Abstract
Trial design
Human papillomavirus infection causes verruca vulgaris. CDK9 inhibitor FIT039 inhibits DNA virus proliferation in animal models. We conducted a multicenter, single-blind, placebo-controlled, randomized phase I/II clinical trial evaluating the safety and efficacy of FIT039 against verruca vulgaris.
Methods
Target lesions were treated with liquid nitrogen once, and a FIT039 patch or placebo patch was applied for 14 days. The primary endpoint was lesion disappearance. The secondary endpoints were safety and changes in dimension, cross-sectional area, and the number of petechial lesions.
Results
A total of 24 participants were randomly allocated to the FIT039 (n = 13, median age, 54 years) and placebo (n = 11, median age, 62 years) groups. Verruca vulgaris did not disappear. FIT039 decreased the dimension to 76% of the initial value on day 29, followed by an increase to 98% on day 57. Placebo showed a monotonic increase to 107% on day 57. Changes in the cross-sectional area and petechiae number were comparable between the groups.
Conclusions
No drug-related adverse reactions occurred. FIT039 efficacy was not determined in this study.
Abbreviations: CI, confidence interval; HPV, human papillomavirus
Introduction
Verruca vulgaris or cutaneous warts is a common disease affecting both children and adults with a prevalence of 3–30% (Beliaeva, 1990; Kilkenny and Marks, 1996; Kyriakis et al., 2007; van Haalen et al., 2009). An immunodeficient state causes extensive and refractory conditions and increases the risk of premalignant dysplasia (Barbosa, 1998; Kirnbauer and Lenz, 2017; Sterling et al., 2014; Viac et al., 1977). Verruca vulgaris can grow, multiply, and persist for years, causing physical and mental burdens on the patients (Ciconte et al., 2003; Kyriakis et al., 2007; Sterling et al., 2014). Infection of the epidermal keratinocytes by the human papillomavirus (HPV) causes verruca vulgaris (Sterling et al., 2014). HPVs are nonenveloped double-stranded DNA viruses approximately 60 nm in diameter (Kirnbauer and Lenz, 2017) with an 8 kb genome (Kirnbauer and Lenz, 2017). Nearly 400 different HPV genotypes have been identified in the skin, and types 1, 2, 4, 27, and 57 are typically associated with verruca vulgaris (Bzhalava et al., 2014; Kirnbauer and Lenz, 2017; Sterling et al., 2014).
Currently, no specific antiviral therapy is available for HPV infection (Kirnbauer and Lenz, 2017; Sterling et al., 2014). The current regimens include chemical or physical destruction of affected keratinocytes (Kirnbauer and Lenz, 2017; Sterling et al., 2014). Caustic chemicals, such as salicylic acid, or physical means, including cryotherapy with liquid nitrogen, have been used (Kirnbauer and Lenz, 2017; Sterling et al., 2014). However, a pooled analysis of randomized controlled trials revealed a cure rate of 23% (range: 5–73%), 52% (range: 0–87%), and 49% (range: 0–69%) in the placebo, salicylic acid, and cryotherapy groups, respectively, indicating that these methods are not always effective (Kwok et al., 2011). Patients should be warned that salicylic acid or cryotherapy can cause chemical burns or blistering. Another approach is to induce an immune response against HPV-infected keratinocytes (Kirnbauer and Lenz, 2017; Sterling et al., 2014). Imiquimod is an agonist of toll-like receptors 7 and 8 and induces proinflammatory responses (involving IFN-α, IL-12, and TNF-α) at the site of application. It is effective against condyloma acuminatum or genital warts caused by HPV, typically types 6 and 11, in the anogenital area (Beutner et al., 1998). However, its effect on verruca vulgaris is unknown (Lynch et al., 2014). Small noncontrolled trials involving 50, 15, and 18 patients have reported complete clearance of verruca vulgaris in 56%, 80%, and 89% of patients after a mean treatment duration of 9.2 weeks with imiquimod (Hengge et al., 2000; Micali et al., 2003). However, a Cochrane review reported an unpublished trial in which imiquimod use for noncondyloma acuminatum failed to show higher efficacy than the placebo (Kwok et al., 2012).
The proliferation of mammalian cells is regulated by cyclin-dependent kinase family members (Yamamoto et al., 2014; Zaborowska et al., 2016). P-TEFb, which consists of CDK9, phosphorylates the C-terminal domain of RNA polymerase II and regulates the synthesis of viral RNA (Zaborowska et al., 2016). FIT039, a specific CDK9 inhibitor, suppresses the replication of a broad spectrum of DNA viruses (including herpes simplex virus 1, herpes simplex virus 2, human adenovirus, human cytomegalovirus, and hepatitis B virus) and HIV in cultured cells in vitro (Okamoto et al., 2015; Tanaka et al., 2016; Yamamoto et al., 2014). Because viral transcription depends more on CDK9 than cellular transcription, FIT039 can be exploited as an antiviral drug (Kirnbauer and Lenz, 2017). Topical application of FIT039 inhibits the development of the skin lesions associated with herpes simplex virus 1 infection in a murine model (Yamamoto et al., 2014). Moreover, FIT039 does not show toxicity at the effective dosages against the viruses mentioned earlier, presumably owing to functional redundancy among cyclin-dependent kinases (Tanaka et al., 2016; Yamamoto et al., 2014). Recently, we found that FIT039 suppresses HPV proliferation in a culture system (Ajiro et al., 2018). Although FIT039 has been shown to effectively resolve cutaneous lesions caused by DNA viruses (Yamamoto et al., 2014), it remains unknown whether it can be used to treat verruca vulgaris. A preliminary proof-of-concept study showed that 1% or 3% FIT039 administered for 24 hours after cryotherapy does not cause topical or systemic adverse reactions (Sumi et al., 2019). To the best of our knowledge, CDK9-specific inhibitors have not been evaluated as a monotherapy for verruca vulgaris. In this study, we evaluated the safety and efficacy of FIT039 monotherapy versus those of placebo against verruca vulgaris in a randomized controlled trial.
