Abstract
Fungal infections are underestimated threats that affect over 1 billion people, and Candida spp., Cryptococcus spp., and Aspergillus spp. are the 3 most fatal fungi. The treatment of these infections is performed with a limited arsenal of antifungal drugs, and the class of the azoles is the most used. Although these drugs present low toxicity for the host, there is an emergence of therapeutic failure due to azole resistance. Drug resistance normally develops in patients undergoing azole long-term therapy, when the fungus in contact with the drug can adapt and survive. Conversely, several reports have been showing that resistant isolates are also recovered from patients with no prior history of azole therapy, suggesting that other routes might be driving antifungal resistance. Intriguingly, antifungal resistance also happens in the environment since resistant strains have been isolated from plant materials, soil, decomposing matter, and compost, where important human fungal pathogens live. As the resistant fungi can be isolated from the environment, in places where agrochemicals are extensively used in agriculture and wood industry, the hypothesis that fungicides could be driving and selecting resistance mechanism in nature, before the contact of the fungus with the host, has gained more attention. The effects of fungicide exposure on fungal resistance have been extensively studied in Aspergillus fumigatus and less investigated in other human fungal pathogens. Here, we discuss not only classic and recent studies showing that environmental azole exposure selects cross-resistance to medical azoles in A. fumigatus, but also how this phenomenon affects Candida and Cryptococcus, other 2 important human fungal pathogens found in the environment. We also examine data showing that fungicide exposure can select relevant changes in the morphophysiology and virulence of those pathogens, suggesting that its effect goes beyond the cross-resistance.
1. Introduction
Candida spp., Cryptococcus spp., and Aspergillus spp. are among the 3 most lethal human pathogenic fungi [1] as they can cause severe systemic infections, which may be fatal even when treated [2]. The treatment relies on a limited arsenal of antifungal drugs from 3 classes: polyenes, echinocandins, and azoles [2,3]. The main antifungal effect of polyenes (for example, amphotericin B) is through binding to a conserved ergosterol region forming large extramembraneous aggregates that remove ergosterol from lipid bilayers [4,5], while echinocandins (caspofungin, anidulafungin, micafungin, and, more recently, rezafungin) disrupt the cell wall as they inhibit noncompetitively the 1,3-D-glucan synthase, an important enzyme for cell wall biosynthesis. Azoles, which are classified as imidazoles (ketoconazole and miconazole), and triazoles (fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole), interrupt ergosterol synthesis by inhibiting lanoterol-14α-D-demethylase encoded by the orthologous ERG11 (in yeasts) and cyp51 (in Aspergillus fumigatus) [2,6,7]. This prevents the conversion of lanosterol into 4,4-dimethyl-8,14,24-trienol, reduces the ergosterol levels on the cell membrane, and accumulates toxic sterols, affecting the membrane integrity and permeability, ultimately inhibiting fungal growth [6–8].
One of the reasons for treatment failure and the high number of deaths caused by systemic mycoses is the emergence of resistance [9–11]. Microbiological resistance is defined as the inability of an antifungal to kill or inhibit the fungal growth in vitro [6,12,13] and can be divided into 2 classes: (i) primary or intrinsic resistance, when a microorganism is naturally resistant to a drug, without previous exposure; and (ii) secondary resistance, when resistance mutations evolve in the population and are selected upon exposure to an antifungal [6].
Several cases of isolation of azole-resistant strains from patients with no prior antifungal therapy have been reported, suggesting that other routes might be driving antifungal resistance [14–18]. Intriguingly, antifungal resistance also happens in the environment since resistant strains have been isolated from plant material, soil, decomposing matter, and compost [19–27]. This fact raises an important question: How does resistance to azoles arise in environmental isolates?
One answer to this question is based on the massive use of fungicides during preharvest in grain- and grass-growing environments and postharvest to prevent spoilage [26,28]. In addition, azoles are used for preserving paintings, coatings, and wallpaper pastes and are typically applied to mattresses to avoid fungal growth [26]. Environmental triazoles also share the same mechanism of action as medical triazoles and have been extensively used for controlling fungal phytopathogens [29,30]. Because of that, and since certain potential human pathogens can be easily isolated from plant material and soil, the most accepted hypothesis is that agrochemicals, especially 14α-demethylase inhibitors (DMIs), operate as a selection pressure for the emergence of resistant strains in the environment (fungicide-driven drug resistance route) [26,31]. Based on that, this review discusses classic and recent studies showing that environmental azole exposure selects cross-resistance to medical azoles in A. fumigatus, with a focus on the mechanisms involved. In addition, we also discuss how this phenomenon can affect Candida and Cryptococcus, other 2 important human fungal pathogens found in the environment.
2. Aspergillus fumigatus
2.1 Habitat, clinical manifestations, treatment, and resistance prevalence
Aspergillus fumigatus is a saprophytic fungus found in soil, crops, seeds, air, leaves, flowers, and indoor environments [15,17,19–21,26,32–36]. It also causes a wide range of chronic and life-threatening infections, such as allergic bronchopulmonary aspergillosis (ABPA), chronic pulmonary aspergillosis (CPA), and invasive pulmonary aspergillosis (IPA) [37]. Such diseases are treated with a restricted arsenal of antifungals from 3 classes: azoles, polyenes, and echinocandins [37–39]. Specifically, the triazoles (voriconazole, itraconazole, posaconazole, and isavulconazole) are the most indicated as the first-line therapy [38,40] and liposomal amphotericin B (polyene) and echinocandins as second-line choices [38,40,41]. Unlike echinocandins and polyenes, resistance to azoles is relatively common and has been increasing since the first A. fumigatus azole-resistant strains were reported in 1997 [42].
The incidence of clinical A. fumigatus triazole resistance varies according to the country and the patient from which it is isolated. In European countries, clinical resistance ranges from 0.6% to 30%, having reached the highest rate (>20%) in the Netherlands, United Kingdom, and Germany [43,44]. Outside Europe, azole resistance has been detected in China (5.5%), India (1.7%), Iran (3.5%), Japan (12.7%), Thailand (3.2%), Australia (2.6%), and the United States (0.6% to 11.8%) [15,32,43,45–48]. In South America, Brazil, Peru, Mexico, and Argentina have also reported triazole-resistant isolates [24,49–53]. The clinical implications of an infection caused by an antifungal-resistant strain are not totally revealed and not always related to therapeutic failure [43]. Nonetheless, some studies have shown that resistance may ultimately lead to a poor outcome [9–11,54].
Triazoles are not mutagenic compounds, which means that resistance occurs when genetic changes in the progeny of A. fumigatus are selected during reproduction. In A. fumigatus, 3 modes of reproduction can happen: asexual, sexual, and parasexual. Through asexual sporulation, common in nature, A. fumigatus produces an abundant number of spores (conidia). Even though the progeny from asexual reproduction is clonal, many conidia may harbor spontaneous mutations, ensuring genetic diversity. If one or more mutations give the conidia a better ability to survive and grow under certain stresses (for example, triazole exposure), the mutant will proliferate and might surpass the growth of the wild-type spore. This selective pressure can happen in any environment containing azoles [55,56].
Although many studies have proved that azole therapy can drive inpatient resistance to emerge in Aspergillus spp. clones [57–66], this route does not explain all cases observed in the genus. Actually, it is estimated that only one-third of the resistant strains arise from in-host adaptation, remarkably those suffering from aspergilloma, allergic or chronic aspergillosis, and predisposing conditions as lung cavities or cystic fibrosis (CF) [11,64]. The main evidence indicating another route is the azole-resistant A. fumigatus isolated from azole-naive patients, which accounts for 64% to 71% of the multiresistant A. fumigatus isolates [16,67,68]. Mellado and colleagues recovered 13 multiple triazole-resistant A. fumigatus strains from patients at different hospitals in the Netherlands—4 of them from individuals with no history of azole treatment [16]. In those cases, the isolates were not only resistant to itraconazole but also had high MIC values of voriconazole, posaconazole, and ravuconazole [16,69]. Subsequently, many studies in different countries have also identified azole-resistant isolates from patients not previously treated with these drugs [46,68,70,71].
Two main hypotheses have been raised to explain this phenomenon: (i) person-to-person transmission of resistant strains; or (ii) infection by an isolate that acquired the resistance mechanism in the environment [26,30]. The first hypothesis has little scientific support because person-to-person or person-to-environment transmissibility has been considered rare or inexistent. In the past, it was thought that transmission happens only through direct donor-to-recipient contact and infected wounds, as most of the transmission happens via aerosolized spores [30]. However, Engel and colleagues proved that A. fumigatus could be recovered from cough aerosols from CF patients [72], thus opening the possibility of patient-to-patient and patient-to-environment transmission. Further experiments, however, are still necessary to better detail the transmission of A. fumigatus by coughing. Nevertheless, aerosolized A. fumigatus conidia from patients could not explain all the resistance found in azole-naive patients due to its frequency, and the aerosolized conidia from environmental sources seem to represent a vaster and more constant source of infection [72].
2.2 Fungicide-driven resistance: Epidemiological, experimental, and field data
Many epidemiological and experimental data corroborate the theory that the DMIs used in the wood and textile industries, and especially those employed in agriculture, may select azole resistance in A. fumigatus in the environment [29,33,46,73,74] (Fig 1A). These studies presenting data supporting fungicide-driven resistance can be categorized into 4 groups: (i) those in which resistant strains were found in both patients and environment [19–24,32–34,49,68,73–81]; (ii) studies attesting cross-resistance between environmental and medical azoles in isolates from both sources [20,22,30,33,46,75,82]; (iii) investigations demonstrating that susceptible isolates could become resistant when exposed to environmental azoles [29,74,83–85]; and (iv) those proving that more resistant strains could be recovered from places or periods at which the fungicides were applied [20,86].
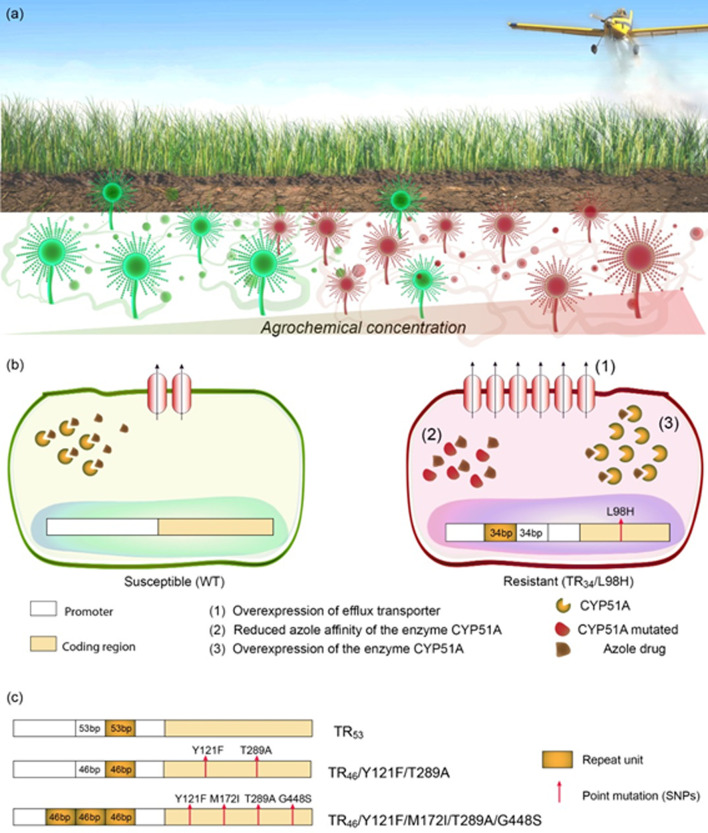
Fig 1. Fungicide exposure effects on Aspergillus fumigatus.
