Abstract
Psoriasis is a chronic inflammatory proliferative skin disease involving various types of chemokines regulating immune cell migration, localization, and activation. Bath psoralen plus UVA (PUVA) treatment is an established phototherapy for psoriasis, but its effects on chemokine levels remain unknown. We investigated the levels of 22 serum chemokines in 20 patients with psoriasis first treated with bath PUVA therapy between 2007 and 2011 in a single center and analyzed the associations between the chemokines and disease severity (PASI) before and after therapy to investigate the mechanisms of action of bath PUVA therapy. Before bath PUVA therapy, the PASI scores correlated with the serum levels of CCL17 (r = 0.581), CCL18 (r = 0.462), CCL19 (r = 0.477), and CXCL16 (r = 0.524). After bath PUVA, the serum levels of CCL17, CCL22, CXCL1, and CXCL9 were significantly decreased. Heatmap clustering and network analysis based on statistically significant Spearman correlations among the chemokines showed distinctive changes in the chemokine signature. Our findings revealed that the levels of several chemokines correlated with the disease state of psoriasis. Furthermore, bath PUVA therapy reduced the secretion of keratinocyte-derived chemokines that induce the migration of immune cells important for psoriasis pathogenesis, partly revealing the mechanism of the therapeutic activity.
Abbreviations: AD, atopic dermatitis; CLA, cutaneous lymphocyte-associated antigen; DC, dendritic cell; KC, keratinocyte; NB-UVB, narrowband UVB; PUVA, psoralen plus UVA; Th, T helper
Introduction
Chemotactic cytokines (i.e., chemokines) represent a large group of small chemotactic proteins (generally 8–11 kDa in size) that recruit circulating leukocytes to sites of inflammation. Approximately 50 different chemokines have been identified, which are classified into four main subfamilies on the basis of their structural differences: CXC, CC, CX3C, and XC. These proteins bind to 7-transmembrane-domain G-protein-coupled receptors on the cell surface to activate signaling pathways (Charo and Ransohoff, 2006).
In psoriasis, various chemokine receptors and their corresponding chemokine ligands are involved in the migration and localization of immune cells (e.g., T helper [Th] 1, Th17, dendritic cells [DCs], monocytes) (Mabuchi et al., 2012). CCL20 is highly produced when keratinocytes (KCs) are stimulated by IL-17, and it attracts CCR6+Th17 cells to local tissues (Furue et al., 2020). Th17 cells play a pivotal role in psoriatic lesions by secreting IL-17, IL-22, and TNF-α (Lowes et al., 2008). IL-17 stimulates KCs not only to promote proliferation and differentiation but also to produce a variety of chemokines. CXCL1, CXCL2, and CXCL8 recruit neutrophils involved in the formation of intraepidermal microabscesses (Chiricozzi et al., 2018). Th1 cells, producing TNF-α and IFN-γ, are recruited by the binding of CXCL9, CXCL10, and CXCL11 to CXCR3, which are secreted mainly from KCs stimulated by IFN-γ (Johansen et al., 2017; Rottman et al., 2001). Cutaneous lymphocyte-associated antigen (CLA)-positive T cells coexpressing CCR4 and CCR10 are induced by CCL17, CCL22, and CCL27 (Homey et al., 2002; Lonsdorf et al., 2009; Rottman et al., 2001). Macrophages derived from monocytes are an important source of TNF-α, a key mediator of chronic inflammation. CCL2, amplified by TNF-α and IFN-γ from KCs, is a chemoattractant of monocytes (Behfar et al., 2018). CCL19 is thought to be produced by DCs in psoriasis and organizes DCs and T cells into formal (lymph node‒like) lymphatic tissue in psoriatic lesions (Mitsui et al., 2012). Interestingly, most chemokines involved in psoriasis are secreted by KCs.
Bath psoralen plus UVA (PUVA) therapy is one of the treatment options for psoriasis. A randomized, open, prospective, multicenter trial performed on an intention-to-treat basis showed that bath PUVA reduced the median PASI score by 74% (16.4 to 4.2) within 6 weeks in 38 patients with psoriasis (Berneburg et al., 2013). However, the detailed mechanisms of action of the therapeutic effects have remained unclear. We have focused on elucidating the mechanisms of action of bath PUVA therapy, particularly with regard to regulatory T cells (Kubo et al., 2017; Saito et al., 2009). To our knowledge, no studies to date have examined the changes in chemokine levels of patients with psoriasis before and after bath PUVA therapy, although some analyses of chemokine levels after narrowband UVB (NB-UVB) therapy have been reported (Coimbra et al., 2010; Ekman et al., 2013; Gao and Wang, 2015). Before NB-UVB therapy, CCL17, CCL20, CCL22, CXCL9, and CXCL10 levels are increased in patients with psoriasis compared with those in healthy controls (Ekman et al., 2013). CCL20 levels also correlate with disease severity. Although NB-UVB therapy reduces skin symptoms at 12 weeks, the chemokine levels are not affected (Ekman et al., 2013). After 15 sessions of NB-UVB phototherapy treatment, serum CCL2 levels are significantly decreased (Gao and Wang, 2015). Plasma IL-22, IL-17, IL-23, and CXCL8 levels are decreased along with a decrease in PASI scores at 12 weeks (Coimbra et al., 2010).
In this study, we evaluated the serum levels of 22 chemokines previously reported to be associated with psoriasis and analyzed the associations among the chemokines before and after bath PUVA therapy to investigate the mechanisms of action of the therapy.
Results
Patient profiles and the efficacy of bath PUVA therapy
A total of 20 patients with psoriasis (14 men, 6 women; aged 27–75 [mean: 52.6] years; disease duration of 0.160–30 [median: 9.5] years) for whom bath PUVA therapy was first initiated between 2007 and 2011 were included in the study (Table 1). Of the 20 patients, 17 had psoriasis vulgaris, and 3 had psoriatic arthritis. The median PASI score before bath PUVA therapy was 23.8 (range: 5.6–69.3). All patients showed a decrease in the PASI score after the treatment (median score: 2.0, range: 0–9.1). The median PASI score was reduced by 91.2%. In total, 90% of the patients achieved PASI 75, 50% achieved PASI 90, and 5% achieved PASI 100. The therapeutic efficacy of bath PUVA therapy in patients with psoriasis was confirmed. We next examined the serum chemokine levels before and after bath PUVA therapy.
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristics | Patients (n = 20) |
|---|---|
| Sex, n (%) | |
| Female | 6 (30) |
| Male | 14 (70) |
| Age, y | |
| Mean (SD) | 52.6 (13.4) |
| Range | 27–75 |
| Type of psoriasis, n (%) | |
| PV | 17 (85) |
| PsA | 3 (15) |
| Duration since onset, y | |
| Median (25‒75%) | 9.5 (3.3‒13.5) |
| Range | 0.16–30 |
| PASI (0‒72) | |
| Median (before bath PUVA) (25‒75%) | 23.8 (13.6‒31.3) |
| Range | 5.6–69.3 |
| Median (after bath PUVA) (25‒75%) | 2.0 (0.9–6.3) |
| Range | 0–9.1 |
| Radiation dose, J/cm2 | |
| Mean (SD) | 58.3 (18.4) |
| Range | 34.5–106 |
| Treatment response (PASI reduction), % | |
| Median (25‒75%) | 91.2 (78.6‒94.6) |
| Range | 40.9–100 |
| Therapy place, n (%) | |
| Outpatient | 1 (5) |
| Inpatient | 19 (95) |
| Body mass index, kg/m2 | |
| Median (25‒75%) | 25.5 (23.6‒28.0) |
| Range | 18.1–41.9 |
| Smoking, n (%) | |
| Current smoker | 7 (35) |
| Previous smoker | 1 (5) |
| Never smoker | 12 (60) |
| Previous treatment, n (%) | |
| Steroid ointment | 13 (65) |
| Vit. D ointment | 8 (40) |
| UV treatment | 3 (15) |
| Immunosuppressive agents | 5 (25) |
| Etretinate | 2 (10) |
| Unknown | 2 (10) |
Abbreviations: PsA, psoriatic arthritis; PUVA, psoralen plus UVA; PV, psoriasis vulgaris; Vit, vitamin.
