Abstract
Bullous pemphigoid (BP) is an autoimmune blistering disease resulting in pruritus and cutaneous blistering. Longitudinal studies characterizing the disease course of patients with BP on conventional therapy are lacking. We sought to characterize the changes in disease activity and pruritus of patients with BP on standard-of-care treatments. We conducted a retrospective cohort study on patients with BP on standard-of-care therapy. Generalized Estimating Equations were used to estimate the mean and standard errors for Bullous Pemphigoid Disease Activity Index (BPDAI) total activity score, BPDAI pruritus component score, and anti-BP180 autoantibody levels (BP180) over time. A total of 80 patients with BP showed consistent reductions in BPDAI total activity score and BPDAI pruritus component score, with a nadir at 4 months. BP180 decreased over time, with the largest reductions at 6 and 9 months. Median partial/complete remission was at 6.7 months, with relapses at a median time of 15.9 months. Receiving operating characteristic analysis determined an optimal BPDAI total activity score cutoff of 3.3 to discriminate partial/complete remission incidence (area under the curve = 0.895, sensitivity = 0.844, specificity = 0.78). In conclusion, in patients with BP on standard-of-care therapy, a natural course of BPDAI total activity score and BPDAI pruritus component score over time was comprehensively projected. BPDAI ≤ 3.3 was associated with partial/complete remission. These results provide reference data to guide future clinical trial design for BP.
Abbreviations: BP, bullous pemphigoid; BPDAI, Bullous Pemphigoid Disease Area Index; GEE, Generalized Estimating Equations; IQR, interquartile range; PCS, pruritus component score; P/C, partial/complete; TAS, total activity score
Introduction
Bullous pemphigoid (BP) is a rare, subepidermal autoimmune blistering disease that results in debilitating symptoms, including pruritus and cutaneous blistering, with a significant impact on QOL. Standard-of-care therapies generally consist of conventional immune-suppressing medications with limited data regarding treatment course and factors influencing disease outcome over time.
The Bullous Pemphigoid Disease Area Index (BPDAI) is a clinical assessment tool that provides objective disease activity and subjective pruritus scores, has demonstrated excellent inter-rater reliability, and correlates with QOL measures specific to autoimmune blistering diseases (Murrell et al., 2012; Patsatsi et al., 2017; Wijayanti et al., 2017). Few studies have evaluated the disease course of BP using a validated clinical measure such as BPDAI, thus making optimal clinical trial designs challenging. In this study, we retrospectively characterized the disease course of patients with BP on standard-of-care therapies evaluating BPDAI and anti-BP180 autoantibodies (BP180) to inform appropriate clinical outcomes.
Results
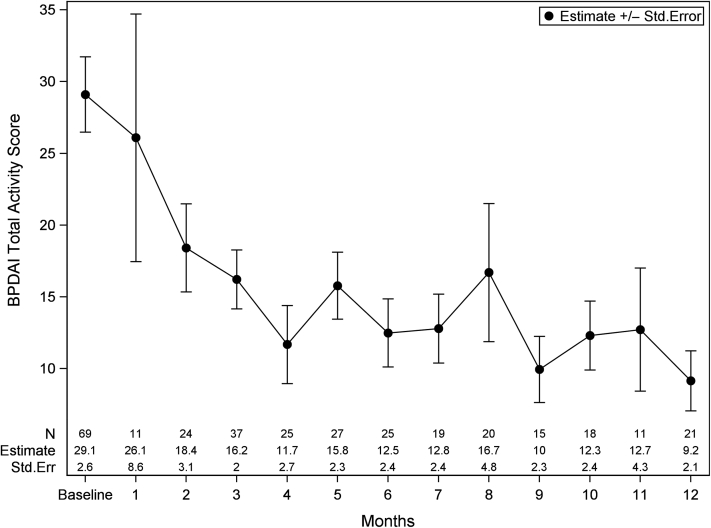
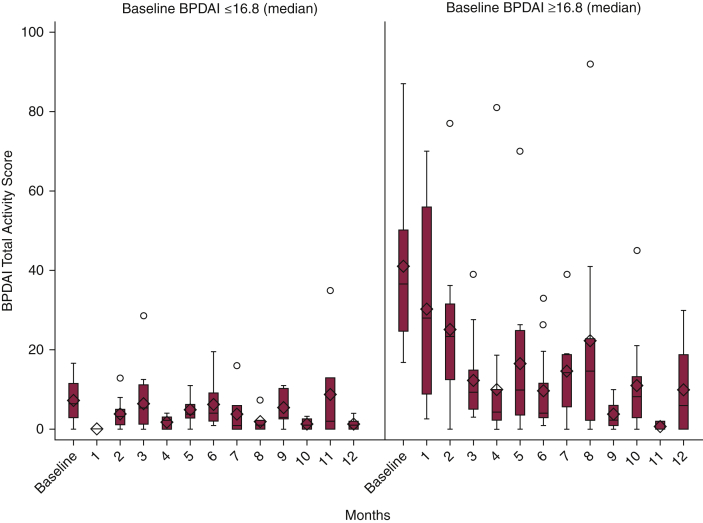
