Abstract
Obesity is a growing epidemic worldwide, and it is also considered a major environmental factor contributing to the pathogenesis of inflammatory skin diseases, including psoriasis (PSO) and atopic dermatitis (AD). Moreover, obesity worsens the course and impairs the treatment response of these inflammatory skin diseases. Emerging evidence highlights that hypertrophied adipocytes and infiltrated immune cells secrete a variety of molecules, including fatty acids and adipokines, such as leptin, adiponectin, and a panel of cytokines/chemokines that modulate our immune system. In this review, we describe how adipose hypertrophy leads to a chronic low-grade inflammatory state in obesity and how obesity-related inflammatory factors are involved in the pathogenesis of PSO and/or AD. Finally, we discuss the potential role of antimicrobial peptides, mechanical stress and impairment of epidermal barrier function mediated by fast expansion, and dermal fat in modulating skin inflammation. Together, this review summarizes the current literature on how obesity is associated with the pathogenesis of PSO and AD, highlighting the potentially important but overlooked immunomodulatory role of adipose tissue in the skin.
Abbreviations: AD, atopic dermatitis; AMP, antimicrobial peptide; AT, adipose tissue; BAT, brown adipose tissue; BMI, body mass index; CI, confidence interval; DC, dendritic cell; dFB, dermal fibroblast; DIO, diet-induced obesity; dWAT, dermal white adipose tissue; FFA, free fatty acid; HFD, high-fat diet; KC, keratinocyte; OA, oleic acid; PA, palmitic acid; PSO, psoriasis; SCORAD, SCORing Atopic Dermatitis; sWAT, subcutaneous white adipose tissue; TC, total cholesterol; TEWL, transepidermal water loss; TG, triglyceride; Th, T helper; TLR, toll-like receptor; WAT, white adipose tissue
Introduction
According to the World Health Organization, worldwide obesity has tripled since 1975, and currently, ∼40% and 13% of adults in the world are overweight and obese, respectively (defined by a body mass index [BMI] ≥ 25 kg/m2 or BMI ≥ 30 kg/m2, respectively), and one in five children and adolescents are overweight globally. Obesity has been increasingly recognized as a major public health problem worldwide, and it was linked with 4.7 million deaths globally in 2017.
A chronic excess of energy intake over energy expenditure results in obesity. In obesity, an abnormal accumulation of excessive body fat that leads to a chronic low-level inflammatory state of the body interferes with the maintenance of an optimal state of heath (Eckel, 2018; Lumeng and Saltiel, 2011). This low-grade systemic inflammation has been recognized as an imbedded mechanism for increased risk of cardiovascular diseases; respiratory diseases; metabolic syndrome; insulin resistance; diabetes mellitus; tumors; cancers; and other inflammatory diseases, including pancreatitis, atherosclerosis, and psoriasis (PSO) in subjects with obesity (Caiazzo et al., 2018; Eckel, 2018; Kim and Bajaj, 2014; Lumeng and Saltiel, 2011; Uzuncakmak et al., 2018; Van Raemdonck et al., 2018; Versini et al., 2014).
Obesity also leads to the manifestation of skin inflammation. Both PSO and atopic dermatitis (AD), two major chronic and recurrent inflammatory skin diseases, are closely associated with obesity (Budu-Aggrey et al., 2019; Yew et al., 2020). It is now known that the skin is affected in obesity through a complex interaction of adipocytokines, hormones, fatty acids, and mechanical factors. In this review, we will first describe the normal function of adipose tissue (AT) and how the dysregulation of adipogenesis leads to adipocyte hypertrophy and inflammation in obesity. We will then review how obesity-related molecules are involved in the pathogenesis of PSO and AD. A systematic literature search was conducted through PubMed and Google scholar regarding original articles or reviews related to adipocytes, inflammation, obesity, PSO, and/or AD.
Obesity, characterized by a pathological expansion of the white AT
Types and distribution of ATs
AT is one of the largest tissue types in the body, comprising 20% of the body weight in healthy individuals and >30% in individuals who are obese. Morphologically, AT can be classified into white, brown, or beige subsets. Whereas the white AT (WAT) is considered a site for energy storage, the brown AT (BAT) functions to dissipate energy through mitochondrial uncoupling and to generate heat, and therefore, BAT is observed mostly in human newborns who do not shiver in the cold (Lidell, 2019). Anatomically, WAT is organized into distinct depots that have unique and location-specific functions, including subcutaneous WAT (sWAT) (located underneath the skin), visceral/omental WAT (located intra-abdominally), and dermal WATs (dWATs) (located within skin dermis) (Chen et al., 2019). In mice, dWAT is separated from sWAT by the panniculus carnosus (a distinct muscle layer), and it develops independently from sWAT and carries out unique nonmetabolic functions, including antimicrobial defense that is not observed in sWAT (Chen et al., 2019; Wojciechowicz et al., 2013). However, because human skin lacks the panniculus carnosus layer, dWAT is not physically separated from sWAT, and human dWAT is presented as a fat island located in the reticular dermis (mostly perifollicular) and superficial region of sWAT (Alexander et al., 2015; Bódis and Roden, 2018). Future studies are still needed to investigate how dWAT and sWAT are developmentally, biochemically, and functionally distinct in humans.
AT, a major secretory organ
Once considered an inert location of energy storage, studies over the last decade have identified AT as a major secretory organ, which plays a central role in a complex network of endocrine and autocrine/paracrine crosstalk between organs and tissues such as the heart, vasculature, liver, muscle, pancreas, and the skin (Eckel, 2018).
The powerful secretory output of WAT is contributed by diverse cell types that are present in AT, including adipocytes; preadipocytes/fibroblasts; endothelial cells; and immune cells, including macrophages and T cells (Ghaben and Scherer, 2019; Kim and Bajaj, 2014). The secretome of WAT comprises a large variety of molecules, including fatty acids, micro RNAs, cytokines/chemokines, and proteins, and these proteins secreted by the WAT are termed adipokines (Eckel, 2018; Ghaben and Scherer, 2019; Kim and Bajaj, 2014). To date, there are >600 putative adipokines that have been reported, and among these proteins, adiponectin, visfatin, and leptin are well-studied adipokines specifically secreted by adipocytes, whereas cytokines and chemokines are mostly secreted by preadipocytes and/or infiltrated immune cells during inflammatory conditions (Eckel, 2018; Kim et al., 2018).
Dysregulated adipocyte hyperplasia and hypertrophy in obesity
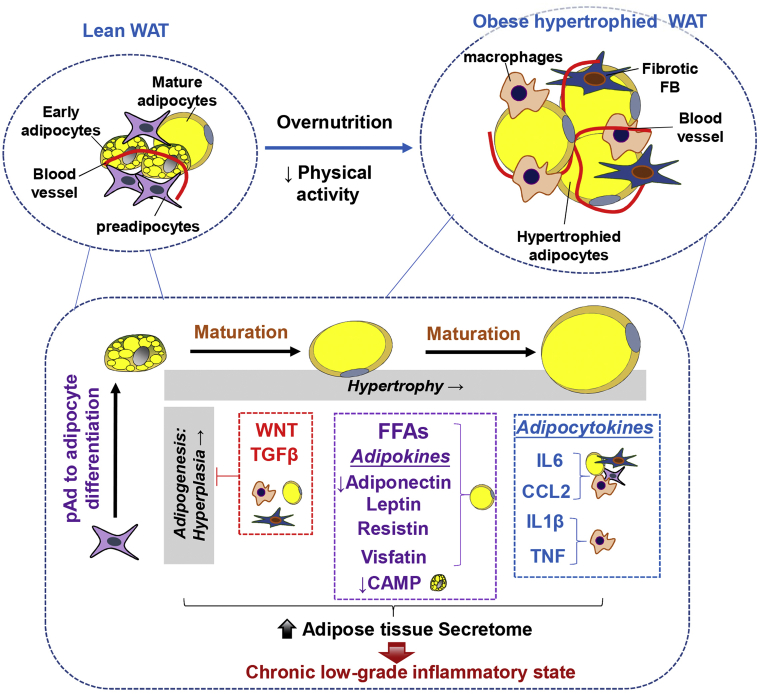
Obesity results from an excessive expansion of WAT by increase in the size of existing adipocytes (hypertrophy) or the formation of new adipocytes from the fibroblast-like preadipocytes (hyperplasia), a process known as adipogenesis (Ghaben and Scherer, 2019) (Figure 1). By lineage tracing experiments in mice, it has been shown that diet-induced obesity (DIO) induces a transient proliferation of preadipocytes to produce new adipocytes (hyperplasia), followed by a hypertrophic growth of adipocytes in the epidydimal WAT (the visceral fat depot in mice), whereas the expansion of sWAT primarily occurs through hypertrophy (Jeffery et al., 2015). Our recent work has shown that DIO triggers an initial hyperplasia growth of dermal adipocytes from adipogenic dermal fibroblasts (dFBs) in mice, but after 2 months of DIO, adipocyte hypertrophy accounts for the majority of dWAT expansion, leading to depletion of adipogenic dFBs and domination of mature adipocytes in the skin dermis during obesity (Zhang et al., 2021a). Therefore, AT expands by hypertrophy with or without hyperplasia in a depot-specific manner during obesity in mice.
