Abstract
Peperomia Ruiz and Pav, the second largest genus of the Piperaceae, has over the years shown potential biological activities. In this sense, the present work aimed to carry out a seasonal and circadian study on the chemical composition of Peperomia circinata essential oils and aromas, as well as to evaluate the preliminary toxicity in Artemia salina Leach and carry out an in silico study on the interaction mechanism. The chemical composition was characterized by gas chromatography (GC/MS and GC-FID). In the seasonal study the essential oil yields had a variation of 1.2–7.9%, and in the circadian study the variation was 1.5–5.6%. The major compounds in the seasonal study were β-phellandrene and elemicin, in the circadian they were β-phellandrene and myrcene, and the aroma was characterized by the presence of β-phellandrene. The multivariate analysis showed that the period and time of collection influenced the essential oil and aroma chemical composition. The highest toxicity value was observed for the essential oil obtained from the dry material, collected in July with a value of 14.45 ± 0.25 μg·mL−1, the in silico study showed that the major compounds may be related to potential biological activity demonstrated by the present study.
Keywords: natural products, volatile compounds, bioactive compounds, molecular docking
1. Introduction
The Piperaceae family, characterized as basal angiosperms [1], has approximately 3600 thousand species distributed both in pantropical and neotropical regions [2]. Many species are described as presenting themselves as herbs, sub-shrubs, shrubs, or arbors, or lianas, epiphytes, rupicolous, or terrestrial [3]. Furthermore, this family is divided into five genera: Macropiper, Zippelia, Piper, Peperomia, and Manekia [4].
Peperomia Ruiz and Pav is the second largest genus of the Piperaceae family, containing about 1600 species [5], and is considered one of the 10 main genus rich in floristic plants species [6]. This genus species are endemic to the Amazon and the Andes, with distribution in tropical and subtropical regions around the world, although these species are more concentrated in the Americas, where there is the greatest habitat diversity, from the southern United States to Argentina and Chile [5]. In Brazil, there are around 162 species, mainly in the Atlantic Forest [7], being morphologically described as presenting opposite or verticillate leaves and webbed, pinnate, or sometimes obscured veins [8].
Many Piperaceae species are aromatic, promising in essential oils production [9], with potential biological activities, such as: antiprotozoal [10], larvicidal [11], psycho-neuropharmacological [12], antifungal [13], insecticidal [14], antibacterial [15], and toxicity [16]. Among these biological activities, it is important to mention that studies focused on essential oils’ toxicity have aroused great interest [17,18,19], due to their biological properties that contribute to the improvement and functioning of several products, especially in food [20], and for that, some studies use in the preliminary assessment of essential oils toxicity against Artemia salina Leach, which is a small crustacean, widely used in these tests, due to its practicality, speed, safety, and economy. In addition, the great importance of this test is related to the acetylcholinesterase (AChE) enzyme, which has been the target of A. salina, through which larvae mortality occurs when they come into contact with the essential oil in the presence of light, and these interactions may indicate a possible biological activity [21].
Regarding the essential oils’ chemical composition from P. circinnata Link var. circinnata, there are only two records in the literature [22,23]; however, in the essential oils from Peperomia genus, a variety of compounds classes can be found, such as: hydrocarbon and oxygenated monoterpenes, hydrocarbon and oxygenated sesquiterpenes, benzenoids, and phenylpropanoids [24], and the diversification in the essential oils’ chemical profile of this genus can be observed in the Peperomia serpens essential oil, which is characterized by the major compounds (E)-nerolidol (38.0%), ledol (27.1%), and α-humulene (11.5%) [25]. Conversely, the P. inaequalifolia essential oil has safrole (32.10%), 11-αH-himachal-4-en-1-β-ol (25.29%), and myristicin (13.29%) as its main chemical constituents [26]. P. pellucida essential oil presents as major compounds carotol (26.6–32.0%), dillapiole (25.1–30.2%), and pygmaein (5.5–10.5%) [27]. In another study carried out with the same species, the predominance of β-farnesene (22.2%), β-bisabolene (14.8%), and β-bergamotene (10.7%) in the essential oil was demonstrated [28].
In this context, the present work aims to carry out a seasonal and circadian study analyzing the chemical composition of essential oils and aromas obtained from P. circinnata Link var. circinnata, and, in addition, to carry out a preliminary toxicity study on Artemia Salina and to study in silico the prediction of potential molecular iteration mechanism.
2. Results and Discussions
Essential oils’ yields (mg/100 g) obtained from the whole plant devoid of spikes for the seasonal study (July/2010 to May/2011) of fresh and dry samples ranged between 3.5–7.9% and 1.2–2.4%, respectively. Variations in the essential oils’ yields obtained from the fresh plant may have been influenced by climatic variations at the collection time (Table 1). Thus, in July and September were the highest levels, which then decreased in January and March, reaching minimum values. It is also observed that the fresh botanical material showed a yield greater than 50% compared to the dry material.
Table 1.
P. circinnata var. circinnata essential oil (EOs) yield from seasonal study in (%).
| July | September | November | January | March | May | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | F | D | F | D | F | D | F | D | F | D | F | |
| % EOs | 2.40 | 7.90 | 2.00 | 4.30 | 2.40 | 5.60 | 1.70 | 3.60 | 1.50 | 3.50 | 1.20 | 4.20 |
F = fresh, D = dry.
In a previous study on the essential oil yield from P. circinnata Link var. circinnata [22], the authors obtained values of 1–2.8%, similar to those observed in this study. In addition, studies have related the maximum and minimum essential oils yields to the rainfall index as one of the factors which added to the period of seasonal collection [29,30,31,32].
The essential oil yields obtained in the months of November and March for the circadian study are shown in Table 2. The botanical material collection was carried out in two periods, evening (M) and afternoon (A). The material was dried by two processes: oven and lyophilization. It is observed that the essential oils’ yields obtained from fresh (F), oven dried (D), and lyophilized (L) materials of collections carried out in the evening and afternoon did not show significant variations owing to the function of time (Table 2). P. circinnata Link var. circinnata essential oils obtained in the rainy season of march showed yields of 1.2–3.5%. For fresh samples collected at night, the best yield was 3.5%. In the period of years considered to have been without rain, the month of November (dry period) produced fresh samples which had the highest yields, as seen in Table 2, with the highest value being 5.6%. In this study, lyophilization was the technique that most influenced the lowest essential oil yields, with a variation in the yield of 1.2–1.8% for the month of March and 1.6% for November; according to Chua et al. [33], drying methods can influence mass yields and no method is 100% effective for dehydrating plants rich in essential oils. As observed by other authors [34,35,36,37], oven drying and lyophilization can change the samples’ morphological characteristics in relation to being fresh or dried at room temperature, which can hinder the essential oils’ extraction, affecting their mass yield and chemical composition.
Table 2.
P. circinnata Link var. circinnata essential oil yield from circadian study.
| March | November | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evening | Afternoon | Evening | Afternoon | |||||||||
| F | D | L | F | D | L | F | D | L | F | D | L | |
| % oil | 3.5 | 1.5 | 1.8 | 2.9 | 1.5 | 1.2 | 5.6 | 2.5 | 1.6 | 5.1 | 2.3 | 1.6 |
| Moisture | 92.4 | 22.0 | 43.5 | 90.9 | 30.6 | 49.2 | 91.1 | 37.4 | 45.0 | 88.9 | 12.7 | 33.6 |
Evening, afternoon, fresh (F), oven drying (D), and lyophilization (L).
2.1. Chemical Composition
The relative chemical composition of P. circinnata Link var. circinnata essential oil whole plant devoid of spikes, fresh, and dry material was different during the seasonal study. The highest concentration of hydrocarbon monoterpenes was obtained from the dry plant, and oxygenated monoterpenes were obtained from fresh samples (Table 3). In total, 38 compounds present in essential oils extracted in the seasonal period were identified, with the majority being myrcene (4.7–16.4%), β-phellandrene (4.3–28.1%), β-elemene (4.3–10%), germacrene D (5.3–13%), and elemicin (1.1–22%). Obtained from collections in July and January (dry and fresh), samples were characterized by the presence of the phenylpropanoid elemicin (13.9–22%), followed by the monoterpene hydrocarbon β-phellandrene (11.1–21.5%), and those obtained by essential oils from dry (September, November, March, and May) and fresh (March and May) botanical materials were characterized by the presence of monoterpene hydrocarbons β-phellandrene (16.5–28.1%) and myrcene (4.7–16.4%), followed by the oxygenated sesquiterpene elemol (4.2–11.2%).
Table 3.
Seasonal variation on the chemical composition of P. circinnata Link var. circinnata essential oils in the months of July, September, November, January, March, and May, fresh (F) and oven dried (D). The concentration values of the compounds are (%).
