Abstract
Monoclonal antibody (MAb)-based capture enzyme-linked immunosorbent assays (ELISAs) for the detection of anti-arboviral immunoglobulin G (IgG ELISAs) were developed for a comprehensive array of medically important arboviruses from the Alphavirus, Flavivirus, and Bunyavirus genera. Tests were optimized and standardized so that maximum homology could be maintained among working parameters for the different viral agents, enabling a wide range of viruses to be easily tested for at one time. MAbs were screened for suitability as capture vehicles for antigens from the three genera. The final test configuration utilized group-reactive MAbs eastern equine encephalitis virus 1A4B-6, dengue 2 virus 4G2, and La Crosse encephalitis virus 10G5.4 to capture the specific inactivated viral antigens. Serum IgG was detected by using alkaline phosphatase-conjugated anti-human IgG (Fc portion). A dilution of 1:400 was chosen as the universal screening serum dilution, with endpoint titrations of serum samples testing positive eliminating occasional false-positive results. IgG ELISA results correlated with those of the standard plaque-reduction neutralization assays. As expected, some test cross-reactivity was encountered within the individual genera, and tests were interpreted within the context of these reactions. The tests were standardized for laboratory diagnosis of arboviral infections, with the intent that they be used in tandem with the corresponding IgM antibody-capture ELISAs.
The U.S. Centers for Disease Control and Prevention considers many diseases caused by arthropod-borne viruses (arboviruses) to be emerging or reemerging. Some of these diseases can be very unpredictable, such as Venezuelan equine encephalitis (VEE), which may emerge in a cyclical fashion and cause widespread disease (16). Others, such as Japanese encephalitis and dengue fever, are endemic and are expanding in certain parts of the world. Moreover, dramatic global increases in human travel and movement of arthropod vectors can result in the introduction of exotic viruses into new areas. Such an introduction occurred in 1999, resulting in an outbreak of West Nile (WN) encephalitis in the New York City area (11). All of these factors mandate that laboratories which provide diagnostic support for arboviruses have rapid and flexible serological techniques available to them. Enzyme-linked immunosorbent assay (ELISA) provides a platform capable of integrating protocols for the wide variety of arboviruses while offering well-documented advantages over more traditional serological methods such as the plaque-reduction neutralization test (PRNT), hemagglutination-inhibition test, and complement fixation test.
Previous publications have described the rapid diagnosis of individual medically important arboviral infections with ELISA (2, 3, 15). Some of these procedures have been adapted and incorporated into comprehensive sets of assays for the detection of anti-arboviral immunoglobulin M (IgM) (IgM antibody-capture [MAC]–ELISA) (14). Here we describe the development of the corresponding IgG ELISAs by using monoclonal antibodies (MAbs) as the antigen capture vehicles. In a diagnostic setting, they are intended for use in tandem with the MAC-ELISAs to produce a clear antibody profile for each specimen.
MATERIALS AND METHODS
Antigens.
Viral antigens (Table 1) were prepared as β-propiolactone-inactivated sucrose-acetone extracts by the method of Clarke and Casals (5) and were obtained from the reference collection at the Division of Vector-Borne Infectious Diseases (DVBID). Normal mouse brain antigen was used as a control in all tests.
TABLE 1.
