Abstract
Purpose
Little is known about the association of psychosocial factors with health-related quality of life (HRQoL) among American Indians with type 2 diabetes (T2D). This study described functional social support, emotional support, coping, resilience, post-traumatic stress disorder, and HRQoL, among American Indians by diabetes status and, among those with diabetes, examined the association of these factors with HRQoL.
Methods
Using data from the Cherokee Nation Health Survey collected between 2017 and 2019, we evaluated differences in each measure of interest according to diabetes status, using t-test and Chi-squared tests of association. We used weighted multiple logistic regression to examine associations between multiple psychosocial factors and HRQoL among those with diabetes.
Results
Compared to individuals without diabetes, participants with diabetes rated their functional social support (4.62 vs. 4.56, respectively) and coping (2.65 vs. 2.61, respectively) slightly lower and were more likely to report ≥ 15 days of poor physical (14% vs. 26%, respectively) and mental health (14% vs. 17%, respectively) in the past month. Odds of reporting poor overall health increased more than sixfold for those who were dissatisfied/very dissatisfied with life (AOR = 6.70). Resilience scores reduced odds of reporting ≥ 15 days with poor physical health, while experiences of post-traumatic stress doubled these odds.
Conclusion
Our study yielded insights into the risk as well as protective factors associated with diabetes outcomes in a large sample of American Indians with T2D. Researchers should design pragmatic trials that deepen understanding of preventive as well as treatment leverage through greater attention to experiences that compromise HRQoL.
Keywords: Type 2 diabetes, Health related quality of life, American Indian, Psychosocial
Introduction
More than 34 million Americans live with diabetes, and approximately 95% have type 2 diabetes (T2D) [1]. Diabetes prevalence in the U.S. is expected to nearly double by 2030 due, in part, to the aging population [2]. American Indian adults have the highest prevalence of diabetes (15%) of any racial or ethnic group [1] and are more than twice as likely to die from diabetes complications compared to their non-Hispanic White counterparts [3]. T2D not only can lead to physical impairment but can also affect health-related quality of life (HRQoL) [4]. HRQoL is a multi-dimensional construct that includes physical, psychological, and social aspects of health, and is known to be poorer in people living with diabetes than in people with other chronic health conditions [5].
Health-related quality of life has been assessed among persons with T2D as noted in a recent systematic review and meta-analysis which included 18 studies and reported the presence of complications, hypertension, depression, and type of diet were associated with worse HRQoL [6]. This review, however, did not include American Indians. Additionally, daily management of T2D, including making dietary changes, initiating physical activity, fluctuating blood glucose levels, and T2D-related complications, is known to negatively impact HRQoL [7–11]. Although little has been published about these issues among Native people [12–17], research indicates older American Indians with T2D experience lower HRQoL compared to Whites or those without T2D [12, 17]. In another study American Indians with comorbid T2D and hypertension had worse HRQoL than those with only T2D or hypertension alone [16]. The association between psychosocial factors and HRQoL should be further examined to optimize health outcomes and improve HRQoL in American Indians with T2D [18].
A number of studies have examined the association of psychosocial factors, such as support, coping, resilience, and mental health [post-traumatic stress disorder (PTSD)] with HRQoL in non-Native populations [19–25]. For example, in a sample of older African Americans and Whites with diabetes, findings suggested that diabetes-specific social support was positively associated with HRQoL [20], and in a systematic review examining the relationship between resilience and chronic diseases findings indicated that lower levels of resilience were associated with poor HRQoL [23]. Individuals with T2D and post-traumatic stress disorder, a serious anxiety disorder, are thought to experience worse health outcomes than those without diabetes [26, 27]. However, when examining the association of post-traumatic stress disorder with HRQoL in American Indians, a paucity of information exists [12, 17, 28] despite the fact that American Indians are more than twice as likely to experience post-traumatic stress disorder compared to the general US population [29]. To date we found only one study that had explored co-occurring T2D and post-traumatic stress disorder and general health status in this population [28]. Aronson et al. found that co-occurring T2D and post-traumatic stress disorder were not associated with overall general health status after controlling for depressive symptoms [28]. Two studies revealed American Indians who experience depressive symptoms have lower physical HRQoL compared to Whites [17] and American Indian adults aged > 50 years who have T2D, compared to those who do not, are more likely to have less social support and poorer overall HRQoL [12].
As summarized above, despite the greater burden of diabetes in American Indians, the association between psychosocial factors and HRQoL has not been fully explored in this particularly vulnerable population. Therefore, drawing upon a large dataset of enrolled citizens of the Cherokee Nation of Oklahoma aged ≥ 18 years, we (1) described functional social support, emotional support, coping, resilience, post-traumatic stress disorder, and HRQoL, by diabetes status, and (2) examined the association of psychosocial factors with HRQoL of those with diabetes.
Research design and methods
Study population and data collection
Cherokee Nation, one of the largest federally recognized tribes in the U.S., has tribal lands that span 6950 square miles across 14 counties in Northeastern Oklahoma and has more than 380,000 tribal citizens [30]. Nearly two-thirds of all Cherokee citizens live in Oklahoma with the majority living within the reservation. In 2019, the unemployment rate among citizens who lived within the reservation was 35% with 27% of the citizens living below the federal poverty line (Personal communication with CN, Jan 2021). Cherokee citizens rely on the large tribally operated healthcare system, including hospitals, outpatient clinics, nursing homes, home health, and wellness programs. Between August 2017 and April 2019, the Tribe’s Public Health Department in partnership with the University of Oklahoma’s Sooner Survey Center conducted a cross-sectional telephone survey of users of its healthcare system. This BRFSS-like survey was used to obtain prevalence data on several key health behaviors and health issues for the tribal population, including tobacco use, other substance use, physical activity, nutrition-related behaviors, and mental health-related measures. These data are used by Cherokee Nation Public Health and Behavioral Health Programs to determine efficacy of current programs and interventions and to improve programs, policies, and interventions. The Tribe’s electronic health record system served as the sampling frame and included potential participants’ county, gender, and age. Three of the co-authors were part of the original study team (AC, AW, and SC).
Study eligibility criteria included Cherokee Nation citizenship, living within the tribe’s service area, aged ≥ 18 years, use of Cherokee Nation Health Services within the past 2 years, English speaking, and providing verbal informed consent. Participants were compensated for their time with a $10 gift card, which was mailed to them after completion of the survey. The study protocol and all survey materials were approved by the Cherokee Nation Institutional Review Board.
