Abstract
Hidradenitis suppurativa (HS) is an inflammatory disease of the skin with a chronic, relapsing-remitting course. The pathogenesis of the disease is poorly understood and involves multiple factors, including genetics, environment, host-microbe interactions, and immune dysregulation. In particular, the composition of the cutaneous microbiome shifts as the disease progresses, although it is unclear whether this is a primary or secondary process. Trials with immunomodulatory therapy elucidate the role of specific immune pathways and cytokine signaling in disease mechanism, such as TNF-α, IL-1β, IL-12, IL-17, IL-23, and complement. Future studies should continue examining the causes of and contributing factors to microbial changes and immune dysregulation in HS pathogenesis.
Abbreviations: AMP, antimicrobial peptide; BD, β-defensin; BMI, body mass index; DC, dendritic cell; DCD, dermcidin; GSC, γ-secretase complex; HiSCR, hidradenitis suppurativa clinical response; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; IHS4, International Hidradenitis Suppurativa Severity Score System; iNOS, inducible nitric oxide synthase; KC, keratinocyte; MMP, matrix metalloproteinase; NET, neutrophil extracellular traps; NMSC, nonmelanoma skin cancer; pDC, plasmacytoid dendritic cell; PG, pyoderma gangrenosum; RCT, randomized controlled trial; SAPHO, synovitis, acne, pustulosis, hyperostosis, and osteitis; Th, T helper type; TLR, toll-like receptor
Introduction
Hidradenitis suppurativa (HS, also called acne inversa) is a chronic inflammatory disorder of the skin. The disease is characterized by painful nodules and abscesses in areas with high hair follicle and sweat gland density, such as the axilla and groin (Coates et al., 2019). These debilitating features can significantly impair QOL and cause psychosocial distress, increasing risk of mood disorders and completed suicide (Anzaldi et al., 2020; Thorlacius et al., 2018a). The prevalence ranges from 0.053% in US studies to as high as 4.1% in European studies, although the latter consisted of relatively small sample sizes (Cosmatos et al., 2013; Miller et al., 2016). The incidence of HS in the US appears to be increasing from 4.3 per 100,000 between 1970 and 1979 to 9.6 per 100,000 from 2000 to 2008 (Vazquez et al., 2013).
The pathogenesis of the disease is complex and multifactorial. This review aims to explore the relationships of risk factors, associated conditions, and mechanisms of cutaneous innate immunology and microbe-host interactions. Furthermore, we review current therapies of HS, with particular focus on drug innovations that reveal mechanisms of immunologic pathophysiology.
Staging
Multiple scoring systems exist to assess the severity of HS disease. Perhaps the most well-known is the Hurley staging system, which consists of three stages. Briefly, stage I is characterized by abscess formation without scarring or sinus tracts, stage II by recurrent abscess(es) with scarring and sinus tract formation, and stage III by diffuse involvement with interconnected sinus tracts and abscesses spanning an entire area (Hurley, 1989).
Various other staging systems exist, including the HS Sartorius score, Physician Global Assessment, and International Hidradenitis Suppurativa Severity Score System (IHS4), among others (Constantinou et al., 2019). It is important to consider the goals of assessment when choosing which scoring system to employ. HS clinical response (HiSCR) has been used in several clinical trials recently and is defined by at least a 50% reduction in abscess and inflammatory nodule count with no increase in abscess and fistula count compared with baseline (Kimball et al., 2016b).
An important potential limitation of using HiSCR to assess clinical efficacy of immunomodulatory therapy is the incomplete understanding of spontaneous progression and remission of disease. Relatively high placebo response rates, sometimes up to 30%, limit interpretation of clinical trial results as there are limited data on placebo-controlled blinded studies (Frew et al., 2019). In addition, HiSCR predominantly assesses the more acute changes of HS, whereas chronic changes such as scarring and sinus tracts are not well captured by this metric. Studies with larger cohorts and subgroup analyses are needed to better understand the effects of immunomodulation on HS when employing HiSCR.
Notably, current staging systems do not account for microbiota composition or dysregulation of immune pathways, which may differ between patients with clinically similar disease. Thus, continued research may augment future staging systems with personalized data regarding each patient’s microbiome and lesional immune profile to choose the optimal personalized treatment regimen.
Risk Factors and Associations
Patient characteristics
Women are more frequently affected by HS, with a female-to-male ratio of 3:1 (Jemec, 2012). Although this disparity may suggest a role for sex hormones in HS pathogenesis, hormone levels of patients with HS appear to lie within normal ranges (Riis et al., 2016b). Symptoms of HS tend to worsen during menses and improve during pregnancy (Vossen et al., 2017).
HS disease has a notable increased prevalence and more severe symptoms in African American and Hispanic populations (Lee et al., 2017; Vlassova et al., 2015). Rigorous and in-depth investigations, including genome-wide association studies and genetic and environmental risk assessments, are needed to better understand the reasons for these disparities. Notably, active smokers are 1.9–14.87 times more likely to be diagnosed with HS than nonsmokers (Garg et al., 2020; Schrader et al., 2014). Former smokers are at a 1.14–1.55 times increased risk of HS (Revuz et al., 2008; Schrader et al., 2014). Studies have demonstrated an increased risk of Hurley stage II or III disease in smokers compared with nonsmokers with HS (Sartorius et al., 2009; Schrader et al., 2014; Vazquez et al., 2013). Smoking is associated with significantly lower rates of disease remission (Kromann et al., 2014). Patients with obesity (body mass index [BMI] ≥ 30 kg/m2) are 2–6 times more likely to be diagnosed with HS (Gold et al., 2014; Sabat et al., 2012). A study suggested that obesity may contribute to HS severity through proinflammatory cytokine production by adipose cells in addition to contributing to ongoing skin breakdown and maceration in the large skin folds, which can trigger inflammation as well (Miller et al., 2016).
There exists an association between metabolic syndrome, a cluster of cardiometabolic conditions including obesity, insulin resistance, hypertension, and lipid abnormalities, and HS (Gold et al., 2014; Sabat et al., 2012). HS is also associated with a significantly increased risk for adverse cardiovascular outcomes and cardiovascular-associated death (Egeberg et al., 2016). Mechanistically, the signaling peptide mTOR is persistently elevated in metabolic syndrome (Das et al., 2017). Likewise, mTOR is increased in the skin of patients with HS, and its gene expression has been shown to correlate with HS severity (Monfrecola et al., 2016). Adipokines are cytokines secreted by adipose tissue whose levels are altered in metabolic syndrome (Francisco et al., 2019). Two recent studies found decreased levels of the anti-inflammatory adipokine adiponectin and increased levels of the proinflammatory adipokines leptin, resistin, and visfatin in patients with HS (González-López et al., 2020; Malara et al., 2018). Because these studies did not identify an association between adipokine expression and disease severity, more research is needed to understand how adipokines relate to HS susceptibility (González-López et al., 2020; Malara et al., 2018).
Genetics
Approximately 34–36% of patients report an affected family member with HS (Canoui-Poitrine et al., 2013). Studies have identified loss-of-function mutations in the PSENEN, NCSTN, and PSEN1 genes, which encode for components of the γ-secretase complex (GSC) (Miskinyte et al., 2012; Pink et al., 2012; Wang et al., 2010). GSC is an intramembranous protease that targets type I transmembrane proteins for cleavage (Pink et al., 2012). An important target of GSC is Notch. The GSC cleaves Notch, resulting in nuclear translocation of the intracellular domain, which affects the expression of factors regulating hair follicle and epidermal cell proliferation (Melnik and Plewig, 2013a). Although impaired Notch signaling is postulated to drive HS pathogenesis, a recent study identified elevated expression of NCSTN, Notch, and their downstream target, PI3K/protein kinase B, in HS lesional skin (Hessam et al., 2020). Because GSC mutations are present in only a minority of patients with familial HS, perhaps loss of Notch may only drive HS pathogenesis in this small subset of patients (Liu et al., 2016; Pink et al., 2012). It is currently unknown which genetic mutations underlie familial HS in patients with absent GSC mutations and how transcriptomic regulation of GSC may affect HS severity (Liu et al., 2016; Pink et al., 2012).
Various studies have identified additional genetic contributors to HS. Increased susceptibility to HS is associated with mutations in the TNF gene promoter region and, recently, the MEFV gene (Savva et al., 2013; Vural et al., 2019). SNPs of the IL12RB1 gene are associated with severity and onset of disease (Giatrakos et al., 2013). There is an increased prevalence of trisomy 21 in patients with HS, and mean age of HS symptom onset occurs earlier in this patient population (Denny and Anadkat, 2016). More studies are needed to elucidate the major loci of genetic susceptibility to HS.
Other associations
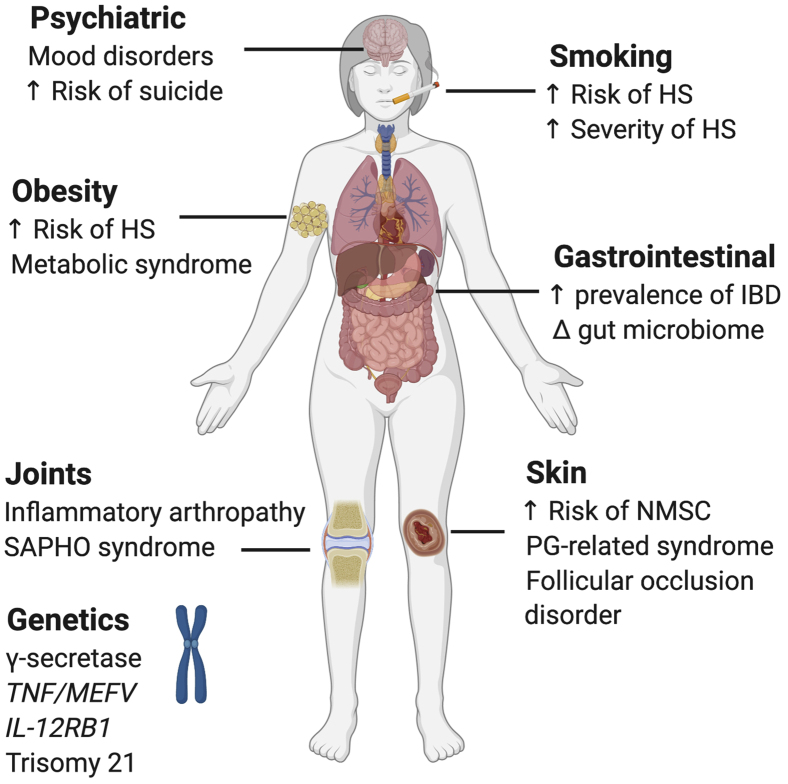
HS is associated with various inflammatory diseases (Figure 1). Inflammatory bowel disease (IBD) frequently co-occurs with HS. One study found the prevalence of HS among patients with Crohn disease and ulcerative colitis to be 26% and 18%, respectively (van der Zee et al., 2014). Pyoderma gangrenosum (PG) is a neutrophil-predominant dermatosis characterized by cutaneous nodules or pustules that rapidly progress to painful deep ulcers. PG has been associated with HS in case reports that describe syndromes consisting of a triad of HS, PG, and acne conglobata with an additional arthritis component (Braun-Falco et al., 2012; Leuenberger et al., 2016; Marzano et al., 2013).
Figure 1.
Risk factors and conditions associated with HS. HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; NMSC, nonmelanocytic skin cancer; PG, pyoderma gangrenosum; SAPHO, synovitis, acne, pustulosis, hyperostosis, and osteitis.
Rosner et al. (1993, 1982) established an association between HS and inflammatory arthropathy in two studies, but did not find a correlation with HLA-B27. A dysregulated immune response and microbial triggers may underlie a shared pathophysiology between HS and spondyloarthropathies (Richette et al., 2014). Another rheumatologic association is synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome (Steinhoff et al., 2002). A case series found that 7 of 12 patients with SAPHO syndrome had evidence of HS (Steinhoff et al., 2002).
In addition to inflammatory disorders, HS is also associated with conditions in which follicular occlusion underlies pathogenesis, such as pilonidal sinuses, pilonidal cysts, and dissecting cellulitis (Miller et al., 2016). Other associations have been described or reported in case reports or smaller studies and are outside the scope of this review article (Kohorst et al., 2015; Miller et al., 2016).
