Abstract
Little is known about how psoriatic disease characteristics and treatment outcomes differ geographically in the United States. Our aim was to explore real-world, geographic variations in the use of biologic classes and outcomes within the Corrona Psoriasis Registry. Patient demographics and disease characteristics were assessed at biologic initiation and at 6 months. Logistic regressions were conducted to evaluate the odds of achieving targeted outcomes for seven United States geographic regions. We examined 737 biologic initiations among 717 patients. IL-17 inhibitors were used most frequently (45%), followed by IL-12‒IL-23 and IL-23 inhibitors (38%) and TNF inhibitors (17%). The proportions of patients with obesity (body mass index > 30) and very severe psoriasis (body surface area > 20) were greatest in the East South Central and West South Central regions. After adjusting for age, sex, race, body mass index, and baseline body surface area, decreased odds of achieving 75% improvement in PASI at 6 months were observed among patients in the East South Central (OR = 0.47, 95% confidence interval = 0.28–0.79, P = 0.004), West South Central (OR = 0.43, 95% confidence interval = 0.22–0.87, P = 0.019), and Pacific (OR = 0.49, 95% confidence interval = 0.28–0.84, P = 0.010) regions compared with those observed among patients in the Northeast. The East South Central and West South Central regions may have the greatest frequencies of very severe disease burden and, along with the Pacific region, may be less likely to achieve targeted response within 6 months of initiating biologic therapy.
Abbreviations: BMI, body mass index; BSA, body surface area; CI, confidence interval; E South Cent, East South Central; IGA, Investigator’s Global Assessment; IL-12/23i, IL-12‒IL-23 inhibitor; IL-17i, IL-17 inhibitor; IL-23i, IL-23 inhibitor; PASI 75, 75% improvement in PASI; TNFi, TNF inhibitor; US, United States; W South Cent, West South Central
Introduction
A decade ago, a geographic pattern of diabetes disease burden was recognized in the southeastern United States (US), deemed the diabetes belt (Barker et al., 2011). Its nomenclature was reminiscent of the stroke belt identified in the 1960s for US states, with increased age-adjusted stroke mortality rates (Borhani, 1965). These studies contributed to focused research on subpopulations within the US burdened with metabolic disease. Psoriasis is an immune-mediated, inflammatory disease affecting 2–3% of adults in the US (Rachakonda et al., 2014) and, similar to diabetes and stroke, is associated with dysmetabolism. Previously, we have shown that within the Corrona Psoriasis Registry, treatment patterns and baseline disease characteristics of psoriasis also vary among geographic regions within the US, with more severe disease noted in the South Central Census divisions (Enos et al., 2020). It is currently not known whether treatment outcomes among patients treated for psoriasis with biologics differ between geographic regions of the US. The treatment of psoriasis with biologics has recently been extensively outlined by the American Academy of Dermatology and National Psoriasis Foundation (Menter et al., 2019), and further recommendations for treatment targets at 3 and 6 months have been set forth by the National Psoriasis Foundation (Armstrong et al., 2017). With these as a guide, an awareness of the potential geographic variations in treatment outcomes could help to improve treatment algorithms and ultimately patient outcomes. The objective of this study was to describe the US geographic variations in the disease response 6 months after initiating biologic therapy for the treatment of psoriasis using data from the Corrona Psoriasis Registry in 2018.
Results
Patient population
There were 717 patients with 737 new biologic initiations at or after enrollment in 2018 with a corresponding 6-month follow-up visit (Table 1). Half of the patients were obese (body mass index [BMI] >30) (51.0%), and the most frequently reported comorbid diseases were hypertension (38.4%), hyperlipidemia (28.5%), and diabetes mellitus (16.0%). At index visit, most patients reported moderate disease activity on the basis of body surface area (BSA), Investigator’s Global Assessment (IGA), and PASI score; the median duration of psoriasis was 11 years; and 38.6% of the patients were biologic naive, whereas 37.0% reported history of >2 previous biologics.
Table 1.
Patient Population and Disease Characteristics of New Biologics Starts in the Corrona Psoriasis Registry, 2018
| Variables | Pacific | Mountain/West North Central | West South Central | East North Central | East South Central | Northeast | South Atlantic | Total | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| Total, n (%) | N = 100 | N = 94 | N = 48 | N = 56 | N = 106 | N = 217 | N = 96 | N = 717 | |
| Age, mean (SD) | 51.9 (15.5) | 49.2 (14.2) | 52.6 (14.4) | 50.2 (16.3) | 50.3 (13.0) | 49.8 (15.4) | 49.9 (14.7) | 50.3 (14.8) | 0.8091 |
| Female sex, n (%) | 36 (36.0) | 39 (41.5) | 24 (50.0) | 23 (41.1) | 63 (59.4) | 105 (48.4) | 50 (52.1) | 340 (47.4) | 0.0232 |