Of the 22 chemokines evaluated, the serum levels of 4 correlated with the PASI score
We assessed the correlation between the serum levels of 22 chemokines and the PASI score. The PASI score before bath PUVA therapy moderately correlated with the serum levels of CCL17 (r = 0.581), CCL18 (r = 0.462), CCL19 (r = 0.477), and CXCL16 (r = 0.524) (Figure 1). After bath PUVA therapy, the serum levels of none of the chemokines evaluated correlated with the PASI score (Table 2). CCL17, a CCR4 ligand expressed in Th2 cells, is a well-known biomarker for atopic dermatitis (AD) that is widely used in clinical practice (Kakinuma et al., 2001). Our results were consistent with recent reports indicating that serum CCL17 levels in patients with psoriasis (especially those with pustular psoriasis) correlated with the PASI score (Kawasaki et al., 2020; Shibuya et al., 2018). CCL19 is assumed to be produced in DCs in psoriasis and induces DCs and T cells to form lymphoid tissue in psoriatic lesions (Mitsui et al., 2012). CXCL16, produced from KCs and antigen-presenting cells (DCs and monocytes), mediates the migration of CD8+ T cells and neutrophils (Günther et al., 2012; Steffen et al., 2018). The roles of these two chemokines could explain the correlation between the PASI score and the serum levels of CCL19 and CXCL16 in patients with psoriasis. Comprehensive gene expression analysis of psoriatic skin lesions revealed an increase in the CCL18 mRNA levels. This chemokine is produced by macrophages or DCs in lymph nodes and acts as a chemoattractant for T cells (Zhou et al., 2003). Expression of CCL18 mRNA in lesional skin is increased compared with that in the nonlesional skin, not only in AD but also in psoriasis (Kim et al., 2012). The results of this study show a positive correlation between CCL18 and the PASI score, although it is unclear how CCL18 affects psoriatic lesions.
Figure 1.
Correlation of pretreatment serum chemokine levels with PASI score. Scatter plots of the pretreatment PASI scores against the levels of each serum chemokine in 20 patients with psoriasis. Red frames indicate a correlation coefficient > 0.4.
Table 2.
Correlation between PASI Score and Serum Chemokines before and after Bath PUVA Therapy
| Chemokines | Before Bath PUVA |
After Bath PUVA |
||
|---|---|---|---|---|
| Correlation Coefficient (95% CI) |
Adjusted P-value |
Correlation Coefficient (95% CI) |
Adjusted P-value |
|
| CCL2 | 0.284 (−0.195 to 0.653) | 0.730 | −0.122 (−0.545 to 0.351) | 0.966 |
| CCL3 | −0.0948 (−0.526 to 0.375) | 0.909 | −0.00527 (−0.458 to 0.450) | 0.982 |
| CCL4 | −0.0639 (−0.503 to 0.401) | 0.909 | −0.0580 (−0.499 to 0.406) | 0.966 |
| CCL5 | −0.0519 (−0.494 to 0.412) | 0.909 | 0.245 (−0.235 to 0.629) | 0.966 |
| CCL11 | 0.0730 (−0.394 to 0.510) | 0.909 | 0.0647 (−0.401 to 0.504) | 0.966 |
| CCL17 | 0.581 (0.173–0.819) | 0.158 | 0.141 (−0.334 to 0.559) | 0.966 |
| CCL18 | 0.462 (0.0102–0.757) | 0.222 | −0.0233 (−0.472 to 0.435) | 0.966 |
| CCL19 | 0.477 (0.0295–0.765) | 0.222 | 0.236 (−0.244 to 0.623) | 0.966 |
| CCL20 | 0.204 (−0.275 to 0.602) | 0.909 | 0.227 (−0.253 to 0.617) | 0.966 |
| CCL21 | 0.0918 (−0.378 to 0.524) | 0.909 | −0.180 (−0.586 to 0.298) | 0.966 |
| CCL22 | −0.0580 (−0.499 to 0.407) | 0.909 | −0.0474 (−0.491 to 0.415) | 0.966 |
| CCL27 | 0.136 (−0.339 to 0.556) | 0.909 | −0.229 (−0.618 to 0.251) | 0.966 |
| CXCL1 | 0.395 (−0.0717 to 0.720) | 0.374 | 0.138 (−0.337 to 0.557) | 0.966 |
| CXCL2 | −0.0150 (−0.466 to 0.442) | 0.950 | 0.305 (−0.172 to 0.667) | 0.966 |
| CXCL5 | 0.192 (−0.287 to 0.594) | 0.909 | −0.0399 (−0.485 to 0.421) | 0.966 |
| CXCL8 | 0.0557 (−0.408 to 0.497) | 0.909 | 0.0354 (−0.425 to 0.481) | 0.966 |
| CXCL9 | 0.0587 (−0.406 to 0.499) | 0.909 | 0.0309 (−0.478 to 0.429) | 0.966 |
| CXCL10 | 0.280 (−0.199 to 0.651) | 0.730 | 0.160 (−0.316 to 0.572) | 0.966 |
| CXCL11 | 0.218 (−0.262 to 0.611) | 0.909 | 0.135 (−0.339 to 0.555) | 0.966 |
| CXCL12 | −0.0399 (−0.485 to 0.421) | 0.909 | 0.266 (−0.214 to 0.642) | 0.966 |
| CXCL16 | 0.524 (0.0925−0.790) | 0.194 | −0.0850 (−0.519 to 0.384) | 0.966 |
| CX3CL1 | 0.151 (−0.325 to 0.556) | 0.909 | −0.0595 (−0.500 to 0.405) | 0.966 |
Abbreviations: CI, confidence interval; PUVA, psoralen plus UVA.
Of the 22 chemokines evaluated, serum levels of 4 were decreased after bath PUVA therapy
The median serum levels of all chemokines evaluated, except for CCL5 and CCL18, were decreased after bath PUVA therapy (Figure 2). The median serum levels of four chemokines were significantly decreased: CCL17 (P = 0.004), CCL22 (P = 0.027), CXCL1 (P = 0.004), and CXCL9 (P = 0.010). CCL17 was the only chemokine whose serum levels correlated with the PASI score before bath PUVA treatment.
Figure 2.
Changes in serum chemokine levels after bath PUVA therapy. The serum levels of 22 chemokines before and after bath PUVA therapy. Red frames and asterisks indicate a statistically significant change (adjusted P < 0.05). PUVA, psoralen plus UVA.
Serum levels of some chemokines correlated with the laboratory data
We analyzed the associations between the pretreatment blood test values, chemokine levels, and PASI score (Table 3). Neutrophil count positively correlated with the PASI score (r = 0.572) and serum CCL17 (r = 0.672) and CXCL1 (r = 0.588) levels. The correlation between neutrophil count and serum CCL17 levels is consistent with recently reported findings (Kawasaki et al., 2020). Serum levels of the 22 chemokines evaluated did not correlate with lymphocyte or eosinophil count. Hemoglobin levels inversely correlated with serum CX3CL1 levels (r = ‒0.605). The serum levels of CCL17 (r = 0.527), CCL20 (r = 0.515), CXCL1 (r = 0.625), CXCL2 (r = 0.488), and CXCL9 (r = 0.578) positively correlated platelet count. Erythrocyte sedimentation rate correlated with serum CXCL1 (r = 0.530), CXCL2 (r = 0.566), and CXCL12 (r = 0.459) levels. CRP protein levels correlated with CCL11 (r = 0.536) and CXCL5 (r = 0.776) levels. No comprehensive analysis of serum chemokines and laboratory data in patients with psoriasis have been reported. Interestingly, the serum levels of three neutrophil attractant chemokines, CXCL1, CXCL2, and CXCL5, correlated with peripheral blood neutrophil count and inflammatory markers (CRP protein and erythrocyte sedimentation rate). It is unclear how changes in other serum chemokine levels reflect their function or disease activity.
Table 3.