The study population included 80 patients with BP with baseline demographic characteristics shown in Table 1. This cohort was predominantly female (57.5%) with a mean age of 73.5 ± 11.8 years. The majority of patients were white (62.5%), and clinical status was divided into those who achieved and maintained partial/complete (P/C) remission (40.0%), those who achieved P/C remission and relapsed (32.5%), and those who never achieved P/C remission (27.5%). The most common systemic therapies were prednisone (85.0%), followed by rituximab (28.8%) and mycophenolate mofetil (30.0%). On the basis of the raw data, the greatest improvement from baseline in BPDAI total activity score (TAS) occurred at month 4 with median scores from 16.8 (interquartile range [IQR] = 6.9–36.6) to 3.6 (IQR = 2.0–5.9) with a percentage reduction of 78.6%. Figure 1 was based on the estimation from the Generalized Estimating Equations (GEE) model; we observed a consistent decline in BPDAI TAS over a 12-month period. The difference between month 4 (11.7 ± 2.7) and baseline (29.1 ± 2.6) was statistically significant (P < 0.001). At the time of this study, there were no validated severity bands for mild to moderate BPDAI TAS; thus, to evaluate the differences in baseline severity, patients were divided into two groups by the median baseline BPDAI TAS. A similar effect was seen but to a greater degree in patients with more severe disease at baseline, with the median group mentioned earlier having an 88.1% BPDAI TAS reduction by 4 months on the basis of raw data versus 66.7% reduction in the group with scores below the median (Figure 2). Overall, 60.6% of patients achieved a 50% reduction in BPDAI TAS, and 45.5% of patients achieved a 75% reduction by 4 months with continued improvement to month 12. No significant differences were noted between treatment modalities. BPDAI TAS was significantly higher at baseline in black patients than in white patients (25.0 [IQR = 8.0–44.3] vs. 12.9 [IQR = 5.3–24.6], P = 0.035) with no significant difference at month 4. The median BPDAI TAS scores at the time of relapse were 7.3 (2.3–12.0) compared with those of one visit before (median 0 [IQR = 0–2.0]). A receiver operating characteristic analysis determined an optimal BPDAI cutoff of 3.3 to discriminate P/C remission incidence (area under the curve = 0.895, sensitivity = 0.844 [95% confidence interval = 0.787–0.891], specificity = 0.780 [95% confidence interval = 0.731–0.824]).
Table 1.
Demographic and Clinical Characteristics of the Study Population at Baseline
| Clinical Values | All Patients (N = 80) |
|---|---|
| Age, y | 73.5 ± 11.8 |
| Sex | |
| Male | 34 (42.5%) |
| Female | 46 (57.5%) |
| Race | |
| White | 50 (62.5%) |
| Black | 27 (33.8%) |
| Asian | 3 (3.7%) |
| Remission status | |
| Achieved and maintained P/C remission | 32 (40.0%) |
| Achieved P/C remission and relapsed | 26 (32.5%) |
| Never achieved P/C remission | 22 (27.5%) |
| Therapies1 | |
| Prednisone | 68 (85.0%) |
| Rituximab | 23 (28.8%) |
| Mycophenolate mofetil | 24 (30.0%) |
| Methotrexate | 9 (11.3%) |
| Dapsone | 9 (11.3%) |
| Azathioprine | 10 (12.5%) |
| Doxycycline | 16 (20.0%) |
| IVIg | 3 (3.8%) |
| Sulfasalazine | 3 (3.8%) |
| Other immune modulating therapy | 12 (15.0%) |
| BPDAI TAS | 16.8 (6.9–36.6) |
| BPDAI PCS | 20.0 (9.0–26.0) |
| BP180, U/ml | 41.0 (0–115.0) |
| BP230, U/ml | 0 (0–38.0) |
| Duration of follow-up, mo | 15.05 (6.0–34.5) |
Abbreviations: BPDAI, Bullous Pemphigoid Disease Area Index; PCS, pruritus component score; P/C, partial/complete; Q, quartile; TAS, total activity score.
