Abstract
Motor control requires precise temporal and spatial encoding across distinct motor centers that is refined through the repetition of learning. The recruitment of motor regions requires modulatory input to shape circuit activity. Here, we identify a role for the baso-cortical cholinergic pathway in the acquisition of a coordinated motor skill in mice. Targeted depletion of basal forebrain cholinergic neurons results in significant impairments in training on the rotarod task of coordinated movement. Cholinergic neuromodulation is required during training sessions as chemogenetic inactivation of cholinergic neurons also impairs task acquisition. Rotarod learning is known to drive refinement of corticostriatal neurons arising in both medial prefrontal cortex (mPFC) and motor cortex, and we have found that cholinergic input to both motor regions is required for task acquisition. Critically, the effects of cholinergic neuromodulation are restricted to the acquisition stage, as depletion of basal forebrain cholinergic neurons after learning does not affect task execution. Our results indicate a critical role for cholinergic neuromodulation of distant cortical motor centers during coordinated motor learning.
SIGNIFICANCE STATEMENT Acetylcholine release from basal forebrain cholinergic neuron terminals rapidly modulates neuronal excitability, circuit dynamics, and cortical coding; all processes required for processing complex sensory information, cognition, and attention. We found that depletion or transient silencing of cholinergic inputs to anatomically isolated motor areas, medial prefrontal cortex (mPFC) and motor cortex, selectively led to significant impairments on coordinated motor learning; disrupting this baso-cortical network after acquisition elicited no effect on task execution. Our results indicate a pivotal role for cholinergic neuromodulation of distant cortical motor centers during coordinated motor learning. These findings support the concept that cognitive components (such as attention) are indispensable in the adjustment of motor output and training-induced improvements in motor performance.
Keywords: acetylcholine, coordination, motor cortex, motor learning, prefrontal cortex, rotarod
Introduction
The basal forebrain is one of the principal sites of acetylcholine synthesis in the brain. Cholinergic projections arising from basal forebrain subregions innervate distinct targets, modulating functions varying from motor control, sensory and perceptual coding, attention, memory, to anxiety (Záborszky et al., 2018; Boskovic et al., 2019). Cholinergic neurons in the nucleus basalis of Meynert (NBM) have widespread projections to the cerebral cortex, while neurons in the diagonal band of Broca (DBB) and substantia innominata (SI) send projections to the prefrontal cortex (PFC; Boskovic et al., 2019). Basal forebrain cholinergic neurons are consistently activated at the onset of movement (Harrison et al., 2016), potentially modulating distant cortical motor centers. Neuromodulation requires temporal precision to modulate target area dynamics and synaptic plasticity, as well as to reinforce cognitive and reward behaviors (Metherate et al., 1992; Gil et al., 1997; Kruglikov and Rudy, 2008). Nicotinic acetylcholine signaling is required for the maturation of top-down attentional circuit development and performance on a visual detection task (Falk et al., 2021). Within humans, numerous studies have suggested a critical role for external focus of attention in skilled motor learning (Wulf, 2013; Lohse et al., 2014; Lewthwaite and Wulf, 2017; Song, 2019). External focus of attention is tightly linked to practice variability and task improvements that occur through movement revision during motor learning (Chua et al., 2019). Meanwhile, divided attention impairs task learning by reducing movement adaptations across trials (Song, 2019). The effects of external focus of attention are robust, driving motor learning both in neurotypical individuals as well as in individuals with intellectual disabilities (Chiviacowsky et al., 2013). Cholinergic innervation of cognition-related frontal cortex may be an attentional mechanism required for the acquisition of coordinated motor behavior.
Cholinergic signaling throughout cortical sensory and motor regions acts to modulate network properties and enhance glutamatergic signaling, driving long-lasting changes in synaptic strength (Rasmusson, 2000). Within primary motor cortex (M1), the maturation of motor representations, or maps, depends on basal forebrain cholinergic input (Ramanathan et al., 2015). In rats, basal forebrain cholinergic input to M1 has been found to support the plasticity of these motor maps as well as the remodeling of corticospinal neuron dendrite morphology (Conner et al., 2003, 2010; Wang et al., 2011). Across sensory modalities, the basal forebrain cholinergic system acts as a rapid and precisely timed reinforcement signal to support fast cortical activation and plasticity in associative learning (Hangya et al., 2015; Liu et al., 2015; Hanson et al., 2021). In primary auditory cortex, pairing electrical stimulation of NBM with a specific auditory stimulus leads to circuit remodeling (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998). Within S1, increased cholinergic signaling suppresses slow spontaneous activity and drives the depolarization of layer 2/3 neurons during whisking (Eggermann et al., 2014). Modulating the activity of basal forebrain cholinergic neurons alters visual and olfactory discrimination capabilities (Goard and Dan, 2009; Ma and Luo, 2012; Pinto et al., 2013). In addition to sensory coding, the cholinergic input to primary visual cortex is also required for the acquisition, but not the expression, of reward timing in the primary visual cortex (Chubykin et al., 2013; Liu et al., 2015). Cholinergic neurons also disinhibit layer 1 interneurons in auditory cortex and mediate associative fear learning (Letzkus et al., 2011). Here, we have assessed the role of cholinergic input to M1 and medial PFC (mPFC) in coordinated motor control.
In the present study, we demonstrate a key role for cholinergic basal forebrain neurons in the acquisition of coordinated motor behavior. We used targeted toxin-mediated and genetic depletion of NBM/SI cholinergic neurons and found that the acquisition of accelerating rotarod behavior was impaired. In order to test the temporal relationship of NBM/SI activity to task acquisition, we used chemogenetics to disrupt cholinergic neuron activity and we observed a similar impairment in coordinated motor performance. As rotarod task acquisition requires coordinated activity across distant motor centers, we then tested whether selectively targeting cholinergic innervation of mPFC or M1 affected performance and found that cholinergic input to both areas was required for coordinated motor learning. The effects of disrupting basal forebrain cholinergic input to the cortex was specific to the acquisition phase of rotarod behavior as we found that cholinergic depletion after training elicited no effect on task execution. Our findings demonstrate a critical role for cholinergic neuromodulation in coordinated motor performance.
Materials and Methods
Animals
All animal experiments and procedures were approved by the Weill Cornell Medicine Institutional Animal Care and Use Committee. All mice were housed on a 12/12 h light/dark cycle from 6 A.M. to 6 P.M. at 25°C with free access to food and water. For skilled pellet reach behavior, animals were calorie restricted to 80–90% of their free-feeding bodyweight. Male and female C57BL/6 animals (8–12 weeks old) were purchased from The Jackson Laboratory. ChAT-Cre mice were originally obtained from The Jackson Laboratory and bred in-house. In our study, hemizygous ChAT-Cre mice were used, which were achieved by back-crossing the homozygous ChAT-Cre line to C57BL/6 mice. In some experiments, hemizygous ChAT-Cre::Ai14 mice were used. This mouse line expresses tdTomato in ChAT-positive neurons.
