Abstract
Background:
The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) has changed in recent years. The present article is intended to establish differences between clinical, laboratory and imaging findings and outcomes of MSSA and MRSA infections, as well as among subgroups of infection such as skin and soft tissue infection, osteoarticular, bacteremia or pneumonia in a pediatric population from Bogota, Colombia.
Methods:
Retrospective cohort study using clinical records of patients under 18 years of age treated at the participating centers in Bogota, Colombia, between 2014 and 2018. The first positive S. aureus culture was studied. MSSA and MRSA were compared. The χ2 test, Fisher exact test, and Kruskal-Wallis test were calculated, and the statistical significance was presented using the difference and its 95% CI.
Results:
Five hundred fifty-one patients were included; 211 (38%) corresponded to MRSA and 340 (62%) to MSSA for a total of 703 cultures. A significantly higher probability of having an MSSA infection than MRSA was found in patients with previous heart disease (3.3% vs. 0.5%), neurologic disease (5.9% vs. 2.5%), recent major surgeries (11% vs. 5%) or who has an implanted device (11% vs. 4%). In contrast, in severe MRSA infections (bacteremia, osteoarticular infections and pneumonia), a higher rate of complications was seen (admission to the pediatric intensive care unit, mechanical ventilation and vasoactive support), and in osteoarticular MRSA, more than 1 surgery per case was seen (89% vs. 61%). Laboratory results and mortality were similar.
Conclusions:
MRSA was associated with a more severe course in bacteremia, osteoarticular infections and pneumonia. Some classical risk factors associated with MRSA infections were found to be related to MSSA. In general, with the exception of skin and soft tissue infection, there was an increased risk of pediatric intensive care unit admission and mechanical and inotropic support with MRSA in a pediatric population.
Keywords: Staphylococcus aureus, staphylococcal infections, pediatrics, bacterial drug resistance, methicillin-resistant Staphylococcus aureus
Unlike methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA) has been associated with increased antibiotic exposure, prolonged hospital stays, and admission to the pediatric intensive care unit (PICU).1–4 Until the 1990s, most MRSA infections occurred in a hospital setting. However, an increase in S. aureus isolates, particularly community-acquired (CA) MRSA, was reported worldwide by 2000.5,6 However, a fall in the incidence of MRSA was reported in Europe where it went from 23% in 2009 to 16% in 2018.7 In the United States of America, a progressive decrease in MRSA isolates in pediatrics has also been reported.8,9 The epidemiologic records for Colombia prepared by the Group for Bacterial Resistance Control in Bogota reported that S. aureus was the second most frequently isolated bacteria in hospitals in 2018 while MRSA frequency was between 40% and 45% from 2010 to 2018.10
CA-MRSA as a cause of infection in children has been associated with greater severity, possibly due to the presence of more virulence factors related to the USA300 strain in the Colombian pediatric population.11 MRSA infections can manifest with skin and soft tissue infection (SSTI),12,13 bacteremia (20%–25%),14,15 osteoarticular infections (OIs), deep infections16–18 or pneumonia.19 Attempts to predict methicillin susceptibility based on clinical or demographic factors have been unsuccessful. Finding the differences between these types of infections will facilitate timely diagnosis, and having this information could help guide the start of timely antibiotic treatment.20–22 The present article is intended to compare clinical, laboratory and imaging findings and outcomes of MSSA and MRSA infections.
MATERIALS AND METHODS
Study Design
This is a multicenter, historical, cohort study that used the medical records of patients with S. aureus infection treated at 6 tertiary-level university centers in Bogota, Colombia, 2 of which were children-only.
Case Selection
Cases were identified based on a positive S. aureus culture, under 18 years of age, and diagnosed between January 1, 2014, and December 31, 2018. The information was extracted from the culture databases of the microbiology laboratories using the WHONET 18 software and automated identification methods that included MicroScan, VITEK 2 XL, VITEK MS, VIRTUO, and Phoenix depending on the center. Patients with cultures that lacked pathologic relevance (colonization or contamination), were less than 1 month old or lacked clinical records (to avoid measurement bias) were excluded.
Infections were classified into (1) SSTI in patients with clinical signs of SSTI and S. aureus in cultures of spontaneous secretion or surgical drainage. In cases of nonsuppurative cutaneous infections, clinical symptoms plus the presence of a positive culture for S. aureus from nasal cavities were considered SSTI; (2) bacteremia in patients with S. aureus in blood cultures (primary) or secondary to another infection (osteoarticular, pneumonia, SSTI or other foci); (3) OI in patients with clinical signs of infection in bones or joints accompanied by suggestive imaging scans and S. aureus in a culture of secretion or blood culture. Finally, (4) pneumonia included patients with clinical signs of lower respiratory tract infection associated with suggestive imaging scans and identification of S. aureus in pleural culture or blood culture. A patient may have multiple cultures from various sites during an infection.
For this analysis, if there was more than 1 positive culture, only the first positive culture from each location was used. Each was categorized as either MSSA or MSRA, and they were compared with each other based on their origin (SSTI, bacteremia, osteoarticular and pneumonia).
Sociodemographic variables (age, sex and center) and underlying diseases (immunodeficiencies, prematurity, neoplasms, autoimmune diseases, congenital heart disease, genetic syndromes, malnutrition, chronic lung disease, neurologic disease, atopic dermatitis or kidney disease) were identified. Risk factors previously described in the literature, such as major surgeries within 6 months of infection, a more than 3-day hospital stay and implanted medical devices (osteosynthesis material, central venous catheter, gastrostomy, ventriculoperitoneal shunt and peritoneal dialysis catheter) were also evaluated. Finally, community-onset infection was defined as a positive culture obtained <72 hours after hospital admission. Clinical and severe manifestations on admission (hypotension, impaired capillary refill or impaired consciousness), lab test results, imaging studies, number of surgeries, and outcomes were also described.
