Abstract
In the forms of either herbs or functional foods, plants and their products have attracted medicinal, culinary, and nutraceutical applications due to their abundance in bioactive phytochemicals. Human beings and other animals have employed those bioactive phytochemicals to improve health quality based on their broad potentials as antioxidant, anti-microbial, anti-carcinogenic, anti-inflammatory, neuroprotective, and anti-aging effects, amongst others. For the past decade and half, efforts to discover bioactive phytochemicals both in pure and crude forms have been intensified using the Caenorhabditis elegans aging model, in which various metabolic pathways in humans are highly conserved. In this review, we summarized the aging and longevity pathways that are common to C. elegans and humans and collated some of the bioactive phytochemicals with health benefits and lifespan extending effects that have been studied in C. elegans. This simple animal model is not only a perfect system for discovering bioactive compounds but is also a research shortcut for elucidating the amelioration mechanisms of aging risk factors and associated diseases.
Keywords: aging, bioactive compounds, Caenorhabditis elegans, longevity pathways, lifespan
1. Introduction
Various plants and their by-products have become a major area of investigation for bioactive compounds with health benefits [1,2]. These bioactive phytochemicals are chemical components (mostly secondary metabolites) that are present in relatively smaller amounts compared to macronutrients such as carbohydrates, proteins, and lipids. Depending on the specific application and benefits, these phytochemicals are classified into various categories such as medicinal, functional foods, nutraceuticals, and botanicals, some of which are closely related or almost convey the same meaning. These compounds function in other processes that are vital to plant survival, such as protection and adaptation, due to their inability to escape several potential categories of ecological and environmental (biotic and abiotic) damaging stresses. However, their benefits extend to humans and other animals that have taken advantage of these properties by sourcing such valuable components from plants for other benefits beyond the basic macronutrients that are essential for life. Most of the bioactive substances that have been discovered from food sources so far are mostly of plant origin and are consumed as fruits, legumes, vegetables, spices, and medicinal herbs [2,3]. These products are available in diverse forms, such as in fresh, raw, or processed forms. Beyond their nutritional values, these food sources contain bioactive compounds that exert anti-microbial, anti-viral, anti-carcinogenic, anti-inflammatory, antioxidants, neuroprotective, and anti-aging effects [2]. Recent research in this field has sought to discover new natural bioactive compounds in food forms that have anti-aging and lifespan extending benefits, examples of which include Silymarin and 6-shogaol [1,3,4,5,6,7].
Aging in humans is closely associated with diverse pathological changes, including cancer, cardiovascular disorders, metabolic diseases such as type II diabetes, and neurodegenerative diseases such as Alzheimer’s disease [1,2]. Conserved in all living forms, aging is a degenerative process that is characterized by a progressive deterioration of cellular components and functions, which in most cases, inevitably leads to mortality [3,4]. In developed countries, aging accounts for 90% of deaths, with about 100,000 cases per day, which makes up approximately two-thirds of deaths globally [5]. The United Nations projects that by 2050, the proportion of the global populace older that is older than 60 years of age will be closely doubled from 962 million to 2.1 billion [6]. Therefore, it is critical to gain an understanding of the molecular mechanism of the aging process along with the search for therapeutic interventions that are capable of extending lifespan and improving health span.
There are nine widely acknowledged hallmarks of aging that feature all of the major alterations of the key biological functions: loss of proteostasis, genomic instability, telomere attrition, mitochondrial dysfunction, epigenetic alterations, cellular senescence, deregulated nutrient-sensing, stem cell exhaustion, and altered intercellular communication [7]. In particular, the proteostasis network, which consists of protein synthesis, folding, secretion, trafficking, disaggregation, and degradation [7,8], is an integral part of the biological quality control systems that ensure cellular homeostasis for the survival and propagation of the organism. During aging, the decline of the proteostasis network contributes to the development of proteotoxicity related disorders such as Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and Amyotrophic lateral sclerosis [1,9]. There are two prominent concepts or theories as to how adverse changes occur in aging at the molecular level [10]. The programmed theory proposes aging as a genetically programmed chronic process, whereas the damage theory puts emphasis on the gradual and cumulative damage to cells and organs derived from internal and external factors [11]. The damage theory largely stems from the free radical theory that primarily focuses on the generation of reactive oxygen species (ROS) as metabolic by-products and their accumulative damage to biomolecules [10,11,12,13] such as DNA/RNA, proteins, and lipids. The antidotes to this fundamental challenge are the antioxidants that provide a counter mechanism to keep the balance of ROS production in check. On one hand, it secures all of the essential ROS-dependent reactions, and on the other, it removes excessive ROS and prevents the undesirable species from being generated, thereby delaying aging-associated changes and increasing longevity [4,11,12,14]. Meanwhile, the search for bioactive compounds (from natural sources as well as synthetic) possessing antioxidant properties has been greatly intensified, which has so far brought to light several candidates with interesting potential in enhancing lifespan and health span.
Many critical aspects of the human aging process have been studied in model organisms, providing us great insight into the individual elements and more importantly the operating and underlying regulatory mechanisms. Amongst these organisms, the Caenorhabditis elegans model has been greatly employed for the discovery of longevity pathways as well as new anti-aging compounds with lifespan extending properties [15,16,17,18]. Here, we briefly introduce C. elegans as an important aging model, highlighting the prominent pathways or factors that are involved in aging process, and discussed the recent discovery of lifespan-extending compounds, plant sources as well as their anti-aging activities in the C. elegans system.
2. C. elegans as a Model for Aging Research
Model organisms have become a crucial part of biomedical research, tackling fundamental biological and medical questions that would otherwise be impossible to study in humans due to the cost, system complexity, and ethical issues [19,20,21]. Any biological system in which aging occurs has the potential of being a model for studying aging. However, the choice of the model is largely dependent on the specific questions to be answered as well as the amenability of the model. C. elegans, a multicellular organism sharing 60–80% similarity with humans at the genomic level, has emerged as an outstanding model for aging research [22,23]. Featured by a highly conserved aging signaling network, C. elegans has a relatively short life cycle and low maintenance and propagation costs [24,25]. More so, sophisticated genetic techniques and manipulations, such as RNAi, CRISPR-cas9, and the auxin-inducible degradation (AID) system, are all applicable to C. elegans for transgenesis as well as forward and reverse genetic screening [26]. The transparent body of C. elegans also serves an ideal system for real-time live imaging of fluorescence-tagged proteins in the whole animal [27,28,29]. Despite the abounding advantages with using C. elegans in aging research, it is not without limitations. On the downside, the advantages that are associated with the small size of this organism, such as ease of handling, can also be a substantial disadvantage, as obtaining a large amount of the same generation is limiting as well as labor intensive. Likewise, in spite of the >60% genetic conservation with humans, C. elegans is indeed a lower invertebrate and is significantly distant from mammals evolutionarily, biochemically, and physiologically [30,31,32].
A series of pioneering works established the biochemical pathways that are associated with aging and longevity in C. elegans, which appear to be conserved through evolution [31,32,33,34,35,36]. Klass isolated eight mutant strains with a remarkable increase in lifespan and correlated this phenomenon with the restriction of caloric intake [33]. Further studies led to the identification of age-1, the first gene linked to lifespan extension [37,38]. Similarly, mutations in daf-2 exhibited more than a double-fold increase in the lifespan through regulating the activity of daf-16 [36]. These discoveries set up the foundation for using the C. elegans model to understand the aging process and to seek opportunities for lifespan extension. More work was inspired, collectively leading to the appreciation of the complex network underpinning the aging process. Meanwhile, this model has also been explored in other dimensions, ranging from assessing the environmental factors for aging (hermetic treatments and caloric restriction), studying the age-related diseases, population and evolutionary studies, and screening of drugs with potential lifespan-extending properties [13,39,40,41,42].
3. Signaling Pathways and Environmental Factors Related to Aging
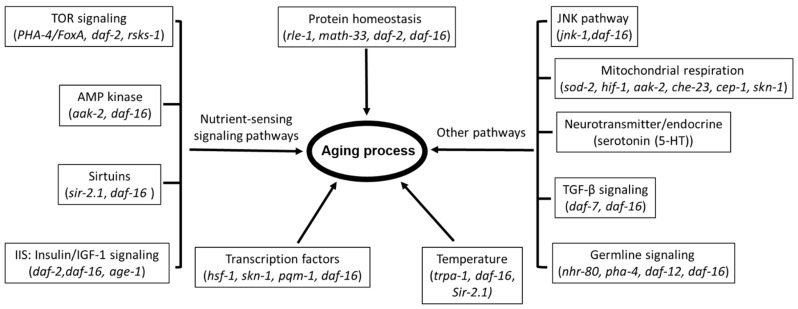
Over 70 genes have been implicated in the pathways regulating the lifespan of C. elegans [38], with a high likelihood of further expansion on the current number. These genes are involved in the nutrient-sensing signaling pathways, including Target of Rapamycin (mTOR) signaling, AMP-activated protein kinase (AMPK)-dependent signaling, sirtuins, and insulin/IGF-1 signaling (IIS). Other implicated pathways include the JNK pathway, TGF-βsignaling, germline signaling, and mitochondrial respiration as well as other factors leading to aging process such as protein homeostasis, temperature, transcription factors, and so on. The representative genes that are involved in those pathways are listed in Figure 1.
Figure 1.
The known signaling pathways and key factors linked to aging process.
Currently, daf-16 is the most central aging related gene and is the downstream target of some of the pathways, most prominently, the IIS pathway. Its activity involves direct interaction with other genes, modulating the nuclear translocation or acting as a transcription factor to numerous target genes for lifespan regulation and stress resistance [43,44,45,46]. Not surprisingly, the pathways or factors that are involved in the aging process interact with each other to mediate lifespan extension.
4. Bioactive Phytochemicals with Health Benefits
Derived from natural sources such as plants, animals, and microorganisms, bioactive compounds exhibit a highly diverse chemical nature. They are capable of interacting with biochemical systems, thereby conferring a wide spectrum of health benefits such as anti-inflammatory, anti-aging, anti-hypertensive, anti-cancer, anti-diabetic, and anti-neurodegenerative properties [47,48,49,50,51,52,53]. Of all of the bioactive compounds, the phytochemicals from plants are the most prominent and have gained more attention since increasing numbers of lifespan-extending candidates have been discovered from phenotypic screening in C. elegans [54]. Reverse genetic screening along with structural studies has elucidated the targets of some of these compounds and the related pathways that are involved. Phytochemical/secondary metabolites can be found in vegetables, fruits, cereals, and inedible plants, and many of them have been identified to possess antioxidant properties [55]. They are chemically categorized into polyphenols, terpenoids, alkaloids, saponins, phytosterols, and organosulfur compounds [55,56,57,58,59]. Here we focused on the application of the most abundant and most chemically diverse phytochemicals (polyphenols, terpenoids, and alkaloids) to aging studies and further highlighted the use of crude extract in aging studies in the subsequent section.