Figure 1.
The cellular transition from lean to obese hypertrophied adipose tissue leads to elevated inflammation and inhibition of adipogenesis. Overnutrition or lack of exercise can lead to hypertrophy growth of adipocytes and eventually obesity. During this process, adipocytes become mature and increase in size. Adipocyte maturation promotes the secretion of FFA and a variety of proinflammatory adipokines (such as leptin, resistin, visfatin) and inhibits the expression of anti-inflammatory adipokine (adiponectin) and antimicrobial peptide CAMP. In addition, increased local levels of WNT and TGFβ inhibit the adipogenic potential of pAds, therefore blocking adipogenesis and adipocyte hyperplasia in obese adipose. Furthermore, the expression of a panel of proinflammatory cytokines and chemokines, including IL-6, CCL2, IL-1β, and TNF-α, are secreted by adipocytes and pAds, and infiltrated macrophages are increased in obese adipose. Together, obesity increases adipose tissue secretome, leading to the development of a chronic low-grade inflammatory state in individuals who are obese. FB, fibroblast; FFA, free fatty acid; pAd, preadipocyte; WAT, white adipose tissue.
Human AT develops early in the second trimester of pregnancy (Spalding et al., 2008), and although the total adipocyte number increases in childhood and adolescence, adipocyte numbers stay constant in lean individuals and individuals who are obese during adulthood (Spalding et al., 2008). By analyzing age-related changes in dermal fat in human skin, we have found that back skin is enriched with dWAT in neonates and that human dWAT becomes gradually lost during postnatal development and aging (Zhang et al., 2019), indicating that adipogenic dFBs are also lost in human skin during aging. We also have found that whereas adipocytes are normally found in the lower dermis of lean subjects, adipocytes infiltrate through the upper dermis in the back skin of subjects who are obese (Zhang et al., 2021a). Another study shows that overfeeding in adult humans leads to adipocyte hyperplasia in the lower body subcutaneous adipose but not in the abdominal subcutaneous adipose (Tchoukalova et al., 2010), suggesting that there is a regional difference in cellular mechanisms of AT growth in obesity.
Adipocyte hypertrophy, adipokine dysregulation, and inflammation
It has been shown that excess AT leads to a chronic low-grade systemic inflammatory state and that this obesity-induced inflammation triggers the development of several chronic conditions (Eckel, 2018; Hafidi et al., 2019; Kim and Bajaj, 2014). As shown in Figure 1, uncontrolled adipocyte hypertrophy leads to abnormal adipokine release, in which there is an increase in proinflammatory adipokines (leptin, visfatin) and a reduction in anti-inflammatory adipokines (adiponectin), leading to local inflammation and immune cell recruitment (Ghaben and Scherer, 2019). Higher levels of inflammatory cytokines/chemokines, including IL6, TNF-α, IL1β, and CCL2, are also secreted from the AT from individuals who are obese, contributing to the development of systemic inflammation (Kim and Bajaj, 2014). The specific role of each adipokine or adipocytokine in driving skin inflammation will be discussed in the section on the potential mechanisms linking obesity and PSO pathogenesis in this article.
The proliferative and adipogenic potentials of preadipocytes were also negatively correlated with both BMI and adipocyte cell size of the donors (Liu et al., 2017; Zhang et al., 2021a). Work from our group or other groups has shown that TNF-α, TGFβ, and/or WNT secreted from enlarging mature adipocytes and/or infiltrated macrophages inhibit the adipogenic potential of preadipocytes and induce a proinflammatory and profibrotic phenotype in these cells, leading to impairment of adipocyte hyperplasia, amplification of tissue inflammation, and fibrosis during the chronic state of obesity (Isakson et al., 2009; Liu et al., 2017; Zhang et al., 2021a). Together, dysregulation of the release of adipokines and/or cytokines driven by adipocyte hypertrophy is a critical event that leads to adipose dysfunction and inflammation in obesity.
Obesity and PSO
PSO is a chronic systemic inflammatory disease associated with several comorbidities, such as cardiovascular diseases, cancer, depression, metabolic syndromes, and obesity, and that severely impacts the quality of patients’ life (Takeshita et al., 2017a; Zusman et al., 2020). PSO has a prevalence of 2‒4% in the United States and Europe up to 9% in Nordic countries and 0.1‒2% in East Asian countries (Armstrong and Read, 2020). Four types of PSO have been reported: PSO vulgaris, pustular PSO, erythrodermic PSO, and psoriatic arthritis; most variants of PSO share three key clinical features of erythema, thickening, and scale (Boehncke and Schön, 2015).
PSO is developed when there is an interaction between keratinocytes (KCs) and immune cells. The initial innate immune activation of KCs in response to damage or infection promotes the subsequent neutrophil recruitment, angiogenesis, and dendritic cell (DC) activation (Sun et al., 2019). IL23, secreted by DCs, differentiates naïve T cells into IL-17‒ and IL-22‒producing T helper (Th) 17 cells. IL-17A and IL-22 then act on KCs, leading to the hyperproliferation and aberrant differentiation of KCs that ultimately leads to psoriatic plaque formation (Sun et al., 2019; Zhang, 2019; Zhang et al., 2016). Therefore, the IL-23/Th17 axis plays a central role in PSO pathogenesis.
Obesity increases the risk for PSO
Obesity has been confirmed to be an independent risk factor for PSO (Budu-Aggrey et al., 2019, Snekvik et al., 2017). A recent published Mendelian randomization study has shown that a 1 kg/m2 increase in BMI is associated with 4% higher odds of PSO (meta-analysis OR 1.04; 95% confidence interval [CI] = 1.03‒1.04; P = 1.73 × 10‒60) (Budu-Aggrey et al., 2019). In addition, BMI is also positively associated with skin disease severity (Fleming et al., 2015).
Obesity impairs the treatment response of PSO drugs
Obesity also reduces the efficacy of conventional PSO medications and increases adverse drug reactions (Carrascosa et al., 2014; Habjanič et al., 2019). Several studies have highlighted a lower response probability to fixed-dose biologic therapies (i.e., adalimumab, etanercept, and ustekinumab 45 mg dose) in patients who are obese and have PSO (Moroni et al., 2020; Shan and Zhang, 2019). A pharmacokinetic study has shown that infliximab (anti‒TNF-α mAb) clearance and serum elimination rates positively correlate with body weights, suggesting that the bioavailability of anti‒TNF-α drugs may be reduced in individuals who are overweight and/or obese compared with that in normal-weight individuals (Passot et al., 2016). Bodyweight has also been recognized as a factor negatively affecting the survival and responsiveness of secukinumab (anti‒IL-17A antibody) (Torres et al., 2019). Moreover, obesity has been recognized as a risk factor for severe hepatic fibrosis, such as nonalcoholic fatty liver disease, in patients with PSO who are taking methotrexate (a commonly prescribed systemic anti-inflammatory PSO drug) (Takeshita et al., 2017b). Interesting, a recent multicenter and cross-sectional study in Italy shows that patients with PSO with a BMI > 30 are less likely to receive biological therapy than patients with BMI >25 and <30 (Scala et al., 2020), indicating that bodyweight may also influence the therapeutic decision-making process in PSO.
