Abstract
The beginning of the end (BOTE) sign has been proposed to describe well-recognized clinical signs of inflammation (including erythema, induration, and scale) that predict imminent resolution of molluscum contagiosum (MC). This phenomenon has never been prospectively studied. An integrated analysis of two prospective, 12-week, randomized, double-blind clinical trials of topical nitric oxide–releasing SB206 gel evaluated an association between BOTE sign and MC lesion reduction. Of 707 randomized patients, ~80% exhibited BOTE signs regardless of treatment assignment. At week 12, MC lesion counts decreased from baseline by 50.7% for baseline BOTE+ versus 29.1% for BOTE– (P = 0.0015) vehicle-treated patients compared with a 63.3% decrease for baseline BOTE+ versus 51.7% for BOTE– (P = 0.0194) SB206-treated patients. Among vehicle-treated patients, 48 (22.3%) who were never BOTE+ had an 18.5% reduction from baseline in MC lesion counts versus a 34.0% reduction in 165 patients (76.7%) who experienced BOTE at any time, suggesting that the projected duration of lesion clearance for patients with 18–20 MC lesions is 15 months for BOTE– versus 6 months for BOTE+ patients. Patients who were both BOTE+ and treated with SB206 had the greatest reduction in MC lesion count. SB206 may trigger BOTE signs and shorten the duration of MC infection. The two studies whose data are analyzed in this study are registered at ClinicalTrials.gov with the identifiers NCT03927703 and NCT03927716
Abbreviations: BOTE, beginning of the end; MC, molluscum contagiosum; NO, nitric oxide; TEAE, treatment-emergent adverse event; Th, T helper
Introduction
Molluscum contagiosum (MC) is a common viral skin infection that most often affects children. Although MC lesions are generally self-limited within 2 years (Olsen et al., 2015), they may develop localized erythema, induration, scale, and even hemorrhagic crusting during the course of the eruption that often prompts urgent evaluation and antibiotic treatment. However, rather than indicating bacterial superinfection, these clinical signs of inflammation are thought to mark a host immune response that heralds the resolution of the viral infection. The term beginning of the end (BOTE), proposed as a name for these localized signs of skin inflammation, is suspected to herald the imminent resolution of the viral infection (Butala et al., 2013). The BOTE sign is characteristically localized to individual lesions and is typically asymptomatic or mildly pruritic (rather than painful). In addition, the BOTE sign is usually self-limited and does not require additional treatment.
Nitric oxide (NO), an endogenous small molecule, plays a role in localized innate immunity by upregulating immediate host responses and has broad-spectrum antimicrobial effects. SB206 is an investigational product that comprises berdazimer sodium, a macromolecule with a polysiloxane backbone covalently bound to N-diazeniumdiolate NO donors, with a hydrogel that promotes NO release on application. In vitro, SB206 demonstrated viricidal effects by significantly reducing human papillomavirus 18 DNA amplification and abrogating progeny virus production (Banerjee et al., 2019). Once-daily topical SB206 12% demonstrated efficacy and tolerability in patients with MC (Hebert et al., 2020) as well as in patients with external genital warts (Tyring et al., 2018). The active ingredient of SB206, berdazimer sodium, has demonstrated significant reductions in T helper (Th)2-, Th22-, Th1-, and Th17-related biomarker expression in patients with atopic dermatitis, suggesting that berdazimer sodium has immune-modulatory functions (Guttman-Yassky et al., 2020) when different concentrations and formulations are used.
The BOTE sign can be modulated by introducing therapy for MC, such as NO-releasing SB206. The analysis presented in this paper used data collected during two randomized controlled trials comparing topical SB206 12% with vehicle applied once daily in children with MC. BOTE status was collected in both trials, and we assessed the impact of BOTE status on the rate of MC lesion clearance (Figure 1). Efficacy and safety data from the two trials are not the focus of the paper; rather, we analyzed data from both studies to evaluate our hypothesis that SB206 promotes the development of BOTE and that the development of BOTE improves MC lesion clearance rates.
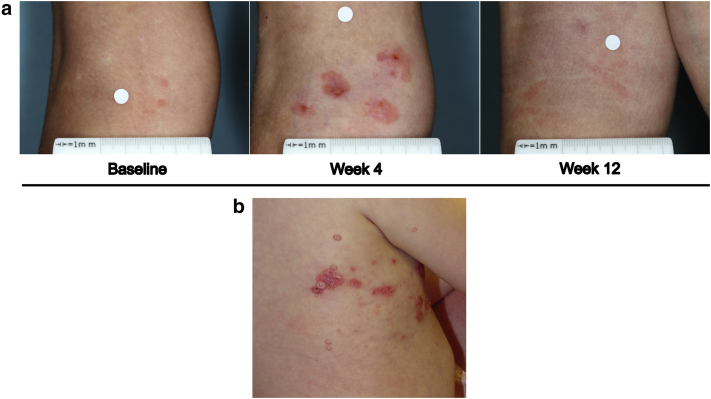
Figure 1.
Example of a BOTE sign. (a) An SB206 clinical trial patient at baseline, week 4, and week 12. Local skin reaction may coexist at week 4. (b) A spontaneous BOTE sign observed in a regular clinical practice patient (not in SB206 MC clinical trials). Patient consent was obtained for the publication of these images. BOTE, beginning of the end; MC, molluscum contagiosum.
