Abstract
Three novel monoterpenoid indole alkaloids gardflorine A (1), gardflorine B (2), and gardflorine C (3) were isolated from the leaves of Gardneria multiflora. Their structures, including absolute configurations, were established on the basis of spectroscopic methods (MS, UV, IR, 1D and 2D NMR) and circular dichroism experiments. All the compounds were evaluated for their vasorelaxant and acetylcholinesterase (AChE) inhibitory activities. Compound 1 exhibited potent vasorelaxant activity, with an EC50 value of 8.7 μM, and compounds 2 and 3 showed moderate acetylcholinesterase (AChE) inhibitory activities, with IC50 values of 26.8 and 29.2 μM, respectively.
Keywords: Gardneria multiflora, monoterpenoid indole alkaloid, vasorelaxant activity, AChE inhibitory activity
1. Introduction
Monoterpenoid indole alkaloids (MIAs) are important secondary metabolites widely distributed in the members of plant families Apocynaceae, Loganiaceae, and Rubiaceae [1]. These compounds have attracted considerable interest in drug research for their complex structures and diverse biological activities, such as ganglion blocking [2], anticancer [3,4,5], anti-inflammatory [6], antibacterial [7], vasorelaxant [8], and neuroprotective activities [9]. The plant Gardneria multiflora (Loganiaceae family) is widely distributed in the south of the Qinling Mountains-Huaihe River Line and north of the Nanling Mountains in China [10]. The roots and leaves of G. multiflora are widely used as medicine for the treatment of arthrophlogosis and sciatica owing to their effects of expelling wind and activating blood flow [10]. Previous phytochemical investigations of plants from this genus led to the isolation of more than 50 compounds [11,12], most of them were identified as MIAs [13,14,15,16,17].
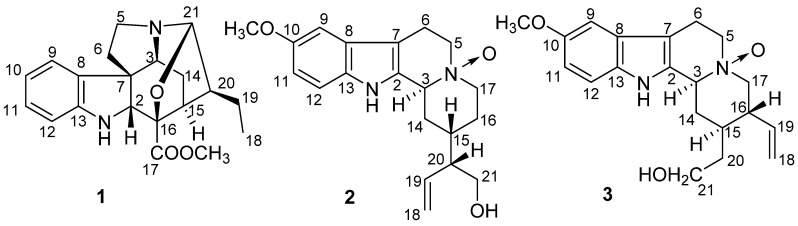
In search of novel and bioactive alkaloids, we carried out a phytochemical investigation on the constituents of the leaves of G. multiflora. In our present study, three new alkaloids (Figure 1), named gardflorine A (1), gardflorine B (2), and gardflorine C (3), were isolated from the leaves of G. multiflora. Their structures were elucidated by using spectroscopic methods and electronic circular dichroism (ECD) calculation. Additionally, compound 1 exhibited potent vasorelaxant activity, with an EC50 value of 8.7 μM, while, compounds 2 and 3 showed moderate AChE inhibitory activities, with IC50 values of 26.8 and 29.2 μM, respectively. Herein, we describe the isolation, structural elucidation, and biological activities of 1–3.
Figure 1.
Chemical structures of compounds 1–3.
2. Results and Discussion
Compound 1 was obtained as white oil, and its molecular formula was established as C20H24N2O3 by HR-ESI-MS at m/z 341.1859 [M + H]+ (calcd for C20H25N2O3, 341.1860). The IR spectrum of 1 indicated the presence of amino group (3355 cm−1), carbonyl group (1736 cm−1), and aromatic ring (1609 and 1464 cm−1). The 1H NMR spectrum of 1 (Table 1) showed signals for an ortho-disubstituted benzene ring [δH 7.08 (1H, dd, J = 7.5, 1.3 Hz), 7.02 (1H, td, J = 7.5, 1.3 Hz), 6.72 (1H, td, J = 7.5, 1.3 Hz), and 6.59 (1H, dd, J = 7.5, 1.3 Hz)], a methyl [δH 0.96 (3H, t, J = 7.4 Hz)], and a methoxy [δH 3.82 (3H, s)]. The 13C NMR and DEPT-135 spectra showed twenty carbon signals, including one carbonyl (δC 174.4), six olefinic carbons (δC 152.7, 133.4, 129.4, 123.6, 120.1, 111.0), two quaternary carbons (δC 85.5, 58.0), five methines (δC 97.5, 73.9, 67.5, 55.0, 39.7), four methylenes (δC 53.2, 44.6, 25.8, 24.5), one methoxy (δC 53.2), and one methyl (δC 11.8). With the aid of 2D NMR spectra, the 1H and 13C NMR signals of 1 were assigned as shown in Table 1.
Table 1.
