Abstract
Several studies have demonstrated that enzyme-linked immunosorbent assay is not a sensitive and specific method to diagnose Helicobacter pylori infection in children, especially in the younger ones. Since serum immune response can also be determined by immunoblotting and it permits the detection of antibodies to virulence factors such as CagA and VacA, we evaluated the accuracy of a commercial immunoblotting test to diagnose H. pylori infection and to assess the humoral immune response to different H. pylori antigens in 122 children who underwent upper gastrointestinal endoscopy. The presence of H. pylori was determined in antral biopsy specimens by culture, preformed urease test, and histological analysis. H. pylori was identified by microbiological and histopathological methods in 66 children (including all of the 21 who had duodenal ulcer). Antibodies to H. pylori were detected in 63 infected children and in 8 noninfected ones. The sensitivity, specificity, and positive and negative predictive values of the immunoblotting test were 95.5, 85.7, 88.7, and 94.1%, respectively. The number of immunoreactive bands increased with age (P = 0.003), and the bands of 35 kDa (P = 0.013); 89 kDa, the VacA antigen (P = 0.001); and 116 kDa, the CagA antigen (P = 0.00004) were more frequently observed in older children. The frequency of the bands of 89 kDa (P = 0.001) and 116 kDa (P = 0.03) was higher in children with duodenal ulcer than in H. pylori-positive children without the disease. In conclusion, the immunoblotting test appears to be useful for the diagnosis of H. pylori infection in children, even in the younger ones.
Helicobacter pylori infection is probably one of the most common chronic bacterial infections worldwide. The infection is predominantly acquired in childhood and in most subjects its course is without complications. Nevertheless, a small percentage of infected individuals develop peptic ulcer disease (17), gastric cancer (26), or mucosa-associated lymphoid tissue lymphoma (2). Once acquired, the infection persists for years and elicits mucosal and serum immune responses in most infected persons (16, 19). Therefore, noninvasive serological tests have been widely used for the diagnosis of H. pylori infection. Among them, enzyme-linked immunosorbent assay (ELISA) is one of the most extensively employed tests because it is relatively inexpensive, quick, easy to perform, and suitable for screening large populations (12). In adults, this method has proved to be highly accurate to diagnose the infection, but in children, especially younger ones, ELISA appears not to be a good screening test. In fact, we observed that a commercial ELISA showed low sensitivity for the diagnosis of H. pylori infection in children aged 2 to 12 years, especially in those without duodenal ulcer. When used in children of different ages, the test presented differences in sensitivity: 44.4% in children 2 to 6 years old; 76.7% in children 7 to 11 years old, and 93.1% in children 12 to 16 years old. We also observed that immunoglobulin G (IgG) antibody levels were higher in older children (25). Similar results were also observed by other investigators (14, 31).
The serum immune response to H. pylori antigens can be also evaluated by immunoblotting (11, 13, 22). Although this test is expensive and time-consuming it appears to be more sensitive, especially with sera with low levels of antibodies that are not detected by ELISA (22). This is probably due to the fact that in immunoblotting, the individual bacterial proteins are better exposed, allowing antibodies to bind more easily (23). Furthermore, it permits detection of antibodies to virulence factors such as CagA and VacA proteins. Therefore, we evaluated the accuracy of a commercial immunoblotting test to diagnose H. pylori infection in children and to assess their humoral immune response to different H. pylori antigens.
MATERIALS AND METHODS
This project was approved by the Ethics Committee of Hospital das Clínicas, Universidade Federal de Minas Gerais, Minas Gerais, Brazil, and informed consent was obtained from children (whenever possible) and their parents.
