Abstract
This study explored mediating pathways, moderating factors, and moderated mediation effects of a web-based, cognitive behavioral therapy (CBT) intervention for chronic pain patients with aberrant drug-related behavior (ADRB). In a 2-arm RCT, patients with chronic pain who screened positive for ADRB received treatment-as-usual (TAU, n = 55) or TAU plus a 12-week, web-based CBT intervention (n = 55). Assessments were conducted at weeks 4, 8, and 12, and at 1- and 3-months post intervention. Web-CBT significantly reduced pain catastrophizing, which, in turn, reduced pain interference and pain severity via a pathway of pain catastrophizing. Web-CBT also significantly reduced ADRB both directly and indirectly by reducing pain catastrophizing. For pain interference and pain severity, web-CBT was more effective than TAU for younger patients (≤ age 50). For pain severity, web-CBT was more effective for both younger patients (≤ age 50), and those with a lifetime substance use disorder. Findings suggest that web-CBT’s positive impact on pain outcomes and ADRB are mediated by its effect on pain catastrophizing, and its treatment effects may be most robust for younger patients and those with histories of substance dependence.
Keywords: Web-based interventions, Opioid use disorder, Chronic pain, Mediation, Moderation and moderated mediation analysis, Cognitive behavior therapy (CBT)
Introduction
In light of the current intersecting epidemics of chronic pain and nonmedical opioid use, there is an urgent need for increased utilization of effective nonpharmacological treatments for chronic pain. This is particularly true among pain patients with histories of, or who are at high risk for, nonmedical opioid use. Psychosocial treatment approaches, such as cognitive behavior therapy (CBT), for chronic pain have broad support in the literature (Institute of Medicine (IOM), 2011), but their uptake is limited by barriers including a lack of qualified providers, poor integration of behavioral therapies into medical practice and inadequate payer coverage for psychosocial treatment (Institute of Medicine (IOM), 2011). Digital technology-based interventions for chronic pain patients who have experienced problems managing their use of opioid medications could be an important means of addressing these barriers and increasing the reach of effective behavioral pain treatments. Technology-based CBT interventions for chronic pain have demonstrated greater effectiveness than wait-list control conditions and comparable effectiveness to other behavioral treatments (Bender et al., 2011; Macea et al., 2010). A recent clinical trial examining the effectiveness of Take Charge of Pain, a web-based CBT intervention for chronic pain patients with aberrant drug-related behavior (ADRB), reported that, when delivered in conjunction with standard specialty pain treatment, this intervention was effective in reducing ADRB, pain catastrophizing and pain-related emergency department visits, compared to standard specialty pain treatment alone (which included opioid prescriptions) (Guarino et al., 2018).
With growing evidence of the effectiveness of web-based interventions for chronic pain, identifying patient characteristics and change processes that are linked to better intervention outcomes is critical. First, identifying patient characteristics associated with better or worse outcomes of technology-based behavioral treatments for pain management can help inform treatment matching. Matching patients to behavioral intervention modalities specific to their characteristics, needs, and conditions can increase satisfaction and reduce patient burden, and may increase the likelihood of long-term gains (Kim et al., 2015, 2016). Second, improvement in psychological and social functioning/processes during the technology-based behavioral intervention period can elucidate the pathways by which these interventions lead to improved outcomes (DasMahapatra et al., 2015). However, little is known about the psychological and social functioning/processes (mechanisms) underlying the effects of technology-based interventions for chronic pain. Of importance, since these pathways can vary by patient subgroup, identifying patient characteristics in relation to these psychological and social mechanisms can provide further insight into strategies for optimizing intervention outcomes for patients with different characteristics (Almirall et al., 2014; Nahum-Shani et al., 2018).
In the present exploratory investigation, we examined baseline characteristics that influenced the effects of the web-based Take Charge of Pain intervention (moderators), as well as the social and psychological mechanisms by which the intervention worked better than standard treatment (mediation effects). We further identified baseline characteristics of patients that interact with differing mediation effects (moderated mediation effects). The primary and selected secondary outcomes of the trial that evaluated Take Charge of Pain are reported elsewhere (Guarino et al., 2018). This secondary analysis focuses on the questions of for whom and how the web-based behavioral intervention enhanced outcomes for chronic pain patients. Note that the program referenced in this report and another program based at Johns Hopkins University with the identical name “Take Charge of Pain” (available at TakeChargeofPain.org) are not the same program.
Methods
The dataset is from a 2-arm, randomized controlled, intention-to-treat clinical trial registered at ClinicalTrials.gov (Protocol Identifier: NCT01498510; see (Guarino et al., 2018) for greater detail regarding the study procedures, web-based intervention and outcome analyses. Eligible participants were required to: be receiving long-term opioid therapy for chronic pain at the study’s collaborating pain specialty practice; report moderate to severe pain (defined as rating one’s worst pain in the past week as ≥ 5 on the 0–10-point Brief Pain Inventory [BPI; Cleeland & Ryan, 1994]) for at least three months; and screen positive for ADRB in the past 30 days (evidenced by endorsing at least four items on the Current Opioid Misuse Measure [COMM; Butler et al., 2007]). Although the cutoff score for clinically significant ADRB on the COMM is 9, we opted to use a lower cutoff score of 4 in order to facilitate participant recruitment and because we felt that the web-CBT program could benefit pain patients with less severe ADRB, potentially delaying or preventing the escalation of their opioid medication misuse.