Cholinergic neuron ablation by p75-saporin
Twenty-eight 10-week-old C57BL/6J mice were anesthetized with 4% isoflurane, maintained during surgery with 1.5–3% isoflurane, and had body temperature maintained at 37°C using a SomnoSuite small animal anesthesia system (Kent Scientific). Subcutaneous injection of buprenorphine (0.1 mg/kg) and meloxicam (2 mg/kg) was given immediately following anesthesia. For global ablation of cholinergic neurons in NBM/SI basal forebrain, anti-p75-conjugated saporin (p75-SAP) or IgG-saporin control (IgG-SAP, n = 8/group, Advanced Targeting Systems) was diluted to final concentration of 0.4 mg/ml in normal saline. Saporin solution (150 nl/site) was bilaterally injected into NBM/SI areas at a rate of 120 nl/min using a glass micropipette filled with mineral oil and attached to a programmable Nanoject III (Drummond Scientific). NBM/SI injection sites: (1) A/P −0.22 mm, M/L ±1.25 mm, D/V −4.7 mm; (2) A/P −0.7 mm, M/L ±1.75 mm, and D/V −4.7 mm. Following each injection, the pipette was held in place for an additional 4 min to prevent backflow. Mice were allowed to recover from surgery for two weeks before behavioral experiments.
In order to selectively ablate basal forebrain cholinergic neurons projecting to specific cortical regions, focal injections were performed in the target areas. IgG-saporin or p75-saporin was diluted to a final concentration of 0.08 mg/ml in normal saline. Saporin solution was bilaterally injected into mPFC, motor cortex, or visual cortex at a rate of 40 nl/min. The following injection volume and coordinates of injection sites were used: mPFC site 1: 200 nl at A/P +2.3 mm, M/L ±0.3 mm, D/V –1.5 mm; mPFC site 2: 200 nl at A/P +1.5 mm, M/L ± 0.3 mm, D/V −2.0 mm; motor cortex site 1: 200 nl at A/P +1.5 mm, M/L ±1.3 mm, D/V −0.6 mm; motor cortex site 2: 200 nl at A/P +0.5 mm, M/L ±1.5 mm, D/V −0.6 mm; motor cortex site 3: 200 nl at A/P −0.5 mm, M/L ±1.2 mm, D/V −0.6 mm; visual cortex site 1: 250 nl at A/P −2.8 mm, M/L ±2.3 mm, D/V −0.6 mm; and visual cortex site 2: 250 nl at A/P −3.8 mm, M/L ±2.3 mm, D/V −0.6 mm.
Cholinergic neuron ablation by diphtheria toxin (DT)
A targeted genetic approach was used to ablate basal forebrain cholinergic neurons. Sixteen eight-week-old transgenic mice expressing Cre recombinase behind the ChAT promoter crossed with Ai14 Rosa-LSL-tdTomato in the C57BL/6J background (ChAT-Cre::Ai14) were transduced with AAV encoding Cre recombinase-dependent DT receptor and the fluorescent reporter eYFP (AAV-DJ/8-EF1A-FLEX-DTR-P2A-EYFP) by bilateral injection into NBM/SI at a rate of 120 nl/min (Herman et al., 2016). AAV expressing only Cre-dependent eYFP fluorescent protein (AAV-DJ/8-EF1A-FLEX-P2A-EYFP) was used as control. NBM/SI was injected with 350 nl of virus (8.73 × 1012 VG/ml) at the following coordinates: site 1: A/P −0.1 mm, M/L ±1.25 mm, D/V −4.7 mm; site 2: A/P −0.45 mm, M/L ±1.5 mm, D/V −4.7 mm; site 3: A/P −0.8 mm, M/L ±1.75 mm, D/V −4.7 mm. The pipette was held in place for an additional 4 min. Two weeks after viral injection, DT (100 μg/kg, Sigma D0564) was intraperitoneally injected to ablate cholinergic neurons expressing DTR. Two weeks later, behavioral tests were performed.
Chemogenetic modulation of cholinergic neuron activity
In order to test the role of cholinergic neuron activity during coordinated motor performance, the designer receptor exclusively activated by designer drug (DREADD) system was used. Twenty hemizygous ChAT-Cre mice were bilaterally injected with Cre-dependent inhibitory DREADD fused with mCherry reporter AAV8-hsyn-DIO-hM4Di-mCherry (1 × 1013 VG/ml; Addgene, 44362; Krashes et al., 2011) or control virus (AAV8-hsyn-DIO-mCherry; 1 × 1013 VG/ml; Addgene, 50459) into three NBM/SI sites (350 nl/site) as with AAV-DTR. After three weeks to allow for viral expression, the DREADD ligand JHU37160 (J60; Hello Bio) was administered via intraperitoneal injection at a dose of 1.0 mg/kg 30 min before rotarod training.
Accelerating rotarod test
Accelerating rotarod is an established behavior to test motor acquisition using coordinated forelimb and hindlimb movements (Deacon, 2013). Mice were habituated on the rotarod (Ugo Basile, 47650) at a speed of 4 rpm for 60 s before testing. For each trial, the rotarod accelerated from 5 to 60 rpm over 300 s. In SAP and DT experiments, mice were trained for three trials per day over 3 d with a 30-min interval between trials. For DREADD experiments, mice were tested 30 min after intraperitoneal injection of DREADD ligand, three trials per day over 3 d without an interval between trials. In alternate rotarod training, mice were habituated at 4 rpm for 60 s on a different rotarod (Med Associates ENV-577 M), with trials accelerating from 4 to 40 rpm over 5 min. The latency to fall after the onset of acceleration during each trial was recorded for each mouse. Individual trials were stopped, and the duration was recorded if mice could not run with consecutive rotations or failed to stay on the rotarod.
Recessed forelimb reach task
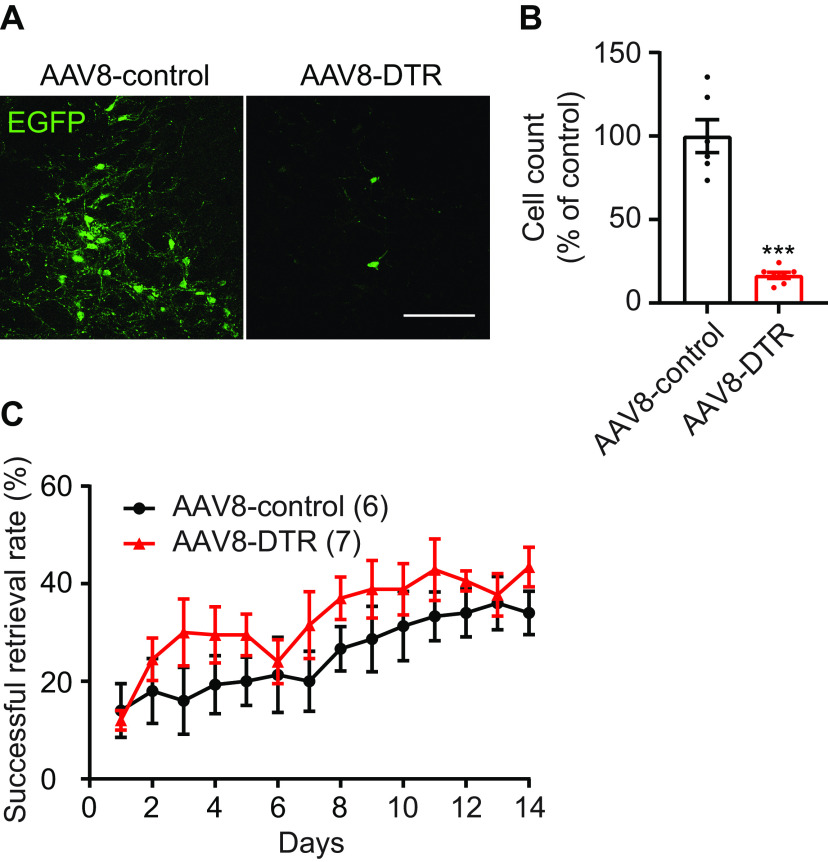
In contrast to the rotarod, single pellet reaching is a stereotyped, skilled behavior used to measure forelimb dexterity of a single forelimb (Whishaw and Pellis, 1990). Mice were food restricted to 80–90% of their free-feeding bodyweight before training. An acrylic behavior box with three slots (7 mm wide) on the left, middle, and right sides of the front wall was used to train the mice. A recessed hole (3 mm wide, 2 mm deep) at 12 mm from the inside wall of the box was used to hold a 20-mg flavored food pellet (Bioserv, F05301). The dominant forelimb for reaching was identified during one session of pellet reaching. Once the dominant forelimb was determined, it was trained over a total of 14 daily sessions consisting of 25 trials each. Mice often become sated and less likely to perform skilled reach after 25 trials. A trial was counted as a success if the mouse successfully grasped, retrieved, and ate the pellet. Only trials with pellet contact were counted. The success rate was defined as the percentage of trials with successful retrieval and eating.