Statistical Analysis
A 40% prevalence reported by Group for Bacterial Resistance Control in Bogota in 2018 was used to estimate sample size.11 Estimated risk factors with frequencies greater than 15% were expected, and calculations were done to detect differences with an odds ratio greater than 2, a power of 80% and an α error of 0.05. As a result, at least 492 cases (351 cases of MSSA and 141 cases of MRSA) were required. A descriptive analysis was carried out, and the qualitative variables showed absolute and relative frequencies. Regarding quantitative variables, central tendency and dispersion measures were used based on the distribution of the data. For comparisons between MSSA and MRSA cultures, the χ2 test was used for categorical variables, Fisher exact test for small sample sizes (n<5) and nonparametric (Kruskal-Wallis) test for continuous variables. Missing data were not imputed for laboratory data, and statistical significance was presented using the difference and its 95% CI. The study was approved by the research committee and the ethics committees for human subject research at all the participating centers.
RESULTS
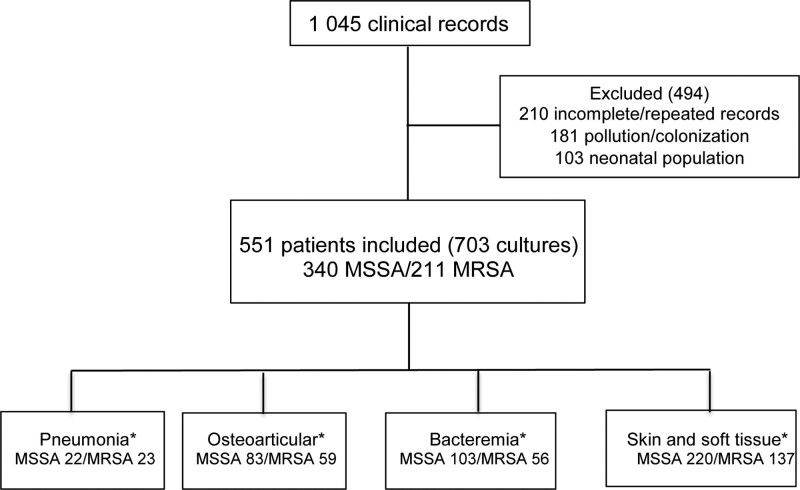
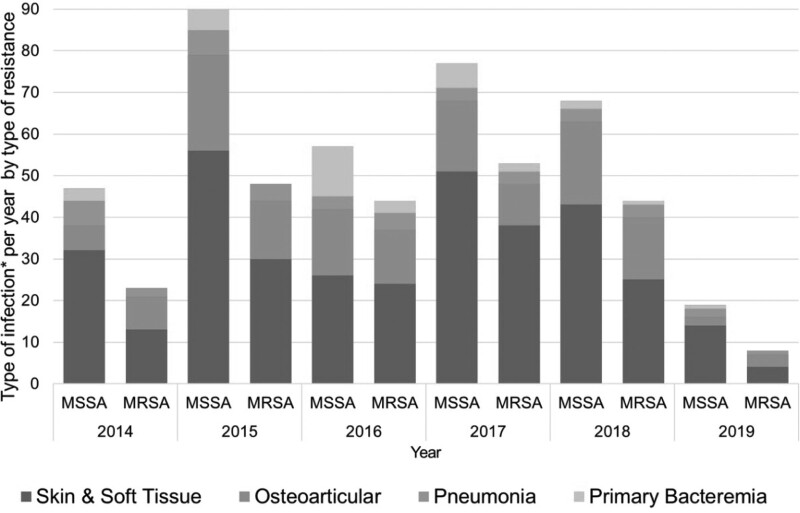
A total sample of 1045 available cases was obtained of which 494 were excluded because 181 corresponded to colonization or contamination, 210 had incomplete information or were repeat cultures, and 103 were neonatal infections. The final analysis included 551 cases with 703 positive cultures. Fig. 1 describes the selection flowchart. Of the cases found, 211 (38%) were MRSA and 340 (62%) were MSSA. These showed slight variations over the years, but the differences were not statistically significant (Table 1; Fig. 2). Age and sex distribution between categories was similar, and no differences were seen in connection with the center. With respect to comorbidities, a statistically significant frequency was seen in heart disease (MRSA 0.5% vs. MSSA 3.3%), neurologic disease (MRSA 2.4% vs. MSSA 5.9%) and other diseases (MRSA 10.9% vs. MSSA 17.7%; Table 1). The presence of major surgery in the last 6 months (MRSA 5% vs. MSSA 11%), device use (MRSA 3% vs. MSSA 11%) and hospital stays of more than 3 days (MRSA 11% vs. MSSA 17%) prior to the current clinical picture were found to be significantly associated with MSSA (Table 2).
FIGURE 1.
Case selection flowchart. *The most important infection was chosen in cases with more than 1 positive culture (osteoarticular > pneumonia > skin and soft tissues > bacteremia).
Table 1.