After completing a baseline assessment, chronic pain patients were randomly assigned to either the treatment-as-usual arm (TAU, n = 55) or the TAU plus web-based CBT intervention arm (web-CBT, n = 55) in a 1-to-1 ratio. During the 12-week intervention period, participants were assessed at weeks 4, 8, and 12; participants were also assessed postintervention, at 1- and 3-month follow-ups. Those assigned to the web-CBT arm were asked to independently complete approximately two modules of the Take Charge of Pain program per week and/or to complete all 27 program modules (see below) over the 12-week intervention period. This self-paced program instructed patients in strategies and skills for effectively coping with pain, pacing activity, restructuring thoughts and feelings about pain, and managing use of opioid medications in order to diminish the negative impact of chronic pain on one’s life. Participants were compensated $50 for the baseline assessment and $40 for assessments at all other timepoints, but were not compensated for completing modules in the web-CBT program (so that compensation amounts were equivalent across trial arms).
Treatment arms
Take charge of pain
The Take Charge of Pain program was based on CBT principles and taught patients strategies for altering dysfunctional thinking about pain and other stressors and skills for coping with pain with the goal of reducing the impact of pain on a patient’s life. Accessible from a computer via the Internet, the program included 27 self-paced modules that each took about 20–30 min to complete. Via text, images and animation, modules were designed to teach a variety of CBT skills (e.g., identifying and challenging automatic negative thinking, controlled breathing, muscle relaxation, attention diversion coping, how to prevent pain from ruining relationships) and to educate about opioids, medication misuse, and strategies for improving medication management (e.g., ‘myths and facts about opioids and addiction,’ and ‘identifying and managing triggers for misusing medication’). Aside from didactic content, interactive features included an activity calendar with progress graphs and pain interference tracking. The web-CBT program automatically tracked participants’ progress through the program, including login dates/times and modules completed. Research staff closely monitored these usage data and periodically provided phone and/or email prompts (according to a standardized schedule) to participants who did not complete sessions on time. While participants could rapidly click through each module’s content-presentation screens, they were unable to advance to the next module until they achieved mastery of the modular content, as demonstrated by 100% accuracy on quiz questions.
Treatment-as-usual
Treatment as usual (TAU) included usual care at the pain management study site: opioid pharmacotherapy, other types of medications, and ther medical interventions (e.g., nerve blocks and injections). A multi-disciplinary medical team consisting of neurologists, anesthesiologists, nurse practitioners, and fellows in pain medicine served the patient population. Notably, psychological and behavioral treatment modalities were not provided at this practice. Each patient’s care was overseen by a physician specializing in pain medicine who both prescribed and monitored adjuvant opioid therapy. Physicians were not blinded to their patients’ trial condition. Those with patients in the web-CBT + TAU arm received reports about their patients’ progress in the web-CBT program, including the specific modules they had completed, in an effort to enhance collaboration between patient and provider.
Variables and measures
Primary outcomes
The present study analyzed three primary outcomes: (1) pain interference (PI), (2) pain severity (PS) and (3) ADRB, as measured by the COMM on a 5-point scale anchored by “Never” and “Very Often.” The COMM™, developed by a group of addiction experts and pain management clinicians, is a brief self-report measurement to help clinicians and other providers monitor aberrant medication-related behaviors of chronic pain patients while being prescribed opioid therapy (Butler et al., 2007). The COMM™ is designed to identify multiple issues such as signs and symptoms of intoxication, emotional volatility, problematic medication behavior, and addiction (Butler et al., 2007). To reduce the likelihood of under-reporting, several items are only subtly related to medication misuse. Pain interference and pain severity were measured with their respective subscales from the Multidimensional Pain Inventory [MPI, (Kerns et al., 1985)]. The Pain Severity subscale, comprised of 3 items rated on a 7-point Likert scale, assesses perceived pain severity in the past week. The 9-item Pain Interference subscale assesses daily interference of pain in relation to work, social activities, and family relationships.
Mediators
We examined five variables as potential mediators of the effects of the web-based CBT intervention on the three primary outcomes. These mediators include: (1) pain catastrophizing, as assessed by the Pain Catastrophizing Scale [PCS; (Sullivan & Hamilton, 2007)]; (2) future orientation, as measured by the Future Scale (Hellström et al., 1999); (3) quality of life, assessed with the WHO-QOL (Skevington et al., 2004; Vahedi, 2010); (4) social support (Medical Outcomes Study Social Support Scale [MOS-SSS (Sherbourne & Stewart, 1991)]; and (5) reward sensitivity, measured with the Sensitivity To Reinforcement of Addictive and other Primary Reward scale [STRAP-R; (Goldstein, 1987)]. In selecting potential mediators, we focused on psychological and social constructs that have been demonstrated in prior research to function as mediators or mechanisms in chronic pain (catastrophizing, future orientation) and/or addictive behaviors (social support, reward sensitivity). Quality of life, which refers to an individual’s subjective sense of well-being across the physical, psychological, social and environmental domains, was selected as a plausible mediator. We hypothesized that the CBT skills trained in the web-based program (e.g., challenging automatic negative thoughts, effective communication skills, identifying and managing triggers for medication misuse) may have improved participants’ psychosocial functioning and adaptive coping, which, in turn, may have led to reductions in pain severity, pain interference and ADRB.
