Abstract
Flagellar (H) antigens are mostly encoded by genes at the fliC locus in E. coli. We have sequenced 11 H7 fliC genes from Escherichia coli strains that belong to seven O serotypes. These sequences, together with those of nine other H7 fliC genes (from strains of three different O serotypes) sequenced recently (S. D. Reid, R. K. Selander, and T. S. Whittam, J. Bacteriol. 181:153–160, 1999), include 10 different sequences. The differences between these 10 sequences range from 0.06 to 3.12%. By comparison with other E. coli flagellin genes, we have identified primer length sequences specific for H7 genes in general and others specific for H7 genes of O157 and O55 strains: the specificity was confirmed by PCR testing the type strains for all 53 E. coli H types. We have previously identified genes specific for the E. coli O157 antigen, and use of the combination of O157- and H7-specific primers allows the sensitive and rapid detection of O157:H7 E. coli strains, which cause the majority of hemorrhagic colitis cases.
Escherichia coli is a clonal species, with clones normally identified by their combination of O and H (and sometimes K) antigens. All enterohemorrhagic E. coli strains produce Shiga-like toxins (Stxs), but E. coli O157:H7 strains are the strains most frequently isolated from humans and are the predominant cause of hemolytic-uremic syndrome (HUS), with the O157:H7 clone having caused approximately two-thirds of all recent cases of HUS in North America and Europe (33). Because of the very low infective dose of this organism (13), bacteria that enter the human food chain can still pose a health problem after enormous dilution. For example, in January 1993 there was an outbreak due to contamination with E. coli O157:H7 at a large meat-processing plant that made over 1 million hamburger patties per day. The affected hamburgers were sold through one retail chain over 4 U.S. states, and 477 people became ill, of whom 3 died (1, 4). The scale of the plant and its operation are such that there would have been great dilution of the contaminated meat, and the highest count of O157:H7 found in hamburger patties with the same production date was 15 organisms per g, although the hamburgers which caused the infection were not tested.
The flagellar protein constitutes the subunit of the helical filament which forms the flagellar organelle. The flagellar protein carries the antigenic determinant(s) for the H antigen. There are 53 H types in E. coli (note that H50 was deleted from the list [6, 21]), and in most cases the flagellin structural genes are located at the fliC locus (17, 18, 30). Studies of E. coli and Salmonella enterica flagellar proteins demonstrated that these proteins are conserved in their terminal regions, while the central region is variable, giving rise to serotype-specific epitopes (12, 18, 22, 35).
Great efforts have been made to develop a method for timely and accurate detection of the O157:H7 strain (see, e.g., references 5, 10, 13, and 23). PCR-based methods are ideal for rapid detection of organisms at low concentrations. PCR detection with probes based on the Stx and eaeA (encoding intimin) genes and an unspecified plasmid gene have been developed, but each probe gave a positive result with some other E. coli strains even when only a small number of strains were tested (10). PCR tests with primers based on the unique substitution in the O157:H7 uidA gene (7) have also been developed.
Serotyping is routinely used to characterize E. coli isolates, and strains with the combination of O157 and H7 antigens all appear to belong to the clone that causes HUS in humans. We suggest that a PCR test based on the antigens will provide the specificity required to detect this high-risk organism. A PCR-restriction fragment length polymorphism test has been developed by using the H7 fliC gene as the target (9), but as discussed below, it has significant disadvantages compared to a PCR test.
Recently, we sequenced the gene cluster that encodes the O157 antigen, and by PCR testing against representatives of all 166 E. coli O serotypes and a range of gram-negative bacterial strains, including some that cross-react serologically with E. coli O157 antisera, we have found that certain O-antigen genes are highly specific for E. coli O157 (34). The object of this study was to identify sequences specific for the H7 fliC gene. To achieve this, we sequenced the fliC genes from 11 H7 strains, compared them with the sequences of fliC genes from 38 E. coli H-antigen type strains, and identified H7-specific sequences. The specificity was confirmed by PCR testing the type strains for all 53 E. coli H antigens. The identification of O157- and H7-specific sequences provides the basis for a sensitive test for rapid detection of E. coli O157:H7. This is important both for decision making related to patient care, as early treatment may reduce the risk of life-threatening complications, and for detection of sources of contamination.
MATERIALS AND METHODS
Bacterial strains.