Data are presented as mean ± SD for normally distributed variables, median (Q1, Q3) for asymmetrically distributed variables, or count (percentage) for categorical variables. BPDAI scores were calculated according to Murrell et al. (2012).
Sums to greater than 100% owing to patients on dual therapy.
Figure 1.
BPDAI TAS from baseline to 12 months. Data are presented as estimate ± Std. Err from the GEE model, which was controlled for age, sex, and race. BPDAI, Bullous Pemphigoid Disease Area Index; GEE, Generalized Estimating Equations; Std. Err, standard error; TAS, total activity score.
Figure 2.
BPDAI TAS from baseline to 12 months by baseline BPDAI TAS. Note: groups were defined as BPDAI total activity score at baseline above and below the median (16.8, IQR = 6.9–36.6); data are presented as estimate ± Std. Err from the raw data. BPDAI, Bullous Pemphigoid Disease Area Index; IQR, interquartile range; Std. Err, standard error; TAS, total activity score.
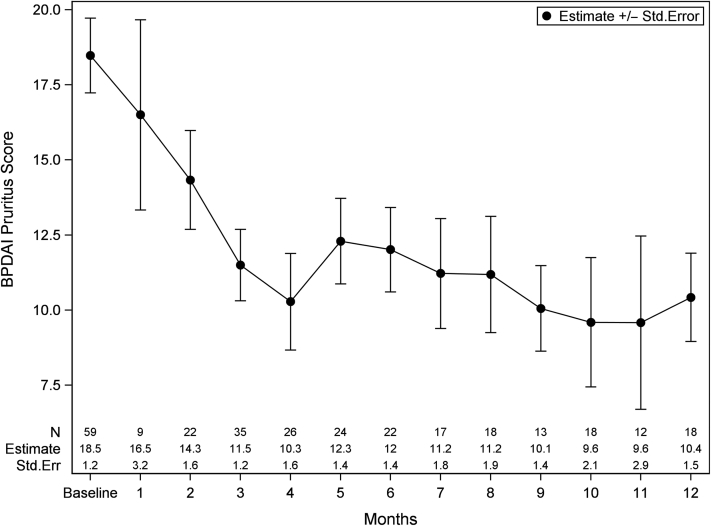
The median BPDAI pruritus component score (PCS) at baseline was 20.0 (IQR = 9.0–26.0) with decreases from baseline until month 4 (8.0 [IQR = 0–15.0]) and subsequent stabilization until 12 months. The percentage reduction of BPDAI PCS at month 4 was 42%, with a similar trend observed in patients above and below the median. On the basis of a GEE model (Figure 3), the estimated mean and standard error were 18.5 (1.2) at baseline and 10.3 (1.6) at month 4 (P < 0.001).
Figure 3.
BPDAI pruritus score from baseline to 12 months. Data are presented as estimate ± Std. Err from the GEE model, which was controlled for age, sex, and race. BPDAI, Bullous Pemphigoid Disease Area Index; GEE, Generalized Estimating Equations; PCS, pruritus component score; Std. Err, standard error.
