Abstract
Objective
To inform quarantine and contact-tracing policies concerning re-positive cases—cases testing positive among those recovered.
Materials and methods
We systematically reviewed and appraised relevant literature from PubMed and Embase for the extent of re-positive cases and their epidemiological characteristics.
Results
In 90 case reports/series, a total of 276 re-positive cases were found. Among confirmed reinfections, 50% occurred within 90 days from recovery. Four reports related onward transmission. In thirty-five observational studies, rate of re-positives ranged from zero to 50% with no onward transmissions reported. In eight reviews, pooled recurrence rate ranged from 12% to 17.7%. Probability of re-positive increased with several factors.
Conclusion
Recurrence of a positive SARS-CoV-2 test is commonly reported within the first weeks following recovery from a first infection.
Introduction
After 14 months in the COVID-19 pandemic, health systems worldwide have still not achieved control of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). SARS-CoV-2 is highly transmissible with a potential secondary attack rate of more than 17% [1]. Further, this rate of transmission has been reported to be even higher in circulating variants of concern such as the B.1.1.7 than in pre-existing variants [2]. Since its emergence in December 2019, the SARS-CoV-2 virus has infected more than 120 million people and led to at least 2.6 million deaths globally [3]. In addition to the high disease burden, the virus has brought an unprecedented downpour of social and economic setbacks, the course of which cannot start to be reversed until herd immunity, natural or artificial, is achieved. While six vaccines are already licensed, we are still far from herd immunity, given that vaccines need to be produced at scale, priced affordably, and allocated globally to be widely deployed [4,5]. Additionally, in consideration of emerging variants and reports of recurrent SARS-CoV-2 infections, the global battle against the virus is far from being over.
Immunological evidence suggests that immune response to post-natural SARS-CoV-2 infection is transient with reported rapid depletion of antibodies in the first four months followed by gradual waning within a year [6–9]. Recent studies have detected viral nucleic acid in previously recovered patients [10] and the first symptomatic reinfection with different viral strains has been confirmed in June 2020 in North America [11]. In the near future, this risk for reinfection is expected to increase due to lack of protective immunity and circulation of new variants. Whereas some studies did not find replication-competent virus in re-positives—a positive test following recovery from a first SARS-COV-2 infection—[12,13], a study in Spain reported onwards transmission [14]. This potential for onwards transmission is of utmost importance as it increases the diseases burden of SARS-CoV-2 and its associated complications. This risk is even higher as previously infected people tend to adhere less to mitigation measures, such as social distancing and public health policies [15]. Adding to the danger of behavioral factors, currently there are no harmonized protocols for contact tracing or isolation for re-positives globally.
Public health policies concerning re-infections vary globally as evidence on the extent and the potential of the consequences is lacking. The European Center for Disease Control (ECDC) recently reported that reinfection with SARS-CoV-2 remains a rare event [16]. They further stated that there is the risk for some re-infected persons to transmit SARS-CoV-2 infection to susceptible contacts as previous infections do not produce sterilizing immunity in all individuals. However, there is insufficient evidence to determine the effect of previous infection on the risk of onward transmission and its impact on public health safety. This is further complicated by the limited evidence on how newly circulating variants of concern affect the probability of reinfection and their role in onward transmission. Currently, COVID-19 mitigation policies in the United States do not recommend retesting or quarantine if a previously recovered patient is exposed to SARS-CoV-2 within 90 days after the date of symptom onset from the initial SARS-CoV-2 infection [17]. In the EU and Germany, public health authorities only impose quaratine in the first 90 days if the person works or lives with a risk group [18] and/or a variant of concern is suspected [19]. If the second exposure occurs more than three months after the first infection, the previously recovered person is considered at the same risk as any other contact person without previous infection [18]. As more recurrent SARS-CoV-2 infections are being reported, whether such episodes are actual reinfections or not, constant evidence on the cause of re-positives and onwards spread is crucial to inform public health policies. We reviewed and synthesized the evidence around the extent and characteristics of reinfections and re-positive rates of SARS-CoV-2, to inform related quarantine and contact-tracing policies.
Materials and methods
We conducted a review of scientific literature following the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) [20]. We performed the literature search using the electronic databases PubMed, Embase and preprint servers (ArRvix, BioRvix, ChemRvix, MedRvix, Preprints.org, ResearchSquare and SSRN). We used novel coronavirus search terms developed by the Robert Koch Institute library, and terms for reinfection, re-positive, reactivation, relapse, recurrence, and secondary infection. The S1 Table provides the detailed search strategy. The search was restricted to SARS-CoV-2 infection in humans, to publication date since 2020 and to English language. The study was exempt from institutional review board approval because no primary data was included. The review protocol has not been previously registered or published.
We organized the search results and removed duplicates using Endnote X7 (Clarivate Analytics) [21]. Title and abstract screening of publications were conducted using Rayyan QCRI web application for systematic reviews [22]. Case reports, case series, observational studies and reviews were included. Publications reporting on cases of one-episode of SARS-CoV-2 infection, manuscripts without primary data (letter to the editor, conference abstract, commentaries), model stimulation studies, laboratory studies, and animal models were excluded. One reviewer (CEB) developed the data extraction forms and two reviewers (SKA and SM) extracted data of the included publications. To minimize potential errors in the content, each reviewer examined a random 20% of data extracted by the other.
The following data were extracted for all included publications: first author, year, country, age study population, number of cases, testing methods, symptomatology, duration between infections and onward transmission of infection. Additional data extracted for the specific study designs included: 1) case reports: comorbidities, reinfection confirmation, infection differentiation method, relaxation of protective behavior, symptomatology of onward infection, 2) observational studies: publication status, country and setting, serology test, evidence for reinfection, total sample size, risk of reinfection, incidence rate, whether the re-positive was identified due to symptoms or not and 3) reviews: publication status, types of studies reviewed, reinfection confirmation, reported association with demographics, necessity of intensive care treatment, and comorbidities.
Three independent reviewers (SKA, SM, AMB) evaluated the quality and risk of bias of included publications. We assessed the publications using Joanna Briggs Institute (JBI) critical appraisal tool for case series and evidence review, and adapted the JBI critical appraisal tool for case reports [23]. For observational cross-sectional and cohort studies, we adapted the National Heart, Lung and Blood institutes protocol [24]. Data from included publications were analyzed descriptively focusing on re-positive rates, epidemiological characteristics of recurrent SARS-CoV-2 infection and onward transmission. The relevant extracted data were organized and presented in tables.
Results
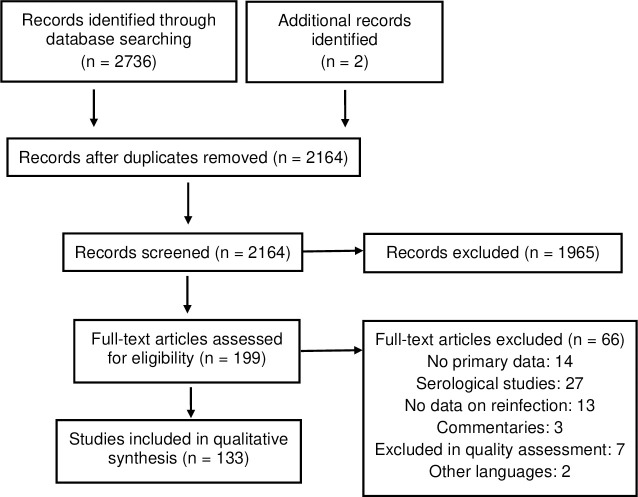
The primary database search on February 2, 2021 yielded 2,736 publications. After removing duplicates, and performing title and abstract screening, we retrieved 199 publications for full text screening (Fig 1).