Standard E. coli H strains (6, 21) were obtained from the Institute of Medical and Veterinary Science, Adelaide, Australia, and Karl Bettelheim of the Victorian Infectious Diseases Reference Laboratory, Victoria, Australia. The other strains used are listed in Table 1, together with the names of those who supplied them.
TABLE 1.
E. coli H7 strains used in this study in addition to the type strains
| Strain name used in this study | GenBank accession no. of fliC gene | H7 allele | Serotype | Original strain name | Sourcea |
|---|---|---|---|---|---|
| M527 | AF228487 | H7-5 | O157:H7 | C664-1992 | a |
| M917 | AF228490 | H7-10 | O18ac:H7 | A57 | IMVS |
| M918 | AF228491 | H7-10 | O18ac:H7 | A62 | IMVS |
| M973 | AF228492 | H7-8 | O2:H7 | A1107 | CDC |
| M1004 | AF228488 | H7-5 | O157:H7 | EH7 | b |
| M1179 | AF228493 | H7-9 | O18ac:H7 | D-M3291/54 | IMVS |
| M1200 | AF228494 | H7-9 | O7:H7 | A64 | c |
| M1211 | AF228495 | H7-7 | O19ab:H7 | F8188-41 | IMVS |
| M1328 | AF228496 | H7-6 | O53:H7 | 14097 | IMVS |
| M1686 | AF228489 | H7-5 | O55:H7 | TB156 | d |
Abbreviations: a, Statens Serum Institut, Copenhagen, Denmark; b, R. Brown, Royal Children's Hospital, Melbourne, Australia; c, Max-Planck Institut für Molekulare Genetik, Berlin, Germany; d, P. Tarr, Children's Hospital and Medical Center, University of Washingtion, Seattle; IMVS, Institute of Medical and Veterinary Science, Adelaide, Australia; CDC, Centers for Disease Control and Prevention, Atlanta, Ga.
Sequencing and analysis.
The fliC gene was first PCR amplified, and the PCR product was sequenced. PCR amplification was performed in a reaction volume of 100 μl consisting of 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 1.5 mM MgCl2; 0.02% (wt/vol) bovine serum albumin; dATP, dTTP, dCTP, and dGTP at a concentration of 20 mM each; 60 pmol of each primer; 1 μl of DNA; and 2.5 U of Taq DNA polymerase. The PCR was generally carried out under the following conditions: denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min for 30 cycles (exceptions are referred to in the Results section). The PCR product was purified with the Promega Wizard PCR purification kit before being sequenced. The sequencing reactions were performed by the DyeDeoxy Terminator Cycle Sequencing method (Applied Biosystems, Foster City, Calif.), and the reaction products were analyzed by using fluorescent dye and an ABI377 automated sequencer (Applied Biosystems). Sequence data were assembled and analyzed by using the Australian National Genomic Information Service, which incorporates several sets of programs (15, 16).
Specificity assay by PCR.
Chromosomal DNA was isolated by using the Promega Genomic isolation kit and was checked by gel electrophoresis and PCR amplification of the mdh gene (which codes for malate dehydrogenase and which is generally present in E. coli) by using the oligonucleotides designed by Boyd et al. (3). PCR was carried out in a total volume of 25 μl, and after PCR 5 μl was run on an agarose gel to check for amplified DNA.
Nucleotide sequence accession numbers.
The DNA sequences have been deposited in GenBank. See Table 1 for GenBank entry numbers.
RESULTS AND DISCUSSION
Sequencing.
Primers flanking the fliC gene were used to amplify PCR by the H7 fliC genes from 10 E. coli strains of O groups O2, O7, O18, O19, O53, O55, and O157. The two primers used are primer 1575 (5′-GGG TGG AAA CCC AAT ACG) and primer 1576 (5′-GCG CAT CAG GCA ATT TGG), which are based on sequences 51 to 34 bp upstream and 37 to 54 bp downstream, respectively, of the E. coli K-12 fliC gene. Each PCR product was first sequenced by using the primers used for the PCR amplification. Primers based on the sequence obtained were then used for further sequencing, and this procedure was repeated until the entire PCR product was sequenced in both orientations.
All 10 strains have a fliC gene of 1,758 bp, and the pairwise differences among these 10 genes ranged from 0 to 2.45%. The fliC gene from the H7 type strain (an O1:K1:H7 E. coli strain) has been sequenced previously (GenBank accession no. L07388), and variation between this sequence and the 10 new sequences is higher (between 0.8 and 3.07%). We resequenced the fliC gene from the H7 type strain and found that its sequence is identical to that of strain M973 but that its sequence differs from the published sequence (GenBank accession no. L07388) at 11 nucleotides.