Correlation between Serum Chemokines and Laboratory Data before Bath PUVA Therapy
| WBC | Neutrophils | Lymphocytes | Monocytes | Eosinophils | Hemoglobin | Platelets | IL-6 | CRP | ESR | |
|---|---|---|---|---|---|---|---|---|---|---|
| PASI | 0.474 (0.0258 – 0.764) | 0.572 (0.160 – 0.815) | 0.157 (–0.319 to 0.570) | 0.314 (–0.163 to 0.672) | –0.0331 (–0.480 to 0.427) | –0.183 (–0.588 to 0.296) | 0.356 (–0.116 to 0.697) | –0.0613 (–0.538 to 0.445) | 0.304 (–0.241 to 0.703) | –0.0939 (–0.536 to 0.389) |
| CCL2 | 0.108 (–0.364 to 0.535) | 0.226 (–0.254 to 0.616) | –0.0962 (–0.527 to 0.374) | –0.00902 (–0.461 to 0.447) | 0.227 (–0.253 to 0.617) | –0.231 (–0.620 to 0.249) | 0.318 (–0.159 to 0.674) | –0.103 (–0.567 to 0.410) | 0.328 (–0.216 to 0.716) | 0.0863 (–0.395 to 0.531) |
| CCL3 | 0.370 (–0.101 to 0.705) | 0.376 (–0.0938 to 0.709) | 0.262 (–0.218 to 0.639) | 0.444 (–0.0127 to 0.747) | –0.0406 (–0.485 to 0.421) | 0.422 (–0.0389 to 0.735) | 0.163 (–0.314 to 0.574) | –0.0588 (–0.536 to 0.447) | 0.154 (–0.384 to 0.613) | 0.117 (–0.369 to 0.553) |
| CCL4 | 0.178 (–0.300 to 0.584) | 0.287 (–0.191 to 0.656) | –0.108 (–0.536 to 0.363) | 0.138 (–0.337 to 0.557) | 0.147 (–0.328 to 0.563) | 0.301 (–0.177 to 0.664) | 0.339 (–0.135 to 0.687) | –0.0686 (–0.543 to 0.439) | –0.0177 (–0.521 to 0.494) | 0.128 (–0.359 to 0.560) |
| CCL5 | –0.138 (–0.557 to 0.337) | – 0.0466 (–0.490 to 0.416) | –0.184 (–0.588 to 0.295) | –0.0541 (–0.496 to 0.410) | –0.171 (–0.580 to 0.306) | 0.257 (–0.223 to 0.636) | 0.0527 (–0.411 to 0.495) | –0.302 (–0.691 to 0.224) | 0.112 (–0.419 to 0.587) | 0.0247 (–0.446 to 0.485) |
| CCL11 | 0.278 (–0.201 to 0.650) | 0.310 (–0.168 to 0.669) | 0.134 (–0.341 to 0.554) | 0.202 (–0.278 to 0.600) | 0.0165 (–0.441 to 0.467) | –0.361 (–0.700 to 0.111) | 0.358 (–0.114 to 0.698) | –0.125 (–0.582 to 0.392) | 0.536 (0.0391 – 0.821) | 0.369 (–0.116 to 0.712) |
| CCL17 | 0.537 (0.110 – 0.797) | 0.672 (0.314 – 0.863) | 0.0872 (–0.382 to 0.520) | 0.343 (–0.131 to 0.689) | 0.334 (–0.141 to 0.684) | –0.434 (–0.742 to 0.0242) | 0.527 (0.0959 – 0.791) | 0.382 (–0.136 to 0.736) | 0.498 (–0.0133 to 0.803) | 0.397 (–0.0848 to 0.728) |
| CCL18 | 0.153 (–0.323 to 0.567) | 0.202 (–0.278 to 0.600) | –0.0346 (–0.481 to 0.426) | –0.119 (–0.543 to 0.354) | 0.0827 (–0.386 to 0.517) | –0.0625 (–0.502 to 0.403) | 0.317 (–0.160 to 0.674) | 0.00980 (–0.485 to 0.500) | –0.0118 (–0.516 to 0.499) | –0.0960 (–0.538 to 0.387) |
| CCL19 | 0.281 (–0.198 to 0.651) | 0.457 (0.00428 – 0.754) | –0.165 (–0.576 to 0.312) | 0.108 (–0.363 to 0.536) | 0.143 (–0.332 to 0.560) | –0.217 (–0.610 to 0.263) | 0.424 (–0.0364 to 0.736) | 0.260 (–0.267 to 0.667) | 0.273 (–0.272 to 0.686) | 0.198 (–0.295 to 0.608) |
| CCL20 | 0.339 (–0.135 to 0.687) | 0.420 (–0.0422 to 0.734) | –0.0436 (–0.488 to 0.418) | 0.0752 (–0.392 to 0.511) | 0.429 (–0.0313 to 0.739) | –0.442 (–0.746 to 0.0149) | 0.515 (0.0795 – 0.785) | 0.378 (–0.141 to 0.734) | 0.387 (–0.150 to 0.748) | 0.432 (–0.0424 to 0.747) |
| CCL21 | 0.152 (–0.324 to 0.567) | 0.0376 (–0.423 to 0.483) | 0.296 (–0.182 to 0.661) | –0.0211 (–0.470 to 0.437) | 0.305 (–0.172 to 0.667) | –0.0459 (–0.489 to 0.417) | 0.0421 (–0.420 to 0.487) | –0.0319 (–0.516 to 0.468) | –0.301 (–0.702 to 0.244) | –0.120 (–0.555 to 0.366) |
| CCL22 | 0.158 (–0.319 to 0.571) | 0.104 (–0.367 to 0.532) | 0.104 (–0.367 to 0.532) | 0.108 (–0.363 to 0.536) | 0.206 (–0.273 to 0.603) | –0.213 (–0.608 to 0.266) | 0.257 (–0.222 to 0.637) | –0.0343 (–0.518 to 0.466) | –0.0148 (–0.519 to 0.497) | 0.238 (–0.256 to 0.633) |
| CCL27 | –0.320 (–0.676 to 0.156) | –0.247 (–0.630 to 0.233) | –0.229 (–0.618 to 0.251) | –0.513 (–0.784 to 0.0769) | 0.286 (–0.193 to 0.655) | –0.0474 (–0.491 to 0.415) | –0.00677 (–0.459 to 0.448) | –0.0392 (–0.522 to 0.462) | –0.207 (–0.647 to 0.336) | –0.214 (–0.618 to 0.279) |
| CXCL1 | 0.458 (0.00545 – 0.755) | 0.588 (0.183 – 0.822) | 0.0376 (–0.423 to 0.483) | 0.289 (–0.190 to 0.656) | 0.0617 (–0.403 to 0.501) | –0.313 (–0.671 to 0.164) | 0.625 (0.239 – 0.841) | 0.206 (–0.319 to 0.634) | 0.282 (–0.263 to 0.691) | 0.530 (0.0848 – 0.798) |
| CXCL2 | 0.195 (–0.284 to 0.596) | 0.253 (–0.227 to 0.634) | –0.00752 (–0.460 to 0.448) | –0.0391 (–0.484 to 0.422) | 0.0466 (–0.416 to 0.490) | –0.354 (–0.696 to 0.119) | 0.488 (0.0444 – 0.771) | –0.0882 (–0.557 to 0.423) | 0.412 (–0.121 to 0.761) | 0.566 (0.136 – 0.816) |
| CXCL5 | 0.379 (–0.0902 to 0.711) | 0.382 (–0.0868 to 0.712) | 0.205 (–0.275 to 0.602) | 0.374 (–0.0955 to 0.708) | –0.00752 (–0.460 to 0.448) | –0.355 (–0.697 to 0.117) | 0.349 (–0.124 to 0.693) | 0.250 (–0.277 to 0.661) | 0.776 (0.442 – 0.921) | 0.451 (–0.0184 to 0.758) |
| CXCL8 | 0.160 (–0.317 to 0.572) | 0.278 (–0.201 to 0.650) | 0.0301 (–0.430 to 0.477) | 0.0737 (–0.393 to 0.510) | 0.287 (–0.191 to 0.656) | 0.294 (–0.185 to 0.659) | 0.147 (–0.329 to 0.563) | 0.260 (–0.267 to 0.667) | –0.0857 (–0.569 to 0.441) | 0.0388 (–0.435 to 0.495) |
| CXCL9 | 0.243 (–0.237 to 0.628) | 0.385 (–0.0833 to 0.714) | –0.199 (–0.598 to 0.281) | 0.0346 (–0.426 to 0.481) | 0.337 (–0.138 to 0.686) | –0.169 (–0.578 to 0.309) | 0.578 (0.168 – 0.817) | 0.304 (–0.222 to 0.693) | 0.421 (–0.110 to 0.765) | 0.298 (–0.195 to 0.670) |
| CXCL10 | –0.0226 (–0.471 to 0.436) | 0.114 (–0.358 to 0.540) | –0.209 (–0.605 to 0.270) | –0.187 (–0.590 to 0.292) | 0.438 (–0.0202 to 0.744) | –0.0580 (–0.499 to 0.406) | 0.0655 (–0.400 to 0.504) | –0.0172 (–0.505 to 0.479) | 0.127 (–0.407 to 0.596) | –0.189 (–0.602 to 0.303) |
| CXCL11 | 0.177 (–0.301 to 0.584) | 0.385 (–0.0833 to 0.714) | –0.161 (–0.573 to 0.316) | –0.0391 (–0.484 to 0.422) | –0.248 (–0.631 to 0.232) | 0.132 (–0.342 to 0.552) | 0.202 (–0.277 to 0.601) | 0.0931 (–0.419 to 0.560) | 0.464 (–0.0574 to 0.786) | 0.0943 (–0.388 to 0.536) |
| CXCL12 | –0.0613 (–0.501 to 0.404) | –0.0873 (–0.520 to 0.382) | 0.0346 (–0.426 to 0.481) | –0.0527 (–0.458 to 0.450) | –0.00827 (–0.460 to 0.447) | –0.422 (–0.735 to 0.0387) | 0.0414 (–0.420 to 0.486) | –0.191 (–0.625 to 0.332) | –0.0480 (–0.543 to 0.471) | 0.459 (–0.00813 to –0.762) |
| CXCL16 | 0.185 (–0.293 to 0.589) | 0.343 (–0.131 to 0.689) | –0.155 (–0.569 to 0.321) | –0.0376 (–0.483 to 0.423) | –0.205 (–0.602 to 0.275) | 0.0940 (–0.376 to 0.525) | 0.411 (–0.0527 to 0.729) | –0.118 (–0.577 to 0.398) | 0.0532 (–0.467 to 0.546) | –0.136 (–0.566 to 0.352) |