| Constituents | RIL | RIC | Jul-D | Jul-F | Set-D | Set-F | Nov-D | Nov-F | Jan-D | Jan-F | Mar-D | Mar-F | May-D | May-F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-pinene | 932 | 932 | 2.9 | 1.4 | 3 | 0.5 | 2.5 | 0.8 | 1.7 | 1.1 | 2.4 | 3.5 | 0.7 | 0.2 |
| β-pinene | 974 | 978 | 3.2 | 2.2 | 3.3 | 0.8 | 2.9 | 1.2 | 2.3 | 1.6 | 3 | 3.2 | 1.4 | 1 |
| mycrene | 988 | 987 | 10.8 | 7 | 15.3 | 6.2 | 10.9 | 5.5 | 12.1 | 7.9 | 13.9 | 16.4 | 9.2 | 4.7 |
| ρ-mentha-1 (7),8-diene | 1003 | 1006 | 0.3 | 0.2 | 0.6 | 0.8 | 0.3 | 0.6 | 0.5 | 0.7 | 1.1 | 1.2 | 0.9 | |
| β-phellandrene | 1025 | 1029 | 21.5 | 14 | 16.5 | 4.3 | 20.6 | 8 | 16.1 | 11.1 | 23.3 | 28.1 | 24.9 | 16.5 |
| terpinolene | 1086 | 1083 | 1.2 | 1 | 1.4 | 1 | 1.8 | 1 | 1.4 | 1.4 | 1.1 | 2.5 | 2.1 | 1 |
| n-decanal | 1201 | 1205 | 1.2 | 1.8 | 1.7 | 2 | 0.7 | 1.4 | 2.2 | 0.9 | 1.7 | 0.7 | ||
| α-ylangene | 1373 | 1366 | 0.4 | 1.9 | 0.3 | 0.4 | 0.2 | 0.4 | 0.2 | 0.2 | 0.5 | 0.7 | 0.5 | 0.8 |
| α-copaene | 1374 | 1373 | 1.6 | 1.5 | 2.8 | 1.3 | 2.3 | 1.5 | 1.9 | 1.6 | 2 | 1.2 | 2.7 | |
| β-elemene | 1389 | 1387 | 5.9 | 7.3 | 6 | 10 | 4.3 | 8.3 | 5.1 | 6.4 | 5.9 | 2.7 | 5.9 | 7.7 |
| methyl eugenol | 1403 | 1401 | 0.2 | 0.3 | 0.3 | 0.4 | 0.1 | |||||||
| dodecanal | 1408 | 1409 | 0.7 | 1.2 | 0.7 | 1.3 | 0.4 | 0.9 | 0.7 | 1.3 | 0.5 | 0.6 | 0.6 | 0.7 |
| β-ylangene | 1419 | 1417 | 1.5 | 2 | 1.5 | 3 | 1.8 | 2 | ||||||
| β-caryophyllene | 1417 | 1418 | 1 | 0.6 | 1 | 1 | 1 | 1.6 | 2.3 | 3.1 | 2.2 | 2.2 | 2.5 | 4.1 |
| β-cedrene | 1419 | 1420 | 0.6 | 0.4 | 0.3 | 0.2 | 0.4 | 0.5 | 0.3 | 0.5 | 0.8 | 0.5 | 1.1 | |
| β-copaene | 1430 | 1426 | 1.8 | 2.6 | 2.3 | 3.7 | 2.3 | 3.2 | 1.7 | 2.2 | 1.6 | 2.1 | 1.4 | 2.3 |
| α-neo-clovene | 1452 | 1447 | 0.6 | 0.7 | 1.1 | 1 | 1 | 0.5 | 1.3 | 0.6 | 0.6 | 0.5 | 1.1 | |
| α-humulene | 1452 | 1451 | 0.6 | 0.4 | 0.5 | 0.8 | 0.6 | 0.9 | 0.5 | 0.7 | 0.5 | 0.3 | 0.6 | 1 |
| Alloaromadendrene | 1458 | 1455 | 0.4 | 0.8 | 0.5 | 0.8 | 0.5 | 0.7 | 0.5 | 0.5 | 1.1 | 0.5 | 0.4 | 0.8 |
| E-β-farnesene | 1454 | 1464 | 0.6 | 0.3 | 0.2 | 0.1 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 1 | |
| γ-muurolene | 1478 | 1471 | 1 | 1.1 | 1.4 | 1.5 | 0.8 | 1.6 | 1.3 | 0.8 | 1.4 | 1.5 | 1.4 | 2.4 |
| germacrene D | 1484 | 1478 | 5.8 | 7.3 | 6.8 | 11.3 | 6.8 | 8.8 | 5.8 | 7.8 | 6.6 | 6.2 | 5.3 | 13 |
| trans-muurola-4 (14),5-diene | 1493 | 1487 | 0.4 | 0.5 | 0.6 | 0.9 | 0.7 | 1.5 | 0.6 | 0.5 | 0.6 | 0.5 | 0.9 | |
| epi-cubebol | 1493 | 1492 | 1 | 1 | 1.2 | 1.2 | 1.1 | 1.2 | 1.4 | 1.8 | 0.8 | 1.5 | 1.8 | |
| α-muurolene | 1500 | 1495 | 1 | 1.3 | 1.2 | 2.5 | 1.4 | 2.2 | 1.1 | 4.1 | 1.2 | 1.4 | 1.1 | 1.9 |
| cubebol | 1514 | 1512 | 5.1 | 0.4 | 4.6 | 3.7 | 3.7 | 4 | 6.4 | 7.5 | 2.8 | 5.6 | 4.9 | |
| δ-cadinene | 1522 | 1515 | 2.3 | 1.8 | 2.3 | 5.7 | 5.2 | 5.5 | 2.1 | 9.4 | 2.1 | 3.6 | 5 | 4.6 |
| zonarene | 1528 | 1518 | 0.4 | 1.3 | 0.3 | |||||||||
| cis-nerolidol | 1531 | 1521 | 0.6 | 0.3 | 1.7 | 0.8 | ||||||||
| elemol | 1548 | 1542 | 0.1 | 2.9 | 10 | 15.1 | 11.2 | 15 | 0.5 | 6 | 4.6 | 4.4 | 4.2 | |
| elemicin | 1555 | 1547 | 13.9 | 22 | 1.1 | 18.3 | 18.1 | |||||||
| germacrene D-4-ol | 1574 | 1573 | 0.6 | 0.7 | 0.4 | 0.2 | 1.4 | 0.2 | 0.4 | 0.1 | 0.6 | 3.9 | ||
| junenol | 1618 | 1603 | 0.7 | 0.7 | 0.7 | 0.9 | 0.6 | 1.2 | 0.8 | 1 | 0.9 | 0.6 | 0.5 | 1.1 |
| 1.10-di-epi-cubenol | 1618 | 1623 | 0.4 | 0.4 | 0.7 | 1.3 | 0.7 | 1.6 | 0.8 | 1.4 | 0.7 | 0.8 | 0.5 | 1.1 |
| epi-α-cadinol | 1638 | 1633 | 0.1 | 0.6 | 0.9 | 0.9 | 0.4 | 0.6 | ||||||
| epi-α-muurolol | 1640 | 1639 | 0.6 | 0.5 | 0.8 | 1.8 | 0.6 | 1.9 | 0.7 | 0.7 | 1 | 0.8 | 0.7 | |
| α-muurolol | 1644 | 1642 | 0.4 | 0.6 | 0.7 | 1 | 0.7 | 1 | 0.2 | 0.8 | 0.5 | 0.5 | ||
| α-cadinol | 1652 | 1651 | 0.8 | 0.7 | 1.2 | 1.5 | 2 | 1.9 | 1.5 | 2 | 0.8 | 0.4 | 1.9 | 1 |
| Monoterpene hydrocarbons | 39.9 | 25.8 | 40.1 | 12.8 | 39.5 | 16.8 | 34.2 | 23.6 | 44.4 | 54.8 | 39.5 | 24.3 | ||
| Oxygenated monoterpenes | 1.2 | 1.8 | 1.7 | 2 | 0.7 | 1.4 | 2.2 | 0.9 | 1.7 | 0.7 | ||||
| Sesquiterpenes Hydrocarbons | 26.1 | 28.6 | 27.1 | 45.6 | 30.2 | 41.6 | 24.1 | 39.1 | 26.3 | 25.9 | 28.5 | 45.7 | ||
| Oxygenated sesquiterpenes | 9.7 | 7.9 | 20.4 | 26.7 | 22.6 | 28 | 13.6 | 6.9 | 18.3 | 11.5 | 20 | 15.4 | ||
| Phenylpropanoids | 13.9 | 22 | 1.1 | 18.5 | 18.4 | 0.3 | 0.4 | 0.1 | ||||||
| Others | 0.7 | 1.2 | 0.7 | 1.3 | 0.4 | 0.9 | 0.7 | 1.3 | 0.5 | 0.6 | 0.6 | 0.7 | ||
| Totals | 91.5 | 87.3 | 91.1 | 88.4 | 93.4 | 87.3 | 92.5 | 91.5 | 90.7 | 93.2 | 90.4 | 86.8 |
RIC: retention index (on DB-5MS column); RIL: literature retention index (Adams [39]).
Essential oils from fresh botanical material collected in September and November were characterized by the presence of oxygenated sesquiterpene elemol (15.0–15.1%); followed by sesquiterpene hydrocarbons germacrene D (8.8–11.3%), and β-elemene (8.3–10.0%) (Table 3). The phenylpropanoid elemicin showed a concentration in essential oils of fresh and dry samples from July and January, with 13.9–22.0% variation, slightly decreasing in September (tr-1.1%), and absent in November, March, and May (Table 3).
In Table 4, showing seasonal variation, the chemical components and retention rates obtained from the aromas of the whole plant devoid of spikes of fresh and dry material are listed, as well as the spikes and samples from November, January, March, and May collections. A total of 37 compounds were identified within the months studied. The aroma of dry (March and November) and fresh (November) whole plants was characterized by the presence of myrcene (13.8–20%), β-phellandrene (13.2–19.0%), cubebol (8–10.7%), and elemol (6.2–9.3%); fresh spikes’ aroma (November) was characterized by the presence of myrcene (20.3%), β-phellandrene (11.5%), elemol (12.7%), and cis-nerolidol (9.3%); the aroma of the whole plant, both dry (January and May) and fresh (May), was characterized by β-phellandrene (8.6–19.1%), myrcene (8.3–11.8%), δ-cadinene (6.8–15.7%), and elemol (5.0–8.4%); the fresh whole plant aromas (January and March) were characterized by β-phellandrene (16.6–18.7%) and myrcene (13.5–16.9%), in addition to the phenylpropanoid elemicin present in the January collection (12.7%) and the sesquiterpene hydrocarbon dauca-4(11),8-diene (9.2%) present in the March collection; the subgroup was obtained from fresh spikes (January, March, and May) characterized by the presence of methyl eugenol (27.4–31.6%), in addition to the monoterpenes myrcene (12.0–31.7%) and β-phellandrene (7.3–24.4%).
Table 4.
Seasonal variation on the chemical constituents obtained from the samples’ (fresh and dry) aroma of Peperomia circinnata collected in the months of November, January, March, and May (fresh (F), oven dried (OD), and fresh spike (S)). The concentration values of the compounds are (%).
| Whole Dry Plant | Whole Fresh Plant | Fresh Spike | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituents | RIL | RIC | Nov-D | Jan-D | Mar-D | May-D | Nov-F | Jan-F | Mar-F | May-F | Nov-F | S-Jan-F | S-Mar-F | S-May-F |
| α-pinene | 932 | 932 | 5.2 | 3.9 | 5.2 | 1.6 | 4.8 | 3 | 5.2 | 1.3 | 3.3 | 1.4 | 1.6 | 1.3 |
| β-pinene | 974 | 978 | 4.3 | 3 | 4.3 | 1.8 | 2.1 | 4.1 | 1.9 | 0.7 | 0.5 | 1 | 0.4 | |
| mycrene | 988 | 987 | 15.4 | 9.5 | 13.8 | 11.8 | 20 | 13.5 | 16.9 | 8.3 | 20.3 | 22.9 | 31.7 | 12 |
| ρ-mentha-1 (7),8-diene | 1003 | 1006 | 1.3 | 1.2 | 2.1 | 1.6 | 1.3 | 0.7 | 1.4 | 1.5 | 0.4 | 0.6 | 1.1 | |
| β-felandrene | 1025 | 1029 | 17.2 | 8.6 | 13.2 | 19.1 | 19 | 16.6 | 18.7 | 14 | 11.5 | 7.3 | 24.4 | 11.2 |