Pathogenesis
Clinical progression of disease
The mechanism by which aberrant inflammation and changes to the cutaneous microbiome (dysbiosis) lead to disease phenotype is not entirely clear and may involve multiple factors. At a subclinical level, HS is characterized by inappropriate antimicrobial peptide (AMP) and proinflammatory cytokine production by keratinocytes (KCs) (van der Zee et al., 2012a). These KCs may be intrinsically deficient in certain inflammatory pathways, such as IL-22 production (Jones et al., 2018). IL-22 induces AMP expression, protects against tissue damage, and maintains the mucosal barrier in the intestine (Sabat and Wolk, 2015; Wang et al., 2014). Inflammatory damage drives infundibular hyperkeratosis, follicular epithelium hyperplasia, and perifolliculitis (von Laffert et al., 2011). These changes may be exacerbated by impaired Notch signaling and smoking (Hana et al., 2007; Melnik and Plewig, 2013a). An influx of T cells and innate immune cells release chemokines and additional proinflammatory cytokines (van der Zee et al., 2012a). Eventually, the hair follicles undergo plugging and enlargement, followed by cyst formation (van der Zee et al., 2012a).
Clinical HS becomes appreciable after rupture of the enlarged hair follicle or cyst and spillage of contents, such as bacteria and keratin. These are released from the pilosebaceous unit to the dermis and trigger a neutrophilic foreign body reaction (van der Zee et al., 2012a). An influx of immune cells and histiocytes ensues, forming multinucleated giant cells that phagocytose the free keratin (van der Zee et al., 2012a). Proliferation of residual KCs and follicular epithelial strands drives chronic inflammation and formation of sinus tracts and fistulae (van der Zee et al., 2012a). Bacterial colonies harbored in these tracts may form biofilm that irreversibly binds to hair follicles and sinus tract epithelium, propagating chronic inflammation (Kathju et al., 2012). Long-standing HS lesions are characterized by B-cell and plasma cell signatures, implicating that later adaptive immune responses may dominate chronic and possibly more severe HS lesions (Gudjonsson et al., 2020).
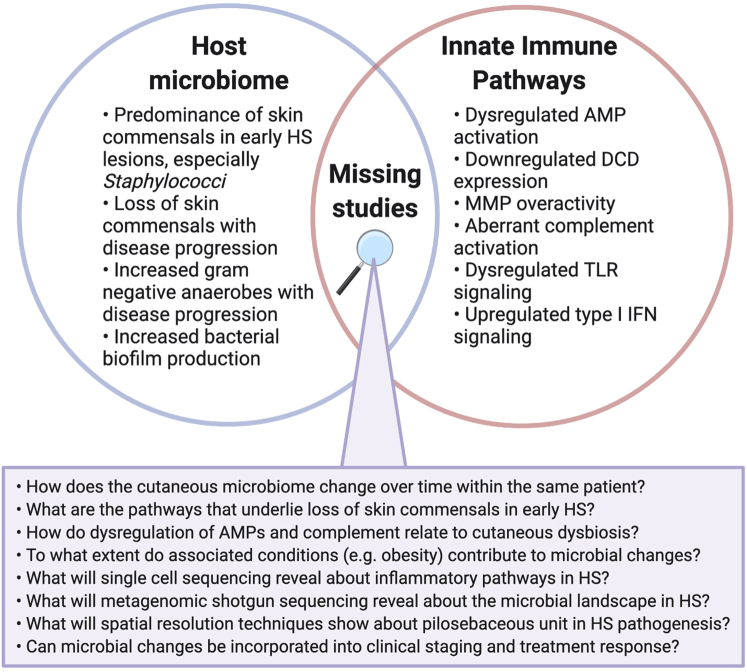
Because HS is often a chronic relapsing-remitting disease, large longitudinal studies examining the flux in dysbiosis and immune dysregulation over time may provide insight into the initiating and sustaining events that characterize HS (Figure 2). A rare complication of chronic HS is the development of nonmelanoma skin cancer (NMSC), which typically occurs 20–30 years after the diagnosis of HS (Lapins et al., 2001; Losanoff et al., 2011). Patients with HS have a 4.6-fold greater risk of developing NMSC, especially in the perianal, perineal, and gluteal regions (Lapins et al., 2001; Losanoff et al., 2011).
Figure 2.
Missing studies at the intersection of cutaneous dysbiosis and innate immunity in HS pathogenesis. AMP, antimicrobial peptide; DCD, dermicidin; HS, hidradenitis suppurativa; MMP, matrix metalloproteinase; TLR, toll-like receptor.
Cutaneous microbiome
The role of dysbiosis in the pathogenesis of HS remains unclear. Many studies of bacterial cultures harvested from HS skin and deep lesions grew normal cutaneous flora. Staphylococcus aureus, coagulase-negative Staphylococci, and Peptostreptococcus spp. are among the most common organisms identified (Brook and Frazier, 1999; Lapins et al., 1999; Matusiak et al., 2014a; Sartorius et al., 2012) (Table 1). Nicotine alters the skin microbiome by inhibiting the growth of various commensals except S. aureus (Pavia et al., 2000). However, traditional methods of bacterial culture may only reflect a small proportion of the cutaneous microbiome—possibly less than 1% (Grice et al., 2008).
Table 1.
Changes in Relative Microbial Abundance Observed in HS Lesional and Nonlesional Skin
| Microbe | Change | Sample | Stage | Method | Reference |
|---|---|---|---|---|---|
| Fusobacterium | Increased | L a, g, gc, o versus NS a, g, gc | I/II/III | 16S and 18S rRNA | (Guet-Revillet et al., 2017; Naik et al., 2020) |
| Increased | L a, g | III | 16S and 18S rRNA | (Ring et al., 2019) | |
| Porphyromonas | Increased | L a, g, gc, o | I/II/III | Culture and 16S rRNA | (Brook and Frazier, 1999; Guet-Revillet et al., 2014) |
| Increased | L a, g, gc, o versus NS a, g, gc | I/II/III | 16S and 18S rRNA | (Guet-Revillet et al., 2017; Ring et al., 2017b) | |
| Increased | L a, g | III | 16S and 18S rRNA | (Ring et al., 2019) | |
| Prevotella | Increased | L a, g, gc, o | I/II/III | Culture and 16S rRNA | (Brook and Frazier, 1999; Guet-Revillet et al., 2014; Lapins et al., 1999) |
| Increased | L a, g, gc, o versus NS a, g, gc | I/II/III | 16S and 18S rRNA | (Guet-Revillet et al., 2017; Ring et al., 2017b) | |
| Increased | L a, g | III | 16S and 18S rRNA | (Ring et al., 2019) | |
| Increased | NL a, g, gc versus NS a, g, gc | I/II/III | 16S rRNA | (Riverain-Gillet et al., 2020) | |
| Staphylococcus aureus | Increased | L a, g, gc, o | I/II/III | Culture and 16S rRNA | (Brook and Frazier, 1999; Guet-Revillet et al., 2014; Lapins et al., 1999; Matusiak et al., 2014a) |
| Corynebacterium | Increased | L a, g, gc, o versus NS a, g, gc | I/II/III | 16S and 18S rRNA | (Guet-Revillet et al., 2017; Ring et al., 2017b) |
| Increased | L and NL a, g versus NS a, g | I/II/III | 16S rRNA | (Schneider et al., 2020) | |
| Decreased | L a, g, gc, o versus NS a, g, gc | III | 16S rRNA | (Naik et al., 2020) | |
| Coagulase-negative Staphylococcus |
Increased | L a, g | I/II/III | Culture | (Lapins et al., 1999; Matusiak et al., 2014a; Sartorius et al., 2012) |
| Decreased1 | L a, g, gc, o versus NS a, g, gc | III | 16S rRNA | (Naik et al., 2020) | |
| Decreased | NL a, g, gc versus NS a, g, gc | I/II/III | 16S rRNA | (Riverain-Gillet et al., 2020) | |
| Cutibacterium | Decreased | L a, g, gc, o versus NS a, g, gc | I/II/III | 16S and 18S rRNA | (Guet-Revillet et al., 2017; Naik et al., 2020; Ring et al., 2017b) |
| Decreased | L a, g | III | 16S and 18S rRNA | (Ring et al., 2019) | |
| Decreased | L and NL a, g versus NS a, g | I/II/III | 16S rRNA | (Schneider et al., 2020) |
Abbreviations: a, axilla; g, groin; gc, gluteal cleft; HS, hidradenitis suppurativa; L, lesional skin; NL, nonlesional skin; NS, normal skin from healthy donor; o, other sites; rRNA, ribosomal RNA.
Major microbes with changes in relative abundance across multiple studies are included in the table. Stage reported as Hurley stage of patient according to study. 16S and 18S rRNA denote sequencing of the 16S and 18S bacterial rRNA.
This study reports commensal Staphylococcus but does not distinguish lower classifications.
More recent studies have utilized metagenomic sequencing to better characterize the cutaneous microbiome (Table 1). Although these studies have similarly identified the predominance of Staphylococcus in early HS lesions, gram-negative anaerobes such as Prevotella and Porphyromonas are predominantly found in chronic suppurating lesions and sinus tracts, hallmarks of moderate to severe disease (Guet-Revillet et al., 2017, 2014; Ring et al., 2019, 2017b).
Despite these advances in understanding bacterial involvement, it remains unknown whether cutaneous dysbiosis plays a primary or secondary role in the development of HS. The predominance of skin commensals in early HS lesions suggests that primary infection is unlikely to be driving pathogenesis (Lapins et al., 1999; Sartorius et al., 2012). Fistulas and sinus tracts encourage formation of bacterial biofilm, which is absent in acute HS lesions but found in a majority of chronic lesions (Okoye et al., 2017; Ring et al., 2017a). Tobacco smoke augments biofilm formation in pathogens such as S. aureus (Hutcherson et al., 2015).
Schneider et al. (2020) analyzed the differentially activated metabolic pathways of microbiota sampled from HS skin. Microbial metabolic differences between HS and normal skin may reflect dysbiosis; for instance, significant reduction of the skin commensal Cutibacterium in HS may account for decreased propionate and retinol metabolism (Schneider et al., 2020). Propionic acid possesses antimicrobial properties that inhibit growth of opportunistic pathogens (Shu et al., 2013). Another metabolomic study found bacterial cell growth and division processes, such as DNA replication and repair, to be strongly associated with HS lesions (Ring et al., 2020). Perhaps the decreased abundance of critical skin commensals such as Cutibacterium permits growth and division of pathogenic bacteria. More research is needed to confirm the directionality of this relationship and pinpoint the mechanisms by which loss of commensal skin organisms occurs, especially in early stages of disease. Further investigation of the HS bacterial metabolome may permit exploration of treatments such as probiotics and microbiome transplants.
Complicating the role of cutaneous dysbiosis in disease pathogenesis is the question of whether the inflammation observed in HS is simply an appropriate response to an overabundance or displacement of microbiota or, rather, an aberrant immune response to cutaneous flora (Coates et al., 2019). Researching nonlesional HS skin, which possesses dysregulated cytokine expression, may illuminate ongoing questions about microbial composition (Kelly et al., 2015). A recent study found that clinically unaffected skinfolds of patients with HS demonstrate a similar abundance of anaerobes such as Prevotella and reduction of skin commensals to lesional skin (Riverain-Gillet et al., 2020). Perilesional skin is also immunologically dysregulated, and more studies are needed to characterize its microbiome (Kelly et al., 2015).
Architectural disruptions to the normal structure of the epidermis, dermis, and adnexa are a hallmark of HS. The influence of these structural changes on the skin microbiome remains unclear. One study found that the relative abundance of mixed anaerobes positively correlates with Hurley stage, whereas abundance of skin commensals such as resident Staphylococci, Cutibacterium, and Corynebacterium spp. negatively correlates with Hurley stage (Naik et al., 2020). This may indicate that an altered skin architecture relates to microbial changes, because sinus tracts are found in severe HS and characterized by subcutaneous cavities filled with gelatinous material (Ring et al., 2019). Observational studies in patients with HS across different Hurley stages is complicated by confounding factors, such as disease chronicity and treatment regimens, that may directly influence the microbiome. Future studies should examine the skin microbiota in structurally distinct HS lesions, such as abscesses and sinus tracts, within the same patients.