Reagent dilutionsa used in the IgG ELISA
| Genus | Viral antigen (strain) | Dilution
|
|
|---|---|---|---|
| Antigen | Positive serum | ||
| Alphavirus | Chikungunya (S27) | 1:5,120 | 1:100 |
| EEE (NJ/60) | 1:1,280 | 1:400 | |
| Evergladesb (Fe3-7c) | 1:10 | 1:400 | |
| Mayaro (D218) | 1:160 | 1:3,200 | |
| Ross River (T48) | 1:160 | 1:200 | |
| Sindbis (EgAr 339) | 1:40 | 1:100 | |
| VEE (TC-83) | 1:120 | 1:400 | |
| WEE (Fleming) | 1:80 | 1:100 | |
| Flavivirus | DEN2c (New Guinea C) | 1:240 | 1:200 |
| Japanese encephalitis (Nakayama) | 1:320 | 1:400 | |
| MVE (Original) | 1:640 | 1:400 | |
| Powassan (64-7483) | 1:40 | 1:1,600 | |
| SLE (TBH-28) | 1:320 | 1:1,600 | |
| WN encephalitis (Eg 101) | 1:100 | 1:1,200 | |
| Yellow fever (17D) | 1:200 | 1:400 | |
| Bunyavirus | California encephalitis (BFS 283) | 1:30 | 1:400 |
| LAC (Original) | 1:20 | 1:400 | |
| Snowshoe hare (Original) | 1:40 | 1:400 | |
| Tahyna (Bardos 92) | 1:20 | 1:400 | |
The reagent dilutions pertain only to the specific lot numbers used in this study. New lots require titration to achieve optimum test performance.
Everglades is a VEE virus, subtype 2.
DEN2 viral antigen was used to represent all four DEN serotypes. The IgG ELISA is not specific enough to be able to differentiate between the serotypes.
Human sera.
Positive control and test sera were selected from the historical collection at the DVBID on the basis of a positive result in a previous serologic test (hemagglutination-inhibition, MAC-ELISA, or PRNT) to the viruses listed in Table 1. All serum specimens were heated at 56°C for 30 min to inactivate complement.
Reagents.
The following reagents were used in all the ELISAs described in this publication unless specifically noted otherwise: coating buffer (0.015 M sodium carbonate, 0.035 M sodium bicarbonate buffer, pH 9.6); blocking buffer (3% goat serum [Colorado Serum Co., Denver, Colo.] in phosphate-buffered saline, 0.1% Tween 20); rinse buffer (phosphate-buffered saline, 0.05% Tween 20); p-nitrophenyl phosphate disodium substrate (Sigma 104; Sigma Diagnostics, St. Louis, Mo.), 3 mg/ml in 1 M Tris, pH 8.0; and stopping reagent (3 M sodium hydroxide). All conjugated antibodies were either commercially available from, or custom conjugated by, Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.
Development of the IgG ELISA.
Several MAbs broadly cross-reactive with viruses of the individual genera were investigated for suitability as capture candidates for the different tests. All hybridomas originated at the DVBID unless otherwise noted. These were as follows: Flavivirus, St. Louis encephalitis (SLE) 6B6C-1 (18), dengue 2 (DEN2) D14G2415 (usually referred to as 4G2) (obtained from the American Type Culture Collection) (9), and Murray Valley encephalitis (MVE) 4A1B-9 (8); Alphavirus, western equine encephalitis (WEE) 2A2C-3 (20), eastern equine encephalitis (EEE) 1A4B-6 (21), and VEE 3A5B-1 (17); and California group viruses, La Crosse encephalitis (LAC) 10G5.4 (obtained as a gift from G. Ludwig) (13). All MAbs were used in the form of clarified ascitic fluid. By applying standard ELISA methodology (22), antigens were box titrated against the capture MAbs of the corresponding genera. The reactions were evaluated using previously titrated horseradish peroxidase-conjugated MAbs, Flavivirus SLE 6B6C-1 at 1:16,000; Alphavirus WEE 2A2C-3 at 1:5,000; and Bunyavirus LAC 10G5.4 at 1:20,000, and results were visualized by using 3,3′,5,5′-tetramethylbenzidine base substrate (TMB-ELISA; Life Technologies, Inc., Gaithersburg, Md.). Colorimetric reactions were compared to those created by box titrating a normal mouse brain antigen against the potential capture MAbs. The MAb for each genus that had the best overall ability to capture the full range of antigens required for the tests was chosen, and dilutions were optimized. Using a battery of previously tested serum samples positive for EEE, SLE, and LAC (as representative members of their genera), the relative suitability of the conjugates anti-human IgG–horseradish peroxidase, anti-human IgG (Fab)2–alkaline phosphatase (AP), and anti-human IgG (Fc)–AP was determined. Optimal concentrations of the antigens were determined via titration. Suitable positive and negative reference serum specimens were chosen from the DVBID archives, and the dilutions of positive control sera and conjugate were also optimized.
