Abstract
PURPOSE
To update recommendations of the ASCO systemic therapy for hormone receptor (HR)-positive metastatic breast cancer (MBC) guideline.
METHODS
An Expert Panel conducted a systematic review to identify new, potentially practice-changing data.
RESULTS
Fifty-one articles met eligibility criteria and form the evidentiary basis for the recommendations.
RECOMMENDATIONS
Alpelisib in combination with endocrine therapy (ET) should be offered to postmenopausal patients, and to male patients, with HR-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, ABC, or MBC following prior endocrine therapy with or without a cyclin-dependent kinase (CDK) 4/6 inhibitor. Clinicians should use next-generation sequencing in tumor tissue or cell-free DNA in plasma to detect PIK3CA mutations. If no mutation is found in cell-free DNA, testing in tumor tissue, if available, should be used as this will detect a small number of additional patients with PIK3CA mutations. There are insufficient data at present to recommend routine testing for ESR1 mutations to guide therapy for HR-positive, HER2-negative MBC. For BRCA1 or BRCA2 mutation carriers with metastatic HER2-negative breast cancer, olaparib or talazoparib should be offered in the 1st-line through 3rd-line setting. A nonsteroidal aromatase inhibitor (AI) and a CDK4/6 inhibitor should be offered to postmenopausal women with treatment-naïve HR-positive MBC. Fulvestrant and a CDK4/6 inhibitor should be offered to patients with progressive disease during treatment with AIs (or who develop a recurrence within 1 year of adjuvant AI therapy) with or without one line of prior chemotherapy for metastatic disease, or as first-line therapy. Treatment should be limited to those without prior exposure to CDK4/6 inhibitors in the metastatic setting.
Additional information can be found at www.asco.org/breast-cancer-guidelines.
INTRODUCTION
ASCO published a guideline in 2016 on endocrine therapy (ET) for hormone receptor (HR)–positive metastatic breast cancer (MBC).1 ASCO updates its guidelines at intervals determined by the Expert Panel, based on targeted literature searching and the expertise of ASCO guideline panel members to identify signals2 in the literature. The present update was prompted by the publication of the SOLAR-1 (Clinical Studies of Alpelisib in Breast Cancer 1) randomized, phase III clinical trial of alpelisib plus fulvestrant in PIK3CA-mutated, HR-positive, HER2-negative advanced breast cancer (ABC),3 and by the publication of trials evaluating the cyclin-dependent kinase (CDK) 4/6 inhibitors palbociclib,4-11 ribociclib,12-17 and abemaciclib.18-22
THE BOTTOM LINE
Endocrine Treatment and Targeted Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: ASCO Guideline Update
Target Population
Women and men with HR-positive, HER2-negative MBC.
Target Audience
Oncology specialists, other health care providers (including primary care physicians, specialists, nurses, social workers, and any other relevant member of a comprehensive multidisciplinary cancer care team), caregivers, and patients.
Methods
An Expert Panel was convened to update clinical practice guideline recommendations based on a systematic review of the medical literature.
UPDATED RECOMMENDATIONS
Clinical Question 1
Should alpelisib be given to postmenopausal women, and to male patients, with HR-positive, HER2-negative, PIK3CA-mutated, ABC, or MBC?
Recommendation 1.1.
Alpelisib in combination with ET should be offered to postmenopausal patients in combination with fulvestrant, and to male patients, with HR-positive, HER2-negative, PIK3CA-mutated, ABC, or MBC following prior ET including an aromatase inhibitor (AI), with or without a CDK4/6 inhibitor. Careful screening for and management of common toxicities are required (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: moderate; Appendix Table A2, online only).
Clinical Question 2
What is the role of biomarkers in treatment selection for patients with HR-positive MBC?
Recommendation 2.1.
To guide the decision to use alpelisib in combination with fulvestrant in postmenopausal patients, and in male patients with HR-positive MBC, clinicians should use next-generation sequencing in tumor tissue or cell-free DNA in plasma to detect PIK3CA mutations. If no mutation is found in cell-free DNA, testing in tumor tissue, if available, should be used as this will detect a small number of additional patients with PIK3CA mutations (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: strong).
Recommendation 2.2.
There are insufficient data at present to recommend routine testing for ESR1 mutations to guide therapy for HR-positive, HER2-negative MBC. Existing data suggest reduced efficacy of AIs compared with the selective estrogen receptor degrader fulvestrant in patients who have tumor or circulating tumor DNA (ctDNA) with ESR1 mutations (type: informal consensus; evidence quality: insufficient; strength of recommendation: moderate).
Recommendation 2.3.
Patients with metastatic HR-positive but HER2-negative breast cancer with germline BRCA1 or 2 mutations who are no longer benefiting from ET may be offered an oral poly (ADP-ribose) polymerase (PARP) inhibitor in the first-line through to third-line setting rather than chemotherapy (type: evidence-based; benefits outweigh harms; evidence quality: intermediate; strength of recommendation: strong).
Qualifying statements: Small single-arm studies show that oral PARP inhibitor therapy demonstrates high response rates in MBC-encoding DNA repair defects, such as germline PALB2 mutation carriers and somatic BRCA mutations. It should also be noted that the randomized PARP inhibitor trials made no direct comparison with taxanes, anthracyclines, or platinums; comparative efficacy against these compounds is unknown.
Clinical Question 3
What is the role of CDK4/6 inhibitors in the treatment of patients with HR-positive MBC?
Recommendation 3.1.
A nonsteroidal AI and a CDK4/6 inhibitor should be offered to postmenopausal patients and to premenopausal patients combined with chemical ovarian function suppression, and to male patients (with a gonadotropin-releasing hormone analog), with treatment-naïve HR-positive MBC (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: strong).
Recommendation 3.2.
Fulvestrant and a CDK4/6 inhibitor should be offered to patients with progressive disease during treatment with AIs (or who develop a recurrence within 1 year of adjuvant AI therapy) with or without one line of prior chemotherapy for metastatic disease, or as first-line therapy. Treatment should be limited to those without prior exposure to CDK4/6 inhibitors in the metastatic setting (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: strong).
Additional Resources
More information, including a supplement with additional evidence tables, slide sets, and clinical tools and resources, is available at www.asco.org/breast-cancer-guidelines. The Methodology Manual (available at www.asco.org/guideline-methodology) provides additional information about the methods used to develop this guideline. Patient information is available at www.cancer.net.
ASCO believes that cancer clinical trials are vital to inform medical decisions and improve cancer care, and that all patients should have the opportunity to participate.
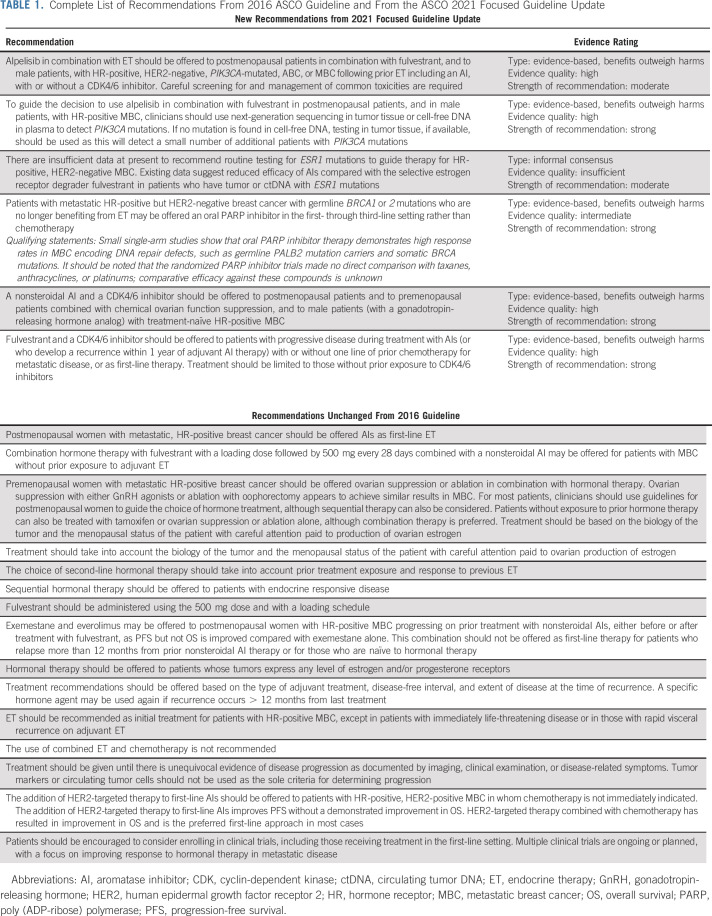
This focused update of the 2016 guideline provides a new recommendation for the use of alpelisib in the treatment of patients with HR-positive MBC; addresses the role of biomarkers in treatment selection for this patient population; and amends prior recommendations concerning the use of CDK4/6 inhibitors in the treatment of these patients. The remaining recommendations from the 2016 guideline are unchanged because there were no new potentially practice-changing data to support substantive revisions (Table 1). The evidence supporting these unchanged recommendations is reviewed in the previous guideline publication.1
TABLE 1.
Complete List of Recommendations From 2016 ASCO Guideline and From the ASCO 2021 Focused Guideline Update
Note that this guideline provides recommendations for ET and targeted therapy, including CDK4/6 and PI3 kinase inhibition for patients with HR-positive MBC. A companion guideline provides recommendations for use of chemotherapy and targeted therapy for patients with HER2-negative MBC that is either endocrine-pretreated or HR-negative.
FOCUSED GUIDELINE QUESTIONS
Clinical Question 1: Should alpelisib be given to postmenopausal women, and to male patients, with HR-positive, HER2-negative, PIK3CA-mutated, ABC, or MBC?
Clinical Question 2: What is the role of biomarkers in treatment selection for patients with HR-positive MBC?
Clinical Question 3: What is the role of CDK4/6 inhibitors in the treatment of patients with HR-positive MBC?
METHODS
Guideline Update Process
ASCO uses a signals approach to facilitate guideline updating.2 This approach identifies new, potentially practice-changing data—signals—that might translate into revised practice recommendations. The approach relies on targeted literature searching and the expertise of ASCO guideline panel members to identify signals. For this focused update, phase III randomized trials on alpelisib and additional CDK4/6 inhibitors provided the signals.
This systematic review-based guideline product was developed by a multidisciplinary Expert Panel, which included a patient representative and an ASCO guidelines staff member with health research methodology expertise. The Expert Panel searched the PubMed database to identify any additional randomized clinical trials (RCTs) that addressed the focused update's three main clinical questions. The electronic searches were supplemented by articles identified by Expert Panel members and by reviews of the bibliographies of relevant articles.
The Methodology Manual available at www.asco.org/guideline-methodology provides additional information about the guideline update approach. Additional information about the results of the updated literature search and search strategy strings is reported in the Data Supplement (online only).
The Expert Panel met by teleconference to consider the evidence for each of the 2021 recommendations. The guideline was circulated in draft form to the Expert Panel. The entire Expert Panel (Appendix Table A1, online only) contributed to the development of the guideline, provided critical review, and finalized the guideline recommendations. The ASCO Clinical Practice Guidelines Committee reviews and approves all ASCO guidelines before publication. All funding for the administration of the project was provided by ASCO.