Another limitation to understanding the cutaneous microbiota in HS is the relative contribution of risk factors and associated conditions to microbial changes. Indeed, smoking, obesity, and diabetes are all associated with unique variations in cutaneous flora (Brandwein et al., 2019; Redel et al., 2013; Thompson et al., 2020). S. aureus is found in higher abundance on the foot skin of patients with diabetes, and Corynebacterium spp. are correlated with smoking status and higher BMI, microbial changes that may overlap with those observed in patients with HS (Brandwein et al., 2019; Redel et al., 2013; Thompson et al., 2020). Diabetic foot ulcers in particular demonstrate increased abundance of gram-negative anaerobes and formation of biofilm (Jneid et al., 2017). However, comparisons between cutaneous microbiota of other conditions and HS are limited by heterogeneity of sampling sites and analysis methods.
To date, there are no longitudinal metagenomic studies that examine the cutaneous microbiota of HS lesions within the same patients over time. Given the relapsing and remitting nature of HS, sampling during disease flares and periods of quiescence may elucidate fluctuations of microbiota and identify dysbiosis predictive of HS disease flares. Additionally, there are currently no studies that have performed metagenomic shotgun sequencing of HS lesional skin. This approach would allow for an unbiased examination of the complete HS microbial landscape, including organisms such as viruses and fungi.
Capturing the dynamics of microbial changes in HS may inform research on how therapeutics target underlying microbial pathology. In particular, patients receiving antimicrobial and/or immunologic therapy may undergo pronounced alterations of the cutaneous and gastrointestinal microbiome, and the latter itself is associated with inflammatory skin disease (Eppinga et al., 2016; Szántó et al., 2019). Recent studies have begun to explore a potential skin-gut axis in HS; more research comparing the altered cutaneous and gut microbiota in patients with HS is needed (Hispán et al., 2020; Lam et al., 2019).
Innate immune pathways
HS is characterized by aberrant activation of the innate immune system, resulting in release of various molecules and upregulation of pathways that function to protect against pathogenic invasion. In the normal innate immune response, AMPs are secreted by KCs and immune cells to kill invasive pathogens and limit inflammation (Coates et al., 2018). Studies have identified increased transcript levels of the AMPs β-defensin (BD) 1 to 3 and S100A7 to A9 in HS skin lesions compared with normal skin (Hofmann et al., 2012; Schlapbach et al., 2009; Wolk et al., 2011). Others have found downregulation of the AMPs BD2 and BD4 in HS lesions relative to normal skin (Dréno et al., 2012; Mozeika et al., 2013). Although Hofmann et al. (2012) demonstrated upregulated BD3 in early HS lesions, they did not identify this increase in Hurley stage III lesions. This relative deficiency of AMP expression may predispose to bacterial infections in severe HS, although there are limited studies on the change in AMP levels with disease progression (Hofmann et al., 2012). Furthermore, the expression profiles of individual AMPs should be clarified.
Recent transcriptome analyses from our group reported genes associated with sweat gland function, including secretoglobins, aquaporin 5, and dermcidin (DCD), to be highly downregulated in HS lesions, whereas S100A8, S100A7, S100A7A, BD2, and other inflammatory factors were upregulated (Coates et al., 2019). DCD, one of the most downregulated genes identified, is an AMP produced by eccrine sweat glands. Its reduction may signify both diminished secretion and an overall decrease in eccrine sweat glands, indicating a perturbation in skin architecture (Coates et al., 2019). It is currently unknown whether the dysregulation of AMP levels, in particular the decrease in DCD, is causative of or reactive to cutaneous dysbiosis. Future studies should clarify the directionality of this relationship and identify pathways through which this is mediated.
Transcriptome analyses of inflammatory pathways in HS skin found upregulation of several genes that encode for molecules in the IFN signaling pathway (Coates et al., 2019; Gudjonsson et al., 2020; Shanmugam et al., 2019). Byrd et al. (2019) demonstrated that these upregulated genes are involved in the type I IFN (α/β) pathway. The authors found the presence of neutrophil extracellular traps (NETs) and plasmacytoid dendritic cells (pDCs) localized to these same skin lesions, postulating that NETs activate pDCs to secrete IFN-α (Byrd et al., 2019). Another recent study corroborated the upregulation of type I IFN responses using single-cell RNA sequencing of lesional skin (Gudjonsson et al., 2020). Relating back to genetics, a recent study found that type I IFN activation and NF-κB signaling are increased in NCSTN knockdown cell lines (Cao et al., 2019). This may indicate enhanced inflammatory responsiveness in individuals haploinsufficient for NCSTN, such as patients with familial HS with GSC mutations (Cao et al., 2019). Future studies should examine if and how GSC mutations regulate type I IFN pathways.
Although limited, data suggest that matrix metalloproteinases (MMPs) contribute to HS pathogenesis. MMPs are produced by activated fibroblasts to degrade the extracellular matrix and induce tissue destruction (Sanchez et al., 2019). MMP-2, MMP-8, and MMP-9 are elevated in the lesional skin of patients with HS (Mozeika et al., 2013; Sanchez et al., 2019; Tsaousi et al., 2016). MMP-8 may offer another mechanistic link to metabolic pathways, as its serum levels negatively correlate with high-density lipoprotein cholesterol, an antiatherogenic lipid, and positively correlate with resistin, a proatherogenic hormone (Tsaousi et al., 2016). A recent ex vivo HS model demonstrated that high levels of MMP-2 and MMP-9 are associated with pathogenic matrix remodeling and elevation of IL-1β but not IL-17, NLRP3, or caspase-1 (Sanchez et al., 2019).
Aberrant complement activation also underlies the innate immune response in HS pathogenesis. Patients with HS have higher serum levels of C5a and C5b-9, and these levels correlate with disease severity (Kanni et al., 2018b). C5a stimulates the production of TNF-α by PBMCs in vitro (Kanni et al., 2018b). A transcriptome and proteome analysis of HS skin and blood also found elevated C5a but reduced C3, C4, and iC3b (Hoffman et al., 2018). Given the neutrophilic infiltrate in HS lesions and peripheral blood, it is possible that progressive dysbiosis drives complement dysregulation or vice versa (Byrd et al., 2019; Hoffman et al., 2018). The role of complement in modulating the skin microbiome in previous studies raises the question of how cutaneous dysbiosis and aberrant complement activation are related in HS (Chehoud et al., 2013).
Expression of toll-like receptors (TLRs), a class of pattern recognition receptors, has been investigated with mixed results and may not fully recapitulate TLR activation or function. Hunger et al. (2008) identified elevated mRNA and protein levels of TLR2 in HS skin lesions. A later study found downregulation of TLR2–4, TLR7, and TLR9 in lesional skin and nonlesional skin, suggesting a deficient innate immune response (Dréno et al., 2012). TLR signaling is modulated by Notch, which regulates proinflammatory patterns of gene expression through NF-κB (Palaga et al., 2008). Cigarette smoke and GSC mutations may potentiate defective Notch signaling, promoting disease development through proinflammatory responses (Melnik and Plewig, 2013a, 2013b). Impaired Notch signaling results in keratin-enriched epidermal cyst formation and impaired apocrine gland homeostasis (Melnik and Plewig, 2013a, 2013b). It may also reduce IL-22 secretion from T cells, although IL-22 production is controlled by additional signaling pathways (Alam et al., 2010).
Cytokine signaling
Although the exact inciting event remains unclear, a proinflammatory milieu underlies perivascular and perifollicular immune cell migration in HS (van der Zee et al., 2012a). TNF-α, a proinflammatory cytokine produced by dendritic cells (DCs) and macrophages, drives chemotaxis of neutrophils, monocytes, and T cells into the skin (Mozeika et al., 2013; van der Zee et al., 2011). Multiple studies demonstrated elevated transcript and protein expression of TNF-α in HS lesions and serum (Kelly et al., 2015; Mozeika et al., 2013; van der Zee et al., 2011). However, decreased levels of TNF-α in HS lesional and nonlesional skin and reduced TNF-α secretion from monocytes compared with healthy controls were seen in other studies (Dréno et al., 2012; Giamarellos-Bourboulis et al., 2007). Trials of anti–TNF-α therapy support the role of elevated rather than diminished TNF-α in HS pathogenesis (Ghias et al., 2020; Kimball et al., 2016a; Oskardmay et al., 2019). TNF-α signaling results in activation of mTOR, a central component of mTOR complex 1 and mTOR complex 2. Anti–TNF-α therapy reduces mTOR expression and quantities of downstream effectors of the mTOR complex 1 pathway in HS lesions (Balato et al., 2019). A small retrospective study found mTOR inhibition effective in combination with TNF-α blockade for severe HS, and future studies should continue exploring mTOR blockade (Bettuzzi et al., 2020).
Across various studies, increases in proinflammatory cytokines IL-1β, IL-17, and IL-23 are consistently observed in HS. Mature IL-1β is produced by the inflammasome, a multiprotein complex assembled through the innate immune response. IL-1β promotes the development of CD4+ T helper type (Th) 17 cells, which secrete IL-17 (Sutton et al., 2006). Stimulation of Th17 cells is enhanced by IL-23, which is produced by macrophages and DCs (Sabat et al., 2019). IL-1β, IL-17, and IL-23 are all elevated at transcript and protein levels in lesional and perilesional HS skin and Th17 cells, the predominant source of IL-17 (Kelly et al., 2015; Schlapbach et al., 2011; van der Zee et al., 2012b, 2011; Wolk et al., 2011). Within the HS lesion, IL-17 stimulates production of chemokines, cytokines, MMPs, and AMPs (Vijatov-Djuric et al., 2017; Wolk et al., 2009). IL-1β and IL-17 may employ a positive feedback loop, as IL-17 stimulates release of IL-1β from KCs via activation of the inflammasome protein NLRP3 and caspase-1 (Cho et al., 2012; Lima et al., 2016).
Subsequent to chemotaxis to the inflammatory environment, CD4+ T cells undergo differentiation to Th1 cells (Sabat et al., 2019). IL-12 is a proinflammatory cytokine elevated in HS skin lesions that is produced by macrophages and DCs and drives this differentiation (Moran et al., 2017; Sabat et al., 2019; Wolk et al., 2011). Th1 cells produce IFN-γ, which activates macrophages and traffics immune cells via upregulation of proinflammatory chemokines and adhesion molecules (Schroder et al., 2004). IFN-γ also establishes a positive feedback loop via induction of CXCR3 ligands, attracting Th1 cells to lesional skin (Van Raemdonck et al., 2015). Not all studies have confirmed elevation of IFN-γ in HS lesions, and more evidence is needed to clarify the role of the type II IFN pathway in disease mechanism (Banerjee et al., 2017; Gudjonsson et al., 2020; Moran et al., 2017).
The pathogenesis of HS also involves the anti-inflammatory cytokine IL-10, which has been found at increased levels in HS lesions (van der Zee et al., 2012b; Wolk et al., 2011). IL-10 is predominantly produced by macrophages and T lymphocytes and acts to limit proinflammatory cytokine production by macrophages and dampen T-cell activation (van der Zee et al., 2012b). It is unclear if its presence is a response to the proinflammatory environment or is exacerbating dysbiosis and inflammation because of its immunosuppressive effects (Kelly and Prens, 2016).
Additional signaling molecules with an emerging role in HS include, but are not limited to, IL-6, IL-22, IL-26, IL-32, and IL-36 (Hessam et al., 2018; Scala et al., 2019; Thomi et al., 2017a, 2017b; Xu et al., 2017). In particular, IL-22 is decreased in HS skin lesions (Hotz et al., 2016; Wolk et al., 2011). Its deficiency may be due to reduced infiltration of IL-22–secreting CD4+ T cells, which are elevated in the serum, but not skin, of patients with HS (Hotz et al., 2016). A recent study found that serum levels of the proinflammatory cytokines IL-36α, -β, and -γ are elevated in patients with HS who smoke compared with those who do not, although the study did not control for disease severity (Hayran et al., 2020). These pathways with emerging roles require more characterization in the preclinical and clinical setting.
Treatment
Lifestyle
Weight loss is recommended for management of HS. A systematic review found that weight loss intervention was associated with decreased HS severity in nine observational studies (Sivanand et al., 2020). A recent crossover study found that weight loss is not associated with a change in IHS4, although this study was limited by small sample size and lack of controls (Damiani et al., 2019). Prospective randomized controlled trials (RCTs) are needed to elucidate the effects of weight loss on HS disease course.
Current guidelines suggest evaluation of smoking status and promotion of smoking cessation in patients with HS (Alikhan et al., 2019; Ingram et al., 2019). Despite these recommendations, there are no RCTs for this intervention. Simonart (2010) described two cases of disease remission in patients who underwent tobacco cessation.
