Abstract
The production of toxic shock syndrome toxin 1 (TSST-1) by Staphylococcus aureus MN8 exposed to a range of oxygen concentrations (0 to 21% [vol/vol]) was examined in batch and thin-film cultures. The response of S. aureus to this range of oxygen concentrations was studied in the absence and in the presence of 7% (vol/vol) carbon dioxide. In the absence of carbon dioxide, TSST-1 production in batch cultures increased from negligible levels in the presence of oxygen concentrations of 1% or less to 500 ng/ml in the presence of 2% oxygen and then decreased to 70 ng/ml or less in the presence of oxygen concentrations of 6% and higher. In the presence of carbon dioxide, however, toxin production increased from negligible levels in the presence of 1% oxygen to 1,900 ng/ml in the presence of 21% oxygen. In thin-film cultures, TSST-1 production increased from nearly undetectable levels under anaerobic conditions to 1 and 10 μg/ml under 21% oxygen in the absence and presence of carbon dioxide, respectively. This study demonstrates the controlling effects of both oxygen and carbon dioxide on TSST-1 production.
Toxic shock syndrome toxin 1 (TSST-1) is a pyrogenic toxin superantigen produced by many pathogenic strains of Staphylococcus aureus. TSST-1 stimulates the proliferation of both CD4+ and CD8+ T cells (5) that display particular Vβ elements in their T-cell receptors (11). TSST-1 is associated with staphylococcal toxic shock syndrome (TSS) and is considered to be the cause of nearly all cases of menstrual TSS and at least 50% of nonmenstrual cases (3). TSS is a severe multisystem condition characterized by high fever, rash, hypotension, and skin desquamation. TSST-1 has also been implicated in recalcitrant, erythematous, desquamating syndrome, which affects AIDS patients (6). TSST-1 is produced by organisms present in 60 to 70% of patients with Kawasaki syndrome, an illness that shares many features with TSS and that is typically seen in children under 4 years of age (12). The role of TSST-1 in Kawasaki syndrome remains controversial, however.
Numerous studies have demonstrated the importance of oxygen tension in the regulation of TSST-1 production by S. aureus. Excess aeration of cultures, as well as complete anaerobiosis, resulted in repression of TSST-1 production, while microaerobic environments appeared to stimulate toxin expression (15, 21, 24). It has been suggested that elevated vaginal oxygen levels associated with the insertion of a tampon stimulate the production of TSST-1 (17). Wagner et al. (23) demonstrated that the vaginal environment is normally anaerobic and that insertion of a tampon dramatically increased the oxygen level on the vaginal mucosal surface. Following tampon insertion, oxygen levels slowly declined throughout the observation period of 8 h.
The response of S. aureus, however, to the full spectrum of physiologically relevant oxygen tensions has never been completely and carefully examined in cultures that mimic physical conditions in vivo. What oxygen levels result in maximum toxin levels and what oxygen levels effectively shut off toxin production have remained unanswered questions. In the study described here, we examined the production of TSST-1 by S. aureus in the presence of a range of oxygen levels relevant to in vivo conditions and discuss their implications for antistaphylococcal chemotherapy. We also characterize the response of S. aureus to various oxygen levels in the presence or absence of carbon dioxide. We introduce the use of thin-film culture to examine the response of S. aureus to oxygen and carbon dioxide.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The S. aureus strain used in this study, MN8, was isolated from the vagina of a TSS patient (4). Strains were grown in beef heart medium prepared as described previously (4) with 1% glucose-phosphate buffer (60 g of glucose, 40 g of NaHCO3, 40 g of NaCl, 30 g of Na2HPO4, and 4 g of l-glutamine in 1 liter of H2O) in either batch or thin-film cultures. For batch cultures, 300 ml of medium in 2-liter flasks was inoculated with S. aureus to achieve an initial density of 107 CFU/ml. Batch cultures were incubated at 37°C while being continuously stirred with a Teflon-coated magnetic stir bar and were flushed at a rate of approximately 5 liters/min with a gas mixture (Praxair, St. Louis, Mo.). The gas mixtures contained a constant percentage of oxygen balanced with nitrogen and, when indicated, 7% carbon dioxide. The effect of stirring in the batch cultures was to entrain completely the atmosphere throughout the culture in the form of small bubbles, exposing the entire culture to atmospheric oxygen levels. In tests with an oxygen probe (model 5300; YSI Inc., Yellow Springs, Ohio), the oxygen concentrations in the headspace of the batch culture container were found to be constant throughout the incubation period, while the dissolved oxygen levels in the liquid medium slowly declined to undetectable levels during exponential growth of the bacteria. Batch cultures were incubated for 6 to 7 h when no carbon dioxide was present or 8 h when 7% carbon dioxide was included in the gas mixture. Samples were removed from the culture at regular time intervals, and readings of the optical density at 600 nm were taken and cell counts were determined prior to ethanol treatment of the culture samples. Proteins were precipitated and cells were killed in 4 volumes of ethanol for a minimum of 12 h. Precipitation by this method has been shown to be complete, and the removal of bacteria prior to treatment with ethanol is not necessary to quantify toxin production (16, 17). The mixtures were centrifuged at 500 × g for 10 min, the supernatant was removed, and the pellet was partially desiccated to remove excess liquid. The pellets were resuspended in 1 ml of phosphate buffer solution containing 1.0% fetal calf serum and 0.05% Tween 20 and were centrifuged at approximately 500 × g for 5 min to remove insoluble cell debris in the preparation for determination of TSST-1 concentrations by enzyme-linked immunosorbent assay (ELISA).