Guideline Disclaimer
The Clinical Practice Guidelines and other guidance published herein are provided by the ASCO to assist providers in clinical decision making. The information herein should not be relied upon as being complete or accurate, nor should it be considered as inclusive of all proper treatments or methods of care or as a statement of the standard of care. With the rapid development of scientific knowledge, new evidence may emerge between the time information is developed and when it is published or read. The information is not continually updated and may not reflect the most recent evidence. The information addresses only the topics specifically identified therein and is not applicable to other interventions, diseases, or stages of diseases. This information does not mandate any particular course of medical care. Further, the information is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. Recommendations specify the level of confidence that the recommendation reflects the net effect of a given course of action. The use of words like “must,” “must not,” “should,” and “should not” indicates that a course of action is recommended or not recommended for either most or many patients, but there is latitude for the treating physician to select other courses of action in individual cases. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. ASCO does not endorse third-party drugs, devices, services, or therapies used to diagnose, treat, monitor, manage, or alleviate health conditions. Any use of a brand or trade name is for identification purposes only. ASCO provides this information on an “as is” basis and makes no warranty, express or implied, regarding the information. ASCO specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. ASCO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information, or for any errors or omissions.
Guideline and Conflicts of Interest
The Expert Panel was assembled in accordance with ASCO's Conflict of Interest Policy Implementation for Clinical Practice Guidelines (“Policy,” found at http://www.asco.org/rwc). All members of the Expert Panel completed ASCO's disclosure form, which requires disclosure of financial and other interests, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as a result of promulgation of the guideline. Categories for disclosure include employment; leadership; stock or other ownership; honoraria, consulting or advisory role; speaker's bureau; research funding; patents, royalties, other intellectual property; expert testimony; travel, accommodations, expenses; and other relationships. In accordance with the Policy, the majority of the members of the Expert Panel did not disclose any relationships constituting a conflict under the Policy.
RESULTS
The PubMed search (from January 1, 2016, to December 31, 2020) conducted to identify publications that reported on studies addressing the clinical questions yielded a total of 265 abstracts; the search string was drawn from the review completed for the 2016 guideline (Data Supplement). Articles were selected for inclusion in the systematic review of the evidence if they were phase III randomized controlled trials, meta-analyses, or pooled analyses of alpelisib or any one of three CDK4/6 inhibitors that evaluated patients with HR-positive, HER2-negative ABC or MBC. Articles were excluded from the systematic review if they were (1) meeting abstracts not subsequently published in peer-reviewed journals; (2) editorials, commentaries, letters, news articles, case reports, or narrative reviews; or (3) published in a non-English language. After review of the identified abstracts, 33 full-text articles were selected for review by the Expert Panel. QUOROM diagrams of the updated searches and the clinical questions are in the Data Supplement.
A total of 30 articles representing eight major RCTs—SOLAR-1,3,23 MONARCH-2,19,20,22 MONARCH-3,18,21,24 PALOMA-2,4-6,25 PALOMA-3,7-11,26,27 MONALEESA-2,13,14,28-31 MONALEESA-3,15,16 and MONALEESA-712,17,32—met eligibility criteria. The results of the phase III RCTs included in the review are summarized in the Data Supplement. Study quality was formally assessed for the eight phase III RCTs identified (Data Supplement 3). Design aspects related to the individual study quality were assessed by one reviewer, with factors such as blinding, allocation concealment, placebo control, intention to treat, and funding sources, etc, generally indicating a low to intermediate potential risk of bias for most of the identified evidence. Refer to the Methodology Manual for definitions of ratings for overall potential risk of bias.
The search also identified 10 meta-analyses33-38 or pooled analyses39-42; these provide confirmatory, supplementary evidence. The main findings of the meta-analysis and pooled analyses are summarized in the Data Supplement. The Data Supplement includes information on the incidence of grade ≥ 3 adverse events (AEs) from reports of the RCTs included in the systematic reviews that provided independent (nonduplicative) AEs data.
Targeted PubMed literature searches were conducted to identify articles on, respectively, relevant biomarkers and health-related quality of life (HRQoL). Five articles from the biomarkers searches met selection criteria and were included in the systematic review.3,23,43-45 Biomarker test articles were limited to studies that provided evidence for the clinical utility of the biomarker in question.46,47 Eleven articles that reported HRQoL data from RCTs of CDK4/6 inhibitors were also included in the systematic review8,19,24-26,28,29,32,41,42,48 (Data Supplement 4); two additional articles reported on HRQoL outcomes from studies of PARP inhibitors among BRCA1 or BRCA2 mutation carriers with MBC.49,50
FOCUSED UPDATE RECOMMENDATIONS
An algorithm for endocrine treatment and targeted therapy for HR-positive, HER2-negative MBC is displayed in Figure 1.
FIG 1.
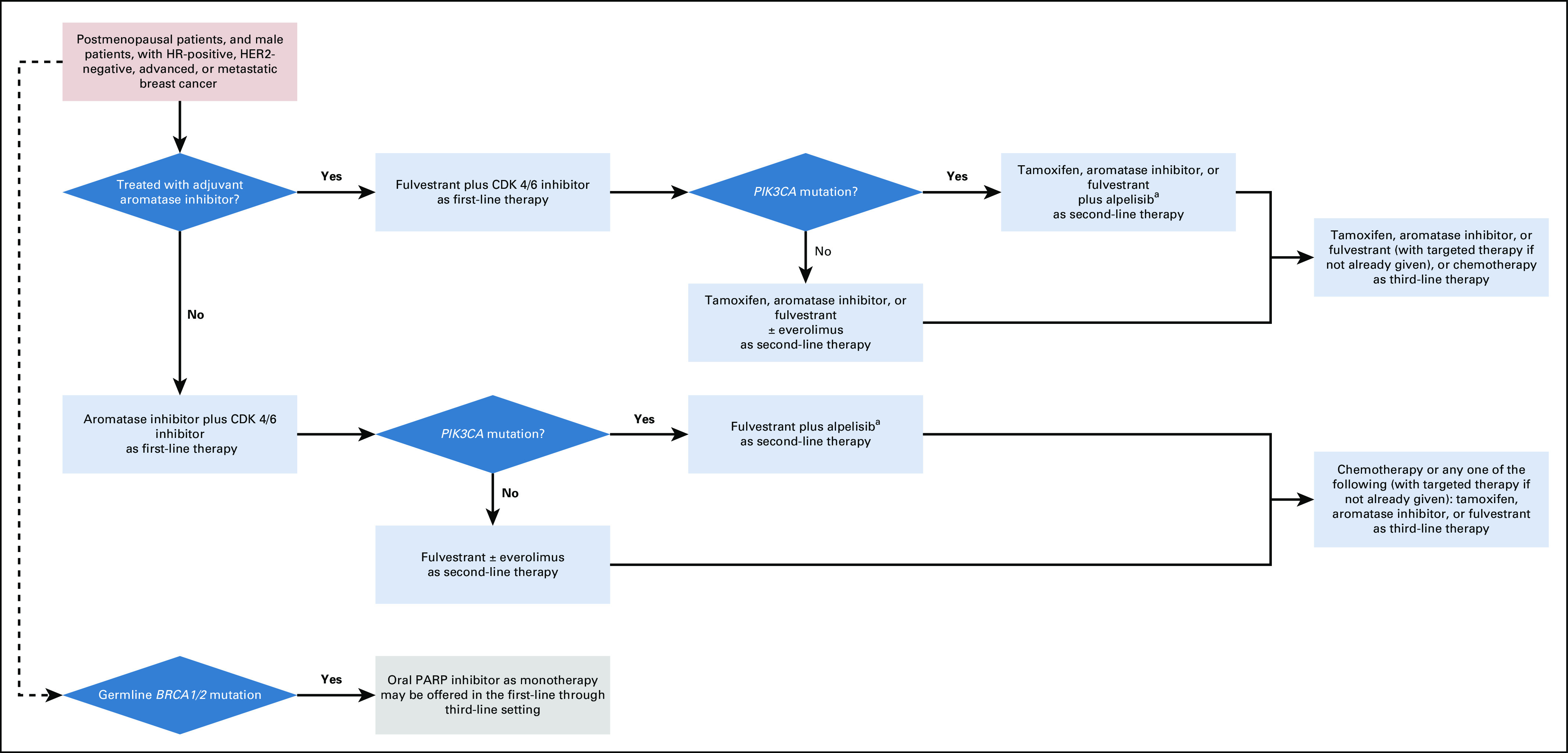
Algorithm for endocrine treatment and targeted therapy for HR-positive, HER2-negative MBC. aPatients receiving alpelisib should have laboratory and symptom monitoring weekly for the first 4 weeks of therapy to avoid serious toxicity. CDK, cyclin-dependent kinase; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MBC, metastatic breast cancer.
Clinical Question 1
Should alpelisib be given to postmenopausal women, and to male patients, with HR-positive, HER2-negative, PIK3CA-mutated, ABC, or MBC?
Recommendation 1.1.
Alpelisib in combination with ET should be offered to postmenopausal patients in combination with fulvestrant, and to male patients, with HR-positive, HER2-negative, PIK3CA-mutated, ABC, or MBC following prior ET including an AI, with or without a CDK4/6 inhibitor. Careful screening for and management of common toxicities are required (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: moderate).
Literature review and analysis.
The systematic review identified two articles reporting on one randomized trial that inform the use of alpelisib in combination with ET. The randomized, double-blind, placebo-controlled phase III clinical trial SOLAR-1 compared the PI3Kα-specific inhibitor alpelisib plus fulvestrant to placebo plus fulvestrant in patients with PIK3CA-mutated, HR-positive, HER2-negative ABC who had received prior ET with or without a CDK4/6 inhibitor.3,23 SOLAR-1 randomly assigned a total of 572 patients. A cohort of 341 patients who had PIK3CA-mutated disease received either alpelisib plus fulvestrant (n = 169) or placebo plus fulvestrant (n = 172). An independent cohort of 231 patients without PIK3CA-mutated cancer also underwent random assignment.
Patients who received alpelisib-fulvestrant had significantly prolonged progression-free survival (PFS), the primary study end point (11.0 months v 5.7 months, P < .001). This benefit was not observed in the group of patients without PIK3CA-mutated breast cancer who received alpelisib-fulvestrant. In safety analyses, the most frequent AEs observed in the overall population were hyperglycemia and rash. Grade 3 hyperglycemia occurred in 36.6% of patients in the alpelisib-fulvestrant group and in 0.7% of patients in the placebo-fulvestrant group; rash occurred in 9.9% of patients in the alpelisib-fulvestrant group and 0.3% of patients in the placebo-fulvestrant group. Grade 3 diarrhea occurred in 6.7% of patients who received alpelisib-fulvestrant versus 0.3% of patients who received placebo-fulvestrant.
In the final overall survival (OS) results from the SOLAR-1 trial, the authors that reported no statistically significant differences in OS were detected between treatment groups. There was an improvement of 7.9 months in OS in the PIK3CA-mutated breast cancer cohort who received alpelisib-fulvestrant (39.3 months; 95% CI, 34.1 to 44.9) compared with patients who received placebo-fulvestrant (31.4 months; 95% CI, 26.8 to 41.3). However, the OS results did not cross the prespecified efficacy boundary. No new safety signals were seen in this follow-up analysis.
The impact of alpelisib-ET on HRQoL, functional status, and pain among patients in SOLAR-1 with PIK3CA-mutated breast cancer was evaluated by Ciruelos et al48 who used the European Organization for Research and Treatment of Cancer Quality of Life of Cancer Patients (EORTC QLQ-C30)51 and the Brief Pain Inventory-Short Form (BPI-SF) questionnaires.52 Global Health Status/QoL was the primary patient-reported outcome (PRO) variable of interest; secondary PRO variables of interest included EORTC QLQ-C30 Physical, Emotional, and Social functioning and the Worst Pain, Pain Severity Index, and Pain Interference Index of the BPI-SF.