NMR data of 1–3 (CD3OD, δ in ppm, J in Hz).
| Position | 1 a | 2 a | 3 b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δ H | δC, Type | δ H | δC, Type | δ H | δC, Type | ||||
| 2 | 4.03, s | 73.9, CH | - | 131.4, C | - | 131.4, C | |||
| 3 | 2.89, d (4.7) | 67.5, CH | 4.61, s | 71.7, CH | 4.60, brs | 71.6, CH | |||
| 5 |
α
β |
3.30, m 3.17, m |
53.2, CH2 | a/b | 3.72, dd (9.2, 4.0) | 69.2, CH2 | a/b | 3.69, m | 69.2, CH2 |
| 6 |
α
β |
2.50, m 2.29, m |
44.6, CH2 | a/b | 3.06, m | 20.6, CH2 |
α
β |
3.07, m 2.98, m |
20.6, CH2 |
| 7 | - | 58.0, C | - | 106.3, C | - | 106.2, C | |||
| 8 | - | 133.4, C | - | 128.0, C | - | 128.0, C | |||
| 9 | 7.08, dd (7.5, 1.3) | 123.6, CH | 6.93, d (2.4) | 101.0, CH | 6.91, d (2.0) | 101.0, CH | |||
| 10 | 6.72, td (7.5, 1.3) | 120.1, CH | - | 155.6, C | - | 155.6, C | |||
| 11 | 7.02, td (7.5, 1.3) | 129.4, CH | 6.78, dd (8.8,2.4) | 113.3, CH | 6.79, dd (8.2,2.0) | 113.3, CH | |||
| 12 | 6.59, dd (7.5, 1.3) | 111.0, CH | 7.24, d (8.8) | 113.2, CH | 7.20, d (8.2) | 113.2, CH | |||
| 13 | - | 152.7, C | - | 133.9, C | - | 133.9, C | |||
| 14 |
α
β |
2.12, m 1.78, m |
25.8, CH2 |
α
β |
2.58, td (13.6,4.9) 2.25, d (13.6) |
28.6, CH2 |
α
β |
2.53, m 2.23, m |
28.4, CH2 |
| 15 | 2.93, d (4.7) | 39.7, CH | 1.54, overlap | 30.7, CH | 1.49, overlap | 30.6, CH | |||
| 16 | - | 85.8, C |
β
α |
2.07, dd (13.4,3.7)1.54, overlap | 23.6, CH2 | 2.13, m | 52.3, CH | ||
| 17 | - | 174.4, C |
α
β |
3.57, m 3.05, m |
59.2, CH2 |
α
β |
3.51, overlap3.01, m | 59.1, CH2 | |
| 18 | 0.96, t (7.4) | 11.8, CH3 | 5.15, m | 118.4, CH2 | 5.11, m | 118.5, CH2 | |||
| 19 | a b |
1.30, m 1.22, m |
24.5, CH2 | 5.69, d (16.9) | 138.3, CH | 5.65, m | 138.3, CH | ||
| 20 | 1.83, t (7.6) | 55.0, CH | 2.17, m | 52.3, CH | a b |
2.03, m 1.49, overlap |
23.6, CH2 | ||
| 21 | 4.59, s | 97.5, CH | 3.59, m | 63.9, CH2 | a b |
3.58, m 3.51, overlap |
63.8, CH2 | ||
| OCH3 | 3.82, s | 53.2, CH3 | 3.80, s | 56.2, CH3 | 3.78, s | 56.2, CH3 | |||
a1H NMR spectra of 1 and 2 were recorded at 600 MHz and 13C NMR was recorded at 150MHz. b 1H NMR spectrum of 3 was recorded at 400 MHz and 13C NMR was recorded at 100 MHz.
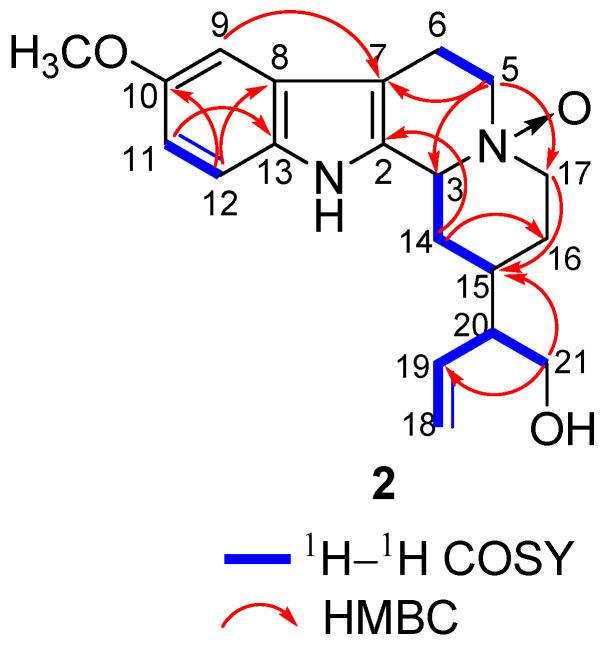
The 1H–1H COSY spectrum revealed the presence of four spin-coupling systems (H-9 to H-12, H2-5 to H2-6, H-3 to H-15, H3-18 to H-20) as shown in Figure 2. The HMBC correlations between H-9 (δH 7.08) and C-7 (δC 58.0)/C-13 (δC 152.7), between H-5β (δH 3.17) and C-3 (δC 67.5)/C-7 (δC 58.0)/C-21 (δC 97.5), between H2-6 (δH 2.50, 2.29) and C-2 (δC 73.9), between H2-19 (δH 1.30, 1.22) and C-15 (δC 39.7)/C-21 (δC 97.5), between H-14β (δH 1.78) and C-7 (δC 58.0)/C-16 (δC 85.8), and between H-2 (δH 4.03)/H-15 (δH 2.93) and C-17 (δC 174.4) indicated the presence of the skeleton of akuammicine alkaloid [18]. Subsequently, the HMBC correlation between OCH3 (δH 3.82) and C-17 (δC 174.4) suggested that the methoxy connected to C-17. Furthermore, HMBC correlation between H-21 (δH 4.59) and C-16 (δC 85.8), combined with obvious downfield NMR shifts of C-16 (δC 85.8) and C-21 (δC 97.5) and the molecular formula of 1, suggested C-16 and C-21 were connected via an oxygen atom. Therefore, the planar structure of 1 was established as shown in Figure 2.