| Race, n (%) | <0.0012 | ||||||||
| White | 31 (31.0) | 84 (89.4) | 39 (81.2) | 52 (92.9) | 102 (96.2) | 180 (82.9) | 81 (84.4) | 569 (79.4) | |
| Other3 | 69 (69.0) | 10 (10.6) | 9 (18.8) | 4 (7.1) | 4 (3.8) | 37 (17.1) | 15 (15.6) | 148 (20.6) | |
| Insurance, n (%)4 | |||||||||
| Private | 45 (45.0) | 65 (69.1) | 35 (72.9) | 46 (82.1) | 71 (67.0) | 173 (79.7) | 78 (81.2) | 513 (71.5) | <0.0012 |
| Medicare | 24 (24.0) | 18 (19.1) | 11 (22.9) | 11 (19.6) | 21 (19.8) | 29 (13.4) | 11 (11.5) | 125 (17.4) | 0.1392 |
| Medicaid | 43 (43.0) | 12 (12.8) | 2 (4.2) | 5 (8.9) | 26 (24.5) | 25 (11.5) | 4 (4.2) | 117 (16.3) | <0.0012 |
| No insurance | 4 (4.0) | 4 (4.3) | 3 (6.2) | 2 (3.6) | 0 (0.0) | 1 (0.5) | 5 (5.2) | 19 (2.6) | 0.0352 |
| BMI, n (%) | <0.0012 | ||||||||
| <25 (underweight/normal) | 35 (35.0) | 13 (13.8) | 6 (12.8) | 7 (12.5) | 17 (16.5) | 57 (26.3) | 22 (23.2) | 157 (22.1) | |
| 25–30 (overweight) | 35 (35.0) | 26 (27.7) | 13 (27.7) | 16 (28.6) | 23 (22.3) | 48 (22.1) | 31 (32.6) | 192 (27.0) | |
| >30 (obese) | 30 (30.0) | 55 (58.5) | 28 (59.6) | 33 (58.9) | 63 (61.2) | 112 (51.6) | 42 (44.2) | 363 (51.0) | |
| Smoking history, n (%) | n = 214 | n = 56 | n = 94 | n = 94 | n = 106 | n = 47 | n = 98 | n = 709 | 0.0012 |
| Never | 99 (46.3) | 21 (37.5) | 42 (44.7) | 59 (62.8) | 45 (42.5) | 26 (55.3) | 61 (62.2) | 353 (49.8) | |
| Former | 88 (41.1) | 23 (41.1) | 27 (28.7) | 22 (23.4) | 30 (28.3) | 16 (34.0) | 16 (16.3) | 222 (31.3) | |
| Current | 27 (12.6) | 12 (21.4) | 25 (26.6) | 13 (13.8) | 31 (29.2) | 5 (10.6) | 21 (21.4) | 134 (18.9) | |
| Current alcohol use, n (%) | n = 217 | n = 56 | n = 94 | n = 96 | n = 106 | n = 48 | n = 100 | n = 717 | 0.0012 |
| None and/or occasional | 114 (52.5) | 36 (64.3) | 40 (42.6) | 59 (61.5) | 81 (76.4) | 27 (56.2) | 78 (78.0) | 435 (60.7) | |
| 1–3 drinks per week | 25 (11.5) | 6 (10.7) | 9 (9.6) | 7 (7.3) | 8 (7.5) | 5 (10.4) | 7 (7.0) | 67 (9.3) | |
| 4–6 drinks per week | 36 (16.6) | 4 (7.1) | 16 (17.0) | 15 (15.6) | 6 (5.7) | 4 (8.3) | 5 (5.0) | 86 (12.0) | |
| 1–2 drinks per day | 29 (13.4) | 4 (7.1) | 16 (17.0) | 8 (8.3) | 4 (3.8) | 4 (8.3) | 8 (8.0) | 73 (10.2) | |
| ≥3 drinks per day | 13 (6.0) | 6 (10.7) | 13 (13.8) | 7 (7.3) | 7 (6.6) | 8 (16.7) | 2 (2.0) | 56 (7.8) | |
| Duration of Psoriasis in years, median (Q1, Q3) | 9.0 (4.0, 18.0) | 13.0 (5.0, 23.8) | 6.5 (2.8, 16.2) | 13.0 (5.0, 25.5) | 7.5 (3.0, 17.0) | 13.0 (6.0, 24.0) | 10.0 (3.5, 25.5) | 11.0 (4.0, 21.2) | <0.0015 |
| Age at onset of psoriasis in years, median (Q1, Q3) | 42.0 (25.8, 53.0) | 33.0 (21.0, 45.8) | 39.5 (25.0, 52.5) | 29.0 (18.0, 51.2) | 41.0 (25.2, 51.8) | 33.0 (19.0, 45.0) | 32.0 (22.0, 47.5) | 35.0 (21.0, 50.0) | <0.0015 |
| BSA—categorical, n (%) | 0.0062 | ||||||||
| Mild (0–3) | 6 (6.0) | 24 (25.5) | 5 (10.4) | 11 (19.6) | 20 (18.9) | 27 (12.4) | 11 (11.5) | 104 (14.5) | |
| Moderate (3–10) | 58 (58.0) | 45 (47.9) | 23 (47.9) | 25 (44.6) | 48 (45.3) | 118 (54.4) | 44 (45.8) | 361 (50.3) | |
| Severe (10–20) | 19 (19.0) | 12 (12.8) | 8 (16.7) | 6 (10.7) | 9 (8.5) | 33 (15.2) | 22 (22.9) | 109 (15.2) | |
| Very severe (>20) | 17 (17.0) | 13 (13.8) | 12 (25.0) | 14 (25.0) | 29 (27.4) | 39 (18.0) | 19 (19.8) | 143 (19.9) | |
| Previous biologic Therapies, n (%) | 0.0162 | ||||||||
| Biologic naive | 37 (37.0) | 39 (41.5) | 18 (37.5) | 18 (32.1) | 35 (33.0) | 88 (40.6) | 42 (43.8) | 277 (38.6) | |
| 1 previous biologic | 36 (36.0) | 20 (21.3) | 15 (31.2) | 20 (35.7) | 18 (17.0) | 46 (21.2) | 20 (20.8) | 175 (24.4) | |
| 2+ previous biologics | 27 (27.0) | 35 (37.2) | 15 (31.2) | 18 (32.1) | 53 (50.0) | 83 (38.2) | 34 (35.4) | 265 (37.0) | |
| PASI, median (Q1, Q3) | 7.2 (4.4, 13.2) | 5.4 (2.4, 9.8) | 5.6 (4.0, 10.7) | 4.3 (2.1, 7.4) | 3.7 (1.9, 7.2) | 7.2 (3.2, 11.4) | 5.7 (2.8, 10.7) | 5.7 (2.8, 10.8) | <0.0015 |
| IGA, n (%) | 0.0802 | ||||||||
| Clear | 1 (1.0) | 2 (2.1) | 1 (2.1) | 6 (10.7) | 4 (3.8) | 7 (3.2) | 4 (4.2) | 25 (3.5) | |
| Almost clear | 2 (2.0) | 11 (11.7) | 3 (6.2) | 3 (5.4) | 0 (0.0) | 14 (6.5) | 4 (4.2) | 37 (5.2) | |
| Mild | 16 (16.0) | 18 (19.1) | 8 (16.7) | 9 (16.1) | 15 (14.2) | 39 (18.0) | 14 (14.6) | 119 (16.6) | |
| Moderate | 64 (64.0) | 46 (48.9) | 28 (58.3) | 33 (58.9) | 70 (66.0) | 124 (57.1) | 56 (58.3) | 421 (58.7) | |
| Severe | 17 (17.0) | 17 (18.1) | 8 (16.7) | 5 (8.9) | 17 (16.0) | 33 (15.2) | 18 (18.8) | 115 (16.0) | |
| Comorbid disease | |||||||||
| Cancer | 2 (2.0) | 9 (9.6) | 3 (6.2) | 1 (1.8) | 8 (7.5) | 21 (9.7) | 8 (8.3) | 52 (7.3) | 0.1482 |
| Cardiovascular disease | 7 (7.0) | 6 (6.4) | 8 (16.7) | 1 (1.8) | 7 (6.6) | 17 (7.8) | 7 (7.3) | 53 (7.4) | 0.1782 |
| Hypertension | 40 (40.0) | 33 (35.1) | 23 (47.9) | 19 (33.9) | 46 (43.4) | 81 (37.3) | 33 (34.4) | 275 (38.4) | 0.5782 |
| Hyperlipidemia | 32 (32.0) | 15 (16.0) | 13 (27.1) | 13 (23.2) | 38 (35.8) | 65 (30.0) | 28 (29.2) | 204 (28.5) | 0.0682 |
| Diabetes mellitus | 19 (19.0) | 12 (12.8) | 8 (16.7) | 4 (7.1) | 25 (23.6) | 36 (16.6) | 11 (11.5) | 115 (16.0) | 0.0972 |
Abbreviations: BMI, body mass index; IGA, Investigator’s Global Assessment; Q, quartile.