| terpinolene | 1086 | 1083 | 2.4 | 2 | 2.1 | 3.1 | 1.9 | 1.9 | 2.6 | 2 | 4.2 | 2.1 | 0.8 | 2.6 |
| Octen-3-yl acetate | 1110 | 1106 | 0.6 | 1.1 | 0.8 | 1.4 | 0.3 | 0.3 | 0.8 | 0.8 | 0.5 | 1.1 | 0.6 | |
| n-decanal | 1201 | 1205 | 0.7 | 2 | 1.7 | 2.3 | 0.7 | 2.4 | 1.1 | 1 | 4.4 | 6.5 | 1.6 | 4.5 |
| α-copaene | 1374 | 1373 | 0.8 | 1.5 | 1.5 | 0.7 | 1.4 | 1.1 | 1.4 | 1.8 | 0.4 | 0.6 | 0.1 | 0.3 |
| β-bourbonene | 1387 | 1379 | 0.3 | 1 | 0.4 | 0.3 | 0.4 | 0.4 | 0.3 | 0.6 | 0.3 | 0.5 | 0.1 | 0.2 |
| β-elemene | 1389 | 1387 | 2.9 | 5.6 | 4.9 | 4.1 | 3.6 | 4.5 | 2.5 | 5.9 | 3.6 | 7.6 | 1.3 | 4.4 |
| methyl eugenol | 1403 | 1399 | 0.1 | 0.3 | 0.1 | 0.2 | 5.4 | 28.8 | 27.4 | 31.6 | ||||
| dodecanal | 1408 | 1409 | 0.4 | 0.9 | 0.8 | 0.4 | 0.5 | 1.2 | 0.1 | 0.7 | 1.2 | 1.8 | 0.2 | 0.9 |
| β-caryophyllene | 1417 | 1415 | 2 | 2.5 | 1.6 | 2.1 | 2.3 | 0.2 | 3.6 | 1.1 | 0.2 | 0.7 | ||
| β-cedrene | 1419 | 1419 | 0.2 | 5.2 | 0.5 | 0.3 | 0.4 | 0.4 | 1.9 | 0.8 | ||||
| β-copaene | 1430 | 1426 | 2.2 | 3.6 | 1.3 | 0.8 | 1.8 | 1.2 | 0.7 | 2.4 | 1.4 | 0.6 | 0.1 | 0.6 |
| α-neo-clovene | 1452 | 1447 | 0.8 | 0.6 | 0.8 | 1 | 0.3 | 1.5 | 0.2 | 0.1 | ||||
| α-humulene | 1452 | 1450 | 0.5 | 1.4 | 1 | 0.4 | 0.4 | 0.5 | 0.8 | 0.2 | 0.1 | 0.1 | ||
| γ-muurolene | 1478 | 1474 | 5 | 1 | 1.3 | 0.6 | 0.4 | 1.9 | 0.3 | 0.1 | 0.2 | |||
| trans-4,10-epoxy-amorphane | 1478 | 1473 | 0.5 | 1.8 | 0.1 | 0.1 | 0.1 | |||||||
| germacrene D | 1484 | 1481 | 0.4 | 4.9 | 3.5 | 5.7 | 5 | 1.7 | 8.6 | 2 | 1.5 | 0.2 | 1.2 | |
| trans-muurola-4 (14),5-diene | 1493 | 1489 | 6.5 | 0.4 | 0.4 | 0.6 | 0.6 | 5.4 | 0.9 | 0.2 | 0.1 | 0.3 | ||
| epi-cubebol | 1493 | 1494 | 1 | 0.6 | 2.5 | 1 | 1.6 | 0.4 | 1.8 | 0.5 | 0.7 | 0.3 | ||
| α-muurolene | 1500 | 1496 | 0.1 | 0.8 | 0.7 | 1 | 1.5 | 1.5 | 0.5 | 0.4 | 0.1 | 0.4 | ||
| trans-β-guaiene | 1502 | 1501 | 0.2 | 1.6 | 0.3 | |||||||||
| β-himachalene | 1500 | 1500 | 3.4 | 0.1 | 1 | 0.1 | 0.1 | |||||||
| cubebol | 1514 | 1518 | 8 | 0.9 | 10.6 | 10.7 | 2.9 | 2.6 | 1.4 | 1.3 | ||||
| δ-cadinene | 1522 | 1515 | 8 | 15.7 | 0.3 | 6.8 | 2.8 | 0.8 | 0.6 | 3.4 | ||||
| zonarene | 1528 | 1518 | 0.2 | 0.4 | 0.1 | 1.5 | ||||||||
| cis-nerolidol | 1531 | 1521 | 3 | 5.4 | 9.3 | 0.1 | ||||||||
| dauca-4 (11),8-diene | 1530 | 1524 | 0.2 | 0.2 | 9.2 | 0.7 | 0.1 | 0.2 | ||||||
| elemol | 1548 | 1547 | 9.3 | 8.4 | 6.2 | 5.4 | 7.6 | 3.2 | 0.2 | 5 | 12.7 | 3.7 | 0.3 | 4.5 |
| elemicin | 1555 | 1555 | 12.7 | 2.4 | 7 | |||||||||
| cis-muurol-5-en-4 α-ol | 1559 | 1572 | 0.4 | 7.7 | 0.7 | 0.2 | 0.1 | 0.2 | ||||||
| germacrene D-4-ol | 1574 | 1575 | 3 | 2.5 | 2.2 | 7.8 | 0.7 | 0.9 | 0.6 | 1.4 | 0.1 | 0.5 | ||
| junenol | 1618 | 1603 | 0.4 | 1.4 | 1.4 | 0.6 | 0.9 | 0.7 | 0.2 | 1.1 | 0.1 | 0.2 | 0.2 | |
| α-cadinol | 1652 | 1651 | 1.2 | 1 | 0.5 | 1 | 1 | 1.5 | 0.6 | 1.8 | 2.9 | 1.3 | 1.6 | |
| Monoterpene hydrocarbons | 45.8 | 28.2 | 40.7 | 39 | 47 | 37.8 | 48.9 | 29 | 40.4 | 34.8 | 60.6 | 27.5 | ||
| Oxygenated monoterpenes | 1.3 | 3.1 | 2.5 | 3.7 | 1 | 2.7 | 1.1 | 1.8 | 5.2 | 7 | 2.7 | 5.1 | ||
| Sesquiterpenes Hydrocarbons | 15.2 | 36.2 | 18.6 | 30.1 | 19.5 | 19.4 | 27.1 | 38.5 | 11.4 | 14 | 3 | 13.6 | ||
| Oxygenated sesquiterpenes | 26.4 | 15.2 | 25.2 | 15.8 | 22.5 | 11.7 | 9.1 | 13.9 | 26.8 | 8.9 | 2.4 | 8.4 | ||
| Phenylpropanoids | 0.1 | 0.3 | 12.8 | 0 | 2.6 | 5.4 | 28.8 | 27.4 | 38.6 | |||||
| Others | 0.4 | 0.9 | 0.8 | 0.4 | 0.5 | 1.2 | 0.1 | 0.7 | 1.2 | 1.8 | 0.2 | 0.9 | ||
| Totals | 89.1 | 83.6 | 87.8 | 89.1 | 90.8 | 85.6 | 86.3 | 86.5 | 90.4 | 95.3 | 96.3 | 94.1 | ||
RIC: retention index (on DB-5MS column); RIL: literature retention index (Adams [39]).
Seasonal variation was observed among the constituents identified in the aromas of the whole plant devoid of spikes in both fresh and dried samples (Table 4), as well as in the whole plant and spikes’ fresh samples obtained from the November, January, March, and May collections. The mono and sesquiterpenes present in the whole plant samples from November showed similar levels in relation to the dry and fresh samples; in January the sesquiterpenes were 50% higher than the monoterpenes in the dry sample, while in the fresh sample the contents were similar, also presenting a higher concentration of phenylpropanoid elemicin (12.7%); in March the sesquiterpenes contents were approximately 15% higher in the dry sample, while in the fresh one the monoterpenes were superior (≈9.0%) to the sesquiterpenes. In May, sesquiterpenes were higher in the fresh samples’ aromas (20%) and predominated in the dry (50%) compared to monoterpenes.
The relationship between the constituents identified in the aromas of the whole plant and the fresh materials’ spikes can be seen in Table 4. The monoterpenes and sesquiterpenes showed similar levels in the months of November, January, and March for the whole plant, the same occurring in the spikes in the months of November and May; the greatest variation in the terpenes class occurred in spikes of March, 7% of sesquiterpenes and 62% of monoterpenes, in the whole plant occurred in May, but there was an inversion, that is, the sesquiterpenes (60%) were superior to the monoterpenes (3%). The presence of the phenylpropanoid class was represented by methyl-eugenol in spikes during the four months of collection (5.4–31.6%) and elemicin in the whole plant in January (12.7%) and in spikes in May (7.0%).
The analysis of essential oil in the circadian study can be seen in (Table 5); in general, 38 compounds were identified in all analyzed fractions, with the predominance of mycrene (4.7–12.1%), β-phellandrene (4.3–28.1%), β-elemene (2.7–10%), germacrene D (5.8–13%), cubebol (0–7.5%) elemol (0–15%), and elemicin with a range of (0–18.3%). P. circinnata whole plant devoid of spikes also revealed circadian variation on the chemical composition of fresh and dry samples, mainly in November. In March, there was no significant variation between monoterpenes and sesquiterpenes. The variation was clearly observed in the November collection, in which sesquiterpenes predominated over mono, mainly in the fresh evening sample (82%), ranging from 59–71% in the others. Phenylpropanoid elemicin only appeared in the month of March, in the afternoon collection (2.5 and 4.0%).
Table 5.
Chemical constituents obtained from the circadian study of P. circinnata Link var. circinnata (fresh (F), oven dried (D), lyophilized (L), evening period (EP), afternoon period (AP), rainy season (rs), and dry season (ds)). The concentration values of the compounds are (%).
| Constituents | RIL | RIC | FEP-rs | FAP-rs | D-EP-rs | D-AP-rs | L-EP-rs | L-AP-rs | FEP-ds | FAP-ds | D-EP-ds | D-AP-ds | L-EP-ds | L-AP-ds |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-pinene | 932 | 932 | 3.5 | 1.8 | 2.4 | 2.4 | 1.1 | 1.8 | 0.8 | 1.1 | 2.5 | 2.1 | 1.7 | 2.7 |
| β-pinene | 974 | 978 | 3.2 | 2.1 | 3 | 2.7 | 1.3 | 2.6 | 1.2 | 1.9 | 2.9 | 2.5 | 2.2 | 3.7 |
| myrcene | 988 | 987 | 16.4 | 13.8 | 13.9 | 11.4 | 6.7 | 9.4 | 5.5 | 6.3 | 10.9 | 9.8 | 9 | 11.3 |
| ρ-mentha-1 (7),8-diene | 1003 | 1006 | 1.1 | 1 | 0.7 | 1 | 0.5 | 0.8 | 0.3 | 0.61 | 0.8 | 0.8 | 0.6 | 1.2 |
| β-phellandrene | 1025 | 1029 | 28.1 | 24.6 | 23.3 | 23.8 | 15.6 | 18.3 | 8 | 15.6 | 20.6 | 18 | 12.6 | 24 |
| terpinolene | 1086 | 1083 | 2.5 | 1.1 | 1.1 | 1.6 | 1.2 | 1.5 | 1 | 1.4 | 1.8 | 1.5 | 1.2 | 1.7 |
| n-decanal | 1201 | 1205 | 1.3 | 1.3 | 0.9 | 0.9 | 1.1 | 1.7 | 0.6 | 0.7 | 0.6 | 0.8 | 0.6 | |