Weight loss improves psoriatic phenotypes
Studies have shown that the psoriatic symptoms can be alleviated during weight loss. Researchers examined the efficacy of weight-loss interventions, both dietary and surgical, on PSO disease course, and their results have suggested that weight loss may be a useful preventative and adjunctive therapy for the treatment of PSO (Debbaneh et al., 2014; Ko et al., 2019; Mahil et al., 2019). Meanwhile, a successful weight loss, especially with fat and sugar intake restricted low-calorie diet, is associated with an improvement in PSO or psoriatic arthritis disease severity and/or responses to medication (Di Minno et al., 2014, Mahil et al., 2019). A large population-based cohort study by Egeberg et al. (2017) has shown that gastric bypass but not gastric banding is associated with a significantly reduced risk and improved prognosis of PSO. Both gastric bypass and gastric banding lead to weight loss, but gastric banding might also increase the secretion of gut hormones that potentially modulate inflammation regardless of weight loss (Egeberg et al., 2017). A randomized and controlled clinical trial has shown that weight loss induced by a low-calorie diet improves the responsiveness of patients who are obese with moderate-to-severe PSO to cyclosporine (Gisondi et al., 2008b). Together, these results suggest that weight loss approaches, including diet modification, may supplement traditional pharmacologic therapies in the treatment of patients who are obese and have PSO.
Potential mechanisms linking obesity and PSO pathogenesis
As we have discussed in the section on adipocyte hypertrophy, adipokine dysregulation, and inflammation, obesity is a proinflammatory condition in which hypertrophied adipocytes, activated preadipocytes/fibroblasts, and infiltrated immune cells all contribute to increased systemic levels of free fatty acid (FFA), adipokines, and/or cytokines. This obesity-associated state of chronic low-grade inflammation may play a role in the pathogenesis of PSO. Next, we will focus on the role of obesity-related FFAs, adipokines (leptin and adiponectin), and adipocytokines (TNF-α, IL-1β, and IL-6) in PSO pathogenesis.
Fatty acids and immune activation
Serum FFAs levels are elevated in individuals who are obese, primarily released from hypertrophied adipocytes (Hafidi et al., 2019). It has been shown that serum FFA levels, including palmitic acid (PA) and oleic acid (OA), correlate with psoriatic inflammation severity in patients with PSO, indicating that FFAs may play a critical role in an obesity-mediated exacerbation of skin inflammation (Herbert et al., 2018; Stelzner et al., 2016). In high-fat diet (HFD)‒fed mice, serum FFA levels correlate with severity of imiquimod-induced PSO-like skin inflammation (Stelzner et al., 2016), and a study by Herbert et al. (2018) has shown that a short-term HFD feeding without an obese phenotype is sufficient to increase FFA levels and amplify psoriasiform skin inflammation. In vitro, FFAs, such as PA and/or OA, can sensitize DCs, resulting in augmented secretion of Th1-/Th17-related cytokines during proinflammatory stimulation (Stelzner et al., 2016), and these FFAs also sensitize myeloid cells to lipopolysaccharide stimulation, resulting in an augmented inflammatory response in activated KCs (Herbert et al., 2018). Results from these studies indicate that FFAs released from hypertrophied adipocytes might be the bridge linking obesity with psoriatic diseases.
PSO and adipokines: leptin and adiponectin
The serum level of leptin is elevated in individuals who are obese, and leptin has recently emerged as a key link between obesity and inflammation. Leptin, mainly secreted from adipocytes, plays an integral role in the suppression of appetite (energy intake) and stimulation of energy expenditure by acting on its receptor (LEPR) expressed on the central and peripheral neurons (Versini et al., 2014). Besides, leptin also acts on the immune system, mainly as a proinflammatory factor. LEPR is ubiquitously expressed on the surface of immune cells such as monocytes/macrophages, T and B cells, and hematopoietic bone marrow precursors as well as skin-resident cells, including KCs, fibroblasts, endothelial cells, and adipocytes (Tadokoro et al., 2015). Activation of leptin signaling in the innate immune cells stimulates the release of proinflammatory mediators (e.g., IL-1, IL-6, TNF-α, and nitric oxide), stimulates phagocytosis on monocytes and macrophages, stimulates chemotaxis and oxygen free radicals synthesis in neutrophils, enhances cytotoxicity and proliferation of NK cells, enhances macrophage phagocytosis, and promotes DC activation and survival (Chiricozzi et al., 2016; Pérez-Pérez et al., 2020). In adaptive immunity, leptin polarizes T cells toward a Th1 response by stimulating the production of IL-2, IL-12, and IFN-γ and inhibiting IL-10 and IL-4, and leptin signaling positively regulates Th17 differentiation and negatively regulates the regulatory T cell function through the mTOR pathway (Dopytalska et al., 2020; Pérez-Pérez et al., 2020). Furthermore, leptin can stimulate the proliferation of KCs, fibroblasts, and endothelial cells, promoting epidermal/dermal hyperplasia and angiogenesis (Dopytalska et al., 2020). Together, elevated serum leptin levels in obesity may contribute to the low-grade inflammatory state, which makes individuals who are obese more susceptible to the development of autoimmune diseases.
In contrast to the blood levels of other adipokines, blood adiponectin levels are decreased in obesity (Hafidi et al., 2019) and have been shown to be decreased in patients with PSO (Kyriakou et al., 2017; Li et al., 2014). Primarily recognized as a metabolic mediator of insulin sensitivity, adiponectin also exerts anti-inflammatory properties by inhibiting the activation of KCs, macrophages, and T cells (Li et al., 2019; Zhang et al., 2021b). In vitro, adiponectin suppresses IL-6, TNF-α, and IL-12 production from activated macrophages and IL-6 production of KCs, and it inhibits the IL-1β/IL-23‒mediated production of IL-17 in dermal γδ T cells (Shibata et al., 2015, 2011). In mice, adiponectin deficiency exacerbates psoriasiform skin inflammation through enhanced infiltration of IL-17‒producing dermal γδ T cells (Shibata et al., 2015). During obesity, adiponectin expression in adipocytes is negatively regulated by inflammatory cytokines, including TNF-α and IL-6 (Lihn et al., 2005), and decreased adiponectin promotes the initiation of the inflammatory state by infiltration of macrophages and the development of Th17 cells, which is central to PSO pathogenesis. Overall, adiponectin is an important anti-inflammatory adipokine in the skin, and dysregulation of adiponectin in obesity may imply a mechanism underlying the relationship between PSO and obesity.
PSO and adipocytokines: IL-6, IL-1β, and TNF-α
IL-6 level is elevated in the plasma of individuals who are obese in proportion to the patient’s BMI (Kim and Bajaj, 2014), and AT is a major source of increased circulating IL-6 in individuals who are obese (Fuster et al., 2016; Mohamed-Ali et al., 1997). In patients with PSO, increased skin and serum IL-6 protein and mRNA levels are also observed, correlating with PSO severity (Coimbra et al., 2016). IL-6 is generally considered a proinflammatory cytokine that promotes inflammation in obesity. IL-6 acting with TGFβ and/or IL-1β is essential for the differentiation of Th17 cells (Chung et al., 2009; Zhang, 2018). Furthermore, IL-6 also acts on other cell types in the pathogenesis of PSO, including (i) KC growth, KC activation, and proinflammatory cytokine/chemokine production (especially in synergy with TNF-α and IL-17A); (ii) macrophage and DC production of proinflammatory cytokines and chemokines; (iii) adhesion molecule expression on endothelial cells; and (iv) neutrophil differentiation (Saggini et al., 2014). However, anti‒IL-6 therapies are ineffective for PSO and can even induce new-onset PSO-like disease (Laurent et al., 2010; Matsushima et al., 2019). In addition, IL-6‒deficient mice when crossed to an IL-17C PSO mouse model display slower onset of psoriatic skin disease phenotype but become worsen over time, suggesting that a lack of IL-6 might lead to compensatory proinflammatory effects by other cytokines, which eventually worsened the psoriatic inflammation (Blauvelt, 2017).