P-value from ANOVA.
P-value from chi-square test.
Race category Other includes African American, Asian, Other; these were collapsed owing to small cell counts (n < 5).
Not mutually exclusive.
P-value from Kruskal‒Wallis test.
Geographic variations within the Corrona Psoriasis Registry
Of the reported enrollees, 30.3% were from the Northeast region, 14.8% were from the East South Central (E South Cent), 13.9% were from the Pacific, 13.4% were from the South Atlantic, 13.1% were from the Mountain/West North Central, 7.8% were from the East North Central, and 6.7% were from the West South Central (W South Cent) (Table 1). Race varied geographically, with the Pacific reporting the greatest proportion of Asian enrollees (52.0%) as well as the lowest proportion of White patients (31.0%).
Obesity (BMI > 30) was most frequently reported among patients in the W South Cent (59.6%), East North Central (58.9%), and E South Cent (61.2%) regions. The Pacific region had the lowest frequency of patients who were obese and the greatest proportion of underweight/normal-weighted (BMI < 25) patients (30.0% and 35.0%, respectively). Hypertension was reported at index visit in 33.9–47.9% of patients across regions, hyperlipidemia in 16.0–32.0% of patients, and diabetes mellitus in 7.1–23.6% of patients; however, these differences were not statistically significant.
Very severe disease (BSA > 20%) was reported most frequently in the E South Cent region (27.4%), followed by the W South Cent (25.0%) and East North Central (25.0%) regions, with a range of 13.8–27.4% across all the regions. The greatest proportion of enrollees reporting a history of >2 previous biologics for the treatment of psoriasis at index visit was in the E South Cent region (50.0%), followed by the Northeast region (38.2%), with a range of 27.0–50.0% across all the regions. The South Atlantic region reported the largest proportion of biologic-naive enrollees (43.8%), with a range of 32.1–43.8% across all the regions.
Geographic variation in biologic class initiation
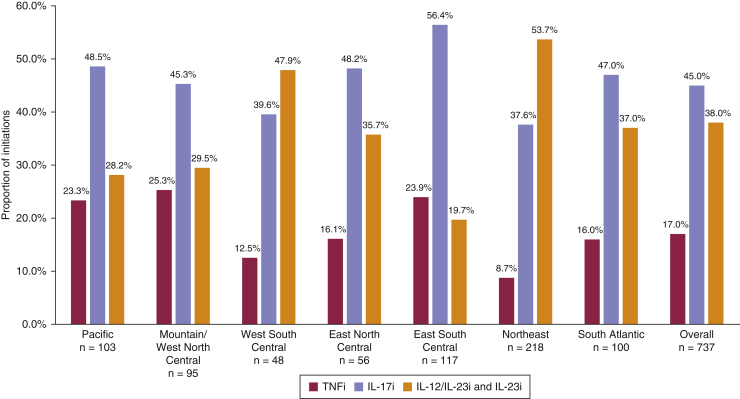
In 2018, the overall patterns of biologic class initiations in the Corrona Psoriasis Registry were IL-17 inhibitor (IL-17i) > IL-12‒IL-23 inhibitor (IL-12/23i) and IL-23 inhibitor (IL-23i) > TNF inhibitor (TNFi) (Figure 1). All census regions mirrored this pattern except the E South Cent (IL-17i > TNFi > IL-12/23i + IL-23i) and the Northeast (IL-12/23i and IL-23i > IL-17i > TNFi) regions.
Figure 1.
Proportion of initiations by biologic drug class, overall and by geographic region. The number and percentage of initiations of TNFi, IL-17i, and IL-12/23i or IL-23i are provided overall and within each geographic region. Univariable multinomial GEE regression yielded P < 0.001. GEE, generalized estimating equation; IL-12/23i, IL-12‒IL-23 inhibitor; IL-17i, IL-17 inhibitor; IL-23i, IL-23 inhibitor; TNFi, TNF inhibitor.
Geographic variation in treatment outcomes at 6 months
At 6 months, 52.2% of all the patients had achieved 75% improvement in PASI (PASI 75), and 47.6% reached a treatment target of BSA ≤ 1 (Tables 2 and 3).
Table 2.
ORs (95% CI) for PASI 75 Response at 6 Months after Biologic Initiation in 2018 by US Geographic Region in the Corrona Psoriasis Registry
| Regions | % (Responders/Total) | Unadjusted OR | Adjusted OR1 |
|---|---|---|---|
| Northeast | 60 (128/212) | Ref | Ref |
| Pacific | 52 (54/103) | 0.74 (0.46, 1.19) | 0.49 (0.28, 0.84)2 |
| Mountain/West North Central | 57 (53/93) | 0.86 (0.53, 1.42) | 0.97 (0.59, 1.59) |
| West South Central | 38 (18/47) | 0.40 (0.21, 0.77)3 | 0.43 (0.22, 0.87)2 |
| East North Central | 51 (27/53) | 0.68 (0.37, 1.24) | 0.69 (0.37, 1.28) |
| East South Central | 41 (47/116) | 0.47 (0.29, 0.76)3 | 0.47 (0.28, 0.79)3 |
| South Atlantic | 51 (50/98) | 0.74 (0.45, 1.20) | 0.70 (0.42, 1.15) |
Abbreviations: BMI, body mass index; BSA, body surface area; CI, confidence interval; PASI 75, 75% improvement in PASI; Ref, reference; US, United States.
Adjusted for age, sex, race, BMI, and baseline BSA.
Significant at P < 0.05.
Significant at P < 0.01.
Table 3.