For thin-film cultures, 1 ml of medium containing 107 S. aureus CFU was placed on the bottom of polystyrene petri dishes (100 by 15 mm; Fisher Scientific, Pittsburgh, Pa.) and was held in place with squares (4 by 4 cm) of polyethylene mesh. The thin-film cultures were placed in sealed, humidified Plexiglas cell culture chambers (as described by Mishell and Mishell [13]; internal dimensions, 20 by 26 by 7.5 cm). The chambers had inlet and exit ports for flushing with the indicated gas mixtures before sealing. The thin-film cultures were incubated at 37°C for 24 h. After removal of the cultures from the chambers, the cells were resuspended by agitation in 3 ml of Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.). Two milliliters of this suspension was immediately treated with 8 ml (4 volumes) of ethanol, and the mixture was incubated at room temperature overnight to precipitate the toxin. As described above, removal of cells prior to ethanol treatment was not necessary for quantitative toxin detection. The mixture was centrifuged at 500 × g for 10 min, the supernatant was removed, and the pellet was desiccated to remove excess liquid. The pellets were resuspended in 1 ml of phosphate buffer solution containing 1.0% fetal calf serum and 0.05% Tween 20 and was centrifuged at approximately 500 × g for 5 min to remove insoluble cell debris in preparation for determination of TSST-1 concentrations by ELISA.
TSST-1 detection.
To measure TSST-1 production, we used a previously described double-antibody sandwich ELISA (7) with minor modifications. Ninety-six-well microtiter plates (NUNC, Roskilde, Denmark) were coated with highly specific rabbit anti-TSST-1 serum in carbonate buffer (0.05M NaCO3 [pH 9.6]) and dried overnight. The wells were washed three times with phosphate buffer solution (1.9 mM NaH2PO4, 3.1 mM NaHPO4, 0.15 M NaCl), 0.05% Tween 20, and 1% fetal calf serum (PTF). Diluted toxin samples (100 μl) were added to the wells, and the plates were incubated for 1.5 h at room temperature. The wells were again washed three times with PTF. A total of 100 μl of a 1:300 dilution in PTF of rabbit anti-TSST conjugated with a horseradish peroxidase label (Toxin Technologies, Sarasota, Fla.) was then added to each well, and the plates were incubated for 1.5 h at room temperature. The wells were washed with PTF three times. Detection substrate (100 μl) (1% [wt/vol] o-phenylenediamine and 0.1% [vol/vol] H2O2 in citrate-phosphate buffer [0.7 M citric acid, 0.3 M Na2HPO4, pH 5.0]) was added to each well, and the plates were incubated until a color change was observed. The reaction was stopped by the addition of 12.5% H2SO4. Colorimetric reactions were detected with a microtiter plate reader (EL 340; Bio-Tek Instruments). TSST-1 concentrations were determined by comparing the absorbance readings for wells that contained samples to those for wells that contained a dilution series of control TSST-1 whose concentration was known. The dilution series of control TSST-1 was included on each microtiter plate. We have consistently been able to detect TSST-1 concentrations as low as 1 ng/ml using this method.