Overall, 40.4% of patients achieved 50% reduction in BPDAI PCS, and 21.15% of patients achieved 75% reduction by 4 months. BPDAI PCS was positively correlated with BPDAI TAS (r = 0.43; P < 0.001), with correlations significant within subgroups of patients who received prednisone (r = 0.43; P < 0.001), adjuvant therapy (r = 0.47; P < 0.001), and previous rituximab infusion (r = 0.46, P < 0.001). Pruritus was significantly higher among black patients at baseline (24.5 [IQR = 17.5–29.5] vs. 17.0 [IQR = 8.0–23.0], P = 0.042) and at month 4 (17.0 [IQR = 15.0–26.0] vs. 3.0 [IQR = 0–10.0], P = 0.010).
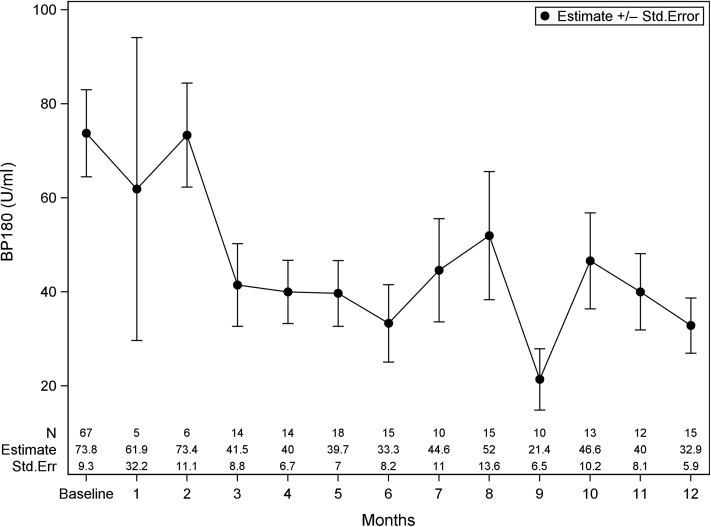
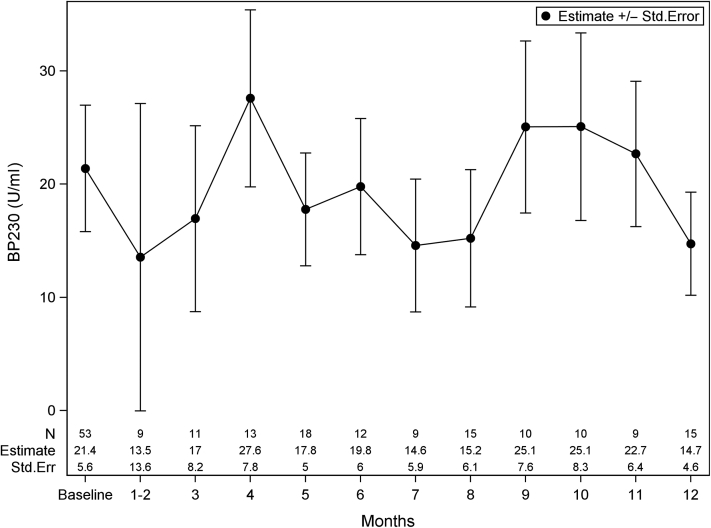
The median baseline BP180 in our cohort was 41.0 U/ml (IQR = 0–115.0). BP180 was found to consistently decrease from baseline, with nadirs at month 6 (21.00 [IQR = 12.0–29.0]) and month 9 (0, IQR = 0–14.0). On the basis of the GEE model, Figure 4 shows these trends in BP180 over time. BP180 significantly correlated with BPDAI TAS (r = 0.62; P < 0.001) and BPDAI PCS (r = 0.31; P < 0.001). BP180 in black patients was significantly higher than that in white patients at baseline (103.0 [IQR = 15.4–175.0] vs. 22.0 [IQR = 0–81.0], P = 0.039) with no difference at month 4. On the basis of the raw data, the median baseline BP230 was 0 (IQR = 0–38.), with no consistent pattern observed in changes over time, likely owing to fewer observations. Figure 5 shows the GEE model for BP230 over time; note that months 1 and 2 were binned owing to scarce data points. There were no differences in BPDAI TAS, BPDAI PCS, BP180, or BP230 by clinical status at baseline or month 4; patients who did not achieve P/C remission trended toward higher BPDAI TAS scores at month 4, although this did not reach statistical significance (Table 2).
Figure 4.
