Abstract
The essential oils of three specimens of Myrcia multiflora (A, B and C) and Eugenia florida were extracted by hydrodistillation, and the chemical compositions from the essential oils were identified by gas chromatography and flame ionization detection (CG/MS and CG-FID). The fungicide potential of the EOs against five fungicide yeasts was assessed: Candida albicans INCQS-40175, C. tropicalis ATCC 6258, C. famata ATCC 62894, C. krusei ATCC 13803 and C. auris IEC-01. The essential oil of the specimen Myrcia multiflora (A) was characterized by the major compounds: α-bulnesene (26.79%), pogostol (21.27%) and δ-amorphene (6.76%). The essential oil of the specimen M. multiflora (B) was rich in (E)-nerolidol (44.4%), (E)-γ-bisabolene (10.64%) and (E,E)-α-farnesene (8.19%), while (E)-nerolidol (92.21%) was the majority of the specimen M. multiflora (C). The sesquiterpenes seline-3,11-dien-6-α-ol (12.93%), eremoligenol (11%) and γ-elemene (10.70%) characterized the chemical profile of the EOs of E. florida. The fungal species were sensitive to the essential oil of M. multiflora (B) (9–11 mm), and the lowest inhibitory concentration (0.07%) was observed in the essential oil of M. multiflora (A) against the yeasts of C. famata. Fungicidal action was observed in the essential oils of M. multiflora (A) against C. famata, with an MIC of 0.78 µL/mL and 3.12 µL/mL; C. albicans, with an MFC of 50 µL/mL and M. multiflora (C) against C. albicans; and C. krusei, with a MFC of 50 µL/mL.
Keywords: essential oil, volatile compounds, pathogens, inhibition potential, antifungal action
1. Introduction
Fungi are defined as eukaryotic beings that cause huge economic and ecological impacts to society [1]. One of the reasons is food contamination. In addition, in some cases they can be pathogenic due to the production of mycotoxins [2], which favor the triggering of relatively mild diseases and infections, such as in the skin and mucosa, and can worsen, affecting multiple organs [3], such as nails, lungs and hair [4]. Among the fungi that cause human pathologies, we have the species of the Candida genus, which are capable of altering, through infections, the immunological suppression or impairment of epithelial barriers [5].
Another concern related to fungi is related to their possible resistance that can be acquired to some commercial drugs. In addition, synthetic products can cause contamination and change food properties. With this, the search for products of natural origin with antimicrobial activity has been increasing over the years [6]. Among the potential bioactive compounds, we highlight those present in essential oils, which can be viable alternatives for the control of fungi [7,8,9,10].
In this context, we can mention the Myrtaceae family, which holds a vast amount of species that produce essential oils [11] and has great antimicrobial potential already described in several species such as Eucalyptus camaldulensis [12], E. lehmannii [13], E. sideroxylon [13], E. leucoxylon [13], E. camaldulensis [13], E. astringens [13], Eugenia jambolana [14], Callistemon citrinus [14], Myrtus communis [14], Melaleuca genistifolia [14] and M. alternifolia [14]. However, the antimicrobial potential of other species, such as Myrcia multiflora and Eugenia florida, is unknown.
Myrcia multiflora is described as a shrub-sized species [11,15] and is popularly known as pedra-ume-caá or insulin plants. Due to traditional and empirical knowledge, it is used as a hypoglycemic agent in the form of infusion or decoction [16,17]. On the other hand, Eugenia florida is a small tree, approximately 3.5 m high, traditionally known as black cherry, whose fruits are edible and tasty. The leaves of this species are used by rural populations as hypotensive and antipyretic agents to reduce cholesterol levels and treat infections, jaundice, heart disease and gastrointestinal disorders [18]. Given the significant importance of these species, the purpose of this study was to assess the chemical composition and the antimicrobial potential of three specimens of M. multiflora and one specimen of E. florida collected in the municipality of Magalhães Barata, State of Pará, Brazil.
2. Results and Discussions
2.1. Yield of the Essential Oils
The essential oil of the specimen Myrcia multiflora (C) showed the highest yield of 0.98 (v/w %), followed by the specimen M. multiflora (A) with 0.60 (v/w %), specimen M. multiflora (B) with 0.28 (v/w %) and Eugenia florida with 0.13 (v/w %). These results demonstrate that the collection periods and ecosystems directly impacted the differences in these contents, as well as environmental factors, such as rainfall, solar incidence, temperature and type of soil [19,20,21].
Comparing our study with other works, the yields of the essential oils found in this research for the three specimens of M. multiflora were higher than those found by [22] in a sample taken in the municipality of Taquari, State of Rio Grande do Sul, Brazil, with a content of 0.20 (v/w %), and lower than the sample of M. multiflora taken in the municipality of Maracanã, State of Pará, Brazil, with a yield of 1.16 (v/w %) [23]. The yield of 0.8–3.1% (v/w %) of the essential oil of E. florida was within the average range found in other studies of species of the genus Eugenia [24].
2.2. Chemical Composition
The essential oils of the studied samples were obtained by hydrodistillation and identified by chromatography coupled to mass spectrometry (GC/MS). Table 1 shows the 100 chemical compounds found in the essential oils of Myrcia multiflora (A), M. multiflora (B), M. multiflora. (C) and Eugenia florida, and the total contents of these essential oils ranged from 88.14 to 98.48%. The essential oil of the specimen Myrcia multiflora (A) was characterized by the major compounds α-bulnesene (26.79%), pogostol (21.27%) and δ-amorphene (6.76%).
Table 1.
Chemical composition found in the essential oils of Myrcia multiflora and Eugenia florida.