Demographic Characteristics Based on the Presence or Absence of Resistance
| MRSA, n = 211 (38%) | MSSA, n = 340 (62%) | Diff (95% CI) [P]* | |
|---|---|---|---|
| Male sex, n (%) | 119 (56%) | 183 (54%) | 3 (−6 to 11)% |
| Age, median (IQR) | 97 (30 to 162) | 92 (23 to 155) | [0.254] |
| 1–12 mo | 30 (14%) | 56 (16%) | −2 (−8 to 4)% |
| 1–4 yr | 47 (22%) | 78 (23%) | −1 (−8 to 6)% |
| 5–11 yr | 63 (30%) | 107 (31%) | −1 (−9 to 7)% |
| 12–17 yr | 71 (34%) | 99 (29%) | 5 (−3 to 13)% |
| Underling diseases, n (%) | 71 (34%) | 140 (42%) | −8 (−16 to 3)% |
| Immunodeficiency | 4 (1.9%) | 10 (3.0%) | −1.1 (−3.7 to 1.5)% |
| Prematurity | 13 (6.2%) | 25 (7.4%) | −1.2 (−5.5 to 3.1)% |
| Malignancy | 6 (2.8%) | 18 (5.3%) | −2.5 (−5.8 to 7.6)% |
| Autoimmune | 4 (1.9%) | 5 (1.0%) | 0.9 (−1.2 to 3.0)% |
| Heart disease | 1 (0.5%) | 11 (3.3%) | −2.8 (−4.9 to −0.7)% |
| Undernutrition | 6 (2.9%) | 12 (3.6%) | −0.7 (−3.4 to 2.3)% |
| Genetic disease | 5 (2.4%) | 16 (4.7%) | −2.3 (−5.4 to 7.5)% |
| Chronic lung disease | 7 (3.3%) | 13 (3.8%) | −0.5 (−3.7 to 2.7)% |
| Neurologic disease | 5 (2.4%) | 20 (5.9%) | −3.5 (−6.7 to −0.3)% |
| Atopic dermatitis | 13 (6.2%) | 26 (7.7%) | −1.5 (−5.8 to 2.8)% |
| Renal disease | 3 (1.4%) | 12 (3.6%) | −2.2 (−4.7 to 0.3)% |
| Other diseases† | 23 (10.9%) | 60 (17.7%) | −6.8 (−12.6 to −1.0)% |
NEC indicates necrotizing enterocolitis. Bold means statistically significant.
*Differences and 95% CIs calculated as the percentage with MRSA minus the percentage with MSSA. Negative values represent a higher proportion of patients with MSSA for the given variable. P values for continuous data using a nonparametric (Kruskal-Wallis) test.
†Other diseases such as the Kawasaki syndrome, achalasia, syphoscoliosis, postextubation laryngeal stenosis, HIV-positive mother, short bowel, history of NEC, history of total colectomy, right perinsular pop hemispherectomy for craniotomy, severe dengue, ileostomy, obesity, polycystic ovary, constipation, allergic rhinitis, hip dysplasia, hypoacusis, headache, giant nevus, sickle cell anemia, psychoactive substance use, esophageal atresia, chicken pox, cirrhosis, hemophilia A and hypothyroidism.
FIGURE 2.
Comparison of MSSA and MRSA cases per type of infection.
Table 2.
Risk Factors Based on the Presence or Absence of Resistance
| MRSA, n = 211 (38%) | MSSA, n = 340 (62%) | Diff (95% CI)* | |
|---|---|---|---|
| Some factor (a, b or c) | 28 (13%) | 83 (24%) | −11 (−17 to −5)% |
| a. Major surgery (n = 549) | 10 (5%) | 37 (11%) | −6 (−10 to −2)% |
| Type of major surgery | |||
| None | 201 (95%) | 303 (89%) | 6 (2 to 10)% |
| Central nervous system | 2 (1.0%) | 4 (1.2%) | −0.2 (−2.0 to 1.6)% |
| Cardiothoracic | 1 (0.5%) | 2 (0.6%) | −0.1 (−1.4 to 1.2)% |
| Abdomen | 3 (1.4%) | 5 (1.5%) | −0.1 (−2.1 to 1.9)% |
| Osteoarticular | 2 (1.0%) | 18 (5.3%) | −4.3 (−7.0 to −1.6)% |
| Skin and soft tissues | 2 (1.0%) | 5 (1.5%) | −0.5 (−2.4 to 1.4)% |
| Others | 0 (0.0%) | 3 (0.9%) | −0.9 (−1.9 to 0.1)% |
| b. Hospital stay (>3 days), n = 527 | 21 (11%) | 54 (17%) | −6 (−11.8 to −0.2)% |
| c. Device use (n = 548) | 7 (4%) | 38 (11%) | −7 (−11 to −3%)% |
| Device type | |||
| None | 204 (97%) | 302 (89%) | 8.0 (4.0 to 12.0)% |
| Osteosynthesis material | 3 (1.4%) | 12 (3.5%) | −2.1 (−4.6 to 0.4)% |
| Central venous catheter | 2 (1.0%) | 16 (4.7%) | −3.7 (−6.3 to −1.0)% |
| Gastrostomy | 1 (0.5%) | 3 (0.9%) | −0.4 (−1.8 to 1.0)% |
| Peritoneal ventricular shunt | 0 (0.0%) | 1 (0.5%) | −0.5 (−1.2 to 0.2)% |
| Peritoneal dialysis catheter | 1 (0.5%) | 2 (0.6%) | −0.1 (−1.4 to 1.2)% |
| Others | 0 (0.0%) | 4 (1.2%) | −1.2 (−2.4 to 0.4)% |
*Differences and 95% CIs calculated as the percentage with MRSA minus the percentage with MSSA. Negative values represent a higher proportion of patients with MSSA for the given variable.
On admission, 27 cases presented some evidence of severity, 14 (4%) had MSSA, and 13 (6%) had MRSA (Diff, 2%; 95% CI −2% to 6%). No differences were found in patients with hypotension [7 (2%) MSSA vs. 7 (3%) MRSA], respiratory distress [5 (1%) MSSA vs. 5 (2%) MRSA −1% to 3%], altered consciousness [0 (0%) MSSA vs. 2 (1%) MRSA 0% to 2%] or prolonged capillary refill [0 (0%) MSSA vs. 1 (0%) MRSA] by type of resistance. Regarding laboratory findings, no major differences were found. There was only a higher number of median absolute neutrophil counts [MRSA 8500 cells/mm3 (interquartile range [IQR], 8720–16,000) vs. MSSA 7522 cells/mm3 (IQR 8720–16,000); P=0.047; Table 3].