4.1. Polyphenolic Compounds
The polyphenolic compounds include flavonoids, tannins, stilbenes, coumarins, lignans, and other phenolic compounds (Table 1) that are mainly involved in curbing oxidative stress and related conditions by providing their reducing power to protect essential cellular components from detrimental oxidative damage [60]. Among the members of this class, the flavonoids are the most abundant group, with over 8000 compounds having been identified [61]. Flavonoids contain a basic flavan nucleus with 15 carbon atoms that are grouped into C6-C3-C6 skeleton that comprises two aromatic C6 rings and a heterocyclic ring with one oxygen atom [62]. The presence of a highly reactive hydroxyl group enables flavonoids to donate the hydrogen atom, thereby reducing highly oxidizing free radicals [62]. The main targeted pathway linked to the anti-aging efficacy by this class of compounds is IIS. For example, Tambulin, as a hydroxy substituted flavanol, enhances stress tolerance and longevity and mitigates the manifestation of Parkinson-like symptoms in C. elegans model, during which the IIS pathway is up-regulated by the expressions of daf-16, sod-1, sod-3, and ctl-2 [22]. Rosmarinic acid (RA) as a natural polyphenol, has been shown to improve the mean lifespan of C. elegans [63] by up-regulating the IIS pathway via ins-18 and daf-16; the MAPK pathway via skn-1 and sek-1; and the stress resistance and antioxidant genes such as ctl-1, sod-3 and sod-5. The lifespan of the worms can also be extended by curcumin [64], which depends on the functions of age-1, skn-1, sir-2.1, sek-1, unc-43, osr-1, and mek-1, which are related to the IIS, MAPKK, and JNK signaling pathways. Polyphenol and chlorogenic acid enhance longevity via the IIS pathway depending on daf-16, skn-1 and hsf-1 [65]. Epigallocatechin gallate and epicatechin (EC) protect and facilitate the expression of major genes of the IIS pathway to protect C. elegans from oxidative stress, thereby enhancing the longevity [66,67,68]. Furthermore, pro-longevity effects have been found in the C. elegans aging models, with a broad variety of polyphenols/flavonoids such as myricetin, resveratrol, quercetin, naringenin, kaempferol, catechin, baicalein, fisetin, caffeic acid, phenethylester, acacetin, and blueberry polyphenols, most of which require daf-16 [42,69,70,71,72,73,74,75,76,77,78,79,80].
Table 1.
Polyphenolic compounds with anti-aging and lifespan extending properties as demonstrated in the C. elegans model.
| Polyphenolic Compounds | Mean Lifespan Extension * | Plant Source # | Ethnobotanical Use | Implicated * Genes | Pathway * | Anti-Aging Effect * | References |
|---|---|---|---|---|---|---|---|
| Flavonoids | |||||||
| Tambulin | 16% at 50 µM | Zanthoxylum armatum (Indian thorny ash, or Nepali Dhania, or Chinese coriander or timur) | Medicinal | daf-16, sod-1, sod-3, ctl-2 | IIS | Anti-Parkinson’s | [22] |
| Rosmarinic acid | 40% at 60 µM 49% at 120 µM 63% at 180 µM |
Rosmarinus officinalis (Rosemary) | Medicinal, Ornamental, Culinary, Source of essential oil |
ins-18, daf-16, sek-1, skn-1, ctl-1, sod-3, sod-5. | IIS and MAPK |
Anti-oxidative, Healthspan extension |
[63] |
| Curcumin | 39% at 20 µΜ | Curcuma longa (Turmeric) | Medicinal, Culinary |
age-1, skn-1, sir-2.1, sek-1, unc-43, osr-1, mek-1genes | IIS, MAPK and JNK | Anti-oxidative, Healthspan extension |
[64] |
| Chlorogenic acid | 20% at 50 µM |
Coffea arabica (Coffee), Camellia sinensis (Tea) |
Medicinal, Beverage |
daf-16, skn-1 and hsf-1 | IIS | Anti-oxidative, Healthspan extension |
[65] |
| Epigallocatechin gallate (EGCG) | 10%–14% at 220 µΜ | Camellia sinensis (Green tea) | Medicinal, Beverage |
daf-16 | IIS |
Anti-oxidative, Anti-Alzheimer’s |
[66,67] |
| 20% at 100 μM | daf-16, sod-3 | IIS | [81] | ||||
| Myricetin | 32% at 100 µM |
Abelmoschus moschatus (musk mallow), Citrus sinensis (Navel oranges), Vaccinium sect. Cyanococcus (Blueberry leaves) |
Medicinal, Food |
daf-16 | IIS | Anti-oxidative | [69] |
| 18% at 100 µM | daf-16, sod-3 | IIS | [74] | ||||
| Quercetin | 15% at 100 µM |
Allium cepa L. (Onions), Malus domestica (Apples), Brassica oleracea (Broccoli), Vitis vinifera (Grape) |
Functional food, Culinary |
daf-16 | IIS | Anti-oxidative, Healthspan extension |
[73] |
| 5% at 100 µM | daf-16 | IIS | [74] | ||||
| 11% at 100 µM 18% at 200 µM |
age-1, daf-2, sek-1 and unc-43 | IIS, CaMKII and p38 MAPK |
[82] | ||||
| Kaempferol | 5% at 100 µM |
Camellia sinensis (Tea), Brassica oleracea (Broccoli), Vitis vinifera (Grape), Solanum lycopersicum (Tomato), Fragaria ananassa (Strawberries), Malus domestica (Apples) |
Beverage, Functional foods |
daf-16 | IIS | Anti-oxidative, Healthspan extension |
[74] |
| 10% at 100 µM | daf-16 | IIS | [77] | ||||
| Fisetin | 6% at 100 µM |
Fragaria ananassa (Strawberries), Malus domestica (Apples), Diospyros kaki (Persimmons), Allium cepa L. (Onions), Cucumis sativus (Cucumbers) |
Functional foods | daf-16 | IIS | Anti-oxidative | [77] |
| Catechin | 8% at 200 µM |
Camellia sinensis (Green tea), Theobroma cacao (Cocoa), Vitis vinifera (Grape), Malus domestica (Apples) |
Medicinal, Functional foods |
akt-2, mev-1, nhr-8 | IIS | Anti-oxidative, Healthspan extension |
[75] |
| Epicatechin (EC) | 15% at 100 µM |
Camellia sinensis (Tea), Theobroma cacao (Cocoa), Vitis vinifera (Grape red wine) |
Functional foods, Beverage |
daf-16, sod-3 | IIS | Anti-oxidative, Healthspan extension |
[81] |
| 47% at 200 µM | daf-2, age-1, akt-1, akt-2, sgk-1, daf-16, skn-1, hsf-1, gst-4, gst-7, hsp-16.2 and hsp-70 | IIS | [68] | ||||
| 3′-O-methylepicatechin | 6% at 200 µM |
Malus domestica (Apples), Vitis vinifera (Grape), Theobroma cacao (Cocoa), Camellia sinensis (Tea) |
Functional foods, Beverage |
- | - | Anti-oxidative, Healthspan extension |
[83] |
| 4′-O-methylepicatechin | 12% at 200 µM | - | - | [83] | |||
| Baicalein | 45% at 100 µM | Scutellaria baicalensis (Baikal skullcap or Chinese skullcap) | Medicinal | skn-1 | IIS | Anti-oxidative | [76] |
| 36% at 0.1% w/v | cbp-1 | - | [84] | ||||
| Caffeic acid | 11% at 300 µM | Coffea arabica (Coffee) | Beverage, Medicinal |
osr-1, sek-1, sir-2.1, unc-43, and daf-16 | Anti-oxidative, | [85] | |
| Acacetin (5,7-dihydroxy-4-methoxyflavone) |
27% at 25 µM | Tephroseris kirilowii (Dog Tongue grass, Cotton bat) | Medicinal | sod-3, gst-4, ctl-1 and hsp-16.2 | IIS | Anti-oxidative, Stress Resistance |
[86] |
| Acacetin 7-O-α-l-rhamnopyranosyl(1–2)β-D-xylopyranoside | 39% at 25 µM | Premna integrifolia (Wind killer) | Medicinal | sir-2.1, skn-1, daf-16, and hsf-1 | Anti-oxidative, Stress Resistance |
[87] | |
| Quercetin-3-O-dirhamnoside | 21% at 200 µM | Curcuma longa (turmeric) | Culinary, Medicinal |
- | - | Anti-oxidative, Stress Resistance |
[39] |
| Quercetin-3-O-glucoside | 23% at 25 µM | Erica multiflora (Winter Heather) | Medicinal | - | - | Anti-oxidative | [88] |
| Isorhamnetin (Quercetin 3′-O-methylether) | 16% at 200 μM |
Ginkgo biloba (ginkgo or gingko also known as the maidenhair tree), Hippophae rhamnoides (Sea buckthorn), Vaccinium sect. Cyanococcus (Blueberry) |
Medicinal, Food |
- | - | Anti-oxidative, Stress Resistance |
[89] |
| Tamarixetin (Quercetin 4′-O-methylether) |
11% at 200 μM | Cyperus teneriffae (Coco-grass) | Medicinal | - | - | Stress resistance, Anti-oxidative |
[89] |
| Icariin | 20% at 45 μM | Herba epimedii (Horny Goat Weed) | Medicinal | daf-2, daf-16, hsf-1 | IIS | Anti-oxidative, Anti-neurodegenerative diseases |
[90] |
| Icariside II | 20% at 20 μM | Herba epimedii (Horny Goat Weed) | Medicinal | daf-2, daf-16, hsf-1 | IIS | Anti-oxidative, Stress resistance, Anti-neurodegenerative diseases |
[90] |
| Isoxanthohumol | 2% at 100 μM | Humulus lupulus (hops) | Medicinal Beverage |
daf-16 | IIS | Anti-oxidative, Stress resistance |
[91] |
| Silymarin | 10% at 25 µM 24% at 50 µM |
Silybum marianum (Milk thistle) | Medicinal | - | - | Anti-oxidative, Stress resistance, Anti- Alzheimer’s |
[1] |
| Genistein | 27% at 100 µM | Vigna angularis (adzuki bean) | Medicinal, Food |
hsp 16.2, sod-3 | Healthspan extension, Stress resistance |
[92] | |
| Taxifolin | 51% at 820 µM |
Silybum marianum (blessed thistle or milk thistle), Carduus marianus (Marian thistle or Our-Lady’s-thistle), Allium cepa L. (Onions) |
Medicinal, Culinary |
- | - | Stress Resistance, Ameliorates Cerebral Amyloid Angiopathy (ACAA) |
[93] |
| Trolox (6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
31% at 0.6 mM–3 mM | Fragaria ananassa (Strawberries) | Functional food | - | - | Anti-oxidative | [93] |
| Chicoric Acid | 20% at 100µM |
Cichorium intybus (Chicory), Echinacea angustifolia (Purple cone flower), Lactuca sativa (Lettuce), Ocimum basilicum (Basil) |
Medicinal, Culinary |
- | AMPK | Anti-oxidative | [94] |
| Naringin | 23% at 50 µM | Citrus grandis (Pomelo), Citrus paradise (grapefruit), and Citrus aurantium (Bitter orange) | Functional food |
daf-16, daf-2, akt-1, akt-2. eat-2, sir-2.1, rsks-1, and clk-1 |
IIS | Anti-oxidative, Anti-Alzheimer’s, Anti-Parkinson’s |
[35] |
| Tannins | |||||||
| Tannic acid | 18% at 100 μM |
Camellia sinensis (Tea), Vitis vinifera (Grape), Arachis hypogaea (Pea nuts) |
Functional food | sek-1 | MAPK | Anti-oxidative, Anti-Alzheimers’, Neuroprotective Others Anti-amyloidogenic, Antimicrobial, Anticancer, Antimutagenic |
[95] |
| 24% at 0.01% w/v | daf-16 | IIS | [84] | ||||