Results
Demographic and clinical characteristics
A total of 24 adults were enrolled and randomized between September 2017 and December 2018 at two institutions. Enrollment was terminated in December 2018 according to the suggestion made in the Data and Safety Monitoring Committee meeting held in October 2018 because it was unlikely to achieve the primary objective (55% disappearance of the target lesion in the FIT039 group). However, the results of the secondary endpoint analysis (the change in the size of the target lesion) indicated certain efficacy (see the Efficacy of FIT039 section of the Result section).
A total of 24 patients with verruca vulgaris were selected for the assessment as indicated in the Consolidated Standards of Reporting Trials diagram (Figure 1). A total of 13 patients (11 men and 2 women with a median age of 54 [range: 22–81] years) and 11 patients (6 men and 5 women with a median age of 62 [range: 39–76] years) were allocated to the FIT039 and placebo groups, respectively (Table 1). Lesions were localized to the upper (seven each in the FIT039 and placebo groups) or lower (six and four in FIT039 and placebo groups, respectively; Table 1) extremities. In the FIT039 group, 1 of the 13 lesions was persistent verruca vulgaris, which was defined as a lesion that existed for at least 6 months at the time of obtaining informed consent, whereas 3 of the 11 lesions were persistent in the placebo group (Table 1). Petechiae were present in 7 of the 13 lesions in the FIT039 group and in 9 of the 11 lesions in the placebo group (Table 1). There were multiple lesions in 7 of the 13 patients in the FIT039 group and in 4 of the 11 patients in the placebo group (Table 1). Treatment, such as conventional cryotherapy and 10% salicylic acid ointment, had been previously applied for >4 weeks to 2 of the 13 patients in the FIT039 group and to 4 of the 11 patients in the placebo group (Table 1).
Figure 1.
CONSORT diagram of the trial. CONSORT, Consolidated Standards of Reporting Trials.
Table 1.
Patient Demography and State of Verruca Vulgaris
| Demography | Classification | FIT039 n = 13 |
Placebo n = 11 |
|---|---|---|---|
| Age (years), mean (SD) | 50.2 (19.1) | 59.9 (9.8) | |
| Median (range) | 54.0 (22, 81) | 62.0 (39, 76) | |
| Sex, n (%) | Men | 11 (84.6) | 6 (54.5) |
| Women | 2 (15.4) | 5 (45.5) | |
| Number of allocated verruca vulgaris cases | N/A | 13 | 11 |
| Anatomical sites of application, n (%) | Palms | 3 (23.1) | 4 (36.4) |
| Upper extremities except palms | 4 (30.8) | 3 (27.3) | |
| Soles | 5 (38.5) | 4 (36.4) | |
| Lower extremities except soles | 1 (7.7) | 0 (0.0) | |
| Number of persistent lesions1, n (%) | Yes | 1 (7.7) | 3 (27.3) |
| No | 12 (92.3) | 8 (72.7) | |
| Patients with petechiae-accompanying lesions2, n (%) | Yes | 7 (53.8) | 9 (81.8) |
| No | 6 (46.2) | 2 (18.2) | |
| Classification of the target lesion, n (%) | Solitary | 6 (46.2) | 7 (63.6) |
| Multiple | 7 (53.8) | 4 (36.4) | |
| Generalized | 0 (0.0) | 0 (0.0) | |
| Previous treatment of the lesion, n (%) | None | 11 (84.6) | 7 (63.6) |
| Yes | 2 (15.4) | 4 (36.4) |
Abbreviation: N/A, not applicable.
Lesions that persisted for at least 6 months at the time of informed consent acquisition since the diagnosis of verruca vulgaris during the first medical examination.
Presence of petechiae is defined by the presence of at least one petechia within the skin area affected by verruca vulgaris.
Summary of the treatment
All participants completed patch application (Figure 1). The median application times of the FIT039 and placebo patches were 324 (range: 281–330) and 320 (range: 284–329) hours, respectively. All allocated participants were included in the safety and efficacy analyses.
