The volume loss of skeletal muscle, including lumbar paravertebral muscles, is negatively correlated with the rate of lumbo-pelvic deformity. Moreover, ectopic fat infiltration with highly expressed adipogenesis promoting genes in the paravertebral muscles may reflect pathology of sarcopenia and may cause age-related lumbo-pelvic deformity.
Keywords: atrogin-1, atrophy, cebpa, ectopic fat infiltration, Goutallier classification, lumbar paravertebral muscles, Murf1, older adults, Pparg, sarcopenia, skeletal muscle volume, spine-pelvic deformity
Abstract
Study Design.
A retrospective analysis of a prospective, non-randomized cohort dataset.
Objective.
To cross-sectionally examine the prevalence of sarcopenia and the association between spine-pelvic deformity and skeletal muscle volume loss and ectopic fat infiltration into lumbar paravertebral muscles (PVMs) in patients who underwent lumbar surgery.
Summary of Background Data.
Muscle quality deterioration has been considered the main pathology of sarcopenia, reducing muscle strength directly. The qualitative deterioration as well as volume loss in PVM, which contributes significantly to core body extension, might cause aging-related spine deformity.
Methods.
In total, 184 patients were included. Sarcopenia was diagnosed at baseline, and all patients underwent whole-body X-ray. The amount of fat in lumbar PVM was evaluated with the Goutallier classification in magnetic resonance imaging findings. The expression of adipogenesis- and atrophy-promoting factors in PVM was evaluated with quantitative polymerase chain reaction.
Results.
In total, 36.1% of adults aged ≥60 years were diagnosed with sarcopenia. The values of skeletal muscle indexes of the limb and trunk were inversely correlated with the sagittal vertical axis, pelvic tilt (PT), and pelvic incidence minus lumbar lordosis (PI-LL) values. The PT and PI-LL were greater, PVM area was smaller, and Goutallier grade was greater in sarcopenic adults than in non-sarcopenic older adults. Additionally, the PVM area correlated with the LL value, and Goutallier's grade correlated with the PT and PI-LL values. Moreover, the amount of ectopic fat in PVMs inversely correlated with skeletal muscle indexes. The expression levels of atrophy gene-1 and muscle ring-finger protein-1 did not differ between the groups and did not correlate with the PVM area. In contrast, the expression of Pparg and Cebpa was upregulated in sarcopenic older adults, where it correlated with Goutallier's grade.
Conclusion.
The volume loss of skeletal muscle, including lumbar PVM, and ectopic fat infiltration into the PVM, may cause the lumbo-pelvic deformity.
Level of Evidence: 3
Sarcopenia is an age-related loss of muscle mass and strength; it has recently become a therapeutic target for efforts aimed at reducing the risk of decreased mobility, serious disability from a fall, and overall physical frailty.1 The prevalence of sarcopenia varies; a previous medical check-up-based survey in Japan estimated it at 8.2%.2 However, estimates obtained from patients who underwent hip surgery or had chronic lumbar pain were 25.7%3 and 40%, respectively.4 The loss of skeletal muscle size and function begins over 40 years of age,5 and muscles responsible for maintaining posture or core body extension are affected earlier.6 In addition, lumbar multifidus contributes to maintaining standing and sitting postures and gait.7 It is currently common knowledge that lumbar kyphosis and posterior pelvic tilt increase lower back pain.8 Therefore, we hypothesized the sarcopenic changes in paravertebral muscle (PVM) holding lumbar lordosis and pelvic antever-sion9,10 might cause age-related spine deformity.
Sarcopenia is an involuntary loss of skeletal muscle mass, slow gait speed, and weak handgrip strength in older adults.11,12 Age-induced muscle weakness precedes muscle volume loss13,14; the deterioration of muscle quality, including ectopic fat infiltration, atrophy, fibrosis, and extracellular fluid volume increase,15 are considered possible causes of sarcopenia and predictors of incident mobility limitations.16 Lipids present in ectopic adipo-cytes are toxic and can cause cell dysfunction and death; similarly, they are associated with insulin resistance and diabetes risk.17 The ectopic fat infiltration into skeletal muscles increases with age, reducing muscle strength and functions directly.18
Recent studies have used computed tomography and magnetic resonance imaging (MRI) scanning to quantify the ectopic fat increase in PVM and its relationship with lower back pain, spinal deformity, and postoperative damage via the posterior approach.19–21 Minimally invasive surgery techniques, such as percutaneous pedicle screw and lateral interbody fusion, have allowed spine surgeons to preserve PVM and its quality. However, even with the minimally invasive techniques, the physical burden of corrective fixation surgery for spinal deformity in the range of thoracic to sacral vertebrae is significant, and the range of motion of the spine is sacrificed.22 Further, elucidation of the pathogenesis of age-related spinal deformities may lead to the development of preventive treatment to avoid surgery and spinal deformity.