Nucleotide sequence variation within the H7 fliC genes.
DNA sequences were aligned by using the CLUSTALW program, and phylogenetic analysis was performed with MULTICOMP software (31).
Reid et al. (32) recently sequenced the H7 fliC genes from nine E. coli strains of three serotypes (O157:H7, O55:H7, and O128:H7). They found four distinct sequences, which were named H7-1 to H7-4 (32). The H7-1 sequence represents the fliC genes from two O55:H7 strains and a nonmotile O157 strain, the H7-2 sequence represents the H7 fliC genes from three O157:H7 strains, and the H7-3 and H7-4 sequences represent the H7 fliC genes from a nonmotile O157 and an O128:H7 strain, respectively (32). Alignment of the 11 newly obtained H7 fliC sequences with the four representative sequences of Reid et al. (32) revealed six more distinct sequences, H7-5 to H7-10 (Table 1).
Sixty-two polymorphic sites are available within the 10 H7 fliC sequences (Fig. 1), with 46 occurring at the third base of a codon, 5 occurring at the second base, and 11 occurring at the first base. Sixteen nonsynonymous substitutions, which represented 16 of the 585 codons, were found. Of the 62 polymorphic sites, 47 were phylogenetically informative (at least 2 bases were present in 2 or more of the 10 sequences).
FIG. 1.
Polymorphic sites within the 10 H7 fliC alleles. Numbering of the polymorphic sites (vertical format) is from the first nucleotide of the gene. The position within the codon for each polymorphic site is shown below the sequences.
H7-1, H7-2, and H7-5 represent H7 sequences of O55 and O157 strains and are almost identical, with 1- or 2-nucleotide pairwise differences between them (Fig. 1). These sequences are distinct from those of other H7 alleles at three “signature” nucleotides at positions 192, 195, and 1683 (Fig. 1). Fields et al. (9) conducted a PCR-restriction fragment length polymorphism analysis of fliC genes from 70 O157:H7 and 18 O55:H7 strains and found that the H7 genes from all 70 O157:H7 strains and 16 of the 18 O55:H7 strains gave identical patterns. Thus, it is highly likely that the H7 genes from all O157:H7 strains are closely related to the H7 gene of the O55:H7 clone. It has been found by multilocus enzyme electrophoresis that the O157:H7 and O55:H7 clones form a small group which stands apart from most other E. coli strains (8, 24, 36–40).
H7-3 (32) was found only in a nonmotile O157 strain. It differs from H7-1 by two frameshift insertions at positions 12 and 17 (not shown in Fig. 1) and seven additional mutations (all of which are nonsynonymous, five of which are clustered at the 5′ end from positions 68 to 133, and two of which are clustered at the 3′ end at positions 1727 and 1728) (Fig. 1). It has been suggested that these seven mutations were fixed after the clone lost motility, which would remove the selective constraints on amino acid-altering mutations (32). However, this does not explain why the mutations are clustered at the ends of fliC, which is indicative of recombination.
Alleles H7-8, H7-9, and H7-10 are closely related, with pairwise differences of 0.06 and 0.46% (Fig. 1), and they differ from other H7 alleles by 2.28 to 3.12%. These three alleles represent H7 sequences from O1, O2, O7, and O10 strains. Alleles H7-4, H7-6, and H7-7 (which represent the H7 genes from O19, O53, and O128 strains) are more similar to O157 and O55:H7 alleles (Fig. 1), with pairwise differences ranging from 0.28 to 0.9%. We suggest that there are two groups of H7 genes and that these comprise alleles H7-1 to H7-6 and alleles H7-8 to H7-10, respectively.
Oligonucleotide primers specific to H7.