| Pentagalloyl Glucose | 18% at 160 μM | Eucalyptus leaves (Southern blue gum or blue gum) |
daf-16, age-1, eat-2, sir-2.1, and isp-1 |
IIS, DR, SIR-2.1 and METC. | Anti-oxidative Others Estrogenic, Anti-inflammatory, Anti-oxidative, Anticancer |
[53] | |
| Stilbene | |||||||
| Resveratrol | Variable effects at 100 μM |
Vitis vinifera (Grape), Peanuts, Theobroma cacao (Cocoa), Vaccinium sect. Cyanococcus (Blueberry), Vaccinium myrtillus (Bilberry), Vaccinium macrocarpon (cranberry) |
Functional food | sir 2.1 | - | Anti-oxidative Others Antiviral, Anti-depressant, Anti-nociceptive, Anti-diabetic activities |
[70] |
| 3% at 5 μM | - | - | [71] | ||||
| 11% at 100 µg/mL | - | [72] | |||||
| OxyResveratrol | 31% at 1000 µM | Morus alba (white mulberry) | Functional food, Medicine |
sir-2.1, aak | AMPK and SIR-2.1 | Anti-oxidative, Neuroprotective |
[96] |
| TSG (2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside) | 23% at 100 μM | Polygonum multiflorum (Tuber fleeceflower) | Medicinal | - | - | Anti-oxidative, Stress resistance |
[97] |
| Polydatin | 30% at 1 mM | Vitis vinifera (Grape) | Functional food | sir-2.1, skn-1, sod-3, and daf-16 | IIS | Anti-oxidative, Stress resistance, Neuroprotective |
[98] |
| Piceatannol | 18% at 50 and 100 µM |
Passiflora edulis (Passion fruit), Camellia sinensis (White Tea), Vitis vinifera (Grape) |
Functional food | daf-16, hsp 16.2, sod-3, sir-2.1 | IIS | Anti-oxidative Others Estrogenic, Anti-inflammatory, Anti-oxidative, Anticancer |
[99] |
| Coumarins | |||||||
| ^ Urolithin A (UA) | 45% at 50 µM | Vaccinium sect. Cyanococcus (Blueberry), Fragaria ananassa (Strawberries), Arachis hypogaea (Pea nuts), Quercus spp (acorns), Punica granatum (pomegranates), Juglans regia (Walnut), Rubus idaeus (raspberries) | Functional food | - | - | Anti-oxidative, Healthspan extension |
[100] |
| ^ Urolithin B (UB) | 36% at 50 µM | Vaccinium sect. Cyanococcus (Blueberry), Fragaria ananassa (Strawberries), Arachis hypogaea (Pea nuts), Quercus spp (acorns), Punica granatum (pomegranates), Juglans regia (Walnut), Rubus idaeus (raspberries) | Functional food | - | - | Anti-oxidative, Healthspan extension |
[100] |
| ^ Urolithin C (UC) | 36% at 50 µM | Vaccinium sect. Cyanococcus (Blueberry), Fragaria ananassa (Strawberries), Arachis hypogaea (Pea nuts), Quercus spp (acorns), Punica granatum (pomegranates), Juglans regia (Walnut), Rubus idaeus (raspberries) | Functional food |
- | - | Anti-oxidative, Healthspan extension |
[100] |
| ^ Urolithin D (UD) | 19% at 50 µM | Vaccinium sect. Cyanococcus (Blueberry), Fragaria ananassa (Strawberries), Arachis hypogaea (Pea nuts), Quercus spp (acorns), Punica granatum (pomegranates), Juglans regia (Walnut), Rubus idaeus (raspberries) | Functional food | - | - | Anti-oxidative, Healthspan extension |
[100] |
| Lignan | |||||||
| Sesamin | 13% at 6.3 µg/plate | Sesamum indicum L. (Sesame Seeds) | Functional Food | daf-2, skn-1, pmk-1, and daf-16 | IIS | Anti-oxidative Others Anti-allergenic, Anti-carcinogenic, Antihypertensive, Hypocholesterolemic |
[101] |
| Vitexin | 17% at 100 mM | Vigna angularis (adzuki beans) | Functional Food | sod-3, hsp-16.2 | IIS | Anti-oxidative Others Antiviral, Anti-depressant, Anti-nociceptive, Anti-diabetic |
[13] |
| Arctigenin | 13% at 100 µM | Arctium lappa (Greater burdock) | Medicinal | daf-16, jnk-1 | IIS | Anti-oxidative, Stress resistance |
[102] |
| Matairesinol | 25% at 100 µM | Arctium lappa (Greater burdock) | Medicinal | daf-16, jnk-1 | IIS | Anti-oxidative, Stress resistance |
[102] |
| Arctiin | 15% at 100 µM | Arctium lappa (Greater burdock) | Medicinal | daf-16, jnk-1 | IIS | Anti-oxidative, Stress resistance |
[102] |
| Lappaol C | 11% at 100 µM | Arctium lappa (Greater burdock) | Medicinal | daf-16, jnk-1 | IIS | Anti-oxidative, Stress resistance |
[102] |
| Lappaol F | 12% at 100 µM | Arctium lappa (Greater burdock) | Medicinal | daf-16, jnk-1 | IIS | Anti-oxidative, Stress resistance |
[102] |
| (Iso) lappaol A | 11% at 100 µM | Arctium lappa (Greater burdock) | Medicinal | daf-16, jnk-1 | IIS | Anti-oxidative, Stress resistance |
[102] |
| Phenolic Compounds | |||||||
| Tryosol | 21% at 250 μM | Olea Europea L. (Olive tree) | Medicinal, Food |
hsf-1, daf-2, daf-16 | IIS | Anti-oxidative, Stress resistance |
[103] |
| 6-Gingerol | 20% at 25 µM | Zingiber officinale (Ginger) | Culinary, Medicinal |
hsp-16.2, sod-3 | Anti-oxidative, Stress resistance |
[5] | |
| 6-Shogaol | 19% at 12.5 µM 25% at 25 µM |
Zingiber officinale (Ginger) | Culinary, Medicinal |
sod-3, hsp-16.2 | Anti-oxidative, Stress resistance |
[5] | |
| Salicyclic Acid | 14% at 1 mM |
Rubus idaeus (raspberries), Salix alba L. (Willow tree) |
Medicinal, Food |
daf-16, sod-3, sod-5, ctl-2, gst-4, gst-10 | IIS | Anti-oxidative, Stress resistance |
[104] |
| Salicylamine | 32% at 100 µM 56% at 500 µM |
Fagopyrum esculentum (Buckwheat) | Culinary | sir-2.1, ets-7 | - | Healthspan extension | [105] |
| Juglone | 29% at 40 µM | Juglans nigra (Black Walnut) | Functional Food, Medicinal |
daf-16, sod-3, hsp-16.2, sir-2.1 | IIS | Anti-oxidative, Stress resistance |
[106] |
| Gallic Acid | 25% at 300 μM |
Punica granatum (Pomegranate), Aspalathus linearis (Rooibos tea), Vitis vinifera (Grape), Raphanus sativus (Black radish), Allium cepa L. (Onions) |
Functional food | - | - | Stress resistance | [17] |
| Ferulic Acid | 9.58% at 500 µM | Beta vulgaris (Beet root), Oryza sativa (Rice), Glycine max (Soyabean), Daucus carota (Carrot), Avena sativa (Oats) | Functional food | daf-2, daf-16, hlh-30, skn-1, and hsf-1 | IIS | Healthspan extension, Stress resistance, Anti- Huntington’s disease |
[107] |
* Mean lifespan and implicated genes, pathways, and anti-aging effects in the table are the specific outcomes from the references indexed directly against the compound at the right-end column. A dash (-) sign in the column for implicated genes and pathways represents where the authors did not proceed to investigate the molecular mechanism beyond the effect observed. Where different mean lifespans are not from the same study, we have referenced the appropriate literature for such. Original units of concentration used by the investigators are reported here. Additionally, where other health benefits besides the anti-aging effect have been noted for the compound in the primary research referenced, we grouped them under the heading “Others” in the column for “Anti-aging-effect”. # Plant sources of the compound in the study indexed to the reference directly at the right-end column are listed. Other sources that have been mentioned by the primary study, and previous studies have also been listed as well. Scientific names and common names (in parenthesis) of the plant sources are provided. ^ Compounds not directly obtained from the plant sources indicated but that are produced by gut microflora from foods rich in ellagitannins such as the plants listed.
4.2. Terpenoids
Terpenoids are the most abundant and structurally diverse phytochemicals. Also known as terpenes, they can be classified based on the number of isoprene units (C5H8)n into various subgroups, including hemiterpenes (C5H8), monoterpenes (C10H16), sesquiterpenes (C15H24), diterpenes (C20H32), sesterterpenes (C25H40), triterpenes (C30H48), tetraterpenes (C40H64), and polyterpenes (C5H8)n. Terpenoids exhibit a broad range of pharmacological activities, ranging from anti-malarial, anti-cancer, anti-inflammatory, anti-bacterial, anti-viral, and anti-aging to anti-neurodegeneration [108]. Some of the notable terpenoids are the chemotherapy medication Taxol® (paclitaxel) and the frontline antimalarial drug Artemisinin [108].
Isoprenol is a hemiterpene-based unsaturated C5 alcohol that confers lifespan extension and stress tolerance in C. elegans via daf-16 and skn-1 through the IIS pathway [23]. Moreover, the expressions of SOD-3 and GST-4 can be boosted by isoprenol through the translocation of DAF-16 from the cytosol into the nucleus [22]. Carnosic acid relies on the MAPK and HSF-1 pathway to up-regulate sod-5, hsp-16.2, hsp-16.1, sek-1, and skn-1 [109]. Carnosol, as a phenolic diterpene, increases the mean lifespan of C. elegans through the HSF-1 signaling pathway to up-regulate sod-3 and sod-5 as antioxidants and hsp-16.1 and hsp-16.2 for heat shock response [110]. Beta-caryophyllene (BCP), a naturally occurring bicyclic sesquiterpene, is capable of extending lifespan as well as of increasing the resistance to oxidative stress by inducing the dietary restriction response and xenobiotic stress response [111]. The compound 4-Hydroxy-E-globularinin, an iridoid isolated from Premma integrifolia, exhibits detoxification activity against ROS and up-regulates hsp-16.2 and sod-3 through daf-16 in the IIS pathway [112]. A similar effect was found with oleanolic acid, where sod-3, hsp-16.2, and ctl-1 were up-regulated via daf-16 [113]. However, α-Tocopherol, either in free form or encapsulated with SDNF (soluble dietary fiber-based nanofibers), may provide the longevity benefits via different routes [114]. Lifespan extension potentials have also been ascribed to additional high molecular weight isoprenoid-based compounds such as withanolide-A, specioside, ursolic acid, and glycyrrhetinic acid [60,115,116,117]. Examples of these terpenoids with longevity-modulating effects are summarized in Table 2.
Table 2.