| CX3CL1 | –0.163 (–0.574 to 0.314) | –0.0466 (–0.490 to 0.416) | –0.218 (–0.611 to 0.262) | –0.164 (–0.575 to 0.313) | –0.158 (–0.571 to 0.319) | –0.605 (–0.831 to –0.209) | –0.0572 (–0.498 to 0.407) | –0.101 (–0.565 to 0.412) | –0.0842 (–0.568 to 0.442) | 0.263 (–0.230 to 0.649) |
Abbreviations: ESR, erythrocyte sedimentation rate; FDR, false discovery rate; WBC, white blood cell.
The P-values were adjusted using an FDR procedure and the bold showed an adjusted P < 0.05.
Changes in the chemokine patterns after bath PUVA therapy
We analyzed the intercorrelations of the 22 chemokines before and after bath PUVA therapy. Heatmap showed that CCL17, CCL18, CCL19, and CXCL16, whose serum levels correlated with the PASI score, and CXCL1 and CXCL9, whose serum levels decreased after treatment, were all in one cluster containing the PASI score (Figure 3). After bath PUVA treatment, CXCL9 moved into another cluster that did not contain the PASI score. CXCL9 is involved in the migration of Th1 cells, which are lymphocytes that play a central role in the pathogenesis of psoriasis. However, serum CXCL9 levels did not correlate with the pretreatment PASI score in this study. A heatmap showed CXCL9 in a cluster containing PASI score before treatment. After treatment, the relationship with the PASI score weakened, which may indicate an effect of treatment.
Figure 3.
Heatmaps of chemokine correlations before and after bath PUVA therapy. Heatmaps and hierarchical cluster analytics (Ward’s method) were performed to depict the overall correlation profiles of chemokines and PASI scores on the basis of Spearman correlation matrices before and after bath PUVA therapy. PUVA, psoralen plus UVA.
Chemokines are intricately involved in the pathogenesis of psoriasis
We examined the relationships between the serum chemokine levels by network analysis on the basis of Spearman correlations greater than an absolute value of r > 0.5 (Figure 4). The network graph is composed of edges and nodes. Edges refer to the connections between the chemokines, as illustrated by the lines. In this study, we defined edges as connections between the chemokines with Spearman correlations between the chemokines. Red edges indicate a positive correlation (r > 0.5), and blue edges indicate a negative correlation (r < –0.5). Nodes refer to objects that are connected together, as illustrated by the circles. The size of each node represents the degree of centrality (i.e., the number of edges from each node). The overall number of network edges was 28. After treatment, the number of edges was reduced to 26, and the negative correlation was increased from 1 to 3. Compared with the other chemokines, the serum levels of CCL17, CCL18, CCL19, and CXCL16, which correlated with the PASI score before treatment, also correlated with the levels of the most other chemokines. Serum CCL19 levels strongly correlated with CCL17, CCL18, CCL20, CXCL1, CXCL8, CXCL9, and CXCL16 levels before treatment, which was the greatest number of chemokines correlated with a single chemokine. After bath PUVA therapy, serum CCL19 levels correlated only with four chemokines: CCL17, CXCL1, CXCL8, and CXCL16. Although serum CCL19 levels were not decreased by treatment, the correlations between the serum CCL19 levels decreased and changed. The largest decrease in the number of correlated chemokines before and after treatment was observed in CCL19 and CCL20 by three; two decreases were observed in CCL4, CCL27, and CXCL2. CCL20 and CCL27 correlated with the serum levels of three chemokines (CCL17, CCL19, CXCL9) and two chemokines (CCL2 and CXCL10). After treatment, the correlation coefficients decreased to below 0.5, and CCL20 and CCL27 did not map with any of the other factors. Bath PUVA therapy changed the correlation patterns between the chemokines. This finding suggests that a complex relationship exists among the chemokines involved in psoriasis. The relationship between the actual changes of each chemokine after bath PUVA therapy with psoriasis pathophysiology is difficult to explain on the basis of network analysis alone.
Figure 4.
Network analysis of chemokine correlation before and after bath PUVA therapy. Network analysis revealed Spearman correlations between the chemokines. Red edges indicate a positive correlation (r > 0.5), and blue edges indicate a negative correlation (r < –0.5). The size of each node represents degree centrality. PUVA, psoralen plus UVA.
Discussion
Psoriasis is a T-cell‒mediated autoimmune disease characterized by increased KC proliferation leading to the formation of distinct erythematous plaques with silver‒white large scales (Boehncke and Schön, 2015). Although the pathophysiology of psoriasis is not yet fully elucidated, several studies have shown significant roles of chemokines, immune cells, KCs, and cytokines in the disease.
This study comprehensively examined the serum levels of 22 chemokines in patients with psoriasis treated with bath PUVA therapy. The PASI score before bath PUVA therapy correlated with the serum levels of CCL17, CCL18, CCL19, and CXCL16. Although CCL18, CCL19, and CXCL16 are involved in the pathogenesis of psoriasis, their correlation with the PASI score has not been evaluated. Comprehensive gene expression analysis of psoriatic skin lesions revealed increased mRNA levels of nine chemokines, including CCL18, CCL19, CCL21, and CXCL12 (Zhou et al., 2003). CCL18 is mainly secreted by antigen-presenting cells such as DCs and monocytes and recruits CLA-positive memory T cells in AD (Günther et al., 2005). Immunostaining of biopsy skin tissue shows CCL18+ cells in AD and psoriatic lesions (Kim et al., 2012). CCL19, secreted mainly from DCs, likely recruits CCR7+ T cells and DCs from the blood to the superficial dermis and may create the same environment for the expansion of effector memory T cells from central memory T cells (Mitsui et al., 2012). Administration of anti-TNF biologics decreases CCL19 gene expression and abolishes dermal lymphoid‒like tissue (Bosè et al., 2013). However, Mitsui et al. (2012) reported that CCL21 transcript level and CCL21 protein level are relatively low in psoriatic dermal aggregates. CXCL16 mediates the homing of CXCR6+ CD8 T cells to human skin (Günther et al., 2012). CXCL16 is secreted by monocytes and DCs by toll-like receptor stimulation and could mediate neutrophil migration through CXCL8 (Steffen et al., 2018).
