Abstract
Selective progesterone receptor modulators (SPRMs) are a new class of compounds developed to target the progesterone receptor (PR) with a mix of agonist and antagonist properties. These compounds have been introduced for the treatment of several gynecological conditions based on the critical role of progesterone in reproduction and reproductive tissues. In patients with uterine fibroids, mifepristone and ulipristal acetate have consistently demonstrated efficacy, and vilaprisan is currently under investigation, while studies of asoprisnil and telapristone were halted for safety concerns. Mifepristone demonstrated utility for the management of endometriosis, while data are limited regarding the efficacy of asoprisnil, ulipristal acetate, telapristone, and vilaprisan for this condition. Currently, none of the SPRMs have shown therapeutic success in treating endometrial cancer. Multiple SPRMs have been assessed for efficacy in treating PR-positive recurrent breast cancer, with in vivo studies suggesting a benefit of mifepristone, and multiple in vitro models suggesting the efficacy of ulipristal acetate and telapristone. Mifepristone, ulipristal acetate, vilaprisan, and asoprisnil effectively treated heavy menstrual bleeding (HBM) in patients with uterine fibroids, but limited data exist regarding the efficacy of SPRMs for HMB outside this context. A notable class effect of SPRMs are benign, PR modulator-associated endometrial changes (PAECs) due to the actions of the compounds on the endometrium. Both mifepristone and ulipristal acetate are effective for emergency contraception, and mifepristone was approved by the US Food and Drug Administration (FDA) in 2012 for the treatment of Cushing’s syndrome due to its additional antiglucocorticoid effect. Based on current evidence, SPRMs show considerable promise for treatment of several gynecologic conditions.
Keywords: mifepristone, ulipristal acetate, vilaprisan, asoprisnil, uterine fibroid, breast cancer
Graphical Abstract
Graphical Abstract.
Essential Points
Progesterone is a factor involved in the development of and treatment of gynecological diseases such as uterine fibroids, endometriosis, endometrial cancer, and breast cancer.
Selective progesterone receptor modulators (SPRMs) are new classes of synthetic compounds that possess agonist and antagonist properties with demonstrated therapeutic potential for uterine fibroids, endometriosis, endometrial cancer, and breast cancer.
Mifepristone is well known for antiglucocorticoid activity (US FDA-approved for the treatment of Cushing’s syndrome in 2012), and its antiprogesterone activity is associated with beneficial effects, such as decreased volume and symptoms of uterine fibroids, but also detrimental effects including endometrial hyperplasia.
The long-term use of asoprisnil is effective in reducing uterine and fibroid size, controlling bleeding, and improving quality of life but may pose a safety concern because of long-term endometrial effects of uninterrupted treatment.
Ulipristal acetate can effectively control bleeding, reduce fibroid size, and improve quality of life without showing significant adverse events except for endometrial hyperplasia without evidence of atypia and has been approved in Canada and Europe as a presurgical therapy for patients with uterine fibroids as well as for emergency contraception in the United States.
Telapristone acetate appears to be effective in fibroid treatment, but the development of the compound was suspended in 2009 due to concerns regarding liver toxicity; recently, studies using lower doses have been restarted.
Vilaprisan is the newest addition of SPRMs that shows efficacy for controlling bleeding and reducing fibroid size and is in clinical trials.
Progesterone
Progesterone (P4; preg-4-ene-3,20-dione) is a natural female sex hormone. It plays essential roles in female reproductive function, including menstruation, implantation, and pregnancy as well as breast development and lactation (1). P4 biosynthesis starts with the common precursor molecule cholesterol (2). Cholesterol is converted to pregnenolone by cytochrome P450scc (CYP11A1) (3), which is then converted to progesterone by the enzyme 3β-hydroxysteroid dehydrogenase (HSD3B) (2). The metabolism of progesterone is rapid and extensive and occurs mainly in the liver. It may form 1 of many different unconjugated metabolites from enzymatic reduction by reductases and hydroxysteroid dehydrogenases (4, 5). Progesterone is metabolized by 5α-reductase and 5β-reductase into dihydrogenated 5α-dihydroprogesterone (5α-DHP) and 5β-dihydroprogesterone (5β-DHP), respectively. These 2 metabolites are further metabolized into tetrahydrogenated allopregnanolone, pregnanolone, isopregnanolone, and epipregnanolone by HSD3A and HSD3B. Progesterone may also be hydroxylated by 17α-hydroxylase (CYP17A1) and 21-hydroxylase (CYP21A2) into 17α-hydroxyprogesterone (17α-OHP) and 21-hydroxyprogesterone, respectively (4, 5). 5α-DHP is an agonist of the progesterone receptor (PR) and shows 82% binding affinity for PR in rhesus monkey uterus (6). Unlike 5α-DHP, 5β-DHP shows very weak binding affinity (1.2%) for the PR (6). In addition, both 5α-DHP and 5β-DHP may act as modulators of γ-aminobutyric acid type A (GABAA) receptors that modify a range of behaviors (7). 5α-DHP has shown potent progestogenic bioactivity in mares; it stimulates endometrial growth and P4-dependent gene expression that maintain equine pregnancy in the absence of luteal P4 (8). 5β-DHP is a potent ligand for an orphan nuclear receptor, pregnane X receptor (PXR), while 5α-DHP shows weak binding affinity for PXR (9). 5β-DHP has been reported to regulate uterine contractility through activation of PXR (10). Allopregnanolone and pregnenolone do not bind to PR, but they are potent modulators of GABAA receptors (7). These 2 metabolites also act as agonists of the PXR. Allopregnanolone plays an important role in mode swings during reproductive events (11). Pregnenolone may exert protective effects against schizophrenia (12) and improve cognitive and memory function (13). In addition, both allopregnanolone and pregnanolone may be involved in sedation and anesthesia of the fetus (14). Two other tetrahydrogenated metabolites, isopregnanolone and epipregnanolone, may act as negative regulators of GABAA receptors and reverse the effect of potentiators, such as allopregnanolone (15,16). The hydroxylated P4 metabolite 17α-OHP is an agonist of the PR but weak in comparison to progesterone. It shows very weak agonism with the glucocorticoid receptor (GR) (17) and antagonism with the mineralocorticoid receptor (MR) (18).
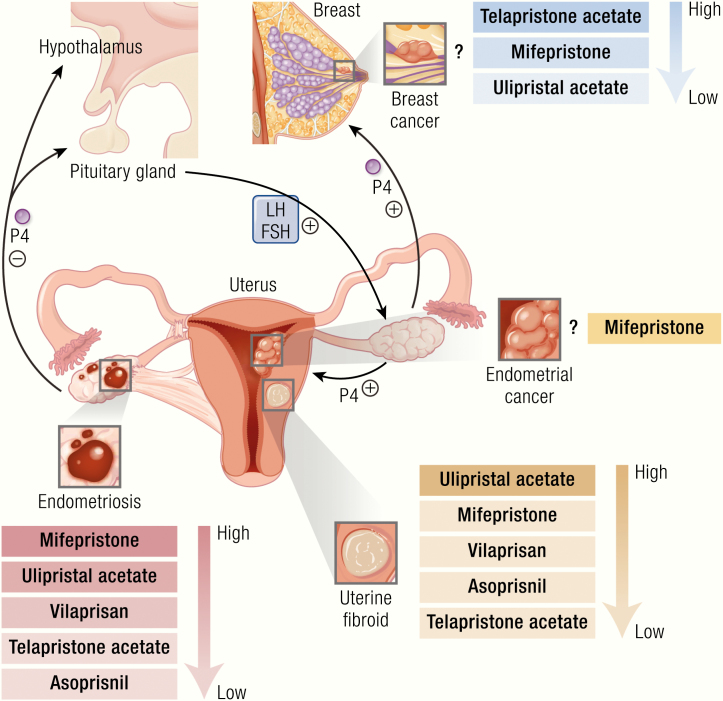
P4 is primarily produced by the ovary, specifically from the corpus luteum, a temporary endocrine gland that develops after ovulation from the ruptured follicle. Placenta and adrenal glands also produce P4. During the menstrual cycle, P4 produced by the corpus luteum converts the endometrium into a secretory state in preparation for implantation of an embryo. If implantation occurs, production of human chorionic gonadotropin hCG) by the embryo supports continued production by the corpus luteum and progesterone levels remain elevated until placental production of progesterone eclipses ovarian production at 6 to 8 weeks of pregnancy. When fertilization does not take place, the P4 levels drop, leading to menstruation via breakdown and shedding of the endometrial tissues. During the normal menstrual cycle, circulating levels of P4 in the body depend upon a normally functioning hypothalamic-pituitary-ovarian axis. The pituitary gland is activated by pulsatile gonadotropin-releasing hormone (GnRH) secretion from the median eminence of the hypothalamus. This leads to production of follicle stimulating hormone (FSH) and luteinizing hormone (LH), which act to stimulate ovulation, leading to ovarian production of P4 (Fig. 1). In addition to the important role of P4 in normal physiological processes of the human body, progesterone is involved as a stimulatory or inhibitory molecule in different pathological conditions, such as uterine fibroids, endometriosis, endometrial cancer, and breast cancer (19) (Fig. 1). Because the role of progesterone in these pathologies is essential yet relatively tissue-specific, selective progesterone receptor modulators (SPRMs) have been developed as therapeutic options for P4 responsive diseases (20) as well as for emergency contraception (EC) (21).
Figure 1.
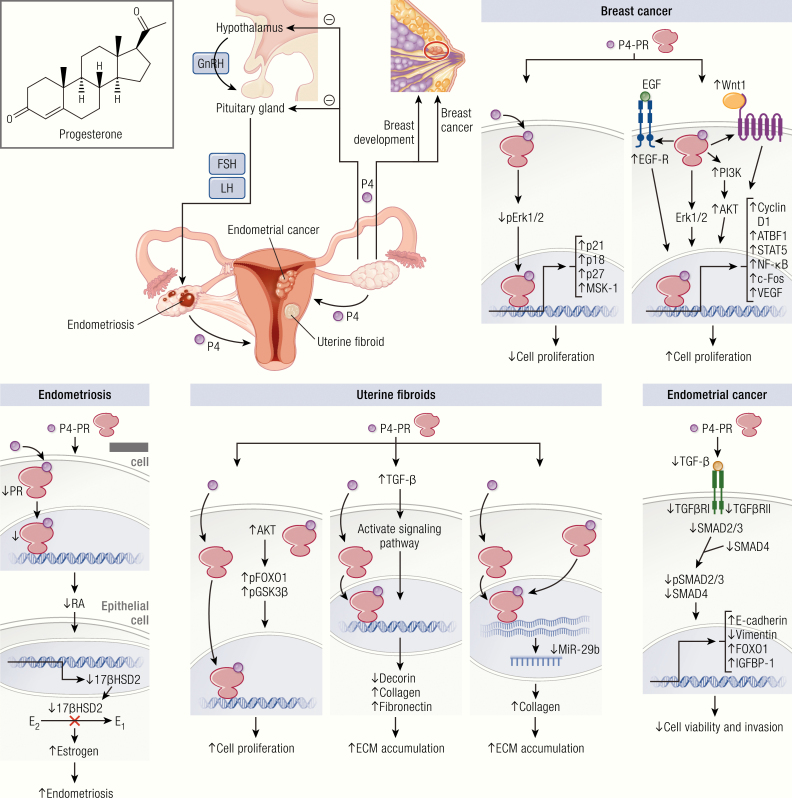
Progesterone role in reproductive diseases. Progesterone synthesis starts with the signal from the hypothalamus to the pituitary gland to release FSH and LH, which further stimulate ovaries to produce progesterone. Progesterone primarily regulates female reproductive function and breast development. Binding of progesterone at its receptor differentially affects tissue growth at its various sites of action. Progesterone is thought to be stimulatory for uterine fibroid growth, while it is protective for endometrial cancer and endometriosis. The effect of progesterone on breast cancer is complex and variable. Progesterone plays a role in the growth and development of uterine fibroids through stimulation of cell proliferation and facilitating extracellular matrix accumulation. This occurs through activation of the AKT and TGF-β3 pathways and the effects of their downstream intermediaries. Progesterone is thought to negatively impact the development of endometriosis; a reduction in P4-regulated genes and PR-B expression in stromal cells has been reported in endometriotic lesions. Progesterone is thought to play a protective role in the development of some endometrial cancers through downregulation of the TGF-β signaling cascades, with the downstream effect inhibiting the growth of endometrial epithelial cells and reducing cancer cell viability and invasion. In the breast, the role of progesterone is complex and controversial. P4 has been shown to drive proliferation, survival, invasion, and angiogenesis of breast cancer cells through the EGF and Wnt-1 pathway, as well as various other intermediaries. In contrast, progestin has also been shown to induce MKP-1 (MAPK phosphatase 1) expression in a PR-dependent fashion as a means of inducing antiproliferative effects.
Progesterone receptors and their activation
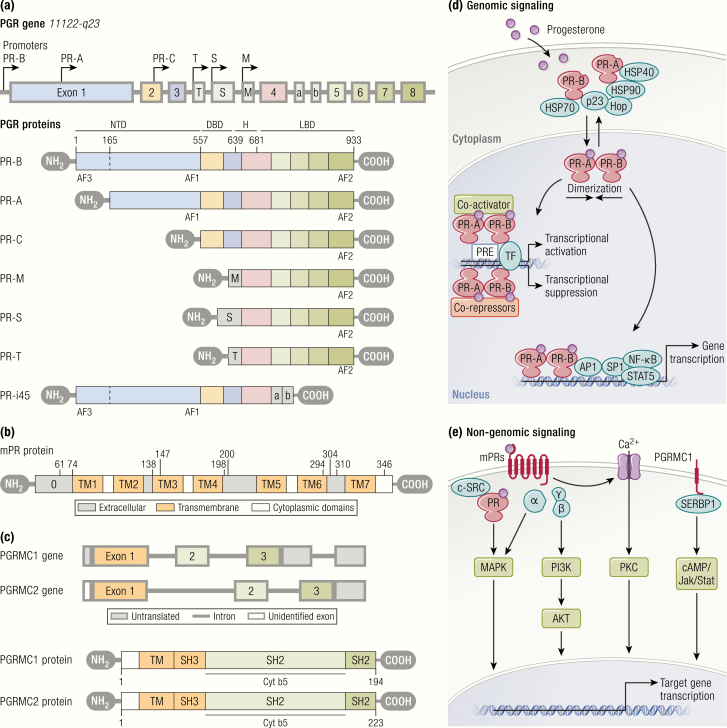
The diverse effects of P4 on target tissues are primarily mediated by the PRs, which are members of the nuclear receptor family of ligand-dependent transcription factors. Progesterone receptors exist as 2 functionally active isoforms, PR-B and PR-A (22) (Fig. 2). Other truncated alternatively spliced isoforms, including PR-C (60 kDa) (23), PR-S (24), PR-T (25), PR-i45 (26), and PR-M (~ 38 kDa) (27) have been reported. Though these isoforms and variants have been shown to have effects, the essential nature of their roles has not been fully elucidated. Progesterone receptor isoforms are encoded by a single gene (HUGO gene symbol = PGR) located at chromosome 11 (11q22-q23). The PGR gene possesses 8 exons and 7 introns. PR-B is a full length protein (116 kDa) of 933 amino acids, while PR-A is truncated (94 kDa) in the N-terminal region, lacking 164 amino acids present in PR-B (28). As a result, the 2 receptors act as functionally distinct transcriptional factors (22). PR-A may act to suppress the function of PR-B, while PR-B often acts as a potent activator for transcription of target genes (29).
Figure 2.
Progesterone receptors and their activation. A: Structural and functional properties of progesterone receptor isoforms. PR-B is a protein of 933 amino acids, while PR-A lacks 164 amino acids of PR-B at N-terminal region. The common structural elements include highly variable NTD, DBD, H region, and LBD. PR-B consists of 2 transcription activation functions, (AF)-1 and AF-3, but PR-A consists of only AF-1 located at NTD. AF-2 located at LBD presents in both PR isoforms. Hinge region is involved in the binding of DNA and coregulators, and the dimerization of receptors following active transport of PR into the nucleus. Other truncated progesterone receptor isoforms are demonstrated below the shaded box. PR-C contains deletions at the amino terminus that likely result from an alternative location for translation initiation. PR-S and PR-T likely give rise to identical proteins that are truncated at the amino-terminus due to retention of an intronic sequence termed exon S or exon T, respectively. They both retain transcription of H and LBD. PR-M contains a novel 16 amino acid amino-terminal sequence encoded by a sequence in the distal third intron of the PR gene, followed by exons 4 through 8 of the original PR gene. PR-i45 retains 2 intronic sequences termed exons i45a and i45b. This leads to a change in the reading frame, which causes a truncated protein that lacks a functional LBD and DBD. B: Schematic diagram of mPR protein showing extracellular (gray), 7-transmembrane (orange), and cytoplasmic (clear) domains predicted by several programs. C: PGRMC1 is comprised of a single N-terminal TM and a Cyt b5 domain. The protein has sites for interaction with SH (Src-homology)-2 and SH-3 domains of Src tyrosine kinases, kinase binding sites, and a phosphorylation site for tyrosine and serine/threonine kinases. D and E: Progesterone receptor-mediated genomic and nongenomic signaling pathways. Genomic signaling begins with progesterone binding to nuclear receptors (PR-A and PR-B), which induces receptor activation and leads to dissociation with heat shock proteins (HSP90, HSP70, and HSP40), following dimerization and translocation into the nucleus where they bind with PREs within the promoter of target genes. It is the subsequent interaction of the transcription complex with specific coregulators and transcription factors that initiate the transcriptional activation or suppression of target genes. Liganded PR can also activate transcription of genes, the promoters of which lack PREs by acting as a bridge between transcription factors and coactivators at promoters containing activator protein 1 (AP-1), specificity protein1 (Sp1), signal transducer and activator of transcription 5 (STAT5), and NF-κB sites. Progesterone elicits nongenomic actions through binding with membrane-bound progesterone receptors (mPRs: mPRα, mPRβ, and mPRγ; and PGRMC1) or cytoplasmic PRs following association with cytoplasmic kinase cascades (such as cSrc) and downstream signaling pathways. These include (MAPK, Ca2+ infiux/PKC activation, and the PI3K/AKT pathway. P4 exerts nongenomic actions through PGRMC1 via association with SERBP1 and downstream signaling through the cAMP and Jak/Stat kinase signaling pathways.
The 2 functionally active PR isoforms (PR-A and PR-B) share some common elements, such as highly variable N-terminal A/B domain (NTD), DNA binding domain (DBD), hinge (H) region, and ligand-binding domain (LBD) (30) (Fig. 2). The NTD component plays an important role in activation of transcription, and both receptors can be conceptualized as ligand-dependent transcript factors. PR-B contains 2 activation functions, (AF)-1 and AF-3, while PR-A contains only AF-1 at the N terminal region (31). The H region is located between DBD and LBD. The H region is involved in the DNA and coregulatory protein binding and dimerization of receptors. This region contains a nuclear localization signal (NLS) for the active transport of PR from the cytoplasm to the nucleus (32) and a site for post-translational modifications (33). The LBD spans most of the C-terminal of PR and contains AF-2 (34). In addition to the binding hormone, the LBD binds coregulators and participates in receptor dimerization (35). PR-C lacks the DBD region as well as the 2 activation function domains AF-3 and AF-1 (23). PR-A and PR-B possess a distinct affinity for specific coregulators (defined as corepressors and coactivators). PR-A shows higher binding affinity for corepressors (such as SMRT [silencing mediator of retinoid and thyroid hormone receptor]), while PR-B exhibits a higher binding affinity for coactivators (such as, SRC-1 [steroid receptor coactivator-1]) (36).
The “classical pathway” of P4 actions includes genomic actions mediated by nuclear PRs (Fig. 2). In the absence of a bound hormone, PRs are complexed with suppressive chaperone molecules such as heat shock proteins (HSPs) HSP90, HSP70, and HSP40, and cochaperone proteins Hop and p23 (37). Binding of P4 to PRs induces receptor activation, a process involving a conformational change of the receptor that leads to the dissociation of chaperones followed by dimerization and translocation of the complex from the cytoplasm into the nucleus. Activated PRs bind to specific PR elements (PREs) within the promoter region of target genes (38) and interacts with specific coregulators (such as SRC-1, SRC-2, and SRC-3, CREB-binding protein (CBP)/p300, and SMRT) (39) and general transcription factors, thereby forming a complex on the target gene promoters. The resulting complex then initiates the transcriptional activation or suppression of target genes. Progesterone receptors can also induce transcription of genes that lack PREs by cooperating with other DNA-bound transcription factors (40), including activator protein 1 (AP-1) (41), specificity protein1 (Sp1) (42), signal transducer and activator of transcription 5 (STAT5) (43), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (44). Progesterone receptors possess numerous sites for post-translational modification, including phosphorylation (45), ubiquitination (46), and sumoylation (47) that serve to influence their stability, trafficking, transcriptional activity, and target gene selectivity.
In addition to genomic actions, P4 can elicit rapid, nongenomic actions (also referred to as “nonclassic” or “extranuclear” effects), which take place within seconds to minutes (48) (Fig. 2). This rapid P4 response can be initiated at the cell surface to activate intracellular signaling pathways through the activation of cytoplasmic PRs or membrane-bound PRs (mPRs) (49). Progesterone receptors can activate mitogen-activated protein kinase (MAPK) signaling pathway through its proline-rich motif by interacting with c-Src tyrosine kinase directly (50) or indirectly (51). The mPRs, also called PAQRs (progestin and adipoQ receptors), were first characterized in fish ovaries (52) and contain 7 transmembrane domains. Three mPR isoforms (~40 kDa), including mPRα (PAQR7), mPRβ (PAQR8), and mPRγ (PAQR5), were subsequently identified in humans and other vertebrate species (53, 54). Two other mPRs, mPRδ (PAQR6) and mPRϵ (PAQR9), have also been proposed as being capable of responding to progesterone (54). The mPR isoforms are encoded by separate genes and possess 7 transmembrane domains. The transcript sizes of the mPR varied from 2.8 to 5.8 kb (53). Dressing et al demonstrated the expression of mPRα, mPRβ, and mPRγ subtypes in human breast tumor biopsies as well as their localization, signaling, and antiapoptotic actions in PR-negative breast cancer cell lines (55). P4 treatment caused rapid activation of p42/44 MAPK in PR-negative breast cancer cell lines (55). The mPRs mediate other nongenomic signaling pathways, including Ca2+ infiux/(protein kinase C) PKC activation (56) and phosphatidylinositol 3-kinases (PI3K)/AKT pathway (55, 57).
In addition to the classical PRs and mPRs, PR membrane component 1 (PGRMC1) (26–28 kDa) (58) and PGRMC2 (59) are 2 other novel membrane proteins belonging to the heme-binding protein family of membrane-associated PR proteins. PGRMC1 consists of 194 amino acids and contains a short N-terminal extracellular domain, a single transmembrane domain, a cytoplasmic domain, and a cytochrome b5 domain (60, 61) (Fig. 2). It is primarily located in the cell membrane but is also expressed in the endoplasmic reticulum and Golgi apparatus (62). It also contains 3 binding interfaces for Src homology domains, which include 2 SH (Src homology)-2 and 1 SH-3 target sequences (63). P4 binds to PGRMC1, which in turn associates with SERBP1 (serpine binding protein 1) and signals through the cyclic adenosine monophosphate (cAMP), and Jak/Stat kinase signaling pathways (64, 65). PGRMC1 can also bind to cytochrome P450 and complex with proteins implicated in cholesterol synthesis, therefore possibly playing a role in progesterone production (66). PGRMC2 is structurally similar to PGRMC1 and likely evolved from a common ancestor (58, 59). However, PGRMC2 differs from PGRMC1 in the N-terminal and transmembrane domains, which indicates that the 2 receptors may interact with different proteins (67).
Progesterone receptors in normal uterine and mammary function
The human uterus is a dynamic organ that is highly responsive to ovarian steroids. It is composed of 2 major compartments, the endometrium and myometrium. The endometrium can be further divided into the luminal and glandular epithelia, surrounded by the stromal layer. The endometrium undergoes dynamic changes during the normal menstrual cycle under the influence of E2 and P4. During the follicular phase of the menstrual cycle, also called the proliferative phase, estrogen levels are high. In this phase the tissue is extensively repaired from the damage caused by menses via E2-mediated proliferation of the epithelial and stromal cells. After ovulation, the endometrium enters into the secretory phase. During this phase, rising circulating P4 antagonizes the proliferative effects of E2, causing differentiation of stromal cells towards decidualization (68). This effect highlights the complex functional interaction between the estrogen receptor (ER) and PR systems. To facilitate this, the relative levels of ER and PR vary between endometrial cell types throughout the menstrual cycle. During the preovulatory phase, ER and PR expression levels were found to be higher in both epithelial and stromal cells, while in the luteal phase, ER staining strongly decreased in both cell types, but stromal cells stained moderately for PR (69, 70). PR-A and PR-B are highly expressed in glandular epithelium during the proliferative phase of the menstrual cycle, which is consistent with the known induction of PR expression by E2. However, there is also predominant expression of PR-B in the glands during the midsecretory phase (71), suggesting its significance in glandular secretion. In the stroma, PR-A is the predominant isoform throughout the menstrual cycle (71, 72), implicating this isoform in postovulatory P4-mediated events. P4 action in the uterus is affected not only by ligand bioavailability but also potentially from the ratio of receptor isoforms in the tissue.
The novel membrane P4 receptors, PGRMC1 and PGRMC2, are differentially expressed in human endometrium (73, 74). PGRMC1 levels are highly expressed in the proliferative phase, but then decline during the secretory phase in an artificial menstrual cycle model in monkeys. PGRMC1 was localized to the stroma, glandular epithelium, and luminal epithelium of the endometrium (74). In contrast, PGRMC2 levels were reduced during the proliferative phases of the cycle and then increased markedly during the secretory phase. PGRMC2 was localized to the luminal and glandular epithelia (74). Bunch et al reported that both PGRMC1 and PGRMC2 levels were significantly lower in the secretory phase endometrium in women with endometriosis (75). These dynamic changes of the PGRMC1: PGRMC2 ratio in the uterus over the reproductive cycle may associate with the development of uterine disease such as endometriosis.
The human myometrium expands through hyperplasia and hypertrophy of smooth muscle cells over the course of pregnancy (76). Both PR-A and PR-B are expressed in the myometrium (77). PR-B is the more abundant isoform in human myometrium at the preterm stage and maintains its levels at the nonlaboring stage, while PR-A is more prominent in the laboring myometrium (77), implicating that regulation of the PR-A:PR-B ratio may contribute to preparation and switch of myometrium into a contractile state. Indeed, recent work showed that the PR-A ligand can independently stimulate the expression of the key labor promoting gene Cx43 (78), while PR-B knockout mice undergo normal pregnancy and parturition (79). In contrast, mice lacking PR-A show ovarian deficiencies and embryos fail to implant (80). The membrane-associated PRs, mPRα and mPRβ, have been detected in human pregnant myometria and in pregnant myometrial cells (81). The activation of mPRs leads to the transactivation of PR-B (81), suggesting cross-talk between mPRs and nuclear PRs, and a possible role in shifting the balance from a quiescent state to one of contraction. Furthermore, Kowalik et al reported the differential expression of mPRα, mPRβ, and mPRγ receptors in the bovine uterus during the reproductive cycle (82), supporting their participation in the regulation of uterine functions.
The mammary gland is a hormonally responsive tissue which undergoes most of its development after birth. It is comprised of 2 major tissue compartments, epithelium and stroma. The epithelial compartment is made up of 2 different cell types, luminal epithelial cells and myoepithelial cells. Three major hormones, estrogens, progesterone, and prolactin, play important roles in the development of the mammary gland (83). In adult ovariectomized BALB/c mice, P4 was found to promote proliferation of luminal and myoepithelial cells, resulting in side branching and alveologenesis. This effect is amplified when E2 is combined with P4 (84). The role of P4 in mammary development are regulated by the ratio of PR-A and PR-B in target tissues. Indeed, mice lacking PR or with an abnormal ratio of PR-A and PR-B exhibit incomplete mammary gland development (85, 86). PR-A levels are induced by E2 and reduced by P4, while PR-B levels are induced by P4 or E2 plus P4 (84). Aupperlee et al reported that PR-A was predominantly found in the virgin mammary gland during active ductal development, while its levels were significantly lower during pregnancy (87). On the other hand, PR-B levels were higher during pregnancy and during alveologenesis (87). A mouse model with deletion of PR-A (80) or PR-B (79) was developed to distinguish the specific role of PR isoforms in uterine and mammary function. PR-A knockout mice show infertility with defects in uterine and ovarian function (80). On the other hand, PR-B knockout mice demonstrate dispensable uterine function and have markedly reduced pregnancy-associated ductal sidebranching and lobuloalveolar development (79). These observations suggest that PR-A may function as the primary driver of uterine function and is sufficient for fertility, while PR-B may be critical for mammary gland development and morphogenesis during pregnancy.
Role of progesterone in reproductive diseases
Uterine fibroids. Uterine fibroids are the most common benign tumors of the female genital tract and are believed to originate from the myometrial smooth muscle cells of the uterus. The prevalence of uterine fibroids is over 3-fold higher in black women compared to white women (88). Several recent studies suggest that vitamin D deficiency is differentially associated with race and fibroid development (89–91). Black women are 10-fold more likely to have vitamin D deficiency compared to white women (92), which may contribute to the higher prevalence of fibroids in this population. While 77% of women will have fibroids detected in the uterus, approximately 25% of women experience associated symptoms (93, 94). Uterine fibroids are primarily associated with abnormal and heavy uterine bleeding, pelvic pain or pressure, as well as bowel and bladder dysfunction. Reduced fertility and a decreased likelihood of clinical pregnancy, implantation, and live birth as well as an increased rate of spontaneous miscarriage have been clearly associated with uterine fibroids (95, 96). Fibroid disease is complicated because the tumors can be located throughout the uterus, seemingly randomly, growing to different sizes and occurring in different locations. For example, fibroids located within the uterine cavity interfere with endometrial development and cause bleeding and infertility, while fibroids located on the outer surface of the uterus do not impair fertility but may be associated with pain or gastrointestinal symptoms when large. This complicated disease exerts an enormous economic burden on the health care system worldwide. Estimates of the financial burden associated with the disease are a total annual cost of $5.89–$34.37 billion in the United States (97).
Various studies suggest that fibroids likely develop from a single myocyte. In analyses of multiple fibroids within the same uterus, chromosomal changes within different fibroids suggests that they develop independently. Further research utilizing X-inactivation concluded that each fibroid is likely clonal in origin. Original studies demonstrating this unicellularity of individual fibroids used isoenzyme analysis of glucose-6phosphate dehydrogenase (98, 99). Mashal et al studied the pattern of X-chromosome-linked inactivation of phosphoglycerokinase in fibroid cells, concluding that the monoclonal pattern of a single inactive allele likely suggested a unicellular origin (100). Fibroids have also been shown to exhibit an intrinsic growth rate independent of other fibroids within the same uterus (101).
Uterine fibroid growth is thought to be dependent on steroid hormones (Fig. 1) since fibroids appear during the reproductive years and regress after menopause. However, the measurement of fibroid growth by MRI revealed that within a single woman, some fibroids grew and others regressed, suggesting that factors in addition to sex steroids contributed to fibroid growth (102). While estrogen (E2) is considered the major mitogenic factor in the uterus, biochemical and clinical studies support the conclusion that P4-PR also plays an important role in fibroid growth and development (103, 104). Higher mitotic activity in uterine fibroids has been observed during the P4 dominance secretory phase compared to the proliferative phase of the menstrual cycle (105, 106), also supporting the P4 involvement in fibroid growth.
