Abstract
Hairpin formation serves an important regulatory role in V(D)J recombination because it requires synapsis of an appropriate pair of recombination sites. How hairpin formation is regulated and which regions of the RAG proteins perform this step remain unknown. We analyzed two conditional RAG-1 mutants that affect residues quite close in the primary sequence to an active site amino acid (D600), and we found that they exhibit severely impaired recombination in the presence of certain cleavage site sequences. These mutants are specifically defective for the formation of hairpins, providing the first identification of a region of the V(D)J recombinase necessary for this reaction. Substrates containing mismatched bases at the cleavage site rescued hairpin formation by both mutants, which suggests that the mutations affect the generation of a distorted or unwound DNA intermediate that has been implicated in hairpin formation. Our results also indicate that this region of RAG-1 may be important for coupling hairpin formation to synapsis.
V(D)J recombination is the primary mechanism responsible for generating antigen receptor diversity. This combinatorial DNA rearrangement process creates the antigen-binding domains of both T-cell receptors and immunoglobulins by bringing together separate variable (V), diversity (D), and joining (J) gene coding segments. Recombination is initiated by the binding of the recombinase (the RAG-1 and RAG-2 proteins) to recombination signal sequences (RSS) adjacent to the coding segments. RSS are composed of highly conserved heptamer and nonamer motifs separated by less-well-conserved spacers of either 12 or 23 nucleotides (12-RSS and 23-RSS, respectively). The RAG proteins introduce a double-strand break precisely at the border between the RSS and the adjacent coding DNA (termed the coding flank sequence), generating two types of broken DNA intermediates: a pair of blunt, 5′-phosphorylated signal ends and a pair of covalently sealed hairpin coding ends. The signal ends are joined, forming a signal joint, while the coding ends are further processed and joined to form a coding joint (see references 11 and 20 for a review).
RAG-mediated DNA cleavage occurs in two steps (24). First, a nick is introduced between the coding flank and the heptamer of the RSS. Nicking liberates a free 3′OH, which serves as the nucleophile in the second step, the formation of a hairpin by transesterification (43). In this reaction, the 3′OH of one DNA strand attacks the opposite strand at the RSS-coding border, forming a hairpin coding end and a blunt signal end. The chemistry of this step—a direct in-line attack by the free 3′OH of the top strand—suggests that some local unpairing or DNA distortion may be necessary to create the proper geometry for transesterification (5, 27, 43). Indeed, DNA distortion facilitates cleavage by some transposases and retroviral integrases (6, 18, 32, 34). Recent work has shown that binding of the RAG proteins to the RSS induces distortion of the DNA near the cleavage site (2, 39). The possibility that DNA distortion promotes hairpin formation is also supported by experiments using substrates with mismatched bases at the cleavage sites (see Discussion). Specific regions of either RAG-1 or RAG-2 responsible for inducing DNA distortion have not been identified.
Efficient recombination between a given pair of gene segments requires that each is flanked by an RSS with a different spacer length (a requirement known as the “12/23 rule”), which prevents inappropriate, immunologically irrelevant recombination events from scrambling the immune receptor loci. The 12/23 rule is enforced at the cleavage step, because efficient cleavage requires the assembly of a synaptic complex involving the RAG proteins, a 12-RSS, and a 23-RSS (9, 44). While nicking occurs in the absence of an appropriate 12/23 RSS pair, hairpin formation does not (44, 47). Under certain conditions, however (such as in the presence of Mn2+, which relaxes the requirement for synapsis), the 12/23 rule can be bypassed, resulting in efficient hairpin formation in the absence of a second RSS (5, 27, 42, 45). The molecular mechanisms linking 12/23 synapsis to hairpin formation remain unknown.
In addition to catalyzing site-specific DNA cleavage, the RAG proteins may also play a role in the joining reaction. In vivo experiments indicated that after cleavage, the coding and signal ends are retained in a postcleavage complex containing the RAG proteins (21) and that this complex plays a critical role in the formation of signal and coding joints (48). These predictions were confirmed by biochemical studies, which identified postcleavage complexes (1, 14), showed that the RAG proteins stimulate coding joint formation in vitro (19, 28), and found that the RAG proteins can open hairpin coding ends (3, 35). These data suggest that the RAG proteins may be important for hairpin end processing in vivo. The RAG proteins may also play other important roles in the joining of both coding and signal ends, such as recruiting other joining and end processing factors to the postcleavage complex (4, 38).
Little is known about the functional anatomy of the V(D)J recombinase. Recently, our laboratory identified three catalytic residues (D600, D708, and E962) in RAG-1 that are critical for both nicking and hairpin formation (17; see also references 12 and 15). Some regions of RAG-1 involved in DNA binding have been mapped; for example, binding to the nonamer requires the hin homology domain of RAG-1 (amino acids 384 to 477 in the murine sequence) (7, 36). Protein-DNA contacts have also been found in the heptamer and proximal coding flank regions of the RSS (10, 26, 39), although specific regions of the RAG proteins responsible for these interactions have not been identified.
Important information about regions of RAG-1 involved in binding and/or catalysis can be gleaned from analysis of mutant proteins that exhibit hypersensitivity to the DNA sequence of the coding flank nucleotides immediately adjacent to the cleavage site. The first such RAG mutant to be isolated, D32, is a combined deletion-insertion mutation in which six amino acids (from S606 to S611 in murine RAG-1) are replaced by a valine and an aspartic acid (30). A second conditional mutant resulting from a single missense mutation at amino acid 609 (H609L) gives a similar but less severe phenotype (29). In vivo analysis using transient-transfection assays shows that both mutants form signal joints and coding joints at or near wild-type levels with certain coding flank sequences (known as “good” coding flanks) but are severely impaired in recombination of other (“bad”) coding flank sequences (29, 31). These data show that the H609 region is important for recombination but do not identify the mechanism of the defect. It is not known how the mutations affect cleavage in vivo, and no biochemical analyses of the purified mutant proteins have been reported.
We considered several hypotheses to explain the recombination defect observed with the D32 and H609L mutants. (i) The most straightforward possibility is that the mutants simply fail to recognize substrates with bad coding flanks (29, 31). (ii) The mutants may fail to interact properly with some other protein factor required for hairpin formation on bad coding flanks (27, 29). (iii) The mutants may not be able to form a synaptic complex using substrates with bad coding flanks (5, 29). (iv) The mutants may affect the coupling of cleavage in the presence of bad coding flanks, thereby hindering the formation of coding and signal joints, since both RSS must be cleaved to allow these joints to be formed. According to this model, single RSS cleavage should not be greatly affected. (v) The mutant proteins may confer defects in one or both of the chemical steps of cleavage (5, 29, 31). These defects could be manifested in cis (at the RSS adjacent to the bad coding flank), in trans (at a partner RSS with a good coding flank), or both. How might the sequence of the coding flank affect catalysis? Alternating pyrimidine and purine sequences, such as those found in the heptamer, assume distorted structures (41); good coding flanks continue the alternating pyrimidine-purine pattern, while bad coding flanks do not (27). Thus, the D32 and H609L mutants might be sensitive to bad coding flanks because these DNA sequences impair the generation of a distorted DNA intermediate critical for hairpin formation (5, 44). (vi) The increasing evidence that the RAG proteins are involved in the joining step (3, 19, 28, 35) led us to also consider the possibility that the D32-H609L recombination defect may be mediated at the level of joining, perhaps by destabilizing the postcleavage complex in the presence of bad coding flanks.
