Abstract
Brain cancers, mainly high-grade gliomas/glioblastoma, are characterized by uncontrolled proliferation and recurrence with an extremely poor prognosis. Despite various conventional treatment strategies, viz., resection, chemotherapy, and radiotherapy, the outcomes are still inefficient against glioblastoma. The blood–brain barrier is one of the major issues that affect the effective delivery of drugs to the brain for glioblastoma therapy. Various studies have been undergone in order to find novel therapeutic strategies for effective glioblastoma treatment. The advent of nanodiagnostics, i.e., imaging combined with therapies termed as nanotheranostics, can improve the therapeutic efficacy by determining the extent of tumour distribution prior to surgery as well as the response to a treatment regimen after surgery. Polymer nanoparticles gain tremendous attention due to their versatile nature for modification that allows precise targeting, diagnosis, and drug delivery to the brain with minimal adverse side effects. This review addresses the advancements of polymer nanoparticles in drug delivery, diagnosis, and therapy against brain cancer. The mechanisms of drug delivery to the brain of these systems and their future directions are also briefly discussed.
Keywords: polymer nanoparticles, glioma/glioblastoma, blood–brain barrier (BBB)/blood brain tumour barrier (BBTB), nanodiagnostics, drug delivery and imaging
1. Introduction
Cancer is one of the serious life-threatening diseases worldwide with a higher risk of mortality, around 10 million new cases are diagnosed every year [1,2]. Among different types of cancer, brain cancer is the most lethal and invasive type of central nervous system (CNS) disorder [3]. Brain cancer is characterised as a heterogeneous group of primary and metastatic cancers in the CNS [4,5]. The average incidence of both malignant and non-malignant brain cancer is reported approximately 28.57 per 100,000 population, mostly affecting 0 to 19 years, with a mean annual morbidity rate of 5.57 per 100,000 population [6,7]. Among these, the malignant primary brain cancers with a 5-year survival rate of less than 33.3–35% and even the rate are still alleviating. The average survival span is still not improved and even lower between 15 to 22 months [8,9]. A recent report from 2020 of the Central Brain Tumor Registry of the United States accounted for primary malignant tumour incidence rate to be 7.08 per 100,000, with 123,484 estimated cases, and 16.71 per 100,000, with 291,927 cases of non-malignant tumour [10]. Malignant primary tumours, i.e., gliomas derived from the glial origin, are newly diagnosed for approximately 70%, mostly in adults [5,11]. The reduced efficacy of brain cancer therapy is mainly attributed to the presence of the blood–brain barrier (BBB) that limits the permeation of systemically applied drugs into the brain [3].
Brain cancers are categorised into two groups, viz., primary brain cancer originated from the brain and resided within the brain, commonly called glioma, and secondary or metastatic brain cancer spreading from primary cancer outside the CNS, originate from systemic neoplasms and further evolved in the interior of brain parenchyma [12,13]. Glial cell originated gliomas include glioblastomas, astrocytomas, schwannomas, oligodendrogliomas, etc. [14]. According to World Health Organization (WHO), glioma tumours of CNS is classified into four grades based on aggressiveness, Grade I pilocytic astrocytoma, Grade II diffuse astrocytoma, Grade III anaplastic astrocytoma, and Grade IV glioblastoma [12]. Glioblastoma (GBM) and its variants were categorised as Grade IV tumours [15]. Grades I and II are considered low-grade glioma, and Grades III and IV are considered high-grade gliomas, i.e., malignant gliomas, and are characterised by poor prognosis [8,16,17]. GBM can either develop from normal brain cells or evolve from pre-existing low-grade astrocytoma [18]. GBM is also termed as glioblastoma multiforme or Grade IV astrocytoma [19]. Excessive penetration and vascular proliferation into brain parenchyma is the indication of aggressive cancer [20].
Conventional glioma therapy includes tumour resection followed by radiotherapy and chemotherapy. Surgical resection is generally considered a standard method for glioblastoma therapy. Yet resection of tumour tissue cannot be entirely removed and hence is limited by the glioblastoma’s aggressiveness caused by penetration into surrounding tissue microenvironment and tumour vascularisation [20,21]. Hence, tumour resection is associated with the administration of chemotherapeutic drugs and/or radiation therapy for enhanced efficiency. Radiation therapy can be delivered internally or externally and is regarded as the standard treatment for high-grade gliomas [22]. Chemotherapy drugs such as carmustine (BCNU) can cross the BBB and target glioma cells directly [20]. Further, chemotherapy has undergone some alteration by replacing the use of some alkylating agents, viz., carmustine (BCNU), nimustine (ACNU), and lomustine (CCNU) with temozolomide (TMZ) [23]. Temozolomide is converted to 5-3-(methyl)-1-(triazen-1-yl) imidazole-4-carboxamide, at physiological pH, damages DNA via methylation of the O6-position of guanines, blocks DNA replication and induces tumour cell death. Presently, TMZ, along with surgical resection and radiotherapy, is applied for glioblastoma therapy [17]. Despite that, all the treatment strategies possess some limitations towards survival and thus, the prognosis still remains poor (Table 1).
Table 1.
Advantages and limitations of conventional glioblastoma therapy.
| Conventional Therapy | Advantage | Limitation |
|---|---|---|
| Resection | Local removal of a tumour |
|
| Radiotherapy | Standard treatment protocol for HGGs |
|
| Chemotherapy | Standard therapy for cancer, cytotoxicity |
|
Although brain cancer resembles to other forms of cancer in the body, the major difference is their intracranial neoplasms, heterogeneity, intricate brain system, and the physiological features of the cranial cavity which restrain the treatment options [10]. Gliomas tend to permeate the surrounding tissue microenvironment, and thereby, it is very difficult to determine the tumour boundaries. This also attributes to several difficulties in conventional therapeutic approaches for a curative outcome. Moreover, the physical and chemical barriers hamper therapeutic drug molecules from reaching tumour locations [11]. The BBB and blood–brain tumour barrier (BBTB) represent the diffusion barrier systems of the brain that regulate the influx of drugs to the brain except owing to certain characteristics [24]. Standard treatments remain ineffective due to poor surgical resection of tumours, mainly the infiltrative ones, poor chemo-therapeutic drug influx to the tumour site, and BBB that restrict them from diffusing toward tumour location [25]. The limitations of radiotherapy also result in incomplete eradication of GBM cells resulting in self-renewal and recurrence [26]. Targeting active anticancer agents to the brain is a challenging task in the area of drug delivery as BBB prevents the transportation of a drug. Hence, higher doses are needed to attain desired therapeutic efficacy which causes undesirable side effects [27].
2. The Blood–Brain Barrier (BBB)
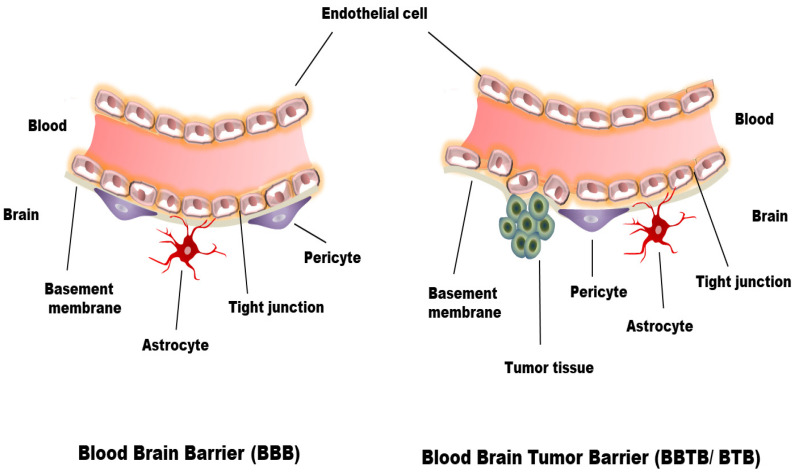
One of the main hurdles for the effective systemic treatment of brain cancer is the presence of the BBB. The BBB is a semipermeable membrane barrier between blood capillaries and cellular components of brain tissues that control the movement of ions, nutrients, and cells. The BBB also serves for the dynamic transport of nutrients, peptides, proteins and immune cells between the brain and blood [28]. The BBB consists of endothelial cells, glial cells (pericytes, astrocytes, and neurons) and basement membrane [29] (Figure 1). The endothelial cells line the interior brain capillaries forming the tight junctions that allow small molecules, gases and curb the influx of harmful toxins or pathogens such as bacteria, lipophilic neurotoxins, xenobiotics and hydrophilic substances from the blood to the brain [30]. Due to the presence of pinocytic vesicles, other carriers, transport proteins, and large numbers of mitochondria, hydrophobic and essential molecules such as O2, CO2, glucose, hormones, etc. can infiltrate either by passive diffusion or active transport mechanisms [29]. The presence of several transmembrane proteins characterises the tight junctions between the inter-endothelial cells. These protein complexes are mainly comprised of occludin, claudin, and junctional adhesion molecules. These three specialised proteins interact to develop an intricate, tight barrier that is exclusive to the cerebro-endothelial cells [31]. The apical part of the endothelial cell is exposed to the brain’s blood capillaries, and the basolateral part is exposed to the cerebrospinal fluid supported by the basement membrane. The basement membrane with 30–40 nm thickness consists of Type IV collagen, fibronectin, laminin, heparin sulfate proteoglycans and other extracellular matrix proteins that completely covers the endothelial cells and limits the movement of the solutes [29,31,32]. Approximately 98% of smaller molecular weight drugs and 100% of larger molecular weight drugs are reported for their inability to cross the intact BBB [33,34]. Under various brain-related pathological conditions, including brain cancers, glioma cells loose the structural integrity and the function of the BBB [35]. BBB is compromised in human glioma cells because of the leaky inter endothelial tight junction and poorly differentiated astrocytes that are unable to release essential components for BBB function [31,36]. In this case, it is termed as blood–brain tumour barrier (BBTB) or blood–tumour barrier (BTB) [14] (Figure 1).
Figure 1.
Schematic representation of blood–brain Barrier (BBB) and the blood–brain tumour barrier (BBTB).