| IRL | IRC | Constituents | M. multiflora (A) | M. multiflora (B) | M. multiflora (C) | E. florida |
|---|---|---|---|---|---|---|
| 1335 | 1334 | δ-elemene | 0.26 | 0.55 | ||
| 1345 | 1345 | α-cubebene | 0.13 | 0.05 | ||
| 1373 | 1369 | α-ylangene | 0.06 | 0.03 | ||
| 1374 | 1374 | α-copaene | 0.59 | 0.03 | 0.26 | |
| 1387 | 1387 | β-bourbonene | 0.04 | 0.05 | ||
| 1389 | 1388 | β-elemene | 2.1 | 0.04 | 1.04 | |
| 1409 | 1405 | α-gurjunene | 0.02 | |||
| 1411 | 1412 | α-cis-bergamotene | 0.06 | |||
| 1417 | 1418 | E-caryophyllene | 3.66 | 2.88 | 1.11 | 2.35 |
| 1430 | 1428 | β-copaene | 0.03 | |||
| 1432 | 1431 | α-trans-bergamotene | 0.76 | 0.05 | 1.78 | |
| 1434 | 1432 | γ-elemene | 10.70 | |||
| 1437 | 1435 | α-guaiene | 2.88 | |||
| 1439 | 1440 | aromadendrene | 0.23 | |||
| 1442 | 1444 | 6,9-guaiadiene | 0.79 | |||
| 1447 | 1448 | isogermacrene D | 0.11 | |||
| 1451 | 1453 | trans-muurola-3,5-diene | 0.31 | |||
| 1452 | 1454 | α-humulene | 3.8 | 0.39 | ||
| 1454 | 1455 | (E)-β-farnesene | 4.38 | 0.59 | ||
| 1457 | 1458 | β-santalene | 0.2 | |||
| 1458 | 1459 | allo-aromadendrene | 0.33 | 0.08 | ||
| 1471 | 1474 | dauca-5,8-diene | 0.03 | 0.64 | ||
| 1478 | 1479 | γ-muurolene | 0.12 | 0.69 | ||
| 1479 | 1480 | amorpha-4,7(11)-diene | 3.1 | |||
| 1481 | 1481 | γ-curcumene | 0.18 | |||
| 1483 | 1482 | β-trans-bergamotene | 0.15 | |||
| 1484 | 1484 | germacrene D | 4.12 | 0.72 | 0.23 | 2.21 |
| 1489 | 1488 | β-selinene | 2.26 | 0.96 | ||
| 1492 | 1490 | β-cis-guaiene | 0.06 | |||
| 1493 | 1492 | α-zingiberene | 1.73 | |||
| 1493 | 1495 | trans-muurola-4(14),5-diene | 0.51 | |||
| 1495 | 1496 | γ-amorphene | 0.12 | |||
| 1496 | 1498 | viridiflorene | 1.49 | 1.55 | ||
| 1500 | 1501 | α-muurolene | 0.12 | 0.47 | ||
| 1505 | 1506 | (E,E)-α-farnesene | 8.19 | 3.28 | ||
| 1505 | 1507 | β-bisabolene | 6.83 | |||
| 1506 | 1508 | (Z)-α-bisabolene | 0.2 | |||
| 1509 | 1512 | α-bulnesene | 26.79 | |||
| 1511 | 1514 | δ-amorphene | 6.76 | 0.54 | 0.31 | |
| 1513 | 1515 | γ-cadinene | 0.61 | |||
| 1514 | 1517 | (Z)-γ-bisabolene | 3.41 | |||
| 1515 | 1518 | sesquicineole | 0.02 | |||
| 1521 | 1522 | β-sesquiphellandrene | 0.79 | |||
| 1522 | 1525 | δ-cadinene | 4.42 | |||
| 1527 | 1528 | (E)-γ-bisabolene | 10.64 | 0.57 | ||
| 1528 | 1529 | zonarene | 0.65 | |||
| 1531 | 1530 | γ-vetivenene | 1.59 | |||
| 1532 | 1531 | γ-cuprenene | 0.05 | |||
| 1533 | 1532 | trans-cadina-1,4-diene | 0.49 | 0.36 | ||
| 1537 | 1535 | α-cadinene | 0.06 | |||
| 1540 | 1539 | selina-4(15),7(11)-diene | 0.99 | |||
| 1540 | 1542 | (E)-α-bisabolene | 0.25 | |||
| 1544 | 1543 | α-calacorene | 0.07 | |||
| 1548 | 1547 | elemol | 0.07 | |||
| 1550 | 1551 | cis-muurol-5-en-4-α-ol | 0.04 | |||
| 1554 | 1553 | β-vetivenene | 4.59 | |||
| 1559 | 1557 | germacrene B | 0.08 | 2.17 | ||
| 1561 | 1565 | (E)-nerolidol | 44.4 | 92.21 | ||
| 1566 | 1568 | maaliol | 0.23 | |||
| 1577 | 1575 | spathulenol | 0.52 | 1.36 | 1.2 | |
| 1582 | 1581 | caryophyllene oxide | 0.87 | |||
| 1590 | 1586 | globulol | 1.16 | 0.12 | ||
| 1592 | 1592 | viridiflorol | 1.82 | 1.23 | ||
| 1596 | 1595 | fokienol | 0.18 | 3.9 | ||
| 1602 | 1602 | ledol | 0.17 | |||
| 1607 | 1605 | 5-epi-7-epi-α-eudesmol | 0.29 | |||
| 1607 | 1606 | (Z)-sesquilavandulol | 0.05 | |||
| 1608 | 1610 | β-atlantol | 0.07 | 0.96 | ||
| 1627 | 1627 | 1-epi-cubenol | 2.39 | |||
| 1629 | 1631 | eremoligenol | 0.31 | 0.4 | 11.0 | |
| 1631 | 1634 | muurola-4,10(14)-dien-1-β-ol | 1.24 | |||
| 1632 | 1635 | α-acorenol | 0.17 | |||
| 1635 | 1636 | cis-cadin-4-en-7-ol | 0.14 | |||
| 1636 | 1637 | gossonorol | 0.6 | 0.01 | ||
| 1640 | 1639 | epi-α-muurolol | 0.14 | 0.04 | ||
| 1642 | 1641 | selina-3,11-dien-6-α-ol | 12.93 | |||
| 1644 | 1645 | α-muurolol | 0.72 | |||
| 1645 | 1646 | cubenol | 3.17 | 0.04 | 2.5 | |
| 1651 | 1653 | pogostol | 21.27 | |||
| 1652 | 1654 | α-cadinol | 0.41 | 3.98 | ||
| 1658 | 1658 | neo-intermedeol | 0.09 | |||
| 1666 | 1665 | 14-hydroxy-(Z)-caryophyllene | 0.35 | |||
| 1670 | 1667 | bulnesol | 0.92 | |||
| 1670 | 1670 | epi-β-bisabolol | 0.64 | |||
| 1674 | 1674 | β-bisabolol | 0.09 | |||
| 1677 | 1678 | mustakone | 0.07 | |||
| 1679 | 1680 | khusinol | 0.96 | |||
| 1683 | 1685 | epi-α-bisabolol | 0.46 | |||
| 1685 | 1688 | α-bisabolol | 1.23 | 0.28 | ||
| 1685 | 1688 | germacra-4(15),5,10(14)-trien-1-α-ol | 0.06 | |||
| 1700 | 1701 | eudesm-7(11)-en-4-ol | 1.91 | |||
| 1713 | 1709 | 14-hydroxy-α-humulene | 0.19 | |||
| 1728 | 1733 | (Z)-γ-curcumen-12-ol | 0.26 | |||
| 1745 | 1740 | γ-costol | 0.45 | |||
| 1754 | 1749 | (Z)-β-curcumen-12-ol | 2.99 | |||
| 1755 | 1752 | α-sinensal | 1.62 | |||
| 1766 | 1666 | β-costol | 0.18 | |||
| 1767 | 1667 | 13-hydroxy-valencene | 1.51 | |||
| 1777 | 1778 | (Z)-α-santalol acetate | 1.47 | |||
| 1794 | 1799 | (Z)-α-trans-bergamotol acetate | 0.46 | |||
| hydrocarbon sesquiterpenes | 59.84 | 39.14 | 5.83 | 44.06 | ||
| oxygenated sesquiterpenes | 32.32 | 59.34 | 92.78 | 44.08 | ||
| Total | 92.16 | 98.48 | 98.61 | 88.14 |
α-bulnesene is described in the literature for presenting antimicrobial activity against fungi and bacteria [25]. In relation to the compound pogostol, studies report its antimicrobial potential [26], and also one of the compounds responsible for the cytotoxic action against cancer cells is present [27]. The antimicrobial, cytotoxic and antioxidant potential of δ-amorphene may be related to its strong presence in research on essential oils [28,29].