To test these hypotheses, we purified the mutant proteins and tested their activities in vitro. Our results demonstrate that the D32 and H609L mutations affect the cleavage step, specifically blocking hairpin formation. Introduction of base mismatches in bad coding flanks rescues hairpin formation, indicating that these mutations interfere with the distortion of DNA at the cleavage site that is important for transesterification. These data provide the first localization of a region of the RAG recombinase that is specifically required for hairpin formation. The close proximity of H609 to an active-site residue (D600), along with the observation that hairpin formation is dependent upon synapsis, suggests that this region of RAG-1 may play a key role in coupling catalysis to synapsis and thus in enforcing the 12/23 rule.
MATERIALS AND METHODS
Plasmid constructs.
RAG-1–H609L was constructed by Kunkel mutagenesis as previously described (17), and the mutation was verified by nucleotide sequencing. The baculovirus transfer vector encoding D32 was constructed by subcloning an approximately 1.5-kb SphI-MluI fragment from pMS132 (a gift of Martin Gellert) (30) into a pFastBac derivative (Bac-to-Bac system; Gibco-BRL, Rockville, Md.) containing the active core of RAG-1 (amino acids 384 to 1008 [30]). This construct contains an N-terminal maltose-binding protein fusion (24), a C-terminal polyhistidine tag (His9), as well as three tandem copies of the c-myc epitope, also on the C terminus (myc3). The transfer vector encoding H609L was constructed as described previously (17). The entire coding region of RAG-1 in each vector was verified by nucleotide sequencing.
The bad coding flank substrate pJH290 contains the sequence 5′-TCGAC in the coding flank adjacent to the 12-RSS and the sequence 5′-GATCC in the coding flank adjacent to the 23-RSS (22, 23). The good coding flank substrate pMS319 contains the sequence 5′-ACCGT in the coding flank adjacent to both the 12-RSS and the 23-RSS (31).
Oligonucleotide substrates.
The pJH290-like 12-RSS substrate was constructed by annealing the top-strand oligonucleotide SK5 (5′-GATCTGGCCTGTCTGCCACAGTGCTACAGACTGGAACAAAAACCCTGCAG-3′) to its complement, SK6. The good 12-RSS oligonucleotide substrate has been previously described (DAR39/40 [24]). The bad coding flank 12-RSS oligonucleotide substrate consists of the top-strand SK3 (5′-GATCTGGCCTGTCTGCCACAGTGCTACAGACTGGAACAAAAACCCTGCAG-3′) annealed to its complement, SK4. All 12-RSS substrates were 32P end labeled on the top strand. The pJH290-like 23-RSS consisted of the top-strand SK38 (5′-ATCGATGAGAGG ATCCCACAGTGGTAGTACTCCACTGTCTGGCTGTACAAAAACCCTC GGG-3′) annealed to its complement, SK39. The good coding flank 23-RSS has been previously described (DG61/62 [14, 24]). The bad 23-RSS substrate consists of the top-strand oligonucleotide SK36 (5′-GATCTGGCCTGTCTGCCACAGTGGTAGTACTCCACTGTCTGGCTGTACAAAAACCCTGCAG-3′) and its complement, SK37. The mispaired 12-RSS substrates were constructed by annealing the top-strand SK3 with either SK40 (5′-CTGCAGGGTTTTTGTTCCAGTCTGTAGCACTGTGCGAGACAGGCCAGATC-3′; bad/good) or SK41 (5′-CTGCAGGGTTTTTGTTCCAGTCTGTAGCACTGTGAAAGACAGGC CAGATC-3′; bad/bad). Nonspecific DNA consists of DAR81/82 and has been previously described (14, 24). All oligonucleotide substrates were annealed in a buffer containing 100 mM potassium glutamate.
Protein purification.
Wild-type and mutant RAG-1 viruses were prepared according to the manufacturer's recommendations (Gibco-BRL), and proteins were purified as described elsewhere (2, 16, 17, 24, 45). Approximately 109 Sf9 cells were infected in monolayer at a multiplicity of infection of 1.0. Cells were harvested ∼60 h postinfection and collected by centrifugation. Cells were then lysed by Dounce homogenization in the presence of 8 ml of lysis buffer (20 mM Tris-Cl [pH 7.9] at 4°C, 0.5 M NaCl, 20% glycerol [vol/vol] 2 mM β-mercaptoethanol) supplemented with 60 mM imidazole. The lysate was cleared by ultracentrifugation at 100,000 × g at 4°C. The cleared lysate was then added to 0.5 ml of metal-chelating Sepharose (Pharmacia Biotech, Piscataway, N.J.), which had been previously charged with 100 mM NiSO4. The mixture was allowed to incubate for at least 2 h at 4°C with gentle agitation. Beads plus bound protein were then washed with 20 column volumes of lysis buffer supplemented with 90 mM imidazole. Bound proteins were eluted in lysis buffer containing 250 mM imidazole in 0.5-ml fractions. Protein containing fractions were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining, combined, and dialyzed against 500 to 1,000 volumes of storage buffer (25 mM HEPES-K, pH 7.5; 150 mM potassium glutamate; 20% glycerol [vol/vol]; 2 mM dithiothreitol) for 3 h at 4°C. Aliquots were frozen in liquid nitrogen and stored at −80°C. Protein concentration of postdialysis aliquots was determined by SDS-PAGE followed by Coomassie blue staining.