Sampling design and weighting
A stratified simple random sampling without replacement design was used [31]. Strata were created based on three variables: county (1–14), gender (male, female), and age (18–34, 35–54, ≥ 55 years) group. Proportional allocation was used due to lack of prior information for study variables of interest. To reduce sampling error, initial design weights were calculated by using strata population sizes divided by the sample sizes [31, 32]. To further reduce coverage error and non-response error and to improve the efficiency of our estimates, we used iterative proportional fitting procedures [33], with trimming to handle extreme weights and to adjust design weights, so the weighted estimates of county, gender, and age group matched with the population quantities obtained from the overall sampling frame. There were 105,990 tribal citizens in the sampling frame file provided to Oklahoma University Health Science Center by the Cherokee Nation. We first drew a stratified sample of 31,008 from the sampling frame. Due to lower than expected response rates in the first sample, we drew an additional sample 14,171 of potential respondents in order to reach our desired number of completed surveys. We attempted to contact 45,179 tribal citizens and obtain completed surveys with 4139 eligible respondents. Potential participants were contacted by telephone up to seven times. Using the American Association of Public Opinion Research Response Rate 3 standard definition, we had a response rate of 17%, assuming 80% eligibility in the sampling frame [34]. To improve the power of statistical analysis, we combined the two samples for the analysis. Since the two samples were selected in the similar time frame and weighting was performed to make sure the combined sample was representative of the sampling frame, it was deemed reasonable to use the combined sample for statistical analysis to reduce selection bias and improve statistical efficiency (Fig. 1).
Fig. 1.
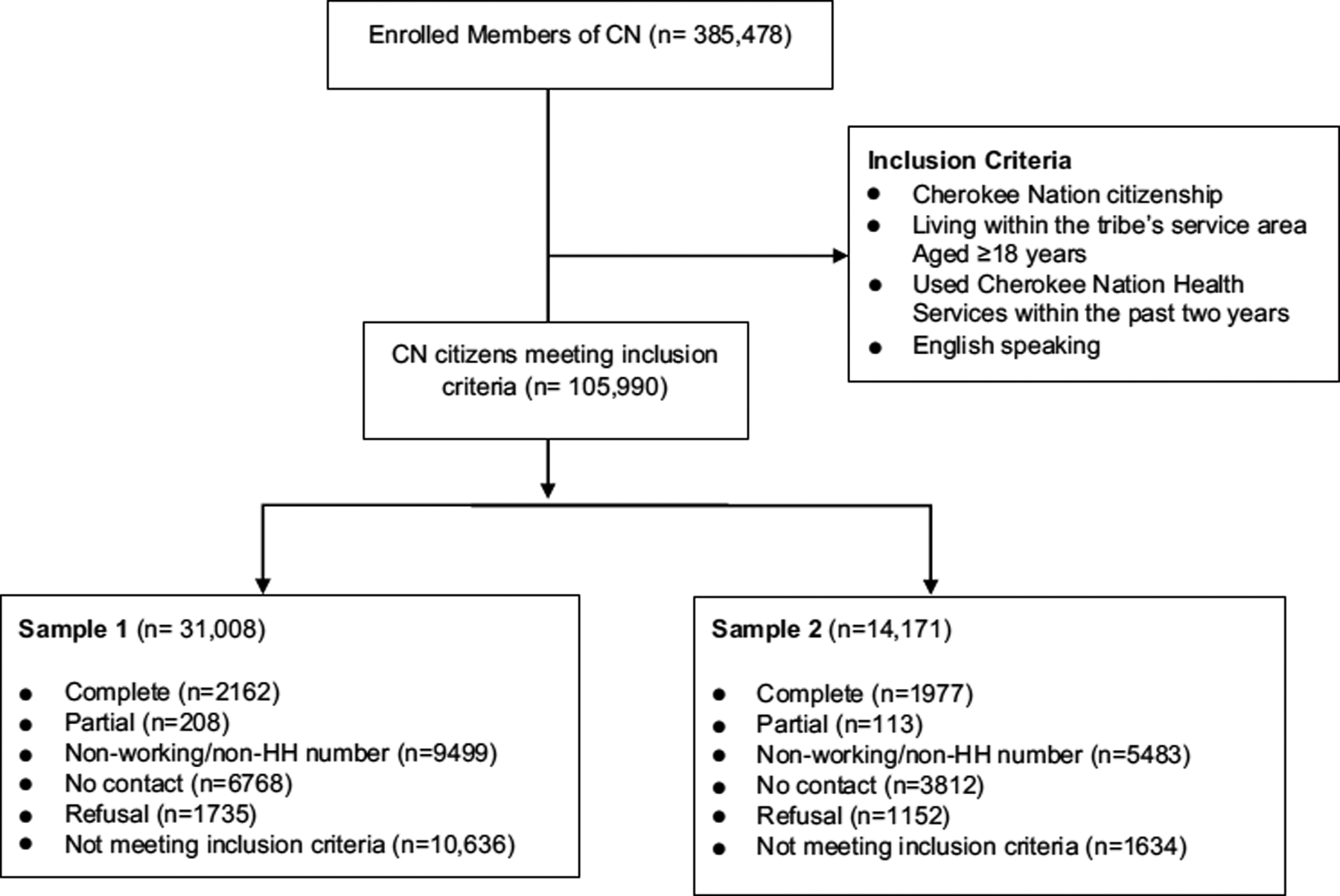
CONSORT flow diagram, Cherokee Nation Health Survey
Measures
Psychosocial factors
We included six different measures of psychosocial factors, including functional social support, emotional support, coping, resilience, life satisfaction, and post-traumatic stress disorder.
Functional social support was operationalized with the Duke UNC Functional Social Support Questionnaire-Short Form (DUFSS-5) [35]. The DUFSS-5 consists of five items measured on a three-point scale ranging from 1 (much less than I would like) to three (as much as I would like). Items are summed together for a summary score ranging from 5 to 15 with higher scores indicating higher levels of support. The DUFSS-5 in this study sample had evidence of reliability with a Cronbach’s alpha of 0.77.