In low-grade gliomas, the structure and function of the BBTB resemble normal BBB, while in hi-grade glioma, BBB is significantly altered, disrupted. Although the degree of BBB disruption varies from the tumour malignancy, low-grade glioma is still a hurdle to treat due to intact BBB. Despite high-grade gliomas, the structural disruption of their vascular density and integrity is negligible to drug permeability in tumour cells [37,38]. However, BBTB is more permeable than the BBB and allows heterogeneous permeability to drugs and other components. Thus, it is a more challenging task to combat the difficulties of brain cancer [39]. Therefore, along with the existing therapeutic regimen, new approaches are required to combat the BBB. To combat these difficulties, various techniques were developed, which are mostly invasive and cause serious side effects. Nanotechnology, especially use of polymer nanoparticles, helps address the major hurdle of glioma therapy non-invasively. Polymer nanoparticles aid in the targeting and delivery of potent drug molecules to the brain. In this review paper, we will briefly summarise the up-to-date existing therapies and diagnoses in brain cancer gliomas using polymer nanoparticles.
3. Polymer Nanoparticles for Drug Delivery Strategy to Overcome the BBB
The BBB is the main problem in the treatment of brain cancer glioma. The chemotherapeutic drugs are mostly ineffective due to limiting permeability to BBB as it allows to pass only low molecular weight (<500 Da), electrically neutral hydrophobic drugs with lipophilicity at log P 2–3 [11,36,40]. The majority of chemotherapeutic drugs are larger in size, ionic, hydrophilic molecules and thus cannot cross the BBB that is attributed to the requirement of a higher systemic dose that results in severe side effects [11]. To overcome these drawbacks, nanoparticles can be utilised for the controlled and sustained delivery of drugs. Biodegradable polymer nanoparticles are extensively studied systems in cancer drug delivery and therapy. These nanoparticles are also highly stable and can be tuned in order to obtain the desirable characteristics for a passive or an active targeting [41]. Polymer nanoparticles can induce selective toxicity and can load ample anticancer drugs or other molecules. Various biodegradable polymeric drug delivery systems include nanogels or hydrogels, poly(ε-caprolactone) (PCL), poly (lactic-co-glycolic acid) (PLGA), chitosan [42,43], dendrimers, etc. [44]. Due to versatile tuneable properties, these nanoparticles can open tight junctions of BBB, shield BBB limiting properties of anticancer drugs, release the drug in a sustainable manner, prolong the systemic circulation, and protect against enzymatic degradation [1,45].
Studies showed that Resveratrol loaded PLGA: D-α-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles (RSV-PLGA-BNPs) displayed significant increasing cytotoxicity and enhanced cell penetration in C6 glioma cells. Haemocompatibility evaluation is one of the critical analyses of interaction between nanoparticles and various blood components that determine any adverse effect upon nanoparticle exposure to blood. The nanoparticles should not cause haemolysis during and after infusions. The haemocompatibility analysis of RSV-PLGA-BNPs revealed safe for i.v. administration. The nanoparticles exhibited prolonged systemic circulation up to 36 h. The nanoparticles also showed higher brain accumulation, suggesting a potential system for the betterment of systemic circulation and plasma half-life with a promising anticancer effect against glioma [1]. In another study, L-carnitine-conjugated PLGA NPs were developed to target glioma cells. These NPs were found to significantly cross the BBB and showed a potential anti-glioma effect [46]. Lactoferrin decorated PEG-PLGA NPs was developed for the delivery of shikonin and the treatment of gliomas [47]. Lactoferrin coating promotes internalisation across the BBB. In vitro and in vivo experiments showed the enhanced nanoparticle uptake and distribution of NPs in the brain with effective treatment of glioblastomas.
4. Polymer Nanoparticles for Anticancer Drug Delivery to the Brain: Mechanism
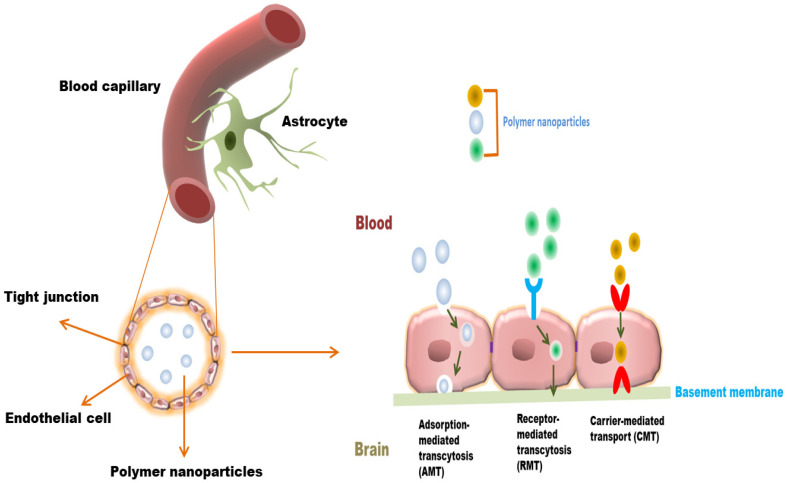
Polymer nanoparticles can cross BBB or BBTB either passively or via active endocytosis mechanisms. The unmodified polymer NPs internalise BBB mainly through passive mechanism, the so-called enhanced permeability and retention (EPR) effect, which depends on nanoparticle size. However, the NPS internalised by a passive mechanism have comparatively lower brain uptake than ligand-functionalised polymer NPs [48]. Various strategies have been undertaken to improve the infiltration of NPs into the brain. These strategies involve modification of NPs with certain moieties or components to take benefit of BBB endocytosis pathways for drug delivery. Polymer nanoparticles are able to cross BBB/BBTB through adsorption-mediated transcytosis (AMT), carrier-mediated transport (CMT), and receptor-mediated transcytosis (RMT) [49,50,51,52] (Figure 2). The internalisation of polymer nanoparticles crossing BBB/BBTB is summarized in Table 2. Polymer nanoparticles with positively charged can electrostatically interact with a negatively charged luminal surface that is attributed to cross the BBB/BBTB. The cationic polymer nanoparticles can be achieved by various surface modification strategies, either by coating or conjugation of cationic polymer or surfactant to non-ionic or neutral polymer. These modifications of NPs have been shown to utilise the AMT mechanisms to improve brain uptake. For example, a study of cationic bovine serum albumin (CBSA) conjugated with poly (ethylene glycol)–b-poly(lactide) (PEG–PLA) nanoparticles (CBSA–NPs), loaded with 6-coumarin was reported for brain delivery. Results revealed that CBSA–NPs uptake in rat brain capillary endothelial cells (BCECs) was enhanced as compared to control group BSA conjugated with pegylated nanoparticles (BSA–NP) BSA–NPs. Fluorescent microscopy of coronal brain sections displayed increased accumulation of CBSA–NPs than of BSA–NPs [53].
Figure 2.
Various transport mechanisms of polymer NPs across blood–brain barrier (BBB).
Table 2.
Summary of BBB permeability based on polymer-based nanoparticles.
| Polymer Nanoparticles | Cargo | Internalisation Mechanism | Cell Line/Animal Model | Remarks | References |
|---|---|---|---|---|---|
| Trimethylated chitosan (TMC)-modified PLGA NPs | Coenzyme Q10 6-coumarin |
AMT | SH-SY5Y cells, AD transgenic mouse brains | Increase uptake of PLGA nanoparticles, neuroprotective effects of Q10 observed in TMC-PLGA NPs than PLGA-NP. |
[57] |
| Angiopep-2 modified PLGA NPs | Doxorubicin (DOX), Epidermal growth factor receptor (EGFR) siRNA |
RMT | U87MG cells, brain orthotopic U87MG glioma xenograft model | Improved DOX and siRNA cellular uptake, NPs able to cross BBB. | [58] |
| Lactoferrin, folic acid modified PLGA NPs | Etoposide | RMT | HBMEC/HA monolayer, U87MG cells | PLGA NPs cross BBB and enhanced 2-fold uptake with Lf-and FA. | [59] |
| RVG29 modified PLGA NPs | Docetaxel | RMT | C6 cells, bEnd3 monolayer BBB model | Better BBB penetration in vitro. | [60] |
| OX26 Mab modified PLGA NPs | Temozolomide (TMZ) | RMT | U215 and U87, in vitro HBLECs monolayer model | Improved TMZ internalisation in glioblastoma cells. | [61] |
| T7- modified, magnetic PLGA nanoparticulate system (MNP/T7- PLGA NPs) |
paclitaxel (PTX) and curcumin (CUR) | RMT | U87 cells and mouse brain endothelial cell line bEnd.3., mice bearing orthotopic glioma (U87-Luc) | >10-fold increase in cellular uptake studies and a >5-fold enhancement in brain delivery compared to the non-functionalized NPs. |
[62] |
| Angiopep conjugated PEG-PCL nanoparticles (ANG-PEG-NP) | paclitaxel (PTX) | RMT (LRP-mediated transcytosis) | U87 MG, Male BALB/c nude mice and ICR mice | The penetration, distribution, and accumulation into 3D glioma spheroid and in vivo glioma region of ANG-PEG-NP was higher than that of plain PEG-PCL nanoparticles (PEG-NP). | [63] |
| dCatAlb encrusted DOX-loaded PLGA nanoparticle | Doxorubicin (DOX) | AMT | monolayer bEnd.3 cells | Enhanced BBB permeation | [64] |
| cRGD/PEG-SS-PCL micelles | Doxorubicin (DOX) | RMT | U87MG glioma xenografts | Efficient accumulation | [65] |
| DOX-loaded cRGD-SS-NGs | Doxorubicin (DOX) | RMT | U87-MG cells, U87-MG glioblastoma xenograft in nude mice | Facilitated cellular uptake and intracellular DOX release | [66] |
| T7–PEG–PLGA micelles | Carmustine (BCNU) | RMT | U87-MG cells, BALB/c nude mice | Accumulation in tumour more efficiently than unconjugated one | [67] |
| PLGA based SSTR2 pep-DIM-NPs | 3,3′-diindolylmethane | RMT | C6 glioma cells, rat Glioma model | Accumulation of the NPs into rat brain tumour sites by crossing the BBB | [68] |
| L-carnitine modified PLGA nanoparticles (LC-PLGA NPs) | Taxol and paclitaxel (PTX) | CMT | hCMEC/D3, T98G cells | Efficient accumulation | [46] |
Abbreviation: Adsorption-mediated transcytosis (AMT), carrier-mediated transport (CMT), and receptor-mediated transcytosis (RMT).