Control TSST-1 for establishing the standard curve was prepared by ethanol precipitation of cell cultures, resolubilization in H2O, centrifugation at 500 × g to remove insoluble cell debris, and successive thin-layer isoelectric focusing in pH gradients of 3 to 10 and then 6 to 8 (4). The TSST-1 preparation was homogeneous when tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (9) with discontinuous 10% acrylamide gels. Control TSST-1 thus prepared has been determined to be homogeneous when subjected to SDS-PAGE and silver staining, and there is no evidence of contamination when the prepared toxin is subjected to automated sequencing. These preparations have also been satisfactory for the preparation of crystals for structure analysis with resolution to 1.8 Å.
To determine our ability to recover TSST-1 from staphylococcal cultures, purified TSST-1 was added to a culture of a TSST-1-negative strain (S. aureus RN4220) to achieve a final concentration of 1.00 μg/ml. The sample was ethanol precipitated and resolubilized in PTF, and insoluble cell debris was removed and subjected to the ELISA as described above. The ELISA indicated a TSST-1 concentration of 1.07 μg/ml, or a recovery of 107%.
RESULTS
Both batch and thin-film cultures were exposed to an atmospheric oxygen range of 0 to 21% (vol/vol) on the basis of the observation that vaginal oxygen concentrations are normally nearly anaerobic and will not exceed ambient oxygen concentrations (21%) upon insertion of a tampon (23).
Batch cultures.
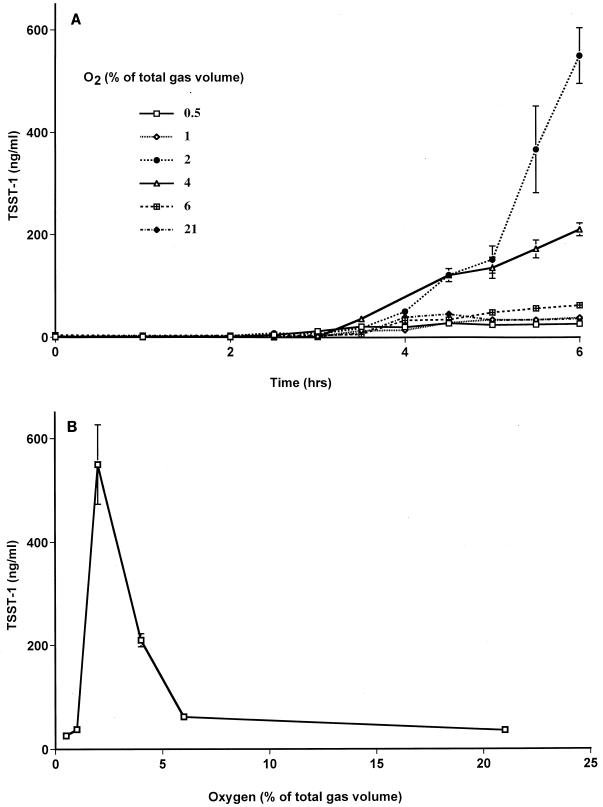
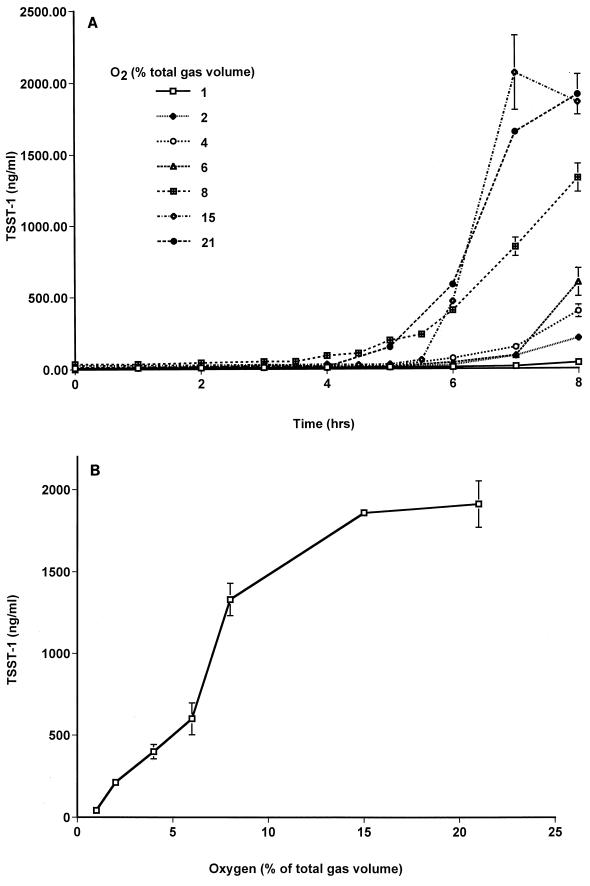
Batch cultures were incubated under continuous gas flow at 37°C until both the cultures had passed postexponential phase and the maximum rate of toxin production was observed. For batch cultures incubated in oxygen and nitrogen mixtures only, these conditions were observed at approximately 6 h. Batch cultures incubated in atmospheres containing 7% carbon dioxide, in addition to oxygen and nitrogen mixtures, were allowed to grow for 8 h until the maximum rate of toxin production was observed. As observed by comparing Fig. 1A and 2A, toxin production by S. aureus incubated in gas mixtures that contained no carbon dioxide was initiated at least 1 h earlier than that in cultures incubated under oxygen balanced with nitrogen and carbon dioxide, supporting the decision to allow the latter to incubate longer. Although minimal toxin was made after the 6- and 8-h time periods reflected in Fig. 1 and 2, respectively, cultures incubated for additional periods of time do not reflect any change in relative toxin production in the presence of different percentages of oxygen.