One distinguishing characteristic of fibroids is their excessive accumulation of extracellular matrix (ECM), including collagens, fibronectin, and proteoglycans (107). The growth of fibroids is influenced by cell-ECM interactions. The ECM of fibroids is abnormal in amount and structure. The rigid structure of the ECM and the abnormal fibrosis are a key pathologic feature. Furthermore, genetic factors have been reported to be associated with uterine fibroids. Mäkinen and colleagues reported that the mediator complex subunit 12 (MED12) gene is mutated at a high frequency (70%) in uterine fibroids (108). A recent study by Paakkola et al reported the biallelic mutations in human NHL repeat-containing protein 2 (NHLRC2) that promoted differentiation of fibroblasts to myofibroblasts in fibrosis, neurodegeneration, and cerebral angiomatosis disease (109). NHLRC2 might be of great interest in uterine fibroids.
P4 may stimulate fibroid growth by regulating growth factor function, ECM activity, and microRNA (miRNA) expression (107, 110) through interactions with PR-A and PR-B. Normal myometrium and uterine fibroids express both PR-A and PR-B, with elevated levels found in fibroids (111). P4 stimulates leiomyoma cell growth and survival through upregulation of antiapoptotic protein B-cell lymphoma (Bcl)-2 (112), epidermal growth factor (EGF) (113), and transforming growth factor-β3 (TGF-β3) (114), and through downregulation of tumor necrosis factor (TNF)-α (115). This stimulatory effect of P4 is mediated, at least in part, by activating the AKT pathway and its downstream effectors, p-GSK3β (phospho-glycogen synthase kinase 3β) and p-FOXO1 (phospho-forkhead Box O1), in fibroid cells (116). Decorin is a collagen-associated ECM within the proteoglycan family. Decorin has been shown to inhibit TGF-β3 (117). The higher levels of TGF-β3 mRNA were observed in leiomyoma (114), and TGF-β3 increased collagen and fibronectin expression (118). It was shown that P4 can decrease mRNA expression of decorin in uterine leiomyoma cells compared to controls (119). This suggests that P4 may influence fibroid growth by inducing TGF-β3 functions through downregulation of decorin activity. miRNAs are short (~22-nucleotide) noncoding RNAs that act as post-transcriptional regulators. The miR-29 family is commonly known as a tumor suppressor (120). It has 3 mature members, miR-29a, miR-29b, and miR-29c. Uterine leiomyoma demonstrate lower expression of miR-29b compared to myometrium (121). In a fibroid xenograft model, restoration of miR-29b resulted in the inhibited accumulation of several collagen subtypes (121). Qiang et al reported that P4 can upregulate mRNA expression of collagens via downregulating miR-29b expression (121). The role of P4 in fibroid growth was further elucidated by the observation that LAT2 (L-type amino acid transporter 2), a PR target gene, was induced by P4 and could be blocked by treatment with mifepristone, a P4 antagonist (122). This may explain the finding in several studies that mifepristone reduces fibroid size and symptoms (123–126). Furthermore, Yin et al reported that krüppel-like transcription factor 11 (KLF11), a known tumor suppressor, was slightly downregulated by P4 but profoundly upregulated by mifepristone treatment in uterine fibroid cells (127).
Endometriosis. Endometriosis is a debilitating condition that negatively affects a woman’s health and quality of life. The disease is characterized by the presence of endometrial glands and stroma located outside the uterine cavity (128). Sampson’s hypothesis postulates that endometriotic lesions may derive from abnormal endometrial cells that access the peritoneum by retrograde menstruation (129). However, endometriosis is actually a complex, protean disease that occurs in several different phenotypes, such as superficial or deeply-invasive disease, and one mechanism may not account for all varieties of disease (128, 130, 131). Endometriosis frequently results in pelvic pain and infertility (128, 132). However, these findings are not very specific; the incidence of endometriosis in reproductive-age women ranges from 6% to 10%, whereas endometriosis may only be present in 50% of women with infertility and 20% of women hospitalized with pelvic pain (131, 133). Endometriosis is a major cause of hysterectomies and hospitalization, and disease-associated costs account for an estimated total annual healthcare cost of $69.4 billion in 2009 in the United States (134).
Endometrial functions are greatly influenced by E2 and P4. These steroid hormones regulate the expression of hundreds to thousands of genes during the menstrual cycle (135). While E2 signaling is considered a major driver for the development and growth of endometriosis (128, 136), P4 plays an opposite role (137) (Fig. 1). Progesterone resistance is believed to play a role in the pathogenesis of endometriosis (138). The ratio of E2:P4 may be altered by the local expression of enzymes, which may in turn alter PR activation or inhibition in the disease state. HSD3B enzyme activity is one of the key mediators in the conversion of dehydroepiandrosterone to androstenedione, a precursor of estrogen production. Higher HSD3B2 mRNA expression and activity was observed in endometriotic tissue compared with normal endometrium (139), supporting the presence of elevated E2 levels in endometriosis. Lower expression of CYP11A1 was seen in the endometriotic lesions (139), indicating the low synthesis of P4 in endometriotic tissues. Huhtinen et al reported that expression of HSD17B2 was significantly lower, while expression of HSD17B6 and CYP19A1 was significantly higher in endometriotic lesions compared to endometrial tissue (140).
Endometriotic lesions demonstrate decreased expression of PR-A and an absence of PR-B compared to eutopic endometrium (141). In addition, it has been reported that several P4‐regulated genes, including glycodelin, N‐acetylglucosamine‐6‐O‐sulfotransferase, and 17β hydroxysteroid dehydrogenase 2 (17βHSD2), were reduced in eutopic endometrium from subjects with endometriosis (142, 143). In the endometrium, P4 induces expression of 17βHSD2, which catalyzes the conversion of biologically potent estradiol to the much less estrogenic estrone (144). P4 may increase the formation of retinoic acid by endometrial stromal cells, which in turn induces 17βHSD2 expression in endometrial epithelial cells in a paracrine manner (145, 146). However, endometriotic stromal cells do not respond to P4 and, therefore, no retinoic acid production occurs in these cells (147). The lack of retinoic acid leads to the decrease of epithelial 17βHSD2 and the failure to inactivate estradiol in endometriotic tissues (147, 148). The inability of endometriotic tissues to upregulate 17βHSD2 in response to P4 may be due to decreased expression of PR-B in stromal cells. Indeed, the loss of PR expression or disturbance of the PR-mediated signaling pathway is often linked with hyperactive E2 action in the endometrium and development of gynecological diseases, including endometriosis (149, 150). In a recent study, it was found that the treatment of female mice with P4 before artificial induction of endometriosis inhibited the development and growth of ectopic lesions, primarily through decreased cell proliferation, inflammation, and angiogenesis (137). Hence, the antiendometriotic nature of P4 has led to progestins as hormonal therapies for clinical treatment of endometriosis (151). Unfortunately, the therapeutic potential of P4 in the management of endometriotic patients remains challenging due to the proliferative role of P4 in endometrial stromal cells (152), which constitutes a major cellular component in the ectopic lesions. Clinical and translational studies suggest that endometriosis is a pleotropic condition and that some ectopic endometrial lesions are responsive to P4 therapy, but others may be resistant (132, 153). Further study is needed to characterize the basis of P4 resistance and to identify the driving factors that downregulate PR signaling pathways in these diseased tissues.
Endometrial cancer. Endometrial cancer is the most common gynecological cancer in the United States and arises in the glandular epithelium (154). In 2019, a total of 61 880 new endometrial cancer cases and 12 160 deaths from endometrial cancer are projected to occur in the United States (155). The primary presenting sign of endometrial cancer is abnormal vaginal bleeding, especially in postmenopausal women. However, endometrial cancer is diagnosed in approximately 3% to 14% of women at or younger than 40 years of age (156, 157). Fortunately, most treatment interventions are curative and the 5-year survival rate approaches 86% after surgical and/or radiation treatment for endometrial cancer (158).
Endometrial carcinoma is classified into histologic categories by cell type; the most common is endometrioid. Most tumors of this type express ERs and PRs. Genomic profiling has further identified various subtypes of endometrial cancer based on copy-number levels, microsatellite instability, and mutations in POLE, a catalytic subunit of DNA polymerase implicated in DNA replication and repair (159). In an analysis by Levine and colleagues, low copy-number levels were associated with increased PR expression. This feature was found in the majority of endometrioid tumors. Of note, the authors found that 25% of endometrioid tumors instead feature high copy-number levels (159), therefore explaining the nonuniversal response of this tumor type to hormonal therapy.
Analogous to their role in normal endometrial function, E2 and P4 govern and participate in growth and development of endometrial cancer. P4 exposure may attenuate endometrial cancer risk (160, 161) (Fig. 1), whereas continued, “unopposed” E2 exposure is strongly associated with increased endometrial cancer risk (162, 163). Hence, P4 has been targeted as a primary treatment for endometrial cancer in premenopausal women. Response rates in this group of women can be as high as 60%, indicating that P4 is a potent inhibitor of endometrial cancer growth (164).
The expression of PRs in endometrial glands can be controlled by both steroid hormones E2 and P4. E2 induces PR production, while P4 downregulates the expression of its own receptor (1). The efficacy of P4 in treating endometrial cancer typically depends on the presence of receptors in target tissues. Expression of PRs was positively correlated with a favorable prognosis and response to P4 treatment (165), whereas loss of PR expression underlies treatment failure. Yang et al examined the possibility of restoring PR expression in endometrial cancer cells by epigenetic modulation and then treating cells with the histone deacetylase inhibitor (HDACi) LBH589 (157). The authors found that treatment of endometrial cancer cells with LBH589 can induce robust upregulation of PR expression, which subsequently upregulates FOXO1, p21, and p27, and downregulates cyclin D1. LBH589 treatment also induces cell cycle arrest in G1; this process is further augmented by P4 (157). This innovative therapeutic approach may be used to sensitize endometrial tumors to progestin therapy.
The variable expression of PRs in endometrial cancer means that the action of P4 in this disease is complex. Ishikawa (well-differentiated) endometrial cancer cells express both PR-A and PR-B, with a predominance of PR-B (166), while poorly differentiated endometrial carcinoma cells express only PR-A (166). By microarray analysis, Jongen et al reported that PR-A and PR-B were associated with lower grade endometrial tumors (167). Advanced endometrial tumors were associated with predominant expression of PR-B (168). In contrast, Arnett-Mansfield et al reported the loss of both PR-A and PR-B isoforms in advanced endometrial cancer (169). Distribution was found to be variable between receptor type, with PR-A predominantly evenly distributed in endometrial cancers contrasting with focal localization of PR-B (170). This apparent inconsistency may relate to the rather dynamic expression of PRs in the endometrial cancers.
P4 exerts antiproliferative effects in endometrial cells through inducing transcription factor FOXO1. Endometrial tumor tissues demonstrate reduced FOXO1 expression compared to normal endometrium (171). Treatment with P4 has been shown to upregulate FOXO1 protein levels in Ishikawa endometrial cancer cells, acting through PR-B (171). FOXO1 can also act as a direct target of progestin in inhibiting growth of endometrial epithelial cells (172). Furthermore, FOXO1 is a known antimitogen and upstream regulator of insulin-like growth factor binding protein 1 (IGFBP-1). P4, through PR-B but not PR-A, can also induce expression of IGFBP-1 in endometrial epithelial cells (173), suggesting the FOXO1/IGFBP-1 axis is important for PR-B-dependent growth inhibition of endometrial epithelial cells. Bokhari et al reported that P4 can also exert an inhibitory effect on endometrial cancer by regulating TGF-β signaling (174). In their analysis, P4 treatment was found to reduce basal- and TGFβ1-induced endometrial cancer cell viability and invasion, which was associated with increased E-cadherin and decreased vimentin expression (174). P4 also inhibited TGF-β signaling cascades, such as TGFβ receptors (TGFβR1, TGFβR3), SMADs (SMAD2/3, pSMAD2/3, and SMAD4), and TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) (174).
Breast cancer. Breast cancer is the most frequent cause of cancer mortality in women worldwide. In the United States, 271 270 new breast cancer cases and 42 260 deaths from breast cancer are projected to occur in 2019 (155). Breast cancer can be categorized into 3 major groups according to the presence or absence of ER and PR, and human EGF receptor 2 (EGFR2 or ERBB2). These include ER-PR positive/ERBB2 negative (70% of patients), ERBB2 positive (15–20%), and triple-negative tumors that lack all receptors (15%) (175).
P4 is critically involved in normal breast development and function (1), and its effects are mostly mediated via PR-A and PR-B (176). The PR-A:PR-B ratio is strongly associated with breast cancer progression (176, 177) and indicates a potential benefit of endocrine therapy (178, 179). Breast cancer patients with high PR-A levels experienced a worse prognosis and resistance to tamoxifen treatment. However, individuals with this subtype did respond to anastrozole (179) and mifepristone (180). The role of P4 in breast cancer progression is complex and remains controversial. P4 may exert stimulatory or inhibitory effects in both breast cancer cells and in animal tumor models (181, 182) (Fig. 1). Progesterone receptor-positive mammary carcinoma cells demonstrated a biphasic cellular response, with an immediate proliferative burst followed by a sustained growth arrest in response to P4 or synthetic progestin treatment (181, 182). The synthetic progestin R5020 has been reported to inhibit the growth of T47D breast cancer cells when stimulated with different mitogens such as serum, estradiol, insulin, and EGF (183). The antiproliferative effect of P4 in breast cancer cells is mediated by the induction of the CDKIs (cyclin-dependent kinase inhibitors), including p21 (184), p18 (185), and p27 (185), as well as PR transcriptional coactivator TReP-132 (186). MAPK phosphatase 1 (MKP-1/DUSP1) is known to act as a counter-regulator of MAPK signaling. Progestin treatment inhibits cell proliferation via inducing MKP-1 expression in a PR-dependent fashion, in association with reduced levels of pERK1/2 in T47D breast cancer cells (187). These results suggest that MKP-1 is one of the critical mediators of the antiproliferative effects of P4-PR in breast cancer cells.
In contrast, clinical studies have reported that women exposed to E2 plus progestin experience an associated increase in breast cancer risk (188). A great deal of evidence supports this clinical observation. P4 drives proliferation, survival, invasion, and angiogenesis of breast cancer cells through multiple signaling cascades (Fig. 1). In human breast cancer cells, progestin is shown to inhibit cell death (189) and stimulate cell proliferation. This proliferation occurs, at least in part, through the upregulation of cyclin D1 expression via PI3K/AKT/NF-κB pathway (190). Carvajal et al reported that P4 alone, or in combination with EGF, induced cell proliferation of ZR-75 breast cancer cells, mediated partly by the EGF/ERK1/2/STAT5 pathway and the transcription factor c-fos (191). The growth of T47D breast cancer cells was also induced by progestin action through the upregulation of Wnt-1 and subsequent robust activation of the EGF-R and ERK1/2 pathways (192). These results demonstrate the physiological role of steroid hormone and growth factor signaling in promoting the survival or proliferation of early breast cancer lesions. P4 also induces expression of vascular endothelial growth factor (VEGF) mRNA and protein in cultured human T47-D breast cancer cells (193), suggesting that angiogenesis may be one mechanism of P4-induced breast cancer cell growth or metastasis. Mammary stem cells are putative targets for cell transformation events leading to breast cancer. P4 was shown to mediate mammary stem cell self-renewal via paracrine mechanisms in which luminal cells signal to basal cells via Wnt4 (wingless-type MMTV [mouse mammary tumor virus] integration site family, member 4) and RANKL (receptor activator of nuclear factor kappa-Β ligand or TNF superfamily member 11) (194). Transcription factor ATBF1 (AT motif-binding factor 1 or zinc finger homeobox 3) is a known prognostic indicator for breast cancer progression (195). Recently, ATBF1 has been reported as a transcriptional target of P4-PR signaling in mammary epithelial cells (196). ATBF1 expression is robustly induced by P4 action through PR in cultured cells and mammary tissues. P4-activated PR binds to the ATBF1 promoter, thus mediating the induction of stem cell marker expression and the expansion of progenitor cells (196).
Selective Progesterone Receptor Modulators
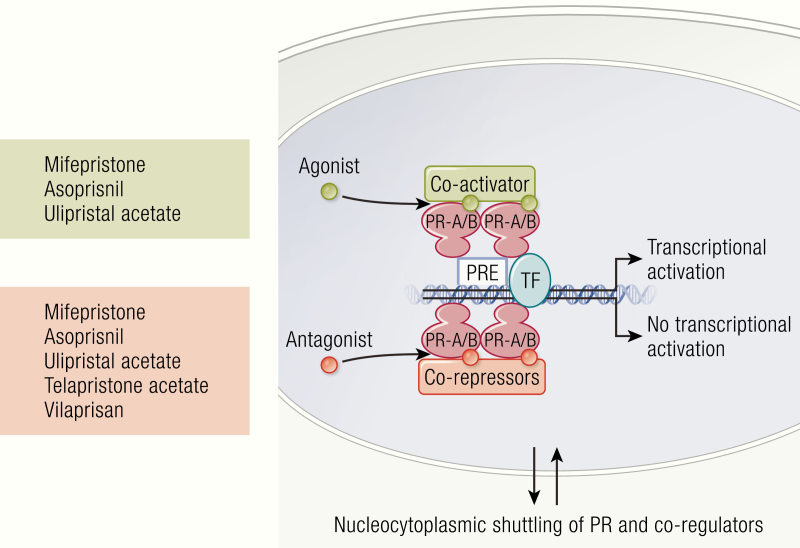
SPRMs are a new class of synthetic steroid ligands (Fig. 3) that are designed to compete at the PR-target site in a tissue-specific manner. This makes them clinically more effective and improves the ability to study progesterone-dependent gynecological diseases. Binding of the SPRM to the PR can induce agonistic, antagonistic, or mixed effects on the PR (197, 198) and persuade a communication for permitting receptor dimerization, binding DNA, and interrelating of coactivators and/or corepressors (Fig. 4). The strength of these divergent effects depends on the ratio of PR-A and PR-B in the tissue and the specific binding affinity of SPRMs for each receptor isoform (199). As agonists, SPRMs recruit more coactivators to induce transcriptional activation (200). However, SPRMs also compete with agonists and stimulate more effective corepressors to induce antagonistic effects (201). Most SPRMs have been developed for clinical application, and they are a desirable class of drugs due to their tissue selectivity and minimal undesirable side effects (20). This section introduces the chemistry and developmental history of the best-studied SPRMs in the context of their pharmacodynamics and mechanisms of action. Since poor oral bioavailability is increasingly an issue in the drug discovery process, the pharmacokinetics and metabolism of SPRMs is also addressed.
Figure 3.
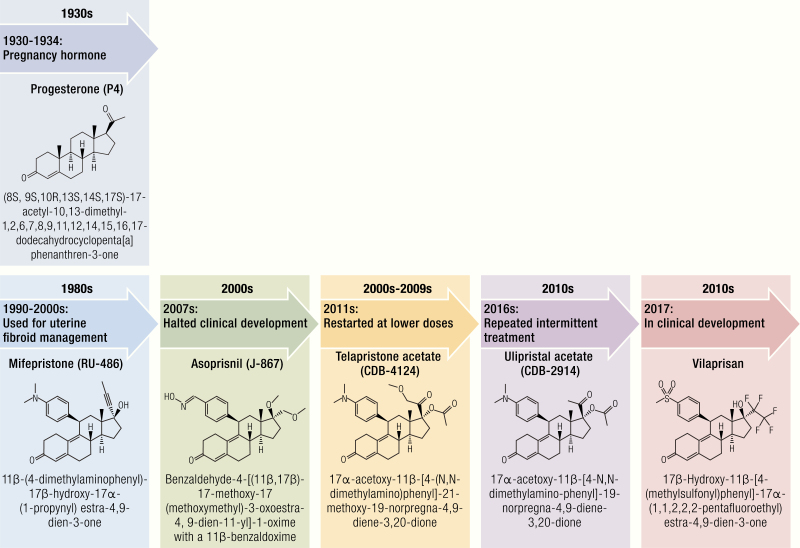
The chemistry and development of selective progesterone receptor modulators. Mifepristone was developed by the French company Roussel Uclaf S.A. It possesses antiprogesterone and antiglucocorticoid effects. Mifepristone has been approved by the FDA in February 2012 for Cushing’s syndrome. Asoprisnil was developing by Schering AG and TAP Pharmaceutical Products. The clinical trial of asoprisnil was suspended in 2007 due to the association with adverse endometrial changes. The development of ulipristal acetate began as CDB-2914 at the NIH and UPA has been approved in Canada and Europe for uterine fibroid treatment, as well as for EC in the United States. Telapristone acetate was also first developed at the NIH in 2000 and is currently under license at Repros Therapeutics Inc. The clinical development of telapristone acetate was suspended in 2009 due to liver toxicity, but studies have been restarted with lower doses. Vilaprisan is under development by Bayer HealthCare Pharmaceuticals. It is currently under investigation in phase III trials for long-term treatment of uterine fibroids.
Figure 4.
Mechanism of action of SPRMs. SPRMs mediate their effects on target cells by binding with PRs at different degrees that induces the recruitment of specific coactivators and corepressors following the modulation of the transcription of target genes.
Mifepristone
Chemistry and development. Mifepristone (RU-486) is a derivative of 19-nor-testosterone with chemical name 11β-(4-dimethylaminophenyl)-17β-hydroxy-17α-(1-propynyl) estra-4,9-dien-3-one (Fig. 3). The key structural elements of mifepristone are the 4-dimethylaminophenyl group at the 11β-position of the 19-nor-steroidal skeleton and 5 chiral centers with the stereochemistry 8S, 11R, 13S, 14S, and 17S. The molecular formula of mifepristone is C29H35NO2, with a molecular weight of 429 g/mol. It has double bonds at the C4 (5) and C9 (10) positions that exchange at the C11β and C17α positions. The substituted radical at position C11 and C17 are crucial of powerful antiprogesterone and antiglucocorticoid activities of mifepristone.
Initially following its discovery in 1980 mifepristone was explored for antiglucocorticoid activities (202). Later, in 1988, significant antiprogesterone activity was observed; due to this property, in 2000 the US government approved mifepristone coadministration with misoprostol for termination of intrauterine pregnancy of fewer than 49 days gestation (203).
Pharmacodynamics and mechanisms of action. Mifepristone shows higher binding affinity (100%) to the human PR than P4 (43%) and its metabolites (monodemethylated [21%], didemethylated [9%], alcoholic metabolites [15%]) in endometrial and myometrial samples (204). Mifepristone also has a 4-fold higher binding affinity to the GR compared to dexamethasone (204). In addition, in vitro receptor binding studies demonstrated that the binding affinities of mifepristone to PR and GR were 5-fold and 2-fold higher than the mifepristone metabolite metapristone (204–206). Mifepristone has a weak binding affinity for the androgen receptor (AR) and no affinity for the ER or MR in humans (207).
Mifepristone efficiently stimulates PR by inducing PR dimerization (as A:A, B:B, or A:B), which permits binding of the PR complex to the progesterone response element (PRE) of DNA (208, 209) (Fig. 4). These dimers have variable effects: A:A dimers are functionally silent, A:B dimers can activate transcription, and A:B dimers markedly inhibit transcriptional activation in P4 responsive cells (210, 211). Furthermore, in the presence of cAMP, mifepristone can convert both A and B receptor isoforms from a translational suppressor to activator (211).
Pharmacokinetics and metabolism. Mifepristone is partially absorbed by the gut following oral administration and is then subject to first-pass metabolism (212). The bioavailability of mifepristone at doses of 20 mg and 100 mg is 69% and 40%, respectively. Peak plasma concentrations of 0.36 to 6.7 μmol/L are achieved after 1 to 2 hours at lower mifepristone doses of 2 to 25 mg (213–215). The steady-state range of plasma concentrations of 1 to 1.5, 1.6 to 2.6, and 2.2 to 3.1 μmol/L were observed after administration of 12.5, 25, and 50 mg of mifepristone for 4 days, with levels still detectable up to 5 days (216). Mifepristone dosages of 25 mg for 14 days and 50 mg for 4 days also produced steady-state plasma concentrations of about 1 μmol/L (212) and 2.9 μmol/L (217). In the healthy human subject, mifepristone administered at doses ranging from 25 to 600 mg was still detectable from a minimum of 4 days (218, 219) to a maximum of 10 days (220). Mifepristone plasma concentrations were extended at 25 to 100 mg, with a half-life over 72 hours (220). In uterine fibroid patients, 12 to 15 hours following oral administration of 200 mg mifepristone, the concentration was 603 to 921 nmol/L in plasma, 195 to 344 pmol/g in myometrial tissue, and 444 to 1040 pmol/g in adipose tissue (216). However, the mono- and di-demethylated metabolites were found at levels 5.2-, 3.1-, and 1.4-times higher in serum, myometrium, and adipose tissues, respectively, compared to parent mifepristone (216).
Mifepristone demonstrates complex pharmacokinetics at higher doses. Within the first 48 hours after ingestion of 100 to 800 mg of mifepristone, no difference was found in the plasma concentration except at 2 hours (221). The disproportionality between the higher doses and achieved plasma concentration may arise due to its extensive metabolism. Similar bioavailability was evaluated in a randomized 2-way crossover study after intake of single doses of mifepristone at 75 mg in capsule or tablet form (222).
Several pharmacokinetic studies examined the metabolism of mifepristone and reported its metabolites, namely metapristone (218, 223), monodemethyl mifepristone, didemethyl mifepristone, and hydroxylated mifepristone (216, 218–220, 224), were rapidly formed within 0.5 to 1 hour after oral administration. Metapristone is the prominent metabolite and can easily be detected at high concentrations in the human body after absorption from the intestine into hepatic circulation (218, 219, 225). The elimination half-life of oral mifepristone was 12.6 to 26.0 hours and 19.8 to 33.1 hours at low doses (25–100 mg, respectively) and 37.6 to 50.9 hours and 40.9 to 124 hours at high doses (200–600 mg, respectively) (219, 225). The plasma concentrations of monodemethylated mifepristone were similar to the parent compounds, but didemethylated and hydroxylated mifepristone were only around 25% of mifepristone and monodemethylated mifepristone (225). Very similar to mifepristone, these metabolites are biologically and immunologically active and might contribute to the antiprogestational (23–30%) and, even more so, the antiglucocorticoid (47–61%) properties of mifepristone (225). Cytochrome P450 (CYP) isoform 3A4 is the main enzyme accountable for the metabolism of mifepristone (226, 227). Recombinant CYP3A4 oxidizes mifepristone to form monodemethylated, didemethylated, and C-hydroxylated metabolites. When metabolized by CYP3A5, only the demethylated metabolite is formed (228) (Fig. 5). Each metabolite may contribute to the drug’s affinity at the PR of 9% to 21% (212). The majority (83%) of mifepristone and its metabolites are eliminated via feces, with a very small fraction (~8.8%) excreted through the kidney over 6 to 7 days (229). The pharmacokinetic variability of mifepristone and its metabolites may differ by tissue dissemination, genetic transformation, and enzymatic polymorphisms among individuals.
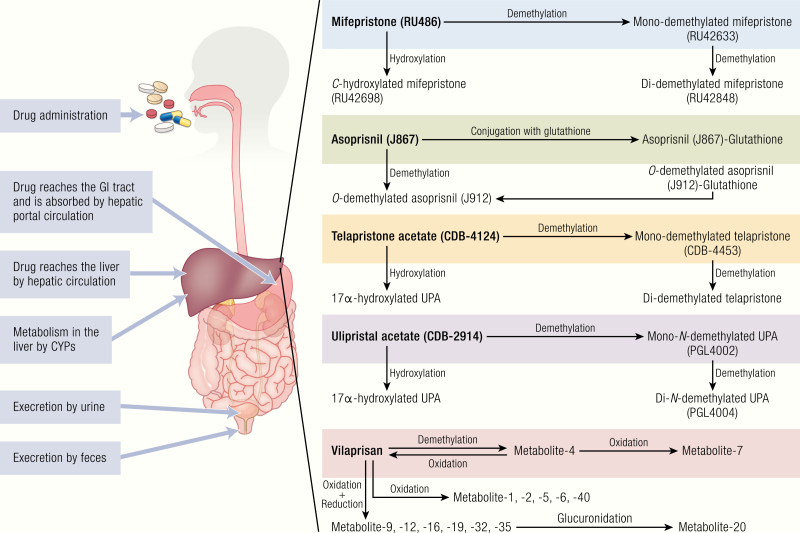
Figure 5.
Pharmacokinetics and metabolism of SPRMs. Oral administration of SPRMs is frequently absorbed in the gastrointestinal tract and transported directly to the liver. All the SPRMs are primarily metabolized in the liver by cytochromes. Monodemethylated, didemethylated, and hydroxylated metabolites of mifepristone are produced by demethylation and hydroxylation metabolic pathway. The asoprisnil metabolites are produced by 17β-O-demethylation, which further conjugated with glutathione (SG). Like mifepristone, telapristone acetate and ulipristal acetate UPA produce mono and didemethylated metabolites, as well as hydroxylated metabolite. It has been proposed that vilaprisan undergoes reduction to produce hydroxyl derivatives, which further oxidate to produce various metabolites. All the SPRM metabolites are further catalyzed by CYP3A4 and aldoketoreductases. The unabsorbed parent compounds and metabolites are eliminated via urine and feces.
Asoprisnil
Chemistry and development. Asoprisnil (J-867) is a hydrophobic oxime, benzaldehyde-4-[(11β,17β)-17-methoxy-17 (methoxymethyl)-3-oxoestra-4, 9-dien-11-yl]-1-oxime belonging to the novel class of 11β-benzaldoxime substituted steroidal SPRMs (Fig. 3). Its molecular formula is C28H35NO4 and it has a molecular weight of 449.591 g/mol. Asoprisnil was under investigation by Schering AG (Wedding, Berlin, Germany) and TAP Pharmaceutical Products (Lake Forest, Illinois) but clinical development was discontinued in 2007 due to the observation of abnormal endometrial changes in patients (230).
Pharmacodynamics and mechanisms of action. Asoprisnil demonstrates a 3-fold greater binding affinity to PR than P4 in the rabbit uterus. Its primary metabolite (demethylated asoprisnil, J912) also exhibits high binding affinity to PR compared to P4 (231). Mixed agonist and antagonist effects have been demonstrated in an animal model by large doses of both asoprisnil and its metabolite.
The ability for the asoprisnil-PR complex to recruit corepressors suggests the complex is able to form an antagonist conformation in addition to the agonist conformation observed when the complex recruits coactivators (232) (Fig. 4). The x-ray structure confirmed that asoprisnil weakly recruits the coactivators AIB1 (amplified in breast cancer 1) and SRC-1 but strongly recruits the corepressor nuclear receptor corepressor (NCoR) (232). In the rabbit uterine epithelium and in the guinea pig uterus and vagina, asoprisnil and its metabolite showed only partial agonist effect (231).
Pharmacokinetics and metabolism. The metabolic profile of asoprisnil has been studied in mouse and guinea pig models as well as monkey hepatocytes and human liver microsomes (233). While some quantitative differences exist between each model system, all have shown a qualitatively similar profile. J912, a product of 17β-O-demethylation, is the major CYP3A4-dependent metabolite of asoprisnil (Fig. 5). Asoprisnil and J912 are further conjugated with glutathione (SG) to J912-SG and J1099-SG. These compounds can be identified in large quantities in rat and mouse bile, and in lesser amounts in human hepatocytes and monkey, mouse, and rat tissue (233) (Fig. 5). The plasma levels of asoprisnil metabolite J912 are found to be 5-fold higher than asoprisnil levels. However, the excretion half-life of asoprisnil and J912 are similar, with mean values of about 4 to 5 hours in animals and humans (233).