Figure 2.
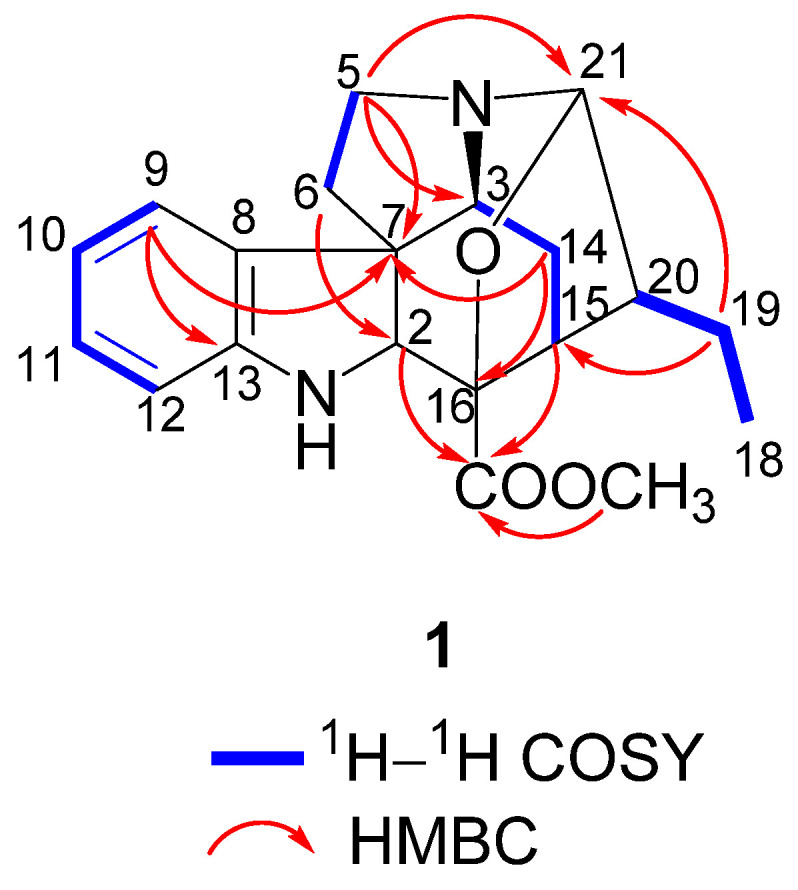
Key 1H–1H COSY and HMBC correlations of 1.
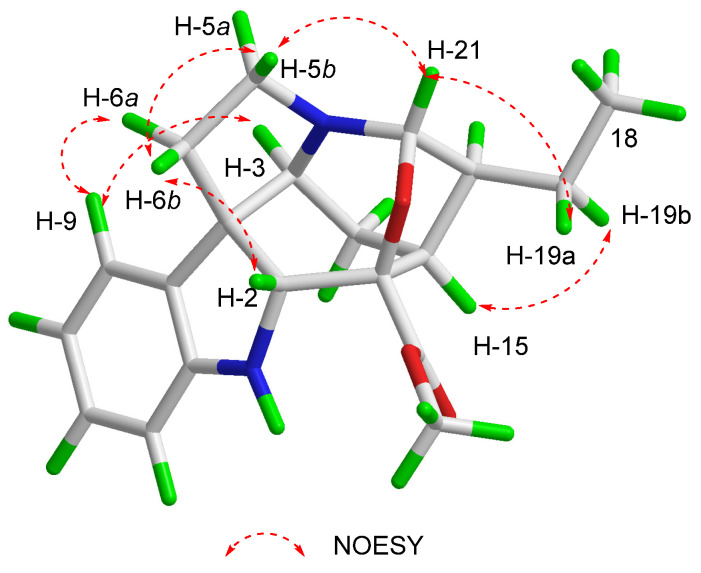
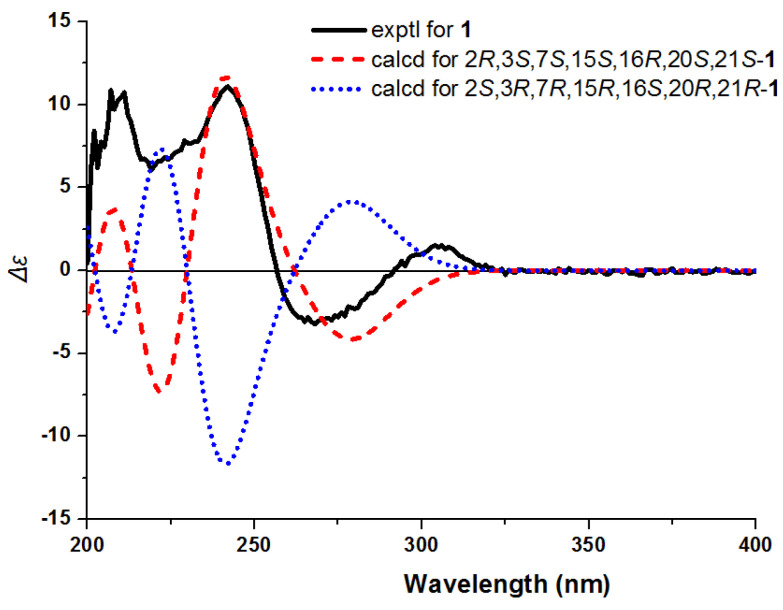
The 2D structure of compound 1 determined by the NOESY cross-peaks (Figure 3) between H-9 and H-3/H-6α, between H-14α and H-3/H-15, and between H-20 and H-15/H-21 indicated the same orientation of these protons. The NOESY cross-peaks between H-6β and H-2 indicated that H-2 was β-oriented. The absolute configuration of 1 was identified by CD experiment (Figure 4), and the negative Cotton effect at λmax 275 (−2.8) nm and the positive Cotton effects at λmax 245 (+11.7) nm and 210 (+11.2) nm in CD spectrum were consistent with the calculated configuration of (2R, 3S, 7S, 15S, 16R, 20S, 21S)-1. Consequently, compound 1 was identified and named as gardflorine A.
Figure 3.
Key NOESY correlations of 1.
Figure 4.
Experimental and calculated ECD spectra of 1.
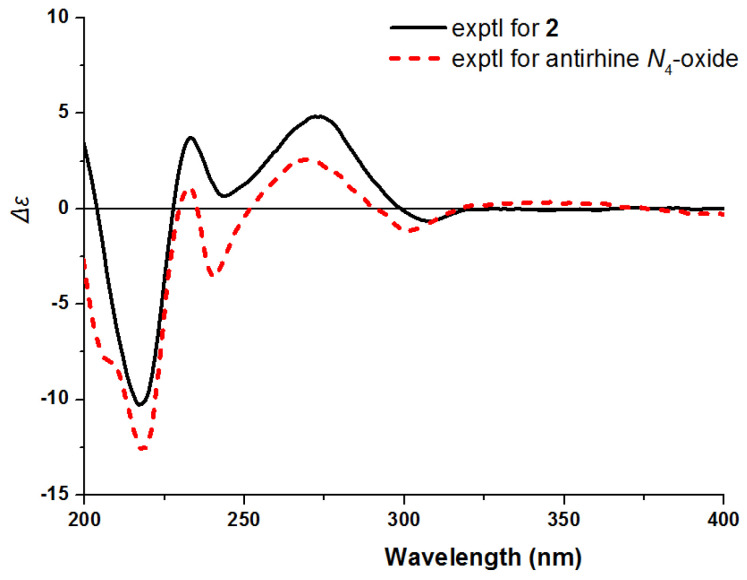
Compound 2 was isolated as yellow powder, and its molecular formula was established as C20H26N2O3 according to HR-ESI-MS at m/z 343.2020 [M + H]+ (calcd for C20H27N2O3, 343.2016). The IR spectrum of 2 revealed the presence of amino group (3404 cm−1), hydroxy group (3215 cm−1) and aromatic ring (1444 cm−1). The 