Global Health Status/QoL scores and functioning and symptom scale scores were similar between the alpelisib and the placebo arms at baseline; and, over time, there was no overall change from baseline in either arm. There was similarly no statistically significant difference between treatment arms in overall treatment effect on Global Health Status/QoL (23.77; 95% CI, 28.35 to 0.80; P = .101), and in time to 10% deterioration for Global Health Status/QoL (hazard ratio, 1.03; 95% CI, 0.72 to 1.48). In the alpelisib arm, there was a larger deterioration in Social functioning (treatment difference, 24.98; 95% CI, 28.86 to 21.09; P = .012), but there were no other differences between arms in overall adjusted mean changes from baseline in other EORTC QLQ-C30 functioning scale scores.
Several differences were observed between treatment arms in overall mean changes from baseline in symptoms scores. Patients who received alpelisib experienced worsening scores from baseline in appetite loss (10.96 v 1.83; P < .001), diarrhea (13.39 v 1.63; P < .001), nausea or vomiting (6.97 v 4.14; P = .019), and fatigue (9.85 v 3.34; P = .014); however, the constipation score (28.54 v 23.61; P = .004) improved from baseline among patients in the alpelisib arm.
Clinical interpretation.
Patients with estrogen receptor–positive (ER+) ABC have multiple hormonal therapy options and, increasingly, have targeted therapy options, to improve important outcomes. Based on the multiple randomized trials of CDK4/6 inhibitors (see section 3, below) showing substantial improvements in PFS and in some instances OS, and the tolerability profile of CDK4/6 inhibitors, patients should receive ET plus a CDK4/6 inhibitor before initiation of PIK3CA- or mammalian target of rapamycin (mTOR)-targeted therapy.
In the SOLAR-1 trial, adding alpelisib yielded improvement in PFS, a trend for improved OS in patients with visceral metastases, and an 8.5-month delay in time to chemotherapy. However, use of alpelisib is associated with significant toxicities that must be carefully monitored and managed. In SOLAR-1, the deterioration in Global Health Status and Quality of Life were similar between the placebo and alpelisib arms, with improvement in Worst Pain Score with alpelisib.48 However, symptom subscales favored placebo for the common side effects seen with alpelisib, diarrhea, appetite loss, nausea or vomiting, and fatigue.
All patients who are being considered for treatment with alpelisib should have a baseline hemoglobin A1c and fasting glucose. SOLAR-1 eligibility was modified part-way through the trial to better manage toxicity, including only patients with baseline hemoglobin A1c < 6.5% (compared with < 8% at study start). Patients with uncontrolled diabetes should not receive alpelisib, although patients with well-controlled type 2 diabetes can be treated. Risk factors such as an elevated baseline hemoglobin A1c and obesity should be considered. The median time to onset of > grade 3 hyperglycemia and rash in SOLAR-1 was 15 and 13 days, respectively. This is critical information, as patients receiving alpelisib should have laboratory and symptom monitoring weekly for the first 4 weeks of therapy to avoid serious toxicity. Interestingly, diarrhea is a later toxicity, with grade 3 events occurring at a median of 139 days.
The majority of patients in SOLAR-1 received metformin alone or in combination with other hypoglycemic agents. Preventive agents appeared to reduce the incidence of higher-grade rash; the most commonly used agents were nonsedating antihistamines or steroids. Preventive agents for rash should be considered in patients who are planned to start alpelisib. In addition to the medications noted above, and antipropulsive agents for diarrhea, dose delays and reductions were commonly used to manage toxicity. In SOLAR-1, using detailed side-effect management guidelines resulted in a decrease in discontinuations for higher-grade AEs.
The SOLAR-1 trial was conducted before CDK4/6 inhibitors were routinely used in combination with ET as treatment for metastatic, HR-positive and HER2-negative breast cancer. Therefore, only 5.9% of patients with PIK3CA-mutated disease enrolled in SOLAR-1 had received prior CDK4/6 inhibitors. Additional data on outcomes with alpelisib after prior treatment with a CDK4/6 inhibitor are available from the nonrandomized BYLIEVE trial, which enrolled 3 cohorts of patients with known PIK3CA-mutated MBC.53 Patients receiving alpelisib and fulvestrant after an AI and a CDK4/6 inhibitor had a median PFS of 7.3 months and 50.4% were alive without disease progression at 6 months (n = 121). These data provide some support for the sequential use of alpelisib after CDK4/6 inhibitors. Based on tolerability and efficacy, the Expert Panel strongly recommends that patients receive CDK4/6 inhibitors in combination with ET before the line of therapy including alpelisib or everolimus.
In the previous guideline,1 the Expert Panel considered the role of the mTOR inhibitor, everolimus, in the management of ER-positive ABC, and recommended that exemestane and everolimus may be offered to postmenopausal women with HR-positive MBC who experience progression during treatment with nonsteroidal AIs, either before or after treatment with fulvestrant, because PFS but not OS was improved compared with exemestane alone. That recommendation is unchanged.
There are limited data for the use of everolimus after CDK4/6 inhibitors. Following CDK4/6 inhibitor therapy, the duration of treatment with everolimus paired with ongoing ET is diminished compared with that seen among patients without prior CDK4/6 inhibitor treatment, with clinical evidence for 4 to 5 months' treatment duration.54 Thus, everolimus may be an option in second or subsequent lines of endocrine-based therapy, although the clinical benefits in contemporary practice in patients treated with CDK4/6 inhibitors are not well defined.
It is not known how the efficacy of everolimus-based therapy compares to that seen with alpelisib; in particular, there are no data for use of everolimus in direct comparison to alpelisib. These targeted agents broadly affect similar PI3K/mTOR pathways in the tumor cell, with overlapping toxicity profiles. If PIK3CA status is not or cannot be determined, if PIK3CA is wild-type, or if the tolerability profile of everolimus in a given patient may be preferable to that of alpelisib, everolimus may be offered as a clinical option. There are no data for the use of alpelisib after everolimus, or vice versa, to guide clinical recommendations.
CLINICAL QUESTION 2
What is the role of biomarkers in treatment selection for patients with HR-positive MBC?
Recommendation 2.1
To guide the decision to use alpelisib in combination with fulvestrant in postmenopausal patients, and in male patients, with HR-positive MBC, clinicians should use next-generation sequencing in tumor tissue or cell-free DNA in plasma to detect PIK3CA mutations. If no mutation is found in cell-free DNA, testing in tumor tissue, if available, should be used as this will detect a small number of additional patients with PIK3CA mutations (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: strong).
Literature review and analysis.
Evidence from the SOLAR-1 trial provides support for the clinical utility of biomarker testing to detect PIK3CA mutations in patients with HR-positive, HER-negative MBC. André et al3,23 evaluated the efficacy of safety of alpelisib-fulvestrant in two cohorts of patients, one cohort with PIK3CA-mutated cancer and one proof-of-concept cohort without PIK3CA-mutated cancer. Patients in both cohorts were randomly assigned to receive either alpelisib-fulvestrant or placebo-fulvestrant. The prolongation of PFS observed with alpelisib-fulvestrant in the cohort of patients with PIK3CA-mutated cancer was not observed in the cohort of patients without PIK3CA-mutated cancer, demonstrating clinical utility as evidenced by improved patient outcomes from the use of a tumor biomarker test result to select treatment strategy.47,55
Analyses of specimens from patients enrolled in SOLAR-1 found low agreement between plasma ctDNA and tumor tissue identification of PIK3CA mutations. Just 177 of 317 (56%) patients with PIK3CA mutations that were confirmed in tumor tissue were found to have PIK3CA mutations identified in the plasma specimen.56 Given the risk of false-negative results and the low agreement between tumor tissue and ctDNA, the US Food and Drug Administration (FDA)-approved labeling recommends a reflex approach in which plasma testing is followed by tissue testing if no PIK3CA mutation is detected in a plasma specimen.56
Clinical interpretation.
Advances in ctDNA technology have increased the sensitivity of plasma screening for tumor-related mutations; however, large data sets comparing plasma and tumor tissue testing with samples obtained at similar times are lacking. Although PIK3CA mutations can be found throughout stages of breast cancer, clearly mutations can be acquired during treatment in the metastatic setting. Therefore, every attempt should be made to test the most recent tumor tissue sample, and if no sample is available, in some cases, plasma testing may be a preferred first step. Testing for PIK3CA mutations in SOLAR-1 focused on specific activating mutations in PIK3CA, including exons 9 and 20 (mutation subtypes E542K, E545X, and H1047X). These mutations are the basis for the regulatory approval of the combination therapy. Alternate mutations or amplifications may be found; response to alpelisib is unknown in these cases.
There are little data regarding PIK3CA mutations or the efficacy of alpelisib in men. It is reasonable, however, to apply the same approach to testing and sequencing of treatment in men with HR-positive and PIK3CA-mutated MBC, as is also recommended for the use of CDK4/6 inhibitors in HR-positive disease.57 Indeed, the current FDA approval for alpelisib and fulvestrant includes a specific statement noting that the current indication includes men.56
Recommendation 2.2
There are insufficient data at present to recommend routine testing for ESR1 mutations to guide therapy for HR-positive, HER2-negative MBC. Existing data suggest reduced efficacy of AIs compared with the selective estrogen receptor degrader fulvestrant in patients who have tumor or ctDNA with ESR1 mutations (type: informal consensus; evidence quality: insufficient; strength of recommendation: moderate).
Literature review and analysis.
The Expert Panel reviewed the available data on ESR1 to guide therapy for HR-positive, HER2-negative MBC and concluded that there is no evidence for the clinical utility of testing for ESR1 mutations.
Clinical interpretation.
ESR1 mutations are uncommon in early-stage breast cancer and also appear to be uncommon in patients with HR-positive metastatic disease without prior exposure to AIs (although they can arise following fulvestrant therapy as well), or when that exposure was more than a year before diagnosis of metastatic disease. A number of trials have demonstrated improved response and PFS with fulvestrant compared with AIs when ESR1 mutations are found in tumor or plasma ctDNA. However, given the infrequency of the mutation in untreated disease, and the sequencing of fulvestrant after disease progression on AIs, routine testing for ESR1 mutations is unlikely to affect either treatment decisions or patient outcomes. Preliminary results from the PARSIFAL trial that randomly assigned 486 patients with HR-positive MBC (de novo or relapsing at least 1 year from adjuvant ET) to receive either letrozole or fulvestrant in combination with the CDK4/6 inhibitor, palbociclib, showed no difference in PFS between the two arms.58 In addition, ESR1 mutations may be acquired or lost during the course of treatment with fulvestrant, making interpretation of archival sample testing complex.
Retrospective studies have suggested that among patients with ER+ ABC and progression on nonsteroidal AI therapy, ESR1 mutations serve as a prognostic marker for survival benefit with use of fulvestrant treatment, as opposed to ongoing AI therapy with steroidal AI therapy using exemestane.59 In clinical practice, it is most common to switch to fulvestrant from AI when patients have had tumor recurrence or progression on AI therapy. These retrospective data provide additional support for that clinical practice, particularly among women whose tumors have acquired ESR1 mutations from AI-exposure, and in whom ongoing AI therapy was of minimal clinical benefit.
Recommendation 2.3
Patients with metastatic HR-positive but HER2-negative breast cancer with germline BRCA1 or 2 mutations who are no longer benefiting from ET may be offered an oral PARP inhibitor in the first-line through to third-line setting rather than chemotherapy (type: evidence-based; benefits outweigh harms; evidence quality: intermediate; strength of recommendation: strong).