| α-copaene | 1374 | 1373 | 2 | 2 | 1.6 | 1.7 | 2.3 | 2 | 2.3 | 1.7 | 1.3 | 1.6 | 2 | 1.2 |
| β-elemene | 1389 | 1387 | 2.7 | 6.3 | 5.9 | 6 | 7.1 | 8.3 | 5.2 | 4.3 | 5 | 6.3 | 3.2 | |
| methyl eugenol | 1403 | 1399 | 0.4 | 0.1 | 0.3 | 0.4 | 0.2 | |||||||
| dodecanal | 1408 | 1409 | 0.6 | 0.7 | 0.5 | 0.5 | 0.7 | 1 | 0.9 | 0.4 | 0.4 | 0.3 | 0.5 | 0.4 |
| β-caryophyllene | 1417 | 1415 | 2.2 | 2.9 | 2.2 | 2.5 | 3.2 | 2.9 | 1 | 1.2 | 0.8 | 0.8 | 1 | 0.8 |
| β-ylangene | 1419 | 1419 | 2.6 | 2 | 2 | 2 | 2.3 | 1.8 | ||||||
| β-copaene | 1430 | 1426 | 2.1 | 1.2 | 1.6 | 1.5 | 2.3 | 1.4 | 3.2 | 2.8 | 2.3 | 2.4 | 2.7 | 3.2 |
| α-neo-clovene | 1452 | 1447 | 0.6 | 1.1 | 0.6 | 0.6 | 0.9 | 0.8 | 1 | 0.9 | 1 | 1.1 | 0.9 | 1 |
| alloaromadendrene | 1458 | 1456 | 0.5 | 0.8 | 1.1 | 0.6 | 0.7 | 1.2 | 0.7 | 0.6 | 0.5 | 0.5 | 0.6 | 1 |
| γ-muurolene | 1478 | 1474 | 1.5 | 2 | 1.4 | 1.4 | 2 | 1.5 | 1.6 | 1.1 | 0.8 | 0.9 | 1.2 | 0.8 |
| germacrene D | 1484 | 1481 | 6.2 | 8.4 | 6.6 | 7.3 | 9.1 | 8.4 | 8.8 | 8 | 6.8 | 7 | 7.9 | 5.9 |
| (E)-muurola-4 (14),5-diene | 1493 | 1489 | 0.6 | 0.6 | 0.5 | 0.7 | 0.7 | 0.7 | 1.5 | 0.9 | 0.7 | 0.7 | 0.9 | 0.4 |
| epi-Cubebol | 1493 | 1494 | 0.8 | 1.5 | 1.8 | 1.8 | 2.2 | 2 | 1.2 | 1.1 | 1.1 | 1.4 | 1.4 | 1.1 |
| α-muurolene | 1500 | 1496 | 1.4 | 1.6 | 1.2 | 1.5 | 2 | 1.8 | 2.3 | 1.7 | 1.4 | 1.5 | 1.9 | 1.2 |
| δ-cadinene | 1522 | 1515 | 3.6 | 3.5 | 2.1 | 3.3 | 4.6 | 3.6 | 5.5 | 6.2 | 5.2 | 4.3 | 4.7 | 5.4 |
| cubebol | 1514 | 1518 | 2.8 | 4.5 | 7.5 | 6.5 | 6.5 | 6.7 | 4 | 3.5 | 3.7 | 5.4 | 3.9 | 1.8 |
| cis-nerolidol | 1531 | 1521 | 0.4 | 0.8 | 1.5 | 1.7 | 1 | 0.4 | 1.2 | |||||
| elemol | 1548 | 1548 | 4.6 | 1 | 6 | 2.4 | 8.8 | 0.5 | 15 | 13.2 | 11.2 | 13.3 | 12.7 | 8.8 |
| elemicin | 1555 | 1555 | 2.5 | 4 | 8 | |||||||||
| germacrene D-4-ol | 1574 | 1575 | 0.6 | 0.4 | 0.4 | 0.4 | 0.3 | 1 | 1.4 | 0.9 | 0.3 | 0.9 | ||
| junenol | 1618 | 1603 | 0.6 | 1 | 0.9 | 1.1 | 1.5 | 1.4 | 1.2 | 0.6 | 0.7 | 1 | 0.6 | |
| 1.10-di-epi-Cubenol | 1618 | 1623 | 0.8 | 0.9 | 0.7 | 0.9 | 1.4 | 1 | 1.6 | 1.2 | 0.7 | 0.9 | 1.5 | 0.9 |
| epi-α-Cadinol | 1638 | 1633 | 0.4 | 0.7 | 1 | 0.7 | 1.9 | 0.6 | 0.8 | |||||
| epi-α-muurolol | 1640 | 1639 | 1 | 0.6 | 0.4 | 0.3 | 0.7 | 0.6 | 1.9 | 0.9 | 0.6 | 1.3 | 1.9 | 1 |
| α-Muurolol | 1644 | 1642 | 0.5 | 0.5 | 0.6 | 0.5 | 1 | 0.6 | 1 | 0.7 | 0.9 | 1.2 | 0.9 | |
| α-Cadinol | 1652 | 1651 | 0.4 | 1 | 0.8 | 0.5 | 2 | 1 | 1.9 | 2.9 | 2 | 1.7 | 2 | 3.4 |
| Monoterpene hydrocarbons | 54.8 | 44.4 | 44.4 | 42.9 | 26.4 | 34.4 | 16.8 | 26.91 | 39.5 | 34.7 | 27.3 | 44.6 | ||
| Oxygenated monoterpenes | 1.3 | 1.3 | 0.9 | 0.9 | 1.1 | 1.7 | 0.6 | 0.7 | 0.6 | 0.8 | 0.6 | |||
| Sesquiterpenes Hydrocarbons | 23.4 | 30.4 | 24.8 | 27.1 | 27.8 | 31.4 | 38.8 | 32.3 | 27.1 | 27.8 | 32.4 | 25.9 | ||
| Oxygenated sesquiterpenes | 11.5 | 11 | 19.7 | 15.1 | 25.9 | 14.9 | 28.9 | 27.2 | 24.3 | 27.5 | 26.3 | 21.4 | ||
| Phenylpropanoids | 0.4 | 2.6 | 0.3 | 4.4 | 0.2 | 8 | ||||||||
| Others | 0.6 | 0.7 | 0.5 | 0.5 | 0.7 | 1 | 0.9 | 0.4 | 0.4 | 0.3 | 0.5 | 0.4 | ||
| Totals | 92 | 90.4 | 90.6 | 90.9 | 82.1 | 91.4 | 85.4 | 87.41 | 92 | 90.9 | 87.3 | 92.9 |
RIC: retention index (on DB-5MS column); RIL: literature retention index (Adams [36]).
In Zoghbi et al.’s [22] study on P. circinnata Link var. circinnata essential oil, the highest concentrations were myrcene (12.2–31.2%) and β-phellandrene (17.5–25.4%); in Silva et al.’s [23] study, the highest concentrations were myrcene 8.3%, limonene 13.5%, cubebol 9.7%, and elemicin 11.5%. Other Peperomia species such as P. rotundifolia, P. pelucida, and P. macrostachya from the Amazon were found to contain major compounds, such as epi-α-bisabolol 15.9%, caryophyllene oxide 12.9%, myristicin 7.6%, aromatic compound 6.6%, and andlimonene 5.4% in P. macrostachya, dillapiole 55.3%, (E)-caryophyllene 14.3%, and carotol (8.1%) in P. pellucida, and decanal (43.3%) in P. rotundifolia [38].
2.1.1. Multivariate Analysis
Chemical Composition of Seasonal Study
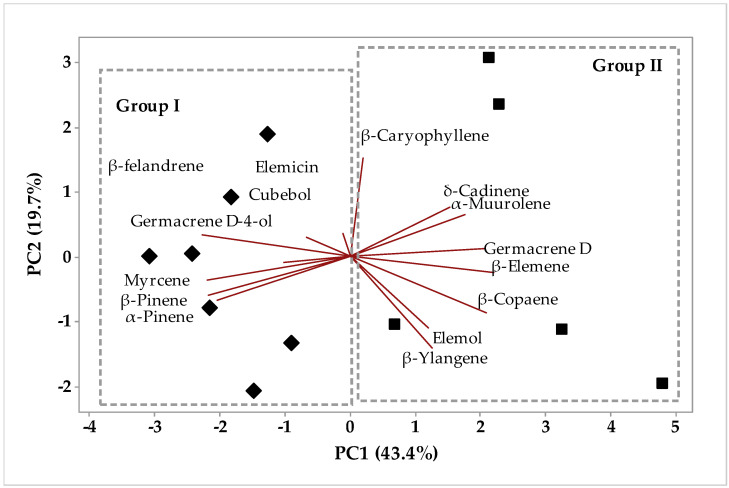
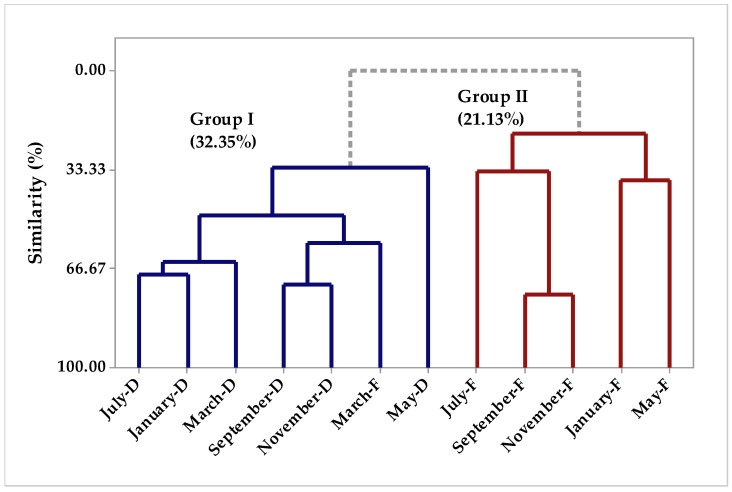
The multivariate analysis PCA (Principal Component Analysis) (Figure 1) and HCA (Hierarchical Cluster Analysis) of the chemical compounds identified in the essential oils different fractions from samples collected between March and November. The first PC1 component explains 43.4% whereas PC2 explains 19.7% of the variations, and the two components add up to 63.1% of variance (Figure 1). The HCA analysis, considering the Euclidean distances and complete bonds (Figure 2), confirmed the formation of two distinct groups. Group 1, with 32.35% similarity, is formed by samples collected from March to November and dried in an oven, plus a sample of fresh P. circinnata Link var. circinnata collected in March.
Figure 1.
Biplot (PCA) resulting from the analysis of compounds identified in P. circinnata Link var. circinnata essential oil in the seasonal study.
Figure 2.
Dendrogram representing the similarity relationship of the compounds identified in P. circinnata Link var. circinnata essential oil in the seasonal study.
Group 2 with 21.13% similarity, shown in Figure 2, was formed by samples collected from May to November without drying treatment (fresh samples). The compounds that characterized group 1 were elemecin, β-phellandrene, germacrene D-4-ol, cubebol, myrcene, β-pineneand, and α-pinene, whereas group 2 was formed by the compounds β-caryophyllene, δ-cadinene, α-muurolene, germacrene D, β-elemene, β-copaene, elemol, and β-ylangene. We also observed that in the present study, seasonality was not the main variable for the groups’ separation, but instead it was the treatment of samples before extraction, which may mean that pre-treatment can be a variable for maintaining the chemical composition without generating losses of compounds by volatilization or degradation.
Multivariate Analysis of the Aroma Chemical Composition
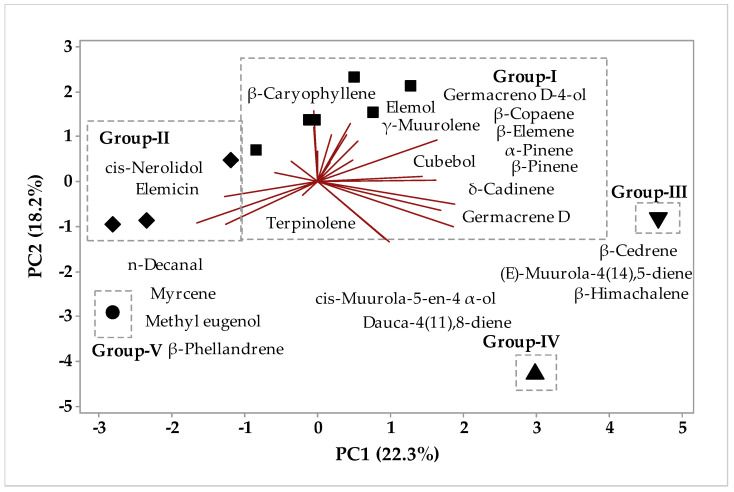
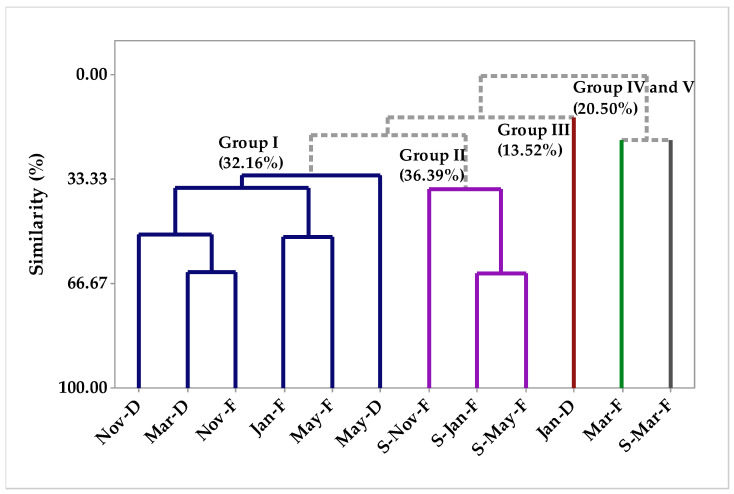
Principal component analysis was applied to the aroma of P. circinnata Link var. circinnata’s whole part and its spikes, and in Figure 3 we observe the principal components analysis, in which the first component explains 23.3% of the variations, whereas the second component explains 18.2%, and the sum of the variances explains 41.1%. Considering the Euclidean distances and complete bonds (Figure 4), in the HCA analysis, the formation of five groups is observed, with group 1 being formed by the samples Nov-D, Mar-D, Nov-F, Jan-F, May-F, and May-D, showing a 32.35% similarity degree, and being characterized by β-caryophyllene, germacrene D, β-elemene, cubebol, γ-muurolene, terpinolene, germacrene D-4ol, β-copaene, δ-cadinene, α-pinene, elemol, and β-pinene compounds. Group 2 (Figure 3), was characterized by the grouping of S-Nov-F, S-Jan-F, and S-May-D samples, showing a similarity of 36.39%, and was characterized by cis-nerolidol and elemecin compounds. Group 3 (Figure 3) was formed only by the Jan-D sample, which in the principal component analysis was characterized by β-cedrene, (E)-muurola-4(14),5-diene, and β-himachalene. Groups 4 and 5 (Figure 3) were formed by Mar-F and S-Mar-F samples, showing a 20.50% similarity degree for the two samples, being characterized by, cis-muurola-5-en-4-α-ol and douca-4(11),8-diene, and β-phellandrene, n-decanal, myrcene and methyl eugenol compounds, respectively.