Antimicrobials
North American and British guidelines recommend antimicrobials for various stages of disease (Alikhan et al., 2019; Ingram et al., 2019). For mild disease, clindamycin may be used as topical therapy for HS as it has demonstrated benefit versus placebo in RCTs (Clemmensen, 1983; Jemec and Wendelboe, 1998). In combination with rifampicin, oral clindamycin is a well-established treatment utilized in cases of tetracycline failure (Alikhan et al., 2019; Gener et al., 2009). Rifampicin possesses potent activity against bacterial biofilm, in contrast to clindamycin (Mandell et al., 2019; Zimmerli and Sendi, 2019). These therapies can also be immunomodulatory by altering NF-κB, activator protein 1, and inducible nitric oxide synthase (iNOS) expression (Frew et al., 2019; Yuhas et al., 2006). Systemic tetracyclines are recommended as monotherapy in Hurley stage I and II disease (Alikhan et al., 2019; Ingram et al., 2019; Zouboulis et al., 2019). Doxycycline in particular has demonstrated efficacy at killing S. aureus biofilms (Mandell et al., 2019). Immunomodulatory effects of tetracyclines include reduction of IL-1, IL-6, IL-8, and TNF-α; downregulation of neutrophil chemotaxis; and suppression of MMP activity and NF-κB signaling (Schmidt et al., 2018; Sun et al., 2015).
The combination of systemic moxifloxacin, metronidazole, and rifampicin is effective for HS refractory to other treatments (Join-Lambert et al., 2011). Moxifloxacin is a fluoroquinolone that reduces IL-1β, IL-8, IL-17A, and TNF-α; stabilizes IXb protein; and inhibits NF-κB signaling (Choi et al., 2003; Weiss et al., 2004). Metronidazole results in alterations of the gastrointestinal microbiome and glucose metabolism (Rodrigues et al., 2017). As IBD and metabolic syndrome are associated with HS, metronidazole may exert indirect anti-inflammatory effects through these changes.
Carbapenems and linezolid are effective for Hurley stage III HS as a 6-week course (Alikhan et al., 2019; Chahine et al., 2018; Scheinfeld, 2015). Both drugs possess anti-inflammatory effects, including reduction of IL-6, IL-12, and TNF-α and inhibition of phagocytosis (Bode et al., 2015; Chahine et al., 2018). Similar to metronidazole, these antibiotics are also associated with changes in the gut microbiome and may indirectly alter disease activity (Dubourg et al., 2014).
Antimicrobial therapy is limited by the relative uncertainty of how much treatment response derives from antibacterial versus anti-inflammatory activity. As such, culture of HS skin lesions for targeted antibiotic therapy is not recommended unless there are signs of secondary infection (Alikhan et al., 2019). Given the high rates of antibiotic resistance, especially among tetracyclines and clindamycin, antimicrobials may primarily manage disease by countering inflammation, at least after prolonged use (Bettoli et al., 2019; Fischer et al., 2017). Antibacterial activity may play a prominent role in limiting progression of early HS, whereas in later stages it may decrease microbial trigger for inflammation and eliminate bacterial biofilm (Bettoli et al., 2019; Fischer et al., 2017). Further studies should examine the antibacterial resistance that may emerge with dual or triple therapy and the effects of gut microbiome changes on HS disease course. Additionally, more randomized prospective studies are needed to guide antimicrobial recommendations, given that current knowledge is mostly limited to retrospective data.
Immunomodulatory drugs
In contrast to antimicrobial drugs, immunologic drugs are directed solely toward immunomodulation. Systemic immunosuppression through small molecule drugs yields mixed results; open-label trials and prospective studies involving methotrexate, cyclosporine, and acitretin demonstrate varying degrees of success or are confounded by use of other concurrent therapies (Anderson et al., 2016; Bianchi et al., 2012; Jemec, 2002; Matusiak et al., 2014b; Tan et al., 2017).
Apremilast is a promising small molecule immunomodulator for management of moderate to severe HS, as a recent RCT with 20 patients found 53% of the treatment arm achieving HiSCR at 16 weeks (Vossen et al., 2019; Weber et al., 2017). This drug is a phosphodiesterase 4 inhibitor that regulates inflammatory mediators, such as TNF-α, IL-10, IL-23, and iNOS (Schafer, 2012).
The efficacy of biologic drugs in inflammatory skin disorders is well documented and may serve as promising treatment for HS (Baker and Isaacs, 2018). Their targeted binding to one or two molecules helps illuminate the roles of different inflammatory pathways in HS pathogenesis based on differential responses to treatment when used empirically and also highlights the heterogeneity of the disease (Figure 3).
Figure 3.
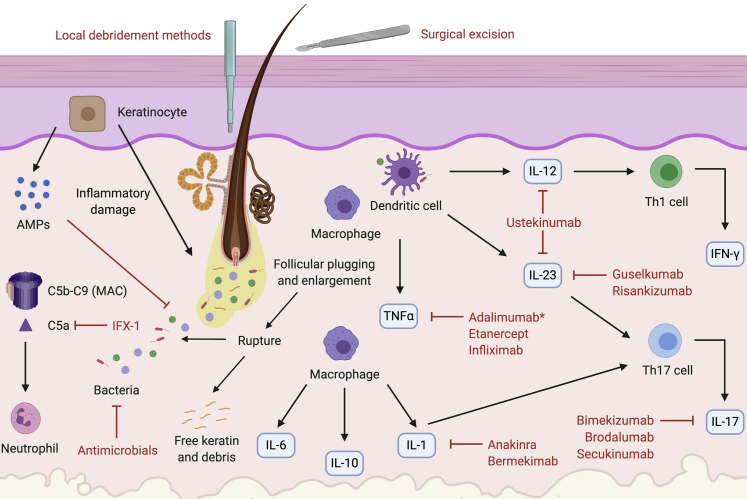
Targeted therapy for HS according to pathogenesis. Keratinocytes inappropriately release AMPs and proinflammatory cytokines, causing infundibular hyperkeratosis and perifolliculitis (von Laffert et al., 2011). The pilosebaceous unit ruptures, releasing keratin and bacteria, the target of antimicrobial drugs (van der Zee et al., 2012a). Complement is aberrantly activated, increasing levels of C5b–C9, which form the MAC, and C5a, a neutrophil chemotaxin targeted by the mAb IFX-1 (Kanni et al., 2018b). Macrophages are recruited and produce IL-6, IL-10, TNF-α, and IL-1 (van der Zee et al., 2012a). TNF-α is also produced by DCs; it is targeted by the mAbs infliximab and adalimumab and the decoy receptor etanercept (Adams et al., 2010; Grant et al., 2010; Kimball et al., 2016a; Pelekanou et al., 2010). Adalimumab is currently the only FDA-approved mAb therapy for HS (Kimball et al., 2016a). IL-1 is blocked by the mAbs anakinra (targeting IL-1α) and bermekimab (targeting IL-1R) (Kanni et al., 2018a; Tzanetakou et al., 2016). Mature IL-1β, synthesized from the inflammasome, stimulates the development of CD4+ Th17 cells. Activation of these cells is also enhanced by IL-23 (Sabat et al., 2019). CD4+ Th17 cells produce IL-17, which is targeted by the mAbs secukinumab (IL-17A), bimekizumab (IL-17A/IL-17F), and brodalumab (IL-17RA) (Baker and Isaacs, 2018; Thorlacius et al., 2018b). Both macrophages and DCs stimulate production of IL-12 and IL-23. IL-12 drives Th1 differentiation to yield IFN-γ production. IL-23 stimulates Th17 cell differentiation and is targeted by the mAbs guselkumab and risankizumab (Baker and Isaacs, 2018; Kearney et al., 2020; Kovacs and Podda, 2019). Both IL-12 and IL-23 are targeted by the mAb ustekinumab (Blok et al., 2016; Sharon et al., 2012). AMP, antimicrobial peptide; DC, dendritic cell; FDA, Food and Drug Administration; HS, hidradenitis suppurativa; MAC, membrane attack complex; Th, T helper type.
Currently, the anti–TNF-α mAb adalimumab is the only Food and Drug Administration–approved biologic therapy for HS, proving effective in two large phase III trials (PIONEER I and II) (Kimball et al., 2016a). Infliximab, another mAb against TNF-α, has demonstrated efficacy in a phase II RCT, although phase III RCTs have yet to be performed (Grant et al., 2010). Retrospective data suggest that doses greater than the manufacturer-recommended 5 mg/kg every 8 weeks may be required; a majority received initial injections at 10 mg/kg and maintenance dose of 10 mg/kg every 6–8 weeks and required dose escalation after one year of therapy (Oskardmay et al., 2019). Another recent study with initial infliximab doses of 7.5 mg/kg every 4 weeks found that 50% of patients who did not achieve clinical response were able to do so after dose escalation to 10 mg/kg every 4 weeks (Ghias et al., 2020). A recent study by Lowe et al. (2020) found that patients with an enriched B-cell signature within lesional skin had significantly lower response rates to anti–TNF-α therapy. Efficacy of the soluble TNF-α decoy receptor etanercept in HS remains inconclusive; although it was ineffective in a RCT, an open phase II trial found improvement in long-term disease course (Adams et al., 2010; Pelekanou et al., 2010).
Other biologic drugs studied or under investigation for HS treatment target cytokines with altered levels in disease. Anakinra and bermekimab, drugs that block IL-1, have demonstrated HiSCR response in 78% and 60% of patients, respectively, at 12 weeks in RCTs (Kanni et al., 2018a; Tzanetakou et al., 2016). Notably, the bermekimab trial recruited patients who failed or were unable to receive adalimumab (Kanni et al., 2018a).
Ustekinumab is an mAb against IL-12 and IL-23 that achieved HiSCR in 47% of patients at week 40 in an open phase II trial and has shown success in numerous case reports (Blok et al., 2016; Gulliver et al., 2012; Santos-Pérez et al., 2014; Sharon et al., 2012). A small retrospective study of guselkumab, an mAb against IL-23, found the drug to be effective in 63% of patients with moderate to severe HS (Casseres et al., 2019), with several case reports or series thereafter confirming successful treatment (Kearney et al., 2020; Kovacs and Podda, 2019). Currently, a phase II trial is being conducted to evaluate the drug (ClinicalTrials.gov, 2018a). A phase II trial is also underway for risankizumab, another mAb that targets IL-23 (ClinicalTrials.gov, 2019a). Clinical responses to these therapies are of particular interest as their results may indicate the relative contributions of the IL-12/Th1 and IL-23/Th17 pathways to HS pathogenesis.
Antibody blockade of IL-17 has also been investigated. There are case reports showing successful treatment of moderate to severe HS with the anti–IL-17A mAb secukinumab, and a recent open pilot trial reported that 67% of patients achieved HiSCR after 24 weeks of therapy (Giuseppe et al., 2018; Jørgensen et al., 2018; Prussick et al., 2019; Thorlacius et al., 2018b). Blinded trials are ongoing for secukinumab; bimekizumab, an mAb targeting IL-17A and IL-17F; and brodalumab, an mAb targeting IL-17RA (ClinicalTrials.gov, 2018c, 2019b, 2017). Results of an open-label cohort study for brodalumab are promising, as 100% of patients achieved HiSCR at 12 weeks of therapy (Frew et al., 2020a, 2020b). Given the association between HS and IBD, a potential concern with IL-17 blockade is paradoxical aggravation of Crohn disease, and more studies are needed to elucidate the effects of this therapy on HS disease course (Hueber et al., 2012).
Recent discovery of the role of complement in HS has driven investigation of the anti-C5a mAb IFX-1. A phase II trial for IFX-1 in patients with moderate to severe HS is underway (ClinicalTrials.gov, 2018b).
Studies of other skin diseases such as psoriasis reveal that biologic drugs may alter microbial diversity and composition, resulting in divergence of lesional and nonlesional skin communities (Langan et al., 2019; Loesche et al., 2018). Research has yet to characterize changes to the cutaneous microbiome in patients with HS undergoing biologic therapy. These shifts may indicate clinical efficacy and/or be used to assess response to treatment.
Surgery
Generally, surgery is recommended in conjunction with medical therapy, as poorly controlled disease may confer greater risk for surgical complications (Alikhan et al., 2019). Small or individual lesions in mild to moderate HS may be managed with local destruction, such as electrosurgery and cryosurgery or punch debridement (Danby et al., 2015). Moderate to severe HS may require surgical management with unroofing or excisional therapy (Danby et al., 2015).