Efficacy of FIT039
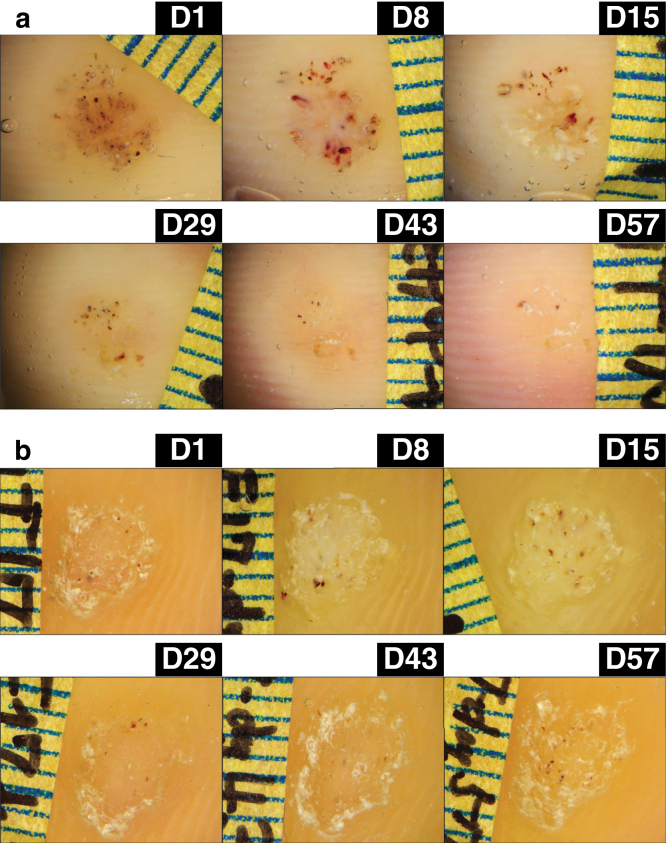
The primary endpoint was the resolution of lesions. The lesions were not resolved on both arms. The proportion of resolution was 0% for both FIT039- and placebo-treated lesions. However, a lesion on the left toe treated with a FIT039 patch started to shrink after day 29 with a decrease in the number of petechiae, and it continued to wane until day 57, although it did not completely disappear during the observational period (Figure 2a). In contrast, the placebo-treated lesions did not show such decreases in dimension, cross-sectional area, or number of petechiae (Figure 2b).
Figure 2.
Representative courses of the lesions. (a) A lesion on the left toe with a FIT039 patch and (b) a lesion on the right sole with a placebo patch are shown. Patches were applied from D1 to D14. The lesions were followed up on D15, D29, D43, and D57. D, day.
We evaluated the changes in the dimension, cross-sectional area, and number of petechiae of FIT039- and placebo-treated lesions to determine treatment efficacy (Table 2 and Figure 3). The dimension of the FIT039-treated lesions decreased to 88% (95% confidence interval [CI]: 77–99), 86% (95% CI: 72–101), and 76% (95% CI: 61–91) of the initial measurements on days 8, 15, and 29, respectively; however, they gradually increased again to 78% (95% CI: 63–94) and 98% (95% CI: 49–146) on days 43 and 57, respectively (Table 3 and Figure 3a; closed circles). In contrast, the placebo-treated lesions showed a slight decrease to 98% (95% CI: 84–112) on day 8 and a continuous increase to 107% (95% CI: 85–130) on day 57 (Table 3 and Figure 3a; open circles). The difference between the FIT039- and placebo-treated groups was –10% (95% CI: –27 to 7; P = 0.223) on day 8, –14% (95% CI: –35 to –7; P = 0.173) on day 15, –25% (95% CI: –46 to 5; P = 0.016) on day 29, –29% (95% CI: –55 to 3; P = 0.028) on day 43, and –10% (95% CI: –64 to 44; P = 0.711) on day 57 (Table 3 and Figure 3a).
Table 2.
Schedule of the Study
| Treatments and Assessments | Day 1 | Day 8 ±2 |
Day 15 –3 |
Day 29 +7 |
Day 43 +7 |
Day 57 +7 |
|---|---|---|---|---|---|---|
| Liquid nitrogen cryotherapy | X | |||||
| Application of patch | ♦-------------------------♦ | |||||
| Measurements of dimension, cross-sectional area, and number of petechiae of verruca vulgaris lesions | X | X | X | X | X | X |
| Symptoms at the application site | X | X | X | X | X | X |
| Scoring of skin reaction1 | X | X | ||||
| Adverse event | ♦------------------------------------♦ | |||||
X indicates the day of the visit of participants.
Dashes with diamonds indicate either the period when the patches were applied or the period of the monitoring for adverse events.
Scoring based on the grading system proposed by the International Contact Dermatitis Research Group.
Figure 3.
Efficacy of FIT039 against verruca vulgaris. (a) Dimensions, (b) cross-sectional area, and (c) number of petechiae of the target lesions treated with FIT039 (closed symbols) or placebo (open symbols) are shown. (d) A pictorial description of the secondary endpoints. Symbols and bars indicate means and the 95% confidential intervals at each time point, respectively. † indicates P < 0.05.
Table 3.
Summary of Changes in Dimensions Based on the Diameters of Verruca Vulgaris Lesions in each Subgroup
| FIT039 (n = 13) | Placebo (n = 11) | Group Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Time Point | n | Mean (SD) (mm2) |
Change from Day 1 (95% CI) (%) | n | Mean (SD) (mm2) |
Change from Day 1 (95% CI) (%) | Difference in Change from Day 1 (95% CI) (%) | P-Value |
| Day 1 | 13 | 26.62 (15.47) | N/A | 11 | 34.34 (25.50) | N/A | N/A | N/A |
| Day 8 | 13 | 24.16 (17.41) | 88.11 (77.08–99.15) | 11 | 34.34 (26.14) | 98.20 (83.9–112.44) | –10.08 (–26.78 to 6.61) | 0.223 |
| Day 15 | 13 | 23.13 (16.92) | 86.16 (71.69–100.62) | 11 | 35.09 (27.44) | 100.33 (83.32–117.34) | –14.17 (–35.0 to –6.71) | 0.173 |
| Day 29 | 13 | 21.01 (17.34) | 75.98 (60.66–91.30) | 11 | 35.85 (27.12) | 101.35 (86.83–115.86) | –25.37 (–45.52 to –5.21) | 0.016 |
| Day 43 | 13 | 21.28 (16.67) | 78.24 (62.72–93.76) | 11 | 36.54 (25.78) | 107.31 (83.96–130.66) | –29.07 (–54.96 to –3.46) | 0.028 |
| Day 57 | 13 | 23.87 (18.32) | 97.62 (49.20–146.03) | 11 | 36.60 (25.20) | 107.35 (84.69–130.00) | –9.73 (–63.59 to 44.12) | 0.711 |
Abbreviations: CI, confidence interval; N/A, not applicable.