Results
Demographic and baseline characteristics for the integrated analysis of both studies are presented in Table 1. At baseline, of 705 patients in the safety population, 454 patients (64.4%) showed no localized BOTE sign (BOTE–), whereas 245 patients (34.8%) showed either mild, moderate, or severe BOTE sign (BOTE = 1, 2, 3; BOTE+). No patients had a very severe BOTE sign (BOTE = 4). The mean duration of MC infection at baseline was approximately 10.8 months for BOTE+ patients, compared with approximately 9.7 months for BOTE– patients.
Table 1.
Demographic and Baseline Characteristics
| Characteristics | Integrated ITT and Safety Population |
||
|---|---|---|---|
| SB206 12% QD | Vehicle QD | Total | |
| ITT population, n | 473 | 234 | 707 |
| Safety population, n | 472 | 233 | 705 |
| Age, y1 | |||
| Mean (SD) | 6.8 (4.8) | 6.8 (4.4) | 6.8 (4.7) |
| Category, n (%) | |||
| <2 | 13 (2.7) | 4 (1.7) | 17 (2.4) |
| ≥2 to <6 | 215 (45.5) | 120 (51.3) | 335 (47.4) |
| ≥6 to <12 | 214 (45.2) | 93 (39.7) | 307 (43.4) |
| ≥12 to <18 | 25 (5.3) | 14 (6.0) | 39 (5.5) |
| ≥18 | 6 (1.3) | 3 (1.3) | 9 (1.3) |
| Baseline lesion count, mean (SD) | 18.4 (14.2) | 17.8 (14.1) | 18.2 (14.2) |
| Baseline BOTE score,2 n (%) | |||
| BOTE– | 311 (65.9) | 143 (61.4) | 454 (64.4) |
| BOTE+ | 157 (33.3) | 88 (37.8) | 245 (34.8) |
| 1 | 112 (23.7) | 60 (25.8) | 172 (24.4) |
| 2 | 43 (9.1) | 21 (9.0) | 64 (9.1) |
| 3 | 2 (0.4) | 7 (3.0) | 9 (1.3) |
| 4 | 0 | 0 | 0 |
| Missing | 4 (0.8) | 2 (0.9) | 6 (0.9) |
Abbreviations: BOTE, beginning of the end; ITT, intention-to-treat; QD, once daily.
Percentages are based on the ITT population.
Percentages are based on the safety population.
BOTE score over time
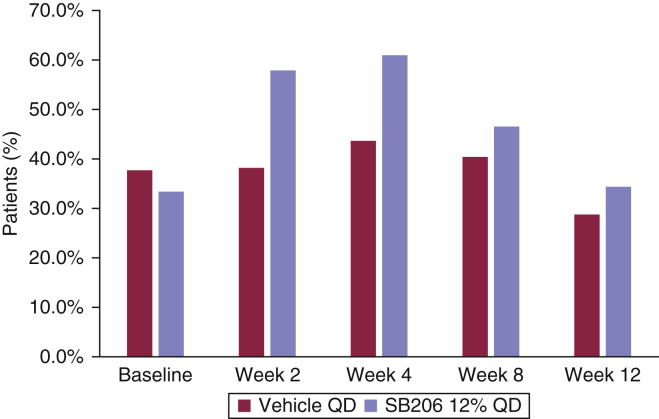
The mean BOTE scores in the vehicle-treated group were lower than those in the SB206-treated group at weeks 2, 4, 8, and 12. The percentage of BOTE+ patients was highest during weeks 2–4 period for SB206 group and during weeks 2–8 period for the vehicle group, with the greatest percentage of BOTE+ patients at week 4 in both groups. The percentage of BOTE+ patients was greater at all time points in the SB206-treated group than in the vehicle-treated group (Figure 2).
Figure 2.
Percentage of BOTE+ patients by week (integrated safety population). The percentage of BOTE+ patients was highest at week 4 for both SB206- and vehicle-treated groups, with a greater percentage of BOTE+ patients in the SB206 group. Integrated safety population: SB206, n = 472; vehicle, n = 233. BOTE, beginning of the end; QD, once daily.
The scoring process for evaluating BOTE signs is described in the Materials and Methods section (Table 2). Grade change in the BOTE score across visits was captured with a shift table (data not shown). No patients in the vehicle-treated group experienced a very severe BOTE score at any time point. Among patients in the SB206-treated group, very severe BOTE scores were reported in three patients (0.7%), in three patients (0.7%), and in two patients (0.5%) at weeks 2, 4, and 8, respectively, but none was reported at week 12. Very severe BOTE scores were reported only once for each of these patients.
Table 2.
BOTE Score
| Score | Global Assessment | Description |
|---|---|---|
| 0 | No inflammation | No evidence of local inflammation |
| 1 | Mild | Minimal erythema and/or edema |
| 2 | Moderate | Definite erythema and/or edema with or without hemorrhagic crusting |
| 3 | Severe | Erythema and edema with definite hemorrhagic crusting |
| 4 | Very Severe | Strong reaction spreading beyond the treated area, bullous reaction, erosions |
Abbreviation: BOTE, beginning of the end.
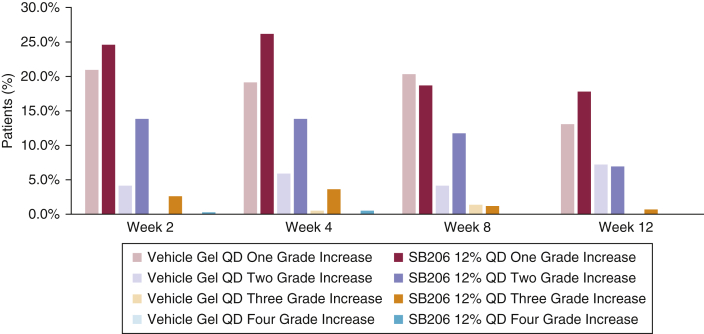
The percentage of patients who showed ≥1-point BOTE score increase from baseline was 28.9%, 31.7%, 31.2% in the vehicle-treated group compared with 57.3%, 61.3%, 43.9% in the SB206-treated group at weeks 2, 4, and 8, respectively. The between-group difference for this parameter decreased by week 12 (27.3% vs. 32.2%), supporting the self-limited nature of the BOTE sign and arguing against cumulative irritant or allergic local skin reactions (Figure 3).