ORs (95% CI) for BSA ≤ 1, BSA 75, IGA 0/1, PASI 90, PASI 100 Response 6 Months after Biologic Initiation in 2018 by US Geographic Region in the Corrona Psoriasis Registry
| Variables | Regions | % Responders | Unadjusted OR | Adjusted OR1 |
|---|---|---|---|---|
| BSA ≤ 1 | ||||
| Northeast | 100/218 (49.3%) | Ref | Ref | |
| Pacific | 55/100 (55.0%) | 1.33 (0.81–2.16) | 1.13 (0.65–1.97) | |
| Mountain/West North Central | 43/95 (54.4%) | 1.22 (0.72–2.06) | 1.22 (0.71–2.09) | |
| West South Central | 18/46 (39.1%) | 0.66 (0.34–1.26) | 0.75 (0.38–1.50) | |
| East North Central | 22/48 (45.8%) | 0.86 (0.46–1.62) | 1.01 (0.53–1.93) | |
| East South Central | 41/107 (38.3%) | 0.71 (0.43–1.15) | 0.86 (0.52–1.43) | |
| South Atlantic | 42/92 (45.7%) | 0.91 (0.55–1.49) | 0.91 (0.55–1.51) | |
| BSA 75 | ||||
| Northeast | 129/213 (60.6%) | Ref | Ref | |
| Pacific | 62/103 (60.2%) | 1.01 (0.62–1.64) | 0.78 (0.45–1.37) | |
| Mountain/West North Central | 53/94 (56.4%) | 0.84 (0.51–1.37) | 0.92 (0.56–1.51) | |
| West South Central | 20/47 (42.6%) | 0.48 (0.25–0.91)2 | 0.48 (0.24–0.95)2 | |
| East North Central | 31/54 (57.4%) | 0.87 (0.48–1.60) | 0.82 (0.45–1.52) | |
| East South Central | 51/115 (44.3%) | 0.55 (0.34–0.87)2 | 0.51 (0.31–0.85)3 | |
| South Atlantic | 56/98 (57.1%) | 0.91 (0.56–1.49) | 0.94 (0.57–1.56) | |
| IGA 0/1 | ||||
| Northeast | 111/197 (56.3%) | Ref | Ref | |
| Pacific | 45/100 (45.0%) | 0.65 (0.40–1.05) | 0.57 (0.33–0.98)2 | |
| Mountain/West North Central | 44/82 (53.7%) | 0.89 (0.53–1.49) | 0.87 (0.51–1.48) | |
| West South Central | 17/44 (38.6%) | 0.48 (0.25–0.94)2 | 0.56 (0.28–1.11) | |
| East North Central | 25/47 (53.2%) | 0.87 (0.46–1.65) | 0.94 (0.49–1.81) | |
| East South Central | 39/113 (34.5%) | 0.44 (0.27–0.71)4 | 0.47 (0.29–0.79)3 | |
| South Atlantic | 49/92 (53.3%) | 0.92 (0.56–1.52) | 0.98 (0.59–1.64) | |
| PASI 90 | ||||
| Northeast | 88/212 (41.5%) | Ref | Ref | |
| Pacific | 40/103 (38.8%) | 0.92 (0.57–1.50) | 0.74 (0.43–1.29) | |
| Mountain/West North Central | 36/93 (38.7%) | 0.88 (0.54–1.45) | 0.94 (0.57–1.55) | |
| West South Central | 15/47 (31.9%) | 0.66 (0.34–1.29) | 0.75 (0.37–1.50) | |
| East North Central | 19/53 (35.8%) | 0.78 (0.42–1.46) | 0.81 (0.43–1.51) | |
| East South Central | 39/116 (33.6%) | 0.77 (0.47–1.25) | 0.81 (0.49–1.35) | |
| South Atlantic | 34/98 (34.7%) | 0.78 (0.47–1.29) | 0.74 (0.45–1.23) | |
| PASI 100 | ||||
| Northeast | 69/212 (32.5%) | Ref | Ref | |
| Pacific | 20/103 (19.4%) | 0.51 (0.29–0.90)2 | 0.45 (0.23–0.86)2 | |
| Mountain/West North Central | 25/93 (26.9%) | 0.75 (0.44–1.29) | 0.75 (0.44–1.30) | |
| West South Central | 11/47 (23.4%) | 0.63 (0.30–1.31) | 0.70 (0.33–1.47) | |
| East North Central | 14/53 (26.4%) | 0.74 (0.38–1.46) | 0.82 (0.42–1.61) | |
| East South Central | 27/116 (23.3%) | 0.66 (0.39–1.11) | 0.71 (0.41–1.23) | |
| South Atlantic | 23/98 (23.5%) | 0.65 (0.38–1.14) | 0.62 (0.35–1.09) | |
Abbreviations: BMI, body mass index; BSA 75, 75% improvement in BSA; BSA, body surface area; CI, confidence interval; IGA, Investigator’s Global Assessment; PASI 100, achieving 100% PASI; PASI 90, achieving 90% PASI; Ref, reference; US, United States.
Adjusted for age, sex, race, BMI, and baseline BSA.
Significant at P < 0.05.
Significant at P < 0.01.
Significant at P < 0.001.
The W South Cent and E South Cent regions had the lowest frequencies of successful treatment outcomes at 6 months on the basis of PASI 75 (38.3% and 40.5%, respectively), BSA 75 (42.6% and 44.3%, respectively), IGA 0/1 (38.6% and 34.5%, respectively), and BSA ≤ 1 (39.1% and 38.3%, respectively). The Northeast region had the highest frequencies of achieving PASI 75, PASI1 00, BSA 75, and IGA 0/1 (60.4%, 32.5%, 60.6%, 56.3%, respectively) (Tables 2 and 3).
The likelihood of achieving treatment outcomes within each region compared with that within the Northeast region was calculated, controlling for age, sex, race, BMI, and baseline BSA (Tables 2 and 3). The E South Cent and W South Cent regions were 53% and 60% less likely, respectively, to achieve PASI 75 than the Northeast region in the unadjusted models (OR = 0.47, 95% confidence interval [CI] = 0.29–0.76, P = 0.002 and OR = 0.40, 95% CI = 0.21–0.77, P = 0.006, respectively), and these associations were similar in adjusted models (OR = 0.47, 95% CI = 0.28–0.79, P = 0.004 and OR = 0.43, 95% CI = 0.22–0.87, P = 0.019, respectively) (Table 2). Although the 95% CI of the OR for the Pacific region included the null in the unadjusted model (OR = 0.74, 95% CI = 0.46–1.19, P = 0.219), after adjusting for age, sex, race, BMI, and baseline BSA, the Pacific emerged to have a 51% decreased likelihood of achieving PASI 75 (OR =0.49, 95% CI = 0.28–0.84, P = 0.010) compared with the Northeast region.