Qualifying statements: Small single-arm studies show that oral PARP inhibitor therapy demonstrates high response rates in MBC-encoding DNA repair defects, such as germline PALB2 mutation carriers and somatic BRCA mutations. It should also be noted that the randomized PARP inhibitor trials made no direct comparison with taxanes, anthracyclines, or platinums; comparative efficacy against these compounds is unknown.
Literature review and analysis.
The systematic literature review identified two RCTs that bear on the question of the role of testing BRCA1/2 testing to guide the use of PARP inhibitors in the treatment of patients with HER2-negative MBC. In an open-label, phase III RCT (OlympiAD), Robson et al43 compared the efficacy and safety of the PARP inhibitor, olaparib (n = 205), with the efficacy and safety of standard therapy with single-agent chemotherapy (capecitabine, eribulin mesylate, or vinorelbine; n = 91) in women with HER2-negative MBC and a germline BRCA mutation. The primary end point, median PFS, was significantly longer in the group that received olaparib monotherapy than in the group that received standard chemotherapy (7.0 months v 4.2 months; hazard ratio for disease progression or death, 0.58; 95% CI, 0.43 to 0.80). The risk of disease progression or death in the olaparib group was 42% lower than in the standard therapy group, and the response rate was almost two times the response rate in the standard therapy group (59.9% v 28.8%). The rate of grade 3 or higher AEs in patients who received olaparib was 36.6%; it was 50.5% in the group that received standard chemotherapy. HRQoL measures were also superior with olaparib than with chemotherapy: treatment with olaparib lead to improvements in the functioning, symptoms, and HRQoL. One exception was the nausea or vomiting symptom score, which was worse among patients who received olaparib.49
In 2019, Robson et al44 reported the results of the prespecified final analysis of OS in the OlympiAD study (at 64% data maturity) and on the long-term tolerability of olaparib. Analyses showed that, compared with chemotherapy treatment of physician's choice (TPC), there was no statistically significant improvement in OS with olaparib: median OS was 19.3 months with olaparib compared to 17.1 months with TPC (hazard ratio, 0.90; 95%, CI 0.66 to 1.23; P = .513). The safety profile in the OS analysis was comparable to that seen in the primary analysis and there was no evidence of cumulative toxicity with extended olaparib exposure.
Litton et al45 reported the results of an open-label, phase III randomized controlled trial (EMBRACA) that compared the efficacy and safety of the PARP inhibitor, talazoparib (n = 287), with standard single-agent chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine; n = 144) for the treatment of ABC in women with a germline BRCA1 or BRCA2 mutation. Median PFS in the talazoparib group was significantly longer than in the standard chemotherapy group (8.6 months v 5.6 months; hazard ratio for disease progression or death, 0.54; 95% CI, 0.41 to 0.71; P < .001). Benefits were seen in patients with either triple-negative or ER-positive breast cancer. There were also differences in the PROs of global health status–quality-of-life and breast symptoms. Compared with standard chemotherapy, talazoparib treatment resulted in a significant delay in the onset of clinically meaningful deterioration; in significant improvement in global health status–quality-of-life; and in improvement in breast symptom scale score from baseline.
In a final analysis of OS, Litton et al60 found that talazoparib did not significantly improve OS over standard, physician's choice of single-agent chemotherapy (hazard ratio, 0.848, 95% CI 0.670 to 1.073; P = .17). Median OS was 19.3 months with talazoparib (95% CI, 16.6 to 22.5 months) compared with 19.5 months (95% CI, 17.4 to 22.4 months) with chemotherapy, although these results were confounded by significant crossover following progression from placebo to PARP inhibitor. Consistent with the primary analysis, the incidence of grade 3-4 AEs was 69.6% among patients who received talazoparib and 64.3% among patients who received chemotherapy. Analyses of PROs50 demonstrated a positive risk-benefit profile of talazoparib. These analyses revealed overall improvement in global health status/quality of life (GHS/QoL) from baseline for talazoparib compared with statistically significant deterioration for physician's choice chemotherapy (3.0 [95% CI 1.2 to 4.8] v −5.4 [95% CI −8.8 to −2.0]; between arms; P < .0001). There was also a statistically significant greater delay in time to deterioration in GHS/QoL in favor of talazoparib (hazard ratio, 0.38; 95% CI, 0.26 to 0.55).
Clinical interpretation.
PARP inhibitors are generally well-tolerated oral agents compared with most chemotherapeutic agents and are an important addition to treatment options for patients with germline mutations in BRCA1 or BRCA2. For patients with HR-positive disease, the optimal sequencing is unknown, and the combination of PARP inhibition and ET has not been evaluated. In general, the combination of ET with a CDK4/6 inhibitor is the preferred first-line treatment in most patients with HR-positive metastatic disease. Treatment decisions should take into account potential toxicities and goals of therapy.
CLINICAL QUESTION 3
What is the role of CDK4/6 inhibitors in the treatment of patients with HR-positive MBC?
Recommendation 3.1
A nonsteroidal AI and a CDK4/6 inhibitor should be offered to postmenopausal patients and to premenopausal patients combined with chemical ovarian function suppression, and to male patients (with a gonadotropin-releasing hormone analog), with treatment-naïve HR-positive MBC (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: strong).
Literature review and analysis.
Use of a nonsteroidal AI and a CDK4/6 inhibitor in postmenopausal women with treatment-naïve HR-positive MBC. The systematic literature review identified 16 articles reporting the results of distinct analyses of data from one of four large-scale phase III RCTs—PALOMA-2, MONALEESA-2, MONALLESA-7, or MONARCH-3—that inform the recommendation on the use of a nonsteroidal AI and a CDK4/6 inhibitor in postmenopausal women with treatment-naïve HR-positive MBC. In what follows, the results of the relevant RCTs are summarized by broad trial end point—PFS and OS; AEs; and PROs, most frequently HRQoL. The detailed efficacy and PRO results from the individual studies are presented in the Data Supplement; data on the incidence of AEs (grade ≥ 3) from reports of the major RCTs are provided in the Data Supplement.
PALOMA-2.
PALOMA (Palbociclib: Ongoing Trials in the Management of Breast Cancer)-2, a double-blind phase III trial, randomly assigned 666 postmenopausal women with previously untreated ER-positive, HER2-negative ABC to receive either palbociclib plus letrozole (n = 444) or placebo plus letrozole (n = 222). PFS was the primary end point of the trial. With a median follow-up of 23 months, the median PFS in the palbociclib-letrozole group was 24.8 months (95% CI, 22.1 to not estimable); in the placebo-letrozole group, the median PFS was 14.5 months (95% CI, 12.9 to 17.1; hazard ratio for disease progression or death, 0.58; 95% CI, 0.46 to 0.72; P < .001).4 After a median follow-up of about 38 months, the median PFS in the group that received palbociclib-letrozole was 27.6 months and the median PFS in the group that received placebo-letrozole was 14.5 months (hazard ratio, 0.563; 1-sided P < .0001).6 An analysis of the efficacy of palbociclib-letrozole in a subgroup of Asian patients enrolled in PALOMA-2 by Im et al5 found that median PFS was significantly longer in Asian patients who received palbociclib-letrozole compared with placebo-letrozole (25.7 months; 95% CI, 19.2 months to not estimable v 13.9 months; 95% CI, 7.4 to 22.0 months; hazard ratio 0.48; 95% CI, 0.27 to 0.87; P = .007).
Consistent with earlier studies of palbociclib, there was a high incidence of hematologic AEs observed in PALOMA-2: neutropenia, leukopenia, anemia, and fatigue were the most common grade 3 or 4 AEs reported.4 Neutropenia occurred in 66.4% of the patients in the palbociclib-letrozole group and in just 1.4% of patients in the placebo-letrozole group, although the incidence of febrile neutropenia was low in patients who received palbociclib and letrozole (1.4% of patients v no patients in the placebo-letrozole group). Permanent discontinuation of any study treatment because of AEs occurred in 9.7% of patients in the palbociclib-letrozole group and 5.9% of patients in the placebo-letrozole group. The safety profile after about 15 additional months of follow-up was consistent with that from the first published report; no new safety signals were observed for palbociclib-letrozole.6 In the analysis of safety data conducted by Im et al,5 the incidence of hematologic toxicities (neutropenia, leukopenia, and thrombocytopenia) was higher in Asians versus non-Asians. However, discontinuation rates attributable to AEs were comparable among Asian and non-Asian patients who received palbociclib-letrozole.
The impact of palbociclib-letrozole on patient-reported QoL in PALOMA-2 was evaluated by Rugo et al25 who found that adding palbociclib to letrozole maintained HRQoL—there were no significant between-arm differences in change from baseline in Functional Assessment of Cancer Therapy (FACT)-Breast Total, FACT-General Total, or EuroQOL 5 dimensions (EQ-5D) scores—and improved pain scores (−0.256 v −0.098; P = .0183). In both trial arms, deterioration of FACT-B Total score was significantly delayed in patients without disease progression versus those with disease progression, as well in patients who had an objective response compared with nonresponders. The FACT-Breast total-assessed HRQoL was maintained with palbociclib-letrozole with extended follow-up (at a median of about 38 months).6 In like manner, QoL was maintained in the analysis of Asian patients enrolled in PALOMA-2: There were no significant differences between treatments from baseline in either the general health status or in breast cancer–specific QoL scores.5 Change from baseline in EQ-5D scores was significantly higher, however, with palbociclib-letrozole compared with placebo-letrozole (0.013 v −0.069; P = .0132).
MONARCH-3.
The double-blind phase III trial, MONARCH 3, randomly assigned 493 postmenopausal women with HR-positive, HER2-negative ABC to receive either the CDK4/6 inhibitor abemaciclib plus a nonsteroidal AI (n = 328), or to placebo plus a nonsteroidal AI (anastrozole or letrozole; n = 165).21 Patients had no prior systemic therapy in the advanced treatment setting. After a median follow-up of 17.8 months, the median PFS was significantly improved in the abemaciclib plus nonsteroidal AI group versus placebo plus a nonsteroidal AI (hazard ratio, 0.54; 95% CI, 0.41 to 0.72; P = .000021; median: not reached in the abemaciclib arm, 14.7 months in the placebo arm). The objective response rate (ORR) was 59% in the abemaciclib-nonsteroidal AI arm and 44% in the placebo-nonsteroidal AI arm (P = .004). After a median follow-up of 26.73 months, the median PFS in the abemaciclib-nonsteroidal AI group was 28.18 months compared with 14.76 months in the placebo-nonsteroidal AI arm (hazard ratio, 0.540; 95% CI, 0.418 to 0.698; P = .000002), and the ORR was 49.7% (95% CI, 44.3 to 55.1) in the abemaciclib arm and 37.0% (95% CI, 29.6 to 44.3) in the placebo arm (P = .005) in the intent-to-treat population.18
Safety data in the interim analysis21 revealed that the most frequent grade 3 or 4 AEs occurring in the abemaciclib and placebo arms were neutropenia (21.1% v 1.2%), diarrhea (9.5% v 1.2%), and leukopenia (7.6% v 0.6%). Diarrhea of any grade was the most frequent AE among patients who received abemaciclib (81.3% v 29.8% in the placebo arm); diarrhea was effectively managed in the majority of cases (83.8%) with abemaciclib dose modifications and antidiarrheal medications. In the final analysis,18 the safety profile was largely consistent with that of the interim analysis; the most frequent grade 3 or 4 AEs in the abemaciclib and placebo arms, respectively, remained neutropenia (23.9% v 1.2%), diarrhea (9.5% v 1.2%), and leukopenia (8.6% v 0.6%).