Figure 3.
Change in BOTE grade by visit (integrated ITT population). The percentage of patients who demonstrated ≥1-point BOTE score increase from baseline was 28.9%, 31.7%, and 31.2% in the vehicle-treated group compared with 57.3%, 61.3%, and 43.9% in the SB206-treated group at weeks 2, 4, and 8, respectively. The between-group difference for this parameter decreased by week 12 (27.3% vs. 32.2%). Integrated ITT population: SB206, n = 473; vehicle, n = 234. BOTE, beginning of the end; ITT, intention-to-treat; QD, once daily.
Baseline BOTE status by maximum postbaseline BOTE status is presented in Table 3. Among patients treated with SB206, 15.9% had no BOTE sign (BOTE–) at baseline or at any visit after baseline compared with 22.3% of vehicle-treated patients, indicating that approximately 80% of patients with MC were BOTE+ regardless of treatment type.
Table 3.
Baseline BOTE Status by Maximum Postbaseline BOTE Status (Integrated ITT Population)
| Maximum Postbaseline BOTE Score, n (%) |
||||||
|---|---|---|---|---|---|---|
| Treatment | Baseline BOTE Status | 0 | 1 | 2 | 3 | 4 |
| SB206 12% QD | 0 | 63 (15.9) | 83 (20.9) | 87 (21.9) | 23 (5.8) | 4 (1.0) |
| 1 | 5 (1.3) | 35 (8.8) | 49 (12.3) | 6 (1.5) | 2 (0.5) | |
| 2 | 1 (0.3) | 9 (2.3) | 20 (5.0) | 3 (0.8) | 3 (0.8) | |
| 3 | 0 | 0 | 0 | 1 (0.3) | 0 | |
| Missing | 3 (0.8) | 0 | 0 | 0 | 0 | |
| Vehicle QD | 0 | 48 (22.3) | 47 (21.9) | 34 (15.8) | 4 (1.9) | 0 |
| 1 | 8 (3.7) | 23 (10.7) | 22 (10.2) | 2 (0.9) | 0 | |
| 2 | 3 (1.4) | 7 (3.3) | 8 (3.7) | 0 | 0 | |
| 3 | 0 | 2 (0.9) | 4 (1.9) | 1 (0.5) | 0 | |
| Missing | 0 | 1 (0.5) | 1 (0.5) | 0 | 0 | |
Abbreviations: BOTE, beginning of the end; ITT, intention-to-treat; QD, once daily.
Differentiation of BOTE from medication-related local skin reactions
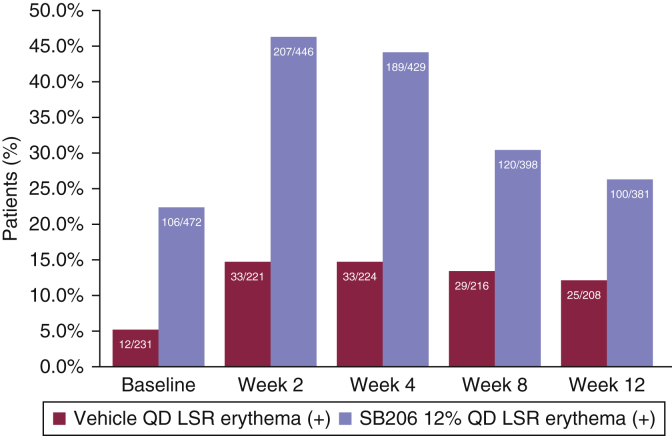
The scoring process for evaluating local skin reactions is described in the Materials and Methods section (Table 4). Erythema was the single major contributor to the total local skin reaction score. Less than 15% of vehicle-treated patients showed a positive erythema component score of local skin reactions, and <50% of SB206-treated patients showed a positive erythema score throughout the studies (Figure 4), suggesting acceptable tolerability of SB206 by 12%. On the other hand, approximately 80% of patients demonstrated BOTE positivity at some point during the studies regardless of the treatment type (Table 3), suggesting that investigators recognized BOTE and tried to differentiate the BOTE inflammatory reaction from local skin reactions.
Table 4.
Local Skin Reaction Score
| Score | Erythema | Flaking and/or Scaling | Crusting | Swelling | Vesiculation and/or Pustulation | Erosion and/or Ulceration |
|---|---|---|---|---|---|---|
| 0 | Not present | Not present | Not present | Not present | Not present | Not present |
| 1 | Slightly pink | Mild, limited | Isolated crusting | Minimal, limited | Fine vesicles | Superficial erosion |
| 2 | Pink or light red | Moderate | Crusting <50% | Mild, palpable | Scant transudate or exudate | Moderate erosion |
| 3 | Red, restricted to the treatment area | Coarse | Crusting >50% | Moderate | Moderate transudate or exudate | Marked, extensive |
| 4 | Red extending outside the treatment area | Scaling extending outside the treatment area | Crusting extending outside the treatment area | Marked swelling extending outside the treatment area | Marked transudate or exudate | Black eschar or ulceration |
Figure 4.