In the CMT mechanism, polymers NPs are designed to deliver drugs in order to take advantage of carrier molecules present in BBB. Polymer NPs are modified or decorated with membrane-penetrating components such as amino acids, peptides, and nutrients capable of transporting cargo across the BBB endothelial cells by utilising systemic transporters. For example, 2-deoxy-D-glucose modified poly (ethylene glycol)-co-poly (trimethylene carbonate) nanoparticles (DGlu-NPs) were studied for targeting the glioma BBB. The internalisation of DGlu-NP on RG-2 rat glioma cells was significantly higher than that of non-modified nanoparticles. This was attributed to the recognition of NPs by GLUT1 leading to enhanced cellular internalisation in glioma cells than in surrounding normal tissue and thus exhibiting promising in vivo anti-glioma activity [54].
Similarly, L-carnitine modified PLGA nanoparticles (LC-PLGA NPs) were designed to utilise the advantage of Na-coupled carnitine transporter 2 (OCTN2) expressions on brain capillary endothelial cells as glioma cells for BBB infiltration and targeting. Results showed increased accumulation of NPs in the BBB endothelial cell line (hCMEC/D3) and the glioma cell line (T98G). This revealed the Na dependent cellular uptake that involves OCTN2 in the NPs internalisation process. Moreover, a higher accumulation of LC-PLGA NPs was also observed in the in vivo mouse model study. Furthermore, loading of drugs Taxol and paclitaxel in the LC-PLGA NPs improved anti-glioma activity in both 2D-cell and 3D-spheroid models [46].
With the RMT mechanism, polymer NPs are decorated/designed with targeting ligands that bind to specific cell surface receptors highly expressed in BBB transport pathways. For example, the Transferrin receptor (TfR) is one of the primary targets for investigating RMT across the BBB because of its high expression on BBB/BBTB endothelium [55]. To evaluate in vivo BBB penetration and targeting efficacy, transferrin modified doxorubicin (DOX) and paclitaxel (PTX) loaded magnetic silica PLGA nanoparticles (MNP-MSN-PLGA-Tf NPs) were developed. The nanoparticles were effectively accumulated in the tumour bearing mice suggesting that Tf facilitates NPs delivery across BBB [56].
5. Polymer Nanoparticles for Brain Cancer Therapy
Polymer NPs are solid colloidal particles that can be utilised as carriers in which the therapeutic drugs or other active components are dissolved, entrapped, encapsulated, or adsorbed on the surface of the polymer matrix [69]. The structure of the polymer NPs can range from nanospheres to nanocapsules depending on the preparation procedure. Various polymers such as chitosan, gelatin, sodium alginate, albumin and polylactides (PLA), polyglycolides (PGA), poly(lactide co-glycolides) (PLGA), polyanhydrides, polyorthoesters, polycyanoacrylates, poly(ε-caprolactone), poly(glutamic acid), poly(malic acid), poly(N-vinyl pyrrolidone), poly(methyl methacrylate), poly(vinyl alcohol), poly(acrylic acid), poly(acrylamide), poly(ethylene glycol), poly(methacrylic acid) are mostly used in nanoparticle formation for both passive and ligand- functionalized actively targeted therapy [70]. Based on the nature of drugs to be loaded and their route of administration, different synthesis methods were implemented for the production of polymer NPs that include solvent evaporation, solvent diffusion, nanoprecipitation, emulsification, reverse salting out, nano-capsules nano-precipitation, layer-by-layer (LbL) method, etc. [71,72,73]. The molecular weight, crystallinity, and stability of polymers and the drug’s physicochemical properties can be analysed to develop polymeric NPs for drug administration to the brain. Polymeric NPs have a unique ability to reach the tumour site through an active targeting route [74]. Researchers have developed docetaxel (DOC)-loaded PCL and its derivative poly (ethylene glycol)-block-poly(ε−caprolactone) methyl ether (mePEG-PCL) nanoparticles that were dispersed in a bioadhesive film and the formulation exhibited sustained release of drugs. Docetaxel-loaded nanoparticles induced more significant cytotoxicity than free docetaxel for glioma treatment [75]. The study reveals that glycopeptide-engineered poly(d,l-lactide-co-glycolide (PLGA) NPs (g7-NPs) provides in vivo evidence of endocytosis of g7-NPs and transported into the endosomes, which help to cross BBB [76]. Gaudin and co-workers have demonstrated the use of convection-enhanced delivery (CED) of NPs for improved chemotherapeutic drugs to the tumour site. They successfully administered gemicitabine, a nucleoside analogue used for the wide range of solid tumours using squalene-based NPs. The study also revealed that PEGylation of the NPs with PEG dramatically improves the distribution of squalene-gemcitabine NPs in the tumours [77].
Most of the current nanomedicines approved by the FDA for clinical use for solid tumour treatment depend on the EPR effect. The enhanced permeability and retention effect or EPR effect is a feature that allows small sized nanoparticles and other active molecules or drugs to pass due to large pore size through leaky vasculature and accumulate in the tumor location.
The brain endothelial cells and glioblastoma cells generally overexpressed a number of receptors, including the low-density lipoprotein receptor, IL-13 receptor, transferrin receptor (TfR), and nicotine acetylcholine receptor that used as drug delivery targets in the brain [78]. Numerous in vivo studies revealed that polymer NPs could circulate for a longer time and accumulate in the tumour site. It is possible to enhance the retention and accumulation of these useful NPs by decorating NPs with tumour-homing ligands such as peptide, aptamer, polysaccharides, saccharides, antibodies, flic acids, etc. [79]. Recently, pluronic micelles (PEG-PPG-PEG) have evolved as perfect candidates for brain therapy, as they can easily cross the BBB and prove their ability to inhibit drug efflux [17]. For instance, Sun et al. developed TfR-T12 peptide-modified PEG-PLA polymer nanoparticle micelles loaded with paclitaxel (PTX) for glioma therapy. They found that the polymeric micelles (TfR-T12-PMs) could be absorbed by tumour cells, cross across BBB monolayers, and inhibit the proliferation of U87MG cells in vitro. A better antiglioma effect with a prolonged median survival of nude mice-bearing glioma was also observed in comparison with unmodified PMs [80]. This suggests that TfR-T12 peptide-modified micelles can cross the BBB system and target glioma cells. In Table 3 and Table 4 are shown various synthetic and natural polymer-based NPs for GBMs therapy or diagnosis.
Table 3.
Synthetic polymer-based nanoparticles for brain cancer glioma therapy.
| Polymers | Method of Preparation | Therapeutic Drug/Other | Targeting Receptor/Molecule | Diagnostic Component | Cell Line/Animal Model | Remark | References |
|---|---|---|---|---|---|---|---|
| Synthetic protein nanoparticle (SPNP) |
Electrohydrodynamic (EHD) jetting | siRNA | STAT3i | Alexa Fluor 647- labeled albumin |
GL26 syngeneic mouse glioma model | Five-fold increase in iRGD loaded SPNP in glioma cell observed in comparison to NPs without iRGD. A total of 87.5% of mice developed anti-GBM immunological memory. | [83] |
| Porphyrin doped conjugated polymer nanoparticles (CPNs) | Controlled nanoaggregation | - | m-RNA | DCF-DA | U-87 MG, T98G and MO59K | NPs enhance the efficacy of PDT to eliminate tumor via ROS generation. MO59K and U-87 MG cells are died with CPN having IC50 values 8 mg/L and 9 mg/L, however, T98G cells are found resistant to CNP-PDT. |
[84] |
| PLGA | Single-emulsion, solvent evaporation technique | Paclitaxel | - | - | U87MG with rats and pigs’ model |
Enhanced in vivo efficacy | [85] |
| PBAEs | Step-wise synthesis | DNA | Cy3 dye | BTICs from patient | More than 60% transfection efficacy is observed. | [86] | |
| cRGD-conjugated PGNRs | Ligand exchange method | - | αv βv- integrin | - | U87MG | cRGD-PGNRs is proved having excellent tumor targeting ability, no cytotoxicity, and sufficient cellular uptake. | [87] |
| Aptamer/gold nanorod conjugate | Step-wise synthesis | Sgc8 aptamer | Cell protein | Fluorescein | Rat or mouse model | A total of 99.09% binding affinity due to the aptamers. Complete destruction of GMB on exposure to LAER is observed. | [88] |
| Poly(N-isopropylacrylamide)-based nanogels and magnetic NPs composite | Co-polymerisation and co-evaporation | Ferrrofluid | - | Sodium fluorescein | Rat model | The drug dose delivered to tumor site is directly proportional to the duration of the “on” pulse. | [89] |
| PEG−PBAE/ePBAE nanoparticles (NPs) | Step wise synthesis, Michael addition |
Plasmid DNA, pHSV-tk, ganciclovir | - | Hoechst 33342 dye | GBM1A and BTIC375 cells/Mice model | PEG−PBAE/ ePBAE NP shows 54 and 82% transfection efficacies in GBM1A and BTIC375 cells while it is 37 and 66% for optimised PBAE NPs without PEG. Death of cancer cell with enhancement of mice life time was observed. |
[90] |
| TEB | Co-precipitation | - | Transferrin (TfR), lactoferrin (LfR) and lipoprotein (LRP) | - | bEnd.3/Mouse model | Ligand-coated TEB nanoparticles are transported across BBB with high efficacy. | [91] |
| PEG-PLA | Emulsion/ solvent evaporation technique |
Neuropilin (NRP), tLyp-1 peptide | Human umbilical vein endothelial cells and Rat C6 glioma cells | tLyp-1 peptide functionalised NPs show better performance in paclitaxel glioma therapy. Observed inhibition of avascular C6 glioma spheroids. Interestingly tLyp-1-NP-PTX formulations shows higher antiproliferation ability with IC50 0.087 mg/mL in comparison to NP-PTX and Taxol. |
[92] | ||
| Transferrin modified PEG-PLA | Double emulsion and solvent evaporation method. | Resveratrol (RSV) | - | - | C6 and U87 glioma cells | RSV-conjugates decreased brain tumor volume and accumulated well in comparison to free RSV. | [93] |
| Polysorbate-coated NPs | Surfactant mediated ultrasonication | Doxorubicin (DOX) | - | Evans Blue solution | Glioblastoma 101/8-bearing rats | Enhanced permeability and retention effect | [94] |
| PCL | Solvent evaporation technique | Irinotecan hydrochloride trihydrate (IRH) | - | - | HGG cells | IRH-loaded PCL NPs has excellent anti-brain tumor activity. PCL shows better drug encapsulation than PLGA. | [95] |
| cRGD-directed AuNR/PEG–PCL hybrid NPs | Nanoprecipitation | Doxorubicin (DOX) | Cy7 | Human U87MG glioma | Controlled release of doxorubicin into human glioblastoma using mice model is achieved that leads to inhibition of 100 % tumour growth. |
[96] | |
| PCL-Diol-b-PU/gold nanofiber composite | Temozolomide (TMZ) | U-87 MG human glioblastoma cells | Slower release of TMZ showing its high potential as implantable device for drug release. Enhanced activity against the U-87 cell. | [97] | |||
| PEG-PCL NPs conjugated with ALMWP | Emulsion/solvent evaporation method | Paclitaxel (PTX), Taxol |
- | coumarin-6 | C6 cells | Animals treated for C6 gliomas with ALMWP-NP-PTX survive longer than those treated with Taxol-NP-PTX. | [98] |
Abbreviation: PLG: poly(lactide-coglycolide), DCF-DA: 2’,7’-dichlorofluorescin-diacetate PBAEs: poly (β-amino ester) s, cRGD: cyclic RGD peptides, PGNRs: PEGylated gold nanorods, PEG: polyethylene glycol, PSMA: prostate-specific membrane antigen, NR: nanorods, PCL-diol: poly (ε-caprolactone diol), PU: polyurethane, ALMWP: activatable low molecular weight protamine.