The essential oil of the specimen Myrcia multiflora (B) was rich in (E)-nerolidol (44.4%), (E)-γ-bisabolene (10.64%) and (E,E)-α-farnesene (8.19%) (Table 1), while (E)-nerolidol (92.21%), (E,E)-α-farnesene (3.28%) and E-caryophyllene (1.11%) were the majority for the specimen Myrcia multiflora (C). Our results differed from those found in two studies with the essential oil of Myrcia multiflora, the first from a sample collected in the municipality of Taquari, State of Rio Grande do Sul, Brazil, which presented β-caryophyllene (7.5%) and germacrene D (8.7%) as the main constituents [22]. The second was from a sample collected in the municipality of Maracanã, State of Pará, Brazil, having as the main compounds β-caryophyllene (10.72%) and selin-11-en-4α-ol (10.67%) [23] (Table 1).
(E)-γ-bisabolene is characterized in studies by demonstrating insecticidal activities against mosquitoes [30], and the sesquiterpene (E,E)-α-farnesene, in the study carried out by [31], as the major constituent demonstrated strong activity antifungal against Trichophyton rubrum- and T. mentagrophytes-type fungi. Regarding the compound E-caryophyllene, studies highlight its fungicidal importance [32] and antioxidant [33], anti-inflammatory [34] and antiprotozoal [35] properties.
The chemical profile of the essential oils of specimen M. multiflora was characterized by hydrocarbon and oxygenated sesquiterpenes, and studies on the essential oils of the Myrcia genus have proven strong the presence of these classes of compounds, as described in two chemical types presented by the essential oils of two specimens of M. tomentosa. Type (A) was characterized by γ-elemene (12.52%), germacrene D (11.45%) and (E)-caryophyllene (10.22%), while type (B) was characterized by the major compounds spathulenol (40.70%), α-zingiberene (9.58%) and γ-elemene (6.89%) [36] (Table 1).
There was a difference in the chemical composition between the studied specimens of M. multiflora, with specimen (A) differing from the other specimens, but the major compound (E)-nerolidol characterized the chemical profile of both specimen (B) and (C). In the latter specimen, the highest content of this sesquiterpene oxygenated was observed (Table 1). This high content was also observed in the essential oil of M. bracteata, which presented 80.8% (E)-nerolidol [37].
The essential oil of Eugenia florida was strongly characterized by the sesquiterpenes selina-3,11-dien-6-α-ol (12.93%), eremoligenol (11%) and γ-elemene (10.70%), very different from what was found in a sample taken in Rio Grande do Sul, Brazil, which presented the major compounds bicyclogermacrene (10.9%), germacrene D (10.4%) and β-caryophyllene (8.1%) [40]. Furthermore, the chemical profile of the essential oil of E. florida differed from that found in the essential oils of the specimens of M. multiflora and that found in the essential oil of Eugenia species.
The compounds 5-hydroxy-cis-calemenene (35.8%), β-caryophyllene (8.9%), trans-cadina-1,4-diene (6.3%), trans-calamenene (6.1%), trans-muurola-3,5-diene (5.9%) and ledol (5.0%) were the main findings in the essential oil of E. egensis. On the other hand, in the essential oil of E polystachya, germacrene D (18.4%), ishwarane (15.7%), 7-epi-α-selinene (7.5%) and bicyclogermacrene (5.1%) predominated [41]. The compounds γ-elemene (25.89%), germacrene B (8.11%) and (E)-caryophyllene (10.76%) were the main compounds of the essential oil of E. patrisii studied by [36], in which, on the other hand, the γ-elemene content was higher than that found in our study. This hydrocarbon sesquiterpene is reported in essential oil research for its antimicrobial and antiproliferative action [42], while the compound seline-3,11-dien-6-α-ol is described in the literature as a possible insecticidal agent [43] and antioxidant [44].
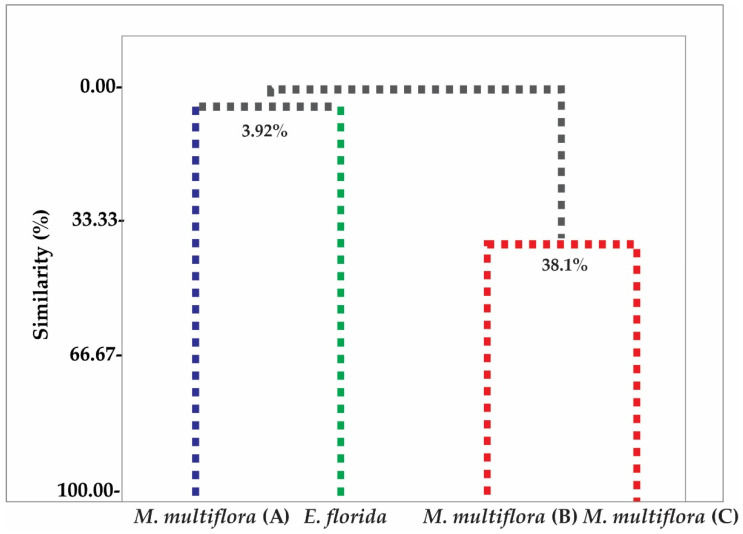
To analyze the possible similarity in the chemical composition of the samples, chemometric analysis of hierarchical clustering analysis (HCA) and principal component analysis (PCA) were applied to the chemical profiles of the essential oils of M. multiflora (A), M. multiflora (B), M. multiflora (C) and E. florida, as shown in Figure 1 and Figure 2, respectively.
Figure 1.
Dendrogram showing relational similarity of compound identified in M. multiflora (A), M. multiflora (B), M. multiflora (C) and E. florida essential oil.
Figure 2.
Biplot (PCA) from analysis of compound identified in M. multiflora (A) M. multiflora (B) M. multiflora (C) E. florida essential oil.
With the HCA cluster analysis using Euclidean distance, it was possible to identify the presence of three distinct groups of chemical profiles: Group I, formed by the sample M. multiflora (A); Group II, formed by the sample E. florida, both with a significant difference of approximately 96.08%; and Group III, formed by the samples M. multiflora (B) and M. multiflora (C) with a degree of similarity of 38.1% (Figure 1).
In Figure 2, it is possible to confirm the formation of the groups. The compounds responsible for the group formations are presented. The eigenvalues corresponding to the principal component analysis PC1 explained 46.8% of the variance, while PC2 explained 39%. When adding the components, they explain 85.8% of the variation. Moreover, when the groups present in Figure 2 are analyzed, we note the compounds that had the highest weights for their formations. Group I, comprising M. multiflora (A), was characterized by the presence of the 1-epi compounds -cubebol, amorpha-4,7(11)-diane, δ-amorphene, α-humulene, (E)-caryophyllene, cubeball, β-selinene, α-bulnesene, β-elemene, germacrene D, α-guaiene and pogostol. Group II, comprising E. florida, was characterized by the presence of germacrene B, (Z)-δ-bisabolene, β-vetivenene, δ-cadinene, α-candinol, eremoligenol, seline-3,11-dien-6-α-ol, fokienol and γ-elemene. Group III, comprising M. multiflora (B) and M. multiflora (C), was characterized by the compounds (Z)-β-curcumen-12-ol, (E)-β-farnesene, (E)-d-bisabolene, β-santalene, (E,E)-α-farnesene, β-bisabolene, (E)-nerolidol and (Z)-α-bisabolene.