Part of the sera tested in the present study were from children included in a previous study for validation of a commercial ELISA (25). We studied sera from 122 children (51 boys and 71 girls; mean age, 9.2 ± 3.4 years; range, 2 to 16 years) who underwent upper gastrointestinal endoscopy for evaluation of symptoms related to the upper gastrointestinal tract, such as recurrent abdominal pain, vomiting, or hematemesis. Among them, 21 presented with a diagnosis of duodenal ulcer (15 boys; mean age, 11.5 ± 1.8 years; range, 8 to 16 years). The children were referred to the Pediatric Digestive Endoscopy Unit of the UFMG University Hospital. Patients less than 2 years of age and those who had received antibiotic therapy for the eradication of H. pylori or antimicrobial drugs during the 6 months before endoscopy, who were taking H2 receptor antagonists, or nonsteroidal anti-inflammatory drugs or who had portal hypertension, coagulation disorders, or anatomical obstacles preventing endoscopy were not included in the study.
At endoscopy, biopsy specimens were obtained from the antral and oxyntic gastric mucosa for microbiological and histological study. One antral fragment was placed in a tube containing Christensen's 2% urea agar and examined within 24 h of incubation at 37°C for urea hydrolysis. For culture, fragments from the antrum and body were kept in thioglycolate broth (Difco Laboratories, Detroit, Mich.) at 4°C for no longer than 5 h before processing. The tissue specimens were ground in a tissue homogenizer and plated onto petri dishes containing Belo Horizonte medium (30), and the plates were incubated at 37°C in a microaerobic environment for up to 7 days. Colonies of H. pylori were identified by spiral gram-negative appearance, positive oxidase and catalase tests, and a rapidly positive urease test. One fragment of the antral mucosa and one fragment of the oxyntic mucosa were fixed in formalin, dehydrated in alcohol and xylene, and embedded in paraffin. Five-micrometer-thick sections were obtained for the preparation of slides which were stained with hematoxylin-eosin for histological examination and with carbolfuchsin for H. pylori identification.
Children were considered to be H. pylori positive if at least two of the three test results were positive or if the culture alone was positive and were considered to be H. pylori negative if the three test results were negative.
Venous blood samples (2 ml) were drawn from each child at the time of endoscopy. The serum was separated, divided into aliquots and stored at −20°C before testing.
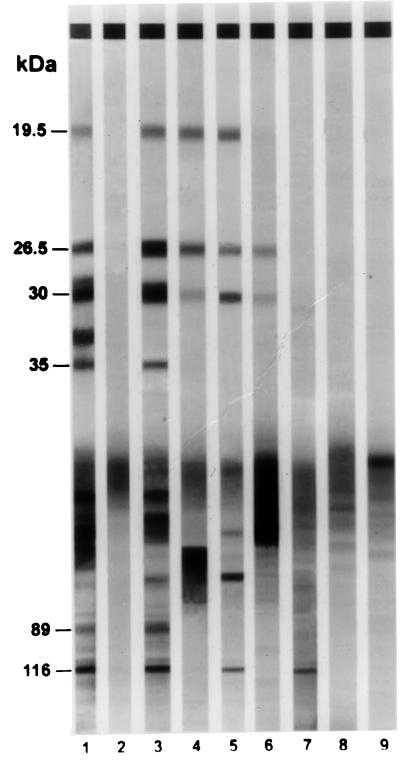
Sera were assayed for H. pylori antibodies using a commercial immunoblotting kit (Helicoblot 2.0; Genelabs Diagnostics, Singapore, Singapore). Helicoblot 2.0 is a qualitative assay used for the detection of IgG antibodies specific for different antigens of H. pylori in human serum or plasma. The antigen is prepared from a lysate of a strain of H. pylori isolated from a patient with peptic ulcer. The proteins from the lysate are electrophoretically separated and transferred to nitrocellulose. The strips contain different H. pylori antigens including those at 116 (CagA), 89 (VacA), 35, 30 (urease H), 26.5 (urease A), and 19.5 (urease E) kDa (8). The assay was performed according to the recommendations of the manufacturer of the kit. Briefly, the strips were washed with wash buffer and each strip was incubated for 1 h with the sample diluted 1:100 in blocking buffer. A serum-positive control and a serum-negative control were used. Following the incubation period, the sera were aspirated and the strips were washed three times. Then, the strips were incubated with an anti-human IgG antibody conjugated with alkaline phosphatase for 1 h. After this step, the conjugate solution was aspirated from each well and the strips were washed three times and incubated with a solution of 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium for 15 min. The reaction was stopped by rinsing the strips several times with distilled water. Finally, the strips were removed from the wells and dried before mounting. Western blotting was considered to be positive for H. pylori if any one band at 116, 89, or 35 kDa, or if any two bands from among bands at 30, 26.5, or 19.5 kDa were present (Fig. 1). The analysis of the test was done by two investigators who were unaware of the H. pylori status of the children.