FIG. 1.
TSST-1 production by S. aureus MN8 in batch cultures flushed with gas mixtures containing various oxygen concentrations balanced with nitrogen. (A) Time course measurements of TSST-1 production in each batch culture. (B) Concentration of TSST-1 in each batch culture at 6 h. Data are means ± standard errors of the means of triplicate ELISA readings for each time point. Toxin production was measured in at least two independent batch cultures at each oxygen concentration, with similar results.
FIG. 2.
TSST-1 production by S. aureus MN8 in batch cultures flushed with gas mixtures containing various oxygen concentrations balanced with nitrogen and 7% carbon dioxide. (A) Time course measurements of TSST-1 production in each batch culture. (B) Concentration of TSST-1 in each batch culture at 8 h. Data are means ± standard errors of the means of triplicate ELISA readings for each time point. Toxin production was measured in at least two independent batch cultures at each oxygen concentration, with similar results.
For all batch cultures, the rate of toxin production was highest during late exponential and postexponential phases and decreased as cultures progressed into stationary phase. The final pH of the cultures ranged from 6.4 to 7.4.
(i) Batch cultures without carbon dioxide.
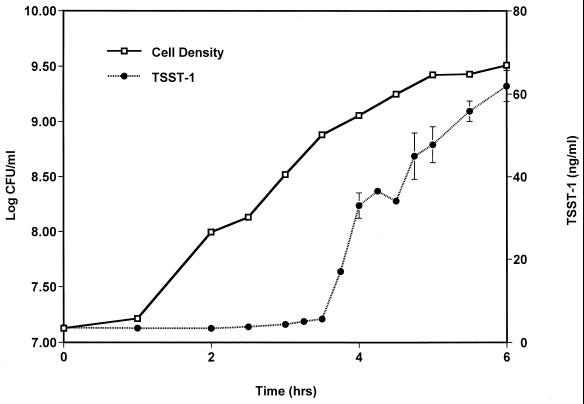
For batch cultures incubated in the presence of oxygen balanced with nitrogen alone, S. aureus began toxin production 3 to 3.5 h after inoculation of 107 CFU/ml of medium (Fig. 1A). This initiation of toxin production corresponded to cell densities of ∼109 CFU/ml. Toxin production at between 6 and 7 h was negligible for cultures incubated in the presence of 0.5, 1, 4, 6, and 21% oxygen. We did not assess TSST-1 production at 7 h in the culture with 2% oxygen; by 6 h toxin production was significantly higher in this culture than in all the other cultures. Total TSST-1 production by S. aureus in batch cultures was negligible under anaerobic conditions, peaked (500 ng/ml) in the presence of 2% oxygen, and declined to only 70 ng/ml in the presence of 6% atmospheric oxygen levels (Fig. 1B). A representative growth curve and a time course measurement of toxin production are shown in Fig. 3. We have chosen to express toxin levels as the amount of TSST-1 produced per milliliter of culture, as the effect on the host will primarily be determined by total toxin levels and not the total amount of toxin produced per cell. With the exception of the batch culture grown in the presence of 0.5% oxygen, however, all cultures demonstrated nearly identical growth patterns and reached similar cell densities.