Emotional support was measured using the Behavioral Risk Factor Surveillance System (BRFSS) question “How often do you get the emotional support you need?” with scores ranging from 1 (Always) to 5 (Never) [36]. The scale was used to examine emotional support in American Indian women aged 18–44 [37].
We operationalized coping and avoidance using the Ayers Coping Scale (ACS) which was divided into two subscales that measured problem focused and avoidance coping strategies [38]. Responses ranged on a three-point scale from 1 (Never) to 3 (Most of the time) with higher scores indicating higher levels of coping or avoidant coping strategies [38]. The ACS has previously been used with American Indians aged 15–54 years [39]. The ACS for this study had evidence of acceptable reliability with a Cronbach’s alpha of 0.60 for the coping subscale and 0.69 for the avoidance coping subscale.
We measured resilience with the Brief Resilience Scale (BRS), which is a six-item scale designed to measure the construct of resilience based on the ability to “bounce back” from stress [40]. Items are scored on a five-point Likert scale from 1 (strongly disagree) to 5 (strongly agree) with higher total scores indicating higher levels of resilience. The BRS with our study sample had evidence of good reliability with a Cronbach’s alpha of 0.83.
Life satisfaction was measured using the BRFSS question “In general, how satisfied are you with your life?” with responses ranging from 1 (Very satisfied) to 4 (Very dissatisfied) [36]. The scale was previously used to examine life satisfaction in American Indian women aged 18–44 [37].
Our sixth psychosocial factor was PTSD measured using the Primary Care Post-Traumatic Stress Disorder-4 Scale (PC-PTSD-4) [41]. The PC-PTSD-4 is a four-item tool that reflects the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, (DSM-IV) PTSD diagnostic criteria. This primary care screening tool, commonly administered to indicate risk of PTSD following exposure to a traumatic event, uses yes/no responses with scores ranging from 0 to 4 based on number of questions answered with “yes.” The screen threshold for the scale is three (answering yes to any three of four questions) indicating probable PTSD. The scale evidenced good test–retest reliability (r = 0.83) [41].
HRQoL was our primary outcome, using the measure developed by the CDC and included in the BRFSS with evidence of test–retest reliability, validity, and responsiveness [42, 43]. The first item measures overall general health by asking “Would you say in general that your health is” with five response options, Excellent, Very good, Good, Fair, or Poor. The other two items measure physical and mental health by asking, “How many days during the last 30 days has your physical or mental health not been good?” Unhealthy days were scored by participants’ self-report of the total number of days during the past 30 days when the person felt his or her physical or mental health was not good. Scores were divided into three categories with higher numbers indicating more days of poor physical (0, 1–14, ≥ 15 days) or mental health (0, 1–14, ≥ 15 days) [42]. This scale has been previously used to good effect among American Indian adults [14, 44].
Demographic and health characteristics
Demographic characteristics for study participants included age (18–44, 45–54, 55–64, ≥ 65 years) and gender (male or female). Marital status was categorized as married, member of unmarried couple, never married, divorced, widowed, or separated. Two measures of socioeconomic status (SES) were used. Annual household income was categorized from $0 to $24,999; $25,000 to $54,999; and ≥ $55,000; however, formal educational attainment was categorized as high school or less and some college or more. Participants were asked if they had ever been told by a healthcare provider they had diabetes. Body Mass Index (BMI) was calculated from self-reported height and weight (BMI = kg/m2).
Statistical methods
Data analysis
We used descriptive statistics to describe the demographic characteristics and each variable of interest for the total survey sample and by diabetes status. Means were calculated with standard deviations for continuous measures and proportions for categorical variables. We evaluated differences in each measure of interest according to diabetes status, using t test and Chi-squared tests of association, as appropriate. Multiple logistic regression was conducted to examine associations between multiple factors (functional social support, emotional support, coping, resilience, life satisfaction, and PTSD) and each HRQoL measure (overall general health, physical health, and mental health) among those with diabetes, adjusting for age, gender, and SES. In these logistic regression models, all HRQoL measures were dichotomized and served as the outcome variables. Poor self-rated overall general health was coded as one if participants endorsed “fair” or “poor” for this question; it was coded as zero otherwise. For the poor physical health measure, it was dichotomized as 1 = “15 or more days” and 0 = “0–14 days” of self-reported days with poor physical health in the past 30 days. Similarly, the poor mental health measure was defined as 1 = “15 or more days” and 0 = “0–14 days” of self-reported days with poor mental health. All analyses were conducted using SAS 9.4 (SAS, Inc. Cary, NC) survey analysis procedures accounting for survey weights and the complex survey design.
Results
Of the 4137 participants who completed the survey, 953 (23%) had diabetes. The demographic characteristics and mean BMI by diabetes status are shown in Table 1. The majority of participants were female (54%), married (61%), had at least some college education (58%), and reported an annual household income of $25,000 or more (61%). Having diabetes was significantly associated with age, education, household income, and marital status (p < 0.0001). Participants with diabetes tended to be older with 60% aged ≥ 55 years compared to 27% among those without diabetes. Those with diabetes were significantly less likely to have a college education compared to individuals without diabetes (50% vs. 60%, respectively). Participants with diabetes also tended to have lower income: 40% reporting a household income of $0–$24,999, compared to just 26% among those without diabetes. Individuals with diabetes had higher mean BMI than those without diabetes (34 vs. 30, respectively). Diabetes status was not associated with gender.
Table 1.