Asoprisnil’s metabolites exhibit different functional activity depending on the presence or absence of P4. J912 displayed partial androgenic, weak antiglucocorticoid, and no estrogenic effects very comparable to asoprisnil and J1042 (231). The antagonistic abilities in endometrial transformation in the rabbit were stronger for J912 compared to asoprisnil or J1042 (234).
Ulipristal acetate
Chemstry and development. Unlike mifepristone, ulipristal acetate (UPA) (CDB-2914) is a derivative of 19-norprogesterone, with 11 β-aryl substituted 17α-acetoxy analogue (Fig. 3). The chemical name of this compound is 17α-acetoxy-11β-[4-N,N-dimethylamino-phenyl]-19-norpregna-4,9-diene-3,20-dione. The molecular formulation is C30H37NO4, with a molecular weight of 475.629 g/mol. CDB-2914 was developed by the Contraceptive Development Branch of the National Institute of Child Health and Human Development (NICHD) (235, 236) and licensed HRA-Pharma in 2006, and the compound was renamed as ulipristal acetate. Ulipristal acetate was approved for EC by the European Medicines Agency (EMA) in May 2009 and by the United States Food and Drug Administration (US FDA) in August 2010. In recent years, UPA has also been used for preoperative and intermittent treatment of moderate to severe uterine fibroids based on its effectiveness shown in phase III trials (237, 238).
Pharmacodynamics and mechanism of action. Ulipristal acetate has strong antagonistic and partial agonistic effects at PR in humans (235). Once this SPRM binds to PR, it reduces the binding capacity of endogenous P4 to its receptor and prevents PR-mediated DNA transcription (235) (Fig. 4). Ulipristal acetate also increases the PR isoform ratio of PR-A to PR-B by decreasing the level of PR-B receptor and increasing PR-A expression. This has been shown to prevent P4-mediated uterine fibroid growth in vitro models (239, 240). Ulipristal acetate has minimal antiglucocorticoid effects in rat and rabbit thymus gland (241) and has a much lower antagonist activity on GR compared to mifepristone (206). Additionally, this compound has little action on the AR and no effect on E2 and mineralocorticoid receptors (242).
Pharmacokinetic and metabolism. The pharmacokinetic profile of UPA was initially examined in female rhesus monkeys (243). The oral and intramuscular bioavailability of UPA equivalents were 56% and 62%, respectively. The mean peak plasma concentration was 192 ± 64 ng/mL after 5 ± 1 hours (243). An incremental oral study examined the pharmacokinetic profile of UPA at 10, 20, or 50 mg for 10 days in healthy females (244). After administration of UPA, the peak plasma concentration was reached within 1 hour, with a median terminal half-life of 0.75 to 0.89 hours and a mean plasma half-life of 38 to 49 hours (244). The maximum plasma concentration for UPA at 10, 20, and 50 mg was 42.2, 130.9, and 354.8 ng/mL after 1 day and 63.7, 169.8, and 454.9 ng/mL after 10 days, respectively. The plasma area under the curve (AUC) values were 216.6, 602.8, and 1655.7 ng/h /mL on day 10. Similar terminal and plasma half-life values were obtained for the principle UPA metabolite (244).
For a 30 mg dose of UPA, the assessed terminal half-life was 32 hours, with a mean clearance of 76.8 L/hour. Ulipristal acetate was detected up to 5 days postadministration in the serum (235). In doses from 1 to 200 mg of UPA, the plasma level was 176 ± 89 ng/mL after 1 hour of intake, with an AUC of 556 ± 260 ng.h/mL (235). The terminal half-life was 32.4 ± 6.3 hours after 30 mg of UPA, with a mean oral clearance of 76.8 ± 64.0 L/h (235). There was linearity between the serum levels with doses up to 50 mg but loss of dose-dependence at 100 and 200 mg, suggesting saturation of carrier sites (235).
The metabolism of UPA is primarily mediated by CYP3A4 in the liver with some CYP1A2 and CYP2D6 involvement (205). Of the metabolites formed by this process, mono-N-demethylated metabolite (PGL4002) is pharmacologically active and has similar pharmacokinetic properties to the parent compound (245), while di-N-demethylated metabolites (PGL4004) are inactive (205) (Fig. 5). Both of these metabolites have 76 and 59% cross-reactivity and function as progesterone antagonists by binding to PRs in vitro (205). Radiochromatographic features of plasma after 1 hour of ingestion of UPA showed that 58% of UPA remains unchanged, with PGL4002 accounting for 21% of metabolites and PGL4004 and PGL4002 + 2H accounting for 8% of metabolites (246). Mono-N-demethylated-UPA reaches a maximum plasma concentration of 9.0 ng/mL, with an AUC value of 26.0 ng.h /mL (235, 243). Ulipristal acetate is mainly eliminated in the feces (90%), with less than 10% eliminated in the urine (247).
Telapristone acetate
Chemistry and development. Telapristone acetate (CDB-4124) is a 21-substituted-19-nor-progestin derivative with the chemical name 17α-acetoxy-11β-[4-(N,N-dimethylamino)phenyl]-21-methoxy-19-norpregna-4,9-diene-3,20-dione (Fig. 3). Like UPA, telapristone acetate is also a 11β-aryl substituted steroidal SPRM. The molecular formula is C31H39NO5, and the compound has a molecular weight of 505.655 g/mol. Telapristone acetate was first developed by the Contraceptive Development Branch of the NICHD in 2000 and is currently under license at Repros Therapeutics Inc. (The Woodlands, Houston, Texas). Data from phase II clinical trials were published (248, 249), but the development of this drug was stopped in 2009 due to hepatic toxicity. Investigations have recently restarted with lower doses of telapristone acetate. In 2013, a phase IIB trial of telapristone acetate was assigned in ClinicalTrials.gov for the treatment of breast cancer (NCT01800422).
Pharmacodynamics and mechanisms of action. The binding affinity of telapristone acetate and its monodemethylated metabolite to PR has been evaluated in human and animal tissue and cells and compared to mifepristone (205, 206). Both telapristone acetate and its metabolite have the same binding affinity as mifepristone to rabbit uterine PR, but mifepristone showed a 3-fold higher binding affinity in recombinant human PR-A and PR-B (205, 206). In the T47D mammary cancer cell model, telapristone acetate and its metabolite were 2- to 3-fold less effective than mifepristone in inducing antagonist effects (Fig. 4). All compounds failed to show any agonist activity in the same model (205, 206). A negligible antiglucocorticoid effect was demonstrated after the addition of telapristone acetate and its metabolite to liver cancer cells (HepG2) (205). Telapristone acetate also has low potency for ER in MCF-7 breast cancer cells (205) and no binding affinity for ER in an in vivo mammary cancer model (250, 251). The A-ring aromatization of telapristone acetate is undetectable in the aromatization of testosterone to E2 (205). Telapristone acetate has also been shown to reduce progestin-driven PR recruitment without altering PR sites on the genome (252) and disturb the PRE reporter activity in the promoter region (253) of breast cancer cells (Fig. 4).
Pharmacokinetics and metabolism. A phase I/II clinical study was conducted in premenopausal women with symptomatic leiomyoma and endometriosis (248, 249) to evaluate the effect of 3 telapristone doses (12.5, 25, and 50 mg daily for 3 and 6 months) (248, 249). Telapristone was quickly absorbed and reached peak levels within 0.5 to 2 hours, exhibiting a biphasic decline in concentration for all subjects (249). According to pharmacokinetic data from in vivo studies, telapristone acetate is primarily changed by CYP3A4 and CYP3A5 in the liver via demethylation to produce monode- and dide-methylated telapristone, and hydroxylation to produce hydroxylated telapristone (250) (Fig. 5). The formation of monodemethylated and hydroxylated metabolites is faster than the development of didemethylated (205). Of the 3 metabolites, monode-methylated telapristone exhibited similar antiprogesterone and less antiglucocorticoid properties than the parent compound in vitro (205, 206).
Alternates to systemic drug therapy for breast cancer prevention are currently undergoing investigation due to the higher side effects with and low adherence to oral medications used for prevention of breast cancer (254, 255). Because prevention is possible through exposure of only the breast to the drug, transdermal approaches to drug administration have been explored with telapristone acetate. Dermal permeation and retention of telapristone acetate in the mammary gland as possible local transdermal therapy for breast cancer prevention has been evaluated in rat models (256). The investigators delivered telapristone acetate via either gel treatment, implant, oral administration, or no treatment control. The mammary levels of telapristone acetate were 7-fold higher in the gel-treated group (1.5 mg/kg/day for 6 weeks) in the axillary glands compared to inguinal glands or with systemic treatment, and 26-fold higher than in the implant group (2.5 mg/kg/day for 6 weeks) (256). Additionally, 3- and 4-fold higher d-telapristone levels were observed in the inguinal and axillary gland in the gel-treated group compared to the implant group (256). Similar plasma concentrations of telapristone were detected in both groups, whereas d-telapristone was significantly higher in the implant group than in the gel group (256). An excellent axillary mammary concentration of telapristone and its metabolite d-telapristone were achieved by transdermal administration. This was not shown to be specific to ER-rich tumors.
Vilaprisan
Chemistry and development. Vilaprisan (BAY 1002670) is a derivative of 17-hydroxy-17-pentafluoroethyl-estra-4,9(10)-dien-11-aryl with the molecular formulation C27H29F5O4S and molecular weight 544.577 g/mol (Fig. 3). Different chemical structure and metabolic excretion pathways differentiates vilaprisan from other SPRMs. Vilaprisan is under development by Bayer HealthCare Pharmaceuticals, Berlin, Germany. Vilaprisan first underwent assessment in 2017 and is currently being examined in large phase III clinical trials to examine the efficacy and safety for the oral treatment of uterine fibroids (257).
Pharmacodynamics and mechanism of action. Vilaprisan demonstrates strong selective binding activity to PR, low binding activity to AR, and moderate to weak binding activity to GR in preclinical models (258) (Fig. 4). The compound exerts strong antagonism activity at doses of 1 to 1.5 mg/kg/day and fails to demonstrate any agonistic effect in vivo models even at higher doses of 10 mg/kg/day (258). Its antagonist effects are found to be 5 times and 10 times more potent compared to UPA and mifepristone, respectively (258). The antagonist activity was confirmed in 2 P4-dependent rat and rabbit animal models through vilaprisan’s ability to interrupt an ongoing early pregnancy through PR blockade in the endometrium and subsequent endometrial gland transformation (258).
Pharmacokinetics and metabolism. Pharmacokinetic effects of vilaprisan in humans were predicted through a single-species scaling method (259). This approach was extrapolated to humans on the basis of body weight. The exposure efficacy during a 24-hour dosing interval in rabbit and rat models was 126 μg x h/L and 116 μg x h/L, respectively. Allowing for a 5% free fraction in human, rabbit, and rat plasma, this predicted efficacy was translated into a daily human dose of 2.5 mg (259). A phase I pharmacokinetic study was conducted in healthy postmenopausal women after oral administration of vilaprisan at doses 1, 5, 15, or 30 mg/day for 28 days (260) and 5 mg of radiolabeled vilaprisan for 12 days (261). Vilaprisan demonstrated approximately 60% oral bioavailability (261). A dose-dependent pharmacokinetic profile was observed by increasing the plasma concentration from 3.74 μg/L to 68.6 μg/L, respectively (260). Vilaprisan did not exhibit any plasma protein binding affinity. Maximum concentrations were found between 1 and 2 hours, with a half-life of 32 to 38 hours (260, 261). Vilaprisan is mainly eliminated through hepatic metabolic pathways by (1) oxidation at the steroid skeleton, (2) reduction in the 3-ketomoiety, or (3) a combination of both pathways (261) (Fig. 5). Vilaprisan administration is associated with only a few minor metabolites created through oxidation and reduction, not exceeding 10% of total drug-related compounds (261). The alpha-hydroxy derivative M-4 is the primary reduction product, which further synthesizes various oxidative metabolites. These oxidative metabolites are catalyzed by CYP3A4, while the reductive metabolites are catalyzed by aldoketoreductases (261). Therefore, the biological contribution of vilaprisan can be assumed to occur mainly by the parent compounds. In terms of excretion, 12% of metabolites were excreted by urine and 71% were excreted by feces (261).
Progesterone receptor modulator-associated endometrial changes
Early studies of SPRMs revealed endometrial changes associated with SPRMs in endometrial tissues due to the antiprogestin actions of the compounds on this tissue. In 2008, Mutter et al first introduced the term “PAECs” (progesterone receptor modulator-associated endometrial changes), which are defined by benign histological changes of the endometrium in response to treatment of PR modulators (262). This study examined 84 endometrial samples from women treated with 4 different PR modulators (mifepristone, UPA, JNJ 17072341, and asoprisnil). Distinct, noncancerous endometrial changes were identified and classified by a group of 7 experienced gynecologic pathologists. Some endometrial samples showed changes characteristic of the normal endometrial cycle or other benign conditions but without detection of atypical hyperplasia. The common features of endometria were identified as novel findings and termed PAECs (262).
Features of PAECs include cystic glandular dilatation, inactive glands, and epithelial lining, with low mitotic activity and increased apoptosis, and rarely decidualized compact stroma (262–264). The changes in the histological patterns of PAEC differ from the normal physiological endometrium and do not resemble histological changes of endometrial hyperplasia (262, 263). Heart and neural crest derivatives expressed 2 (HAND2), a PR-regulated gene in the stromal cells, mediates the antiproliferative action of P4 to regulate endometrial epithelial function (265). The HAND2 gene is found to be hypermethylated and silenced in endometrial hyperplasia and cancer (266). Kannan et al investigated if altered expression of HAND2 is associated with UPA or mifepristone-induced PAECs. The investigators found that mifepristone, but not UPA, induced suppression of HAND2 expression in human endometrial biopsies (263). Dysregulation of HAND2 has been linked to complex atypical endometrial hyperplasia and cancer. These results suggest that UPA does not alter the HAND2 pathway. In a recent study, Berger et al partly explained PAEC features through microarray gene expression analysis (267). The authors found upregulation of THY1 (Thy-1 cell surface antigen), ADAM12 (ADAM metallopeptidase domain 12), and TN-C (tenascin C) after mifepristone treatment in endometrium with PAECs compared to endometrium without PAECs. This set of genes are involved in the structural architecture of tissues (268–270). This group also demonstrated that the proliferation marker Ki-67 was not affected and none of the differentially regulated genes were involved in the endometrial cancer-signaling pathway (267). These results suggest that differentially expressed genes are primarily involved in modifying tissue architecture of endometrium without promoting malignant transformation.
Molecular Mechanisms and Clinical Efficacy of Selective Progesterone Receptor Modulators in Progesterone-responsive Reproductive Disorders
Each SPRM imparts its clinical effect through action on various P4-dependent mediators (Fig. 6). Involvement of multiple mediators allows SPRMs to target disease through a variety of pathways that result in decreased cell proliferation, angiogenesis, ECM deposition and accumulation, and mechanical signaling, in addition to increased apoptosis. SPRMs often demonstrate a dose-dependent effect on objective signs of disease and are associated with significant improvements in health-related quality of life. This favorable clinical efficacy, in combination with a low side-effect profile, makes them an attractive option for the various pathologic processes demonstrated in Fig. 6 and discussed below.
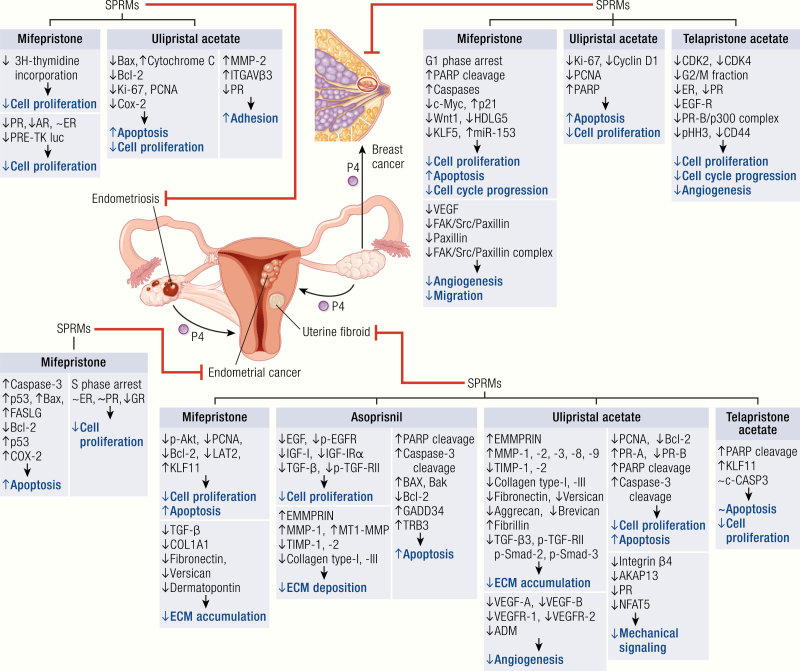
Figure 6.
Molecular mechanisms of clinical efficacy of SPRMs in uterine fibroid, endometriosis, endometrial cancer, and breast cancer. SPRMs effectively inhibit the development of various disease processes through the involvement of multiple mediators (black) that are implicated in various mechanisms of growth (blue). The growth of uterine fibroids is inhibited by all 4 SPRMs (ulipristal acetate, mifepristone, telapristone, and asoprisnil). The mechanism of this effect is multifactorial, involving reduction in cell proliferation, extracellular matrix deposition, angiogenesis, mechanical signaling, as well as induction of apoptosis. Mifepristone and ulipristal acetate may induce an antiendometriosis effect through modulation of cell proliferation, apoptosis, and adhesion. Mifepristone also may induce inhibitory effects on endometrial cancer by downregulation of cell proliferation and upregulation of apoptosis. Three SPRMs, including mifepristone, telapristone acetate, and ulipristal acetate, are thought to exert a therapeutic effect on breast cancer, at least in part, by downregulation of cell proliferation, angiogenesis, and migration, as well as the induction of apoptosis.
Uterine fibroids
Mifepristone
Molecular mechanisms
Ishikawa et al demonstrated that E2 plus P4-induced fibroid growth in a xenograft mice model but that this process could be blocked by mifepristone treatment (271). Mifepristone-induced fibroid regression is partly mediated by the alteration of cell growth and the suppression of TGF-β activity (272), AKT levels (116), as well as expression of proliferating cell nuclear antigen (PCNA) and the antiapoptotic protein Bcl-2 (273). LAT2 is a Na+-independent neutral amino acid transporter that functions as an oncogenic protein to promote glycolysis (274). In primary fibroid cells, P4 significantly induced LAT2 mRNA levels, which were blocked by cotreatment with mifepristone (122). KLF11 is known as a tumor suppressor, expressed at low levels in fibroid compared to myometrium (127). P4 minimally downregulated KLF11 mRNA levels but was robustly upregulated by mifepristone (127), suggesting that KLF11 is one of the critical regulators of mifepristone-mediated fibroid regression. Recently, Engman et al reported that GSTM1 (glutathione-s transferase mu 1), an enzyme that catalyzes the conjugation of glutathione to activated carcinogens, appeared to be correlated with mifepristone-induced fibroid regression (275). P4 agonist R5020 was found to increase ECM components collagen1A1, fibronectin, versican, and dermatopontin production in human fibroid cells, whereas mifepristone decreased protein production of these genes (276). Collectively, these results suggest that mifepristone mediates fibroid regression by alteration of genes involved in cell proliferation and fibrosis (Fig. 6).
Clinical efficacy
Several clinical trials of mifepristone at an ultra-low dose (2.5 mg) to a high dose (50 mg) for a 3- to 12-month treatment period has been studied in women with symptomatic fibroids (123–126, 277–286). Mifepristone appears to be well tolerated by phase I-III studies (Table 1). Here we discuss some major placebo-controlled, double blind, randomized studies.
Table 1.
Clinical efficacy of SPRMs in uterine fibroids, endometriosis, endometrial cancer, and breast cancer.
| SPRMs | Pathological Conditions | Clinical Trial | Treatment Dose | Duration | Clinical Efficacy | Tolerability and Safety Concerns |
|---|---|---|---|---|---|---|
| Mifepristone | Uterine fibroid | Phase I (NCT00579475) | 50 mg | 3 months | ||
| Phase II | 10 mg | 3 months |
|
|||
| Phase II (NCT00881140) | 10 mg (vaginal) | 3 months |
|
|||
| Phase II | 5 mg | 3 months | ||||
| Phase II | 5 mg or 10 mg | 6 months and follow-up over 12 months |
|
|||
| Phase III (NCT00133705) | 5 mg | 6 months |
|
|||
| Phase III (NCT01786226) | 2.5 or 5 mg | 3 months |
|
|||
| Endometriosis | Phase I | 5 or 100 mg | 3–6 months | — | ||
| Phase I | 50 mg | 6 months |
|
|||
| Phase I | 5 mg | 6 months | — | |||
| Phase II/III (NCT02271958) | 5 or 10 mg | 6 months | ||||
| Endometrial cancer | Phase II (NCT00505739) | 200 mg | 8 weeks to 2 years |
|
||
| Breast cancer | Phase II | 200 mg | 16 weeks |
|
|
|
| Asoprisnil | Uterine fibroid | Phase II (NCT0016045) | 5, 10, or 25 mg | 12 weeks |
|
|
| Phase II | 10 or 20 mg | 12 weeks |
|
|||
| Phase III ((NCT00152269, NCT00160381) | 10 or 25 mg | 12 months | ||||
| Endometriosis | Phase II (NCT00160446) | 5, 10, or 25 mg | 12 weeks |
|
|
|
| Phase II (NCT00160433) | 0.5, 1.5, or 5 mg | 3 months |
|
— | ||
| Telapristone | Uterine fibroid | Phase I/II | 12.5, 25, or 50 mg | 3 months |
|
|
| Endometriosis | Phase II (NCT00958412) | 25 mg | 4 months | — |
|
|
| Phase II (NCT01728454) | 6 or 12 mg | 18 weeks |
|
— | ||
| Phase II (NCT00556075) | 25 or 50 mg | 4 months | — |
|
||
| Ulipristal acetate | Uterine fibroid | Phase II (NCT00290251) | 10 or 20 mg or placebo | 90–120 days |
|
|
| Phase II | 2.5, 5, or 10 mg | 84 days |
|
|
||
| Phase II | 10 or 20 mg or placebo | 12 weeks |
|
|||
| Phase III, PEARL I (NCT00755755) | 5 or 10 mg or placebo | 13 weeks | ||||
| Phase III, PEARL II (NCT00740831) | 5 or 10 mg or placebo | 3 months | ||||
| Phase III, PEARL III (NCT01156857), and PEARL III extension (NCT01252069) | 10 mg or placebo | 12 months (four 3-month courses) | ||||
| Phase III, PEARL IV (NCT01629563) | 5 or 10 mg | Repeated 12 weeks | ||||
| Phase III, PREMYA (NCT01635452) | 5 mg | 3 months with 12 months follow-up |
|
— | ||
| Phase III, VENUS I (NCT02147197) | 5 or 10 mg or placebo | 12 weeks with 12 weeks drug-free follow-up |
|
|||
| Phase III, VENUS II (NCT02147158) | 5 or 10 mg or placebo | 12 weeks with 12 weeks drug-free follow-up | ||||
| Endometriosis | Phase IV (NCT02213081) | 15 mg | 3 months |
|
|
|
| Vilaprisan | Uterine fibroid | Phase I (NCT01816815) | 0.1, 0.5, 1, 2, or 5 mg | 12 weeks |
|
|
| Phase I (NCT02262663) | 0.5, 1, 2, or 4 mg | 12 weeks |
|
|
||
| Phase II, ASTEROID 1 (NCT02131662) | 0.5, 1, 2, or 4 mg or placebo | 12 weeks |
Phase I
A prospective randomized placebo-controlled trial (NCT00579475) was designed to evaluate the effect of mifepristone at a high dose (50 mg) on uterine blood flow, fibroid size, and endometrial status (125). A total of 30 women with symptomatic uterine fibroids were given mifepristone (50 mg) or placebo daily for 3 months. A complete lack of vaginal bleeding was observed in 86% of women during the second month of mifepristone treatment and in close to 100% during the third month of mifepristone treatment, compared with no significant changes in the placebo group (125). The percentage of total fibroid volume was decreased by 28% in the mifepristone-treated group, compared with placebo (6%). Mifepristone treatment increased hemoglobin levels and decreased serum E2 levels in the mifepristone-treated group, while P4 levels were decreased in both groups. No significant changes were observed in the liver transferase enzyme profile (except for one patient in the mifepristone-treated group). Endometrial biopsies showed no evidence of hyperplasia or malignancy. Nonphysiological appearances or cccc in 7 of 8 cases of the mifepristone-treated group, compared to placebo (4 of 11). Cystic glandular dilatation was significantly more frequent (5 of 8 cases) in the mifepristone-treated group compared to 1 of 11 placebo cases (125).
Phase II
A comparatively low dose (10 mg) of mifepristone was studied for 3 months in 40 women with symptomatic fibroids, compared to placebo (124). After the treatment period, the rates of amenorrhea were reduced to 84.2% in the mifepristone-treated group versus none in the placebo group. The complete relief of dysmenorrhea occurred in 80% of women with mifepristone treatment but only 33% of patients were relieved of pelvic pain. The uterine and fibroid volume was reduced by 26% to 30% in the mifepristone-treated group, as compared to none in the placebo group. Hemoglobin levels were increased in the mifepristone-treated group but decreased in the placebo group. All other biochemical parameters such as serum bilirubin, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, alkaline phosphatase, blood urea, and serum creatinine were in the normal range in both treatment groups. No side effects, such as nausea, vomiting, fatigue, diarrhea, headache, weakness, hot flushes, and loss of libido, were reported in either treatment group. Endometrial hyperplasia without atypia was observed in 63.1% of women compared to none in the placebo group (124).
Vaginal administration of mifepristone at a dose of 10 mg for 3 months has been studied in a prospective, open-label, phase II clinical trial (NCT00881140) at 2 tertiary centers as a treatment for women with symptomatic uterine fibroids (n = 33) (287). Mifepristone treatment induced amenorrhea in 44.8% of women by the end of treatment. The symptom severity of uterine fibroids was assessed with the score of the Uterine Fibroid Symptoms Quality of Life Questionnaire (UFS-QoL). The UFS-QoL score was significantly reduced from 20.7 to 14.0 after 3 months of mifepristone treatment. Mifepristone treatment was also associated with reduction of fibroid volume from 135 cc to 101.2 cc. No significant differences were observed in the blood hemoglobin values, serum creatinine, liver transferase enzymes, or lipid levels after mifepristone treatment. Mifepristone treatment induced adverse events such as hot flushes by 10.3%, nausea by 6.9%, feeling of weakness by 6.9%, abdominal pain by 24.1%, and vaginal discharge by 20.7%. Similar to other studies, endometrial biopsies showed no evidence of endometrial hyperplasia or cellular atypia (287).
The efficacy, safety, and quality of life of a single (5 mg) dose of mifepristone for 3 months have been studied in women with uterine fibroids, compared to placebo (126). This study (126), included 124 women with symptomatic uterine fibroids. After the treatment period, amenorrhea rates were reported in 93.1% women with mifepristone treatment, compared to placebo (4.3%). Fibroid volume was reduced by 28.5% in the mifepristone-treated group, compared with an increase of 1.8% in the placebo group (126). E2 levels were not greatly varied between the placebo and mifepristone groups. Hot flashes were reported by 24.1% and 8.5% women in the mifepristone-treated and placebo group, respectively. In addition, the prevalence of nausea and fatigue was very low but significant differences were observed between these 2 groups. PAECs were reported in 24.5% of women in the mifepristone-treated group, compared to 2.4% with placebo (126).
The long-term efficacy and safety of 5 mg and 10 mg doses of mifepristone for 6 months, and post-treatment evolution over 12 months, have been studied through a randomized double-blind clinical trial (280). This study included 176 women with symptomatic uterine fibroids who were randomized to receive 5 mg or 10 mg mifepristone daily for 6 months. At the end of the treatment period, amenorrhea rates were observed in 90.2% and 94.8% women with 5 mg and 10 mg mifepristone, respectively. Mifepristone treatment reduced fibroid volume by 48.1% and 39.1% with 5 mg and 10 mg, respectively. After 6 months of treatment, hepatic transaminases were elevated in 7.3% and 5.1% patients in the 5 mg and 10 mg groups, respectively. Adverse events were reported by 9.8% and 20.5% of women experiencing hot flashes, 3.7% experiencing nausea, 3.8% experiencing vomiting, and 8.5% and 12.8% experiencing fatigue with a mifepristone dose of 5 mg and 10 mg, respectively or in either dose or both. After 3 months of treatment, PAECs were reported by 26.8% and 42.9% of women in the 5 mg and 10 mg treated groups, respectively (280).
Phase III
A placebo-controlled study (NCT00133705) evaluated the effect of low-dose (5 mg) mifepristone on quality of life, pain, bleeding, and uterine size in women with symptomatic uterine fibroids (123). A total of 42 women with symptomatic fibroids were randomized to receive 5 mg mifepristone or placebo daily for 26 weeks. This study observed that amenorrhea occurred in 41% of patients with an improvement of anemia rates. The mean hemoglobin levels were increased in the mifepristone-treated group and decreased in the placebo group. Uterine size was reduced by an average of 47% in the mifepristone-treated group and increased by an average of 10% in the placebo group. No endometrial hyperplasia was noted in any participant (123).
Three months of mifepristone at an ultra-low dose (2.5 mg) or low dose (5 mg) has been studied with a 9-month follow-up period in women with symptomatic uterine fibroids (NCT01786226) (285). The rates of amenorrhea observed were in 78.3% and 93.6% women in 2.5 mg and 5 mg mifepristone-treated groups, respectively. After 3 months of the treatment period, fibroid volume was decreased by 27.9% and 45.5%, with 2.5 mg and 5 mg mifepristone, respectively. The improvement in the quality of life was similar in both treatment groups. Adverse events were observed by 9.4% and 15.6% (hot flashes), 1.9% and 3.7% (nausea), 3.8% and 2.7% (vomiting), and 1.9% and 3.7% (feeling of fatigue) in the 2.5 mg and 5 mg mifepristone-treated groups, respectively. Aspartate amino transferase (ASAT) or alanine amino transferase (ALAT) or both were increased in 12.7% and 6.6% women with 2.5 mg and 5 mg mifepristone, respectively. Endometrial biopsies showed no evidence of endometrial hyperplasia (285).
Asoprisnil
Molecular mechanisms
Asoprisnil has been shown to inhibit uterine fibroid cell proliferation with and without growth factor involvement (288). Specifically, asoprisnil inhibits EGF-, IGF-I- and TGFβ3-induced uterine fibroid cell proliferation (289) (Fig. 6). Furthermore, asoprisnil has been shown to downregulate mRNA and protein expression of EGF, IGF-I, and TGF-β3, as well as protein expression of pEGFR, IGF-IRα, and pTGF-RII in leiomyoma cells (289). Asoprisnil has also been shown to induce apoptosis in fibroid cells as measured by an increased TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling)-positive rate, cleaved caspase-3, and cleaved PARP expression as well as decreased Bcl-2 protein expression (288). Xu et al demonstrated that asoprisnil can elicit endoplasmic reticulum stress-induced apoptosis in cultured fibroid cells through a variety of mechanisms (290). The GADD153 (DNA-damage-inducible gene 153) transcription factor plays a vital role in promoting asoprisnil-induced apoptosis in cultured leiomyoma cells through upregulating GADD34, TRB3 (tribbles-related protein 3), Bax, and Bak, as well as downregulating Bcl-2 protein levels (290). The basal levels of ECM-remodeling enzymes EMMPRIN (extracellular matrix metalloproteinase inducer), MMP (matrix metalloproteinase)-1, and MT1-MMP (membrane type 1-MMP) proteins were found to be decreased in fibroid cells compared to myometrial cells, whereas TIMP (tissue inhibitor of MMP)-1, TIMP-2, and type I and type III collagen protein were found to be increased (291). Asoprisnil treatment reversed the protein contents of ECM-remodeling enzymes and collagens in cultured leiomyoma cells (291). Remarkably, asoprisnil did not affect cell proliferation and apoptosis, expression of growth factors and their receptors, or ECM-remodeling enzymes and collagen contents in myometrial cells (288, 289, 291). The cell type-specific effects of asoprisnil may be due to the differential expression of PR-B in leiomyoma versus normal myometrial cells (288).