Wild-type RAG-2 protein (amino acids 1 to 383) was purified from the Chinese hamster ovary-derived RMP-41 cells (25) as an N-terminal glutathione S-transferase fusion protein as described (33, 36). Briefly, ∼109 cells were transiently transfected with the expression plasmid pEBG-RAG2ΔC (a gift of David Schatz). Cells were harvested ∼60 h posttransfection, collected by centrifugation, and resuspended in RSB (10 mM Tris [pH 7.4 at 4°C], 10 mM NaCl, 5 mM MgCl2, 0.5% NP-40). Then, 1.5 volumes of LSB (20 mM Tris [pH 7.4 at 4°C], 1 M NaCl, 0.2% NP-40, 0.2 mM MgCl2) was added, and the mixture was allowed to incubate at 4°C with gentle agitation for 2 h. Next, 0.4 ml of glutathione-agarose beads (Stratagene, La Jolla, Calif.) was added, and the mixture was incubated for 1 h at 4°C with gentle agitation. The column matrix plus bound proteins were then collected by centrifugation and washed five times in a 2:3 RSB-LSB solution. Proteins were recovered by at least five rounds of elution with 0.3 ml of elution buffer (50 mM Tris [pH 8.3 at 4°C], 20 mM glutathione, 1 M NaCl, 10% glycerol) and incubation at 4°C with gentle agitation for at least 15 min. Protein-containing fractions were identified by SDS-PAGE followed by Coomassie staining and dialyzed for 3 h against 500 to 1,000 volumes of storage buffer.
Recombinant human HMG-1 (amino acids 1 to 162) was purified from Escherichia coli by differential trichloroacetic acid precipitation (40% followed by 10%). The dried pellet was resuspended in storage buffer (25 mM Tris [pH 8.0 at 4°C], 1 mM EDTA, 1 mM dithiothreitol, 150 mM KCl, 10% glycerol [vol/vol]). The protein concentration was determined by the Bradford assay.
Plasmid cleavage assay.
Approximately 100 ng each of RAG-1 and RAG-2 (as determined by Coomassie staining) were incubated with 50 ng of the plasmid recombination substrates pJH290 (22) or pMS319 (31) in 10 μl containing 50 mM HEPES-K (pH 8.0), 26 mM KCl, 4 mM NaCl, 5 mM MgCl2, 100 μg of bovine serum albumin (BSA) per ml, 1 mM ATP, and 200 ng of HMG-1. Storage buffer was substituted for RAG-1 in control incubations. Reactions were incubated at 30°C for 3 h and were terminated by the addition of 90 μl of stop buffer (100 mM Tris-Cl, pH 8.0; 0.2% SDS; 0.25 mg of proteinase K per ml; 10 mM EDTA) followed by incubation at 55°C for at least 1 h. Samples were then phenol-chloroform extracted, and DNA was recovered by ethanol precipitation. DNA was then digested with 1 U of PvuII (Gibco-BRL) and separated by 4.5% PAGE in a Tris-borate buffer system. Separated products were then transferred to membrane (GeneScreen Plus; NEN Life Sciences, Boston, Mass.) and probed with the random-primed, 32P-labeled 693-bp PvuII fragment from pJH290 as described elsewhere (37). The probe is complementary to all indicated products from both plasmids. Products were visualized by PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and analyzed by using ImageQuant software (v4.2).
Oligonucleotide cleavage and gel retardation assays.
Gel retardation assays were carried out in a 10-μl reaction volume in a buffer containing 25 fmol of oligonucleotide substrate, 37.8 mM HEPES-K (pH 7.5), 51 mM potassium glutamate, 10% glycerol (vol/vol), 3 mM dithiothreitol, 2.5 pmol of nonspecific competitor (FM117 [24]), 5 mM CaCl2, 60 μg of BSA per ml, 0.006% NP-40, and approximately 100 ng each of RAG-1 and RAG-2. Storage buffer was substituted for RAG-1 in control samples. Samples were incubated for 30 min at 37°C, followed by fixation with glutaraldehyde at a 0.1% final concentration for 10 min at 37°C. Complexes were separated by electrophoresis through a 4 to 20% polyacrylamide gradient gel (Novex, San Diego, Calif.) in a Tris-borate buffer system. Dried gels were visualized by using a PhosphorImager and analyzed with ImageQuant software.
Oligonucleotide cleavage assays were performed as described earlier (14). Briefly, approximately 100 ng each of RAG-1 and RAG-2 were added to a reaction mixture containing 25 mM morpholinepropanesulfonic acid, 2 mM dithiothreitol, 100 μg of BSA per ml, 5 mM CaCl2, 19 mM potassium acetate, 25 fmol of 32P-end-labeled 12-RSS, 250 fmol of unlabeled 23-RSS, and 200 ng of HMG-1. Samples were incubated for 10 min at 37°C. MgCl2 was then added to a final concentration of 5 mM, and samples were incubated for a further 20 to 45 min at 37°C. Reactions were terminated by the addition of an equal volume of formamide loading dye (95% [vol/vol] formamide, 10 mM EDTA, 0.05% bromophenol blue). Products were resolved on a 10% acrylamide gel containing 30% formamide, 0.67× Tris-borate-EDTA, 7 M urea, and 12.5 mM HEPES-K (pH 7.5) that was run for approximately 100 min at 75 W. Bands were visualized by using a PhosphorImager.
RESULTS
The D32 and H609L mutations block recombination at the cleavage step.
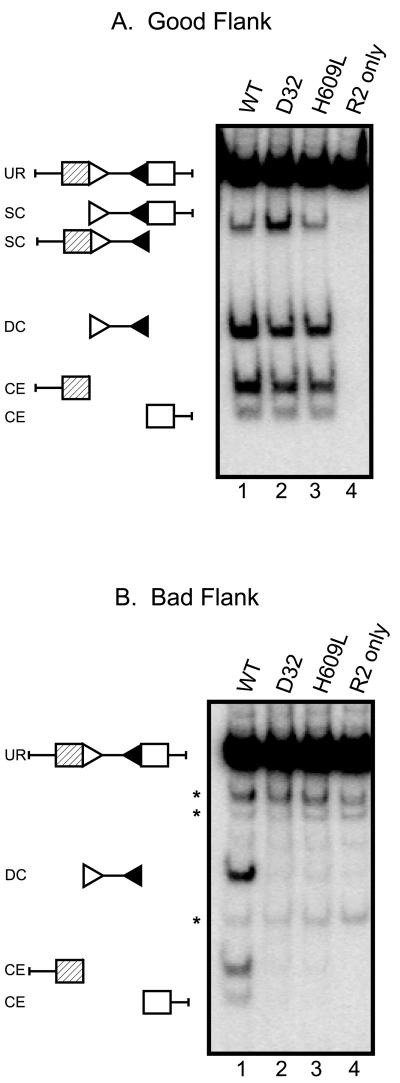
Previous work showed that both the D32 and H609L mutants form coding and signal joints at normal levels using substrates with good coding flank sequences, such as pMS319 (5′-ACCGT-heptamer), but are severely defective for formation of both types of joints using substrates with bad flanks, such as pJH290 (5′-TCGAC-heptamer) (29, 31). To determine whether the impaired joint formation is caused by a defect in cleavage, we performed a series of in vitro plasmid cleavage assays. Mutant (D32 and H690L) and wild-type RAG-1 proteins were purified from a baculovirus expression system. These proteins, along with separately purified RAG-2, were incubated with pMS319 (good flanks) or pJH290 (bad flanks), the same plasmid substrates used previously to test recombination in vivo (27, 31, 45). All proteins cleaved pMS319 with comparable efficiency (compare levels of double-cut signal end product and levels of coding ends; Fig. 1A, lanes 1 to 3). Only wild-type RAG-1, however, performed robust cleavage of pJH290 (Fig. 1B, lane 1). Neither the D32 nor the H609L mutants produced detectable double RSS cleavage or coding ends (lanes 2 and 3, respectively). In vivo measurements of cleavage using these substrates obtained similar results (data not shown). Together, these data demonstrate that the D32 and H609L mutations specifically affect the cleavage step.