Standardization of test serum dilution.
A panel of convalescent-phase serum samples from the DVBID collection were examined to ascertain the optimal test serum dilution for use in a diagnostic setting. Each genus was represented by one virus: Alphavirus, EEE; Flavivirus, SLE; and Bunyavirus, LAC. Six positive and four negative serum specimens were used for EEE, nine positive and eight negative serum specimens were used for SLE, and six positive and five negative sera were used for LAC. The serum samples were titrated against viral and normal antigens by using twofold serial dilutions from 1:400 to 1:6,400 and a 1:1,000 dilution of goat anti-human IgG (Fc portion)–AP. The positive-to-negative (P/N) ratio for each dilution in the standardization, and subsequently in diagnostic tests, was determined as follows: arithmetic mean optical density (OD) at 405 nm of test or positive control serum reacted with viral antigen divided by arithmetic mean OD at 405 nm of normal serum reacted with viral antigen.
Configuration of the diagnostic screening IgG ELISA.
The inner 60 wells of an Immulon II 96-well microtiter plate were coated with 75 μl of capture MAb per well in coating buffer as follows: EEE 1A4B-6 at 1:10,000 for alphaviruses, DEN2 4G2 at 1:8,000 for flaviviruses, and LAC 10G5.4 at 1:8,000 for California serogroup viruses. Wells were incubated overnight at 4°C and then washed five times with 250 μl of wash buffer by using an automatic plate washer. Two hundred microliters of blocking buffer per well was applied and incubated for 30 min at room temperature. After a wash series, titrated antigens (Table 1) were diluted in wash buffer. Fifty microliters of diluted viral antigen was added to half of the wells, followed by an overnight incubation at 4°C. Normal mouse brain antigen was diluted in an identical manner and added to the wells adjacent to the viral antigens, so that each test serum, positive control serum, and negative control serum sample would be tested in triplicate on both viral and normal mouse brain antigens. Test and negative human control sera were diluted to 1:400 with wash buffer; previously titrated positive human control sera were diluted as shown in Table 1. Plates were washed, and 50 μl of serum per well was added. Each microtiter plate had space for eight test sera in addition to the control sera. After a 1-h incubation at 37°C, plates were washed, and 50 μl of goat anti-human IgG (Fc portion)–AP conjugate per well was added at dilutions of 1:2,000 for alphaviruses, 1:8,000 for flaviviruses, and 1:8,000 for California serogroup viruses in blocking buffer. Plates were incubated at 37°C for 1 h and then washed 10 times. Seventy-five microliters of Sigma 104 substrate was added to all wells. Plates were incubated at room temperature for exactly 30 min, and the reaction was stopped with 35 μl of 3 M sodium hydroxide. Absorbance at 405 nm was read with a microtiter plate reader, and P/N ratios were calculated. In order for a test to be valid, it was necessary for the P/N ratio of the positive control serum to be ≥2.0. A patient serum sample was considered IgG positive to the virus tested for when its P/N ratio was ≥2.0 and the mean OD of the patient serum reacted with viral antigen was at least twofold greater than the mean OD of the patient serum reacted with normal antigen; if the latter criterion was not met, the result was regarded as uninterpretable. A range of acceptable ODs was determined empirically for the normal serum controls for each viral antigen. If a test exhibited normal serum ODs that fell outside of this range, the test was repeated.
Test comparison and cross-reactivity.