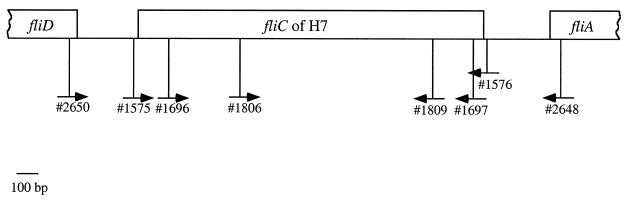
We have sequenced the fliC genes from 39 H type strains (unpublished data). Comparing the sequences of H7 strains with those of the other 38 H type strains, we have identified two primer sequences (primer 1806 [5′-GCT GCA ACG GTA AGT GAT] at positions 553 to 570 of H7-10 and primer 1809 [5′-GGC AGC AAG CGG GTT GGT] at positions 1500 to 1483 of H7-10) (Fig. 2) which are conserved in all the 10 H7 alleles but which have less than 85% similarity with any other fliC gene of the 39 H type strains.
FIG. 2.
Diagram showing locations of oligonucleotide primers.
Primers 1806 and 1809 were tested by PCR against all 53 standard H type strains and the strains listed in Table 1. The PCR was carried out as described above with an annealing temperature of 58°C. A PCR product of about 1 kb was generated from all the H7 strains, and none was generated with these primers by using DNA from any other H type strain (data not shown).
Until recently, all 53 forms of the H antigen in E. coli were thought to be encoded at the fliC locus, as has been shown for E. coli K-12. However, Ratiner (26–30) showed that some H-antigen genes are at loci other than fliC, and fllA, fllK, and fllM have been identified. We have confirmed that at least 39 of the 53 H-antigen genes from type strains map to fliC (unpublished data), and the primers were chosen after comparison of the primer sequences with the sequences of the 39 H-antigen genes. The PCR result included all H-antigen specificities and indicates that even when tested with type strains whose H-antigen genes we have not sequenced and/or whose H-antigen gene is at a locus other than fliC, these primers are still specific for H7.
Oligonucleotide primers specific for H7 genes of O157 and O55 strains.
The fliC genes of O55:H7 and O157:H7 strains are closely related (see above), and we have identified two oligonucleotides (oligonucleotides 1696 [5′-GGC CTG ACT CAG GCG GCC] at positions 178 to 195 in H7-5 and oligonucleotide 1697 [5′-GAG TTA CCG GCC TGC TGA] at positions 1700 to 1683 in H7-5) (Fig. 2) which are unique to H7 of O157 and O55. Although not identical to any part of the fliC sequences of any other H7 strain, the sequences of these two primers are identical or have high-level similarity to the sequences of fliC genes of some other H types. However, a combination of one of these primers with one of the H7-specific primers can give specificity for the H7 genes of E. coli O157 and O55.
Primer pairs 1696-1809 and 1697-1806 were used to carry out PCR with chromosomal DNA samples of all the H type strains and the H7 strains listed in Table 1. PCRs were carried out as described above with annealing temperatures of 61°C (for 1696-1809) and 59°C (for 1697-1806). Both primer pairs produced a band of the predicted size with the O157:H7 strains (strains M1004 and M527; see Table 1) and the O55:H7 strain (strain M1686; see Table 1) but gave no band with the other strains tested (data not shown).
Oligonucleotide primers based on genes flanking fliC.
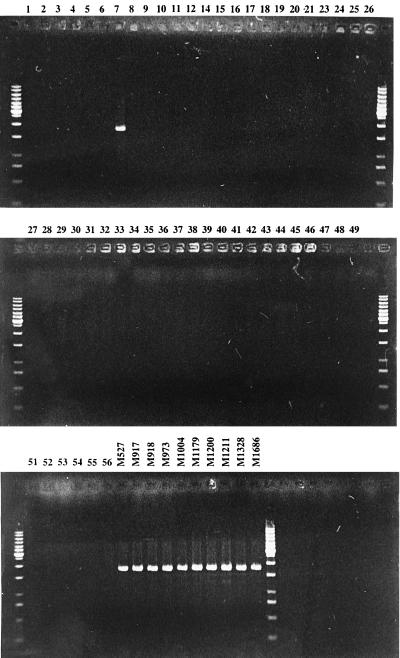
We have also chosen two primers (primer 2648 [5′-CAA TGC TTC GTG ACG CAC] and primer 2650 [5′-CAG CGA TGA AAT ACT TGC CAT]) (Fig. 2) which were based on the E. coli K-12 fliA and fliD genes (19, 25), respectively. fliA and fliD flank the fliC gene in E. coli K-12 (2, 19, 25) and encode the ς factor for transcription initiation of class 3 operons and a filament cap for filament assembly, respectively (18). Each of these two primers was paired with the one of the two H7-specific primers (2648-1806 and 2650-1809) to carry out PCR with chromosomal DNA samples of all the H type strains and the H7 strains listed in Table 1. PCRs were carried out as described above with annealing temperatures of 56°C (for 2648-1806) and 58°C (for 2650-1809). Both primer pairs produced bands of the predicted size with all of the H7 strains but gave no band with the other strains tested (see Fig. 3 for the result obtained with primer pair 2648-1806).
