopathic knee OA severity48 and future studies may seek to evaluate associations between biomarkers and frontal plane moments and angles following ACLR. Additionally, future studies should evaluate the effect of changes in joint tissue metabolism on muscle activation patterns and estimates of tibiofemoral contact forces. Future research should investigate the interactions between concentrations of other biomarkers related to PTOA early following ACL injury and gait biomechanics following ACLR. Additionally, 4 of the 37 individuals in the study underwent previous reconstruction of the contralateral ACL (Table I). Though the IL-6 and MMP-3 concentrations were collected from joint specific synovial fluid samples, and only the biomechanics of the involved limb were analyzed, the gait biomechanics of these individuals may have been influenced by the previous contralateral ACLR12. Although we included a control group to help provide as a reference for uninjured biomechanics, we did not measure gait biomechanics prior to ACL injury and as such, it is unclear if aberrant gait biomechanics found at 6 months post-ACLR existed prior to ACL injury. Rather than prescribing a walking speed to all participants, we chose to collect gait biomechanics at the participants’ preferred walking speed to maintain the ecological validity of the study. Additionally, while the functional waveform gait analysis allows for detection of differences throughout the entirety of stance, it does not account for possible phase shifts. Finally, the functional waveform gait analyses that we conducted to evaluate differences between quartiles across the entire stance phase does not lend itself to the adjustment of the biomechanical outcomes while accounting for potential confounding variables. Larger studies should seek to elucidate the confounding effect of potential variables (e.g. sex, walking speed, concomitant injuries, or pain ) on the relationship between early biochemical changes following ACLR and gait biomechanics.
In conclusion, individuals with the highest SF concentrations of IL-6 and MMP-3 early following ACL injury demonstrated a greater degree of underloading and utilization of the stiffened-knee gait strategy during gait at 6 months following ACLR compared to individuals with the lowest concentrations of IL-6 and MMP-3 and Uninjured Controls. Furthermore, individuals with the lowest SF concentrations of IL-6 and MMP-3 demonstrated gait patterns similar to Uninjured Controls at 6 months post-ACLR compared to individuals with the highest concentrations of IL-6 and MMP-3. Additional research is required to establish a causal link between early knee SF biomarker concentrations and aberrant gait biomechanics post-ACLR.
Supplementary Material
Acknowledgments
Role of the Funding Source:
The current study was funded by grants from: 1) National Athletic Trainers Association Research and Education Foundation (New Investigator Research Grant Award [#14NewInv001]); 2) North Carolina Translational and Clinical Sciences (TraCS) Institute Planning Grant, 3) National Institutes of Health National Institute of Arthritis & Musculoskeletal and Skin Diseases (1R03AR066840–01A1)
Conflict of Interest:
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. There are no professional relationships with companies or manufacturers who will benefit from the results of the present study to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friel NA, Chu CR. The Role of ACL Injury in the Development of Posttraumatic Knee Osteoarthritis. Clin Sports Med. 2013;32(1): 1–12. doi: 10.1016/j.csm.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am J Sports Med. 2016;44(6):1502–1507. doi: 10.1177/0363546516629944 [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396 [DOI] [PubMed] [Google Scholar]
- 4.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. doi: 10.4085/1062-6050-49.3.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Favre J, Erhart-Hledik JC, Chu CR. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann Biomed Eng. 2015;43(2):376–387. doi: 10.1007/s10439-014-1117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: A systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015;33(7):939–947. doi: 10.1002/jor.22817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–2297. doi: 10.1002/jor.23534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrosimone B, Blackburn JT, Harkey MS, et al. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44(2):425–432. doi: 10.1177/0363546515618380 [DOI] [PubMed] [Google Scholar]