FIG. 1.
Examples of the immunoblot pattern obtained with sera from children by employing Helicoblot 2.0. Lane 1, positive control; lane 2, negative control; lane 3, serum from child with duodenal ulcer; lanes 4 to 7, sera from H. pylori-positive children without duodenal ulcer; lanes 8 and 9, sera from H. pylori-negative children.
The performance of the test was evaluated by determining the sensitivity, specificity, and positive and negative predictive values with the 95% confidence interval (CI).
In order to compare the number of immunoreactive bands between groups, the children with duodenal ulcer were age matched with 21 H. pylori-positive children without duodenal ulcer.
The χ2 test and Fisher's exact test were used to compare proportions between the groups. Analysis of variance for normal distribution and the Kruskal-Wallis test for variables of asymmetrical distribution were employed to evaluate differences in the number of immunoreactive bands and differences in age among the groups. The Wilcoxon test was used to analyze the age-matched groups, and Spearman's correlation was used to analyze the association between age and number of bands. The level of significance was set at P < 0.05.
RESULTS
H. pylori was identified by microbiological and histopathological methods in 66 (54.1%) of 122 children. All duodenal ulcer patients were H. pylori positive. The characteristics of the children studied are listed in Table 1. The H. pylori-positive and -negative groups were similar in gender (P = 0.97), but the number of boys was proportionally higher in the group of children with duodenal ulcer than in the group of H. pylori-positive children without duodenal ulcer (P = 0.002). The mean age of H. pylori-negative children was significantly lower (P = 0.0008) than that of H. pylori-positive ones, and the age of children with duodenal ulcer was significantly higher than that of H. pylori-positive children without duodenal ulcer (P = 0.03).
TABLE 1.
Characteristics of the children studied
| H. pylori status | No. of children | No. of boys/ no. of girls | Mean age ± SD (yr) | Age range (yr) |
|---|---|---|---|---|
| Positive | 66 | 28/38 | 10.2 ± 2.8 | 3–16 |
| With duodenal ulcer | 21 | 15/6 | 11.5 ± 1.8 | 8–16 |
| Without duodenal ulcer | 45 | 13/32 | 9.6 ± 2.9 | 3–13 |
| Negative | 56 | 23/33 | 8.0 ± 3.6 | 2–14 |
| Total | 122 | 51/71 | 9.2 ± 3.3 | 2–16 |
Western blotting was positive in 63 of the 66 infected children and in 8 of the 56 noninfected ones. The sensitivity, specificity, and positive and negative predictive values of the test were 95.5% (95% CI, 86.4 to 98.8%), 85.7% (95% CI, 73.2 to 93.2%), 88.7% (95% CI, 78.5 to 94.7%), and 94.1% (95% CI, 82.8 to 98.5%), respectively. In H. pylori-positive patients the sensitivities of the test were similar in those with (95.2%) and without (95.5%) duodenal ulcer (P = 1.0). When children were stratified by age (2 to 6, 7 to 11, and 12 to 16 years old), no difference was observed in test sensitivity (87.5, 96.6, and 96.4%, respectively; P = 0.51). The number of immunoreactive bands increased with age (P = 0.003), and the bands of 35 (P = 0.01), 89 (P = 0.001), and 116 (P = 0.00004) kDa were more frequently observed in older children. The increase in the frequency of the bands of 89 and 116 kDa was observed even when children with duodenal ulcer were excluded from the analysis (P = 0.004 and P = 0.0005, respectively) (Table 2). Children with duodenal ulcer presented more bands than age-matched H. pylori-positive children without duodenal ulcer, although it had not reached statistical significance (P = 0.08). The frequency of the bands of 89 and 116 kDa was higher in children with duodenal ulcer than in children without the disease (P = 0.001 and P = 0.03, respectively) (Table 3).