Terpenoids with anti-aging and lifespan extending properties as demonstrated in the C. elegans model.
| Terpenoids | Mean Lifespan Extension * | Plant Source # | Ethnobotanical Use | Implicated * Genes | Pathway * | Anti-Aging Effect * | References |
|---|---|---|---|---|---|---|---|
| Carnosic Acid | 3% at 60 µM 8% at 120 µM 16% at 180 µM |
Rosmarinus officinalis L (Rosemary) | Food, Medicinal |
sod-5, hsp-16.2, hsp-16.1, sek-1, skn-1 | MAPK and HSF-1 | Anti-oxidant, Anti-inflammatory, Antibacterial, Anti-cancer, Neuroprotective |
[109] |
| Carnosol | 19% at 180 µM | Rosmarinus officinalis L (Rosemary) | Medicinal, Culinary |
sod-3, sod-5, hsp-16.1, hsp-16.2, hsf-1, daf-16. | IIS | Antioxidant, Anticancer, Antimicrobial, Anti-inflammatory |
[110] |
| Beta-Caryophyllene | >22% at 50 μM |
Syzygium aromaticum (Clove), Cannabis sativa (hemp), Rosmarinus officinalis L (Rosemary, Humulus lupulus (Hops) |
Culinary, Beverages |
pha-4, sir-2.1, hsf-1, skn-1, daf-16, gst-4, gst-7, hsp-70, sod-2, sod-3 and daf-9 | IIS | Anti-oxidant, Anti-inflammatory, anti-biotic, Anti-carcinogenic, local anesthetic |
[111] |
| 4-Hydroxy-E-globularinin | 18% at 20 μM | Premna integrifolia (Wind killer) | Medicinal | daf-16, hsp-16.2, sod-3 | IIS | Anti-oxidant | [112] |
| 10-O-trans-p-Coumaroylcatalpol | 17% at 20 μM | Premna integrifolia (Wind killer) | Medicinal | daf-16 | IIS | Anti-oxidant, Anti-parkinson’s disease |
[118] |
| Oleanolic acid | 16% at 300 μM | Constituent of the leaves and roots of more than 120 plant species such as Olea europaea (olive tree), Viscum album (European mistletoe or common mistletoe), Aralia chinensis (Chinese Angelica Tree) |
Food, Medicinal |
sod-3, hsp-16.2, ctl-1, daf-16 | IIS | Anti-oxidant, Hepatoprotective, Hypoglycemic, Anti-inflammatory |
[113] |
| α-Tocopherol | 7% at 50 µg/mL 15% at 100 µg/mL 17% at 200 µg/mL |
Sunflower seeds (Helianthus annuus), Prunus dulcis (Almonds), Corylus avellana L. (Hazelnuts), Arachis hypogaea (Pea nuts), Spinacia oleracea (Spinach), Brassica oleracea var. italica (Broccoli), Actinidia deliciosa (Kiwifruit), Mangifera indica (Mango) |
Functional food | - | - | Anti-oxidant | [114] |
| Withanolide-A | 29% at 5 μM | Withania Somnifera (Ashwagandha) | Medicinal | sgk-1, daf-16, sod-3, skn-1, hsf-1, gst-4, hsp-16.2 | IIS | Neuroprotective, Stress resistance |
[115] |
| Specioside | 15% at 25 μM | Stereospermum suaveolens (Patala) | Medicinal | sod-1, sod-2, sod-3, gst-4, gst-7, hsp-16.2, hsp-70, clt-1 | IIS | Antioxidant, Stress resistance |
[87] |
| Ursolic acid | 32% at 25 μM |
Malus domestica (Apple peels), , rosemary, Lavandula angustifolia (lavender), Mentha piperita (Peppermint), Thymus vulgaris (thyme), Ocimum basilicum (Basil), Vaccinium myrtillus (Bilberry) |
Medicinal, Culinary |
jnk-1, jkk-1 | JNK-1 | Antioxidant, Stress resistance |
[117] |
| 18α-Glycyrrhetinic acid | 17% at 20 μg/mL | Glycyrrhiza glabra (Licorice) | Medicinal, Culinary |
skn-1, daf-16 | p38 MAPK | Neuroprotective | [59] |
| Glaucarubinone | 1.9 days at 100 nM | Simaroubaceae spp. (Amargo, Bitterwood, Marupa, or Quassia) | Ornamental, Medicinal |
- | - | Anti-oxidant Others Antimalarial |
[71] |
| Fucoxanthin | 14% at 5 μM |
Undaria pinnatifida (Wakame), Hijikia fusiformis (Hijiki) |
Medicinal | - | - | Antioxidant, Stress resistance |
[119] |
| Catalpol | 28% at 25 μM | Rehmannia glutinosa (Chinese foxglove) | Medicinal plant | mek-1, daf-2, age-1, daf-16, and skn-1 | IIS | Anti-oxidant, Anti- Alzheimer’s, Anti-Parkinson’s, Anti-stroke Others Anticancer, Anti-diabetes |
[120] |
| Ferulsinaic acid | 20% at 100 µM | Ferula communis (Giant Fennel) | Medicinal, Culinary |
- | - | Anti-oxidant | [121] |
| Verminoside | 20% at 25 µM | Stereospermum suaveolens (Patala) | Medicinal | daf-16 | - | Antioxidant, Stress resistance |
[116] |
| Dehydroabietic acid | 15% at 10 µM |
Pinus densiflora (Japanese red pine), Pinus sylvestris (Scots pine), Abies grandis(Grand fir) |
Medicinal | sir-2.1 | - | Healthspan extension | [122] |
| Secoisolariciresinol Diglucoside | 22% at 500 µM |
Linum usitatissimum (Flaxseed) |
Food, Medicine | daf-16, hsf-1, nhr-80, daf-12, glp-1, eat-2, and aak-2. | IIS | Anti-oxidant, Anti-Alzheimer’s, Anti-Parkinson’s |
[123] |
* Mean lifespan and implicated genes, pathways, and anti-aging effects in the table are the specific outcomes from the references indexed directly against the compound at the right-end column. A dash (-) sign in the column for implicated genes and pathways represents where authors did not proceed to investigate the molecular mechanism beyond the effect observed. Where different mean lifespans are not from the same study, we have referenced the appropriate literature for such. Original units of concentration used by the investigators are reported here. Additionally, where other health benefits besides the anti-aging effect have been noted for the compound in the primary research referenced, we grouped them under the heading “Others” in the column for “Anti-aging-effect”. # Plant sources of the compound in the study indexed to the reference directly at the right-end column are listed. Other sources that have been mentioned by the primary study and previous studies have also been listed as well. Scientific names and common names (in parenthesis) of the plant sources are provided.
4.3. Alkaloids
Alkaloids represent a class of nitrogenous chemicals that are not only derived from plants but also from fungi, bacteria, and animals [124]. According to their heterocyclic ring system and biosynthetic precursor, this class of compounds are categorized into eight subgroups, including tropanes, indoles, imidazoles, piperidines, isoquinolines, pyrrolizidines, quinolozidines, and pyrrolidine alkaloids [124]. Historically, some alkaloids have been used as poisons, whereas others have been used as remedies against fever and snakebites [124,125,126]. Though a bit under the shadow of toxicity, alkaloids elicit great potential in pharmaceutical development, primarily as analgesic, antioxidant, anti-inflammatory, anti-bacterial, anti-spasmodic, anti-cancer, anti-hypertensive, and stimulants to the central nervous system [56,124,127]. Particularly, over 300 compounds of this class have been shown to possess some degree of anti-aging property [40]. Reserpine confers significant thermo tolerance and longevity benefits to the worms, which is likely independent of daf-16 and partially rely on serotonin [126]. Enhanced longevity effects are also offered by the methylxanthine alkaloid caffeine, which is able to induce the nuclear translocation of daf-16 but does not requires its activity [128]. The action of pentagalloyl glucose (a gallotannin) on lifespan requires the cooperation between four pathways, including the IIS pathway, the mitochondrial ETC, the Sir-2.1 signaling, and the dietary restriction pathway [53]. The studied alkaloids with lifespan extending potentials in C. elegans are summarized in Table 3.
Table 3.
Alkaloids with anti-aging and lifespan extending properties as demonstrated in the C. elegans model.
| Alkaloids | Mean Lifespan Extension * | Plant Source # | Ethnobotanical Use | Implicated * Genes | Pathway * | Anti-Aging Effect * | References |
|---|---|---|---|---|---|---|---|
| Reserpine | 31% at 30 μM |
Rauwolfia serpentine (Indian snakeroot), Rauwolfia vomitoria (the poison devil’s-pepper) |
Medicinal | tph-1 (serotonin) | Serotonin pathway | Anti-oxidant, Antipsychotic, Anti-hypertensive |
[126] |
| Tomatidine | 7% at 25 μM | Solanum lycopersicum (Unripe tomato fruits, leaves and stems) | Medicinal, Functional food |
skn-1 | IIS | Anti-inflammatory, Anti- tumorigenic, Lipid-lowering activities | [129] |
| Spermidine | 18% at 0.2 mM | Glycine max (soy bean), Pisum sativum (green peas), Zea mays (Maize corn) | Functional food | - | - | Enhanced autophagy | [130] |
| 15% at 0.2 mM | - | - | [131] | ||||
| Caffeine | 29% at 0.1% w/v |
Theobroma cacao (Cocoa beans), Cola acuminata (kola nuts), Camellia sinensis (Tea leaves), Coffea arabica (coffee beans) |
Beverages, Medicinal |
daf-16 | IIS | Antioxidant, Stress resistance, Neuroprotective, Anti-Alzheimer’s |
[84] |
| 16% at 10 mM | daf-16 | IIS | [3] | ||||
| 80% at 5 mM | daf-2 | IIS | [128] | ||||
| 31.9% at 5 mM | skn-1, gst-4 | IIS | [127] | ||||
| Theophylline | 25% at 5 mM |
Camellia sinensis (Tea), Coffea arabica (Coffea) |
Beverages, Medicinal |
skn-1, gst-4 | IIS | Antioxidant, Stress resistance |
[127] |
| Chlorophyll | 23% at 10 µg/mL 25% at 40 µg/mL | Spinacia oleracea (Spinach) | Food, Medicinal |
daf-16, sod-3 | IIS | Antioxidant | [132] |
| Pyrroloquinoline quinone | 33% at 0.5 mM | Actinidia deliciosa (Kiwifruit), Petroselinum crispum (Parsley), Capsicum annuum (Green bell pepper), Carica papaya (Pawpaw) | Functional food, Culinary |
daf-16,skn-1, sod-3, hsp 16.2, gst-1 and gst-10 | IIS | Antioxidant, Stress resistance |
[133] |
| Calycosin | 21% at 200 µM | Astragalus mongholicus Bunge (membranous milk-vetch) | Medicinal | daf-16, hsp-16.2, ctl-1, sod-3 | IIS | Antioxidant, Stress resistance |
[134] |
* Mean lifespan and implicated genes, pathways, and anti-aging effects in the table are the specific outcomes from the references indexed directly against the compound at the right-end column. A dash (-) sign in the column for implicated genes and pathways represents where authors did not proceed to investigate the molecular mechanism beyond the effect observed. Where different mean lifespans are not from the same study, we have referenced the appropriate literature for such. Original units of concentration used by the investigators are reported here. Additionally, where other health benefits besides the anti-aging effect have been noted for the compound in the primary research referenced, we grouped them under the heading “Others” in the column for “Anti-aging-effect”. # Plant sources of the compound in the study indexed to the reference directly at the right-end column are listed. Other sources that have been mentioned by the primary study and previous studies have also been listed as well. Scientific names and common names (in parenthesis) of the plant sources are provided.