A recent systematic review found the overall mean complication rate to be 24% and mean recurrence rate to be 20% (Bouazzi et al., 2020). The authors did not find a significant association between HS comorbidities that are surgical risk factors and complication and/or recurrence rates of surgery. However, studies vary widely in their follow-up times and clinical definitions of complication and recurrence. Future studies should clarify this heterogeneity and standardize postsurgical evaluation.
Other therapies
Given that HS is more common in females and symptoms may vary during pregnancy and the perimenstrual period, antiandrogenic therapies have been used, although there are limited data to support their use (Vossen et al., 2017). Such therapies tested in case series, retrospective studies, and one RCT include ethinyl estradiol and norgestrel, ethinyl estradiol and cyproterone acetate, and spironolactone (Kraft and Searles, 2007; Lee and Fischer, 2015; Mortimer et al., 1986). There is evidence that patients taking the 5α-reductase inhibitor finasteride achieve clinical improvement, although the exact mechanism by which this occurs is unclear and its use is off-label (Clark et al., 2017). These therapies may be more beneficial for patients presenting with flares during their menstrual period and/or those who have features of polycystic ovarian syndrome (Alikhan et al., 2019).
Pain associated with HS appears to be the greatest source of morbidity in patients afflicted by the disease (von der Werth and Jemec, 2001). Commonly utilized therapies include topical anesthetics, nonsteroidal anti-inflammatory drugs, and opioids (Alikhan et al., 2019; Puza et al., 2019). One study found that opioids were prescribed in over one fifth of patients with HS who presented to the emergency department (Puza et al., 2019). Prospective studies are needed to construct a more standardized approach toward pain management in HS.
Other medical therapies, such as peeling agents, intralesional steroids, and topical retinoids, can be used as adjuvants or for acute relief, but there is a lack of data supporting their use as monotherapy (Alikhan et al., 2019; Frew et al., 2019; Riis et al., 2016a). Oral retinoids have yielded varying levels of success mostly limited to Hurley stage I and II disease (Blok et al., 2013; Huang and Kirchhof, 2017).
Conclusion and Future Directions
HS is a chronic inflammatory disorder whose pathogenesis is still poorly understood and involves multiple factors. Although it is known that a shift in predominance of skin commensals to gram-negative anaerobes occurs as HS lesions progress, it remains elusive how this change occurs and whether it is a primary or secondary process. More research is needed to examine the early innate immune response and identify key molecules and pathways that are causative of or reactive to cutaneous dysbiosis. Studies of immunomodulatory therapies support the role of altered cytokine signaling and dysregulated innate immune pathways, such as TNF-α, IL-1β, IL-12, IL-17, IL-23, and complement. The use of next-generation sequencing technologies, specifically single-cell sequencing, can be employed to provide rich descriptions of the pathways that are perturbed in HS and can thereby be targeted for treatment. Spatial transcriptomics is another novel technique that is of special value when studying a complicated organ such as the skin. This may allow unique insight into the role of the pilosebaceous unit in driving HS disease.
Guidelines for HS treatment recommend lifestyle modification and antibiotics as first line therapy because smoking, obesity, and bacterial involvement have been established as contributors to disease. However, there is a relative dearth of RCTs examining the efficacy of these recommendations and, in the case of antibiotics, uncertainty about the importance of anti-inflammatory versus antimicrobial responses. Surgery and other treatments function as useful adjuvants to medical therapy for HS. These should continue to be explored to enhance pain and cosmetic management. Current research investigating the skin microbiome, transcriptome, and metabolome of diverse patient cohorts with HS will continue to illuminate pathogenesis and inform more personalized approaches toward management of comorbidities and treatment of disease.
Data availability statement
No datasets were generated or analyzed during the current study.
ORCIDs
Simon Jiang: https://orcid.org/0000-0002-9509-9001
Melodi Javid Whitley: https://orcid.org/0000-0002-8163-6881
Paula Mariottoni: https://orcid.org/0000-0002-1561-3489
Tarannum Jaleel: https://orcid.org/0000-0002-2362-5121
Amanda S. MacLeod: https://orcid.org/0000-0001-8139-9110
Acknowledgments
Author Contributions
Conceptualization: SWJ, MJW, PM, ASM; Investigation: SWJ, MJW, PM, TJ, ASM; Supervision: ASM; Visualization: SWJ; Writing - Original Draft Preparation: SWJ, MJW, PM, TJ, ASM; Writing - Review and Editing: SWJ, MJW, PM, TJ, ASM
Acknowledgments
The authors would like to acknowledge BioRender, which was used for figure illustration. This work was funded in part by support from the Department of Dermatology (to ASM). ASM is supported by NIH R01 AI139207. SWJ received support through the Burroughs Wellcome Fund Physician-Scientist Institutional Award. MJW received support through the Duke Office of Physician Scientist Development Technician Support Grant and the Duke-UNC Immunotherapy Training Grant.
Conflict of Interest
ASM consults for the company Silab and is on the scientific evaluation committee of the LEO Foundation. The spouse of ASM is employed by Precision BioSciences and holds stock and stock options. All authors confirm and declare that this review and literature included were performed in the absence of any commercial or financial relationships that would present a potential conflict of interest.
accepted manuscript published online 12 January 2020
References
- Adams D.R., Yankura J.A., Fogelberg A.C., Anderson B.E. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501–504. doi: 10.1001/archdermatol.2010.72. [DOI] [PubMed] [Google Scholar]
- Alam M.S., Maekawa Y., Kitamura A., Tanigaki K., Yoshimoto T., Kishihara K., et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2010;107:5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhan A., Sayed C., Alavi A., Alhusayen R., Brassard A., Burkhart C., et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76–90. doi: 10.1016/j.jaad.2019.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.D., Zauli S., Bettoli V., Boer J., Jemec G.B.E. Cyclosporine treatment of severe hidradenitis suppurativa--a case series. J Dermatolog Treat. 2016;27:247–250. doi: 10.3109/09546634.2015.1088128. [DOI] [PubMed] [Google Scholar]
- Anzaldi L., Perkins J.A., Byrd A.S., Kharrazi H., Okoye G.A. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510–513. doi: 10.1016/j.jaad.2019.09.019. [DOI] [PubMed] [Google Scholar]
- Baker K.F., Isaacs J.D. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77:175–187. doi: 10.1136/annrheumdis-2017-211555. [DOI] [PubMed] [Google Scholar]
- Balato A., Caiazzo G., Annunziata M.C., Marasca C., Scala E., Cacciapuoti S., et al. Anti-TNF-α therapy modulates mTORC1 signalling in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33:e43–e45. doi: 10.1111/jdv.15160. [DOI] [PubMed] [Google Scholar]
- Banerjee A., McNish S., Shanmugam V.K. Interferon-gamma (IFN-γ) is elevated in wound exudate from hidradenitis suppurativa. Immunol Invest. 2017;46:149–158. doi: 10.1080/08820139.2016.1230867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettoli V., Manfredini M., Massoli L., Carillo C., Barozzi A., Amendolagine G., et al. Rates of antibiotic resistance/sensitivity in bacterial cultures of hidradenitis suppurativa patients. J Eur Acad Dermatol Venereol. 2019;33:930–936. doi: 10.1111/jdv.15332. [DOI] [PubMed] [Google Scholar]
- Bettuzzi T., Frumholtz L., Jachiet M., Lepelletier C., Djermane M., Cordoliani F., et al. Sirolimus as combination rescue therapy with tumor necrosis alpha inhibitors for severe, refractory hidradenitis suppurativa. J Am Acad Dermatol. 2020;83:1441–1444. doi: 10.1016/j.jaad.2020.06.042. [DOI] [PubMed] [Google Scholar]
- Bianchi L., Hansel K., Stingeni L. Recalcitrant severe hidradenitis suppurativa successfully treated with cyclosporine A. J Am Acad Dermatol. 2012;67:e278–e279. doi: 10.1016/j.jaad.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Blok J.L., Li K., Brodmerkel C., Horvátovich P., Jonkman M.F., Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839–846. doi: 10.1111/bjd.14338. [DOI] [PubMed] [Google Scholar]
- Blok J.L., van Hattem S., Jonkman M.F., Horváth B. Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. Br J Dermatol. 2013;168:243–252. doi: 10.1111/bjd.12104. [DOI] [PubMed] [Google Scholar]
- Bode C., Muenster S., Diedrich B., Jahnert S., Weisheit C., Steinhagen F., et al. Linezolid, vancomycin and daptomycin modulate cytokine production, toll-like receptors and phagocytosis in a human in vitro model of sepsis. J Antibiot (Tokyo) 2015;68:485–490. doi: 10.1038/ja.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouazzi D., Chafranska L., Saunte D.M.L., Jemec G.B.E. Systematic review of complications and recurrences after surgical interventions in hidradenitis suppurativa. Dermatol Surg. 2020;46:914–921. doi: 10.1097/DSS.0000000000002323. [DOI] [PubMed] [Google Scholar]
- Brandwein M., Katz I., Katz A., Kohen R. Beyond the gut: skin microbiome compositional changes are associated with BMI. Hum Microbiome J. 2019;13:100063. [Google Scholar]
- Braun-Falco M., Kovnerystyy O., Lohse P., Ruzicka T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)--a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409–415. doi: 10.1016/j.jaad.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Brook I., Frazier E.H. Aerobic and anaerobic microbiology of axillary hidradenitis suppurativa. J Med Microbiol. 1999;48:103–105. doi: 10.1099/00222615-48-1-103. [DOI] [PubMed] [Google Scholar]
- Byrd A.S., Carmona-Rivera C., O’Neil L.J., Carlucci P.M., Cisar C., Rosenberg A.Z., et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aav5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoui-Poitrine F., Le Thuaut A., Revuz J.E., Viallette C., Gabison G., Poli F., et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506–1511. doi: 10.1038/jid.2012.472. [DOI] [PubMed] [Google Scholar]
- Cao L., Morales-Heil D.J., Roberson E.D.O. Nicastrin haploinsufficiency alters expression of type I interferon-stimulated genes: the relationship to familial hidradenitis suppurativa. Clin Exp Dermatol. 2019;44:e118–e125. doi: 10.1111/ced.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseres R.G., Kahn J.S., Her M.J., Rosmarin D. Guselkumab in the treatment of hidradenitis suppurativa: a retrospective chart review. J Am Acad Dermatol. 2019;81:265–267. doi: 10.1016/j.jaad.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Chahine A.A., Nahhas A.F., Braunberger T.L., Rambhatla P.V., Hamzavi I.H. Ertapenem rescue therapy in hidradenitis suppurativa. JAAD Case Rep. 2018;4:482–483. doi: 10.1016/j.jdcr.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehoud C., Rafail S., Tyldsley A.S., Seykora J.T., Lambris J.D., Grice E.A. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc Natl Acad Sci USA. 2013;110:15061–15066. doi: 10.1073/pnas.1307855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.A., Suh J.W., Lee K.H., Kang J.L., Woo S.Y. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int Immunol. 2012;24:147–158. doi: 10.1093/intimm/dxr110. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Song M.J., Kim S.H., Choi S.M., Lee D.G., Yoo J.H., et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704–3707. doi: 10.1128/AAC.47.12.3704-3707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.K., Quinonez R.L., Saric S., Sivamani R.K. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23 [PubMed] [Google Scholar]
- Clemmensen O.J. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325–328. doi: 10.1111/j.1365-4362.1983.tb02150.x. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov A study to test the efficacy, safety and pharmacokinetics of bimekizumab in subjects with moderate to severe hidradenitis suppurativa. 2017. https://clinicaltrials.gov/ct2/show/NCT03248531