Bath PUVA treatment significantly decreased the serum levels of CCL17, CCL22, CXCL1, and CXCL9. Among these, CCL17 was the only chemokine whose serum levels correlated with the pretreatment PASI score and were decreased further by phototherapy. CCL17, a CCR4 ligand expressed in Th2 cells, is a well-known biomarker for AD (Kakinuma et al., 2001). Serum CCL17 levels in patients with psoriasis (especially those with pustular psoriasis) correlate with PASI score (Kawasaki et al., 2020; Shibuya et al., 2018). Immunohistologic staining showed that epidermal KCs in skin biopsy tissues obtained from patients with psoriasis are slightly positive for CCL17 but not at the same level as in AD (Kakinuma et al., 2001), and CCL17 mRNA expression is increased in psoriasis lesions compared with that in nonlesional areas (Rottman et al., 2001). In normal epidermal KCs from patients without psoriasis, IFN-γ combined with TNF-α stimulation significantly increases CCL17 concentration in the culture supernatant (Vestergaard et al., 2000) and the CCL17 mRNA expression in cultured dermal vascular endothelial cells (Rottman et al., 2001). CCR4 is also expressed on Th17 cells (Lim et al., 2008). On the other hand, CCL17 is abundant in platelets, and platelet activation leads to the release of α-granule chemokines, such as CCL3, CCL5, CCL7, CCL17, CXCL1, CXCL5, and CXCL8, which attract leukocytes and further activate other platelets (Fujisawa et al., 2002; Gear and Camerini, 2003). In this study, serum CCL17 levels correlated with platelet count before and after treatment. We cannot rule out the effects of CCL17 on platelets. Skin-tropic memory T cells are positive for CLA and skin-homing receptors such as CCR4, CCR6, CCR8, and CCR10 (Casciano et al., 2020; Homey et al., 2000; Hudak et al., 2002; Schaerli et al., 2004). In patients with psoriasis, CLA, CCR4, and CCR6 expressions are decreased, and CXCR3 and CCL5 expression is significantly increased because CCR7+CD45RA‒CD4+/CD8+ central memory T cells differentiate into CCR7‒CD45RA‒ effector memory T cells (Casciano et al., 2020).
Similar to CCL17, CCL22 is a CCR4 ligand. In patients with psoriasis, plasma CCL22 levels are increased (Ekman et al., 2013). CCL22 is thought to be secreted from KCs and endothelium by stimulation with IFN-γ and TNF-α and to mediate the migration of CLA+ T cells (Rottman et al., 2001). CCL17 and CCL22 may play important roles in the migration of circulating central memory T cells from the blood to the skin. CXCL1 is secreted by epidermal KCs by IL-17 stimulation and promotes CXCR2+ neutrophil migration (Gillitzer et al., 1996; Takei-Taniguchi et al., 2012). Neutrophils migrate into the epidermis through proinflammatory mediator signaling, including IL-17, and form Munro’s microabscesses. Our study showed that serum CXCL1 levels correlated with neutrophil counts. CXCL2, CXCL5, and CXCL8 have similar neutrophil migration‒promoting functions (Chiricozzi et al., 2018; Lorscheid et al., 2019), but bath PUVA treatment did not significantly decrease these chemokines.
Th1 cells expressing chemokine receptor CXCR3 have a crucial role in the pathogenesis of psoriasis and are recruited by CXCL9, CXCL10, and CXCL11 from KCs by IFN-γ and TNF-α (Johansen et al., 2017; Rottman et al., 2001). However, serum levels of CCL3, CCL4, CCL5, and CX3CL1, which are also involved in Th1 cell migration (Dai et al., 2014; Fraticelli et al., 2001; Giustizieri et al., 2001), were unchanged.
Although serum levels of CCL20 and CCL27 were not significantly decreased, their correlation with the disease state (r > 0.5) before and after treatment disappeared. CCL20 is secreted by epidermal KCs in response to IL-17 stimulation and recruits CCR6+ Th17 cells (Furue et al., 2020). Resident memory T cells are implicated in the maintenance and recurrence of psoriatic lesions. In the epidermis of never-lesional skin from patients with psoriasis, CCR6+CD4-resident T cells, which produce IL-17, IFN-γ, and IL-22, are enriched, in addition to CD103+CD49a‒CD8 T cells. KCs from never-lesional skin have an increased CCL20 response to microbial organisms, leading to the accumulation of CCR6+ T cells able to produce disease-inducing cytokines. As a result, new psoriatic lesions develop (Gallais Sérézal et al., 2019).
CCL27, secreted from KCs by IL-1β and TNF-α stimulation, mediates the migration of CCR10+/CLA+ lymphocytes to the epidermis (Homey et al., 2002). Serum CCL27 levels are increased in patients with psoriasis vulgaris compared with those in healthy people (Kakinuma et al., 2003) and correlate with PASI score (Chen et al., 2019). On the other hand, the CCL27‒CCR10 axis, which regulates the activation of γδT and αβT cells, however, is severely suppressed in patients with psoriasis (Li et al., 2021; Riis et al., 2011).
Considering the multiple chemokine receptors reduced by bath PUVA therapy in this study, Th22 cells, which comprise a subset of CCR4+CCR6+CCR10+CD4 T cells that secrete IL-22 but not IL-17 or IFN γ (Duhen et al., 2009), may also be reduced by bath PUVA therapy. Th22 cells are increased in the peripheral blood (Kagami et al., 2010) and skin lesions (Nograles et al., 2009) of patients with psoriasis. Th22 cells as well as Th17 cells produce IL-22, which upregulates signal transducer and activator of transcription 3, thereby promoting the proliferation of dermal KCs (Zheng et al., 2007). Bath PUVA therapy may also inhibit Th22 migration.
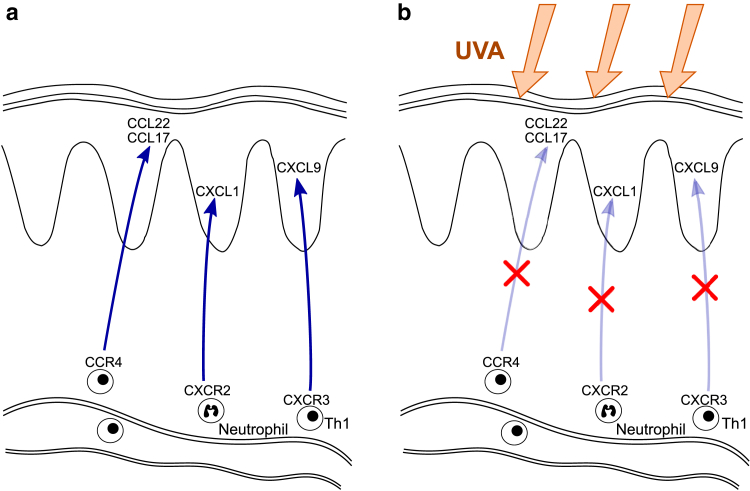
Chemokines whose serum levels were reduced after bath PUVA therapy (CCL17, CCL22, CXCL1, CXCL9) are produced by epidermal KCs and act as immune cell chemoattractants (Tables 4 and 5), suggesting that decreasing the serum levels of these chemokines by bath PUVA would reduce immune cell recruitment (Figure 5). CCR4, chemokine receptor‒binding chemokines reduced by bath PUVA therapy, is expressed in central memory. Therefore, bath PUVA therapy may reduce disease recurrence. Although biologics have made remarkable contributions to the treatment of psoriasis, phototherapy should still be considered an essential treatment option.
Table 4.