Clinical efficacy. Phase II
To study the efficacy and safety of asoprisnil in women with uterine fibroids, a placebo-controlled trial (NCT00160459) was conducted in the United States and Canada (292). A total of 121 women with fibroids were randomized to receive asoprisnil daily at 5 mg, 10 mg, and 25 mg or placebo for 12 weeks. Asoprisnil treatment suppressed uterine bleeding by 28%, 64%, and 83% in women treated with 5 mg, 10 mg, and 25 mg, respectively, compared to none in the placebo group. The amenorrhea rates were reported by 16%, 36%, and 70% at 5 mg, 10 mg, and 25 mg asoprisnil doses, respectively, but by no patient in the placebo group. After 12 weeks of asoprisnil treatment, the median percentage of uterine volume was reduced by 14%, 9%, and 17% at 5 mg, 10 mg, and 25 mg doses of asoprisnil, respectively, with a slight increase in the placebo group (1%). Fibroid volume was reduced by 36% at 25 mg of asoprisnil treatment, compared to placebo (4%). A significant reduction was observed in bloating and in pelvic pressure compared to placebo (292). Hemoglobin levels were increased in the asoprisnil-treated group and decreased in the placebo group. No statistically significant differences were observed in E2 levels, N-telopeptide (a biomarker of bone resorption), and cortisol concentrations compared to placebo. Asoprisnil was appeared to be well tolerated, and adverse events were mild or moderate. Vasomotor symptoms were reported by 3%, 10%, and 8% at 5 mg, 10 mg, and 25 mg, respectively, compared with 0 in the placebo group. After 12 weeks of treatment, no adverse endometrial effects, such as hyperplasia or neoplasms were detected. Nonphysiologic endometrial changes or “PAECs” were detected in 43%, 58%, and 58% of women administered 5 mg, 10 mg, and 25 mg of asoprisnil, respectively (292) (Table 1).
Another phase II placebo-controlled trial evaluated the effect of asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in women with symptomatic fibroids (293). A total of 33 premenopausal patients were recruited and administered asoprisnil at 10 mg or 25 mg or placebo for 12 weeks. Suppression of uterine bleeding was experienced by 33% and 91% of women with 10 mg and 25 mg asoprisnil treatment, respectively, compared with none of the women in the placebo group. According to the UFS-QOL score, reduced symptom severity was observed in both asoprisnil-treated groups compared with placebo. The median percent change in largest fibroid volume with 10 mg and 25 mg asoprisnil was a reduction of 0.4% and 25.8%, respectively, compared with a small increase in size seen in the placebo group (4.9%) (293). No serious adverse events were observed in asoprisnil-treated groups. The most common adverse events were headache, nasopharyngitis, nausea, back pain, perioperative complications, and abdominal pain that were experienced by at least 4 patients in any treatment groups (293).
Phase III
Recently, Stewart et al performed a pooled analysis from two 12-month, placebo-controlled, randomized trials (NCT00152269, NCT00160381) (294). In these trials, asoprisnil was given at 10 mg, 25 mg, or placebo daily for 12 months in women with heavy menstrual bleeding associated with uterine fibroids. The authors found a significantly higher number of amenorrheic patients in the 10-mg group (66–78%) and in the 25-mg group (83–93%), compared to placebo (3–12%). Fibroid and uterine sizes were also significantly decreased by 48% and 28% with 10 mg and 63% and 39% with 25 mg asoprisnil dose, compared with increased 16% and 13% in the placebo group. Hemoglobin levels were increased and significant improvements in HRQL (health-related quality of life) were observed with asoprisnil treatment. Endometrial biopsies showed significant changes in endometrial thickness (~2 mm increase) at 12 months of asoprisnil treatment. SPRM-specific categories (“nonphysiologic secretory effect” and “secretory pattern, mixed type”) were significantly increased with asoprisnil treatment at 6 (8%) and 12 months (19%) compared to placebo (1–4%). Two adverse endometrial findings were observed: complex hyperplasia without atypia after 6 months at 10 mg, and 1 low-grade endometrial adenosarcoma after 9 months at 25 mg of asoprisnil treatment. The adenosarcoma was focal, and interpreted by the investigators as possibly pre-existing. Nevertheless, the finding suggested that uninterrupted treatment may pose safety concerns, “because of unknown long-term endometrial effects.” (294). A small increase in the rate of vasomotor symptoms was also observed, which could be attributed to the reduction in E2 levels (294).
Ulipristal acetate
Molecular mechanisms
Ulipristal acetate exerts antiproliferative, proapoptotic, antiangiogenic, and antifibrotic effects in uterine fibroid cells in a cell-type specific manner (240, 295) (Fig. 6). Ulipristal acetate inhibited cell proliferation and induced apoptosis, evidenced by the decreased expression of PCNA and Bcl-2 protein as well as increased expression of cleaved caspase-3 and cleaved PARP in cultured fibroid cells, compared to untreated controls (295). The proapoptotic effects were further confirmed through an increased TUNEL-positive rate in cultured fibroid cells (295). The antiangiogenic effects of UPA were demonstrated by decreased VEGF-A and VEGF-B, their receptors VEGFR-1, VEGFR-2, as well as adrenomedullin protein contents in cultured leiomyoma cells (239, 296) but not in normal myometrial cells (239). Notably, UPA treatment increased PR-A and decreased PR-B in cultured leiomyoma cells without significant changes in normal myometrial cells, compared to their untreated control groups (239). The antifibrotic effects of UPA in fibroid cells have been demonstrated by several groups. Ulipristal acetate significantly increases EMMPRIN, MMP-1, and MMP-8 protein and MMP-1, MMP-2, MMP-3, and MMP-9 mRNA levels, while decreasing mRNA and protein levels of TIMP-1 and TIMP-2, as well as type I and type III collagen in cultured fibroid cells, without comparable effects in normal myometrial cells (297). Ulipristal acetate can also block profibrotic growth factor activin-A-induced mRNA expression of fibronectin in myometrial and leiomyoma cultured cells (296). Courtoy et al reported that UPA reduced ECM volume and increased MMP-2 expression in women with symptomatic fibroids compared to untreated groups (298). In a later study, they reported that the reduction of fibroid volume after UPA treatment was correlated with an increase in MMP-1 and MMP-2 activity as well as a decrease in TIMP-1 activity (299). A placebo-controlled study also showed that amounts of versican, fibronectin, and MMP-9 were reduced while MMP-2 was increased in response to UPA treatment in fibroid surgical specimens compared to placebo groups (300).
Basal mRNA levels of TGF-β3 and TGFR2 are reported to be elevated in normal fibroid compared to myometrium (301). Ulipristal acetate significantly reduced protein levels of active TGF-β3, p-TGFR2, pSmad2, and pSmad3 as well as ECM collagen 1, fibronectin, and versican in fibroids (301). Notably, there was a statistically significant increase of the ECM protein fibrillin in fibroid treated with UPA (301). Furthermore, UPA was shown to decrease serum and tumor tissue concentrations of TGF-β3 in patients with uterine fibroids (302).
Mechanical signaling of uterine fibroids may be affected by UPA treatment. Compared with untreated fibroids, integrin subunit beta 4 transcript levels were reduced by UPA treatment (303). A-kinase anchoring protein 13 (AKAP13) was found to augment P4 signaling in uterine fibroid cells (304), while UPA effectively decrease protein expression levels of AKAP13 and PR in fibroids compared to placebo-treated tissues (304). Nuclear factor of activated T-cells (NFAT5) is known as an osmoadaptive transcription factor that maintains fluid equilibrium in cells by regulating osmolyte transporters Aldo-keto reductase family 1 member B1 (AKR1B1) and solute carrier family 5 member 3 (SLC5A3). Uterine fibroids demonstrate increased NFAT5 mRNA levels compared to myometrium from placebo subjects (305). Ulipristal acetate decreased expression levels of NFAT5 protein, solute transporters AKR1B1 and SLC5A3, and caused an associated decline in the expression of proteoglycans, versican, aggrecan, and brevican in fibroids tissue and fibroid cells grown in 3-dimensional cultures, compared to untreated groups (305).
Clinical efficacy
Several clinical trials indicate that UPA is well tolerated and effective in reducing fibroid size (238, 306). Ulipristal acetate treatment reduced fibroid-associated symptoms and improved quality of life (237, 307). Some studies reported adverse events, including hot flashes, headache and pain, discomfort, or tenderness in the breasts, nasopharyngitis and abdominal pain (237, 238, 306, 308). PAECs were reported occasionally as well, but not endometrial hyperplasia (103, 238, 306, 308, 309). No concerns were initially raised for safety regarding liver function (238, 310) (Table 1).
Phase II
In a randomized placebo-controlled trial (NCT00290251), UPA was given to premenopausal women with symptomatic fibroids (n = 18) at 10 mg or 20 mg daily for 90 to 120 days (311). Fibroid volume was reduced by 36% and 21%, with 10 mg and 20 mg UPA, respectively, compared to placebo (6%). Hemoglobin was unchanged, but median E2 levels were greater in all groups. Ulipristal acetate also improved scores on the uterine leiomyoma symptom quality-of-life subscale. Endometrial cystic hyperplasia was observed in one woman without evidence of atypia (311).
Previously, Chabbert-Buffet et al also reported similar findings in a study of UPA given to 46 women at 2.5 mg, 5 mg, or 10 mg daily for 84 days (312). Amenorrhea was reported in 81% and 90% of women with 5 mg and 10 mg, respectively. E2 levels remained in the normal range. At the end of the treatment period, endometrial hyperplasia was not detected in UPA-treated groups (312). Another phase IIB study evaluated the efficacy and tolerability of UPA in premenopausal women with symptomatic uterine fibroids (313). Ulipristal acetate was given at 10 mg or 20 mg or placebo for 12 weeks. Total fibroid volume increased by 7% in the placebo group but decreased by 17% and 24% with 10 mg and 20 mg UPA, respectively. Amenorrhea was observed in 20/26 (77%) women treated with UPA but in none of the women in the placebo group. In this study, UPA was associated with an improvement in hemoglobin levels but had no effect on E2 levels. Cystic glandular dilatation, simple hyperplasia, or complex hyperplasia without cellular atypia or endometrial intraepithelial neoplasia was detected in 4/33 women treated with UPA (313). These small studies suggest that UPA was well tolerated, with no serious adverse events.
Phase III
Several phase III studies of UPA for women with symptomatic fibroids have been reported. These include PEARL I (NCT00755755) (103), PEARL II (NCT00740831) (306), PEARL III (NCT01156857) and PEARL III extension (NCT01252069) (238), PEARL IV (NCT01629563) (237), PREMYA (NCT01635452) (307), VENUS I (NCT02147197) (309), and VENUS II (NCT02147158) (308).
In the PEARL I trial (NCT00755755), women with symptomatic fibroids and heavy uterine bleeding were given UPA at 5 mg (n = 96) or 10 mg (n = 98) or placebo (n = 48) daily for up to 13 weeks (103). Uterine bleeding was controlled in 91% and 92% of the women with 5 mg and 10 mg UPA, respectively, compared to the placebo group (19%). UPA treatment-induced amenorrhea was seen in 73%, and 82% of women with 5 mg and 10 mg, respectively, compared to placebo (6%). After 13 weeks, fibroid volume was decreased by 21% and 12% in 5 mg and 10 mg UPA-treated groups, respectively, but increased by 3% in the placebo group. The reduction in the uterine volume was achieved by 25% in both UPA-treated groups. Higher levels of hemoglobin and hematocrit levels were observed in both UPA-treated groups compared to placebo. The levels of glucose, estradiol, corticotropin, or prolactin were not consistently different between the UPA-treated groups and the placebo group. Adverse events, including headache and pain, discomfort, or tenderness in the breasts did not occur at a significantly higher frequency in the UPA-treated groups than in the placebo group. The incidence of hot flashes was low (<3%) in all 3 groups. PAECs were observed in 62%, 57%, and 6% of women with 5 mg, 10 mg, and placebo, respectively, but these changes were disappeared after 6 months of post-treatment period (103).
In the PEARL II trial (NCT00740831), 307 patients with symptomatic fibroids and heavy uterine bleeding were randomly assigned to receive UPA daily at 5 mg or 10 mg or monthly intramuscular injections of leuprolide acetate (3.75 mg) for 3 months (306). Ulipristal acetate at 5 mg and 10 mg controlled uterine bleeding in 90% and 98% of women, respectively, while leuprolide acetate controlled uterine bleeding in 89% of women. The regression in the volume of the 3 largest fibroids was observed in 36% and 42% of women with 5 mg and 10 mg UPA treatment, but UPA induced regression in 53% of women. The reduction in the uterine volume was lower in the UPA-treated groups (20–22%) compared to groups receiving leuprolide acetate (47%). The levels of corticotropin, thyrotropin, prolactin, or aminotransferase levels were significantly changed in amongst groups. Hot flashes (moderate to severe) were reported in 11% and 10% of women treated with 5 mg and 10 mg of UPA and in 40% of women treated with leuprolide acetate. PAECs were observed in 58% and 59% of women receiving 5 mg and 10 mg of UPA, respectively, and in 12% of those receiving leuprolide acetate. After the 6-month post-treatment period, the presence of PAECs were low and at similar rates between the 3 treatment groups (6–7%) (306).
In the PEARL III (NCT01156857) and PEARL III extension (NCT01252069) trials, the efficacy and safety of long-term UPA treatment was investigated in 209 women with symptomatic fibroids associated with heavy menstrual bleeding (238). Women received repeated intermittent open-label UPA treatment courses (10 mg daily) for up to four 3-month intervals, immediately followed by 10-days of randomized norethisterone acetate (NETA) or placebo. Amenorrhea was reported in 79% (n = 164) of women after the first UPA course. In the next 2, 3, and 4 UPA courses, the amenorrhea rates were 89% (n = 131), 88% (n = 119), and 90% (n = 107), respectively. The regression rates in the volume of the 3 largest fibroids after UPA treatment were 45.1% and 72.1% in the PEARL III (after the first treatment course) and PEARL III extension study (after 4 treatment courses), respectively. TEAEs were reported in 120 women (57.4%) during the first course of UPA treatment, but only 8 women (3.8%) experienced severe adverse events. TEAEs occurring in >5% of women were headache (16.3%), nasopharyngitis (6.7%), and abdominal pain (5.3%). The results from physical examination, vital signs, liver function, hormone levels, ovarian ultrasound, or electrocardiograms showed no evidence of safety concerns. PAECs were reported in 18/171 (11%), 45/176 (26%), and 22/87 (25%) women at the time of screening and at approximately 6 weeks after the first and fourth UPA courses, respectively. After 3 months post-treatment, PAECs were reduced (3/15 biopsies) (238).
In the PEARL IV trial (NCT01629563), a total of 451 patients were recruited to investigate the efficacy and safety of repeated 12-week courses of 5 mg or 10 mg of UPA for intermittent treatment of women with symptomatic fibroids associated with heavy uterine bleeding (237). At the end of the treatment course, amenorrhea was observed in 62% and 73% women with 5 mg and 10 mg UPA, respectively. The median reductions in fibroid volume from baseline were 38% and 38% after the first course of treatment, and 54% and 58% after the second course of treatment with 5 mg and 10 mg of UPA, respectively. Pain improved in both treatment groups. The median visual analogue scale pain scores were reduced from baseline values of 39.5 and 43.0 to 6.0 and 6.0 with 5 mg and 10 mg of UPA treatment, respectively. Quality of life was severely impaired at baseline but was substantially improved at the end of both treatment courses for treatment groups. No evidence of safety issues were observed among physical examination, vital signs, laboratory safety tests, ovarian ultrasound, or electrocardiograms. Adverse events were reported in 44% of women after the first treatment courses, and in 27% and 30% of women after 5 mg and 10 mg UPA treatment, respectively. Headaches and hot flashes were the most frequently reported adverse events but occurred in ≤10% of women. PAECs were reported in 17 biopsies (8%) at screening in each treatment group. After the second course of treatment, PAECs were observed in 29 (16%) and 35 biopsies (19%) with 5 mg and 10 mg UPA treatment, respectively (237).
In the PREMYA trial (NCT01635452), the safety, efficacy, and quality of life of patients taking UPA have been studied in women with moderate to severe symptoms of uterine fibroids (n = 1568) in the preoperative setting within the European Union (Germany, France, UK, Romania, Portugal, Sweden, Poland, Hungary, Slovenia, and Austria) (307). Ulipristal acetate was given at 5 mg once daily for 3 months. Patients were followed during 3 months of UPA treatment and for another 12 months after treatment discontinuation. Only 38.8% of patients underwent surgery, of which the majority were conservative or minimally invasive. The Clinical Global Impression-Improvement scale indicated that UPA treatment was associated with improvement in 60% of patients at 3 months. After treatment discontinuation pain and quality of life were remained lower than baseline during the entire period of follow-up (307).
The VENUS I trial (NCT02147197) was conducted to evaluate the efficacy and tolerability of UPA for treatment women with symptomatic uterine fibroids. A total of 157 women with abnormal uterine bleeding, and one or more discrete fibroid were randomized to receive 5 mg or 10 mg UPA, or placebo once daily for 12 weeks followed by a 12-week drug-free follow-up (309). This is the first US-based phase III study that included a large proportion of black and obese (BMI ≥ 30 kg/m2) patients. Amenorrhea rates were reported at 47.2% and 58.3% in the 5 mg and 10 mg UPA-treated groups, respectively, compared to placebo (1.8%). After 3 months of the treatment period, fibroid volume was increased by 7.2% from baseline in the placebo group (n = 41) and reduced by 9.6% and 16.3% in the 5 mg (n = 39) and 10 mg (n = 33) UPA-treated groups, respectively. At the end of follow-up (12 weeks post-treatment), fibroid volume was increased by 5.7% from baseline in the placebo group (n = 31) and decreased by 2.3% and 17.4% from baseline in the 5 mg (n = 36) and 10 mg (n = 30) UPA-treated groups, respectively. TEAEs were reported by 43.4% and 54.2% with 5 mg and 10 mg UPA-treated groups, respectively, and were 28.6% in the placebo group. The most common TEAEs (5% or greater in either UPA group during treatment) were hot flashes, blood creatine phosphokinase elevation, and hypertension. All endometrial biopsies were benign. The proportion of PAECs were similar for all treatment groups at baseline and at the end of treatment: 5 mg UPA (28.3 and 26.2%), 10 mg UPA (22.9 and 29.7%), and placebo (23.6 and 13.6%), and decreased by end of follow-up: 5 mg UPA (19.0%), 10 mg UPA (12.1%), and placebo (7.1%) (309).
The VENUS II trial (NCT02147158) also evaluated the efficacy and tolerability of UPA for the treatment of symptomatic uterine fibroids (308). A total of 432 women with fibroids and abnormal uterine bleeding received 5 mg or 10 mg of UPA or placebo daily for two 12 weeks. Treatment courses were separated by a drug-free interval of 2 menses and followed by a 12-week drug-free follow-up period. This trial was conducted at 51 research centers in the United States and 2 in Canada. After the first course of treatment, amenorrhea was reported by 42.0% and 54.8% of women with 5 mg and 10 mg UPA, respectively, compared to placebo (0.0%), suggesting that both 5 mg and 10 mg were superior to placebo. The percent change from baseline in fibroid volume was reduced by 11.9% and 13.5% with 5 mg and 10 mg UPA, respectively, and increased by 5.8% with placebo. Fibroid volume was further reduced with UPA after the second course of UPA treatment. At the end of the first course of treatment, TEAEs were reported by 46.6%, 43.2%, and 41.4% of women receiving 5 mg UPA, 10 mg UPA, and placebo, respectively. The common TEAEs (5% or greater of patients in any treatment group) were hot flashes, headache, fatigue, and nausea; however, only hot flashes occurred more frequently in both UPA groups compared to placebo. During the second course of treatment, headache was the only adverse event reported by 5% or greater of patients in any treatment group. PAECs were similar among treatment groups at baseline and after each treatment course (308).
Phase IV
Several phase IV clinical trials have been listed in ClinicalTrials.gov but their results have not yet been published. An interventional, randomized trial (NCT02361879) evaluated the efficacy of UPA versus leuprolide acetate treatment before hysteroscopic resection of uterine fibroids. Women with symptomatic submucosal leiomyomas received UPA at 5 mg daily for 3 months or leuprolide acetate at 11.25 mg during the luteal phase of the menstrual cycle. The primary outcome measure of the trial was to compare the proportion of controlled uterine bleeding by pictorial blood assessment chart (PBAC) (314). The estimated completion date of this trial was September 2017. Another interventional, randomized trial (NCT02357563) compared the proportion of restored uterine cavity 1 year after UPA or leuprolide acetate treatment in patients with submucosal uterine leiomyoma. Women received UPA at 5 mg/day for 3 months or leuprolide acetate at 11.25 mg the luteal phase of the menstrual cycle. This study was expected to finish in September 2017. A double-blind, randomized, controlled trial (NCT02288130) investigated if UPA was as effective as GnRHa in terms of surgical outcome. Women with symptomatic uterine submucosal fibroids eligible for laparoscopic myomectomy were given UPA at 5 mg/day for 3 months or leuprolide acetate at 11.25 mg in the luteal phase of the menstrual cycle. The primary outcome was to assess intraoperative blood loss during laparoscopic myomectomy. This trial was expected to finish in November 2017. An open-label study (NCT02601196) has been designed to study in vitro fertilization (IVF) outcome following treatment with UPA for women with a myomatous uterus after at least one IVF failure. Ulipristal acetate was given at 5 mg/day for 3 months to women with fibroids and at least 1 failure in IVF treatment. This study was expected to finish in January 2018. A Chinese population-based study (NCT02825719) is evaluating the effect and safety of UPA in controlling uterine bleeding (evaluated by PBAC) in Chinese women using 5 mg of UPA daily for 12 weeks before surgery. This trial is expected to finish in December 2019. An interventional, randomized, parallel assignment study is evaluating the effectiveness of UPA versus an aromatase inhibitor plus a GnRH analog in controlling uterine size and abnormal uterine bleeding for women with symptomatic fibroids (NCT03421639). The selected dose for UPA is 5 mg/day for 3 months, and aromatase inhibitor plus GnRH analog is 1 mg/day of anastrozole plus 3.6 mg/monthly for 3 months. This trial is expected to finish in February 2020. The results of these studies will further augment the clinical evidence for this SPRM.
Telapristone acetate
Molecular mechanisms
The effect of telapristone acetate on uterine fibroid cell proliferation and apoptosis has been variable. Luo et al reported that telapristone acetate can significantly decrease cell proliferation, and induced apoptosis in uterine fibroid cells, (315) (Fig. 6). Telapristone acetate significantly upregulated levels of the apoptosis marker cleaved PARP and the tumor suppressor KLF11 in leiomyoma cells. Interestingly, cell proliferation or apoptosis in myometrial cells was not affected by telapristone treatment (315). A study by Roeder et al reported that telapristone acetate was not able to significantly induce apoptosis in cultured fibroid cells, since c-CASP3 levels were not elevated (316).
Clinical efficacy
A number trials, including phase I (NCT01069094), phase II (NCT02811159, NCT01739621, NCT02301897, NCT02323646, NCT00882258, NCT01451424, and NCT01069094), and phase III (NCT00737282, NCT00874302, NCT00853567, NCT00683917, NCT00735553, NCT00785356, NCT01069120, and NCT00702702) of telapristone acetate have been listed in ClinicalTrials.gov and examine the safety, pharmacokinetics, and efficacy of telapristone for the long-term treatment of uterine fibroids. The results from many of the trials have not been published, and several of the phase III clinical trials have been terminated or withdrawn due to concerns for patient safety.
Phase I/II
In 30 women with symptomatic uterine fibroids, Wiehle et al examined the effect of 3 doses of telapristone acetate (12.5, 25, or 50 mg) for 3 months on fibroid size and uterine bleeding compared to either placebo or leuprolide acetate (3.75 mg). Telapristone acetate at 12.5, 25, or 50 mg reduced fibroid size by 10.6%, 32.6%, and 40.3%, respectively, compared to leuprolide acetate (32.6%) and placebo (10.6%) (249). The number and intensity of uterine bleeding days were also reduced by telapristone acetate, compared to placebo. After 3 months of treatment, the overall incidence of TEAEs was lower among the telapristone acetate-treated subjects compared to leuprolide acetate and placebo (249) (Table 1).
Vilaprisan
Clinical efficacy. Phase I
The menstrual bleeding pattern in healthy women was investigated through vilaprisan treatment at the dose of 0.1, 0.5, 1, 2, and 5 mg daily for 12 weeks (NCT01816815) (317). The number of bleeding days was significantly decreased in the vilaprisan-treated groups, compared with placebo, and maximal nonbleeding rates of 100% and 90.9% were observed in 2 and 5 mg vilaprisan doses, respectively. All women resumed menstruation within 52 days of vilaprisan discontinuation. Follicular growth and E2 concentrations were not suppressed in response to vilaprisan treatment. Changes in endometrial thickness were not reported by the end of vilaprisan treatment. In addition, no serious TEAEs or study discontinuations due to adverse events were reported (317) (Table 1).
The ovarian function in healthy young women was investigated by vilaprisan treatment at doses 0.5, 1, 2, or 4 mg/day for 12 weeks (NCT02262663) (318). This study demonstrated a pronounced effect of vilaprisan on ovarian activity. Utilizing the Hoogland score for ovarian activity, the authors observed ovulation inhibition in >80% of the women receiving vilaprisan ≥1 mg/day. Through a Bayesian approach, the authors concluded that ovulation inhibition was estimated to increase from 37% in women receiving 0.5 mg/day vilaprisan to 76%, 86%, and 88% in women receiving 1, 2, and 4 mg/day, respectively. Amenorrhea occurred in 75% of women at doses of vilaprisan ≥1 mg. After the end of treatment, menstrual bleeding resumed within 51 days for women, and ovulation was observed in a high proportion of women during the first (76%) and second (98%) follow-up cycles. Vilaprisan treatment at doses ≥1 mg/day decreased mean average E2 and mean maximum P4 concentrations, compared to pretreatment values. After the end of vilaprisan treatment, both E2 and P4 levels were returned to pretreatment condition, suggesting a rapid resumption of normal ovarian activity. Ovarian follicle growth was not entirely suppressed during vilaprisan treatment and thus is not a suspected cause of this hypoestrogenism. Serum FSH and LH levels were reduced at vilaprisan dosages ≥1 mg/day compared to pretreatment levels but returned to pretreatment levels after the end of treatment. The incidence of PAECs were increased in a dose-dependent fashion, reaching values between 70% and 95% after doses ≥1 mg vilaprisan, but most changes disappeared after the first post-treatment bleeding (318).
Phase II
A placebo-controlled trial (ASTEROID 1) (NCT02131662) was designed to study the safety and efficacy of different vilaprisan doses in women with uterine fibroids (319). A total of 300 women diagnosed with at least one fibroid 3 to10 cm in diameter received vilaprisan at doses 0.5 mg, 1.0 mg, 2.0 mg, or 4.0 mg or placebo daily for 12 weeks, taking a 24-week follow-up. The complete absence of bleeding or spotting was observed in 60% of patients with vilaprisan dose 4.0 mg, and in 30%, 56%, and 54% of patients taking vilaprisan dose 0.5, 1.0, and 2.0 mg, respectively, compared with 1.7% in placebo groups. After 12 weeks, vilaprisan at ≥1.0 mg induced amenorrhea (<2 mL/28 days) in >83% of women compared with placebo (9%). After treatment cessation, menstruation returned to 75% of patients within 24 to 31 days in the vilaprisan treatment groups. The reduction of median fibroid volume was 14.9% to 41.4% by the end of vilaprisan treatment compared with the placebo group (4.9%) (319). PAECs were reported in 33% to 58% of women treated with vilaprisan compared to 20.0% in the placebo group. In some patients, liver transaminases were slightly elevated but were mostly mild and were present in the vilaprisan and placebo groups at similar levels. There was a trend toward decreasing E2 levels with longer duration of vilaprisan treatment, but levels essentially returned to baseline at the end of treatment. FSH levels were moderately decreased after 1 month of vilaprisan treatment but returned to near baseline levels after the end of treatment. At least 1 TEAE was reported by 73.4% of patients. The most frequent TEAEs were ovarian cyst (11.0%), headache (9.7%), and hot flashes (9.3%). Adverse events were reported similar in all vilaprisan and placebo groups. Three women discontinued the trial due to TEAEs: 2 women were in the placebo group and 1 women in the 0.5-mg vilaprisan group (319).
The other phase II study (ASTEROID2) (NCT02465814) evaluated the efficacy and safety of different treatment regimens with vilaprisan at 2 mg daily doses (12 weeks repeated cycles or 24 weeks continuous treatment) compared to both placebo and UPA in patients with heavy bleeding associated with uterine fibroids (257). This study used amenorrhea as the primary measure of efficacy. The secondary measures included time to normalized menstrual bleeding and percent change in uterine fibroid volume, as well as endometrial changes. This study was completed at the end of 2016, but the results have not been published yet.
Phase III
Vilaprisan is currently under investigation in phase III studies for long-term treatment of uterine fibroids in the trials ASTEROID4 (NCT03400956), ASTEROID5 (NCT03240523), ASTEROID6 (NCT03194646), ASTEROID7 (NCT03699176), and ASTEROID8 (NCT03476928). These study programs have been designed to evaluate the efficacy and safety of vilaprisan at doses of 2 mg for 12 weeks or 24 weeks. Amenorrhea, the incidence of TEAEs, and the percent change in bone mineral density (BMD) of the lumbar spine will be assessed as primary outcome measures. Secondary measures include the number of bleeding days, heavy menstrual bleeding (HMB) response, time to onset of amenorrhea, time to onset of controlled bleeding, absence of bleeding (spotting allowed), endometrial histology, and endometrial thickness.
Clinical takeaway
The majority of studies involving the efficacy of SPRMs for managing uterine fibroids have been done with mifepristone, the original SPRM, and ulipristal acetate, for its higher selectivity. While early, small studies (123) failed to demonstrate a reduction in fibroid volume with mifepristone use, recent larger-powered studies (280, 285) have demonstrated this benefit at a 27.9% to 48.1% decrease in fibroid volume. These studies have also shown significant reduction in bleeding and improvement in quality of life. However, phase III trials showing significant rates of amenorrhea (up to 93.6%) (285) and other adverse events, including hot flushes, nausea, vomiting, and fatigue have led to preferential analysis of alternate SPRMs in further analyses (285). Ulipristal acetate has significantly reduced binding and antagonist potency at the glucocorticoid receptor compared to mifepristone (206). Women treated with UPA in the PEARL II study experienced controlled uterine bleeding in >90% of women by week 13 (306), with rates of amenorrhea that have been shown to occur within the first 10 days of therapy (103). Women treated with UPA also experienced a high reduction in fibroid volume of up to 21% (306). It is well tolerated, with <5% of patients in the PEARL IV study discontinuing treatment (237). While PAECs were occasionally reported, no endometrial atypia was reported and PAECs were found to be reversible after 1 to 2 months of treatment cessation (237).