Anti-BP180 IgG antibody titer from baseline to 12 months. Note: data are presented as estimate ± Std. Err from the GEE model, which was controlled for age, sex, and race. GEE, Generalized Estimating Equations; Std. Err, standard error.
Figure 5.
Anti-BP230 IgG antibody titer from baseline to 12 months. Note: data are presented as estimate ± Std. Err from the GEE model, which was controlled for age, sex, and race. Months 1 and 2 were binned owing to the scarce data points. GEE, Generalized Estimating Equations; Std. Err, standard error.
Table 2.
Univariate Associations of Clinical Factors by Clinical Status at Baseline and Month 4
|
Clinical Factor |
Baseline |
Month 4 |
||||||
|---|---|---|---|---|---|---|---|---|
| Never Achieved P/C Remission | Achieved P/C Remission and Relapsed | Achieved and Maintained P/C Remission | P-Value | Never Achieved P/C Remission | Achieved P/C Remission and Relapsed | Achieved and Maintained P/C Remission | P-Value | |
| BPDAI TAS | 21 (7–47.6) | 16.1 (8–35) | 16.9 (5–40) | 0.816 | 5.9 (5–81) | 3.9 (3–5) | 2.9 (1–4.8) | 0.080 |
| BPDAI PCS | 18 (11–26) | 21 (9–28) | 19 (8–24) | 0.620 | 0 (0–30) | 9.5 (6–15) | 3 (0–15) | 0.592 |
| Anti-BP180 IgG (U/ml) | 50 (0–88) | 62 (12–147) | 19 (0–107) | 0.573 | 18.5 (10–27) | 12.5 (10–15) | 45 (26–54) | 0.288 |
| Anti-BP230 IgG (U/ml) | 0 (0–0) | 0 (0–29) | 0 (0–50) | 0.071 | 0 (0–0) | 36.5 (19–54) | 21 (10–40) | 0.351 |
Abbreviations: BPDAI, Bullous Pemphigoid Disease Area Index; PCS, pruritus component score; P/C, partial/complete; Q, quartile; TAS, total activity score.
At baseline: never achieved P/C remission, n = 22; achieved P/C remission and relapsed, n = 26; and achieved and maintained P/C remission, n = 32. Data are presented as median (Q1, Q3); nonparametric values calculated using the Kruskal‒Wallis test.
Discussion
Owing to the limited number of longitudinal studies evaluating BPDAI over time, this study sought to characterize the disease course of patients with BP on standard-of-care therapies. In this retrospective longitudinal study of 80 patients with BP, we observed consistent reductions in BPDAI TAS/PCS and BP180 over a 12-month period, with initial nadirs at 4 and 6 months, respectively. This trend was consistently observed despite the use of different systemic therapies, including rituximab. Patients with more severe baseline disease tended to have greater and more rapid reductions in BPDAI TAS, likely influenced by the use of more aggressive immunomodulatory therapies; however, the nadir at 4 months was consistently noted (Figure 2). These findings suggest that 16 weeks is an appropriate duration to detect a clinical response.
Patients with BP describe significant QOL impact from itching, and the use of itching as an outcome in BP studies is lacking (Cole et al., 2020; Sebaratnam et al., 2013). BPDAI PCS is a subjective measurement of itch using a visual analog scale and is a summary of scores over the past 24 hours, week, and month. Similar to BPDAI TAS, we found consistent reductions in BPDAI PCS by 4 months. However, over the 12-month period, patients with low BPDAI TAS surprisingly still experienced a persistent itch burden. This result suggests possible consequences of other factors in addition to disease activity, but future studies will need to clarify this discrepancy. In general, BPDAI PCS is a simple measurement tool to provide information on subjective itch burden and may be appropriate as secondary outcomes in clinical trials.