Fig 1. Selection of studies.
After applying the eligibility criteria in the full text assessment, 133 publications were included. The included publications comprised 75 case reports, 15 case series, 35 observational studies and 8 reviews. This includes two additional manuscripts published after the initial database research but deemed essential given the relevance of their content [25,26].
In case reports and case series, a total of 276 cases between the age of three [27] and 93 [28] years were recorded as re-positive by PCR. About 101 cases had one or more comorbidities. The characteristics of cases are displayed in the S2 Table. The duration between the two infection episodes varied between one [29–33] and 32 weeks [34]. Applying the Robert Koch Institute’s (RKI) definition for probability of reinfection [35], 217 non-previously confirmed reinfection cases were classified as possible reinfection and eight case as probable reinfection [27,36–41] as shown in S2 Table. Thirty-eight cases were not classified as either possible, probable or confirmed reinfections as not all case definition elements were reported as required by RKI criteria. Twelve cases were confirmed to be reinfections through whole genome sequencing of viral material in both episodes [11,42–50]. Fifty percent of all confirmed reinfections were reported to have occurred within 90 days after the initial disease. Phylogenetic analysis in one re-positive case sample, identified new viral strain which was absent in the location of exposure during patient’s first episode [14]. As this finding did not meet the RKI criteria, this re-positivity could not be classified as confirmed reinfection. Clinical characteristics of confirmed reinfections and the re-positive case with new viral strain are detailed in Table 1. Seven studies, including one study of confirmed reinfection, reported follow-up testing of contacts of re-positives [14,39,47,51–54]. Four of these studies identified positive contacts. The positive contacts from three studies included family members in two studies [14,55], and one treating physician in another study [51]. In the fourth study, viral genomic materials were identified to be identical in a re-infected health care worker (HCW) and three patients. The clustered nature of the transmission suggested a possible index case, however as symptoms of COVID-19 infection was first observed in a patient who received no care from re-infected HCW, it was unclear whether the re-infected HCW was the index patient [47]. The quality assessment of case reports and case series were rated on a scale of zero to nine and ten respectively as shown S3 and S4 Tables. Case reports with evidence of confirmed reinfections were among the highly rated publications. The top-rated case series reported re-positivity occurring between three to six weeks after initial disease [56,57]. One of the top-rated case series reported high rate of re-positivity to be associated with younger age, low body mass index and moderate disease severity [57].
Table 1. Characteristics of confirmed SARS-COV-2 reinfection cases.
| Author | Country | Number of cases | Age in years | Co-morbidities | Symptoms at 1st episode | Time (weeks) between 1st and 2nd episode | Symptoms at 2nd episode | Onward transmission from 2nd episode |
|---|---|---|---|---|---|---|---|---|
| Tillett RL et al. [11] | USA | 1 | 25 | No | Sore throat, cough, headache, nausea, diarrhoea | 6 | Fever, headache, dizziness, cough, nausea, diarrhoea, shortness of breath | NR |
| Mulder M et al. [58] | Netherlands | 1 | 89 | Yes | Fever, severe cough | 8 | Fever, cough, dyspnoea, tachypnoea | NR |
| Prado-Vivar B et al. [59] | Ecuador | 1 | 46 | No | Headache, drowsiness | 9 | Odynophagia, nasal congestion, fever, back pain, productive cough, dyspnoea | NR |
| To KK et al. [49] | Hong Kong | 1 | 33 | No | Productive cough, sore throat, fever, headache | 10 | Asymptomatic | NR |
| Van Elslande J et al. [48] | Belgium | 1 | 51 | Yes | Headache, fever, myalgia, cough, chest pain, dyspnoea, anosmia, change of taste | 12 | Headache, cough, fatigue | NR |
| Colson P et al. [50] | France | 1 | 70 | Yes | Fever, cough | 15 | Asymptomatic | NR |
| Goldman JD et al. [42] | USA | 1 | 60–69 | Yes | Fever, chills, productive cough, dyspnoea, chest pain | 20 | Dyspnoea, dry cough, weakness | NR |
| Harrington D et al. [34] | UK | 1 | 78 | Yes | Fever | 32 | Shortness of breath | NR |
| Lee JS et al. [41] | South Korea | 1 | 21 | No | Sore throat, cough | 3 | Sore throat, cough | NR |
| Gupta V et al. [43] | India | 2 | 25–28 | No | Asymptomatic | 9–14 | Asymptomatic | NR |
| Selhorst P et al. [60] | Belgium | 1 | 39 | NR | Cough, dyspnoea, headache, fever, general malaise | 26.5 | Mild symptoms | Unclear |
| Pérez-Lago L et al* [14] | Spain | 1 | 53 | Yes | Dyspnoea, fever, cough | 20 | Respiratory failure | Yes |
* Phylogenetically confirmed but does not meet RKI confirmed reinfection criteria,
NR not reported.
The 35 observational studies, including four preprints [61–64], were predominantly conducted in China and focused on healthcare settings as shown in Table 2. A total of 1,100 re-positives were reported out of 180,185 previously recovered patients, whereby one study reported 44 re-positives of an unknown total assessed [65]. Reported re-positive rates ranged from zero to 50% [66,67] in patients aged between two months [68] and 95 years [69]. At least 40% of re-positives were found to be symptomatic at the second episode (451/1,046). The duration between discharge/negative test/completion of therapy and re-positivity varied from less than one [12] to 33 weeks [62]. Highest re-positive rates of more than 20% were reported to occur in a follow- up period between one to seven weeks [64,66,70,71], while low re-positive rates occurred in a follow-up period of more than nine weeks [25,62,63,67]. Only two studies performed genome sequencing from naso-/oropharyngeal samples, but full-length viral genomes could not be obtained [12,72]. Eight studies reported testing or follow-up of contacts, but no onward transmission was identified [70,72–78]. The quality of observational studies was assessed on a scale of zero to 14. Included publications were rated between 3 [79] and 11 [65,78,80], as shown in S5 Table. Two of the three top rated publications reported re-positive rates of 6.25% within five weeks [80] and 19.81% within three to five weeks [78] after initial infection. The third top-rated study reported 44 re-positive cases two weeks post-discharge [65]. The two studies, that included healthcare workers were scored 10/14 and had one of the lowest re-positive rates of 0% and 0.32% [62,67].