Goetz et al,24 using the EORTC Quality of Life Questionnaire Core 30 (QLQC30)51 and the Breast Cancer Questionnaire (BR23),61 evaluated the impact of receiving abemaciclib plus a nonsteroidal AI on global HRQoL, functioning, and symptoms. A clinically meaningful between-treatment difference was defined as a 10-point difference compared with a patient's baseline score. Analyses revealed that, with one exception, symptom and functioning scores on the EORTC QLQ-C30 or BR23 did not satisfy the threshold for clinically meaningful differences between the two treatment arms. The exception was diarrhea, for which there was both a statistically significant and clinically meaningful difference from baseline score in favor of the placebo arm of the trial (18.68 ± 1.80; P < .001). Similarly, except for diarrhea, there were no differences in time to sustained deterioration between the two treatment arms for functioning, global HRQoL, and most symptoms. Compared with patients in the placebo arm of the trial, patients who received abemaciclib plus a nonsteroidal AI reported a shorter time to deterioration for diarrhea (hazard ratio, 1.74; 95% CI, 1.25 to 2).
MONALESSA-2.
The Mammary Oncology Assessment of LEE011's (Ribociclib's) Efficacy and Safety (MONALEESA-2) trial evaluated the efficacy and safety of ribociclib plus letrozole for the first-line treatment in postmenopausal women with HR-positive, HER2-negative recurrent or MBC. Hortobagyi et al13 randomly assigned 668 patients to receive either ribociclib and letrozole (n = 334) or placebo and letrozole (n = 334). Patients had not received prior systemic therapy for ABC. The median PFS rate after the first interim analysis at a median duration of follow-up of 15.3 months in the ribociclib-letrozole group was 63.0% (95% CI 54.6 to 70.3); the median PFS rate in the placebo group was 42.2% (95% CI, 34.8 to 49.5). The PFS duration was significantly longer in the ribociclib-letrozole group versus the placebo group (hazard ratio, 0.56; 95% CI, 0.43 to 0.72; P = 3.29 × 10−6 for superiority). The median PFS after the second interim analysis at a median duration of follow-up of 26.4 months was 25.3 months (95% CI, 23.0 to 30.3) for ribociclib-letrozole and 16.0 months (95% CI, 13.4 to 18.2) for placebo-letrozole (hazard ratio, 0.568; 95% CI, 0.457 to 0.704; log-rank P = 9.63 × 10−8).
In a predefined subgroup analysis that evaluated PFS in 227 patients enrolled in MONALEESA-2 who presented with de novo ABC,30 the median PFS in the ribociclib-letrozole group was not reached compared with 16.4 months in the placebo-letrozole group. The estimated PFS rate after 12 months in the ribociclib-letrozole arm was 82% and was 66% in the placebo-letrozole arm. The PFS duration was significantly longer in the ribociclib-letrozole group versus the placebo group in this subset of patients with de novo ABC (hazard ratio, 0.45, 95% CI 0.27 to 0.75). In a separate prespecified subgroup analysis of the efficacy and safety of ribociclib-letrozole in elderly (≥ 65 years) patients enrolled in MONALEESA-2, Sonke et al31 found no significant difference in the ribociclib-letrozole PFS benefit between elderly patients (hazard ratio, 0.608; 95% CI, 0.394 to 0.937) and younger patients (hazard ratio, 0.523; 95% CI, 0.378 to 0.723).
At the first interim analysis,13 grade 3 or 4 AEs that occurred in ≥ 5% of patients in either arm were neutropenia, leukopenia, hypertension, increased ALT level, lymphopenia, and increased AST level. Grade 3 or 4 neutropenia occurred in 59.3% in the ribociclib group compared with 0.9% in the placebo group; leukopenia occurred in 21.0% versus 0.6% of patients in the ribociclib and placebo groups, respectively. Of the serious AEs observed, 7.5% in the ribociclib arm and 1.5% in the placebo arm were judged to be because of the study regimen. AE data were comparable at the updated analysis14; no new or unexpected toxicities were seen and there was no evidence of cumulative toxicity with extended follow-up. The safety profile in the study of patients in MONALEESA-2 who presented with de novo ABC30 was also comparable to that reported by Hortobagyi et al13 in 2016 for the overall population; the safety profile of ribociclib-letrozole was similar between elderly patients and younger patients in the prespecified subgroup analysis conducted by Sonke et al.31
Two articles reported on PROs from MONALEESA-2. In an exploratory analysis, Janni et al28 found that the mean reduction in the EORTC QLQ-C30–measured pain score was greater and clinically meaningful (> 5 points) in the ribociclib-letrozole group compared with the placebo-letrozole group (26% v 15%). Verma et al29 reported that on-study global health status/QoL, as measured by the EORTC QLQ-C30, were maintained from baseline and were similar across treatment arms. Time to definitive deterioration in overall HRQoL (EORTC QLQC30) was similar between arms as well (hazard ratio, 0.944; 95% CI, 0.720 to 1.237). Symptom scores were higher in general in the ribociclib-letrozole group, but average changes from the baseline assessment were lower than the minimally important difference, defined as a change of 5-10 points from baseline. Finally, the authors confirmed that ribociclib-letrozole was associated with a clinically meaningful pain reduction.
MONALESSA-7.
The double-blind phase III trial, MONALEESA-7, randomly assigned 672 premenopausal women with HR-positive, HER2-negative ABC to receive either the CDK4/6 inhibitor ribociclib and ET (tamoxifen or a nonsteroidal AI) plus ovarian suppression with goserelin (n = 335), or placebo and ET (tamoxifen or a nonsteroidal AI) plus ovarian suppression with goserelin (n = 337). The results of the primary efficacy analysis17 revealed that the addition of ribociclib to ET significantly improved PFS. In the ribociclib group, the median PFS was 23.8 months (95% CI 19.2 to not reached); the median PFS in the placebo group was 13.0 months (11.0 to 16.4; hazard ratio, 0.55, 95% CI 0.44 to 0.69; P < .0001). In a protocol-specified interim analysis of OS, Im et al12 reported that adding ribociclib to ET significantly improved OS compared with ET alone: the estimated OS at 42 months was 70.2% in the ribociclib group (95% CI, 63.5 to 76.0) and 46.0% (95% CI, 32.0 to 58.9) in the placebo group (hazard ratio for death, 0.71; 95% CI, 0.54 to 0.95; P = .00973).
Analyses of safety data for the two study arms revealed that neutropenia, leukopenia, and increased ALT level were the most common (reported in > 10% of patients) grade 3 or 4 AEs. Neutropenia occurred in 61% of patients in the ribociclib group and 4% of patients in the placebo group; leukopenia in 14% and 1%; and increased ALT in 5% and 1%. Serious AEs deemed to be because of the trial regimen occurred in 4% of patients in the ribociclib group and 3% of the patients in the placebo group. The safety profile observed in the OS analysis report was comparable.12 The most common grade 3 or 4 AEs in the ribociclib and placebo after a median of 2 years of treatment exposure were neutropenia (63.5% and 4.5%, respectively), hepatobiliary toxic effects (11% and 6.8%), and prolonged QT interval (1.8% and 1.2%). No new safety signals were evident.
Harbeck et al32 evaluated HRQoL outcomes from patient-reported data collected from patients enrolled in MONALEESA-7 using the EORTC QLQ-C30 and the EQ-5D-5L. The results indicated that HRQoL was maintained for a longer time among patients in the ribociclib-ET arm of the study. Patients treated with ribociclib-ET had a longer TTD ≥ 10% in HRQoL than patients treated with placebo-ET (hazard ratio, 0.67, 95% CI 0.52 to 0.86). The TTD ≥ 10% in pain was also longer in patients treated with ribociclib-ET compared with those treated with placebo-ET (hazard ratio, 0.65; 95% CI 0.45 to 0.92).
Clinical interpretation.
The efficacy and overall tolerability of CDK4/6 inhibitors in combination with ET have changed treatment options for patients with HR-positive MBC. Marked PFS benefits in the first-line setting in postmenopausal as well as premenopausal and perimenopausal women receiving AIs and all three CDK4/6 inhibitors, including patients with visceral disease and high risk features, as well as OS benefit in premenopausal and perimenopausal women receiving AIs and CDK4/6 inhibitors, suggest that in most patients, these combinations are the preferred first-line treatment. Survival data from the majority of first-line studies evaluating AIs in combination with CDK4/6 inhibitors are still awaited, but crossover to CDK4/6 inhibitors from placebo following disease progression may affect these results.
The MONALEESA-3 trial also evaluated fulvestrant in the first-line setting in a combined study including patients with early relapse or in the second-line setting (see full results below). However, given the efficacy data of fulvestrant in the second-line setting, the difficulty separating patients treated in the first-line setting, and the convenience of oral therapy with AIs, the Panel recommends that first-line therapy in patients either naïve to prior ET, or with recurrent disease at least 1 year from prior exposure to an AI, include an AI as the endocrine partner with CDK4/6 inhibition.
The large number of randomized trials of ET+/− CDK4/6 inhibitor therapy has allowed the US FDA to do pooled analyses of subsets of patients. The efficacy benefits of adding CDK4/6 inhibitor therapy were similar in younger (< 70 years) and older (> 70 years) women, including women > 75 years.41 However, in the analysis of older patients (≥ 75 years), there was more toxicity among women age ≥ 75 years, including greater risks of fatigue, diarrhea, neutropenia, and hepatotoxicity. Older patients were more likely to have dose reductions or treatment interruptions because of side effects. Patients > 75 years were also more likely to have decreased quality of life, with less mobility, self-care, and activity, while on CDK4/6 inhibitors than were younger patients. Clinicians and patients should be aware of the greater toxicity experience and greater risk of adverse impact on quality of life in older patients receiving CDK4/6 inhibitors, and factor that into decision making along with the documented improvement in PFS seen with this class of drugs among elderly patients with breast cancer.
Although the majority of patients appear to benefit from combination therapy, there are postmenopausal women for whom endocrine monotherapy may be the best choice for first-line therapy. This decision should be influenced by limited disease burden, long disease-free interval, patient age, patient choice, and other factors such as treatment tolerance. In this case, it is recommended that CDK4/6 inhibitors be combined with second-line ET. Optimal sequencing is an ongoing research question.
Recommendation 3.2
Fulvestrant and a CDK4/6 inhibitor should be offered to patients with progressive disease during treatment with AIs (or who develop a recurrence within 1 year of adjuvant AI therapy) with or without one line of prior chemotherapy for metastatic disease, or as first-line therapy. Treatment should be limited to those without prior exposure to CDK4/6 inhibitors in the metastatic setting (type: evidence-based, benefits outweigh harms; evidence quality: high; strength of recommendation: strong).
Literature review and analysis.
Use of fulvestrant and a CDK4/6 inhibitor in patients with progressive disease during treatment with AIs (or who develop a recurrence within 1 year of adjuvant AI therapy) with or without one line of prior chemotherapy for metastatic disease, or as first-line therapy. The systematic literature review identified 11 articles reporting the results of analyses of data from one of three large-scale phase III RCTs—PALOMA-3, MONALEESA-3, or MONARCH-2—that inform the recommendation concerning the use of fulvestrant and a CDK4/6 inhibitor in patients with progressive disease during treatment with AIs, or who develop a recurrence within 1 year of adjuvant AI therapy, either with or without one line of prior chemotherapy for metastatic disease or as first-line therapy. The results of the relevant RCTs are summarized by broad trial end point—PFS and OS; AEs; and PROs, most frequently HRQoL. The efficacy and PRO results from the individual studies are presented in the Data Supplement; data on the incidence of AEs (grade ≥ 3) from reports of the major RCTs are provided in the Data Supplement.