The PCS (Cronbach’s alpha = 0.87) is designed to measure excessive negative thoughts or feelings about pain in three domains—helplessness, rumination and magnification—on a 5-point scale. The Future Scale measures expectations about the future in two subscales (a 5-item explicit and a 5-item implicit subscale) using a 7-point response scale. The WHO-QOL measures quality of life, life satisfaction, and personal well-being with 26-items assessing the broad domains of physical health, psychological health, social relationships and environmental factors [e.g., finances, safety (Skevington et al., 2004; Vahedi, 2010)]. The 19-item MOS-SSS measures how often relevant forms of social support are available to participants, with a 5-point response scale. The STRAP-R scale consists of four subscales covering reward sensitivity, and, in our analysis, total average scores were used (Goldstein, 1987).
Moderators
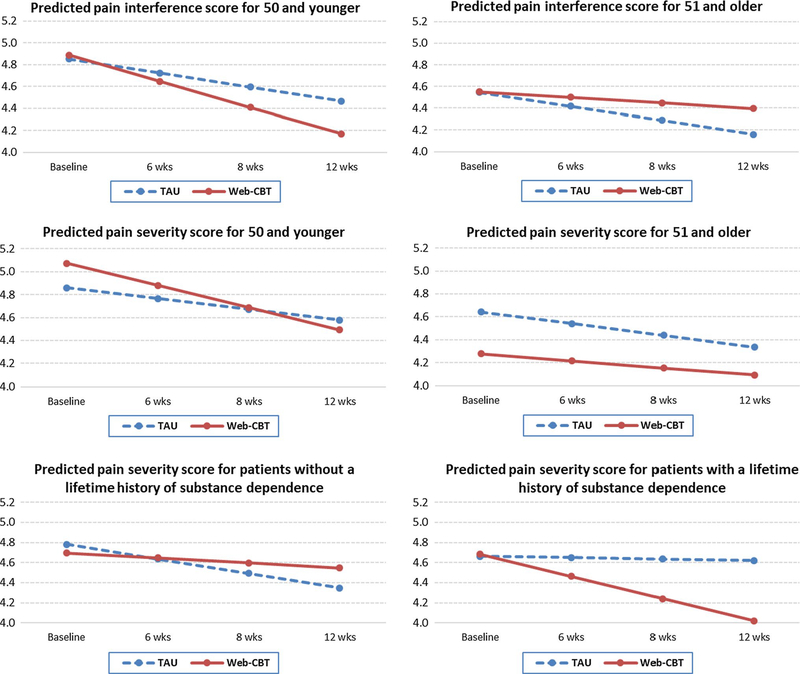
For moderation analyses, nine demographic and other baseline variables were chosen as potential moderators. These include age, race, gender, marital status, lifetime mental health diagnosis, lifetime substance dependence disorder [measured by the DSM-IV-based criteria within the MINI Neuropsychiatric Interview; see Guarino et al. (2018)], chronic pain duration, past 30-day ADRB (measured by the COMM score at baseline), and fixed daily opioid dose (in morphine milligram equivalents). For meaningful interpretations, non-categorical variables—age, pain duration, ADRB and fixed daily opioid dose—were all mean-centered prior to analysis. To illustrate significant moderation effects over time, we plotted the predicted outcomes by time and moderators. For ease of reading the patterns, we dichotomized significant continuous or other non-categorical moderators by using median split. For example, after mean-centering, patients’ age was divided into younger versus older groups (a median split) for illustration purposes in Fig. 1.
Fig. 1.
Three-way interaction of study arm, time, and baseline characteristics on pain outcomes. Note: Substance dependence was assessed at baseline with the MINI Neuropsychiatric Interview that is based on DSM-IV criteria. The Y-axis is based on a 7-point Likert scale, from the pain severity and interference scales of the MPI, ranging from 0 to 6
Analytic methods
During the 12-week intervention period, all three primary outcomes and three of the mediators (pain catastrophizing [PCS], future orientation [the Future Scale], and quality of life [WHO-QOL]) were measured at four-time points—base-line and 4-, 8- and 12-weeks. The other two mediators (social support [MOS-SSS] and reward sensitivity [STRAP-R]) were measured only at baseline and 12-weeks. Therefore, two sets of mediation analyses were conducted depending on data availability. Longitudinal mediation analyses using 4 data points were conducted for three mediators, PCS, FS and WHO-QOL, and cross-sectional mediation analyses using difference scores (baseline—12 weeks) were conducted for MOS-SSS and STRAP-R. Mixed-effects models via SAS PROC MIXED were used for longitudinal mediation analyses (equivalent to parallel process of latent growth curve models in MPLUS, except separate steps were taken to fit mediation model) and generalized linear models (GLM) in SAS were used for cross-sectional mediation analyses. In terms of mediation analytic methods, a causal mediation method based on potential outcomes and a counterfactual framework is a new development in the field (VanderWeele, 2015). This new approach has a potential advantage for better detecting and controlling for possible confounding. However, we did not adopt this approach because (1) this method is still under development for longitudinal data and (2) the extended conventional analytic method we used is justified.