Change from day 1 (%) = ([value at each time point]/[value on day 1]) × 100.
Day 1 is defined as the day of application of the skin patch.
The cross-sectional area of the FIT039-treated lesions showed negligible change until day 29, decreased to 81% (95% CI: 60–102) on day 43, and increased to 86% (95% CI: 64–108) on day 57 (Table 4 and Figure 3b; closed boxes). The cross-sectional area of the placebo-treated lesions increased to 110% (95% CI: 97–124) on day 8, decreased to 87% (95% CI: 67–107) on day 29, increased again to 98% (95% CI: 73–122) on day 43, and decreased again to 91% (95% CI: 74–108) on day 57 (Table 4 and Figure 3b; open boxes). The difference between the FIT039- and placebo-treated groups was –13% (95% CI: –30 to 4; P = 0.122) on day 8, –7% (95% CI: −24 to 9; P = 0.362) on day 15, 10% (95% CI: –13 to 33; P = 0.389) on day 29, –17% (95% CI: –47 to 13; P = 0.261) on day 43, and –5% (95% CI: –32 to 22; P = 0.699) on day 57 (Table 4 and Figure 3b).
Table 4.
Summary of Changes in the Cross-Sectional Area of Lesions Based on the Pixel of the Digital Image in each Subgroup
| FIT039 (n = 13) | Placebo (n = 11) | Group Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Time Point | n | Mean (SD) (mm2) |
Change from Day 1 (95% CI) (%) | n | Mean (SD) (mm2) |
Change from Day 1 (95% CI) (%) | Difference in Change from Day 1 (95% CI) (%) | P-Value |
| Day 1 | 13 | 21.41 (13.55) | N/A | 11 | 28.10 (18.47) | N/A | N/A | N/A |
| Day 8 | 13 | 20.33 (11.80) | 97.35 (85.63–109.08) | 11 | 31.84 (25.08) | 110.33 (96.83–123.82) | –12.97 (–29.72 to 3.78) | 0.122 |
| Day 15 | 13 | 20.86 (12.15) | 98.93 (90.60–107.26) | 11 | 31.18 (25.89) | 106.40 (89.73–123.07) | –7.47 (–24.11 to 9.17) | 0.362 |
| Day 29 | 13 | 20.50 (12.42) | 97.08 (82.06–121.11) | 11 | 23.54 (13.43) | 87.32 (67.41–107.22) | 9.77 (–13.29 to 32.82) | 0.389 |
| Day 43 | 13 | 17.70 (13.54) | 80.76 (59.70–101.82) | 11 | 26.35 (15.80) | 97.48 (73.24–121.72) | –16.72 (–46.82 to 13.37) | 0.261 |
| Day 57 | 13 | 18.68 (14.30) | 85.80 (63.63–107.97) | 11 | 24.70 (13.46) | 90.93 (73.99–107.88) | –5.13 (–32.31 to 22.04) | 0.699 |
Abbreviations: CI, confidence interval; N/A, not applicable.
Change from day 1 (%) = ([value at each time point]/[value on day 1]) × 100.
Day 1 is defined as the day of application of the skin patch.
Petechiae existed on 7 of the 13 and 9 of the 11 FIT039- and placebo-treated lesions, respectively (Table 5 and Figure 3c). The number of petechiae on the FIT039-treated lesions decreased to 59% compared with that in the original lesion (95% CI: 21–97) on day 8 and was maintained at 60% thereafter (Table 5 and Figure 3c; closed triangles). The number of petechiae on the placebo-treated lesions decreased to 29% (95% CI: 5–53) on day 15, increased to 91% (95% CI: –12 to 194) on day 43, and dropped to 52% (95% CI: 19–85) on day 57 (Table 5 and Figure 3c; open triangles). The difference between the FIT039- and placebo-treated groups was –13% (95% CI: –89 to 64; P = 0.731) on day 8, 36% (95% CI: –17 to 90; P = 0.168) on day 15, –1% (95% CI: –83 to 82; P = 0.987) on day 29, –25% (95% CI: –138 to 89; P = 0.651) on day 43, and 14% (95% CI: –37 to 65; P = 0.560) on day 57 (Table 5 and Figure 3c).
Table 5.