FIG. 1.
Mutant proteins do not cleave a plasmid substrate with bad coding flanks. (A) Plasmid substrate containing two good coding flanks was incubated with RAG proteins as indicated. The positions of the unrearranged substrate (UR), double-cut product (DC), single-cut (SC), and coding ends (CE) are indicated. A control incubation lacking RAG-1 protein was loaded in lane 4. (B) Plasmid substrate containing two bad coding flanks was assayed as in panel A. Asterisks indicate background bands generated by PvuII digestion at “star” sites (see Materials and Methods). The positions of substrate and cleavage products are as in panel A. Panels A and B show the same exposure of the same blot.
If defective recombination were to result from a defect in the coupling of cleavage at the two RSS, one would expect to see high levels of single-cut signal ends, as well as coding ends, when the mutant proteins are incubated with a substrate containing bad coding flanks. We noted that the D32 mutant yielded a slight increase in single RSS cleavage events at good coding flanks (Fig. 1A, lane 2); this was not observed with H609L. Importantly, however, no increase in single signal cleavage was observed at bad coding flanks (Fig. 1B), and the mutant proteins produced no detectable coding ends (Fig. 1B, lanes 2 and 3). Thus, defective joint formation by the mutants cannot be attributed to uncoupling cleavage in the presence of bad coding flanks.
DNA binding by D32 and H609L is not affected by the coding flank sequence.
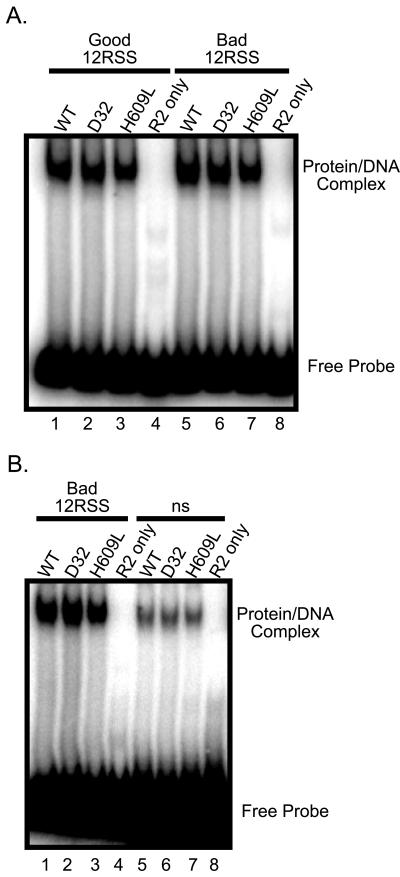
The cleavage defect described above could result from effects of the D32 and H609L mutations on the recognition of substrates bearing bad coding flanks, as suggested previously (29, 31). To test this hypothesis, we performed standard electrophoretic mobility shift assays (13) using radiolabeled oligonucleotide substrates containing either good or bad coding flanks (see below for a more detailed description of these substrates). Wild-type and mutant proteins bound similarly to good and bad 12-RSS (Fig. 2A, compare lanes 1 to 3 and lanes 5 to 7) and to good and bad 23-RSS (data not shown). Note that a slight degree of shift is seen with a nspecific oligonucleotide (Fig. 2B, lanes 5 to 7), a finding in agreement with previously published data showing that there is only a 50-fold preference for an authentic RSS sequence (13). These binding data, along with the ability of the mutant proteins to nick substrates with good and bad coding flanks with equal efficiency (see below), demonstrate that neither the D32 nor the H609L mutants are appreciably defective for recognition of substrates with bad coding flanks.
FIG. 2.
Wild-type and mutant RAG-1 proteins bind to good and bad flanks similarly. Radiolabeled oligonucleotide substrates were incubated in Ca2+ with RAG-1 proteins as indicated. The positions of the unbound substrate and the protein-bound complex are indicated. (A) Binding to a good flank 12-RSS substrate (lanes 1 to 4) and a bad coding flank 12-RSS substrate (lanes 5 to 8). (B) Binding to a bad coding flank substrate (lanes 1 to 4) and a nonspecific oligonucleotide control (lanes 5 to 8).
Cleavage is blocked specifically at the hairpin formation step.
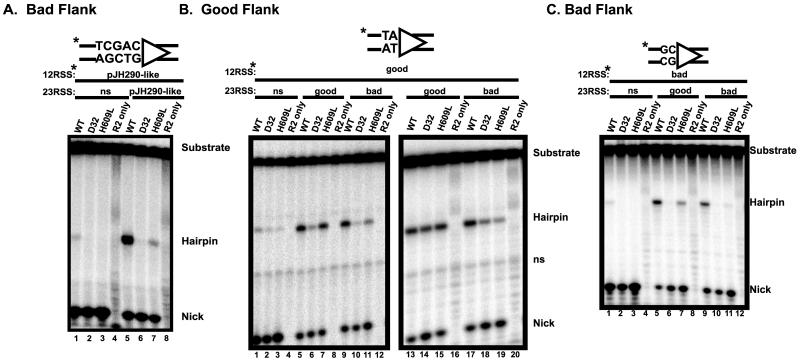
To examine the cleavage defect in more detail, we employed a standard oligonucleotide cleavage assay that allows concurrent examination of both nicking and hairpin formation (14, 24). We designed bad 12- and 23-RSS oligonucleotide substrates in which the sequences encompassing the cleavage sites (the RSS and 16 nucleotides of the coding flank) are identical to the corresponding sequences in pJH290. Cleavage of a radiolabeled 12-RSS was assayed under coupled cleavage conditions (in which both 12- and 23-RSS are required for efficient hairpin formation) in the presence of either an unlabeled nonspecific oligonucleotide (Fig. 3A, lanes 1 to 4) or an unlabeled bad 23-RSS (lanes 5 to 8). All three proteins (wild-type, D32, and H609L) efficiently nicked the 12-RSS substrate (lanes 1 to 3). As expected, neither the wild-type RAG proteins nor the mutant proteins formed hairpins efficiently in the presence of a nonspecific partner oligonucleotide (lanes 1 to 3). In the presence of an unlabeled 23-RSS partner, wild-type RAG-1 catalyzed robust hairpin formation (lane 5), while the D32 and H609L proteins were severely defective (lanes 6 to 7). Unlike the D32 mutant, H609L did form trace levels of hairpins; this is consistent with the latter's milder phenotype in vivo (29). These data demonstrate that the cleavage defect conferred by the D32 and H609L mutations is due to a specific deficit in hairpin formation. Furthermore, the ability of the mutant proteins to nick bad coding flank substrates at wild-type levels provides additional evidence that these proteins are able to bind productively to such substrates.