4.4. Plants Crude Drugs and Extracts with Lifespan Extending Abilities in C. elegans
Medicinal plants are essential sources of bioactive therapeutic compounds. Therapeutic studies using pure isolated phytochemicals are commonly preceded by preliminary studies using crude plant extracts to confirm the therapeutic potentials. Several studies have reported that some plant extracts show a lifespan prolonging effect on C. elegans. For instance, the aqueous stem bark extract from Endopleura uchi was reported to alter the DAF-16/FOXO pathway and to enhance the expression of the stress response genes such as hsp-16.2 and sod-3 [135]. Through High Performance Liquid Chromatography/Ultraviolet-Visible (HPLC UV/VIS) analysis, phenolic bergenin has been proposed as the major active component, potentially paving a new path for further developing compounds with similar and even more potent effects [135]. Calycophyllum spruceanum water extract has been shown to modulate the DAF-16/FOXO pathway, and five secondary metabolites have been identified via HPLC/Mass Spectrometry (MS) analyses, including 5-hydroxy-6-methoxycoumarin-7-glucoside, cyanidin, gardenoside, taxifolin, and 5-hydroxymorin [136]. Both the leaf and fruit extract of Caesalpinia mimosoides and Eugenia uniflora possess longevity enhancing activity via the IIS pathway with sod-3, gst-4, and hsp-16.2 as targets [137,138]. Glochidion zeylanicum and Anacardium occidentale leaf extracts have also been reported to display longevity enhancing and oxidative stress resistance activities in C. elegans via the DAF-16/FOXO and SKN-1/Nrf-2 signaling pathways [139,140]. Furthermore, phytochemical analysis narrowed down on the active compounds, revealing benzoic acid, pentadecanoic acid, octadecatrienoic acid, n-hexadecanoic acid, β-caryophyllene, palmitic acid, and α-linolenic acid as prominent metabolites. Extract from Hibiscus sabdariffa L. exhibits significant lifespan extension activities alongside curbing amyloid-β toxicity in C. elegans, which is mediated by the IIS pathway through the activation of the DAF-16 and SKN-1 transcription factors [141]. An ayurvedic polyherbal extract (PHE) derived from six herbs, including Berberis aristata, Emblica officinalis, Cyperus rotundus, Terminalia chebula Cedrus deodara, and Terminalia bellirica, has been shown to enhance the expressions of daf-16, daf-2, skn-1, sod-3, and gst-4, all of which are associated with longevity and stress response [142]. As mentioned in the foregoing, although crude extracts may exert therapeutic effects, the main ingredients that are responsible are pure chemical components that serve specific effects. It is therefore important to elucidate and characterize the specific components, as this will eventually pave the way for new strategic pharmacological designs. Examples of these plant extracts with longevity-modulating effects are summarized in Table 4.
Table 4.
Plant crude extracts with anti-aging and lifespan-extending properties as demonstrated in the C. elegans model.
| Plant Source # | Mean Lifespan Extension * | Ethnobotanical Use | Implicated Genes * | Pathway * | Anti-Aging Effect * | References |
|---|---|---|---|---|---|---|
| Endopleura uchi (Uxi) | 33% at 300 µg/mL | Medicinal | daf-16, hsp-16.2 and sod-3 | IIS | Antioxidant, Stress resistance, Anti-Huntington’s disease |
[135] |
| Calycophyllum spruceanum (capirona) | 16% at 300 µg/mL | Medicinal | daf-16 | IIS | Antioxidant, Stress resistance, Healthspan extension |
[136] |
| Caesalpinia mimosoides (Pansi) | 4% at 50 µg/mL | Food vegetable | daf-16, sod-3, gst-4 | IIS | Antioxidant, Stress resistance |
[137] |
| Eugenia uniflora (Surinam cherry) | Significant increase at 500 µg/mL | Food, Medicinal |
daf-16, hsp-16.2 and sod-3 | IIS | Antioxidant, Stress resistance, Healthspan extension |
[138] |
| Anacardium occidentale (Cashew) | 20% by 50 μg/mL | Medicinal, Functional food |
daf-16, skn-1, sod-3, gst-4 | IIS | Antioxidant, Stress Resistance, Healthspan extension |
[139] |
| Glochidion zeylanicum (Umbrella Cheese) | 10% at 100 µg/mL | Medicinal, Food |
daf-16, skn-1, sod-3, gst-4 | IIS | Antioxidant, Stress Resistance, Healthspan extension |
[140] |
| Hibiscus sabdariffa L. (Roselle) | 24% at 1 mg/mL | Medicinal, Beverage, Food supplement |
daf-16, skn-1 | IIS | Antioxidant, Stress Resistance, Anti-Neurodegenerative |
[141] |
| Polyherbal extract of Berberis aristata (Indian barberry); Emblica officinalis (Indian gooseberry or amla); Cyperus rotundus (Purple Nutsedge); Terminalia chebula (gall nut); Cedrus deodara(Himalayan cedar); Terminalia bellirica (beleric myrobalan) | 16% at 0.01 µg/mL | Medicinal | daf-16, daf-2, skn-1, sod-3 and gst-4 | IIS | Antioxidant, Stress Resistance, Anti-Neurodegenerative |
[142] |
|
Betula utilis (Himalayan Silver Birch) |
35.99 % at 50 μg/mL |
Medicinal | daf-16, hsf-1, skn-1, sod-3 and gst-4. | IIS | Antioxidant, Healthspan extension |
[143] |
| Citrus sinensis (Orange extracts) | 10.5%, 18.0%, and 26.2% at 100, 200, and 400 mg/mL, respectively | Functional food | daf-16, sod-3, gst-4, sek-1, and skn-1 | IIS | Antioxidant, Healthspan extension |
[35] |
|
Cuscuta chinensis (Chinese Dodder) |
24% at 30 µg/mL | Medicinal | hsp-16.1 and hsp-12.6 | IIS | Stress Resistance, Healthspan extension |
[144] |
|
Eucommia ulmoides (Hardy Rubber Tree) |
9% at 30 µg/mL | - | - | Stress resistance | [144] |
* Mean lifespan and implicated genes, pathways, and anti-aging effects in the table are the specific outcomes from the references indexed directly against the compound at the right-end column. A dash (-) sign in the column for implicated genes and pathways represents where authors did not proceed to investigate the molecular mechanism beyond the effect observed. Where different mean lifespans are not from the same study, we have referenced the appropriate literature for such. Original units of concentration used by the investigators are reported here. Additionally, where other health benefits besides the ant-aging effect have been noted for the compound in the primary research referenced, we grouped them under the heading “Others” in the column for “Anti-aging-effect”. # Plant sources of the compound in the study indexed to the reference directly at the right-end column are listed. Other sources that have been mentioned by the primary study and previous studies have also been listed along. Scientific names and common names (in parenthesis) of the plant sources are provided.
5. Summary and Perspectives
Globally, the significant rise in the aging populace is imposing a great economic and social burden. It is necessary to conduct more research focusing on the biological process of aging, with the aim of facilitating the development of potential interventions to alleviate the adverse health impact of aging-associated medical conditions such as neurodegenerative disorders, cancer, diabetes, and cardiovascular diseases. Suitable model organisms such as C. elegans will continuously provide more unique insight into this complex process as well as in the discovery of new bioactive compounds with pharmaceutical efficacy. Plant polyphenols including flavonoids, tannins, stilbene, coumarins, lignan, and other phenolic compounds as well as terpenoids and alkaloids have been a major source of these bioactive compounds, either as single purified compound or in crude extract mixtures. The disadvantage of crude extracts is that a complex interaction between all of the components of the crude and the biological system of the model may produce inhibitory, antagonistic, or synergistic effects. This makes it impossible to know exactly which component is inducing the particular effect being observed. Despite this challenge, crude the extracts of medicinal plants such as Endopleura uchi, Calycophyllum spruceanum, Caesalpinia mimosoides, Eugenia uniflora, Glochidion zeylanicum, Anacardium occidentale, Hibiscus sabdariffa L, and an ayurvedic polyherbal extract (PHE) derived from six herbs has demonstrated efficacy in extending lifespan in C. elegans [135,136,137,138,140,141,142]. However, purified compounds are better alternatives, as they provide the advantages of elucidating the structure, targets, mechanism of action, and pathways involved as well as the modification of the compound for improved activity.
In the future, discovering novel and effective natural candidates that extend lifespan and that delay aging and related diseases still depends on advancements in state-of-the-art high-throughput screening techniques [145,146]. Combining this effort with studies in suitable model organisms such as C. elegans [147,148] will provide a better platform for understanding the aging process as well as facilitating healthy aging to improve the quality of life of the elderly population.