Summary of the Number of Petechiae in Verruca Vulgaris Lesions in Each Subgroup
| FIT039 (n = 13) | Placebo (n = 11) | Group Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | n | n1 | Mean (SD) | Change from Day 1 (95% CI) (%) | n | n1 | Mean (SD) | Change from Day 1 (95% CI) (%) | Difference in Change from Day 1 (95% CI) (%) | P-Value |
| Day 1 | 13 | 7 | 15.35 (24.16) | N/A | 11 | 9 | 9.68 (11.41) | N/A | N/A | N/A |
| Day 8 | 13 | 7 | 10.04 (16.15) | 59.04 (20.92–97.16) | 11 | 9 | 5.45 (8.84) | 71.56 (5.16–137.96) | –12.51 (–88.88 to 63.86) | 0.731 |
| Day 15 | 13 | 7 | 8.65 (12.97) | 65.24 (3.87–126.61) | 11 | 9 | 3.32 (4.83) | 28.96 (4.93–52.99) | 36.28 (–17.17 to 89.73) | 0.168 |
| Day 29 | 13 | 7 | 15.42 (30.13) | 66.10 (17.00–115.20) | 11 | 9 | 4.59 (6.63) | 66.76 (–2.58 to 136.10) | –0.66 (–83.38 to 82.06) | 0.987 |
| Day 43 | 13 | 7 | 12.81 (23.62) | 66.58 (27.56–105.61) | 11 | 9 | 6.00 (8.93) | 91.12 (–12.11 to 194.35) | –24.54 (–138.26 to 89.18) | 0.651 |
| Day 57 | 13 | 7 | 11.27 (19.14) | 66.09 (17.55–114.63) | 11 | 9 | 5.77 (8.79) | 51.91 (19.05–84.78) | 14.18 (–35.81 to 65.16) | 0.560 |
Abbreviations: CI, confidence interval; N/A, not applicable.
Change from day 1 (%) = ([value at each time point]/[value on day 1]) × 100.
Day 1 is defined as the day of application of the skin patch.
Target verruca vulgaris lesions having petechiae were subjected to analysis.
Safety
In total, 7 of the 24 patients reported adverse events: 3 in the FIT039 arm and 4 in the placebo arm. All events were classified as mild. One patient in the placebo arm reported pain and itching related to the placebo patch without cutaneous reactions. No adverse reactions were observed in the FIT039 arm. No serious adverse events were observed in either arm.
Discussion
Verruca vulgaris is a common cutaneous disease caused by HPV infection. The existing therapies focus on the destruction of infected keratinocytes. Currently, there is no specific antiviral therapy for HPV infection. To address this, we conducted a phase I/II trial of FIT039, a CDK9 inhibitor, against verruca vulgaris.
In this study, the application of FIT039 for 2 weeks decreased the dimension of lesions, which might have been influenced by physicians’ bias because they were aware of the assigned treatment owing to the single-blind protocol. Warts on the palms, soles, and other parts of the body were treated and evaluated in the same manner; however, different body parts could respond differently. Treatment efficacy could have been determined if optimal treatment and evaluation were carried out for each body part. Digital images were evaluated by evaluators who were blinded to the treatment. However, evaluators of digital images were at a disadvantage because the image displayed on a computer monitor lacked information on skin surface texture, making it difficult to determine the true boundaries of the lesions. Furthermore, assessments of verruca vulgaris are difficult owing to variability in their clinical appearance (Hogendoorn et al., 2018). A new clinical measure, the Cutaneous WARTS diagnostic tool, might improve the assessment of FIT039 efficacy in the future (Hogendoorn et al., 2018).
In this study, skin patches were simply applied to the target lesion. The physicians in charge and the participants were not allowed to perform any other interventions on the target lesion, such as scraping or removing the hyperkeratotic corneum, to prevent any bias that might affect the disease course. However, this strict condition hampered the accurate assessment of the lesion because of the thick hyperkeratotic areas over the target lesions. Furthermore, a thickened corneum could have impeded the penetration of FIT039 into the lesion. Despite these constraints, FIT039-treated lesions showed a reduction in dimension, indicating the efficacy of FIT039 in verruca vulgaris.
Patients with immunodeficient conditions often experience multiple verruca vulgaris on the extremities caused by HPV infection (Kirnbauer and Lenz, 2017). Verruca vulgaris deteriorates the QOL by causing physical pain, nail destruction, deformity of the skin appearance, psychological burden, and negative social impressions. Effective and noninvasive regimens that control HPV replication comprise an important unmet need for immunocompromised patients as well as for patients with congenital immunodeficiency and those undergoing post-transplantation care, those receiving immunosuppressants to treat autoimmune diseases, and those receiving anticancer chemotherapy. Thus, FIT039 matches this purpose. Practically, FIT039 can enhance the efficacy of conventional regimens, such as scraping, salicylic acid application, and cryotherapy.
Nonetheless, this study has several limitations. The number of subjects was small and did not meet the initial enrollment goals; hence, a subgroup analysis was not feasible. The target lesions comprised different subtypes of verruca vulgaris, that is, palmoplantar and nonpalmoplantar types, which responded differently to FIT039. The results from the single-blind analysis regarding the minimal changes in the lesion dimension remain to be confirmed. There were only two timepoints for dimension measurements during the patch application, and because the pharmacological effect of FIT039 is nonvirucidal, more frequent assessment of the dimensions and longer FIT039 treatment duration remain warranted.
FIT039 is a previously unreported agent that inhibits the proliferation of several viruses that use CDK9 for replication. Our study confirmed the safety of the FIT039 skin patch against verruca vulgaris after 2 weeks of application. However, the efficacy of FIT039 monotherapy could not be clearly demonstrated owing to the limitations described earlier. Therefore, the development of a novel regimen that can cure a broad spectrum of HPV-related mucocutaneous diseases, including verruca vulgaris, high-risk HPV infection, and cervical carcinoma, remains warranted.
Materials and Methods
Study design and treatments
Details of the protocol have been published previously (Nomura et al., 2019). This was a multicenter, single-blind, placebo-controlled, randomized phase I/II clinical trial. The study was approved by the institutional review board of Kyoto University Hospital (Japan) on 18 October 2017 (K037, protocol version 1.0, September 2017) and the National Hospital Organization Kyoto Medical Center (Japan) on 22 November 2017. The study was conducted in compliance with the protocol of the Ministerial Ordinance on Good Clinical Practice for Drugs and the Helsinki Declaration (World Medical Association, 2001). The patients provided written informed consent. This study was registered at the University Medical Information Network Clinical Trials Registry under UMIN000029695 (http://www.umin.ac.jp/ctr/index-j.htm).