Nestlet shredding
This task has been developed to test innate, repetitive, compulsive-like behaviors in mice (Angoa-Perez et al., 2013); 1.2-g nestlets were placed into each test cage and the mass of remaining intact nestlet was measured at several time points over a one-week period.
Open field test
Open field testing allows for an assessment of overall activity and anxiety levels. Mice were placed in a chamber (length × width × height: 30 × 22.5 × 25 cm) and allowed to explore for 5 min. Behavior was recorded from the top of the chamber at 48 fps (GoPro, HERO3+) and analyzed by MATLAB software (Autotyping15.04 source code; Patel et al., 2014). Thigmotaxis was defined as the percentage of time that mice spent within two inches of the arena walls.
Wire hanging test
Mice were individually placed on a wire mesh grid to test grip strength. Once the animal grasped the grid with all four paws and appeared stable, the mesh was inverted and placed atop an open chamber. The duration that the mice were able to hang from the grid was recorded. A soft blanket was placed at the bottom of the chamber to avoid animal injury. Each animal was tested three times and the longest duration was used as the animal's latency to fall.
Retrograde NBM/SI labeling
Retrograde tracing was performed in eight-week-old C57BL/6J mice (n = 4) to label basal forebrain neurons projecting to specific cortical areas. Alexa Fluor 488-conjugated, 555-conjugated, or 647-conjugated cholera toxin B subunit (CTB; 1% wt/vol, Invitrogen) was bilaterally injected to mPFC (A/P +1.9 mm, M/L ±0.3 mm, D/V −1.5 mm), motor cortex (+0.5 mm, ±1.5 mm, −0.6 mm), or visual cortex (−3.3 mm, ±2.3 mm, −0.6 mm), respectively. A burr hole was drilled over each corresponding area and 100-nl tracer was injected at each site at a rate of 40 nl/min. To prevent backflow, the pipette was left in the brain for 5 min after injection. Mice were killed one week later. Transverse brain sections were cut coronally at 40 μm of thickness. ChAT immunostaining was performed to label cholinergic neurons. Some sections were counterstained with DAPI. Sections containing injection sites were imaged at 10× on a Leica SP8 confocal microscopy with parameters adjusted based on the intensity of expression and background fluorescence (tile scan). Images containing cholinergic neurons were acquired using 10× objective.
Histology
Mice were anesthetized with ketamine/xylazine cocktail (150 mg/kg; 15 mg/kg) and transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde (PFA). Brains were harvested and postfixed in 4% PFA overnight at 4°C, cryoprotected by immersion in 30% sucrose in 0.1 m PBS for 2 d, and transversely cryosectioned (40 μm thick) using a Leica cryostat. Free floating sections were permeabilized with 0.3% Triton X-100 in PBS for 30 min at room temperature. After blocking with 10% donkey serum, sections were incubated with primary antibodies for 2 d at 4°C. Primary antibodies used: goat anti-ChAT (1:100, Millipore, AB144P), rabbit anti-p75 (1:100, Advanced Targeting Systems, AB-N01AP), rabbit anti-GFP (1:1500, Invitrogen, A6455), rabbit anti-DsRed antibodies (1:3000, Takara, 632496). Sections were washed three times in PBS, followed by incubation with fluorescently conjugated secondary antibodies (1:200, Jackson ImmunoResearch) for 1.5 h at room temperature. Nuclei were labeled with a 10 min incubation with DAPI (1 μg/ml) in PBS. Images were acquired on a Leica SP8 confocal microscope with 10× or 20× objectives under constant imaging parameters. Total CTB-labeled NBM/SI neurons in serial sections were manually counted.
Experimental design and statistical analysis
Rotarod, skilled pellet reach, and nestlet shredding behavior tests were analyzed using two-way repeated measures ANOVA with post hoc Sidak's comparison test using GraphPad Prism 9.0. The differences between two groups were compared by two-tailed t tests, if they met criteria for normality (Shapiro–Wilk test) and by Mann–Whitney test if not. Two-way ANOVA with post hoc Dunnett's multiple comparisons test was used to compare cholinergic fibers among different groups. One-way ANOVA with post hoc Dunnett's test was used to compare body mass among animals with p75-SAP injection into different cortices. All behavior and analysis were performed in a double-blinded manner. For all figures, *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Ablation of basal forebrain cholinergic neurons impairs coordinated motor training
In order to ablate basal forebrain cholinergic neurons, we injected C57BL/6J mice bilaterally with anti-p75-conjugated saporin (p75-SAP) into the NBM and SI, control mice were injected with IgG-conjugated saporin (IgG-SAP; Fig. 1A). p75-SAP is an established approach to target cholinergic neurons in the basal forebrain as they selectively express the low-affinity nerve growth factor p75NTR receptor. p75-SAP infusion resulted in nearly complete loss of NBM/SI cholinergic neurons (n = 6, two-tailed t test, p < 0.001; Fig. 1B,C). Motor coordination was tested using an accelerating rotarod, a task to test motor acquisition using coordinated forelimb and hindlimb movements (Deacon, 2013). Mice were trained over nine trials starting at 5 rpm with a constant acceleration over 5 min to 60 rpm (Fig. 1D,J). Both male and female mice injected with p75-SAP exhibited worse performance on accelerating rotarod compared with IgG-injected controls, with significantly shorter latency to fall and no effect of training on performance, indicating that this was a robust effect independent of sex differences (n = 8/treatment group, four male and four female animals per treatment group, two-way repeated measures ANOVA, p = 0.004; Fig. 1E,F). These results confirmed the findings from a pilot study in which mice trained under alternate conditions (4–40 rpm over 5 min) on a different rotarod showed impaired performance and attenuated learning following depletion of basal forebrain cholinergic neurons by p75-SAP injection, relative to IgG-SAP controls (n = 6/treatment group, two-way ANOVA, p < 0.001; Fig. 1G–I). In addition to rotarod deficits, animals with cholinergic neuron ablation showed a reduction in nestlet-shredding behavior, a measure of innate, repetitive, compulsive-like behaviors in mice (Angoa-Perez et al., 2013). Control mice quickly tear up nestlet bedding material placed in their cages while p75-SAP-injected mice showed dramatically reduced nesting behavior as determined by measuring the mass of nestlets over 7 d (two-way ANOVA, p < 0.003; Fig. 1K,L).