FIG. 3.
Growth curve and time course measurement of TSST-1 production by S. aureus MN8 representative of cultures incubated in the presence of oxygen balanced with nitrogen. The data shown here are for the batch culture incubated in the presence of 6% oxygen balanced with nitrogen. All cultures, with the exception of the culture incubated in the presence of 0.5% oxygen, exhibited similar growth patterns and toxin production profiles. TSST-1 production data are means ± standard errors of the means of triplicate ELISA readings for each time point.
(ii) Batch cultures with carbon dioxide.
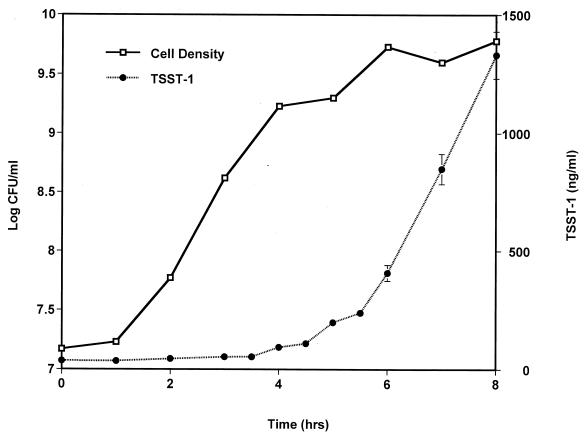
In batch cultures incubated in the presence of 7% carbon dioxide, S. aureus initiated toxin production 4 to 4.5 h after inoculation (Fig. 2A), 1 h later than did S. aureus incubated without carbon dioxide. In addition, S. aureus responded to oxygen levels that increased from 0 to 21% by producing increasing amounts of TSST-1 (40 to 1,900 ng/ml, respectively) (Fig. 2B). Cultures incubated in the presence of oxygen balanced with nitrogen and carbon dioxide also exhibited highly similar growth patterns, and initiation of toxin production corresponded to densities of ∼109 CFU/ml. A representative growth curve and a time course measurement of toxin production are shown in Fig. 4.
FIG. 4.
Growth curve and time course measurement of TSST-1 production by S. aureus MN8 representative of cultures incubated in the presence of oxygen balanced with nitrogen and 7% carbon dioxide. The data shown here are for the batch culture incubated in the presence of 8% oxygen balanced with nitrogen and 7% carbon dioxide. All cultures, with the exception of the culture incubated in the presence of 1% oxygen, exhibited similar growth patterns and toxin production profiles. TSST-1 production data are means ± standard errors of the means of triplicate ELISA readings for each time point.
(iii) Batch cultures with controlled pH.
Decreasing levels of oxygen result in slightly lower pH levels, likely due to the buildup of by-products of homolactic fermentation by S. aureus. It was possible that, for batch cultures incubated in the presence of carbon dioxide-containing gas mixtures, lower pH conditions resulted in decreased levels of dissolved carbon dioxide and thus lower levels of TSST-1 production. To address this possibility, we conducted a set of batch cultures identical to those described earlier (including batch cultures in the presence of 7% carbon dioxide), except that the pH was maintained at 7.4 to 7.5 with 10 N NaOH. As expected, TSST-1 production remained virtually undetectable under anaerobic conditions and reached 2 μg/ml in the presence of 8% oxygen (data not shown).
Thin films.
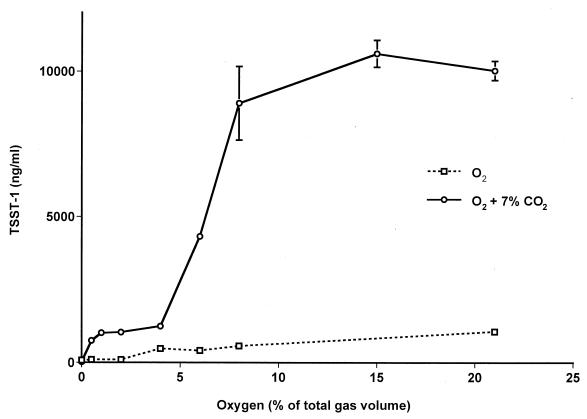
Staphylococci grown in thin-film cultures formed a visible, opaque film covering the area (4 by 4 cm) of the petri dish. Without carbon dioxide, the production of TSST-1 by S. aureus in these cultures increased as oxygen levels were increased from 0 to 21% (Fig. 5). Cell densities were highly similar in these cultures, with an average of 3.6 × 109 CFU/ml. In the presence of carbon dioxide, TSST-1 production began to level off in the presence of approximately 8% oxygen and remained high in the presence of oxygen at up to 21% (Fig. 5). Cell densities ranged from 2.3 × 109 CFU/ml under anaerobic conditions to 5.8 × 109 CFU/ml in the presence of 21% oxygen. TSST-1 production was significantly enhanced in the presence of carbon dioxide (approximately 1 log unit).