- 9.Luc-Harkey BA, Franz JR, Hackney AC, Blackburn JT, Padua DA, Pietrosimone B. Lesser lower extremity mechanical loading associates with a greater increase in serum cartilage oligomeric matrix protein following walking in individuals with anterior cruciate ligament reconstruction. Clin Biomech. 2018;60:13–19. doi: 10.1016/j.clinbiomech.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 10.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am J Sports Med. 2016;44(1):143–151. doi: 10.1177/0363546515608475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans-Pickett A, Davis-Wilson HC, Luc-Harkey BA, et al. Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6–12 months after anterior cruciate ligament reconstruction. Clin Biomech. 2020;76:105014. doi: 10.1016/j.clinbiomech.2020.105014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis HC, Pfeiffer SJ, Johnston CD, et al. Walking Biomechanics Six and Twelve Months Following Anterior Cruciate Ligament Reconstruction Compared to Healthy Controls. Med Sci Sport Exerc. 2019;51(Supplement):265. doi: 10.1249/01.mss.0000561300.58804.30 [DOI] [Google Scholar]
- 13.Pfeiffer SJ, Spang JT, Nissman D, et al. Gait Mechanics and T1ρ MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction. Med Sci Sport Exerc. 2019;51(4):630–639. doi: 10.1249/MSS.0000000000001834 [DOI] [PubMed] [Google Scholar]
- 14.Pietrosimone B, Blackburn JT, Padua DA, et al. Walking gait asymmetries 6 months following anterior cruciate ligament reconstruction predict 12-month patient-reported outcomes. J Orthop Res. 2018;36(11):2932–2940. doi: 10.1002/jor.24056 [DOI] [PubMed] [Google Scholar]
- 15.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech. 2004;19(1):44–49. doi: 10.1016/j.clinbiomech.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 16.Khandha A, Manal K, Wellsandt E, Capin J, Snyder-Mackler L, Buchanan TS. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(3):625–633. doi: 10.1002/jor.23261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26(3):332–341. doi: 10.1002/jor.20496 [DOI] [PubMed] [Google Scholar]
- 18.Sparkes V, Whatling GM, Biggs P, et al. Comparison of gait, functional activities, and patient-reported outcome measures in patients with knee osteoarthritis and healthy adults using 3D motion analysis and activity monitoring: An exploratory case-control analysis. Orthop Res Rev. 2019;11:129–140. doi: 10.2147/ORR.S199107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatfield GL, Stanish WD, Hubley-Kozey CL. Three-dimensional biomechanical gait characteristics at baseline are associated with progression to total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67(7):1004–1014. doi: 10.1002/acr.22564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edd SN, Favre J, Blazek K, Omoumi P, Asay JL, Andriacchi TP. Altered gait mechanics and elevated serum pro-inflammatory cytokines in asymptomatic patients with MRI evidence of knee cartilage loss. Osteoarthr Cartil. 2017;25(6):899–906. doi: 10.1016/j.joca.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 21.Lohmander LS, Hoerrner LA, Lark MW. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993;36(2):181–189. doi: 10.1002/art.1780360207 [DOI] [PubMed] [Google Scholar]
- 22.Pedersen BK, Brandt C. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010. doi: 10.1155/2010/520258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron M, Buchgraber A, Passler H, et al. The Natural History of the Anterior Cruciate Ligament-Deficient Knee. Am J Sports Med. 1997;25(6):751–754. doi: 10.1177/036354659702500605 [DOI] [PubMed] [Google Scholar]
- 24.Lohmander LS, Roos H, Dahlberg L, Hoerrner LA, Lark MW. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res. 1994;12(1):21–28. doi: 10.1002/jor.1100120104 [DOI] [PubMed] [Google Scholar]