(a) Azole-susceptible and azole-resistant A. fumigatus can be identified in both fungicide-free and fungicide-containing soils and plant-based materials. There is an enrichment, however, of azole-resistant A. fumigatus in niches containing fungicides. (b) Azole-resistant A. fumigatus isolated from places holding fungicides may present some alterations compared to susceptible isolates that confer them cross-resistance with medical azoles, such as overexpression of efflux pumps and the azole-target enzyme, CYP51A, and CYP51A with a reduced azole affinity. The last 2 physiological changes are due to mutations in the gene cyp51A. The most common mutations are a pair of 34-bp sequence (in tandem) in the gene promoter (TR34), which lead to overexpression of cyp51A, together with a mutation that results in leucine replacement by histidine at position 98 (L98H) in the enzyme CYP51A, reducing the affinity of the enzyme to the azole drugs. (c) Other tandem repeat mutations combined or not with point mutations in the gene cyp51A conferring cross-resistance between environmental and medical azoles also can be detected in azole-resistant A. fumigatus isolated from fungicides-containing places. It is important to notice that the alterations represented correspond to amino acids and not in the DNA and that other tandem repeat mutations have already been observed in the clinical sets, but only TR34, TR46, and TR53 have been describing in environmental strains.
Classically, the studies in the Netherlands started to shed light on how environmental azole exposure could lead to cross-resistance to medical azoles [26,30]. First, they demonstrated that itraconazole-resistant A. fumigatus could be isolated from indoor environments (including patient rooms at hospitals), as well as from cultivable soils, seeds, leaves, and compost—but never from azole-naive soils. These resistant strains also posed high resistance to 2 fungicides, metconazole and tebuconazole, thus demonstrating cross-resistance between medical and environmental azoles [26]. Interestingly, 13 out of the 15 resistant strains isolated from the environment had the same mutation in the gene that encodes the azole-target enzyme (cyp51A) [26], which was identical to the isolate identified in the clinical isolates [14]. Such mutations led to a leucine replaced by histidine at position 98 (L98H) in the enzyme CYP51A, along with a pair of 34-base pair (bp) sequence (in tandem) in the gene promoter region (TR34) (TR34/L98H) [16]. The 34-bp sequence in tandem in the cyp51A promoter induces overexpression of cyp51A (about 8-fold) [16], and the point mutation hinders the interaction between the drug and the target enzyme [30] (Fig 1B). This combination of mechanisms results in a consistent itraconazole resistance and variable voriconazole, posaconazole, and isavuconazole susceptibility [30,34,68]. Frequently, TR34/L98H also confers a pan-azole resistance, both to medical and environmental azoles [26,30]. Coincidentally, the first resistant clinical isolate carrying TR34/L98H was reported infecting a patient in 1998 [14,30], just a few years after triazole fungicides had been introduced into the Netherlands [30], which suggests that this mutant could had emerged after azole fungicide contact in the field. Eventually, the TR34/L98H mutation was identified in many other European countries, and also in Asia, North and South America, Australia, and Africa [25,43].
The tandem repeat mutation was also identified in DMI-resistant phytopathogens [82,87], strongly suggesting that this is a common resistance mechanism among molds exposed to these fungicides. Penicillium digitatum, for example, contains tandem repeat mutations varying from 126 bp to 199 bp, which have been associated with DMI resistance [88,89]. However, other resistant isolates of plant pathogens, such as Pyrenopeziza brassicae, Monilinia fructicola, and Venturia inaequalis, have fragments inserted in cyp51A promotor (fragments from 65 bp to 553 bp) [90–92] instead of tandem repeat alteration. In general, both genetic variations result in overexpression of cyp51A as in A. fumigatus [82,87].
If DMIs are really the stressors leading to selection of these mutations in the environment, they should probably share similar molecular structures to clinical azoles and dock similarly to them at the azole-target enzyme in A. fumigatus. In order to address these questions, Snelders and colleagues carried out molecule alignment and docking studies using homology modeling of cyp51A. They identified 5 DMIs, propiconazole, bromuconazole, tebuconazole, epoxiconazole, and difenoconazole, which share structural molecular characteristics to medical triazoles, suggesting that they could select cross-resistance in A. fumigatus. These DMIs also assume a similar configuration when docking to the target enzyme and act against wild-type but not against multi-triazole-resistant A. fumigatus [30], further supporting the idea of DMI as a selection pressure.
Other resistance mechanisms involving promotor duplications, either combined or not with single nucleotide polymorphisms (SNPs), have been described in clinical and environmental strains (Fig 1C). TR53 (2 copies of a 53-bp sequence in tandem in cyp51A) was the second mechanism discovered [30] and thought to be restricted to clinical isolates until it was identified in resistant A. fumigatus strains isolated from flower fields in Colombia [49]. TR46/Y121F/T289A (with 2 copies of a 46-bp sequence in tandem in cyp51A, combined with 2 SNPs) (Fig 1C) was also identified in both clinical and environmental isolates [20,48,49,51,71,73,74,93,94]. This mutation provides resistance especially to voriconazole and in some cases to other medical azoles and environmental fungicides [73]. TR46/Y121F/T289A was first reported in the Netherlands [93] and subsequently in Belgium [95], India [73], Denmark [71], Germany [96], Colombia [24,49], and China [86]. The spreading of TR46/Y121F/T289A is worrisome, as it can cause high resistance to voriconazole, which is recommended as the first-line therapy for many aspergillosis [97].
Recently, another promoter-repeat mutation (a triple 46-bp promoter repeat), combined with 4 SNPs (TR463/Y121F/M172I/T289A/G448S), which leads to a pan-triazole resistance, was discovered (Fig 1C) [20]. The isolates harboring these mutations came from compost heaps containing azole fungicides and A. fumigatus clinical isolates from the Netherlands [20]. Moreover, additional tandem repeats in cyp51A gene, either combined or not with SNPs, were reported in environmental azole-resistant strains, such as TR464/Y121F/M172I/T289A/G448S [20], TR34/L98H/S297T/F495I [22,86], TR46/Y121F/M172I/T289A/G448S [19], TR92/Y121F/M172I/ T289A/G448S [19], and point mutations without tandem repeat alterations, for example, P216L [33], A284T, G448S, P222Q [74], G54R [34], G138S, Y433N, and N248K [85].
In the environment, azole-resistant isolates harboring the aforementioned genetic modifications have been isolated from several places and materials, including leaves, plant seeds, soil samples, flowerbeds, compost, hospital surroundings, and air samples [19,20,22,24,26,34,49,93,98]. In this way, some researchers have been reporting potential hotspot to isolate those mutants (especially TR34/L98H and TR46/Y121F/T289A), including soils from strawberry fields in China [22]; azole-exposed compost [20], flower bulb waste, green waste material, and wood chippings in the Netherlands [19]. These environments contain several characteristics that may facilitate not only the emergence of azole-resistant strains, but also their maintenance, and spread [19,20]. Such chacharacteristic are beyond the scopus of this review and has been recentely well discussed by Burks and colleagues [98]. Besides the fact that not all the soil or culture seems to be favorable for the emergence of resistant strains, it appears that are some DMIs more prone to select mutations in A. fumigatus and cause cross-resistance with medical azoles, such as propiconazole, bromuconazole, tebuconazole, epoxiconazole, difenoconazole, prothioconazole, and azaconazole [19,30].
Mutations in the cyp51A promoter and its open reading frame (ORF) causing overexpression and/or significant changes in the conformation of lanosterol 14α-demethylase are the primary azole resistance mechanisms in clinical and environmental A. fumigatus isolates. However, azole-resistant strains with wild-type cyp51A have been found, suggesting other resistance means unrelated to cyp51A modifications [29,81,86]. Cui and colleagues exposed azole-susceptible strains to liquid culture medium and soil treated with tebuconazole and then recovered 12 resistant isolates without any alteration in the cyp51A gene [29]. The mRNA quantitative analysis showed that some of these isolates overexpressed the genes encoding a transcription factor involved in resistance (AtrF), 2 efflux pumps (AfuMDR1, AfuMDR2), and paralogue genes for the azole-target enzyme (cyp51A and cyp51B) [29]. Another study also demonstrated that the fungicide propiconazole could select resistance by causing overexpression of cyp51A and the efflux pump genes AfuMDR3 and AfuMDR4 [85]. Overall, these data show how diverse the mechanism behind azole resistance in A. fumigatus is (Fig 1B) and that researchers should also look for alterations beyond the cyp51A gene.
The role of asexual reproduction and in vitro and in vivo resistance acquisition in A. fumigatus is already well defined and discussed in this paper. In contrast, the importance of sexual and parasexual cycles are not totally revealed. There is building evidence showing that sexual cycle of A. fumigatus plays a vital part in its resistance development, thus accounting for the genetic diversity. In this sense, Camps and colleagues verified that TR34/L98H strains could outcross with wild-type isolates with diverse genetic backgrounds [99], and Zhang and colleagues obtained TR463 mutation outcrossing 2 TR46 strains that were isolated from the same azole-containing compost, possibly through unequal crossing over between the double tandem repeats (TRs) during meiosis [20]. Sexual reproduction, which requires 2 different mating types, results in new genotypes, which may be a source of diversity within azole-resistant isolates in vitro [86]. In turn, the parasexual cycle, performed through the hyphal plasmogamy, nuclear exchange and fusion, and subsequent haploidization, plays a role in azole resistance development in diploid A. fumigatus isolated from CF patients [100]. Nevertheless, its function in environmental resistance acquisition is still unknown.
3. The other side of the story
The hypothesis that DMI could be prompting resistance in A. fumigatus is not unanimously accepted. Hollomon, for instance, stated that it was unlikely that selection for resistance occurred in soil [28]. He verified that the levels of fungicides available at the upper 10 cm of soil were very low (maximum exposure concentrations (MECs), between 0.3 and 0.4 mg/kg), especially when compared to the exposure concentrations of triazole drugs in patients (approximately 11 mg/L of blood serum) [28]. Indeed, some studies have proved that higher concentrations of fungicides are required to obtain resistant isolates from azole-contaminated soils (1.0 to 10.0 mg/kg of propiconazole and 0.5 to 5.0 mg/kg of tebuconazole) [29,85]. Nevertheless, in his critical analysis, Hollomon considered the results from a single-spray application [28]. In turn, other authors demonstrated that, for example, the level of propiconazole deposited in the soil was approximately 0.5 to 2.0 mg/kg when it was sprayed on plants 2 to 3 times, with an interval of 7 to 10 days, which is the recommended application regimen for this DMI [101]. Therefore, it is plausible to imagine that the residual DMI in the soil might be enough to select resistant isolates.
Another critical point raised by Hollomon was the lack of experimental data detecting any preexisting resistant isolates in the cultivable fields and showing how their frequency rose after the azole spraying [28]. Recently, Barber and colleagues conducted a systematic study, in which they sampled 10 agricultural sites in Germany over 3 years [102]. In their research, they consider both conventionally managed fields, where azole fungicides were applied, and those in organic farming systems, which did not use these compounds. Although they were able to isolate azole-resistant strains carrying the most common mutations, the results exhibited only a modest decrease in azole susceptibility after the growing season and azole exposure [102]. Hence, this study did not prove a direct and incontestable link between azole application in the field and increased azole resistance in A. fumigatus. Other studies have also failed in connecting fungicide usage and A. fumgiatus increasing resistance. van der Torre and colleagues recovered over 86 A. fumigatus from soil-covered root vegetables and other fresh produce in the UK, and none was azole resistant [103,104]. Similarly, Astvad did not detect resistance from any of the 113 isolates from soil in Denmark. Additionally, no pan-azole-resistant mutant (TR34 or TR46) was found from 180 strains isolated from soil samples in UK (90 from untreated wheat crops and 90 from plots sprayed with foliar fungicides), neither other 30 strains isolated from permanent grass land [104].
On the other hand, some authors showed consistent data attesting that azole-resistant isolates are significantly more common in DMI-containing places, such as sawmills that use fungicides to preserve wood compared to the ones that do not [33], soils from azole-treated agricultural sites versus urban areas [23], and compost heaps containing azoles in relation to azole-free ones [20]. Furthermore, Cao and colleagues, in a comprehensive study aiming to isolate resistant A. fumigatus from paddy soils, found that the prevalence of azole-resistant isolates is positively correlated with the residual levels of azole fungicides in the soil [86] (Fig 1A).