Table 3.
Laboratory Findings at the Time of Infection Based on the Presence or Absence of Resistance
| MRSA, n = 211 (38%) | MSSA, n = 340 (62%) | Diff (95% CI) [P]* | |
|---|---|---|---|
| Complete blood count performed | 177 (84%) | 300 (88%) | −4 (−10 to 2)% |
| Missing | 1 | 0 | |
| Leukocytes (cell/mm3), median (IQR) | 13,104 (9200 to 18,035) | 11,990 (8720 to 16,000) | [0.061] |
| Neutrophils (cell/mm3), median (IQR) | 8500 (8720 to 16,000) | 7522 (8720 to 16,000) | [0.047] |
| Platelets (cell/mm3), median (IQR) | 344,000 (8720 to 16,000) | 338,000 (8720 to 16,000) | [0.947] |
| C-reactive protein (mg/L), median (IQR) | 53.7 (12.8 to 167.8) | 39.0 (9.6 to 105) | [0.057] |
| Not done | 61 | 83 | |
| ESR (mm/h), median (IQR) | 31 (16 to 45) | 30 (13 to 48) | [0.669] |
| Blood cultures done | 86 (41%) | 153 (45%) | −1 (−12 to 4)% |
| Positive | 56 (65%)† | 103 (67%)† | −2 (−15 to 11)% |
*Differences and 95% CIs calculated as the percentage with MRSA minus the percentage with MSSA. Negative values represent a higher proportion of patients with MSSA for the given variable. P values for continuous data using a nonparametric (Kruskal-Wallis) test.
†Percentage of the complete blood count or blood cultures drawn.
The median overall hospital stay was 10 days (IQR 6–17 days) for MRSA and 8 days (IQR 5–15 days) for MSSA; 14% (77/551) of infections required admission to PICU with stays of 8 days (IQR 6–11 days) for MRSA and 6 days (IQR 3–13 days) for MSSA. Of the total number of patients, 53% (41/77) received assisted ventilation with a median of 74–9 days for MRSA and 94–11 days for MSSA while inotropic agents were used in 77% (48/62) of the patients for 43–7 days with MRSA and 62–9 days with MSSA. With respect to the number of days of hospitalization, a longer stay in hospital was only found in the group of MRSA OIs (P = 0.0006), and no significant differences were found in the number of days spent in the PICU. Mortality was reported in 3 MRSA cases (1%) and 7 MSSA cases (2%). None of these differences were significant. Regarding the type of infection, 356 cases presented with SSTI infection (associated or not with bacteremia), 136 with OIs that may or may not have been associated with other infections, 45 with pneumonia not associated with OI and 21 with bacteremia not associated with any of the infections described above. More than one focus of infection was identified in 22 cases: 13 cases presented with OI and SSTI simultaneously; 5 cases with pneumonia and OI; 1 case with pneumonia and SSTI and 3 cases had OI, pneumonia, and SSTI infection. Each group is described below based on the type of infection; a case may be included in more than 1 group (Table 4).
Table 4.
Infection Groups Depending on the Presence or Not of Resistance
| MRSA, n (%)211 | MSSA, n (%)340 | Diff (95% CI)*/P value† | |
|---|---|---|---|
| Skin and soft tissues | 137 (65%) | 219 (64%) | 1 (−9 to 8)% |
| Blood cultures done | 25 (18%) | 52 (24%) | −6 (−15 to 3)% |
| Positive blood cultures | 6 (24%)‡ | 25 (48%)‡ | −24 (−46 to −2)% |
| Treatment | |||
| Surgical treatment only | 61 (46%) | 89 (42%) | 4 (−7 to 15)% |
| Spontaneous drainage only | 60 (45%) | 86 (40%) | 5 (−6 to 16)% |
| Surgical and spontaneous drainage | 4 (3%) | 9 (4%) | −1 (−5 to 3)% |
| No drainage | 9 (7%) | 29 (14%) | −7 (−13 to −1)% |
| Missing | 3 | 6 | |
| Localization | |||
| Face, head and neck | 28 (21%) | 71 (33%) | −12 (−21 to −3)% |
| Upper limbs | 25 (19%) | 45 (21%) | −2 (−11 to 5)% |
| Lower limbs | 51 (38%) | 57 (26%) | 12 (2 to 22)% |
| Chest | 17 (13%) | 23 (11%) | 2 (−5 to 9)% |
| Abdomen | 4 (3%) | 8 (4%) | −1 (−5 to 3)% |
| Hip | 6 (4%) | 7 (3%) | 1 (−3 to 5)% |
| Genitalia | 3 (2%) | 7 (3%) | −1 (−4 to 2)% |
| Missing | 2 | 3 | |
| Duration of hospital stay (days), median (IQR) | 7 (5 to 9) | 5 (4 to 8) | [P = 0.1175] |
| PICU admission | 7 (4%) | 8 (5%) | −1 (−5 to 3)% |
| Length of stay (days), median (IQR) | 8 (5 to 51) | 9 (8 to 12) | [P = 0.8975] |
| Mechanical ventilation | 5 (4%) | 3 (1%) | 3% (−1 to 7)% |
| Vasoactive support | 5 (4%) | 5 (2%) | 2% (−2 to 6)% |
| Bacteremia | 56 (27%) | 103 (30%) | −3 (−11 to 4) % |
| Origin | |||
| Health care–associated infection | 8 (14%) | 22 (21%) | −7 (−20 to 5)% |
| Community-onset infection | 46 (85%) | 76 (78%) | 7 (−6 to 20)% |
| Missing | 2 | 5 | — |
| Type | |||
| Primary | 4 (7%) | 17 (17%) | −10 (−20 to 0)% |
| Secondary | 52 (93%) | 86 (84%) | 9 (−1 to 19) % |
| Secondary infection | |||