The IL-1β‒IL-1R signaling plays a critical role in PSO pathogenesis by regulating dermal IL-17‒producing cells and stimulating KCs (Cai et al., 2019). IL-1 signaling in T cells has been shown to be required for Th17 cell differentiation from naïve T cells in both humans and mice, and IL-1β combined with IL-6 and IL-23 effectively induces IL-17 production from naïve T cells synergies, generating pathogenic Th17 cells that promote autoimmunity (Acosta-Rodriguez et al., 2007; Chung et al., 2009; Lee et al., 2012). It has been suggested that the pronounced proinflammatory signature of AT is mainly driven by IL-1β released from AT macrophages in patients who are obese and have type 2 diabetes (Dalmas et al., 2014). In addition, macrophage-derived IL-1β promotes the production of IL-17 and IL-22 from CD4+ T cells, which can in turn act on the IL-17 and IL-22 receptors on macrophages, leading to a further increase in IL-1β release (Dalmas et al., 2014). These data suggest that IL-1β and the T-cell cytokines IL-17 and IL-22 may be key players driving autoimmune amplification that promotes obesity-induced type 2 diabetes.
In addition to IL-6 and IL-1β, TNF-α is an important adipocytokine linking obesity and PSO. Overproduction of TNF-α in the AT or in the skin is an important feature for obesity and PSO, respectively (Rodríguez-Cerdeira et al., 2019). Etanercept, a TNF-α‒blocking agent, has been proven to be effective in treating both PSO and obesity-related type 2 diabetes, suggesting that TNF-α may play a central role in regulating inflammation in PSO and obesity (Hamminga et al., 2006). Interestingly, anti-TNF treatment but not anti‒IL-17, anti‒IL-12, or anti‒IL-23 treatments can induce a bodyweight increase in patients with PSO (Esposito et al., 2009; Gisondi et al., 2013, 2008a; Patsalos et al., 2020; Shi et al., 2021; Wu et al., 2020), indicating that TNF-α may play a unique role in suppressing adipocyte growth. Indeed, TNF-α is known to inhibit adipogenesis and induce adipocyte lipolysis (Cawthorn and Sethi, 2008), and therefore, it is likely that TNF blockage may promote weight gain by restoring adipogenesis and inhibiting lipolysis.
Obesity and AD
AD is a chronic, relapsing, inflammatory skin disease characterized by eczema-like lesions with intense pruritis and high serum IgE. It is a major skin disease affecting ∼20% of children and ∼3% of adults worldwide (Avena-Woods, 2017). Whereas the association between obesity and PSO has been intensively studied, how obesity is associated with AD is less understood (Hirt et al., 2019).
Different from PSO, which is an autoimmune disease driven by a Th1-/Th17-dominant immune response, AD is considered as an allergic skin disease in which the activation of Th2 immune response is considered to be the principal initiating mechanism of the disease pathology (Guttman-Yassky et al., 2018; Sun et al., 2019). The AD pathogenesis involves both inside-out and outside-in theories (Zhang, 2021). The outside-in theory suggests that the systemic activation of Th2 and Th22 cells initiates the acute AD cutaneous pathology, whereas the outside-in theory indicates that a defective epidermal barrier allows entry of allergen and pathogen, which then promote the development of a Th2 immune response (Elias, 2018; Guttman-Yassky et al., 2018). Transition from the acute to the chronic stage of AD is marked by the recruitment of Th1- and Th17-producing T cells (Guttman-Yassky et al., 2018).
Overweight/obesity increases the propensity for AD
Studies have shown that children and adults who are overweight/obese had a higher risk of developing AD than normal-weight individuals (Ali et al., 2018; Zhang and Silverberg, 2015). A recent study involving 86,969 pediatric patients with AD and 116,564 matched controls from the United States has shown that AD is significantly associated with metabolic syndrome (OR = 1.61) and obesity (OR = 1.81) (Huang et al., 2021). A meta-analysis has confirmed that overweight/obesity is associated with increased prevalence and severity of AD in North America and Asia but not in Europe, and this discrepancy is likely because of the varied definition of obesity and AD used in European studies (Zhang and Silverberg, 2015). Although there are inconsistencies in whether AD is associated with obesity, it appears that AD is more consistently associated with obesity in younger children rather than in older patients. In addition to the age factor, the inconsistency may also be explained by a difference in study design, AD diagnostic criteria, variable definitions of overweight or obesity, and sex (Ali et al., 2018). Large prospective cohort studies are still required to confirm the association between AD and obesity, especially in adult patients.
The causal relationship between AD and adiposity
To investigate the causal relationship between AD and obesity, Yew et al. (2020) conducted a Mendelian randomization analysis using data extracted from GWASs of BMI and AD. They found that increase in obesity determined by susceptible genes is associated with increased risk of AD (OR of AD = 1.08 [95% CI = 1.01‒1.14; P = 0.015] per unit increase in BMI). In contrast, increased risk of AD determined by relevant susceptibility gene is not associated with a higher BMI (change in BMI attributable to AD based on genetic information: 0.00; 95% CI = 0.02‒0.02; P = 0.862). This study indicates a causal role of adiposity in the development of AD, and obesity may be a risk factor for AD, facilitating the development of AD.
Potential mechanisms linking obesity and AD
As we have described in the section on the potential mechanisms linking obesity and PSO pathogenesis, in the obese state, there is a systemic increase in the expression of proinflammatory adipokines such as leptin and a decrease in the anti-inflammatory adipokines such as adiponectin. Assessment of blood levels of a panel of adipokines in adult patients without obese suffering from chronic extrinsic childhood-onset AD found that adiponectin decreases and leptin increases in patients with AD compared with those in healthy subjects, and the levels of adiponectin are inversely associated with the disease severity (Jaworek et al., 2020). This study suggests that adipokines may be the potential biomarkers to determine eczema intensity and severity. In line with this, adiponectin levels are found inversely associated with the prevalence of AD (Nagel et al., 2009). However, a small study conducted among Korean patients with AD has shown that although BMI is positively associated with SCORing Atopic Dermatitis (SCORAD) index and total serum IgE levels, there is no significant difference in the serum levels of leptin or adiponectin with SCORAD index (Han et al., 2016). Further analysis found that adiponectin levels are significantly lower in extrinsic AD than in intrinsic AD, indicating that adiponectin may play a role in the pathogenesis in extrinsic AD but not in intrinsic AD (Han et al., 2016). These conflicting results suggest that change in the expression levels of adipokine alone is not sufficient to trigger AD, and future studies are needed to clarify the role of obesity in AD pathogenesis.
The direct role of adipokines in AD pathogenesis is not fully understood. Studies have shown that activation of adiponectin receptor signaling pathway in myeloid or lymphoid immune cells suppresses proinflammatory cytokine expression, promotes anti-inflammatory cytokine (IL-10) expression, blocks the activation of NF-kB in DCs, and inhibits antigen-specific T-cell proliferation and cell survival (Masaki et al., 2004; Tan et al., 2014; Wilk et al., 2011). In a three-dimensional in vitro epidermal equivalent AD model, it has also been shown that adiponectin suppresses the expression of thymic stromal lymphopoietin and IL-8 and enhances the expression of lipid biosynthetic enzymes and differentiation factors such as FLG (Seo et al., 2019), suggesting that decreased expression of adiponectin may suppress the development of Th2 immune response and enhance epidermal differentiation and barrier function.