Table 2. Characteristics of confirmed SARS-COV-2 reinfection cases as reported in observational studies analysed.
| Author | Preprint | Country and Setting | Total | Reinfection cases | Age in years | Re-positive rate (%) | Time in weeks between 1st and 2nd episode | Symptomatic at reinfection (%) | Onward transmission from 2nd episode |
|---|---|---|---|---|---|---|---|---|---|
| Hanrath AT et al. [67] | No | UK, healthcare workers | 1038 | 0 | NR | 0.00% | 24 | NA | NR |
| Abu-Raddad LJA et al. [63] | Yes | Qatar, surveillance | 133266 | 54 | median 33 range (16–57) |
0.04% | median 9.3 (6.4–18.4) | 42.6% | NR |
| Pilz et al. [25] | No | Austria, surveillance | 14840 | 40 | median 39.8 range (26–55) |
0.27% | 30±4 | 7.5% hospitalisation | NR |
| Lumley SF et al. [62] | Yes | UK, healthcare workers | 1246 | 4 | NR | 0.32% | 22.8–33 | 25.0% | NR |
| Patwardhan A [68] | No | USA, children | 989 | 4 | median 3.55 range (0.2–13) |
0.40% | 1–3 after last negative | 50.0% | NR |
| Hansen CH et al. [26] | No | Denmark, surveillance | 11068 | 72 | NR | 0.65% | >12 | NR | NR |
| Luo S et al. [81] | No | China, hospital patients | 1673 | 13 | NR | 0.78% | NR | 100% | NR |
| Pan L et al. [73] | No | China, hospital patients | 1350 | 14 | 44.4 ± 15 | 1.04% | 1.7 ± 0.7 after discharge | 7.1% | not found |
| Kang YJ et al. [82] | No | South Korea | 7829 | 163 | in groups | 2.08% | >1–5 after discharge | 43.9% mild | NR |
| Du HW et al. [74] | No | China, hospital patients | 126 | 3 | NR | 2.38% | 1–3 after treatment | >66.7% | not found |
| Ali AM et al. [61] | Yes | Iraq, hospital patients | 829 | 26 | range 10–60 | 3.14% | 3.7–19.7 after recovery | 96.2% | NR |
| Wang X et al. [83] | No | China, hospital patients | 193 | 12 | 55.5 ±13.7 | 6.22% | 4–8 | 83.3% | NR |
| Zhou J et al. [80] | No | China, hospital patients | 368 | 23 | 51±16 | 6.25% | 5 | 82.1% | NR |
| Qiao XM et al. [75] | No | China, hospital patients | 15 | 1 | NR | 6.67% | 2 | 100% | not found |
| Hu J et al. [84] | No | China, hospital patients | 117 | 8 | 46.25 ± 17.7 | 6.84% | median 1.8 (1.7–2.3) after discharge | 100% | NR |
| Tao W et al. [85] | No | China, hospital patients | 173 | 12 | NR | 6.94% | 5 | 0% | NR |
| Liu T et al. [86] | No | China, hospital patients | 150 | 11 | median 49 range (37–62) |
7.33% | 2 | NR | NR |
| Chen J et al. [87] | No | China, hospital patients | 1087 | 81 | median 62 range (16–90) |
7.45% | median 1.3 (0.4–2.6) | 84%mild; 16% moderate/severe | NR |
| Zheng J et al. [88] | No | China, hospital patients | 285 | 27 | median 44 range (32–62) |
9.47% | 1 (0.7–1.1) | 37.1% | NR |
| Bongiovanni M et al. [69] | No | Italy, hospital patients | 1146 | 125 | mean 65.7 95%CI (26–95) | 10.91% | 2.9 (95% CI 0.4–6.1) after discharge | 23.2% | NR |
| Zhang K et al. [89] | No | China, hospital patients | 220 | 27 | 22–78 | 12.27% | 1–8 | 22% mild; 77% moderate | NR |
| Tian M et al. [90] | No | China, hospital patients | 147 | 20 | mean 37.2 range (4–80) |
13.61% | 1–6.7 after discharge | 0% | NR |
| Lu J et al. [12] | No | China, hospital patients | 619 | 87 | median 28 range (0.3–69) |
14.05% | 1 (0.3–2.7) after discharge | 0% | NR |
| An J et al. [76] | No | China, hospital patients | 262 | 38 | 94.7% < 60y | 14.50% | 2 | 29% mild; 71% moderate | not found |
| Yuan J et al. [91] | No | China, hospital patients | 172 | 25 | median 28 range 16.3–42 |
14.53% | 1 week ± 0.55 after last negative | 32% mild | NR |
| Wu J et al. [92] | No | China, hospital patients | 60 | 10 | NR | 16.67% | 1–8 | 20% mild | NR |
| Yang C et al. [72] | No | China, hospital patients | 479 | 93 | median 34 95%CI (29–38) | 19.42% | 2–6 | 28% mild | not found |
| Abdullah MS et al. [77] | No | China, hospital patients | 138 | 27 | 41.3 ± 17.0 | 19.57% | 1.5 after discharge | 22% mild | not found |
| Wong J et al. [78] | No | Brunei, hospital patients | 106 | 21 | median 47 | 19.81% | 3–5 | 5% | not found |
| Xiao AT [64] | Yes | China, hospital patients | 70 | 15 | median 64 range (51–73) |
21.43% | 3–7 | NR | NR |
| Li Y et al. [71] | No | China, hospital patients | 13 | 4 | median 37 range (1–73) |
30.77% | 0.7–2 after discharge | 100% | NR |
| Zhang X et al. [93] | No | China, hospital patients | 59 | 19 | NR | 32.20% | NR | 0% | NR |
| Peng D et al. [70] | No | China, hospital patients | 38 | 14 | 7.2±4.8 | 36.84% | 1–5 | 71.4% | not found |
| Zhao W et al. [66] | No | China, hospital patients | 14 | 7 | median 5.7 range (3–7) |
50.00% | median 2 (1–2.4) after discharge | 14.3% | NR |
| Chen LZ et al. [65] | No | China, hospital patients | NA | 44 | 49.68 ± 16.80 | NA | 2 after discharge | >63.6% | NR |
NR not reported, CI confidence interval.
Table 3 details the eight selected literature reviews including five meta-analyses. The quality and risk of bias assessment of the reviews ranked between four and 11 on a scale of 11 as shown in S6 Table. The largest review included 85 publications and primary data on nine patients [94]. In this review, a total of 1,350 re-positive cases were identified, and a mean duration of re-positivity of 34.5 days after initial infection was observed in 123 cases, that were confirmed recovered after two negative PCR tests. Two high-rated reviews reported pooled recurrence rate of SARS-CoV-2 to be 14.6% (95%CI: 11.1–18.1%) [95] and 17.7% (95% CI: 12.4%-25.2%) [96]. Regarding duration between initial and recurrent episode, the top-rated study reported pooled estimate of the interval of disease onset to recurrence to be 35.4 days (95% CI 32.65–38.24 days) and the pooled estimate from last negative test to recurrence of infection to be 9.8 days (95% CI 7.31–12.22 days) [95]. Additionally, the time from discharge to recurrence of SARS-CoV-2 was reported by two other high-rated reviews to be 13.4 days (12.1–14.7) [96], and between two and 22 days. No cause of re-positivity was identified in the reviews, although the probability of recurrent SARS-CoV-2 infection increased with prolonged initial illness, moderate disease severity, decreased leucocytes, low platelets and low CD4 count [96]. The association of recurrent SARS-CoV-2 infection with age was controversial. Both, young and old age were identified as risk factors for recurrent SARS-CoV-2 infection [95,96]. None of the reviews reported on onwards transmission. Within the reviewed studies, in a report from Korea CDC where 790 contacts of 285 re-positive cases were monitored, no case was identified as newly infected from contact with re-positive cases during the re-positive period [97].