PALOMA-3.
The double-blind phase III trial, PALOMA-3, randomly assigned 521 patients with HR-positive, HER2-negative ABC that had relapsed or progressed during prior ET, to receive either palbociclib plus fulvestrant (n = 347) or placebo plus fulvestrant (n = 174).9 Unique to the fulvestrant combination trials, patients were eligible for PALOMA-3 who had received prior chemotherapy for metastatic disease and this represented 34% of the trial population. The median PFS at the preplanned interim analysis in the palbociclib-fulvestrant group was 9.2 months (95% CI 7.5 to not estimable); the median PFS in the placebo-fulvestrant group was 3.8 months (95% CI, 3.5 to 5.5; hazard ratio for disease progression or death, 0.42; 95% CI, 0.32 to 0.56; P < .001). This improvement in PFS with palbociclib plus fulvestrant was confirmed in an updated analysis conducted by Cristofanilli et al:7 median PFS was 9.5 months (95% CI, 9.2 to 11.0) in the palbociclib-fulvestrant group versus 4.6 months (95% CI, 3.5 to 5.6) in the placebo-fulvestrant group (hazard ratio, 0.46; 95% CI, 0.36 to 0.59). The longer PFS with palbociclib-fulvestrant was also seen in a subset analysis of the cohort of 108 premenopausal women enrolled in the trial.27 For premenopausal women who received palbociclib plus fulvestrant (n = 72), the median PFS was 9.5 months; for premenopausal women who received placebo-fulvestrant (n = 36), the median PFS was 5.6 months (hazard ratio, 0.50; 95% CI, 0.29 to 0.87).
In a prespecified analysis of OS, Turner et al10 reported that the difference in OS in the entire trial population was not statistically significant: hazard ratio for death, 0.81; 95% CI, 0.64 to 1.03; P = .09; absolute difference, 6.9 months. However, palbociclib-fulvestrant treatment resulted in longer OS than placebo-fulvestrant treatment among patients with sensitivity to prior ET. In this subpopulation, the median OS was 39.7 months (95% CI, 34.8 to 45.7) in the group treated with palbociclib-fulvestrant and 29.7 months (95% CI, 23.8 to 37.9) in the group treated with placebo-fulvestrant (hazard ratio, 0.72; 95% CI, 0.55 to 0.94; absolute difference, 10.0 months). A subsequent retrospective analysis suggested that OS benefit was seen only in those patients who had not received prior chemotherapy for metastatic disease.
In analyses of safety data from the initial PALOMA-3 trial report,9 the most common grade 3 or 4 AEs in the palbociclib-fulvestrant and placebo-fulvestrant groups were neutropenia (62.0%, v 0.6%, respectively), leukopenia (25.2% v 0.6%), anemia (2.6% v 1.7%), thrombocytopenia (2.3% v 0%), and fatigue (2.0% v 1.2%). Grade 3 or 4 neutropenia are effectively managed by dose modification.11 However, rates of febrile neutropenia were relatively low—0.6% in the palbociclib group and 0.6% in the placebo group. Serious AEs from any cause were seen in 9.6% of patients treated with palbociclib-fulvestrant and in 14.0% of the patients treated with placebo-fulvestrant. The safety profile of fulvestrant plus palbociclib was consistent in follow-up analyses after a median follow-up of 8.9 months7 and 44.8 months,10 with no new safety signals identified. Finally, the incidence of grade 3-4 AEs and serious AEs was comparable between premenopausal and postmenopausal women who were treated with palbociclib plus fulvestrant.27
Two studies that reported the results of PROs assessments used the EORTC QLQ-C30 and the EORTC QLQ-BR23 (the breast cancer module).8,9 Turner et al9 found that among patients treated with palbociclib-fulvestrant, global QoL was maintained; however, among patient treated with placebo-fulvestrant, global QoL deteriorated significantly (mean overall change from baseline in QLQ-C30 score [range, 0-100, with higher scores indicating a higher quality of life], −0.9 points v −4.0 points; P = .03). Patients in the palbociclib-fulvestrant group also demonstrated significant improvement in emotional functioning versus patients in the placebo group. Harbeck et al8 found a significant difference between the two study arms in overall global QoL scores (66.1, 95% CI, 64.5-67.7 v 63.0, 95% CI, 60.6 to 65.3; P = .0313) and in delay in QoL deterioration (median not reached; hazard ratio, 0.641; 95% CI, 0.451 to 0.910; one-sided P = .0065) that favored the palbociclib-fulvestrant group. The palbociclib-fulvestrant group also experienced a significantly greater improvement in pain from baseline assessment (−3.3; 95% CI, −5.1 to −1.5 v 2.0; 95% CI, −0.6 to 4.6; P = .0011), and this palbociclib group had significantly less deterioration for nausea or vomiting from baseline (1.7; 95% CI, 0.4 to 3.0 v 4.2; 95% CI, 2.3 to 6.1; P = .0369).
MONARCH-2.
MONARCH-2 was a double-blind, phase III trial that randomly assigned 669 women with HR-positive, HER2-negative ABC that had progressed on neoadjuvant or adjuvant ET, to abemaciclib plus fulvestrant (n = 446) or to placebo plus fulvestrant (n = 223).22 Abemaciclib-fulvestrant significantly improved PFS and ORR versus placebo-fulvestrant. The median PFS in the abemaciclib group was 16.4 months and the median PFS in the placebo groups was 9.3 months (hazard ratio, 0.553; 95% CI, 0.449 to 0.681; P = .001); the ORR in the abemaciclib group was 48.1% (95% CI, 42.6% to 53.6%) versus 21.3% (95% CI, 15.1% to 27.6%) in the placebo group. In a prespecified interim analysis of OS, median OS was 46.7 months for patients in the abemaciclib-fulvestrant arm and 37.3 months for patients in the placebo-fulvestrant arm (hazard ratio, 0.757; 95% CI, 0.606 to 0.945; P = .01). Subgroup analyses of OS revealed stronger effects in patients with visceral disease at baseline (hazard ratio, 0.675; 95% CI, 0.511 to 0.891) and in patients with primary resistance to prior ET (hazard ratio, 0.686; 95% CI, 0.451 to 1.043).
In the primary analysis,22 the most common all-grade AEs observed in the abemaciclib-fulvestrant and placebo-fulvestrant groups were diarrhea (86.4% v 24.7%, respectively), neutropenia (46.0% v 4.0%), nausea (45.1% v 22.9%), and fatigue (39.9% v 26.9%). The three most common grade 3 or 4 AEs in the abemaciclib-fulvestrant versus placebo-fulvestrant arms were neutropenia (26.5% and 1.7%), diarrhea (13.4% and 0.4%), and anemia (7.2% and 0.9%). Serious AEs likely because of study drug were occurred in 8.8% of patients who received abemaciclib and in 1.3% of patients who received placebo. The safety profile seen in the OS analysis was consistent with that in the primary analysis with no new safety signals reported.20
PROs in MONARCH-2 were assessed using the modified Brief Pain Inventory, Short Form (mBPI-sf); the EORTC QoL Core 30 (QLQ-C30); and the Breast Cancer Questionnaire (QLQ-BR23). HRQoL scores were maintained from baseline and comparable between study arms. Compared with the placebo-fulvestrant arm, patients in the abemaciclib-fulvestrant arm experienced a 4.9-month delay in pain deterioration. The abemaciclib-fulvestrant also had significantly greater time to sustained deterioration (TTSD) on the mBPI-sf and in use of analgesics (hazard ratio, 0.76, 95% CI, 0.59 to 0.98) and on the QLQ-C30 pain item (hazard ratio, 0.62; 95% CI, 0.48 to 0.79). Also, compared with the control arm, patients in the abemaciclib arm experienced a significant delayed TTSD in fatigue, pain, physical and social functioning, and nausea and vomiting. The exception was diarrhea, for which the TTSD favored the placebo-fulvestrant group (hazard ratio, 1.60; 95% CI, 1.20 to 2.10).
MONALEESA-3.
The phase III, double-blind, placebo-controlled trial, MONALEESA-3, randomly assigned 726 postmenopausal women with HR-positive, HER2-negative ABC to ribociclib plus fulvestrant (n = 484) or to placebo plus fulvestrant (n = 242).16 Patients were either treatment-naïve or had received up to one line of previous ET in the advanced treatment setting. Median PFS was significantly longer in the ribociclib-fulvestrant group compared with the placebo-fulvestrant group. In the ribociclib arm, the median PFS was 20.5 months (95% CI, 18.5 to 23.5 months); it was 12.8 months in the placebo arm (95% CI, 10.9 to 16.3 months; hazard ratio, 0.593; 95% CI, 0.480 to 0.732; P < .001). For patients treated with ribociclib-fulvestrant, the ORR was 40.9%. The ORR was 28.7% for patients treated with placebo-fulvestrant. OS data were immature at the first protocol-specified interim analysis.
The second protocol-specified analysis of OS found a significant OS benefit of treatment with ribociclib-fulvestrant over placebo-fulvestrant.15 In the ribociclib-fulvestrant group, the estimated OS at 42 months was 57.8% (95% CI, 52.0 to 63.2); in the placebo-fulvestrant group, the estimated OS was 45.9% (95% CI, 36.9 to 54.5). This is a 28% difference in the relative risk of death (hazard ratio, 0.72; 95% CI, 0.57 to 0.92; P = .00455).
Safety analyses from the primary report16 revealed two grade 3 AEs that were reported in ≥ 10% of patients in either study arm. These AEs were neutropenia (46.6% in the ribociclib arm v 0% in the placebo arm) and leukopenia (13.5% v 0%). One grade 4 AE that was reported in ≥ 5% of patients was neutropenia, which occurred in 6.8% of patients in the ribociclib group as compared to 0% of placebo group patients. Serious AEs that were deemed because of the study medication occurred in 11.2% and 2.5% of patients in the ribociclib and placebo groups, respectively. The safety analysis conducted for the second interim analysis found no new safety signals, and the safety profile was consistent with that seen in the primary report.15
Clinical interpretation.
The survival benefits seen with the addition of CDK4/6 inhibitors to fulvestrant in the chemotherapy naïve second-line setting are impressive, and along with tolerability and maintained or improved quality of life, have further solidified the role of these targeted agents in the treatment of metastatic HR-positive breast cancer. For the majority of patients, treatment with CDK4/6 inhibitors in the first-line setting is preferable, but combinations with fulvestrant may be optimal for those intolerant to AIs; for those who have developed recurrent disease within 1 year of last adjuvant AI therapy; or for those for whom single-agent ET is the preferred first-line treatment.
We learned inadvertently from these trials that prior chemotherapy affects PFS and OS in response to subsequent ET. In PALOMA-3, approximately one third of patients had received prior chemotherapy, compared with none in MONARCH-2 and MONALEESA-3. Interestingly, the PFS to fulvestrant alone was shorter in PALOMA-3 compared with the other two trials, although the impact of adding the CDK4/6 inhibitor was similar by hazard ratios across all three trials. A subset analysis also suggests that the survival impact in PALOMA-3 was limited to those patients who had not received prior chemotherapy. These data serve to further emphasize the importance of sequential ET before use of chemotherapy for the treatment of HR-positive MBC, except in situations with primary endocrine resistance or immediately life-threatening visceral disease.
Given the extensive efficacy data, there has been interest in the use of CDK4/6 inhibitors following progression on the same or different CDK4/6 inhibitor, given either alone or in combination with the same or sequential ET. To date, retrospective data suggest potential efficacy confounded by the nature of the analyses, but support future study. Several prospective randomized phase II trials are evaluating this question.