Table 4.
Natural polymer-based nanoparticles for brain cancer glioma therapy.
| Natural Polymer-Based Nanoparticles | Method of Preparation | Therapeutic Drug/Other | Targeting Receptor/Molecule | Diagnostic Component | Cell Line/Animal Model | Remark | References |
|---|---|---|---|---|---|---|---|
| Den-angio nanoprobe | Step-wise synthesis | - | LRP receptor-mediated endocytosis | U87MG | Den-Angio shows localisation in the brain tumours and makes image-guided tumour resection possible. | [99] | |
| CDP-NP | Single-step synthesis at room temperature, self-assembly method | - | Proteins | e-GFP, luciferin | BV2, N9 microglia (MG) cells and GL261 glioma cells/mice model | CDP-NPs were efficiently taken up by BV2 and N9 microglia (MG) cells compared to GL261 glioma cells. | [100] |
| Silver NPs impregnated alginate–chitosan-blended nanocarrier | Polyelectrolyte complex formation reaction | DNA | Acridine Orange/Ethidium Bromide dual stain | U87MG | Extensive DNA damage was observed on cell cycle analysis. | [101] | |
| Hyaluronan (HA)-grafted lipid-based NPs (LNPs) | Amine coupling strategy | rRNA interference (RNAi), doxorubicin and BCNU | CD44 receptor | DAPI (blue) | T98G, U87MG, and U251 | Prolonged survival of treated mice in the orthotopic model was observed. | [102] |
| Cardamom extract-loaded gelatine NPs (CE-loaded GNPs) | Two-step de-solvation method | Cardamom extract | - | - | U87MG | Extract to polymer ratio as 1:20 was found to be the best with entrapment. efficiency close to 70% | [103] |
| NK@AIEdots (natural-killer-cell-mimic nanorobots with aggregation-induced emission) |
Step-wise synthesis, assembly process | - | - | - | U-87 MG, bEnd.3 | The tumour growth was also successfully inhibited by NK@AIEdots on exposure to NIR light. | [104] |
| Heparin-based polymer)–SWL–(cRGD) NPs (S = serine, W = tryptophan, L = leucine) |
Coupling reaction | αv βv and EphA2 in glioma | f Oregon-green488 | U87 and U251 | NPs easily pass-through BBB to the tumour site. In addition, inhibition of glioma cell proliferation is noticed. | [105] | |
| poly-L-arginine-chitosan-triphosphate matrix (ACSD) | Green co-precipitation method | Doxorubicin, SPIONs | - | Prussian blue staining and inductively coupled plasma | Rat glioma C6 cells | ACSD NPs are proved as promising theranostic formulation MRI analysis shows uptake of NPs in C6 glioma cells. There observed 38.6% drug release in neutral pH while 58% in acidic pH. A 44-fold increase in IC50 value of doxorubicin was found when the drug was loaded in NPs. |
[106] |
| Albumin nanoparticles (NPs) | Two-step synthesis, grafting | Paclitaxel (PTX) | Substance P (SP) peptide | Cou-6 dye | Glioma U87 cells | Albumin nanoparticles are found satisfactory for drug delivery vehicles for the treatment of GBM. The targeting effect of SP, and efficient cellular uptake of SP-HSA-PTX NPs into brain capillary endothelial cells (BCECs) and U87 cells is improved. | [107] |
| Human serum albumin (HSA) NPs | High-pressure homogeniser technique | Doxorubicin | - | LysoTracker | bEnd.3 cells as well as U87MG | Anti-glioma efficacy is improved due to the dual-enhanced system of dual cationic absorptive transcytosis and glucose-transport by using c- and m-HSA together. | [108] |
| Albumin NPs | Green synthesis | Paclitaxel and fenretinide | - | CY5 dye | Human glioma U87, U251 cells, mouse glioma C6, GL261 cells, |
The albumin-binding proteins are found to be overexpressed in the tumour/glioma cells, where epithelium cells are responsible for delivering NPs to brain tumours. | [109] |
| Menthol-modified casein NPs(M-CA-NP) | Self-assembled micelle formation | 10-Hydroxycamptothecin, methanol |
- | Cou-6 | C6 cells | Resulted in enhanced drug accumulation in the tumour site. | [110] |
| Transferrin-functionalised NPs (Tf-NP) | Functionalisation | Temozolomide and the bromodomain | Cy5.5 | U87MG and GL261 cells | Therapy showed 1.5- to 2-fold decrease in tumor burden and corresponding increase in survival in tumor bearing mice | [111] |
Abbreviation: Den: dendrimer, Angio: angiopep-2, PDT: photodynamic therapy, CDP-NP: cyclodextrin-based nanoparticle, TEB: triphenylamine-4-vinyl- (P-methoxy-benzene), DAPI: 4′,6-diamidino-2-phenylindole, SPIONs: superparamagnetic iron oxide nanoparticles.
Recently, much research has been carried out on combined photo-based therapy along with other conventional therapy or imaging for glioblastoma treatment. For example, a novel photoacoustic and photothermal guided semiconducting polymer nanoparticles (SPNs) using poly (ethylene glycol)-block-poly (propylene glycol)-block-poly (ethylene glycol) (PEG-b-PPG-b-PEG) and SP were reported. The SPNs displayed efficient cellular internalisation for PAI and PTT toward U87 cells and accumulated in subcutaneous as well as brain tumours upon intravenous injection and induced efficient cell death upon NIR-II light irradiation [81].
The recent updates reveal that conjugated polymer nanoparticles (CPNs) are performing well as photosensitiser (PS) in photodynamic therapy (PDT). This efficiency is achieved by CPNs due to their uniform size, biocompatibility, and outstanding ROS production due to extraordinary photo-physical properties as well as fluorescence emission. It is found that porphyrin doped CPNs can eliminate GBMs through ROS-induced apoptotic damage [82].
6. Polymer Nanoparticles in the Diagnosis of Brain Cancer
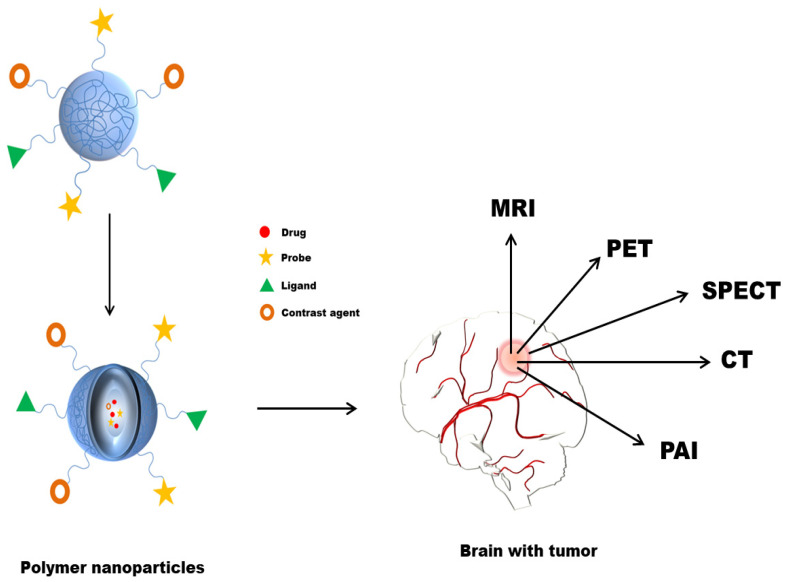
Before surgery, a high-resolution image using imaging modalities is required for glioma detection. Owing to the invasiveness of glioma cells, determining the exact tumour boundary by eye is challenging. Proper imaging of a tumour is essential for assessing the extent of tumour distribution before surgery and the response to a treatment regimen after surgery [5]. Several available techniques for visualisation and diagnosis of brain cancer glioma include optical and ultrasound (US) imaging, photoacoustic (PA) imaging, computed tomography (CT), positron emission tomography (PET), single-photon emission computed tomography (SPECT) and fluorescence (FL) imaging techniques (Figure 3) [112]. Currently, magnetic resonance imaging (MRI), a non-invasive technique that can detect the size, shape, and tumour location, is initially employed diagnostic method for patients with suspected GBM [113]. MRI can determine the boundaries of the tumour tissues and/or intraoperative to elucidate tumour outline during surgical resection by applying gadolinium (Gd). Due to a shorter half-life, Gd must be administered often to maintain blood levels for efficient scanning. The use of intraoperative ultrasonography to obtain integrated brain tissue imaging is another non-optical method. However, this approach does not provide enough information for detecting smaller or superficial brain tumours. Other invasive techniques for analysing brain tumour tissues include Raman spectroscopy, optical coherence tomography, fluorescence spectroscopy, and thermal imaging [114]. Computed tomography (CT) can also be used to determine the presence of the tumour. Still, its use is relatively lesser in clinics for diagnosing GBM due to poor resolution compared to MRI [115]. Likewise, positron emission tomography (PET) imaging with 11C-methionine could be an effective diagnostic tool for GBM patients’ prognosis [116,117]. To understand cancer tumours, precise preoperative imaging and painless sensitive post-imaging techniques to provide real-time data are demanded. Current imaging modalities, however, lack accuracy, sensitivity, and specificity. Nanotechnology has sparked interest in bioimaging and biosensing in recent years.