Overall, these data indicate that the DMI used in the agriculture and wood industry could be the main responsible for selecting resistant strains of A. fumigatus. Nonetheless, this process depends on some factors, such as the amount of azole applied and remaining in the environment (residual azole), the frequency of application, the type of azole employed, whether the azoles are used in a mixture or as an individual drug, and the interval between applications [29,33,86].
3.1 Fungicide effects on morphology, physiology, and virulence: What we know and it is missing?
Other aspects of A. fumigatus exposure to fungicides have been scarcely studied, such as its effect on virulence, metabolism, morphology, and fitness cost. Resistance mutations usually happen at a cost, as in the absence of an antifungal drug, the resistant genotype is less fit than the wild-type isolates [56]. Consequently, the mutant can disappear in the drug-free environment or become less virulent due to the fitness cost. Faria-Ramos reported that prochloraz-adapted colonies of A. fumigatus macroscopically became mostly white, losing the typical pigmentation due to nonconidiation, which must affect spreading and infectiveness [83,105]. In contrast, strains carrying cyp51A mutations, as TR34/L98H and TR46/Y121F/T289A, apparently do not have any fitness cost, as they are found dispersed worldwide in both azole-containing and azole-naive environments, coexisting with wild-type strains [105,106].
Nonetheless, little is known about the apparent absence of fitness cost in these and other fungicide-exposed mutants. Some hypotheses that still need scientific proof have been raised as follows: (i) resistant strains exhibit fitness cost in some particular environments, and the strains have only been tested under optimal laboratory conditions; (ii), TR34/L98H and TR46/Y121F/T289A could have developed a compensatory evolutionary mechanism, meaning that mutations might have counterbalanced any fitness cost by exposition to an azole-free environment; and (iii) tandem repetitions in the promoter could have been the compensatory mutation for the point mutations in cyp51A [56].
In summary, recent data have filled some gaps and reinforced the theory of fungicide-driven azole resistance in A. fumigatus. However, future research should also consider cyp51A-independent mechanisms and other fungal aspects (fitness cost, virulence, and metabolism) of azole resistance development.
4. Candida spp.
4.1 Habitat, clinical manifestations, treatment, and resistance incidence
Candida is a medically important polyphyletic fungal genus with more than 300 different species, of which 20 are potentially pathogenic to humans and other mammals [107,108]. Candida albicans, Candida glabrata, Candida parapsilosis, and Candida. tropicalis are part of human microbiota responsible for most of infections involving this fungus [109–113]. These infections, collectively called candidiasis, range from superficial mycoses and deep-seated (intra-abdominal abscesses, peritonitis, and osteomyelitis) to invasive infections (candidemia) [110,114].
Candidiasis can be treated with polyenes, echinocandins, and, especially, azoles [115]. However, the azole therapy has been presenting an increasing limitation due the number of clinical azole-resistant strains that have been isolated lately, especially among the non-albicans Candida species [111,116–118]. This can be linked to the massive use of fluconazole as prophylaxis (in patients considered at risk of infection) that could be selecting secondary resistance [119–122]. Intriguingly, although in-host resistance acquisition is the main route of azole resistance development in Candida spp., the isolation of azole-resistant strains from patients with no prior history of antifungal treatment has become common [123–128]. One of the explanations for this phenomenon may be in the environment [129].
Although the environment is not the primary reservoir for most of the Candida spp., they are also found in soils, trees, fruits, and water [129–132]. Indeed, it seems that some species are more related to specific niches, as C. tropicalis in soils, while others, such as C. albicans, can be found in multiple niches (fruits, soil, and plant matter) [129].
4.2 Fungicide-driven resistance: Epidemiological, experimental, and field data
Similar to A. fumigatus, Candida isolates from the environment may present reduced susceptibility or resistance to clinical azoles [129,131]. This fact raises the question if any environmental factors exist acting as a selecting pressure and affecting the fungus before contact with the host. Considering that Candida spp. is found in the environment and may acquire resistance in that place, the hypothesis that fungicides, especially environmental azoles, could be the stressor-selecting pressure has gained more attention.
Some observations support the link between the agricultural use of azole agrochemicals and the emergence of Candida spp. resistance [133]. First, it has been shown that the fluconazole MIC values are higher in Candida isolated from the surface of nonorganic fruits (sprayed with fungicides) compared to those collected from organic ones (without agrochemical) (16 to 64 g/L versus 1 to 8 mg/L) [134]. Secondly, C. tropicalis from the soil of Taiwan had a reduction in fluconazole susceptibility and showed genetical relatedness with clinical and less azole-susceptible strains. In addition, these isolates were more resistant to agricultural azoles, suggesting a cross-resistance between environmental and clinical azoles [131]. The cross-resistance between these substances has been also shown in C. albicans obtained from the oropharynx of HIV–positive people, which had resistance to fluconazole and high MIC to agricultural azoles (fluquinconazole, penconazole, tebuconazole, and triadimenol) [135].
Obtention of cross-resistance can also be achieved in vitro to exposing yeasts to agricultural azoles. Fluconazole and posaconazole resistance, for example, were selected in C. glabrata after a previous exposure to the fungicide prochloraz [136]. In addition, susceptible C. parapsilosis species complex became more resistant to fluconazole, itraconazole, and voriconazole after being cultured in a medium supplemented with the fungicides tetraconazole and tebuconazle, similarly as happened in the positive control using fluconazole [137,138].
The idea that fungicide-driven resistance in human pathogens has also been used to explain the origin of new multidrug resistance in Candida species, such as Candida auris [139,140]. C. auris is an emerging yeast, frequently resistant to fluconazole, and recently reported in clinical settings worldwide that may have its origin in the environment [141]. This hypothesis is supported by the new study of Arora and colleagues, who, for the first time, isolated this species from the environment. C. auris was found in salt marsh virgin habitats (areas with no human activity) and sandy beaches, which suggests that prior to its recognition as a human pathogen, it existed as an environmental fungus [141]. One isolate demonstrated to be less antifungal resistant, which could reinforce the hypothesis that drug resistance in clinical strains isolated in other parts of the world emerged from induction by fungicides [130]. However, so far, it is not known if C. auris lives in cultivable soils or in plant materials, where they could be in contact with fungicides. Even though, due its multidrug resistance, it has been proposed that agrochemical exposure may be related to the C. auris resistance [139,140]. In fact, distribution maps of azole fungicides usage within the US matched the reported scattering of C. auris [142]. More experiments and field data are necessary to test such hypothesis.
Primary and secondary azole resistance mechanisms are well studied and understood in Candida spp. Several mechanisms have been described, being the most important the overexpression of ERG11 and efflux pumps (MDR, CDRs) genes and alterations in ERG11p [111]. Coincidently, agricultural azoles select cross-resistance by using the exact mechanisms underlying fluconazole resistance (Fig 2A) [137,138]. Prochloraz induces the up-regulation of the ATP binding cassette multidrug transporter genes (PDH1) and the transcription factor that may regulate them (PDR1) but seems to not select any important mutation in ERG11 [136]. Alike, Rocha and colleagues demonstrated that C. parapsilosis exposed to tetraconazole and with cross-resistance to clinical azoles increased drug efflux through pumps, such as MDR1p and CDRp [143] (Fig 2A). Lately, Brilhante and colleagues showed that tebuconazole- and tetraconazole-exposed C. parapsilosis species complex strains had cross-resistance due to overexpression of ERG11 but not of efflux pump genes [137]. Also, sterol composition in C. parapsilosis (sensu stricto) and Candida orthopsilosis tend to be different after fungicide exposure [137], what may be related to azole resistance if it supports the membrane integrity. Altogether, these data show the diverse azole mechanisms that can be selected by fungicides (Fig 2A).
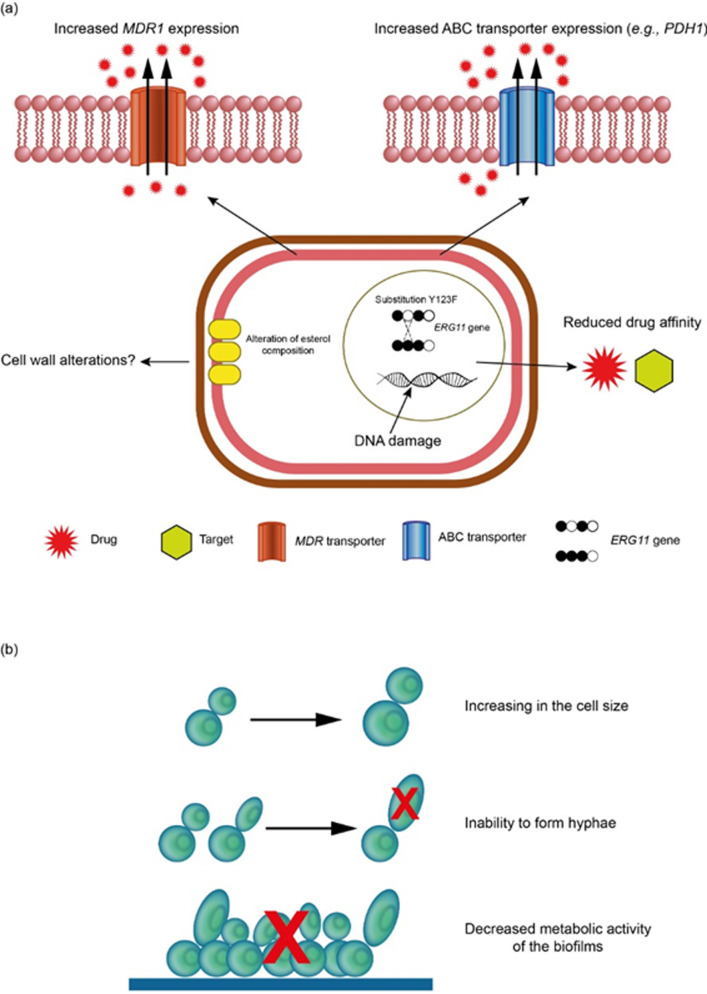
Fig 2. Cellular alterations induced by fungicides exposure in Candida spp.
(a) Mechanisms of resistance induced by fungicides in Candida spp. Azole resistance triggered by fungicide exposure shows up-regulation of ABC multidrug transporters, such as PDH1. In addition, amino acid substitution Y132F in the erg11 gene can occur, suggesting that this selected resistance is mainly associated with increased drug efflux through ATP-dependent pumps. Sterol composition and DNA damage are also consequences of fungicide exposure. (b) Alterations in morphophysiology and virulence of Candida spp. caused by fungicides. Candida spp. exposure to fungicides showed an expanded cell size, inability to form hyphae, and significantly altered time of adhesion and decreased the metabolic activity of biofilms. ABC, ATP-binding cassette.
4.3 Fungicide effects on morphology, physiology, and virulence: What we know and it is missing?
In addition to cross-resistance, agrochemicals can affect the morphophysiology and virulence of Candida spp. (Fig 2B). Tebuconazole altered the metabolism of C. parapsilosis (sensu stricto) at the time of adhesion and decreased the metabolic activity of biofilms [137]. Species of azole-tolerant biofilm-producing non-wild-type C. albicans were found colonizing agricultural soils cultivated with azole fungicides [144]. The influence of fungicides on the development phases of Candida spp. may mimic the state of an in vivo infection of yeast colonies occurring in a natural environment. Specifically, C. albicans and Candida pulcherrima showed an expanded cell size after exposure to different concentrations of Tango Star (epoxiconazole and fenpropimorph), and C. albicans was not able to form hyphae (Fig 2B). Tango Star, which inhibits ergosterol synthesis, may contribute to depleting the intracellular pool of ergosterol while blocking the transition of blastospores during hyphae formation [145]. The overall response to agrochemical stress in C. glabrata and to a lesser extent in C. tropicalis was the selection of subpopulations with increased fatty acid unsaturation rates [145]. Treatments with Tango Star also aggravated the total DNA damage in C. pulcherrima cells (Fig 2B) [146].