1H NMR spectrum of 2 (Table 1) showed three aromatic protons [δH 7.24 (1H, d, J = 8.8 Hz), 6.93 (1H, d, J = 2.4 Hz), and 6.79 (1H, dd, J = 8.8, 2.4 Hz)], three olefinic protons [δH 5.69 (1H, d, J = 16.9 Hz) and 5.15 (2H, m)], and one methoxy proton [δH 3.80 (3H, s)]. The 13C NMR and DEPT-135 spectra showed twenty carbon signals due to ten olefinic carbons (δC 155.6, 138.3, 133.9, 131.4, 128.0, 118.4, 113.3, 113.2, 106.3, 101.0), three methines (δC 71.7, 52.3, 30.7), six methylenes (δC 69.2, 63.9, 59.2, 28.6, 23.6, 20.6), and one methoxy (δC 56.2). Comparison of the NMR data of 2 with those of the known compound antirhine N4-oxide [19] showed that they were very similar except for the presence of an additional methoxy group in 2. The chemical shifts of C-10, C-9, C-11, and C-13 shifted from δC 120.6, 119.0, 123.2, and 138.8 in antirhine N4-oxide to δC 155.6, 101.0, 113.3, and 128.0 in 2, suggesting that the methoxy group might be connected to C-10. This was confirmed by HMBC correlations from δH 3.80 (OCH3) to δC 155.6 (C-10) (Figure 5). The absolute configuration of 2 was determined by comparing the ECD spectrum of 2 with that of antirhine N4-oxide (Figure 6). Therefore, compound 2 was identified as 10-methoxyantirhine N4-oxide and named as gardflorine B.
Figure 5.