TABLE 2.
Frequency of immunoreactive bands as detected by immunoblotting in sera of 45 H. pylori-positive children without duodenal ulcer
| Age range (yr) | n | % of sera immunoreactive bands at:
|
|||||
|---|---|---|---|---|---|---|---|
| 19.5 kDa | 26.5 kDa | 30 kDa | 35 kDaa | 89 kDab | 116 kDac | ||
| 02–06 | 8 | 87.5 | 62.5 | 75.0 | 12.5 | 0 | 12.5 |
| 07–11 | 20 | 90.0 | 80.0 | 90.0 | 45.0 | 45.0 | 75.0 |
| 12–16 | 17 | 82.3 | 94.1 | 94.1 | 58.8 | 70.5 | 88.2 |
P = 0.09.
P = 0.004.
P = 0.0005.
TABLE 3.
Frequency of immunoreactive bands as detected by immunoblotting in sera of 56 H. pylori-negative and 66 H. pylori-positive children with (n = 21) and without (n = 45) duodenal ulcer
| H. pylori status | % of sera with immunoreactive bands at:
|
|||||
|---|---|---|---|---|---|---|
| 19.5 kDa | 26.5 kDa | 30 kDa | 35 kDa | 89 kDa | 116 kDa | |
| Positive | 81.8 | 84.8 | 86.7 | 50.0 | 59.1 | 75.8 |
| With duodenal ulcer | 71.7 | 90.5 | 85.7 | 57.1 | 90.5a | 95.2b |
| Without duodenal ulcer | 86.7 | 82.2 | 86.7 | 46.7 | 44.4a | 66.7b |
| Negative | 3.6 | 14.3 | 7.1 | 3.6 | 1.8 | 8.9 |
P = 0.001.
P = 0.03.
Sera from eight H. pylori-negative children aged 2 to 14 years showed positive immunoblotting results, with the following immunoreactive bands being detected: 19.5 kDa (n = 2), 26.5 kDa (n = 5), 30 kDa (n = 3), 35 kDa (n = 2), 89 kDa (n = 1), and 116 kDa (n = 5) (Table 3). Bands of 26.5 kDa (n = 3) and 30 kDa (n = 1) were observed in the sera of H. pylori-negative children that did not fulfill the immunoblotting positivity criterion.
DISCUSSION
Although ELISA is accurate for the diagnosis of H. pylori infection in adults, in children this fact does not appear to be confirmed (14, 25, 31). This may be due to differences in quantitative and qualitative immune response between adults and children. Younger children may be expected to have infections of short duration and to have more frequently acute primary infections (32, 34), showing lower H. pylori serum antibody titers than adults (7). These differences may also be caused by differences in antigen recognition that may occur in different age groups (21, 36).
Immunoblotting is an alternative serologic assay available to diagnose H. pylori infection (22, 37). It appears to be more sensitive for the detection of acute H. pylori infection than ELISA, but, as also required for other serologic tests, the antigen preparation must be standardized (22) and the test must be validated for the population being studied (37).
Although several authors have employed immunoblotting to detect the antibodies anti-CagA and anti-VacA (3, 9, 24) and to determine the antibody profile in pediatric patients (8, 20), none of them have determined the specificity and the positive and negative predictive values of the test used to diagnose H. pylori infection. In a study conducted with 49 H. pylori-positive Belgian and Moroccan children, the blot was 89.9% sensitive compared with histology and culture. However, the authors did not evaluate H. pylori-negative children (35). In another study evaluating 68 Bangladeshi children between 4 and 24 months of age, it was observed that blotting was positive in 6 of 53 H. pylori-positive children by urea breath test or PCR of stool samples (4).