Demographic characteristics and mean BMI of participants with and without diabetes
| Total % (n) | Diabetes % (n) | No diabetes % (n) | p value (Wald chi-square test) | |
|---|---|---|---|---|
| Age | ||||
| 18–44 | 49.3 (1632) | 18.9 (138) | 57.1 (1494) | < .0001 |
| 45–54 | 16.6 (719) | 20.7 (179) | 15.6 (540) | |
| 55–64 | 15.7 (829) | 26.7 (285) | 12.9 (544) | |
| 65+ | 18.3 (957) | 33.7 (351) | 14.4 (606) | |
| Gender | ||||
| Female | 54.4 (2478) | 52.5 (548) | 54.9 (1930) | 0.2158 |
| Male | 45.6 (1659) | 47.5 (405) | 45.1 (1254) | |
| Education | ||||
| High school or less | 41.9 (1757) | 50.2 (470) | 39.8 (1287) | < .0001 |
| Some college or more | 58.1 (2367) | 49.8 (479) | 60.2 (1888) | |
| Household income | ||||
| $0–$24,999 | 28.5 (1232) | 40.0 (384) | 25.5 (848) | < .0001 |
| $25,000–$54,999 | 27.8 (1145) | 25.7 (248) | 28.3 (897) | |
| $55,000+ | 33.3 (1317) | 22.8 (205) | 36.0 (1112) | |
| Missing | 10.5 (443) | 11.5 (116) | 10.2 (327) | |
| Marital status | ||||
| Never married | 15.4 (505) | 8.7 (72) | 17.1 (433) | |
| Divorced, widowed, separated | 23.3 (1073) | 32.9 (323) | 20.9 (750) | |
| Body mass index, mean (SE) | 30.6 (0.1) | 33.9 (0.3) | 29.7 (0.1) | < .0001 |
Table 2 summarizes the differences in HRQoL and other psychosocial factors by diabetes status. Compared to those without diabetes, participants with diabetes rated their functional social support (4.62 vs. 4.56, respectively; p = 0.0063) and coping (2.65 vs. 2.61, respectively; p = 0.0053) slightly lower and avoidance coping (1.74 vs. 1.78, respectively; p = 0.039) slightly higher. Similarly, 47% of participants with diabetes rated their health as fair/poor compared to just 20% among those without diabetes (p < 0.0001). Compared to those without diabetes, participants with diabetes were more likely to report ≥ 15 days of poor physical health (14% vs 26%, respectively; p < 0.0001) and poor mental health (14% vs. 17%, respectively; p = 0.0021). Participants with diabetes tended to report less emotional support; 9% reported rarely/never receiving emotional support when they needed it compared to 6% among those without diabetes (p = 0.0003). Participants with diabetes were less likely to be very satisfied with life compared to those without diabetes (40% vs. 47%, respectively; p = 0.0003). There were no statistically significant differences in resilience and post-traumatic stress disorder scores between the two groups.
Table 2.
Summary of the differences in HRQoL and functional social support, coping, resilience, PTSD, emotional support, life satisfaction by diabetes status
| Total | Diabetes | No diabetes | p value | |
|---|---|---|---|---|
| Functional social support | ||||
| Mean (SD) | 4.61 (.01) | 4.56 (.02) | 4.62 (.01) | 0.0063 |
| Coping | ||||
| Mean (SD) | 2.65 (.01) | 2.61 (.01) | 2.65 (.01) | 0.0053 |
| Avoidance coping | ||||
| Mean (SD) | 1.75 (.01) | 1.78 (.02) | 1.74 (.01) | 0.0391 |
| Resilience | ||||
| Mean (SD) | 3.74 (.01) | 3.70 (.02) | 3.75 (.01) | 0.0858 |
| Post-traumatic stress disorder | ||||
| Mean (SD) | 0.95 (.02) | 0.93 (.05) | 0.96 (.03) | 0.6208 |
| 0–2% (n) | 79.6 (3264) | 80.8 (758) | 79.4 (2506) | 0.3527 |
| 3 or more % (n) | 20.4 (823) | 19.2 (179) | 20.6 (644) | |
| Emotional support needed | ||||
| Always/usually % (n) | 81.1 (3305) | 76.1 (718) | 82.3 (2587) | 0.0003 |
| Sometimes % (n) | 12.2 (507) | 14.6 (133) | 11.6 (374) | |
| Rarely/never % (n) | 6.7 (279) | 9.3 (90) | 6.1 (189) | |
| Life satisfaction | ||||
| Very satisfied % (n) | 45.2 (1852) | 39.7 (372) | 46.6 (1480) | 0.0003 |
| Satisfied % (n) | 48.8 (2010) | 52.2 (495) | 47.9 (1515) | |
| Dissatisfied/very dissatisfied % (n) | 6.0 (248) | 8.1 (74) | 5.5 (174) | |
| Health-related quality of life | ||||
| Overall general health | ||||
| Excellent/very good % (n) | 40.6 (1549) | 15.0 (141) | 47.1 (1408) | < .0001 |
| Good % (n) | 34.1 (1439) | 37.6 (348) | 33.2 (1091) | |
| Fair/poor % (n) | 25.3 (1138) | 47.4 (458) | 19.7 (680) | |
| Healthy days | ||||
| Physical health not good past 30 days | ||||
| 0 days % (n) | 54.6 (2117) | 41.6 (375) | 57.9 (1742) | < .0001 |
| 1–14 days % (n) | 29.3 (1219) | 32.3 (307) | 28.5 (912) | |
| 15 or more days % (n) | 16.1 (717) | 26.1 (248) 13.6 (469) | 13.6 (469) | |
| Mental health not good past 30 days | ||||
| 0 days % (n) | 61.5 (2496) | 63.1 (590) | 61.1 (1906) | 0.0021 |
| 1–14 days % (n) | 24.4 (977) | 20.4 (193) | 25.4 (784) | |
| 15 or more days % (n) | 14.1 (585) | 16.5 (152) 13.5 (433) | 13.5 (433) |
Table 3 examines only participants with diabetes and summarizes the results of multiple logistic regression analyses estimating the association of psychosocial factors with each measure of HRQoL, adjusting for age, gender, SES, BMI, and marital status. Life dissatisfaction is strongly associated with poor overall general health. Specifically, compared to those who were very satisfied with life, individuals who were dissatisfied/very dissatisfied with life had more than sixfold higher odds of reporting poor overall general health (AOR = 6.70; 95% CI 2.83–15.88). Functional social support, emotional support, coping, avoidance coping, resilience, PTSD, marital status, and gender were not significantly associated with poor overall general health.
Table 3.