Figure 1.
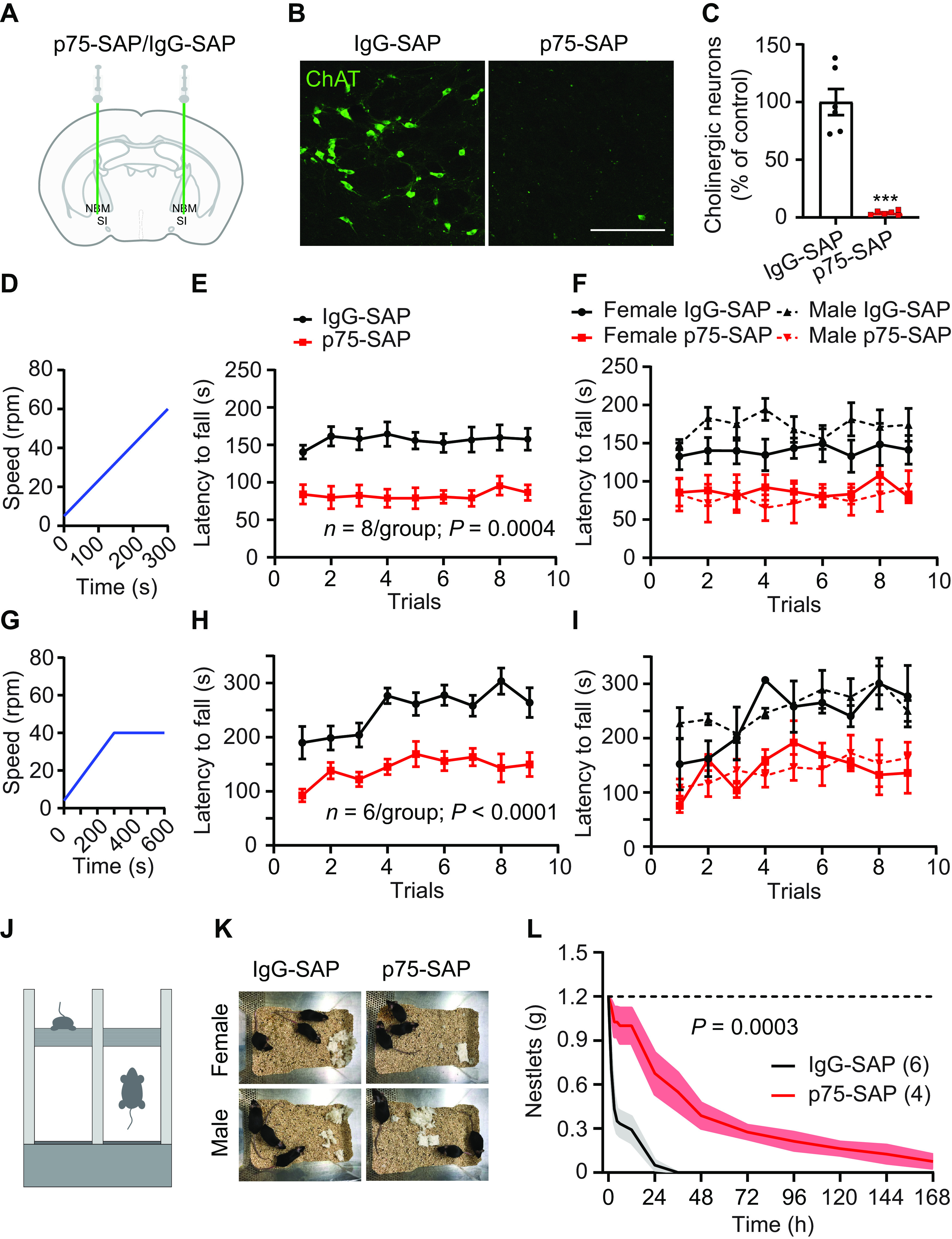
Pharmacological ablation of basal forebrain cholinergic neurons impairs coordinated motor learning. A, NBM/SI basal forebrain cholinergic neuron targeting with anti-p75-conjugated saporin or control IgG-saporin. B, NBM/SI cholinergic neurons following IgG-SAP or p75-SAP injection (scale bar: 200 μm). C, Quantification of ChAT immunostained neurons in NBM/SI basal forebrain in animals with IgG-SAP (control) or p75-SAP injection (two-tailed t test, ***p < 0.001). D–F, The training paradigm was continuous acceleration from 5 to 60 rpm over 5 min (D). Ablation of cholinergic neurons by p75-SAP severely impaired rotarod training performance (E, repeated measures ANOVA, p = 0.0004, F(1,14) = 21.31). p75-SAP effects were independent of sex (F). G–I, In an alternate rotarod training paradigm, C57BL/6J mice were trained on a Med Associates ENV-577 M rotarod with trials accelerating from 4 to 40 rpm over 5 min followed by an additional 5 min at 40 rpm (G). Ablation of cholinergic neurons by p75-SAP severely impaired alternate rotarod training performance (H, repeated-measures ANOVA, p < 0.0001, F(1,10) = 42.74). p75-SAP effects were independent of sex (I). J, Schematic of rotarod training. K, Nestlet shredding behavior in female and male mice injected with IgG-SAP (control) or p75-SAP. L, Quantification of remaining, intact nestlets over time (n is number of cages, repeated measures ANOVA, p = 0.0003, F(1,8) = 35.79). Data presented as mean ± SEM, n in parentheses is number of mice unless otherwise indicated.
Mice were also trained to perform a recessed version of the single pellet reach task over two weeks. In contrast to the rotarod, single pellet reaching is a stereotyped, skilled behavior used to measure forelimb dexterity of a single forelimb (Whishaw and Pellis, 1990). The single pellet reach task is frequently used in rat models to study motor learning (Zemmar et al., 2015; Bova et al., 2019); however, many mice fail to show an improvement on the standard pellet reach task, exhibiting an essentially flat learning curve across training (Chen et al., 2014). We therefore used a modified, recessed version of the skilled reach task in which mice retrieve a food pellet from a concave depression (Fig. 2A,B). On the standard task, the initial success rate was 27 ± 5%, while after two weeks of training this had only increased to 37 ± 4% (Fig. 2C). The resulting increase was not statistically significant (paired t test, p = 0.15). Separate mice trained on the recessed forelimb reach task had a lower initial success rate at 17 ± 3%, but a more consistent improvement, increasing to 41 ± 3% with training (paired t test, p < 0.0001). 43% of mice trained on the standard forelimb reach task failed to improve by >15% over the course of two weeks (Fig. 2D), while 93% of mice trained on the recessed forelimb reach task demonstrated improvements with training. This recessed version of the single pellet reach task allowed us to assess skilled motor learning in our study. We found that p75-SAP injection did not impair the learning of the recessed forelimb reach task, as both groups exhibited a similar improvement in performance over the course of training (Fig. 2E).
Figure 2.