In the present study, we validated the Helicoblot kit to diagnose H. pylori infection in children. In contrast to what we have previously observed for ELISA, the assay showed high sensitivity (95.4%) for the diagnosis of H. pylori infection in this age group, even among the younger children and those without duodenal ulcer. The sensitivity observed was similar to that reported by Vandenborre et al. (35), who employed the same test using Belgian and Moroccan children. This may be due to the fact that immunoblotting allows the detection of low concentrations of antibodies or that different antigen preparations are used in ELISA (21, 22). The results of the present study are also similar to those reported by others in adults. Faulde et al. (11) observed a sensitivity of 100% for an in-house Western blotting test. Nilsson et al. (22) also used an in-house blotting test and found a sensitivity of 97.5%. More recently, Yamaoka et al. (37) validated two commercial immunoblotting tests for the Japanese adult population and observed a sensitivity of 100% for both Helicoblot 2.0 and RIDA Blot Helicobacter.
Regarding the specificity of the test, false-positive results were observed for 8 of 56 noninfected children. This finding is in agreement with data previously reported by others for adults. Nilsson et al. (22) observed a specificity of 92.5% for an in-house blot and Yamaoka et al. (37) found specificities of 90 and 80% for the Helicoblot 2.0 and RIDA Blot Helicobacter, respectively. The relatively low specificity observed by us and others may be due to the fact that the patients may have taken antimicrobial drugs for other purposes, with a resulting decrease in the bacterial load, or the bacterium may be eliminated spontaneously, a fact more frequently observed in young children (15, 28), and the antibody titers can take a long time to decrease. Sörberg et al. (33) observed that IgG antibodies to 19.5- and 120-kDa antigens were detected up to 32 months after the eradication of the microorganism with antimicrobial drugs. In the present study immunoreactive bands to 26.5- and 30-kDa H. pylori antigens were observed in 14.3 and 7.1% of H. pylori-negative children, respectively. Although most of the studies have demonstrated that cross-reacting antibodies detected by blotting were to medium-sized antigens of H. pylori (11, 21, 37), Nilsson et al. (22) observed that 3 (7.5%) of 40 H. pylori-negative adults had antibodies to small size antigens.
In the present study we observed that older children showed a higher number of immunoreactive bands. It is known that H. pylori infection is acquired predominantly in childhood, and consequently at this age the chance of having acute infection with low levels of antibodies is greater. We also observed that the bands of 35, 89, and 116 kDa were more frequently detected in sera of older children than of younger ones. Differences in antigen recognition were also reported by Mitchell et al. (21). These authors evaluated by immunoblotting the progression of the acute infection in two adults and two children from the same family and in an adult of another family and observed that the initial antibody responses in the children were to small-molecular-size antigens and in the adults the initial responses were to larger-molecular-size antigens. It is also possible that children of different ages are colonized by different H. pylori strains. In fact, we observed that older children without duodenal ulcer are infected more frequently by H. pylori strains that present the cagA gene detected by PCR than young children (29).
As described for adults, a variety of immunoblot patterns were observed in H. pylori-positive children. It has been suggested that this polymorphism in serum immune response to H. pylori reflects either an evolution of immune response (21) or the diversity of H. pylori infecting strains (1), which has been suspected to be correlated to a predisposition for severe diseases (1). In the present study we observed that antibodies to CagA and VacA proteins were significantly more frequent in H. pylori-positive children with duodenal ulcer (95.2 and 90.5%, respectively) than in H. pylori-positive children without duodenal ulcer (66.7 and 44.4%, respectively). The high frequency of antibodies to CagA which we observed in children with duodenal ulcer in contrast with the children without duodenal ulcer is similar to that reported by several authors for adults from Western countries (5, 6, 10). In children there are few studies on this subject (20, 24). Although in all of them almost all children with duodenal ulcer presented antibodies against CagA; only one study detected differences in CagA positivity between children with and without duodenal ulcer (24). These differences may be explained by the fact that in most studies few children with duodenal ulcer were evaluated (20), or geographic differences may occur as reported for adults (18).