Summary of the association of psychosocial factors with each measure of HRQoL: poor overall general health, no. of days with poor physical health, and no. of days with poor mental health among those with diabetes
| Poor overall general healtha | No. of days with poor physical healthb | No. of days with poor mental healthc | ||||
|---|---|---|---|---|---|---|
| Adj. OR | 95% CI | Adj. OR | 95% CI | Adj. OR | 95% CI | |
| Functional social support | ||||||
| Mean | 0.87 | (0.62–1.20) | 0.89 | (0.62–1.28) | 0.95 | (0.62–1.46) |
| Coping | ||||||
| Mean | 1.05 | (0.68–1.60) | 0.84 | (0.53–1.35) | 0.57 | (0.30–1.08) |
| Avoidance coping | ||||||
| Mean | 0.99 | (0.73–1.34) | 1.05 | (0.74–1.49) | 1.01 | (0.64–1.60) |
| Resilience | ||||||
| Mean | 0.82 | (0.63–1.08) | 0.7 | (0.52–0.93) | 0.6 | (0.41–0.87) |
| Post-traumatic stress disorder | ||||||
| 0–2 | Ref | Ref | Ref | Ref | Ref | Ref |
| 3 or more | 1.3 | (0.85–1.97) | 2.01 | (1.30–3.12) | 3.7 | (2.21–6.19) |
| Emotional support needed | ||||||
| Always/usually | Ref | Ref | Ref | Ref | Ref | Ref |
| Sometimes | 1.26 | (0.77–2.06) | 0.94 | (0.55–1.63) | 1.73 | (0.88–3.40) |
| Rarely/never | 1.51 | (0.81–2.82) | 0.55 | (0.29–1.04) | 1.31 | (0.63–2.70) |
| Life satisfaction | ||||||
| Very satisfied | Ref | Ref | Ref | Ref | Ref | Ref |
| Satisfied | 1.51 | (1.07–2.13) | 1.07 | (0.71–1.63) | 1.9 | (1.04–3.50) |
| Dissatisfied/very dissatisfied | 6.7 | (2.83–15.88) | 3.28 | (1.55–6.95) | 8.4 | (3.39–20.77) |
| Age | ||||||
| 18–44 | Ref | Ref | Ref | Ref | Ref | Ref |
| 45–54 | 1.49 | (0.88–2.53) | 1.51 | (0.82–2.76) | 0.96 | (0.48–1.95) |
| 55–64 | 2.27 | (1.38–3.74) | 1.71 | (0.96–3.03) | 0.58 | (0.29–1.19) |
| 65+ | 1.43 | (0.86–2.38) | 1.01 | (0.55–1.86) | 0.39 | (0.18–0.82) |
| Gender | ||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 0.84 | (0.61–1.16) | 0.98 | (0.68–1.40) | 0.68 | (0.42–1.10) |
| Education | ||||||
| High school or less | Ref | Ref | Ref | Ref | Ref | Ref |
| Some college or more | 0.5 | (0.36–0.69) | 0.85 | (0.59–1.22) | 0.6 | (0.35–1.04) |
| Marital status | ||||||
| Married, member of unmarried couple | Ref | Ref | Ref | Ref | Ref | Ref |
| Divorced, widowed, separated | 0.86 | (0.60–1.22) | 0.9 | (0.61–1.33) | 1.1 | (0.66–1.83) |
| Never married | 1 | (0.56–1.82) | 0.52 | (0.24–1.09) | 0.81 | (0.32–2.06) |
| Body mass index | 1.03 | (1.00–1.05) | 1.04 | (1.01–1.06) | 1.03 | (1.00–1.06) |
Adjusted for household income
Self-rate: 0 = “excellent/very good/good”; 1 = “fair/poor”
Poor physical health: 0 = ““0–14 days”; 1 = “15 or more days”
Poor mental health: 0 = “0–14 days”; 1 = “15 or more days”
The psychosocial factors that are significantly associated with poor physical health include lower levels of resilience, post-traumatic stress disorder, and life dissatisfaction. A one unit increase in resilience score was associated with 30% reduced odds of reporting ≥ 15 days with poor physical health (AOR = 0.70; 95% CI 0.52–0.93). Experience of post-traumatic stress disorder (i.e., reporting three or more traumatic events) doubled the odds of reporting poor physical health (AOR = 2.01; 95% CI 1.30–3.12). People with lower life satisfaction were more than three times as likely to report having 15 or more days of poor physical health in the last month (AOR = 3.28; for dissatisfied/very dissatisfied vs. very satisfied; 95% CI 1.55–6.95). Functional social support, emotional support, coping, avoidance coping, age, gender, marital status, and education were not significantly associated with ≥ 15 poor physical health days.
Similar to physical health, the psychosocial factors that are significantly associated with poor mental health include lower level of resilience, post-traumatic stress disorder, and life dissatisfaction. A one unit increase in resilience score was associated with a 40% reduction in the odds of reporting ≥ 15 days of poor mental health (AOR = 0.60; 95% CI 0.41–0.87). Experience of post-traumatic stress disorder tripled more than the odds of reporting ≥ 15 days of poor mental health (AOR = 3.70; 95% CI 2.21–6.19). Compared to those who are very satisfied with life, individuals dissatisfied/very dissatisfied with life are more than eight times more likely to report ≥ 15 days of poor mental (AOR = 8.40; 95% CI 3.39–20.77). Functional social support, emotional support, coping, avoidance coping, gender, marital status, and education were not significantly associated with poor mental health.
Discussion
Consistent with prior studies [10, 45], we found that participants with diabetes were more likely to rate their overall general health as fair/poor and rated their functional social support, emotional support, and coping slightly lower than those without diabetes.
We also found that HRQoL was poorer among participants with diabetes, again consistent with prior studies that showed a negative impact of diabetes on physical health and emotional well-being [10, 13, 17]. For instance, one study examined HRQoL among African American (n = 200), American Indian (n = 181), and White (n = 212) rural adults aged ≥ 60 years with diabetes and found American Indians scored poorer on the physical and mental components of HRQoL compared to Whites [17]. Given that HRQoL is affected by diabetes-related complications (i.e., stroke, cardiovascular disease) and lifestyles (i.e., physical activity, diet) [6], and the high rates of these complications among American Indians with diabetes, it is not surprising participants in our study reported poorer HRQoL compared to those without diabetes.
Resilience, the ability to adapt to stress and thrive in the face of adversity, is an important factor to consider related to diabetes management and outcomes. Lower levels of resilience were associated with poorer mental and physical health, which suggests that resilience may be a protective factor of good HRQoL. These findings are similar to a previous study that examined the association of resilience with mental and physical health among older American Indians [46] and a systematic review that examined resilience in persons with chronic diseases [23]. Of the 12 studies in the systematic review none included American Indian populations [23]. A more recent systematic literature review also failed to identify any publications with respect to the association of resilience and obesity prevention in American Indians [47]. Likewise, to our knowledge, no studies have examined resilience as a potential protective factor in American Indians with T2D. However, based on findings from other American Indian resilience research [46, 48–50] and from the fact that American Indian communities continue to thrive despite enduring both historical and contemporary trauma, loss, and oppression, we know that American Indians have been and continue to be resilient. The role resilience plays in regard to HRQoL among American Indians promises to fill an important knowledge gap and may yield important insights into the underlying psychosocial mechanisms. This line of inquiry may also suggest points of resilience-based interventions that can improve health outcomes among American Indians with T2D.