2.3. Antimicrobial Activity
The in vitro antifungal activity of the four oils was assessed against five fungal species (Candida albicans INCQS-40175, C. tropicalis ATCC 6258, C. famata ATCC 62894, C. krusei ATCC 13803 and C. auris IEC-01) of medical importance. All tested oils showed an inhibition zone for all assessed species. However, the inhibitory effect varied among the species. The diameter of the halos was between 6 and 11 mm (Table 2).
Table 2.
In vitro effect of the botanical extracts on medically important yeasts assessed by the agar disc diffusion method.
| Halo Diameter (mm) | ||||
|---|---|---|---|---|
| Fungal Species | Myrcia multiflora (A) | Eugenia florida | M. multiflora (B) | M. multiflora (C) |
| C. albicans | 8 | 7 | 9 | 7 |
| C. tropicalis | 8 | 8 | 11 | 8 |
| C. krusei | 6 | 6 | 10 | 8 |
| C. famata | 9 | 8 | 10 | 8 |
| C. auris | 9 | 8 | 10 | 8 |
The studied fungal species showed sensitivity in the presence of the essential oil of the specimen M. multiflora (B), which showed higher inhibition power (9–11 mm), based on the size of the halos. These values are consistent with the inhibition potential presented by the antifungal agent amphotericin B against Candida albicans (10–19 mm), C. tropicalis (10–20 mm) and C. krusei (9–20 mm) [45]. The fungal species C. krusei showed resistance (6 mm) in contact with the essential oil of the specimen M. multiflora (A), in contrast to the other fungal species. The essential oil showed an inhibition potential of 8–9 mm. The species C. albicans and C. krusei showed resistance to the essential oil of E. florida, with inhibition potentials of 7 mm and 6 mm, respectively. The essential oil of the specimen M. multiflora (C) showed low inhibition potential (7 mm) against C. albicans.
The essential oils from Myrcia and Eugenia showed high inhibition potential against different types of fungi. The essential oil of Myrcia ovata showed significant antimicrobial activity against C. albicans (30 mm) [46]. In another study, the essential oil of E. caryophyllata was effective against C. albicans (18.4 mm) and C. tropicalis (19 mm) [47]. These inhibition potentials were higher than those found in our study, which may be somehow related to the chemical type presented in each of the essential oils [48].
By analyzing the minimum inhibitory concentration (MIC), the essential oil of the specimen M. multiflora (A) presented 0.78 µL/mL, according to Table 3. This was the lowest MIC found and was able to inhibit the growth of Candida famata. This high capacity to inhibit the fungal yeast (C. famata) may be related to the main constituents of the essential oil of M. multiflora (A): α-bulnesene (26.79%), pogostol (21.27%) and δ-amorphene (6.76%), as well as other compounds with lower essential oil [49]. In turn, the essential oil of the specimen M. multiflora (C) showed a minimum inhibitory concentration of 12 µL/mL for both C. krusei and C. famata. The MIC values found in this work differed from those observed by [50] in the essential oil of Siparuna guianensis, which was able to inhibit the growth of C. albicans, with an MIC of 125 µL/mL. Likewise, the essential oil of Eugenia caryophyllus was able to inhibit the growth of the same fungal species, with an MIC of 0.017 µL/mL [51].
Table 3.
Minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of extracts on medically important yeasts; data expressed in µL/mL.
| Myrcia multiflora (A) | M. multiflora (C) | |||
|---|---|---|---|---|
| Fungal Species | MIC | MFC | MIC | MFC |
| C. albicans | 50 | 50 | 50 | 50 |
| C. tropicalis | 50 | a | 50 | >50 |
| C. krusei | 6.25 | >25 | 12.5 | 50 |
| C. famata | 0.78 | 3.12 | 12.5 | >50 |
| C. auris | 3.12 | >12.5 | 5 | >50 |
A reduction in colonial growth was observed after plating.
In this study, it was possible to observe that some essential oils were able to inhibit up to two fungal species. However, others were able to reduce fungal growth, such as the essential oil of the specimen M. multiflora (A), which had fungicidal action on C. albicans and C. famata, with an MFC of 50 µL/mL and 3.12 µL/mL, respectively. On the other hand, the essential oil from the specimen M. multiflora (C) had fungicidal action on C. albicans and C. krusei, with an MFC of 50 µL/mL. It is important to mention that this essential oil is characterized by a high content of (E)-nerolidol (92.21%), and this compound is even described in the literature for presenting antimicrobial activity, mainly antifungal [52,53,54]. The MFC result found in the EOs of M. multiflora (A) against C. famata is within that observed in the essential oil of the leaves of Myrtus communis against the fungal species Candida albicans (ATCC 10261), C. tropicalis (ATCC 750), C. krusei (ATCC 6258), C. glabrata (ATCC 90030) and C. parapsilosis (ATCC 4344), with MFC values ≤8 µL/mL [55].
No report on the antimicrobial activity of the essential oil of M. multiflora and Eugenia florida was found in the literature. However, studies were observed within the genera Eugenia and Myrcia, species with antimicrobial properties, of which we can highlight Eugenia uniflora [56], E. involucrata [57], E. caryophyllata [58], Myrcia palustres [48], M. ovata [59] and M. splendens [60].
Other analyses shall be made in order to assess the degree of involvement of the fungal cell better after being challenged with the botanical extracts. Therefore, the antimicrobial properties of the essential oils may be associated with terpene compounds, which, due to their highly lipophilic nature and low molecular weight, are capable of interrupting the cell membrane, causing cell death or inhibiting the sporulation and germination of fungi that deteriorate food products [61].
3. Materials and Methods
3.1. Botanic Material
Aerial parts of three specimens of Myrcia multiflora and one specimen of Eugenia florida were collected in the coastal region of the State of Pará, in the city of Magalhães Barata, Brazil. Specimens (A) and (B) of M. multiflora were collected on 12/07/2017, the first in an area of secondary forest (capoeira), and the second in areas of swidden planting exposed to sunlight, the geographic coordinates of which are 00°48′20.9″ S, 47°33′57.3″ W. Specimen (C) of M. multiflora was collected on 06/18/2019 in an area of secondary forest (capoeira) and the species of E. florida on 09/21/2019 in a lowland area on the banks of the Curral river. The exsiccate was incorporated in the collection of the João Murça Pires Herbarium (MG) of Museu Paraense Emílio Goeldi, in the collection of Aromatic Plants of the Amazon, Belém, Pará, and were registered under Voucher number MG231882 (M. multiflora A), MG231881 (M. multiflora B), MG231882 (M. multiflora C) and MG237472 (E. florida).
3.2. Preparation of Botanical Material
The leaves of E. florida and M. multiflora (A, B and C) were dried for 5 days at 35 °C in an oven with air circulation before being crushed in a knife mill. The moisture content was analyzed using an ID50 infrared humidity determiner in the temperature range of 60–180 °C, with a 1 °C increment and bidirectional RT-232 °C output.
3.3. Extraction of Essential Oils
The samples were subjected to hydrodistillation in modified Clevenger-type glass systems for 3 h, coupled to a refrigeration system to maintain the condensation water at around 12 °C. After the extraction, the oils were centrifuged for 5 min at 3000 rpm, dehydrated with anhydrous sodium sulfate and centrifuged again under the same conditions. Oil yield was calculated in mL/100 g. The oils were stored in amber glass ampoules, sealed with flame and stored in a refrigerator at 5 °C.