Key 1H–1H COSY and HMBC correlations of 2.
Figure 6.
Experimental ECD spectra of 2 and antirhine N4-oxide.
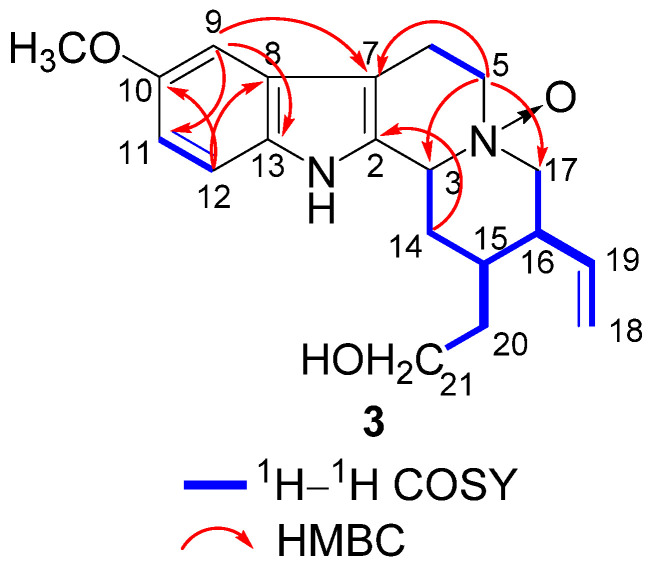
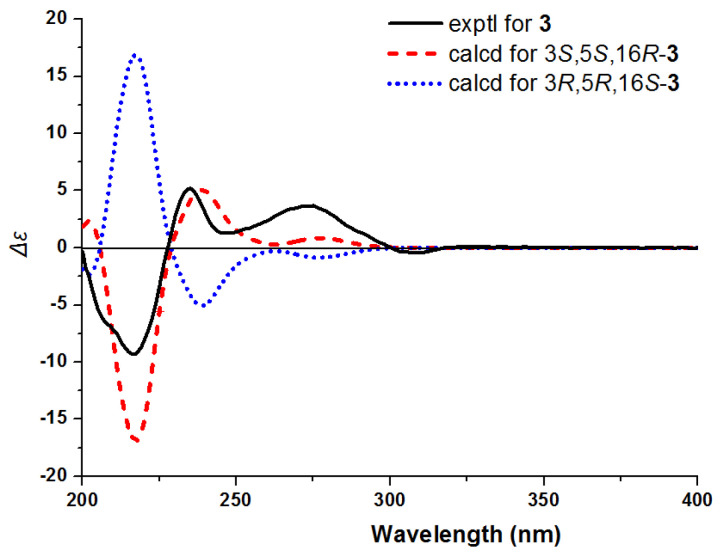
The molecular formula of 3 was established as C20H26N2O3 according to an [M + H]+ ion peak at m/z 343.2012 [M + H]+ (calcd for C20H27N2O3, 343.2016) in the HR-ESI-MS spectrum. Its IR spectrum suggested the presence of amino group (3396 cm−1), hydroxy group (3230 cm−1), and aromatic ring (1461 cm−1). The 1H and 13C NMR spectrum of 3 (Table 1) showed the presence of a 1,2,4-trisubstituted benzene ring [δH 7.20 (1H, d, J = 8.2 Hz), 6.91 (1H, d, J = 2.0 Hz), 6.79 (1H, dd, J = 8.2, 2.0 Hz); δC 155.6, 133.9, 128.0, 113.3, 113.2, 101.0], a terminal double bond [δH 5.65 (1H, m), 5.11 (2H, m); δC 138.3, 118.5], and one methoxy signal [δH 3.78 (3H, s); δC 56.2]. The 1D NMR data of 3 (Table 1) closely resembled to those of 2; however, their 2D NMR data showed many differences. The 1H–1H COSY correlations of H-3 (δH 4.60)/H2-14 (δH 2.53, 2.23)/H-15 (δH 1.49)/H-16 (δH 2.13)/H2-17 (δH 3.51, 3.01), H-15/H2-20 (δH 2.03, 1.49)/H2-21 (δH 3.58, 3.51), and H-16/H-19 (δH 5.65)/H2-18 (δH 5.11), together with the HMBC correlations between H2-5 (δH 3.69) and C-17 (δC 59.1), between H2-17 and C-19 (δC 138.3), between H2-18 and C-16 (δC 52.3), and between H2-21 and C-15 (δC 30.6) revealed that the terminal double bond was linked to C-16 (Figure 7). The NOESY correlations between H-15 and H-3/H-19 established the relative configuration of 3. Subsequently, the absolute configuration of 3 was identified by CD experiment (Figure 8). The positive Cotton effect at λmax 272 (+3.7) nm and 235 (+5.2) nm and the negative Cotton effect at λmax 217 (−9.3) nm displayed good agreement with the calculated ECD curve for (3S, 15S, 16R)-3. Consequently, compound 3 was identified as 10-methoxycorynantheol N4-oxide and named as gardflorine C.
Figure 7.
Key 1H–1H COSY and HMBC correlations of 3.
Figure 8.
Experimental and calculated ECD spectra of 3.