Participants with diabetes who endorsed ≥ 3 traumatic events had doubled the odds of reporting ≥ 15 poor physical health days and tripled more than the odds of reporting ≥ 15 poor mental health days. Compared to non-Hispanic Whites, American Indians experience higher rates of post-traumatic stress disorder [29]. The co-occurrence of T2D and post-traumatic stress disorder is associated with worse diabetes-related health outcomes compared to T2D alone [25, 26, 28]. To date, only one study has examined post-traumatic stress and HRQoL among American Indians with T2D [28]. According to findings from a sample of 218 American Indian participants, 21.8% who screened positive for post-traumatic stress (≥ 3 traumatic events) revealed no association with overall general health status when controlling for depressive symptoms [28]. The reason for this finding may derive from the co-linearity between post-traumatic stress disorder and depressive symptoms. While this study examined overall general health, it did not explore poor physical or mental health. Post-traumatic stress disorder causes tremendous mental health burden and is a major risk factor for developing chronic health disease, such as T2D [51]. Our study indicated that post-traumatic stress disorder was negatively associated with HRQoL, but further research is warranted to examine the pathways of this association (i.e., biologic changes, health behaviors, etc.). Future research is needed to determine if treating post-traumatic stress symptoms can improve HRQoL in American Indians with T2D.
Life satisfaction has been found to be an indicator of HRQoL [52, 53]. Our study demonstrated that being dissatisfied/very dissatisfied with life (compared to very satisfied) increased odds by threefold for poor physical health and by more than eightfold for poor mental health. These findings were similar to a prior study that examined the associations between life satisfaction and HRQoL and chronic illnesses among adults using the 2005 BRFSS data [53]. In that study, individuals who were dissatisfied/very dissatisfied with their lives had 9.9 poor physical health days and 13.7 poor mental health days per month compared to those who were very satisfied with their lives (1.5 and 2.4 days, respectively) [53]. Life satisfaction is an important contributor to health and well-being. Low life satisfaction is associated with not only increased risk of developing a chronic disease, such as diabetes, but increased risk of death [54]. Future studies should more carefully consider this association to better understand how to enhance life satisfaction and thereby improve diabetes-related outcomes in American Indians with T2D.
When examining the association of psychosocial factors with HRQoL in minority populations, it is important to address potential concerns that arise when instruments used are primarily developed and tested with non-minority populations. All but three of the instruments used in our study (DUFSS-5, PC-PTSD, BRS) have been used with American Indian samples, but to our knowledge there is no evidence to support content validity for these instruments among American Indians. Examining content validity is important for ensuring elements of an instrument are relevant and representative of the construct [55]. Racial and ethnic groups have unique cultural characteristics that may affect instrument accuracy. For example, American Indians may hold different perspectives and values related to social support compared to other racial and ethnic groups. Future studies should explore whether these instruments are culturally relevant and if the meaning and intent of the questions apply to the American Indian population.
Limitations
Our study has several noteworthy limitations. First, the data are cross sectional; therefore, we cannot draw conclusions about causality, although there is evidence of a number of strong associations that deserve further attention. Second, the findings are specific to the Cherokee Nation, and may not be generalizable to other American Indian communities. However, the results are likely to be instructive for other similar tribes, particularly those in Oklahoma. Third, the data were collected via a telephone survey, which may have introduced bias since characteristics of non-participants differed substantially from those who responded. For example, respondents reported higher household income and higher levels of education than Cherokee citizens as a whole. Additionally, the data were derived from self-report questionnaires, which can introduce recall and social desirability biases, or inaccuracies due to difficulty of comprehension, latency of events, age, education, and SES of the respondents. Weighting was used to minimize these biases, and ensures that the survey sample more closely resembled the actual Cherokee Nation population. Interviewer debriefing revealed few problems in this regard, though both remain of concern. Fourth, although the response rate was 17%, this rate is actually high given the national average based on the American Association for Public Opinion Research response rate of 9% for random digit dial telephone surveys [34, 56]. Fifth, the data did not distinguish between specific types of diabetes (i.e., Type 1 vs. Type 2); however, type 1 diabetes is not as common in American Indian populations [1]. Last, not all measures had been validated in the American Indian population or in populations with T2D and these measures lack evidence to support content validity within American Indian populations, although there is substantial evidence as to their reliability in other populations.
Conclusion
Our study yielded important insights into the risk as well as protective factors associated with diabetes outcomes in a large sample of American Indians with T2D. These insights help fill the gaps in our knowledge regarding linkages between such psychosocial factors and HRQoL in this particularly vulnerable segment of the population. Our next steps should move, as possible, from cross-sectional to longitudinal examinations of these associations. Researchers should design relevant pragmatic trials that can provide a much deeper understanding of the preventive and treatment leverage through greater attention to resilience, traumatic experiences, and other experiences that compromise HRQoL.
Acknowledgements
This research is supported by the National Institute on Aging under award numbers P30AG059295 and P30AG15297, the National Center for Minority Health and Health Disparities under award number P60 MD000507, and by the National Institute of Diabetes, Digestive, and Kidney Diseases under award number P30DK092923. Dr. Sixia Chen was partially supported by the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with and Institutional Development Award (IDeA) from National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Centers for Disease Control and Prevention. (2020). National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2020. U.S. Department of Health and Human Services. [Google Scholar]
- 2.Rowley WR, Bezold C, Arikan Y, Byrne E, & Krohe S (2017). Diabetes 2030: Insights from yesterday, today, and future trends. Population Health Management, 20(1), 6–12. 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heron M (2018). Deaths: Leading causes for 2016. National Vital Statistics Reports, 67(6), 1–77. [PubMed] [Google Scholar]
- 4.Healthy People 2020 Foundation Health Measure Report Health-Related Quality of Life and Well-Being (2010). Retrived from https://www.healthypeople.gov/2020/about/foundation-health-measures/Health-Related-Quality-of-Life-and-Well-Being. Accessed 12 Jan 2020.