In regard to anti-VacA antibodies, the data in the literature are more inconsistent. Although adult patients with duodenal ulcer are more frequently colonized by an H. pylori strain that presents s1m1 or s1m2 vacA genotypes (10) that are considered cytotoxigenic, the presence of anti-VacA antibodies is not consistently observed more frequently in these patients than in those without duodenal ulcer. Only 47.8% of our adult patients with duodenal ulcer evaluated by the same blotting test presented antibodies to VacA protein (G. A. Rocha, A. M. R. Oliveira, and D. M. M. Queiroz, unpublished data). These data suggest that different responses to VacA protein may occur between adults and children with duodenal ulcer. Another explanation is that children with duodenal ulcer carry only the s1m1 vacA strain that produces more cytotoxin, differing from adults with duodenal ulcer, who may carry s1m1 or s1m2 vacA strains (10). Recently, Perez-Perez et al. (27) demonstrated that serum IgG anti-VacA antibodies were present more frequently in patients carrying s1m1 strains than in other H. pylori-positive patients.
In summary, Western blotting appears to be useful for the diagnosis of H. pylori infection in children. Furthermore, we demonstrated that most children with duodenal ulcer present antibodies to CagA and VacA proteins, a fact suggesting that children infected with strains possessing these virulent markers are more prone to developing duodenal ulcer.
ACKNOWLEDGMENTS
This work was supported by grants from the CNPq, FINEP, FAPEMIG, and PRPq/UFMG, Brazil.
REFERENCES
- 1.Aucher P, Petit M L, Mannant P R, Pezennec L, Babin P, Fauchere J L. Use of immunoblot assay to define serum antibody patterns associated with Helicobacter pylori infection and with H. pylori-related ulcers. J Clin Microbiol. 1998;36:931–936. doi: 10.1128/jcm.36.4.931-936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blecker U, McKeithan T W, Hart J, Kirschner B S. Resolution of Helicobacter pylori-associated gastric lymphoproliferative disease in a child. Gastroenterology. 1995;109:973–977. doi: 10.1016/0016-5085(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Carmolinga-Ponce M, Torres J, Perez-Perez G, Leal-Herrera Y, Gonzalez-Ortiz B, Madrazo de la Garza A, Gomez A, Munhoz O. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am J Gastroenterol. 1998;93:1264–1270. doi: 10.1111/j.1572-0241.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 4.Casswall T, Hans-Olof Nilsson H, Bergström M, Aleljung P, Wadström T, Dahlström A K, Albert M J, Sarker S A. Evaluation of serology, 13C-urea breath test, and polymerase chain reaction of stool samples to detect Helicobacter pylori in Bangladeshi children. J Pediatr Gastroenterol Nutr. 1999;28:31–36. doi: 10.1097/00005176-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ching C K, Wong C Y, Kwok E, Ong L, Covacci A, Lam S K. Prevalence of CagA-bearing Helicobacter pylori strains detected by anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996;91:949–953. [PubMed] [Google Scholar]
- 6.Cover T L, Glupczynski Y, Lage A P, Burette A, Tummuru M K R, Perez-Perez G I, Blaser M J. Serologic detection of infection with cagA+Helicobacter pylori strains. J Clin Microbiol. 1995;33:1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czinn S J. Serodiagnosis of Helicobacter pylori in pediatric patients. J Pediatr Gastroenterol Nutr. 1999;28:132–134. doi: 10.1097/00005176-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Elitsur Y, Adkins L, Saeed D, Neace C. Helicobacter pylori antibody profile in household members of children with H. pylori infection. J Clin Gastroenterol. 1999;29:178–182. doi: 10.1097/00004836-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Elitsur Y, Neace C, Werthammer M C, Triest W E. Prevalence of CagA, VacA antibodies in symptomatic and asymptomatic children with Helicobacter pylori infection. Helicobacter. 1999;4:100–105. doi: 10.1046/j.1523-5378.1999.98530.x. [DOI] [PubMed] [Google Scholar]