Erythema component score of medication-related LSRs over time. Patients treated with SB206 showed higher LSR composite scores than patients treated with vehicle. The LSR composite score, primarily driven by erythema, peaked at week 2 or 4 and began to steadily decline through week 12. Integrated safety population: SB206, n = 472; vehicle, n = 233. The number of observed patients in each group at each time point is shown within each bar (n/N). The positive sign (+) includes erythema scores of 1, 2, 3, or 4. LSR, local skin reaction; QD, once daily.
Baseline BOTE status and MC lesion reduction
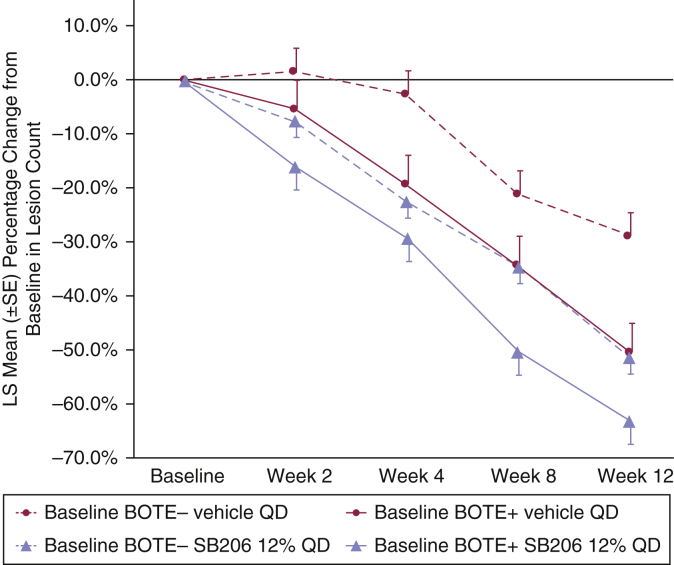
After 12 weeks of vehicle treatment, baseline BOTE+ patients showed 50.7% least-squares mean lesion count reduction from baseline compared with 29.1% for BOTE– patients (P = 0.0015). A slightly smaller rank-order difference in baseline versus that at 12-week lesion counts was seen in the SB206-treated groups by BOTE status: 63.3% for baseline BOTE+ compared with 51.7% for baseline BOTE– patients (P = 0.0194). The magnitude of the differences between the baseline BOTE– and baseline BOTE+ patients in relation to the percentage reduction from baseline in lesion count was greater in the vehicle-treated group than in the SB206-treated group (Figure 5). Over time, patients treated with SB206 showed greater lesion reduction than those treated with vehicle regardless of BOTE status at baseline, whereas SB206-treated patients who were BOTE+ at baseline showed greater lesion reduction than SB206-treated patients who were BOTE– at baseline (Figure 5).
Figure 5.
LS mean percentage change from baseline in MC lesion count over time by baseline BOTE score for integrated ITT population. Vehicle-treated baseline BOTE+ patients showed a greater reduction in lesion count than vehicle-treated baseline BOTE– patients. Over time, patients treated with SB206 showed a greater reduction in lesion count than those treated with vehicle regardless of BOTE status at baseline, whereas SB206-treated patients who were BOTE+ at baseline showed a greater reduction in lesion count than SB206-treated patients who were BOTE– at baseline. Integrated ITT population: SB206, n = 473; vehicle, n = 234. BOTE, beginning of the end; ITT, intention-to-treat; LS, least square; MC, molluscum contagiosum; QD, once daily; SE, standard error.
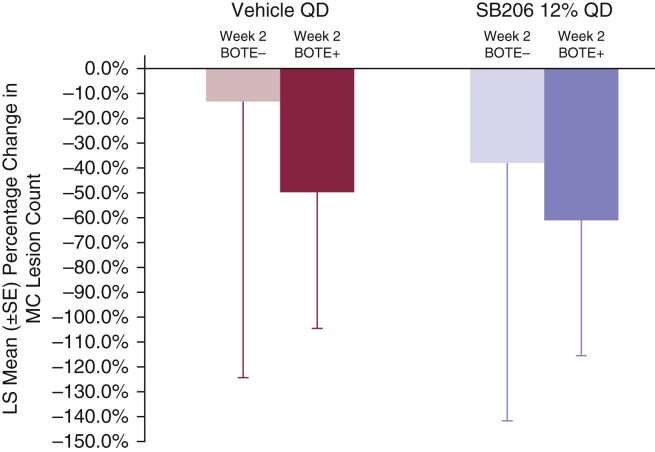
Week 2 BOTE status and MC lesion reduction
The percentage change from baseline in lesion count at week 12 was compared on the basis of the week 2 BOTE status (Figure 6). In the vehicle-treated group, the mean percentage lesion count reduction at week 12 was 13.6% for BOTE– patients (n = 115; 54.2%) compared with 49.4% for BOTE+ patients (n = 97; 45.8%) at week 2. In the SB206-treated group, the mean percentage lesion count reduction at week 12 was 38.2% for BOTE– (n = 164; 42.1%) and 61.4% for BOTE+ (n = 226; 57.9%) patients at week 2. The magnitude of percentage reduction from baseline in MC counts at week 12 was greater for patients who were BOTE+ at week 2 in both groups but highest in the SB206-treated group, regardless of week 2 BOTE status.
Figure 6.