As the newest SPRM, vilaprisan use has been demonstrated to induce amenorrhea in >83% of women in phase II trials with a dose-dependent reduction in fibroid volume of 14.9% to 41.4% (319). Vilaprisan is currently being investigated in multiple phase III studies for safety and efficacy of long-term treatment for uterine fibroids.
Asoprisnil has demonstrated rates of amenorrhea and fibroid volume reduction at the highest tested doses compared to mifepristone, but is no longer a preferred option due to concerns for potential adverse long-term endometrial effects (294). While telapristone has been shown to reduce fibroid size and number, as well as the intensity of uterine bleeding days by selectively inhibiting fibroid cell proliferation (315), many phase III clinical trials studying this compound have been withdrawn due to concerns for patient safety.
Endometriosis
Mifepristone
Molecular mechanisms
Murphy et al investigated the antiproliferative and antiprogesterone effect of mifepristone on primary endometrial and endometriosis cells (320). Mifepristone reduced cell growth as evidenced by decreased in 3H-thymidine incorporation of endometrial and endometriosis cells (320). Mifepristone also inhibited ER and PR (321) as well as P4-mediated induction of the PRE-TK luciferase activity (320) (Fig. 6), suggesting that the therapeutic effects of mifepristone on endometriosis were partly mediated by alteration of P4-PR induced transcriptional response. The antiproliferative effect of mifepristone in endometrial cells was further demonstrated by Narvekar et al (322). This group found decreased expression of PR, increased expression of AR, and no change of ER expression levels. Since androgens can act as an antagonist of E2 action, enhanced AR expression induced by mifepristone could play a role in its antiproliferative effects.
Clinical efficacy
Mifepristone-induced regression of endometriotic lesions has been variable and appears to be dependent on the duration of treatment (Table 1). Mifepristone failed to produce regression of endometriotic lesions in a rodent model after 2 months (323) and in humans after 3 months of treatment (324). However, 6 months of mifepristone treatment was associated with less visible disease in women (325). A small prospective open-label trial suggested possible efficacy for endometriosis-associated pain (324). A phase II/III trial observed that mifepristone was effective in symptomatic improvement but adverse events were reported in a significant number of patients (326).
Phase I
Kettel and coworkers completed 3 small clinical trials with low dose (5 mg) to high dose (100 mg) mifepristone for a treatment period of 3 to 6 months in a total of 22 patients (324, 325, 327). In the first small prospective open trial, the higher dose of mifepristone (100 mg/d or approximately 2 mg/kg) for 3 months was studied in patients with endometriosis (n = 6) (324). After the end of the treatment period, pelvic pain was improved in all 6 patients, but the regression of endometriosis was not evident (324). In the next trial, authors administered mifepristone daily at 50 mg for 6 months in women with pelvic pain due to endometriosis (n = 9) (325). Mifepristone treatment induced amenorrhea in all patients and improved pelvic pain and uterine cramping. Visible endometriosis was reduced by 55% with mifepristone treatment. One patient experienced elevated levels of liver transaminases during the last month of treatment (325). In an uncontrolled pilot study, mifepristone at a lower dose (5 mg) was given daily to women with endometriosis for 6 months (n = 7) (327). Cyclic bleeding ceased in all 7 patients, while pelvic pain was improved in 6 of 7 patients. No significant change was found in mean endometriosis score at the end of treatment (327), suggesting this dosage is too low to achieve acceptable efficacy.
Phase II/III
To study the effectiveness and safety of mifepristone in women with the laparoscopic diagnostic of endometriosis, a total of 360 patients were enrolled and treated with mifepristone at 2.5, 5, or 10 mg daily for 6 months or daily placebo for 3 months (NCT02271958) (326). The changes in prevalence and intensity of dysmenorrhea, the incidence of hot flashes, nausea, vomiting, fatigue/tiredness, elevated hepatic transaminases, and histological alterations of the endometrium were assessed. After 3 months of treatment, the prevalence of dysmenorrhea decreased to 10.2%, 1.1%, and 1.1% with 2.5, 5, and 10 mg mifepristone treatment, respectively, compared to placebo (39.3%). Amenorrhea was reported in 78.7%, 97.8%, and 98.9% of patients with 2.5, 5, and 10 mg mifepristone treatment, respectively, compared to placebo (1.1%). Adverse events such as hot flashes, nausea, vomiting, and fatigue/tiredness were reported in a significant number of patients, compared to placebo groups, after 3 months of treatment. In the mifepristone-treated groups, 3.4% of subjects developed elevated hepatic transaminases up to 99 IU. PAECs were reported in 19.7%, 9.7%, and 13.0% of patients receiving 2.5, 5, and 10 mg mifepristone treatment, respectively (326).
Asoprisnil
Clinical efficacy: phase II
The efficacy and safety of asoprisnil in women with endometriosis has been studied by a double-blind trial (NCT00160446) (328). Patients were randomized to receive asoprisnil at 5 mg (n = 31), 10 mg (n = 33), 25 mg (n = 32), or placebo (n = 34) daily for 12 weeks. After the end of the treatment period, significant reductions in the average daily combined pelvic pain/dysmenorrhea scores were observed in asoprisnil-treated groups with all 3 asoprisnil doses, compared to placebo (328). Asoprisnil also induced amenorrhea at rates of 50%, 71%, and 93% with 5, 10, and 25 mg, respectively, compared with no change in the placebo group. E2 serum concentrations were not affected by asoprisnil treatment. No TEAEs (treatment-emergent adverse effects) were observed during treatment or follow-up period. (TEAEs are defined by all adverse effects occurring after the first dose of the treatment drug until the end of the study.) Adverse events were evenly distributed among treatment and placebo groups and were generally mild and self-limiting (328) (Table 1).
Treatment of women with endometriosis with asoprisnil at 0.5, 1.5, and 5 mg for 3 months demonstrate that 5 mg was only the effective dose for pain relief compared to placebo (20). Later, asoprisnil was studied for the long-term safety in women with endometriosis by an open-label extension trial (NCT00160420). Asoprisnil was given at 5 mg daily for 12 months. This study included the assessments of the endometrium, adverse events, lipid profiles, and changes of laboratory values and vital signs. The results of the study have not yet been published.
Ulipristal acetate
Molecular mechanisms
The antiendometriosis effect of UPA has been reported in female Wistar albino rats (329) and female C57BL/6 mice (330). Mechanistically, UPA-induced regression of endometriotic lesions in female Wistar albino rats was mediated by induction of apoptosis (as revealed by a higher positive rate of Bax and cytochrome C, while a lower positive rate of Bcl-2), reduction of cell proliferation (as indicated by a decrease in Ki-67 expression), and anti-inflammatory effect (as demonstrated by a decrease in COX-2 expression) (329). In an experimental endometriosis model in female C57BL/6 mice, UPA significantly decreased expression of proliferation marker PCNA and increased expression of adhesion molecules MMP-2 and integrin alpha V (ITGAV)β3 (330). PLAU (plasminogen activator, urokinase), HIF (hypoxia-inducible factor)-1α and VEGFA expression, peritoneal fluid PGE2 (prostaglandin E2) levels, and ERα and ERβ expression were not affected by UPA, while PR expression was significantly lower in UPA-treated groups (330). The above results suggest that the therapeutic effects of UPA are mediated by proliferation, apoptosis, and adhesion (Fig. 6).
Clinical efficacy: phase IV
An open-label, interventional study (NCT02213081) has been launched to investigate if UPA can decrease pain associated with endometriosis. Part of the results from this trial has been published (331). A 25-year-old nulligravida with endometriosis-related pelvic pain refractory to medical and surgical intervention was administered 15 mg UPA daily for 3 months. Ulipristal acetate treatment substantially improved pain symptoms. PAECs were detected after less than 3 months of UPA treatment and resolved after cessation of treatment and induction of withdrawal bleed (331) (Table 1).
Telapristone acetate
Clinical efficacy: phase II
To study the safety and effectiveness of telapristone acetate at 25 mg (daily for 4 months) for the treatment of premenopausal women with symptomatic endometriosis, a randomized, double-blind trial (NCT00958412) was listed in ClinicalTrials.gov but was terminated due to concerns for patient safety. Another randomized, double-blind trial (NCT01728454) was listed in ClinicalTrials.gov to evaluate the safety and efficacy of telapristone acetate for the treatment of premenopausal women with symptomatic endometriosis. A total of 60 patients were recruited and telapristone acetate was given at 6 or 12 mg daily for 18 weeks. The primary outcome was to determine the change in individual Biberoglu & Behrman Symptom Severity (BBSS) pain scores after telapristone acetate treatment. This study has been completed, but the results are not yet published. Another trial (NCT00556075) was initiated to study the safety and efficacy of telapristone acetate of 25 or 50 mg (daily for 4 months) for the treatment of premenopausal women with symptomatic endometriosis but was terminated due to safety concerns.
Vilaprisan
Clinical efficacy: phase II
To study the efficacy and safety of 2 different doses (2 and 4 mg) of vilaprisan for 24 weeks in women with symptomatic endometriosis, a placebo-controlled trial (NCT03573336) has been listed in ClinicalTrials.gov. Changes in the worst pain from baseline will be measured. This study is expected to be finished on August 17, 2022.
Clinical takeaway
Mifepristone has been shown to be capable of inducing regression of endometriotic lesions when administered over a period of months. A 2017 Cochrane review indicated that it is associated with a 0.08 OR (0.04, 0.17) reduction in dysmenorrhea and a 0.23 odds ratio (OR) (0.1, 0.51) reduction in dyspareunia at 3 months compared to placebo (332). It is associated with amenorrhea in nearly 90% of women (332), with insufficient evidence to show differences in rates of nausea, vomiting, and fatigue. Of concern, a phase II/III trial of 360 women has demonstrated as high as 3.4% of patients receiving 3 months of daily mifepristone have developed elevated hepatic transaminases (326). Better quality evidence is required to best understand the efficacy, safety, and appropriate dosing of this SPRM for management of endometriosis.
Data on asoprisnil and ulipristal acetate are limited; while both asoprisnil and UPA have demonstrated promise in their ability to reduce dysmenorrhea and pelvic pain, results from additional studies are required to draw any conclusions about their efficacy and potential adverse effects. Similarly, only a few trials have begun assessing the safety and effectiveness of telapristone and vilaprisan for treatment of endometriosis.
Endometrial cancer
Mifepristone
Molecular mechanisms
In Ishikawa cells, a well-differentiated endometrial adenocarcinoma cell line, mifepristone-inhibited cell proliferation by arresting cell cycle progression at S phase (333), induced apoptosis through activation of caspase-3, and increased Bax (BCL2 associated X, apoptosis regulator), and FASLG (Fas ligand) expression (333), demonstraing its antiproliferative and apoptotic effects. The ability of mifepristone to induce apoptosis and growth inhibition in 3 endometrial cancer cell lines (HEC-1A, KLE, and RL95-2) was further explained by decreased expression of ER, PR, and GR (321, 334). In contrast, other studies reported that mifepristone increased expression of p53 and COX-2 (cyclooxygenase-2) and decreased levels of Bcl-2 with no changes of ERα/β or PR-A/PR-B levels in HEC-1-A and/or Ishikawa cells (335, 336) (Fig. 6). This suggests that apoptosis may be due to p53 activation rather than steroid receptor modulation.
Clinical efficacy: phase II
Mifepristone has been studied for the treatment of PR-positive advanced or recurrent endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma (NCT00505739) (337). Mifepristone was given at 200 mg daily for 8 weeks to 2 years to 13 patients with low grade endometrial stromal sarcoma (n = 2) and endometrioid adenocarcinoma (n = 11). Mifepristone treatment induced a stable disease rate of 25% that lasted from 8 weeks up to ≥77 weeks. No significant changes were observed in quality of life (Table 1). Adverse events were anorexia, fatigue, and mood alterations observed in 50%, 50%, and 58% of patients, respectively. Mifepristone was appeared to be well tolerated, as no dose reductions and no delays due to drug toxicity were observed during the treatment period (337).
Clinical takeaway
Mifepristone has shown to be unsuccessful in treating PR-positive advanced or recurrent endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma. While a phase II clinical trial showed a stable disease rate of 25% (337), a later phase II clinical trial by the gynecologic oncology group assessing the utility of mifepristone in ovarian, peritoneal, and fallopian tube cancers showed a partial response in only 1 of 22 study patients (338). Partial affinity of mifepristone for steroid receptors may explain the limited efficacy and significant toxicity in these clinical trials.
Breast cancer
Mifepristone
Molecular mechanisms
Mifepristone induced cell growth inhibition by arresting G1 phase and apoptosis by poly(ADP-ribose) polymerase cleavage (PARP) and caspase activation in ER-expressing, antiestrogen-resistant breast cancer cells (339). Mifepristone also induced apoptosis in MDA-231 human breast cancer cells (340, 341). The inhibition of cell cycle progression in T-47D breast cancer cells by mifepristone was accompanied by a marked decrease in c-myc expression (342) and induction of p21 (343). Mifepristone selectively inhibited the proliferation of the PR-positive breast cancer MCF-7 and T-47D cells but not MDA-MB-231 cells (344). WNT1 was identified as a novel mediator of the antiproliferative effects of mifepristone in MCF-7 cells (344). HDLG5 functions as a target of progestin in MCF-7 breast cancer cells. Synthetic progestin medroxyprogesterone acetate (MPA) induced a rapid and strong upregulation of HDLG5 mRNA in MCF-7, T47D, and ZR-75-1 cells, which was further abrogated by mifepristone treatment (345). KLF5 is known to exert functions in promoting breast cell proliferation, survival, and tumor growth (346, 347). Liu et al reported that mifepristone can suppress the tumor growth of triple-negative breast cancer cells and patient-derived xenografts in NOD-SCID mice through inhibiting KLF5 expression via induction of miR-153 (348). In a human breast cancer xenograft model, mifepristone induced tumor regression, which was associated with a PR-A:PR-B ratio that leads to recruitment of coregulators AIB1 or SMRT to the Cyclin D1 and MYC promoters (349). The angiogenic molecule VEGF mRNA and protein expression were induced by P4 in cultured human T47-D breast cancer cells (193), which was effectively blocked by mifepristone treatment (350). The blockage of progestin-induced VEGF by mifepristone abolished the proliferation of endothelial and tumor epithelial cells (351). Exposure of xenografts to mifepristone induced growth inhibition of xenograft progestin-dependent tumors. Tumor progression was dependent on expression of VEGF (352). A systems pharmacology analysis presented that mifepristone can prevent breast cancer cells from migration and interfere with their adhesion to endothelial cells, which were associated with the inhibition of focal adhesion kinase (FAK), paxillin, and the formation of FAK/Src/paxillin complex (353) (Fig. 6).
Clinical efficacy: phase I
The effect of mifepristone on cell proliferation in human breast tissue from premenopausal women has been evaluated (354). In a placebo-controlled trial, a total of 30 women scheduled for surgical treatment of leiomyomas were enrolled to receive 50 mg mifepristone or placebo daily for 3 months. The proliferation maker Ki-67 index was found to be significantly reduced in mifepristone-treated groups, compared with placebo (354). The cortisol levels were not affected by mifepristone treatment, whereas an increase in serum testosterone was noted. The authors also identified a reduction in breast symptoms, including soreness and swelling, and an increase in the incidence of hot fiashes increased (354). Of note, the study was limited by the fact that interpretable biopsies were only available from 19% of the original study population. Furthermore, as fine needle aspiration cannot distinguish between ductal and stromal tissue, further analysis is required to assess whether this antiproliferative effect of mifepristone confers possible protection against malignant versus benign tumors.
Recently, an open-label study has been listed in ClinicalTrials.gov to study the effect of mifepristone in breast cancer patients with higher levels of PR-A than PR-B (NCT02651844). The changes in tumor cell proliferation, apoptosis, downstream signaling pathways of PR, tumor size, gene expression, and serum mifepristone levels will be compared from baseline to the time of surgery. This study is slated to be completed in March 2020.
Phase II
The toxic effects and response rate of mifepristone have been studied in women with PR-positive recurrent breast cancer (355). A total of 28 patients were enrolled and given 200 mg mifepristone daily. Throughout this treatment, disease was reassessed every 4 weeks. Mifepristone produced only 3 partial responses in all patients (response rate 10.7%) (Table 1). Toxic effects of mifepristone were generally mild to moderate and consisted of nausea, lethargy, anorexia, and hot flashes (355). Recently, another trial (NCT01898312) is recruiting patients with mutations in the breast cancer susceptibility genes BRCA1 or BRCA2. The primary outcome was to study epithelial cell proliferation in breast tissue. This study is expected to be completed in 2019.
Ulipristal acetate
Molecular mechanisms
In a patient-derived breast cancer xenograft model, cell proliferation was clearly reduced in the UPA treated group compared to vehicle, as revealed by the significant reduction in Ki-67, cyclin D1, and PCNA expression (356). Apoptosis was also observed, as demonstrated by an increase in activated PARP expression in response to UPA treatment (356).
Telapristone acetate
Molecular mechanisms
Mammary carcinogenesis was inhibited by telapristone acetate through the reduction of cell proliferation and induction of apoptosis (251, 357) (Fig. 6). An extensive study by Wiehle et al investigated the therapeutic effect of telapristone acetate in an animal model as well as in human T47D breast cancer cells (251). Telapristone acetate prevented the development of spontaneous mammary hyperplastic and premalignant lesions in Sprague-Dawley (Hsp: SD/BR) rats, compared to untreated groups. Telapristone acetate also suppressed N-methyl-N-nitrosourea (MNU)-induced mammary carcinogenesis (251). The anticarcinogenesis effect of telapristone in MNU-induced mammary tumors was mediated by inhibiting cell proliferation and inducing apoptosis, which was correlated with a decreased proportion of PR+ tumor cells and with decreased serum P4 but not E2. Treatment of T47D breast cancer cells with telapristone acetate also inhibited cell proliferation by inhibiting CDK2, CDK4, cell cycle progression, and downregulation of ER and PR signaling (251). Clare et al also reported that blockade of P4 signaling by telapristone resulted in a decreased G2/M fraction, caused by decreased expression of genes that facilitate the G2/M transition in T47D breast cancer cells (253). Tumors were induced with MPA or P4 plus MNU in rats. Telapristone decreased tumor incidence and tumor burden, with a significant decrease in pHH3- (proliferation) and CD34- (angiogenesis) positive cells. Telapristone was more effective than mifepristone in inducing growth inhibition of T47D spheroids (358). Telapristone was more effective than mifepristone for inhibiting growth of T47D spheroids (358). Telapristone acetate and some known aromatase inhibitors, including letrozole, anastrozole, and exemestane were tested to determine their ability to regulate testosterone-induced cell proliferation and anchorage-independent growth of T47Darom cells. Telapristone acetate was more effective alone or in combination with aromatase inhibitors in reducing testosterone-induced cell proliferation and anchorage-independent growth of T47Darom cells, compared to an aromatase alone. Telapristone acetate inhibited expression levels of PR-B and EGF-R and reduced the formation of the complex PRB/p300 in the nucleus of T47Darom cells (359). Davaadelger et al investigated the molecular mechanism by which telapristone acetate antagonizes PR action in T47D breast cancer cells (252). The investigators observed that telapristone acetate decreased PR occupancy on chromatin and recruits coregulators such as TRPS1 (transcriptional repressor GATA binding 1) to the PR complex, thereby regulating PR target gene expression and associated cellular responses (252).
Clinical efficacy: phase II
An active phase IIB trial of telapristone acetate has been listed in ClinicalTrials.gov for the treatment of stage I-II primary breast cancer prior to surgery (NCT01800422). This study has been designed to study the effect of telapristone acetate (administered orally once daily for 2–10 weeks) on the growth rate of breast cancer cells, compared to placebo. The primary measured outcome is observed tumor growth from baseline to the time of surgery. Secondary outcome measures include changes in expression of apoptosis markers, blood estradiol and P4 levels, and breast tissue concentrations of the study drug and its metabolite (CDB4453) compared to plasma concentrations and liver and renal function. This trial is expected to be finished in March 2020.
Clinical takeaway
Mifepristone has been shown to significantly reduce a proliferation marker in breast tissue but has only been associated with a response rate of 10.7% when studied in women with PR-positive recurrent breast cancer. Multiple additional studies are currently underway to further study the effect of mifepristone in patients based on BRCA status and levels of PR-A versus PR-B. The role of ulipristal acetate in reducing cell proliferation is currently undergoing investigation in cell models. Telapristone has been found in vitro to be more effective than mifepristone in inhibiting growth (358), and clinical trials assessing the in vivo effect of telapristone on breast cancer are currently underway.
Heavy menstrual bleeding
Heavy menstrual bleeding is defined as heavy bleeding that is cyclic due to its relationship with ovulation. This term has replaced the prior, less precise term “menorrhagia,” which had been used to describe heavy or prolonged uterine bleeding but fails to differentiate between volume and duration of bleeding or between cyclic and anovulatory bleeding. Chronic heavy bleeding can result in anemia and is often exceptionally disruptive to daily activities, independent of the quantity of blood lost. When a woman presents with HMB, the differential is vast, and is explored using the PALM-COEIN system (structural causes: Polyp, Adenomyosis, Leiomyoma, Malignancy & Hyperplasia; non-structural causes: Coagulopathy, Ovulatory Dysfunction, Endometrial, Iatrogenic, Not Yet Classified). Women presenting with HMB should first be evaluated to exclude the possibility of premalignant or malignant disease. The mechanism behind the effect SPRMs have on HMB is yet to be fully elucidated but may relate to the distinct changes in histological morphology (PAECs) that have been identified in association with their use (230, 262, 293, 360).
Ulipristal acetate
Molecular mechanisms
Three months of UPA use has been shown to be associated with altered architectural features, including dilation of the epithelial gland inactivity or features of abortive subnuclear vacuolization, and occasional mitoses and apoptosis. This distinctive histology was found to have reversed to normal after the discontinuation of UPA treatment (262, 360).
Clinical efficacy: phase I
A clinical trial to assess the efficacy of UPA in managing HMB in women between the ages of 18 and 51 who do not have uterine fibroids is currently listed in clinicaltrials.gov (NCT03027973), but it has not yet begun recruiting patients. Participants will also be excluded if they have a history of any known causes of HMB, including endometrial hyperplasia, cervical dysplasia, uterine or cervical polyps, adenomyosis, ovulatory dysfunction, any coagulopathy, or untreated thyroid disease.
Phase IV
An experimental, double-blind, randomized, parallel group study is being conducted to assess the efficacy of UPA in women with abnormal bleeding while on levonorgestrel-releasing intrauterine system (NCT03186586). The pilot population contains 26 women aged 18–45 years old randomized to receive 5mg/day of UPA for 5 days or placebo. The prevalence of bleeding will be assessed for a period of 3 months.
Asoprisnil
Molecular mechanisms
Histologically, asoprisnil is associated with the development of thick muscular walls within the endometrial spiral arteries (230) and decreased uterine artery blood flow (293). This effect appears to be unique to asoprisnil within the class of SPRMs. In the setting of high PR expression in the endometrial stroma (361), it is possible that the effect of asoprisnil occurs through affecting stromal PR expression. Wilkens et al have proposed a mechanism through indirect alteration of uterine natural killer (NK) function via PR mediation of IL-15 levels (362). In normal endometrium, IL-15 levels are responsive to P4 in that PR stromal cells transcribe IL-15. When exposed to P4, endometrial stromal cells will increase IL-15 transpresentation to NK cells and will expand and differentiate. Wilkens et al demonstrated that asoprisnil-treated endometrium demonstrates downregulation of stromal PR expression, upregulation of glandular PR expression, and reduction in uterine NK cells (362). They also found that asoprisnil caused suppression of IL-15 at a mRNA level. These findings may support a role of interleukin-15 (IL-15), an inflammatory mediator that has been shown to act between endometrial stromal cells, uterine NK cells, and endometrial spiral arteries (362).
Clinical efficacy: phase II
Bayer and TAP Pharmaceutical Products Inc. performed a multicenter, double-blind, placebo-controlled, randomized, parallel group study to evaluate the effects of different doses of asoprisnil in managing treatment-resistant HMB without an organic cause (NCT00288691). A total of 26 women aged 30–55 years were given daily oral administration of 5, 10, or 25 mg of asoprisnil or placebo for 35 to 50 days. The trial was completed in January 2004, but the results have not been made publicly available.
Clinical takeaway
SPRMs have been used for the treatment of HMB within the context of their use for management of uterine fibroids. The studies presented within section Uterine fibroids includes multiple studies (103, 123, 124, 126, 237, 238, 257, 280, 285, 287, 292, 308, 309, 312, 313, 318, 319) demonstrating the efficacy of mifepristone, ulipristal acetate, vilaprisan, and asoprisnil in achieving amenorrhea in patients with symptomatic fibroids. Currently, studies evaluating the role of SPRMs for HMB outside of this context are limited. Only 3 studies (NCT03027973, NCT03186586, and NCT00288691) have been initiated to assess the role of SPRMs in women with HMB unrelated to uterine fibroids. The results of these studies are yet to be published and made publicly available.
Tolerability and safety concerns of SPRMs
Adverse events
All the SPRMs are well tolerated by most patients. TEAEs were generally mild to moderate and were not associated with dose reduction or any delay of trials, with the exception of trials stopped due to elevated liver enzymes for telapristone. Adverse events including hot flashes, nausea, vomiting, lethargy, anorexia, fatigue/tiredness, abdominal pain, and vaginal discharge were associated with mifepristone (126, 280, 285, 287, 326, 337, 355), asoprisnil (292, 293), UPA (103, 237, 238, 306, 308, 309), telapristone acetate (249), and vilaprisan (319).
Liver toxicity
Associated with their primary metabolism in the liver (Fig. 6), some SPRMs have inconsistently been associated with liver toxicity. The effect of mifepristone on liver transferase enzyme profile has been variable (125, 285, 287). Yerushalmi et al found no significant differences in the liver transferase enzyme profile (287), while other studies found elevated levels of transaminases in a small number of patients (125, 285). Available clinical trials suggest no evidence of liver toxicity in asoprisnil-treated patients (292–294). For UPA, over 750 000 patients have been treated and 5 cases of drug-induced liver injury (DILI) have been reported as possibly linked to UPA treatment (238, 310). Four of these cases ended in liver transplantation. In February 2018 the EMA announced temporary restrictive measures for the compound, and the Pharmacovigilance Risk Assessment Committee (PRAC) subsequently issued temporary recommendations advising physicians not to initiate new treatment courses. In May 2018, the PRAC made recommendations for reducing the risk of liver injury and allowed treatments to resume overseas. Ongoing UPA trials in the United States were halted and have not yet resumed. As noted above, clinical trials of telapristone acetate were suspended in 2009 due to liver toxicity concerns (199), but these have been restarted with lower doses of the compound. Levels of liver transaminases were found to be slightly increased in both vilaprisan and placebo groups at similar levels (319).
Endometrial hyperplasia and neoplasms
Endometrial hyperplasia is a thickening of the lining of the uterus caused by excessive proliferation of the endometrial cells. It is typically diagnosed as a noncancerous entity, but in some cases can it can be associated with the risk of progression to endometrial cancer (363). Chronic exposure to estrogens unopposed by progesterone is considered a key component in the development of endometrial hyperplasia. Cyclic progestin or hysterectomy constitutes the major treatment option for endometrial hyperplasia (364). The use of SPRMs for the treatment of reproductive disorders has also been associated with the development of endometrial hyperplasia. Bagaria et al reported that mifepristone can induce endometrial hyperplasia without atypia in 63.1% of women (124), while other studies found no evidence of endometrial hyperplasia with mifepristone (123, 125, 285, 287). The other SPRM asoprisnil was shown to induce adverse endometrial changes (complex hyperplasia without atypia or with adenosarcoma) (294). Chabbert-Buffet et al found no endometrial hyperplasia in the UPA-treated group (312), while other studies found endometrial hyperplasia without evidence of atypia in some UPA-treated patients (311, 313). While the effect of SPRMs on endometrial hyperplasia is quite variable, SPRMs are consistently associated with PAECs and nonphysiologic endometrial changes. A significant number of patients developed PAECs following treatment with SPRMs. Available evidence indicates that these changes regress within a few cycles of menstrual bleeding off the medication.
Pregnancy outcome following SPRM use
To date, only UPA has been evaluated in the context of pregnancy outcomes following use for uterine fibroids. This data is still limited in quantity but suggests that UPA use can allow for favorable pregnancy outcomes, with or without interval myomectomy. Recently, Pécout et al reported that UPA treatment is associated with the disappearance of a fibroid after pregnancy in a 38-year-old patient (365). In 2018, De Gasperis-Brigante et al published the first and only systemic review to date evaluating pregnancy outcomes following UPA treatment for uterine fibroids (366). In their evaluation of 71 post-UPA pregnancies, there were 50 live births, 19 spontaneous abortions, 1 fetal death, 2 terminations, and 1 ongoing pregnancy at the time of their review. A total of 44 of the pregnancies occurred in women who underwent myomectomy following UPA use. Five women who became pregnant and did not undergo myomectomy following UPA use experienced delivery complications related to fibroids (366).
The literature regarding teratogenicity of SPRMs following failed use as an emergency contraceptive is limited because many women in this situation elect to undergo an induced abortion. However, published data suggests that this risk is likely minimal to none. In a prospective study by Bernard et al of 105 pregnancies exposed to mifepristone in the first 12 weeks of pregnancy, the overall rate of major congenital malformations was 4.2% compared to the expected rate of 2% to 4% seen in the general population (367, 368). In combined data from postmarketing reports and developmental studies of UPA, there were 376 exposed pregnancies reported, 232 of which had a known outcome (369). These pregnancies resulted in 28 live births, 34 first-trimester spontaneous miscarriages, and 151 elective terminations. While it is important to consider the large percent of women lost to follow-up, the observed rate of miscarriages (13.8%) is comparable to the rate of 20% seen in the general population. Of the elective terminations, 1 was a case of a trisomy 21 fetus in a 42-year-old woman that was thought to be unrelated to the exposure, as she took the drug following 6 weeks of amenorrhea. There was also a case of a fetal cardiac defect identified at 12 weeks gestation, which was found by the reporter to have an uncertain relationship to the exposure (369). There were no reported complications during the pregnancy or delivery and no increased rate of ectopic pregnancy with exposure to 30 mg UPA.
Selective Progesterone Receptor Modulators as Emergency Contraceptives
Emergency contraception
EC is defined as the use of a device or drug as an emergency measure to prevent pregnancy after unprotected intercourse, contraceptive failure or sexual assault. In the United States, approximately 60% of all women of reproductive age use contraceptive methods (370), indicating a need for accessible, safe, and effective EC. Emergency contraception is currently administered through the Cu-IUD (copper intrauterine device) and through emergency contraceptive pills. Historically, emergency contraceptive pills have taken 3 forms: progestin and estrogen, progestin only, and SPRMs. Emergency contraceptive pills with both estrogen and progestin are no longer marketed due to lower efficacy and higher adverse effects. The Cu-IUD has remained the most effective mechanism but is a less-preferred option for many women, likely due to factors such as need for a trained provider, high out-of-pocket cost, and availability of appointments for IUD placement (371). SPRMs are a promising new alternative to current commercially available ECs, as they are increasingly demonstrating better efficacy.
Mifepristone was the first SPRM studied for use as EC in 1992 (372). It is currently available as EC at doses of 10 to 25 mg for up to 120 hours after unprotected sexual intercourse in a small number of countries, including Armenia, Moldova, Ukraine, China, Russia, and Vietnam, but is not currently available for use as EC in the United States.
Ulipristal acetate is a new class of the second generation of SPRM, which has been approved for EC for use up to 5 days after unprotected intercourse. A single dose of 30 mg UPA for EC (ellaOne; HRA-Pharma, Paris, France) was approved by the EMA in 2009 and by the FDA in the United States in 2010 (Ella).