- 10.Evans D G, Queiroz D M M, Mendes E N, Evans D J., Jr Helicobacter pylori cagA status and s and m alleles of vacA in isolates from individuals with a variety of H. pylori-associated gastric disease. J Clin Microbiol. 1998;36:3435–3437. doi: 10.1128/jcm.36.11.3435-3437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulde M, Cremer J, Zöller L. Humoral immune response against Helicobacter pylori as determined by immunoblot. Electrophoresis. 1993;14:945–951. doi: 10.1002/elps.11501401150. [DOI] [PubMed] [Google Scholar]
- 12.Goossens H, Glupczynski Y, Burette A, Van den Borre C, Butzler J P. Evaluation of a commercially available second-generation immunoglobulin G enzyme immunoassay for the detection of Helicobacter pylori infection. J Clin Microbiol. 1992;30:176–180. doi: 10.1128/jcm.30.1.176-180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karvar S, Helge K, Frosch M, Burghardt W, Gross U. Use of serum-specific immunoglobulins A and G for detection of Helicobacter pylori infection in patients with chronic gastritis by immunoblot analysis. J Clin Microbiol. 1997;35:3058–3061. doi: 10.1128/jcm.35.12.3058-3061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna B, Cutler A, Israel N R, Perry M, Lastovica A, Fields P I, Gold B. Use caution with serologic testing for Helicobacter pylori infection in children. J Infect Dis. 1998;178:460–465. doi: 10.1086/515634. [DOI] [PubMed] [Google Scholar]
- 15.Klein P D, Gilman R H, Leon-Barua R, Diaz F, Smith E A, Graham D Y. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol. 1994;12:2196–2200. [PubMed] [Google Scholar]
- 16.Mégraud F. Advantages and disadvantages of current diagnostic tests for the detection of Helicobacter pylori. Scand J Gastroenterol. 1996;31(Suppl. 215):57–62. doi: 10.3109/00365529609094536. [DOI] [PubMed] [Google Scholar]
- 17.Mégraud F, Lamouliatte H. Helicobacter pylori and duodenal ulcer. Dig Dis Sci. 1992;37:769–772. doi: 10.1007/BF01296437. [DOI] [PubMed] [Google Scholar]
- 18.Miehlke S, Kibler K, Kim J G, Figura N, Small S M, Graham D Y, Go M F. Allelic variation in the cagA gene of Helicobacter pylori obtained from patients from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 19.Mitchell H M, Lee A, Bukowickz J, Borody T. The use of serology to diagnose active Campylobacter pylori infection. Med J Aust. 1988;149:604–609. doi: 10.5694/j.1326-5377.1988.tb120800.x. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell H M, Hazell S L, Bohane T D, Hu P, Chen M, Li Y Y. The prevalence of antibody to CagA in children is not a marker for specific disease. J Pediatr Gastroenterol Nutr. 1999;28:71–75. doi: 10.1097/00005176-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell H M, Hazell S L, Kolesnikow T, Mitchell J, Frommer D. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori. Infect Immun. 1996;64:1166–1172. doi: 10.1128/iai.64.4.1166-1172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson I, Ljungh A, Aleljung P, Waström T. Immunoblot assay serodiagnosis of Helicobacter pylori infections. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson I, Lindkvist P, Wretlind B, Wadström T, Giesecke J. Immunoblot is superior to EIA for anti-Helicobacter pylori antibody determinations in young children from both high and low endemic areas. Gut. 1999;45(Suppl. 111):A97. [Google Scholar]