Week 12 mean percentage change from baseline in MC lesion count by week 2 BOTE status. Percentage change from baseline in lesion count at week 12 was compared on the basis of the week 2 BOTE status (Figure 6). In the vehicle-treated group, the mean percentage lesion count reduction at week 12 was 13.6% for BOTE– patients (n = 115; 54.2%) compared with 49.4% for BOTE+ patients (n = 97; 45.8%) at week 2. In the SB206-treated group, the mean percentage lesion count reduction at week 12 was 38.2% for BOTE– patients (n = 164; 42.1%) and 61.4% for BOTE+ patients (n = 226; 57.9%) at week 2. The magnitude of the percentage reduction from baseline in MC counts at week 12 was greater for patients who were BOTE+ at week 2 in both groups but highest in the SB206-treated group, regardless of week 2 BOTE status. Integrated ITT population: SB206, n = 473; vehicle, n = 234. BOTE, beginning of the end; ITT, intention-to-treat; LS, least square; MC, molluscum contagiosum; QD, once daily; SE, standard error.
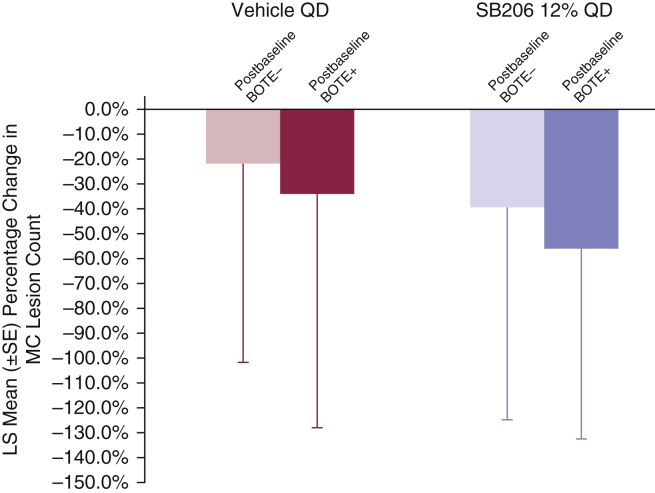
Postbaseline BOTE status and MC lesion reduction
The percentage change in lesion counts between baseline and week 12 was compared by the highest postbaseline BOTE status at any visit (Figure 7). In the vehicle-treated group, there was a 22.0% mean percentage reduction from baseline in lesion counts for patients who were BOTE– after baseline (n = 59; 27.4%), compared with a 33.8% mean reduction for patients with any detectable postbaseline BOTE inflammation (n = 156; 72.6%). In the SB206-treated group, these mean percentage reductions and patient numbers were 39.6% (n = 72; 18.1%) and 54.9% (n = 325; 81.9%) for BOTE– and BOTE+ patients, respectively.
Figure 7.
Week 12 mean percentage change in MC lesion count by maximum postbaseline BOTE status. Percentage change in lesion counts between baseline and week 12 was compared by the highest postbaseline BOTE status at any visit. In the vehicle-treated group, there was a 22.0% mean percentage reduction from baseline in lesion counts for patients who were BOTE– after baseline (n = 59; 27.4%), compared with a 33.8% mean reduction for patients with any detectable postbaseline BOTE inflammation (n = 156; 72.6%). In the SB206-treated group, these mean percentage reductions and patient numbers were 39.6% (n = 72; 18.1%) and 54.9% (n = 325; 81.9%) for BOTE– and BOTE+ patients, respectively. Integrated ITT population: SB206, n = 473; vehicle, n = 234. BOTE, beginning of the end; ITT, intention-to-treat; LS, least square; MC, molluscum contagiosum; QD, once daily; SE, standard error.
Safety
For the safety population, the treatment-emergent adverse events (TEAEs) reported by a total of ≥5% of patients in either treatment group included application-site pain (18.7%) and application-site erythema (11.6%) in the SB206-treated group versus 4.3% and 1.3%, respectively, in the vehicle-treated group. The TEAEs reported by a total of ≥5% of patients in either treatment group are shown by severity in Table 5.
Table 5.
Treatment-Emergent Adverse Events ≥5% in any Treatment Group
| Adverse Events | Integrated Safety Population |
|||||
|---|---|---|---|---|---|---|
| Vehicle QD (n = 233) |
SB206 12% QD (n = 472) |
|||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Application-site pain | 9 (3.9) | 1 (0.4) | 0 | 49 (10.4) | 38 (8.1) | 1 (0.2) |
| Application-site erythema | 2 (0.9) | 1 (0.4) | 0 | 23 (4.9) | 29 (6.1) | 3 (0.6) |
| Application-site scar1 | 19 (8.2) | 0 | 0 | 16 (3.4) | 0 | 0 |
Abbreviations: MC, molluscum contagiosum; QD, once daily.
Values are given as n (%).
Scar includes a small indentation after MC lesion resolution.
Discussion
The BOTE sign is a well-recognized phenomenon that features the clinical signs mimicking secondary bacterial infection but likely represents a host response against the primary viral infection, frequently heralding the sign of imminent resolution of MC lesions. With the development of topical treatment for MC, it is important to recognize the impact of this phenomenon on the natural history of MC infection and distinguish it from a medication-related adverse effect (Butala et al., 2013; Olsen et al., 2015). To our knowledge, the BOTE sign has never been prospectively and systematically evaluated. Our data demonstrated an 80% incidence of the BOTE sign during the 12-week study periods, regardless of active or vehicle treatment, and patients with a longer duration of MC lesions before study entry were more likely to exhibit the BOTE sign.