Table 3. Characteristics of confirmed SARS-COV-2 reinfection cases as reported in reviews analysed.
| Author | Preprint | Studies reviewed | Total studies | New positive respiratory samples after recovery | Duration from symptoms to 1st +, 1st -, 2nd +, 2nd–(days) | % cases with ≥1 comorbidity | Associated demographics | Cases requiring admission to ICU | Confirmed reinfection | Onward transmission from 2nd episode |
|---|---|---|---|---|---|---|---|---|---|---|
| Arafkas M et al. [98] | No | Literature review + meta-analysis | 7 (3 case reports, 1 case series, 2 clinical studies, 1 in-vivo study) | 15 + 9 persistent positive | NR | NR | None | 6 deaths | No | NR |
| Azam M et al. [95] | No | Systematic review + meta-analysis | 14 (8 cohort, 6 cross-sectional) | 14.6% (95% CI 11.1–18.1%) | 35.4 (95% CI 32.65–38.24) 9.8 (95% CI 7.31–12.22) |
NR | Risk factor: young age, long initial illness; protective factor: diabetes, severe disease, low lymphocyte | NR | No | NR |
| Dao TL et al. [97] | No | Narrative review | 62 | Few hundred | NR | 1 study: 64% cases with comorbidities | None | 3 | 1 study | Not found |
| Elsayed SM et al. [99] | No | Systematic review | 11 case reports | 11 | 1st negative to 2nd positive: 2–22 | NR | NR | NR | NR | NR |
| Gidari A et al. [94] | No | Systematic review + primary data | 85 (32 case reports, 50 case series, 5 reviews) | 1341 + 9 primary data | 6.2(4.7), 19.1(10.2), 34.5(18.7), 41.2(21.5) | 34.5% | None | 2 ICU cases | No | NR |
| Hoang T et al. [100] | Yes | Literature review + meta-analysis | 37 (14 case reports, 5 case series, 18 observational studies) |
16% (95%CI 12–20) | Disease onset to admission 17.3, admission to discharge 16.7, discharge to re-positive 10.5 | 43% (95% CI 31–55) | NR | NR | No | NR |
| Mattiuzzi C et al. [101] | No | Literature review + meta-analysis | 17 clinical studies | 12% (95%CI 12–13) | Discharge to re-positive 1–60 | NR | NR | NR | No | NR |
| Yao MQ et al. [96] | No | Systematic review + meta-analysis | 10 | 17.7% (95% CI: 12.4%-25.2%) | Discharge to re-positive 13.38 (95% CI: 12.08–14.69) |
NR | Age, moderate severity, bilateral pulmonary infiltration, low leucocytes/platelets/CD4 | NR | No | NR |
NR not reported, CI confidence interval, ICU intensive care unit.
Discussion
To the best of our knowledge, this is the first literature review rating evidence on more than 1349 recurrent SARS-CoV-2 infections worldwide. In this review, we found that recurrence of a positive SARS-CoV-2 test among previously recovered patients is common. Some re-positives follow exposure and/or present severe illness including death. The incidence of re-positivity varied with duration after initial disease, high rates of re-positivity with pooled incidence of 12.0% to 17.7% was observed within the first 90 days after initial infection as compared to rates (less than 1%) after 90 days or more. High re-positive rates within 3 months after initial infection raise questions on the cause of re-positivity, such as potential of prolonged viral shedding, testing errors or actual re-infections. Certainly, the high rates of re-positivity are of concern for current COVID-19 public health policies.
Current international policies base their recommendations for contract tracing and travel restrictions on a duration of 90 days after initial infection [17,18]. The US Centers for Disease Prevention and Control (CDC) does not impose quarantine measures within 90 days of re-exposure. The CDC regards re-positivity within 90 days of re-exposure more likely as persistent shedding of viral RNA than reinfection and states that the risk of potential SARS-CoV-2 transmission are likely outweighed by the personal and societal benefits of avoiding unnecessary quarantine [17]. The ECDC also identified in their assessment that ongoing vaccine trials have been focused mainly on their efficacy and effectiveness in reducing disease outcome such as severity of disease or induced mortality and not on their ability to reduce the risk of SARS-CoV-2 transmission from infected vaccinated individuals to susceptible contacts [16]. The ECDC therefore underlines the need for follow-up studies to better assess the potency and duration of protection from reinfection and their effect against further transmission of contacts [16].
Whole genome sequencing is key to identifying the causes for re-positivity. However, confirming re-infections through genome sequencing is rarely performed given the difficulty in ascertaining the first infection in the absence of stored genetic material and given the large number of infected people worldwide [3]. Only 15 out of 124 included publications with primary data reported on whole genome sequencing.
In the absence of a validated re-infection definition, often a clinical perspective is applied. By applying the clinical definition of RKI, the majority of re-positives in our review were classified as possible reinfections and only 12 were confirmed reinfections as stated in the original studies. Notwithstanding the limited number of confirmed reinfections, we showed in the present review that 50% of genetically confirmed cases of re-infection were observed within 90 days after initial infection. This finding questions the current recommendation on contract tracing and travel restrictions. In view of our results, application of the current regulations could lead to an underestimation of re-infections and their potential threat to public health measures. There is therefore the need to continuously update current policies to respond to the dynamic situation of the global pandemic. Most especially, as our review has identified that the duration between infection episodes can be shorter than suggested in the 90 days regulation.
We found limited evidence on onwards transmission of recurrent SARS-CoV-2 infection. In total, only 15 studies assessed contact tracing or follow-up of re-positives, but four of these found evidence for onward transmission from re-positives [14,51,55,60]. This potential infectiousness in addition to the known reluctance of recovered patients to adhere to mitigation measures [15] emphasizes the need for further studies on onward transmission. Evidence from these studies can strongly impact on testing and tracing regulations, as well as on quarantine and isolation requirements.
As the pandemic progresses and as re-positive cases are reportedly increasing, it is essential to identify individuals who are at most risk of reinfection. In our review, we did not find conclusive evidence on risk factors, timing and mechanism of re-infection, nor a cause of re-positivity. In terms of risk factors, no reliable predictive marker was found, but prolonged initial illness [95], moderate disease severity, decreased leucocytes, low platelets and low CD4 count [96] were associated with re-positivity. The association of recurrent SARS-CoV-2 infection with age was controversial.