FIG. 3.
Agarose gel electrophoresis of PCR product obtained by PCR assay with oligonucleotide primer 2648-1806 with DNAs from E. coli strains. DNAs from E. coli H type strains are labeled with the H type numbers, and DNAs from other strains are labeled with the strain numbers. GeneRuler (1-kb DNA ladder) from Gibco Life Technologies was used as the molecular size marker.
The variation among the 10 H7 alleles is very low, but it is nevertheless possible that our H7-specific primers may fail to give positive results with some H7 isolates because of the low level of variation that affects a primer site. The low level of variation makes it extremely unlikely that the two H7-specific regions (primers 1806 and 1809) in any H7 isolate would both have undergone change, and thus other primers (whether or not they are H7 specific, such as primers 2648 and primers 2650) can be used to pair with each of 1806 and 1809 to confirm a negative result. H7-specific sequences other than those for the two primers that we have chosen are also located in the H7 fliC central region, and thus, other H7-specific primers can also be used.
There have been previous reports of primers specific for H7. However, the primers were based on many fewer sequence data and were not tested against all E. coli H-antigen type strains. For example, Gannon et al. (11) reported the use of H7 as a target in a PCR assay system and used two H7-based primers (primers flicH7-F and flicH7-R) based on the alignment of two H7 sequences. We compared the sequences of these two primers with the 39 fliC sequences: the sequences of primer flicH7-F is identical to the sequence of a region present in 24 fliC genes that encode other H antigens but is different from the sequence of one of the H7 alleles (H7-4) at one base; the sequence of primer flicH7-R is different from those of four H7 alleles (H7-5 to H7-8) at 1 base. The first primer is not H7 specific, and the second one is specific to 4 of the 10 H7 sequences, including 2 of the 3 sequences found in O157:H7 strains, making neither suitable for a routine assay.
Our sequence data confirm that the PCR-restriction fragment length polymorphism test for H7 fliC genes developed by Fields et al. (9) is specific for the H7 genes of O157 and O55 strains. However, because this test is based on the restriction pattern of the PCR-amplified fliC gene, it can be applied only to pure bacterial cultures and is not suitable for use with environmental samples, in which there could be several E. coli strains with the O157:H7 strain in the minority. Also, the restriction enzyme-digested PCR fragments need to be run on an agarose gel to see the pattern, which makes this test not amenable to the systems currently used in clinical laboratories, such as the TaqMan assay (14, 20).
The comprehensive analysis that we have undertaken, which involved more fliC genes and all H type strains, has shown that sequences previously thought to be specific for H7 do not have the desired specificity and has identified primers with better specificity for H7 genes and the H7 genes of O157 and O55 strains. It is nevertheless possible that with the routine use of the primers identified in this study, other discrepancies would be found. However, we believe that the use of two H7-specific primers, each of which can be used with one of two nonspecific primers, will ensure that the proposed test will have a very high degree of reliability in detecting the H7 gene of the O157:H7 clone.
There are many O157 or H7 clones, and it is for the O157:H7 clone for which there is a major need for detection methods. We have previously identified O-antigen genes specific for O157 and here have identified H7-specific primers. Primers specific for these two genes provide a good basis for the development of a PCR-based method that can be used to identify O157:H7 strains and that can replace the time-consuming plating and serotyping methods. The use of O157- and H7-specific tests for screening for this organism is highly desirable, as in the traditional serologic tests for detection of O157 and H7 strains, tests specific for the combination of O157 and H7 are used for identification of this clone.
For example, in routine use, separate PCRs with H7- and O157-specific primers can be carried out. For H7, one can use primer pairs 2648-1806, 2650-1809, and 1806-1809 separately. Positive PCR results with all the three primer pairs would indicate the presence of strains that carry the H7 antigen. Positive PCR results with one or two primer pairs would indicate the presence of strains that carry the H7 antigen and that have a variation(s) at a priming site(s). Negative PCR results with all three primer pairs would indicate the absence of strains that carry the H7 antigen. The second result is predicted to be very rare and could be confirmed by sequencing. For O157, one can use previously identified O157-specific primers (34), and the test can be carried out by the method of Wang and Reeves (34).