Figure 3.
Biplot (PCA) resulting from the analysis of compounds identified in P. circinnata Link var. circinnata aroma in the seasonal study.
Figure 4.
Dendrogram representing the similarity relationship of the compounds identified in P. circinnata Link var. circinnata aroma in the seasonal study.
Multivariate Analysis of the Circadian Study Chemical Composition
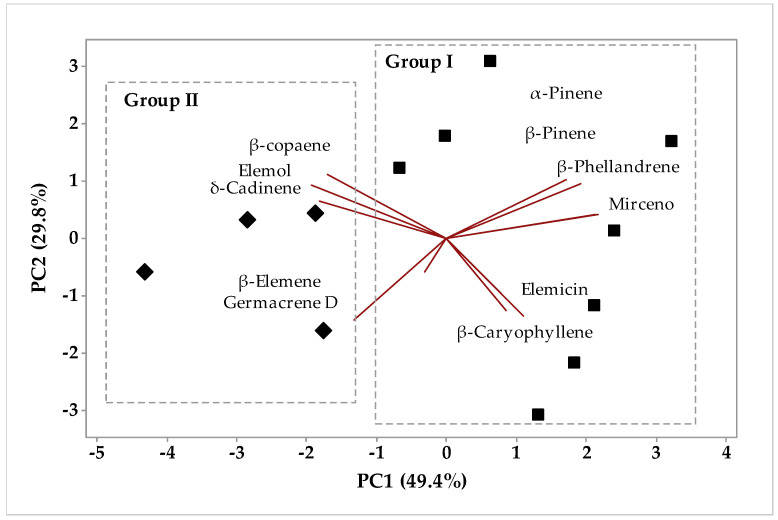
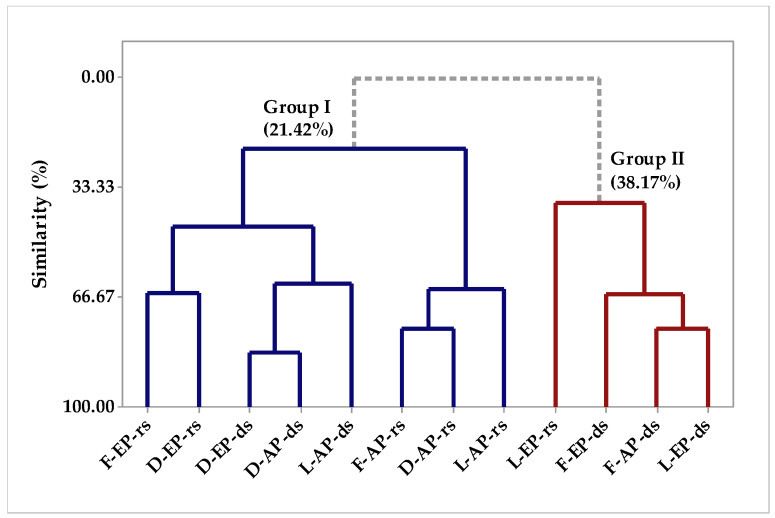
In the circadian study, the essential oils of P. circinnata Link var. circinnata collected in the evening and afternoon, in the winter and summer, when applying principal component analysis (PCA), it is observed that PC1 explained 49.4% and PC2 explained 29.8% of the analyzed variables, while the sum of the variances PC1 and PC2 added up to 79.2% (Figure 5). Figure 6 brings the Hierarchical Cluster Analysis (HCA); the formation of two groups can be observed, group 1 being formed by the grouping of samples collected in the evening and afternoon, fresh and dried in greenhouses as F-EP-rs, D-EP-rs, D-EP-ds, D-AP-ds, L-AP-ds, F-AP-rs, D-AP-rs, and L-AP-rs. In Figure 5, we can see that group 1 was characterized by the compounds that had the highest weights for analysis, such as β-caryophyllene, elemecin, mircene, β-phellandrene, β-pineneand, and α-pinene. Group 2 (Figure 6) was formed by agglutination of L-EP-rs, F-EP-ds, F-AP-ds, and L-EP-ds samples. In Figure 5, using PCA, group 1 was characterized by the presence of the components β-copaene, elemol, δ-cadinene, β-elemene, and germacrene D, and group 2 was characterized by α-pinene, β-pinene, β-phellandrene, mircene, elemecin, and β-caryophyllene. In Pirbalouti’s [40] work, the increase in drying temperature decreased the concentrations of α-pinene, sabinene, β-myrcene, and β-phellandrene in the basil essential oil.
Figure 5.
Biplot (PCA) resulting from the analysis of compounds identified in P. circinnata Link var. circinnata essential oil in the circadian study.
Figure 6.
Dendrogram representing the similarity relationship of the compounds identified in P. circinnata Link var. circinnata oil in the circadian study (fresh (F), dried (D), lyophilized (L), evening period (EP), afternoon period (AP), rainy season (rs), and dry season (ds)).
2.2. Cytotoxicity Bioassay in Artemia Salina
In the control group tests there was no mortality; therefore, the use of DMSO is viable as a solvent for the assay. LC50 values were calculated by converting the percentage of larvae mortality into probits, thus making it possible to trace the equation as a function of concentration values on a logarithmic scale (Table 6). Furthermore, studies have shown that there is a strong correlation between in vitro toxicity results using A. salina and in vivo study using natural products [41,42].
Table 6.
Preliminary toxicity of P. circinnata Link var. circinnata essential oils with A. salina.
| Essential Oil | Concentration (μg·mL−1) | Mortality (%) | LC50 (μg·mL−1) |
|---|---|---|---|
| 50 | 100 | ||
| JulF | 25 | 80 | 17.66 ± 0.33 |
| 10 | 10 | ||
| 5 | 0 | ||
| 50 | 100 | ||
| JulD | 25 | 100 | 14.45 ± 0.25 |
| 10 | 20 | ||
| 5 | 0 | ||
| 100 | 100 | ||
| SetD | 50 | 80 | 35.11 ± 0.93 |
| 25 | 40 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| NovD | 50 | 100 | 26.32 ± 0.00 |
| 25 | 90 | ||
| 10 | 0 | ||
| 50 | 100 | ||
| JanF | 25 | 70 | 18.29 ± 0.00 |
| 10 | 10 | ||
| 5 | 0 | ||
| 100 | 100 | ||
| JanD | 50 | 100 | 23.37 ± 2.55 |
| 25 | 100 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| MarF | 50 | 100 | 26.87 ± 0.47 |
| 25 | 80 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| MarD | 50 | 100 | 21.90 ± 0.00 |
| 25 | 100 | ||
| 10 | 0 | ||
| 100 | 80 | ||
| MayF | 50 | 50 | 51.55 ± 2.12 |
| 25 | 40 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| MayD | 50 | 80 | 33.07 ± 3.80 |
| 25 | 50 | ||
| 10 | 0 |
The tested oils showed a mortality rate ranging from 100 to 10%, according to the concentration range. These concentrations ranged from 50 to 5 μg·mL−1 for samples from July (fresh and dry) and January (fresh), from 100 to 10 μg·mL−1 for samples from September, November, and January (dry material), and March and May (fresh and dry) (Table 6). The sample with the lowest toxicity was from the month of May (fresh) which had LC50 (51.55 ± 2.12 μg·mL−1) (Table 6), which presented sesquiterpenic hydrocarbons as its major constituents; the results of the present work were close to those obtained for Schinusmolle L. essential oil rich in α-phellandrene, β-phellandrene, β-myrcene, limonene, and α-pinene, where it presented LC50 47 and 67 μg·mL−1 for leaf and fruit. In the present work, the sample that had the highest toxicity was from July (dry), that had LC50 (14.45 ± 0.25 μg·mL−1) (Table 6), which presented as main constituents β-phellalandrene and elemicin (Table 3). In general, all the essential oil fractions tested had LC50 < 100 μg·mL−1 values, which means that essential oils have high toxicity [43], and values > 1000 μg·mL−1 can be considered of low toxicity [44].
Toxicity tests in A. Salina performed using Hyptis suaveolens (L.) Poiteau (Lamiaceae) essential oil [45] presented LC50 values similar to those obtained in the present work, whereas the Garcinia mangostana essential oil obtained LC50 of 1.70 µg·mL−1 and 5.15 μg·mL−1 for leaves and stem [46], and Ferulago trifida had LC50 1.1 ± 0.3 μg·mL−1 [47]; these toxicities were higher than those presented by P. circinnata Link var. circinnata essential oils. Essential oils rich in phenylpropanoids such as cloves, demonstrated LC50 value of 0.5993 ± 0.0464 μg·mL–1 [48], which is higher than the tests of the major substances tested separately; this may be related to the synergistic effect of the substances present in the essential oil [49,50,51]. According to Radulović et al. [52], essential oils that have high toxicity values must be carefully managed to avoid intoxication. In a study carried out with three species of Peperomia, the authors obtained LC50 results of 1.9 ± 0.1 μg·mL−1 for P. rotundifolia essential oil, 2.4 ± 0.5 μg·mL−1 for P. pellucida extract, and 9.0 ± 0.4 μg·mL−1 for P. macrostachya essential oils.
2.3. In Silico Evaluation of Interaction with AChE
Previous studies have successfully reported the use of in silico approaches to evaluate the interaction of naturally occurring compounds that have molecular targets of pharmacological and toxicological interest [53,54,55,56]. For this reason, molecular docking was used to investigate the interactions established between β-elemene and elemicin with AChE. This enzyme has been reported as a target for A. salina [21,57]. The binding mode and interactions established in the complex can be seen in Figure 7.
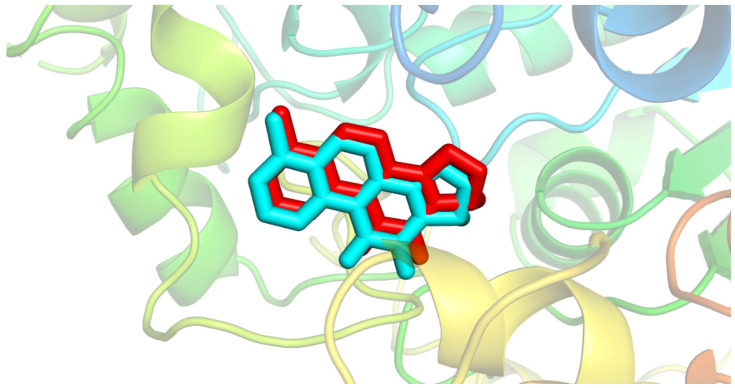
Figure 7.