Mediation effects
Conventional mediation analysis consists of 4 steps (Baron & Kenny, 1986): (1) The intervention (X) must significantly affect an outcome, Y (X → Y, called total effect, or c path); (2) X must significantly affect a mediator, M (X → M, called a path); (3) M must significantly affect Y (M → Y, called b path); and (4) mediation effect is assessed by introducing a mediator (M) into the X and Y relationship. If the effect of X on Y after including M (called direct effect, c’ path coefficient) becomes nonsignificant, it is evidence of total mediation; if the magnitude of the direct effect, c’ becomes smaller than the total effect (c path coefficient) but remains significant, it is evidence of partial mediation. However, later development of this analytic framework has demonstrated that the first step, a significant effect of X on Y, does not have to be present for mediation analysis (Hayes, 2017; MacKinnon, 2008), and a key to demonstrate a mediation effect is to test the indirect effect from X to Y via pathway of M (the product of ab paths). Because the product term ab has asymetric distribution, to test this indirect effect, either asymmetric confidence interval, or bootstrapping or Monte Carlo simulation methods (Hayes & Rockwood, 2016; Tofighi & MacKinnon, 2011) should be used to estimate confidence intervals for mediated effect. For our analysis, we focused on the significance testing of the indirect effect (ab product) by estimating an asymmetric confidence interval around this term to assess the mediation effect. To gain a comprehsensive understanding of the process of mediation or mechanism of change for each outcome, we also examined the effect of a path (X → M), b path (M → Y) and c’ path (X → Y controlling for M) coefficients estimated from the models.
Moderation effects
Moderation analyses were conducted using a mixed-effects modeling framework for the three primary outcomes. Treatment (web-CBT vs. TAU), time (baseline, 4, 8 and 12 weeks), treatment x time interaction, moderator, moderator x treatment interaction, moderator x time interaction, and treatment x moderator x time (3-way) interaction were specified as fixed effects, and the intercept and slope for time variables were specified as random effects to take the correlated nature of longitudinal data into account. A significant treatment x moderator x time (3-way) interaction indicates the existence of moderation effects, i.e., treatment effects on an outcome may differ at a specific level of a moderation variable.
Moderated mediation effects
There are several ways in which moderated mediation effects can occur. First, intervention effects on a mediator (X → M, or the “a” path) may be changed by a moderator. Second, the effect of a mediator on an outcome (M → Y, or the “b” path) may be influenced by a moderator. Third, both the “a” path and the “b” path can be moderated by the same moderator or by two different moderators (Hayes, 2017). Last, indirect mediation effects (the product of ab) may be moderated by a moderator (Fairchild & MacKinnon, 2009; Krull & MacKinnon, 2001; Krull et al., 2016; MacKinnon, 2008). In this study, we tested moderation of indirect mediation effects using Mplus.
Results
Participants
The majority of participants were female (64%), not married (82%), unemployed (78%), and racially diverse (45% White, 35% Black/African American, and 20% ‘Other’), with almost 70% reporting pain duration of 6 years or more, and about one-half reporting one or more pain surgeries in their lifetime. The mean age of participants was 51.3 years (SD = 11 years). Participants reported high fixed daily doses of prescribed opioid analgesics (mean morphine milligram equivalent dose = 297 mg [SD = 545 mg], median = 124 mg).
Mediation effects
Longitudinal mediation analyses with the three primary outcomes [pain interference (PI), pain severity (PS) and ADRB (COMM)] and three mediators [pain catastrophizing (PCS), Future Scale (FS) and WHO-QOL] indicate that pain catastrophizing had significant mediation effects for all primary outcomes (Table 1). Indirect treatment effects via the mediator PCS were significant for both pain interference (PI) and pain severity (PS) outcomes: the mediated indirect path coefficient for X → PCS → PI was - 0.028 (confidence interval: - 0.06, - 0.002), and the indirect path coefficient for X → PCS → PS was - 0.022 (confidence interval: - 0.050, - 0.001). The total treatment effect on COMM was significant (- 1.461, p = 0.004), and the direct effect of the web-based intervention was reduced after including the PCS mediator in the model but remained significant (- 1.113, p = 0.022). Interestingly, the indirect or mediated effect was also significant: the indirect path coefficient was - 0.32 (confidence interval: - 0.644, - 0.022]. That is, ADRB was significantly reduced both directly due to the web-based CBT intervention, and also indirectly via reduced pain catastrophizing as a result of the web-based CBT intervention.
Table 1.
Results of mediation analyses
| Outcomes | Mediators | Total effect of treatment on outcome (y) |
Effect of treatment on mediator |
Effect of mediator on outcome |
Effect of treatment on outcome when mediator is included |
Mediation effect (indirect effect) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (x to y) | c | a (x to m) |