- 24.Oderda G, Figura N, Bayeli P F, Basagni C, Bugnoli M, Armellini D, Altare F, Ansaldi N. Serologic recognition of Helicobacter pylori cytotoxin-associated protein, peptic ulcer and gastroduodenal pathology in childhood. Eur J Gastroenterol Hepatol. 1993;5:695–699. [Google Scholar]
- 25.Oliveira A M R, Rocha G A, Queiroz D M M, Mendes E N, Carvalho A S T, Ferrari T C A, Nogueira A M M F. Evaluation of enzyme-linked immunosorbent assay for the diagnosis of Helicobacter pylori infection in children from different age groups with and without duodenal ulcer. J Pediatr Gastroenterol Nutr. 1999;28:157–161. doi: 10.1097/00005176-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Perez G I, Peek R M, Jr, Atherton J C, Blaser M J, Cover T L. Detection of anti-VacA antibody responses in serum and gastric juice samples using type s1m1, s2m2 Helicobacter pylori VacA antigens. Clin Diagn Lab Immunol. 1999;6:489–493. doi: 10.1128/cdli.6.4.489-493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perri F, Pastore M, Clemente R, Festa V, Quitadamo M, Niro G, Conoscitore P, Rutgeerts P, Andriulli A. Helicobacter pylori infection may undergo spontaneous eradication in children: a 2-year follow-up study. J Pediatr Gastroenterol Nutr. 1998;27:181–183. doi: 10.1097/00005176-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Queiroz D M M, Mendes E N, Carvalho A S T, Rocha G A, Oliveira A M R, Soares T F, Santos A, Cabral M M D A, Nogueira A M M F. J. Infect Dis. 2000;181:626–630. doi: 10.1086/315262. [DOI] [PubMed] [Google Scholar]
- 30.Queiroz D M M, Mendes E N, Rocha G A. Indicator medium for isolation of Campylobacter pylori. J Clin Microbiol. 1987;25:2378–2379. doi: 10.1128/jcm.25.12.2378-2379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raymond J, Kalach N, Bergeret M, Barbet J P, Benhamou P H, Gendrel D, Dupont C. Evaluation of a serological test for diagnosis of Helicobacter pylori infection in children. Eur J Clin Microbiol Infect Dis. 1996;15:415–417. doi: 10.1007/BF01690102. [DOI] [PubMed] [Google Scholar]
- 32.Sim J G, Kim E C, Seo J K. The role of serology in the diagnosis of Helicobacter pylori infection in children. Clin Pediatr. 1995;34:458–462. doi: 10.1177/000992289503400901. [DOI] [PubMed] [Google Scholar]
- 33.Sörberg M, Engstrand L, Ström M, Jönsson K A, Jörbeck H, Granström M. The diagnostic value of enzyme immunoassay and immunoblot in monitoring eradication of Helicobacter pylori. Scand J Infect Dis. 1997;29:147–151. doi: 10.3109/00365549709035875. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J E, Whatmore A M, Barer M R, Eastham E J, Kehoe M A. Serodiagnosis of Helicobacter pylori infection in childhood. J Clin Microbiol. 1990;28:2641–2646. doi: 10.1128/jcm.28.12.2641-2646.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenborre C, Cadranel S, Butzler J P, Glupczynski Y. Serum reactivity to Helicobacter pylori antigens assessed by Helico Blot 2.0 in H. pylori-positive children from different ethnic groups. Gut. 1995;37(Suppl. 1):A79. [Google Scholar]
- 36.Westblom T U, Madan E, Gudipati S, Midkiff B R, Czinn S J. Diagnosis of Helicobacter pylori infection in adult and pediatric patients by using Pyloriset, a rapid latex agglutination test. J Clin Microbiol. 1992;30:96–98. doi: 10.1128/jcm.30.1.96-98.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaoka Y, Kodama T, Graham D Y, Kashima K. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J Clin Microbiol. 1998;36:3433–3434. doi: 10.1128/jcm.36.11.3433-3434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]