FIG. 3.
D32 and H609L are specifically defective for hairpin formation. (A) 12-RSS oligonucleotide substrates that duplicate the cleavage site of the bad coding flank plasmid were used in an oligonucleotide cleavage assay. The sequence of the coding flank is depicted above. Radiolabeled 12-RSS, RAG proteins, HMG-1 protein, and either a nonspecific DNA (ns) oligonucleotide or a 23-RSS were incubated in Mg2+. The positions of the uncleaved substrate, the nicked intermediate, and the hairpin product are indicated. Control incubations lacking RAG-1 were loaded in lanes 4 and 8. (B) Oligonucleotide cleavage assays utilizing radiolabeled 12-RSS substrates with a good coding flank were performed as in panel A. The pertinent sequence of the coding flank is depicted above. Where indicated, unlabeled nonspecific DNA (ns), good coding flank 23-RSS, or bad coding flank 23-RSS was added. The positions of the uncleaved substrate, nicked intermediate, and hairpin product are given, as is the position of a nonspecific band (ns) present in the unreacted substrate. Control incubations lacking RAG-1 were loaded in lanes 4, 8, and 12. Lanes 13 to 20 show longer time points of cleavage of a radiolabeled good flank 12-RSS with either an unlabeled good flank 23-RSS (lanes 13 to 16) or a bad flank 23RSS (lanes 17 to 20). (C) Oligonucleotide cleavage assays with radiolabeled bad coding flank 12-RSS were performed as described above.
Coding flank sequences affect cleavage both in cis and trans.
In vivo studies have suggested that the coding sequence sensitivity of the D32 mutation is determined by the heptamer-proximal dinucleotide of the coding flank (31). To directly compare the effects of these two nucleotides in the absence of other potentially confounding sequence variations, we chose well-characterized 12- and 23-RSS oligonucleotide substrates with good coding flanks (13, 14, 24). We generated bad 12- and 23- substrates from these sequences by changing only the first two nucleotides of the coding flank from TA to GC. These substrates were then used in coupled cleavage assays in order to dissect the effects of bad coding flanks in cis (cleavage site adjacent to the bad flank) and in trans (the partner RSS bears the bad flank).
As expected, all three proteins efficiently nicked a radiolabeled 12-RSS good substrate, whether paired with an unlabeled nonspecific oligonucleotide (Fig. 3B, lanes 1 to 3), an unlabeled good 23-RSS (lanes 5 to 7), or an unlabeled bad 23-RSS (lanes 9 to 11). Very little hairpin formation was catalyzed by the mutant proteins in the presence of an unlabeled nonspecific partner oligonucleotide (lanes 2 to 3); the addition of a good 23-RSS (lane 7) markedly improved hairpin formation by H609L. This mutant, however, was sensitive to the presence of a bad partner RSS, yielding fewer hairpins (compare lane 7 with lane 11). This effect, though not large, was reproduced consistently in several experiments. Hairpin formation by the D32 protein is impaired under the conditions used for this assay (a 20-min incubation) because this mutant shows a mild kinetic defect in this reaction (data not shown and see below). To assess the effect of D32 under more optimal conditions for this mutant, we repeated the experiment with a longer incubation time (45 min) (Fig. 3B, lanes 13 to 21). Under these conditions, the D32 and H609L mutants efficiently formed hairpins in the presence of an unlabeled good 23-RSS (lanes 14 to 15), but not with a bad 23-RSS partner (lanes 18 to 19). These data demonstrate that cleavage at a good flank by both mutant proteins is impaired by the presence of bad coding flanks in trans.
Cleavage of a labeled bad 12-RSS is shown in Fig. 3C. Hairpin formation by the wild-type RAG proteins was barely detectable in the presence of a nonspecific partner (lane 1), and neither mutant formed hairpins (lanes 2 and 3). All three proteins exhibited robust nicking activity. With a good partner RSS, wild-type RAG proteins yielded high levels of hairpins, but no hairpin formation was seen with D32, and only weak activity was seen with H609L (lanes 5 to 7). In the presence of a bad partner RSS, wild-type RAG proteins exhibited robust hairpin formation (lane 9). The D32 mutant, on the other hand, formed no detectable hairpins (lane 10), and H609L yielded only traces of hairpins (lane 11). Similar results were obtained in parallel experiments using a labeled 23-RSS (data not shown). Furthermore, prenicked substrates failed to rescue the hairpin formation defect for either D32 or H609L (data not shown), providing further evidence that these mutations specifically affect the hairpin formation step.
We draw four main conclusions from these data. (i) The two coding flank nucleotides proximal to the heptamer are sufficient to cause a profound cleavage defect. (ii) Bad coding flanks affect cleavage by the mutant proteins at the second step, hairpin formation. (iii) The effects of bad coding flanks are most pronounced in cis. (iv) The coding flank sequence of the partner RSS also affects cleavage in trans, albeit to a lesser extent than in cis; this indicates that the mutant proteins are capable of synaptic interactions.
Introduction of mispaired bases adjacent to the cleavage site rescues hairpin formation.
We hypothesized that the effect of bad coding flanks on hairpin formation might result from an inability to create DNA distortion near the cleavage site. Such distortions have been postulated to facilitate the transesterification reaction by creating a geometry that favors an in-line attack by the 3′OH (5, 27, 43). We therefore sought to determine whether unpairing the first two nucleotides of the coding flank could rescue hairpin formation by creating a more favorable substrate geometry. This approach had previously been successful with wild-type RAG proteins under conditions that support uncoupled cleavage (5, 27).