We attempted to compare the clinical factors associated with P/C remission and relapse at baseline and at the 4-month time period (Table 2). The median time to relapse was 15.9 months (IQR = 12.4–26.9), and no correlations were observed between baseline characteristics and predictive of relapse, including sex, age, BPDAI PCS, BP180, and BP230, which is in contrast to the findings of a previous study suggesting increased baseline BP180 being predictive of relapse (Koga et al., 2018). The BPDAI TAS was noted to increase at the time of relapse from a median of 0 to 7.3, which is consistent with the findings of previous studies showing worsening disease associated with an increase in BPDAI TAS greater than 3 (Wijayanti et al., 2017). The median time to P/C remission was 6.7 months (IQR = 3.9–11.5), and BPDAI TAS at 4 months trended toward significance in those patients who achieved P/C remission (P = 0.080). Additional studies with larger sample sizes are needed to clarify this observation (Patsatsi et al., 2017; Sebaratnam et al., 2013; Wijayanti et al., 2017). Previous studies evaluating change in BPDAI over time had defined a cut-off value for severe disease as BPDAI TAS > 56, and more recently, an international validation study refined further cutoffs for both mild (0–20) and moderate (20–56) disease, whereas banding for remission was lacking (Lévy-Sitbon et al., 2014; Masmoudi et al., 2021). Although our dataset did not allow for defining specific cutoffs for BPDAI severity, our data do suggest a BPDAI TAS score of 3.3 as a precise predictor of P/C remission for potential use in clinical trials.
Regarding race, BPDAI TAS and BP180 were significantly higher in black patients at baseline than in white patients, with normalization by 4 months with treatment. Interestingly, BPDAI PCS was persistently elevated in black patients compared with that in whites, independent of therapy. These findings are to our knowledge previously unreported in BP and mirror previous reports suggesting a greater burden of pruritus in non-whites, particularly in African Americans (Shaw et al., 2017). Additional studies are warranted to explore the impacts of race on clinical outcomes in BP.
Limitations of this study included a single site in a tertiary referral academic center, the retrospective nature of the study with missing data, and a relatively small number of patients with severe BP. However, the relative diversity of our patient population was informative. In conclusion, we observed consistent reductions in BPDAI TAS/PCS and BP180 in patients treated with standard-of-care therapy, and this study provides important data to further inform the study design of much-needed future clinical trials.
Materials and Methods
This study was approved by the Emory Institutional Review Board (#IRB00054860). After obtaining Institutional Review Board approval, a retrospective chart review was performed on 142 patients with a clinical diagnosis of BP treated at the Autoimmune Blistering Disease clinic, Emory University between June 15, 2012 (when the BPDAI was adopted) and February 1, 2019. All subjects were aged ≥18 years with a histopathologic and/or serologic diagnosis of BP. In addition, patients were required to have at least two visits to be included in this study. Some patients were initially seen at our institution but not at the autoimmune blistering disease specialty clinic and did not have documented BPDAI scores at baseline. Clinical records were queried for demographic information, treatments, clinical parameters (BPDAI TAS and BPDAI PCS), and anti-BP180/-BP230 autoantibodies (BP180/BP230). BPDAI and clinical status (baseline, flare, or P/C remission) were scored in accordance with consensus definitions (Murrell et al., 2012). A total of 80 patients with BP on standard-of-care therapies were included in the study. Of note, 20 of these patients treated with rituximab were included in a previous publication (Polansky et al., 2019). All patients included in this study were prescribed medium-to-high potency topical corticosteroids in addition to any systemic therapies as is standard in our clinical practice.
Statistical analysis was conducted using SAS, version 9.4, and SAS macros developed by Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute (Atlanta, GA) (Liu et al., 2018). Descriptive statistics (mean, SD, median, 25th and 75th percentile (quartile 1–quartile 3), range, count, and percentage) were summarized for baseline characteristics. Univariate analysis comparing clinical factors by clinical status at baseline and month 4 was performed using Kruskal‒Wallis test. Because clinical parameters were measured longitudinally over multiple clinical visits, GEE (Hardin, 2003), a marginal model with a robust sandwich variance estimator, was used to estimate the population-averaged means and standard errors at each time, controlling for baseline race, sex, and age. The correlation structure among the repeated visits for the same patients was determined that minimized the Akaike’s information criterion. A receiver operating characteristic analysis (Fawcett, 2006) was calculated to determine an optimum cutoff (Youden’s index) for BPDAI TAS using P/C remission status as the outcome. Pearson’s correlation coefficient was used to describe the association between two continuous measurements, for example, BPDAI TAS and BP180. P ≤ 0.05 was considered statistically significant.