A Review of the 22 Chemokines Analyzed in this Study
| Chemokine | Receptor | Secreted Stimulation (In Vitro or In Vivo) | Putative Functions | Reference |
|---|---|---|---|---|
| Secreted from KCs | ||||
| CCL2 | CCR2 | IFN-γ, TNF-α | Monocyte and macrophage trafficking | Behfar et al. (2018) |
| CCL3 | CCR5 | Unknown | Th1 cell trafficking | Dai et al. (2014) |
| CCL4 | CCR5 | Unknown | Th1 cell trafficking | Dai et al. (2014) |
| CCL5 | CCR5 | TNF-α, IFN-γ | Th1 cell trafficking | Dai et al. (2014); Giustizieri et al. (2001) |
| CCL11 | CCR3 | Unknown | Unknown | No papers |
| CCL17 | CCR4 | TNF-α, IFN-γ | Skin homing and T-cell trafficking | Rottman et al. (2001) |
| CCL20 | CCR6 | TNF-α, IL-17 | Th17 cell and DC trafficking | Furue et al. (2020) |
| CCL22 | CCR4 | TNF-α, IFN-γ | Skin homing and T-cell trafficking | Rottman et al. (2001) |
| CCL27 | CCR10 | TNF-α, IL-1 | Skin homing and T-cell trafficking | Chen et al. (2019); Homey et al. (2002); Kakinuma et al. (2003) |
| CXCL1 | CXCR2 | IL-17 | Neutrophil trafficking | Gillitzer et al. (1996); Takei-Taniguchi et al. (2012) |
| CXCL2 | CXCR2 | IL-17, IL-36 | Neutrophil trafficking | Chiricozzi et al. (2018) |
| CXCL5 | CXCR2 | IL-17, IL-36 | Neutrophil trafficking | Chiricozzi et al. (2018) |
| CXCL8 | CXCR1,2 | IL-17 | Neutrophil trafficking | Takei-Taniguchi et al. (2012) |
| CXCL9 | CXCR3 | TNF-α, IFN-γ | Th1 cell trafficking | Rottman et al. (2001) |
| CXCL10 | CXCR3 | TNF-α, IFN-γ | Th1 cell trafficking | Rottman et al. (2001) |
| CXCL11 | CXCR3 | IFN-α | Th1 cell trafficking | Johansen et al. (2017) |
| CXCL16 | CXCR6 | TNF-α, IFN-γ | CD8+ T-cell trafficking Neutrophil trafficking |
Günther et al. (2012); Steffen et al. (2018) |
| Secreted from DCs | ||||
| CCL18 | CCR8 | Unknown | T-cell trafficking | Zhou et al. (2003) |
| CCL19 | CCR7 | Unknown | DC and T-cell trafficking and promoting the formation of secondary lymphoid tissue | Mitsui et al. (2012); Zhou et al. (2003) |
| CCL21 | CCR7 | Unknown | DC and T-cell trafficking and promoting the formation of secondary lymphoid tissue | Zhou et al. (2003) |
| CXCL16 | CXCR6 | Type-I IFN, TNF-α | CD8+ T-cell trafficking Neutrophil trafficking |
Günther et al. (2012); Steffen et al. (2018) |
| Secreted from monocytes/macrophages | ||||
| CCL18 | CCR8 | Unknown | T-cell trafficking | Zhou et al. (2003) |
| CXCL16 | CXCR6 | Type-I IFN, TNF-α | CD8+ T-cell trafficking Neutrophil trafficking |
Günther et al. (2012); Steffen et al. (2018) |
| Secreted from ECs | ||||
| CCL17 | CCR4 | TNF-α, IFN-γ | Skin homing and T-cell trafficking | Rottman et al. (2001) |
| CX3CL1 | CX3CR1 | TNF-α, IFN-γ | Th1 polarization, NK cells trafficking | Fraticelli et al. (2001) |
| Secreted from fibroblasts | ||||
| CXCL12 | CXCR4 | Unknown | Keratinocyte proliferation | Quan et al. (2015) |
Abbreviations: DC, dendritic cell; EC, endothelial cell, KC, keratinocyte; Th, T helper.
Table 5.
Receptors Expressed in Immune Cells Associated with Psoriasis
| Receptor | Immune Cells |
|---|---|
| CXCR2 | Neutrophils |
| CXCR3 | Th1, TEM cells |
| CCR4 | TCM cells, Th17, Th22 |
| CCR6 | Th17, DCs |
| CCR7 | TCM cells |
| CCR8 | CLA+ T cells |
| CCR10 | TCM cells, Th17, Th22 |
| CX3CR1 | Th1, NK cells |
Abbreviations: CLA, cutaneous lymphocyte-associated antigen; DC, dendritic cell; TCM, central memory T cell; TEM, effector memory T cell; Th, T helper.
Figure 5.
Schematic model of the effect of bath PUVA therapy on chemokines and immune cells in psoriatic lesions. (a) In psoriatic skin lesions, various types of immune cells with specific chemokine receptors are recruited by the corresponding chemokine ligands secreted from KCs. (b) After bath PUVA therapy, the serum levels of chemokines secreted from KCs are reduced, which suppresses the recruitment of immune cells. KC, keratinocyte; PUVA, psoralen plus UVA; Th1, T helper type 1.
Previous researchers revealed a decrease in plasma CXCL8 levels and serum CCL2 levels in patients with psoriasis after NB-UVB therapy (Coimbra et al., 2010; Gao and Wang, 2015). Our results showed no significant decrease in the serum CXCL8 and CCL2 levels after bath PUVA therapy. Considering the neutrophil migration‒promoting functions of CXCL8, both NB-UVB and bath PUVA therapy may have similar suppressive effects on neutrophil recruitment in the skin lesion. In addition, the type of KC-derived chemokines that are suppressed by the therapies may differ depending on the wavelength. On the other hand, plasma CCL17, CCL20, CCL22, CXCL8, CXCL9, and CXCL10 levels were not changed significantly at 12 weeks after NB-UVB (Ekman et al., 2013). Contrary to NB-UVB, UVA reaches the dermis in normal human skin (Meinhardt et al., 2008). Therefore, even in psoriatic rashes with a thick stratum corneum, bath PUVA therapy may more fully reach the epidermal KCs, DCs, and monocytes in the dermis.
In summary, several chemokines correlated with the disease state of psoriasis in our patient population. In addition, chemokines secreted by KCs, which induce the migration of immune cells important for the pathogenesis of psoriasis, were reduced by bath PUVA therapy, partly revealing the mechanism of action of the therapy.
Materials and Methods
Human study
All experiments with human samples were conducted in accordance with the ethical principles of the Declaration of Helsinki. The Institutional Review Board of Nagoya City University Graduate School of Medical Sciences (Japan) approved our protocols (approval numbers 312, 325-4, and 325-5). All participants provided written informed consent for the use of their serum. This single-center, retrospective cross-sectional observational study was conducted at Nagoya City University Hospital (Japan).
Study design and blood samples
A total of 20 patients with psoriasis (mean age of 52.6 years, range: 27–75 years; 14 men) for whom bath PUVA therapy was initiated between 2007 and 2011 were enrolled in the study (Table 1). Of the 20 patients, 17 had psoriasis vulgaris, and 3 had psoriatic arthritis. Only 1 patient was treated as an outpatient, and the other 19 patients were treated in the hospital. We obtained some peripheral blood before beginning the bath PUVA therapy and after approximately 20 radiation sessions. Serum was purified from blood.
Bath PUVA therapy protocol
During hospitalization, the patients were bathed with a 0.0001% 8-methoxy psoralen dilution at 37 °C for 15 minutes, followed by systemic UVA radiation immediately thereafter, five sessions per week. The UVA radiation was applied using a Dermaray TS (Eisai-Toshiba, Tokyo, Japan) with FLR100HBL/A/DMR fluorescent tubes. UVA radiation was started at 0.5 J/cm2 and was increased by increments of 0.5 J/cm2 with a maximum dose of 4.0 J/cm2. When symptoms such as strong erythema, erosion, or skin pain occurred during UVA radiation, the UVA dose was not further increased, and UVA radiation was continued at a dose below 4.0 J/cm2. In the outpatient clinic, UVA radiation was conducted for one session per week. UVA radiation was initiated at 0.2 J/cm2, and radiation doses were increased as follows: 0.5 J/cm2, 0.8 J/cm2, 1.1 J/cm2, 1.5 J/cm2, 1.9 J/cm2, 2.3 J/cm2, 2.8 J/cm2, 3.3 J/cm2, and 4.0 J/cm2.