Figure 3.
Polymer NPs in imaging for improved diagnosis of brain cancer.
‘Nanodiagnostics’ combined with nanotechnology could provide a drug delivery system with traditional diagnostic and imaging procedures [118,119]. Nanotechnology has made it easier to acquire data with great precision and accuracy while avoiding invasive procedures. NPs with tunable optical, magnetic, and electrical properties are able to provide diagnostic tools for detection and imaging brain cancer/tumours [120]. Biocompatible NPs owing ideal physical characteristics, such as surface chemistry, morphology, solubility, stability, etc., facilitate drug delivery and imaging as it acts as image contrast agents [121]. Polymer NPs could be a good reservoir system for drugs and a platform for additional modification for efficient tumour targeting or imaging [122]. Polymer NPs possess various advantages in drug delivery to the brain that can entrap or carry drugs that prevent them from metabolism and excretion. Moreover, NPs can easily transport drugs across the BBB without changing the barrier properties [31,123,124]. In this section, polymer NPs utilised in the diagnosis and detection of brain cancer glioma until now are primarily focused. The imaging and diagnosis techniques currently being investigated with reference to polymer NPs are listed in Table 5.
Table 5.
Polymer nanoparticles in imaging and diagnosis of brain cancer therapy.
| Nanoparticles | Detection Method | Cell Line | Animal Model | Therapy/Drug | References |
|---|---|---|---|---|---|
| SPIONs and DOX loaded poly-l-arginine-chitosan-triphosphate matrix (ACSD) NPs | MRI | C6 glioma cells | - | DOX | [106] |
| P80- TMZ/SPIO-NPs (PLGA coating) | MRI | C6 glioma cells | - | TMZ | [128] |
| Micelles SPION and Au NPs (PEG-PCL coating) | MRI, CT | - | U251 xenograft and orthotopic brain tumour models. | Radiotherapy | [129] |
| Chitosan-dextran superparamagnetic NPs (CS-DX-SPIONs) | MRI | C6 glioma, U87 | orthotopic C6 gliomas in rats | - | [130] |
| DOX-Ps@80-SPIONs | MRI | glioblastoma C6 cells | Glioma-bearing rats | DOX | [131] |
| Paclitaxel (PTX) and superparamagnetic iron oxide (SPIO)-loaded PEGylated poly (lactic-co-glycolic acid) (PLGA)-based NPs(PTX/SPIONPs) | MRI | - | orthotopic U87MG model | PTX | [126] |
| SPIO-loaded brain penetrating PLGA NPs | PET, MRI | - | rat model | - | [127] |
| [18F] NPB4-labeled and C6-loaded PLGA NPs | PET | - | rats bearing BCSC-derived xenografts | - | [85] |
| TMZ and iron oxide-containing polymer NPs(PMNPs) | MRI | U87 glioma cells | rodent model | TMZ | [132] |
Abbreviation: N-(4-[18F] fluorobenzyl) propanamido-PEG4-Biotin, brain cancer stem cells (BCSCs).
Polymer-based superparamagnetic NPs have mainly been employed as drug delivery systems and contrast agents in MRI imaging. These NPs are highly stable and biocompatible, can prolong systemic circulation time, have drug loading ability and control of drug release, and combine with their magnetic performance for MRI [125]. Ganipineni et al. synthesised paclitaxel (PTX) and superparamagnetic iron oxide (SPIO)-loaded PEGylated PLGA-based NPs (PTX/SPIONPs) and analysed for therapeutic efficacy in an orthotopic U87MG model. The cellular internalisation of these NPs was found to be concentration dependent. The MRI scanning displayed the blood–brain barrier disruption in the glioma affected location. Moreover, enhanced accumulation was also observed in ex vivo bio-distribution analysis of GBM-bearing mice with magnetic targeting [126]. Researchers have evaluated SPIO-loaded brain penetrating PLGA NPs by CED administration on rat models and visualised using positron emission tomography (PET) and MRI [127]. SPIO-loaded NPs showed excellent transverse (T2) relaxivity. After CED of NPs, the biodistribution in the brain was analysed using MRI, which revealed a period of one month longer signal attenuation of SPIO-loaded brain-penetrating PLGA NPs. The co-administration of SPIO-loaded PLGA NPs allows intraoperative monitoring of biodistribution in the brain in order to ensure the delivery to tumour location and therapeutic effect over time [127]. Researchers have developed Polysorbate 80 coated temozolomide-loaded PLGA-based superparamagnetic nanoparticles (P80- TMZ/SPIO-NPs), evaluated for anti-glioma activity and analysed as a diagnostic agent for MRI [128]. The superparamagnetic P80-TMZ/SPIO-NPs showed a significant antiproliferative effect and remarkable cellular internalisation on C6 glioma cells. Moreover, the in vitro MRI scanning revealed that P80-TMZ/SPIO-NPs could also serve as a good contrast agent [128].
7. Limitations and Challenges
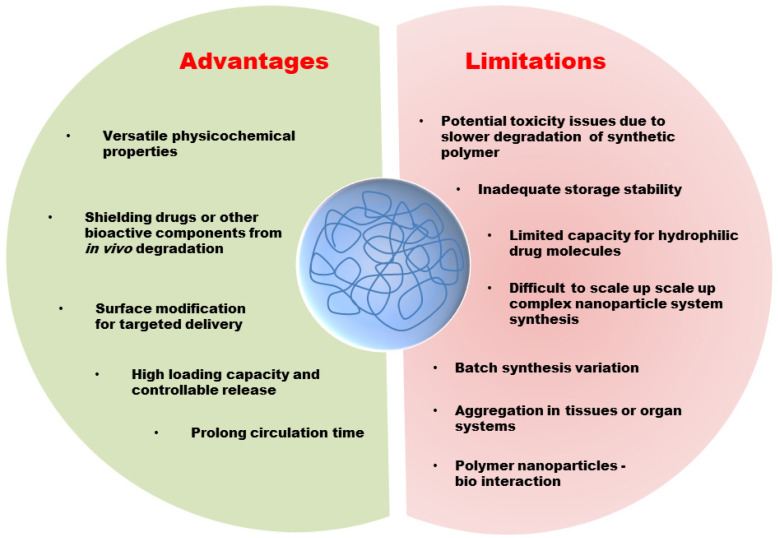
From the past times, tremendous developments have been evidenced in brain cancer therapy. Yet, there have not been emerged significant changes in mortality rate and improving patients’ quality of life. Although nanoparticle-based drug delivery systems have brought a new horizon, many challenges remain and need to be solved in the future. The development of effective polymeric NPs for drug delivery and targeting is a challenging task for clinical translations. The advantage and limitations are summarised in Figure 4. The toxicity of these systems is one of the main challenges. The slow degradation rate of polymer NPs induce a longer circulation time in the body and could cause unknown complications.
Figure 4.
The advantages and limitations of polymer NPs in drug delivery and therapy.
Further, extensive investigations are required for optimization of the NPs. One of the major obstacles in clinical translation is the interaction of NPs and biological systems. Upon entering the complicated biological system, the designed polymeric NPs will instantly interact with neighboring biomolecules, leading to the formation of protein corona that alters their properties. This affects NPs size, stability, surface properties and determines the pharmacokinetics, biodistribution, cellular internalisation, intracellular trafficking, immune system, and toxicity [133,134,135,136]. In addition, more in vitro and in vivo studies are required to better understand the mechanisms in targeted nanoparticle-based therapy. Several essential factors related to the in vivo behaviour of NPs and their effect on other healthy brain cells are hence required to be extensively examined. Currently, there is still insufficient pre-clinical data of polymer-based NPs on brain delivery, data to correlate in vitro-in vivo observation, which makes it difficult to conclude about their therapeutic efficacy.
8. Future Perspective and Conclusions
Glial originated brain cancers are the most aggressive gliomas that depict a threat to humans. The conventional therapies are still inefficient to overcome due to tumor heterogeneity and, specifically, the blood–brain barrier (BBB) of malignant gliomas. The polymeric nanoparticles-based brain cancer therapy approaches are currently gaining interest due to the drug safety, controllable drug release, and efficient targeting in tumors. Most importantly, reports revealed that polymer NPs could even transport across BBB. In this review article, we summarize the newest breakthroughs in the use of polymer nanocarriers for drug delivery, therapy and diagnosis of brain cancer are explored, emphasizing how they are a critical aspect of modern anticancer drug delivery strategies. Various polymer NPs have been generated to reduce anticancer drug losses, premature degradation, enhance drug availability, and reduce drug toxicity by improving drug accumulation in specific organs and tissues. Although the potential impact of polymer NPs in cancer therapy is exceedingly promising, numerous obstacles that currently limit their widespread clinical usage must be solved. For polymer NPs to be used in clinical trials, long-term safety investigations must be conducted in various animal models to eliminate the possibility of non-endogenous components accumulating in the body causing any harm. As a result, huge costs must be provided when conducting in vivo pharmacokinetic studies to evaluate the applicability in the human body. Another factor to consider is the challenges that may arise when transitioning from laboratory to large-scale production. The scaling up of the preparatory process is a major obstacle that must be surmounted. A significant number of polymer NPs are currently in the pre-clinical stage of development, but only one system has entered a clinical study. This is primarily because several challenges impede further development, such as a lack of potency in animal models and toxicity concerns. To overcome the aforementioned concerns, researchers need to focus more on new therapeutic innovations such as revising fabrication processes to modify and improve polymeric NPs in order to accommodate the demand for various anticancer drugs for effective clinical feasibility. New therapeutic innovations also include novel therapeutic strategies for combination therapy and stimuli-activated drug delivery. For example, delivering two or more anticancer drugs simultaneously might enhance the treatment of various cancer developments by targeting different tumour related signalling pathways, resulting in a synergistic therapeutic impact. In addition, the researcher needs to improve the targeting of cancer stem cells (CSCs) for effective cancer therapeutic effect as CSCs is a critical factor for tumour recurrence. In conclusion, pre-clinical experimentation and clinical trials are mandatory for an efficient polymer nanoparticle-based anticancer therapy. Hopefully, all of these developments will lead to more patient-specific and targeted anticancer therapies.