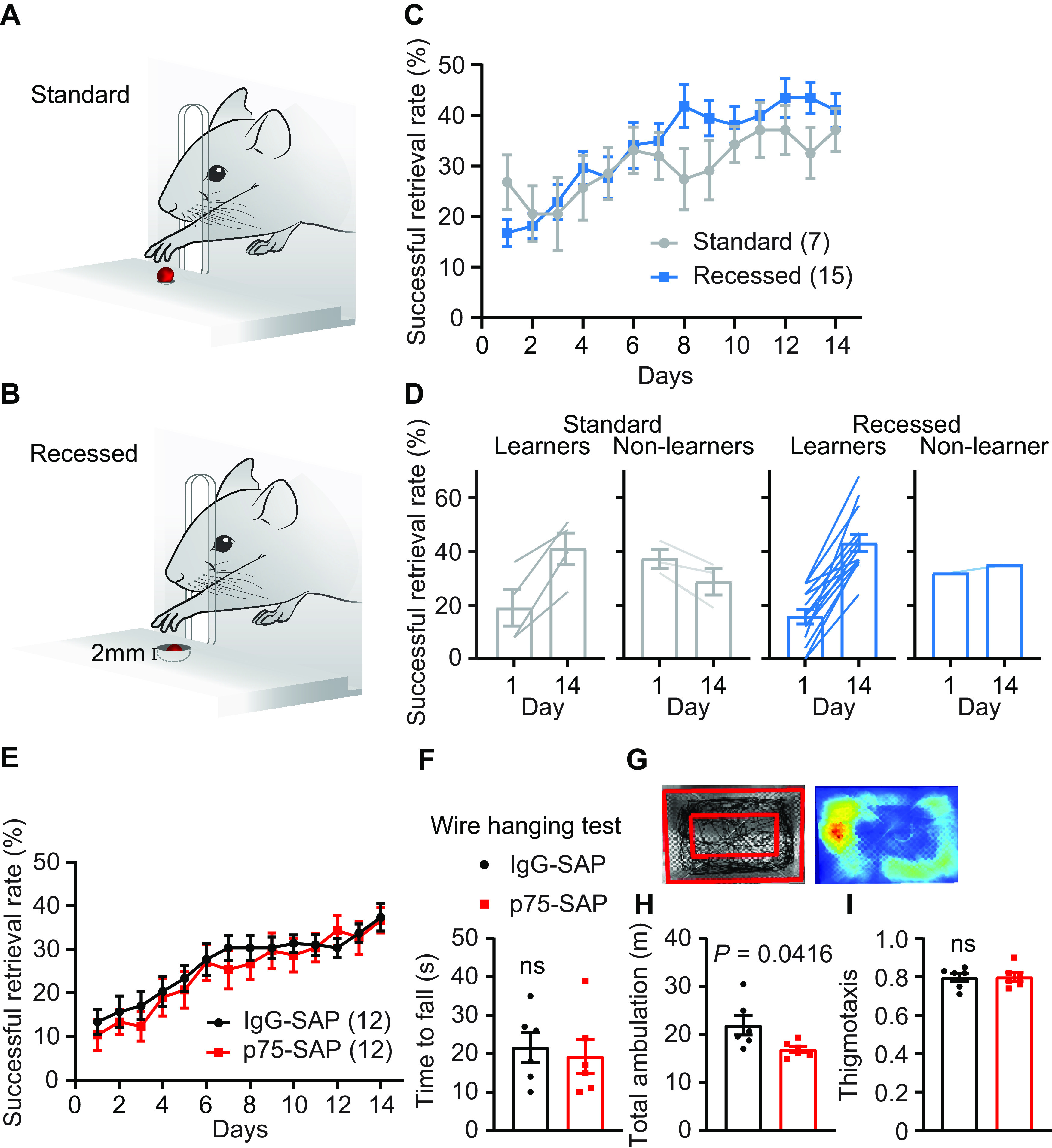
Mice exhibit more consistent learning on the recessed forelimb reach task. A, B, Illustration depicting the standard (A) and recessed (B) skilled pellet reach behavior. C, Successful retrieval rate significantly improves over two weeks of behavioral training on the recessed version compared with the standard task. D, The majority of mice exhibit learning over the course of training on the recessed version of the task (14/15), in contrast to the standard task (4/7). E, Learning of the recessed forelimb reach task was unimpaired following p75-SAP injection (repeated measures ANOVA, p > 0.05, F(1,22) = 0.2983). F, Animal strength was similar between treatment groups (two-tailed t test, p = 0.70). G, Representative sample of the open field test showing walking trace of one mouse (left panel); outer red rectangle marked the boundary of walking space. The distance between two rectangles is 2 inches. Heat map of walking trace (right panel). H, Total walking distance was shorter in mice injected with p75-SAP (two-tailed t test, p = 0.042). I, Thigmotaxis was similar between treatment groups. Data presented as mean ± SEM, n in parentheses is number of mice unless otherwise indicated.
We tested the overall health of mice after cholinergic ablation. At two weeks after saporin injection, mice injected with p75-SAP exhibited a slight but significant decrease in the body mass (pre: 21.7 ± 1.3 g; post: 19.8 ± 1.4 g; n = 8, paired t test, p = 0.002). Overall animal strength was unaffected as determined using a wire hanging test. Both groups showed a similar time to fall, regardless of gender (Fig. 2F). General activity was tested in an open field (Fig. 2G). p75-SAP-injected mice exhibited a reduced total walking distance compared with IgG-injected controls (two-tailed t test, p = 0.042; Fig. 2H). As mice navigated the open field, they largely remained close to the walls (thigmotaxis), indicative of a normal level of anxiety. No difference in thigmotaxis was observed between groups (Fig. 2I).
Genetic lesion of basal forebrain cholinergic neurons impairs coordinated motor training
In order to confirm the selectivity of p75-SAP findings, we next used a targeted genetic approach to deplete basal forebrain cholinergic neurons. ChAT-Cre::Ai14 mice were bilaterally injected into NBM/SI with AAV encoding the DT receptor in a Cre-dependent manner (AAV/DJ8-FLEX-DTR-EYFP). Control mice were injected with AAV expressing only Cre-dependent EYFP fluorescent reporter (Fig. 3A). DT injection ablated nearly all cholinergic neurons in the NBM and SI areas in mice transduced with AAV-FLEX-DTR-EYFP (Fig. 3B,C). Motor cortex and mPFC received strong cholinergic innervation from NBM/SI areas, as exhibited in mice injected with control virus (Fig. 3D). Two weeks after DT injection, AAV-DIO-DTR-EYFP mice showed a mild but significant decrease in body mass (pre: 22.4 ± 1.0 g; post: 20.1 ± 1.0 g; n = 8, paired t test, p < 0.001). DT-injected control animals showed no reduction in body mass. Similar to the effects of p75-SAP NBM/SI lesion, DT injection into AAV-DIO-DTR-EYFP-transduced ChAT-Cre::Ai14 mice resulted in severely impaired performance on the accelerating rotarod with a significantly shifted learning curve (5–60 rpm over 5 min; n = 8, ANOVA, p = 0.001; Fig. 3E,F). Furthermore, ChAT-Cre::Ai14 mice transduced with DTR virus exhibited dramatically reduced nestlet shredding behavior after DT treatment (ANOVA, p < 0.001; Fig. 3G). DT injection had no effect on the learning of the recessed forelimb reach task (Fig. 4A–C).
Figure 3.