Study participants
We enrolled men and women aged >20 years with verruca vulgaris or verruca plantaris lesions measuring 4–10 mm on the major axis on the extremities. Patients were excluded if they had been treated with cryotherapy for the target lesion or had taken systemic antiviral within 4 weeks before the acquisition of written informed consent (Nomura et al., 2019). The target sample size was 44, and patients were recruited between November 2017 and December 2018. Participants were randomly allocated to the FIT039-patch or placebo group at a ratio of 1:1 according to the allocation sequences obtained when the investigators sent the patient registration forms to the data center by facsimile. The centralized, computer-generated allocation sequences were prepared by an external contract research organization.
Intervention
On day 1, the target lesions in the FIT039 group were treated with liquid nitrogen cryotherapy to damage the epidermis and facilitate percutaneous absorption of FIT039. Next, a FIT039 patch was applied for 14 days. The evaluation period was up to day 57 (Table 1). The placebo group was treated and evaluated in the same way, except that the patch applied was a placebo. Participants were not informed about whether they were applied with FIT039 or placebo patches.
Evaluation of verrucous lesions
Digital dermatoscopic images of the target verruca vulgaris or verruca plantaris (verruca vulgaris on the palms and soles) lesions were captured using Derma9500S-GR (Derma Medical, Yokohama, Japan) with and without echo gel on days 1, 8, 15, 29, 43, and 57 (Table 3). The primary endpoint of efficacy was the disappearance of the treated lesions. This was evaluated on the basis of the examination performed by the physicians in charge of the treatment.
The secondary endpoint was morphological changes. Dimensions, cross-sectional areas, and the number of petechiae of the lesions were assessed. The dimension of a verruca lesion was defined as the product of the largest diameter in millimeter on an axis line of the lesion and the largest diameter on a line normal to the axis line. Dimensions were measured during examination by the physicians in charge. The cross-sectional area was defined as the area (in mm2) of the target lesion, calculated on the basis of the corresponding pixel count of a circumscribed area of the digital image. The cross-sectional area and the number of petechiae were determined by two independent dermatologists in a blinded manner.
Safety was another secondary endpoint, involving the evaluation of the incidence of adverse events and adverse drug reactions. Frequency counts and percentages were summarized. Adverse skin reactions were evaluated according to the criteria of the International Contact Dermatitis Research Group.
Statistical analysis
The primary endpoint of efficacy was the proportion of patients in whom the treated lesions disappeared, as evaluated by the investigators. A sample size of 44 was set to achieve 80% power at a one-sided significance level of 10%, considering ineligible patients and under the assumption of at least 55% disappearance of lesions in the FIT039 group, compared with 15% disappearance of lesions in the placebo group (Berman et al., 1986; Bunney et al., 1984; Kwok et al., 2011; Schmidt and Jacobsen, 1981; Spanos et al., 1990). The safety endpoint was a skin reaction to the FIT039 transdermal patch, according to the scoring system devised by the International Contact Dermatitis Research Group: 0 = no reaction,?1 = doubtful reaction (faint erythema only), 1 = weak positive reaction (palpable erythema, infiltration, possibly papules), 2 = strong positive reaction (erythema, infiltration, papules, vesicles), and 3 = extreme positive reaction (intense erythema and infiltration and coalescent vesicles) (Wilkinson et al., 1970).
Analyses of efficacy and safety were based on a March 2019 database lock. All analyses were conducted in accordance with the intention-to-treatment principle. The primary efficacy analysis was carried out by analysis of the images of the lesions by the investigators to estimate the proportion of resolved lesions in each arm and perform group comparisons using Fisher’s exact test at a one-sided significance level of 10%, if available. The remaining efficacy analyses with point estimates and 95% CI (corresponding to Student’s t-test at a two-sided significance level of 5%) were exploratory and were not adjusted for multiple testing. Thus, inferences from the CIs might not be reproducible. For patients who discontinued this trial with respect to the primary efficacy endpoint of the resolution of lesions, the missing values were imputed as failure-to-resolve lesions (baseline observation carried forward approach). As for the secondary efficacy endpoints, we devised three measures: dimension (the product of lesion diameters), cross-sectional area (pixel count of the lesion area), and number of petechiae (number of clots in the lesion) (Nomura et al., 2019). Missing values were not imputed. Standard descriptive statistics were used to summarize the demographic characteristics and safety results. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
Statement regarding patient involvement in the research
Patients were first involved in this research in the phase I stage. Patients can access complete information of this clinical trial using relevant keywords, including verruca, human papillomavirus, FIT039 transdermal patch, or UMIN000029695 on the Japan Primary Registries Network (https://rctportal.niph.go.jp/en/), which meets the WHO Registry Criteria (version 2.1, April 2009).
Data availability statement
Because we continue to develop the FIT039 patch for verrucae by modifying the formulation and/or dosage and administration, the dataset cannot be shared at present because it may interfere with the transfer of technology to marketing companies. Please contact the corresponding author for additional information.