Data availability statement
No large datasets were generated or analyzed during this study. Limited datasets necessary to interpret and/or replicate data in this paper are available on request to the corresponding author.
ORCIDs
Emily F. Cole: http://orcid.org/0000-0002-3043-3152
Taryn DeGrazia: http://orcid.org/0000-0002-1537-3374
Yuxian Sun: http://orcid.org/0000-0002-6061-8457
Yuan Liu: http://orcid.org/0000-0001-8926-3058
Ron Feldman: http://orcid.org/0000-0001-5766-5132
Author Contributions
Conceptualization: RJF, EFC, TD; Data Curation: TD, EFC, YL; Formal Analysis: YL, YS; Investigation: TD, EFC; Methodology: RJF, EFC; Project Administration: RJF, EFC; Resources: RJF, YL; Software: YL; Supervision: RJF; Validation: YL, YS; Visualization: YL, YS; Writing - Original Draft Preparation: EFC, RJF; Writing - Review and Editing: EFC, RJF, YL
Conflict of Interest
The authors state no conflict of interest.
accepted manuscript published online XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2021;X:100050
References
- Cole E.F., DeGrazia T., AlShamekh S., Feldman R. Itch-related quality of life impact across 3 autoimmune blistering diseases: a retrospective cohort study. Itch. 2020;5:e39. [Google Scholar]
- Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;27:861–874. [Google Scholar]
- Hardin J.W., Hibe J.M. Chapman and Hall/CRC; London: 2003. Generalized estimating equations. [Google Scholar]
- Koga H., Teye K., Ishii N., Ohata C., Nakama T. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne) 2018;5:139. doi: 10.3389/fmed.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Sitbon C., Barbe C., Plee J., Goeldel A.L., Antonicelli F., Reguiaï Z., et al. Assessment of bullous pemphigoid disease area index during treatment: a prospective study of 30 patients. Dermatology. 2014;229:116–122. doi: 10.1159/000362717. [DOI] [PubMed] [Google Scholar]
- Liu Y., Nickleach D.C., Zhang C., Switchenko J.M., Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros. F1000Res. 2018;7:1955. doi: 10.12688/f1000research.16866.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi W., Vaillant M., Vassileva S., Patsatsi A., Quereux G., Moltrasio C., et al. International validation of the Bullous pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106–1112. doi: 10.1111/bjd.19611. [DOI] [PubMed] [Google Scholar]
- Murrell D.F., Daniel B.S., Joly P., Borradori L., Amagai M., Hashimoto T., et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66:479–485. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsatsi A., Kokolios M., Kyriakou A., Lamprou F., Stylianidou D., Tsapas A., et al. Quality of life in Greek patients with autoimmune bullous diseases assessed with ABQOL and TABQOL indexes. Acta Derm Venereol. 2017;97:1145–1147. doi: 10.2340/00015555-2737. [DOI] [PubMed] [Google Scholar]
- Polansky M., Eisenstadt R., DeGrazia T., Zhao X., Liu Y., Feldman R. Rituximab therapy in patients with bullous pemphigoid: a retrospective study of 20 patients. J Am Acad Dermatol. 2019;81:179–186. doi: 10.1016/j.jaad.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Sebaratnam D.F., Hanna A.M., Chee S.N., Frew J.W., Venugopal S.S., Daniel B.S., et al. Development of a quality-of-life instrument for autoimmune bullous disease: the Autoimmune Bullous Disease Quality of Life Questionnaire. JAMA Dermatol. 2013;149:1186–1191. doi: 10.1001/jamadermatol.2013.4972. [DOI] [PubMed] [Google Scholar]
- Shaw F.M., Luk K.M.H., Chen K.H., Wrenn G., Chen S.C. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63–69. doi: 10.1016/j.jaad.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Wijayanti A., Zhao C.Y., Boettiger D., Chiang Y.Z., Ishii N., Hashimoto T., et al. The reliability, validity and responsiveness of two disease scores (BPDAI and ABSIS) for bullous pemphigoid: which one to use? Acta Derm Venereol. 2017;97:24–31. doi: 10.2340/00015555-2473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No large datasets were generated or analyzed during this study. Limited datasets necessary to interpret and/or replicate data in this paper are available on request to the corresponding author.