In summary, there is some field and experimental data demonstrating that fungicides may be inducing resistance to clinical azoles in Candida spp. mainly through activation of overexpression of efflux pumps and ERG11 genes. They are also affecting its morphophysiology; however, it is unclear, if those alterations impact Candida virulence. Based on that, the use of azoles in human medicine and the environment requires surveillance and restrictions to minimize the risk of selecting azole resistance in Candida.
5. Cryptococcus spp.
5.1 Habitat, clinical manifestations, treatment, and resistance incidence
Cryptococcus neoformans and Cryptococcus gattii (also called C. neoformans and C. gattii complex) are encapsulated basidiomycetous yeasts and the most medically relevant species within the genus Cryptococcus, causing infections called cryptococcosis [147]. Although C. neoformans has been typically found in association with birds, isolated from their nests and excrements [147–149], both species live predominately in niches related to plant material, such as bark and trunk cavities of trees, fruits, underlaying soil, and decaying wood. They can be isolated from trees of Eucalyptus spp. (eucalyptus), Olea (olive trees), Ceratonia (carob trees), Pinus, Aesculus, and several others [149–151]. From the environment, patients inhale basidiospores or desiccated yeasts. Once the propagules reach the lungs, they might develop, multiplicate, and disseminate to other organs, especially to the central nervous system [148,152–154].
The treatment for cryptococcosis is performed with amphotericin B combined with fluconazole and/or 5-flucytosine [148,155–157]. Although resistance is not considered an issue in Cryptococcus spp. [157], secondary resistance to azoles has been recurrently reported [149,158–164]. The observations include a study showing that the MIC50 and MIC90 values of fluconazole have increased 2-fold in a comparison between C. neoformans isolated in 2017 and strains obtained 10 years earlier in Africa [159], and another that reported that the mean MIC50 of fluconazole for clinical cryptococcal isolates increased 2-fold over time, from 4 μg/mL in 2000 to 2012 to 8 μg/mL in 2014 to 2018 [165].
5.2 Fungicide-driven resistance: Epidemiological, experimental, and field data
Differently from A. fumigatus and Candida spp., C. gattii and C. neoformans do not usually occur in crops, flower beds, and commercial plant-based products. They are found in association with Eucalyptus and other trees, especially in trunk hollows [151,166,167]. Thus, it is unusual to link these species with fungicide exposure in the environment, as these chemicals are often employed to preserve and treat plant diseases of commercial relevance [26,28,33]. Nonetheless, it is worth remarking that Eucalyptus and other trees are valuable assets for the wood industry, which also uses fungicides for wood preservation [33]. Moreover, Chowdhary and colleagues isolated azole-resistant A. fumigatus from trunk hollows in Tanzania [75], the same niche of pathogenic Cryptococcus [151,166]. On that account, Del Poeta and Casadevall hypothesized that fungicides could also be driving cryptococcus virulence and resistance evolution [168].
Trying to prove this hypothesis, Bastos and colleagues evaluated the effect of the environmental antifungals tebuconazole and pyraclostrobin (a strobilurin that acts as mitochondrial respiration inhibitor) on C. gattii and C. neoformans strains. The exposure to agrochemicals caused cross-resistance to medical azoles, remarkably fluconazole. The cross-resistance was permanent in some exposed strains, lasting even after several cultures in agrochemical-free media, and temporary in others, then returning to the original susceptibility when the contact with the fungicide ceased [31,169]. Other studies using a similar methodology and the same strains also demonstrated that exposure to the fungicide benomyl (mitotic inhibitor) and the herbicides flumioxazin (inhibits protoporphyrinogen oxidase, an enzyme that is important for the synthesis of chlorophyll), isoxaflutole (inhibits the 4-hydroxyphenyl pyruvate dioxygenase), and pendimethalin (inhibits root and shoot growth by preventing plant cell division and elongation) reduced the susceptibility to agrochemicals and clinical antifungals (https://www.epa.gov/caddis-vol2/caddis-volume-2-sources-stressors-responses-herbicides) [170,171]. Although herbicides have different mechanisms of action compared to fungicides, they may activate pathways that increase fungal fitness, which probably alter the way that fungal cells behave in the presence of clinical antifungals. Cross-resistance to fluconazole was also verified in an in vivo murine model for cryptococcosis. The drug proved ineffective in controlling the infection caused by cells previously adapted to tebuconazole, pyraclostrobin, and benomyl, compared to cells nonexposed to fungicides [31,169,171].
Cryptococcus spp. usually become more tolerant to azoles through 3 mechanisms: (i) enhanced expression of ERG11p; (ii) mutation in the ERG11 gene; and (iii) overexpression of efflux pumps [155,172–175]. The molecular mechanism behind cross-resistance selected by environmental azoles, strobilurins, and benzimidazoles, however, has not been fully uncovered. Nonetheless, it seems that fungicide exposure selects mutations in some strains whose resistance strengthens permanently [31,169,171]. It is still unclear the role of mutations in these phenotypes. Epigenetic changes cannot be ruled out since in C. neoformans, for example, they can remain for a long time in the absence of a stressor agent [176]. On the other hand, the expression analysis of ERG11 and the efflux pump genes AFR1, PDR11, and MDR11 revealed that exposure to tebuconazole, pyraclostrobin, and benomyl boosted their expression in C. gattii and C. neoformans (Fig 3A) [31,169,171]. Besides, Carneiro and colleagues performed a rhodamine 6G assay and observed that benomyl-exposed cells pumped out the dye more than the nonexposed control, thus reinforcing this mechanism as a probable factor in the cross-resistance to medical azoles [171].
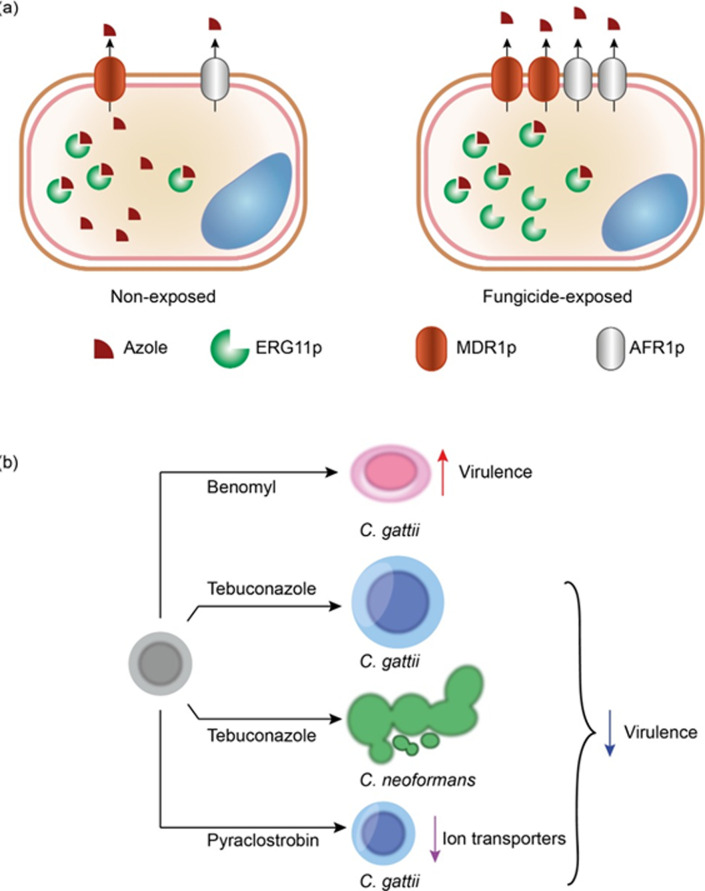
Fig 3. Fungicide exposure effects on Cryptococcus spp.
(a) Exposure to fungicides can select cross-resistance to clinical azoles especially through overexpression of efflux pumps (MDR11 and AFR1) and ERG11 genes, the azole target. (b) Different fungicides also can induce important alterations in the cell morphophysiology of Cryptococcus cells that may be related to virulence.
These data demonstrate that not only DMIs structurally similar to medical azoles select cross-resistance to clinical drugs in Cryptococcus spp., but also other fungicides with different targets, and herbicides [31,169–171]. However, a question remains: If fungicide-driven resistance occurs in Cryptococcus spp., which are widely spread over natural areas, why has such a small number of azole-resistant Cryptococcus been isolated from the environment? The answer may be related to specific conditions that apparently select cross-resistance between fungicides and clinical azoles, such as the temperature [31,169].
The role of temperature in the antifungal tolerance process becomes evident when analyzing cross-resistance. In this case, Bastos and colleagues observed that exposing C. gattii and C. neoformans strains to fungicides at 30°C increased the number of colonies that became more resistant to fungicides, compared to when this process was executed at 37°C [31,169]. In addition, the temperature influenced the MIC of azoles used as clinical drugs and fungicides. When the MIC of drugs was determined at 37°C using colonies previously exposed to fungicides at 30°C, the MIC values was lower than when the experiment was carried out at 30°C [31,169]. Another study recently confirmed the connection between resistance acquisition and lower temperature as they proved that adaptation in drugs as fluconazole and amphotericin B at lower temperatures selects resistance to these drugs in C. neoformans, which does not happen at a higher temperature [177]. Overall, temperature probably influences the survival and adaptation of Cryptococcus spp. in the presence of fungicides and clinical drugs, as well as the manifestation of this resistance in a host with high body temperature. It suggests that if the resistance acquisition happens in the environment due to fungicide, Cryptococcus may not express it in vivo [31,169].
5.3 Fungicide effects on morphology, physiology, and virulence: What we know and it is missing?
It has been proved that fungicides also affect the morphology and virulence of Cryptococcus spp [31,169]. As in other fungi, the cell morphology of Cryptococcus is crucial to resist environmental stresses and for virulence. Remarkably, the capsule, which is very characteristic of this genus, is deemed as the primary virulence factor [178]. In general, cells with a large capsule tend to be more virulent than those with a small one or acapsular mutants [179]. The surface–volume (S/V) ratio of the yeast is another factor that plays an essential role in the pathogenesis of these species. Yeasts with a high S/V also appear to be more virulent since they replicate fast and migrate to the CNS to a great extent [171,180].
When C. gattii R265 was exposed to tebuconazole, the cell body expanded (decreased S/V), compared to nonexposed controls (Fig 3B). This phenomenon coincided with a reduced virulence of these cells in the murine model for cryptococcosis, achieving an avirulent status since they were unable to kill any mice [31]. Tebuconazole-exposed C. neoformans H99 [31] and pyraclostrobin-exposed C. gattii R265 [169] were also less virulent than non-fungicide-exposed cells, which demonstrated that there is a fitness cost of being more resistant to drugs. In those cases, the decrease in virulence was related to pseudohyphae formation in tebuconazole-adapted C. neoformans H99 [31] and a reduced expression of ion transporters in pyraclostrobin-exposed C. gattii (Fig 3B) [169]. Conversely, C. gattii L24/01, previously nonvirulent, became hypervirulent after exposure to benomyl (Fig 3B). It rapidly translocates to the brain, survives and multiply inside macrophages, and kills mice. This phenotype was associated with the inscrease in the S/V ratio, and an improved replicative capacity, both in vitro and inside phagocytes [171]. Together, these results show how complex could be the fungicide exposure effects on C. neoformans and C. gattii morphophysiology and virulence, besides its effect on antifungal resistance.
In summary, these data indicate that fungicide exposure affects the resistance, morphology, and virulence of Cryptococcus spp. in a fungicide- and strain-dependent manner. There is also a fitness cost translated as a decrease or loss of virulence in some strains. In contrast, others can become surprisingly more adapted to the host, resulting in a virulence boost.