| Osteoarticular | 38 (73%)‡ | 46 (53%)‡ | 20 (4 to 36)% |
| Pulmonary | 9 (17%)‡ | 10 (12%)‡ | 5 (−7 to 17)% |
| SSTI | 3 (6%)‡ | 18 (21%)‡ | −15% (−26 to −4)% |
| Others | 2 (4%)‡ | 12 (14%)‡ | −10 (−19 to −1)% |
| PICU admission | 29 (52%) | 29 (28%) | 24 (8 to 40)% |
| Length of stay (days), median (IQR) | 14 (8 to 23) | 5 (2 to 15) | [P = 0.2429] |
| Mechanical ventilation | 17 (30%) | 13 (13%) | 17 (3 to 31)% |
| Vasoactive support | 21 (38%) | 18 (17%) | 21 (6 to 36)% |
| Osteoarticular | 59 (28%) | 77 (23%) | 5 (−3 to 13)% |
| Diagnosis | |||
| OM | 24 (41%) | 36 (43%) | −2 (−19 to 15) % |
| SA | 18 (31%) | 27 (33%) | −2 (−18 to 14)% |
| Both (SA and OM) | 16 (27%) | 13 (16%) | 11 (−3 to 25)% |
| Missing | 4 | 9 | |
| ICU admission | 21 (36%) | 9 (12%) | 24 (10 to 38)% |
| Length of stay (days), median (IQR) | 8 (6 to 10) | 4 (2 to 15) | [P = 0.648] |
| Mechanical ventilation | 11 (19%) | 2 (3%) | 16 (5 to 27)% |
| Vasoactive support | 16 (27%) | 4 (5%) | 22 (10 to 34)% |
| Surgical treatment | 55 (93%) | 70 (91%) | 2 (−7 to 11)% |
| More than 1 surgical procedure‡ | 49 (89%)‡ | 43 (61%)‡ | 28 (14 to 42)% |
| More than 3 surgical procedures‡ | 25 (45%)‡ | 17 (24%)‡ | 21 (4 to 38)% |
| Pneumonia | 23 (11%) | 22 (6%) | 5 (0 to 10)% |
| Chest radiographs | |||
| Interstitial and alveolar pattern | 1 (4%) | 8 (38%) | −34 (−56 to −12)% |
| Lobar and multilobar consolidation | 22 (96%) | 13 (62%) | 34 (12 to 56)% |
| Complicated pneumonia§ | 13 (57%) | 5 (24%) | 33 (6 to 60) % |
| Missing | 0 | 1 | |
| Duration of hospital stay (days), median (IQR) | 17.5 (13.5 to 24) | 18 (12 to 35) | [P = 0.7244] |
| ICU admission | 17 (74%) | 11 (50%) | 24 (−3 to 52)% |
| Length of stay (days), median (IQR) | 8 (4 to 13) | 6 (5 to 10) | [P = 0.7612] |
| Mechanical ventilation | 14 (61%) | 7 (32%) | 29 (1 to 57)% |
| Vasoactive support | 12 (52%) | 6 (27%) | 25 (−3 to 53)% |
OM indicates osteomyelitis; SA, septic arthritis.
*Differences and 95% CIs calculated as the percentage with MRSA minus the percentage with MSSA. Negative values represent a higher proportion of patients with MSSA for the given variable.
†P value by Kruskal-Wallis; statistical significance, P<0.05.
‡Percentage of the blood cultures, secondary bacteremia or surgical treatment.
§Complicated pneumonia defined as the presence of empyema, pneumatoceles, pleural effusion, necrotizing pneumonia or cavitation.
Skin and Soft Tissue Infections
There were 356 cases of SSTI of which 137 (38.5%) were MRSA and 219 (61.5%) MSSA. In the MRSA group, 137 (65%) presented with SSTI and 219 (64%) in the MSSA group (Table 4). No differences were found between the type of infection either between surgical drainage and spontaneous drainage based on susceptibility. The presence of nonsuppurative infections (without drainage) was statistically more frequent in MSSA (14%) than in MRSA (7%; Table 4).
Bacteremia
One hundred fifty-nine (159) cases of bacteremia were found. Of these, 84 (52%) were associated with OI, 19 (13%) with pneumonia, 21 (13%) with SSTI and 14 (9%) with other foci; 21 (13%) had no identified focus (primary). In the MSSA group, 103 cases (30%) had bacteriemia and 56 (27%) did in the MRSA group. Bacteremia secondary to OI was more frequently associated with methicillin resistance (MRSA 73% vs. MSSA 53%) and SSTIs with methicillin-sensitive S. aureus (MRSA 6% vs. MSSA 21%). PICU admission (MRSA 52% vs. MSSA 28%), mechanical ventilation (MRSA 30% vs. MSSA 13%), and vasoactive support (MRSA 38% vs. MSSA 17%) were statistically more frequent in the MRSA group (Table 4).
OIs
One hundred thirty-six (136) cases were found: 59 (43.4%) due to MRSA and 77 (56.6%) to MSSA. In the MRSA group, 59 (28%) had OI and 77 (23%) did in the MSSA group with no differences regarding the type of infection [osteomyelitis (OM), AS or septic arthritis, and OM; Table 4]. Positive blood cultures in 38 (64%) were associated with MRSA and 40 (52%) with MSSA (Diff, 12%; 95% CI −5% to 29%). In 116 (92.8%), tissue cultures were positive, with no differences between MRSA [52 (96.3%)] and MSSA [64 (90.4%); Diff, 6%; 95% CI −3% to 15%]. It is significant that patients with MRSA isolation required more than 3 surgical procedures (MRSA 45% vs. MSSA 24%). In addition, there were significantly more PICU admissions (36% vs. 12%), more mechanical ventilation (19% vs. 3%), more vasoactive support (27% vs. 5%) and more days of hospitalization [20 (15–31) vs. 15 (11–21)] related to MRSA.