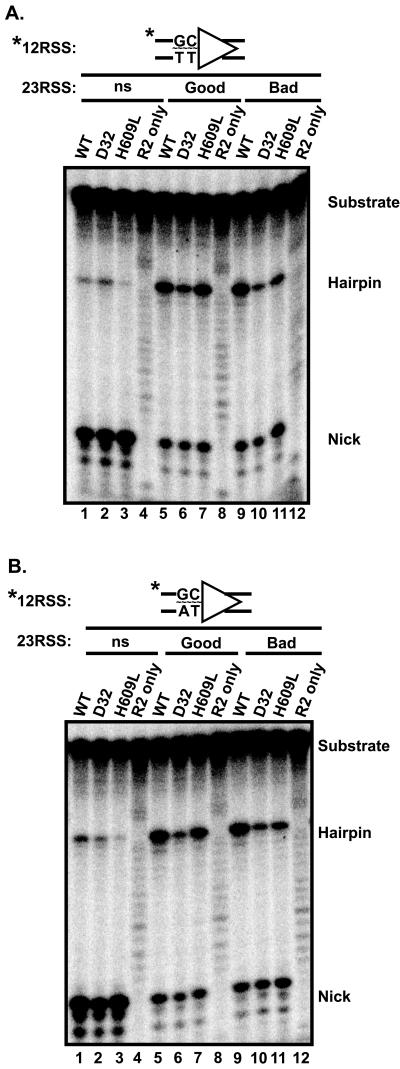
We tested several radiolabeled 12-RSS oligonucleotide substrates containing different base mismatches in the first two nucleotides of the coding flank. The results of coupled cleavage assays obtained with a substrate containing G-T and C-T mismatches are shown in Fig. 4A. Note that the two heptamer proximal nucleotides on each strand of this substrate constitute bad coding flanks (31). When paired with an unlabeled nonspecific partner oligonucleotide, both wild-type and mutant proteins nicked efficiently (Fig. 4A, lanes 1 to 3). Notably, some hairpin formation was observed with all three proteins. The formation of hairpins by D32 and H609L is in stark contrast to the situation observed with the fully base-paired bad coding flank substrate (compare with Fig. 3C, lanes 2 and 3). When paired with an unlabeled, fully base paired 23-RSS, all three proteins were able to efficiently form hairpins on the mispaired substrate (lanes 5 to 11), regardless of whether the partner RSS bore good or bad coding flanks. Once again, the behavior of the mutant proteins in this situation contrasts sharply with their behavior with the fully base paired bad substrate (Fig. 3C, lanes 10 to 11). Thus, unpairing the bad coding flanks rescues hairpin formation.
FIG. 4.
Mispaired coding flanks rescue the cleavage defect of D32 and H609L. (A) Oligonucleotide cleavage assays were performed using radiolabeled 12-RSS substrates containing a 2-bp mismatch in the coding flank. Note that both strands, although mismatched, would be considered a bad coding flank sequence (37). Unlabeled nonspecific DNA (ns), good coding flank 23-RSS, or bad coding flank 23-RSS were incubated with RAG proteins in Mg2+. The positions of the uncleaved substrate, the hairpin product, and the nicked intermediate are indicated. Control incubations lacking RAG-1 were loaded in lanes 4, 8, and 12. (B) Oligonucleotide cleavage assay with a 2-bp-mismatched substrate containing a bad coding flank on the top strand and a good coding flank on the bottom strand as pictured above. Except for the nucleotides indicated, the mispaired substrates are otherwise identical between panels A and B. Second signals, band labels, and control samples are as in panel B. Panels A and B represent the same exposure of the same gel.
We also tested a different unpaired substrate, constructed by annealing a bad coding flank on the top strand and a good coding flank on the bottom strand (Fig. 4B). This substrate also rescued hairpin formation, with results virtually identical to those shown in Fig. 4A. Similar results were also obtained with the converse substrate, a mispaired 12-RSS substrate containing a good coding flank on the top strand and a bad coding flank on the bottom strand (data not shown). These data indicate that introduction of unpaired bases at the coding flank- heptamer border rescues the defect in hairpin formation on a bad coding flank seen with D32 and H609L. Unpairing these nucleotides does not, however, completely bypass the 12/23 rule, since both a 12-and a 23-RSS are required for efficient hairpin formation in Mg2+ (compare lanes 1 to 3 with lanes 5 to 11 in Fig. 4A or B).
DISCUSSION
We have elucidated the precise molecular step at which the D32 and H609L mutant proteins are defective for V(D)J recombination. Our data are the first to identify a region of the V(D)J recombinase specifically required for hairpin formation and provide strong support for a model in which the cleavage site, including coding flank DNA, must be distorted in order for hairpins to be formed.
Cleavage site distortion promotes hairpin formation under coupled cleavage conditions.
Hairpin formation by one-step transesterification requires that the 3′OH of one strand directly attack the opposite strand, a reaction that has been hypothesized to require distortion of the DNA near the cleavage site (5, 27, 43). This hypothesis has received some support from studies of single RSS cleavage by the wild-type RAG proteins. Binding of the RAG complex induces structural alterations near the cleavage site (2, 39). In addition, when incubated in Mn2+ with a substrate containing a bad coding flank, the RAG proteins introduce a nick but fail to form hairpins (5, 27). Introducing mismatched base pairs in the coding flank immediately adjacent to the cleavage site restores hairpin formation, providing support for the notion that bad coding sequences might impede this putative structural distortion (5, 27). It is interesting to note that this defect in hairpin formation is also rescued by incubation in Mg2+ under coupled cleavage conditions (44), which suggests that synapsis may help induce the required DNA distortion at the cleavage site.
It is important to note that the defects in hairpin formation by wild-type RAG proteins were observed using a single RSS under nonphysiologic conditions that bypass the 12/23 rule. Moreover, they were conducted in the presence of Mn2+, a divalent metal ion known to have effects on both DNA structure (8) and the behavior of DNA-protein interactions. Mn2+ relaxes the specificity of many nucleases (46), including the RAG proteins (44), and, by unknown mechanisms, nonspecifically rescues the activity of a number of RAG-1 mutants (17). Our data show that bad coding flanks block hairpin formation by the D32 and H609L mutants under coupled conditions, in Mg2+. We have further shown that, under coupled cleavage conditions, unpaired bad coding flanks rescue hairpin formation. These results strongly support a requirement for DNA distortion in the formation of hairpins.
Synapsis, hairpin formation, and the 12/23 rule.
What is the role of synapsis in hairpin formation? Without synapsis, hairpin formation does not occur, leading to the suggestion that the role of synapsis is to facilitate the required distortion at the cleavage site (5, 44). This is not the whole story, however, since unpairing the coding flank nucleotides does not restore hairpin formation by either the wild-type or mutant proteins in the absence of an appropriate synaptic partner (Fig. 4). Thus, synaptic interactions have additional functions, which may include bringing the appropriate active site into the proper position to catalyze hairpin formation.