6. Conclusions and perspectives
Several studies have demonstrated that there is an environmental route driving resistance to medical azoles in A. fumigatus due to fungicide use, especially the use of DMIs. Field and laboratory data revealed that resistant strains found in patients and in the environment could develop cross-resistance to environmental and medical azoles via the same mechanism. Likewise, susceptible isolates can become resistant when exposed to environmental azoles. However, the existing literature is not unanimous on whether or not resistant A. fumigatus strains hold predominance in azole-contaminated or fungicide-sprayed soils [20,86,102]. One theory rejects the possibility of spontaneous emergence of azole resistance in the soil by suggesting that the phenomenon would be triggered by crop waste gathered up in the surroundings. This hypothesis explains the findings of some authors who observed the prevalence of resistant strains in compost material [20] but not in arable soils [104]. In fact, A. fumigatus is commonly found in compost piles, plant material in decomposition, and wastewater from urban areas [98]. Thus, studies that did not detect resistant strains eventually assume the soil as hotspot of resistance emergence, when it might actually be importing this condition.
Further studies should clarify why there is an enrichement of resistant isolates in some places containing azoles but not in others. In addition, they should provide a better understanding of the roles of the fungicide application regimen, the accumulation of these substances in the soil, and their influence on resistance development. Other unanswered questions, such as the importance of sexual and parasexual cycles in the process of resistance acquisition, the reasons why TR34/L98H and other mutants do not seem to present fitness costs, and how fungicide exposure affects the physiology and virulence of A. fumigatus strains should also be adressed.
Despite scarce, the existing evidence of an environmental route triggering resistance in pathogenic yeasts (such as Candida and Cryptococcus) should not be neglected. Most of the current data are based on in vitro studies pointing out that agrochemicals could select cross-resistance to medical azoles. Nonetheless, comprehensive fieldwork comparing the isolation of resistant strains from azole-containing environments versus azole-free ones is still necessary. The studies must also focus on revealing the molecular mechanisms of resistance selected by fungicides and how extrinsic and intrinsic conditions interfere with this phenomenon.
One of the main problems with the environmental drug acquisition is that measures to prevent and control the emergence of resistant strains in clinical practice, including the rational use of drugs, have overall proved to be inefficient, which reinforces the need for new perspectives. The one-health approach has been successful in dealing with antibiotic resistance, as indiscriminate use of these drugs in veterinary medicine and especially as growth promoters for animals has been perceived as a source of acquired bacterial resistance. Thus, there is a great international effort and pressure for the rational use of antibiotics in animal medicine and restriction of their use as growth promoters [181]. In this case, antifungal resistance should also be looked after since the origin of this problem could be in the environment outside the hospital.
Fungicides and other pesticides are indivisible parts of current food production and supply, but assuring human health is paramount, despite productivity claims. Therefore, the sensible use of fungicides with the potential for selecting cross-resistance with clinical drugs is a top priority in future discussions.
Acknowledgments
We thank to Florent Morio and Debora Castelo-Branco for reading this manuscript and providing critical comments.
Funding Statement
RWB is supported by the grant 2017/19821-5, São Paulo Research Foundation (FAPESP). GHG research is supported by FAPESP (grants 2016/07870-9 and 2018/10962-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. DAS is a research fellow of the CNPq (303762/2020-9) and his research is supported by Minas Gerais Research Foundation (FAMIG) (Grant PPM-00061-18). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden Killers: Human Fungal Infections. Sci Transl Med. 2012;4. doi: 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 2.Perfect JR. The antifungal pipeline: a reality check. Nat Rev Drug Discov. 2017/05/13. 2017;16:603–616. doi: 10.1038/nrd.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman J, Krysan DJ. Drug resistance and tolerance in fungi. Nat Rev Microbiol. 2020;18. doi: 10.1038/s41579-019-0322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol. 2014;10:400–6. doi: 10.1038/nchembio.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo X, Zhang J, Li X, Xiao E, Lange JD, Rienstra CM, et al. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Central Science. 2021;7. doi: 10.1021/acscentsci.1c00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Ami R, Kontoyiannis DP. Resistance to Antifungal Drugs. Infect Dis Clin North Am. 2021;35. doi: 10.1016/j.idc.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 7.Nett JE, Andes DR. Antifungal Agents. Infect Dis Clin North Am. 2016;30. doi: 10.1016/j.idc.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 8.Akins RA. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med Mycol. 2005;43. doi: 10.1080/13693780500138971 [DOI] [PubMed] [Google Scholar]
- 9.Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect. 2019;25. doi: 10.1016/j.cmi.2018.11.027 [DOI] [PubMed] [Google Scholar]
- 10.Steinmann J, Hamprecht A, Vehreschild MJGT, Cornely OA, Buchheidt D, Spiess B, et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70. doi: 10.1093/jac/dku566 [DOI] [PubMed] [Google Scholar]
- 11.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, et al. Frequency and Evolution of Azole Resistance in Aspergillus fumigatus Associated with Treatment Failure1. Emerg Infect Dis. 2009;15. doi: 10.3201/eid1507.090043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcazar-Fuoli L, Mellado E. Current status of antifungal resistance and its impact on clinical practice. Br J Haematol. 2014;166. doi: 10.1111/bjh.12896 [DOI] [PubMed] [Google Scholar]
- 13.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360. doi: 10.1126/science.aap7999 [DOI] [PubMed] [Google Scholar]
- 14.Snelders E, van der Lee HAL, Kuijpers J, Rijs AJMM, Varga J, Samson RA, et al. Emergence of Azole Resistance in Aspergillus fumigatus and Spread of a Single Resistance Mechanism. PLoS Med. 2008;5. doi: 10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, et al. Clonal Expansion and Emergence of Environmental Multiple-Triazole-Resistant Aspergillus fumigatus Strains Carrying the TR34/L98H Mutations in the cyp51A Gene in India. PLoS ONE. 2012;7. doi: 10.1371/journal.pone.0052871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellado E, Garcia-Effron G, Alcázar-Fuoli L, Melchers WJG, Verweij PE, Cuenca-Estrella M, et al. A New Aspergillus fumigatus Resistance Mechanism Conferring In Vitro Cross-Resistance to Azole Antifungals Involves a Combination of cyp51A Alterations. Antimicrob Agents Chemother. 2007;51. doi: 10.1128/AAC.01092-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Linden JWM, Snelders E, Kampinga GA, Rijnders BJA, Mattsson E, Debets-Ossenkopp YJ, et al. Clinical Implications of Azole Resistance in Aspergillus fumigatus, the Netherlands, 2007–2009. Emerg Infect Dis. 2011;17. doi: 10.3201/eid1710.110226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhary A, Sharma C, Meis JF. Azole-Resistant Aspergillosis: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis. 2017;216. doi: 10.1093/infdis/jix210 [DOI] [PubMed] [Google Scholar]
- 19.Schoustra SE, Debets AJM, Rijs AJMM, Zhang J, Snelders E, Leendertse PC, et al. Environmental hotspots for azole resistance selection of aspergillus fumigatus, the netherlands. Emerg Infect Dis. 2019;25:1347–53. doi: 10.3201/eid2507.181625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Snelders E, Zwaan BJ, Schoustra SE, Meis JF, van Dijk K, et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio. 2017;8. doi: 10.1128/mBio.00791-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst SF, Berkow EL, Stevenson KL, Litvintseva AP, Lockhart SR. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J Antimicrob Chemother. 2017;72:2443–6. doi: 10.1093/jac/dkx168 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Dong F, Zhao J, Fan H, Qin C, Li R, et al. High azole resistance in aspergillus fumigatus isolates from Strawberry Fields, China, 2018. Emerg Infect Dis. 2020;26:81–9. doi: 10.3201/eid2601.190885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bromley MJ, van Muijlwijk G, Fraczek MG, Robson G, Verweij PE, Denning DW, et al. Occurrence of azole-resistant species of Aspergillus in the UK environment. J Glob Antimicrob Resist. 2014;2:276–9. doi: 10.1016/j.jgar.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Moreno C, Lavergne RA, Hagen F, Morio F, Meis JF, le Pape P. Fungicide-driven alterations in azole-resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia. 2019;111:217–24. doi: 10.1080/00275514.2018.1557796 [DOI] [PubMed] [Google Scholar]
- 25.Resendiz-Sharpe A, Dewaele K, Merckx R, Bustamante B, Vega-Gomez MC, Rolon M, et al. Triazole-Resistance in Environmental Aspergillus fumigatus in Latin American and African Countries. J Fungi. 2021;7. doi: 10.3390/jof7040292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelders E, Huis in’t Veld RAG, Rijs AJMM, Kema GHJ, Melchers WJG, Verweij PE. Possible Environmental Origin of Resistance of Aspergillus fumigatus to Medical Triazoles. Appl Environ Microbiol. 2009;75. doi: 10.1128/AEM.02217-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis. 2014;33:919–26. doi: 10.1007/s10096-013-2027-1 [DOI] [PubMed] [Google Scholar]
- 28.Hollomon D. Does agricultural use of azole fungicides contribute to resistance in the human pathogen Aspergillus fumigatus? Pest Manag Sci. 2017;73:1987–93. doi: 10.1002/ps.4607 [DOI] [PubMed] [Google Scholar]
- 29.Cui N, He Y, Yao S, Zhang H, Ren J, Fang H, et al. Tebuconazole induces triazole-resistance in Aspergillus fumigatus in liquid medium and soil. Sci Total Environ. 2019;648:1237–43. doi: 10.1016/j.scitotenv.2018.08.247 [DOI] [PubMed] [Google Scholar]
- 30.Snelders E, Camps SMT, Karawajczyk A, Schaftenaar G, Kema GHJ, van der Lee HA, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE. 2012;7. doi: 10.1371/journal.pone.0031801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastos RW, Carneiro HCS, Oliveira LVN, Rocha KM, Freitas GJC, Costa MC, et al. Environmental triazole induces cross-resistance to clinical drugs and affects morphophysiology and virulence of Cryptococcus gattii and C. neoformans. Antimicrob Agents Chemother. 2018;62. doi: 10.1128/AAC.01179-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nabili M, Shokohi T, Moazeni M, Khodavaisy S, Aliyali M, Badiee P, et al. High prevalence of clinical and environmental triazole-resistant Aspergillus fumigatus in Iran: Is it a challenging issue? J Med Microbiol. 2016;65:468–75. doi: 10.1099/jmm.0.000255 [DOI] [PubMed] [Google Scholar]
- 33.Jeanvoine A, Rocchi S, Reboux G, Crini N, Crini G, Millon L. Azole-resistant Aspergillus fumigatus in sawmills of Eastern France. J Appl Microbiol. 2017;123:172–84. doi: 10.1111/jam.13488 [DOI] [PubMed] [Google Scholar]