- ClinicalTrials.gov A study to evaluate the efficacy, safety, and tolerability of guselkumab for the treatment of participants with moderate to severe hidradenitis suppurativa (HS) (NOVA) 2018. https://clinicaltrials.gov/ct2/show/NCT03628924 (accessed 28 March 2020)
- ClinicalTrials.gov Efficacy and safety study of IFX-1 in patients with moderate to severe hidradenitis suppurativa (HS) (SHINE) 2018. https://clinicaltrials.gov/ct2/show/NCT03487276 (accessed 9 November 2020)
- ClinicalTrials.gov Study of Efficacy and Safety of Two Secukinumab Dose Regimens in Subjects With Moderate to Severe Hidradenitis Suppurativa (HS) (SUNRISE) 2018. https://clinicaltrials.gov/ct2/show/NCT03713632 (accessed 9 November 2020)
- ClinicalTrials.gov A global study comparing risankizumab to placebo in adult participants with moderate to severe hidradenitis suppurativa (DETERMINED 1) 2019. https://clinicaltrials.gov/ct2/show/NCT03926169 (accessed July 27, 2020)
- ClinicalTrials.gov Treatment of moderate hidradenitis suppurativa. 2019. https://clinicaltrials.gov/ct2/show/NCT03910803 (accessed July 1, 2020)
- Coates M., Blanchard S., MacLeod A.S. Innate antimicrobial immunity in the skin: a protective barrier against bacteria, viruses, and fungi. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates M., Mariottoni P., Corcoran D.L., Kirshner H.F., Jaleel T., Brown D.A., et al. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C.A., Fragoulis G.E., Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both? Ther Adv Musculoskelet Dis. 2019;11 doi: 10.1177/1759720X19895488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmatos I., Matcho A., Weinstein R., Montgomery M.O., Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;69:819. doi: 10.1016/j.jaad.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Damiani G., Mahroum N., Pigatto P.D.M., Pacifico A., Malagoli P., Tiodorovic D., et al. The safety and impact of a model of intermittent, time-restricted circadian fasting (“Ramadan Fasting”) on hidradenitis suppurativa: insights from a multicenter, observational, cross-over, pilot, exploratory study. Nutrients. 2019;11:1781. doi: 10.3390/nu11081781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danby F.W., Hazen P.G., Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(Suppl. 1):S62–S65. doi: 10.1016/j.jaad.2015.07.043. [DOI] [PubMed] [Google Scholar]
- Das A., Reis F., Maejima Y., Cai Z., Ren J. mTOR signaling in cardiometabolic disease, cancer, and aging. Oxid Med Cell Longev. 2017;2017:6018675. doi: 10.1155/2017/6018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny G., Anadkat M.J. Hidradenitis suppurativa (HS) and Down syndrome (DS): increased prevalence and a younger age of hidradenitis symptom onset. J Am Acad Dermatol. 2016;75:632–634. doi: 10.1016/j.jaad.2016.04.045. [DOI] [PubMed] [Google Scholar]
- Dréno B., Khammari A., Brocard A., Moyse D., Blouin E., Guillet G., et al. Hidradenitis suppurativa: the role of deficient cutaneous innate immunity. Arch Dermatol. 2012;148:182–186. doi: 10.1001/archdermatol.2011.315. [DOI] [PubMed] [Google Scholar]
- Dubourg G., Lagier J.C., Robert C., Armougom F., Hugon P., Metidji S., et al. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. Int J Antimicrob Agents. 2014;44:117–124. doi: 10.1016/j.ijantimicag.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Egeberg A., Gislason G.H., Hansen P.R. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429–434. doi: 10.1001/jamadermatol.2015.6264. [DOI] [PubMed] [Google Scholar]
- Eppinga H., Sperna Weiland C.J., Thio H.B., van der Woude C.J., Nijsten T.E.C., Peppelenbosch M.P., et al. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease, but not in hidradenitis suppurativa. J Crohns Colitis. 2016;10:1067–1075. doi: 10.1093/ecco-jcc/jjw070. [DOI] [PubMed] [Google Scholar]
- Fischer A.H., Haskin A., Okoye G.A. Patterns of antimicrobial resistance in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2017;76:309–313.e2. doi: 10.1016/j.jaad.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Francisco V., Ruiz-Fernández C., Pino J., Mera A., González-Gay M.A., Gómez R., et al. Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases. Biochem Pharmacol. 2019;165:196–206. doi: 10.1016/j.bcp.2019.03.030. [DOI] [PubMed] [Google Scholar]
- Frew J.W., Hawkes J.E., Krueger J.G. Topical, systemic and biologic therapies in hidradenitis suppurativa: pathogenic insights by examining therapeutic mechanisms. Ther Adv Chronic Dis. 2019;10 doi: 10.1177/2040622319830646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew J.W., Navrazhina K., Grand D., Sullivan-Whalen M., Gilleaudeau P., Garcet S., et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: an open-label cohort study. J Am Acad Dermatol. 2020;83:1341–1348. doi: 10.1016/j.jaad.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew J.W., Navrazhina K., Sullivan-Whalen M., Gilleaudeau P., Garcet S., Krueger J.G. Weekly administration of brodalumab in hidradenitis suppurativa: an open-label cohort study [e-pub ahead of print] Br J Dermatol. 2020 doi: 10.1111/bjd.19478. [DOI] [PubMed] [Google Scholar]
- Garg A., Neuren E., Cha D., Kirby J.S., Ingram J.R., Jemec G.B.E., et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey of Impact and Healthcare Needs (VOICE) project. J Am Acad Dermatol. 2020;82:366–376. doi: 10.1016/j.jaad.2019.06.1301. [DOI] [PubMed] [Google Scholar]
- Gener G., Canoui-Poitrine F., Revuz J.E., Faye O., Poli F., Gabison G., et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148–154. doi: 10.1159/000228334. [DOI] [PubMed] [Google Scholar]
- Ghias M.H., Johnston A.D., Kutner A.J., Micheletti R.G., Hosgood H.D., Cohen S.R. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094–1101. doi: 10.1016/j.jaad.2019.09.071. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Antonopoulou A., Petropoulou C., Mouktaroudi M., Spyridaki E., Baziaka F., et al. Altered innate and adaptive immune responses in patients with hidradenitis suppurativa. Br J Dermatol. 2007;156:51–56. doi: 10.1111/j.1365-2133.2006.07556.x. [DOI] [PubMed] [Google Scholar]
- Giatrakos S., Huse K., Kanni T., Tzanetakou V., Kramer M., Grech I., et al. Haplotypes of IL-12Rβ1 impact on the clinical phenotype of hidradenitis suppurativa. Cytokine. 2013;62:297–301. doi: 10.1016/j.cyto.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Giuseppe P., Nicola P., Valentina C., Elena C., Salvatrice C., Rosario G., et al. A case of moderate hidradenitis suppurativa and psoriasis treated with secukinumab. Ann Dermatol. 2018;30:462–464. doi: 10.5021/ad.2018.30.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D.A., Reeder V.J., Mahan M.G., Hamzavi I.H. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699–703. doi: 10.1016/j.jaad.2013.11.014. [DOI] [PubMed] [Google Scholar]
- González-López M.A., Vilanova I., Ocejo-Viñals G., Arlegui R., Navarro I., Guiral S., et al. Circulating levels of adiponectin, leptin, resistin and visfatin in non-diabetics patients with hidradenitis suppurativa. Arch Dermatol Res. 2020;312:595–600. doi: 10.1007/s00403-019-02018-4. [DOI] [PubMed] [Google Scholar]
- Grant A., Gonzalez T., Montgomery M.O., Cardenas V., Kerdel F.A. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205–217. doi: 10.1016/j.jaad.2009.06.050. [DOI] [PubMed] [Google Scholar]
- Grice E.A., Kong H.H., Renaud G., Young A.C. NISC Comparative Sequencing Program, Bouffard GG, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson J.E., Tsoi L.C., Ma F., Billi A.C., van Straalen K.R., Vossen A.R.J.V., et al. Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guet-Revillet H., Coignard-Biehler H., Jais J.P., Quesne G., Frapy E., Poirée S., et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990–1998. doi: 10.3201/eid2012.140064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guet-Revillet H., Jais J.P., Ungeheuer M.N., Coignard-Biehler H., Duchatelet S., Delage M., et al. The microbiological landscape of anaerobic infections in hidradenitis suppurativa: a prospective metagenomic study. Clin Infect Dis. 2017;65:282–291. doi: 10.1093/cid/cix285. [DOI] [PubMed] [Google Scholar]
- Gulliver W.P., Jemec G.B.E., Baker K.A. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911–914. doi: 10.1111/j.1468-3083.2011.04123.x. [DOI] [PubMed] [Google Scholar]
- Hana A., Booken D., Henrich C., Gratchev A., Maas-Szabowski N., Goerdt S., et al. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007;80:2214–2220. doi: 10.1016/j.lfs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Hayran Y., Alli N., Yücel C., Akdoğan N., Turhan T. Serum IL-36α, IL-36β, and IL-36γ levels in patients with hidradenitis suppurativa: association with disease characteristics, smoking, obesity, and metabolic syndrome. Arch Dermatol Res. 2020;312:187–196. doi: 10.1007/s00403-019-02012-w. [DOI] [PubMed] [Google Scholar]
- Hessam S., Gambichler T., Skrygan M., Scholl L., Sand M., Meyer T., et al. Increased expression profile of NCSTN, Notch and PI3K/AKT3 in hidradenitis suppurativa [e-pub ahead of print] J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16962. (accessed 9 November 2020) [DOI] [PubMed] [Google Scholar]
- Hessam S., Sand M., Gambichler T., Skrygan M., Rüddel I., Bechara F.G. Interleukin-36 in hidradenitis suppurativa: evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br J Dermatol. 2018;178:761–767. doi: 10.1111/bjd.16019. [DOI] [PubMed] [Google Scholar]
- Hispán P., Murcia O., Gonzalez-Villanueva I., Francés R., Giménez P., Riquelme J., et al. Identification of bacterial DNA in the peripheral blood of patients with active hidradenitis suppurativa. Arch Dermatol Res. 2020;312:159–163. doi: 10.1007/s00403-019-01965-2. [DOI] [PubMed] [Google Scholar]
- Hoffman L.K., Tomalin L.E., Schultz G., Howell M.D., Anandasabapathy N., Alavi A., et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S.C., Saborowski V., Lange S., Kern W.V., Bruckner-Tuderman L., Rieg S. Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol. 2012;66:966–974. doi: 10.1016/j.jaad.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Hotz C., Boniotto M., Guguin A., Surenaud M., Jean-Louis F., Tisserand P., et al. Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J Invest Dermatol. 2016;136:1768–1780. doi: 10.1016/j.jid.2016.04.036. [DOI] [PubMed] [Google Scholar]
- Huang C.M., Kirchhof M.G. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120–125. doi: 10.1159/000477207. [DOI] [PubMed] [Google Scholar]
- Hueber W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D.R., et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger R.E., Surovy A.M., Hassan A.S., Braathen L.R., Yawalkar N. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br J Dermatol. 2008;158:691–697. doi: 10.1111/j.1365-2133.2007.08425.x. [DOI] [PubMed] [Google Scholar]
- Hurley H. In: Dermatologic Surgery: Principles and Practice. Roenigk R.K., Roenigk H.H. Jr., editors. Marcel Dekker Inc; New York: 1989. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach; pp. 729–739. [Google Scholar]
- Hutcherson J.A., Scott D.A., Bagaitkar J. Scratching the surface – tobacco-induced bacterial biofilms. Tob Induc Dis. 2015;13:1. doi: 10.1186/s12971-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J.R., Collier F., Brown D., Burton T., Burton J., Chin M.F., et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019;180:1009–1017. doi: 10.1111/bjd.17537. [DOI] [PubMed] [Google Scholar]
- Jemec G.B.E. Methotrexate is of limited value in the treatment of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:528–529. doi: 10.1046/j.1365-2230.2002.11125.x. [DOI] [PubMed] [Google Scholar]
- Jemec G.B.E. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366:158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- Jemec G.B., Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971–974. doi: 10.1016/s0190-9622(98)70272-5. [DOI] [PubMed] [Google Scholar]
- Jneid J., Lavigne J.P., La Scola B., Cassir N. The diabetic foot microbiota: a review. Hum Microbiome J. 2017;5–6:1–6. [Google Scholar]
- Join-Lambert O., Coignard H., Jais J.P., Guet-Revillet H., Poirée S., Fraitag S., et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49–58. doi: 10.1159/000321716. [DOI] [PubMed] [Google Scholar]