b (m to y) |
c’ |
a*b |
||||||
| Estimate (SE) | p value | Estimate (SE) | p value | Estimate (SE) | p value | Estimate (SE) | p value | Estimate | (CI) | ||
|
| |||||||||||
| Longitudinal (baseline, 4, 8 and 12 weeks) | |||||||||||
| Pain interference | |||||||||||
| PCS | −0.018 (.061) | 0.772 | −1.416 (.671) | 0.036 | 0.020 (.004) | <.0001 | 0.014 (.060) | 0.863 | −0.028 | (−0.060, −0.002) | |
| FS | −0.018 (.061) | 0.772 | −0.455 (.561) | 0.419 | −0.004 (.006) | 0.432 | −0.020 (.061) | 0.745 | 0.002 | (0.006, −0.001) | |
| WHO-QOL | −0.018 (.061) | 0.772 | 0.677 (.546) | 0.217 | −0.026 (.004) | <.0001 | −0.001 (.060) | 0.994 | −0.018 | (−0.048, 0.010) | |
| Pain severity | |||||||||||
| PCS | −0.027 (.065) | 0.672 | −1.416 (.671) | 0.036 | 0.016 (.004) | 0.001 | −0.005 (.064) | 0.937 | −0.022 | (−0.050, −0.001) | |
| FS | −0.027 (.065) | 0.672 | −0.455 (.561) | 0.419 | −0.014 (.006) | 0.014 | −0.033 (.064) | 0.607 | 0.006 | (−1.183, 1.868) | |
| WHO-QOL | −0.027 (.065) | 0.672 | 0.677 (.546) | 0.217 | −0.016 (.005) | 0.000 | −0.016 (.065) | 0.806 | −0.011 | (−0.044, 0.015) | |
| Aberrant drug-related behavior | |||||||||||
| PCS | −1.461 (.495) | 0.004 | −1.416 (.671) | 0.036 | 0.226 (.029) | <.0001 | −1.113 (.482) | 0.022 | −0.320 | (−0.644, −0.022) | |
| FS | −1.461 (.495) | 0.004 | −0.455 (.561) | 0.419 | −0.217 (.037) | <.0001 | −1.531 (.485) | 0.002 | 0.099 | (−0.188, 0.401) | |
| WHO-QOL | −1.461 (.495) | 0.004 | 0.677 (.546) | 0.217 | −0.144 (.031) | <.0001 | −1.347 (.482) | 0.006 | −0.097 | (−0.272, 0.056) | |
| Difference Score (Baseline-12 weeks) | |||||||||||
| Pain interference | |||||||||||
| MOS-SSS | 0.012 (.189) | 0.951 | −0.162 (.142) | 0.257 | −0.153 (.136) | 0.266 | 0.023 (.193) | 0.905 | 0.025 | (−0.031, 0.115) | |
| STRAP-R | 0.012 (.189) | 0.951 | 0.079 (.135) | 0.558 | −0.076 (.150) | 0.613 | 0.103 (.197) | 0.601 | −0.006 | (−0.068, 0.041) | |
| Pain severity | |||||||||||
| MOS-SSS | 0.036 (.203) | 0.859 | −0.162 (.142) | 0.257 | 0.063 (.149) | 0.674 | 0.050 (.210) | 0.812 | −0.001 | (−0.090, 0.052) | |
| STRAP-R | 0.036 (.203) | 0.859 | 0.079 (.135) | 0.558 | 0.163 (.164) | 0.323 | 0.055 (.216) | 0.799 | 0.013 | (−0.046, 0.096) | |
| Aberrant drug-related behavior | |||||||||||
| MOS-SSS | 4.024 (1.531) | 0.010 | −0.162 (.142) | 0.257 | 1.206 (1.101) | 0.276 | 3.892 (1.557) | 0.014 | −0.195 | (−0.919, 0.152) | |
| STRAP-R | 4.024 (1.531) | 0.010 | 0.079 (.135) | 0.558 | −1.323 (1.212) | 0.279 | 3.328 (1.592) | 0.039 | −0.105 | (−0.742, 0.349) | |
Pain Catastrophizing Scale = PCS, FS = Future Scale, WHO-QOL = Quality of Life scale, Medical Outcomes Study Social Support Scale = MOS-SSS, Sensitivity to Reinforcement of Addictive and other Primary Reward = STRAP-R. Two separate analyses were conducted because the two mediators, MOS-SSS and STRAP-R, were measured only at baseline and 12 weeks
The Future Scale had no mediation effect on any of the outcomes. However, for the WHO-QOL scale, the total treatment effect on all three outcomes was reduced after including WHO-QOL in the model as a mediator, but none of the indirect or mediated effects were significant (Table 1).
For the mediators examined with the baseline-to-12-week difference score (MOS-social support and STRAP-R), only STRAP-R had a partial mediation effect on the COMM outcome. Specifically, the total treatment effect on COMM was 4.024 (p = 0.01), and the direct effect was reduced to 3.328 after including STRAP-R as a mediator (p = 0.039), but the indirect effect was not significant (- 0.105, confidence interval: - 0.742, 0.349).
Moderation effects
Figure 1 illustrates the significant moderator effects. Age had a significant moderation effect on both pain interference (PI, p = 0.005) and pain severity (PS, p = 0.007). For PI, web-CBT was more effective than TAU for younger patients (age 50 or younger), but less effective than TAU for patients in the older age group (age 51 and older). For PS, there was a similar pattern, i.e., the effect of web-CBT was much stronger than that of TAU for the younger group, but there was almost no difference in the relative effectiveness of the two treatment conditions for the older group. Lifetime substance dependence disorder (assessed by the MINI International Neuropsychiatric Interview (MINI) as yes vs. no) (Sheehan et al., 1998) significantly moderated the treatment effect on pain severity (p = 0.024). Compared to TAU alone, web-based CBT significantly improved PS for patients with lifetime substance dependence disorder, but had no observed effect on those without a dependence disorder (Fig. 1). None of the other moderators showed significant effects on any of the three outcomes.
Moderated mediation effects
We conducted exploratory analyses of moderated mediation effects by examining the difference in indirect or mediated effect between different levels of each moderator. We did not find any significant moderated mediation effects. We observed a trend showing that marital status had a marginal moderated mediation effect: the indirect effect of web-CBT on COMM via PCS (mediator) was marginally stronger for ever-married than never married patients (p = 0.063, confidence interval: - 0.147, 7.909). The lack of statistically significant moderated mediation effects is likely attributable to inadequate statistical power.