3.4. Chemical Composition Analysis
The chemical compositions of the EOs of M. multiflora (A, B and C) and E. florida, were analyzed using a Shimadzu QP-2010 plus (Kyoto, Japan), a gas chromatography system equipped with an Rtx-5MS capillary column (30 m × 0.25 mm; 0.25 µm film thickness) (Restek Corporation, Bellefonte, PA, USA) coupled to a mass spectrometer (GC/MS) (Shimadzu, Kyoto, Japan). The programmed temperature was maintained at 60–240 °C at a rate of 3 °C/min, with an injector temperature of 250 °C, helium as the carrier gas (linear velocity of 32 cm/s, measured at 100 °C) and a splitless injection (1 μL of a 2:1000 hexane solution), using the same operating conditions as described in the literature [62]. Except for the carrier hydrogen gas, the components were quantified using gas chromatography (CG) on a Shimadzu QP-2010 system (Kyoto, Japan), equipped with a flame ionization detector (FID) (Kyoto, Japan), under the same operating conditions as before. The retention index for all volatile constituents was calculated using a homologous series of n-alkanes (C8–C40), Sigma-Aldrich (San Luis, MO, USA), according to van den Dool and Kratz [63]. The components were identified by comparison (i) of the experimental mass spectra with those compiled in libraries (reference) and (ii) their retention indices to those found in the literature [38,39].
3.5. Antimicrobial Activity of the Essential Oils
3.5.1. Microorganisms
To analyze the antifungal activity, 5 fungal species were used (Candida albicans INCQS-40175, C. tropicalis ATCC 6258, C. famata ATCC 62894, C. krusei ATCC 13803 and C. auris IEC-01). All isolates were cultivated in Sabouraud agar at 37 °C for 48 h before the beginning of the tests. These tests were performed at the Laboratory of Superficial and Systemic Mycoses of Instituto Evandro Chagas (IEC).
3.5.2. Agar Disc Diffusion Test
The antifungal activity of the essential oils was assessed using the agar disc diffusion method [64] with modifications. The paper filter discs (6 mm) were impregnated with 20 μL of each extract. The suspensions of the test microorganisms were prepared with 0.45% saline solution (0.5 on the McFarland scale). Each microorganism suspension was spread on the surface of Sabouraud agar culture medium in Petri dishes (15 × 90 mm). Then, the paper discs impregnated with the extracts were placed on the surface of the plates inoculated with the microorganisms. The plates were incubated for 48 h at 37 °C. After this period, visual readings were taken, observing the presence of growth inhibition zone measured in millimeters, with the help of a millimeter ruler. As a positive control, paper discs were impregnated with nystatin solution. All tests were carried out in duplicate. Inhibition zones ≥8 mm indicated that the microorganism was sensitive to the tested essential oil, according to the classification proposed by the authors [65,66,67].
3.5.3. Broth Microdilution: Determination of MIC
To determine the minimum inhibitory concentration (MIC), the oils from specimens A and C of M. multiflora were selected (they presented sufficient quantities to carry out all tests). The susceptibility of the microorganisms to the 5 extracts was determined by the broth microdilution method recommended by the “US National Committee for Clinical Laboratory Standards” (NCCLS), with adaptations. Five microorganisms were tested (Candida albicans INCQS-40175, C. tropicalis ATCC 6258, C. famata ATCC 62894, C. krusei ATCC 13803 and C. auris IEC-01). The microorganisms were cultivated in Sabouraud agar at 37 °C for 48 h. From these cultures, cellular suspensions similar to the McFarland scale 0.5 were prepared. In a 96-well plate, serial dilution at a ratio of 2 of each extract to be tested was performed, starting from 10% (20/180 μL), in a final volume of 100 μL. Then, 100 µL of the yeast suspension was added. The final concentration in each well reached 50% µL/mL, 25% µL/mL, 12.5 µL/mL, 6.2 µL/mL, 3.1 µL/mL, 1.5 µL/mL, 0.7 µL/mL, 0.3 µL/mL, 0.19 µL/mL and 0.0 µL/mL. After adding the yeast to the previously diluted oils, the plate was incubated in a bacteriological incubator at 37 °C for 48 h, and at the end, the readings were taken visually, and the lowest concentration of extract capable of inhibiting visible fungal growth was recorded. The test was carried out in duplicate [68].
3.5.4. Broth Microdilution: Determination of MFC
After completion of the test and visual reading of the broth microdilution to determine MIC, the test to determine the minimum fungicidal concentration (MFC) was carried out. The test consisted of plating 10 μL of each dilution in Sabouraud agar and incubating them in a bacteriological incubator at 37 °C for 48 h. After this period, the lowest dilution capable of killing 99.5% of the original inocula was recorded. The test was carried out in duplicate [68].
3.6. Statistical Analysis
The EO composition data required hierarchical cluster analysis (HCA) and principal component analysis (PCA) to establish composition patterns between the species. The data were processed by Minitab Statistical Software 17 trial version (State College, PA, USA). The HCA was carried out by adopting the similarity using Euclidean distance through a 4 × 28 correlation matrix, and the PCA was carried out using the covariance matrix of the samples since all input data were presented as the mass percentage (≥2%). Both HCA and PCA were carried out using all the identified compounds as variables [36,69].
4. Conclusions
The chemical profile of the essential oils was strongly characterized by the sesquiterpene class, with α-bulnesene (26.79%), (E)-nerolidol (44.4%) and (E)-nerolidol (92.21%) in the essential oils of Myrcia multiflora (A, B and C), and selina-3,11-dien-6-α-ol (12.93%) in Eugenia florida as main constituents, and there was a significant difference between the chemical profiles of the studied species, which in a way is associated with different types of ecosystems and collection periods.
By chemometric analysis of hierarchical clustering analysis (HCA) and principal component analysis (PCA), it was possible to verify this expressive difference in the chemical composition of essential oils. In HCA and PCA there was the formation of three distinct groups of chemical profiles: Group I being formed by the sample M. multiflora (A) and Group II by the sample E. florida, both with a significant difference of approximately 96.08%. Group III was formed by the samples M. multiflora (B) and M. multiflora (C), with a similarity degree of 38.1%. This formation of groups is related to the presence of chemical compounds present in the essential oils of each studied species.
The essential oils of Myrcia multiflora showed high inhibition potential against the fungal species C. albicans, C. famata and C. krusei, which may be related to the hydrophobicity of the terpenoid class, mainly of the compounds α-bulnesene, pogostol, δ-amorphene and (E)-nerolidol. However, new studies have to be carried out to prove the antimicrobial potential of the studied species and their actual impact on the obstruction of fungal cells.
In view of the search for new natural pathogens, the fungicidal potential presented in M. multiflora essential oils may be promising for the development of natural agents that inhibit the action of diseases caused by these phytopathogens.
Acknowledgments
The author Mozaniel Santana de Oliveira thanks PCI-MCTIC/MPEG, as well as CNPq, for the scholarship process number 302050/2021-3. The authors would like to thank the Universidade Federal do Pará.