- 25.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D, et al. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJP. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248(1):265–268. doi: 10.1042/bj2480265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: A criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT, Blackburn JT. Walking Ground Reaction Force Post-ACL Reconstruction: Analysis of Time and Symptoms. 2018. doi: 10.1249/MSS.0000000000001776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J, Seeley MK, Francom D, Reese CS, Hopkins JT. Functional vs. Traditional Analysis in Biomechanical Gait Data: An Alternative Statistical Approach. J Hum Kinet. 2017;60:39–49. doi: 10.1515/hukin-2017-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 31.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1):64. doi: 10.1186/1477-7525-1-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luc-Harkey BA, Harkey MS, Stanley LE, Blackburn JT, Padua DA, Pietrosimone B. Sagittal plane kinematics predict kinetics during walking gait in individuals with anterior cruciate ligament reconstruction. Clin Biomech. 2016;39:9–13. doi: 10.1016/J.CLINBIOMECH.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 33.Kowalski E, Catelli DS, Lamontagne M. Side does not matter in healthy young and older individuals – Examining the importance of how we match limbs during gait studies. Gait Posture. 2019;67:133–136. doi: 10.1016/j.gaitpost.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 34.Bell AL, Brand RA, Pedersen DR. Prediction of hip joint centre location from external landmarks. Hum Mov Sci. 1989;8(1):3–16. doi: 10.1016/0167-9457(89)90020-1 [DOI] [Google Scholar]
- 35.Vanwanseele B, Lucchinetti E, Stussi E. The effects of immobilization on the characteristics of articular cartilage: Current concepts and future directions. Osteoarthr Cartil. 2002;10(5):408–419. doi: 10.1053/joca.2002.0529 [DOI] [PubMed] [Google Scholar]
- 36.Van Rossom S, Smith CR, Zevenbergen L, et al. Knee Cartilage Thickness, T1ρ and T2 Relaxation Time Are Related to Articular Cartilage Loading in Healthy Adults. PLoS One. 2017;12(1):e0170002. doi: 10.1371/journal.pone.0170002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17(1):56–63. doi: 10.1016/S0268-0033(01)00097-3 [DOI] [PubMed] [Google Scholar]
- 38.Zeni JA, Higginson JS, Higginson JS. Dynamic knee joint stiffness in subjects with a progressive increase in severity of knee osteoarthritis. Clin Biomech (Bristol, Avon). 2009;24(4):366–371. doi: 10.1016/j.clinbiomech.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz RJ, Harrison D, Wang HM, Shultz SJ. Sagittal-plane knee moment during gait and knee cartilage thickness. J Athl Train. 2017;52(6):560–566. doi: 10.4085/1062-2050-52.4.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook TM, Farrell KP, Carey IA, Gibbs JM, Wiger GE. Effects of Restricted Knee Flexion and Walking Speed on the Vertical Ground Reaction Force During Gait. J Orthop Sport Phys Ther. 1997;25(4):236–244. doi: 10.2519/jospt.1997.25.4.236 [DOI] [PubMed] [Google Scholar]
- 41.Punzi L, Galozzi P, Luisetto R, et al. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2(2):e000279. doi: 10.1136/rmdopen-2016-000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthr Cartil. 2015;23(1):1–12. doi: 10.1016/j.joca.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Seeley MK, Park J, King D, Hopkins JT. A novel experimental knee-pain model affects perceived pain and movement biomechanics. J Athl Train. 2013;48(3):337–345. doi: 10.4085/1062-6050-48.2.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietrosimone B, Lepley AS, Murray AM, Thomas AC, Bahhur NO, Schwartz TA. Changes in voluntary quadriceps activation predict changes in muscle strength and gait biomechanics following knee joint effusion. Clin Biomech. 2014;29(8):923–929. doi: 10.1016/j.clinbiomech.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 45.Torry MR, Decker MJ, Viola RW, O’Connor DD, Richard Steadman J. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech. 2000;15(3):147–159. doi: 10.1016/S0268-0033(99)00083-2 [DOI] [PubMed] [Google Scholar]
- 46.Lattermann C, Jacobs CA, Proffitt Bunnell M, et al. A Multicenter Study of Early Anti-inflammatory Treatment in Patients with Acute Anterior Cruciate Ligament Tear. Am J Sports Med. 2017;45(2):325–333. doi: 10.1177/0363546516666818 [DOI] [PubMed] [Google Scholar]
- 47.Kraus VB, Birmingham J, Stabler TV, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial (NCT00332254). Osteoarthr Cartil. 2012;20(4):271–278. doi: 10.1016/j.joca.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 48.Erhart-Hledik JC, Favre J, Andriacchi TP. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. J Biomech. 2015;48(14):3868–3875. doi: 10.1016/j.jbiomech.2015.09.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