FIG. 5.
TSST-1 production by S. aureus MN8 in thin-film cultures incubated in atmospheres containing various oxygen levels balanced with nitrogen only or with nitrogen and 7% CO2. TSST-1 concentrations are shown for cultures incubated for 24 h in chambers initially flushed with the indicated gas mixture and then sealed. Data are means ± standard errors of the means of values from three separate thin-film cultures for which triplicate ELISA readings were performed for each culture.
DISCUSSION
TSS emerged as a recognized disease in the late 1970s and early 1980s. Early in the study of TSS, a correlation between the use of certain types of tampons and incidence of the disease was demonstrated (19). Numerous studies have attempted to determine the nature of this association. In 1983, our laboratory proposed that the presence of oxygen in the vaginal environment played a pivotal role in the induction of TSST-1 production by S. aureus (17). Several studies have shown that elevated oxygen, carbon dioxide, and protein levels, along with a relatively neutral pH, are required for TSST-1 production by S. aureus (8, 17, 24). Todd et al. (22) showed that altering any of these conditions greatly reduced the amount of TSST-1 synthesized by S. aureus in vitro and, furthermore, that these conditions are present in patients who experience TSS. Previous studies have not thoroughly examined the role of oxygen in the regulation of TSST-1 production, however, across the entire range of oxygen concentrations relevant to in vivo conditions. In this study, we approximate in vivo conditions using a high-protein medium (beef heart) at neutral pH and controlled levels of carbon dioxide and oxygen. We fully characterize TSST-1 production by S. aureus exposed to the full range of oxygen concentrations expected to occur in vivo (0 to 21% [vol/vol] oxygen) in the presence and absence of carbon dioxide.
Both oxygen and carbon dioxide exhibit controlling effects on TSST-1 production in batch and thin-film cultures. In both culture types, anaerobiosis led to a reduction in the level of toxin production to nearly undetectable levels (Fig. 1B, 2B, and 5). Similarly, batch cultures incubated without carbon dioxide in the presence of oxygen levels above 6% exhibited greatly reduced levels of toxin production (Fig. 1B). In contrast, the presence of carbon dioxide in either the batch or the thin-film cultures greatly increased the level of TSST-1 production (Fig. 2B and 5). This is consistent with previous studies that showed that both elevated oxygen and elevated carbon dioxide levels were required for significant toxin production (8, 22). (TSST-1 production remained high even in the presence of normal atmospheric levels of oxygen [21%] in the presence of carbon dioxide. This suggests that previous studies that demonstrated an inhibitory effect of excess aeration [15, 24] either maintained culture oxygen levels well above those for cultures in our study or lacked sufficient carbon dioxide concentrations in the cultures.) These observations suggest sensory mechanisms that act in the regulation of virulence factors in S. aureus.
Wagner et al. (23) showed that carbon dioxide levels on the vaginal surface recovered from near atmospheric levels (<1% [vol/vol]) to normal in vivo levels (5 to 7% [vol/vol]) within a half hour after insertion of a tampon, whereas oxygen levels remained well above normal in vivo levels for the entire 8-h observation period. This suggests that vaginal S. aureus will encounter both elevated carbon dioxide and oxygen levels concurrently, conditions which this study demonstrates are optimal for TSST-1 production.