When considering each biologic class (Tables 4 and 5), of those treated with IL-12/23i and IL-23i, those from the E South Cent, W South Cent, and Pacific regions were less likely to achieve PASI 75 than those from the Northeast region (OR = 0.30, 95% CI = 0.11–0.80, P = 0.016; OR = 0.24, 95% CI = 0.08–0.67, P = 0.007; OR = 0.26, 95% CI = 0.10–0.65, P = 0.004, respectively) after adjusting for age, sex, race, BMI, and baseline BSA (Table 4). For patients initiating TNFi and IL-17i, all CIs were consistent with no associations of regions with PASI 75 response.
Table 4.
ORs (95% CI) for PASI 75 Response 6 Months after Biologic Initiation in 2018 by US Geographic Region in the Corrona Psoriasis Registry, Stratified by Biologic Class
| Regions | TNFi |
IL-17i |
IL-12/23i + IL-23i |
|||
|---|---|---|---|---|---|---|
| % Responders | Adjusted OR1 | % Responders | Adjusted OR1 | % Responders | Adjusted OR1 | |
| Northeast | 47 (9/19) | Ref | 58 (45/77) | Ref | 64 (74/116) | Ref |
| Pacific | 63 (15/24) | 0.73 (0.16–3.23) | 56 (28/50) | 0.66 (0.29–1.52) | 38 (11/29) | 0.26 (0.10–0.65)2 |
| Mountain/West North Central | 59 (13/22) | 1.31 (0.34–5.06) | 65 (28/43) | 1.46 (0.65–3.27) | 43 (12/28) | 0.46 (0.19–1.11) |
| West South Central | 33 (2/6) | 0.49 (0.06–3.95) | 53 (10/19) | 0.94 (0.32–2.74) | 27 (6/22) | 0.24 (0.08–0.67)2 |
| East North Central | 44 (4/9) | 0.68 (0.11–4.19) | 63 (15/24) | 1.24 (0.47–3.28) | 40 (8/20) | 0.39 (0.14–1.06) |
| East South Central | 29 (8/28) | 0.44 (0.11–1.72) | 48 (31/65) | 0.63 (0.31–1.27) | 35 (8/23) | 0.30 (0.11–0.80)3 |
| South Atlantic | 38 (6/16) | 0.50 (0.12–2.13) | 52 (24/46) | 0.72 (0.34–1.54) | 56 (20/36) | 0.69 (0.31–1.52) |
Abbreviations: CI, confidence interval; IL-12/23i, IL-12‒IL-23 inhibitor; IL-17i, IL-17 inhibitor; IL-23i, IL-23 inhibitor; PASI 75, 75% improvement in PASI; TNFi, TNF inhibitor; Ref, reference; US, United States.
Adjusted for age, sex, race, BMI, and baseline BSA.
Significant at P < 0.01.
Significant at P < 0.05.
Table 5.
ORs (95% CI) for BSA 75, BSA ≤ 1, IGA 0/1 Response 6 Months after Biologic Initiation in 2018 by US Geographic Region in the Corrona Psoriasis Registry, Stratified by Biologic Class
| Variables | Region | TNF % Responders | IL-17 |
IL-12/IL-23 + IL-23 |
|||
|---|---|---|---|---|---|---|---|
| Adjusted OR1 | % Responders | Adjusted OR1 | % Responders | Adjusted OR1 | |||
| BSA 75 | |||||||
| Northeast | 9/19 (47.4%) | Ref | 47/78 (64.0%) | Ref | 73/116 (62.9%) | Ref | |
| Pacific | 14/24 (58.3%) | 0.62 (0.14–2.77) | 34/50 (68.0%) | 1.02 (0.44–2.40) | 14/29 (48.3%) | 0.45 (0.18–1.12) | |
| Mountain/West North Central | 12/23 (52.2%) | 0.96 (0.25–3.67) | 29/43 (67.4%) | 1.60 (0.71–3.62) | 12/28 (42.9%) | 0.53 (0.21–1.31) | |
| West South Central | 2/6 (33.3%) | 0.42 (0.05–3.50) | 9/19 (47.4%) | 0.71 (0.24–2.06) | 9/22 (40.9%) | 0.48 (0.18–1.32) | |
| East North Central | 5/9 (55.6%) | 0.77 (0.12–4.79) | 16/25 (64.0%) | 1.23 (0.47–3.25) | 10/20 (50.0%) | 0.59 (0.22–1.63) | |
| East South Central | 8/27 (29.6%) | 0.35 (0.09–1.41) | 34/65 (52.3%) | 0.74 (0.36–1.49) | 9/23 (39.1%) | 0.26 (0.09–0.75)2 | |
| South Atlantic | 8/16 (50.0%) | 0.78 (0.19–3.21) | 26/46 (56.5%) | 0.79 (0.37–1.71) | 22/36 (61.1%) | 1.05 (0.46–2.39) | |
| BSA ≤ 1 | |||||||
| Northeast | 10/18 (55.6%) | Ref | 32/72 (44.4%) | Ref | 58/113 (51.3%) | Ref | |
| Pacific | 14/24 (58.3%) | 0.62 (0.14–2.82) | 26/48 (54.2%) | 1.29 (0.55–3.02) | 15/28 (53.6%) | 1.01 (0.40–2.51) | |
| Mountain/West North Central | 8/20 (40.0%) | 0.46 (0.11–1.90) | 27/35 (77.1%) | 3.63 (1.43–9.21)3 | 8/24 (33.3%) | 0.55 (0.21–1.43) | |
| West South Central | 2/6 (33.3%) | 0.46 (0.05–4.11) | 9/19 (47.4%) | 1.23 (0.42–3.58) | 7/21 (33.3%) | 0.62 (0.23–1.71) | |
| East North Central | 3/9 (33.3%) | 0.66 (0.10–4.37) | 12/21 (57.1%) | 1.81 (0.65–5.01) | 7/18 (38.9%) | 0.62 (0.21–1.81) | |
| East South Central | 8/26 (30.8%) | 0.36 (0.09–1.46) | 29/59 (49.2%) | 1.39 (0.68–2.86) | 4/22 (18.2%) | 0.33 (0.10–1.04) | |
| South Atlantic | 4/15 (26.7%) | 0.25 (0.05–1.27) | 21/43 (48.8%) | 1.10 (0.50–2.39) | 17/34 (50.0%) | 1.05 (0.46–2.39) | |
| IGA 0/1 | |||||||
| Northeast | 9/17 (52.9%) | Ref | 35/68 (51.5%) | Ref | 67/112 (59.8%) | Ref | |
| Pacific | 12/24 (50.0%) | 0.61 (0.14–2.67) | 21/49 (42.9%) | 0.63 (0.27–1.48) | 12/27 (44.4%) | 0.46 (0.18–1.17) | |
| Mountain/West North Central | 11/19 (57.9%) | 1.05 (0.26–4.29) | 23/37 (62.2%) | 1.37 (0.59–3.17) | 10/26 (38.5%) | 0.49 (0.19–1.22) | |
| West South Central | 3/6 (50.0%) | 0.89 (0.11–6.94) | 8/18 (44.4%) | 0.84 (0.28–2.47) | 6/20 (30.0%) | 0.40 (0.14–1.16) | |
| East North Central | 3/8 (37.5%) | 0.63 (0.10–4.07) | 13/21 (61.9%) | 1.50 (0.54–4.15) | 9/18 (50.0%) | 0.68 (0.24–1.93) | |
| East South Central | 5/26 (19.2%) | 0.25 (0.06–1.07) | 27/64 (42.2%) | 0.66 (0.33–1.35) | 7/23 (30.4%) | 0.38 (0.14–1.04) | |
| South Atlantic | 6/15 (40.0%) | 0.52 (0.12–2.38) | 24/44 (54.5%) | 1.07 (0.49–2.30) | 19/33 (57.6%) | 1.12 (0.48–2.59) | |
Abbreviations: BMI, body mass index; BSA 75, 75% improvement in BSA; BSA, body surface area; CI, confidence interval; IGA, Investigator’s Global Assessment; Ref, reference; US, United States.