Lesion count reduction was analyzed in association with BOTE status at different time points, including at baseline; at week 2; and at any visit during the studies, including or excluding the baseline, with consistent results: BOTE+ patients showed more lesion reductions than BOTE– patients. We observed significantly greater reductions in MC lesions among baseline BOTE+ and earlier-onset BOTE+ patients (i.e., patients who were BOTE+ at baseline or at week 2) than among BOTE– patients regardless of treatment. The highest number of patients who achieved lesion reduction was found in those who were BOTE+ and were treated with SB206. SB206-treated patients who were BOTE– at week 2 had a 40% reduction in MC lesions from baseline (Figure 6), and patients who were BOTE– at baseline had a 50% reduction from baseline (Figure 5), suggesting that patients who were BOTE– at baseline may have become BOTE+ between the baseline and week 2 owing to the effect of SB206 (Figure 2), resulting in a greater reduction of MC lesions.
These results support the importance of the BOTE sign as a marker of host immunity against MC infection rather than as a secondary bacterial infection or as a TEAE. Treatment with SB206 may trigger or augment the BOTE sign, with corresponding reductions in MC lesion counts that are highest for patients with baseline or early (as seen at week 2)-onset BOTE sign.
This cohort of patients had an average number of 18‒20 MC lesions at baseline. A large majority experienced the BOTE sign during the 12-week clinical trials. Vehicle-treated patients who did not develop the BOTE sign within 12 weeks experienced an average of 20% reduction in MC lesion count compared with an average of 50% reduction for those who developed a BOTE sign. A higher percentage lesion reduction may predict a shorter duration of MC infection, leading to faster lesion clearance: for BOTE– patients, a 20% reduction in MC lesions within 3 months can be extrapolated to a 100% reduction within 15 months; for BOTE+ patients, a 50% reduction within 3 months predicts a 100% reduction within 6 months, assuming a linear reduction of MC lesions.
Once-daily application of SB206 for up to 12 weeks was associated with an average of 40% MC lesion count reduction for BOTE– patients, suggesting a 7- to 8-month duration to complete resolution. For patients who developed the BOTE sign at any time during the treatment period, lesion reduction averaged 55%, suggesting less than a 6-month duration to complete resolution. In clinical practice, the BOTE sign should be viewed as a switch that portends a rapid improvement at any point the switch is activated. The accelerated resolution of MC lesions associated with SB206 treatment may be due to either immune-enhancing and viricidal effects of NO or due to both. There was a small percentage of patients who discontinued treatment before completing the study at week 12 owing to relatively severe BOTE or local skin reaction and achieved complete MC lesion clearance. Additional studies may help to clarify the need for continued treatment after the emergence of the BOTE sign.
Collective BOTE severity was assessed per patient rather than quantified by the number of lesions that showed the BOTE sign. The results do not provide definitive evidence that the BOTE signs predict complete resolution of MC lesions given the short (12-week) treatment duration. The percentage reduction of MC lesion count was not assessed beyond 12 weeks. It is not known whether 12 weeks of treatment can continuously reduce the lesion counts after treatment discontinuation or whether a longer treatment duration is necessary to achieve complete clearance.
The majority of patients with MC likely experienced mild-to-moderate BOTE signs during the course of this viral skin infection that did not represent secondary bacterial infection or a TEAE in this prospective clinical trial, regardless of active or vehicle treatment. Recognition of the BOTE sign will help clinicians to provide anticipatory guidance for patients with MC, regardless of treatment. Absence of the BOTE sign may be associated with a longer duration of MC infection. Therefore, baseline and emerging BOTE status is an important factor to consider when designing future studies to assess the efficacy and safety of any treatment for MC. Our data indicate that SB206 shortens the duration of MC infection regardless of BOTE status and suggest that this topical NO-releasing treatment may trigger the BOTE sign, thereby enhancing effectiveness.
Materials and Methods
Patients and study designs
Participants in two identical phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group studies (NCT03927716 [NI-MC301] and NCT03927703 [NI-MC302]) were included in this analysis. The patients, aged ≥6 months (MC301 [n = 352] and MC302 [n = 355]), were randomized in a 2:1 ratio to either SB206 12% or vehicle and were treated for 12 weeks. Patients who were receiving treatment for MC entered a washout period of up to 14 days before randomization. Patients from the same household received the same treatment assignment.
Patients or their caregivers applied SB206 or vehicle once daily for 12 weeks to all the MC lesions identified at baseline and to any new lesions that arose during either study. If the investigator determined that all the MC lesions were cleared at a clinic visit, the study drug was discontinued.
Study visits occurred at baseline and at weeks 2, 4, 8, 12, and 24. No study drug treatments were provided after the week 12 visits.
Ethics
Both studies were conducted in accordance with the regulations stated in the Federal Code of Regulations for Good Clinical Practices and the International Conference on Harmonization guidelines and in accordance with all local and national regulations. Institutional review boards reviewed and approved all study materials (Advarra centrally approved NI-MC301 and 302 studies. The Ann & Robert H. Lurie Children’s Hospital of Chicago Institutional Review Board approved the conduct of NI-MC301 study for the institution), and patients and/or their caregivers provided written informed consent and/or assent before screening procedures were initiated in either study. Patient consent was obtained for publication of the images in Figure 1a and b. Both studies were prospectively registered on ClinicalTrials.gov, as follows: NCT03927703 (April 25, 2019) and NCT03927716 (April 25, 2019).