- Jones D., Banerjee A., Berger P.Z., Gross A., McNish S., Amdur R., et al. Inherent differences in keratinocyte function in hidradenitis suppurativa: evidence for the role of IL-22 in disease pathogenesis. Immunol Invest. 2018;47:57–70. doi: 10.1080/08820139.2017.1377227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A.H.R., Yao Y., Thomsen S.F. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med. 2018;2018 doi: 10.1155/2018/8685136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanni T., Argyropoulou M., Spyridopoulos T., Pistiki A., Stecher M., Dinarello C.A., et al. MABp1 targeting IL-1α for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randomized study. J Invest Dermatol. 2018;138:795–801. doi: 10.1016/j.jid.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Kanni T., Zenker O., Habel M., Riedemann N., Giamarellos-Bourboulis E.J. Complement activation in hidradenitis suppurativa: a new pathway of pathogenesis? Br J Dermatol. 2018;179:413–419. doi: 10.1111/bjd.16428. [DOI] [PubMed] [Google Scholar]
- Kathju S., Lasko L.A., Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385–389. doi: 10.1111/j.1574-695X.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- Kearney N., Byrne N., Kirby B., Hughes R. Successful use of guselkumab in the treatment of severe hidradenitis suppurativa. Clin Exp Dermatol. 2020;45:618–619. doi: 10.1111/ced.14199. [DOI] [PubMed] [Google Scholar]
- Kelly G., Hughes R., McGarry T., van den Born M., Adamzik K., Fitzgerald R., et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431–1439. doi: 10.1111/bjd.14075. [DOI] [PubMed] [Google Scholar]
- Kelly G., Prens E.P. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51–58. doi: 10.1016/j.det.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Kimball A.B., Okun M.M., Williams D.A., Gottlieb A.B., Papp K.A., Zouboulis C.C., et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]
- Kimball A.B., Sobell J.M., Zouboulis C.C., Gu Y., Williams D.A., Sundaram M., et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989–994. doi: 10.1111/jdv.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorst J.J., Kimball A.B., Davis M.D.P. Systemic associations of hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(Suppl. 1):S27–S35. doi: 10.1016/j.jaad.2015.07.055. [DOI] [PubMed] [Google Scholar]
- Kovacs M., Podda M. Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33:e140–e141. doi: 10.1111/jdv.15368. [DOI] [PubMed] [Google Scholar]
- Kraft J.N., Searles G.E. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125–131. doi: 10.2310/7750.2007.00019. [DOI] [PubMed] [Google Scholar]
- Kromann C.B., Deckers I.E., Esmann S., Boer J., Prens E.P., Jemec G.B.E. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819–824. doi: 10.1111/bjd.13090. [DOI] [PubMed] [Google Scholar]
- Lam S.Y., Radjabzadeh D., Eppinga H., van der Zee H.H., Kraaij R., Konstantinov S.R., et al. IDDF2019-ABS-0293 A microbiome pilot study: the exploration of the gut-skin axis in hidradenitis suppurativa. Gut. 2019;68(Suppl. 1):A31. [Google Scholar]
- Langan E.A., Künstner A., Miodovnik M., Zillikens D., Thaçi D., Baines J.F., et al. Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. Br J Dermatol. 2019;181:1254–1264. doi: 10.1111/bjd.17989. [DOI] [PubMed] [Google Scholar]
- Lapins J., Jarstrand C., Emtestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol. 1999;140:90–95. doi: 10.1046/j.1365-2133.1999.02613.x. [DOI] [PubMed] [Google Scholar]
- Lapins J., Ye W., Nyrén O., Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730–734. [PubMed] [Google Scholar]
- Lee A., Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192–196. doi: 10.1111/ajd.12362. [DOI] [PubMed] [Google Scholar]
- Lee D.E., Clark A.K., Shi V.Y. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456–461. doi: 10.1159/000486741. [DOI] [PubMed] [Google Scholar]
- Leuenberger M., Berner J., Di Lucca J., Fischer L., Kaparos N., Conrad C., et al. PASS syndrome: an IL-1-driven autoinflammatory disease. Dermatology. 2016;232:254–258. doi: 10.1159/000443648. [DOI] [PubMed] [Google Scholar]
- Lima A.L., Karl I., Giner T., Poppe H., Schmidt M., Presser D., et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514–521. doi: 10.1111/bjd.14214. [DOI] [PubMed] [Google Scholar]
- Liu M., Davis J.W., Idler K.B., Mostafa N.M., Okun M.M., Waring J.F. Genetic analysis of NCSTN for potential association with hidradenitis suppurativa in familial and nonfamilial patients. Br J Dermatol. 2016;175:414–416. doi: 10.1111/bjd.14482. [DOI] [PubMed] [Google Scholar]
- Loesche M.A., Farahi K., Capone K., Fakharzadeh S., Blauvelt A., Duffin K.C., et al. Longitudinal study of the psoriasis-associated skin microbiome during therapy with ustekinumab in a randomized phase 3b clinical trial. J Invest Dermatol. 2018;138:1973–1981. doi: 10.1016/j.jid.2018.03.1501. [DOI] [PubMed] [Google Scholar]
- Losanoff J.E., Sochaki P., Khoury N., Levi E., Salwen W.A., Basson M.D. Squamous cell carcinoma complicating chronic suppurative hidradenitis. Am Surg. 2011;77:1449–1453. [PubMed] [Google Scholar]
- Lowe M.M., Naik H.B., Clancy S., Pauli M., Smith K.M., Bi Y., et al. Immunopathogenesis of hidradenitis suppurativa and response to anti–TNF-α therapy. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malara A., Hughes R., Jennings L., Sweeney C.M., Lynch M., Awdeh F., et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br J Dermatol. 2018;178:792–793. doi: 10.1111/bjd.15904. [DOI] [PubMed] [Google Scholar]
- Mandell J.B., Orr S., Koch J., Nourie B., Ma D., Bonar D.D., et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604–1609. doi: 10.1002/jor.24291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano A.V., Trevisan V., Gattorno M., Ceccherini I., De Simone C., Crosti C. Pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa (PAPASH): a new autoinflammatory syndrome associated with a novel mutation of the PSTPIP1 gene. JAMA Dermatol. 2013;149:762–764. doi: 10.1001/jamadermatol.2013.2907. [DOI] [PubMed] [Google Scholar]
- Matusiak Ł., Bieniek A., Szepietowski J.C. Bacteriology of hidradenitis suppurativa - which antibiotics are the treatment of choice? Acta Derm Venereol. 2014;94:699–702. doi: 10.2340/00015555-1841. [DOI] [PubMed] [Google Scholar]
- Matusiak L., Bieniek A., Szepietowski J.C. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170–174. doi: 10.1111/bjd.12884. [DOI] [PubMed] [Google Scholar]
- Melnik B.C., Plewig G. Impaired Notch signalling: the unifying mechanism explaining the pathogenesis of hidradenitis suppurativa (acne inversa) Br J Dermatol. 2013;168:876–878. doi: 10.1111/bjd.12068. [DOI] [PubMed] [Google Scholar]
- Melnik B.C., Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol. 2013;22:172–177. doi: 10.1111/exd.12098. [DOI] [PubMed] [Google Scholar]
- Miller I.M., McAndrew R.J., Hamzavi I.H. Prevalence, risk factors, and comorbidities of hidradenitis suppurativa. Dermatol Clin. 2016;34:7–16. doi: 10.1016/j.det.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Miskinyte S., Nassif A., Merabtene F., Ungeheuer M.N., Join-Lambert O., Jais J.P., et al. Nicastrin mutations in French families with hidradenitis suppurativa. J Invest Dermatol. 2012;132:1728–1730. doi: 10.1038/jid.2012.23. [DOI] [PubMed] [Google Scholar]
- Monfrecola G., Balato A., Caiazzo G., De Vita V., Di Caprio R., Donnarumma M., et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631–1633. doi: 10.1111/jdv.13233. [DOI] [PubMed] [Google Scholar]
- Moran B., Sweeney C.M., Hughes R., Malara A., Kirthi S., Tobin A.M., et al. Hidradenitis suppurativa is characterized by dysregulation of the Th17:Treg cell axis, which is corrected by anti-TNF therapy. J Invest Dermatol. 2017;137:2389–2395. doi: 10.1016/j.jid.2017.05.033. [DOI] [PubMed] [Google Scholar]
- Mortimer P.S., Dawber R.P., Gales M.A., Moore R.A. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263–268. doi: 10.1111/j.1365-2133.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- Mozeika E., Pilmane M., Nürnberg B.M., Jemec G.B.E. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm Venereol. 2013;93:301–304. doi: 10.2340/00015555-1492. [DOI] [PubMed] [Google Scholar]
- Naik H.B., Jo J.H., Paul M., Kong H.H. Skin microbiota perturbations are distinct and disease severity–dependent in hidradenitis suppurativa. J Invest Dermatol. 2020;140:922–925.e3. doi: 10.1016/j.jid.2019.08.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye G.A., Vlassova N., Olowoyeye O., Agostinho A., James G., Stewart P.S., et al. Bacterial biofilm in acute lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:241–243. doi: 10.1111/bjd.14805. [DOI] [PubMed] [Google Scholar]
- Oskardmay A.N., Miles J.A., Sayed C.J. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702–708. doi: 10.1016/j.jaad.2019.05.022. [DOI] [PubMed] [Google Scholar]
- Palaga T., Buranaruk C., Rengpipat S., Fauq A.H., Golde T.E., Kaufmann S.H.E., et al. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol. 2008;38:174–183. doi: 10.1002/eji.200636999. [DOI] [PubMed] [Google Scholar]
- Pavia C.S., Pierre A., Nowakowski J. Antimicrobial activity of nicotine against a spectrum of bacterial and fungal pathogens. J Med Microbiol. 2000;49:675–676. doi: 10.1099/0022-1317-49-7-675. [DOI] [PubMed] [Google Scholar]
- Pelekanou A., Kanni T., Savva A., Mouktaroudi M., Raftogiannis M., Kotsaki A., et al. Long-term efficacy of etanercept in hidradenitis suppurativa: results from an open-label phase II prospective trial. Exp Dermatol. 2010;19:538–540. doi: 10.1111/j.1600-0625.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- Pink A.E., Simpson M.A., Desai N., Dafou D., Hills A., Mortimer P., et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa) J Invest Dermatol. 2012;132:2459–2461. doi: 10.1038/jid.2012.162. [DOI] [PubMed] [Google Scholar]
- Prussick L., Rothstein B., Joshipura D., Saraiya A., Turkowski Y., Abdat R., et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609–611. doi: 10.1111/bjd.17822. [DOI] [PubMed] [Google Scholar]
- Puza C.J., Wolfe S.A., Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatol Surg. 2019;45:1327–1330. doi: 10.1097/DSS.0000000000001693. [DOI] [PubMed] [Google Scholar]
- Redel H., Gao Z., Li H., Alekseyenko A.V., Zhou Y., Perez-Perez G.I., et al. Quantitation and composition of cutaneous microbiota in diabetic and nondiabetic men. J Infect Dis. 2013;207:1105–1114. doi: 10.1093/infdis/jit005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuz J.E., Canoui-Poitrine F., Wolkenstein P., Viallette C., Gabison G., Pouget F., et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Richette P., Molto A., Viguier M., Dawidowicz K., Hayem G., Nassif A., et al. Hidradenitis suppurativa associated with spondyloarthritis — results from a multicenter national prospective study. J Rheumatol. 2014;41:490–494. doi: 10.3899/jrheum.130977. [DOI] [PubMed] [Google Scholar]
- Riis P.T., Boer J., Prens E.P., Saunte D.M.L., Deckers I.E., Emtestam L., et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151–1155. doi: 10.1016/j.jaad.2016.06.049. [DOI] [PubMed] [Google Scholar]
- Riis P.T., Ring H.C., Themstrup L., Jemec G.B. The role of androgens and estrogens in hidradenitis suppurativa - a systematic review. Acta Dermatovenerol Croat. 2016;24:239–249. [PubMed] [Google Scholar]
- Ring H.C., Bay L., Nilsson M., Kallenbach K., Miller I.M., Saunte D.M., et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993–1000. doi: 10.1111/bjd.15007. [DOI] [PubMed] [Google Scholar]
- Ring H.C., Sigsgaard V., Thorsen J., Fuursted K., Fabricius S., Saunte D.M., et al. The microbiome of tunnels in hidradenitis suppurativa patients. J Eur Acad Dermatol Venereol. 2019;33:1775–1780. doi: 10.1111/jdv.15597. [DOI] [PubMed] [Google Scholar]
- Ring H.C., Thorsen J., Jørgensen A.H., Bay L., Bjarnsholt T., Fuursted K., et al. Predictive metagenomic analysis reveals a role of cutaneous dysbiosis in the development of hidradenitis suppurativa. J Invest Dermatol. 2020;140:1473–1476. doi: 10.1016/j.jid.2019.11.011. [DOI] [PubMed] [Google Scholar]
- Ring H.C., Thorsen J., Saunte D.M., Lilje B., Bay L., Riis P.T., et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017;153:897–905. doi: 10.1001/jamadermatol.2017.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riverain-Gillet É., Guet-Revillet H., Jais J.P., Ungeheuer M.N., Duchatelet S., Delage M., et al. The surface microbiome of clinically unaffected skinfolds in hidradenitis suppurativa: A cross-sectional culture-based and 16S rRNA gene amplicon sequencing study in 60 patients. J Invest Dermatol. 2020;140:1847–1855.e6. doi: 10.1016/j.jid.2020.02.046. [DOI] [PubMed] [Google Scholar]