Adjusted for age, sex, race, BMI, and baseline BSA.
Significant at P < 0.05.
Significant at P < 0.01.
Biologic discontinuation and switching among census regions in the Corrona Psoriasis Registry reported at 6 months
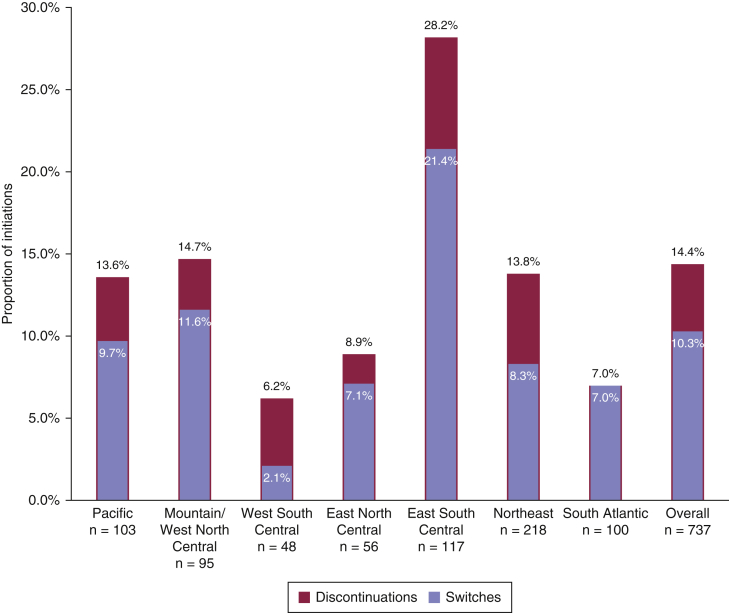
At 6 months, 10.3% of all the patients had switched biologic classes, and 14.4% had discontinued therapy (Figure 2). The E South Cent region had the greatest frequency of patients that switched (21.4%) as well as the greatest frequency of those discontinuing therapy (28.2%) (Figure 2). The W South Cent region reported the lowest frequency of patients both switching biologics (2.1%) and discontinuing therapy (6.2%).
Figure 2.
Proportion of discontinuations and switches of biologic drugs at 6-month follow-up, overall and by geographic region. The number and percentage of discontinuations and switches of all biologic therapies at 6-month follow-up are provided overall and within each geographic region. Switches are a subset of discontinuations. Univariable logistic GEE regressions used to test the associations among the geographic region and discontinuations and among geographic regions and switches were both significant (P < 0.001 and P = 0.009, respectively). GEE, generalized estimating equation.
Overall, efficacy was the most frequently reported reason for discontinuation of therapy (57 of 106, 53.8%) than safety (15 of 106, 14.2%), insurance (11 of 106, 10.4%), and other reasons (23 of 106, 21.7%) (P = 0.013, data not shown).
Discussion
In this study, we have shown regional differences in baseline disease characteristics, in choice of biologic class, in treatment patterns, and in treatment outcomes at 6 months after biologic therapy initiation within the Corrona Psoriasis Registry.
Census regions with more severe baseline disease characteristics had poorer treatment outcomes at 6 months. Of note, the E South Cent and W South Cent census regions reported the greatest proportion of patients with obesity (BMI > 30) as well as a greater proportional burden of very severe disease (BSA > 20%), and nearly half had used >2 biologics. In unadjusted and adjusted models, these census regions had the lowest frequencies of patients achieving successful treatment outcomes. Unlike patients in the E South Cent and W South Cent regions, patients in the Pacific census region had the lowest proportion of obesity, had the second-lowest proportion of very severe psoriasis, and were a majority (51.5%) Asian. Interestingly, when adjusted for age, sex, race, BMI, and baseline BSA, they too did not achieve successful treatment outcomes, compared with those in the Northeast region at 6 months. Frequencies of diabetes, hypertension, and hyperlipidemia were greater in the E South Cent, W South Cent, and Pacific regions than in other census regions in this study; however, these differences were not statistically significant. In an analysis of all the 2018 enrollees to the Corrona Psoriasis Registry (n = 2,896), we have observed significantly increased frequencies of patients with BMI > 30, diabetes, and hyperlipidemia in the E South Cent region (Enos et al., 2020). Although TNFα inhibitors and ustekinumab (IL-12/23i) have shown decreased efficacy in patients with obesity (Højgaard et al., 2016; Lebwohl et al., 2010; Shan and Zhang, 2019; Singh et al., 2018), our findings were independent of BMI. Therefore, it is possible that concurrent comorbid diseases, known to be elevated in this same geographic distribution, may yet be contributory (Barker et al., 2011; Gurka et al., 2018). Furthermore, the role of genetics in treatment response is not fully understood, and because psoriasis is a polygenic disease, despite controlling for race, the heterogeneity of ethnic groups both within and between each geographic region could be at play (Guðjónsson et al., 2002; Shen et al., 2019).