Author Contributions
Conceptualization, D.B., K.K.B. and B.R.; methodology, K.K.B., B.R., T.S., and S.P.; formal analysis, K.K.B., B.R., T.S., B.K.C. and D.B.; investigation S.P., T.S., K.K.B. and L.I.A.; writing—original draft preparation, K.K.B., B.R., T.S., S.P., B.K.C., D.B., Z.A.K., H.A.E. writing—review and editing, K.K.B., B.R., T.S., S.P., B.K.C., D.B. and L.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a research grant from the University Grants Commission (NFST) vide Grant No. F1-17.1/2015-16/NFST-2015-17-ST-ASS-3863.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vijayakumar M.R., Kosuru R., Singh S.K., Prasad C.B., Narayan G., Muthu M.S., Singh S. Resveratrol loaded PLGA:d-α-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv. 2016;6:74254–74268. doi: 10.1039/C6RA15408E. [DOI] [Google Scholar]
- 2.Cadinoiu A.N., Rata D.M., Atanase L.I., Mihai C.T., Bacaita S.E., Popa M. Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests. Pharmaceutics. 2021;13:886. doi: 10.3390/pharmaceutics13060866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saenz del Burgo L., Hernández R.M., Orive G., Pedraz J.L. Nanotherapeutic approaches for brain cancer management. Nanomedicine. 2014;10:905–919. doi: 10.1016/j.nano.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Dolecek T.A., Propp J.M., Stroup N.E., Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-Oncology. 2012;14((Suppl. 5)):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y., Morshed R.A., Auffinger B., Tobias A.L., Lesniak M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrom Q.T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N.E., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncol. 2013;15((Suppl. 2)):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atanase L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers. 2021;13:447. doi: 10.3390/polym13030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sona M.M., Viswanadh M.K., Singh R.P., Agrawal P., Mehata A.K., Pawde D.M., Narendra, Sonkar R., Muthu M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics. 2018;2:70–86. doi: 10.7150/ntno.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapointe S., Perry A., Butowski N.A. Primary brain tumours in adults. Lancet. 2018;392:432–446. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
- 10.Stawicki B., Schacher T., Cho H. Nanogels as a Versatile Drug Delivery System for Brain Cancer. Gels. 2021;7:63. doi: 10.3390/gels7020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoud B.S., AlAmri A.H., McConville C. Polymeric Nanoparticles for the Treatment of Malignant Gliomas. Cancers. 2020;12:175. doi: 10.3390/cancers12010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerna T., Stiborova M., Adam V., Kizek R., Eckschlager T. Nanocarrier drugs in the treatment of brain tumors. J. Cancer Metastasis Treat. 2016;2:407–416. doi: 10.20517/2394-4722.2015.95. [DOI] [Google Scholar]
- 13.Kabitha K., Rajan M.S., Hegde K., Koshy S., Shenoy A. A comprehensive review on brain tumor. Int. J. Pharm. Chem. Biol. Sci. 2013;3:1165–1171. [Google Scholar]
- 14.ELAmrawy F., Othman A.A., Adkins C., Helmy A., Nounou M.I. Tailored nanocarriers and bioconjugates for combating glioblastoma and other brain tumors. J. Cancer Metastasis Treat. 2016;2:112–122. doi: 10.20517/2394-4722.2015.78. [DOI] [Google Scholar]
- 15.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paw I., Carpenter R.C., Watabe K., Debinski W., Lo H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362:1–7. doi: 10.1016/j.canlet.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaser T., Han I., Wu L., Zeng X. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017;8:166. doi: 10.3389/fphar.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taiarol L., Formicola B., Magro R.D., Sesana S., Re F. An update of nanoparticle-based approaches for glioblastoma multiforme immunotherapy. Nanomedicine. 2020;15:1861–1871. doi: 10.2217/nnm-2020-0132. [DOI] [PubMed] [Google Scholar]
- 19.Scheithauer B.W. Development of the WHO classification of tumors of the central nervous system: A historical perspective. Brain Pathol. 2009;19:551–564. doi: 10.1111/j.1750-3639.2008.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozdemir-Kaynak E., Qutub A.A., Yesil-Celiktas O. Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front. Physiol. 2018;9:170. doi: 10.3389/fphys.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alifieris C., Trafalis D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 22.van den Bent M.J., Afra D., de Witte O., Ben Hassel M., Schraub S., Hoang-Xuan K., Malmström P.-O., Collette L., Piérart M., Mirimanoff R., et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 23.Beier D., Schulz J.B., Beier C.P. Chemoresistance of glioblastoma cancer stem cells—Much more complex than expected. Mol. Cancer. 2011;10:128. doi: 10.1186/1476-4598-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook L., Freedman J. Brain Tumors. The Rosen Publishing Group; New York, NY, USA: 2012. [Google Scholar]
- 25.Kim S.-S., Harford J.B., Pirollo K.F., Chang E.H. Effective treatment of glioblastoma requires crossing the blood-brain barrier and targeting tumors including cancer stem cells: The promise of nanomedicine. Biochem. Biophys. Res. Commun. 2015;468:485–489. doi: 10.1016/j.bbrc.2015.06.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binello E., Germano I.M. Targeting glioma stem cells: A novel framework for brain tumors. Cancer Sci. 2011;102:1958–1966. doi: 10.1111/j.1349-7006.2011.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthu M.S., Mei L., Feng S.-S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine. 2014;9:1277–1280. doi: 10.2217/nnm.14.83. [DOI] [PubMed] [Google Scholar]
- 28.Jain K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine. 2012;7:1225–1233. doi: 10.2217/nnm.12.86. [DOI] [PubMed] [Google Scholar]
- 29.Mohanta B.C., Palei N.N., Surendran V., Dinda S.C., Rajangam J., Deb J., Sahoo B.M. Lipid Based Nanoparticles: Current Strategies for Brain Tumor Targeting. Curr. Nanomater. 2019;4:84–100. doi: 10.2174/2405461504666190510121911. [DOI] [Google Scholar]
- 30.Dinda S.C., Pattnaik G. Nanobiotechnology-based drug delivery in brain targeting. Curr. Pharm. Biotechnol. 2013;14:1264–1274. doi: 10.2174/1389201015666140608143719. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W., Mehta A., Tong Z., Esser L., Voelcker N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021;8:2003937. doi: 10.1002/advs.202003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serlin Y., Shelef I., Knyazer B., Friedman A. Anatomy and physiology of the blood-brain barrier. Semin. Cell Dev. Biol. 2015;38:2–6. doi: 10.1016/j.semcdb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardridge W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jena L., McErlean E., McCarthy H. Delivery across the blood-brain barrier: Nanomedicine for glioblastoma multiforme. Drug Deliv. Transl. Res. 2020;10:304–318. doi: 10.1007/s13346-019-00679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ising C., Heneka M.T. Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis. 2018;9:120. doi: 10.1038/s41419-017-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhowmik A., Khan R., Ghosh M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. BioMed Res. Int. 2015;2015:320941. doi: 10.1155/2015/320941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephenson J., Nutma E., van der Valk P., Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belykh E., Shaffer K.V., Lin C., Byvaltsev V.A., Preul M.C., Chen L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020;10:739. doi: 10.3389/fonc.2020.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy S., Tatiparti K., Sau S., Iyer A.K. Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov. Today. 2021;26:1944–1952. doi: 10.1016/j.drudis.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Wei X., Chen X., Ying M., Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm. Sin. B. 2014;4:193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latasha L.P. Nanomedicines and the future of glioma. Neuro. Oncol. 2015;10:16–22. [Google Scholar]
- 42.Pati S., Chatterji A., Dash B.P., Nelson B.R., Sarkar T., Shahimi S., Edinur H.A., Abd Manan T.S.B., Jena P., Mohanta Y.K., et al. Structural characterization and antioxidant potential of chitosan by γ-irradiation from the carapace of horseshoe crab. Polymers. 2020;12:2361. doi: 10.3390/polym12102361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pati S., Sarkar T., Sheikh H.I., Bharadwaj K.K., Mohapatra P.K., Chatterji A., Dash B.P., Edinur H.A., Nelson B.R. γ-Irradiated Chitosan from Carcinoscorpius rotundicauda (Latreille, 1802) Improves the Shelf Life of Refrigerated Aquatic Products. Front. Mar. Sci. 2021;8:498. doi: 10.3389/fmars.2021.664961. [DOI] [Google Scholar]
- 44.Rabha B., Bharadwaj K.K., Baishya D., Sarkar T., Edinur H.A., Pati S. Synthesis and Characterization of Diosgenin Encapsulated Poly-ε-Caprolactone-Pluronic Nanoparticles and Its Effect on Brain Cancer Cells. Polymers. 2021;13:1322. doi: 10.3390/polym13081322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo Y.-L., Lin H.-C., Hong S.-T., Chang C.-H., Wang C.-S., Lin A.M.-Y. Lipid polymeric nanoparticles modified with tight junction-modulating peptides promote afatinib delivery across a blood–brain barrier model. Cancer Nanotechnol. 2021;12:13. doi: 10.1186/s12645-021-00084-w. [DOI] [Google Scholar]
- 46.Kou L., Hou Y., Yao Q., Guo W., Wang G., Wang M., Fu Q., He Z., Ganapathy V., Sun J. L-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif. Cells Nanomed. Biotechnol. 2018;46:1605–1616. doi: 10.1080/21691401.2017.1384385. [DOI] [PubMed] [Google Scholar]
- 47.Li H., Tong Y., Bai L., Ye L., Zhong L., Duan X., Zhu Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018;107:204–211. doi: 10.1016/j.ijbiomac.2017.08.155. [DOI] [PubMed] [Google Scholar]