It is not readily apparent why the thin-film cultures responded differently than the batch cultures to various oxygen levels when carbon dioxide was not present (Fig. 1B and 5). At least two factors might be responsible for the observed differences in the response to oxygen levels. First, the physical environments of the batch and thin-film cultures are quite different. Unlike the constantly stirred cells in the batch culture, which are evenly exposed to atmospheric gases, the relatively stationary cells of the thin films are possibly exposed to concentration gradients of oxygen and carbon dioxide. Cells near the surface of the bacterial film are exposed to atmospheric levels of these gasses, while cells closer to the polystyrene base may experience reduced oxygen and elevated carbon dioxide levels due to the aerobic metabolism of cells nearer the surface. Second, the batch cultures were continuously flushed with gas mixtures, whereas the thin-film cultures were placed into chambers initially flushed with the gas mixture and then sealed for the duration of the experiment. Atmosphere replacement may have prevented the accumulation of some metabolic by-products in the batch cultures, while lack of atmosphere replacement allowed accumulation of by-products in the thin-film cultures. This accumulation may have significantly altered the response of S. aureus in the thin-film cultures by inducing toxin production in the presence of high oxygen levels.
Oxygen-scavenging agents might make effective antitoxigenic compounds, as anaerobic conditions inhibit TSST-1 production by S. aureus. It would be necessary, however, to find one that is both nontoxic to humans and a particularly effective scavenging agent, as even low levels of oxygen permit toxin production. A more effective approach to combating staphylococcal infection might be to target the oxygen-sensing mechanism that staphylococci use to regulate virulence factor production. It is not difficult to envision a two-component histidine kinase (HK)-response regulator (RR) pair that is sensitive to oxygen levels and that interacts with S. aureus global regulators of virulence factors, including toxins. A search of the S. aureus genome databases at TIGR and at the University of Oklahoma with a TBlastN search program (1) revealed the presence of putative homologs to the ResDE HK-RR system that has been implicated in global regulation of aerobic and anaerobic respiration in Bacillus subtilis (14, 20). The putative S. aureus RR (PorA) is 68% identical to ResD, while the putative S. aureus HK (PorB) is 34% identical to ResE. The C terminus of PorB, which contains the conserved regions integral to HK activity, is 45% identical to the ResE C-terminal region. The N terminus has less identity (26%) with the corresponding region in ResE, but this region contains transmembrane helices that are less highly conserved among members of the HK family. We have confirmed the presence of these ResDE homolog genes in MN8 using PCR methods. Our laboratory is investigating the role of this putative two-component system in the control of virulence factors in S. aureus.
Indeed, the targeting of two-component systems has been shown to be effective against gram-positive pathogens, including methicillin-resistant S. aureus and vancomycin-resistant Enterococcus faecium (2, 10). It is conceivable that such a targeted drug may inhibit oxygen sensing by staphylococci through two-component systems and thus prevent toxin production. Although these specialized drugs remain toxic to humans, future efforts are likely to produce safe and effective compounds for use in antistaphylococcal chemotherapy. Glycerol monolaurate has also been shown to have antitoxigenic effects, while it has only weak antimicrobial action (18). A possible mechanism of glycerol monolaurate, which acts at the lipid-water interface, is to interfere with the activity of membrane-bound sensor proteins that are used by S. aureus and that sense environmental conditions and regulate toxin production. Inhibition of toxin production would reduce systemic disorders in patients, while they would help to prevent further spread of the organism. Treatment with combinations of drugs that interfere with staphylococcal environmental sensing mechanisms and standard antibiotics may provide an effective two-pronged approach by quickly eliminating toxin production and clearing the organism from the patient.
ACKNOWLEDGMENTS
This study was supported by a research grant from The Procter and Gamble Co. J.M.Y. was supported by a Howard Hughes Medical Institute Predoctoral Fellowship in the Biological Sciences and a research grant from the National Institute of Allergy and Infectious Diseases (grant AI22159).