Regarding the mechanism of qualitative deterioration in muscles, several animal-based studies and studies of mesenchymal stem cell differentiation have reported that the levels of adipogenesis promoting factor expression, for example, peroxisome proliferator-activated receptor gamma (Pparg) and CCAAT/enhancer-binding protein alpha (Cebpa),21 increase during ectopic fat infiltration in skeletal muscles.23 In contrast, muscle atrophy is induced by muscle ring-finger protein-1 (Murf1) and atrophy gene-1 (Atrogin-1) expression, regulated by forkhead box O or nuclear factor kappa B signals.24,25
This study aimed to investigate the influence of PVM quality and muscular volume on the prevalence of sarcopenia and lumbo-pelvic deformity. Furthermore, the extent of atrophy and ectopic fat infiltration of PVM was evaluated by analyzing relevant gene expression levels along with MRI findings in patients who underwent lumbar surgeries.
MATERIALS AND METHODS
Patients were eligible for this study if they were aged >20 years and underwent lumbar surgery at our institution between March 2019 and January 2021. Patients were excluded from this study if they were diagnosed with skeletal dysplasia, chromosomal abnormality, tumor, infection, acute trauma, nonunion vertebral body, or if they had a history of spinal surgery or an existing implant, or if they could not undergo MRI scanning, skeletal muscle index (SMI) evaluation, or a standing whole-spine X-ray.
Diagnosis of Sarcopenia
Sarcopenia was diagnosed based on the Asian Working Group for Sarcopenia 2019 guidelines,11 which stipulates low limb muscle-mass (SMI of <7.0 kg/m2 in men and <5.7 kg/m2 in women) with low muscle strength (handgrip strength of <28 kg in men and <18 kg in women), and/or low physical performance (6 m walking speed of <1.0 m/s). SMI was evaluated in all patients with InBody720 (InBody Co, Ltd, Seoul, Korea), a multifrequency bioelectrical impedance analyzer.
Radiographic Imaging
All patients underwent a full-length coronal and lateral X-ray in a free-standing position before surgery. The following parameters were examined using the digital imaging and communications in medicine (DICOM) system ShadeQuest/ViewR (Yokogawa Medical Solutions Corporation, Tokyo, Japan): Cobb angle, sagittal vertical axis (SVA), lumbar lordosis (LL, L1S1), pelvic tilt (PT), pelvic incidence minus lumbar lordosis (PI-LL), and thoracic kyphosis (TK, T5T12) values.8
Magnetic Resonance Imaging
MRI scans of the lumbar spine were obtained. MRI scanners with a static magnetic field strength of 1.5 or 3 T were used. The imaging protocol included T1- and T2-weighted transverse fast spin-echo images. Observers reviewed the images using the DICOM system ShadeQuest/ViewR. Cross-sectional areas of individual PVM and intervertebral disc at levels L4–5 were measured on the axial T2-weighted image by constructing polygon points around the outer margins of the targets. Bilateral PVM areas relative to the disc area were analyzed as PVM area.
Fat content in the PVM at levels L4–5 was graded by the Goutallier classification with T1-weighted axial images.20,26 Grades 0, 1, 2, 3, and 4 corresponded to no intramuscular fat, some fatty streaks present, fat present at a volume lower than that of muscle tissue, fat volume equal to muscle volume, and fat volume greater than muscle volume, respectively (Figure 1). Grades assigned during two rounds of assessment by two examiners 2 weeks apart were averaged. The kappa value calculated using all estimates from both observers (DL and TK) was 0.736 (95% confidence interval [CI]: 0.681–0.791). The corresponding within-observer values were 0.771 (95% CI 0.696–0.847) and 0.798 (95% CI 0.727–0.868) for DL and TK, respectively.
Figure 1.
Representative images of each Goutallier classification grade in lumbar paravertebral muscles (PVMs). PVMs are indicated by the red lines.
Quantitative Polymerase Chain Reaction Analysis of Paraspinal Muscles
PVM samples (5–10 mg) were collected surgically and preserved in RNAlater (Thermo Fisher Scientific, Waltham, MA). Total RNA was extracted using ReliaPrep RNA Tissue Miniprep System (Promega, Madison, WI) and reverse transcribed into complementary DNA, using Moloney Murine Leukemia Virus Reverse Transcriptase (Invitrogen, Carlsbad, CA). Gene expression levels were analyzed by the ΔΔthreshold cycle (CT) method, using the SYBR Green and Step One Plus Real-time polymerase chain reaction (PCR) system (Thermo Fisher Scientific, Waltham, MA). All the samples were assayed in triplicate, and the CT values were averaged. Glyceraldehyde-3-phosphate dehydrogenase was employed as the internal control, and the CT values of the targets were normalized by those of the control group. Primer sequences are presented in Table 1.