Structure obtained by redocking (blue) overlaying the crystallographic binder (red).
Before performing the docking of the molecules of interest, it was necessary to validate the protocol described in the methodology. To develop the methodology, we first tried to reproduce the binding mode of the crystallographic ligand performing its redocking. For this, the ligand of the PDB 4M0E was deleted [58] and then it was redocked. To assess the binding mode’s reproducibility, the ligand interacting conformation was evaluated by comparing the redocked structure with the crystal structure. The evaluation of the obtained complexes was carried out using the root-mean-square deviation RMSD between the ligands. According to the literature, for the docking protocol to be validated, the RMSD between the redocked and the crystallographic ligand must be less than 2 Å [59,60,61,62]. In our results, an RMSD of 1.42 Å was obtained. In Figure 7, it is possible to visualize the compounds overlapping. After protocol validation, the docking between β-elemene and elemicin was performed.
The MolDock Score obtained was −91.61 Kcal/mol for the complex formed by β-elemene and −90.19 Kcal/mol for the system established with elemicin. In these binding poses the ligands were able to form an interaction with residues from the enzyme catalytic cavity. These interactions are able to favor the compounds inhibitory capacity.
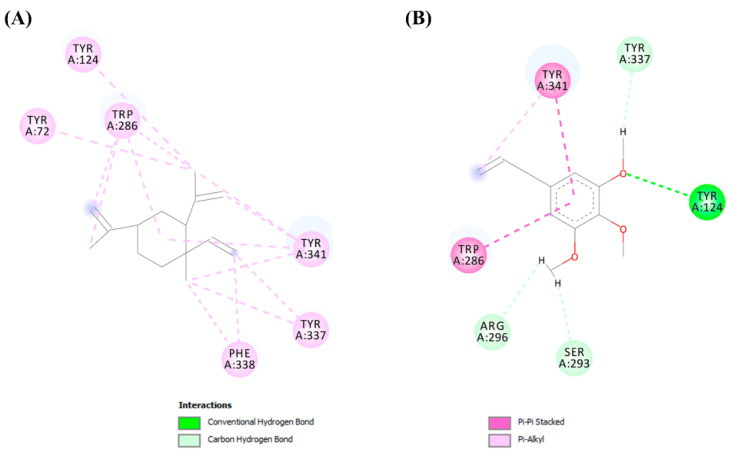
The AChE binding cavity is formed by three subsites, the anionic subsite (Trp86, Tyr133, Tyr337, and Phe338), acyl pocket (Phe295 and Phe297), and oxyanion hole (Gly121, Gly122, and Ala204) [63,64]. In Figure 8A,B, it shows that the two ligands are able to interact with residues present in the anionic subsite. With residues from this subsite, the β-elemene compound interacted with Tyr337 and Phe338 through hydrophobic interactions of the pi-alkyl type. The elemicin ligand interacted with Tyr337 through hydrogen bonds. Besides these, other interactions were established with the ligands in the binding pocket. The β-elemene also interacted with Tyr124, Tyr72, Trp286, and Tyr341. All these interactions were hydrophobic of the pi-alkyl type. Elemicin formed pi-pi type hydrophobic interactions with Tyr341 and Trp286, as well as hydrogen bonds with Tyr124, Figure 8A,B.
Figure 8.
Interactions established in the complexes established with (A) β-elemene and (B) elemicin.
3. Materials and Methods
3.1. Collection of Botanical Material for Seasonal and Circadian Study
P. circinnata Link var. circinnata collections occurred bimonthly, from July to May, in the evening, always on the 15th day at 8 am in Belém, Pará, and the samples were removed from the mango tree trunks near the Emílio Goeldi Zoo and Botanical Park. In November and March, collections were carried out in the evening and afternoon (8 am and 5 pm) for the circadian study. This specimen was identified by comparison with an authentic voucher (MG 172736) deposited in the Emılio Goeldi Museum herbarium, city of Belém, Pará state, Brazil.
3.2. Processing of Botanical Material
Whole plants devoid of spikes were divided into two parts; the fresh material was cut into small parts and subjected to the hydrodistillation process. The remainder was dried in an air circulation oven at room temperature for five days, then ground, homogenized, weighed, and subjected to hydrodistillation. Drying for the circadian study was achieved by two processes: oven (ventilation) and lyophilization.
3.2.1. Extraction Methods
Hydrodistillation
For the essential oil extraction process in circadian and seasonal studies, 40 g of fresh and dry sample from P. circinnata Link var. circinnata were dried in an air circulation oven and then subjected to hydrodistillation. The same proportion of water in relation to plant material was used, according to the methodology described by [53,65].
Simultaneous Distillation–Extraction
For aroma extraction, 10 g of sample from P. circinnata Link var. circinnata was used, then mixed with water (20 mL) and subjected to simultaneous distillation–extraction (SDE) for 3 h, using a Chrompack Micro-Steam Distillation Extractor (Likens-Nickerson) and pentane (2 mL) as organic mobile phase, as described by [66].
3.3. Identification of Chemical Constituents
The chemical composition of P. circinnata Link var. circinnata essential oils and aromas was analyzed by Gas Chromatography coupled with Mass Spectrometry, using a Thermo DSQ-II system equipped with a DB-5MS silica capillary column (30 m × 0.25 mm; 0.25 μm) with temperature program: 60–240 °C, using gradient of 3 °C/min; injector temperature: 240 °C; carrier gas: helium (linear velocity of 32 cm/s, measured at 100 °C); splitless injection flow (0.1 mL of a 2:1000 sol. of n-hexane); temperature of ion source and other parts at 200 °C. The quadrupole filter swept in the range of 39 to 500 daltons every second. Ionization was achieved by the electronic impact technique at 70 eV. Volatile components identification was based on the linear retention index (Kováts Index) calculated in relation to the retention times of a homologous series of n-alkanes (C8–C40) according Van den Dool and Kratz [67] and on the fragmentation pattern observed in the mass spectra, by comparison in the data system and literature libraries [39,68]. Quantitative data regarding the volatile constituents were obtained by peak-area normalization using a FOCUS GC/FID, as previously reported by our research group [69].
3.4. Preliminary Toxicity Bioassay with Artemia Salina Leach
For the toxicity tests, the sample with the highest mass yield was selected. The preliminary toxicity bioassay test of P. circinnata Link var. circinnata essential oil in A. salina Leach was performed as described in the literature [57,70,71]. The essential oil was prepared at concentrations of 100, 50, 20, 10, 5, and 1 μg·mL−1 were used, from fresh and dry samples obtained in the seasonal study in the months of July, September, November, January, March, and May. A total of ten Artemia salina larvae were added to each test flask with the aid of automatic micropipettes. Brine water (artificial) and DMSO were used as solvents with a 95:5 ratio. In the control group and the positive group with lapachol, the same solvent was used for the samples and larvae under the same conditions as the bioassay. After 24 h of contact between the larvae and the sample solution, the dead larvae were counted (in each concentration), and the mortality rate and the IC50 value were calculated using the Probitos statistical method. All the experiments were performed in triplicate (n = 3).
3.5. Molecular Docking
For molecular docking studies, the compounds β-elemene (PubChem CID 9859094) and elemicin (PubChem CID 10248) were obtained from Pub Chem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 20 May 2021). Then, their structures were optimized with B3LYP/6-31G* [72] using Gaussian 09 software [73].
We used the molecular method to evaluate the compounds interaction mode with Acetylcholinesterase (AChE). For this we used the Molegro Virtual Docker (MVD) 5.5 software [74], and the crystal structure used as a molecular target can be found in the Protein Data Bank (http://www.rcsb.org, accessed on 20 May 2021) using the ID: 4M0E [58].
The MolDock Score (GRID) scoring function was used with Grid resolution of 0.30Å and 5Å radius encompassing the entire connection cavity. The MolDock SE algorithm was used with number of runs equal to 10, 1500 max interactions, and max population size equal to 50. The maximum evaluation of 300 steps with neighbor distance factor equal to 1 and energy threshold equal to 100 was used during the molecular docking simulation.
3.6. Statistical Analysis
Multivariate analysis was performed according to the methodology described by [69,75], where Minitab 17® software (free version, Minitab Inc., State College, PA, USA) was used. The variables were the essential oils’ chemical constituents. The raw data were first standardized to have the same “weight”. The Principal Component Analysis (PCA) was obtained using the software configuration “correlation” of the matrix type. In the Hierarchical Cluster Analysis (HCA) of the samples, the Euclidean distance options were used for distance measurement and complete connection for connection method.
4. Conclusions
The highest essential oil yield from the seasonal analysis of P. circinnata Link var. circinnata during the study period was found in the fresh plant, especially in July, that is, in the dry season, which may mean that low humidity contributes to oil production. Multivariate analysis allowed to observe the similarities and differences between the chemical compositions in the different periods studied, showing that, according to the period and time of collection, there is a qualitative and quantitative change in the chemical composition. In the circadian study of the months of November and March, there were no significant variations in oil yield in the evening and afternoon collections. The essential oils obtained from fresh and dry plants showed a quantitative difference, in relation to the more volatile constituents (monoterpenes). Seasonal variation can be seen in relation to the presence of the phenylpropanoid elemicin in the months of July and January; as well as the oxygenated sesquiterpene elemol. These qualitative and quantitative variations of the oil are affected by seasonality and, consequently, are reflected in the biological activities evaluated, as at all concentrations we can observe that there was a low LC50 representing a potential biological activity of the samples. In the in silico study, it was observed that in the complex formed between AChE-β-elemene and AChE-elemicin, the interactions formed had residues present in the anionic subsite of the enzyme, in addition to interactions with other residues of the enzyme. Most of these interactions were hydrophobic and helped to maintain the systems formed by molecular interactions.
Acknowledgments
The author Márcia Moraes Cascaes thanks CAPES for the Phd scholarship process number: [88887.497476/2020-00]. The author Mozaniel Santana de Oliveira thanks PCI-MCTIC/MPEG, as well as CNPq for the scholarship process number: [302050/2021-3]. The authors would like to thank the Universidade Federal do Pará.