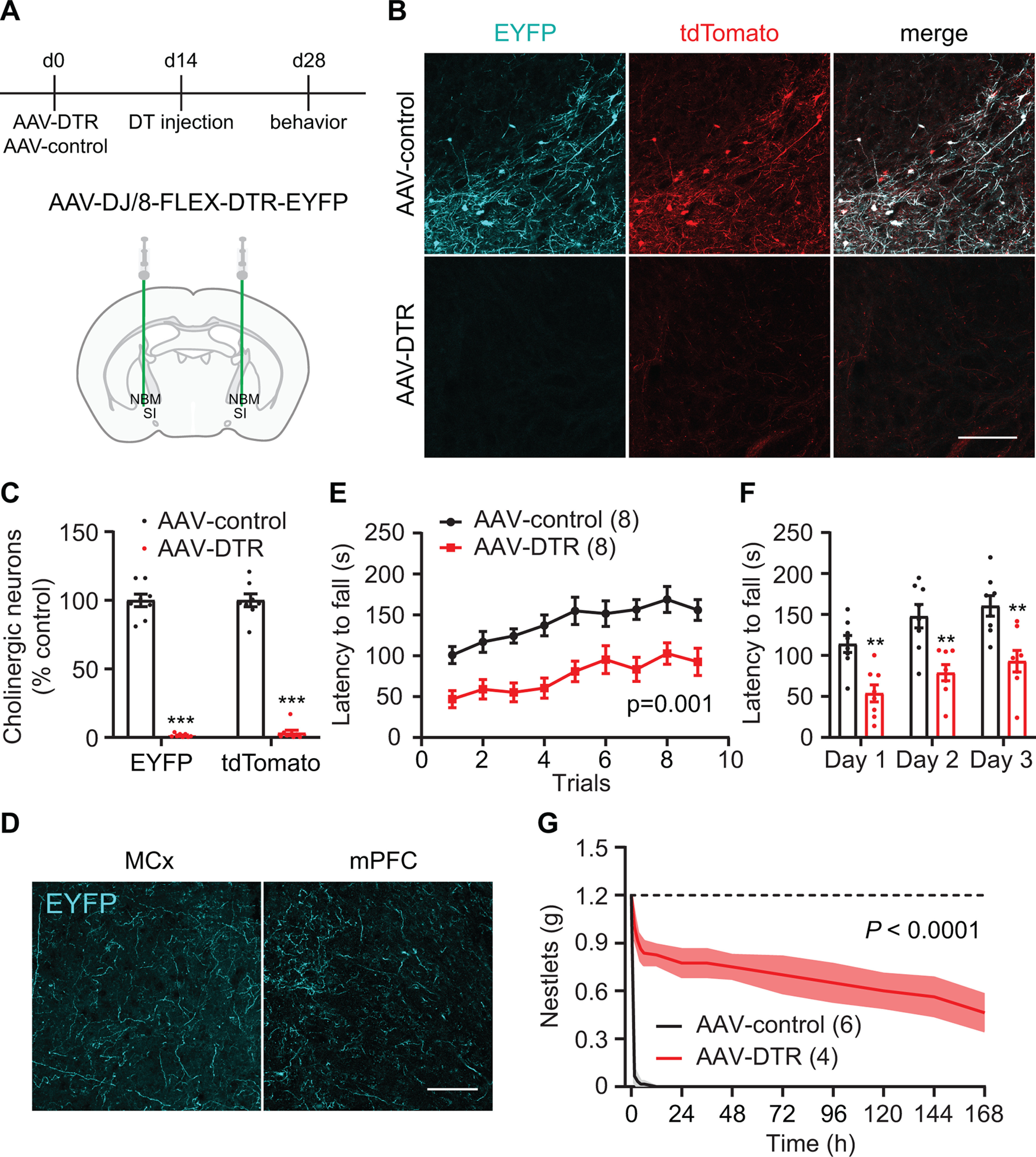
Genetic depletion of cholinergic neurons impairs coordinated motor learning. A, Timeline outlining genetic ablation of cholinergic neurons in hemizygous ChAT-Cre::Ai14 mice followed by behavior tests (top panel). NBM/SI basal forebrain cholinergic neuron transduction with AAV-DTR or control EYFP. B, NBM/SI cholinergic neurons expressing tdTomato in mice injected with AAV-FLEX-EYFP (control) or AAV-FLEX-DTR-EYFP (scale bar: 200 μm). C, Quantification of EYFP or tdTomato-positive neurons in NBM/SI basal forebrain (EYFP: two-tailed t test; tdTomato: Mann–Whitney test; ***p < 0.001). D, EYFP-labeled cholinergic fibers in motor cortex and mPFC after AAV-FLEX-EYFP transduction of cholinergic neurons in ChAT-Cre mice (scale bar: 100 μm). E, Genetic ablation of cholinergic neurons in basal forebrain severely impaired performance on rotarod training (repeated-measures ANOVA, p = 0.001, F(1,14) = 17.30). F, Rotarod latencies averaged for three trials each day (repeated measures ANOVA with post hoc Sidak's comparison test, **p < 0.01). G, Quantification of remaining, intact nestlets over time (n is number of cages, repeated measures ANOVA, p < 0.0001, F(1,8) = 117.4). Data presented as mean ± SEM, n in parentheses is number of mice unless otherwise indicated.
Figure 4.
Learning of the recessed forelimb reach task was unimpaired following DT-induced ablation of DTR-expressing cholinergic neurons. In a subset of experiments, hemizygous ChAT-Cre mice were co-injected with AAV8-syn-FLEX-DTR and AAV2-syn-FLEX-EGFP. Two weeks later, mice were injected with DT. Confocal images showed EGFP expression in mice with or without transduction of DTR virus (A). Scale bar: 200 μm. B, Quantification of the number of EGFP-positive neurons in basal forebrain of animals injected with AAV8-DTR as compared with control animals (unpaired t test, ***p < 0.001). C, Genetic ablation of cholinergic neurons did not impair learning of recessed forelimb reach task (repeated-measures ANOVA, p = 0.1429, F(1,11) = 2.49). Data presented as mean ± SEM.
Chemogenetic silencing of cholinergic neurons impairs coordinated motor training
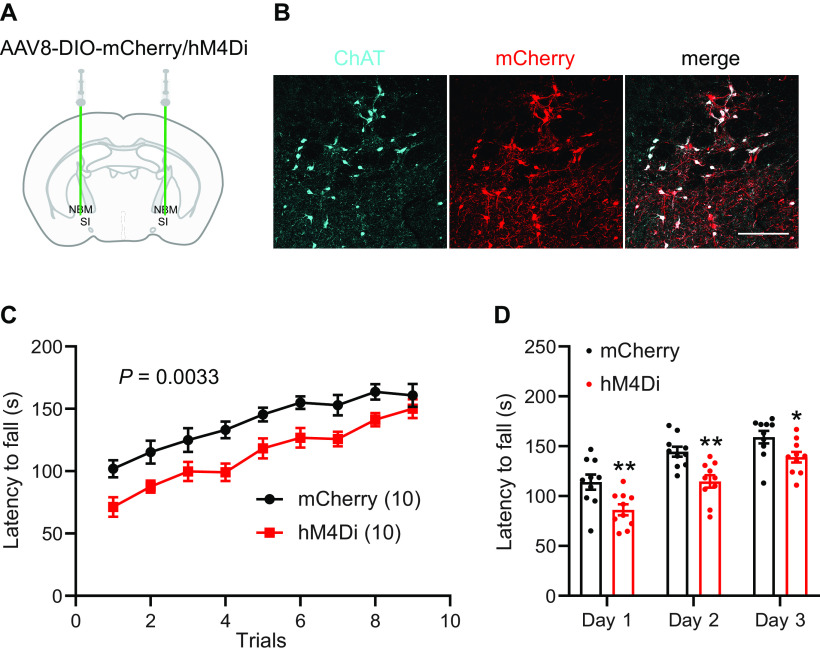
As neuron depletion is a permanent disruption of the NBM/SI circuit and did not inform us of the temporal relationship of cholinergic neuron activity to the acquisition of coordinated motor behavior, we then used the DREADD system to limit cholinergic neuron activity during behavior. ChAT-Cre mice were bilaterally injected into NBM/SI with Cre-dependent inhibitory DREADD AAV-DIO-hM4Di-mCherry or control virus (AAV-DIO-mCherry; Fig. 5A). After three weeks to allow for viral expression (Fig. 5B), rotarod training was performed 30 min after intraperitoneal injection of the DREADD ligand J60 (1 mg/kg). A total of nine trials were performed on the accelerating rotarod (5–60 rpm over 5 min). Inactivation of cholinergic neurons during behavior significantly impaired rotarod performance and shifted the learning curve (n = 10, ANOVA, p = 0.0033; Fig. 5C,D).