TABLE 1.
Sequences of the Primers Used in Quantitative Polymerase Chain Reaction Analysis
| Gapdh | F: TGAGAAGTATGACAACAGCCTC R: CATGGACTGTGGTCATGAG |
| Pparg | F: GTCTCATAATGCCATCAGGTTTG R: GATAACGAATGGTGATTTGTCTG |
| Cebpa | F: GTCACACCAGAAAGCTAG GTC R: GGCATACAGTACAAACAAGGC |
| Atrogin-1 | F: CTGCTGTGGAAGAAACTCTG R: ATCTTCTTCCAATCCAGCTG |
| Murf1 | F: ATCACTCAGCTGGAGGATTC R: AACTTCTGGCTCAGCTCTTC |
Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analyses
Statistical analyses were performed using SPSS version 21 (IBM, Armonk, NY). Differences in the average values were assessed using Student t test. Pearson's correlation coefficients (r) were assessed in the correlation analysis; |r| >0.20 was considered indicative of a correlation. Estimate uncertainty was expressed using 95% CI, and _P values <0.05 were considered statistically significant.
RESULTS
Overall, of 234 patients in whom lumbar surgeries were performed during the study period, 184 were included in this study. Among 147 patients aged ≥60 years, 28.0% and 46.2% of men and women were diagnosed with sarcopenia, respectively (Table 2). Fourteen men and 23 women aged <60 years were categorized as young control patients. The impact of age on the decrease in the limb and trunk SMI was stronger in men than in women (Figure 2). Patients with sarcopenia were significantly older than those without sarcopenia (men 78.6 vs. 70.3, P < 0.001, women 75.6 vs. 72.0, P < 0.05, t test). There was no significant difference in body fat percentage between the sarcopenic and non-sarco-penic groups; however, the former group had lower BMI (men 22.7 vs. 25.3, P = 0.001, women 23.0 vs. 25.6, P < 0.05) and SMI of the upper and lower limbs and trunk than the latter group (men 1.7/4.7/7.2 vs. 2.2/5.7/7.8, respectively, P < 0.001, women 1.4/3.7/5.1 vs. 1.6/4.5/ 6.1, respectively, P < 0.001), suggesting an age-related muscle loss without obesity in patients with sarcopenia. In addition, sarcopenic men had both lower walking speed and grip strength than their counterparts.
TABLE 2.
Comparison of Sarcopenia Diagnostic Factors and Body Composition Between Young Controls, Nonsarcopenic, and Sarcopenic Older Adults
| Young Controls | Nonsarcopenic Older Adults | Sarcopenic Older Adults | P Value | ||
| Number patients | M | 14 | 59 | 23 | |
| W | 23 | 35 | 30 | ||
| Age (yrs) | M | 54.2 (±3.8) | 70.3 (±6.5) | 78.6 (±5.2) | <0.001 |
| W | 50.4 (±8.0) | 72.0 (±6.3) | 75.6 (±7.1) | 0.034 | |
| Body mass index (kg/ m2) | M | 27.1 (±4.7) | 25.3 (±3.0) | 22.7 (±2.9) | 0.001 |
| W | 23.8 (±3.2) | 25.6 (±3.9) | 23.0 (±3.4) | 0.005 | |
| Body fat percentage (%) | M | 29.1 (±0.1) | 27.0 (±0.1) | 25.6 (±0.1) | 0.515 |
| W | 30.1 (±0.1) | 36.3 (±0.1) | 34.5 (±0.1) | 0.452 | |
| Upper limb SMI (kg/ m2) | M | 2.30 (±0.4) | 2.17 (±0.3) | 1.68 (±0.3) | <0.001 |
| W | 1.57 (±0.3) | 1.63 (±0.3) | 1.36 (±0.2) | <0.001 | |
| Lower limb SMI (kg/ m2) | M | 5.74 (±0.7) | 5.68 (±0.7) | 4.69 (±0.5) | <0.001 |
| W | 4.35 (±1.1) | 4.52 (±0.7) | 3.73 (±0.5) | <0.001 | |
| Limb SMI (kg/m2) | M | 8.04 (±1.0) | 7.84 (±0.8) | 6.37 (±0.7) | <0.001 |
| W | 5.92 (±1.3) | 6.14 (±0.9) | 5.08 (±0.6) | <0.001 | |
| Trunk SMI (kg/m2) | M | 9.04 (±1.2) | 8.61 (±0.8) | 7.22 (±0.9) | <0.001 |
| W | 7.13 (±0.8) | 7.34 (±0.7) | 6.48 (±0.7) | <0.001 | |
| Gait speed (m/s) | M | 0.96 (±0.2) | 0.97 (±0.4) | 0.67 (±0.3) | <0.001 |
| W | 0.81 (±0.2) | 0.83 (±0.4) | 0.71 (±0.3) | <0.001 | |
| Grip power (kg) | M | 35.0 (±8.9) | 34.2 (±7.0) | 25.3 (±7.2) | <0.001 |
| W | 23.5 (±5.4) | 19.2 (±3.9) | 16.7 (±5.0) | 0.206 |
The average values presented with standard deviation. The P values represent the statistical significance of Student t test in the difference between the average of nonsarcopenic and sarcopenic older adults.