What is the nature of the defect in the D32 and H609L mutants? Hairpin formation requires (i) synapsis with an appropriate partner RSS; (ii) transmission of a signal indicating that appropriate synapsis has occurred, such as induction of a conformational change; and (iii) execution of the chemical step of hairpin formation. In principle, the mutants could be defective for any or all of these processes. Our data provide three lines of evidence that both D32 and H609L are capable of synapsis. First, hairpin formation at a good flank by both mutants is assisted by the presence of a partner RSS containing either a good or a bad flank. Second, hairpin formation by H609L at a bad flank is substantially improved by the presence of a good partner RSS, clearly indicating that synaptic interactions can occur. Third, as noted above, both mutants are capable of efficiently forming hairpins from substrates containing unpaired coding flanks, but only in the presence of a second RSS. Based on these data, we conclude that both the D32 and H609L mutants are capable of synapsis (and are also capable of transmitting at least a partial synapsis signal) when bound to RSS with bad flanks.
Our data suggest that the mutants are incapable of achieving the proper distorted DNA configuration with a bad coding flank (presumably because bad flanks disrupt the natural tendency of the sequences near the cleavage site to adopt a distorted DNA structure). This leads to a specific defect in hairpin formation. The mutations could either directly affect the process of DNA distortion or cause quantitative or qualitative defects in the transmission of the synapsis signal, leading to a secondary defect in hairpin formation. It is particularly interesting to note that both mutants affect residues quite close in the primary sequence to an active-site amino acid (D600). This region could be important for coupling hairpin formation to synapsis and thus be vital to the enforcement of the 12/23 rule.
ACKNOWLEDGMENTS
We thank L. Huye for assistance with the plasmid cleavage assay, the oligonucleotide cleavage assay, and the provision of HMG-1 protein. We are grateful to M. Estes and S. Crawford for expert advice and assistance on baculovirus purification. L. Huye, V. Brandt, M. Purugganan, M. Neiditch, J. Qiu, and S. Shah provided helpful comments on the manuscript. Members of the Roth lab provided many helpful discussions. W. Kan, S. Gillenwater, and M. Calicchio provided technical assistance. S. Robertson and M. Lowe provided administrative support.
This work was supported by a grant from the National Institute of Health (AI-36420). M.A.L. was supported by a National Institutes of Health Predoctoral Fellowship (T32-AI07495). D.B.R. is an Assistant Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Agrawal A, Schatz D G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- 2.Akamatsu Y, Oettinger M A. Distinct roles of RAG1 and RAG2 in binding the V(D)J recombination signal sequences. Mol Cell Biol. 1998;18:4670–4678. doi: 10.1128/mcb.18.8.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besmer E, Mansilia-Soto J, Cassard S, Sawchuk D J, Brown G, Sadofsky M, Lewis S M, Nussenzweig M C, Cortes P. Hairpin coding end opening is mediated by RAG1 and RAG2 proteins. Mol Cell. 1998;2:817–828. doi: 10.1016/s1097-2765(00)80296-8. [DOI] [PubMed] [Google Scholar]
- 4.Bogue M A, Wang C, Zhu C, Roth D B. V(D)J recombination in Ku86-deficient mice: distinct effects on coding, signal, and hybrid joint formation. Immunity. 1997;7:37–47. doi: 10.1016/s1074-7613(00)80508-7. [DOI] [PubMed] [Google Scholar]
- 5.Cuomo C A, Mundy C L, Oettinger M A. DNA sequence and structure requirements for cleavage of V(D)J recombination signal sequences. Mol Cell Biol. 1996;16:5683–5690. doi: 10.1128/mcb.16.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies D R, Goryshin I Y, Reznikoff W S, Rayment I. Three-dimensional structure of the Tn5 synaptic complex transposition intermediate. Science. 2000;289:77–85. doi: 10.1126/science.289.5476.77. [DOI] [PubMed] [Google Scholar]
- 7.Difilippantonio M J, McMahan C J, Eastman Q M, Spanopoulou E, Schatz D G. RAG1 mediates signal sequence recognition and recruitment of RAG2 in V(D)J recombination. Cell. 1996;87:253–262. doi: 10.1016/s0092-8674(00)81343-4. [DOI] [PubMed] [Google Scholar]
- 8.Duckett D R, Murchie A I H, Lilley D M J. The role of metal ions in the conformation of the four-way DNA junction. EMBO J. 1990;9:583–590. doi: 10.1002/j.1460-2075.1990.tb08146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastman Q M, Leu T M J, Schatz D G. Initiation of V(D)J recombination in vitro obeying the 12/23 rule. Nature. 1996;380:85–88. doi: 10.1038/380085a0. [DOI] [PubMed] [Google Scholar]
- 10.Eastman Q M, Villey I J, Schatz D G. Detection of RAG protein-V(D)J recombination signal interactions near the site of DNA cleavage by UV cross-linking. Mol Cell Biol. 1999;19:3788–3797. doi: 10.1128/mcb.19.5.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fugmann S D, Lee A I, Shockett P E, Villey I J, Schatz D G. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 12.Fugmann S D, Villey I J, Ptaszek L M, Schatz D G. Identification of two catalytic residues in RAG1 that define a single active site within the RAG1/RAG2 protein complex. Mol Cell. 2000;5:97–107. doi: 10.1016/s1097-2765(00)80406-2. [DOI] [PubMed] [Google Scholar]
- 13.Hiom K, Gellert M. A stable RAG1-RAG2-DNA complex that is active in V(D)J cleavage. Cell. 1997;88:65–72. doi: 10.1016/s0092-8674(00)81859-0. [DOI] [PubMed] [Google Scholar]
- 14.Hiom K, Gellert M. Assembly of a 12/23 paired signal complex: a critical control point in V(D)J recombination. Mol Cell. 1998;1:1011–1019. doi: 10.1016/s1097-2765(00)80101-x. [DOI] [PubMed] [Google Scholar]
- 15.Kim D R, Dai Y, Mundy C L, Yang W, Oettinger M A. Mutations of acidic residues in RAG1 define the active site of the V(D)J recombinase. Genes Dev. 1999;13:3070–3080. doi: 10.1101/gad.13.23.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D R, Oettinger M A. Functional analysis of coordinated cleavage in V(D)J recombination. Mol Cell Biol. 1998;18:4679–4688. doi: 10.1128/mcb.18.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landree M A, Wibbenmeyer J A, Roth D B. Mutational analysis of RAG-1 and RAG-2 identifies three active site amino acids in RAG-1 critical for both cleavage steps of V(D)J recombination. Genes Dev. 1999;13:3059–3069. doi: 10.1101/gad.13.23.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavoie B D, Chan B S, Allison R G, Chaconas G. Structural aspects of a higher order nucleoprotein complex: induction of an altered DNA structure at the Mu-host junction of the Mu type 1 transpososome. EMBO J. 1991;10:3051–3059. doi: 10.1002/j.1460-2075.1991.tb07856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leu T M, Eastman Q M, Schatz D G. Coding joint formation in a cell-free V(D)J recombination system. Immunity. 1997;7:303–314. doi: 10.1016/s1074-7613(00)80532-4. [DOI] [PubMed] [Google Scholar]