Assessments
BOTE
A training program for investigators included the recognition of MC lesions by visual inspection and palpation, systematic counting of active MC lesions, identification of signs that distinguished medication-related local skin reactions from the BOTE sign, and the use of a 5-point severity scale to rate BOTE components (erythema, edema, crusting, bullous reaction, erosion) (Table 2). The investigator training program included a differentiation between medication-related local skin reactions and BOTE. If there was clinically significant pain (e.g., burning sensation, tickling sensation), it was classified as a local skin reaction. However, local skin reactions and BOTE are not mutually exclusive; thus, there may be an overlap. The BOTE scale was a secondary assessment in the clinical trials and was evaluated at baseline and at weeks 2, 4, 8, and 12.
MC lesion count
Active MC lesions were identified as discrete, waxy, palpable skin-colored, dome-shaped papules averaging 3–5 mm in diameter, often umbilicated, and sometimes with a caseous plug. All active (treatable, raised, and palpable) MC lesions were counted at baseline and at weeks 2, 4, 8, and 12, except those located within 2 cm from eyelid margins owing to safety concerns with an alcohol-based formulation. Lesions were counted using a body map as a tool to document the details of the lesion count. If the investigator could not clearly differentiate the lesions as would be the case for agminated (clustered) lesions, then this type of lesion was counted as one lesion. If the investigator could differentiate and count the umbilical tops of the lesions separately, then each lesion was counted separately. Lesion clearance was defined as complete resolution of active (treatable, raised, and palpable) features. For a given patient, the same evaluator completed the lesion counts at every visit when possible; at a minimum, the same evaluator performed the lesion counts at baseline and at week 12.
Safety and local skin reactions
TEAEs were assessed throughout the study. Local skin reactions were evaluated as the composite score (sum of the component scores), and each component (erythema, flaking and/or scaling, crusting, swelling, vesiculation and/or pustulation, erosion and/or ulceration) was graded 0–4 at baseline and at weeks 2, 4, 8, and 12 (Table 4). When local skin reactions were considered clinically significant, the investigator reported the condition as a TEAE.
Statistical analysis
Statistical analyses were conducted on the integrated data from the intention-to-treat and safety populations of the MC301 and MC302 studies. The intention-to-treat population included all randomized patients, and the safety population included all randomized patients who received at least one application of the study drug.
An overall BOTE status for each patient was categorized as follows: no BOTE sign (BOTE = 0), represented as BOTE–, and mild to very severe BOTE sign (BOTE = 1, 2, 3, and 4), represented as BOTE+. The number of BOTE+ lesions per patient and individual lesion severity were not analyzed.
Continuous variable summaries included the number of patients (n) with nonmissing values, mean, SD, median, and minimum and maximum values. Categorical variable summaries included the frequency and percentage of patients who were in the category or each possible value. In general, the denominator for the percentage calculation was based on the total number of patients in the analysis population within each treatment group, unless otherwise specified. The denominator for by-visit displays was the number of patients in the relevant analysis population with nonmissing data at each visit.
The objective of this integrated analysis was to evaluate our hypothesis that SB206 promotes the development of BOTE and that the development of BOTE improves MC lesion clearance. Efficacy analyses of SB206 versus that of the vehicle will be reported in an upcoming publication. The association between percentage change in lesion count over time and patient BOTE status (BOTE– or BOTE+) was assessed as follows: percentage change in lesion count from baseline in relation to the baseline BOTE status within the treatment groups was evaluated using a repeated-measures mixed model, which included the baseline BOTE status, visit, investigator type (dermatologist vs. other specialty), age, and baseline lesion count as factors with a compound symmetry covariance matrix (the model-based estimated least-squares means were provided). Descriptive statistics were used to summarize the following variables: percentage change in lesion count from baseline in relation to week 2 BOTE status; percentage change in lesion count from baseline in relation to maximum postbaseline BOTE status; and percentage change in lesion count from baseline in relation to maximum BOTE status at any visit, including baseline. Comparisons of MC lesion count reduction from baseline by BOTE positivity with that by BOTE negativity status were made within the treatment groups (either vehicle or SB206); no statistical comparisons between treatment groups were performed.
The incidence of TEAEs ≥5% in any treatment group was summarized for the safety population.
Data availability statement
Datasets related to this article can be requested from Novan, Inc by contacting TMC at tmaeda-chubachi@novan.com. Novan, Inc will assess each request on a case-by-case basis.