M indicates men; W, women.
Figure 2.
Correlation between the patients’ age and skeletal muscle index (SMI). n = 96 in men, 88 in women.
Radiographic Analysis of the Spine
Among the X-ray parameters of the whole subjects shown in Figure 3, only TK values were greater in non-sarcopenic older adults than in young controls (22.1 vs. 17.6, P < 0.05, t test). In contrast, SVA, PT, PI-LL, and TK values were significantly greater in sarcopenic older adults than in young controls (59.6 vs. 38.1,24.5 vs. 20.7,17.7 vs. 10.2, and 24.0 vs. 17.6, respectively, P < 0.05). Moreover, PT and PI-LL values were significantly greater in sarcopenic older adults than in non-sarcopenic older adults (24.5 vs. 18.7 and 17.7 vs. 7.8, respectively, P < 0.001).
Figure 3.
Comparison of X-ray parameters between young controls, non-sarcopenic older adults, and sarcopenic older adults. Results presented as boxplots and crosses represent median values, and differences in the average values presented in the graphs assessed using Student t test. N.S. indicates not significant. ∗P < 0.05. ∗∗P < 0.001.
There was an inverse correlation between limb SMI and SVA values in men (r – 0.25, P) and between limb and trunk SMI and PT (r = –0.24, P = 0.01 and r = –0.35, P = 0.001) and PI-LL values (r = –0.26 and –0.32, respectively, P < 0.05) in women. (Table 3).
TABLE 3.
Correlation Between Limb or Trunk Skeletal Mass Index (SMI) and the Sagittal X-ray Parameters
| Men | SVA (mm) | LL (°) | PT (°) | PI-LL (°) | TK (°) |
| Limb SMI (kg/m2) | r = –0.25 P = 0.01 | r = 0.13P = 0.21 | r = 0.13P = 0.21 | r = –0.19P = 0.07 | r = –0.09P = 0.40 |
| Trunk SMI (kg/m2) | r = –0.11P = 0.29 | r = 0.10P = 0.32 | r = 0.10P = 0.32 | r = –0.15vP = 0.15 | r = –0.07P = 0.49 |
| Women | |||||
| Limb SMI (kg/m2) | r = –0.12P = 0.28 | r = 0.12P = 0.28 | r = –0.21 P = 0.04 | r = –0.26 P = 0.02 | r = –0.01P = 0.89 |
| Trunk SMI (kg/m2) | r = –0.08P = 0.44 | r = 0.10P = 0.34 | r = –0.35 P = 0.001 | r = –0.32 P = 0.002 | r = –0.01P = 0.93 |
The factors of |Pearson correlation coefficients (r)|>0.20 are illustrated in bold, along with those P values. n = 96 in men, 88 in women.