ORCIDs
Takashi Nomura: http://orcid.org/0000-0002-4004-1339
Eriko Sumi: http://orcid.org/0000-0002-5028-0499
Gyohei Egawa : http://orcid.org/0000-0002-6101-4719
Saeko Nakajima: http://orcid.org/0000-0003-0831-1447
Eiko Toichi: http://orcid.org/0000-0001-6301-8556
Nana Inoue: http://orcid.org/0000-0001-7617-7249
Mami Shibuya : http://orcid.org/0000-0003-4735-2938
Natsuko Okamoto : http://orcid.org/0000-0003-3102-901X
Tsuyoshi Mitsuishi: http://orcid.org/0000-0001-6696-5524
Ryuji Uozumi: http://orcid.org/0000-0002-9546-9869
Harue Tada: http://orcid.org/0000-0001-8634-5592
Takayuki Nakagawa: http://orcid.org/0000-0003-1890-0843
Nobuhiro Kusuba: http://orcid.org/0000-0002-2238-5517
Aika Okuno: http://orcid.org/0000-0002-0201-1112
Chihiro Shimizuhira: http://orcid.org/0000-0001-5567-1950
Makiko Ishikawa: http://orcid.org/0000-0002-7622-7960
Shiro Tanaka: http://orcid.org/0000-0001-6817-5235
Masatoshi Hagiwara: http://orcid.org/0000-0003-1193-7571
Kenji Kabashima: http://orcid.org/0000-0002-0773-0554
Author Contributions
Conceptualization: TNo, ES, MH, KK; Data Curation: RU, HT; Formal Analysis: RU, HT; Funding Acquisition: MH; Investigation: TNo, GE, SN, ET, NI, MS, NO, NK, AO, CS, MI; Methodology: TNo, ES, GE, TM, RU, HT, MH, KK; Project Administration: TNo, ES, MH, KK; Resources: TNo, GE, SN, ET, NI, MS, NO, TNa, CS, MI, ST, MH, KK; Software: RU, HT; Supervision: TNo, ES, MH, KK; Validation: RU, HT; Visualization: TNo, ES, RU, HT; Writing - Original Draft Preparation: TNo, ES; Writing - Review and Editing: TNo, ES, GE, SN, ET, NI, MS, NO, TM, RU, HT, TNa, NK, AO, CS, MI, ST, MH, KK
Acknowledgments
This study was partially funded by the Project Promoting Clinical Trials for Development of New Drugs (19lk0201061t0004) from Japan Agency for Medical Research and Development (AMED) to ST. We express our gratitude to the participants in this study. We would like to thank Editage (http://www.editage.com) for English language editing. This study is registered at the University Medical Information Network Clinical Trials Registry under UMIN000029695 (http://www.umin.ac.jp/ctr/index-j.htm). This investigator-initiated study was supported by KinoPharma (Tokyo, Japan).
Conflict of Interest
MH owns equity in and is a scientific advisor at KinoPharma. The remaining authors state no conflict of interest.
accepted manuscript published online 20 May 2021; corrected proof published online 20 July 2021
Footnotes
Cite this article as: JID Innovations 2021;X:100026
References
- Ajiro M., Sakai H., Onogi H., Yamamoto M., Sumi E., Sawada T., et al. CDK9 inhibitor FIT-039 suppresses viral oncogenes E6 and E7 and has a therapeutic effect on HPV-induced neoplasia. Clin Cancer Res. 2018;24:4518–4528. doi: 10.1158/1078-0432.CCR-17-3119. [DOI] [PubMed] [Google Scholar]
- Barbosa P. Plantar verrucae and HIV infection. Clin Podiatr Med Surg. 1998;15:317–327. [PubMed] [Google Scholar]
- Beliaeva T.L. Populiatsionnaia chastota borodavok [The population incidence of warts] Vestn Dermatol Venerol. 1990;2:55–58. [in Russian] [PubMed] [Google Scholar]
- Berman B., Davis-Reed L., Silverstein L., Jaliman D., France D., Lebwohl M. Treatment of verrucae vulgaris with alpha 2 interferon. J Infect Dis. 1986;154:328–330. doi: 10.1093/infdis/154.2.328. [DOI] [PubMed] [Google Scholar]
- Beutner K.R., Spruance S.L., Hougham A.J., Fox T.L., Owens M.L., Douglas J.M., Jr. Treatment of genital warts with an immune-response modifier (imiquimod) J Am Acad Dermatol. 1998;38:230–239. doi: 10.1016/s0190-9622(98)70243-9. [DOI] [PubMed] [Google Scholar]
- Bunney M.H., Nolan M.W., Buxton P.K., Going S.M., Prescott R.J. The treatment of resistant warts with intralesional bleomycin: a controlled clinical trial. Br J Dermatol. 1984;111:197–207. doi: 10.1111/j.1365-2133.1984.tb04044.x. [DOI] [PubMed] [Google Scholar]
- Bzhalava D., Mühr L.S., Lagheden C., Ekström J., Forslund O., Dillner J., et al. Deep sequencing extends the diversity of human papillomaviruses in human skin. Sci Rep. 2014;4:5807. doi: 10.1038/srep05807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciconte A., Campbell J., Tabrizi S., Garland S., Marks R. Warts are not merely blemishes on the skin: a study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol. 2003;44:169–173. doi: 10.1046/j.1440-0960.2003.00672.x. [DOI] [PubMed] [Google Scholar]
- Hengge U.R., Esser S., Schultewolter T., Behrendt C., Meyer T., Stockfleth E., et al. Self-administered topical 5% imiquimod for the treatment of common warts and molluscum contagiosum. Br J Dermatol. 2000;143:1026–1031. doi: 10.1046/j.1365-2133.2000.03777.x. [DOI] [PubMed] [Google Scholar]
- Hogendoorn G.K., Bruggink S.C., Hermans K.E., Kouwenhoven S.T.P., Quint K.D., Wolterbeek R., et al. Developing and validating the cutaneous WARTS (CWARTS) diagnostic tool: a novel clinical assessment and classification system for cutaneous warts. Br J Dermatol. 2018;178:527–534. doi: 10.1111/bjd.15999. [DOI] [PubMed] [Google Scholar]