Pneumonia
Forty-five (45) cases were identified of which 23 (51%) were associated with MRSA and 22 (49%) with MSSA. Chest radiographs showed differences in lobar and multilobar consolidation patterns (MRSA 96% vs. MSSA 62%) and complicated pneumonia (MRSA 57% vs. MSSA 24%). Admission to PICU (74% vs. 50%) and the need for mechanical ventilation (61% vs. 32%) was more frequent in the MRSA group.
DISCUSSION
The present study found a relationship between OIs, secondary bacteremia, complicated pneumonia, and the presence of MRSA with no differences in SSTI. Some of the risk factors previously described in MRSA infections in this series were interestingly associated with MSSA infections (previous major surgery, use of any implanted device and recent hospitalization).
Finding risk factors associated with MSSA such as major surgery in the last 6 months or the presence of implanted medical devices was rare,23 but this is consistent with the most recent study by Vallejo et al of neurosurgical patients, where greater susceptibility to oxacillin was observed in infections in patients with ventriculoperitoneal shunts (70%).24 Likewise, in 2019, Foster et al reported that MSSA was the primary agent in infections associated with orthopedic implants (72%).25
Regarding SSTI, a higher frequency of MSSA was identified in the study population while no significant differences were found by year and demographic profile compared with MRSA. The Texas Children’s Hospital demonstrated a significant decline in invasive and SSTI from CA-MRSA and suggested that CA-MSSA could be a reemerging pathogen in this type of infection.26 This decrease in CA-MRSA is partially attributed to the possible acquisition of herd immunity to the endemic clone USA300 of CA-MSSA, which could protect against invasive infections.26 In this study, no differences were identified based on the type of infection (abscess or cellulitis). However, as Elliott et al described, MSSA was more frequent in nonsuppurative skin infections in accordance with recent studies in which no differences were seen in the treatment with β-lactam versus other antibiotics with MRSA activity.27 This is similar to our findings that nonsuppurative skin infections seem to be more frequently associated with MSSA or Streptococcus pyogenes.28,29
Most cases of bacteremia were caused by CA-MSSA and, to a lesser extent, by hospital-acquired MRSA. These findings are consistent with the usual behavior in bacteremia due to MSSA30 and MRSA.31 In children, community-based S. aureus bacteremia is more frequent and is associated with concomitant foci of infection30,32 while healthcare-associated bacteremia is more commonly observed in neonates under the age of one and usually related to intravascular devices.33 Significant risk factors were found based on the origin of secondary bacteremia; osteoarticular and pulmonary foci are at increased risk of secondary bacteremia due to MRSA. Hamdy et al also demonstrated that foci such as OM (31%), catheter-related infection (22%), SSTI (16%) and pneumonia (9%) are predominant31 in a cohort of children with bacteremia caused by MRSA. Abernethy et al, in turn, reported that most bacteremia cases caused by MSSA originated from SSTI33; this is similar to what has been reported in this study.
OIs due to MRSA were more severe than MSSA infections. They required more than 3 surgical interventions, coinciding with Arnold et al, and others, who stated that patients with MRSA needed surgical procedures more frequently (91%) than cases with MSSA (62%; P < 0.001).16,34–36 In contrast to the findings of this study, reports from Finland and France, where MSSA predominates, describe less need for surgical procedures.37,38 Kaplan reported differences in the United States (minor surgeries, shorter duration of antibiotics) possibly due to the predominance of MSSA and the lower frequency of virulence factors, particularly the presence of Panton-Valentine leucocidin.39 Longer hospital stays for MRSA compared with MSSA are described in the literature as generally associated with greater medical complications,16 which is similar to our findings (MRSA 2015–31 vs. MSSA 1511–21; P < 0.001). In this study, MRSA significantly predicted the requirement of additional surgical procedures for the treatment of OIs and a more severe clinical course.
In our study, MRSA was slightly more frequent in bacteremia secondary to pneumonia, and this coincides with other populations where higher rates of complications and mortality have also been described.40 Pneumonia caused by MRSA is associated with a higher rate of complications such as pleural effusion, pleural thickening, increased requirement for tube placement, pneumatoceles and high mortality rates with no differences in demographic data, days with fever, tachypnea, hypoxia, and length of hospital stay.41,42 In this study, a higher frequency of complicated pneumonia was found [MRSA 13 (57%) vs. MSSA 5 (24%)]. Some studies have already described a particularly higher frequency of lung abscess and necrotizing pneumonia.43–45 The literature describes a higher frequency of complications, need for PICU, hospital stay, etc. in MRSA infections that are attributed, in part, to the presence of Panton-Valentine leucocidin, a cytotoxin that destroys leukocytes and causes tissue necrosis.38 Panton-Valentine leucocidin has been most frequently associated with abscess formation, tissue inflammation, pulmonary complications and the possibility of septic thrombophlebitis, especially in OM.46 It is most commonly found in MRSA cases, especially in the USA300 strain47 or in others depending on the geographical region.10,48
Finally, regarding the behavior of S. aureus in the study period, no differences were found in the presence of MRSA by year or by type of infection studied. This contrasts with Sutter et al, who, from 2005 to 2014, described a decrease in the frequency of MRSA in 39,207 children in the United States while reporting 59.4% susceptibility to oxacillin in 2005 and 68.4% in 2014.7 Similarly, Hulten et al reported a 60.4% drop in MRSA infections in the Texas Children’s Hospital between 2007 and 2014.23 Considering the current findings, future changes in the behavior of MRSA in children may be expected. It is, therefore, essential to promote surveillance of S. aureus infections in the pediatric population across the country.