Lumbar Magnetic Resonance Imaging Findings
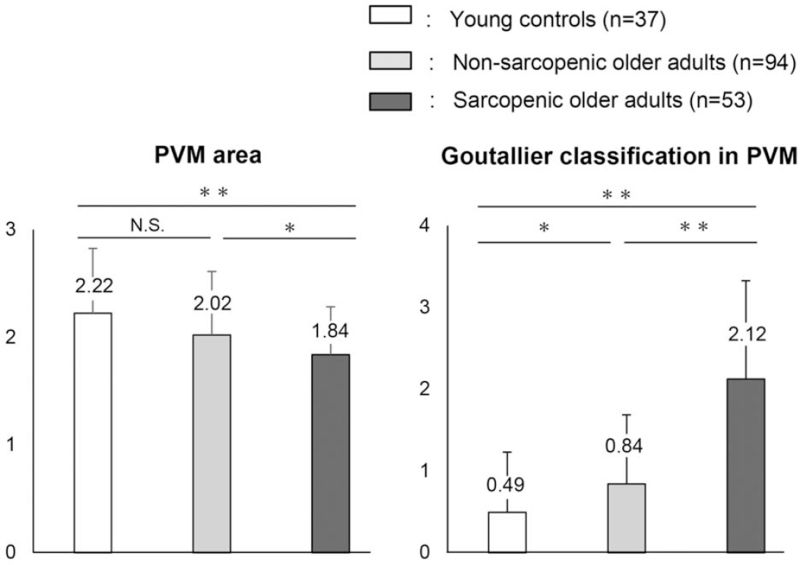
The PVM area relative to the disc area at L4-5 levels was significantly smaller in sarcopenic than in non-sarcopenic older adults (1.84 vs. 2.02, P < 0.05, t test, Figure 4) as skeletal muscle mass of limb and trunk decreased in sarcopenia. The grade of Goutallier classification in PVM at this level was significantly greater in sarcopenic older adults than in their counterparts (2.12 vs. 0.84, P < 0.001). Additionally, the PVM area in women correlated with the limb (r = 0.29, P < 0.01) and trunk SMI (r = 0.24, P < 0.05); meanwhile, the amount of fat in PVM inversely correlated with the limb and trunk SMI (men r = –0.36, P = 0.001 and –0.29, P < 0.01, women r = –0.28, P = 0.01 and –0.27, P = 0.01, Figure 5).
Figure 4.
Comparison of the paravertebral muscle (PVM) area and Goutallier classification in PVM at L4-5 level. Results presented as means ± stan-standard deviations, and differences in the average values assessed using Student t test. N.S. indicates not significant. ∗P < 0.05. ∗∗P < 0.001.
Figure 5.
Correlation between the skeletal muscle index (SMI) and paravertebral muscle (PVM) area or Goutallier classification in PVM. n = 96 in men, 88 in women.
Furthermore, the PVM area correlated directly and inversely with LL (r = 0.42, P < 0.001) and PI-LL values (r = –0.28, P < 0.001), respectively; additionally, the grade of Goutallier classification significantly correlated with PT (r = –0.29, P = 0.01) and PI-LL values (r = 0.31, P < 0.001), and the patients’ age (r = 0.27, P < 0.001, Table 4).
TABLE 4.
Correlation Between Paravertebral Muscle (PVM) Area or the Grade of Goutallier Classification in PVM and Age or the Sagittal X-Ray Parameters
| Age (yrs Old) | SVA (mm) | LL (°) | PT (°) | PI-LL (°) | TK(°) | |
| PVM area | r = –0.19P = 0.06 | r = –0.20P = 0.007 | r = 0.42 P < 0.001 | r = –0.17P = 0.03 | r = –0.28 P < 0.001 | r = 0.10P = 0.19 |
| Goutallier's grade | r = –0.29 P = 0.004 | r = 0.15P = 0.05 | r = –0.11P = 0.13 | r = 0.31 P < 0.001 | r = 0.27 P < 0.001 | r = 0.09P = 0.25 |
Both the area and the Goutallier grade analyzed at L4–5 levels. The factors of |Pearson correlation coefficients (r)|>0.20 are illustrated in bold, along with those P values. n = 184.
Polymerase Chain Reaction Analysis of Lumbar PVM
Levels L4–5 were included in the surgical range of 144 patients. PCR-analyzable PVM samples were obtained from 25, 57, and 35 young controls and non-sarcopenic and sarcopenic older adults, respectively. Although the average PVM area was smaller in sarcopenic than in non-sarcopenic older adults, there was no difference among patients of the groups with PCR-analyzable samples in the levels of Atrogin-1 and Murf1 expression. Meanwhile, the levels of adipogenesis promoter expression did not differ between young controls and non-sarcopenic older adults; however, the levels of Pparg and Cebpa expression were approximately four- and three-fold higher (P < 0.05, t test), respectively, in sarcopenic than in non-sarcopenic older adults (Figure 6).
Figure 6.
Among-group comparisons of gene expression in paravertebral muscle (PVM). Results presented as means ± standard deviations, and differences in the average values assessed using Student t test. N.S. indicates not significant. ∗P < 0.05. ∗∗P < 0.001.
The muscle atrophy promoter expression levels did not correlate with the PVM area or X-ray parameters, while those of adipogenesis promoter expression correlated with the grade of Goutallier classification (Pparg: r = 0.44, P < 0.001 and Cebpa: r = 0.52, P < 0.001) and PT values (Pparg: r = 0.23, P < 0.05) in X-ray findings (Figure 7 and Table 5).
Figure 7.
Correlation between muscle atrophy promoter expression levels and paravertebral muscle (PVM) area, and levels of adipogenesis promoter expression and Goutallier's grade in PVM. n = 117.