Although studies have indicated that obesity promotes the expansion of Th17 cell lineage, how obesity may impact the initiation of AD pathogenesis by directly altering the Th2 cell development remains largely unknown. A study by Bapat et al. has shown that obesity-associated dysregulation of peroxisome proliferator–activated receptor-γ in CD4+ T cells drives the obesity-associated Th2 immunopathology (Bapat et al., 20191). In addition, a recent study found that children with AD had significantly higher levels of total cholesterol (TC) and triglycerides (TG), and higher levels of TC and TG are also significantly associated with the SCORAD index, indicating that abnormal blood lipid profile may contribute to AD pathogenesis in children (Kim et al., 2021). Future studies are still needed to determine whether obesity increases the risk of AD pathogenesis by a general increase of the systemic inflammation by lipid and/or adipokines secreted from hypertrophied adipocytes or by directly influencing T-cell priming toward the Th2 immune response.
A potential link between obesity-related mechanical stretching and PSO and AD pathogenesis
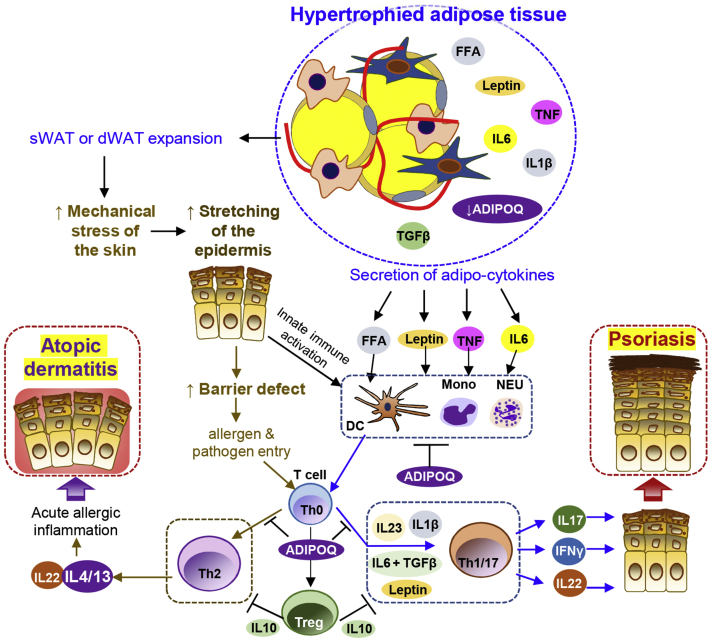
Striae, also known as stretch marks, are characterized by linear, atrophic, and white-colored plaques; are commonly found in subjects who are obese; and are often found on surfaces that have the most tension, including abdomen, thighs, buttocks, and breasts (Uzuncakmak et al., 2018). It has been suggested that expansion of the subcutaneous fat tissue is the major factor in the pathogenesis of Striae (Uzuncakmak et al., 2018). In addition, PSO often occurs in areas that have strong tension, and the relapses of PSO are often triggered by infection, wounding, and mechanical stress (Kamiya et al., 2019; Zhang, 2019). A recent study has shown that skin stretching by implanting a dilator in mice impairs skin barrier, upregulates the expression of PSO-related cytokines in epidermal KCs, and aggravates IL-23‒triggered psoriatic phenotype (Qiao et al., 2019). Indeed, it has been reported that plaque PSO can develop along the striae distensae, suggesting that striae may be a potential precipitating factor for the Koebner phenomenon in PSO (Morais et al., 2013; Verma, 2009).
Excessive skin fat tissue expansion may impair the barrier function of the skin epidermis. A study to determine the impact of obesity in epidermal permeability barrier status in children has found that skin transepidermal water loss (TEWL) values are significantly higher in children who are obese than in normal-weight children (Nino et al., 2012). In adults, the TEWL values are also significantly higher in individuals who are obese than in lean controls (Löffler et al., 2002), suggesting that obesity may aggravate the severity of AD by impairing the barrier integrity and therefore allowing allergen entry, promoting an allergic response that leads to AD. Although the link between the mechanical stress caused by fat expansion and PSO and/or AD pathogenesis is yet to be determined, it is likely that obesity-related mechanical stretching contributes to skin inflammation by impairing the epidermal barrier function and predepositing KCs under activation state (Figure 2).
Figure 2.
Proposed model for how adipose tissue hypertrophy promotes the development of inflammatory cascade that leads to the pathogenesis of psoriasis and/or atopic dermatitis. The mechanism stress during adipose tissue expansion in obesity may stretch the epidermis, leading to impairment of the epidermal barrier function and activation of the innate immune cascade that trigger autoimmune activation. Proinflammatory molecules (such as FFA, adipokines, and cytokines) released from expanding fat tissue can activate the innate immune cells (such as NEUs, macrophages, and DCs), followed by activation of the Th1, Th17, and/or Th22 cells, which in term act on epidermal keratinocytes, ultimately leading to epidermal hyperplasia and psoriasis pathogenesis. Adiponectin (ADIPOQ) is an anti-inflammatory adipokine that can inhibit myeloid and lymphocyte activation, and the expression of ADIPOQ is lost in the hypertrophied adipocytes in individuals who are obese, which may contribute to psoriasis and/or atopic dermatitis pathogenesis. Defective skin barrier may allow entry of allergens and/or pathogens into the skin, which promotes the development of Th2 cell immune response that drives acute allergic inflammation and ultimately pathogenesis of atopic dermatitis. DC, dendritic cell; dWAT, dermal white adipose tissue; FFA, free fatty acid; Mono, monocyte; NEU, neutrophil; sWAT, subcutaneous white adipose tissue; Th, T helper; Treg, regulatory T cell.
Future perspective: The role of dWAT in PSO or AD pathogenesis?
Recent studies from our group have identified dermal fat (dWAT) as a newly recognized layer of skin defense (Chen et al., 2019), but how dWAT is related to PSO and/or AD remains largely unexplored. We have shown that antimicrobial peptide (AMP) cathelicidin is abundantly secreted from differentiating early adipocytes during adipogenesis contributing to skin innate defense against bacterial infection, and loss of adipogenesis or adipocyte hypertrophy during aging and/or obesity impairs this antimicrobial function of dWAT (Zhang et al., 2021a, 2019, 2015). AMPs represent a class of endogenous antibiotics-like proteins induced during infection or injury in host cells (Zhang and Gallo, 2016). The expression of cathelicidin is elevated in psoriatic skin (Ong et al., 2002), and cathelicidin peptide LL37 promotes autoimmunity by activating the DNA toll-like receptor (TLR) 9 or single-strand RNA TLR7 pathway in plasmacytoid DCs (Ganguly et al., 2009; Gregorio et al., 2010) or the double-strand RNA MAVS/TLR3 pathway in KCs (Zhang, 2019; Zhang et al., 2016). It is possible that AMP represents a novel class of adipokine mediating the cross-talk between obesity and skin inflammation. Future studies will be needed to determine how adipokines, including AMPs and cytokines, derived from dWAT, the AT residing in close proximity to skin epidermis, may influence local immune microenvironment and ultimately the development of PSO or AD.
Conclusion
Obesity is closely related to PSO and AD, and chronic low-grade inflammatory state driven by abnormal secretion of FFAs as well as adipokines and/or adipocytokines from hypertrophied adipocytes and other local cells may be the underlying mechanism for increased risk of the development of these inflammatory skin diseases in subjects who are obese. Systemic inflammation that is present in both AD and PSO may in turn increase the risk of obesity and metabolic disorders, both of which inflammatory component plays a key role in their pathological pathways. Future studies are still needed to determine how PSO and AD, two inflammatory skin diseases driven by distinct immune pathways, are both associated with obesity. Overall, obesity may facilitate PSO or AD pathogenesis by secreting adipokines, but adipokines alone are not sufficient to trigger PSO or AD. It is likely that whether obesity exacerbates PSO or AD-like skin inflammation may depend on the coexistence of additional factors, such as genetic factors, environmental factors, and skin microbiota composition. Finally, it is important for dermatologists to recognize the contribution of obesity in driving skin inflammation and to propose that both patients with AD and PSO change their life and diet habits accordingly in addition to the conventional pharmacologic or biological treatments.