Some of the limitations of the present study include the retrospective nature of the data collection process and the failure to carry out molecular studies to describe virulence genes.
Conclusion
This study raises the importance of active monitoring of S. aureus infections in pediatrics in our region.
Footnotes
The authors have no conflicts of interest to disclose.
Ethics Committee: This study was submitted and approved by the ethics and the research on humans committees in all hospitals. Hospital ethics review board (Comité de ética en investigación con seres humanos Hospital de San José, CEISH-HSJ). 26/Junio/2019. N 0272-2019
References
- 1.Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong SY, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zervou FN, Zacharioudakis IM, Ziakas PD, et al. MRSA colonization and risk of infection in the neonatal and pediatric ICU: a meta-analysis. Pediatrics. 2014;133:e1015–e1023. [DOI] [PubMed] [Google Scholar]
- 4.Senthilkumar K, Biswal N, Sistla S. Risk factors associated with methicillin-resistant Staphylococcus aureus infection in children. Indian Pediatr. 2015;52:31–33. [DOI] [PubMed] [Google Scholar]
- 5.David MZ, Daum RS. Update on epidemiology and treatment of MRSA infections in children. Curr Pediatr Rep. 2013;1:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber JS, Coffin SE, Smathers SA, et al. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2019. Stockholm: ECDC; 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf. Accessed November 30, 2020. [Google Scholar]
- 8.Sutter DE, Milburn E, Chukwuma U, et al. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137:e20153099. [DOI] [PubMed] [Google Scholar]
- 9.Song X, Cogen J, Singh N. Incidence of methicillin-resistant Staphylococcus aureus infection in a children’s hospital in the Washington metropolitan area of the United States, 2003 - 2010. Emerg microbes & infect. 2013;2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sáenz Moncaleano V. Boletín Informativo: resultados de la vigilancia de la resistencia bacteriana Año 2018 componente pediátrico y adulto. Universidad Nacional de Colombia; 2018:26. [Google Scholar]
- 11.Márquez-Ortiz RA, Álvarez-Olmos MI, Escobar Pérez JA, et al. ; Research Group of Pediatric Infectious Diseases. USA300-related methicillin-resistant Staphylococcus aureus clone is the predominant cause of community and hospital MRSA infections in Colombian children. Int J Infect Dis. 2014;25:88–93. [DOI] [PubMed] [Google Scholar]
- 12.Rojo P, Barrios M, Palacios A, et al. Community-associated Staphylococcus aureus infections in children. Expert Rev Anti Infect Ther. 2010;8:541–554. [DOI] [PubMed] [Google Scholar]
- 13.John CC, Schreiber JR. Therapies and vaccines for emerging bacterial infections: learning from methicillin-resistant Staphylococcus aureus. Pediatr Clin North Am. 2006;53:699–713. [DOI] [PubMed] [Google Scholar]
- 14.Mejer N, Westh H, Schonheyder HC, et al. Stable incidence and continued improvement in short term mortality of Staphylococcus aureus bacteraemia between 1995 and 2008. BMC infectious diseases. 2012;12:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klieger SB, Vendetti ND, Fisher BT, et al. Staphylococcus aureus bacteremia in hospitalized children: incidence and outcomes. Infect Control Hosp Epidemiol. 2015;36:603–605. [DOI] [PubMed] [Google Scholar]
- 16.Sattler CA, Mason EO, Jr, Kaplan SL. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr Infect Dis J. 2002;21:910–917. [DOI] [PubMed] [Google Scholar]
- 17.Davis WT, Gilbert SR. Comparison of methicillin-resistant versus Susceptible Staphylococcus aureus pediatric osteomyelitis. J Pediatr Orthop. 2018;38:e285–e291. [DOI] [PubMed] [Google Scholar]
- 18.Bouras D, Doudoulakakis A, Tsolia M, et al. Staphylococcus aureus osteoarticular infections in children: an 8-year review of molecular microbiology, antibiotic resistance and clinical characteristics. J Med Microbiol. 2018;67:1753–1760. [DOI] [PubMed] [Google Scholar]
- 19.Doudoulakakis AG, Bouras D, Drougka E, et al. Community-associated Staphylococcus aureus pneumonia among Greek children: epidemiology, molecular characteristics, treatment, and outcome. Eur J Clin Microbiol Infect Dis. 2016;35:1177–1185. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich LN, Reid D, Doo D, et al. Predicting MSSA in acute hematogenous osteomyelitis in a setting with MRSA prevalence. J Pediatr Orthop. 2015;35:426–430. [DOI] [PubMed] [Google Scholar]
- 21.Wade Shrader M, Nowlin M, Segal LS. Independent analysis of a clinical predictive algorithm to identify methicillin-resistant Staphylococcus aureus osteomyelitis in children. J Pediatr Orthop. 2013;33:759–762. [DOI] [PubMed] [Google Scholar]
- 22.An TJ, Benvenuti MA, Mignemi ME, et al. Similar clinical severity and outcomes for methicillin-resistant and methicillin-Susceptible Staphylococcus aureus pediatric musculoskeletal infections. Open Forum Infect Dis. 2017;4:ofx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue S, Moriyama T, Horinouchi Y, et al. Comparison of clinical features and outcomes of staphylococcus aureus vertebral osteomyelitis caused by methicillin-resistant and methicillin-sensitive strains. Springerplus. 2013;2:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallejo JG, Cain AN, Mason EO, et al. Staphylococcus aureus central nervous system infections in children. Pediatr Infect Dis J. 2017;36:947–951. [DOI] [PubMed] [Google Scholar]
- 25.Foster CE, Lamberth LB, Kaplan SL, et al. Clinical characteristics and outcomes of Staphylococcus aureus implant-associated infections in children. Pediatr Infect Dis J. 2019;38:808–811. [DOI] [PubMed] [Google Scholar]