TABLE 5.
Correlation Between the Gene Expression Levels in Paravertebral Muscles and Sagittal X-Ray Parameters
| SVA (mm) | LL (°) | PT (°) | PI-LL (°) | TK (°) | |
| Atrogin-1 | r = –0.07 | r = 0.05 | r = –0.12 | r = –0.09 | r = –0.17 |
| P = 0.43 | P = 0.59 | P = 0.18 | P = 0.34 | P = 0.08 | |
| Murf1 | r = –0.02 | r = –0.04 | r = 0.03 | r = 0.09 | r = –0.12 |
| P = 0.85 | P = 0.69 | P = 0.72 | P = 0.33 | P = 0.20 | |
| Pparg | r = –0.03 | r = 0.03 | r = 0.23 | r = 0.15 | r = 0.17 |
| P = 0.75 | P = 0.76 | P = 0.01 | P = 0.11 | P = 0.06 | |
| Cebpa | r = –0.01 | r = 0.07 | r = 0.19 | r = 0.11 | r = 0.20 |
| P = 0.92 | P = 0.48 | P = 0.04 | P = 0.25 | P = 0.03 |
The gene expression levels evaluated by quantitative polymerase chain reaction.
The factors of |Pearson correlation coefficients (r)|>0.20 are illustrated in bold, along with those P values. n = 117.
DISCUSSION
This study investigated the clinical relevance of ectopic fat infiltration into lumbar PVM on sarcopenia and spinal deformity rates in patients who underwent lumbar surgery. The prevalence of sarcopenia in patients aged >60years was 36.1%. Kyphosis and posterior PT were observed in patients with sarcopenia. To the best of our knowledge, this is the first study presenting genetic evidence that the increase in ectopic fat in PVM was negatively associated with skeletal muscle mass loss, sarcopenia prevalence, and lumbo-pelvic deformity.
Association between sarcopenia and spinal deformity has been scarcely studied previously; Eguchi reported a negative influence of trunk SMI loss on cobb angle, SVA, and PT, and that of limb SMI loss on Roland-Morris Disability Questionnaire of lower back pain.27,28 In this study, SMI decreased with age; there was a correlation between the lower trunk and limb SMI with the greater SVA, PT, and PI-LL values. Moreover, lumbar PVM size in MRI findings, which was small in sarcopenic older adults, could equally be an indicator of lumbar kyphosis. Our results indicated that the limb and trunk muscle mass and the PVM volume, a lumbar extensor for posture hold-ing,10 were important to maintain the lumbar lordosis and anterior pelvic tilt.
However, Murf1 and Atrogin-1 expression levels did not reveal any age- or sarcopenia-related changes, contrary to the adipogenesis promotors. Muscle atrophy-inducing factors did not correlate with the SMI, PVM area, or X-ray parameters. Age-dependent muscle volume loss, primary sarcopenia, was reportedly normally caused by a decrease in satellite cell number and suppression of the differentiation from satellite cells to myoblasts.29 Muscular atrophy caused by disuse or denervation (secondary sarcopenia) may be an acute proteolytic change induced by inflammatory cyto-kines, such as tumor necrosis factor-a and interleukin-6, that activate Murf1 and Atrogin-1 expression.30 Muscle degeneration associated with age-dependent hormonal changes is a long-term process that may differ from abnormal acute muscular loss (atrophy).31
In our analysis, ectopic fat infiltration into PVM reflected the pathological changes associated with age-and sarcopenia-related qualitative deterioration rather than atrophy of the muscle groups responsible for the lumbo-pelvis alignment. The European Working Group on Sarcopenia noted that muscle strength is not determined by muscle mass only, and that muscle quality may determine muscle strength; low muscle strength is the key characteristic of sarcopenia.12 This suggestion was based on studies demonstratingthat muscle massandstrength do not share a linear relationship,14 and muscle strength rather than mass was associated with mortality.32 Additionally, fat infiltration into skeletal muscles is reportedly a predictor of immobility in well-functioning older adults.16 An animal model-based study suggested that the presence of ectopic fat in interstitial muscle spaces directly decreased muscle strength and tension.33 The present findings similarly suggest that the amount of ectopic fat co-occurring with high levels of adipogenesis promoter expression in lumbar PVM could be a direct indicator of total body skeletal muscle mass loss.