Author Contributions
N.O.O. and A.S.O. wrote the initial manuscript. P.O.O., E.O.O., G.L., C.J., W.F. and B.W. revised the manuscript. B.W. supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Start-up Funding of Guangxi Academy of Sciences (2017YJJ026), Collaborative Innovation Project of the Agricultural Science and Technology Innovation Program (CAAS-XTCX20190025-6), and Science and Technology Service Network Program of Chinese Academy of Sciences (KFJ-STS-QYZD-201-5-3) to B.W. and the Bagui Scholar Program Fund (2016A24) of Guangxi Zhuang Autonomous Region to C.J.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar J., Park K.-C., Awasthi A., Prasad B. Silymarin Extends Lifespan and Reduces Proteotoxicity in C. elegans Alzheimer’s Model. CNS Neurol. Disord.-Drug Targets. 2015;14:295–302. doi: 10.2174/1871527314666150116110212. [DOI] [PubMed] [Google Scholar]
- 2.Sahardi N.F.N.M., Makpol S. Ginger (Zingiber officinale Roscoe) in the Prevention of Ageing and Degenerative Diseases: Review of Current Evidence. Evid.-Based Complement. Altern. Med. 2019;2019:5054395. doi: 10.1155/2019/5054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutphin G.L., Bishop E., Yanos M.E., Moller R.M., Kaeberlein M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev. Healthspan. 2012;1:9. doi: 10.1186/2046-2395-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadowska-Bartosz I., Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed Res. Int. 2014;2014:404680. doi: 10.1155/2014/404680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee E.B., Kim J.H., Kim Y.J., Noh Y.J., Kim S.J., Hwang I.H., Kim D.K. Lifespan-extending property of 6-shogaol from Zingiber officinale Roscoe in Caenorhabditis elegans. Arch. Pharm. Res. 2018;41:743–752. doi: 10.1007/s12272-018-1052-0. [DOI] [PubMed] [Google Scholar]
- 6.United Nations Department of Economic and Social Affairs: World Population Prospects. United Nations Department of Economic and Social Affairs; New York, NY, USA: 2017. pp. 1–25. [Google Scholar]
- 7.López-otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The Hallmarks of Aging Longevity. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Li J., Huang K.X., Le W.D. Establishing a novel C. elegans model to investigate the role of autophagy in amyotrophic lateral sclerosis. Acta Pharmacol. Sin. 2013;34:644–650. doi: 10.1038/aps.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung A. Damage-Based Theories of Aging and Future Treatment Schemes. Int. J. Sci. Eng. Res. 2011;2:1–4. [Google Scholar]
- 11.Gems D. An integrated theory of ageing in the nematode Caenorhabditis elegans. J. Anat. 2000;197:521–528. doi: 10.1046/j.1469-7580.2000.19740521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Back P., Braeckman B.P., Matthijssens F. ROS in aging Caenorhabditis elegans: Damage or signaling? Oxid. Med. Cell. Longev. 2012;2012:608478. doi: 10.1155/2012/608478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee E.B., Kim J.H., Cha Y.S., Kim M., Song S.B., Cha D.S., Jeon H., Eun J.S., Han S., Kim D.K. Lifespan extending and stress resistant properties of vitexin from Vigna angularis in Caenorhabditis elegans. Biomol. Ther. 2015;23:582–589. doi: 10.4062/biomolther.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins J.J., Evason K., Kornfeld K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Exp. Gerontol. 2006;41:1032–1039. doi: 10.1016/j.exger.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Davies S.K., Bundy J.G., Leroi A.M. Metabolic youth in middle age: Predicting aging in Caenorhabditis elegans using metabolomics. J. Proteome Res. 2015;14:4603–4609. doi: 10.1021/acs.jproteome.5b00442. [DOI] [PubMed] [Google Scholar]
- 16.Jones L.M., Staffa K., Perally S., LaCourse E.J., Brophy P.M., Hamilton J.V. Proteomic analyses of Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates a shared detoxification system in longevity assurance. J. Proteome Res. 2010;9:2871–2881. doi: 10.1021/pr9009639. [DOI] [PubMed] [Google Scholar]
- 17.Saul N., Pietsch K., Stürzenbaum S.R., Menzel R., Steinberg C.E.W. Diversity of polyphenol action in Caenorhabditis elegans: Between toxicity and longevity. J. Nat. Prod. 2011;74:1713–1720. doi: 10.1021/np200011a. [DOI] [PubMed] [Google Scholar]
- 18.Taira N., Nguyen B.C.Q., Be Tu P.T., Tawata S. Effect of Okinawa Propolis on PAK1 Activity, Caenorhabditis elegans Longevity, Melanogenesis, and Growth of Cancer Cells. J. Agric. Food Chem. 2016;64:5484–5489. doi: 10.1021/acs.jafc.6b01785. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary M.K., Rizvi S.I. Invertebrate and vertebrate models in aging research. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2019;163:114–121. doi: 10.5507/bp.2019.003. [DOI] [PubMed] [Google Scholar]
- 20.Miller D.L. There Are Worms in My Aging Research. J. Gerontol. Biol. Sci. 2019;74:1170–1172. doi: 10.1093/gerona/glz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell S.J., Scheibye-Knudsen M., Longo D.L., De Cabo R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829. [DOI] [PubMed] [Google Scholar]
- 22.Pandey T., Sammi S.R., Nooreen Z., Mishra A., Ahmad A., Bhatta R.S., Pandey R. Anti-ageing and anti-Parkinsonian effects of natural flavonol, tambulin from Zanthoxyllum aramatum promotes longevity in Caenorhabditis elegans. Exp. Gerontol. 2019;120:50–61. doi: 10.1016/j.exger.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Pandey S., Phulara S.C., Jha A., Chauhan P.S., Gupta P., Shukla V. 3-Methyl-3-buten-1-ol (isoprenol) confers longevity and stress tolerance in Caenorhabditis elegans. Int. J. Food Sci. Nutr. 2019;70:595–602. doi: 10.1080/09637486.2018.1554031. [DOI] [PubMed] [Google Scholar]
- 24.Tatar M., Kopelman A., Epstein D., Tu M.P., Yin C.M., Garofalo R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 25.Altintas O., Park S., Lee S.J.V. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016;49:81–92. doi: 10.5483/BMBRep.2016.49.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan J., Rollins J.A., Zang X., Wu D., Zou L., Wang Z., Ye C., Wu Z., Kapahi P., Rogers A.N., et al. Translational Regulation of Non-autonomous Mitochondrial Stress Response Promotes Longevity. Cell Rep. 2019;28:1050–1062.e6. doi: 10.1016/j.celrep.2019.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bras A. Protein aggregation contributes to aging in C. elegans. Lab Anim. 2019;48:200. doi: 10.1038/s41684-019-0335-5. [DOI] [Google Scholar]
- 28.Pigazzini M.L., Gallrein C., Iburg M., Kaminski Schierle G., Kirstein J. Characterization of Amyloid Structures in Aging C. elegans Using Fluorescence Lifetime Imaging. J. Vis. Exp. 2020;157:e61004. doi: 10.3791/61004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai Y., Li W., Feng G., Yang Y., Wang X., Ou G. Live imaging of cellular dynamics during Caenorhabditis elegans postembryonic development. Nat. Protoc. 2012;7:2090–2102. doi: 10.1038/nprot.2012.128. [DOI] [PubMed] [Google Scholar]
- 30.Uno M., Nishida E. Lifespan-regulating genes in C. elegans. Npj Aging Mech. Dis. 2016;2:16010. doi: 10.1038/npjamd.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen A., Vantipalli M.C., Lithgow G.J. Using Caenorhabditis elegans as a model for aging and age-related diseases. Ann. N. Y. Acad. Sci. 2006;1067:120–128. doi: 10.1196/annals.1354.015. [DOI] [PubMed] [Google Scholar]
- 32.Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 33.KLASS M.R. Culture methods Labeling. Mech. Ageing Dev. 1983;22:279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- 34.Schaffitzel E., Hertweck M. Recent aging research in Caenorhabditis elegans. Exp. Gerontol. 2006;41:557–563. doi: 10.1016/j.exger.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Tissenbaum H.A. Using C. elegans for aging research. Invertebr. Reprod. Dev. 2015;59:59–63. doi: 10.1080/07924259.2014.940470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy C.T., McCarroll S.A., Bargmann C.I., Fraser A., Kamath R.S., Ahringer J., Li H., Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–284. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 37.Johnson T.E. Subfield History: Caenorhabditis elegans as a System for Analysis of the Genetics of Aging. Sci. Aging Knowl. Environ. 2002;2002:re4. doi: 10.1126/sageke.2002.34.re4. [DOI] [PubMed] [Google Scholar]
- 38.Johnson T.E. Advantages and disadvantages of Caenorhabditis elegans for aging research. Exp. Gerontol. 2003;38:1329–1332. doi: 10.1016/j.exger.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Ahn D., Lee E.B., Kim B.J., Lee S.Y., Lee T.G., Ahn M.S., Lim H.W., Cha D.S., Jeon H., Kim D.K. Antioxidant and lifespan extending property of quercetin-3-O-dirhamnoside from Curcuma longa L. in Caenorhabditis elegans. J. Korean Soc. Appl. Biol. Chem. 2014;57:709–714. doi: 10.1007/s13765-014-4200-3. [DOI] [Google Scholar]
- 40.Ding A.J., Zheng S.Q., Huang X.B., Xing T.K., Wu G.S., Sun H.Y., Qi S.H., Luo H.R. Current Perspective in the Discovery of Anti-aging Agents from Natural Products. Nat. Products Bioprospect. 2017;7:335–404. doi: 10.1007/s13659-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broughton S.J., Piper M.D.W., Ikeya T., Bass T.M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D.J., Leevers S.J., et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powers R.W., Kaeberlein M., Caldwell S.D., Kennedy B.K., Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu A.L., Murphy C.T., Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay A., Oh S.W., Tissenbaum H.A. Worming pathways to and from DAF-16/FOXO. Exp. Gerontol. 2006;41:928–934. doi: 10.1016/j.exger.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Kwon E.S., Narasimhan S.D., Yen K., Tissenbaum H.A. A new DAF-16 isoform regulates longevity. Nature. 2010;466:498–502. doi: 10.1038/nature09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolff S., Ma H., Burch D., Maciel G.A., Hunter T., Dillin A. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell. 2006;124:1039–1053. doi: 10.1016/j.cell.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 47.Kamath R.S., Martinez-Campos M., Zipperlen P., Fraser A.G., Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2:1–10. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukitt-Hale B., Lau F.C., Josep J.A. Berry fruit supplementation and the aging brain. J. Agric. Food Chem. 2008;56:636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- 49.Kaunda J.S., Zhang Y.J. The Genus Solanum: An Ethnopharmacological, Phytochemical and Biological Properties Review. Volume 9. Springer; Singapore: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dehghan E., Zhang Y., Saremi B., Yadavali S., Hakimi A., Dehghani M., Goodarzi M., Tu X., Robertson S., Lin R., et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nat. Commun. 2017;8:2223. doi: 10.1038/s41467-017-02394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dehghan E., Goodarzi M., Saremi B., Lin R., Mirzaei H. Hydralazine targets cAMP-dependent protein kinase leading to sirtuin1/5 activation and lifespan extension in C. elegans. Nat. Commun. 2019;10:4905. doi: 10.1038/s41467-019-12425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gürbüz N., Uluişik S., Frary A., Frary A., Doğanlar S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018;268:602–610. doi: 10.1016/j.foodchem.2018.06.093. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Onken B., Chen H., Xiao S., Liu X., Driscoll M., Cao Y., Huang Q. Mechanism of longevity extension of Caenorhabditis elegans induced by pentagalloyl glucose isolated from eucalyptus leaves. J. Agric. Food Chem. 2014;62:3422–3431. doi: 10.1021/jf500210p. [DOI] [PubMed] [Google Scholar]