Treatment patterns differed across the US within the Corrona Psoriasis Registry at both index visits and at 6 months. At index visit, a greater proportion of patients in the E South Cent and the Northeast regions had already been treated with >2 biologics. Although stopping or changing therapies could be due to any number of reasons, it is well-documented that efficacy is a primary driver (Levin et al., 2014). Yet, these two census regions were often at opposite ends of the spectrum for treatment outcomes in our analysis. A recent study using the Medical Expenditure Panel Survey showed regional differences in healthcare expenditures and access to biologic medications in the US: the Northeast had significantly increased total healthcare costs per patient per year and higher ambulatory care costs, whereas patients in the southern US had the greatest access to biologic medications (Nguyen et al., 2020). Similarly, the South census region has been shown to have the most biologic prescriptions, whereas the Northeast region had the least in a commercial claims and encounters database (Galli et al., 2018). Given these findings, our data showing comparatively poorer treatment outcomes in the E South Cent region would suggest that medication access may not equate to achieving psoriasis treatment targets within 6 months, and more emphasis may need to be placed on comorbid disease/ethnicity/heritage. Future research is needed to clarify the reasons behind this disconnect.
Limitations to this study include that the Corrona registry is not a random, population-based, representative sample; the registry cohort comprises patients invited to participate in the registry by their dermatologists. Regions are derived from the practice location, not from the residential addresses of the patients. Some regions are under-represented compared with others, and patients seen at participating sites may not represent all the patients in the overall region. Nevertheless, a major strength of this study is that it is among the first to describe the regional differences in response to biologic therapies among real-world patients with psoriasis. Evaluation of severity measures may vary among providers within the registry, although Corrona provides rigorous registry training to all investigators and support staff at each participating site before patient enrollment and registry data collection. Insurance variation and out-of-pocket expenses were not accounted for and may represent possible limitations. There were some limitations owing to small sample sizes. Biologics were grouped by class, and we were unable to analyze the IL-12/23i and IL-23i classes individually. When analyzing outcomes by drug class, the input sample sizes were small, resulting in wide CIs; thus, inferences based on effect estimates within biologic class strata are limited. Models were adjusted for few covariates and may be subject to omitted-variable bias because it is likely that not every important variable was included. In addition, there were no ad-hoc adjustments for multiple comparisons; thus, the results should be interpreted with caution.
Our findings suggest that there is a geographic variation in the achievement of treatment response at 6 months among patients with psoriasis treated with biologics in the US. The E South Cent and W South Cent regions may have the greatest frequencies of very severe disease burden and, along with the Pacific region, may be less likely to achieve targeted response within 6 months of initiating biologic therapy. It is important for clinicians to be aware of the geographic trends in their region because a further understanding of the potential factors driving these regional differences (e.g., comorbid disease, genetic heterogeneity) could help improve treatment algorithms to ultimately advance patient care.
Materials and Methods
Study setting
This analysis used data from the Corrona Psoriasis Registry, a prospective, multicenter, observational, disease-based registry, the design of which has been previously described (Strober et al., 2018). At the time of the analysis, 248 US sites were active (Figure 3), and biologic therapies available included adalimumab, brodalumab, certolizumab pegol, etanercept, etanercept biosimilar, guselkumab, infliximab, infliximab biosimilars, ixekizumab, secukinumab, tildrakizumab, and ustekinumab.
Figure 3.
Distribution of Corrona Psoriasis Registry sites across the US within the designated census regions as of January 2020. E North Cent, East North Central; E South Cent, East South Central; Mount, Mountain; N East, Northeast; S Atl, South Atlantic; US, United States; W North Cent, West North Central; W South Cent, West South Central.
Study population
In 2018, there were 737 biologic initiations with both an index and 6-month follow-up visit (window of 4–9 months); these 737 biologic initiations were among 717 patients. Patients could initiate >1 biologic in a calendar year, and those who did initiate >1 biologic contributed multiple initiations to the analysis. Patient initiations were categorized by drug class into one of the following three groups: TNFis, IL-17is, and IL-12/23i and IL-23i. IL-12/23i and IL-23i were collapsed into a single class owing to small sample size. Patients were categorized into seven different geographic regions: Northeast, East North Central, Mountain/West North Central, South Atlantic, E South Cent, W South Cent, and Pacific regions. These geographic regions were derived from the nine US census divisions (US Census Bureau, 2019), with New England and Mid-Atlantic being combined into the Northeast region and Mountain and West North Central region being combined into Mountain/West North Central owing to limited sample sizes in these regions.
Outcomes
Disease severity measures included the percentage of affected BSA, IGA, and PASI (Bożek and Reich, 2017). The following outcomes were calculated at the 6-month follow-up visit: PASI 75, achievement of 90% PASI, and 100% improvement in PASI (achievement of 100% PASI), BSA of 1 or lower (BSA ≤1), 75% improvement in BSA, and clear-to-minimal status for IGA (score of 0 or 1, IGA 0/1). Treatment patterns at the time of the 6-month follow-up visit included the frequency of discontinuations and/or switch of index biologic therapy. In rank order, physicians record up to three reasons (efficacy, safety, insurance, and other reasons) for discontinuation/switch of therapy; primary reasons are required whenever a discontinuation is observed.
Covariates
Baseline characteristics included demographics (age, sex, race, health insurance type, BMI category [underweight/normal-weighted, <25 kg/m2; overweight, 25–30 kg/m2; obese, >30 kg/m2]), smoking history, current alcohol use, history of comorbidities (cancer, cardiovascular disease, hypertension, hyperlipidemia, diabetes mellitus), duration of psoriasis, age at onset of psoriasis, and the number of previous biologic therapies (zero, one, more than two).
Statistical analysis
Patient characteristics at index visit were described overall and stratified by geographic region using frequency counts and percentages for categorical variables and means and SDs for continuous variables; for patients with >1 initiation, the characteristics from the patient’s first observed initiation were reported. Patient characteristics were compared across the geographic regions using ANOVA for normal continuous variables, Kruskal‒Wallis test for non-normal continuous variables, and chi-square tests for categorical variables. Treatment patterns at follow-up were assessed with logistic regressions. To determine whether the geographic region was associated with outcomes at 6 months after biologic initiation, we used logistic regression models to calculate the odds of achieving each outcome within each geographic region compared with that within the Northeast region (reference group) and reported the ORs and 95% CIs. Unadjusted models were calculated, followed by adjustment for age, sex, race, BMI, and baseline BSA. Because patients could contribute multiple initiations to the analysis, generalized estimating equations with an unstructured covariance matrix were used to account for correlated observations (Liang and Zeger, 1986). Patients were further stratified by drug class, and models were repeated within these subgroups. We implemented Firth’s bias reduction method in these stratified models to address problems encountered with quasi-complete separation (Firth, 1993). R, version 3.5.2, was used for statistical analysis (R Core Team, 2018). The significance level was set at α = 0.05 for type 1 error; there were no adjustments for multiple testing.