Limitations
Synthesizing data from different study designs including preprints to respond to the pressing needs for scientific evidence, enabled us to provide robust evidence on the extent and characteristics of reinfections. However, there are some limitations to the review. Firstly, some included studies were lacking important information including details on timing of testing and definition of re-infection. Critical appraisal was applied to take quality of studies into account. Secondly, restricting the language to English, could decrease generalizability of results as included studies may not cover all studies on recurrent SARS-CoV-2 infection. Thirdly, we did not perform double data extraction. But, in order to minimize data extraction error, a sample of 20% of extracted data was randomly cross checked. Finally, the RKI definition of reinfection probability has some limitations as confirmed infection considers viral load. But most included studies did not provide detailed information on the number of genetic copies of SARS-CoV-2 or perform virus cultures.
The present review has shown that re-positivity rates are high, but data on cause of re-positivity, infectivity and predictive markers are scarce. However, this review emphasizes the continuous need to update policies on contact tracing and quarantine regulations. Only by taking re-infections into account, it is possible to respond to the COVID-19 strategic preparedness and response plan of the WHO [102] and get in control of the global pandemic.
Conclusion
In this review, we found that recurrence of a positive SARS-CoV-2 test among previously recovered cases is a commonly-reported phenomenon within the first few weeks from recovery. While some of these cases follow exposure, confirmed SARS-CoV-2 re-infections are rare. Fifty percent of genetically confirmed cases of re-infection were observed within 90 days after initial disease. Evidence on onwards transmission and predictive markers is limited but existent. With this high rate of recurrence of SARS-CoV-2, and mixed evidence of the risk to public health, policy makers need to re-consider current policies of contact tracing and quarantine regulations.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the team at RKI library for their support and for contribution to the search tools.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Jing Q-L, Liu M-J, Zhang Z-B, Fang L-Q, Yuan J, Zhang A-R, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. The Lancet Infectious Diseases. 2020;20(10):1141–50. doi: 10.1016/S1473-3099(20)30471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021:eabg3055. doi: 10.1126/science.abg3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer. COVID-19 Coronavirus Pandemic 2021 [cited 2021 17 March]. Available from: https://www.worldometers.info/coronavirus/.
- 4.Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. The Lancet. doi: 10.1016/S0140-6736(21)00306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. COVID-19 vaccines [updated 13.08.2021; cited 2021 26.08.2021]. Available from: https://www.who.int/westernpacific/emergencies/covid-19/covid-19-vaccines.
- 6.Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421–5. doi: 10.1038/s41586-021-03647-4 [DOI] [PubMed] [Google Scholar]
- 7.Vanshylla K, Di Cristanziano V, Kleipass F, Dewald F, Schommers P, Gieselmann L, et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host & Microbe. 2021;29(6):917-29.e4. 10.1016/j.chom.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfego D, Sullivan A, Poirier B, Williams J, Grover A, Gillim L, et al. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine. 2021;36:100902. 10.1016/j.eclinm.2021.100902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siggins MK, Thwaites RS, Openshaw PJM. Durability of Immunity to SARS-CoV-2 and Other Respiratory Viruses. Trends in Microbiology. 2021;29(7):648–62. 10.1016/j.tim.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SeyedAlinaghi S, Oliaei S, Kianzad S, Afsahi AM, MohsseniPour M, Barzegary A, et al. Reinfection risk of novel coronavirus (CoVID-19): A systematic review of current evidence. World journal of virology. 2020;9(5):79. doi: 10.5501/wjv.v9.i5.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. The Lancet Infectious diseases. 2021;21(1):52–8. Epub 2020/10/16. doi: 10.1016/S1473-3099(20)30764-7 ; PubMed Central PMCID: PMC7550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, et al. Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. EBioMedicine. 2020;59:102960. Epub 2020/08/28. doi: 10.1016/j.ebiom.2020.102960 ; PubMed Central PMCID: PMC7444471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC K. Findings from investigation and analysis of re-positive cases 2020 [cited 2021 09 Feb]. Available from: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1externalicon.
- 14.Pérez-Lago L, Pérez Latorre L, Herranz M, Tejerina F, Sola-Campoy PJ, Sicilia J, et al. A complete analysis of the epidemiological scenario around a SARS-CoV-2 reinfection: previous infection events and subsequent transmission. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith LE, Mottershaw AL, Egan M, Waller J, Marteau TM, Rubin GJ. The impact of believing you have had COVID-19 on self-reported behaviour: Cross-sectional survey. PloS one. 2020;15(11):e0240399. doi: 10.1371/journal.pone.0240399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ECDC. Risk of SARS-CoV-2 transmission from newly-infected individuals with documented previous infection or vaccination 2021 [cited 2021 30 March]. Available from: https://www.ecdc.europa.eu/en/publications-data/sars-cov-2-transmission-newly-infected-individuals-previous-infection.
- 17.CDC. Duration of Isolation and Precautions for Adults with COVID-19 2021 [cited 2021 15 Feb]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html.
- 18.ECDC. Contact tracing: public health management of persons, including healthcare workers, who havehad contact with COVID-19 cases in the European Union–thirdupdate 2020 [cited 2021 09 Feb]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-contact-tracing-public-health-management-third-update.pdf.
- 19.RKI. Kontaktpersonen-Nachverfolgung bei SARS-CoV-2-Infektionen 2021 [cited 2021 09 Feb]. Available from: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Kontaktperson/Management.html.
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The EndNote Team. EndNote. Philadelphia, PA: Clarivate Analytics. [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic reviews. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moola S MZ, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer’s Manual.The Joanna Briggs Institute, 2017. Available fromhttps://reviewersmanual.joannabriggs. doi: 10.11124/JBISRIR-2016-003247 [DOI] [Google Scholar]
- 24.NIH. Study Quality Assessment Tools 2021 [cited 24 Feb 2021]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 25.Pilz S, Chakeri A, Ioannidis JP, Richter L, Theiler-Schwetz V, Trummer C, et al. SARS-CoV-2 re-infection risk in Austria. European journal of clinical investigation. n/a(n/a):e13520. 10.1111/eci.13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. The Lancet. 2021. 10.1016/S0140-6736(21)00575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav SP, Thakkar D, Bhoyar RC, Jain A, Wadhwa T, Imran M, et al. Asymptomatic reactivation of SARS-CoV-2 in a child with neuroblastoma characterised by whole genome sequencing. IDCases. 2021;23:e01018. PubMed PMID: rayyan-123531947 doi: 10.1016/j.idcr.2020.e01018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS-CoV-2 reinfection—six possible cases. The Journal of infection. 2020. Epub 2020/08/18. doi: 10.1016/j.jinf.2020.08.011 ; PubMed Central PMCID: PMC7422822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geling T, Huaizheng G, Ying C, Hua H. Recurrent positive nucleic acid detection in a recovered COVID-19 patient: A case report and literature review. Respiratory medicine case reports. 2020;31:101152. Epub 2020/07/25. doi: 10.1016/j.rmcr.2020.101152 ; PubMed Central PMCID: PMC7351047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahin M, Khan MH, Hossain I, Mullick AR. RISK OF REACTIVATION OR REINFECTION OF NOVEL CORONAVIRUS: A STUDY BASED ON CASE REPORTS. International Journal of Public Health and Clinical Sciences. 2020;7(5):107–11. [Google Scholar]
- 31.Zheng SL, Sun WL, Sun LN, Zeng XH, Wang QX. Negative viral nucleic acid test of induced sputum: an additional criteria for COVID-19 patient’s discharge. European review for medical and pharmacological sciences. 2020;24(22):11934–8. Epub 2020/12/05. doi: 10.26355/eurrev_202011_23853 . [DOI] [PubMed] [Google Scholar]
- 32.Gousseff M, Penot P, Gallay L, Batisse D, Benech N, Bouiller K, et al. Clinical recurrences of COVID-19 symptoms after recovery: Viral relapse, reinfection or inflammatory rebound? The Journal of infection. 2020;81(5):816–46. Epub 2020/07/04. doi: 10.1016/j.jinf.2020.06.073 ; PubMed Central PMCID: PMC7326402 to declare regarding this subject. This work had no financial support. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao j, Hu Z, Liu J, Pang P, Fu G, Qian A, et al. Positive RT-PCR Test Results in Discharged COVID-19 Patients: Reinfection or Residual?: Research Square; 2020. doi: 10.3389/fneur.2020.606924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington D, Kele B, Pereira S, Couto-Parada X, Riddell A, Forbes S, et al. Confirmed Reinfection with SARS-CoV-2 Variant VOC-202012/01. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2021. Epub 2021/01/10. doi: 10.1093/cid/ciab014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert Koch Institut. Definition für die Reinfektion mit SARS-CoV-2 2021 [cited 2021 31 March]. Available from: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Reinfektion.html.