By using the diagnostic IgG ELISA configuration, P/N ratios for clinical specimens in representative ELISAs were compared to endpoint titers yielded by PRNT. The PRNTs were performed with Vero cells as previously described for EEE (19) and for SLE and LAC (12). The following strains were used in all neutralization tests: EEE strain NJ/60, SLE strain TBH-28, and LAC strain Original. Cross-reactivities of the IgG ELISAs were determined by reacting positive control sera against multiple antigens within the genus.
Accuracy of P/N ratios compared to titrated endpoints.
A total of 142 diagnostic serum specimens were tested for antibodies to a variety of arboviral agents by using the diagnostic configuration of the IgG ELISA at the 1:400 screening dilution and by endpoint titration via a twofold dilution series, ranging from 1:400 to 1:128,000. Normal serum samples were diluted in an identical manner, and the endpoints were determined by calculating the P/N ratio of the test sera at each dilution. Specimens were chosen so that approximately equal numbers of antibody-positive and -negative serum specimens were included in the comparison for each virus tested for, according to the appropriate locality batteries (14) that were determined at the time of submission of the specimens.
RESULTS
Reagent dilutions.
In the initial stages of the IgG ELISA development, the prospective capture MAbs were box titrated against viral and negative control antigens. The antibody of choice showed the best overall ability to capture all the antigens of interest within the genus while producing a low background reaction with the normal antigen. The working dilution was chosen so that the P/N ratio in the majority of tests for each genus was maximized. By using these criteria, the following capture MAbs were chosen: Flavivirus DEN2 (strain New Guinea C) 4G2 (mixed isotypes IgG1 and IgG2a) used at a 1:8,000 dilution, Alphavirus EEE (strain NJ/60) 1A4B-6 (isotype IgG2b) used at a 1:10,000 dilution, and Bunyavirus LAC (strain Original) 10G5.4 (isotype IgG2a) used at a 1:8,000 dilution. The detection conjugate was chosen by using a similar approach. The product that provided the highest P/N ratio was goat anti-human IgG (Fc)–AP used at a 1:1,000 dilution in the development stages of the tests. This dilution was subsequently adjusted for each genus group of diagnostic test procedures after some experience with the tests had been gained.
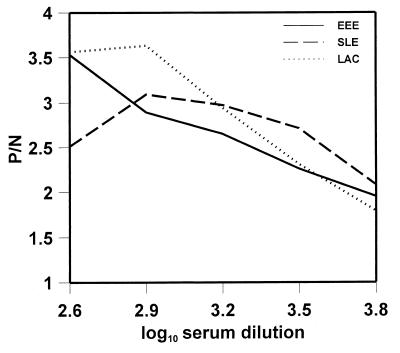
Antigen and positive control serum dilutions were determined by titration. The results for the specific lot numbers tested are listed in Table 1. Figure 1 illustrates the relationship of test serum dilution to P/N ratio for the three represented genera. The ODs of antibody-positive sera reacted on normal mouse brain antigen (background) became a significant problem at serum concentrations greater than 1:400 (data not shown). The serum dilutions that gave the best P/N ratios were 1:400 for EEE, 1:400 or 1:800 for LAC, and 1:800 for SLE. To simplify the test setup of multiple arbovirus IgG ELISAs, it was preferable to choose a single serum dilution that could be used in all the assays. The 1:400 serum dilution was chosen, as this gave acceptable P/N ratios for all tests and had the advantage of being the same serum dilution that is used in the MAC-ELISA (14). The final configuration for the diagnostic IgG ELISA was formulated from these results and is set forth in Materials and Methods.
FIG. 1.
Standardization of serum dilution for the arboviral IgG ELISAs. Antisera to EEE, SLE, and LAC were used to represent their respective viral genera. P/N ratios for each serum dilution were derived by dividing the arithmetic mean OD of the positive serum specimens reacted with viral antigen by the mean OD of the known negative serum specimens reacted with viral antigen.
Test comparison and cross-reactivity.