Chemokine analysis
Chemokine analysis was performed on the blood serum of 20 patients. A total of 13 chemokines—CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL11 (Eotaxin), CCL17 (thymus and activation-regulated chemokine), CCL20 (MIP-3α), CXCL1 (GROα), CXCL5 (ENA-78), CXCL8 (IL-8), CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (I-TAC)—were analyzed using a flow cytometry bead‒based immunoassay (LEGENDplex Human Proinflammatory Chemokine Panel [13-plex], BioLegend, San Diego, CA, #740003) according to the manufacturer’s instructions. Nine other chemokines were analyzed using a flow cytometry bead‒based immunoassay (Aimplex Premixed Analyte Kit, Human CCL21, 6Ckine; AimPlex Biosciences, Pomona, CA, #B112464) and a human custom 8-Plex panel, including CCL18 (PARC), CCL19 (MIP-3β), CCL22 (MDC), CCL27 (CTACK), CXCL2 (GROβ), CXCL12 (SDF-1), CXCL16, and CX3CL1 (fractalkine) (AimPlex Biosciences, #T1C080627). The samples were acquired on a FACSVerse equipped with BD FACSuite Software (BD Bioscience, San Jose, CA) and were analyzed with FCAP Array Software Version 3.0 (BD Bioscience).
Laboratory data
Peripheral blood from the same study-enrolled participants was collected before starting the bath PUVA therapy as part of a routine blood test. White blood cell counts; the number of circulating neutrophils, lymphocytes, monocytes, eosinophils, hemoglobin, and platelets; CRP protein; erythrocyte sedimentation rate; and IL-6 were measured in the central laboratory at our hospital.
Statistical analyses
All statistical analyses were performed using Prism 8.4.1 (GraphPad Software, San Diego, CA). Before applying parametric analysis methods, we examined the normality of the data using the Shapiro‒Wilk normality test. Normally distributed numerical data are summarized using mean and SD, and non-normally distributed data are summarized using median and 25–75% percentile (Table 1). Serum chemokines and laboratory data mainly deviated from the normal distribution; therefore, nonparametric methods were used. Data shown in Figure 1 and Tables 2 and 3 were analyzed by Spearman rank correlation coefficients to examine the associations between pretreatment/post-treatment serum chemokine levels, laboratory data, and PASI scores. Data shown in Figure 2 were analyzed by Wilcoxon matched-pairs signed-rank test. P-values were adjusted using the Benjamini–Hochberg procedure to produce a false discovery rate. Significant results were determined on the basis of a false discovery rate‒adjusted P < 0.05.
The heatmaps and network analysis presented in Figures 3 and 4 were generated from Spearman correlation matrices containing values of serum chemokine levels before and after bath PUVA treatment. The heatmaps were generated with Prism (GraphPad Software), and hierarchical clustering was carried out using Ward’s method with R language, version 3.6.3 (www.r-project.org). The network among serum chemokines was visualized with Cytoscape software, version 3.8.0 (https://cytoscape.org/). In the network graphs, edges were limited to Spearman correlations greater than an absolute value of r > 0.5.
Data availability statement
No datasets were generated or analyzed during this study.
ORCIDs
Yoshifumi Kanayama: http://orcid.org/0000-0002-7882-3002
Kan Torii: http://orcid.org/0000-0003-1964-0529
Kyoko Ikumi: http://orcid.org/0000-0003-1401-0966
Akimichi Morita: http://orcid.org/0000-0001-8372-3754
Author Contributions
Conceptualization: YK, KT, KI, AM; Investigation: YK, KT; Supervision: AM; Visualization: YK, KT, KI; Writing - Original Draft Preparation: YK; Writing - Review and Editing: YK, KT, KI, AM
Disclaimer
Study funding bodies played no role in the study design, data collection or analysis, decision to publish the study, or the preparation of the manuscript.
Acknowledgments
We thank Akiko Nishioka and Saori Kasuya for their technical assistance. This work was funded by a Grant-in-Aid for Scientific Research B 17H04242 (AM) from the Japan Society for the Promotion of Science.
Conflict of Interest
AM has received research grants, consulting fees, and/or speaker’s fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Nichi-Iko Pharmaceutical, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and Ushio. The remaining authors state no conflict of interest.
accepted manuscript published online 24 May 2021; corrected proof published online 22 June 2021
Footnotes
Cite this article as: JID Innovations 2021;X:100027
References
- Behfar S., Hassanshahi G., Nazari A., Khorramdelazad H. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine. 2018;110:226–231. doi: 10.1016/j.cyto.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Berneburg M., Herzinger T., Rampf J., Hoetzenecker W., Guenova E., Meisner C., et al. Efficacy of bath psoralen plus ultraviolet A (PUVA) vs. system PUVA in psoriasis: a prospective, open, randomized, multicentre study. Br J Dermatol. 2013;169:704–708. doi: 10.1111/bjd.12466. [DOI] [PubMed] [Google Scholar]
- Boehncke W.H., Schön M.P. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- Bosè F., Petti L., Diani M., Moscheni C., Molteni S., Altomare A., et al. Inhibition of CCR7/CCL19 axis in lesional skin is a critical event for clinical remission induced by TNF blockade in patients with psoriasis. Am J Pathol. 2013;183:413–421. doi: 10.1016/j.ajpath.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Casciano F., Diani M., Altomare A., Granucci F., Secchiero P., Banfi G., et al. CCR4+ skin-tropic phenotype as a feature of central memory CD8+ T cells in healthy subjects and psoriasis patients. Front Immunol. 2020;11:529. doi: 10.3389/fimmu.2020.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chen J., Du J., Han Y., Wei Z. Correlation analysis between IL-35, IL-36γ, CCL27 and psoriasis vulgaris [e-pub ahead of print] J Dermatolog Treat. 2019 doi: 10.1080/09546634.2019.1689226. (accessed 20 January 2021) [DOI] [PubMed] [Google Scholar]
- Chiricozzi A., Romanelli P., Volpe E., Borsellino G., Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19:179. doi: 10.3390/ijms19010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra S., Oliveira H., Reis F., Belo L., Rocha S., Quintanilha A., et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-α levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010;163:1282–1290. doi: 10.1111/j.1365-2133.2010.09992.x. [DOI] [PubMed] [Google Scholar]
- Dai Y.J., Li Y.Y., Zeng H.M., Liang X.A., Xie Z.J., Zheng Z.A., et al. Effect of pharmacological intervention on MIP-1α, MIP-1β and MCP-1 expression in patients with psoriasis vulgaris. Asian Pac J Trop Med. 2014;7:582–584. doi: 10.1016/S1995-7645(14)60098-5. [DOI] [PubMed] [Google Scholar]
- Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- Ekman A.K., Sigurdardottir G., Carlström M., Kartul N., Jenmalm M.C., Enerbäck C. Systemically elevated Th1-, Th2- and Th17-associated chemokines in psoriasis vulgaris before and after ultraviolet B treatment. Acta Derm Venereol. 2013;93:527–531. doi: 10.2340/00015555-1545. [DOI] [PubMed] [Google Scholar]
- Fraticelli P., Sironi M., Bianchi G., D’Ambrosio D., Albanesi C., Stoppacciaro A., et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Fujisawa R., Kato Y., Nakayama T., Morita A., Katsumata H., et al. Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:139–146. doi: 10.1067/mai.2002.126079. [DOI] [PubMed] [Google Scholar]
- Furue K., Ito T., Tsuji G., Nakahara T., Furue M. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol. 2020;91:e12846. doi: 10.1111/sji.12846. [DOI] [PubMed] [Google Scholar]
- Gallais Sérézal I., Hoffer E., Ignatov B., Martini E., Zitti B., Ehrström M., et al. A skewed pool of resident T cells triggers psoriasis-associated tissue responses in never-lesional skin from patients with psoriasis. J Allergy Clin Immunol. 2019;143:1444–1454. doi: 10.1016/j.jaci.2018.08.048. [DOI] [PubMed] [Google Scholar]