A new question is likely to arise in the near future. Recent preliminary data have demonstrated potential efficacy of the CDK4/6 inhibitor, abemaciclib, in the adjuvant high-risk setting in combination with ET.62 If these data are confirmed with longer follow-up, we will need to understand the efficacy of CDK4/6 inhibitors in the metastatic setting in patients who received adjuvant CDK4/6 inhibition, and what the optimal time from last exposure is to see efficacy in the metastatic setting. At the moment, there are no data to inform this question, and there is no current approved indication for CDK4/6 inhibitors in early-stage disease.
GAPS IN THE LITERATURE AND FUTURE RESEARCH DIRECTIONS
There are a number of gaps in our current understanding of treatment options for HR-positive MBC. Optimal sequencing, differences among CDK4/6 inhibitors, and the potential for combining ET with PARP inhibitors are all areas that remain to be investigated.
The systematic review did not identify any studies, either RCTs or prospective-retrospective studies, that investigated the use of biomarker results to inform the recommendation for use of PARP inhibitors in patients with PALB2 germline mutations and HR-positive, HER-negative MBC. Evaluating PARP inhibitors in patients with germline mutations resulting in defective DNA repair other than BRCA1 or BRCA2 is extremely challenging because of the low prevalence of these mutations; randomized trials are not feasible. The data from Tung et al,63 albeit from a single-arm, phase II trial, are quite striking in patients with germline PALB2 mutations, with 10 of 11 patients having at least some tumor shrinkage and one patient with no change in tumor size. In addition, other case reports support the efficacy of PARP inhibition in patients with germline PALB2 mutations. The original trial has been expanded to include an additional 30 patients with germline PALB2 mutations and 30 patients with somatic BRCA mutations, in whom encouraging responses were also seen.63
Treatment postprogression data from the phase III CDK4/6 inhibitor trials provide some support for sequential use of combinations such as everolimus and exemestane following progression on CDK4/6 inhibitor and ET combinations. The studies leading to approval of everolimus (and alpelisib) occurred before CDK4/6 inhibitors were available for clinical use. Retrospective studies suggest that some efficacy may be maintained with sequenced therapy, but there are no data from prospective studies.
Future research is focused on a number of areas including novel endocrine therapies, new targeted agents, and novel combinations. Novel endocrine therapies are of great interest. These include oral selective estrogen receptor downregulators, currently in phase III trials; estrogen receptor covalent antagonists; novel selective estrogen receptor modulators; and selective androgen receptor modulators. Several of these studies are focusing on patients whose tumors have specific ESR1 mutations. New targeted agents focusing on pathways known to be associated with endocrine resistance include AKT inhibitors, now in several phase III trials, as well as fibroblast growth factor receptor inhibitors, aurora kinase A inhibitors, and immune checkpoint inhibitors. Somatic ERBB2 mutations have been observed, particularly in HR-positive metastatic lobular cancers with encouraging responses seen in single-arm trials combining the oral tyrosine kinase inhibitor neratinib with fulvestrant, and more recently, trastuzumab. These studies are ongoing.
Based on preclinical data, combinations of CDK4/6 inhibitors and AKT inhibitors are being evaluated in phase III trials with the goal of delaying or preventing resistance. As noted above, an additional area for study includes continuing CDK4/6 inhibitors following progression of disease, either with a change in the inhibitor, the ET, or both.
PATIENT AND CLINICIAN COMMUNICATION
MBC presents complex and evolving treatment options as well as quality-of-life decisions for patients. Whether a patient is progressing from first-line to second-line treatment, or experiencing de novo metastatic disease, it is important that clinicians practice communication skills and tasks to optimize the patient-clinician relationship, and foster patient and clinician well-being and family well-being. See Patient-Clinician Communication: ASCO Consensus Guideline for recommendations and strategies to optimize patient-provider communication.64 For example, clinicians should:
Clearly define goals of treatment and communicate treatment options.
Check for patient understanding.
Include significant others in the conversation per patient preference.
Reassess patient goals of care, quality of life priorities, and risk tolerance.
Check for alignment with goals of treatment.
Discuss end-of-life care early in incurable illness. For example, involve palliative care teams; ask about Advanced Directive status, and encourage completion and submission to medical file.
Discuss out-of-pocket costs and indirect costs (ie, patient energy, transportation, and child care) of additional therapy.
Patient understanding is a key building block to optimizing communication. Clinicians can work with an interdisciplinary team to use the knowledge and skills of nursing, social work, and/or palliative care colleagues to support enhanced patient understanding. In addition, decision aids designed to clarify a patient's options and involve patients in treatment decisions are being studied and are potentially effective in increasing patient understanding.65,66 A recent White Paper from the Consistent Testing Terminology Working Group recommends using consistent terminology to maximize communication and understanding between patient and clinician.67 In particular, a clear definition and understanding about known outcomes such as PFS is needed to ensure patients have the needed information to participate in decision making.68
It is important to recognize barriers to effective communication. Biases (both conscious and unconscious) may exist for both clinicians and patients. Clinicians may have preconceived ideas for how many lines of therapy a patient should be willing to try, or thoughts about what a person can or cannot afford or what a person can or cannot understand.69,70 Patients may have had previous negative interactions with health care providers or the health care system. Willingness to become aware of one's biases is the first step in learning how to remove these barriers to effective communication.
OPEN COMMENT
The draft recommendations were released to the public for open comment from March 17, 2021, through March 31, 2021. Response categories of “Agree as written,” “Agree with suggested modifications,” and “Disagree, see comments” were captured for each of the seven proposed recommendations with 18 written comments received across draft recommendations. A total of 64% of the respondents (7 of 11) either agreed or agreed with slight modifications with the recommendations and 36% (4 of 11) of the respondents disagreed with selected recommendations and offered comments and suggested revisions. The Expert Panel reviewed comments from all sources and determined whether to maintain the original draft recommendations; to revise with minor language changes; or to consider major recommendation revisions. All changes were incorporated before CPGC final review and approval.
ADDITIONAL RESOURCES
Additional information including a supplements, evidence tables, and clinical tools and resources can be found at www.asco.org/breast-cancer-guidelines. Patient information is available there and at www.cancer.net.
Related ASCO Guidelines
Patient-Clinician Communication64 (http://ascopubs.org/doi/10.1200/JCO.2017.75.2311)
Chemo- and Targeted Therapy for Patients With HER2-Negative Metastatic Breast Cancer That is Either Endocrine-Pretreated or Hormone Receptor-Negative: ASCO Guideline Update71
ACKNOWLEDGMENT
The Expert Panel wishes to thank Drs Zoneddy Dayao and Bruno Ferrari, the ASCO Clinical Practice Guidelines Committee, Drs Beverly Moy and Lisa Carey, and ASCO's Nofisat Ismaila and Bryan Rumble for their thoughtful reviews and insightful comments on this guideline. The Expert Panel also thanks Brittany E. Harvey for assistance with construction of evidence summary tables and the figure, and for editorial review of earlier drafts of the guideline manuscript. The Expert Panel is grateful to Drs Miguel Gil-Gil, Shilpi Gupta, Asha Karippot, Tara Kaufmann, Jeffrey Kirshner, Lisa Law, Ingrid Mayer, Aki Morikawa, Maria Raquel Nunes, Ilana Schlam, and Peter Van Veldhuizen for participating in external review of the draft practice recommendations. Special thanks to Hope S. Rugo, MD, who served as Expert Panel Co-Chair for this update from April 2020 to April 2021.
APPENDIX
TABLE A1.
Endocrine Treatment and Targeted Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer Expert Panel Membership
TABLE A2.
Recommendation Rating Definitions
Debra L. Barton
Research Funding: Merck
Lesley J. Fallowfield
Honoraria: Voluntis, Genomic Health, NanoString Technologies, Novartis, Pfizer, MSD, Novartis, AbbVie, Clovis Oncology
Consulting or Advisory Role: Puma Biotechnology, Voluntis, AstraZeneca, Takeda, Genomic Health/Exact Sciences, Lilly, Seagen, Roche
Research Funding: Bristol Myers Squibb, Novartis, Lilly
Travel, Accommodations, Expenses: Genomic Health
Stephen R. D. Johnston
Honoraria: Pfizer, Novartis, Eisai, AstraZeneca, Roche, Lilly
Consulting or Advisory Role: Lilly, Puma Biotechnology, Novartis, Pfizer
Research Funding: Pfizer, Puma Biotechnology
Expert Testimony: Novartis
Jennifer K. Litton
Honoraria: UpToDate
Consulting or Advisory Role: Pfizer, AstraZeneca, Medivation/Pfizer, Ayala Pharmaceuticals
Speakers' Bureau: Physicans' Education Resource, UpToDate, Med Learning Group, Medscape, Prime Oncology, Clinical Care Options, Medpage
Research Funding: Genentech, Pfizer, EMD Serono, AstraZeneca, Zenith Epigenetics, Merck
Patents, Royalties, Other Intellectual Property: UptoDate
Travel, Accommodations, Expenses: Physicans' Education Resource, Med Learning Group, Medscape, Clinical Care Options
Other Relationship: Medivation/Pfizer
Praveen Vikas
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax
Research Funding: Sanofi
Rachel L. Yung
Research Funding: Novartis, Odonate Therapeutics, American Cancer Society/Pfizer
Hope S. Rugo
Honoraria: Puma Biotechnology, Mylan
Consulting or Advisory Role: Samsung
Research Funding: Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Lilly, Genentech, Merck, Immunomedics, Odonate Therapeutics, Daiichi Sankyo, Seattle Genetics, Sermonix Pharmaceuticals, AstraZeneca
Travel, Accommodations, Expenses: Pfizer, Novartis, Macrogenics, Mylan, Daiichi Sankyo, AstraZeneca Spain, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/summary
No other potential conflicts of interest were reported.
Clinical Practice Guidelines Committee approval: June 4, 2021
Reprint Requests: 2318 Mill Road, Suite 800, Alexandria, VA 22314; guidelines@asco.org
DISCLAIMER
Dr Harold J. Burstein, MD, PhD, first author on this paper, is also a Consultant Editor for Journal of Clinical Oncology.
CORRESPONDING AUTHOR
American Society of Clinical Oncology, 2318 Mill Rd, Suite 800, Alexandria, VA 22314; e-mail: guidelines@asco.org.