Figure 5.
Chemogenetic silencing of cholinergic neurons impairs coordinated motor performance. A, NBM/SI basal forebrain cholinergic neuron transduction with AAV-DIO-hM4Di-mCherry in hemizygous ChAT-Cre mice. B, hM4Di-mCherry fusion protein expression in ChAT-positive neurons (scale bar: 200 μm). C, J60 delivered 30 min before behavior severely impaired performance on rotarod training in mice expressing hM4Di in NBM/SI cholinergic neurons (repeated-measures ANOVA, p = 0.0033, F(1,18) = 11.44). D, Rotarod latencies averaged for three trials each day (repeated measures ANOVA with post hoc Sidak's comparison test, *p < 0.05, **p < 0.01). Data presented as mean ± SEM.
Cholinergic projections to both motor cortex and mPFC are required for coordinated motor training
Basal forebrain cholinergic neurons project extensively throughout the cortex; therefore, we selectively targeted cholinergic inputs to distinct cortical areas to determine the target locus of acetylcholine action during coordinated motor learning. We injected p75-SAP directly into motor areas required for coordinated motor learning, mPFC and motor cortex, as well as into visual cortex, which is not anticipated to be involved in the task (Fig. 6A–C). Control animals were injected with IgG-SAP into mPFC, motor, or visual cortices. IgG-SAP injection into different targets had no effect on rotarod behavior and data from these animals were pooled. Two weeks after SAP injections, mice were trained on the accelerating rotarod. Depletion of cholinergic input to both mPFC and motor cortex produced significant deficits in rotarod performance, compared with control IgG-SAP-injected animals (ANOVA, p = 0.0011; Fig. 6D,E). Visual cortex injection of p75-SAP had mild but not significant effects on behavior. Injection of p75-SAP into mPFC or motor cortex had no impact on body weight (IgG-SAP, 27.2 ± 0.5, n = 6; p75-SAP mPFC, 29.2 ± 1.0, n = 5; motor cortex, 27.1 ± 0.6, n = 5; one-way ANOVA, p = 0.122, F(2,13) = 2.485). These data indicate that cholinergic basal forebrain neurons regulate motor coordination through modulation of both primary and associative motor circuits (Fig. 6C).
Figure 6.
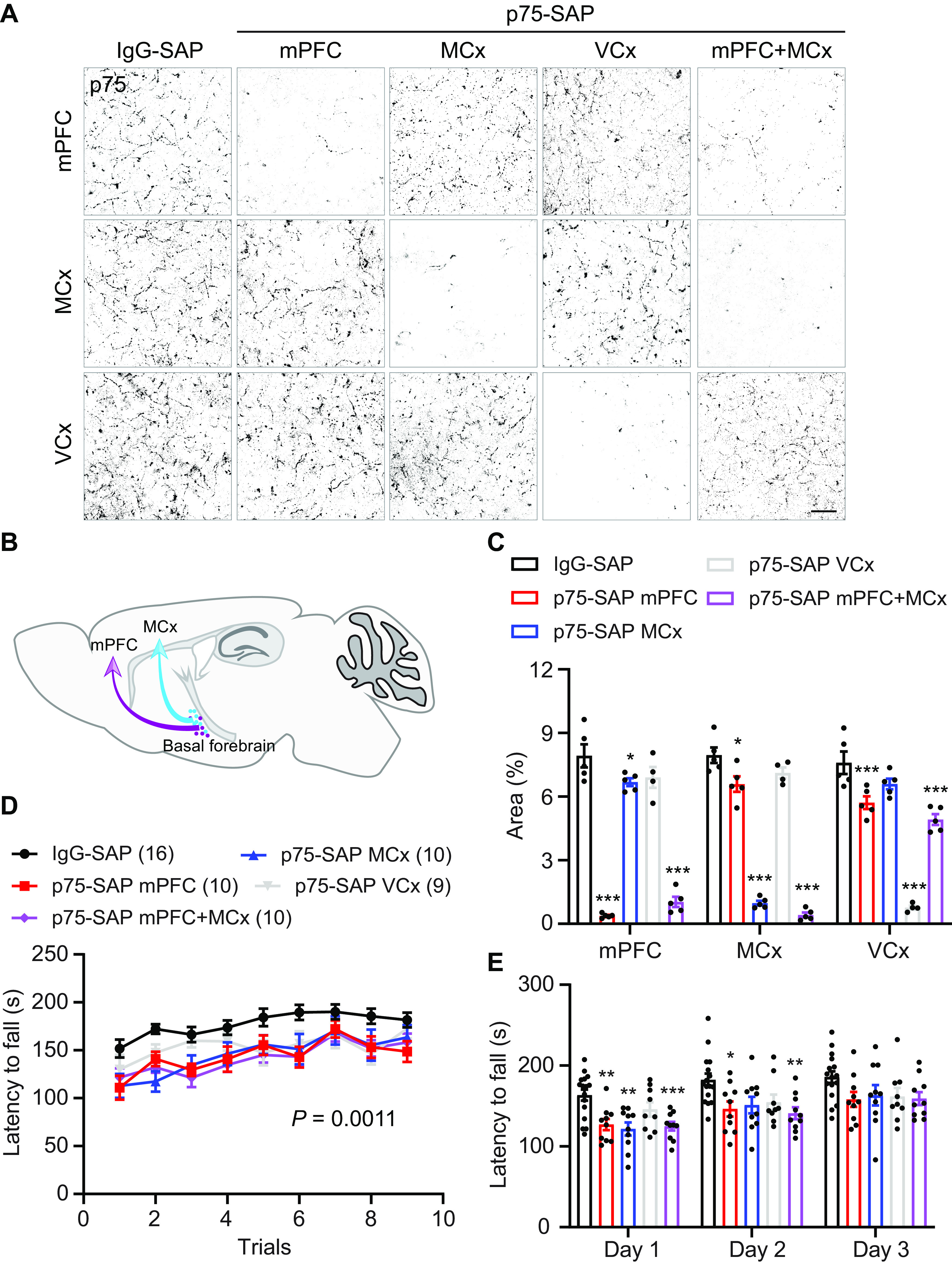
Cholinergic inputs to both mPFC and motor cortex (MCx) are required for coordinated motor training. A, Cholinergic innervation in mPFC, MCx, and visual cortex (VCx) after selective targeting with IgG-SAP or p75-SAP (scale bar: 20 μm). B, Schematic illustrating cholinergic innervation of distant motor centers involved in coordinated motor learning. C, Quantification of p75-positive cholinergic fibers in mPFC, MCx, and VCx in response to selective ablation of cholinergic inputs (two-way ANOVA with post hoc Dunnett's multiple comparisons test, *p < 0.05, ***p < 0.01). D, Depletion of cholinergic inputs to either mPFC, MCx, or both mPFC and MCx significantly impaired performance on rotarod behavior training. Depletion of cholinergic input to VCx had a partial impact on rotarod performance (repeated-measures ANOVA, p = 0.0011, F(4,50) = 5.382.). E, Rotarod latencies averaged for three trials each day (repeated measures ANOVA with post hoc Sidak's comparison test; *p < 0.05, **p < 0.01, ***p < 0.001). Data presented as mean ± SEM.