In order to explore the scientific connotation of the traditional use of G. multiflora, the vasorelaxant and AChE inhibitory activities in vitro of compounds 1–3 were evaluated. Among them, compound 1 exhibited potent vasorelaxant activity, with an EC50 value of 8.7 μM (EC50 = 0.1 μM for positive control phentolamine mesylate). Moreover, compounds 2 and 3 exhibited moderate AChE inhibitory activities, with IC50 values of 26.8 and 29.2 μM, respectively (Supplementary Materials).
3. Materials and Methods
3.1. General Experimental Procedures
UV and IR spectra were obtained on a JASCO V-550 spectrophotometer and a JASCO FI/IR-480 Plus Fourier transform infrared spectrometer, respectively. CD spectra were recorded on a Chirascan spectropolarimeter. Optical rotations were determined with a JASCO P-1020 Automatic Polarimeter. HR-ESI-MS data were obtained using an Agilent 6210 ESI/TOF mass spectrometer. NMR experiments were performed on Bruker AV-600 and AV-400 spectrometers. HPLC was carried out on an Agilent 1260 chromatograph and a semi-preparative chromatograph with a DAD detector.
Column chromatography (CC) was performed on silica gel (60–80 mesh, 200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (Pharmacia Biotech AB), and YMC-Pack ODS (Merck). TLC was carried out on glass precoated silica gel GF254 plates. Waters X-bridge C18 column (250 × 4.6 mm, 5 μm; 250 × 10 mm, 5 μm) was used to analyze and isolate the compounds.
3.2. Plant Materials
The leaves of Gardneria multiflora were collected in Yangchang Town, Longli County, Guizhou province, in June 2018 and identified by Dr. Ying Zhang of Jinan University. A voucher specimen has been deposited at the Medical College of Jiaying University (No. MCJU-021).
3.3. Extraction and Isolation
The dried, powdered leaves of G. multiflora (20 kg) were percolated with 95% ethanol (100 L×3). After evaporation of solvent in vacuum, the residue (1.5 kg) was suspended in water, and the pH was adjusted to 2–3 by 5% HCl and then partitioned with chloroform; thus, the chloroform layer and acid water layer were obtained. The acid water layer was then adjusted to pH 9–10 by ammonia water, chloroform extraction was carried out, and the crude total alkaloid (chloroform part) was obtained. The chloroform extract (31.3 g) was subjected to a silica gel column eluting with chloroform/methanol (100:0 to 0:100, v/v) to afford 8 fractions (A1–A8). Then, fraction A4 was further separated by a silica gel, ODS, Sephadex LH-20 columns, and preparative HPLC to afford 1 (2.7 mg). Fraction A6 was successively separated on Sephadex LH-20 (MeOH) a20nd purified by preparative HPLC with MeOH–H2O–Et2NH (60:40:0.0002) to afford 2 (6.6 mg) and 3 (5.9 mg).
3.4. Spectral Data
3.4.1. Gardflorine A (1)
White oil, [α +36.1 (c 0.92, MeOH); UV(MeOH) λmax (log ε) 209 (4.31), 245 (3.22), 299 (4.05); IR (KBr) νmax 3355, 2957, 2877, 1736, 1674, 1609, 1485, 1464, 1383, 1308, 750 cm−1; HR-ESI-MS m/z 341.1859 [M + H]+ (calcd for C20H25N2O3, 341.1860); 1H and 13C NMR data, see Table 1.
3.4.2. Gardflorine B (2)
Yellow powder, [α +49.0 (c 1.01, MeOH); UV(MeOH) λmax (log ε) 211 (4.33), 275 (3.81); IR (KBr) νmax 3404, 3215, 2929, 1622, 1444, 1327, 1227, 1115, 742, 630 cm−1; HR-ESI-MS m/z 343.2020 [M + H]+ (calcd for C20H27N2O3, 343.2016); 1H and 13C NMR data, see Table 1.
3.4.3. Gardflorine C (3)
Yellow powder, [α −17.4 (c 0.70, MeOH); UV(MeOH) λmax (log ε) 206 (5.02), 274 (4.45); IR (KBr) νmax 3396, 3230, 3056, 2925, 1638, 1461, 1330, 1234, 1156, 1079, 742, 512 cm−1; HR-ESI-MS m/z 343.2012 [M + H]+ (calcd for C20H27N2O3, 343.2016); 1H and 13C NMR data, see Table 1.
3.5. Vasorelaxant Assay
The vasorelaxant activity of these isolates against KCl-induced contractions of rat renal artery rings was measured as described previously [20,21,22]. Renal arteries were removed rapidly out from SD rats, immediately placed into 4 °C oxygenated K-H solution, cleaned of its surrounding fat and connective tissues, and then cut into portions of about 2 mm in length. Each segment was mounted in a Multi Myograph System (Danish Myo Technology A/S, Denmark) and then bathed in K-H solution [composition (in mM): NaCl, 120; KCl, 4.6; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 25; glucose, 10; CaCl2, 2.5], bubbled with 95% O2–5% CO2, and maintained at 37 °C. The isometric tension of renal artery rings was collected by four-channel physiological force transducers. All the rings were set to an optimal tension of 2 g and stabilized in normal K-H solution for 90 min. The rings were then contracted by 0.5 μM phenylephrine and challenged with 3 μM acetylcholine to confirm the integrity of the endothelium. Endothelium-intact rings contraction was evoked by a depolarizing KCl (60 mM) solution. The EC50 values of the test compounds and the positive control (phentolamine mesylate) were calculated from cumulative concentration–tension curves by linear regression.