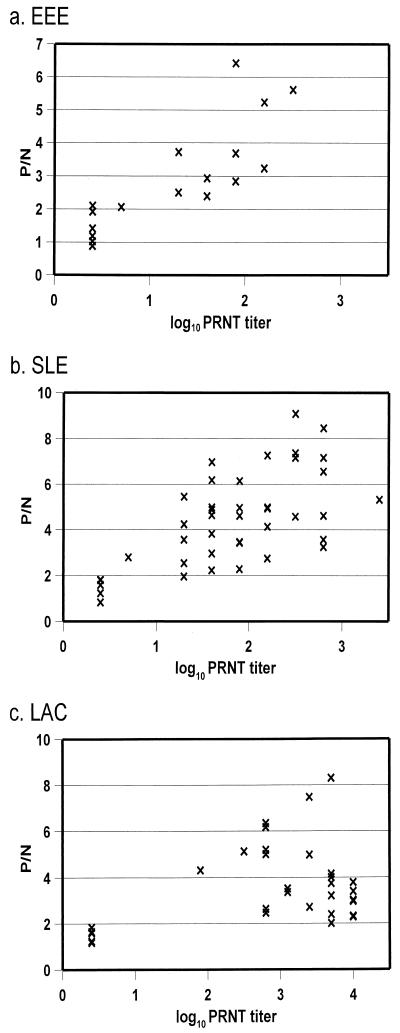
To evaluate the performance of the IgG ELISA by using patient serum at a screening dilution of 1:400, P/N ratios were compared to PRNT results. Antisera to EEE, SLE, and LAC viruses were used to represent their respective viral genera. ELISA P/N ratios versus log10 PRNT endpoint titers were represented on scatter plots (Fig. 2). The IgG ELISA results compared favorably to those of the PRNT, with a good correlation between results in most instances. The only exception occurred in the SLE tests, where a single serum tested negative in the IgG ELISA but had a positive result by PRNT. A number of LAC antisera exhibited high PRNT titers but low ELISA P/N ratios (Fig. 2c). When these serum samples were titrated against viral antigen, flat curves were obtained, indicating that, while ELISA endpoints were consistent with PRNT, the ELISA lacked sensitivity at the higher antibody levels for some sera only. Nevertheless, no false negatives occurred.
FIG. 2.
Comparison of representative IgG ELISA and PRNT results. Scatter plots are of EEE, SLE, and LAC. P/N ratios were determined at a serum dilution of 1:400. A P/N ratio of <2.0 was considered to be a negative result. The PRNT values plotted are of the log10 endpoint titers. A PRNT titer of <5 (<log10 0.7) was considered to be a negative result.
Some positive control sera were cross-reactive with multiple antigens in the IgG ELISA (data not shown). The same positive control serum was used in the DEN, WN, and MVE IgG ELISAs. In addition, the LAC positive control serum was used for all the California group virus tests. The SLE reference serum was also found to be cross-reactive in the MVE and WN tests, and the EEE serum reacted in the WEE and VEE tests. By taking advantage of these cross-reactions, the need to find homologous positive control sera for the rarer agents was mitigated.
Relative accuracy of the screening P/N ratios compared to endpoint titration results.
The IgG ELISA screening P/N ratios were determined for 142 serum samples and compared to the corresponding titration endpoint values. The majority of the negative sera that were chosen for the analysis had screening P/N ratios between 1.5 and 1.9. Samples were separated into those tested for a variety of alphaviruses, flaviviruses, and California group viruses, according to the relevant locality batteries. To the extent possible, the negative sera had originally been tested for a mix of viruses similar to those for which the positive sera were tested. Of a total of 39 sera tested for IgG to alphaviruses, 34 (87%) results were in agreement by the two methods. Four serum samples tested positive by using the single screening dilution but negative by endpoint titration, and one sample was negative by screening P/N ratio while being positive by titration. For the flavivirus tests, 67 results out of 70 (96%) were in agreement. Three samples tested positive by screening P/N ratio but were negative by endpoint titration. Of the sera tested for California group viruses, 32 results out of 33 (97%) were in agreement. One sample tested positive by screening P/N ratio while testing negative by endpoint titration.