We thank John McCormick for special assistance in developing laboratory techniques. We gratefully acknowledge the Staphylococcus aureus Genome Project and B. A. Roe, A. Dorman, F. Z. Najar, S. Clifton, and J. Iandolo, who received funding from the National Institutes of Health and the Merck Genome Research Institute.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett J F, Goldschmidt R M, Lawrence L E, Foleno B, Chen R, Demers J P, Johnson S, Kanojia R, Fernandez J, Bernstein J, Licata L, Donetz A, Huang S, Hlasta D J, Macielag M J, Ohemeng K, Frechette R, Frosco M B, Klaubert D H, Whiteley J M, Wang L, Hoch J A. Antibacterial agents that inhibit two-component signal transduction systems. Proc Natl Acad Sci USA. 1998;95:5317–5322. doi: 10.1073/pnas.95.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergdoll M S, Schlievert P M. Toxic shock syndrome toxin. Lancet. 1984;ii:691. [Google Scholar]
- 4.Blomster-Hautamaa D A, Schlievert P M. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 5.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 6.Cone L A, Woodard D R, Byrd R G, Schulz K, Kopp S M, Schlievert P M. A recalcitrant, erythematous, desquamating disorder associated with toxin-producing staphylococci in patients with AIDS. J Infect Dis. 1992;165:638–643. doi: 10.1093/infdis/165.4.638. [DOI] [PubMed] [Google Scholar]
- 7.Freed R C, Evenson M L, Reiser R F, Bergdoll M S. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol. 1982;44:1349–1355. doi: 10.1128/aem.44.6.1349-1355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kass E H, Kendrick M I, Tsai Y C, Parsonnet J. Interaction of magnesium ion, oxygen tension, and temperature in the production of toxic-shock-syndrome toxin-1 by Staphylococcus aureus. J Infect Dis. 1987;155:812–815. doi: 10.1093/infdis/155.4.812. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Macielag M J, Demers J P, Fraga-Spano S A, Hlasta D J, Johnson S G, Kanojia R M, Russell R K, Sui Z, Weidner-Wells M A, Werblood H, Foleno B D, Goldschmidt R M, Loeloff M J, Webb G C, Barrett J F. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J Med Chem. 1998;41:2939–2945. doi: 10.1021/jm9803572. [DOI] [PubMed] [Google Scholar]
- 11.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. [PubMed] [Google Scholar]
- 12.Meissner H C, Leung D Y M. Kawasaki syndrome. In: Gorbach S L, Bartlett J G, Blackow N R, editors. Infectious diseases. Philadelphia, Pa: The W. B. Saunders Co.; 1998. pp. 1662–1666. [Google Scholar]
- 13.Mishell B B, Mishell R I. Primary immunization in suspension cultures. In: Mishell B B, Shiigi S M, editors. Selected methods in cellular immunology. W. H. San Francisco, Calif: Freeman & Co.; 1980. pp. 30–37. [Google Scholar]
- 14.Nakano M M, Zuber P, Glaser P, Danchin A, Hulett F M. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J Bacteriol. 1996;178:3796–3802. doi: 10.1128/jb.178.13.3796-3802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarafian S K, Morse S A. Environmental factors affecting toxic shock syndrome toxin-1 (TSST-1) synthesis. J Med Microbiol. 1987;24:75–81. doi: 10.1099/00222615-24-1-75. [DOI] [PubMed] [Google Scholar]
- 16.Schlievert P M. Immunochemical assays for toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:339–344. doi: 10.1016/s0076-6879(88)65050-6. [DOI] [PubMed] [Google Scholar]
- 17.Schlievert P M, Blomster D A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983;147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 18.Schlievert P M, Deringer J R, Kim M H, Projan S J, Novick R P. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob Agents Chemother. 1992;36:626–631. doi: 10.1128/aac.36.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shands K N, Schmid G P, Dan B B, Blum D, Guidotti R J, Hargrett N T, Anderson R L, Hill D L, Broome C V, Band J D, Fraser D W. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 20.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor D, Holland K T. Effect of dilution rate and Mg2+ limitation on toxic shock syndrome toxin-1 production by Staphylococcus aureus grown in defined continuous culture. J Gen Microbiol. 1988;134:719–723. doi: 10.1099/00221287-134-3-719. [DOI] [PubMed] [Google Scholar]
- 22.Todd J K, Todd B H, Franco-Buff A, Smith C M, Lawellin D W. Influence of focal growth conditions on the pathogenesis of toxic shock syndrome. J Infect Dis. 1987;155:673–681. doi: 10.1093/infdis/155.4.673. [DOI] [PubMed] [Google Scholar]
- 23.Wagner G, Bohr L, Wagner P, Petersen L N. Tampon-induced changes in vaginal oxygen and carbon dioxide tensions. Am J Obstet Gynecol. 1984;148:147–150. doi: 10.1016/s0002-9378(84)80165-9. [DOI] [PubMed] [Google Scholar]
- 24.Wong A C, Bergdoll M S. Effect of environmental conditions on production of toxic shock syndrome toxin 1 by Staphylococcus aureus. Infect Immun. 1990;58:1026–1029. doi: 10.1128/iai.58.4.1026-1029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]