- 34.Riat A, Plojoux J, Gindro K, Schrenzel J, Sanglard D. Azole resistance of environmental and clinical Aspergillus fumigatus isolates from Switzerland. Antimicrob Agents Chemother. 2018;62. doi: 10.1128/AAC.02088-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaezi A, Fakhim H, Javidnia J, Khodavaisy S, Abtahian Z, Vojoodi M, et al. Pesticide behavior in paddy fields and development of azole-resistant Aspergillus fumigatus: Should we be concerned? J Mycol Med. 2018;28:59–64. doi: 10.1016/j.mycmed.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 36.Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, et al. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. J Microbial Biotechnol. 2017;10. doi: 10.1111/1751-7915.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latgé J-P, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33. doi: 10.1128/CMR.00140-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. Oxford University Press; 2016. p. e1–e60. doi: 10.1093/cid/ciw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Cantero A, López-Fernández L, Guarro J, Capilla J. Azole resistance mechanisms in Aspergillus: update and recent advances. Int J Antimicrob Agents. Elsevier B.V.; 2020. doi: 10.1016/j.ijantimicag.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 40.Jenks J, Hoenigl M. Treatment of Aspergillosis. J Fungi. 2018;4. doi: 10.3390/jof4030098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med. W.B. Saunders Ltd; 2018. p. 121–131. doi: 10.1016/j.rmed.2018.06.029 [DOI] [PubMed] [Google Scholar]
- 42.Denning DW, Venkateswarlu K, Oakley KL, Anderson MJ, Manning NJ, Stevens DA, et al. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41. doi: 10.1128/AAC.41.6.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharpe AR, Lagrou K, Meis JF, Chowdhary A, Lockhart SR, Verweij PE. Triazole resistance surveillance in Aspergillus fumigatus. Med Mycol. Oxford University Press; 2018. p. S83–S92. doi: 10.1093/mmy/myx144 [DOI] [PubMed] [Google Scholar]
- 44.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, et al. Prospective Multicenter International Surveillance of Azole Resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21. doi: 10.3201/eid2106.140717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchido Y, Tanaka M, Nakano S, Yamamoto M, Matsumura Y, Nagao M. Prospective multicenter surveillance of clinically isolated Aspergillus species revealed azole-resistant Aspergillus fumigatus isolates with TR34/L98H mutation in the Kyoto and Shiga regions of Japan. Med Mycol. 2019;57:997–1003. doi: 10.1093/mmy/myz003 [DOI] [PubMed] [Google Scholar]
- 46.Wu CJ, Wang HC, Lee JC, Lo HJ, Dai CT, Chou PH, et al. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses. 2015;58:544–9. doi: 10.1111/myc.12354 [DOI] [PubMed] [Google Scholar]
- 47.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses. 2015;58:350–5. doi: 10.1111/myc.12324 [DOI] [PubMed] [Google Scholar]
- 48.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, et al. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in aspergillus fumigatus isolates in the United States. J Clin Microbiol. 2016;54:168–71. doi: 10.1128/JCM.02478-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Moreno C, Lavergne RA, Hagen F, Morio F, Meis JF, le Pape P. Azole-resistant Aspergillus fumigatus harboring TR 34 /L98H, TR 46 /Y121F/T289A and TR 53 mutations related to flower fields in Colombia. Sci Rep. 2017;7. doi: 10.1038/s41598-017-00035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontes L, Augusto C, Beraquet G, Arai T, Pigolli GL, Lyra L, et al. Aspergillus fumigatus Clinical Isolates Carrying CYP51A with TR34/L98H/S297T/F495I Substitutions Detected after Four-Year Retrospective Azole Resistance Screening in Brazil. 2020. doi: 10.1128/AAC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isla G, Leonardelli F, Tiraboschi IN, Refojo N, Hevia A, Vivot W, et al. First Clinical Isolation of an Azole-Resistant Aspergillus fumigatus Isolate Harboring a TR46 Y121F T289A Mutation in South America. 2018. Available from: http://aac.asm.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macedo D, Leonardelli F, Gamarra S, Garcia-Effron G. Emergence of Triazole Resistance in Aspergillus spp. in Latin America. Curr Fungal Infect Rep. 2021. doi: 10.1007/s12281-021-00418-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonçalves SS. Global aspects of triazole resistance in Aspergillus fumigatus with focus on latin American Countries. J Fungi (Basel). 2017. doi: 10.3390/jof3010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh A, Sharma B, Mahto KK, Meis JF, Chowdhary A. High-frequency direct detection of triazole resistance in aspergillus fumigatus from patients with chronic pulmonary fungal diseases in India. J Fungi (Basel). 2020;6. doi: 10.3390/jof6020067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. Lancet Publishing Group; 2009. p. 789–795. doi: 10.1016/S1473-3099(09)70265-8 [DOI] [PubMed] [Google Scholar]
- 56.Verweij PE, Zhang J, Debets AJM, Meis JF, van de Veerdonk FL, Schoustra SE, et al. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect Dis. Lancet Publishing Group; 2016. p. e251–e260. doi: 10.1016/S1473-3099(16)30138-4 [DOI] [PubMed] [Google Scholar]
- 57.Guegan H, Prat E, Robert-Gangneux F, Gangneux JP. Azole Resistance in Aspergillus fumigatus: A Five-Year Follow Up Experience in a Tertiary Hospital With a Special Focus on Cystic Fibrosis. Front Cell Infect Microbiol. 2021;10. doi: 10.3389/fcimb.2020.613774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Li H, Li R, Bu D, Wan Z. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother. 2005;55:31–7. doi: 10.1093/jac/dkh507 [DOI] [PubMed] [Google Scholar]
- 59.Denning DW, Venkateswarlu K, Oakley KL, Anderson MJ, Manning NJ, Stevens DA, et al. Itraconazole Resistance in Aspergillus fumigatus. 1997. Available from: http://aac.asm.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tashiro M, Izumikawa K, Hirano K, Ide S, Mihara T, Hosogaya N, et al. Correlation between Triazole Treatment History and Susceptibility in Clinically Isolated Aspergillus fumigatus. Antimicrob Agents Chemother. 2012;56. doi: 10.1128/AAC.01449-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camps SMT, van der Linden JWM, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, et al. Rapid Induction of Multiple Resistance Mechanisms in Aspergillus fumigatus during Azole Therapy: a Case Study and Review of the Literature. Antimicrob Agents Chemother. 2012;56. doi: 10.1128/AAC.01449-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagiwara D, Takahashi H, Watanabe A, Takahashi-Nakaguchi A, Kawamoto S, Kamei K, et al. Whole-Genome Comparison of Aspergillus fumigatus Strains Serially Isolated from Patients with Aspergillosis. J Clin Microbiol. 2014;52. doi: 10.1128/JCM.01105-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howard SJ, Pasqualotto AC, Anderson MJ, Leatherbarrow H, Albarrag AM, Harrison E, et al. Major variations in Aspergillus fumigatus arising within aspergillomas in chronic pulmonary aspergillosis. Mycoses. 2013;56. doi: 10.1111/myc.12047 [DOI] [PubMed] [Google Scholar]
- 64.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol. 2015;06. doi: 10.3389/fmicb.2015.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dannaoui E. Acquired itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2001;47. doi: 10.1093/jac/48.1.47 [DOI] [PubMed] [Google Scholar]
- 66.Hare RK, Gertsen JB, Astvad KMT, Degn KB, Løkke A, Stegger M, et al. In vivo selection of a unique tandem repeat mediated azole resistance mechanism (TR 120) in aspergillus fumigatus cyp51A, Denmark. Emerg Infect Dis. 2019;25: 577–580. doi: 10.3201/eid2503.180297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Møller N, Khan H, et al. Development of azole resistance in aspergillus fumigatus during azole therapy associated with change in virulence. PLoS ONE. 2010;5. doi: 10.1371/journal.pone.0010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother. 2012;67:362–6. doi: 10.1093/jac/dkr443 [DOI] [PubMed] [Google Scholar]
- 69.Verweij PE, Mellado E, Melchers WJG. Multiple-Triazole–Resistant Aspergillosis. N Engl J Med. 2007;356. doi: 10.1056/NEJMc061720 [DOI] [PubMed] [Google Scholar]
- 70.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother. 2011;55:4465–8. doi: 10.1128/AAC.00185-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Astvad KMT, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, et al. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother. 2014;58:5096–101. doi: 10.1128/AAC.02855-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engel TGP, Erren E, van den Driessche KSJ, Melchers WJG, Reijers MH, Merkus P, et al. Aerosol Transmission of Aspergillus fumigatus in Cystic Fibrosis Patients in the Netherlands. Emerg Infect Dis. 2019;25. doi: 10.3201/eid2504.181110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Azole-resistant aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother. 2014:555–557. doi: 10.1093/jac/dkt397 [DOI] [PubMed] [Google Scholar]
- 74.Ren J, Jin X, Zhang Q, Zheng Y, Lin D, Yu Y. Fungicides induced triazole-resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J Hazard Mater. 2017;326:54–60. doi: 10.1016/j.jhazmat.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 75.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, et al. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother. 2014;69:2979–83. doi: 10.1093/jac/dku259 [DOI] [PubMed] [Google Scholar]
- 76.Prigitano A, Esposto MC, Grancini A, Biffi A, Innocenti P, Cavanna C, et al. Azole resistance in Aspergillus isolates by different types of patients and correlation with environment—An Italian prospective multicentre study (ARiA study). Mycoses. 2021;64:528–36. doi: 10.1111/myc.13241 [DOI] [PubMed] [Google Scholar]
- 77.Rocchi S, Daguindau E, Grenouillet F, Deconinck E, Bellanger AP, Garcia-Hermoso D, et al. Azole-resistant Aspergillus fumigatus isolate with the TR34/L98H mutation in both a fungicide-sprayed field and the lung of a hematopoietic stem cell transplant recipient with invasive aspergillosis. J Clin Microbiol. 2014;52:1724–6. doi: 10.1128/JCM.03182-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharma C, Hagen F, Moroti R, Meis JF, Chowdhary A. Triazole-resistant Aspergillus fumigatus harbouring G54 mutation: Is it de novo or environmentally acquired? J Glob Antimicrob Resist. 2015;3:69–74. doi: 10.1016/j.jgar.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 79.Dunne K, Hagen F, Pomeroy N, Meis JF, Rogers TR. Intercountry Transfer of Triazole-Resistant Aspergillus fumigatus on Plant Bulbs. Clin Infect Dis. 2017;65:147–9. doi: 10.1093/cid/cix257 [DOI] [PubMed] [Google Scholar]
- 80.Tsitsopoulou A, Posso R, Vale L, Bebb S, Johnson E, White PL. Determination of the prevalence of triazole resistance in environmental Aspergillus fumigatus strains isolated in South Wales, UK. Front Microbiol. 2018;9. doi: 10.3389/fmicb.2018.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahangarkani F, Badali H, Abbasi K, Nabili M, Khodavaisy S, de Groot T, et al. Clonal expansion of environmental triazole resistant aspergillus fumigatus in Iran. J Fungi (Basel). 2020;6:1–9. doi: 10.3390/jof6040199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia-Rubio R, Gonzalez-Jimenez I, Lucio J, Mellado E. Aspergillus fumigatus Cross-Resistance between Clinical and Demethylase Inhibitor Azole Drugs. Appl Environ Microbiol. 2021;87:1–12. doi: 10.1128/AEM.02539-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faria-Ramos I, Farinha S, Neves-Maia J, Tavares PR, Miranda IM, Estevinho LM, et al. Development of cross-resistance by Aspergillus fumigatus to clinical azoles following exposure to prochloraz, an agricultural azole. BMC Microbiol. 2014;14. doi: 10.1186/1471-2180-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, van den Heuvel J, Debets AJM, Verweij PE, Melchers WJG, Zwaan BJ, et al. Evolution of cross-resistance to medical triazoles in Aspergillus fumigatus through selection pressure of environmental fungicides. Proc R Soc B Biol Sci. 2017;284. doi: 10.1098/rspb.2017.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao D, Yao S, Zhang H, Wang S, Jin X, Lin D, et al. Mutation in cyp51A and high expression of efflux pump gene of Aspergillus fumigatus induced by propiconazole in liquid medium and soil. Environ Pollut. 2020;256. doi: 10.1016/j.envpol.2019.113385 [DOI] [PubMed] [Google Scholar]