Author Contributions
Conceptualization, O.O.F.; formal analysis, M.S.d.O., S.H.M.d.S. and E.H.d.A.A.; investigation, O.O.F., S.H.M.d.S. and M.S.d.O.; writing—review and editing, E.H.d.A.A.; visualization, E.H.d.A.A.; supervision, E.H.d.A.A.; project administration, E.H.d.A.A.; All authors have read and agreed to the published version of the manuscript.
Funding
Universidade Federal do Pará/Proposp/PROGRAMA DE APOIO À PUBLICAÇÃO QUALIFICADA—PAPQ-EDITAL 06/2021. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All supporting data can be obtained from the corresponding author upon formal request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of Myrcia multiflora and Eugenia florida. The essential oil of the Museu Paraense Emílio Goeldi is available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hibbett D., Abarenkov K., Kõljalg U., Öpik M., Chai B., Cole J., Wang Q., Crous P., Robert V., Helgason T., et al. Sequence-based classification and identification of Fungi. Mycologia. 2016;108:1049–1068. doi: 10.3852/16-130. [DOI] [PubMed] [Google Scholar]
- 2.Wang L., Jiang N., Wang D., Wang M. Effects of essential oil citral on the growth, mycotoxin biosynthesis and transcriptomic profile of alternaria alternata. Toxins. 2019;11:553. doi: 10.3390/toxins11100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict K., Jackson B.R., Chiller T., Beer K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019;68:1791–1797. doi: 10.1093/cid/ciy776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albuquerque P., Casadevall A. Quorum sensing in fungi a review. Med. Mycol. 2012;50:337–345. doi: 10.3109/13693786.2011.652201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasheminejad N., Khodaiyan F., Safari M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019;275:113–122. doi: 10.1016/j.foodchem.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 7.Zuzarte M., Gonçalves M.J., Cavaleiro C., Canhoto J., Vale-Silva L., Silva M.J., Pinto E., Salgueiro L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J. Med. Microbiol. 2011;60:612–618. doi: 10.1099/jmm.0.027748-0. [DOI] [PubMed] [Google Scholar]
- 8.Damjanović-Vratnica B., Dakov T., Šuković D., Damjanović J. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from Montenegro. Czech J. Food Sci. 2011;29:277–284. doi: 10.17221/114/2009-CJFS. [DOI] [Google Scholar]
- 9.Orhan I.E., Özçelik B., Kartal M., Kan Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012;36:239–246. doi: 10.3906/biy-0912-30. [DOI] [Google Scholar]
- 10.Rana I.S., Rana A.S., Rajak R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011;42:1269–1277. doi: 10.1590/S1517-83822011000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato A., Morretes B. Morfo-anatomia foliar de Myrcia multiflora (Lam.) DC.—Myrtaceae. Rev. Bras. Plantas Med. 2011;13:43–51. doi: 10.1590/S1516-05722011000100007. [DOI] [Google Scholar]
- 12.Gakuubi M.M., Maina A.W., Wagacha J.M. Antifungal Activity of Essential Oil of Eucalyptus camaldulensis Dehnh. against Selected Fusarium spp. Int. J. Microbiol. 2017;2017:8761610. doi: 10.1155/2017/8761610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palareti G., Legnani C., Cosmi B., Antonucci E., Erba N., Poli D., Testa S., Tosetto A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016;38:42–49. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- 14.Siddique S., Perveen Z., Nawaz S., Shahzad K., Ali Z. Chemical Composition and Antimicrobial Activities of Essential Oils of Six Species from Family Myrtaceae. J. Essent. Oil Bear. Plants. 2015;18:950–956. doi: 10.1080/0972060X.2014.935020. [DOI] [Google Scholar]
- 15.Cascaes M.M., Guilhon G.M.S.P., Zoghbi M.d.G., Andrade E.H.A., Santos L.S., da Silva J.K.R., Uetanabaro A.P.T., Araújo I.S. Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh leaves (myrtaceae) Nat. Prod. Res. 2019;35:1717–1721. doi: 10.1080/14786419.2019.1629912. [DOI] [PubMed] [Google Scholar]
- 16.Vareda P.M.P., Saldanha L.L., Camaforte N.A.d.P., Violato N.M., Dokkedal A.L., Bosqueiro J.R. Myrcia bella Leaf Extract Presents Hypoglycemic Activity via PI3k/Akt Insulin Signaling Pathway. Evid. Based Complement. Altern. Med. 2014;2014:5436061. doi: 10.1155/2014/543606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moresco H.H., Pereira M., Bretanha L.C., Micke G.A., Pizzolatti M.G., Brighente I.M.C. Myricitrin as the main constituent of two species of Myrcia. J. Appl. Pharm. Sci. 2014;4:1–7. doi: 10.7324/JAPS.2014.40201. [DOI] [Google Scholar]
- 18.Bastos R.G., Rosa C.P., Oliver J.C., Silva N.C., Dias A.L.T., Da Rocha C.Q., Vilegas W., Da Silva G.A., Da Silva M.A. Chemical characterization and antimicrobial activity of hydroethanolic crude extract of Eugenia florida DC (Myrtaceae) leaves. Int. J. Pharm. Pharm. Sci. 2016;8:110–115. [Google Scholar]
- 19.do Nascimento L.D., Cascaes M.M., Oliveira L.H.A., de Aguiar A.E.H. Pesquisa na Cadeia de Suprimentos de Plantas Aromáticas. Atena Editora; Ponta Grossa, Brazil: 2019. Rendimento e Composição Química do Óleo Essencial das Folhas de Eugenia uniflora L. em Diferentes Tempos de Extração; pp. 48–58. [Google Scholar]
- 20.Pegoraro R.L., de Falkenberg M.B., Voltolini C.H., Santos M., Paulilo M.T.S. Produção de óleos essenciais em plantas de Mentha x piperita L. var. piperita (Lamiaceae) submetidas a diferentes níveis de luz e nutrição do substrato. Rev. Bras. Bot. 2010;33:631–637. doi: 10.1590/S0100-84042010000400011. [DOI] [Google Scholar]
- 21.Silva I.G.R., Sousa E.M., Moraes A.A.B., Sarges M.d.S.R., Cascaes M.M., Nascimento L.D., Andrade E.H.d.A. Avaliação sazonal do rendimento e composição química do óleo essencial das folhas de Aniba parviflora (Meisn) Mez. (Lauraceae) Braz. J. Dev. 2020;6:41334–41345. doi: 10.34117/bjdv6n6-610. [DOI] [Google Scholar]
- 22.Henriques A.T., Sobral M., Bridi R., Vérin P., Menut C., Lamaty G., Bessière J.M. Essential Oils from Five Southern Brazilian Species of Myrcia (Myrtaceae) J. Essent. Oil Res. 1997;9:13–18. doi: 10.1080/10412905.1997.9700707. [DOI] [Google Scholar]
- 23.Pereira R.A., Zoghbi M.D.G.B., Bastos M.D.N.D.C. Essential Oils of Twelve Species of Myrtaceae Growing Wild in the Sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants. 2010;13:440–450. doi: 10.1080/0972060X.2010.10643847. [DOI] [Google Scholar]