DISCUSSION
Analysis of anti-arboviral IgG in patient sera by using the ELISA format has previously been reported (2). When inactivated antigens are applied as a coating directly onto a plate, nonspecific background reactions occur (7). To avoid this problem, IgG ELISAs have been developed as either indirect assays, in which purified virus is applied as a coating onto the microtiter plate (2), or antibody-capture methods, where the plates are coated with anti-human IgG (6). Both of these approaches have disadvantages associated with them. The use of purified live virus is undesirable, both from a safety standpoint and because the preparation of multiple purified viruses is impractical. The antibody-capture method employs inactivated viral antigen and is therefore convenient; however, the anti-human IgG captures all IgG present in the sera. IgG persists long after an infection, and therefore this method is likely to suffer from a lack of sensitivity due to competition from nonspecific IgG. Barry et al. (1) used the MAb 4G2 to capture inactivated yellow fever antigen for the detection of serum IgG. Part of the method described here is a modification of that technique. This MAb-based capture ELISA has been expanded to accommodate a wide variety of arboviruses from three different genera. It has the advantage of detecting IgG to specific antigens (or those antigens with which the sera cross-react) while avoiding many of the disadvantages that are inherent in a number of the other ELISA formats.
The majority of serum specimens are suitable candidates for analysis by ELISA, and the MAC- and IgG ELISAs are currently the initial serologic tests employed in our laboratory. The use of PRNT is now reserved for positive ELISA result confirmation, for instances where an ELISA result is masked by high background, or for cases where the high specificity of the PRNT is required. For very acute-phase serum specimens where subsequent specimens are unavailable, methods such as viral isolation or reverse transcriptase PCR (10) may be indicated, provided that a cold chain has been maintained. Immunoglobulin G to arboviruses generally rises by day 12 after onset of symptoms and may persist for years following infection. A specimen that tests positive for IgG but negative for IgM, and that was obtained <45 days after onset of symptoms, may indicate the presence of IgG to a past infection.
The IgG ELISAs were designed to be operationally efficient and to minimize the number of false-positive and -negative results. Nevertheless, the compromise with respect to choice of serum dilution may have been responsible for the occurrence of occasional incorrect results. The single false-negative result in Fig. 2b that was produced in the SLE test (log10 PRNT titer of 1.3, P/N ratio of <2) was probably caused by the slight prozone effect, as seen at the 1:400 dilution for SLE in Fig. 1. The reason for the presence of a few borderline results (P/N ratio, 2.0 to 2.5), in conjunction with high PRNT titers in the LAC IgG ELISA (Fig. 2c), is unclear. The occasional disparity observed between the ELISA and PRNT indicated the need to resolve equivocal ELISA results. The ability of the screening P/N ratios to give an accurate result is imperative: a false negative dictates that no endpoint titration is performed and the result is reported as being negative. Therefore, a bias toward false-positive screening P/N ratios is preferable compared to a false-negative bias. The combined false-positive rate of the serum samples tested by screening dilution compared to endpoint dilution shown is 5.6%. The corresponding false-negative rate is low (0.7%). This indicates that the bias leans toward false-positive result reporting when using the standard diagnostic screening serum dilution. To reduce the number of false-positive results, our laboratory now routinely performs endpoint titrations on all specimens that yield a P/N ratio of 2.0 or greater at the 1:400 screening dilution. It should be noted that the absolute P/N ratio at the screening dilution of 1:400 is not necessarily predictive of the antibody concentration in the sample. A quantitative result could be obtained only if both the normal serum control absorbance and the test sera absorbances could always be guaranteed to fall within the linear portion of an absorbance-versus-antibody concentration curve. This is not always the case with the screening IgG ELISA.