- Kilkenny M., Marks R. The descriptive epidemiology of warts in the community. Australas J Dermatol. 1996;37:80–86. doi: 10.1111/j.1440-0960.1996.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R., Lenz P. In: Dermatology. Fourth edition. Bolognia J.L., Schaffer J.V., Cerroni L., editors. Elsevier; Amsterdam, Netherlands: 2017. Human papillomaviruses; pp. 1383–1399. [Google Scholar]
- Kwok C.S., Gibbs S., Bennett C., Holland R., Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2012;2012 doi: 10.1002/14651858.CD001781.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C.S., Holland R., Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233–246. doi: 10.1111/j.1365-2133.2011.10218.x. [DOI] [PubMed] [Google Scholar]
- Kyriakis K., Pagana G., Michailides C., Emmanuelides S., Palamaras I., Terzoudi S. Lifetime prevalence fluctuations of common and plane viral warts. J Eur Acad Dermatol Venereol. 2007;21:260–262. doi: 10.1111/j.1468-3083.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- Lynch M.D., Cliffe J., Morris-Jones R. Management of cutaneous viral warts. BMJ. 2014;348:g3339. doi: 10.1136/bmj.g3339. [DOI] [PubMed] [Google Scholar]
- Micali G., Dall'Oglio F., Nasca M.R. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233–236. doi: 10.1080/09546630310016763. [DOI] [PubMed] [Google Scholar]
- Nomura T., Sumi E., Egawa G., Nakajima S., Toichi E., Uozumi R., et al. The efficacy of a cyclin dependent kinase 9 (CDK9) inhibitor, FIT039, on verruca vulgaris: study protocol for a randomized controlled trial. Trials. 2019;20:489. doi: 10.1186/s13063-019-3570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Hidaka A., Toyama M., Hosoya T., Yamamoto M., Hagiwara M., et al. Selective inhibition of HIV-1 replication by the CDK9 inhibitor FIT-039. Antiviral Res. 2015;123:1–4. doi: 10.1016/j.antiviral.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Jacobsen F.K. Ergebnis einer doppelblind und randomisiert durchgeführten klinischen Studie eines neuen Warzenmittels auf Basis Fluorouracil [Double-blind randomized clinical study on treatment of warts with a fluorouracil-containing topical preparation (author's transl)] Z Hautkr. 1981;56:41–43. [in German] [PubMed] [Google Scholar]
- Spanos N.P., Williams V., Gwynn M.I. Effects of hypnotic, placebo, and salicylic acid treatments on wart regression. Psychosom Med. 1990;52:109–114. doi: 10.1097/00006842-199001000-00009. [DOI] [PubMed] [Google Scholar]
- Sterling J.C., Gibbs S., Haque Hussain S.S., Mohd Mustapa M.F., Handfield-Jones S.E. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171:696–712. doi: 10.1111/bjd.13310. [DOI] [PubMed] [Google Scholar]
- Sumi E., Nomura T., Asada R., Uozumi R., Tada H., Amino Y., et al. Safety and plasma concentrations of a cyclin-dependent kinase 9 (CDK9) inhibitor, FIT039, administered by a single adhesive skin patch applied on normal skin and cutaneous warts. Clin Drug Investig. 2019;39:55–61. doi: 10.1007/s40261-018-0712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Okuyama-Dobashi K., Murakami S., Chen W., Okamoto T., Ueda K., et al. Inhibitory effect of CDK9 inhibitor FIT-039 on hepatitis B virus propagation. Antiviral Res. 2016;133:156–164. doi: 10.1016/j.antiviral.2016.08.008. [DOI] [PubMed] [Google Scholar]
- van Haalen F.M., Bruggink S.C., Gussekloo J., Assendelft W.J., Eekhof J.A. Warts in primary schoolchildren: prevalence and relation with environmental factors. Br J Dermatol. 2009;161:148–152. doi: 10.1111/j.1365-2133.2009.09160.x. [DOI] [PubMed] [Google Scholar]
- Viac J., Thivolet J., Chardonnet Y. Specific immunity in patients suffering from recurring warts before and after repetitive intradermal tests with human papilloma virus. Br J Dermatol. 1977;97:365–370. doi: 10.1111/j.1365-2133.1977.tb14243.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.S., Fregert S., Magnusson B., Bandmann H.J., Calnan C.D., Cronin E., et al. Terminology of contact dermatitis. Acta Derm Venereol. 1970;50:287–292. [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–374. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Onogi H., Kii I., Yoshida S., Iida K., Sakai H., et al. CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses. J Clin Invest. 2014;124:3479–3488. doi: 10.1172/JCI73805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowska J., Isa N.F., Murphy S. P-TEFb goes viral. Inside Cell. 2016;1:106–116. doi: 10.1002/icl3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because we continue to develop the FIT039 patch for verrucae by modifying the formulation and/or dosage and administration, the dataset cannot be shared at present because it may interfere with the transfer of technology to marketing companies. Please contact the corresponding author for additional information.