- 24.da Costa J.S., Barroso A.S., Mourão R.H.V., da Silva J.K.R., Maia J.G.S., Figueiredo P.L.B. Seasonal and antioxidant evaluation of essential oil from Eugenia uniflora L., curzerene-rich, thermally produced in situ. Biomolecules. 2020;10:328. doi: 10.3390/biom10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi R., Pande V., Thakuri B. Antimicrobial activity of the essential oil of Phlomis bracteosa. Sci. World. 2011;9:63–65. doi: 10.3126/sw.v9i9.5521. [DOI] [Google Scholar]
- 26.Urban A.M., Swiech J.N.D., Moraes G.S., Paludo K., Ito C.A.S., Dias J.D.F.G., Montrucchio D.P., Miguel O.G., Miguel M.D. Cantinoa althaeifolia essential oil: Chemical composition and biological, antioxidant, antimicrobial, and antitumor activities. Res. Soc. Dev. 2021;10:e9910212040. doi: 10.33448/rsd-v10i2.12040. [DOI] [Google Scholar]
- 27.Da Silva J.K.R., Andrade E.H.A., Mourão R.H.V., Maia J.G.S., Dosoky N.S., Setzer W.N. Chemical profile and in vitro biological activities of essential oils of Nectandra puberula and N. cuspidata from the Amazon. Nat. Prod. Commun. 2017;12:131–134. doi: 10.1177/1934578X1701200137. [DOI] [PubMed] [Google Scholar]
- 28.Setzer W.N., Park G., Agius B.R., Stokes S.L., Walker T.M., Haber W.A. Chemical Compositions and Biological Activities of Leaf Essential Oils of Twelve Species of Piper from Monteverde, Costa Rica. Nat. Prod. Commun. 2008;3:1934578X0800300. doi: 10.1177/1934578X0800300823. [DOI] [Google Scholar]
- 29.Deng G.B., Zhang H.B., Xue H.F., Chen S.N., Chen X.L. Chemical Composition and Biological Activities of Essential Oil from the Rhizomes of Iris bulleyana. Agric. Sci. China. 2009;8:691–696. doi: 10.1016/S1671-2927(08)60266-7. [DOI] [Google Scholar]
- 30.Govindarajan M., Vaseeharan B., Alharbi N.S., Kadaikunnan S., Khaled J.M., Al-anbr M.N., Alyahya S.A., Maggi F., Benelli G. High efficacy of (Z)-γ-bisabolene from the essential oil of Galinsoga parviflora (Asteraceae) as larvicide and oviposition deterrent against six mosquito vectors. Environ. Sci. Pollut. Res. 2018;25:10555–10566. doi: 10.1007/s11356-018-1203-3. [DOI] [PubMed] [Google Scholar]
- 31.Morocho V., Toro M.L., Cartuche L., Guaya D., Valarezo E., Malagón O., Ramírez J. Chemical composition and antimicrobial activity of essential oil of Lepechinia radula benth epling. Rec. Nat. Prod. 2017;11:57–62. [Google Scholar]
- 32.Langat M.K., Mayowa Y., Sadgrove N., Danyaal M., Prescott T.A.K., Kami T., Schwikkard S., Barker J., Cheek M. Multi-layered antimicrobial synergism of (E)-caryophyllene with minor compounds, tecleanatalensine B and normelicopine, from the leaves of Vepris gossweileri (I. Verd.) Mziray. Nat. Prod. Res. 2021;2021:1899176. doi: 10.1080/14786419.2021.1899176. [DOI] [PubMed] [Google Scholar]
- 33.Caroline D.S.F., Alcy F.R., Natale C.C.C., Odair S.M., Joyce K.R.D.S., Eloisa H.A.A., Jos G.S.M. Composition and antioxidant and antifungal activities of the essential oil from Lippia gracilis Schauer. Afr. J. Biotechnol. 2014;13:3107–3113. doi: 10.5897/AJB2012.2941. [DOI] [Google Scholar]
- 34.Sabulal B., Dan M., Anil J.J., Kurup R., Pradeep N.S., Valsamma R.K., George V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry. 2006;67:2469–2473. doi: 10.1016/j.phytochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Moreira R.R.D., dos Santos A.G., Carvalho F.A., Perego C.H., Crevelin E.J., Crotti A.E.M., Cogo J., Cardoso M.L.C., Nakamura C.V. Antileishmanial activity of Melampodium divaricatum and Casearia sylvestris essential oils on Leishmania amazonensis. Rev. Inst. Med. Trop. Sao Paulo. 2019;61:e33. doi: 10.1590/s1678-9946201961033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jesus Pereira Franco C., Oliveira Ferreira O., Barbosa De Moraes Â.A., Pompeu Varela E.L., Diniz Do Nascimento L., Percário S., Santana De Oliveira M., De Aguiar Andrade E.H. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia patrisii Vahl, E. punicifolia (Kunth) DC., and Myrcia tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Molecules. 2021;26:3292. doi: 10.3390/molecules26113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoghbi M.d.G.B., Andrade E.H.A., da Silva M.H.L., Carreira L.M.M., Maia J.G.S. Essential oils from three Myrcia species. Flavour Fragr. J. 2003;18:421–424. doi: 10.1002/ffj.1242. [DOI] [Google Scholar]
- 38.Mondello L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds. Volume 10. Wiley-Blackwell; Hoboken, NJ, USA: 2015. pp. 4–5. [Google Scholar]
- 39.Adams R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. pp. 804–806. [Google Scholar]
- 40.Apel M.A., Sobral M., Schapoval E.E.S., Henriques A.T., Menut C., Bessiere J.M. Essential oil composition of Eugenia florida and Eugenia mansoi. J. Essent. Oil Res. 2004;16:321–322. doi: 10.1080/10412905.2004.9698732. [DOI] [Google Scholar]
- 41.da Silva J., Andrade E., Barreto L., da Silva N., Ribeiro A., Montenegro R., Maia J. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines. 2017;4:51. doi: 10.3390/medicines4030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni J., Mahdavi B., Ghezi S. Chemical Composition, Antimicrobial, Hemolytic, and Antiproliferative Activity of Essential Oils from Ephedra intermedia Schrenk & Mey. J. Essent. Oil Bear. Plants. 2019;22:1562–1570. doi: 10.1080/0972060X.2019.1707717. [DOI] [Google Scholar]
- 43.Urzúa A., Modak B., Santander R., Heit C., Echeverría J. Insecticidal properties of Heliotropium stenophyllum essential oil on the house fly, Musca domestica L. Boletín Latinoam. Caribe Plantas Med. Aromáticas. 2013;12:196–200. [Google Scholar]
- 44.Vidic D., Ćavar Zeljković S., Dizdar M., Maksimović M. Essential oil composition and antioxidant activity of four Asteraceae species from Bosnia. J. Essent. Oil Res. 2016;28:445–457. doi: 10.1080/10412905.2016.1150216. [DOI] [Google Scholar]
- 45.Pedroso R.d.S., Menezes R.d.P., Ferreira J.C., Penatti M.P.A., de Sá W.M., Malvino L.D.S., Candido R.C., Moreira T.d.A. Sensibilidade de isolados de Candida spp. a antifúngicos por disco-difusão em ágar e microdiluição em caldo. BioSci. J. 2014;30:304–311. [Google Scholar]