- Gao M.L., Wang A.G. Effect of NB-UVB on levels of MCP-1 and CCR6 mRNA in patients with psoriasis vulgaris. Genet Mol Res. 2015;14:12137–12144. doi: 10.4238/2015.October.9.1. [DOI] [PubMed] [Google Scholar]
- Gear A.R., Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- Gillitzer R., Ritter U., Spandau U., Goebeler M., Bröcker E.B. Differential expression of GRO-alpha and IL-8 mRNA in psoriasis: a model for neutrophil migration and accumulation in vivo. J Invest Dermatol. 1996;107:778–782. doi: 10.1111/1523-1747.ep12371803. [DOI] [PubMed] [Google Scholar]
- Giustizieri M.L., Mascia F., Frezzolini A., De Pità O., Chinni L.M., Giannetti A., et al. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol. 2001;107:871–877. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- Günther C., Bello-Fernandez C., Kopp T., Kund J., Carballido-Perrig N., Hinteregger S., et al. CCL18 is expressed in atopic dermatitis and mediates skin homing of human memory T cells. J Immunol. 2005;174:1723–1728. doi: 10.4049/jimmunol.174.3.1723. [DOI] [PubMed] [Google Scholar]
- Günther C., Carballido-Perrig N., Kaesler S., Carballido J.M., Biedermann T. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J Invest Dermatol. 2012;132:626–634. doi: 10.1038/jid.2011.371. [DOI] [PubMed] [Google Scholar]
- Homey B., Alenius H., Müller A., Soto H., Bowman E.P., Yuan W., et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Homey B., Dieu-Nosjean M.C., Wiesenborn A., Massacrier C., Pin J.J., Oldham E., et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- Hudak S., Hagen M., Liu Y., Catron D., Oldham E., McEvoy L.M., et al. Immune surveillance and effector functions of CCR10(+) skin homing T cells. J Immunol. 2002;169:1189–1196. doi: 10.4049/jimmunol.169.3.1189. [DOI] [PubMed] [Google Scholar]
- Johansen C., Rittig A.H., Mose M., Bertelsen T., Weimar I., Nielsen J., et al. STAT2 is involved in the pathogenesis of psoriasis by promoting CXCL11 and CCL5 production by keratinocytes. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S., Rizzo H.L., Lee J.J., Koguchi Y., Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma T., Nakamura K., Wakugawa M., Mitsui H., Tada Y., Saeki H., et al. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- Kakinuma T., Saeki H., Tsunemi Y., Fujita H., Asano N., Mitsui H., et al. Increased serum cutaneous T cell-attracting chemokine (CCL27) levels in patients with atopic dermatitis and psoriasis vulgaris. J Allergy Clin Immunol. 2003;111:592–597. doi: 10.1067/mai.2003.114. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Kamata M., Shimizu T., Nagata M., Fukaya S., Hayashi K., et al. Thymus and activation-regulated chemokine (TARC) in patients with psoriasis: increased serum TARC levels in patients with generalized pustular psoriasis. J Dermatol. 2020;47:1149–1156. doi: 10.1111/1346-8138.15511. [DOI] [PubMed] [Google Scholar]
- Kim H.O., Cho S.I., Chung B.Y., Ahn H.K., Park C.W., Lee C.H. Expression of CCL1 and CCL18 in atopic dermatitis and psoriasis. Clin Exp Dermatol. 2012;37:521–526. doi: 10.1111/j.1365-2230.2011.04295.x. [DOI] [PubMed] [Google Scholar]
- Kubo R., Muramatsu S., Sagawa Y., Saito C., Kasuya S., Nishioka A., et al. Bath-PUVA therapy improves impaired resting regulatory T cells and increases activated regulatory T cells in psoriasis. J Dermatol Sci. 2017;86:46–53. doi: 10.1016/j.jdermsci.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Li C., Xu M., Coyne J., Wang W.B., Davila M.L., Wang Y., et al. Psoriasis-associated impairment of CCL27/CCR10-derived regulation leads to IL-17A/IL-22-producing skin T-cell overactivation. J Allergy Clin Immunol. 2021;147:759–763.e9. doi: 10.1016/j.jaci.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.W., Lee J., Hillsamer P., Kim C.H. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- Lonsdorf A.S., Hwang S.T., Enk A.H. Chemokine receptors in T-cell-mediated diseases of the skin. J Invest Dermatol. 2009;129:2552–2566. doi: 10.1038/jid.2009.122. [DOI] [PubMed] [Google Scholar]
- Lorscheid S., Müller A., Löffler J., Resch C., Bucher P., Kurschus F.C., et al. Keratinocyte-derived IκBζ drives psoriasis and associated systemic inflammation. JCI Insight. 2019;4 doi: 10.1172/jci.insight.130835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes M.A., Kikuchi T., Fuentes-Duculan J., Cardinale I., Zaba L.C., Haider A.S., et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Mabuchi T., Chang T.W., Quinter S., Hwang S.T. Chemokine receptors in the pathogenesis and therapy of psoriasis. J Dermatol Sci. 2012;65:4–11. doi: 10.1016/j.jdermsci.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Meinhardt M., Krebs R., Anders A., Heinrich U., Tronnier H. Wavelength-dependent penetration depths of ultraviolet radiation in human skin. J Biomed Opt. 2008;13:044030. doi: 10.1117/1.2957970. [DOI] [PubMed] [Google Scholar]
- Mitsui H., Suárez-Fariñas M., Belkin D.A., Levenkova N., Fuentes-Duculan J., Coats I., et al. Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J Invest Dermatol. 2012;132:1615–1626. doi: 10.1038/jid.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles K.E., Zaba L.C., Shemer A., Fuentes-Duculan J., Cardinale I., Kikuchi T., et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252.e2. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C., Cho M.K., Shao Y., Mianecki L.E., Liao E., Perry D., et al. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell. 2015;6:890–903. doi: 10.1007/s13238-015-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis J.L., Johansen C., Vestergaard C., Bech R., Kragballe K., Iversen L. Kinetics and differential expression of the skin-related chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Exp Dermatol. 2011;20:789–794. doi: 10.1111/j.1600-0625.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- Rottman J.B., Smith T.L., Ganley K.G., Kikuchi T., Krueger J.G. Potential role of the chemokine receptors CXCR3, CCR4, and the integrin alphaEbeta7 in the pathogenesis of psoriasis vulgaris. Lab Invest. 2001;81:335–347. doi: 10.1038/labinvest.3780242. [DOI] [PubMed] [Google Scholar]
- Saito C., Maeda A., Morita A. Bath-PUVA therapy induces circulating regulatory T cells in patients with psoriasis. J Dermatol Sci. 2009;53:231–233. doi: 10.1016/j.jdermsci.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Schaerli P., Ebert L., Willimann K., Blaser A., Roos R.S., Loetscher P., et al. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199:1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya T., Honma M., Iinuma S., Iwasaki T., Takahashi H., Ishida-Yamamoto A. Alteration of serum thymus and activation-regulated chemokine level during biologic therapy for psoriasis: possibility as a marker reflecting favorable response to anti-interleukin-17A agents. J Dermatol. 2018;45:710–714. doi: 10.1111/1346-8138.14308. [DOI] [PubMed] [Google Scholar]
- Steffen S., Abraham S., Herbig M., Schmidt F., Blau K., Meisterfeld S., et al. Toll-like receptor-mediated upregulation of CXCL16 in psoriasis orchestrates neutrophil activation. J Invest Dermatol. 2018;138:344–354. doi: 10.1016/j.jid.2017.08.041. [DOI] [PubMed] [Google Scholar]
- Takei-Taniguchi R., Imai Y., Ishikawa C., Sakaguchi Y., Nakagawa N., Tsuda T., et al. Interleukin-17- and protease-activated receptor 2-mediated production of CXCL1 and CXCL8 modulated by cyclosporine A, vitamin D 3 and glucocorticoids in human keratinocytes. J Dermatol. 2012;39:625–631. doi: 10.1111/j.1346-8138.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard C., Bang K., Gesser B., Yoneyama H., Matsushima K., Larsen C.G. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhou X., Krueger J.G., Kao M.C., Lee E., Du F., Menter A., et al. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol Genomics. 2003;13:69–78. doi: 10.1152/physiolgenomics.00157.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during this study.