- 48.Golombek S.K., May J.-N., Theek B., Appold L., Drude N., Kiessling F., Lammers T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018;130:17–38. doi: 10.1016/j.addr.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel T., Zhou J., Piepmeier J.M., Saltzman W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulgar V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2018;12:1019. doi: 10.3389/fnins.2018.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kou L., Bhutia Y.D., Yao Q., He Z., Sun J., Ganapathy V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018;9:27. doi: 10.3389/fphar.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhi K., Raji B., Nookala A.R., Khan M.M., Nguyen X.H., Sakshi S., Pourmotabbed T., Yallapu M.M., Kochat H., Tadrous E., et al. PLGA Nanoparticle-Based Formulations to Cross the Blood-Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics. 2021;13:500. doi: 10.3390/pharmaceutics13040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu W., Zhang Y., Tan Y.-Z., Hu K.-L., Jiang X.-G., Fu S.-K. Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J. Control. Release. 2005;107:428–448. doi: 10.1016/j.jconrel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X., Xin H., Ren Q., Gu J., Zhu L., Du F., Feng C., Xie Y., Sha X., Fang X. Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35:518–529. doi: 10.1016/j.biomaterials.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 55.Bray N. Biologics: Transferrin’ bispecific antibodies across the blood-brain barrier. Nat. Rev. Drug Discov. 2015;14:14–15. doi: 10.1038/nrd4522. [DOI] [PubMed] [Google Scholar]
- 56.Cui Y., Xu Q., Chow P.K.-H., Wang D., Wang C.-H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–8520. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z.H., Wang Z.Y., Sun C.S., Wang C.Y., Jiang T.Y., Wang S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs t the brain. Biomaterials. 2010;31:908–915. doi: 10.1016/j.biomaterials.2009.09.104. [DOI] [PubMed] [Google Scholar]
- 58.Wang L., Hao Y., Li H., Zhao Y., Meng D., Li D., Shi J., Zhang H., Zhang Z., Zhang Y. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: Angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J. Drug Target. 2015;23:832–846. doi: 10.3109/1061186X.2015.1025077. [DOI] [PubMed] [Google Scholar]
- 59.Kuo Y.-C., Chen Y.-C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015;479:138–149. doi: 10.1016/j.ijpharm.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 60.Hua H., Zhang X., Mu H., Meng Q., Jiang Y., Wang Y., Lu X., Wang A., Liu S., Zhang Y., et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018;543:179–189. doi: 10.1016/j.ijpharm.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 61.Ramalho M.J., Sevin E., Gosselet F., Lima J., Coelho M.A.N., Loureiro J.A., Pereira M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018;545:84–92. doi: 10.1016/j.ijpharm.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y., Zhang M., Zeng F., Jin H., Xu Q., Huang Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces. 2016;8:32159–32169. doi: 10.1021/acsami.6b10175. [DOI] [PubMed] [Google Scholar]
- 63.Xin H., Sha X., Jiang X., Zhang W., Chen L., Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33:8167–8176. doi: 10.1016/j.biomaterials.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 64.Muniswamy V.J., Raval N., Gondaliya P., Tambe V., Kalia K., Tekade R.K. ‘Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019;555:77–99. doi: 10.1016/j.ijpharm.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y., Zhang J., Meng F., Deng C., Cheng R., Feijen J., Zhong Z. cRGD-functionalized reduction-sensitive shell-sheddable biodegradable micelles mediate enhanced doxorubicin delivery to human glioma xenografts in vivo. J. Control. Release. 2016;233:29–38. doi: 10.1016/j.jconrel.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Chen W., Zou Y., Zhong Z., Haag R. Cyclo(RGD)-Decorated Reduction-Responsive Nanogels Mediate Targeted Chemotherapy of Integrin Overexpressing Human Glioblastoma In Vivo. Small. 2017;13:1601997. doi: 10.1002/smll.201601997. [DOI] [PubMed] [Google Scholar]
- 67.Bi Y., Liu L., Lu Y., Sun T., Shen C., Chen X., Chen Q., An S., He X., Ruan C., et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces. 2016;8:27465–27473. doi: 10.1021/acsami.6b05572. [DOI] [PubMed] [Google Scholar]
- 68.Bhowmik A., Chakravarti S., Ghosh A., Shaw R., Bhandary S., Bhattacharyya S., Sen P.C., Ghosh M.K. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3′-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget. 2017;8:65339–65358. doi: 10.18632/oncotarget.18689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prabhu R.H., Patravale V.B., Joshi M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015;10:1001–1018. doi: 10.2147/IJN.S56932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Roemeling C., Jiang W., Chan C.K., Weissman I.L., Kim B.Y.S. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017;35:159–171. doi: 10.1016/j.tibtech.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Zielińska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020;25:3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guzmán E., Mateos-Maroto A., Ruano M., Ortega F., Rubio R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017;249:290–307. doi: 10.1016/j.cis.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Mateos-Maroto A., Abelenda-Núñez I., Ortega F., Rubio R.G., Guzmán E. Polyelectrolyte Multilayers on Soft Colloidal Nanosurfaces: A New Life for the Layer-By-Layer Method. Polymer. 2021;13:1221. doi: 10.3390/polym13081221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 75.Varan C., Bilensoy E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017;8:1446–1456. doi: 10.3762/bjnano.8.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinto M.P., Arce M., Yameen B., Vilos C. Targeted brain delivery nanoparticles for malignant gliomas. Nanomedicine. 2017;12:59–72. doi: 10.2217/nnm-2016-0307. [DOI] [PubMed] [Google Scholar]
- 77.Gaudin A., Song E., King A.R., Saucier-Sawyer J.K., Bindra R., Desmaële D., Couvreur P., Saltzman W.M. PEGylated squalenoyl-gemcitabine nanoparticles for the treatment of glioblastoma. Biomaterials. 2016;105:136–144. doi: 10.1016/j.biomaterials.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J., Li Y., Zhang T., Zhang X. Development of bioactive materials for glioblastoma therapy. Bioact. Mater. 2016;1:29–38. doi: 10.1016/j.bioactmat.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong Y., Meng F., Deng C., Zhong Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules. 2014;15:1955–1969. doi: 10.1021/bm5003009. [DOI] [PubMed] [Google Scholar]
- 80.Sun P., Xiao Y., Di Q., Ma W., Ma X., Wang Q., Chen W. Transferrin Receptor-Targeted PEG-PLA Polymeric Micelles for Chemotherapy Against Glioblastoma Multiforme. Int. J. Nanomed. 2020;15:6673–6688. doi: 10.2147/IJN.S257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wen G., Li X., Zhang Y., Han X., Xu X., Liu C., Chan K.W.Y., Lee C.-S., Yin C., Bian L., et al. Effective Phototheranostics of Brain Tumor Assisted by Near-Infrared-II Light-Responsive Semiconducting Polymer Nanoparticles. ACS Appl. Mater. Interfaces. 2020;12:33492–33499. doi: 10.1021/acsami.0c08562. [DOI] [PubMed] [Google Scholar]
- 82.Ibarra L.E., Beaugé L., Arias-Ramos N., Rivarola V.A., Chesta C.A., López-Larrubia P., Palacios R.E. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine. 2020;15:1687–1707. doi: 10.2217/nnm-2020-0106. [DOI] [PubMed] [Google Scholar]
- 83.Gregory J.V., Kadiyala P., Doherty R., Cadena M., Habeel S., Ruoslahti E., Lowenstein P.R., Castro M.G., Lahann J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020;11:5687. doi: 10.1038/s41467-020-19225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caverzán M.D., Beaugé L., Chesta C.A., Palacios R.E., Ibarra L.E. Photodynamic therapy of Glioblastoma cells using doped conjugated polymer nanoparticles: An in vitro comparative study based on redox status. J. Photochem. Photobiol. B Biol. 2020;212:112045. doi: 10.1016/j.jphotobiol.2020.112045. [DOI] [PubMed] [Google Scholar]
- 85.Zhou J., Patel T.R., Sirianni R.W., Strohbehn G., Zheng M.-Q., Duong N., Schafbauer T., Huttner A.J., Huang Y., Carson R.E., et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. USA. 2013;110:11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guerrero-Cázares H., Tzeng S.Y., Young N.P., Abutaleb A.O., Quiñones-Hinojosa A., Green J.J. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. ACS Nano. 2014;8:5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi J., Yang J., Park J., Kim E., Suh J.-S., Huh Y.-M., Haam S. Specific Near-IR Absorption Imaging of Glioblastomas Using Integrin-Targeting Gold Nanorods. Adv. Funct. Mater. 2011;21:1082–1088. doi: 10.1002/adfm.201002253. [DOI] [Google Scholar]
- 88.Oli M. Aptamer conjugated gold nanorods for targeted nanothermal radiation of Glioblastoma cancer cells (A novel selective targeted approach to cancer treatment) Young Sci. J. 2009;2:18. doi: 10.4103/0974-6102.68740. [DOI] [Google Scholar]
- 89.Hoare T., Santamaria J., Goya G.F., Irusta S., Lin D., Lau S., Padera R., Langer R., Kohane D.S. A Magnetically Triggered Composite Membrane for On-Demand Drug Delivery. Nano Lett. 2009;9:3651–3657. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim J., Mondal S.K., Tzeng S.Y., Rui Y., Al-kharboosh R., Kozielski K.K., Bhargav A.G., Garcia C.A., Quiñones-Hinojosa A., Green J.J. Poly(ethylene glycol)–Poly(beta-amino ester)-Based Nanoparticles for Suicide Gene Therapy Enhance Brain Penetration and Extend Survival in a Preclinical Human Glioblastoma Orthotopic Xenograft Model. ACS Biomater. Sci. Eng. 2020;6:2943–2955. doi: 10.1021/acsbiomaterials.0c00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu Q., Cai X., Zhang X., Li S., Song Y., Du D., Dutta P., Lin Y. Synthetic Polymer Nanoparticles Functionalized with Different Ligands for Receptor-mediated Transcytosis across Blood-Brain Barrier. ACS Appl. Bio Mater. 2018;1:1687–1694. doi: 10.1021/acsabm.8b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu Q., Gao X., Gu G., Kang T., Tu Y., Liu Z., Song Q., Yao L., Pang Z., Jiang X., et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG–PLA nanoparticles loaded with paclitaxel. Biomaterials. 2013;34:5640–5650. doi: 10.1016/j.biomaterials.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 93.Guo W., Li A., Jia Z., Yuan Y., Dai H., Li H. Transferrin modified PEG-PLA-resveratrol conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharmacol. 2013;718:41–47. doi: 10.1016/j.ejphar.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 94.Ambruosi A., Khalansky A.S., Yamamoto H., Gelperina S.E., Begley D.J., Kreuter J. Biodistribution of polysorbate 80-coated doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J. Drug Target. 2006;14:97–105. doi: 10.1080/10611860600636135. [DOI] [PubMed] [Google Scholar]