- 5.Chen HY, Baumgardner DJ, & Rice JP (2011). Health-related quality of life among adults with multiple chronic conditions in the United States, Behavioral Risk Factor Surveillance System, 2007. Preventing Chronic Disease, 8(1), A09. [PMC free article] [PubMed] [Google Scholar]
- 6.Jing X, Chen J, Dong Y, Han D, Zhao H, Wang X, et al. (2018). Related factors of quality of life of type 2 diabetes patients: A systematic review and meta-analysis. Health and Quality of Life Outcomes, 16(1), 189. 10.1186/s12955-018-1021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin RR, & Peyrot M (1999). Quality of life and diabetes. Diabetes/Metabolism Research and Reviews, 15(3), 205–218. . [DOI] [PubMed] [Google Scholar]
- 8.Solli O, Stavem K, & Kristiansen IS (2010). Health-related quality of life in diabetes: The associations of complications with EQ-5D scores. Health and Quaility of Life Outcomes, 8, 18–18. 10.1186/1477-7525-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman K, Wee HL, Leow MKS, Tai ES, Lee J, Lim SC, et al. (2013). Associations between complications and health-related quality of life in individuals with diabetes. Clinical Endocrinology, 78(6), 865–873. 10.1111/j.1365-2265.2012.04480.x. [DOI] [PubMed] [Google Scholar]
- 10.Thommasen HV, Berkowitz J, Thommasen AT, & Michalos AC (2005). Understanding relationships between diabetes mellitus and health-related quality of life in a rural community. Rural and Remote Health, 5(3), 441. [PubMed] [Google Scholar]
- 11.Aschalew AY, Yitayal M, & Minyihun A (2020). Health-related quality of life and associated factors among patients with diabetes mellitus at the University of Gondar referral hospital. Health and Qualilty of Life Outcomes, 18(1), 62–62. 10.1186/s12955-020-01311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFall SL, Solomon TGA, & Smith DW (2000). Health-related quality of life of older Native American primary care patients. Research on Aging, 22(6), 692–714. 10.1177/0164027500226005. [DOI] [Google Scholar]
- 13.Johnson JA, Nowatzki TE, & Coons SJ (1996). Health-related quality of life of diabetic Pima Indians. Medical Care, 34(2), 97–102. 10.1097/00005650-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Goins RT, John R, Hennessy CH, Denny CH, & Buchwald D (2006). Determinants of health-related quality of life among older American Indians and Alaska Natives. Journal of Applied Gerontology, 25(1_suppl), 73S–88S. 10.1177/0733464805283037. [DOI] [Google Scholar]
- 15.Gilliland FD, Mahler R, & Davis SM (1998). Health-related quality of life for rural American Indians in New Mexico. Ethnicity and Health, 3(3), 223–229. 10.1080/13557858.1998.9961864. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Beals J, Whitesell NR, Roubideaux Y, & Manson SM (2009). Health-related quality of life and help seeking among American Indians with diabetes and hypertension. Quality of Life Research, 18(6), 709–718. 10.1007/s11136-009-9495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quandt SA, Graham CN, Bell RA, Snively BM, Golden SL, Stafford JM, et al. (2007). Ethnic disparities in health-related quality of life among older rural adults with diabetes. Ethnicity and Disease, 17(3), 471–476. [PMC free article] [PubMed] [Google Scholar]
- 18.Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, & Peyrot M (2016). Psychosocial care for people with diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39(12), 2126–2140. 10.2337/dc16-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawaz A, Malik JA, & Batool A (2014). Relationship between resilience and quality of life in diabetics. Journal of College of Physicians Surgeons Pakistan, 24(9), 670–675. [PubMed] [Google Scholar]
- 20.Bowen PG, Clay OJ, Lee LT, Vice J, Ovalle F, & Crowe M (2015). Associations of social support and self-efficacy with quality of life in older adults with diabetes. Journal of Gerontological Nursing, 41(12), 21–31. 10.3928/00989134-20151008-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zurita-Cruz JN, Manuel-Apolinar L, Arellano-Flores ML, Gutierrez-Gonzalez A, Najera-Ahumada AG, & Cisneros-Gonzalez N (2018). Health and quality of life outcomes impairment of quality of life in type 2 diabetes mellitus: A cross-sectional study. Health and Quality of Life Outcomes, 16(1), 94. 10.1186/s12955-018-0906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali S, Stone M, Skinner T, Robertson N, Davies M, & Khunti K (2010). The association between depression and health-related quality of life in people with type 2 diabetes: A systematic literature review. Diabetes/Metabolism Research and Reviews, 26, 75–89. 10.1002/dmrr.1065. [DOI] [PubMed] [Google Scholar]
- 23.Cal SF, Sá LRD, Glustak ME, & Santiago MB (2015). Resilience in chronic diseases: A systematic review. Cogent Psychology, 2(1), 1024928. 10.1080/23311908.2015.1024928. [DOI] [Google Scholar]
- 24.Tang TS, Brown MB, Funnell MM, & Anderson RM (2008). Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. Diabetes Educator, 34(2), 266–276. 10.1177/0145721708315680. [DOI] [PubMed] [Google Scholar]
- 25.Dixon HD, Michopoulos V, Gluck RL, Mendoza H, Munoz AP, Wilson JG, et al. (2020). Trauma exposure and stress-related disorders in African-American women with diabetes mellitus. Endocrinology, Diabetes and Metabolism,. 10.1002/edm2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller SA, Mancuso CA, Boutin-Foster C, Michelen W, McLean-Long C, Foote B, et al. (2011). Associations between posttraumatic stress disorder and hemoglobin A1(C) in low-income minority patients with diabetes. General Hospital Psychiatry, 33(2), 116–122. 10.1016/j.genhosppsych.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trief PM, Ouimette P, Wade M, Shanahan P, & Weinstock RS (2006). Post-traumatic stress disorder and diabetes: Comorbidity and outcomes in a male veterans sample. Journal of Behavorial Medicine, 29(5), 411–418. 10.1007/s10865-006-9067-2. [DOI] [PubMed] [Google Scholar]
- 28.Aronson BD, Palombi LC, & Walls ML (2016). Rates and consequences of posttraumatic distress among American Indian adults with type 2 diabetes. Journal of Behavioral Medicine, 39(4), 694–703. 10.1007/s10865-016-9733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassett D, Buchwald D, & Manson S (2014). Posttraumatic stress disorder and symptoms among American Indians and Alaska Natives: A review of the literature. Social Psychiatry and Psychiatric Epidemiology, 49(3), 417–433. 10.1007/s00127-013-0759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osiyo! (2020, November). Retrieved from https://www.cherokee.org/. Accessed 12 Jan 2020.