ORCIDs
Zhuolin Guo: http://orcid.org/0000-0001-8660-9279
Yichun Yang: http://orcid.org/0000-0001-8043-6581
Yanhang Liao: http://orcid.org/0000-0003-1142-1196
Yuling Shi: http://orcid.org/0000-0002-1273-7881
Ling-juan Zhang: http://orcid.org/0000-0001-6937-4578
Author Contributions
Conceptualization: ZG, YS, LJZ; Resources: ZG, YY; Supervision: YS, LJZ; Writing - Original Draft Preparation: ZG, LJZ; Writing - Review and Editing: ZG, YY, YL, YS, LJZ
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
This work is supported by National Key R&D Program of China (2020YFA0112900), National Natural Science Foundation of China grants (81971551 and K2918001), Natural Science Foundation of Fujian (2020J06006) and Xiamen University grant X2123303 to LJZ and grants from National Natural Science Foundation of China (numbers 81872522 and 82073429), Innovation Program of Shanghai Municipal Education Commission (number 2019-01-07-00-07-E00046), the Clinical Research Plan of SHDC (number SHDC2020CR1014B), and Program of Shanghai Academic Research Leader (number 20XD1403300) to YS.
accepted XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2021;X:100064
Bapat SP, Liang Y, Liu S, Zhang L-j, Vogel I, Mar DJ, et al. Obesity potentiates TH2 immunopathology via dysregulation of PPARγ. bioRxiv 2019.
References
- Acosta-Rodriguez E.V., Napolitani G., Lanzavecchia A., Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Alexander C.M., Kasza I., Yen C.L., Reeder S.B., Hernando D., Gallo R.L., et al. Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res. 2015;56:2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z., Suppli Ulrik C., Agner T., Thomsen S.F. Is atopic dermatitis associated with obesity? A systematic review of observational studies. J Eur Acad Dermatol Venereol. 2018;32:1246–1255. doi: 10.1111/jdv.14879. [DOI] [PubMed] [Google Scholar]
- Armstrong A.W., Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(Suppl. 8):S115–S123. [PubMed] [Google Scholar]
- Blauvelt A. IL-6 differs from TNF-α: unpredicted clinical effects caused by IL-6 blockade in psoriasis. J Invest Dermatol. 2017;137:541–542. doi: 10.1016/j.jid.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Bódis K., Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Investwe. 2018;48:e13017. doi: 10.1111/eci.13017. [DOI] [PubMed] [Google Scholar]
- Boehncke W.-H., Schön M.P. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- Budu-Aggrey A., Brumpton B., Tyrrell J., Watkins S., Modalsli E.H., Celis-Morales C., et al. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Xue F., Quan C., Qu M., Liu N., Zhang Y., et al. A critical role of the IL-1β-IL-1R signaling pathway in skin inflammation and psoriasis pathogenesis. J Invest Dermatol. 2019;139:146–156. doi: 10.1016/j.jid.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo G., Fabbrocini G., Di Caprio R., Raimondo A., Scala E., Balato N., et al. Psoriasis, cardiovascular events, and biologics: lights and shadows. Front Immunol. 2018;9:1668. doi: 10.3389/fimmu.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J.M., Vilavella M., Garcia-Doval I., Carretero G., Vanaclocha F., Daudén E., et al. Body mass index in patients with moderate-to-severe psoriasis in Spain and its impact as an independent risk factor for therapy withdrawal: results of the Biobadaderm Registry. J Eur Acad Dermatol Venereol. 2014;28:907–914. doi: 10.1111/jdv.12208. [DOI] [PubMed] [Google Scholar]
- Cawthorn W.P., Sethi J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.X., Zhang L.J., Gallo R.L. Dermal white adipose tissue: a newly recognized layer of skin innate defense. J Invest Dermatol. 2019;139:1002–1009. doi: 10.1016/j.jid.2018.12.031. [DOI] [PubMed] [Google Scholar]
- Chiricozzi A., Raimondo A., Lembo S., Fausti F., Dini V., Costanzo A., et al. Crosstalk between skin inflammation and adipose tissue-derived products: pathogenic evidence linking psoriasis to increased adiposity. Expert Rev Clin Immunol. 2016;12:1299–1308. doi: 10.1080/1744666X.2016.1201423. [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra S., Catarino C., Santos-Silva A. The triad psoriasis-obesity-adipokine profile. J Eur Acad Dermatol Venereol. 2016;30:1876–1885. doi: 10.1111/jdv.13701. [DOI] [PubMed] [Google Scholar]
- Dalmas E., Venteclef N., Caer C., Poitou C., Cremer I., Aron-Wisnewsky J., et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63:1966–1977. doi: 10.2337/db13-1511. [DOI] [PubMed] [Google Scholar]
- Di Minno M.N., Peluso R., Iervolino S., Russolillo A., Lupoli R., Scarpa R., et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis. 2014;73:1157–1162. doi: 10.1136/annrheumdis-2012-202812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbaneh M., Millsop J.W., Bhatia B.K., Koo J., Liao W. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133–140. doi: 10.1016/j.jaad.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopytalska K., Baranowska-Bik A., Roszkiewicz M., Bik W., Walecka I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020;19:215. doi: 10.1186/s12944-020-01391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J. In: The cellular secretome and organ crosstalk. Eckel J., editor. Academic Press; Cambridge: 2018. Adipose issue: major secretory organ; pp. 9–63. [Google Scholar]
- Egeberg A., Sørensen J.A., Gislason G.H., Knop F.K., Skov L. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery [published correction apears in JAMA Surg 2018;153:692] JAMA Surg. 2017;152:344–349. doi: 10.1001/jamasurg.2016.4610. [DOI] [PubMed] [Google Scholar]
- Elias P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp Dermatol. 2018;27:847–851. doi: 10.1111/exd.13693. [DOI] [PubMed] [Google Scholar]
- Esposito M., Mazzotta A., Saraceno R., Schipani C., Chimenti S. Influence and variation of the body mass index in patients treated with etanercept for plaque-type psoriasis. Int J Immunopathol Pharmacol. 2009;22:219–225. doi: 10.1177/039463200902200124. [DOI] [PubMed] [Google Scholar]
- Fleming P., Kraft J., Gulliver W.P., Lynde C. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg. 2015;19:450–456. doi: 10.1177/1203475415586332. [DOI] [PubMed] [Google Scholar]
- Fuster J.J., Ouchi N., Gokce N., Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly D., Chamilos G., Lande R., Gregorio J., Meller S., Facchinetti V., et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- Gisondi P., Conti A., Galdo G., Piaserico S., De Simone C., Girolomoni G. Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: a prospective cohort study. Br J Dermatol. 2013;168:1124–1127. doi: 10.1111/bjd.12235. [DOI] [PubMed] [Google Scholar]
- Gisondi P., Cotena C., Tessari G., Girolomoni G. Anti-tumour necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J Eur Acad Dermatol Venereol. 2008;22:341–344. doi: 10.1111/j.1468-3083.2007.02429.x. [DOI] [PubMed] [Google Scholar]
- Gisondi P., Del Giglio M., Di Francesco V., Zamboni M., Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242–1247. doi: 10.3945/ajcn.2008.26427. [DOI] [PubMed] [Google Scholar]
- Gregorio J., Meller S., Conrad C., Di Nardo A., Homey B., Lauerma A., et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Yassky E., Krueger J.G., Lebwohl M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp Dermatol. 2018;27:409–417. doi: 10.1111/exd.13336. [DOI] [PubMed] [Google Scholar]
- Habjanič N., Lužar-Stiffler V., Kerec-Kos M., Grabnar Peklar D. Efficacy of calcipotriol-betamethasone ointment in patients with mild to moderate plaque psoriasis: subgroup analyses. Dermatology. 2019;235:501–508. doi: 10.1159/000502516. [DOI] [PubMed] [Google Scholar]