Author Contributions
Conceptualization, K.d.S.M.M., B.d.S.F., J.N.C., O.O.F., C.d.J.P.F. and M.M.C.; methodology, J.N.C., O.O.F., C.d.J.P.F. and M.M.C.; formal analysis, M.S.d.O. and E.H.d.A.A.; investigation, K.d.S.M.M., B.d.S.F., J.N.C., O.O.F., C.d.J.P.F., M.M.C. and M.S.d.O.; writing—review and editing, E.H.d.A.A.; visualization, E.H.d.A.A.; supervision, E.H.d.A.A.; project administration, E.H.d.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001. Universidade Federal do Pará/Proposp/PROGRAMA DE APOIO À PUBLICAÇÃO QUALIFICADA–PAPQ-EDITAL 06/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Samples of Peperomia circinnata Link var. circinnata. (Piperaceae). The essential oil of the Museu Paraense Emílio Goeldi is available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jaramillo M.A., Manos P.S., Zimmer E.A. Phylogenetic Relationships of the Perianthless Piperales: Reconstructing the Evolution of Floral Development. Int. J. Plant Sci. 2004;165:403–416. doi: 10.1086/382803. [DOI] [Google Scholar]
- 2.Melo A., Guimarães E.F., Alves M. Synopsis of the genus Peperomia Ruiz & Pav. (Piperaceae) in Roraima State, Brazil. Hoehnea. 2016;43:119–134. doi: 10.1590/2236-8906-75/2015. [DOI] [Google Scholar]
- 3.Wanke S., Samain M.-S., Vanderschaeve L., Mathieu G., Goetghebeur P., Neinhuis C. Phylogeny of the Genus Peperomia (Piperaceae) Inferred from the trnK/matK Region (cpDNA) Plant Biol. 2006;8:93–102. doi: 10.1055/s-2005-873060. [DOI] [PubMed] [Google Scholar]
- 4.Paz R.F., Guimarães E.F., Ramos C.S. The occurrence of phenylpropanoids in the saps of six Piper species (Piperaceae) from Brazil. Gayana Bot. 2017;74:236–239. doi: 10.4067/S0717-66432017005000109. [DOI] [Google Scholar]
- 5.Vergara-Rodrígue D., Mathieu G., Samain M.S., Armenta-Montero S., Krömer T. Diversity, distribution, and conservation status of Peperomia (Piperaceae) in the state of Veracruz, Mexico. Trop. Conserv. Sci. 2017;10:1940082917702383. doi: 10.1177/1940082917702383. [DOI] [Google Scholar]
- 6.Frenzke L., Scheiris E., Pino G., Symmank L., Goetghebeur P., Neinhuis C., Wanke S., Samain M.S. A revised infrageneric classification of the genus Peperomia (Piperaceae) Taxon. 2015;64:424–444. doi: 10.12705/643.4. [DOI] [Google Scholar]
- 7.Sarnaglia Junior V.B., De Lírio E.J., Freitas J., Guimarães E.F. New records of Peperomia armondii Yunck, Peperomia hispidula (Sw.) A. Dietr., and Peperomia mandioccana Miq. for the state of Espírito Santo, southeastern Brazil. Check List. 2015;11:1580. doi: 10.15560/11.2.1580. [DOI] [Google Scholar]
- 8.Carvalho-Silva M., Guimarães E.F., Sarnaglia V.B. Two new species of Peperomia Ruiz & Pavon (Piperaceae) from southeastern Brazil and four new synonymies. Phytotaxa. 2019;422:225–232. doi: 10.11646/phytotaxa.422.3.2. [DOI] [Google Scholar]
- 9.Salehi B., Zakaria Z.A., Gyawali R., Ibrahim S.A., Rajkovic J., Shinwari Z.K., Khan T., Sharifi-Rad J., Ozleyen A., Turkdonmez E., et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauter I.P., Rossa G.E., Lucas A.M., Cibulski S.P., Roehe P.M., da Silva L.A.A., Rott M.B., Vargas R.M.F., Cassel E., von Poser G.L. Chemical composition and amoebicidal activity of Piper hispidinervum (Piperaceae) essential oil. Ind. Crops Prod. 2012;40:292–295. doi: 10.1016/j.indcrop.2012.03.025. [DOI] [Google Scholar]
- 11.Da Silva M.F.R., Bezerra-Silva P.C., de Lira C.S., de Lima Albuquerque B.N., Agra Neto A.C., Pontual E.V., Maciel J.R., Paiva P.M.G., Navarro D.M.d.A.F. Composition and biological activities of the essential oil of Piper corcovadensis (Miq.) C. DC (Piperaceae) Exp. Parasitol. 2016;165:64–70. doi: 10.1016/j.exppara.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Oyemitan I.A., Olayera O.A., Alabi A., Abass L.A., Elusiyan C.A., Oyedeji A.O., Akanmu M.A. Psychoneuropharmacological activities and chemical composition of essential oil of fresh fruits of Piper guineense (Piperaceae) in mice. J. Ethnopharmacol. 2015;166:240–249. doi: 10.1016/j.jep.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Bezerra J.W.A., Rodrigues F.C., Pereira da Cruz R., da Silva L.E., do Amaral W., Andrade Rebelo R., Begnini I.M., Fonseca Bezerra C., Iriti M., Varoni E.M., et al. Antibiotic Potential and Chemical Composition of the Essential Oil of Piper caldense C. DC. (Piperaceae) Appl. Sci. 2020;10:631. doi: 10.3390/app10020631. [DOI] [Google Scholar]
- 14.Andrés M.F., Rossa G.E., Cassel E., Vargas R.M.F., Santana O., Díaz C.E., González-Coloma A. Biocidal effects of Piper hispidinervum (Piperaceae) essential oil and synergism among its main components. Food Chem. Toxicol. 2017;109:1086–1092. doi: 10.1016/j.fct.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Salleha W.M.N.H.W., Ahmada F., Yenb K.H. Chemical compositions and antimicrobial activity of the essential oils of piper abbreviatum, P. Erecticaule and P. Lanatum (Piperaceae) Nat. Prod. Commun. 2014;9:1795–1798. doi: 10.1177/1934578x1400901235. [DOI] [PubMed] [Google Scholar]
- 16.Turchen L.M., Piton L.P., Dall’Oglio E.L., Butnariu A.R., Pereira M.J.B. Toxicity of Piper aduncum (Piperaceae) Essential Oil Against Euschistus heros (F.) (Hemiptera: Pentatomidae) and Non-Effect on Egg Parasitoids. Neotrop. Entomol. 2016;45:604–611. doi: 10.1007/s13744-016-0409-7. [DOI] [PubMed] [Google Scholar]
- 17.Kpadonou Kpoviessi B.G.H., Ladekan E.Y., Kpoviessi D.S.S., Gbaguidi F., Yehouenou B., Quetin-Leclercq J., Figueredo G., Moudachirou M., Accrombessi G.C. Chemical Variation of Essential Oil Constituents of Ocimum gratissimum L. from Benin, and Impact on Antimicrobial Properties and Toxicity against Artemia salinaLeach. Chem. Biodivers. 2012;9:139–150. doi: 10.1002/cbdv.201100194. [DOI] [PubMed] [Google Scholar]
- 18.Oliva M.D.L.M., Gallucci N., Zygadlo J.A., Demo M.S. Cytotoxic activity of Argentinean essential oils on Artemia salina. Pharm. Biol. 2007;45:259–262. doi: 10.1080/13880200701214557. [DOI] [Google Scholar]
- 19.Soares B.V., Morais S.M., dos Santos Fontenelle R.O., Queiroz V.A., Vila-Nova N.S., Pereira C.M.C., Brito E.S., Neto M.A.S., Brito E.H.S., Cavalcante C.S.P., et al. Antifungal Activity, Toxicity and Chemical Composition of the Essential Oil of Coriandrum sativum L. Fruits. Molecules. 2012;17:8439–8448. doi: 10.3390/molecules17078439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dima C., Dima S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015;5:29–35. doi: 10.1016/j.cofs.2015.07.003. [DOI] [Google Scholar]
- 21.Lima L.R., Andrade F.K., Alves D.R., de Morais S.M., Vieira R.S. Anti-acetylcholinesterase and toxicity against Artemia salina of chitosan microparticles loaded with essential oils of Cymbopogon flexuosus, Pelargonium x ssp and Copaifera officinalis. Int. J. Biol. Macromol. 2021;167:1361–1370. doi: 10.1016/j.ijbiomac.2020.11.090. [DOI] [PubMed] [Google Scholar]
- 22.Zoghbi M.G.B., Andrade E.H.A., Lobato R.C.L., Tavares A.C.C., Souza A.P.S., Conceição C.C.C., Guimarães E.F. Peperomia circinnata Link and Peperomia rotundifolia (L.) Kunth growing on different host-trees in Amazon: Volatiles and relationship with bryophytes. Biochem. Syst. Ecol. 2005;33:269–274. doi: 10.1016/j.bse.2004.09.006. [DOI] [Google Scholar]
- 23.Da Silva M.H.L., Zoghbi M.D.G.B., Andrade E.H.A., Maia J.G.S. The essential oils ofPeperomia pellucida Kunth andP. circinnata Link var.circinnata. Flavour Fragr. J. 1999;14:312–314. doi: 10.1002/(SICI)1099-1026(199909/10)14:5<312::AID-FFJ835>3.0.CO;2-B. [DOI] [Google Scholar]
- 24.Alves N.S.F., Setzer W.N., da Silva J.K.R. The chemistry and biological activities of Peperomia pellucida (Piperaceae): A critical review. J. Ethnopharmacol. 2019;232:90–102. doi: 10.1016/j.jep.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro B.G., Silva A.S.B., Souza G.E.P., Figueiredo J.G., Cunha F.Q., Lahlou S., Da Silva J.K.R., Maia J.G.S., Sousa P.J.C. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J. Ethnopharmacol. 2011;138:479–486. doi: 10.1016/j.jep.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 26.Noriega Rivera P., Mosquera T., Baldisserotto A., Abad J., Aillon C., Cabezas D., Piedra J., Coronel I., Manfredini S. Chemical Composition and in-vitro biological activities of the essential oil from leaves of Peperomia inaequalifolia Ruiz & Pav. Am. J. Essent. Oil Nat. Prod. 2015;2:29–31. [Google Scholar]
- 27.Verma R.S., Padalia R.C., Goswami P., Chauhan A. Essential oil composition of Peperomia pellucida (L.) Kunth from India. J. Essent. Oil Res. 2015;27:89–95. doi: 10.1080/10412905.2014.982878. [DOI] [Google Scholar]
- 28.Usman L.A., Ismaeel R.O. Chemical Composition of Root Essential oil of Peperomia pellucida (L.) Kunth. Grown in Nigeria. J. Essent. Oil Bear. Plants. 2020;23:628–632. doi: 10.1080/0972060X.2020.1794983. [DOI] [Google Scholar]
- 29.Matias E.F.F., Alves E.F., Silva M.K.N., Carvalho V.R.A., Figueredo F.G., Ferreira J.V.A., Coutinho H.D.M., Silva J.M.F.L., Ribeiro-Filho J., Costa J.G.M. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 2016;87:45–53. doi: 10.1016/j.indcrop.2016.04.028. [DOI] [Google Scholar]
- 30.Ribeiro P.H.S., dos Santos M.L., da Camara C.A.G., Born F.S., Fagg C.W. Seasonal chemical compositions of the essential oils of two eugenia species and their acaricidal proPERTIES. Quim. Nova. 2015;39:38–43. doi: 10.5935/0100-4042.20150161. [DOI] [Google Scholar]
- 31.Sarrazin S., da Silva L., de Assunção A., Oliveira R., Calao V., da Silva R., Stashenko E., Maia J., Mourão R. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules. 2015;20:1860–1871. doi: 10.3390/molecules20021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain A.I., Anwar F., Hussain Sherazi S.T., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Chua L.Y.W., Chong C.H., Chua B.L., Figiel A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019;12:450–476. doi: 10.1007/s11947-018-2227-x. [DOI] [Google Scholar]
- 34.Hazarika U., Gosztola B. Lyophilization and its effects on the essential oil content and composition of herbs and spices—A review. Acta Sci. Pol. Technol. Aliment. 2020;19:467–473. doi: 10.17306/J.AFS.2020.0853. [DOI] [PubMed] [Google Scholar]
- 35.Díaz-Maroto M.C., Pérez-Coello M.S., Cabezudo M.D. Effect of Drying Method on the Volatiles in Bay Leaf (Laurus nobilis L.) J. Agric. Food Chem. 2002;50:4520–4524. doi: 10.1021/jf011573d. [DOI] [PubMed] [Google Scholar]
- 36.Díaz-Maroto M.C., González Viñas M.A., Cabezudo M.D. Evaluation of the effect of drying on aroma of parsley by free choice profiling. Eur. Food Res. Technol. 2003;216:227–232. doi: 10.1007/s00217-002-0643-6. [DOI] [Google Scholar]
- 37.Yousif A.N., Scaman C.H., Durance T.D., Girard B. Flavor Volatiles and Physical Properties of Vacuum-Microwave- and Air-Dried Sweet Basil (Ocimum basilicum L.) J. Agric. Food Chem. 1999;47:4777–4781. doi: 10.1021/jf990484m. [DOI] [PubMed] [Google Scholar]