Mifepristone
Molecular mechanisms
The efficacy of mifepristone as EC appears to be dependent on the dose and the time of treatment during the menstrual cycle via its effects on the endometrium. In the follicular phase of the menstrual cycle, mifepristone treatment interrupts normal follicular development, resulting in a delay or inhibition of ovulation (373). This effect may not persist at all points throughout the ovulation cycle; a small pilot study showed that ovulation is not consistently blocked even when mifepristone is administered at a dose of 200 mg after the onset of the LH surge (374). Mifepristone has been reported to inhibit the human embryo implantation process in a three-dimensional human endometrial cell culture model (375). Zhou et al reported that mifepristone may negatively affect implantation by increasing the cytotoxicity of NK cells (376). Mifepristone treatment upregulates PR-B concentration in the epithelial and stromal cells of the fallopian tube as well as glandular cells of the endometrium during the midluteal phase (377), which is consistent with the high efficacy of this compound to prevent pregnancy when used as a postovulatory contraceptive method. Mifepristone may reduce fertilization by impairment of sperm function since it inhibits P4-induced calcium influx and acrosome reaction in human spermatozoa (378), as well as suppresses the rate of penetration of human spermatozoa into zona‐free hamster oocytes (379).
Clinical efficacy
A recent Cochrane review (14 RCTs, n = 8752, high-quality evidence or 27 RCTs, n = 6052, moderate-quality evidence) suggested that both middose (25–50 mg) and low-dose (less than 25 mg) mifepristone were probably more effective in preventing pregnancy than levonorgestrel (1.5 mg) (21). Common adverse effects with mifepristone include nausea, headache, dizziness, breast tenderness, abdominal pain, and spotting/bleeding after treatment. Of these, only spotting/bleeding after treatment was seen at a significantly reduced rate in women using mifepristone low- or mid-dose compared to levonorgestrel 1.5 mg (21). Mifepristone has been shown to have a minimal risk associated with breastfeeding, as peak levels in breast milk are <1.5% of maternal levels after administration of 4 times the EC dose of mifepristone (380).
A randomized controlled trial (n = 2065) was conducted in the United Kingdom to compare the efficacy between mifepristone (10 mg) and levonorgestrel (2 doses of 750 µg given 12 hours apart) in the context of EC within 120 hours of unprotected intercourse (381). Pregnancy rates were 1.3% and 2.0% for mifepristone and levonorgestrel, with prevention of expected pregnancies by 77% and 64%, respectively. This trial also demonstrated variation in the timing of a woman’s subsequent menstrual cycle after treatment, with women receiving mifepristone experiencing a delayed onset of menses and women receiving levonorgestrel experiencing an early onset. Women reported similar levels of satisfaction between the 2 pills (mifepristone, 94%; levonorgestrel, 91%) (381).
In another study, 398 women and adolescents were enrolled and given 100 mg of ethinyl estradiol and 1 mg of norgestrel, each given twice 12 hours apart (standard therapy) and were compared to 402 women and adolescents who received mifepristone at 600 mg. Mifepristone treatment was associated without pregnancy, while 4 cases were reported in the standard therapy group. The subjects treated with mifepristone reported less nausea (40% vs. 60%) and vomiting (3% vs. 17%) (372).
In a double-blind, randomized, controlled trial conducted in Australia, a total of 150 healthy women with regular menstrual cycles were enrolled to receive 1 of the 3 doses (10, 50, or 600 mg) of mifepristone (382). Pregnancy rates were observed at 2.0%, 2.1%, and 2.1% with 10, 50, and 600 mg of mifepristone, respectively. The common side effects, including nausea, headache, dizziness, fatigue/weakness, breast tenderness, diarrhea, lower abdominal pain, vaginal bleeding, or spotting were reported by participants within 7 days of mifepristone treatment (382).
A single-arm trial was conducted in Cuba to evaluate the efficacy of 10 mg mifepristone for EC up to 6 days after unprotected coitus (383). A total of 635 women were included in this trial. Pregnancy rates were observed in 7/635 women (1.1%) after mifepristone treatment. Adverse events were reported by 10.7% (fatigue), 6.1% (dizziness), 4.9% (nausea), and by 0.6% (vomiting) with mifepristone treatment. Menstruation was delayed more than 7 days in 38/635 (6.0%) of women (383).
A randomized double-blind trial was conducted in 5 family-planning clinics in China (384). A total of 998 healthy women were assigned and given single-dose 10 mg gestrinone (n = 499) or 10 mg mifepristone (n = 499) after unprotected coitus up to 72 hours. Pregnancy rates were observed in 1.8% and 2.4% of the cases with mifepristone and gestrinone group, respectively (384).
Ulipristal acetate
Molecular mechanisms
Ulipristal acetate acts by preventing or delaying ovulation (385). Ulipristal acetate may be taken as EC up to 120 hours after unprotected sex for a 70% reduction in unintended pregnancy risk (386). This effect persists even when UPA is administered after the onset of the LH surge, but before the peak (387). When UPA is administered before the onset of the LH surge, follicular rupture is delayed by at least 5 days in 100% of cases (385). When UPA is administered after the LH surge but before the LH peak, it delays follicular rupture in 79% of cases. However, only 8% of women experience delayed follicular rupture if UPA is taken after the LH peak (385). A recent study concluded that UPA may also inhibit uterine decidualization by downregulating expression of several key genes, including interleukin 15 (IL-15), STAT3, prolactin (PRL), IGFBP1, and HAND2, involved in this process (388). Ulipristal acetate at midfollicular doses of 10 to 100 mg caused a dose-dependent delay in ovulation and also inhibited luteal phase endometrial maturation, which consequently inhibits implantation (236). A single early luteal dose of up to 100 mg UPA decreased endometrial thickness with increased glandular PRs, without altering corpus luteal function (389). Ulipristal acetate does not appear to impair sperm function (390, 391). Munuce et al showed that the sperm functional parameters, including vitality, sperm protein tyrosine phosphorylation, as well as spontaneous and human follicular fluid‐induced acrosome reaction, were not affected by UPA treatment (391). In addition, it was shown that UPA did not affect the ability of human sperm to bind to the surface of human tubal tissue explants or to penetrate the mouse cumulus mass and the zona-free hamster eggs (390). An in vitro study has shown that UPA does not interfere with embryo attachment to the endometrium (392). Ulipristal acetate also suppresses ciliary beating and muscular contraction in the fallopian tube (393). However, the in vivo efficacy of these postovulatory mechanisms remains uncertain, as a recent clinical study indicated that UPA has significantly higher efficacy when administered before versus after ovulation (394).
Clinical efficacy
A recent Cochrane review analysis using 2 randomized controlled trials (n = 3448) (395, 396) presented that UPA was associated with fewer pregnancies compared to levonorgestrel when administered within 120 hours after unprotected intercourse (21). These 2 randomized trials compared the efficacy of UPA to levonorgestrel or placebo for women seeking EC within 72 hours of unprotected intercourse (395) and up to 120 hours (396). In the first study (395), healthy women were randomly assigned to receive a single dose of 50 mg of UPA, plus either a placebo 12 hours later or 2 doses of 0.75 mg of levonorgestrel taken 12 hours separately. Pregnancies rates were 0.9% in the UPA group and 1.7% in the levonorgestrel group, respectively. The reduction in the number of pregnancies from placebo was 85% in the UPA group and 69% in the levonorgestrel group. A somewhat greater percentage of UPA (29%) compared to the levonorgestrel group (24%) experienced nausea, whereas the similar incidence of bleeding and spotting was observed in both groups (395). In the second study (NCT00551616), a total of 2221 women were randomly assigned to receive a single dose of 30 mg UPA (n = 1104) or 1.5 mg levonorgestrel (n = 1117) (396). The pregnancy rates were 1.8% (n = 844) and 2.6% (n = 852) in the UPA group and levonorgestrel group, respectively, in women who received EC within 72 hours of sexual intercourse. In the 203 women who received EC between 72 and 120 hours after sexual intercourse, 3 pregnancies occurred, all of which were in the levonorgestrel group. The common adverse event was headache 19.3% (n = 1104) in the UPA group and 18.9% (n = 1117) in the levonorgestrel group, with other observed events, including dizziness and molar pregnancy (396).
A large US-based prospective clinical trial showed that UPA at 30 mg single dose was very effective and well tolerated for EC when used 48 to 120 hours following unprotected intercourse (397). This trial included a total of 1241 women with regular cycles. The pregnancy rates were observed at 2.3% (1.4–3.8%) for 48 to 72 hours of intervals, 2.1% (1.0–4.1%) for ≥72 to 96 hours of intervals, and 1.3% (0.1–4.8%) for ≥ 96 to 120 hours of intervals. Adverse events were generally mild or moderate. The most common adverse events were headache, nausea, and abdominal pain. The length of menstrual cycle was increased by a mean of 2.8 days, whereas the duration of menstrual bleeding did not change (397).
Selective Progesterone Receptor Modulators as Anti-glucocorticoid Agents
Mifepristone
Cushing’s syndrome
Cushing’s syndrome is a rare endocrine disorder characterized by elevated levels of cortisol (398). Iatrogenic (exogenous or drug-related) Cushing’s syndrome is generally seen in clinical practice and is associated with exogenous use of glucocorticoid products. Endogenous Cushing’s syndrome is rare and is associated with the body’s own overproduction of cortisol.
Most cases are caused by an adrenocorticotropic hormone (ACTH)-secreting tumor of the pituitary gland that produces an excess amount of ACTH, which in turn stimulates the adrenal glands to release more cortisol. When this form of the syndrome develops, it is referred to as Cushing’s disease. Excess cortisol can also be caused by an ACTH-secreting tumor located in the lungs, pancreas, thyroid, or thymus gland. Cushing’s syndrome is the moniker used for excess cortisol resulting from benign, malignant, or hyperplastic diseases of the adrenal glands causing secretion of cortisol in the absence of ACTH stimulation (399, 400). The excess cortisol seen in Cushing’s syndrome results in hypertension, hyperglycemia, obesity, and a myriad of other problems (400). These complications lead to significant morbidity related to illness and twice the mortality rate in patients with Cushing’s syndrome compared to the general population (401). The presence of diabetes mellitus and hypertension in these patients are the most important predictors of death (401). Surgery remains the first-line treatment for Cushing’s syndrome, but mifepristone is used as a second-line or third-line therapy for patients who have failed to achieve remission with other treatment modalities (399, 402). Mifepristone has been approved by the FDA in February 2012 for patients with hyperglycemia secondary to Cushing’s syndrome who are not surgical candidates or whose disease is refractory to surgery (403, 404). The starting dosage of mifepristone is 300 mg/day, which can be increased every 2 to 4 weeks up to a maximum of 1200 mg/day. Mifepristone is commercially available in the United States as Korlym as of May 1, 2012.
Several recent clinical trials (405–410) have evaluated the clinical efficacy and safety of mifepristone for patients with Cushing’s syndrome. A Europe-based retrospective study was conducted with mifepristone for patients with malignant and benign Cushing’s syndrome. This study enrolled 15 patients with malignant conditions associated with adrenocortical carcinoma (n = 12) and ectopic ACTH secretion (n = 3), and 5 patients with benign conditions associated with Cushing’s disease (n = 4) and bilateral adrenal hyperplasia (n = 1). Mifepristone was given at a starting dose of 400 mg daily (200–1000 mg) for 2 months (for malignant Cushing’s syndrome) or 6 months (for benign Cushing’s syndrome) (405). Clinical (signs of hypercortisolism, elevated blood pressure, adrenal insufficiency) and biochemical (abnormal serum potassium and glucose) parameters were evaluated. Mifepristone treatment improved clinical signs in 73% of patients with malignant Cushing’s syndrome patients and 80% of patients with benign Cushing’s syndrome (405). Signs of adrenal insufficiency were observed in 15% of patients and increased blood pressure levels were observed in 15% of patients. (405).
A large US-based multicenter, open-label trial (NCT00569582) was conducted to assess the efficacy of administering mifepristone in patients (n = 50) with endogenous Cushing’s syndrome along with type 2 diabetes mellitus or impaired glucose tolerance or hypertension (406). Mifepristone was given at doses of 300 to 1200 mg daily for 24 weeks. Mifepristone treatment improved the clinical status in 87% of patients. Glucose profiles were improved in 60% of patients with diabetes mellitus or glucose intolerance. Insulin resistance, depression, cognition, and quality of life also improved. Mifepristone treatment induced adverse events, including fatigue, nausea, headache, low potassium, arthralgia, vomiting, edema, and endometrial thickening (406).
Katznelson et al performed a post hoc analysis of secondary end-point data from a multicenter, open-label trial (407). In this trial, mifepristone was given at 300 to 1200 mg doses daily for 24 weeks to patients (n = 46) with refractory Cushing’s syndrome associated with type 2 diabetes mellitus or impaired glucose tolerance or hypertension (407). Global clinical assessment included 8 broad clinical categories, including glucose control, lipid levels, blood pressure, body composition, clinical appearance, strength, psychiatric or cognitive symptoms and quality of life. Data showed that mifepristone treatment induced significant improvements in global clinical response assessments in 88% of patients (407).
Bilateral macronodular adrenal hyperplasia (BMAH) is a cause of primary adrenal hypercortisolism, a rare form of Cushing’s syndrome. In a retrospective review, Cohan and colleagues reported that mifepristone was given to 4 patients with hypercortisolism due to BMAH (348). Clinical improvement was observed in all 4 patients after mifepristone treatment. Improvement in cardiometabolic parameters, glycemic control, and hypertension was also observed. All patients experienced significant weight loss. Mifepristone treatment was associated with adverse event fatigue (408).
In the US-based SEISMIC (Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing’s Syndrome) trial, 43 patients with Cushing’s disease were treated with mifepristone at 300 to 1200 mg for 24 weeks (409). Mifepristone treatment was associated with an increase in ACTH levels in 72% of patients, as well as tumor progression in 4 cases (409). Consistent weight loss was observed in the mifepristone-treated patients at a 2 year follow-up (410).
Psychiatric conditions
Psychotic depression is a severe mental disorder characterized by delusions and/or hallucinations. This condition occurs at a prevalence of 0.4% (411). Dysregulation and hyperactivity of the hypothalamic-pituitary-adrenal axis, with subsequent elevation in circulating cortisol levels, are thought to be involved in the development of psychotic depression (412). Due to the antiglucocorticoid effects of mifepristone, mifepristone has recently been tested for use in the treatment of various mood disorders, including psychotic depression (413–416).
In 2006, DeBattista et al reported that mifepristone is well tolerated and can be effective for the treatment of psychotic depression (416). Later, Blasey et al designed a study with 433 patients diagnosed with psychotic depression who were randomly assigned to placebo or mifepristone at 300, 600, or 1200 mg for 7 days (413). This group also reported that mifepristone was well tolerated at all 3 doses. Mifepristone effectively reduced psychotic characteristics in patients with psychotic depression. The rapid and sustained reduction in psychotic features were associated with mifepristone plasma levels ≥ 1660 ng/mL (413). A US-based double-blind, randomized placebo-controlled trial (NCT00637494) also reported that mifepristone at 1200 mg daily for 7 days was safe and well-tolerated. Mifepristone plasma levels of 1637 ng/mL or greater was reported in 66.7% of patients experiencing significant reductions in psychotic symptoms compared to the placebo group (414). Recently, Block et al performed a combined analysis from 5 similarly designed phase II or III studies on treatment with mifepristone (n = 833) or placebo (n = 627) in patients with psychotic depression. They found that mifepristone at a 1200 mg daily dose allowed most patients to achieve a therapeutic mifepristone plasma level of ≥ 1637 ng/mL, which was effective to reduce psychotic features with wide safety margins (415).
Conclusion and Future Perspectives
Significant advancement has been achieved in the use of SPRMs for human gynecological and nongynecological diseases. Substantial research in this area has led to the US FDA approval of mifepristone in 2012 for Cushing’s syndrome and the approval of UPA in Canada and Europe as a presurgical therapy for patients with uterine fibroids, as well as for emergency contraception in the United States. A small number of trials suggest that the clinical efficacy of mifepristone is not evident or somewhat variable in endometriosis, endometrial cancer, and breast cancer (Table 1 and Fig. 6). A therapeutic effect of SPRMs in uterine fibroids is clearly evident (Table 1 and Fig. 6). Notably, heavy menstrual bleeding is a frequent symptom of uterine fibroids, and SPRMs cause a rapid cessation of bleeding, often in only a few days in women with very heavy bleeding due to their effects on the endometrium. The mechanism of therapeutic benefit for women with fibroids also includes a reduction in fibroid size that occurs during treatment. Mifepristone-induced fibroid regression is partly mediated by the inhibition of cell proliferation and oncogenic gene expression (LAT2), induction of apoptosis and tumor suppressor gene expression (KLF11), as well as suppression of AKT, TGF-β activity, and ECM accumulation (Fig. 6). Asoprisnil appears to be effective in reducing uterine bleeding and fibroid volume, partially due to its role in the induction of apoptosis, suppression of proliferation, ECM accumulation, and molecular pathways involving IL-15, as well as growth factors (EGF, IGF-I, and TGF-β) and their receptors (p-EGFR, IGF-IRα, and p-TGF-RII) (Fig. 6). Ulipristal acetate is effective for treatment of uterine fibroids by inducing antiproliferative, proapoptotic, antiangiogenic, and antifibrotic effects (Fig. 6), and it appears to reduce pain associated with endometriosis by inhibiting cell proliferation, inhibiting cell adhesion, and inducing apoptosis (Fig. 6). While the clinical efficacy of SPRMs is promising, safety concerns have been raised due to some patients experiencing complex hyperplasia or liver toxicity during the course of SPRM treatment (Table 1). Mifepristone and UPA can cause atypical endometrial hyperplasia, and 1 report noted possible safety concerns because of unknown long-term endometrial effects with uninterrupted treatment with asoprisnil. Current evidence is reassuring regarding liver toxicity with UPA and asoprisnil treatment, but the development of telapristone acetate was suspended due to concerns regarding liver toxicity. Some trials have reported slightly elevated levels of transaminases in a small number of vilaprisan- and mifepristone-treated patients. Finally, all SPRMs have been associated with PAECs. While gene expression studies indicate that PAECs do not correlate with endometrial hyperplasia or neoplasms, these features may require interrupted treatment cycles in women. In conclusion, current evidence suggests that SPRMs are emerging therapies that show immense promise as therapeutic options for gynecologic diseases and nongynecologic conditions.
Acknowledgements
Financial Support: M. S. I. is supported, in part, by the Ines Mandl Research Foundation. J. S. is supported, in part, by the Howard and Georgeanna Jones Foundation.
Disclosure Summary: J. H. S. is, or has served, as a principal investigator on research sponsored by Bayer, Abbvie, Biospecifics, Allergan, Inc., and Myovant.
Glossary
Abbreviations
- 17α-OHP
17α-hydroxyprogesterone
- 17βHSD2
17β hydroxysteroid dehydrogenase 2
- 5α-DHP
5α-dihydroprogesterone
- 5β-DHP
5β-dihydroprogesterone
- ACTH
adrenocorticotropic hormone
- ADAM12
ADAM metallopeptidase domain 12
- AF
activation function
- AIB1
amplified in breast cancer 1
- AKAP13
A-Kinase Anchoring Protein 13
- AKR1B1
Aldo-keto reductase family 1 member B1
- AP-1
activator protein 1
- ATBF1
AT Motif-Binding Factor 1 or zinc finger homeobox 3
- Bax
BCL2 associated X, apoptosis regulator
- BBSS
Biberoglu & Behrman Symptom Severity
- Bcl-2
B-cell lymphoma
- BMAH
bilateral macronodular adrenal hyperplasia
- cAMP
cyclic adenosine monophosphate
- CDKI
cyclin-dependent kinase inhibitor
- COX-2
cyclooxygenase-2
- Cu-IUD
copper intrauterine device
- CYP
cytochrome P450
- DBD
DNA binding domain
- E2
estrogen
- EC
emergency contraception
- ECM
extracellular matrix
- EMA
European Medicines Agency
- EMMPRIN
extracellular matrix metalloproteinase inducer
- ERBB2
epidermal growth factor 2
- FAK
focal adhesion kinase
- FASLG
Fas ligand
- FDA
Food and Drug Administration
- FOXO1
forkhead Box O1
- FSH
follicle stimulating hormone
- GABAA
aminobutyric acid type A
- GADD153
DNA-damage-inducible gene 153
- GSK3β
glycogen synthase kinase 3β
- GSTM1
glutathione-s transferase mu 1
- H
hinge region
- HAND2
heart and neural crest derivatives expressed 2
- HDACi
histone deacetylase inhibitor
- HIF-1α
hypoxia-inducible factor-1alpha
- HMB
heavy menstrual bleeding
- HRQL
health-related quality of life
- HSP
heat shock protein
- IGFBP-1
insulin-like growth factor binding protein 1
- IL-15
interleukin 15
- KLF11
krüppel-like transcription factor 11
- LAT2
L-type amino acid transporter 2
- LBD
ligand-binding domain
- LGESS
low grade endometrial stromal sarcoma
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- MKP-1/DUSP1
MAPK phosphatase 1
- MMP
matrix metalloproteinase
- MPA
medroxyprogesterone acetate
- mPR
membrane-bound progesterone receptors
- MR
mineralocorticoid receptor
- MT1-MMP
membrane type 1-MMP
- NETA
norethisterone acetate
- NFAT5
nuclear factor of activated T-cells
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLS
nuclear localization signal
- NTD
N-terminal A/B domain
- P4
progesterone
- PAEC
progesterone receptor modulator-associated endometrial change
- PARP
poly(ADP-ribose) polymerase cleavage
- PBAC
pictorial blood assessment chart
- PCNA
proliferating cell nuclear antigen
- PGE2
prostaglandin E2
- PGL4002
mono-N-demethylated metabolite
- PGL4004
di-N-demethylated metabolite
- PI3K/AKT
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLAU
plasminogen Activator, Urokinase
- PR
progesterone receptor
- PRE
progesterone response elements
- PRL
prolactin
- PXR
pregnane X receptor
- RANKL
Receptor activator of nuclear factor kappa-Β ligand or TNF superfamily member 11
- SEISMIC
Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing’s Syndrome
- SLC5A3
solute carrier family 5 member 3
- SMRT
silencing mediator of retinoid and thyroid hormone receptor
- Sp1
specificity protein1
- SPRM
selective progesterone receptor modulator
- SRC
steroid receptor coactivator-1
- STAT5
signal transducer and activator of transcription 5
- TEAE
Treatment-emergent adverse effect
- TGF-β
transforming growth factor-β
- THY1
Thy-1 cell surface antigen
- TIMP
tissue inhibitor of MMP
- TNBC
Triple-negative breast cancer
- TN-C
tenascin C
- TNF-α
tumor necrosis factor-α
- TRPS1
Transcriptional Repressor GATA Binding 1
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- UFS-QoL
Uterine Fibroid Symptoms Quality of Life Questionnaire
- UPA
ulipristal acetate
- VEGF
vascular endothelial growth factor
Additional Information
Disclosure Summary: J.S. is or was a PI on research sponsored by Bayer, Abbvie, Biospecifics, Allergan, Inc., and Myovant. J.S. serves on boards of the American Society for Reproductive Medicine, Society for Reproductive Investigation, the American Board of Obstetrics and Gynecology, and the American Gynecological and Obstetrical Society. M.S.I. has nothing to disclose. S.A. has nothing to disclose. S.J. has nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. [DOI] [PubMed] [Google Scholar]
- 2. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–970. [DOI] [PubMed] [Google Scholar]
- 3. Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking thematic review series: genetics of human lipid diseases. J Lipid Res. 2011;52(12):2111–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8 (Suppl 1):3–63. [DOI] [PubMed] [Google Scholar]
- 5. Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68(10–13):879–890. [DOI] [PubMed] [Google Scholar]
- 6. Illingworth DV, Elsner C, De Groot K, Flickinger GL, Mikhail G. A specific progesterone receptor of myometrial cytosol from the rhesus monkey. J Steroid Biochem. 1977;8(2):157–160. [DOI] [PubMed] [Google Scholar]
- 7. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA A receptor. Nat Rev Neurol. 2005;6(7)::565–575. [DOI] [PubMed] [Google Scholar]
- 8. Scholtz EL, Krishnan S, Ball BA, et al. Pregnancy without progesterone in horses defines a second endogenous biopotent progesterone receptor agonist, 5α-dihydroprogesterone. Proc Natl Acad Sci U S A. 2014;111(9):3365–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92(1):73–82. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell BF, Mitchell JM, Chowdhury J, et al. Metabolites of progesterone and the pregnane X receptor: a novel pathway regulating uterine contractility in pregnancy? Am J Obstet Gynecol. 2005;192(4):1304–1313; discussion 1313. [DOI] [PubMed] [Google Scholar]
- 11. McEvoy K, Osborne LM. Allopregnanolone and reproductive psychiatry: an overview. Int Rev Psychiatry. 2019;31(3):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marx CE, Bradford DW, Hamer RM, et al. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78–90. [DOI] [PubMed] [Google Scholar]
- 13. Vallée M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Brain Res Rev. 2001;37(1–3):301–312. [DOI] [PubMed] [Google Scholar]
- 14. Mellor DJ, Diesch TJ, Gunn AJ, Bennet L. The importance of “awareness” for understanding fetal pain. Brain Res Rev. 2005;49(3):455–471. [DOI] [PubMed] [Google Scholar]
- 15. Bäckström T, Wahlström G, Wahlström K, Zhu D, Wang M-D. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur J Pharmacol. 2005;512(1):15–21. [DOI] [PubMed] [Google Scholar]
- 16. Woodward RM, Polenzani L, Miledi R. Effects of steroids on gamma-aminobutyric acid receptors expressed in Xenopus oocytes by poly(A)+ RNA from mammalian brain and retina. Mol Pharmacol. 1992;41(1):89–103. [PubMed] [Google Scholar]
- 17. Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN, Network OFPRU . Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins. Am J Obstet Gynecol. 2007;197(6):599.e591–599.e597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mooij CF, Parajes S, Pijnenburg-Kleizen KJ, Arlt W, Krone N, Claahsen-van Der Grinten HL. Influence of 17-hydroxyprogesterone, progesterone and sex steroids on mineralocorticoid receptor transactivation in congenital adrenal hyperplasia. Horm Res Paediatr. 2015;83(6):414–421. [DOI] [PubMed] [Google Scholar]
- 19. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26(3):423–438. [DOI] [PubMed] [Google Scholar]
- 21. Shen J, Che Y, Showell E, Chen K, Cheng L. Interventions for emergency contraception. Cochrane Database Syst Rev. 2019;1(1):CD001324.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313; discussion 313–294. [PubMed] [Google Scholar]
- 23. Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 5’-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol. 1990;4(12):1833–1840. [DOI] [PubMed] [Google Scholar]
- 24. Hirata S, Shoda T, Kato J, Hoshi K. The novel isoform of the progesterone receptor cDNA in the human testis and detection of its mRNA in the human uterine endometrium. Oncology. 2000;59(Suppl 1):39–44. [DOI] [PubMed] [Google Scholar]
- 25. Hirata S, Shoda T, Kato J, Hoshi K. The novel exon, exon T, of the human progesterone receptor gene and the genomic organization of the gene. J Steroid Biochem Mol Biol. 2002;80(3):365–367. [DOI] [PubMed] [Google Scholar]
- 26. Yamanaka T, Hirata S, Shoda T, Hoshi K. Progesterone receptor mRNA variant containing novel exon insertions between exon 4 and exon 5 in human uterine endometrium. Endocr J. 2002;49(4):473–482. [DOI] [PubMed] [Google Scholar]
- 27. Saner KJ, Welter BH, Zhang F, et al. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200(1–2):155–163. [DOI] [PubMed] [Google Scholar]
- 28. Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9(5):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244–1255. [DOI] [PubMed] [Google Scholar]
- 30. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278(41):39261–39264. [DOI] [PubMed] [Google Scholar]
- 32. Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. Embo J. 1992;11(10):3681–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill KK, Roemer SC, Churchill ME, Edwards DP. Structural and functional analysis of domains of the progesterone receptor. Mol Cell Endocrinol. 2012;348(2):418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darimont BD, Wagner RL, Apriletti JW, et al. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998;12(21):3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tetel MJ, Jung S, Carbajo P, Ladtkow T, Skafar DF, Edwards DP. Hinge and amino-terminal sequences contribute to solution dimerization of human progesterone receptor. Mol Endocrinol. 1997;11(8):1114–1128. [DOI] [PubMed] [Google Scholar]
- 36. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20(9):3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14(7):939–946. [DOI] [PubMed] [Google Scholar]
- 38. Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357(1–2):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lonard DM, O’Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8(10):598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68(10–13):761–770. [DOI] [PubMed] [Google Scholar]
- 41. Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci U S A. 1996;93(12):6169–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22(4):823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cerliani JP, Guillardoy T, Giulianelli S, et al. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer Res. 2011;71(10):3720–3731. [DOI] [PubMed] [Google Scholar]
- 44. Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271(11):6217–6224. [DOI] [PubMed] [Google Scholar]
- 45. Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276(11):8475–8483. [DOI] [PubMed] [Google Scholar]
- 46. Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97(3):1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277(37):33950–33956. [DOI] [PubMed] [Google Scholar]
- 48. Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46–56. [DOI] [PubMed] [Google Scholar]
- 49. Garg D, Ng SSM, Baig KM, Driggers P, Segars J. Progesterone-mediated non-classical signaling. Trends Endocrinol Metab. 2017;28(9):656–668. [DOI] [PubMed] [Google Scholar]
- 50. Boonyaratanakornkit V, Scott MP, Ribon V, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8(2):269–280. [DOI] [PubMed] [Google Scholar]
- 51. Migliaccio A, Piccolo D, Castoria G, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. Embo J. 1998;17(7):2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100(5):2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100(5):2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith JL, Kupchak BR, Garitaonandia I, et al. Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73(11):1160–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dressing GE, Alyea R, Pang Y, Thomas P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm Cancer. 2012;3(3):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Swiatek‐De Lange M, Stampfl A, Hauck SM, et al. Membrane‐initiated effects of progesterone on calcium dependent signaling and activation of VEGF gene expression in retinal glial cells. Glia. 2007;55:1061–1073. [DOI] [PubMed] [Google Scholar]