3.6. AChE Inhibitory Activity Assay
The AChE inhibitory activities of the isolated compounds were assayed by a modified Ellman’s method [6,23]. Compounds and positive control were dissolved in 1% DMSO. The phosphate buffer (pH 8.0), tacrine, test compounds, and acetylcholinesterase (0.02 μM) were added, in sequence to 96-well plates and incubated for 20 min (30 °C). The reaction was initiated by the addition of 20 μL of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (0.625 mM) and 20 μL of acetylthiocholine iodide (0.625 mM) for the AChE inhibitory activity assay, respectively. The optical density was measured at 405 nm by an ELISA microplate reader. Tacrine (IC50 0.33 μM) was used as positive control. All the reactions were performed in triplicate. The percentage inhibition (I%) was calculated as follows: I% = (1 − S)/E × 100 (S is the absorbance of the test compound-containing reaction, and E is the absorbance of the control reaction).
4. Conclusions
In summary, three new monoterpenoid indole alkaloids (1–3) were isolated and identified from the leaves of G. multiflora. The new compounds were elucidated by spectroscopic analyses and computational calculation. Moreover, the vasorelaxant and AChE inhibitory activities of all isolates were tested. Compound 1 exhibited potent vasorelaxant activity, with an EC50 value of 8.7 μM, while compounds 2 and 3 exhibited AChE inhibitory activities, with IC50 values of 26.8 and 29.2 μM, respectively. The discovery of the new alkaloids expands the family of MIAs and provides reference for further structure–activity discussions in future research.
Supplementary Materials
The following are available online. Figure S1: Leaves of G. multiflora, Tables S1 and S2: biological activities of compounds 1–3, Figures S2–S10: HR-ESI-MS, UV, IR, 1D and 2D NMR spectra of compound 1, Figures S11–S19: HR-ESI-MS, UV, IR, 1D and 2D NMR spectra of compound 2, Figures S20–S28: HR-ESI-MS, UV, IR, 1D and 2D NMR spectra of compound 3.
Author Contributions
S.-Y.Z., Z.-W.L. and Q.-L.C. conducted the isolation, purification, and identification of compounds. J.X. and M.S. carried out the vasorelaxant assay and AChE inhibitory activity assay. Q.-W.Z. designed and supervised the study and wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. U1801287 and 82073712) and by the University of Macau (MYRG2019-00150-ICMS).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
The data of the NMR and vasorelaxant and AchE inhibitory activity presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1–3 are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang J., Yuan M.F., Li S.T., Sang C.C., Chen M.F., Ao Y.L., Li Z.W., Xie J., Ye W.C., Zhang X.Q. Hunzeylanines A–E, five bisindole alkaloids tethered with a methylene group from the roots of Hunteria zeylanica. Org. Chem. 2020;85:10884–10890. doi: 10.1021/acs.joc.0c01448. [DOI] [PubMed] [Google Scholar]
- 2.Masatoshi H., Yukihiro O. Effect of gardneria alkaloids on ganglionic transmission in the rabbit and rat superior cervical ganglia in situ. Chem. Pharm. Bull. 1978;26:48–52. doi: 10.1248/cpb.26.48. [DOI] [PubMed] [Google Scholar]
- 3.Harada M., Ozaki Y. Effect of indole alkaloids from Gardneria genus and Uncaria genus on neuromuscular transmission in the rat limb in situ. Chem. Pharm. Bull. 1976;24:211–214. doi: 10.1248/cpb.24.211. [DOI] [PubMed] [Google Scholar]
- 4.Feng T., Li X.L., Zhang B.H., Li Y., Cai X.H., Liu Y.P., Luo X.D. Gardovatine, a novel Strychnos-Strychnos bisindole alkaloid with cytotoxicity from Gardneria oveta. Bioorg. Med. Chem. Lett. 2013;23:5563–5565. doi: 10.1016/j.bmcl.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 5.Yan H.S., Yan H.D. Research advance on Dai medicine Gardneria multiflora. J. Med. Pharm.Chin. Minorities. 2019;25:40–41. [Google Scholar]
- 6.Zhang W., Xu W., Wang G.Y., Gong X.Y., Li N.P., Wang L., Ye W.C. Gelsekoumidines A and B: two pairs of atropisomeric bisindole alkaloids from the roots of Gelsemium elegans. Org. Lett. 2017;19:5194–5197. doi: 10.1021/acs.orglett.7b02463. [DOI] [PubMed] [Google Scholar]