- 20.Lewis S M. The mechanism of V(D)J joining: lessons from molecular, immunological and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- 21.Lewis S M, Hesse J E, Mizuuchi K, Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988;55:1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- 22.Lieber M R, Hesse J E, Lewis S, Bosma G C, Rosenberg N, Mizuuchi K, Bosma M J, Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 23.Lieber M R, Hesse J E, Mizuuchi K, Gellert M. Lymphoid V(D)J recombination: nucleotide insertion at signal joints as well as coding joints. Proc Natl Acad Sci USA. 1988;85:8588–8592. doi: 10.1073/pnas.85.22.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBlane J F, van Gent D C, Ramsden D A, Romeo C, Cuomo C A, Gellert M, Oettinger M A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 25.Merrihew R V, Sargent R G, Wilson J H. Efficient modification of the APRT gene by FLP/FRT site-specific targeting. Somat Cell Mol Genet. 1995;21:299–307. doi: 10.1007/BF02257465. [DOI] [PubMed] [Google Scholar]
- 26.Mo X, Bailin T, Noggle S, Sadofsky M J. A highly ordered structure in V(D)J recombination cleavage complexes is facilitated by HMG1. Nucleic Acids Res. 1999;28:1228–1236. doi: 10.1093/nar/28.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsden D A, McBlane J F, van Gent D C, Gellert M. Distinct DNA sequence and structure requirements for the two steps of V(D)J recombination signal cleavage. EMBO J. 1996;15:3197–3206. [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsden D A, Paull T T, Gellert M. Cell-free V(D)J recombination. Nature. 1997;388:488–491. doi: 10.1038/41351. [DOI] [PubMed] [Google Scholar]
- 29.Roman C A J, Baltimore D. Genetic evidence that the RAG1 protein directly participates in V(D)J recombination through substrate recognition. Proc Natl Acad Sci USA. 1996;93:2333–2338. doi: 10.1073/pnas.93.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadofsky M J, Hesse J E, McBlane J F, Gellert M. Expression and V(D)J recombination activity of mutated RAG-1 proteins. Nucleic Acids Res. 1993;21:5644–5650. doi: 10.1093/nar/21.24.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadofsky M J, Hesse J E, van Gent D C, Gellert M. RAG-1 mutations that affect the target specificity of V(D)J recombination: a possible direct role of RAG-1 in site recognition. Genes Dev. 1995;9:2193–2199. doi: 10.1101/gad.9.17.2193. [DOI] [PubMed] [Google Scholar]
- 32.Savilahti H, Rice P A, Mizuuchi K. The phage Mu transpososome core: DNA requirements for assembly and function. EMBO J. 1995;14:4893–4903. doi: 10.1002/j.1460-2075.1995.tb00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawchuk D J, Weis-Garcia F, Malik S, Besmer E, Bustin M, Nussenzweig M C, Cortes P. V(D)J recombination: modulation of RAG1 and RAG2 cleavage activity on 12/23 substrates by whole cell extract and DNA-bending proteins. J Exp Med. 1997;185:2025–2031. doi: 10.1084/jem.185.11.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scottoline B P, Chow S, Ellison V, Brown P O. Disruption of the terminal base pairs of retroviral DNA during integration. Genes Dev. 1997;11:371–382. doi: 10.1101/gad.11.3.371. [DOI] [PubMed] [Google Scholar]
- 35.Shockett P E, Schatz D G. DNA hairpin opening mediated by the RAG1 and RAG2 proteins. Mol Cell Biol. 1999;19:4159–4166. doi: 10.1128/mcb.19.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spanopoulou E, Zaitseva F, Wang F-H, Santagata S, Baltimore D, Panayotou G. The homeodomain region of Rag-1 reveals the parallel mechanisms of bacterial and V(D)J recombination. Cell. 1996;87:263–276. doi: 10.1016/s0092-8674(00)81344-6. [DOI] [PubMed] [Google Scholar]
- 37.Steen S B, Gomelsky L, Speidel S L, Roth D B. Initiation of V(D)J recombination in vivo: role of recombination signal sequences in formation of single and paired double-strand breaks. EMBO J. 1997;16:2656–2664. doi: 10.1093/emboj/16.10.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steen S B, Han J-O, Mundy C, Oettinger M A, Roth D B. Roles of the “dispensable” portions of RAG-1 and RAG-2 in V(D)J recombination. Mol Cell Biol. 1999;19:3010–3017. doi: 10.1128/mcb.19.4.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swanson P C, Desiderio S. V(D)J recombination signal recognition: distinct, overlapping DNA-protein contacts in complexes containing RAG1 with and without RAG2. Immunity. 1998;9:115–125. doi: 10.1016/s1074-7613(00)80593-2. [DOI] [PubMed] [Google Scholar]
- 40.Swanson P C, Desiderio S. RAG-2 promotes heptamer occupancy by RAG-1 in the assembly of a V(D)J initiation complex. Mol Cell Biol. 1999;19:3674–3683. doi: 10.1128/mcb.19.5.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timsit Y, Vilbois E, Moras D. Base-pairing shift in the major groove of (CA)n tracts by B-DNA crystal structures. Nature. 1991;354:167–170. doi: 10.1038/354167a0. [DOI] [PubMed] [Google Scholar]
- 42.van Gent D C, Hiom K, Paull T T, Gellert M. Stimulation of V(D)J cleavage by high mobility group proteins. EMBO J. 1997;16:2665–2670. doi: 10.1093/emboj/16.10.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gent D C, Mizuuchi K, Gellert M. Similarities between initiation of V(D)J recombination and retroviral integration. Science. 1996;271:1592–1594. doi: 10.1126/science.271.5255.1592. [DOI] [PubMed] [Google Scholar]
- 44.van Gent D C, Ramsden D A, Gellert M. The RAG1 and RAG2 proteins establish the 12/23 rule in V(D)J recombination. Cell. 1996;85:107–113. doi: 10.1016/s0092-8674(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 45.van Gent D C, McBlane J F, Ramsden D A, Sadofsky M J, Hesse J E, Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995;81:925–934. doi: 10.1016/0092-8674(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 46.Vermote C L M, Vipond B, Halford S E. EcoRV restriction endonuclease: communication between DNA recognition and catalysis. Biochemistry. 1992;31:6089–6097. doi: 10.1021/bi00141a019. [DOI] [PubMed] [Google Scholar]
- 47.West R B, Lieber M R. The RAG-HMG1 complex enforces the 12/23 rule of V(D)J recombination specifically at the double-hairpin formation step. Mol Cell Biol. 1998;18:6408–6415. doi: 10.1128/mcb.18.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu C, Bogue M A, Lim D-S, Hasty P, Roth D B. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]