Data availability statement
No datasets were generated or analyzed during this study.
Ethics statement
The investigators received de-identified data from the CorEvitas registry (formerly, the Corrona Psoriasis Registry), whose participating sites obtain written, informed patient consent and IRB approval; therefore, no further institutional approval or patient consent was required.
ORCIDs
Clinton W. Enos: http://orcid.org/0000-0002-6783-1904
Katie A. O’Connell: http://orcid.org/0000-0003-4999-0458
Ryan W. Harrison: http://orcid.org/0000-0003-4575-006X
Robert R. McLean: http://orcid.org/0000-0001-5352-3794
Blessing Dube: http://orcid.org/0000-0003-0056-997X
Abby S. Van Voorhees: http://orcid.org/0000-0003-3863-3842
Author Contributions
Conceptualization: CWE, ASVV; Data Curation: RWH, RRM, BD; Formal Analysis: RWH, RRM, BD; Funding Acquisition: CWE, ASVV; Investigation: CWE, KAO, ASVV; Methodology: RWH, RRM, BD; Writing - Original Draft Preparation: CWE, KAO, ASVV; Writing - Review and Editing: CWE, KAO, RWH, RRM, BD, ASVV
Acknowledgments
This study was sponsored by CorEvitas, LLC (formerly known as Corrona), and the analysis was funded by CorEvitas LLC. Access to study data was limited to CorEvitas, and CorEvitas statisticians completed all of the analysis; all authors contributed to the interpretation of the results. CorEvitas, LLC has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Chugai Pharmaceutical, Eli Lilly and Company, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Novartis, Ortho Dermatologics, Pfizer, Regeneron Pharmaceuticals, Sanofi, Sun, and UCB. We thank the participating providers and patients for contributing data to the Corrona Psoriasis Registry. This study was supported through a partnership between the Corrona Psoriasis Registry and the National Psoriasis Foundation Medical Board.
Conflict of Interest
CWE has served as a consultant on an advisory board for UCB. RWH, RRM, and BD are employees at CorEvitas, LLC. ASVV has received grants and/or research support from Celgene, Eli Lilly and Company, and AbbVie and has served as a consultant with Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, UCB, Novartis, Pfizer, Valeant Pharmaceuticals, Merck, Dermira, and Allergan. KAO states no conflict of interest.
accepted manuscript published online 6 May 2021; corrected proof published online 1 June 2021
Footnotes
Cite this article as: JID Innovations 2021;X:100025
References
- Armstrong A.W., Siegel M.P., Bagel J., Boh E.E., Buell M., Cooper K.D., et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290–298. doi: 10.1016/j.jaad.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Barker L.E., Kirtland K.A., Gregg E.W., Geiss L.S., Thompson T.J. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40:434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Borhani N.O. Changes and geographic distribution of mortality from cerebrovascular disease. Am J Public Health Nations Health. 1965;55:673–681. doi: 10.2105/ajph.55.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bożek A., Reich A. The reliability of three psoriasis assessment tools: psoriasis area and severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851–856. doi: 10.17219/acem/69804. [DOI] [PubMed] [Google Scholar]
- Enos C.W., O'Connell K.A., Harrison R.W., McLean R.R., Dude B., Van Voorhees A.S. Geographic variations in biologic therapy and disease characteristics: a pilot-study in the Corrona Psoriasis Registry. J Drugs Dermatol. 2020;19:1119–1122. doi: 10.36849/JDD.2020.5366. [DOI] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- Galli E., Liu G., Leslie D., Kirby J., Miller J.J. Prescription pattern variability of biologic therapies in treating psoriasis. J Psoriasis Psoriatic Arthritis. 2018;3:84–87. [Google Scholar]
- Guðjónsson J.E., Valdimarsson H., Kárason A., Antonsdóttir A.A., Hjaltey Rúnarsdóttir E., Gulcher J.R., et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362–365. doi: 10.1046/j.0022-202x.2001.01656.x. [DOI] [PubMed] [Google Scholar]
- Gurka M.J., Filipp S.L., DeBoer M.D. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutr Diabetes. 2018;8:14. doi: 10.1038/s41387-018-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højgaard P., Glintborg B., Kristensen L.E., Gudbjornsson B., Love T.J., Dreyer L. The influence of obesity on response to tumour necrosis factor-alpha inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology (Oxford) 2016;55:2191–2199. doi: 10.1093/rheumatology/kew326. [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Yeilding N., Szapary P., Wang Y., Li S., Zhu Y., et al. Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol. 2010;63:571–579. doi: 10.1016/j.jaad.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Levin A.A., Gottlieb A.B., Au S.C. A comparison of psoriasis drug failure rates and reasons for discontinuation in biologics vs conventional systemic therapies. J Drugs Dermatol. 2014;13:848–853. [PubMed] [Google Scholar]
- Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Menter A., Strober B.E., Kaplan D.H., Kivelevitch D., Prater E.F., Stoff B., et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- Nguyen K.B., Read C., Wu K.K., Armstrong A.W. Where you live matters: regional differences in health care resource use for psoriasis in the United States. J Am Acad Dermatol. 2020;82:1360–1367. doi: 10.1016/j.jaad.2019.10.014. [DOI] [PubMed] [Google Scholar]
- R Core Team R: a language and environment for statistical computing. 2018. https://ww.R-project.org/
- Rachakonda T.D., Schupp C.W., Armstrong A.W. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Shan J., Zhang J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: a systematic review and meta-analysis. Joint Bone Spine. 2019;86:173–183. doi: 10.1016/j.jbspin.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Shen M., Lim S.W.D., Tan E.S., Oon H.H., Ren E.C. HLA correlations with clinical phenotypes and risk of metabolic comorbidities in Singapore Chinese psoriasis patients. Mol Diagn Ther. 2019;23:751–760. doi: 10.1007/s40291-019-00423-z. [DOI] [PubMed] [Google Scholar]
- Singh S., Facciorusso A., Singh A.G., Vande Casteele N., Zarrinpar A., Prokop L.J., et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober B., Karki C., Mason M., Guo N., Holmgren S.H., Greenberg J.D., et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78:323–332. doi: 10.1016/j.jaad.2017.10.012. [DOI] [PubMed] [Google Scholar]
- US Census Bureau Census regions and divisions of the United States. 2019. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during this study.