- Rodrigues R.R., Greer R.L., Dong X., DSouza K.N., Gurung M., Wu J.Y., et al. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017;8:2306. doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner I.A., Burg C.G., Wisnieski J.J., Schacter B.Z., Richter D.E. The clinical spectrum of the arthropathy associated with hidradenitis suppurativa and acne conglobata. J Rheumatol. 1993;20:684–687. [PubMed] [Google Scholar]
- Rosner I.A., Richter D.E., Huettner T.L., Kuffner G.H., Wisnieski J.J., Burg C.G. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520–525. doi: 10.7326/0003-4819-97-4-520. [DOI] [PubMed] [Google Scholar]
- Sabat R., Chanwangpong A., Schneider-Burrus S., Metternich D., Kokolakis G., Kurek A., et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat R., Wolk K. Deciphering the role of interleukin-22 in metabolic alterations. Cell Biosci. 2015;5:68. doi: 10.1186/s13578-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat R., Wolk K., Loyal L., Döcke W.D., Ghoreschi K. T cell pathology in skin inflammation. Semin Immunopathol. 2019;41:359–377. doi: 10.1007/s00281-019-00742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J., Le Jan S., Muller C., François C., Renard Y., Durlach A., et al. Matrix remodelling and MMP expression/activation are associated with hidradenitis suppurativa skin inflammation. Exp Dermatol. 2019;28:593–600. doi: 10.1111/exd.13919. [DOI] [PubMed] [Google Scholar]
- Santos-Pérez M.I., García-Rodicio S., Del Olmo-Revuelto M.A., Pozo-Román T. Ustekinumab for hidradenitis suppurativa: a case report. Actas Dermosifiliogr. 2014;105:720–722. doi: 10.1016/j.ad.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Sartorius K., Emtestam L., Jemec G.B.E., Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- Sartorius K., Killasli H., Oprica C., Sullivan A., Lapins J. Bacteriology of hidradenitis suppurativa exacerbations and deep tissue cultures obtained during carbon dioxide laser treatment. Br J Dermatol. 2012;166:879–883. doi: 10.1111/j.1365-2133.2011.10747.x. [DOI] [PubMed] [Google Scholar]
- Savva A., Kanni T., Damoraki G., Kotsaki A., Giatrakou S., Grech I., et al. Impact of Toll-like receptor-4 and tumour necrosis factor gene polymorphisms in patients with hidradenitis suppurativa. Br J Dermatol. 2013;168:311–317. doi: 10.1111/bjd.12105. [DOI] [PubMed] [Google Scholar]
- Scala E., Di Caprio R., Cacciapuoti S., Caiazzo G., Fusco A., Tortorella E., et al. A new T helper 17 cytokine in hidradenitis suppurativa: antimicrobial and proinflammatory role of interleukin-26. Br J Dermatol. 2019;181:1038–1045. doi: 10.1111/bjd.17854. [DOI] [PubMed] [Google Scholar]
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583–1590. doi: 10.1016/j.bcp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Scheinfeld N. Extensive hidradenitis suppurativa (HS) Hurly stage III disease treated with intravenous (IV) linezolid and meropenem with rapid remission. Dermatol Online J. 2015;21 [PubMed] [Google Scholar]
- Schlapbach C., Hänni T., Yawalkar N., Hunger R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Schlapbach C., Yawalkar N., Hunger R.E. Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. J Am Acad Dermatol. 2009;61:58–65. doi: 10.1016/j.jaad.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Schmidt K.E., Kuepper J.M., Schumak B., Alferink J., Hofmann A., Howland S.W., et al. Doxycycline inhibits experimental cerebral malaria by reducing inflammatory immune reactions and tissue-degrading mediators. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A.M., Cook L.C., Zhan X., Banerjee K., Cong Z., Imamura-Kawasawa Y., et al. Loss of skin microbial diversity and alteration of bacterial metabolic function in hidradenitis suppurativa. J Invest Dermatol. 2020;140:716–720. doi: 10.1016/j.jid.2019.06.151. [DOI] [PubMed] [Google Scholar]
- Schrader A.M.R., Deckers I.E., van der Zee H.H., Boer J., Prens E.P. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460–467. doi: 10.1016/j.jaad.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Shanmugam V.K., Jones D., McNish S., Bendall M.L., Crandall K.A. Transcriptome patterns in hidradenitis suppurativa: support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clin Exp Dermatol. 2019;44:882–892. doi: 10.1111/ced.13959. [DOI] [PubMed] [Google Scholar]
- Sharon V.R., Garcia M.S., Bagheri S., Goodarzi H., Yang C., Ono Y., et al. Management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92:320–321. doi: 10.2340/00015555-1229. [DOI] [PubMed] [Google Scholar]
- Shu M., Wang Y., Yu J., Kuo S., Coda A., Jiang Y., et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One. 2013;8:e55380. doi: 10.1371/journal.pone.0055380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonart T. Hidradenitis suppurativa and smoking. J Am Acad Dermatol. 2010;62:149–150. doi: 10.1016/j.jaad.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sivanand A., Gulliver W.P., Josan C.K., Alhusayen R., Fleming P.J. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64–72. doi: 10.1177/1203475419874412. [DOI] [PubMed] [Google Scholar]
- Steinhoff J.P., Cilursu A., Falasca G.F., Guzman L., Reginato A.J. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13–22. doi: 10.1097/00124743-200202000-00005. [DOI] [PubMed] [Google Scholar]
- Sun J., Shigemi H., Tanaka Y., Yamauchi T., Ueda T., Iwasaki H. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397–404. doi: 10.1016/j.bbrep.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H.G., Lavelle E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szántó M., Dózsa A., Antal D., Szabó K., Kemény L., Bai P. Targeting the gut-skin axis—probiotics as new tools for skin disorder management? Exp Dermatol. 2019;28:1210–1218. doi: 10.1111/exd.14016. [DOI] [PubMed] [Google Scholar]
- Tan M.G., Shear N.H., Walsh S., Alhusayen R. Acitretin. J Cutan Med Surg. 2017;21:48–53. doi: 10.1177/1203475416659858. [DOI] [PubMed] [Google Scholar]
- Thomi R., Kakeda M., Yawalkar N., Schlapbach C., Hunger R.E. Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2017;31:2091–2096. doi: 10.1111/jdv.14389. [DOI] [PubMed] [Google Scholar]
- Thomi R., Yerly D., Yawalkar N., Simon D., Schlapbach C., Hunger R.E. Interleukin-32 is highly expressed in lesions of hidradenitis suppurativa. Br J Dermatol. 2017;177:1358–1366. doi: 10.1111/bjd.15458. [DOI] [PubMed] [Google Scholar]
- Thompson K.G., Shuster M., Ly B.C., Antonescu C., Florea L., Chien A.L., et al. Variability in skin microbiota between smokers, former smokers, and nonsmokers. J Am Acad Dermatol. 2020;83:942–944. doi: 10.1016/j.jaad.2020.01.042. [DOI] [PubMed] [Google Scholar]
- Thorlacius L., Cohen A.D., Gislason G.H., Jemec G.B.E., Egeberg A. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52–57. doi: 10.1016/j.jid.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Thorlacius L., Theut Riis P., Jemec G.B.E. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 2018;179:182–185. doi: 10.1111/bjd.15769. [DOI] [PubMed] [Google Scholar]
- Tsaousi A., Witte E., Witte K., Röwert-Huber H.J., Volk H.D., Sterry W., et al. MMP8 is increased in lesions and blood of acne inversa patients: a potential link to skin destruction and metabolic alterations. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/4097574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanetakou V., Kanni T., Giatrakou S., Katoulis A., Papadavid E., Netea M.G., et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52–59. doi: 10.1001/jamadermatol.2015.3903. [DOI] [PubMed] [Google Scholar]
- van der Zee H.H., de Ruiter L., van den Broecke D.G., Dik W.A., Laman J.D., Prens E.P. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- van der Zee H.H., de Winter K., van der Woude C.J., Prens E.P. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. Br J Dermatol. 2014;171:673–675. doi: 10.1111/bjd.13002. [DOI] [PubMed] [Google Scholar]
- van der Zee H.H., Laman J.D., Boer J., Prens E.P. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol. 2012;21:735–739. doi: 10.1111/j.1600-0625.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- van der Zee H.H., Laman J.D., de Ruiter L., Dik W.A., Prens E.P. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol. 2012;166:298–305. doi: 10.1111/j.1365-2133.2011.10698.x. [DOI] [PubMed] [Google Scholar]
- Van Raemdonck K., Van den Steen P.E., Liekens S., Van Damme J., Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26:311–327. doi: 10.1016/j.cytogfr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Vazquez B.G., Alikhan A., Weaver A.L., Wetter D.A., Davis M.D. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97–103. doi: 10.1038/jid.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijatov-Djuric G., Doronjski A., Mitic I., Brkic S., Barisic N. Interleukin-17A levels increase in serum of children with juvenile idiopathic arthritis. Arch Rheumatol. 2017;32:234–243. doi: 10.5606/ArchRheumatol.2017.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassova N., Kuhn D., Okoye G.A. Hidradenitis suppurativa disproportionately affects African Americans: a single-center retrospective analysis. Acta Derm Venereol. 2015;95:990–991. doi: 10.2340/00015555-2176. [DOI] [PubMed] [Google Scholar]
- von der Werth J.M., Jemec G.B. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809–813. doi: 10.1046/j.1365-2133.2001.04137.x. [DOI] [PubMed] [Google Scholar]
- von Laffert M., Stadie V., Wohlrab J., Marsch W.C. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164:367–371. doi: 10.1111/j.1365-2133.2010.10034.x. [DOI] [PubMed] [Google Scholar]
- Vossen A.R.J.V., van Doorn M.B.A., van der Zee H.H., Prens E.P. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80–88. doi: 10.1016/j.jaad.2018.06.046. [DOI] [PubMed] [Google Scholar]
- Vossen A.R.J.V., van Straalen K.R., Prens E.P., van der Zee H.H. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155–156. doi: 10.1016/j.jaad.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Vural S., Gündoğdu M., Gökpınar İli E., Durmaz C.D., Vural A., Steinmüller-Magin L., et al. Association of pyrin mutations and autoinflammation with complex phenotype hidradenitis suppurativa: a case-control study. Br J Dermatol. 2019;180:1459–1467. doi: 10.1111/bjd.17466. [DOI] [PubMed] [Google Scholar]
- Wang B., Yang W., Wen W., Sun J., Su B., Liu B., et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- Wang X., Ota N., Manzanillo P., Kates L., Zavala-Solorio J., Eidenschenk C., et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- Weber P., Seyed Jafari S.M., Yawalkar N., Hunger R.E. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: a case series of 9 patients. J Am Acad Dermatol. 2017;76:1189–1191. doi: 10.1016/j.jaad.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Weiss T., Shalit I., Blau H., Werber S., Halperin D., Levitov A., et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob Agents Chemother. 2004;48:1974–1982. doi: 10.1128/AAC.48.6.1974-1982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K., Warszawska K., Hoeflich C., Witte E., Schneider-Burrus S., Witte K., et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228–1239. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]
- Wolk K., Witte E., Warszawska K., Schulze-Tanzil G., Witte K., Philipp S., et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–3581. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- Xu H., Xiao X., He Y., Zhang X., Li C., Mao Q., et al. Increased serum interleukin-6 levels in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2017;34:82–84. doi: 10.5114/ada.2017.65626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas Y., Berent E., Ovadiah H., Azoulay I., Ashkenazi S. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396–398. doi: 10.1128/AAC.50.1.396-398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01746-18. e01746-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis C.C., Bechara F.G., Dickinson-Blok J.L., Gulliver W., Horváth B., Hughes R., et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19–31. doi: 10.1111/jdv.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.