- 7.Ding C.F., Ma H.X., Yang J., Qin X.J., Njateng G.S.S., Yu H.F., Wei X., Liu Y.P., Huang W.Y., Yang Z.F., et al. Antibacterial indole alkaloids with complex heterocycles from Voacanga africana. Org. Lett. 2018;20:2702–2706. doi: 10.1021/acs.orglett.8b00913. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Song M., Ao Y.L., Li Y., Zou X.Y., Xu J., Wang Y., Zhang D.M., Zhang X.Q., Ye W.C. Alstolarines A and B, two unusual monoterpenoid indole alkaloids with acetal moiety from Alstonia scholaris. Org. Chem. Front. 2020;7:3468–3473. doi: 10.1039/D0QO00751J. [DOI] [Google Scholar]
- 9.He Q.F., Wu Z.L., Li L., Sun W.Y., Wang G.Y., Jiang R.W., Hu L.J., Shi L., He R.R., Wang Y., et al. Discovery of neuritogenic securinega alkaloids from Flueggea suffruticosa by a building blocks-based molecular network strategy. Angew. Chem. Int. Ed. 2021;60:19609–19613. doi: 10.1002/anie.202103878. [DOI] [PubMed] [Google Scholar]
- 10.Editorial Committee of Flora of China . Flora of China. Volume 61. Science and Technology Press; Beijing, China: 1992. p. 243. [Google Scholar]
- 11.Xie G.H., Ma L., Zheng Z.P., Hu L.H. Lignans from Gardneria multiflor. Chin. J. Nat. Med. 2007;5:255–258. [Google Scholar]
- 12.Akayama H., Nitta W., Kitajima M., Aimi N., Sakai S. A new Gardneria alkaloid, gardquinolone, having a novel 4-quinolone skeleton. J. Nat. Prod. 1994;57:521–523. doi: 10.1021/np50106a014. [DOI] [Google Scholar]
- 13.Li X.N., Cai X.H., Feng T., Li Y., Liu Y.P., Luo X.D. Monoterpenoid Indole Alkaloids from Gardneria ovata. J. Nat. Prod. 2011;74:1073–1078. doi: 10.1021/np2000254. [DOI] [PubMed] [Google Scholar]
- 14.Zhong X.H., Xiao L., Wang Q., Zhang B.J., Bao M.F., Cai X.H., Peng L. Cytotoxic 7S-oxindole alkaloids from Gardneria multiflora. Phytochem. Lett. 2014;10:55–59. doi: 10.1016/j.phytol.2014.08.001. [DOI] [Google Scholar]
- 15.Yang W.X., Huang T., Zhang J.X., Liu J.H., Hao X.J., Zhang Y.H. A New Monoterpenoid Indole Alkaloid from Gardneria multiflora Makino. Chin. Pharm. J. 2016;51:1113–1115. [Google Scholar]
- 16.Yang W.X., Chen Y.F., Yang J., Huang T., Wu L.L., Xiao N., Hao X.J., Zhang Y.H. Monoterpenoid indole alkaloids from Gardneria multiflora. Fitoterapia. 2018;124:8–11. doi: 10.1016/j.fitote.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Si Y.Y., Wang W.W., Feng Q.M., Zhao Z.Z., Xue G.M., Sun Y.J., Feng W.S., Young J.I., Wang X.S. Neuroinflammatory inhibitors from Gardneria nutans Siebold & Zuccarini. RSC Adv. 2021;11:27085–27091. doi: 10.1039/d1ra05204g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan L.S., Yang S.P., Wu Y., Ding J., Yue J.M. Terpenoid Indole Alkaloids from Winchia calophylla. J. Nat. Prod. 2006;69:18–22. doi: 10.1021/np0502701. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H., Liu Y.B., Li Y., Li L., Ma S.G., Qu J., Yu S.S. Analgesic corynanthe-type alkaloids from Strychnos angustiflora. Tetrahedron. 2016;72:1276–1284. doi: 10.1016/j.tet.2015.11.011. [DOI] [Google Scholar]
- 20.Hu G.Y., Li X.X., Zhang S.Y., Wang X. Association of rat thoracic aorta dilatation by astragaloside IV with the generation of endothelium-derived hyperpolarizing factors and nitric oxide, and the blockade of Ca2+ channels. Biomed. Rep. 2016;5:27–34. doi: 10.3892/br.2016.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J., Zou X.Y., Sang C.C., Song M., Chen Q.L., Zhang J. Three new monoterpenoid indole alkaloids from Alstonia rostrata. Tetrahedron Lett. 2021;75:153180. doi: 10.1016/j.tetlet.2021.153180. [DOI] [Google Scholar]
- 22.Zhang J., Liu Z.W., Li Y., Wei C.J., Xie J., Yuan M.F., Zhang D.M., Ye W.C., Zhang X.Q. Structurally Diverse Indole Alkaloids with Vasorelaxant Activity from Melodinus hemsleyanus. J. Nat. Prod. 2020;83:2313–2319. doi: 10.1021/acs.jnatprod.9b00925. [DOI] [PubMed] [Google Scholar]
- 23.Lou H.Y., Yi P., Hu Z.X., Li Y.N., Zeng Y.R., Gu W., Huang L.J., Yuan C.M., Hao X.J. Polycyclic polyprenylated acylphloroglucinols with acetylcholinesterase inhibitory activities from Hypericum perforatum. Fitoterapia. 2020;143:104550. doi: 10.1016/j.fitote.2020.104550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of the NMR and vasorelaxant and AchE inhibitory activity presented in this study are available in Supplementary Materials.