- 86.Cao D, Wang F, Yu S, Dong S, Wu R, Cui N, et al. Prevalence of azole-resistant aspergillus fumigatus is highly associated with azole fungicide residues in the fields. Environ Sci Technol. 2021;55:3041–9. doi: 10.1021/acs.est.0c03958 [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Rubio R, Cuenca-Estrella M, Mellado E. Triazole Resistance in Aspergillus Species: An Emerging Problem. Drugs. 2017;77. doi: 10.1007/s40265-017-0714-4 [DOI] [PubMed] [Google Scholar]
- 88.Ghosoph JM, Schmidt LS, Margosan DA, Smilanick JL. Imazalil resistance linked to a unique insertion sequence in the PdCYP51 promoter region of Penicillium digitatum. Postharvest Biol Technol. 2007;44. doi: 10.1016/j.postharvbio.2006.11.008 [DOI] [Google Scholar]
- 89.Hamamoto H, Hasegawa K, Nakaune R, Lee YJ, Makizumi Y, Akutsu K, et al. Tandem Repeat of a Transcriptional Enhancer Upstream of the Sterol 14α-Demethylase Gene (CYP51) in Penicillium digitatum. Appl Environ Microbiol. 2000;66. doi: 10.1128/AEM.66.8.3421-3426.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carter HE, Fraaije BA, West JS, Kelly SL, Mehl A, Shaw MW, et al. Alterations in the predicted regulatory and coding regions of the sterol 14α-demethylase gene (CYP51) confer decreased azole sensitivity in the oilseed rape pathogen Pyrenopeziza brassicae. Mol Plant Pathol. 2014;15. doi: 10.1111/mpp.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schnabel G, Jones AL. The 14α-Demethylasse (CYP51A1) Gene is Overexpressed in Venturia inaequalis Strains Resistant to Myclobutanil. Phytopathology. 2001;91. doi: 10.1094/PHYTO.2001.91.1.102 [DOI] [PubMed] [Google Scholar]
- 92.Luo C-X, Schnabel G. The Cytochrome P450 Lanosterol 14α-Demethylase Gene Is a Demethylation Inhibitor Fungicide Resistance Determinant in Monilinia fructicola Field Isolates from Georgia. Appl Environ Microbiol. 2008;74. doi: 10.1128/AEM.02159-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Linden JWM, Camps SMT, Kampinga GA, Arends JPA, Debets-Ossenkopp YJ, Haas PJA, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57:513–20. doi: 10.1093/cid/cit320 [DOI] [PubMed] [Google Scholar]
- 94.Lavergne RA, Morio F, Favennec L, Dominique S, Meis JF, Gargala G, et al. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother. 2015;59:4331–5. doi: 10.1128/AAC.00127-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vermeulen E, Maertens J, de Bel A, Nulens E, Boelens J, Surmont I, et al. Nationwide Surveillance of Azole Resistance in Aspergillus Diseases. Antimicrob Agents Chemother. 2015;59. doi: 10.1128/AAC.03906-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischer J, van Koningsbruggen-Rietschel S, Rietschel E, Vehreschild MJGT, Wisplinghoff H, Kronke M, et al. Prevalence and molecular characterization of azole resistance in Aspergillus spp. isolates from German cystic fibrosis patients. J Antimicrob Chemother. 2014;69. doi: 10.1093/jac/dku009 [DOI] [PubMed] [Google Scholar]
- 97.Snelders E, Camps SMT, Karawajczyk A, Rijs AJMM, Zoll J, Verweij PE, et al. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet Biol. 2015;82:129–35. doi: 10.1016/j.fgb.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 98.Burks C, Darby A, Londoño LG, Momany M, Brewer MT. Azole-resistant Aspergillus fumigatus in the environment: Identifying key reservoirs and hotspots of antifungal resistance. PLoS Pathog. 2021. doi: 10.1371/journal.ppat.1009711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camps SMT, Rijs AJMM, Klaassen CHW, Meis JF, O’Gorman CM, Dyer PS, et al. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J Clin Microbiol. 2012;50:2674–80. doi: 10.1128/JCM.00335-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engel T, Verweij PE, van den Heuvel J, Wangmo D, Zhang J, Debets AJM, et al. Parasexual recombination enables Aspergillus fumigatus to persist in cystic fibrosis. ERJ Open Res. 2020;6. doi: 10.1183/23120541.00020-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Z, Jiang W, Jian Q, Song W, Zheng Z, Wang D, et al. Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese fields. Food Chem. 2015;168. doi: 10.1016/j.foodchem.2014.07.087 [DOI] [PubMed] [Google Scholar]
- 102.Barber AE, Riedel J, Sae-Ong T, Kang K, Brabetz W, Panagiotou G, et al. Effects of Agricultural Fungicide Use on Aspergillus fumigatus Abundance, Antifungal Susceptibility, and Population Structure. mBio. 2020;11. doi: 10.1128/mbio.02213-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van der Torre MH, Whitby C, Eades CP, Moore CB, Novak-Frazer L, Richardson MD, et al. Absence of Azole Antifungal Resistance in Aspergillus fumigatus Isolated from Root Vegetables Harvested from UK Arable and Horticultural Soils. J Fungi (Basel). 2020;6. doi: 10.3390/jof6040208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fraaije B, Atkins S, Hanley S, Macdonald A, Lucas J. The Multi-Fungicide Resistance Status of Aspergillus fumigatus Populations in Arable Soils and the Wider European Environment. Front Microbiol. 2020;11. doi: 10.3389/fmicb.2020.599233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mavridou E, Bruggemann RJM, Melchers WJG, Verweij PE, Mouton JW. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 2010;54:4758–64. doi: 10.1128/AAC.00606-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chowdhary A, Kathuria S, Xu J, Meis JF. Emergence of Azole-Resistant Aspergillus fumigatus Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013;9. doi: 10.1371/journal.ppat.1003633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shan Y, Fan S, Liu X, Li J. Prevalence of Candida albicans-closely related yeasts, Candida africana and Candida dubliniensis, in vulvovaginal candidiasis. Med Mycol. 2014;52:636–40. doi: 10.1093/mmy/myu003 [DOI] [PubMed] [Google Scholar]
- 108.Theill L, Dudiuk C, Morano S, Gamarra S, Nardin ME, Méndez E, et al. Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina. Rev Argent Microbiol. 2016;48:43–9. 10.1016/j.ram.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 109.McCarty TP, Pappas PG. Invasive Candidiasis. Infect Dis Clin North Am. 2016/01/08. 2016;30:103–124. doi: 10.1016/j.idc.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 110.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018/05/12. 2018;4:18026. doi: 10.1038/nrdp.2018.26 [DOI] [PubMed] [Google Scholar]
- 111.Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25. doi: 10.1016/j.cmi.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 112.Neufeld PM, Melhem M de SC, Szeszs MW, Ribeiro MD, Amorim E de LT, da Silva M, et al. Nosocomial candidiasis in Rio de Janeiro State: Distribution and fluconazole susceptibility profile. Braz J Microbiol. 2015;46. doi: 10.1590/S1517-838246220120023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ulu Kilic A, Alp E, Cevahir F, Ture Z, Yozgat N. Epidemiology and cost implications of candidemia, a 6-year analysis from a developing country. Mycoses. 2017;60. doi: 10.1111/myc.12582 [DOI] [PubMed] [Google Scholar]
- 114.Brown GD, Denning DW, Levitz SM. Tackling Human Fungal Infections. Science. 2012;336. doi: 10.1126/science.1228998 [DOI] [PubMed] [Google Scholar]
- 115.Bhattacharya S, Sae-Tia S, Fries BC. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics (Basel). 2020;9:312. doi: 10.3390/antibiotics9060312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. 2010/11/06. 2010;13:180–195. doi: 10.1016/j.drup.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 117.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, et al. Wild-type MIC distributions and epidemiological cutoff values for posaconazole and voriconazole and Candida spp. as determined by 24-hour CLSI broth microdilution. J Clin Microbiol. 2010/12/17. 2011;49: 630–637. doi: 10.1128/JCM.02161-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pfaller MA, Espinel-Ingroff A, Canton E, Castanheira M, Cuenca-Estrella M, Diekema DJ, et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J Clin Microbiol. 2012/03/31. 2012;50: 2040–2046. doi: 10.1128/JCM.00248-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Autmizguine J, Smith PB, Prather K, Bendel C, Natarajan G, Bidegain M, et al. Effect of fluconazole prophylaxis on Candida fluconazole susceptibility in premature infants. J Antimicrob Chemother. 2018/09/25. 2018;73: 3482–3487. doi: 10.1093/jac/dky353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bennett JE, Izumikawa K, Marr KA. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother. 2004/04/24. 2004;48: 1773–1777. doi: 10.1128/AAC.48.5.1773-1777.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldman M, Cloud GA, Smedema M, LeMonte A, Connolly P, McKinsey DS, et al. Does long-term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? The National Institute of Allergy and Infectious Diseases Mycoses study group. Antimicrob Agents Chemother. 2000/05/19. 2000;44: 1585–1587. doi: 10.1128/AAC.44.6.1585-1587.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991/10/31. 1991;325: 1274–1277. doi: 10.1056/NEJM199110313251803 [DOI] [PubMed] [Google Scholar]
- 123.Pfaller MA, Diekema DJ. Rare and Emerging Opportunistic Fungal Pathogens: Concern for Resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–31. doi: 10.1128/JCM.42.10.4419-4431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004/01/30. 2004;10 Suppl 1:11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x [DOI] [PubMed] [Google Scholar]
- 125.Thomaz DY, de Almeida JN, Lima GME, de Nunes MO, Camargo CH, de C Grenfell R, et al. An Azole-Resistant Candida parapsilosis Outbreak: Clonal Persistence in the Intensive Care Unit of a Brazilian Teaching Hospital. Front Microbiol. 2018;9. doi: 10.3389/fmicb.2018.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arastehfar A, Daneshnia F, Hafez A, Khodavaisy S, Najafzadeh M-J, Charsizadeh A, et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med Mycol. 2020;58. doi: 10.1093/mmy/myz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen P-Y, Chuang Y-C, Wu U-I, Sun H-Y, Wang J-T, Sheng W-H, et al. Clonality of Fluconazole-Nonsusceptible Candida tropicalis in Bloodstream Infections, Taiwan, 2011–2017. Emerg Infect Dis. 2019;25. doi: 10.3201/eid2509.190520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chong Y, Ito Y, Kamimura T, Shimoda S, Miyamoto T, Akashi K, et al. Fatal candidemia caused by azole-resistant Candida tropicalis in patients with hematological malignancies. J Infect Chemother. 2012;18. doi: 10.1007/s10156-012-0412-9 [DOI] [PubMed] [Google Scholar]
- 129.Morio F. Dear medical mycologists, it is time to look outside the box. FEMS Yeast Res. 2020;20. doi: 10.1093/femsyr/foz080 [DOI] [PubMed] [Google Scholar]
- 130.Opulente DA, Langdon QK, Buh Kv, Haase MAB, Sylvester K, Moriarty Rv, et al. Pathogenic budding yeasts isolated outside of clinical settings. FEMS Yeast Res. 2019/05/12. 2019;19. doi: 10.1093/femsyr/foz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y-L, Lin C-C, Chang T-P, Lauderdale T-L, Chen H-T, Lee C-F, et al. Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PLoS ONE. 04/05. 2012;7: e34609–e34609. doi: 10.1371/journal.pone.0034609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vogel C, Rogerson A, Schatz S, Laubach H, Tallman A, Fell J. Prevalence of yeasts in beach sand at three bathing beaches in South Florida. Water Res. 2007;41. doi: 10.1016/j.watres.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect. 2014;20:42–8. 10.1111/1469-0691.12513 [DOI] [PubMed] [Google Scholar]
- 134.Crump KR, Edlinds TD. Agricultural Fungicides May Select for Azole Antifungal Resistance in Pathogenic Candida. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington; 2004.