- 57. Zheng S, Huang J, Zhou K, et al. Progesterone enhances vascular endothelial cell migration via activation of focal adhesion kinase. J Cell Mol Med. 2012;16(2):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239(3):726–731. [DOI] [PubMed] [Google Scholar]
- 59. Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379(7):907–911. [DOI] [PubMed] [Google Scholar]
- 60. Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;229(1):86–89. [DOI] [PubMed] [Google Scholar]
- 61. Kowalik MK, Rekawiecki R, Kotwica J. The putative roles of nuclear and membrane-bound progesterone receptors in the female reproductive tract. Reprod Biol. 2013;13(4):279–289. [DOI] [PubMed] [Google Scholar]
- 62. Peluso JJ. Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol Reprod. 2006;75(1):2–8. [DOI] [PubMed] [Google Scholar]
- 63. Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105(1–5):16–36. [DOI] [PubMed] [Google Scholar]
- 64. Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA. Nonclassical progesterone signalling molecules in the nervous system. J Neuroendocrinol. 2013;25(11):991–1001. [DOI] [PubMed] [Google Scholar]
- 65. Pru JK, Clark NC. PGRMC1 and PGRMC2 in uterine physiology and disease. Front Neurosci. 2013;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hughes AL, Powell DW, Bard M, et al. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5(2):143–149. [DOI] [PubMed] [Google Scholar]
- 67. Wendler A, Wehling M. PGRMC2, a yet uncharacterized protein with potential as tumor suppressor, migration inhibitor, and regulator of cytochrome P450 enzyme activity. Steroids. 2013;78(6):555–558. [DOI] [PubMed] [Google Scholar]
- 68. Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am J Reprod Immunol. 2016;75(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garcia E, Bouchard P, De Brux J, et al. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab. 1988;67(1):80–87. [DOI] [PubMed] [Google Scholar]
- 70. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334–340. [DOI] [PubMed] [Google Scholar]
- 71. Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84(8):2963–2971. [DOI] [PubMed] [Google Scholar]
- 72. Mote PA, Balleine RL, McGowan EM, Clarke CL. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod. 2000;15(Suppl 3):48–56. [DOI] [PubMed] [Google Scholar]
- 73. Zhang L, Kanda Y, Roberts DJ, et al. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol. 2008;287(1–2):81–89. [DOI] [PubMed] [Google Scholar]
- 74. Keator CS, Mah K, Slayden OD. Alterations in progesterone receptor membrane component 2 (PGRMC2) in the endometrium of macaques afflicted with advanced endometriosis. Mol Hum Reprod. 2012;18(6):308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bunch K, Tinnemore D, Huff S, Hoffer ZS, Burney RO, Stallings JD. Expression patterns of progesterone receptor membrane components 1 and 2 in endometria from women with and without endometriosis. Reprod Sci. 2014;21(2):190–197. [DOI] [PubMed] [Google Scholar]
- 76. Shynlova O, Kwong R, Lye SJ. Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy. Reproduction. 2010;139(1):247–253. [DOI] [PubMed] [Google Scholar]
- 77. Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92(5):1927–1933. [DOI] [PubMed] [Google Scholar]
- 78. Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 81. Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20(7):1519–1534. [DOI] [PubMed] [Google Scholar]
- 82. Kowalik MK, Dobrzyn K, Rekawiecki R, Kotwica J. Expression of membrane progestin receptors (mPRs) α, β and γ in the bovine uterus during the oestrous cycle and pregnancy. Theriogenology. 2019;140:171–179. [DOI] [PubMed] [Google Scholar]
- 83. Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2(12):a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aupperlee MD, Haslam SZ. Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology. 2007;148(5):2290–2300. [DOI] [PubMed] [Google Scholar]
- 85. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 86. Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95(2):696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146(8):3577–3588. [DOI] [PubMed] [Google Scholar]
- 88. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967–973. [DOI] [PubMed] [Google Scholar]
- 89. Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. 1, 25 Dihydroxyvitamin D3 enhances the antifibroid effects of ulipristal acetate in human uterine fibroids. Reprod Sci. 2018;26(6):812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oskovi Kaplan ZA, Taşçi Y, Topçu HO, Erkaya S. 25-Hydroxy vitamin D levels in premenopausal Turkish women with uterine leiomyoma. Gynecol Endocrinol. 2018;34(3):261–264. [DOI] [PubMed] [Google Scholar]
- 91. Ciavattini A, Delli Carpini G, Serri M, et al. Hypovitaminosis D and “small burden” uterine fibroids: opportunity for a vitamin D supplementation. Medicine (Baltimore). 2016;95(52):e5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2002;76:187–192. [DOI] [PubMed] [Google Scholar]
- 93. Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–438. [DOI] [PubMed] [Google Scholar]
- 94. Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433–445. [DOI] [PubMed] [Google Scholar]
- 95. Saravelos SH, Yan J, Rehmani H, Li TC. The prevalence and impact of fibroids and their treatment on the outcome of pregnancy in women with recurrent miscarriage. Hum Reprod. 2011;26(12):3274–3279. [DOI] [PubMed] [Google Scholar]
- 96. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223. [DOI] [PubMed] [Google Scholar]
- 97. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012; 206(6):211.e211-211.e219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Townsend DE, Sparkes RS, Baluda MC, McClelland G. Unicellular histogenesis of uterine leiomyomas as determined by electrophoresis by glucose-6-phosphate dehydrogenase. Am J Obstet Gynecol. 1970;107(8):1168–1173. [DOI] [PubMed] [Google Scholar]
- 99. Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965;150(3692):67–69. [DOI] [PubMed] [Google Scholar]
- 100. Mashal RD, Fejzo ML, Friedman AJ, et al. Analysis of androgen receptor DNA reveals the independent clonal origins of uterine leiomyomata and the secondary nature of cytogenetic aberrations in the development of leiomyomata. Genes Chromosomes Cancer. 1994;11(1):1–6. [DOI] [PubMed] [Google Scholar]
- 101. Cai YR, Diao XL, Wang SF, Zhang W, Zhang HT, Su Q. X-chromosomal inactivation analysis of uterine leiomyomas reveals a common clonal origin of different tumor nodules in some multiple leiomyomas. Int J Oncol. 2007;31(6):1379–1389. [PubMed] [Google Scholar]
- 102. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105(50):19887–19892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Donnez J, Tatarchuk TF, Bouchard P, et al. ; PEARL I Study Group . Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. [DOI] [PubMed] [Google Scholar]
- 104. Friedman AJ, Daly M, Juneau-Norcross M, Gleason R, Rein MS, LeBoff M. Long-term medical therapy for leiomyomata uteri: a prospective, randomized study of leuprolide acetate depot plus either oestrogen-progestin or progestin ‘add-back’ for 2 years. Hum Reprod. 1994;9(9):1618–1625. [DOI] [PubMed] [Google Scholar]
- 105. Kawaguchi K, Fujii S, Konishi I, et al. Immunohistochemical analysis of oestrogen receptors, progesterone receptors and Ki-67 in leiomyoma and myometrium during the menstrual cycle and pregnancy. Virchows Arch A Pathol Anat Histopathol. 1991;419(4):309–315. [DOI] [PubMed] [Google Scholar]
- 106. Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160(3):637–641. [DOI] [PubMed] [Google Scholar]
- 107. Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59–85. [DOI] [PubMed] [Google Scholar]
- 108. Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–255. [DOI] [PubMed] [Google Scholar]
- 109. Paakkola T, Salokas K, Miinalainen I, et al. Biallelic mutations in human NHLRC2 enhance myofibroblast differentiation in FINCA disease. Hum Mol Genet. 2018;27(24):4288–4302. [DOI] [PubMed] [Google Scholar]
- 110. Islam MS, Protic O, Stortoni P, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril. 2013;100(1):178–193. [DOI] [PubMed] [Google Scholar]
- 111. Brandon DD, Bethea CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169(1):78–85. [DOI] [PubMed] [Google Scholar]
- 112. Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82(1):293–299. [DOI] [PubMed] [Google Scholar]
- 113. Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83(6):2192–2198. [DOI] [PubMed] [Google Scholar]
- 114. Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73(5):1006–1011. [DOI] [PubMed] [Google Scholar]
- 115. Kurachi O, Matsuo H, Samoto T, Maruo T. Tumor necrosis factor-alpha expression in human uterine leiomyoma and its down-regulation by progesterone. J Clin Endocrinol Metab. 2001;86(5):2275–2280. [DOI] [PubMed] [Google Scholar]
- 116. Hoekstra AV, Sefton EC, Berry E, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94(5):1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–284. [DOI] [PubMed] [Google Scholar]
- 118. Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93(5):1500–1508. [DOI] [PubMed] [Google Scholar]
- 119. Barker NM, Carrino DA, Caplan AI, et al. Proteoglycans in leiomyoma and normal myometrium: abundance, steroid hormone control, and implications for pathophysiology. Reprod Sci. 2016;23(3):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kwon JJ, Factora TD, Dey S, Kota J. Systematic review of miR-29 in cancer. Mol. Ther. Oncolytics. 2019;12:173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Qiang W, Liu Z, Serna VA, et al. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155(3):663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Luo X, Yin P, Reierstad S, et al. Progesterone and mifepristone regulate L-type amino acid transporter 2 and 4F2 heavy chain expression in uterine leiomyoma cells. J Clin Endocrinol Metab. 2009;94(11):4533–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1381–1387. [DOI] [PubMed] [Google Scholar]
- 124. Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K. Low-dose mifepristone in treatment of uterine leiomyoma: a randomised double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynaecol. 2009;49(1):77–83. [DOI] [PubMed] [Google Scholar]
- 125. Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24(8):1870–1879. [DOI] [PubMed] [Google Scholar]
- 126. Esteve JL, Acosta R, Pérez Y, et al. Mifepristone versus placebo to treat uterine myoma: a double-blind, randomized clinical trial. Int J Womens Health. 2013;5:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yin P, Lin Z, Reierstad S, et al. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res. 2010;70(4):1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev. 2019;40(4):1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422–469. [Google Scholar]
- 130. Painter JN, O’Mara TA, Morris AP, et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018;7(5):1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- 132. Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 2017;108(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Spaczynski, RZ and Duleba AJ. Diagnosis of endometriosis . Semin Reprod Med. 2003;21(02):193–208. [DOI] [PubMed] [Google Scholar]
- 134. Rogers PAW, Adamson GD, Al-Jefout M, et al. Research priorities for endometriosis. Reprod Sci. 2017;24:202–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kao LC, Tulac S, Lobo S, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138. [DOI] [PubMed] [Google Scholar]
- 136. Yilmaz BD, Bulun SE. Endometriosis and nuclear receptors. Hum Reprod Update. 2019;25(4):473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Li Y, Adur MK, Kannan A, et al. Progesterone alleviates endometriosis via inhibition of uterine cell proliferation, inflammation and angiogenesis in an immunocompetent mouse model. Plos One. 2016;11(10):e0165347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McKinnon B, Mueller M, Montgomery G. Progesterone resistance in endometriosis: an acquired property? Trends Endocrinol Metab. 2018;29(8):535–548. [DOI] [PubMed] [Google Scholar]
- 139. Huhtinen K, Saloniemi-Heinonen T, Keski-Rahkonen P, et al. Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. J Clin Endocrinol Metab. 2014;99(11):E2188–E2197. [DOI] [PubMed] [Google Scholar]
- 140. Huhtinen K, Desai R, Ståhle M, et al. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. 2012;97(11):4228–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 142. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 143. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. [DOI] [PubMed] [Google Scholar]
- 144. Yang S, Fang Z, Gurates B, et al. Stromal PRs mediate induction of 17beta-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol. 2001;15(12):2093–2105. [DOI] [PubMed] [Google Scholar]
- 145. Cheng YH, Yin P, Xue Q, Yilmaz B, Dawson MI, Bulun SE. Retinoic acid (RA) regulates 17beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J Clin Endocrinol Metab. 2008;93(5):1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Casey ML, MacDonald PC, Andersson S. 17 beta-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J Clin Invest. 1994;94(5):2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cheng Y-H, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol. 2007; 196(4):391.e391-391.e398 [DOI] [PubMed] [Google Scholar]
- 148. Zeitoun K, Takayama K, Sasano H, et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab. 1998;83(12):4474–4480. [DOI] [PubMed] [Google Scholar]
- 149. Fang Z, Yang S, Lydon JP, et al. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil Steril. 2004;82(3):673–678. [DOI] [PubMed] [Google Scholar]
- 150. Tangen IL, Werner HM, Berg A, et al. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer. 2014;50(17):3003–3010. [DOI] [PubMed] [Google Scholar]
- 151. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. [DOI] [PubMed] [Google Scholar]
- 152. Vallejo G, La Greca AD, Tarifa-Reischle IC, et al. CDC2 mediates progestin initiated endometrial stromal cell proliferation: a PR signaling to gene expression independently of its binding to chromatin. Plos One. 2014;9(5):e97311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone receptor status predicts response to progestin therapy in endometriosis. J Clin Endocrinol Metab. 2018;103(12):4561–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. [DOI] [PubMed] [Google Scholar]
- 155. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 156. Crissman JD, Azoury RS, Barnes AE, Schellhas HF. Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol. 1981;57(6):699–704. [PubMed] [Google Scholar]
- 157. Yang S, Xiao X, Jia Y, et al. Epigenetic modification restores functional PR expression in endometrial cancer cells. Curr Pharm Des. 2014;20(11):1874–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5(6):533–538. [DOI] [PubMed] [Google Scholar]
- 159. Levine DA, Network CGAR . Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Allen N, Yang T. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070. [DOI] [PubMed] [Google Scholar]
- 161. Wei J, Zhang W, Feng L, Gao W. Comparison of fertility-sparing treatments in patients with early endometrial cancer and atypical complex hyperplasia: a meta-analysis and systematic review. Medicine (Baltimore). 2017;96(37):e8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Beral V, Bull D, Reeves G; Million Women Study Collaborators . Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–1551. [DOI] [PubMed] [Google Scholar]
- 163. Sjögren LL, Mørch LS, Løkkegaard E. Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas. 2016;91:25–35. [DOI] [PubMed] [Google Scholar]
- 164. Kim YB, Holschneider CH, Ghosh K, Nieberg RK, Montz FJ. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer. 1997;79(2):320–327. [DOI] [PubMed] [Google Scholar]
- 165. Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106(2):325–333. [DOI] [PubMed] [Google Scholar]
- 166. Kumar NS, Richer J, Owen G, Litman E, Horwitz KB, Leslie KK. Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: implications for unopposed estrogen action. Cancer Res. 1998;58(9):1860–1865. [PubMed] [Google Scholar]
- 167. Jongen V, Briët J, de Jong R, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol. 2009;112(3):537–542. [DOI] [PubMed] [Google Scholar]
- 168. Fujimoto J, Ichigo S, Hori M, Nishigaki M, Tamaya T. Expression of progesterone receptor form A and B mRNAs in gynecologic malignant tumors. Tumour Biol. 1995;16(4):254–260. [DOI] [PubMed] [Google Scholar]
- 169. Arnett-Mansfield RL, deFazio A, Wain GV, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001;61(11):4576–4582. [PubMed] [Google Scholar]
- 170. Arnett-Mansfield RL, DeFazio A, Mote PA, Clarke CL. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab. 2004;89(3):1429–1442. [DOI] [PubMed] [Google Scholar]
- 171. Ward EC, Hoekstra AV, Blok LJ, et al. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149(4):1942–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kyo S, Sakaguchi J, Kiyono T, et al. Forkhead transcription factor FOXO1 is a direct target of progestin to inhibit endometrial epithelial cell growth. Clin Cancer Res. 2011;17(3):525–537. [DOI] [PubMed] [Google Scholar]
- 173. Nakamura M, Takakura M, Fujii R, et al. The PRB-dependent FOXO1/IGFBP-1 axis is essential for progestin to inhibit endometrial epithelial growth. Cancer Lett. 2013;336(1):68–75. [DOI] [PubMed] [Google Scholar]
- 174. Bokhari AA, Lee LR, Raboteau D, et al. Progesterone inhibits endometrial cancer invasiveness by inhibiting the TGFβ pathway. Cancer Prev Res (Phila). 2014;7(10):1045–1055. [DOI] [PubMed] [Google Scholar]
- 175. Waks AG, Winer EP. Breast cancer treatment: a review. Jama. 2019;321(3):288–300. [DOI] [PubMed] [Google Scholar]
- 176. Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–172. [DOI] [PubMed] [Google Scholar]
- 177. Graham JD, Yeates C, Balleine RL, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;55(21):5063–5068. [PubMed] [Google Scholar]
- 178. Hopp TA, Weiss HL, Hilsenbeck SG, et al. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10(8):2751–2760. [DOI] [PubMed] [Google Scholar]
- 179. Mote PA, Gompel A, Howe C, et al. Progesterone receptor A predominance is a discriminator of benefit from endocrine therapy in the ATAC trial. Breast Cancer Res Treat. 2015;151(2):309–318. [DOI] [PubMed] [Google Scholar]
- 180. Rojas PA, May M, Sequeira GR, et al. Progesterone receptor isoform ratio: a breast cancer prognostic and predictive factor for antiprogestin responsiveness. J Natl Cancer Inst. 2017;109(7):djw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Musgrove EA, Lee CS, Sutherland RL. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991;11(10):5032–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Groshong SD, Owen GI, Grimison B, et al. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol. 1997;11(11):1593–1607. [DOI] [PubMed] [Google Scholar]
- 183. Gill PG, Tilley WD, De Young NJ, Lensink IL, Dixon PD, Horsfall DJ. Inhibition of T47D human breast cancer cell growth by the synthetic progestin R5020: effects of serum, estradiol, insulin, and EGF. Breast Cancer Res Treat. 1991;20(1):53–62. [DOI] [PubMed] [Google Scholar]
- 184. Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273(17):10696–10701. [DOI] [PubMed] [Google Scholar]
- 185. Swarbrick A, Lee CS, Sutherland RL, Musgrove EA. Cooperation of p27(Kip1) and p18(INK4c) in progestin-mediated cell cycle arrest in T-47D breast cancer cells. Mol Cell Biol. 2000;20(7):2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gizard F, Robillard R, Gross B, et al. TReP-132 is a novel progesterone receptor coactivator required for the inhibition of breast cancer cell growth and enhancement of differentiation by progesterone. Mol Cell Biol. 2006;26(20):7632–7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chen CC, Hardy DB, Mendelson CR. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J Biol Chem. 2011;286(50):43091–43102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Moore MR, Spence JB, Kiningham KK, Dillon JL. Progestin inhibition of cell death in human breast cancer cell lines. J Steroid Biochem Mol Biol. 2006;98(4–5):218–227. [DOI] [PubMed] [Google Scholar]
- 190. Saitoh M, Ohmichi M, Takahashi K, et al. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology. 2005;146(11): 4917–4925. [DOI] [PubMed] [Google Scholar]
- 191. Carvajal A, Espinoza N, Kato S, et al. Progesterone pre-treatment potentiates EGF pathway signaling in the breast cancer cell line ZR-75. Breast Cancer Res Treat. 2005;94(2):171–183. [DOI] [PubMed] [Google Scholar]
- 192. Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27(2):466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hyder SM, Huang JC, Nawaz Z, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–790. [DOI] [PubMed] [Google Scholar]
- 194. Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 195. Zhang Z, Yamashita H, Toyama T, et al. ATBF1-a messenger RNA expression is correlated with better prognosis in breast cancer. Clin Cancer Res. 2005;11(1):193–198. [PubMed] [Google Scholar]
- 196. Li M, Zhao D, Ma G, et al. Upregulation of ATBF1 by progesterone-PR signaling and its functional implication in mammary epithelial cells. Biochem Biophys Res Commun. 2013;430(1):358–363. [DOI] [PubMed] [Google Scholar]
- 197. Madauss KP, Stewart EL, Williams SP. The evolution of progesterone receptor ligands. Med Res Rev. 2007;27(3):374–400. [DOI] [PubMed] [Google Scholar]
- 198. Ali M, Al-Hendy A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol Reprod. 2017;97(3):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Bouchard P, Chabbert-Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril. 2011;96(5):1175–1189. [DOI] [PubMed] [Google Scholar]
- 200. Allan GF, Leng X, Tsai SY, et al. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992;267(27):19513–19520. [PubMed] [Google Scholar]
- 201. Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP, O’Malley BW. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69(4):703–713. [DOI] [PubMed] [Google Scholar]
- 202. Laue L, Lotze MT, Chrousos GP, Barnes K, Loriaux DL, Fleisher TA. Effect of chronic treatment with the glucocorticoid antagonist RU 486 in man: toxicity, immunological, and hormonal aspects. J Clin Endocrinol Metab. 1990;71(6):1474–1480. [DOI] [PubMed] [Google Scholar]
- 203. Vastag B. Effect of mifepristone approval on research remains to be seen. J Natl Cancer Inst. 2000;92(24):1970–1971. [DOI] [PubMed] [Google Scholar]
- 204. Oskari H, Kimmo K, Horacio C, Irving S, Tapani L, Pekka L. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. J Steroid Biochem. 1987;26(2):279–284. [DOI] [PubMed] [Google Scholar]
- 205. Attardi BJ, Burgenson J, Hild SA, Reel JR. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. 2004;88(3):277–288. [DOI] [PubMed] [Google Scholar]
- 206. Attardi BJ, Burgenson J, Hild SA, Reel JR, Blye RP. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188(1–2):111–123. [DOI] [PubMed] [Google Scholar]
- 207. Heikinheimo O, Kekkonen R, Lähteenmäki P. The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action. Contraception. 2003;68(6):421–426. [DOI] [PubMed] [Google Scholar]
- 208. Chen J, Wang J, Shao J, et al. The unique pharmacological characteristics of mifepristone (RU486): from terminating pregnancy to preventing cancer metastasis. Med Res Rev. 2014;34(5):979–1000. [DOI] [PubMed] [Google Scholar]
- 209. Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. [DOI] [PubMed] [Google Scholar]
- 210. Robbins A, Spitz IM. Mifepristone: clinical pharmacology. Clin Obstet Gynecol. 1996;39(2):436–450. [DOI] [PubMed] [Google Scholar]
- 211. Sartorius CA, Groshong SD, Miller LA, et al. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54(14):3868–3877. [PubMed] [Google Scholar]
- 212. Heikinheimo O, Kekkonen R. Dose-response relationships of RU 486. Ann Med. 1993;25(1):71–76. [DOI] [PubMed] [Google Scholar]
- 213. Földesi I, Falkay G, Kovács L. Determination of RU486 (mifepristone) in blood by radioreceptorassay; a pharmacokinetic study. Contraception. 1996;54(1):27–32. [DOI] [PubMed] [Google Scholar]
- 214. Kekkonen R, Heikinheimo O, Mandelin E, Lähteenmäki P. Pharmacokinetics of mifepristone after low oral doses. Contraception. 1996;54(4):229–234. [DOI] [PubMed] [Google Scholar]
- 215. Leminen R, Ranta S, von Hertzen H, Oehler J, Heikinheimo O. Pharmacokinetics of 10 mg of mifepristone. Contraception. 2003;68(6):427–429. [DOI] [PubMed] [Google Scholar]
- 216. Heikinheimo O. Pharmacokinetics of the antiprogesterone RU 486 in women during multiple dose administration. J Steroid Biochem. 1989;32(1A):21–25. [DOI] [PubMed] [Google Scholar]
- 217. Swahn ML, Wang G, Aedo AR, Cekan SZ, Bygdeman M. Plasma levels of antiprogestin RU 486 following oral administration to non-pregnant and early pregnant women. Contraception. 1986;34(5):469–481. [DOI] [PubMed] [Google Scholar]
- 218. Tang C, Bi HC, Zhong GP, Chen X, Huang ZY, Huang M. Simultaneous determination of mifepristone and monodemethyl-mifepristone in human plasma by liquid chromatography-tandem mass spectrometry method using levonorgestrel as an internal standard: application to a pharmacokinetic study. Biomed Chromatogr. 2009;23(1):71–80. [DOI] [PubMed] [Google Scholar]
- 219. Teng YN, Dong RQ, Wang BJ, et al. Determinations of mifepristone and its metabolites and their pharmacokinetics in healthy female Chinese subjects. Yao Xue Xue Bao. 2011;46(10):1241–1245. [PubMed] [Google Scholar]
- 220. Lähteenmäki P, Heikinheimo O, Croxatto H, et al. Pharmacokinetics and metabolism of RU 486. J Steroid Biochem. 1987;27(4–6):859–863. [DOI] [PubMed] [Google Scholar]
- 221. Heikinheimo O. Clinical pharmacokinetics of mifepristone. Clin Pharmacokinet. 1997;33(1):7–17. [DOI] [PubMed] [Google Scholar]
- 222. Liao A, Pang X, Li H, Xiong Z, Wu X. Bioavailability of mifepristone in capsule versus tablet form in healthy nonpregnant women. Contraception. 2008;77(6):431–434. [DOI] [PubMed] [Google Scholar]
- 223. Wang J, Chen J, Wan L, et al. Synthesis, spectral characterization, and in vitro cellular activities of metapristone, a potential cancer metastatic chemopreventive agent derived from mifepristone (RU486). Aaps J. 2014;16(2):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cekan S, Aedo AR, Segerstéen E, Van Look P, Messinis I, Templeton A. Levels of the antiprogestin RU 486 and its metabolites in human blood and follicular fluid following oral administration of a single dose. Hum Reprod. 1989;4(2):131–135. [DOI] [PubMed] [Google Scholar]
- 225. Shi YE, Ye ZH, He CH, et al. Pharmacokinetic study of RU 486 and its metabolites after oral administration of single doses to pregnant and non-pregnant women. Contraception. 1993;48(2):133–149. [DOI] [PubMed] [Google Scholar]
- 226. Jang GR, Wrighton SA, Benet LZ. Identification of CYP3A4 as the principal enzyme catalyzing mifepristone (RU 486) oxidation in human liver microsomes. Biochem Pharmacol. 1996;52(5):753–761. [DOI] [PubMed] [Google Scholar]
- 227. Reilly PE, Gomi RJ, Mason SR. Mechanism-based inhibition of rat liver microsomal diazepam C3-hydroxylase by mifepristone associated with loss of spectrally detectable cytochrome P450. Chem Biol Interact. 1999;118(1):39–49. [DOI] [PubMed] [Google Scholar]
- 228. Khan KK, He YQ, Correia MA, Halpert JR. Differential oxidation of mifepristone by cytochromes P450 3A4 and 3A5: selective inactivation of P450 3A4. Drug Metab Dispos. 2002;30(9):985–990. [DOI] [PubMed] [Google Scholar]
- 229. Spitz IM, Bardin CW. Clinical pharmacology of RU 486–an antiprogestin and antiglucocorticoid. Contraception. 1993;48(5):403–444. [DOI] [PubMed] [Google Scholar]
- 230. Williams AR, Critchley HO, Osei J, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007;22(6):1696–1704. [DOI] [PubMed] [Google Scholar]
- 231. DeManno D, Elger W, Garg R, et al. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68(10–13):1019–1032. [DOI] [PubMed] [Google Scholar]
- 232. Madauss KP, Grygielko ET, Deng SJ, et al. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21(5):1066–1081. [DOI] [PubMed] [Google Scholar]
- 233. Bilyeu T, Morris E, Lee R, Premkumar N, Cole K, Velagaleti P, Bopp B, Castagnoli N, Mulford D. Interspecies comparison: in vitro metabolism of J867 by hepatocytes and microsomes from male and female human, and various animal species. Drug Metab Rev 2002; 34:46–46 [Google Scholar]
- 234. Chwalisz K, Brenner RM, Fuhrmann UU, Hess-Stumpp H, Elger W. Antiproliferative effects of progesterone antagonists and progesterone receptor modulators on the endometrium. Steroids. 2000;65(10–11):741–751. [DOI] [PubMed] [Google Scholar]
- 235. Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68(10–13):1013–1017. [DOI] [PubMed] [Google Scholar]
- 236. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;15(5):1092–1099. [DOI] [PubMed] [Google Scholar]
- 237. Donnez Z, Hudecek R, Donnez O, et al . Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103(2):519–527.e513. [DOI] [PubMed] [Google Scholar]
- 238. Donnez J, Vázquez F, Tomaszewski J, et al . Long-term treatment of uterine fibroids with ulipristal acetate☆. Fertil Steril. 2014;101(6):1565–1573.e1518. [DOI] [PubMed] [Google Scholar]
- 239. Xu Q, Ohara N, Chen W, et al. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21(9):2408-2416. [DOI] [PubMed] [Google Scholar]
- 240. Yoshida S, Ohara N, Xu Q, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28(3):260-273. [DOI] [PubMed] [Google Scholar]
- 241. Hild SA, Reel JR, Hoffman LH, Blye RP. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000;15(4):822–829. [DOI] [PubMed] [Google Scholar]
- 242. Orihuela PA. Ulipristal, a progesterone receptor antagonist as a contraceptive and for the treatment of uterine fibroids. Curr Opin Investig Drugs. 2007;8(10):859–866. [PubMed] [Google Scholar]
- 243. Larner JM, Reel JR, Blye RP. Circulating concentrations of the antiprogestins CDB-2914 and mifepristone in the female rhesus monkey following various routes of administration. Hum Reprod. 2000;15(5):1100–1106. [DOI] [PubMed] [Google Scholar]
- 244. Pohl O, Osterloh I, Gotteland JP. Ulipristal acetate - safety and pharmacokinetics following multiple doses of 10-50 mg per day. J Clin Pharm Ther. 2013;38(4):314–320. [DOI] [PubMed] [Google Scholar]
- 245. Pohl O, Osterloh I, Lecomte V, Gotteland JP. Changes in gastric pH and in pharmacokinetics of ulipristal acetate - a drug-drug interaction study using the proton pump inhibitor esomeprazole. Int J Clin Pharmacol Ther. 2013;51(1):26–33. [DOI] [PubMed] [Google Scholar]
- 246. Pohl O, Kendrick J, Gotteland J. Metabolic disposition of [14C] ulipristal acetate in healthy premenopausal women. J Bioequiv Availab 2013;5:177–184. [Google Scholar]
- 247. Pohl O, Zobrist RH, Gotteland JP. The clinical pharmacology and pharmacokinetics of ulipristal acetate for the treatment of uterine fibroids. Reprod Sci. 2015;22(4):476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Ioffe OB, Zaino RJ, Mutter GL. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol. 2009;22(3):450–459. [DOI] [PubMed] [Google Scholar]
- 249. Wiehle RD, Goldberg J, Brodniewicz T, Jarus-Dziedzic K, Jabiry-Zieniewicz Z. Effects of a new progesterone receptor modulator, CDB-4124, on fibroid size and uterine bleeding. US Obstet Gynecol. 2008;3(1):17–20. [Google Scholar]
- 250. Morris D, Podolski J, Kirsch A, Wiehle R, Fleckenstein L. Population pharmacokinetics of telapristone (CDB-4124) and its active monodemethylated metabolite CDB-4453, with a mixture model for total clearance. Aaps J. 2011;13(4):665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Wiehle R, Lantvit D, Yamada T, Christov K. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila). 2011;4(3):414–424. [DOI] [PubMed] [Google Scholar]
- 252. Davaadelger B, Murphy AR, Clare SE, Lee O, Khan SA, Kim JJ. Mechanism of telapristone acetate (CDB4124) on progesterone receptor action in breast cancer cells. Endocrinology. 2018;159(10):3581-–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Clare SE, Gupta A, Choi M, et al. Progesterone receptor blockade in human breast cancer cells decreases cell cycle progression through G2/M by repressing G2/M genes. BMC Cancer. 2016;16(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–585. [DOI] [PubMed] [Google Scholar]