- Hafidi M.E., Buelna-Chontal M., Sánchez-Muñoz F., Carbó R. Adipogenesis: a necessary but harmful strategy. Int J Mol Sci. 2019;20:3657. doi: 10.3390/ijms20153657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamminga E.A., van der Lely A.J., Neumann H.A., Thio H.B. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768–773. doi: 10.1016/j.mehy.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Han B., Wu W.H., Bae J.M., Son S.J., Lee J.H., Han T.Y. Serum leptin and adiponectin levels in atopic dermatitis (AD) and their relation to disease severity. J Am Acad Dermatol. 2016;75:629–631. doi: 10.1016/j.jaad.2016.04.036. [DOI] [PubMed] [Google Scholar]
- Herbert D., Franz S., Popkova Y., Anderegg U., Schiller J., Schwede K., et al. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. J Invest Dermatol. 2018;138:1999–2009. doi: 10.1016/j.jid.2018.03.1522. [DOI] [PubMed] [Google Scholar]
- Hirt P.A., Castillo D.E., Yosipovitch G., Keri J.E. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037–1057. doi: 10.1016/j.jaad.2018.12.070. [DOI] [PubMed] [Google Scholar]
- Huang A.H., Roh Y.S., Sutaria N., Choi J., Williams K.A., Canner J.K., et al. Real-world comorbidities of atopic dermatitis in the pediatric ambulatory population in the United States. J Am Acad Dermatol. 2021;85:893–900. doi: 10.1016/j.jaad.2021.03.016. [DOI] [PubMed] [Google Scholar]
- Isakson P., Hammarstedt A., Gustafson B., Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworek A.K., Szepietowski J.C., Szafraniec K., Jaworek M., Hałubiec P., Wojas-Pelc A., et al. Adipokines as biomarkers of atopic dermatitis in adults. J Clin Med. 2020;9:2858. doi: 10.3390/jcm9092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E., Church C.D., Holtrup B., Colman L., Rodeheffer M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K., Kishimoto M., Sugai J., Komine M., Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20:4347. doi: 10.3390/ijms20184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Kim Y.K., Kim S., Kim J.E., Tian Y.D., Doh E.J., et al. Adipochemokines induced by ultraviolet irradiation contribute to impaired fat metabolism in subcutaneous fat cells. Br J Dermatol. 2018;178:492–501. doi: 10.1111/bjd.15907. [DOI] [PubMed] [Google Scholar]
- Kim J., Bajaj M. In: Pathobiology of human disease: a dynamic encyclopedia of disease mechanisms. Mitchell R., editor. Elsevier Science; Amsterdam, Netherlands: 2014. Normal adipose tissue biology: adipocytokines and inflammation; pp. 488–497. [Google Scholar]
- Kim J.H., Lee S.W., Yon D.K., Ha E.K., Jee H.M., Sung M., et al. Association of serum lipid parameters with the SCORAD index and onset of atopic dermatitis in children. Pediatr Allergy Immunol. 2021;32:322–330. doi: 10.1111/pai.13391. [DOI] [PubMed] [Google Scholar]
- Ko S.H., Chi C.C., Yeh M.L., Wang S.H., Tsai Y.S., Hsu M.Y. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD011972.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakou A., Patsatsi A., Sotiriadis D., Goulis D.G. Serum leptin, resistin, and adiponectin concentrations in psoriasis: a meta-analysis of observational studies. Dermatology. 2017;233:378–389. doi: 10.1159/000481882. [DOI] [PubMed] [Google Scholar]
- Laurent S., Le Parc J.M., Clérici T., Bréban M., Mahé E. Onset of psoriasis following treatment with tocilizumab. Br J Dermatol. 2010;163:1364–1365. doi: 10.1111/j.1365-2133.2010.10005.x. [DOI] [PubMed] [Google Scholar]
- Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.C., Krishnamoorthy P., DerOhannessian S., Doveikis J., Wilcox M., Thomas P., et al. Psoriasis is associated with decreased plasma adiponectin levels independently of cardiometabolic risk factors. Clin Exp Dermatol. 2014;39:19–24. doi: 10.1111/ced.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Geng L., Liu X., Gui W., Qi H. Recombinant adiponectin alleviates abortion in mice by regulating Th17/Treg imbalance via p38MAPK-STAT5 pathway. Biol Reprod. 2019;100:1008–1017. doi: 10.1093/biolre/ioy251. [DOI] [PubMed] [Google Scholar]
- Lidell M.E. Brown adipose tissue in human infants. Handb Exp Pharmacol. 2019;251:107–123. doi: 10.1007/164_2018_118. [DOI] [PubMed] [Google Scholar]
- Lihn A.S., Pedersen S.B., Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- Liu L.F., Craig C.M., Tolentino L.L., Choi O., Morton J., Rivas H., et al. Adipose tissue macrophages impair preadipocyte differentiation in humans. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler H., Aramaki J.U., Effendy I. The influence of body mass index on skin susceptibility to sodium lauryl sulphate. Skin Res Technol. 2002;8:19–22. doi: 10.1046/j.0909-752x. [DOI] [PubMed] [Google Scholar]
- Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahil S.K., McSweeney S.M., Kloczko E., McGowan B., Barker J.N., Smith C.H. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? A critically appraised topic. Br J Dermatol. 2019;181:946–953. doi: 10.1111/bjd.17741. [DOI] [PubMed] [Google Scholar]
- Masaki T., Chiba S., Tatsukawa H., Yasuda T., Noguchi H., Seike M., et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- Matsushima Y., Hayashi A., Mizutani K., Kondo M., Nakai Y., Habe K., et al. Psoriasiform dermatitis developing during treatment of juvenile idiopathic arthritis with tocilizumab. Case Rep Dermatol. 2019;11:317–321. doi: 10.1159/000504429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V., Goodrick S., Rawesh A., Katz D.R., Miles J.M., Yudkin J.S., et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Morais P., Oliveira M., Matos J. Striae: a potential precipitating factor for Koebner phenomenon in psoriasis? Dermatol Online J. 2013;19:18186. [PubMed] [Google Scholar]
- Moroni L., Farina N., Dagna L. Obesity and its role in the management of rheumatoid and psoriatic arthritis. Clin Rheumatol. 2020;39:1039–1047. doi: 10.1007/s10067-020-04963-2. [DOI] [PubMed] [Google Scholar]
- Nagel G., Koenig W., Rapp K., Wabitsch M., Zoellner I., Weiland S.K. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20:81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- Nino M., Franzese A., Ruggiero Perrino N., Balato N. The effect of obesity on skin disease and epidermal permeability barrier status in children. Pediatr Dermatol. 2012;29:567–570. doi: 10.1111/j.1525-1470.2012.01738.x. [DOI] [PubMed] [Google Scholar]
- Ong P.Y., Ohtake T., Brandt C., Strickland I., Boguniewicz M., Ganz T., et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Passot C., Mulleman D., Bejan-Angoulvant T., Aubourg A., Willot S., Lecomte T., et al. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. MAbs. 2016;8:1407–1416. doi: 10.1080/19420862.2016.1216741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsalos O., Dalton B., Leppanen J., Ibrahim M.A.A., Himmerich H. Impact of TNF-α inhibitors on body weight and BMI: a systematic review and meta-analysis. Front Pharmacol. 2020;11:481. doi: 10.3389/fphar.2020.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez A., Sánchez-Jiménez F., Vilariño-García T., Sánchez-Margalet V. Role of leptin in inflammation and vice versa. Int J Mol Sci. 2020;21:5887. doi: 10.3390/ijms21165887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao P., Guo W., Ke Y., Fang H., Zhuang Y., Jiang M., et al. Mechanical stretch exacerbates psoriasis by stimulating keratinocyte proliferation and cytokine production. J Invest Dermatol. 2019;139:1470–1479. doi: 10.1016/j.jid.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cerdeira C., Cordeiro-Rodríguez M., Carnero-Gregorio M., López-Barcenas A., Martínez-Herrera E., Fabbrocini G., et al. Biomarkers of inflammation in obesity-psoriatic patients. Mediators Inflamm. 