- 26.Hultén KG, Mason EO, Lamberth LB, et al. Analysis of invasive community-acquired methicillin-Susceptible Staphylococcus aureus infections during a period of declining community acquired methicillin-resistant Staphylococcus aureus infections at a large children’s hospital. Pediatr Infect Dis J. 2018;37:235–241. [DOI] [PubMed] [Google Scholar]
- 27.Elliott DJ, Zaoutis TE, Troxel AB, et al. Empiric antimicrobial therapy for pediatric skin and soft-tissue infections in the era of methicillin-resistant Staphylococcus aureus. Pediatrics. 2009;123:e959–e966. [DOI] [PubMed] [Google Scholar]
- 28.Shuman EK, Malani PN. Empirical MRSA coverage for nonpurulent cellulitis: swinging the pendulum away from routine use. JAMA. 2017;317:2070–2071. [DOI] [PubMed] [Google Scholar]
- 29.Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs cephalexin alone on clinical cure of uncomplicated cellulitis: a randomized clinical trial. JAMA. 2017;317:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMullan BJ, Bowen A, Blyth CC, et al. Epidemiology and mortality of Staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatr. 2016;170:979–986. [DOI] [PubMed] [Google Scholar]
- 31.Hamdy RF, Hsu AJ, Stockmann C, et al. Epidemiology of methicillin-resistant Staphylococcus aureus bacteremia in children. Pediatrics. 2017;139:e20170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles F, Voss L, Segedin E, et al. Review of Staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Arch Dis Child. 2005;90:1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abernethy J, Sharland M, Johnson AP, et al. How do the epidemiology of paediatric methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus bacteraemia differ? J Med Microbiol. 2017;66:737–743. [DOI] [PubMed] [Google Scholar]
- 34.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–708. [DOI] [PubMed] [Google Scholar]
- 35.Sarkissian EJ, Gans I, Gunderson MA, et al. Community-acquired Methicillin-resistant Staphylococcus aureus Musculoskeletal Infections: Emerging Trends Over the Past Decade. J Pediatr Orthop. 2016;36:323–327. [DOI] [PubMed] [Google Scholar]
- 36.Jain MJ, Bradko V, Zhu H, Inneh I, Shinava VR. Pediatric osteoarticular infection: trend in surgically treated patients and association of methicillin-resistant Staphylococcus aureus with requirement of secondary procedures [published online ahead of print October 8, 2020]. J Pediatr Orthop B. 2020. Available at: https://journals.lww.com/jpo-b/Abstract/9000/Pediatric_osteoarticular_infection__trend_in.98734.aspx. [DOI] [PubMed] [Google Scholar]
- 37.Peltola H, Pääkkönen M, Kallio P, et al. ; Osteomyelitis-Septic Arthritis Study Group. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J. 2010;29:1123–1128. [DOI] [PubMed] [Google Scholar]
- 38.Gillet Y, Dohin B, Dumitrescu O, et al. Infections ostéoarticulaires à staphylocoques dorés sécréteurs de la leucocidine de Panton-Valentine [Osteoarticular infections with Staphylococcus aureus secreting Panton-Valentine leucocidin]. Arch Pediatr. 2007;14(suppl 2):S102–S107. French. doi: 10.1016/s0929-693x(07)80043-1 [DOI] [PubMed] [Google Scholar]
- 39.Kaplan SL. Acute hematogenous osteomyelitis in children: differences in clinical manifestations and management. Pediatr Infect Dis J. 2010;29:1128–1129. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber MP, Chan CM, Shorr AF. Bacteremia in Staphylococcus aureus pneumonia: outcomes and epidemiology. J Crit Care. 2011;26:395–401. [DOI] [PubMed] [Google Scholar]
- 41.Martín Espín I, Aguilera-Alonso D, García-Perea A, et al. Methicillin-resistant Staphylococcus aureus community-acquired pneumonia in infants. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37:551–552. [DOI] [PubMed] [Google Scholar]
- 42.Len KA, Bergert L, Patel S, et al. Community-acquired Staphylococcus aureus pneumonia among hospitalized children in Hawaii. Pediatr Pulmonol. 2010;45:898–905. [DOI] [PubMed] [Google Scholar]
- 43.Erdem G, Bergert L, Len K, et al. Radiological findings of community-acquired methicillin-resistant and methicillin-susceptible Staphylococcus aureus pediatric pneumonia in Hawaii. Pediatr Radiol. 2010;40:1768–1773. [DOI] [PubMed] [Google Scholar]
- 44.Vardakas KZ, Matthaiou DK, Falagas ME. Comparison of community-acquired pneumonia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus producing the Panton-Valentine leukocidin. Int J Tuberc Lung Dis. 2009;13:1476–1485. [PubMed] [Google Scholar]
- 45.Morikawa K, Okada F, Ando Y, et al. Meticillin-resistant Staphylococcus aureus and meticillin-susceptible S. aureus pneumonia: comparison of clinical and thin-section CT findings. Br J Radiol. 2012;85:e168–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John JF, Jr, Lindsay JA. Clones and drones: do variants of panton-valentine leukocidin extend the reach of community-associated methicillin-resistant Staphylococcus aureus?. J Infect Dis. 2008;197:175–178. [DOI] [PubMed] [Google Scholar]
- 47.Brown ML, O'Hara FP, Close NM, et al. Prevalence and sequence variation of panton-valentine leukocidin in methicillin-resistant and methicillin-susceptible staphylococcus aureus strains in the United States. J Clin Microbiol. 2012;50:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aires-de-Sousa M, Conceicao T, de Lencastre H. Unusually high prevalence of nosocomial Panton-Valentine leukocidin-positive Staphylococcus aureus isolates in Cape Verde Islands. J Clin Microbiol. 2006;44:3790–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]