- 36.AlFehaidi A, Ahmad SA, Hamed E. SARS-CoV-2 re-infection: a case report from Qatar. The Journal of infection. PubMed PMID: rayyan-123529938. doi: 10.1016/j.jinf.2020.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso FOM, Sabino BD, Guimarães M, Varella RB. Recurrence of SARS-CoV-2 infection with a more severe case after mild COVID-19, reversion of RT-qPCR for positive and late antibody response: Case report. Journal of medical virology. 93(2):655–6. PubMed PMID: rayyan-123529953. doi: 10.1002/jmv.26432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao G, Zhu Z, Fan L, Ye S, Huang Z, Shi Q, et al. Absent immune response to SARS-CoV-2 in a 3-month recurrence of coronavirus disease 2019 (COVID-19) case. Infection.1–5. PubMed PMID: rayyan-123530538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrocínio de Jesus R, Silva R, Aliyeva E, Lopes L, Portugalyan M, Antunes L, et al. Reactivation of SARS-CoV-2 after Asymptomatic Infection while on High-Dose Corticosteroids. Case Report. SN comprehensive clinical medicine. 1–4. PubMed PMID: rayyan-123531375. doi: 10.1007/s42399-020-00548-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tehrani HA, Darnahal M, Nadji SA, Haghighi S. COVID-19 re-infection or persistent infection in patient with acute myeloid leukaemia M3: a mini review. New microbes and new infections. 39:100830. PubMed PMID: rayyan-123531765. doi: 10.1016/j.nmni.2020.100830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JS, Kim SY, Kim TS, Hong KH, Ryoo NH, Lee J, et al. Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Reinfection After Recovery from Mild Coronavirus Disease 2019. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. doi: 10.1093/cid/ciaa1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman JD, Wang K, Roltgen K, Nielsen SCA, Roach JC, Naccache SN, et al. Reinfection with SARS-CoV-2 and Failure of Humoral Immunity: a case report. medRxiv: the preprint server for health sciences. 2020. Epub 2020/10/01. doi: 10.1101/2020.09.22.20192443 ; PubMed Central PMCID: PMC7523175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta V, Bhoyar RC, Jain A, Srivastava S, Upadhayay R, Imran M, et al. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. doi: 10.1093/cid/ciaa1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington D, Kele B, Pereira S, Couto-Parada X, Riddell A, Forbes S, et al. Confirmed Reinfection with SARS-CoV-2 Variant VOC-202012/01. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. PubMed PMID: rayyan-123530661. doi: 10.1093/cid/ciab014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulder M, van der Vegt D, Oude Munnink BB, GeurtsvanKessel CH, van de Bovenkamp J, Sikkema RS, et al. Reinfection of SARS-CoV-2 in an immunocompromised patient: a case report. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. PubMed PMID: rayyan-123531220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prado-Vivar B, Becerra-Wong M, Guadalupe JJ, Márquez S, Gutierrez B, Rojas-Silva P, et al. A case of SARS-CoV-2 reinfection in Ecuador. The Lancet Infectious diseases. PubMed PMID: rayyan-123531420. doi: 10.1016/S1473-3099(20)30910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selhorst P, Van Ierssel S, Michiels J, Mariën J, Bartholomeeusen K, Dirinck E, et al. Symptomatic SARS-CoV-2 reinfection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. PubMed PMID: rayyan-123531606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Elslande J, Vermeersch P, Vandervoort K, Wawina-Bokalanga T, Vanmechelen B, Wollants E, et al. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/09/06. doi: 10.1093/cid/ciaa1330 ; PubMed Central PMCID: PMC7499557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/08/26. doi: 10.1093/cid/ciaa1275 ; PubMed Central PMCID: PMC7499500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colson P, Finaud M, Levy N, Lagier JC, Raoult D. Evidence of SARS-CoV-2 re-infection with a different genotype. The Journal of infection. 2020. Epub 2020/11/19. doi: 10.1016/j.jinf.2020.11.011 ; PubMed Central PMCID: PMC7666873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonifácio LP, Pereira APS, Araújo D, Balbão V, Fonseca B, Passos ADC, et al. Are SARS-CoV-2 reinfection and Covid-19 recurrence possible? a case report from Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2020;53:e20200619. PubMed PMID: rayyan-123530121. doi: 10.1590/0037-8682-0619-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loconsole D, Passerini F, Palmieri VO, Centrone F, Sallustio A, Pugliese S, et al. Recurrence of COVID-19 after recovery: a case report from Italy. Infection. 48(6):965–7. PubMed PMID: rayyan-123531036. doi: 10.1007/s15010-020-01444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogawa Y, Nishida K, Gohma I, Kasahara K, Yano H. Assessing the effects of exposure to a SARS-CoV-2 re-positive patient in healthcare personnel. BMC research notes. 13(1):511. PubMed PMID: rayyan-123531304. doi: 10.1186/s13104-020-05365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P. Recurrent presence of SARS-CoV-2 RNA in a 33-year-old man. Journal of medical virology. 93(2):592–4. PubMed PMID: rayyan-123531878. doi: 10.1002/jmv.26334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patrocínio de Jesus R, Silva R, Aliyeva E, Lopes L, Portugalyan M, Antunes L, et al. Reactivation of SARS-CoV-2 after Asymptomatic Infection while on High-Dose Corticosteroids. Case Report. SN comprehensive clinical medicine. 2020:1–4. Epub 2020/10/13. doi: 10.1007/s42399-020-00548-x ; PubMed Central PMCID: PMC7531809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu R, Jiang Z, Gao H, Huang D, Jiang D, Chen F, et al. Recurrent Positive Reverse Transcriptase-Polymerase Chain Reaction Results for Coronavirus Disease 2019 in Patients Discharged From a Hospital in China. JAMA network open. 3(5):e2010475. PubMed PMID: rayyan-123530709. doi: 10.1001/jamanetworkopen.2020.10475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J, Zheng L, Li Z, Hao S, Ye F, Chen J, et al. Kinetics of SARS-CoV-2 positivity of infected and recovered patients from a single center. Scientific reports. 10(1):18629. PubMed PMID: rayyan-123530717. doi: 10.1038/s41598-020-75629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulder M, van der Vegt D, Oude Munnink BB, GeurtsvanKessel CH, van de Bovenkamp J, Sikkema RS, et al. Reinfection of SARS-CoV-2 in an immunocompromised patient: a case report. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/10/13. doi: 10.1093/cid/ciaa1538 ; PubMed Central PMCID: PMC7665355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prado-Vivar B, Becerra-Wong M, Guadalupe JJ, Márquez S, Gutierrez B, Rojas-Silva P, et al. A case of SARS-CoV-2 reinfection in Ecuador. The Lancet Infectious diseases. 2020. Epub 2020/11/27. doi: 10.1016/S1473-3099(20)30910-5 ; PubMed Central PMCID: PMC7833993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selhorst P, Van Ierssel S, Michiels J, Mariën J, Bartholomeeusen K, Dirinck E, et al. Symptomatic SARS-CoV-2 reinfection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/12/15. doi: 10.1093/cid/ciaa1850 ; PubMed Central PMCID: PMC7799230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali AM, Ali KM, Fatah MH, Tawfeeq HM, Rostam HM. SARS-CoV-2 Reinfection in Patients Negative for Immunoglobulin G Following Recovery from COVID-19. medRxiv: the preprint server for health sciences. 2020:2020.11.20.20234385. doi: 10.1101/2020.11.20.20234385 [DOI] [Google Scholar]