Discussion and conclusion
Prior studies have reported that computerized psychosocial interventions can produce comparable or better treatment outcomes than face-to-face interventions for a range of behavioral health problems, including chronic pain and substance misuse, e.g., Marsch et al. (2014). Further investigation to identify mechanisms of behavior change and subgroups of patients who respond better to digital interventions (Ehde et al., 2014) as well as other formats (Broderick et al., 2016) is critical, but has been little explored. Understanding these mechanisms and conditional effects of treatment could help inform the development of effective interventions that target salient change processes and are tailored for specific subgroups, and could provide guidance for clinicians, allowing them to recommend technology-based interventions to those patients most likely to benefit from these easily accessible treatments. We conducted mediation analyses, as well as exploratory moderation and moderated mediation analyses, to identify subgroups of chronic pain patients with ADRB that may have different processes for improving their pain management outcomes while receiving web-CBT as part of their care. Given the early stage of research in this area, we examined a broad range of psychological and social processes as potential mediators, and patient demographic and health behavior characteristics as potential moderators that may yield different effects of web-based CBT on pain severity, pain interference and ADRB.
We found that when pain catastrophizing was well managed, the web-CBT intervention had significant indirect effects on pain severity, pain interference and ADRB. This mediation analysis outcome indicates that even though the intervention itself did not significantly reduce pain severity or pain interference when directly compared to TAU alone (see total effects in Table 1), this lack of an observed group difference may be due to the intervention’s effects on these outcomes being fully mediated by pain catastrophizing. This is consistent with prior work showing that decreases in pain catastrophizing mediate relationships between traditional (non-digital) CBT interventions and improvements in pain-related outcomes (e.g., Smeets et al., 2006; Turner et al., 2007). Many have argued (e.g., Ballantyne & Sullivan, 2015) and demonstrated (Williams et al., 2012) that the degree of distress or suffering experienced as a result of chronic pain (i.e., catastrophizing) can be improved even in the absence of a reduction in pain severity. As a result, pain reduction is not a primary focus for many CBT studies, but rather, such interventions (as was the case with Take Charge of Pain) explicitly target automatic negative thinking and maladaptive behaviors associated with pain with an aim to help patients more effectively cope with pain.
With a large body of evidence suggesting that pain catastrophizing is one of the strongest psychological predictors of pain outcomes and a significant risk marker for adverse pain and health outcomes (e.g., greater disabilty, pain intensity, depression, anxiety, suicidal ideation, work absenteeism, opioid misuse, and healthcare utilization) (Quartana et al., 2009), a recent systematic review and meta-analysis (Schütze et al., 2018) sought to answer the question of how to best reduce pain catastrophizing with chronic non-cancer pain. The best evidence was found for cognitive-behavioral therapy, multimodal treatment, and acceptance and commitment therapy. However, treatments were deemed most likely to produce clinically significant benefits when they were targeted to people with high levels of catastrophizing and CBT was found to have the best evidence in these cohorts. Thus, it is a very positive finding that the web-based Take Charge of Pain program impacted pain catastrophizing consistent with this review.
Perceived quality of life showed a similar pattern, but with partially mediated effects, indicating that there was an additional mechanism functioning in the intervention outcome pathways. Participants’ reward sensitivity also partially mediated the intervention’s effects on ADRB. Given that self-regulation entails suppressing immediate gratification to achieve long-term goals (Job et al., 2015; Leventhal et al., 1998), this pathway in particular implies that psychosocial interventions that teach self-regulatory skills and thought processes, and emphasize the salience of expected future rewards (e.g., improvement in quality of life) may be especially effective in helping opioid-treated chronic pain patients with demonstrated evidence of ADRB.
One of the largest randomized controlled effectiveness trials of CBT (delivered in-person or on the phone) for chronic pain with a focus on moderator analyses found that the oldest patients (M = 76.7) showed the most robust treatment effects for pain and daily quality of life compared with younger patients (M = 57.7). As shown in the moderation analysis outcomes in this study, younger participants (age 50 or younger) assigned to web-based CBT reported significantly greater reductions in pain interference and pain severity relative to older patients in the same condition. This age group difference implies that perhaps younger patients were more likely to benefit from the technology-mediated therapeutic tools compared to older patients which may be related to greater familiarity/comfort with digital technologies among younger generations. The prevalence of chronic pain conditions in adults increases across the lifespan (Tsang et al., 2008) and there is a shortage of well-designed research concerning the effectiveness of different chronic pain treatments in older patients (Park & Hughes, 2012). A recent study (Tse et al., 2020) explored the use of a digital-based pain management program using visually appealing contexts to reduce stress, promote positive feelings, and potentially reduce pain thresholds/increase pain tolerance among older adults (M age = 85.6). The study found that participants preferred to view the pictures using digital devices (ipad/iphone) rather than hard copy versions. Studies like this suggest that improving training or simplifying procedures related to use of web-based CBT tools like Take Charge of Pain may be of considerable utility to our aging populace with chronic pain (rates of approximately 47–63% among adults over 65 in developed countries) (Kneeland et al., 2019).
Interestingly, patients with lifetime substance dependence disorder at baseline also reported significantly greater improvement in pain severity when receiving the web-CBT intervention than did patients without a history of substance dependence. The selective effectiveness of the web-based program in this regard could be related to the explicit substance/medication misuse content included in the intervention (i.e., the web-CBT intervention may have had greater relevance for those with a history of substance dependence). This finding indicates that patients with lifetime substance dependence at baseline considerably benefited from web-based CBT in learning about coping skills and knowledge important for diminishing the negative impact of chronic pain on their lives. This finding is aligned with prior work reporting that persons with more severe substance use risk profiles may benefit more from digital behavioral treatments (Campbell et al., 2014; Kim et al., 2015, 2016). Also, most empirical work investigating the relationship between pain catastrophizing and substance use has focused on nonmedical opioid use among people with chronic pain who are not seeking treatment. However, among those with comorbid substance use disorder and chronic pain, there is good evidence that catastrophizing offers a potential therapeutic target to improve substance use treatment outcomes (Kneeland et al., 2019).