2019;2019:7353420. doi: 10.1155/2019/7353420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggini A., Chimenti S., Chiricozzi A. IL-6 as a druggable target in psoriasis: focus on pustular variants. J Immunol Res. 2014;2014:964069. doi: 10.1155/2014/964069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala E., Megna M., Amerio P., Argenziano G., Babino G., Bardazzi F., et al. Patients' demographic and socioeconomic characteristics influence the therapeutic decision-making process in psoriasis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S., Seong K.H., Kim C.D., Seo S.J., Park B.C., Kim M.H., et al. Adiponectin attenuates the inflammation in atopic dermatitis-like reconstructed human epidermis. Ann Dermatol. 2019;31:186–195. doi: 10.5021/ad.2019.31.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J., Zhang J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: a systematic review and meta-analysis. Joint Bone Spine. 2019;86:173–183. doi: 10.1016/j.jbspin.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Shi L.Q., Lian N., Sun J.T., Liu L.H., Chen M. Association between the systemic treatment of psoriasis and cardiovascular risk. Chin Med J (Engl) 2021;134:518–520. doi: 10.1097/CM9.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Tada Y., Hau C., Tatsuta A., Yamamoto M., Kamata M., et al. Adiponectin as an anti-inflammatory factor in the pathogenesis of psoriasis: induction of elevated serum adiponectin levels following therapy. Br J Dermatol. 2011;164:667–670. doi: 10.1111/j.1365-2133.2010.10123.x. [DOI] [PubMed] [Google Scholar]
- Shibata S., Tada Y., Hau C.S., Mitsui A., Kamata M., Asano Y., et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun. 2015;6:7687. doi: 10.1038/ncomms8687. [DOI] [PubMed] [Google Scholar]
- Snekvik I., Smith C.H., Nilsen T.I.L., Langan S.M., Modalsli E.H., Romundstad P.R., et al. Obesity, waist circumference, weight change, and risk of incident psoriasis: prospective data from the HUNT study. J Invest Dermatol. 2017;137:2484–2490. doi: 10.1016/j.jid.2017.07.822. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Arner E., Westermark P.O., Bernard S., Buchholz B.A., Bergmann O., et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Stelzner K., Herbert D., Popkova Y., Lorz A., Schiller J., Gericke M., et al. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur J Immunol. 2016;46:2043–2053. doi: 10.1002/eji.201546263. [DOI] [PubMed] [Google Scholar]
- Sun L., Liu W., Zhang L.J. The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J Immunol Res. 2019;2019:1824624. doi: 10.1155/2019/1824624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S., Ide S., Tokuyama R., Umeki H., Tatehara S., Kataoka S., et al. Leptin promotes wound healing in the skin. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita J., Grewal S., Langan S.M., Mehta N.N., Ogdie A., Van Voorhees A.S., et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita J., Grewal S., Langan S.M., Mehta N.N., Ogdie A., Van Voorhees A.S., et al. Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol. 2017;76:393–403. doi: 10.1016/j.jaad.2016.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P.H., Tyrrell H.E., Gao L., Xu D., Quan J., Gill D., et al. Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res. 2014;74:5711–5722. doi: 10.1158/0008-5472.CAN-13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova Y.D., Votruba S.B., Tchkonia T., Giorgadze N., Kirkland J.L., Jensen M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres T., Balato A., Conrad C., Conti A., Dapavo P., Ferreira P., et al. Secukinumab drug survival in patients with psoriasis: a multicenter, real-world, retrospective study. J Am Acad Dermatol. 2019;81:273–275. doi: 10.1016/j.jaad.2019.02.031. [DOI] [PubMed] [Google Scholar]
- Uzuncakmak T.K., Akdeniz N., Karadag A.S. Cutaneous manifestations of obesity and themetabolic syndrome. Clin Dermatol. 2018;36:81–88. doi: 10.1016/j.clindermatol.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Van Raemdonck K., Umar S., Szekanecz Z., Zomorrodi R.K., Shahrara S. Impact of obesity on autoimmune arthritis and its cardiovascular complications. Autoimmun Rev. 2018;17:821–835. doi: 10.1016/j.autrev.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.B. Striae: stretching the long list of precipitating factors for 'true koebnerization' of vitiligo, lichen planus and psoriasis. Clin Exp Dermatol. 2009;34:880–883. doi: 10.1111/j.1365-2230.2009.03312.x. [DOI] [PubMed] [Google Scholar]
- Versini M., Jeandel P.Y., Rosenthal E., Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Wilk S., Scheibenbogen C., Bauer S., Jenke A., Rother M., Guerreiro M. Adiponectin is a negative regulator of antigen-activated T cells. Eur J Immunol. 2011;41:2323–2332. doi: 10.1002/eji.201041349. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz K., Gledhill K., Ambler C.A., Manning C.B., Jahoda C.A. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS One. 2013;8:e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.Y., Yu C.L., Yang S.J., Chi C.C. Change in body weight and body mass index in psoriasis patients receiving biologics: a systematic review and network meta-analysis. J Am Acad Dermatol. 2020;82:101–109. doi: 10.1016/j.jaad.2019.07.103. [DOI] [PubMed] [Google Scholar]
- Yew Y.W., Loh M., Thng S.T.G., Chambers J.C. Investigating causal relationships between body mass index and risk of atopic dermatitis: a Mendelian randomization analysis. Sci Rep. 2020;10:15279. doi: 10.1038/s41598-020-72301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Silverberg J.I. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606–616.e4. doi: 10.1016/j.jaad.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang L.J. Type1 interferons potential initiating factors linking skin wounds with psoriasis pathogenesis. Front Immunol. 2019;10:1440. doi: 10.3389/fimmu.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.J. Recent progress at the psoriasis and atopic dermatitis research front: an experimental dermatology perspective. Exp Dermatol. 2021;30:756–764. doi: 10.1111/exd.14388. [DOI] [PubMed] [Google Scholar]
- Zhang L.J., Chen S.X., Guerrero-Juarez C.F., Li F., Tong Y., Liang Y., et al. Age-related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor beta. Immunity. 2019;50:121–136.e5. doi: 10.1016/j.immuni.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.J., Gallo R.L. Antimicrobial peptides. Curr Biol. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Zhang L.J., Guerrero-Juarez C.F., Chen S.X., Zhang X., Yin M., Li F., et al. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abb5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.J., Guerrero-Juarez C.F., Hata T., Bapat S.P., Ramos R., Plikus M.V., et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.J., Sen G.L., Ward N.L., Johnston A., Chun K., Chen Y., et al. Antimicrobial peptide LL37 and MAVS signaling drive interferon-β production by epidermal keratinocytes during skin injury. Immunity. 2016;45:119–130. doi: 10.1016/j.immuni.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. The role of transforming growth factor β in T helper 17 differentiation. Immunology. 2018;155:24–35. doi: 10.1111/imm.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cao H., Chen J., Li Y., Xu A., Wang Y. Adiponectin-expressing Treg facilitate T lymphocyte development in thymic nurse cell complexes. Commun Biol. 2021;4:344. doi: 10.1038/s42003-021-01877-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman E.Z., Howren A.M., Park J.Y.E., Dutz J., De Vera M.A. Epidemiology of depression and anxiety in patients with psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50:1481–1488. doi: 10.1016/j.semarthrit.2020.02.001. [DOI] [PubMed] [Google Scholar]