- 95.Mahmoud B.S., McConville C. Development and Optimization of Irinotecan-Loaded PCL Nanoparticles and Their Cytotoxicity against Primary High-Grade Glioma Cells. Pharmaceutics. 2021;13:541. doi: 10.3390/pharmaceutics13040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhong Y., Wang C., Cheng R., Cheng L., Meng F., Liu Z., Zhong Z. cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J. Control. Release. 2014;195:63–71. doi: 10.1016/j.jconrel.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 97.Irani M., Sadeghi G.M.M., Haririan I. The sustained delivery of temozolomide from electrospun PCL-Diol-b-PU/gold nanocompsite nanofibers to treat glioblastoma tumors. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017;75:165–174. doi: 10.1016/j.msec.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 98.Gu G., Xia H., Hu Q., Liu Z., Jiang M., Kang T., Miao D., Tu Y., Pang Z., Song Q., et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34:196–208. doi: 10.1016/j.biomaterials.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 99.Yan H., Wang J., Yi P., Lei H., Zhan C., Xie C., Feng L., Qian J., Zhu J., Lu W., et al. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood-brain barrier. Chem. Commun. 2011;47:8130–8132. doi: 10.1039/c1cc12007g. [DOI] [PubMed] [Google Scholar]
- 100.Alizadeh D., Zhang L., Hwang J., Schluep T., Badie B. Tumor-associated macrophages are predominant carriers of cyclodextrin-based nanoparticles into gliomas. Nanomedicine. 2010;6:382–390. doi: 10.1016/j.nano.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma S., Chockalingam S., Sanpui P., Chattopadhyay A., Ghosh S.S. Silver nanoparticles impregnated alginate-chitosan-blended nanocarrier induces apoptosis in human glioblastoma cells. Adv. Healthc. Mater. 2014;3:106–114. doi: 10.1002/adhm.201300090. [DOI] [PubMed] [Google Scholar]
- 102.Cohen Z.R., Ramishetti S., Peshes-Yaloz N., Goldsmith M., Wohl A., Zibly Z., Peer D. Localized RNAi Therapeutics of Chemoresistant Grade IV Glioma Using Hyaluronan-Grafted Lipid-Based Nanoparticles. ACS Nano. 2015;9:1581–1591. doi: 10.1021/nn506248s. [DOI] [PubMed] [Google Scholar]
- 103.Nejat H., Rabiee M., Varshochian R., Tahriri M., Jazayeri H.E., Rajadas J., Ye H., Cui Z., Tayebi L. Preparation and characterization of cardamom extract-loaded gelatin nanoparticles as effective targeted drug delivery system to treat glioblastoma. React. Funct. Polym. 2017;120:46–56. doi: 10.1016/j.reactfunctpolym.2017.09.008. [DOI] [Google Scholar]
- 104.Deng G., Peng X., Sun Z., Zheng W., Yu J., Du L., Chen H., Gong P., Zhang P., Cai L., et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano. 2020;14:11452–11462. doi: 10.1021/acsnano.0c03824. [DOI] [PubMed] [Google Scholar]
- 105.Wang J., Yang Y., Zhang Y., Huang M., Zhou Z., Luo W., Tang J., Wang J., Xiao Q., Chen H., et al. Dual-Targeting Heparin-Based Nanoparticles that Re-Assemble in Blood for Glioma Therapy through Both Anti-Proliferation and Anti-Angiogenesis. Adv. Funct. Mater. 2016;26:7873–7885. doi: 10.1002/adfm.201602810. [DOI] [Google Scholar]
- 106.Gholami L., Tafaghodi M., Abbasi B., Daroudi M., Kazemi Oskuee R. Preparation of superparamagnetic iron oxide/doxorubicin loaded chitosan nanoparticles as a promising glioblastoma theranostic tool. J. Cell. Physiol. 2019;234:1547–1559. doi: 10.1002/jcp.27019. [DOI] [PubMed] [Google Scholar]
- 107.Ruan C., Liu L., Lu Y., Zhang Y., He X., Chen X., Zhang Y., Chen Q., Guo Q., Sun T., et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B. 2018;8:85–96. doi: 10.1016/j.apsb.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Byeon H.J., Thao L.Q., Lee S., Min S.Y., Lee E.S., Shin B.S., Choi H.-G., Youn Y.S. Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J. Control. Release. 2016;225:301–313. doi: 10.1016/j.jconrel.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 109.Lin T., Zhao P., Jiang Y., Tang Y., Jin H., Pan Z., He H., Yang V.C., Huang Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano. 2016;10:9999–10012. doi: 10.1021/acsnano.6b04268. [DOI] [PubMed] [Google Scholar]
- 110.Gao C., Liang J., Zhu Y., Ling C., Cheng Z., Li R., Qin J., Lu W., Wang J. Menthol-modified casein nanoparticles loading 10-hydroxycamptothecin for glioma targeting therapy. Acta Pharm. Sin. B. 2019;9:843–857. doi: 10.1016/j.apsb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lam F.C., Morton S.W., Wyckoff J., Vu Han T.-L., Hwang M.K., Maffa A., Balkanska-Sinclair E., Yaffe M.B., Floyd S.R., Hammond P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018;9:1991. doi: 10.1038/s41467-018-04315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuhnline Sloan C.D., Nandi P., Linz T.H., Aldrich J.V., Audus K.L., Lunte S.M. Analytical and biological methods for probing the blood-brain barrier. Annu. Rev. Anal. Chem. 2012;5:505–531. doi: 10.1146/annurev-anchem-062011-143002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carlsson S.K., Brothers S.P., Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014;6:1359–1370. doi: 10.15252/emmm.201302627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vasefi F., MacKinnon N., Farkas D.L., Kateb B. Review of the potential of optical technologies for cancer diagnosis in neurosurgery: A step toward intraoperative neurophotonics. Neurophotonics. 2017;4:11010. doi: 10.1117/1.NPh.4.1.011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fakhoury M. Drug delivery approaches for the treatment of glioblastoma multiforme. Artif. Cells Nanomed. Biotechnol. 2016;44:1365–1373. doi: 10.3109/21691401.2015.1052467. [DOI] [PubMed] [Google Scholar]
- 116.Dhermain F.G., Hau P., Lanfermann H., Jacobs A.H., van den Bent M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet. Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 117.Galldiks N., Dunkl V., Kracht L.W., Vollmar S., Jacobs A.H., Fink G.R., Schroeter M. Volumetry of [11C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol. Imaging. 2012;11:516–527. doi: 10.2310/7290.2012.00022. [DOI] [PubMed] [Google Scholar]
- 118.Zottel A., Videtič Paska A., Jovčevska I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials. 2019;12:1588. doi: 10.3390/ma12101588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.d’Angelo M., Castelli V., Benedetti E., Antonosante A., Catanesi M., Dominguez-Benot R., Pitari G., Ippoliti R., Cimini A. Theranostic Nanomedicine for Malignant Gliomas. Front. Bioeng. Biotechnol. 2019;7:325. doi: 10.3389/fbioe.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang W., Fan W., Lau J., Deng L., Shen Z., Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019;48:2967–3014. doi: 10.1039/C8CS00805A. [DOI] [PubMed] [Google Scholar]
- 121.Reddy L.H., Couvreur P. Nanotechnology for therapy and imaging of liver diseases. J. Hepatol. 2011;55:1461–1466. doi: 10.1016/j.jhep.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 122.Gauger A.J., Hershberger K.K., Bronstein L.M. Theranostics Based on Magnetic Nanoparticles and Polymers: Intelligent Design for Efficient Diagnostics and Therapy. Front. Chem. 2020;8:561. doi: 10.3389/fchem.2020.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Vlerken L.E., Amiji M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006;3:205–216. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- 124.Qu J., Zhang L., Chen Z., Mao G., Gao Z., Lai X., Zhu X., Zhu J. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016;23:3408–3416. doi: 10.1080/10717544.2016.1189465. [DOI] [PubMed] [Google Scholar]
- 125.Luk B.T., Zhang L. Current Advances in Polymer-Based Nanotheranostics for Cancer Treatment and Diagnosis. ACS Appl. Mater. Interfaces. 2014;6:21859–21873. doi: 10.1021/am5036225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ganipineni L.P., Ucakar B., Joudiou N., Bianco J., Danhier P., Zhao M., Bastiancich C., Gallez B., Danhier F., Préat V. Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma. Int. J. Nanomed. 2018;13:4509–4521. doi: 10.2147/IJN.S165184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strohbehn G., Coman D., Han L., Ragheb R.R.T., Fahmy T.M., Huttner A.J., Hyder F., Piepmeier J.M., Saltzman W.M., Zhou J. Imaging the delivery of brain-penetrating PLGA nanoparticles in the brain using magnetic resonance. J. Neurooncol. 2015;121:441–449. doi: 10.1007/s11060-014-1658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ling Y., Wei K., Zou F., Zhong S. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Int. J. Pharm. 2012;430:266–275. doi: 10.1016/j.ijpharm.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 129.Sun L., Joh D.Y., Al-Zaki A., Stangl M., Murty S., Davis J.J., Baumann B.C., Alonso-Basanta M., Kaol G.D., Tsourkas A., et al. Theranostic Application of Mixed Gold and Superparamagnetic Iron Oxide Nanoparticle Micelles in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2016;12:347–356. doi: 10.1166/jbn.2016.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shevtsov M., Nikolaev B., Marchenko Y., Yakovleva L., Skvortsov N., Mazur A., Tolstoy P., Ryzhov V., Multhoff G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs) Int. J. Nanomed. 2018;13:1471–1482. doi: 10.2147/IJN.S152461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu H.-L., Mao K.-L., Huang Y.-P., Yang J.-J., Xu J., Chen P.-P., Fan Z.-L., Zou S., Gao Z.-Z., Yin J.-Y., et al. Glioma-targeted superparamagnetic iron oxide nanoparticles as drug-carrying vehicles for theranostic effects. Nanoscale. 2016;8:14222–14236. doi: 10.1039/C6NR02448C. [DOI] [PubMed] [Google Scholar]
- 132.Bernal G.M., LaRiviere M.J., Mansour N., Pytel P., Cahill K.E., Voce D.J., Kang S., Spretz R., Welp U., Noriega S.E., et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine. 2014;10:149–157. doi: 10.1016/j.nano.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahmoudi M., Bertrand N., Zope H., Farokhzad O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today. 2016;11:817–832. doi: 10.1016/j.nantod.2016.10.005. [DOI] [Google Scholar]
- 134.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9:1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neerooa B.N., Ooi L.-T., Shameli K., Dahlan N.A., Islam J.M.M., Pushpamalar J., Teow S.-Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels. 2021;7:60. doi: 10.3390/gels7020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bharadwaj K.K., Rabha B., Pati S., Sarkar T., Choudhury B.K., Barman A., Bhattacharjya D., Srivastava A., Baishya D., Edinur H.A., et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules. 2021;26:6389. doi: 10.3390/molecules26216389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study did not report any data.