- 54.Ye X., Linton J.M., Schork N.J., Buck L.B., Petrascheck M. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell. 2014;13:206–215. doi: 10.1111/acel.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenzer Bern H., Sadeghi Bern L. Phytochemicals: Sources and biological functions. J. Pharmacogn. Phytochem. JPP. 2016;339:339–341. [Google Scholar]
- 57.Banu K.S., Cathrine L. General Techniques Involved in Phytochemical Analysis. Int. J. Adv. Res. Chem. Sci. 2015;2:25–32. [Google Scholar]
- 58.Tiwari P., Kumar B., Mandeep K., Kaur G., Kaur H. Phytochemical screening and Extraction: A Review. Int. Pharm. Sci. 2011;1:98–106. [Google Scholar]
- 59.Papaevgeniou N., Sakellari M., Jha S., Tavernarakis N., Holmberg C.I., Gonos E.S., Chondrogianni N. 18α-Glycyrrhetinic acid proteasome activator decelerates aging and Alzheimer’s disease progression in Caenorhabditis elegans and neuronal cultures. Antioxid. Redox Signal. 2016;25:855–869. doi: 10.1089/ars.2015.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papaevgeniou N., Chondrogianni N. Anti-aging and Anti-aggregation Properties of Polyphenolic Compounds in C. elegans. Curr. Pharm. Des. 2018;24:2107–2120. doi: 10.2174/1381612824666180515145652. [DOI] [PubMed] [Google Scholar]
- 61.Abdel-Moneim A.M.E., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Swelum A.A., Arif M., Fayyaz M., Abd El-Hack M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020;104:1851–1866. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- 62.Banjarnahor S.D.S., Artanti N. Antioxidant properties of flavonoids. Med. J. Indones. 2014;23:239–244. doi: 10.13181/mji.v23i4.1015. [DOI] [Google Scholar]
- 63.Lin C., Xiao J., Xi Y., Zhang X., Zhong Q., Zheng H., Cao Y., Chen Y. Rosmarinic acid improved antioxidant properties and healthspan via the IIS and MAPK pathways in Caenorhabditis elegans. BioFactors. 2019;45:774–787. doi: 10.1002/biof.1536. [DOI] [PubMed] [Google Scholar]
- 64.Liao V.H.C., Yu C.W., Chu Y.J., Li W.H., Hsieh Y.C., Wang T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011;132:480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Zheng S.Q., Huang X.B., Xing T.K., Ding A.J., Wu G.S., Luo H.R. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via Insulin/IGF-1 signaling pathway. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017;72:464–472. doi: 10.1093/gerona/glw105. [DOI] [PubMed] [Google Scholar]
- 66.Abbas S., Wink M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine. 2010;17:902–909. doi: 10.1016/j.phymed.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Abbas S., Wink M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009;75:216–221. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- 68.Ayuda-Durán B., González-Manzano S., Miranda-Vizuete A., Dueñas M., Santos-Buelga C., González-Paramás A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE. 2019;14:e0199483. doi: 10.1371/journal.pone.0199483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Büchter C., Ackermann D., Havermann S., Honnen S., Chovolou Y., Fritz G., Kampkötter A., Wätjen W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int. J. Mol. Sci. 2013;14:11895–11914. doi: 10.3390/ijms140611895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Havermann S., Chovolou Y., Humpf H.U., Wätjen W. Caffeic acid phenethylester increases stress resistance and enhances lifespan in Caenorhabditis elegans by modulation of the insulin-like DAF-16 signalling pathway. PLoS ONE. 2014;9:e100256. doi: 10.1371/journal.pone.0100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asthana J., Yadav D., Pant A., Yadav A.K., Gupta M.M., Pandey R. Acacetin 7-O-α-l-rhamnopyranosyl (1-2) β-D-xylopyranoside elicits life-span extension and stress resistance in Caenorhabditis elegans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016;71:1160–1168. doi: 10.1093/gerona/glv173. [DOI] [PubMed] [Google Scholar]
- 72.Sater H.M., Bizzio L.N., Tieman D.M., Muñoz P.D. A Review of the Fruit Volatiles Found in Blueberry and Other Vaccinium Species. J. Agric. Food Chem. 2020;68:5777–5786. doi: 10.1021/acs.jafc.0c01445. [DOI] [PubMed] [Google Scholar]
- 73.Bass T.M., Weinkove D., Houthoofd K., Gems D., Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Zarse K., Bossecker A., Müller-Kuhrt L., Siems K., Hernandez M.A., Berendsohn W.G., Birringer M., Ristow M. The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans. Horm. Metab. Res. 2011;43:241–243. doi: 10.1055/s-0030-1270524. [DOI] [PubMed] [Google Scholar]
- 75.Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K., Criollo A., Galluzzi L., Malik S.A., Vitale I., et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kampkötter A., Timpel C., Zurawski R.F., Ruhl S., Chovolou Y., Proksch P., Wätjen W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Grünz G., Haas K., Soukup S., Klingenspor M., Kulling S.E., Daniel H., Spanier B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012;133:1–10. doi: 10.1016/j.mad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Saul N., Pietsch K., Menzel R., Stürzenbaum S.R., Steinberg C.E.W. Catechin induced longevity in C. elegans: From key regulator genes to disposable soma. Mech. Ageing Dev. 2009;130:477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Havermann S., Rohrig R., Chovolou Y., Humpf H. Molecular Effects of Baicalein in Hct116 Cells and Caenorhabditis elegans: Activation of the Nrf2 Signaling Pathway and Prolongation of Lifespan. J. Agric. Food Chem. 2013;61:2158–2164. doi: 10.1021/jf304553g. [DOI] [PubMed] [Google Scholar]
- 80.Kampkötter A., Gombitang Nkwonkam C., Zurawski R.F., Timpel C., Chovolou Y., Wätjen W., Kahl R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007;81:849–858. doi: 10.1007/s00204-007-0215-4. [DOI] [PubMed] [Google Scholar]
- 81.Bartholome A., Kampkötter A., Tanner S., Sies H., Klotz L.O. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys. 2010;501:58–64. doi: 10.1016/j.abb.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Pietsch K., Saul N., Menzel R., Stürzenbaum S.R., Steinberg C.E.W. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology. 2009;10:565–578. doi: 10.1007/s10522-008-9199-6. [DOI] [PubMed] [Google Scholar]
- 83.Surco-Laos F., Dueñas M., González-Manzano S., Cabello J., Santos-Buelga C., González-Paramás A.M. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res. Int. 2012;46:514–521. doi: 10.1016/j.foodres.2011.10.014. [DOI] [Google Scholar]
- 84.Lublin A., Isoda F., Patel H., Yen K., Nguyen L., Hajje D., Schwartz M., Mobbs C. FDA-approved drugs that protect mammalian neurons from glucose toxicity slow aging dependent on Cbp and protect against proteotoxicity. PLoS ONE. 2011;6:e27762. doi: 10.1371/journal.pone.0027762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pietsch K., Saul N., Chakrabarti S., Stürzenbaum S.R., Menzel R., Steinberg C.E.W. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology. 2011;12:329–347. doi: 10.1007/s10522-011-9334-7. [DOI] [PubMed] [Google Scholar]
- 86.Asthana J., Mishra B.N., Pandey R. Acacetin promotes healthy aging by altering stress response in Caenorhabditis elegans. Free Radic. Res. 2016;50:861–874. doi: 10.1080/10715762.2016.1187268. [DOI] [PubMed] [Google Scholar]
- 87.Asthana J., Yadav A.K., Pant A., Pandey S., Gupta M.M., Pandey R. Specioside ameliorates oxidative stress and promotes longevity in Caenorhabditis elegans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015;169:25–34. doi: 10.1016/j.cbpc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Dueñas M., Surco-Laos F., González-Manzano S., González-Paramás A.M., Gómez-Orte E., Cabello J., Santos-Buelga C. Deglycosylation is a key step in biotransformation and lifespan effects of quercetin-3-O-glucoside in Caenorhabditis elegans. Pharmacol. Res. 2013;76:41–48. doi: 10.1016/j.phrs.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Surco-Laos F., Cabello J., Gómez-Orte E., González-Manzano S., González-Paramás A.M., Santos-Buelga C., Dueñas M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011;2:445–456. doi: 10.1039/c1fo10049a. [DOI] [PubMed] [Google Scholar]
- 90.Cai W.J., Huang J.H., Zhang S.Q., Wu B., Kapahi P., Zhang X.M., Shen Z.Y. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans. PLoS ONE. 2011;6:e28835. doi: 10.1371/journal.pone.0028835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Büchter C., Havermann S., Koch K., Wätjen W. Isoxanthohumol, a constituent of hop (Humulus lupulus L.), increases stress resistance in Caenorhabditis elegans dependent on the transcription factor DAF-16. Eur. J. Nutr. 2016;55:257–265. doi: 10.1007/s00394-015-0843-z. [DOI] [PubMed] [Google Scholar]
- 92.Lee E.B., Ahn D., Kim B.J., Lee S.Y., Seo H.W., Cha Y.S., Jeon H., Eun J.S., Cha D.S., Kim D.K. Genistein from vigna angularis extends lifespan in Caenorhabditis elegans. Biomol. Ther. 2015;23:77–83. doi: 10.4062/biomolther.2014.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benedetti M.G., Foster A.L., Vantipalli M.C., White M.P., Sampayo J.N., Gill M.S., Olsen A., Lithgow G.J. Compounds that confer thermal stress resistance and extended lifespan. Exp. Gerontol. 2008;43:882–891. doi: 10.1016/j.exger.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlernitzauer A., Oiry C., Hamad R., Galas S., Cortade F., Chabi B., Casas F., Pessemesse L., Fouret G., Feillet-Coudray C., et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS ONE. 2013;8:e78788. doi: 10.1371/journal.pone.0078788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saul N., Pietsch K., Menzel R., Stürzenbaum S.R., Steinberg C.E.W. The longevity effect of tannic acid in Caenorhabditis elegans: Disposable soma meets hormesis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010;65 A:626–635. doi: 10.1093/gerona/glq051. [DOI] [PubMed] [Google Scholar]
- 96.Lee J., Kwon G., Park J., Kim J.K., Lim Y.H. Brief Communication: SIR-2.1-dependent lifespan extension of Caenorhabditis elegans by oxyresveratrol and resveratrol. Exp. Biol. Med. 2016;241:1757–1763. doi: 10.1177/1535370216650054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Büchter C., Zhao L., Havermann S., Honnen S., Fritz G., Proksch P., Wätjen W. TSG (2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside) from the Chinese herb Polygonum multiflorum increases lifespan and stress resistance of Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2015;2015:124357. doi: 10.1155/2015/124357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wen H., Gao X., Qin J. Probing the anti-aging role of polydatin in Caenorhabditis elegans on a chip. Integr. Biol. (UK) 2014;6:35–43. doi: 10.1039/C3IB40191J. [DOI] [PubMed] [Google Scholar]
- 99.Shen P., Yue Y., Sun Q., Kasireddy N., Kim K.H., Park Y. Piceatannol extends the lifespan of Caenorhabditis elegans via DAF-16. BioFactors. 2017;43:379–387. doi: 10.1002/biof.1346. [DOI] [PubMed] [Google Scholar]
- 100.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Félix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 101.Yaguchi Y., Komura T., Kashima N., Tamura M., Kage-Nakadai E., Saeki S., Terao K., Nishikawa Y. Influence of oral supplementation with sesamin on longevity of Caenorhabditis elegans and the host defense. Eur. J. Nutr. 2014;53:1659–1668. doi: 10.1007/s00394-014-0671-6. [DOI] [PubMed] [Google Scholar]