ACKNOWLEDGMENTS
We thank all the people and institutes listed in Table 1 and Karl Bettelheim for kindly supplying strains.
This study was supported by the Australian Research Council.
REFERENCES
- 1.Bell B P, Goldoft M, Griffin P M, Davis M A, Gordon D C, Tarr P I, Bartleson C A, Lewis J H, Barrett T J, Wells J G, Baron R, Kobayashi J. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA. 1994;272:1349–1353. [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett G I, Bloch C A, Perna N T, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Boyd E F, Nelson K, Wang F-S, Whittam T S, Selander R K. Molecular genetic basis of allelic polymorphism in malate dehydrogenase (mdh) in natural populations of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci USA. 1994;91:1280–1284. doi: 10.1073/pnas.91.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States, 1992–1993. Morbid Mortal Weekly Rep. 1993;42:258–263. [PubMed] [Google Scholar]
- 5.Cubbon M D, Coia J E, Hanson M F, Thomson-Carter F M. A comparison of immunomagnetic separation, direct culture and polymerase chain reaction for the detection of verocytotoxin-producing Escherichia coli O157 in human faeces. J Med Microbiol. 1996;44:219–222. doi: 10.1099/00222615-44-3-219. [DOI] [PubMed] [Google Scholar]
- 6.Ewing W H. Edwards and Ewing's identification of the Enterobacteriaceae. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. [Google Scholar]
- 7.Feng P. Identification of Escherichia coli O157:H7 by DNA probe specific for an allele of uidA gene. Mol Cell Probes. 1993;7:151–154. doi: 10.1006/mcpr.1993.1021. [DOI] [PubMed] [Google Scholar]
- 8.Feng P, Lampel K A, Karch H, Whittam T S. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J Infect Dis. 1998;177:1750–1753. doi: 10.1086/517438. [DOI] [PubMed] [Google Scholar]
- 9.Fields P I, Blom K, Hughes H J, Helsel L O, Feng P, Swaminathan B. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol. 1997;35:1066–1070. doi: 10.1128/jcm.35.5.1066-1070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fratamico P M, Sackitey S K, Wiedmann M, Deng M Y. Detection of Escherichia coli O157:H7 by multiplex PCR. J Clin Microbiol. 1995;33:2188–2191. doi: 10.1128/jcm.33.8.2188-2191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gannon V P J, D'Souza S, Graham T, King R K, Rahn K, Read S. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J Clin Microbiol. 1997;35:656–662. doi: 10.1128/jcm.35.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joys T M. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985;260:15758–15761. [PubMed] [Google Scholar]
- 13.Keene W E, McAnulty J M, Hoesly F C, Williams L P, Hedberg K, Oxman G L, Barrett T J, Pfaller M A, Fleming D W. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N Engl J Med. 1994;331:579–584. doi: 10.1056/NEJM199409013310904. [DOI] [PubMed] [Google Scholar]
- 14.Kimura B, Kawasaki S, Fujii T, Kusunoki J, Itoh T, Flood S J. Evaluation of TaqMan PCR assay for detecting Salmonella in raw meat and shrimp. J Food Prot. 1999;62:329–335. doi: 10.4315/0362-028x-62.4.329. [DOI] [PubMed] [Google Scholar]
- 15.Littlejohn T G. Bioinformatics: the essential ingredient. Today's Life Sci. 1996;8:28–33. [Google Scholar]
- 16.Littlejohn T G, Bucholtz C A, Campbell R M M, Gata B A, Huynh C, Kim S H. Computing for biotechnology—WebANGIS. Australasian Biotechnology. 1996;6:211–217. [Google Scholar]
- 17.Mabeck C E, Ørskov F, Ørskov I. Escherichia coli serotypes and renal involvement in urinary-tract infections. Lancet. 1971;i:1312–1314. doi: 10.1016/s0140-6736(71)91884-8. [DOI] [PubMed] [Google Scholar]