- 255. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. [DOI] [PubMed] [Google Scholar]
- 256. Lee O, Ivancic D, Allu S, et al. Local transdermal therapy to the breast for breast cancer prevention and DCIS therapy: preclinical and clinical evaluation. Cancer Chemother Pharmacol. 2015;76(6):1235–1246. [DOI] [PubMed] [Google Scholar]
- 257. Seitz C, Bumbuliene Ž, Costa AR, et al. Rationale and design of ASTEROID 2, a randomized, placebo- and active comparator-controlled study to assess the efficacy and safety of vilaprisan in patients with uterine fibroids. Contemp Clin Trials. 2017;55:56–62. [DOI] [PubMed] [Google Scholar]
- 258. Wagenfeld A, Bone W, Schwede W, Fritsch M, Fischer OM, Moeller C. BAY 1002670: a novel, highly potent and selective progesterone receptor modulator for gynaecological therapies. Hum Reprod. 2013;28(8):2253–2264. [DOI] [PubMed] [Google Scholar]
- 259. Möller C, Bone W, Cleve A, et al. Discovery of vilaprisan (BAY 1002670): a highly potent and selective progesterone receptor modulator optimized for gynecologic therapies. Chemmedchem. 2018;13(21):2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Schultze-Mosgau MH, Schuett B, Hafner FT, et al. Pharmacokinetics and safety of the selective progesterone receptor modulator vilaprisan in healthy postmenopausal women. Int J Clin Pharmacol Ther. 2017;55(1):16–24. [DOI] [PubMed] [Google Scholar]
- 261. Schultze-Mosgau MH, Höchel J, Prien O, et al. Characterization of the pharmacokinetics of vilaprisan: bioavailability, excretion, biotransformation, and drug-drug interaction potential. Clin Pharmacokinet. 2018;57(8):1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21(5):591–598. [DOI] [PubMed] [Google Scholar]
- 263. Kannan A, Bhurke A, Sitruk-Ware R, et al. Characterization of molecular changes in endometrium associated with chronic use of progesterone receptor modulators: ulipristal acetate versus mifepristone. Reprod Sci. 2018;25(3):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Fiscella J, Bonfiglio T, Winters P, Eisinger SH, Fiscella K. Distinguishing features of endometrial pathology after exposure to the progesterone receptor modulator mifepristone. Hum Pathol. 2011;42(7):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Jones A, Teschendorff AE, Li Q, et al. Role of DNA methylation and epigenetic silencing of HAND2 in endometrial cancer development. Plos Med. 2013;10(11):e1001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Berger C, Boggavarapu N, Norlin E, et al. Molecular characterization of PRM-associated endometrial changes, PAEC, following mifepristone treatment. Contraception. 2018;98(4):317–322. [DOI] [PubMed] [Google Scholar]
- 268. Nyren-Erickson EK, Jones JM, Srivastava DK, Mallik S. A disintegrin and metalloproteinase-12 (ADAM12): function, roles in disease progression, and clinical implications. Biochim Biophys Acta. 2013;1830(10):4445–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Barker TH, Grenett HE, MacEwen MW, et al. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295(2):488–496. [DOI] [PubMed] [Google Scholar]
- 270. Chiquet‐Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200(4):488–499. [DOI] [PubMed] [Google Scholar]
- 271. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Chegini N, Ma C, Tang XM, Williams RS. Effects of GnRH analogues, ‘add-back’ steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-beta expression. Mol Hum Reprod. 2002;8(12):1071–1078. [DOI] [PubMed] [Google Scholar]
- 273. Chung YJ, Chae B, Kwak SH, et al. Comparison of the inhibitory effect of gonadotropin releasing hormone (GnRH) agonist, selective estrogen receptor modulator (SERM), antiprogesterone on myoma cell proliferation in vitro. Int J Med Sci. 2014;11(3):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Feng M, Xiong G, Cao Z, et al. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Engman M, Varghese S, Lagerstedt Robinson K, et al. GSTM1 gene expression correlates to leiomyoma volume regression in response to mifepristone treatment. Plos One. 2013;8(12):e80114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Patel A, Malik M, Britten J, Cox J, Catherino WH. Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fertil Steril. 2016;105(4):1102–1110. [DOI] [PubMed] [Google Scholar]
- 277. Reinsch RC, Murphy AA, Morales AJ, Yen SS. The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: a prospective, randomized study. Am J Obstet Gynecol. 1994;170(6):1623–162 7; discussion 1627. [PubMed] [Google Scholar]
- 278. Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol. 2005;12(3):227–233. [DOI] [PubMed] [Google Scholar]
- 279. Carbonell Esteve JL, Acosta R, Heredia B, Pérez Y, Castañeda MC, Hernández AV. Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2008;112(5):1029–1036. [DOI] [PubMed] [Google Scholar]
- 280. Esteve JL, Acosta R, Pérez Y, Campos R, Hernández AV, Texidó CS. Treatment of uterine myoma with 5 or 10mg mifepristone daily during 6 months, post-treatment evolution over 12 months: double-blind randomised clinical trial. Eur J Obstet Gynecol Reprod Biol. 2012;161(2):202–208. [DOI] [PubMed] [Google Scholar]
- 281. Carbonell JL, Acosta R, Pérez Y, et al. Safety and effectiveness of different dosage of mifepristone for the treatment of uterine fibroids: a double-blind randomized clinical trial. Int J Womens Health. 2013;5:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Eisinger SH, Fiscella J, Bonfiglio T, Meldrum S, Fiscella K. Open-label study of ultra low-dose mifepristone for the treatment of uterine leiomyomata. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):215–218. [DOI] [PubMed] [Google Scholar]
- 283. Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993;76(2):513–517. [DOI] [PubMed] [Google Scholar]
- 284. Esteve JLC, Riverón AM, Cano M, et al. Mifepristone 2.5 mg versus 5 mg daily in the treatment of leiomyoma before surgery. Int J Womens Health. 2012;4:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Carbonell JL, Acosta R, Pérez Y, Garcés R, Sánchez C, Tomasi G. Treatment of uterine myoma with 2.5 or 5 mg mifepristone daily during 3 months with 9 months posttreatment followup: randomized clinical trial. ISRN Obstet Gynecol. 2013;2013:649030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol. 2003;101(2):243–250. [DOI] [PubMed] [Google Scholar]
- 287. Yerushalmi GM, Gilboa Y, Jakobson-Setton A, et al. Vaginal mifepristone for the treatment of symptomatic uterine leiomyomata: an open-label study. Fertil Steril. 2014;101(2):496–500. [DOI] [PubMed] [Google Scholar]
- 288. Chen W, Ohara N, Wang J, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91(4):1296–1304. [DOI] [PubMed] [Google Scholar]
- 289. Wang J, Ohara N, Wang Z, et al. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21(7):1869–1877. [DOI] [PubMed] [Google Scholar]
- 290. Xu Q, Ohara N, Liu J, et al. Selective progesterone receptor modulator asoprisnil induces endoplasmic reticulum stress in cultured human uterine leiomyoma cells. Am J Physiol Endocrinol Metab. 2007;293(4):E1002–E1011. [DOI] [PubMed] [Google Scholar]
- 291. Morikawa A, Ohara N, Xu Q, et al. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod. 2008;23(4):944–951. [DOI] [PubMed] [Google Scholar]
- 292. Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril. 2007;87(6):1399–1412. [DOI] [PubMed] [Google Scholar]
- 293. Wilkens J, Chwalisz K, Han C, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab. 2008;93(12):4664–4671. [DOI] [PubMed] [Google Scholar]
- 294. Stewart EA, Diamond MP, Williams ARW, et al. Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: pooled analysis of two 12-month, placebo-controlled, randomized trials. Hum Reprod. 2019;34(4):623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Xu Q, Takekida S, Ohara N, et al. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5’-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metab. 2005;90(2):953–961. [DOI] [PubMed] [Google Scholar]
- 296. Ciarmela P, Carrarelli P, Islam MS, et al. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod Sci. 2014;21(9):1120–1125. [DOI] [PubMed] [Google Scholar]
- 297. Xu Q, Ohara N, Liu J, et al. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod. 2008;14(3):181–191. [DOI] [PubMed] [Google Scholar]
- 298. Courtoy GE, Donnez J, Marbaix E, Dolmans M-M. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril. 2015;104(2):426–434.e421. [DOI] [PubMed] [Google Scholar]
- 299. Courtoy GE, Henriet P, Marbaix E, et al. Matrix metalloproteinase activity correlates with uterine myoma volume reduction after ulipristal acetate treatment. J Clin Endocrinol Metab. 2018;103(4):1566–1573. [DOI] [PubMed] [Google Scholar]
- 300. Cox J, Malik M, Britten J, Lewis T, Catherino WH. Ulipristal acetate and extracellular matrix production in human leiomyomas in vivo: a laboratory analysis of a randomized placebo controlled trial. Reprod Sci. 2018;25(2):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Lewis TD, Malik M, Britten J, Parikh T, Cox J, Catherino WH. Ulipristal acetate decreases active TGF-β3 and its canonical signaling in uterine leiomyoma via two novel mechanisms. Fertil Steril. 2019;111(4):683–684. [DOI] [PubMed] [Google Scholar]
- 302. Ciebiera M, Włodarczyk M, Wrzosek M, Słabuszewska-Jóźwiak A, Nowicka G, Jakiel G. Ulipristal acetate decreases transforming growth factor β3 serum and tumor tissue concentrations in patients with uterine fibroids. Fertil Steril. 2018;109(3):501–507.e502. [DOI] [PubMed] [Google Scholar]
- 303. Courtoy GE, Donnez J, Ambroise J, et al. Gene expression changes in uterine myomas in response to ulipristal acetate treatment. Reprod Biomed Online. 2018;37(2):224–233. [DOI] [PubMed] [Google Scholar]
- 304. Ng SSM, Jorge S, Malik M, et al. A-kinase anchoring protein 13 (AKAP13) augments progesterone signaling in uterine fibroid cells. J Clin Endocrinol Metab. 2018;104(4):970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Britten JL, Malik M, Lewis TD, Catherino WH. Ulipristal acetate mediates decreased proteoglycan expression through regulation of nuclear factor of activated T-Cells (NFAT5). Reprod Sci. 2019;26(2):184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Donnez J, Tomaszewski J, Vázquez F, et al. ; PEARL II Study Group . Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. [DOI] [PubMed] [Google Scholar]
- 307. Fernandez H, Schmidt T, Powell M, Costa AP, Arriagada P, Thaler C. Real world data of 1473 patients treated with ulipristal acetate for uterine fibroids: premya study results. Eur J Obstet Gynecol Reprod Biol. 2017;208:91–96. [DOI] [PubMed] [Google Scholar]
- 308. Liu JH, Soper D, Lukes A, et al. Ulipristal acetate for treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;132(5):1241–1251. [DOI] [PubMed] [Google Scholar]
- 309. Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Donnez J. Liver injury and ulipristal acetate: an overstated tragedy? Fertil Steril. 2018;110(4):593–595. [DOI] [PubMed] [Google Scholar]
- 311. Levens ED, Potlog-Nahari C, Armstrong AY, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P; VA2914 Study Group . Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;92(9):3582–3589. [DOI] [PubMed] [Google Scholar]
- 313. Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, Wesley R, Armstrong A. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril 2011;95(2):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97(8):734–739. [DOI] [PubMed] [Google Scholar]
- 315. Luo X, Yin P, Coon V JS, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93(8):2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Roeder H, Jayes F, Feng L, Leppert PC. CDB-4124 does not cause apoptosis in cultured fibroid cells. Reprod Sci. 2011;18(9):850–857. [DOI] [PubMed] [Google Scholar]
- 317. Schütt B, Kaiser A, Schultze-Mosgau MH, et al. Pharmacodynamics and safety of the novel selective progesterone receptor modulator vilaprisan: a double-blind, randomized, placebo-controlled phase 1 trial in healthy women. Hum Reprod. 2016;31(8):1703–1712. [DOI] [PubMed] [Google Scholar]
- 318. Schütt B, Schultze‐Mosgau MH, Draeger C, et al. Effect of the novel selective progesterone receptor modulator vilaprisan on ovarian activity in healthy women. J Clin Pharmacol. 2018;58(2):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Bradley LD, Singh SS, Simon J, et al. Vilaprisan in women with uterine fibroids: the randomized phase 2b ASTEROID 1 study. Fertil Steril. 2019;111(2):240–248. [DOI] [PubMed] [Google Scholar]
- 320. Murphy AA, Zhou MH, Malkapuram S, Santanam N, Parthasarathy S, Sidell N. RU486-induced growth inhibition of human endometrial cells. Fertil Steril. 2000;74(5):1014–1019. [DOI] [PubMed] [Google Scholar]
- 321. Jiang J, Wu RF, Wang ZH, Sun HC, Xu Z, Xiu HM. Effect of mifepristone on estrogen and progesterone receptors in human endometrial and endometriotic cells in vitro. Fertil Steril. 2002;77(5):995–1000. [DOI] [PubMed] [Google Scholar]
- 322. Narvekar N, Cameron S, Critchley HO, Lin S, Cheng L, Baird DT. Low-dose mifepristone inhibits endometrial proliferation and up-regulates androgen receptor. J Clin Endocrinol Metab. 2004;89(5):2491–2497. [DOI] [PubMed] [Google Scholar]
- 323. Tjaden B, Galetto D, Woodruff JD, Rock JA. Time-related effects of RU486 treatment in experimentally induced endometriosis in the rat. Fertil Steril. 1993;59(2):437–440. [DOI] [PubMed] [Google Scholar]
- 324. Kettel LM, Murphy AA, Mortola JF, Liu JH, Ulmann A, Yen SS. Endocrine responses to long-term administration of the antiprogesterone RU486 in patients with pelvic endometriosis. Fertil Steril. 1991;56(3):402–407. [DOI] [PubMed] [Google Scholar]
- 325. Kettel LM, Murphy AA, Morales AJ, Ulmann A, Baulieu EE, Yen SS. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril. 1996;65(1):23–28. [DOI] [PubMed] [Google Scholar]
- 326. Carbonell JL, Riverón AM, Leonard Y, González J, Heredia B, Sánchez C. Mifepristone 2.5, 5, 10 mg versus placebo in the treatment of endometriosis. J Reprod Health Med. 2016; 2(1):17–25 [Google Scholar]
- 327. Kettel LM, Murphy AA, Morales AJ, Yen SS. Preliminary report on the treatment of endometriosis with low-dose mifepristone (RU 486). Am J Obstet Gynecol. 1998;178(6):1151–1156. [DOI] [PubMed] [Google Scholar]
- 328. Mattia-Goldberg KC, Lee M, Elger W, Edmonds A. Treatment of endometriosis with the novel selective progesterone receptor modulator (SPRM) asoprisnil. Fertil Steril. 2004;82 (Supplement 2): S83–S84 [DOI] [PubMed] [Google Scholar]
- 329. Huniadi CA, Pop OL, Antal TA, Stamatian F. The effects of ulipristal on Bax/Bcl-2, cytochrome c, Ki-67 and cyclooxygenase-2 expression in a rat model with surgically induced endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):360–365. [DOI] [PubMed] [Google Scholar]
- 330. Liang B, Wu L, Xu H, et al. Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: a comparison study in mice. Reprod Biol Endocrinol. 2018;16(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Bressler LH, Bernardi LA, Snyder MA, Wei J-J, Bulun S. Treatment of endometriosis-related chronic pelvic pain with Ulipristal Acetate and associated endometrial changes. HSOA J Reprod Med Gynaecol Obstet. 2017;2:008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Fu J, Song H, Zhou M, et al. Progesterone receptor modulators for endometriosis. Cochrane Database Syst Rev. 2017;7(7):CD009881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Li A, Felix JC, Minoo P, Amezcua CA, Jain JK. Effect of mifepristone on proliferation and apoptosis of Ishikawa endometrial adenocarcinoma cells. Fertil Steril. 2005;84(1):202–211. [DOI] [PubMed] [Google Scholar]
- 334. Schneider CC, Gibb RK, Taylor DD, Wan T, Gerçel-Taylor C. Inhibition of endometrial cancer cell lines by mifepristone (RU 486). J Soc Gynecol Investig. 1998;5(6):334–338. [DOI] [PubMed] [Google Scholar]
- 335. Navo MA, Smith JA, Gaikwad A, Burke T, Brown J, Ramondetta LM. In vitro evaluation of the growth inhibition and apoptosis effect of mifepristone (RU486) in human Ishikawa and HEC1A endometrial cancer cell lines. Cancer Chemother Pharmacol. 2008;62(3):483–489. [DOI] [PubMed] [Google Scholar]
- 336. Ørbo A, Moe BT, Grønaas H, Paulssen RH. Early effects of high concentrations of progesterone and mifepristone A gene expression study of endometrial cancer cells (Ishikawa). J Steroid Biochem Mol Biol. 2009;113(1–2):139–149. [DOI] [PubMed] [Google Scholar]
- 337. Ramondetta LM, Johnson AJ, Sun CC, et al. Phase 2 trial of mifepristone (RU-486) in advanced or recurrent endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma. Cancer. 2009;115(9):1867–1874. [DOI] [PubMed] [Google Scholar]
- 338. Rocereto TF, Brady WE, Shahin MS, et al. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol. 2010;116(3):332–334. [DOI] [PubMed] [Google Scholar]
- 339. Gaddy VT, Barrett JT, Delk JN, Kallab AM, Porter AG, Schoenlein PV. Mifepristone induces growth arrest, caspase activation, and apoptosis of estrogen receptor-expressing, antiestrogen-resistant breast cancer cells. Clin Cancer Res. 2004;10(15):5215–5225. [DOI] [PubMed] [Google Scholar]
- 340. Jang JH, Woo SM, Um HJ, et al. RU486, a glucocorticoid receptor antagonist, induces apoptosis in U937 human lymphoma cells through reduction in mitochondrial membrane potential and activation of p38 MAPK. Oncol Rep. 2013;30(1):506–512. [DOI] [PubMed] [Google Scholar]
- 341. Liang Y, Hou M, Kallab AM, Barrett JT, El Etreby F, Schoenlein PV. Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta1. Int J Oncol. 2003;23(2):369–380. [PubMed] [Google Scholar]
- 342. Musgrove EA, Sutherland RL. Effects of the progestin antagonist RU 486 on T-47D breast cancer cell cycle kinetics and cell cycle regulatory genes. Biochem Biophys Res Commun. 1993;195(3):1184–1190. [DOI] [PubMed] [Google Scholar]
- 343. Musgrove EA, Lee CS, Cornish AL, Swarbrick A, Sutherland RL. Antiprogestin inhibition of cell cycle progression in T-47D breast cancer cells is accompanied by induction of the cyclin-dependent kinase inhibitor p21. Mol Endocrinol. 1997;11(1):54–66. [DOI] [PubMed] [Google Scholar]
- 344. Benad P, Rauner M, Rachner TD, Hofbauer LC. The anti-progestin RU-486 inhibits viability of MCF-7 breast cancer cells by suppressing WNT1. Cancer Lett. 2011;312(1):101–108. [DOI] [PubMed] [Google Scholar]
- 345. Purmonen S, Ahola TM, Pennanen P, et al. HDLG5/KIAA0583, encoding a MAGUK-family protein, is a primary progesterone target gene in breast cancer cells. Int J Cancer. 2002;102(1):1–6. [DOI] [PubMed] [Google Scholar]
- 346. Liu R, Zheng HQ, Zhou Z, Dong JT, Chen C. KLF5 promotes breast cell survival partially through fibroblast growth factor-binding protein 1-pERK-mediated dual specificity MKP-1 protein phosphorylation and stabilization. J Biol Chem. 2009;284(25):16791–16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Zheng HQ, Zhou Z, Huang J, Chaudhury L, Dong JT, Chen C. Krüppel-like factor 5 promotes breast cell proliferation partially through upregulating the transcription of fibroblast growth factor binding protein 1. Oncogene. 2009;28(42):3702–3713. [DOI] [PubMed] [Google Scholar]
- 348. Liu R, Shi P, Nie Z, et al. Mifepristone suppresses basal triple-negative breast cancer stem cells by down-regulating KLF5 expression. Theranostics. 2016;6(4):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Wargon V, Riggio M, Giulianelli S, et al. Progestin and antiprogestin responsiveness in breast cancer is driven by the PRA/PRB ratio via AIB1 or SMRT recruitment to the CCND1 and MYC promoters. Int J Cancer. 2015;136(11):2680–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Hyder SM, Chiappetta C, Stancel GM. Pharmacological and endogenous progestins induce vascular endothelial growth factor expression in human breast cancer cells. Int J Cancer. 2001;92(4):469–473. [DOI] [PubMed] [Google Scholar]
- 351. Liang Y, Hyder SM. Proliferation of endothelial and tumor epithelial cells by progestin-induced vascular endothelial growth factor from human breast cancer cells: paracrine and autocrine effects. Endocrinology. 2005;146(8):3632–3641. [DOI] [PubMed] [Google Scholar]
- 352. Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res. 2007;67(20):9929–9936. [DOI] [PubMed] [Google Scholar]
- 353. Yu S, Yang X, Zhu Y, et al. Systems pharmacology of mifepristone (RU486) reveals its 47 hub targets and network: comprehensive analysis and pharmacological focus on FAK-Src-Paxillin complex. Sci Rep. 2015;5:7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Engman M, Skoog L, Söderqvist G, Gemzell-Danielsson K. The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology. Hum Reprod. 2008;23(9):2072–2079. [DOI] [PubMed] [Google Scholar]
- 355. Perrault D, Eisenhauer EA, Pritchard KI, et al. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. 1996;14(10):2709–2712. [DOI] [PubMed] [Google Scholar]
- 356. Esber N, Cherbonnier C, Resche-Rigon M, et al. Anti-tumoral effects of anti-progestins in a patient-derived breast cancer xenograft model. Horm Cancer. 2016;7(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Wiehle RD, Christov K, Mehta R. Anti-progestins suppress the growth of established tumors induced by 7,12-dimethylbenz(a)anthracene: comparison between RU486 and a new 21-substituted-19-nor-progestin. Oncol Rep. 2007;18(1):167–174. [PubMed] [Google Scholar]
- 358. Lee O, Choi MR, Christov K, Ivancic D, Khan SA. Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer Lett. 2016;376(2):310–317. [DOI] [PubMed] [Google Scholar]
- 359. Gupta A, Mehta R, Alimirah F, et al. Efficacy and mechanism of action of Proellex, an antiprogestin in aromatase overexpressing and Letrozole resistant T47D breast cancer cells. J Steroid Biochem Mol Biol. 2013;133:30–42. [DOI] [PubMed] [Google Scholar]
- 360. Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol. 2012;31(6):556–569. [DOI] [PubMed] [Google Scholar]
- 361. Brenner RM, Slayden OD. Steroid receptors in blood vessels of the rhesus macaque endometrium: a review. Arch Histol Cytol. 2004;67(5):411–416. [DOI] [PubMed] [Google Scholar]
- 362. Wilkens J, Male V, Ghazal P, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol. 2013;191(5):2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Sanderson PA, Critchley HO, Williams AR, Arends MJ, Saunders PT. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017;23(2):232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Chandra V, Kim JJ, Benbrook DM, Dwivedi A, Rai R. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol 2015;27(1): e8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Pécout M, Cosson M, Collinet P, Rubod C, Giraudet G. Disappearance of a myoma after pregnancy in a 38 years old patient, treated by ulipristal acetate without success before getting pregnant. J Gynecol Obstet Hum Reprod. 2019;48(9):781–783. [DOI] [PubMed] [Google Scholar]
- 366. De Gasperis-Brigante C, Singh SS, Vilos G, Kives S, Murji A. Pregnancy outcomes following ulipristal acetate for uterine fibroids: a systematic review. J Obstet Gynaecol Can. 2018;40:1066–1076.e1062. [DOI] [PubMed] [Google Scholar]
- 367. Bernard N, Elefant E, Carlier P, et al. Continuation of pregnancy after first-trimester exposure to mifepristone: an observational prospective study. Bjog. 2013;120(5):568–574. [DOI] [PubMed] [Google Scholar]
- 368. .Sutcliffe L. Current concepts in genetics. Congenital malformations. N Engl J Med. 1976;295(4):204–207. [DOI] [PubMed] [Google Scholar]
- 369. Levy DP, Jager M, Kapp N, Abitbol JL. Ulipristal acetate for emergency contraception: postmarketing experience after use by more than 1 million women. Contraception. 2014;89(5):431–433. [DOI] [PubMed] [Google Scholar]
- 370. Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Haeger KO, Lamme J, Cleland K. State of emergency contraception in the U.S., 2018. Contracept Reprod Med. 2018;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med. 1992;327(15):1041–1044. [DOI] [PubMed] [Google Scholar]
- 373. Liu JH, Garzo G, Morris S, Stuenkel C, Ulmann A, Yen SS. Disruption of follicular maturation and delay of ovulation after administration of the antiprogesterone RU486. J Clin Endocrinol Metab. 1987;65(6):1135–1140. [DOI] [PubMed] [Google Scholar]
- 374. Brown A, Williams A, Cameron S, Morrow S, Baird DT. A single dose of mifepristone (200 mg) in the immediate preovulatory phase offers contraceptive potential without cycle disruption. Contraception. 2003;68(3):203–209. [DOI] [PubMed] [Google Scholar]
- 375. Boggavarapu NR, Berger C, von Grothusen C, Menezes J, Gemzell-Danielsson K, Lalitkumar PG. Effects of low doses of mifepristone on human embryo implantation process in a three-dimensional human endometrial in vitro co-culture system. Contraception. 2016;94(2):143–151. [DOI] [PubMed] [Google Scholar]
- 376. Zhou F, Chen XY, Zhuang YL, Chen YZ, Huang LL. Low-dose mifepristone increases uterine natural killer cell cytotoxicity and perforin expression during the receptive phase. Fertil Steril. 2011;96(3):649–653. [DOI] [PubMed] [Google Scholar]
- 377. Sun X, Christow A, Marions L, Gemzell-Danielsson K. Progesterone receptor isoform B in the human fallopian tube and endometrium following mifepristone. Contraception. 2003;67(4):319–326. [DOI] [PubMed] [Google Scholar]
- 378. Yang J, Serres C, Philibert D, Robel P, Baulieu EE, Jouannet P. Progesterone and RU486: opposing effects on human sperm. Proc Natl Acad Sci U S A. 1994;91(2):529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Yang J, Serres C, Baulieu EE, Jouannet P. RU486 inhibits penetration of human spermatozoa into zona-free hamster oocytes. Int J Androl. 1996;19(1):61–66. [DOI] [PubMed] [Google Scholar]
- 380. Sääv I, Fiala C, Hämäläinen JM, Heikinheimo O, Gemzell-Danielsson K. Medical abortion in lactating women–low levels of mifepristone in breast milk. Acta Obstet Gynecol Scand. 2010;89(5):618–622. [DOI] [PubMed] [Google Scholar]
- 381. Hamoda H, Ashok PW, Stalder C, Flett GM, Kennedy E, Templeton A. A randomized trial of mifepristone (10 mg) and levonorgestrel for emergency contraception. Obstet Gynecol. 2004;104(6):1307–1313. [DOI] [PubMed] [Google Scholar]
- 382. Jin J, Weisberg E, Fraser IS. Comparison of three single doses of mifepristone as emergency contraception: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2005;45(6):489–494. [DOI] [PubMed] [Google Scholar]
- 383. Carbonell Esteve J, García R, Breto A, Llorente M. Emergency contraception in Cuba with 10 mg of mifepristone. Eur J Contracept Reprod Health Care. 2007;12(2):162–167. [DOI] [PubMed] [Google Scholar]
- 384. Wu S, Dong J, Cong J, Wang C, VonHertzen H, Godfrey EM. Gestrinone compared with mifepristone for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2010;115(4):740–744. [DOI] [PubMed] [Google Scholar]
- 385. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25(9):2256–2263. [DOI] [PubMed] [Google Scholar]
- 386. Davis AR, Praditpan P. Emergency contraception: two steps forward, one step back. Semin Reprod Med. 2016;34(3):152–158. [DOI] [PubMed] [Google Scholar]
- 387. Brache V, Cochon L, Deniaud M, Croxatto HB. Ulipristal acetate prevents ovulation more effectively than levonorgestrel: analysis of pooled data from three randomized trials of emergency contraception regimens. Contraception. 2013;88(5):611–618. [DOI] [PubMed] [Google Scholar]
- 388. Lira-Albarrán S, Durand M, Barrera D, et al. A single preovulatory administration of ulipristal acetate affects the decidualization process of the human endometrium during the receptive period of the menstrual cycle. Mol Cell Endocrinol. 2018;476:70–78. [DOI] [PubMed] [Google Scholar]
- 389. Stratton P, Levens ED, Hartog B, et al. Endometrial effects of a single early luteal dose of the selective progesterone receptor modulator CDB-2914. Fertil Steril. 2010;93(6):2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Zumoffen C, Gómez-Elías MD, Caille AM, et al. Study of the effect of ulipristal acetate on human sperm ability to interact with tubal tissue and cumulus-oocyte-complexes. Contraception. 2017;95(6):586–591. [DOI] [PubMed] [Google Scholar]
- 391. Munuce MJ, Zumoffen C, Cicaré J, Caille A, Ghersevich S, Bahamondes L. Effect of exposure to ulipristal acetate on sperm function. Eur J Contracept Reprod Health Care. 2012;17(6):428–437. [DOI] [PubMed] [Google Scholar]
- 392. Berger C, Boggavarapu NR, Menezes J, Lalitkumar PG, Gemzell-Danielsson K. Effects of ulipristal acetate on human embryo attachment and endometrial cell gene expression in an in vitro co-culture system. Hum Reprod. 2015;30(4):800–811. [DOI] [PubMed] [Google Scholar]
- 393. Li HW, Liao SB, Yeung WS, Ng EH, O WS, Ho PC. Ulipristal acetate resembles mifepristone in modulating human fallopian tube function. Hum Reprod. 2014;29(10):2156–2162. [DOI] [PubMed] [Google Scholar]
- 394. .Li HWR, Lo SST, Ng EHY, Ho PC. Efficacy of ulipristal acetate for emergency contraception and its effect on the subsequent bleeding pattern when administered before or after ovulation. Hum Reprod 2016;31:1200–1207. [DOI] [PubMed] [Google Scholar]
- 395. Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562. [DOI] [PubMed] [Google Scholar]
- 397. Fine P, Mathé H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 Pt 1):257–263. [DOI] [PubMed] [Google Scholar]
- 398. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of cushing’s disease. Endocr Rev. 2015;36(4):385–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Biller BM, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Mazziotti G, Gazzaruso C, Giustina A. Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol Metab. 2011;22(12):499–506. [DOI] [PubMed] [Google Scholar]
- 402. Castinetti F, Brue T, Conte-Devolx B. The use of the glucocorticoid receptor antagonist mifepristone in Cushing’s syndrome. Curr Opin Endocrinol Diabetes Obes. 2012;19(4):295–299. [DOI] [PubMed] [Google Scholar]
- 403. Morgan FH, Laufgraben MJ. Mifepristone for management of Cushing’s syndrome. Pharmacotherapy. 2013;33(3):319–329. [DOI] [PubMed] [Google Scholar]
- 404. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Castinetti F, Fassnacht M, Johanssen S, et al. Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur J Endocrinol. 2009;160(6):1003–1010. [DOI] [PubMed] [Google Scholar]
- 406. Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C; SEISMIC Study Investigators . Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. [DOI] [PubMed] [Google Scholar]
- 407. Katznelson L, Loriaux DL, Feldman D, Braunstein GD, Schteingart DE, Gross C. Global clinical response in Cushing’s syndrome patients treated with mifepristone. Clin Endocrinol (Oxf). 2014;80(4):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Cohan P, East HE, Galati SJ, et al. Mifepristone treatment in four cases of primary bilateral macronodular adrenal hyperplasia (BMAH). J Clin Endocrinol Metab. 2019;104(12):6279–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Fleseriu M, Findling JW, Koch CA, Schlaffer SM, Buchfelder M, Gross C. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing’s disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J Clin Endocrinol Metab. 2014;99(10):3718–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. .Fein HG, Vaughan TB 3rd, Kushner H, Cram D, Nguyen D. Sustained weight loss in patients treated with mifepristone for Cushing’s syndrome: a follow-up analysis of the SEISMIC study and long-term extension. BMC Endocr Disord. 2015;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Ohayon MM, Schatzberg AF. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002;159(11):1855–1861. [DOI] [PubMed] [Google Scholar]
- 412. Keller J, Flores B, Gomez RG, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60(3):275–281. [DOI] [PubMed] [Google Scholar]
- 413. Blasey CM, Block TS, Belanoff JK, Roe RL. Efficacy and safety of mifepristone for the treatment of psychotic depression. J Clin Psychopharmacol. 2011;31(4):436–440. [DOI] [PubMed] [Google Scholar]
- 414. Block T, Petrides G, Kushner H, Kalin N, Belanoff J, Schatzberg A. Mifepristone plasma level and glucocorticoid receptor antagonism associated with response in patients with psychotic depression. J Clin Psychopharmacol. 2017;37(5):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Block TS, Kushner H, Kalin N, Nelson C, Belanoff J, Schatzberg A. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54. [DOI] [PubMed] [Google Scholar]
- 416. DeBattista C, Belanoff J, Glass S, et al. Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry. 2006;60(12):1343–1349. [DOI] [PubMed] [Google Scholar]