- 62.Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibodies to SARS-CoV-2 are associated with protection against reinfection. medRxiv: the preprint server for health sciences. 2020:2020.11.18.20234369. doi: 10.1101/2020.11.18.20234369 [DOI] [Google Scholar]
- 63.Abu-Raddad LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. doi: 10.1093/cid/ciaa1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao AT, Tong YX, Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. Journal of medical virology. 2020;92(10):1755–6. Epub 2020/04/10. doi: 10.1002/jmv.25855 ; PubMed Central PMCID: PMC7262304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen LZ, Lin ZH, Chen J, Liu SS, Shi T, Xin YN. Can elevated concentrations of ALT and AST predict the risk of ’recurrence’ of COVID-19? Epidemiology and infection. 2020;148:e218. Epub 2020/09/22. doi: 10.1017/S0950268820002186 ; PubMed Central PMCID: PMC7522471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao W, Wang Y, Tang Y, Zhao W, Fan Y, Liu G, et al. Characteristics of Children With Reactivation of SARS-CoV-2 Infection After Hospital Discharge. Clinical pediatrics. 2020;59(9–10):929–32. Epub 2020/05/29. doi: 10.1177/0009922820928057 . [DOI] [PubMed] [Google Scholar]
- 67.Hanrath AT, Payne BAI, Duncan CJA. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. The Journal of infection. 2020. Epub 2020/12/30. doi: 10.1016/j.jinf.2020.12.023 ; PubMed Central PMCID: PMC7832116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patwardhan A. Sustained Positivity and Reinfection With SARS-CoV-2 in Children: Does Quarantine/Isolation Period Need Reconsideration in a Pediatric Population? Cureus. 2020;12(12):e12012. Epub 2020/12/17. doi: 10.7759/cureus.12012 ; PubMed Central PMCID: PMC7732141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bongiovanni M, Vignati M, Giuliani G, Manes G, Arienti S, Pelucchi L, et al. The dilemma of COVID-19 recurrence after clinical recovery. The Journal of infection. 2020;81(6):979–97. Epub 2020/08/19. doi: 10.1016/j.jinf.2020.08.019 ; PubMed Central PMCID: PMC7428731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng D, Zhang J, Ji Y, Pan D. Risk factors for redetectable positivity in recovered COVID-19 children. Pediatric pulmonology. 2020;55(12):3602–9. Epub 2020/10/14. doi: 10.1002/ppul.25116 ; PubMed Central PMCID: PMC7675449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Hu Y, Yu Y, Zhang X, Li B, Wu J, et al. Positive result of Sars-Cov-2 in faeces and sputum from discharged patients with COVID-19 in Yiwu, China. Journal of medical virology. 2020;92(10):1938–47. Epub 2020/04/21. doi: 10.1002/jmv.25905 ; PubMed Central PMCID: PMC7264799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang C, Jiang M, Wang X, Tang X, Fang S, Li H, et al. Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study. Emerging microbes & infections. 2020;9(1):2368–78. Epub 2020/11/06. doi: 10.1080/22221751.2020.1837018 ; PubMed Central PMCID: PMC7655076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan L, Wang R, Yu N, Hu C, Yan J, Zhang X, et al. Clinical characteristics of re-hospitalized COVID-19 patients with recurrent positive SARS-CoV-2 RNA: a retrospective study. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2021:1–8. Epub 2021/01/16. doi: 10.1007/s10096-020-04151-9 ; PubMed Central PMCID: PMC7808928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du HW, Chen JN, Pan XB, Chen XL, Yixian Z, Fang SF, et al. Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2021;40(2):413–7. Epub 2020/09/01. doi: 10.1007/s10096-020-04024-1 ; PubMed Central PMCID: PMC7456660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiao XM, Xu XF, Zi H, Liu GX, Li BH, Du X, et al. Re-positive Cases of Nucleic Acid Tests in Discharged Patients With COVID-19: A Follow-Up Study. Frontiers in medicine. 2020;7:349. Epub 2020/07/14. doi: 10.3389/fmed.2020.00349 ; PubMed Central PMCID: PMC7324684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.An J, Liao X, Xiao T, Qian S, Yuan J, Ye H, et al. Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Annals of translational medicine. 2020;8(17):1084. Epub 2020/11/05. doi: 10.21037/atm-20-5602 ; PubMed Central PMCID: PMC7575971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdullah MS, Chong PL, Asli R, Momin RN, Mani BI, Metussin D, et al. Post discharge positive re-tests in COVID-19: common but clinically non-significant. Infectious diseases (London, England). 2020;52(10):743–5. Epub 2020/06/25. doi: 10.1080/23744235.2020.1780309 . [DOI] [PubMed] [Google Scholar]
- 78.Wong J, Koh WC, Momin RN, Alikhan MF, Fadillah N, Naing L. Probable causes and risk factors for positive SARS-CoV-2 test in recovered patients: Evidence from Brunei Darussalam. Journal of medical virology. 2020;92(11):2847–51. Epub 2020/06/20. doi: 10.1002/jmv.26199 ; PubMed Central PMCID: PMC7323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang YJ. South Korea’s COVID-19 Infection Status: From the Perspective of Re-positive Test Results After Viral Clearance Evidenced by Negative Test Results. Disaster medicine and public health preparedness. 2020:1–3. Epub 2020/05/23. doi: 10.1017/dmp.2020.168 ; PubMed Central PMCID: PMC7298089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou J, Zhang J, Zhou J, Yi H, Lin Z, Liu Y, et al. Clinical characteristics of re-positive COVID-19 patients in Huangshi, China: A retrospective cohort study. PloS one. 2020;15(11):e0241896. Epub 2020/11/05. doi: 10.1371/journal.pone.0241896 ; PubMed Central PMCID: PMC7641455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo S, Guo Y, Zhang X, Xu H. A follow-up study of recovered patients with COVID-19 in Wuhan, China. International Journal of Infectious Diseases. 2020;99:408–9. doi: 10.1016/j.ijid.2020.05.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang YJ, Joo H. South Korea’s COVID-19 infection status: From the perspective of reconfirmation after complete recovery. Journal of Pure and Applied Microbiology. 2020;14:1073–5. PubMed PMID: rayyan-123530817. [Google Scholar]