- 38.De Lira P.N.B., da Silva J.K.R., Andrade E.H.A., Sousa P.J.C., Silva N.N.S., Maia J.G.S. Essential Oil Composition of Three Peperomia Species from the Amazon, Brazil. Nat. Prod. Commun. 2009;4:1934578X0900400. doi: 10.1177/1934578X0900400323. [DOI] [PubMed] [Google Scholar]
- 39.Adams R.P. In: Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4th ed. Adams R.P., editor. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 40.Ghasemi Pirbalouti A., Mahdad E., Craker L. Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem. 2013;141:2440–2449. doi: 10.1016/j.foodchem.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 41.Lagartoparra A. Comparative study of the assay of and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 42.Stojanović N.M., Randjelović P.J., Mladenović M.Z., Ilić I.R., Petrović V., Stojiljković N., Ilić S., Radulović N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019;133:110794. doi: 10.1016/j.fct.2019.110794. [DOI] [PubMed] [Google Scholar]
- 43.Nguta J.M., Mbaria J.M., Gakuya D.W., Gathumbi P.K., Kabasa J.D., Kiama S.G. Cytotoxicity of antimalarial plant extracts from Kenyan biodiversity to the brine shrimp, Artemia salina L. (Artemiidae) Drugs Ther. Stud. 2012;2:12. doi: 10.4081/dts.2012.e12. [DOI] [Google Scholar]
- 44.Costa W.K., de Oliveira J.R.S., de Oliveira A.M., Santos I.B.D.S., da Cunha R.X., de Freitas A.F.S., da Silva J.W.L.M., Silva V.B.G., Aguiar J.C.R.D.O.F.D., da Silva A.G., et al. Essential oil from Eugenia stipitata McVaugh leaves has antinociceptive, anti-inflammatory and antipyretic activities without showing toxicity in mice. Ind. Crops Prod. 2020;144:112059. doi: 10.1016/j.indcrop.2019.112059. [DOI] [Google Scholar]
- 45.Bezerra J.W.A., Costa A.R., da Silva M.A.P., Rocha M.I., Boligon A.A., da Rocha J.B.T., Barros L.M., Kamdem J.P. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (LAMIACEAE) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017;113:437–442. doi: 10.1016/j.sajb.2017.10.003. [DOI] [Google Scholar]
- 46.Aboaba S., Akande A., Flamini G. Chemical Composition, Toxicity and Antibacterial activity of the Essential Oils of Garcinia mangostana from Nigeria. J. Essent. Oil Bear. Plants. 2014;17:78–86. doi: 10.1080/0972060X.2014.884759. [DOI] [Google Scholar]
- 47.Tavakoli S., Vatandoost H., Zeidabadinezhad R., Hajiaghaee R., Hadjiakhoondi A., Abai M.R., Yassa N. Gas Chromatography, GC/Mass analysis and bioactivity of essential oil from aerial parts of Ferulago trifida: Antimicrobial, antioxidant, AChE inhibitory, general toxicity, MTT assay and larvicidal activities. J. Arthropod-Borne Dis. 2017;11:414–426. [PMC free article] [PubMed] [Google Scholar]
- 48.Cansian R.L., Vanin A.B., Orlando T., Piazza S.P., Puton B.M.S., Cardoso R.I., Gonçalves I.L., Honaiser T.C., Paroul N., Oliveira D. Toxicity of clove essential oil and its ester eugenyl acetate against Artemia salina. Braz. J. Biol. 2017;77:155–161. doi: 10.1590/1519-6984.12215. [DOI] [PubMed] [Google Scholar]
- 49.Benelli G., Flamini G., Canale A., Cioni P.L., Conti B. Toxicity of some essential oil formulations against the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Diptera Tephritidae) Crop Prot. 2012;42:223–229. doi: 10.1016/j.cropro.2012.05.024. [DOI] [Google Scholar]
- 50.Betim F.C.M., de Oliveira C.F., de Souza A.M., Szabo E.M., Zanin S.M.W., Miguel O.G., Miguel M.D., Dias J.D.F.G. Ocotea nutans (Nees) Mez (Lauraceae): Chemical composition, antioxidant capacity and biological properties of essential oil. Braz. J. Pharm. Sci. 2019;55 doi: 10.1590/s2175-97902019000118284. [DOI] [Google Scholar]
- 51.Retnowati R., Rahman M.F., Yulia D. Chemical Constituents of the Essential Oils of White Turmeric (Curcuma zedoaria (Christm.) Roscoe) from Indonesia and its Toxicity toward Artemia salina Leach. J. Essent. Oil Bear. Plants. 2014;17:393–396. doi: 10.1080/0972060X.2014.895196. [DOI] [Google Scholar]
- 52.Radulović N.S., Genčić M.S., Stojanović N.M., Randjelović P.J., Stojanović-Radić Z.Z., Stojiljković N.I. Toxic essential oils. Part V: Behaviour modulating and toxic properties of thujones and thujone-containing essential oils of Salvia officinalis L., Artemisia absinthium L., Thuja occidentalis L. and Tanacetum vulgare L. Food Chem. Toxicol. 2017;105:355–369. doi: 10.1016/j.fct.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 53.De Oliveira M.S., Da Cruz J.N., Da Costa W.A., Silva S.G., Brito M.D.P., De Menezes S.A.F., Neto A.M.D.J.C., Andrade E.H.D.A., Junior R.N.D.C. Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules. 2020;25:3852. doi: 10.3390/molecules25173852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro A.L.G., Cruz J.N., Sodré D.F., Correa-Barbosa J., Azonsivo R., de Oliveira M.S., de Sousa Siqueira J.E., da Rocha Galucio N.C., de Oliveira Bahia M., Burbano R.M.R., et al. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated from Eleutherine plicata herb. using bioassays and in silico approaches. Arab. J. Chem. 2021;14:103084. doi: 10.1016/j.arabjc.2021.103084. [DOI] [Google Scholar]
- 55.Souza da Silva Júnior O., de Jesus Pereira Franco C., Barbosa de Moraes A.A., Cruz J.N., Santana da Costa K., Diniz do Nascimento L., Helena de Aguiar Andrade E. In silico analyses of toxicity of the major constituents of essential oils from two Ipomoea L. species. Toxicon. 2021;195:111–118. doi: 10.1016/j.toxicon.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Cascaes M.M., Silva S.G., Cruz J.N., de Oliveira M.S., Oliveira J., de Moraes A.A.B., da Costa F.A.M., da Costa K.S., Nascimento L.D.D., Andrade E.H.D.A. First report on the Annona exsucca DC. Essential oil and in silico identification of potential biological targets of its major compounds. Nat. Prod. Res. 2021;35:1–4. doi: 10.1080/14786419.2021.1893724. [DOI] [PubMed] [Google Scholar]
- 57.Santana de Oliveira M., Pereira da Silva V.M., Cantão Freitas L., Gomes Silva S., Nevez Cruz J., de Aguiar Andrade E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers. 2021;15:e2000982. doi: 10.1002/cbdv.202000982. [DOI] [PubMed] [Google Scholar]
- 58.Cheung J., Gary E.N., Shiomi K., Rosenberry T.L. Structures of human acetylcholinesterase bound to dihydrotanshinone i and territrem B show peripheral site flexibility. ACS Med. Chem. Lett. 2013;4:1091–1096. doi: 10.1021/ml400304w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leão R.P., Cruz J.V., da Costa G.V., Cruz J.N., Ferreira E.F.B., Silva R.C., de Lima L.R., Borges R.S., dos Santos G.B., Santos C.B.R. Identification of New Rofecoxib-Based Cyclooxygenase-2 Inhibitors: A Bioinformatics Approach. Pharmaceuticals. 2020;13:209. doi: 10.3390/ph13090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Araújo P.H.F., Ramos R.S., da Cruz J.N., Silva S.G., Ferreira E.F.B., de Lima L.R., Macêdo W.J.C., Espejo-Román J.M., Campos J.M., Santos C.B.R. Identification of potential COX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules. 2020;25:4183. doi: 10.3390/molecules25184183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costa E., Silva R., Espejo-Román J., Neto M.D.A., Cruz J., Leite F., Silva C., Pinheiro J., Macêdo W., Santos C. Chemometric methods in antimalarial drug design from 1,2,4,5-tetraoxanes analogues. SAR QSAR Environ. Res. 2020;31:677–695. doi: 10.1080/1062936X.2020.1803961. [DOI] [PubMed] [Google Scholar]
- 62.Mascarenhas A.M.S., de Almeida R.B.M., de Araujo Neto M.F., Mendes G.O., da Cruz J.N., dos Santos C.B.R., Botura M.B., Leite F.H.A. Pharmacophore-based virtual screening and molecular docking to identify promising dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. J. Biomol. Struct. Dyn. 2020;39:6021–6030. doi: 10.1080/07391102.2020.1796791. [DOI] [PubMed] [Google Scholar]
- 63.Ordentlich A., Barak D., Kronman C., Flashner Y., Leitner M., Segall Y., Ariel N., Cohen S., Velan B., Shafferman A. Dissection of the human acetylcholinesterase active center determinants of substrate specificity. Identification of residues constituting the anionic site, the hydrophobic site, and the acyl pocket. J. Biol. Chem. 1993;268:17083–17095. doi: 10.1016/S0021-9258(19)85305-X. [DOI] [PubMed] [Google Scholar]
- 64.Xu Y., Colletier J.P., Weik M., Jiang H., Moult J., Silman I., Sussman J.L. Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray versus molecular dynamics. Biophys. J. 2008;95:2500–2511. doi: 10.1529/biophysj.108.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira O.O., Da Cruz J.N., Franco C.D.J.P., Silva S.G., Da Costa W.A., De Oliveira M.S., Andrade E.H.D.A. First report on yield and chemical composition of essential oil extracted from myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules. 2020;25:783. doi: 10.3390/molecules25040783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maia J.G.S., Andrade E.H.A., da Silva M.H.L. Aroma volatiles of pequi fruit (Caryocar brasiliense Camb.) J. Food Compos. Anal. 2008;21:574–576. doi: 10.1016/j.jfca.2008.05.006. [DOI] [Google Scholar]
- 67.Van Den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 68.Stein S., Mirokhin D., Tchekhovskoi D., Mallard G., Mikaia A., Zaikin V., Sparkmanm D. Standard Reference Data Program of the National Institute of Standards and Technology. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2011. The NIST mass spectral search program for the nist/epa/nih mass spectra library. [Google Scholar]
- 69.Silva S.G., Figueiredo P.L.B., Nascimento L.D., da Costa W.A., Maia J.G.S., Andrade E.H.A. Planting and seasonal and circadian evaluation of a thymol-type oil from Lippia thymoides Mart. & Schauer. Chem. Cent. J. 2018;12:113. doi: 10.1186/s13065-018-0484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finney D.J. Probit Analysis. J. Pharm. Sci. 1971;60:1432. doi: 10.1002/jps.2600600940. [DOI] [Google Scholar]
- 71.Meyer B., Ferrigni N., Putnam J., Jacobsen L., Nichols D., McLaughlin J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 72.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 73.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., et al. DJ Gaussian 09. Contacting Gaussian, Inc.; Wallingford, CT, USA: 2009. pp. 2–3. Revision E.01. [Google Scholar]
- 74.Thomsen R., Christensen M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 75.De Oliveira M., Cruz J., Ferreira O., Pereira D., Pereira N., Oliveira M., Venturieri G., Guilhon G., Filho A.S., Andrade E. Chemical Composition of Volatile Compounds in Apis mellifera Propolis from the Northeast Region of Pará State, Brazil. Molecules. 2021;26:3462. doi: 10.3390/molecules26113462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Samples of Peperomia circinnata Link var. circinnata. (Piperaceae). The essential oil of the Museu Paraense Emílio Goeldi is available from the authors.