There are a number of practical considerations related to the IgG ELISA. We determined that some reagents could be diluted ahead of time and stored for future use. The capture MAb-coated microtiter plates were stable for up to 9 days when the plates were stored wet and covered at 4°C. Patient serum, positive control serum, and normal human serum could also be diluted appropriately and stored for up to 1 week at 4°C. This was not true for antigens, conjugates, or substrates. These required preparation immediately prior to use for optimum performance. For consistency of results, careful maintenance of the test schedule was important; the conjugate and substrate incubation times were the most critical.
It is well documented that arboviral IgM is more specific than IgG for arboviral antigens (23). As expected, significant cross-reactivity between antisera and related heterologous antigens was observed in the IgG ELISA. This raised the question of whether the test format could be condensed. Rather than performing individual IgG ELISAs for each virus, it might be reasonable to employ a single antigen to represent a number of viruses with which the serum cross-reacts. Limited experiments with patient sera have been performed (data not shown), which indicate the potential feasibility of this approach with groups of flaviviruses. Data suggest, however, that this method may not be suitable for alphaviruses. While this approach deserves further investigation, the current lack of multiple antisera to several of the viruses precludes the possibility of generating any statistically significant data. Therefore, the most reliable method is to continue the use of homologous antigens in the IgG ELISA.
The development of the IgG ELISA has allowed for a significant streamlining of diagnostic testing at the DVBID laboratory. Viruses from the same genera can easily be tested for by substituting only the specific viral antigens and positive serum controls in the test formats. The advantages are numerous. The rapid turnaround of results in conjunction with the MAC-ELISA and the ease of test validation because all of the necessary controls are incorporated on one plate are especially beneficial. Quick and meaningful results are obtained where large serum sets need to be evaluated, such as during an epidemic. The WN MAC- and IgG ELISAs were successfully used to confirm clinical diagnoses in the 1999 outbreak of WN encephalitis in New York. Most importantly, the MAC- and the IgG ELISAs used in tandem surpass the diagnostic capabilities of the more traditional methods, allowing complete serum antibody profiles to be derived.
ACKNOWLEDGMENTS
We thank Roy Campbell and Rebecca Deavours for their help with the preparation of the manuscript.
REFERENCES
- 1.Barry M, Patterson J E, Tirrell S, Cullen M R, Shope R E. The effect of chloroquine prophylaxis on yellow fever antibody response: comparison of plaque reduction neutralization test and enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1991;44:79–82. doi: 10.4269/ajtmh.1991.44.79. [DOI] [PubMed] [Google Scholar]
- 2.Calisher C H, Berardi V P, Muth D J, Buff E E. Specificity of immunoglobulin M and G antibody response in humans infected with eastern and western equine encephalitis viruses: application to rapid serodiagnosis. J Clin Microbiol. 1986;23:369–372. doi: 10.1128/jcm.23.2.369-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calisher C H, Pretzman C I, Muth D J, Parsons M A, Peterson E D. Serodiagnosis of La Crosse virus infections in humans by detection of immunoglobulin M class antibodies. J Clin Microbiol. 1986;23:667–671. doi: 10.1128/jcm.23.4.667-671.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Addressing emerging infectious disease threats: a prevention strategy for the United States. Morbid Mortal Weekly Rep. 1994;43:RR-5. [PubMed] [Google Scholar]