- 31.Lohr S (2010). Sampling: Design and analysis. Cengage Learning. [Google Scholar]
- 32.Cochran WG (1977). Sampling Techniques. Wiley. [Google Scholar]
- 33.Deming WE, & Stephan FF (1940). On a least squares adjustment of a sampled frequency table when the expected marginal totals are known. Annals of Mathematical Statistics, 11(4), 427–444. 10.1214/aoms/1177731829. [DOI] [Google Scholar]
- 34.The American Association for Public Opinion Research. (2015). Standard definitions: Final dispositions of case codes and outcome rates for surveys. (8th ed.). AAPOR. [Google Scholar]
- 35.Saracino R, Kolva E, Rosenfeld B, & Breitbart W (2015). Measuring social support in patients with advanced medical illnesses: An analysis of the Duke-UNC functional social support questionnaire. Palliative Support Care, 13(5), 1153–1163. 10.1017/s1478951514000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. (2011). National Center for Chronic Disease Prevention and Health Promotion: Behavioral Risk Factor Surveillance System. Centers for Disease Control and Preventio. [Google Scholar]
- 37.Willet MN, Hayes DK, Zaha RL, & Fuddy LJ (2012). Social-emotional support, life satisfaction, and mental health on reproductive age women’s health utilization, US, 2009. Materneral Child Health Journal, 16(Suppl 2), 203–212. 10.1007/s10995-012-1096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayers TS, Sandier IN, West SG, & Roosa MW (1996). A dispositional and situational assessment of children’s coping: Testing alternative models of coping. Journal of Personality, 64(4), 923–958. 10.1111/j.1467-6494.1996.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 39.Buchwald D, Goldberg J, Noonan C, Beals J, & Manson S (2005). Relationship between post-traumatic stress disorder and pain in two American Indian tribes. Pain Medicine, 6(1), 72–79. 10.1111/j.1526-4637.2005.05005.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, & Bernard J (2008). The brief resilience scale: Assessing the ability to bounce back. International Journal of Behavioral Medicine, 15(3), 194–200. 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 41.Prins A, & Ouimette P (2004). The primary care PTSD screen (PC-PTSD): Development and operating characteristics. Primary Care Psychiatry, 9, 151–151. [Google Scholar]
- 42.Centers for Disease Control and Prevention. (2000). Measuring healthy days. CDC. [Google Scholar]
- 43.Moriarty DG, Kobau R, Zack MM, & Zahran HS (2005). Tracking healthy days: A window on the health of older adults. Preventing Chronic Disease, 2(3), A16. [PMC free article] [PubMed] [Google Scholar]
- 44.Zahran HS, Kobau R, Moriarty DG, Zack MM, Holt J, & Donehoo R (2005). Health-related quality of life surveillance–United States, 1993–2002. MMWR Surveillance Summaries, 54(4), 1–35. [PubMed] [Google Scholar]
- 45.Holt R, & Kalra S (2013). A new DAWN: Improving the psychosocial management of diabetes. Indian Journal of Endocrinology and Metabolism, 17(7), 95–99. 10.4103/2230-8210.119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schure MB, Odden M, & Goins RT (2013). The association of resilience with mental and physical health among older American Indians: The Native Elder Care Study. American Indian Alaska Native Mental Health Research, 20(2), 27–41. 10.5820/aian.2002.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamora-Kapoor A, Sinclair K, Nelson L, Lee H, & Buchwald D (2019). Obesity risk factors in American Indians and Alaska Natives: A systematic review. Public Health, 174, 85–96. 10.1016/j.puhe.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Grandbois DM, & Sanders GF (2009). The resilience of Native American elders. Issues in Mental Health Nursing, 30(9), 569–580. 10.1080/01612840902916151. [DOI] [PubMed] [Google Scholar]
- 49.Denham AR (2008). Rethinking historical trauma: Narratives of resilience. Transcultural Psychiatry, 45(3), 391–414. 10.1177/1363461508094673. [DOI] [PubMed] [Google Scholar]
- 50.Stumblingbear-Riddle G, & Romans JS (2012). Resilience among urban American Indian adolescents: Exploration into the role of culture, self-esteem, subjective well-being, and social support. American Indian Alaska Native Mental Health Research, 19(2), 1–19. 10.5820/aian.1902.2012.1. [DOI] [PubMed] [Google Scholar]
- 51.Vancampfort D, Rosenbaum S, Ward PB, Steel Z, Lederman O, Lamwaka AV, et al. (2016). Type 2 diabetes among people with posttraumatic stress disorder: Systematic review and meta-analysis. Psychosomatic Medicine, 78(4), 465–473. 10.1097/psy.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 52.Moons P, Budts W, & De Geest S (2006). Critique on the conceptualisation of quality of life: A review and evaluation of different conceptual approaches. International Journal of Nursing Studies, 43(7), 891–901. 10.1016/j.ijnurstu.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Strine TW, Chapman DP, Balluz LS, Moriarty DG, & Mokdad AH (2008). The associations between life satisfaction and health-related quality of life, chronic illness, and health behaviors among U.S. community-dwelling adults. Journal of Community Health, 33(1), 40–50. 10.1007/s10900-007-9066-4. [DOI] [PubMed] [Google Scholar]
- 54.Rosella LC, Fu L, Buajitti E, & Goel V (2018). Death and chronic disease risk associated with poor life satisfaction: A population-based cohort study. American Journal of Epidemiology, 188(2), 323–331. 10.1093/aje/kwy245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haynes S, Richard D, & Kubany E (1995). Content validity in psychological assessment: A functional approach to concepts and methods. Psychological Assessment, 7, 238–247. 10.1037/1040-3590.7.3.238. [DOI] [Google Scholar]
- 56.Pew Research Center. (2017). What low response rates mean for telephone surveys. Pew Research Center. [Google Scholar]