In summary, the results of the present study identify subgroups of chronic pain patients with ADRB that can benefit from web-based CBT interventions. Offering Take Charge of Pain as part of biopsychosocial integrated treatment can enhance treatment outcomes and may be especially helpful for younger patients and those who have a history of substance dependence. Our mechanism pathways also indicate that our previously reported finding (that the web-based CBT intervention reduced ADRB), was mediated by reductions in patients’ pain catastrophizing (which was also directly reduced by the web-CBT intervention).
Limitations
Study limitations include that the sample was drawn from a single urban pain specialty program. Future studies in this area would benefit from a sample drawn from multiple sites with greater variability on the rural–urban continuum especially due to the relative lack of in-person psychosocial supports for people with chronic pain in rual settings. The economic disadvantage of the participants in the study sample may also have led to self-selection bias. Another limitation is that two of the mediators (social support [MOS-SSS] and reward sensitivity [STRAP-R]) were measured only at baseline and 12-weeks. The results are still valid, but analytic models for these two mediators were not consistent with models for the other mediators. The relatively small sample size is an additional limitation. The observed trend showing that marital status had a marginal moderated mediation effect could simply be a chance finding. However, given the relatively small sample size in this trial it is not surprising to find non-significant moderated mediation effects. Relatedly, the study could have benefited from collecting mediator data at more timepoints such that we might have increased confidence in the validity of our findings.
Implications.
Practice
The web-based, cognitive behavior therapy (CBT) intervention Take Charge of Pain can improve pain-related outcomes among chronic pain patients and may be particularly effective for younger patients (≤ age 50) and those with lifetime substance dependence.
Policy
Our study is based on one clinical trial. Although presented findings are statistically valid within our study sample, we believe it is premature to make a definitive recommendation for policy makers. However, our findings do contribute to the accumulating data supporting the efficacy of web-based CBT interventions for chronic pain patients. As described in our paper, we suggest policy makers gather evidence from multiple clinical trials to determine which sub-groups of patients respond favorably to technology-based therapeutic tools and how those tools can be modified to benefit diverse cohorts of chronic pain patients.
Research
Future research may benefit from longitudinal assessments of psychosocial factors that may play a mediating role in improving chronic pain outcomes.
Acknowledgments
Funding This research was supported by grants from the US National Institute on Drug Abuse (NIDA) of the National Institutes of Health (R01DA026887 and P30DA029926).
Footnotes
Conflict of interest Lisa A. Marsch is affiliated with HealthSim, LLC, the business that developed the web-based intervention platform used in this study. This relationship is extensively managed by Dr. Marsch and her academic institution. No additional conflicts of interest.
Compliance with ethical standards
Human and animal rights and Informed consent All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board for the protection of human subjects at National Development and Research Institutes (NDRI), Inc. and Mount Sinai Health System (formally Continuum Health Partners, Inc.) approved the study design and procedure. Dartmouth’s IRB agreed to abide by the decisions of NDRI’s IRB. Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affliations.
References
- Almirall D, Nahum-Shani I, Sherwood NE, & Murphy SA (2014). Introduction to SMART designs for the development of adaptive interventions: With application to weight loss research. Translational Behavioral Medicine, 4, 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, & Sullivan MD (2015). Intensity of chronic pain—The wrong metric? The New England Journal of Medicine, 373, 2098–2099. 10.1056/NEJMp1507136. [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173. [DOI] [PubMed] [Google Scholar]
- Bender JL, Radhakrishnan A, Diorio C, Englesakis M, & Jadad AR (2011). Can pain be managed through the Internet? A systematic review of randomized controlled trials. Pain®, 152, 1740–1750. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Keefe FJ, Schneider S, Junghaenel DU, Bruckenthal P, Schwartz JE, Kaell AT, Caldwell DS, McKee D, & Gould E (2016). Cognitive behavioral therapy for chronic pain is effective, but for whom? Pain, 157, 2115–2123. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, & Jamison RN (2007). Development and validation of the current opioid misuse measure. Pain, 130, 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, Matthews AG, Stitzer M, Miele GM, Polsky D, Turrigiano E, Walters S, McClure EA, & Kyle TL (2014). Internet-delivered treatment for substance abuse: A multisite randomized controlled trial. American Journal of Psychiatry, 171, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, & Ryan KM (1994). Pain assessment: Global use of the Brief Pain Inventory. Annals, Academy of Medicine, Singapore, 23, 129–138. [PubMed] [Google Scholar]
- DasMahapatra P, Chiauzzi E, Pujol LM, Los C, & Trudeau KJ (2015). Mediators and moderators of chronic pain outcomes in an online self-management program. The Clinical Journal of Pain, 31, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, & Turner JA (2014). Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. American Psychologist, 69, 153. [DOI] [PubMed] [Google Scholar]
- Fairchild AJ, & MacKinnon DP (2009). A general model for testing mediation and moderation effects. Prevention Science, 10, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H (1987). Multilevel statistical models. (2nd ed.). London: Edward Arnold. [Google Scholar]
- Guarino H, Fong C, Marsch LA, Acosta MC, Syckes C, Moore SK, Cruciani RA, Portenoy RK, Turk DC, & Rosenblum A (2018). Web-based cognitive behavior therapy for chronic pain patients with aberrant drug-related behavior: Outcomes from a randomized controlled trial. Pain Medicine, 19, 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. (2nd ed.). New York: Guilford Press. [Google Scholar]