- 102.Su S., Wink M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry. 2015;117:340–350. doi: 10.1016/j.phytochem.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 103.Cañuelo A., Gilbert-López B., Pacheco-Liñán P., Martínez-Lara E., Siles E., Miranda-Vizuete A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech. Ageing Dev. 2012;133:563–574. doi: 10.1016/j.mad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Ayyadevara S., Bharill P., Dandapat A., Hu C., Khaidakov M., Mitra S., Shmookler Reis R.J., Mehta J.L. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Sign. 2013;18:481–490. doi: 10.1089/ars.2011.4151. [DOI] [PubMed] [Google Scholar]
- 105.Nguyen T.T., Caito S.W., Zackert W.E., West J.D., Zhu S., Aschner M., Fessel J.P., Roberts L.J. Scavengers of reactive γ-ketoaldehydes extend Caenorhabditis elegans lifespan and healthspan through protein-level interactions with SIR-2.1 and ETS-7. Aging (Albany NY) 2016;8:1759–1780. doi: 10.18632/aging.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heidler T., Hartwig K., Daniel H., Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 107.Li H., Yu X., Meng F., Zhao Z., Guan S., Wang L. Ferulic acid supplementation increases lifespan and stress resistance via insulin/IGF-1 signaling pathway in C. elegans. Int. J. Mol. Sci. 2021;22:4279. doi: 10.3390/ijms22084279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang G., Tang W., Bidigare R.R. Natural Products: Drug Discovery and Therapeutic Medicine. Humana Press; Totowa, NJ, USA: 2005. Terpenoids as therapeutic drugs and pharmaceutical agents; pp. 197–227. [Google Scholar]
- 109.Lin C., Zhang X., Xiao J., Zhong Q., Kuang Y., Cao Y., Chen Y. Effects on longevity extension and mechanism of action of carnosic acid in: Caenorhabditis elegans. Food Funct. 2019;10:1398–1410. doi: 10.1039/C8FO02371A. [DOI] [PubMed] [Google Scholar]
- 110.Lin C., Zhang X., Su Z., Xiao J., Lv M., Cao Y., Chen Y. Carnosol improved lifespan and healthspan by promoting antioxidant capacity in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019;2019:5958043. doi: 10.1155/2019/5958043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pant A., Saikia S.K., Shukla V., Asthana J., Akhoon B.A., Pandey R. Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Exp. Gerontol. 2014;57:81–95. doi: 10.1016/j.exger.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Shukla V., Yadav D., Phulara S.C., Gupta M.M., Saikia S.K., Pandey R. Longevity-promoting effects of 4-hydroxy-E-globularinin in Caenorhabditis elegans. Free Radic. Biol. Med. 2012;53:1848–1856. doi: 10.1016/j.freeradbiomed.2012.08.594. [DOI] [PubMed] [Google Scholar]
- 113.Zhang J., Lu L., Zhou L. Oleanolic acid activates daf-16 to increase lifespan in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2015;468:843–849. doi: 10.1016/j.bbrc.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 114.Li J., Chotiko A., Chouljenko A., Gao C., Zheng J., Sathivel S. Delivery of alpha-tocopherol through soluble dietary fibre-based nanofibres for improving the life span of Caenorhabditis elegans. Int. J. Food Sci. Nutr. 2019;70:172–181. doi: 10.1080/09637486.2018.1489785. [DOI] [PubMed] [Google Scholar]
- 115.Akhoon B.A., Pandey S., Tiwari S., Pandey R. Withanolide A Offers Neuroprotection, Ameliorates Stress Resistance and Prolongs the Life Expectancy of Caenorhabditis Elegans. Volume 78. Elsevier B.V.; Amsterdam, The Netherlands: 2016. [DOI] [PubMed] [Google Scholar]
- 116.Pant A., Asthana J., Yadav A.K., Rathor L., Srivastava S., Gupta M.M., Pandey R. Verminoside mediates life span extension and alleviates stress in Caenorhabditis elegans. Free Radic. Res. 2015;49:1384–1392. doi: 10.3109/10715762.2015.1075017. [DOI] [PubMed] [Google Scholar]
- 117.Negi H., Shukla A., Khan F., Pandey R. 3β-Hydroxy-urs-12-en-28-oic acid prolongs lifespan in C. elegans by modulating JNK-1. Biochem. Biophys. Res. Commun. 2016;480:539–543. doi: 10.1016/j.bbrc.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 118.Shukla V., Phulara S.C., Yadav D., Tiwari S., Kaur S., Gupta M.M., Nazir A., Pandey R. Iridoid Compound 10-O-trans-p-Coumaroylcatalpol Extends Longevity and Reduces Alpha Synuclein Aggregation in Caenorhabditis elegans. CNS Neurol. Disord.-Drug Targets. 2013;11:984–992. doi: 10.2174/1871527311211080007. [DOI] [PubMed] [Google Scholar]
- 119.Lashmanova E., Proshkina E., Zhikrivetskaya S., Shevchenko O., Marusich E., Leonov S., Melerzanov A., Zhavoronkov A., Moskalev A. Fucoxanthin increases lifespan of Drosophila melanogaster and Caenorhabditis elegans. Pharmacol. Res. 2015;100:228–241. doi: 10.1016/j.phrs.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 120.Seo H.W., Cheon S.M., Lee M.H., Kim H.J., Jeon H., Cha D.S. Catalpol modulates lifespan via DAF-16/FOXO and SKN-1/Nrf2 activation in Caenorhabditis elegans. Evidence-Based Complement. Altern. Med. 2015;2015:524878. doi: 10.1155/2015/524878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sayed A.A.R. Ferulsinaic acid attenuation of advanced glycation end products extends the lifespan of Caenorhabditis elegans. J. Pharm. Pharmacol. 2011;63:423–428. doi: 10.1111/j.2042-7158.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 122.Kim J., Kang Y.-G., Lee J., Choi D., Cho Y., Shin J.-M., Park J.S., Lee J.H., Kim W.G., Seo D.B., et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015;412:216–225. doi: 10.1016/j.mce.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Lu M., Tan L., Zhou X.G., Yang Z.L., Zhu Q., Chen J.N., Luo H.R., Wu G.S. Secoisolariciresinol Diglucoside Delays the Progression of Aging-Related Diseases and Extends the Lifespan of Caenorhabditis elegans via DAF-16 and HSF-1. Oxid. Med. Cell. Longev. 2020;2020:1293935. doi: 10.1155/2020/1293935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roy A. A review on the alkaloids an important therapeutic compound from plants. Int. J. Plant Biotechnol. 2017;3:1–9. doi: 10.231/JIM.0b013e3181948b37. [DOI] [Google Scholar]
- 125.Hardie D.G. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Srivastava D., Arya U., SoundaraRajan T., Dwivedi H., Kumar S., Subramaniam J.R. Reserpine can confer stress tolerance and lifespan extension in the nematode C. elegans. Biogerontology. 2008;9:309–316. doi: 10.1007/s10522-008-9139-5. [DOI] [PubMed] [Google Scholar]
- 127.Li H., Roxo M., Cheng X., Zhang S., Cheng H., Wink M. Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans. Food Chem. X. 2019;1:100005. doi: 10.1016/j.fochx.2019.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bridi J.C., de Almeida Barros A.G., Sampaio L.R., Damásio Ferreira J.C., Antunes Soares F.A., Romano-Silva M.A. Lifespan extension induced by caffeine in Caenorhabditis elegans is partially dependent on adenosine signaling. Front. Aging Neurosci. 2015;7:220. doi: 10.3389/fnagi.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fang E.F., Waltz T.B., Kassahun H., Lu Q., Kerr J.S., Morevati M., Fivenson E.M., Wollman B.N., Marosi K., Wilson M.A., et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017;7:46208. doi: 10.1038/srep46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morselli E., Mariño G., Bennetzen M.V., Eisenberg T., Megalou E., Schroeder S., Cabrera S., Bénit P., Rustin P., Criollo A., et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 132.Wang E., Wink M. Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan. PeerJ. 2016;4:e1879. doi: 10.7717/peerj.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu J.Z., Huang J.H., Khanabdali R., Kalionis B., Xia S.J., Cai W.J. Pyrroloquinoline quinone enhances the resistance to oxidative stress and extends lifespan upon DAF-16 and SKN-1 activities in C. elegans. Exp. Gerontol. 2016;80:43–50. doi: 10.1016/j.exger.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 134.Lu L., Zhao X., Zhang J., Li M., Qi Y., Zhou L. Calycosin promotes lifespan in Caenorhabditis elegans through insulin signaling pathway via daf-16, age-1 and daf-2. J. Biosci. Bioeng. 2017;124:1–7. doi: 10.1016/j.jbiosc.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 135.Peixoto H., Roxo M., Silva E., Valente K., Braun M., Wang X., Wink M. Bark extract of the amazonian tree endopleura uchi (humiriaceae) extends lifespan and enhances stress resistance in Caenorhabditis elegans. Molecules. 2019;24:915. doi: 10.3390/molecules24050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peixoto H., Roxo M., Koolen H., Da Silva F., Silva E., Braun M.S., Wang X., Wink M. Calycophyllum spruceanum (Benth.), the amazonian “tree of youth” prolongs longevity and enhances stress resistance in Caenorhabditis elegans. Molecules. 2018;23:534. doi: 10.3390/molecules23030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rangsinth P., Prasansuklab A., Duangjan C., Gu X., Meemon K., Wink M., Tencomnao T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019;19:164. doi: 10.1186/s12906-019-2578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tambara A.L., de Los Santos Moraes L., Dal Forno A.H., Boldori J.R., Gonçalves Soares A.T., de Freitas Rodrigues C., Mariutti L.R.B., Mercadante A.Z., de Ávila D.S., Denardin C.C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018;120:639–650. doi: 10.1016/j.fct.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 139.Duangjan C., Rangsinth P., Gu X., Wink M., Tencomnao T. Lifespan extending and oxidative stress resistance properties of a leaf extracts from anacardium occidentale L. in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019;2019:9012396. doi: 10.1155/2019/9012396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duangjan C., Rangsinth P., Gu X., Zhang S., Wink M., Tencomnao T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine. 2019;64:153061. doi: 10.1016/j.phymed.2019.153061. [DOI] [PubMed] [Google Scholar]
- 141.Koch K., Weldle N., Baier S., Büchter C., Wätjen W. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020;59:137–150. doi: 10.1007/s00394-019-01894-w. [DOI] [PubMed] [Google Scholar]
- 142.Rathor L., Pant A., Awasthi H., Mani D., Pandey R. An antidiabetic polyherbal phytomedicine confers stress resistance and extends lifespan in Caenorhabditis elegans. Biogerontology. 2017;18:131–147. doi: 10.1007/s10522-016-9668-2. [DOI] [PubMed] [Google Scholar]
- 143.Pandey S., Phulara S.C., Mishra S.K., Bajpai R., Kumar A., Niranjan A., Lehri A., Upreti D.K., Chauhan P.S. Betula utilis extract prolongs life expectancy, protects against amyloid-β toxicity and reduces Alpha Synuclien in Caenorhabditis elegans via DAF-16 and SKN-1. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2020;228:108647. doi: 10.1016/j.cbpc.2019.108647. [DOI] [PubMed] [Google Scholar]
- 144.Sayed S.M.A., Siems K., Schmitz-Linneweber C., Luyten W., Saul N. Enhanced healthspan in Caenorhabditis elegans treated with extracts from the traditional chinese medicine plants Cuscuta chinensis Lam. and Eucommia ulmoides Oliv. Front. Pharmacol. 2021;12:604435. doi: 10.3389/fphar.2021.604435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Reilly L.P., Luke C.J., Perlmutter D.H., Silverman A.G., Pak S.C. C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014;23:247–253. doi: 10.1016/j.addr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ben-Yakar A. High-content and high-throughput in vivo drug screening platforms using microfluidics. Assay Drug Dev. Technol. 2019;17:8–13. doi: 10.1089/adt.2018.908. [DOI] [PubMed] [Google Scholar]
- 147.Banerjee S., Riordan M., Bhat M.A. Genetic aspects of autism spectrum disorders: Insights from animal models. Front. Cell. Neurosci. 2014;8:58. doi: 10.3389/fncel.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmeisser K., Parker J.A. Worms on the spectrum-C. elegans models in autism research. Exp. Neurol. 2018;299:199–206. doi: 10.1016/j.expneurol.2017.04.007. [DOI] [PubMed] [Google Scholar]