- 135.Müller FM, Staudigel A, Salvenmoser S, Tredup A, Miltenberger R, Herrmann Jv. Cross-resistance to medical and agricultural azole drugs in yeasts from the oropharynx of human immunodeficiency virus patients and from environmental Bavarian vine grapes. Antimicrob Agents Chemother. 2007/06/06. 2007;51:3014–3016. doi: 10.1128/AAC.00459-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Faria-Ramos I, Tavares PR, Farinha S, Neves-Maia J, Miranda IM, Silva RM, et al. Environmental azole fungicide, prochloraz, can induce cross-resistance to medical triazoles in Candida glabrata. FEMS Yeast Res. 2014/08/19. 2014;14:1119–1123. doi: 10.1111/1567-1364.12193 [DOI] [PubMed] [Google Scholar]
- 137.Brilhante RSN, Alencar LP, Bandeira SP, Sales JA, Evangelista AJJ, Serpa R, et al. Exposure of Candida parapsilosis complex to agricultural azoles: An overview of the role of environmental determinants for the development of resistance. Sci Total Environ. 2018/10/13. 2019;650: 1231–1238. doi: 10.1016/j.scitotenv.2018.09.096 [DOI] [PubMed] [Google Scholar]
- 138.Rocha MFG, Alencar LP, Paiva MAN, Melo LM, Bandeira SP, Ponte YB, et al. Cross-resistance to fluconazole induced by exposure to the agricultural azole tetraconazole: An environmental resistance school? Mycoses. 2016;59:281–90. doi: 10.1111/myc.12457 [DOI] [PubMed] [Google Scholar]
- 139.Casadevall A, Kontoyiannis DP, Robert V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio. 2019;10:e01397–19. doi: 10.1128/mBio.01397-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Casadevall A, Kontoyiannis DP, Robert V. Environmental Candida auris and the Global Warming Emergence Hypothesis. mBio. 2021;12. doi: 10.1128/mBio.00360-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arora P, Singh P, Wang Y, Yadav A, Pawar K, Singh A, et al. Environmental Isolation of Candida auris from the Coastal Wetlands of Andaman Islands, India. mBio. 2021;12:e03181–20. doi: 10.1128/mBio.03181-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rhodes J. Rapid Worldwide Emergence of Pathogenic Fungi. Cell Host Microbe. 2019/07/12. 2019;26:12–14. doi: 10.1016/j.chom.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 143.Rocha MF, Alencar LP, Paiva MA, Melo LM, Bandeira SP, Ponte YB, et al. Cross-resistance to fluconazole induced by exposure to the agricultural azole tetraconazole: an environmental resistance school? Mycoses. 2016/02/13. 2016;59:281–290. doi: 10.1111/myc.12457 [DOI] [PubMed] [Google Scholar]
- 144.Sidrim JJC, de Maria GL, de AN Paiva M, dos S Araújo G, da Graça-Filho RV, de Oliveira JS, et al. Azole-Resilient Biofilms and Non-wild Type C. albicans Among Candida Species Isolated from Agricultural Soils Cultivated with Azole Fungicides: an Environmental Issue? Microb Ecol. 2021. doi: 10.1007/s00248-021-01694-y [DOI] [PubMed] [Google Scholar]
- 145.Potocki L, Baran A, Oklejewicz B, Szpyrka E, Podbielska M, Schwarzbacherová V. Synthetic Pesticides Used in Agricultural Production Promote Genetic Instability and Metabolic Variability in Candida spp. Genes (Basel). 2020/07/30. 2020;11. doi: 10.3390/genes11080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Potocki L, Depciuch J, Kuna E, Worek M, Lewinska A, Wnuk M. FTIR and Raman Spectroscopy-Based Biochemical Profiling Reflects Genomic Diversity of Clinical Candida Isolates That May Be Useful for Diagnosis and Targeted Therapy of Candidiasis. Int J Mol Sci. 2019/03/03. 2019;20. doi: 10.3390/ijms20040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen SC-A, Meyer W, Sorrell TC. Cryptococcus gattii Infections. Clin Microbiol Rev. 2014;27. doi: 10.1128/CMR.00126-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zavala S, Baddley JW. Cryptococcosis. Semin Respir Crit Care Med. 2020;41. doi: 10.1055/s-0039-3400280 [DOI] [PubMed] [Google Scholar]
- 149.Chen M, Wang Y, Li Y, Hong N, Zhu X, Pan W, et al. Genotypic diversity and antifungal susceptibility of environmental isolates of Cryptococcus neoformans from the Yangtze River Delta region of East China. Med Mycol. 2020. doi: 10.1093/mmy/myaa096 [DOI] [PubMed] [Google Scholar]
- 150.Cogliati M, Puccianti E, Montagna MT, de Donno A, Susever S, Ergin C, et al. Fundamental niche prediction of the pathogenic yeasts Cryptococcus neoformans and Cryptococcus gattii in Europe. Environ Microbiol. 2017;19:4318–25. doi: 10.1111/1462-2920.13915 [DOI] [PubMed] [Google Scholar]
- 151.Cogliati M, D’Amicis R, Zani A, Montagna MT, Caggiano G, de Giglio O, et al. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016;16. doi: 10.1093/femsyr/fow086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: A review of pathobiology and clinical aspects. Med Mycol. 2019;57. doi: 10.1093/mmy/myy086 [DOI] [PubMed] [Google Scholar]
- 153.Kronstad JW, Attarian R, Cadieux B, Choi J, Douza CA, Griffiths EJ, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9. doi: 10.1038/nrmicro2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen Y, Toffaletti DL, Tenor JL. The Cryptococcus neoformans Transcriptome at the Site of Human. mBio. 2014;5:1–10. doi: 10.1128/mBio.01087-13.Editor [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iyer KR, Revie NM, Fu C, Robbins N, Cowen LE. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat Rev Microbiol. 2021. doi: 10.1038/s41579-021-00511-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Day JN, Chau TTH, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination Antifungal Therapy for Cryptococcal Meningitis. N Engl J Med. 2013;368:1291–302. doi: 10.1056/NEJMoa1110404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nasri H, Kabbani S, Bou Alwan M, Wang YF, Rebolledo PA, Kraft CS, et al. Retrospective Study of Cryptococcal Meningitis With Elevated Minimum Inhibitory Concentration to Fluconazole in Immunocompromised Patients. Open Forum Infect Dis. 2016;3. doi: 10.1093/ofid/ofw076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Naicker SD, Mpembe RS, Maphanga TG, Zulu TG, Desanto D, Wadula J, et al. Decreasing fluconazole susceptibility of clinical south african Cryptococcus neoformans isolates over a decade. PLoS Negl Trop Dis. 2020;14. doi: 10.1371/journal.pntd.0008137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gutch RS, Nawange SR, Singh SM, Yadu R, Tiwari A, Gumasta R, et al. Antifungal susceptibility of clinical and environmental Cryptococcus neoformans and Cryptococcus gattii isolates in Jabalpur, a city of Madhya Pradesh in Central India. Braz J Microbiol. 2015;46. doi: 10.1590/S1517-838246420140564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Silva DC, Martins MA, Szeszs MW, Bonfietti LX, Matos D, Melhem MSC. Susceptibility to antifungal agents and genotypes of Brazilian clinical and environmental Cryptococcus gattii strains. Diagn Microbiol Infect Dis. 2012;72. doi: 10.1016/j.diagmicrobio.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 162.Smith KD, Achan B, Hullsiek KH, Mcdonald TR, Okagaki LH, Alhadab AA, et al. Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother. 2015;59:7197–204. doi: 10.1128/AAC.01299-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen YC, Chang TY, Liu JW, Chen FJ, Chien CC, Lee CH, et al. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: A 12-year longitudinal study. BMC Infect Dis. 2015;15:1–7. doi: 10.1186/s12879-014-0722-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li Y, Zou M, Yin J, Liu Z, Lu B. Microbiological, Epidemiological, and Clinical Characteristics of Patients With Cryptococcal Meningitis at a Tertiary Hospital in China: A 6-Year Retrospective Analysis. Front Microbiol. 2020;11. doi: 10.3389/fmicb.2020.01837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chesdachai S, Rajasingham R, Nicol MR, Meya DB, Bongomin F, Abassi M, et al. Minimum Inhibitory Concentration Distribution of Fluconazole Against Cryptococcus Species and the Fluconazole Exposure Prediction Model. Open Forum Infect Dis. 2019;6. doi: 10.1093/ofid/ofz369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schmertmann LJ, Irinyi L, Malik R, Powell JR, Meyer W, Krockenberger MB. The mycobiome of Australian tree hollows in relation to the Cryptococcus gattii and C. neoformans species complexes. Ecol Evol. 2019;9. doi: 10.1002/ece3.5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mseddi F, Sellami A, Jarboui MA, Sellami H, Makni F, Ayadi A. First Environmental Isolations of Cryptococcus neoformans and Cryptococcus gattii in Tunisia and Review of Published Studies on Environmental Isolations in Africa. Mycopathologia 2011;171. doi: 10.1007/s11046-010-9364-8 [DOI] [PubMed] [Google Scholar]
- 168.del Poeta M, Casadevall A. Ten Challenges on Cryptococcus and Cryptococcosis. Mycopathologia. 2012;173. doi: 10.1007/s11046-011-9469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bastos RW, Freitas GJC, Carneiro HCS, Oliveira LVN, Gouveia-Eufrasio L, Santos APN, et al. From the environment to the host: How non-azole agrochemical exposure affects the antifungal susceptibility and virulence of Cryptococcus gattii. Sci Total Environ. 2019;681. doi: 10.1016/j.scitotenv.2019.05.094 [DOI] [PubMed] [Google Scholar]
- 170.Carneiro HCS, Ribeiro NQ, Bastos RW, Santos DA. Effect of non-antifungal agrochemicals on the pathogenic fungus Cryptococcus gattii. Med Mycol. 2020;58:47–53. doi: 10.1093/mmy/myz018 [DOI] [PubMed] [Google Scholar]
- 171.Carneiro HCS, Bastos RW, Ribeiro NQ, Gouveia-Eufrasio L, Costa MC, Magalhães TFF, et al. Hypervirulence and cross-resistance to a clinical antifungal are induced by an environmental fungicide in Cryptococcus gattii. Sci Total Environ. 2020;740. doi: 10.1016/j.scitotenv.2020.140135 [DOI] [PubMed] [Google Scholar]
- 172.Sionov E, Lee H, Chang YC, Kwon-Chung KJ. Cryptococcus neoformans Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes. PLoS Pathog. 2010;6:e1000848. doi: 10.1371/journal.ppat.1000848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Basso LR, Gast CE, Bruzual I, Wong B. Identification and properties of plasma membrane azole efflux pumps from the pathogenic fungi Cryptococcus gattii and Cryptococcus neoformans. J Antimicrob Chemother. 2014;70:1396–407. doi: 10.1093/jac/dku554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Santos JRA, Holanda RA, Frases S, Bravim M, Araujo GDS, Santos PC, et al. Fluconazole alters the polysaccharide capsule of cryptococcus gattii and leads to distinct behaviors in murine cryptococcosis. PLoS ONE. 2014;9:1–14. doi: 10.1371/journal.pone.0112669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang ML, Uhrig J, Vu K, Singapuri A, Dennis M, Gelli A, et al. Fluconazole susceptibility in Cryptococcus gattii is dependent on the ABC transporter Pdr11. Antimicrob Agents Chemother. 2016;60:1202–7. doi: 10.1128/AAC.01777-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sionov E, Chang YC, Kwon-Chung KJ. Azole heteroresistance in Cryptococcus neoformans: Emergence of resistant clones with chromosomal disomy in the mouse brain during fluconazole treatment. Antimicrob Agents Chemother. 2013;57:5127–30. doi: 10.1128/AAC.00694-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Carlson T, Lupinacci E, Moseley K, Chandrasekaran S. Effects of environmental factors on sensitivity of Cryptococcus neoformans to fluconazole and amphotericin B. FEMS Microbiol Lett. 2021;368. doi: 10.1093/femsle/fnab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bielska E, May RC. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016;16. doi: 10.1093/femsyr/fov106 [DOI] [PubMed] [Google Scholar]
- 179.Chang YC, Chung KJK. Complementation of a Capsule-Deficient Mutation of Cryptococcus neoformans Restores Its Virulence. Mol Cell Biol. 1994. Available from: https://journals.asm.org/journal/mcb. doi: 10.1128/mcb.14.7.4912-4919.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ferreira GF, Santos JRA, da Costa MC, de Holanda RA, et al. Heteroresistance to itraconazole alters the morphology and increases the virulence of Cryptococcus gattii. Antimicrob Agents Chemother. 2015;59:4600–9. doi: 10.1128/AAC.00466-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McEwen SA, Collignon PJ. Antimicrobial Resistance: a One Health Perspective. Microbiology Spectrum. 2018;6. doi: 10.1128/microbiolspec.ARBA-0009-2017 [DOI] [PubMed] [Google Scholar]