ORCIDs
Tomoko Maeda-Chubachi: https://orcid.org/0000-0002-3614-1505
David Hebert: https://orcid.org/0000-0002-4904-6929
Elizabeth Messersmith: https://orcid.org/0000-0002-6731-0770
Elaine C. Siegfried: https://orcid.org/0000-0002-4794-9905
Author Contributions
Conceptualization: TMC, ECS; Data Curation: DH; Formal Analysis: TMC, DH, ECS, EM; Funding Acquisition: EM; Investigation: TMC, ECS; Methodology: TMC, DH, ECS; Project Administration: TMC, DH, EM; Resources: TMC, EM; Software: DH; Supervision: TMC, ECS, EM; Validation: DH; Visualization: TMC, DH; Writing - Original Draft Preparation: TMC; Writing - Review and Editing: TMC, DH, ECS, EM
Acknowledgments
Novan was involved in the design and conduct of both clinical trials; the collection, management, analysis, and interpretation of the integrated data set; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication. We are grateful to the patients and caregivers who gave the gift of clinical research participation. In addition, we thank all the principal investigators for their contributions to the studies: Mark Ling (Newnan, GA), Cindy Owen (Louisville, KY), Marina Raihkel (Lomita, CA), James Del Rosso (Las Vegas, NV), Janet Dubois (Austin, TX), Aldo Trovato (Mobile, AL), Loretta Gremillion (Monroe, LA), John Browning Jr (San Antonio, TX), Frank Calcagno (Gresham, OR), Daniel Stewart (Clinton Township, MI), Scott Fretzin (Indianapolis, IN), Paul Gillum (Norman, OK), Robert Clifford (Charleston, SC), Ana Elosegui (Miami, FL), Suzanne Bruce (Katy, TX), Jess DeMaria (Dublin, OH), Cheryl Hull (Rogers, AR), Barry Kuttner (West Palm Beach, FL), Todd Bertoch (Salt Lake City, UT), Walter Pharr (Greensboro, NC), Amy Paller (Chicago, IL), Kappa Peddy (Lynchburg, VA), Angel Rico (Miami Lakes, FL), Ann Reed (Boca Raton, FL), David Brougher (Evansville, IN), Joseph Ley (Kingsport, TN), Matthew Miller (Fountain Inn, SC), David Greenstein (Beverly, MA), Andrea Mabry (Little Rock, AR), Darvy Mann (Longview, TX), Ryan Harris (Boise, ID), Pearl Kwong (Jacksonville, FL), Gabrielle Virgo (Silver Spring, MD), John Kelly (Warwick, RI), and James Pehoushek (Glendale, AZ) for NI-MC301. Dowling Stough (Bryant, AR), John Tu (Rochester, NY), Walter Nahm (San Diego, CA), Steven Kempers (Fridley, MN), Stephen Tyring (Houston, TX), Adelaide Hebert (Houston, TX), Megan Landis (New Albany, IN), Michael Hassman (Berlin, NJ), Joel Schlessinger (Omaha, NE), Seemal Desai (Plano, TX), Stephen Stripling (Mt. Pleasant, SC), Joe Williams (Thornton, CO), Brenda LaTowsky (Scottsdale, AZ), John Scott (Richmond, VA), Joshua Berlin (Boynton Beach, FL), Kimberly Grande (Knoxville, TN), Wendy McFalda (Clarkston, MI), Sharleen St. Surin-Lord (Largo, MD), Lazaro Nunez (Miami, FL), Peter Silas (Syracuse, UT), David Stoll (Beverly Hills, CA), William Coleman IV (Metairie, LA), Theresa Knoepp (Anderson, SC), Michael Bell (Deland, FL), Scott Katz (Plano, TX), Christopher Sartore (Owensboro, KY), Mary Lupo (New Orleans, LA), Rania Agha (Oakbrook Terrace, IL), Kimberly Brown-Gullatt (Columbus, GA), Abel Jarell (Portsmouth, NH), Seth Forman (Tampa, FL), Anton Duke (Bryant, AR), Fredric Garner (Burke, VA), Benjamin Ehst (Portland, OR), and Scott Guenthner (Plainfield, IN) for NI-MC302. This study was reviewed and approved by Advarra IRB (Pro00033404 for NI-MC301 and Pro00033266 for NI-MC302). The studies were funded by Novan, Inc.
Conflict of Interest
ECS has served as a consultant for Dermavant Sciences, GlaxoSmithKline, LEO Pharma, Novan, Pierre Fabre Dermo-Cosmétique US, Pfizer, Regeneron, Sanofi, UCB, Valeant Pharmaceuticals, and Verrica Pharmaceuticals; as a principal investigator for Allergan, Amgen, Eli Lilly, Janssen Pharmaceuticals, Mayne Pharma Group, Medimetriks Pharmaceuticals, Pfizer, Pierre Fabre Dermo-Cosmétique US, Regeneron, and Verrica Pharmaceuticals; as a Data Safety Monitoring Board member for LEO Pharma, Pfizer, and Novan; and as an advisory board member for Verrica Pharmaceuticals. DH and TMC are employees and stockholders in Novan. EM was an employee of Novan at the time the analysis was completed and the first draft of the manuscript was submitted and is a stockholder in Novan.
accepted manuscript published online 5 May 2021; corrected proof published online 12 June 2021
Footnotes
Cite this article as: JID Innovations 2021;X:100019
References
- Banerjee N.S., Moore D.W., Wang H.K., Broker T.R., Chow L.T. NVN1000, a novel nitric oxide-releasing compound, inhibits HPV-18 virus production by interfering with E6 and E7 oncoprotein functions. Antiviral Res. 2019;170:104559. doi: 10.1016/j.antiviral.2019.104559. [DOI] [PubMed] [Google Scholar]
- Butala N., Siegfried E., Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:e1650–e1653. doi: 10.1542/peds.2012-2933. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E., Gallo R.L., Pavel A.B., Nakatsuji T., Li R., Zhang N., et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531–2535.e2. doi: 10.1016/j.jid.2020.04.013. [DOI] [PubMed] [Google Scholar]
- Hebert A.A., Siegfried E.C., Durham T., de León E.N., Reams T., Messersmith E., et al. Efficacy and tolerability of an investigational nitric oxide-releasing topical gel in patients with molluscum contagiosum: a randomized clinical trial. J Am Acad Dermatol. 2020;82:887–894. doi: 10.1016/j.jaad.2019.09.064. [DOI] [PubMed] [Google Scholar]
- Olsen J.R., Gallacher J., Finlay A.Y., Piguet V., Francis N.A. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190–195. doi: 10.1016/S1473-3099(14)71053-9. [DOI] [PubMed] [Google Scholar]
- Tyring S.K., Rosen T., Berman B., Stasko N., Durham T., Maeda-Chubachi T. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100–1105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets related to this article can be requested from Novan, Inc by contacting TMC at tmaeda-chubachi@novan.com. Novan, Inc will assess each request on a case-by-case basis.