Previous studies have examined the association between the extent of fat infiltration into chronically ruptured rotator cuffs and clinical outcomes, such as post-repair re-tear rate.26 The loss of sustainable mechanical stimulations of compression, tension, and shearing in skeletal muscles may stimulate the adipogenesis pathways, for example, the inhibition of the Wnt signal, which suppresses adipogenesis.34–36 These genetic changes likely promote the differentiation of ectopic adipocytes from mesenchymal stem cells in the intra-muscular spaces.23,37 During fat infiltration (in an animal model) and adipocyte maturation from mesenchymal stem cells, the levels of Pparg and Cebpa expression, which are master regulators of adipocyte differentiation, initially increase and subsequently decrease.23,36 This evidence suggests that the highly expressed adipogenesis promoters in PVM may indicate further fat infiltration even in muscles that are already severely infiltrated, creating the vicious cycle of sarcopenia: the loss of PVM strength reduces lumbar stability and extension due to ectopic fat infiltration, leading to lumbar kyphosis and reduced spinal plasticity and range of motion, which, in turn, causes greater ectopic fat infiltration into the PVM.
Overall, interventions for sarcopenia and ectopic fat infiltration into PVM should be implemented, following the precedent of osteoporosis. Supplementation with essential amino acids or vitamin D in older adults may improve walking speed and muscular function and reduce the risk of falling.38,39 Treatments with myostatin inhibitors, which suppress muscle volume regulation, may be useful.40 A basic research study of myostatin inhibition indicated that it suppresses the reduction of type II muscle fibers, which is supposedly the main mechanism of sarcopenia.41 Continuous resistance training in senescent mice helps maintain muscle quality via the Wnt signal activation35; in fact, resistance training in older adults is more effective at improving muscle mass and strength than treatment with testosterone or growth hormone.42
This study has several limitations, which should be considered when interpreting its findings. First, a control group of participants without lumbar diseases was lacking due to ethical restrictions associated with obtaining muscle samples from healthy people. Second, toclarify the complex pathology of sarcopenia and an association between deterioration of muscle quality and spine deformity, some of the correlations observed were statistically significant but lower to than the others. Therefore, more patients of further studies will be needed to confirm and/or strengthen the results of this study. Third, devices used and static magnetic field strength of MRI scanners were not standardizedin this study. As our hospital is the highest-tier care providerinthe prefecture, patients usually present at the clinic with previously acquired MRI scans and other test findings. Performing another MRI scan for such patients may cause them additional physical and financial burdens. Moreover, MRI data of <1.5T static magnetic field strength were excluded from the analysis. Fourth, while evaluating the association between sarcopenia and lumbar canal stenosis or deformity, accurate diagnosis of sarcopenia, excluding the effects of pain and neuropathy, is difficult to obtain. However, more than 64% of sarcopenia cases in this study were diagnosed based on low muscle (grip) strength; the SMI values of the lower and upper limbs and trunk were lower in sarcopenic than in non-sarcopenic older adults. Finally, this study was a cross-sectional analysis. Future longitudinal studies should examine medium- and long-term postoperative spinal alignment in this patient group.
In conclusion, sarcopenia and loss of limb or trunk muscle mass, including PVM, can cause lumbo-pelvic deformity. The present findings suggest that ectopic fat infiltration into lumbar PVM may be a therapeutic target for sarcopenia-related lumbo-pelvic deformity. Prevention and treatment strategies for sarcopenia and fat infiltration into PVM should be investigated further.
Key Points
Volume loss of skeletal muscle, including paravertebral muscle, in sarcopenic patients is negatively associated with lumbar kyphosis and posterior pelvic tilt.
Ectopic fat infiltrates lumbar paravertebral muscles in sarcopenic patients, and is negatively associated with lumbar kyphosis and posterior pelvic tilt.
Ectopic fat infiltration in lumbar paravertebral muscles better reflects sarcopenia-related qualitative muscle deterioration than does muscle atrophy.
Acknowledgments
The authors are grateful to Ikuyo Tsuchimochi, Momoe Yano, Naoko Sakamoto, and Michiko Sugita for providing technical assistance.
Footnotes
The manuscript submitted does not contain information about medical device/drug.
Clinical Research from Miyazaki University Hospital grant funds were received in support of this work.
No relevant financial activities outside the submitted work.