- Hayes AF, & Rockwood NJ (2016). Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behaviour Research and Therapy, 98, 1–19. [DOI] [PubMed] [Google Scholar]
- Hellström C, Jansson B, & Carlsson SG (1999). Subjective future as a mediating factor in the relation between pain, pain-related distress and depression. European Journal of Pain, 3, 221–233. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM). (2011). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington: The National Academies Press. [PubMed] [Google Scholar]
- Job V, Bernecker K, Miketta S, & Friese M (2015). Implicit theories about willpower predict the activation of a rest goal following self-control exertion. Journal of Personality and Social Psychology, 109(4), 694–706. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Turk DC, & Rudy TE (1985). The west haven-yale multidimensional pain inventory (WHYMPI). Pain, 23, 345–356. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Marsch LA, Acosta MC, Guarino H, & Aponte-Melendez Y (2016). Can persons with a history of multiple addiction treatment episodes benefit from technology delivered behavior therapy? A moderating role of treatment history at baseline. Addictive Behaviors, 54, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Marsch LA, Guarino H, Acosta MC, & Aponte-Melendez Y (2015). Predictors of outcome from computer-based treatment for substance use disorders: Results from a randomized clinical trial. Drug and Alcohol Dependence, 157, 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneeland ET, Griffin ML, Taghian N, Weiss RD, & McHugh RK (2019). Associations between pain catastrophizing and clinical characteristics in adults with substance use disorders and co-occurring chronic pain. The American Journal of Drug and Alcohol Abuse, 45, 488–494. 10.1080/00952990.2019.1581793. [DOI] [PubMed] [Google Scholar]
- Krull JL, Cheong J, Fritz MS, & MacKinnon DP (2016). Moderation and mediation in interindividual longitudinal analysis. Developmental Psychopathology, Journal Article. 10.1002/9781119125556.devpsy121. [DOI] [Google Scholar]
- Krull JL, & MacKinnon DP (2001). Multilevel modeling of individual and group level mediated effects. Multivariate Behavioral Research, 36, 249–277. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Leventhal EA, & Contrada RJ (1998). Self-regulation, health, and behavior: A perceptual–cognitive approach. Psychology & Health, 13(4), 717–733. [Google Scholar]
- Macea DD, Gajos K, Calil YAD, & Fregni F (2010). The efficacy of Web-based cognitive behavioral interventions for chronic pain: A systematic review and meta-analysis. The Journal of Pain, 11, 917–929. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP (2008). Introduction to statistical mediation analysis. Milton Park: Taylor & Francis Group LLC. [Google Scholar]
- Marsch LA, Guarino H, Acosta M, Aponte-Melendez Y, Cleland C, Grabinski M, Brady R, & Edwards J (2014). Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. Journal of Substance Abuse Treatment, 46, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkie-witz K, Tewari A, & Murphy SA (2018). Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52, 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, & Hughes AK (2012). Nonpharmacological approaches to the management of chronic pain in community-dwelling older adults: A review of empirical evidence. Journal of the American Geriatrics Society, 60, 555–568. 10.1111/j.1532-5415.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, & Edwards RR (2009). Pain catastrophizing: A critical review. Expert Review of Neurotherapeutics, 9, 745–758. 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze R, Rees C, Smith A, Slater H, Campbell JM, & O’Sullivan P (2018). How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. The Journal of Pain: Official Journal of the American Pain Society, 19, 233–256. 10.1016/j.jpain.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33 (quiz 34–57). [PubMed] [Google Scholar]
- Sherbourne CD, & Stewart AL (1991). The MOS social support survey. Social Science and Medicine, 32, 705–714. [DOI] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, & O’Connell KA (2004). The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research, 13, 299–310. [DOI] [PubMed] [Google Scholar]
- Smeets RJEM, Vlaeyen JWS, Kester ADM, & Knottnerus JA (2006). Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. The Journal of Pain: Official Journal of the American Pain Society, 7, 261–271. 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Sullivan CJ, & Hamilton ZK (2007). Exploring carrers in deviance: A joint trajectory analysis of criminal behavior and substance use in an offender population. Deviant Behavior, 28, 497–523. [Google Scholar]
- Tofighi D, & MacKinnon DP (2011). RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods, 43(3), 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GLG, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine J-P, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, & Watanabe M (2008). Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. The Journal of Pain: Official Journal of the American Pain Society, 9, 883–891. 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Tse MMY, Ng SSM, Bai X, Lee PH, Lo R, Yeung SSY, Li Y, & Tang SK (2020). An exploration of the use of visually appealing contexts in a pain management program. European Geriatric Medicine. 10.1007/s41999-020-00339-6. [DOI] [PubMed] [Google Scholar]
- Turner JA, Holtzman S, & Mancl L (2007). Mediators, moderators, and predictors of therapeutic change in cognitive–behavioral therapy for chronic pain. Pain, 127, 276–286. 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Vahedi S (2010). World Health Organization Quality-of-Life Scale (WHOQOL-BREF): Analyses of their item response theory properties based on the graded responses model. Iranian Journal of Psychiatry, 5, 140. [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T (2015). Explanation in causal inference: Methods for mediation and interaction. Oxford: Oxford University Press. [Google Scholar]
- Williams AC, Eccleston C, & Morley S (2012). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews, 11, CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]