- 18.Macnab R M. Flagella and motility. In: Neidhardt F D, editor. Escherichia and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 19.Mytelka D S, Chamberlin M J. Escherichia coli fliAZY operon. J Bacteriol. 1996;178:24–34. doi: 10.1128/jb.178.1.24-34.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberst R D, Hays M P, Bohra L K, Phebus R K, Yamashiro C T, Paszko-Kolva C, Flood S J, Sargeant J M, Gillespie J R. PCR-based DNA amplification and presumptive detection of Escherichia coli O157:H7 with an internal fluorogenic probe and the 5′ nuclease (TaqMan) assay. Appl Environ Microbiol. 1998;64:3389–3396. doi: 10.1128/aem.64.9.3389-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ørskov I, Ørskov F, Bettelheim K A, Chandler M E. Two new Escherichia coli O antigens, O162 and O163, and one new H antigen, H56. Withdrawal of H antigen H50. Acta Pathol Microbiol Scand. 1975;83:121–124. doi: 10.1111/j.1699-0463.1975.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 22.Parish C R, Wistar R, Ada G L. Cleavage of bacterial flagellin with cyanogen bromide: antigenic properties of the protein fragments. Biochem J. 1969;113:501–506. doi: 10.1042/bj1130501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park C H, Vandel N M, Hixon D L. Rapid immunoassay for detection of Escherichia coli O157 directly from stool specimens. J Clin Microbiol. 1996;34:988–990. doi: 10.1128/jcm.34.4.988-990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pupo G M, Karaolis D K R, Lan R, Reeves P R. Evolutionary relationships among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocus enzyme electrophoresis and mdh sequence studies. Infect Immun. 1997;65:2685–2692. doi: 10.1128/iai.65.7.2685-2692.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raha M, Kawagishi I, Muller V, Kihara M, Macnab R M. Escherichia coli produces a cytoplasmic alpha-amylase, AmyA. J Bacteriol. 1992;174:6644–6652. doi: 10.1128/jb.174.20.6644-6652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratiner Y A. Phase variation of the H antigen in Escherichia coli strain Bi327-41, the standard strain for Escherichia coli flagellar antigen H3. FEMS Microbiol Lett. 1982;15:33–36. [Google Scholar]
- 27.Ratiner Y A. Presence of two structural genes determining antigenically different phase-specific flagellins in some Escherichia coli strains. FEMS Microbiol Lett. 1983;19:37–41. [Google Scholar]
- 28.Ratiner Y A. Two genetic arrangements determining flagellar antigen specificities in two diphasic Escherichia coli strains. FEMS Microbiol Lett. 1985;29:317–323. [Google Scholar]
- 29.Ratiner Y A. Different alleles of the flagellin gene hagB in Escherichia coli standard H test strains. FEMS Microbiol Lett. 1987;48:97–104. [Google Scholar]
- 30.Ratiner Y A. New flagellin-specifying genes in some Escherichia coli strains. J Bacteriol. 1998;180:979–984. doi: 10.1128/jb.180.4.979-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves P R, Farnell L, Lan R. MULTICOMP: a program for preparing sequence data for phylogenetic analysis. Comput Appl Biosci. 1994;10:281–284. doi: 10.1093/bioinformatics/10.3.281. [DOI] [PubMed] [Google Scholar]
- 32.Reid S D, Selander R K, Whittam T S. Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J Bacteriol. 1999;181:153–160. doi: 10.1128/jb.181.1.153-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarr P I. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–8. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Reeves P R. Organization of Escherichia coli O157 O antigen gene cluster and identification of its specific genes. Infect Immun. 1998;66:3545–3551. doi: 10.1128/iai.66.8.3545-3551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei L, Joys T M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985;186:791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- 36.Whittam T S, Ochman H, Selander R K. Multilocus genetic structure in natural populations of Escherichia coli. Proc Natl Acad Sci USA. 1983;80:1751–1755. doi: 10.1073/pnas.80.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whittam T S, Wachsmuth I K, Wilson R A. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1988;157:1124–1133. doi: 10.1093/infdis/157.6.1124. [DOI] [PubMed] [Google Scholar]
- 38.Whittam T S, Wilson R A. Genetic relationships among pathogenic Escherichia coli of serogroup O157. Infect Immun. 1988;56:2467–2473. doi: 10.1128/iai.56.9.2467-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittam T S, Wilson R A. Genetic relationships among pathogenic strains of avian Escherichia coli. Infect Immun. 1988;56:2458–2466. doi: 10.1128/iai.56.9.2458-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittam T S, Wolfe M L, Wachsmuth I K, Ørskov F, Ørskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]