- 46.Cândido C.S., Portella C.S.A., Laranjeira B.J., da Silva S.S., Arriaga A.M.C., Santiago G.M.P., Gomes G.A., Almeida P.C., Carvalho C.B.M. Effects of Myrcia ovata Cambess. essential oil on planktonic growth of gastrointestinal microorganisms and biofilm formation of Enterococcus faecalis. Braz. J. Microbiol. 2010;41:621–627. doi: 10.1590/S1517-83822010000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kouidhi B., Zmantar T., Bakhrouf A. Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann. Microbiol. 2010;60:599–604. doi: 10.1007/s13213-010-0092-6. [DOI] [Google Scholar]
- 48.Dos Santos C.V., Mallmann A.P., Toledo A.G., Bandeira D.M., da Costa W.F., Marins D.M.Á., Corrêa J.M., Pinto F.G.d.S. Composição química, atividade antimicrobiana e antioxidante do óleo essencial de folhas Myrcia palustris DC. (MYRTACEAE) Res. Soc. Dev. 2021;10:e20510313303. doi: 10.33448/rsd-v10i3.13303. [DOI] [Google Scholar]
- 49.dos Santos J.F.S., Rocha J.E., Bezerra C.F., do Nascimento Silva M.K., de Matos Y.M.L.S., de Freitas T.S., dos Santos A.T.L., da Cruz R.P., Machado A.J.T., Rodrigues T.H.S., et al. Chemical composition, antifungal activity and potential anti-virulence evaluation of the Eugenia uniflora essential oil against Candida spp. Food Chem. 2018;261:233–239. doi: 10.1016/j.foodchem.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira M.S., da Cruz J.N., da Costa W.A., Silva S.G., Brito M.d.P., de Menezes S.A.F., de Jesus A.M.C.N., de Aguiar A.E.H., de Carvalho R.N., Jr. Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules. 2020;25:3852. doi: 10.3390/molecules25173852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisin G., Safiyev S., Craker L.E. Antimicrobial Activity of Some Essential Oils. Acta Hortic. 1999;501:283–288. doi: 10.17660/ActaHortic.1999.501.45. [DOI] [Google Scholar]
- 52.Krist S., Banovac D., Tabanca N., Wedge D.E., Gochev V.K., Wanner J., Schmidt E., Jirovetz L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015;10:143–148. doi: 10.1177/1934578X1501000133. [DOI] [PubMed] [Google Scholar]
- 53.Parreira N.A., Magalhães L.G., Morais D.R., Caixeta S.C., De Sousa J.P.B., Bastos J.K., Cunha W.R., Silva M.L.A., Nanayakkara N.P.D., Rodrigues V., et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem. Biodivers. 2010;7:993–1001. doi: 10.1002/cbdv.200900292. [DOI] [PubMed] [Google Scholar]
- 54.Passos J.L., Barbosa L.C.A., Demuner A.J., Alvarenga E.S., Da Silva C.M., Barreto R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules. 2012;17:11447–11455. doi: 10.3390/molecules171011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zomorodian K., Moein M., Lori Z.G., Ghasemi Y., Rahimi M.J., Bandegani A., Pakshir K., Bazargani A., Mirzamohammadi S., Abbasi N. Chemical Composition and Antimicrobial Activities of the Essential Oil from Myrtus communis Leaves. J. Essent. Oil Bear. Plants. 2013;16:76–84. doi: 10.1080/0972060X.2013.764183. [DOI] [Google Scholar]
- 56.Victoria F.N., Lenardão E.J., Savegnago L., Perin G., Jacob R.G., Alves D., da Silva W.P., da Motta A.d.S., Nascente P.d.S. Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 2012;50:2668–2674. doi: 10.1016/j.fct.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Toledo A.G., de Souza J.G.d.L., da Silva J.P.B., Favreto W.A.J., da Costa W.F., Pinto F.G.d.S. Chemical composition, antimicrobial and antioxidant activity of the essential oil of leaves of Eugenia involucrata DC. Biosci. J. 2020;36:568–577. doi: 10.14393/BJ-v36n2a2020-48096. [DOI] [Google Scholar]
- 58.Mendes J.M., Guerra F.Q.S., de Oliveira P.F., de Sousa J.P., Trajano V.N. Actividad antifúngica del aceite esencial de Eugenia caryophyllata sobre cepas de Candida tropicalis de aislados clínicos [Antifungal activity of the essential oil of Eugenia caryophyllata on Candida tropicalis strains from clinical isolates] Plantas Med. Aromáticas. 2012;11:208–217. [Google Scholar]
- 59.Sampaio T.S., Blank A.F., Gagliardi P.R., Wisniewski A., Arrigoni-Blank M.d.F., Nizio D.A.d.C., Alves M.F., de Nascimento A.F., Jr. Antifungal activity of essential oils of myrcia ovata chemotypes and their major compounds on phytopathogenic fungi. BioSci. J. 2020;36:364–375. doi: 10.14393/BJ-v36n2a2020-42315. [DOI] [Google Scholar]
- 60.Pontes F.C., Abdalla V.C.P., Imatomi M., Fuentes L.F.G., Gualtieri S.C.J. Antifungal and antioxidant activities of mature leaves of Myrcia splendens (Sw.) DC. Braz. J. Biol. 2019;79:127–132. doi: 10.1590/1519-6984.179829. [DOI] [PubMed] [Google Scholar]
- 61.Nazzaro F., Fratianni F., Coppola R., De Feo V. Essential oils and antifungal activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Oliveira M.S., da Silva V.M.P., Freitas L.C., Silva S.G., Cruz J.N., de Aguiar A.E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers. 2021;18:cbdv.202000982. doi: 10.1002/cbdv.202000982. [DOI] [PubMed] [Google Scholar]
- 63.van Den D.H., Dec Kratz P. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 64.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing. Volume 25. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2005. Fifteenth Informational Supplement. [Google Scholar]
- 65.Ponce A.G., Fritz R., Del Valle C., Roura S.I. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT Food Sci. Technol. 2003;36:679–684. doi: 10.1016/S0023-6438(03)00088-4. [DOI] [Google Scholar]
- 66.Moreira M.R., Ponce A.G., Del Valle C.E., Roura S.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT Food Sci. Technol. 2005;38:565–570. doi: 10.1016/j.lwt.2004.07.012. [DOI] [Google Scholar]
- 67.Celikel N., Kavas G. Antimicrobial properties of some essential oils against some pathogenic microorganisms. Czech J. Food Sci. 2008;26:174–181. doi: 10.17221/1603-CJFS. [DOI] [Google Scholar]
- 68.de Castro R.D., de Oliveira Lima E. Screening da atividade antifúngica de óleos essenciais sobre cepas de Candida. Pesqui. Bras. Odontopediatria Clin. Integr. 2011;11:341–345. doi: 10.4034/PBOCI.2011.113.06. [DOI] [Google Scholar]
- 69.de Oliveira M.S., Cruz J.N., Ferreira O.O., Pereira D.S., Pereira N.S., Oliveira M.E.C., Venturieri G.C., Guilhon G.M.S.P., Souza F.A.P.d.S., Andrade E.H.d.A. Chemical Composition of Volatile Compounds in Apis mellifera Propolis from the Northeast Region of Pará State, Brazil. Molecules. 2021;26:3462. doi: 10.3390/molecules26113462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data can be obtained from the corresponding author upon formal request.