- 83.Wang X, Zhang P, Xiao J, Zhu Y, Zhang L, Chen L. Positive virus detection in patients who recovered from COVID-19 during quarantine period between discharge and home: a two-center experience in Wuhan, China. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2020:1–5. Epub 2020/11/26. doi: 10.1007/s10096-020-03918-4 ; PubMed Central PMCID: PMC7685185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu J, Li S, Wu Y, Xiong Z, Yang Y, Gong L, et al. Surveillance and re-positive RNA test in patients recovered from COVID-19. Journal of medical virology. 2020. Epub 2020/09/30. doi: 10.1002/jmv.26568 ; PubMed Central PMCID: PMC7646640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tao W, Wang X, Zhang G, Guo M, Ma H, Zhao D, et al. Re-detectable positive SARS-CoV-2 RNA tests in patients who recovered from COVID-19 with intestinal infection. Protein & cell. 2020:1–6. Epub 2020/09/27. doi: 10.1007/s13238-020-00778-8 ; PubMed Central PMCID: PMC7518948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu T, Wu S, Zeng G, Zhou F, Li Y, Guo F, et al. Recurrent positive SARS-CoV-2: Immune certificate may not be valid. Journal of medical virology. 2020;92(11):2384–6. Epub 2020/05/30. doi: 10.1002/jmv.26074 ; PubMed Central PMCID: PMC7283767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J, Xu X, Hu J, Chen Q, Xu F, Liang H, et al. Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan, China. Aging. 2020;12(17):16675–89. Epub 2020/09/11. doi: 10.18632/aging.103795 ; PubMed Central PMCID: PMC7521537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng J, Zhou R, Chen F, Tang G, Wu K, Li F, et al. Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: A prospective cohort study. PLoS neglected tropical diseases. 2020;14(8):e0008648. Epub 2020/09/01. doi: 10.1371/journal.pntd.0008648 ; PubMed Central PMCID: PMC7505432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang K, Lau JY-N, Yang L, Ma Z-G. SARS-CoV-2 reinfection in two patients who have recovered from COVID-19. Precision Clinical Medicine. 2020;3(4):292–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian M, Long Y, Hong Y, Zhang X, Zha Y. The treatment and follow-up of ’recurrence’ with discharged COVID-19 patients: data from Guizhou, China. Environmental microbiology. 2020;22(8):3588–92. Epub 2020/07/08. doi: 10.1111/1462-2920.15156 ; PubMed Central PMCID: PMC7361525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. Polymerase Chain Reaction Assays Reverted to Positive in 25 Discharged Patients With COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020;71(16):2230–2. Epub 2020/04/09. doi: 10.1093/cid/ciaa398 ; PubMed Central PMCID: PMC7184423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J, Cheng J, Shi X, Liu J, Huang B, Zhao X, et al. Recurrence of SARS-CoV-2 nucleic acid positive test in patients with COVID-19: a report of two cases. BMC pulmonary medicine. 20(1):308. PubMed PMID: rayyan-123531929. doi: 10.1186/s12890-020-01348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X, Li M, Zhang B, Chen T, Lv D, Xia P, et al. The N gene of SARS-CoV-2 was the main positive component in repositive samples from a cohort of COVID-19 patients in Wuhan, China. Clinica chimica acta; international journal of clinical chemistry. 511:291–7. PubMed PMID: rayyan-123532016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gidari A, Nofri M, Saccarelli L, Bastianelli S, Sabbatini S, Bozza S, et al. Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2021;40(1):1–12. Epub 2020/10/11. doi: 10.1007/s10096-020-04057-6 ; PubMed Central PMCID: PMC7547550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azam M, Sulistiana R, Ratnawati M, Fibriana AI, Bahrudin U, Widyaningrum D, et al. Recurrent SARS-CoV-2 RNA positivity after COVID-19: a systematic review and meta-analysis. Scientific reports. 2020;10(1):20692. Epub 2020/11/28. doi: 10.1038/s41598-020-77739-y ; PubMed Central PMCID: PMC7691365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao MQ, Zheng QX, Xu J, Deng JW, Ge TT, Zhou HB, et al. Factors associated with a SARS-CoV-2 recurrence after hospital discharge among patients with COVID-19: systematic review and meta-analysis. Journal of Zhejiang University: Science B. 2020;21(12):940–7. doi: 10.1631/jzus.B2000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dao TL, Hoang VT, Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2021;40(1):13–25. Epub 2020/10/29. doi: 10.1007/s10096-020-04088-z ; PubMed Central PMCID: PMC7592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arafkas M, Khosrawipour T, Kocbach P, Zielinski K, Schubert J, Mikolajczyk A, et al. Current meta-analysis does not support the possibility of COVID-19 reinfections. Journal of medical virology. 2020. doi: 10.1002/jmv.26496 [DOI] [PubMed] [Google Scholar]
- 99.Elsayed SM, Reddy MK, Murthy PM, Gupta I, Valiuskyte M, Sánchez DF, et al. The Possibility and Cause of Relapse After Previously Recovering From COVID-19: A Systematic Review. Cureus. 2020;12(9):e10264. Epub 2020/10/13. doi: 10.7759/cureus.10264 ; PubMed Central PMCID: PMC7537484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoang T. Systematic review and meta-analysis of factors associated with re-positive viral RNA after recovery from COVID-19. Journal of medical virology. 2020. Epub 2020/11/03. doi: 10.1002/jmv.26648 . [DOI] [PubMed] [Google Scholar]
- 101.Mattiuzzi C, Henry BM, Sanchis-Gomar F, Lippi G. SARS-CoV-2 recurrent RNA positivity after recovering from coronavirus disease 2019 (COVID-19): a meta-analysis. Acta bio-medica: Atenei Parmensis. 2020;91(3):e2020014. Epub 2020/09/15. doi: 10.23750/abm.v91i3.10303 ; PubMed Central PMCID: PMC7717013 consultancies, stock ownership, equity interest, patent/licensing arrangement et al.) that might pose a conflict of interest in connection with the submitted article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.WHO. COVID-19STRATEGIC PREPAREDNESS AND RESPONSE PLAN 2021 [cited 2021 March 03]. Available from: https://cdn.who.int/media/docs/default-source/3rd-edl-submissions/who_sprp-2021final18022021.pdf?sfvrsn=ce5092f9_1&download=true.