EDITOR'S NOTE
This ASCO Clinical Practice Guideline provides recommendations, with comprehensive review and analyses of the relevant literature for each recommendation. Additional information, including a supplement with additional evidence tables, slide sets, clinical tools and resources, and links to patient information at www.cancer.net, is available at www.asco.org/breast-cancer-guidelines.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Mark R. Somerfield
Collection and assembly of data: Mark R. Somerfield
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Endocrine Treatment and Targeted Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: ASCO Guideline Update
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Debra L. Barton
Research Funding: Merck
Lesley J. Fallowfield
Honoraria: Voluntis, Genomic Health, NanoString Technologies, Novartis, Pfizer, MSD, Novartis, AbbVie, Clovis Oncology
Consulting or Advisory Role: Puma Biotechnology, Voluntis, AstraZeneca, Takeda, Genomic Health/Exact Sciences, Lilly, Seagen, Roche
Research Funding: Bristol Myers Squibb, Novartis, Lilly
Travel, Accommodations, Expenses: Genomic Health
Stephen R. D. Johnston
Honoraria: Pfizer, Novartis, Eisai, AstraZeneca, Roche, Lilly
Consulting or Advisory Role: Lilly, Puma Biotechnology, Novartis, Pfizer
Research Funding: Pfizer, Puma Biotechnology
Expert Testimony: Novartis
Jennifer K. Litton
Honoraria: UpToDate
Consulting or Advisory Role: Pfizer, AstraZeneca, Medivation/Pfizer, Ayala Pharmaceuticals
Speakers' Bureau: Physicans' Education Resource, UpToDate, Med Learning Group, Medscape, Prime Oncology, Clinical Care Options, Medpage
Research Funding: Genentech, Pfizer, EMD Serono, AstraZeneca, Zenith Epigenetics, Merck
Patents, Royalties, Other Intellectual Property: UptoDate
Travel, Accommodations, Expenses: Physicans' Education Resource, Med Learning Group, Medscape, Clinical Care Options
Other Relationship: Medivation/Pfizer
Praveen Vikas
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax
Research Funding: Sanofi
Rachel L. Yung
Research Funding: Novartis, Odonate Therapeutics, American Cancer Society/Pfizer
Hope S. Rugo
Honoraria: Puma Biotechnology, Mylan
Consulting or Advisory Role: Samsung
Research Funding: Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Lilly, Genentech, Merck, Immunomedics, Odonate Therapeutics, Daiichi Sankyo, Seattle Genetics, Sermonix Pharmaceuticals, AstraZeneca
Travel, Accommodations, Expenses: Pfizer, Novartis, Macrogenics, Mylan, Daiichi Sankyo, AstraZeneca Spain, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/summary
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline J Clin Oncol 343069–31032016 [DOI] [PubMed] [Google Scholar]
- 2.Shojania KG, Sampson M, Ansari MT, et al. How quickly do systematic reviews go out of date? A survival analysis Ann Intern Med 147224–2332007 [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer N Engl J Med 3801929–19402019 [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer N Engl J Med 3751925–19362016 [DOI] [PubMed] [Google Scholar]
- 5.Im SA, Mukai H, Park IH, et al. Palbociclib plus letrozole as first-line therapy in postmenopausal Asian women with metastatic breast cancer: Results from the phase III, randomized PALOMA-2 study JCO Glob Oncol 51–192019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugo HS, Finn RS, Dieras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up Breast Cancer Res Treat 174719–7292019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial Lancet Oncol 17425–4392016 [DOI] [PubMed] [Google Scholar]
- 8.Harbeck N, Iyer S, Turner N, et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: Patient-reported outcomes from the PALOMA-3 trial Ann Oncol 271047–10542016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer N Engl J Med 373209–2192015 [DOI] [PubMed] [Google Scholar]
- 10.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer N Engl J Med 3791926–19362018 [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3) Oncologist 211165–11752016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer N Engl J Med 381307–3162019 [DOI] [PubMed] [Google Scholar]
- 13.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer N Engl J Med 3751738–17482016 [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer Ann Oncol 291541–15472018 [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer N Engl J Med 382514–5242020 [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3 J Clin Oncol 362465–24722018 [DOI] [PubMed] [Google Scholar]
- 17.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial Lancet Oncol 19904–9152018 [DOI] [PubMed] [Google Scholar]
- 18. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman PA, Toi M, Neven P, et al. Health-related quality of life in MONARCH 2: Abemaciclib plus fulvestrant in hormone receptor-positive, HER2-negative advanced breast cancer after endocrine therapy Oncologist 25e243–e2512020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sledge GW, Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial JAMA Oncol 6116–1242020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer J Clin Oncol 353638–36462017 [DOI] [PubMed] [Google Scholar]
- 22.Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy J Clin Oncol 352875–28842017 [DOI] [PubMed] [Google Scholar]
- 23.Andre F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1 Ann Oncol 32208–2172021 [DOI] [PubMed] [Google Scholar]
- 24.Goetz MP, Martin M, Tokunaga E, et al. Health-related quality of life in MONARCH 3: Abemaciclib plus an aromatase inhibitor as initial therapy in HR+, HER2- advanced breast cancer Oncologist 25e1346–e13542020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugo HS, Dieras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial Ann Oncol 29888–8942018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner NC, Finn RS, Martin M, et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases Ann Oncol 29669–6802018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loibl S, Turner NC, Ro J, et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results Oncologist 221028–10382017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janni W, Alba E, Bachelot T, et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2- advanced breast cancer: Tumor response and pain reduction in the phase 3 MONALEESA-2 trial Breast Cancer Res Treat 169469–4792018 [DOI] [PubMed] [Google Scholar]
- 29.Verma S, O'Shaughnessy J, Burris HA, et al. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA-2 Breast Cancer Res Treat 170535–5452018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial Breast Cancer Res Treat 168127–1342018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonke GS, Hart LL, Campone M, et al. Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial Breast Cancer Res Treat 167659–6692018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harbeck N, Franke F, Villanueva-Vazquez R, et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: Results from a phase III randomized clinical trial (MONALEESA-7) Ther Adv Med Oncol. 2020;12:1758835920943065. doi: 10.1177/1758835920943065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ding W, Li Z, Wang C, et al. The CDK4/6 inhibitor in HR-positive advanced breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e10746. doi: 10.1097/MD.0000000000010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Ma G, Li W, et al. CDK4/6 inhibitors in combination with hormone therapy for HR(+)/HER2(-) advanced breast cancer: A systematic review and meta-analysis of randomized controlled trials Clin Breast Cancer 18e943–e9532018 [DOI] [PubMed] [Google Scholar]
- 35.Li J, Fu F, Yu L, et al. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: A meta-analysis of randomized clinical trials Breast Cancer Res Treat 18021–322020 [DOI] [PubMed] [Google Scholar]
- 36.Messina C, Cattrini C, Buzzatti G, et al. CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: A systematic review and meta-analysis of randomized trials Breast Cancer Res Treat 1729–212018 [DOI] [PubMed] [Google Scholar]
- 37. Piezzo M, Chiodini P, Riemma M, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: A systematic review and meta-analysis. Int J Mol Sci. 2020;21:6400. doi: 10.3390/ijms21176400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng J, Wu J, Wang C, et al. Combination cyclin-dependent kinase 4/6 inhibitors and endocrine therapy versus endocrine monotherapy for hormonal receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: A systematic review and meta-analysis. PLoS One. 2020;15:e0233571. doi: 10.1371/journal.pone.0233571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer J Natl Cancer Inst 111419–4302019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis Lancet Oncol 21250–2602020 [DOI] [PubMed] [Google Scholar]
- 41.Howie LJ, Singh H, Bloomquist E, et al. Outcomes of older women with hormone receptor-positive, human epidermal growth factor receptor-negative metastatic breast cancer treated with a CDK4/6 inhibitor and an aromatase inhibitor: An FDA pooled analysis J Clin Oncol 373475–34832019 [DOI] [PubMed] [Google Scholar]
- 42.Rugo HS, Turner NC, Finn RS, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: A pooled analysis of randomised PALOMA clinical studies Eur J Cancer 101123–1332018 [DOI] [PubMed] [Google Scholar]
- 43.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N Engl J Med 377523–5332017 [DOI] [PubMed] [Google Scholar]
- 44.Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer Ann Oncol 30558–5662019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation N Engl J Med 379753–7632018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon RM, Paik S, Hayes DF.Use of archived specimens in evaluation of prognostic and predictive biomarkers J Natl Cancer Inst 1011446–14522009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline J Clin Oncol 332695–27042015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciruelos EM, Rugo HS, Mayer IA, et al. Patient-reported outcomes in patients with PIK3CA-mutated hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer from SOLAR-1 J Clin Oncol 392005–20152021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robson M, Ruddy KJ, Im SA, et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial Eur J Cancer 12020–302019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ettl J, Quek RGW, Lee KH, et al. Quality of life with talazoparib versus physician's choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: Patient-reported outcomes from the EMBRACA phase III trial Ann Oncol 291939–19472018 [DOI] [PubMed] [Google Scholar]
- 51.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology J Natl Cancer Inst 85365–3761993 [DOI] [PubMed] [Google Scholar]
- 52.Cleeland CS, Ryan KM.Pain assessment: Global use of the Brief pain Inventory Ann Acad Med Singap 23129–1381994 [PubMed] [Google Scholar]
- 53.Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study Lancet Oncol 22489–4982021 [DOI] [PubMed] [Google Scholar]
- 54. Rozenblit M, Mun S, Soulos P, et al. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23:14. doi: 10.1186/s13058-021-01394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes DF.Defining clinical utility of tumor biomarker tests: A clinician's viewpoint J Clin Oncol 39238–2482021 [DOI] [PubMed] [Google Scholar]
- 56.Narayan P, Prowell TM, Gao JJ, et al. FDA approval summary: Alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer Clin Cancer Res 271842–18492021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassett MJ, Somerfield MR, Baker ER, et al. Management of male breast cancer: ASCO guideline J Clin Oncol 381849–18632020 [DOI] [PubMed] [Google Scholar]
- 58. Llombart-Cussac A, Perez-Garcia JM, Bellet M, et al. PARSIFAL: A randomized, multicenter, open-label, phase II trial to evaluate palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with estrogen receptor (ER)[+]/HER2[-] metastatic breast cancer. J Clin Oncol. 2020;38 suppl; abstr 1007. [Google Scholar]
- 59.Turner NC, Swift C, Kilburn L, et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: A combined analysis of the phase III SoFEA and EFECT trials Clin Cancer Res 265172–51772020 [DOI] [PubMed] [Google Scholar]
- 60.Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial Ann Oncol 311526–15352020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality-of-Life questionnaire module: First results from a three-country field study J Clin Oncol 142756–27681996 [DOI] [PubMed] [Google Scholar]
- 62.Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol 383987–39982020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tung NM, Robson ME, Ventz S, et al. TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes J Clin Oncol 384274–42822020 [DOI] [PubMed] [Google Scholar]
- 64.Gilligan T, Coyle N, Frankel RM, et al. Patient-clinician communication: American Society of Clinical Oncology consensus guideline J Clin Oncol 353618–36322017 [DOI] [PubMed] [Google Scholar]
- 65.O'Brien MA, Whelan TJ, Villasis-Keever M, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis J Clin Oncol 27974–9852009 [DOI] [PubMed] [Google Scholar]
- 66. Herrmann A, Mansfield E, Hall AE, et al. Wilfully out of sight? A literature review on the effectiveness of cancer-related decision aids and implementation strategies. BMC Med Inform Decis Mak. 2016;16:36. doi: 10.1186/s12911-016-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.The Clearity Foundation https://www.clearityfoundation.org/wp-content/uploads/2020/07/Consistent-Testing-Terminology-Whitepaper-Final-070720.pdf
- 68.Fallowfield LJ, Catt SL, May SF, et al. Therapeutic aims of drugs offering only progression-free survival are misunderstood by patients, and oncologists may be overly optimistic about likely benefits Support Care Cancer 25237–2442017 [DOI] [PubMed] [Google Scholar]
- 69.Yanez B, Stanton AL, Maly RC.Breast cancer treatment decision making among Latinas and non-Latina Whites: A communication model predicting decisional outcomes and quality of life Health Psychol 31552–5612012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marcelin JR, Siraj DS, Victor R, et al. The impact of unconscious bias in healthcare: How to recognize and mitigate it J Infect Dis 220S62–S732019 [DOI] [PubMed] [Google Scholar]
- 71.Moy B, Rumble BR, Come SE, et al. Chemotherapy and targeted therapy for patients with HER2–negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor–negative: ASCO guideline update J Clin Oncol 393938–39582021 [DOI] [PubMed] [Google Scholar]