References
- 1.Rubio-Maicas C, Duarte-Alfonso E, Beseler-Soto MR, et al. Prevalence of sarcopenia in a media and long stay Unit. Rev Clin Esp 2014; 214:303–308. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int 2017; 28:189–199. [DOI] [PubMed] [Google Scholar]
- 3.Ji HM, Han J, Jin DS, et al. Sarcopenia and sarcopenic obesity in patients undergoing orthopedic surgery. Clin Orthop Surg 2016; 8:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai Y, Matsui H, Ito S, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia 2017; 3:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada M, Moriguch Y, Mitani T, et al. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr Gerontol Int 2014; 14: (suppl 1): 8–14. [DOI] [PubMed] [Google Scholar]
- 6.Viitasalo JT, Era P, Leskinen AL, et al. Muscular strength profiles and anthropometry in random samples of men aged 31-35, 51-55 and 71-75 years. Ergonomics 1985; 28:1563–1574. [Google Scholar]
- 7.Banno T, Arima H, Hasegawa T, et al. The effect of paravertebral muscle on the maintenance of upright posture in patients with adult spinal deformity. Spine Deform 2019; 7:125–131. [DOI] [PubMed] [Google Scholar]
- 8.Schwab F, Ungar B, Blondel B, et al. Scoliosis Research SocietySchwab adult spinal deformity classification: a validation study. Spine (Phila Pa 1976) 2012; 37:1077–1082. [DOI] [PubMed] [Google Scholar]
- 9.Takaki SMP, Kaneoka KPM, Okubo YPP, et al. Analysis of muscle activity during active pelvic tilting in sagittal plane. Phys Ther Res 2016; 19:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn SE, Portney LG. Electromyographic activity of back musculature during Williams’ flexion exercises. Phys Ther 1981; 61:878–885. [DOI] [PubMed] [Google Scholar]
- 11.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21:300–307. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009; 90:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006; 61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 15.Yamada Y, Schoeller DA, Nakamura E, et al. Extracellular water may mask actual muscle atrophy during aging. J Gerontol A Biol Sci Med Sci 2010; 65:510–516. [DOI] [PubMed] [Google Scholar]
- 16.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005; 60:324–333. [DOI] [PubMed] [Google Scholar]
- 17.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999; 48:1600–1606. [DOI] [PubMed] [Google Scholar]
- 18.Thornell LE. Sarcopenic obesity: satellite cells in the aging muscle. Curr Opin Clin Nutr Metab Care 2011; 14:22–27. [DOI] [PubMed] [Google Scholar]
- 19.Kalichman L, Hodges P, Li L, et al. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J 2010; 19:1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamai K, Chen J, Stone M, et al. The evaluation of lumbar paraspinal muscle quantity and quality using the Goutallier classification and lumbar indentation value. Eur Spine J 2018; 27:1005–1012. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Rosen ED, Brun R, et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipo-genesis and insulin sensitivity. Mol Cell 1999; 3:151–158. [DOI] [PubMed] [Google Scholar]
- 22.Bae J, Lee SH. Minimally invasive spinal surgery for adult spinal deformity. Neurospine 2018; 15:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D, Taniguchi N, Sato K, et al. HMGB2 is a novel adipogenic factor that regulates ectopic fat infiltration in skeletal muscles. Sci Rep 2018; 8:9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 2001; 98:14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiq-uitin ligases required for skeletal muscle atrophy. Science 2001; 294:1704–1708. [DOI] [PubMed] [Google Scholar]
- 26.Goutallier D, Postel JM, Gleyze P, et al. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg 2003; 12:550–554. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi Y, Suzuki M, Yamanaka H, et al. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord 2017; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meakin JR, Fulford J, Seymour R, et al. The relationship between sagittal curvature and extensor muscle volume in the lumbar spine. J Anat 2013; 222:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa-Victor P, Gutarra S, Garcı’a-Prat L, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014; 506:316–321. [DOI] [PubMed] [Google Scholar]
- 30.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 19852005; 98:1154–1162. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev 2013; 41:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006; 61:72–77. [DOI] [PubMed] [Google Scholar]
- 33.Biltz NK, Collins KH, Shen KC, et al. Infiltration of intramuscular adipose tissue impairs skeletal muscle contraction. J Physiol 2020; 598:2669–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christodoulides C, Lagathu C, Sethi JK, et al. Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009; 20:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horii N, Uchida M, Hasegawa N, et al. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J 2018; 32:3547–3559. [DOI] [PubMed] [Google Scholar]
- 36.Itoigawa Y, Kishimoto KN, SanoH, et al. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res 2011; 29:861–866. [DOI] [PubMed] [Google Scholar]
- 37.Uezumi A, Fukada S, Yamamoto N, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010; 12:143–152. [DOI] [PubMed] [Google Scholar]
- 38.Børsheim E, Bui QU, Tissier S, et al. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr 2008; 27:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 2004; 33:589–595. [DOI] [PubMed] [Google Scholar]
- 40.Trendelenburg AU, Meyer A, Rohner D, et al. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 2009; 296:C1258–C1270. [DOI] [PubMed] [Google Scholar]
- 41.Murphy KT, Koopman R, Naim T, et al. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J 2010; 24:4433–4442. [DOI] [PubMed] [Google Scholar]
- 42.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 2011; 43:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]