- 5.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 6.Erdman D D, Anderson L J. Monoclonal antibody-based capture enzyme immunoassays for specific immunoglobulin G (IgG), IgA, and IgM antibodies to respiratory syncytial virus. J Clin Microbiol. 1990;28:2744–2749. doi: 10.1128/jcm.28.12.2744-2749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazier C L, Shope R E. Detection of antibodies to alphaviruses by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979;10:583–585. doi: 10.1128/jcm.10.4.583-585.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkes R A, Roehrig J T, Hunt A R, Moore G A. Antigenic structure of the Murray Valley encephalitis virus E glycoprotein. J Gen Virol. 1988;69:1105–1109. doi: 10.1099/0022-1317-69-5-1105. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman B M, Summers P L, Dubois D R, Eckels K H. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1987;36:427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- 10.Lanciotti R S, Calisher C H, Gubler D J, Chang G-J, Vorndam A V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanciotti R S, Roehrig J T, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe K E, Crabtree M B, Scherret J H, Hall R A, Mackenzie J S, Cropp C B, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage H M, Stone W, McNamara T, Gubler D J. Origin of the West Nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 12.Lindsey H S, Calisher C H, Mathews J H. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol. 1976;4:503–510. doi: 10.1128/jcm.4.6.503-510.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig G V, Israel B A, Christensen B M, Yuill T M, Schultz K T. Monoclonal antibodies directed against the envelope glycoproteins of La Crosse virus. Microb Pathog. 1991;11:411–421. doi: 10.1016/0882-4010(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 14.Martin D A, Muth D A, Brown T, Johnson A J, Karabatsos N, Roehrig J T. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monath T P, Nystrom R R, Bailey R E, Calisher C H, Muth D J. Immunoglobulin M antibody capture enzyme-linked immunosorbent assay for diagnosis of St. Louis encephalitis. J Clin Microbiol. 1984;20:784–790. doi: 10.1128/jcm.20.4.784-790.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivas F, Diaz L A, Cardenas V M, Daza E, Bruzon L, Alcala A, De la Hoz O, Cacares F M, Aristizabal G, Martinez J W, Revelo D, De la Hoz F, Boshell J, Camacho T, Calderon L, Olano V A, Villarreal L I, Roselli D, Alvarez G, Ludwig G, Tsai T. Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J Infect Dis. 1997;175:828–832. doi: 10.1086/513978. [DOI] [PubMed] [Google Scholar]
- 17.Roehrig J T, Day J W, Kinney R M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology. 1982;118:269–278. doi: 10.1016/0042-6822(82)90346-4. [DOI] [PubMed] [Google Scholar]
- 18.Roehrig J T, Mathews J H, Trent D W. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology. 1983;128:118–126. doi: 10.1016/0042-6822(83)90323-9. [DOI] [PubMed] [Google Scholar]
- 19.Roehrig J T, Mathews J H. The neutralization site on the E2 glycoprotein of Venezuelan equine encephalomyelitis (TC-83) virus is composed of multiple conformationally stable epitopes. Virology. 1985;142:334–346. doi: 10.1016/0042-6822(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 20.Roehrig J T. The use of monoclonal antibodies in studies of the structural proteins of Togaviruses and Flaviviruses. In: Schlesinger S, Schlesinger M J, editors. The Togaviridae and Flaviviridae. New York, N.Y: Plenum Publishing Corp.; 1986. pp. 251–278. [Google Scholar]
- 21.Roehrig J T, Hunt A R, Chang G-J, Sheik B, Bolin R A, Tsai T F, Trent D W. Identification of monoclonal antibodies capable of differentiating antigenic varieties of eastern equine encephalitis viruses. Am J Trop Med Hyg. 1990;42:394–398. doi: 10.4269/ajtmh.1990.42.394. [DOI] [PubMed] [Google Scholar]
- 22.Tsai T F, Happ C M, Bolin R A, Montoya M, Campos E, Francy D B, Hawkes R A, Roehrig J T. Stability of St. Louis encephalitis viral antigen detected by enzyme immunoassay in infected mosquitoes. J Clin Microbiol. 1988;26:2620–2625. doi: 10.1128/jcm.26.12.2620-2625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westaway E G, Della-Porta A J, Reedman B M. Specificity of IgM and IgG antibodies after challenge with antigenically related togaviruses. J Immunol. 1974;112:656–663. [PubMed] [Google Scholar]