In order to determine whether collateral branches of NBM/SI neurons innervate the anatomically isolated regions tested above, we injected unique fluorescently conjugated retrograde tracer CTB into mPFC (488 nm), motor cortex (555 nm), and visual cortex (647 nm; Fig. 7A,B). Overall, 58% of labeled neurons in basal forebrain were ChAT-positive. The majority of CTB-labeled basal forebrain input to motor and visual cortices were from cholinergic neurons at 71.2 ± 6.1 and 57.2 ± 2.0%, respectively. In mPFC, the percentage of cholinergic neurons was 49.4 ± 1.4% (Fig. 7C). Retrogradely labeled, noncholinergic, basal forebrain neurons include GABAergic or glutamatergic neurons (Gritti et al., 2003; Henny and Jones, 2008; Kim et al., 2015). The majority of cholinergic neurons (84.4 ± 2.0%) were labeled with a single CTB tracer, indicating that there was no overlap in their axonal projections within the labeled regions (Fig. 7D,E). Although the sites of retrograde tracer injection were distal to each other, we did observe overlapping labeling of a small population of basal forebrain cholinergic neurons. Double-labeled neurons projecting to mPFC and motor cortex represented 4.1 ± 1.4% of NBM/SI cholinergic neurons, motor and visual cortices 3.6 ± 1.4%, and mPFC and visual cortex 5.9 ± 2.4%. Cholinergic neurons labeled by three colors of CTB were observed rarely (2.1 ± 1.5%). The shared innervation may, at least in part, explain the mild effects of cholinergic input to visual cortex on rotarod training.
Figure 7.
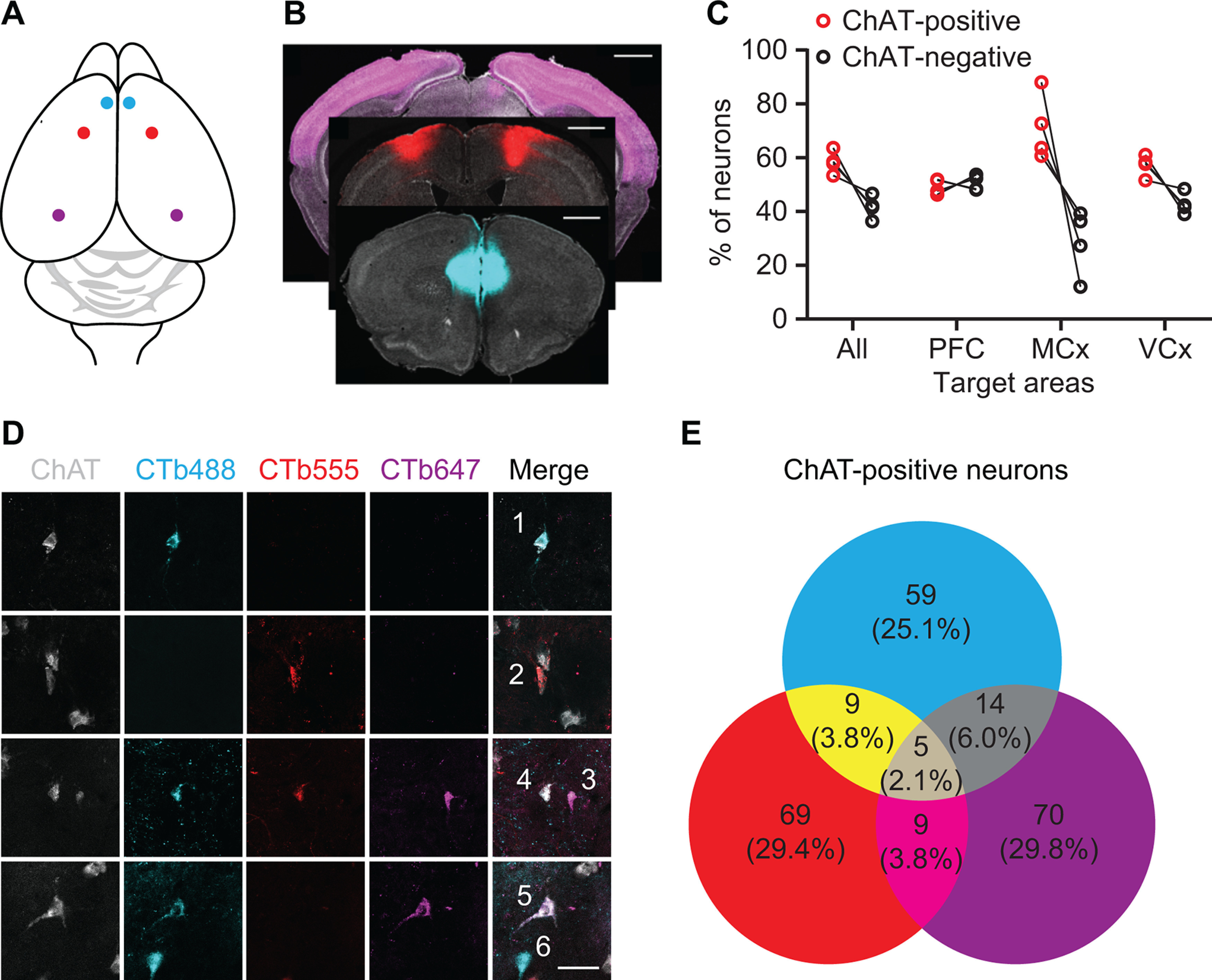
Retrograde labeling of NBM/SI cholinergic projections to cortex. A, Illustration of retrograde tracing strategy: CTB conjugated to Alexa Fluor 488, 555, or 647 was bilaterally injected to mPFC (cyan), motor cortex (red), or visual cortex (purple), respectively. B, CTB injection sites: mPFC (cyan), motor cortex (red), and visual cortex (purple). Sections were stained with DAPI (gray; scale bar: 1 mm). C, Quantification of cholinergic and noncholinergic neurons projecting to cerebral cortices. D, Retrograde labeling of NBM/SI with CTB conjugates. Neurons 1, 2, and 3 projected to one of these three cortices. Neuron 4 projected to mPFC and motor cortex. Neuron 5 projected to mPFC and visual cortex. Neuron 6 is noncholinergic (scale bar: 40 μm). E, Quantification of total CTB-labeled cholinergic NBM/SI neurons.
Basal forebrain cholinergic neurons are not required for execution of previously trained coordinated motor behavior
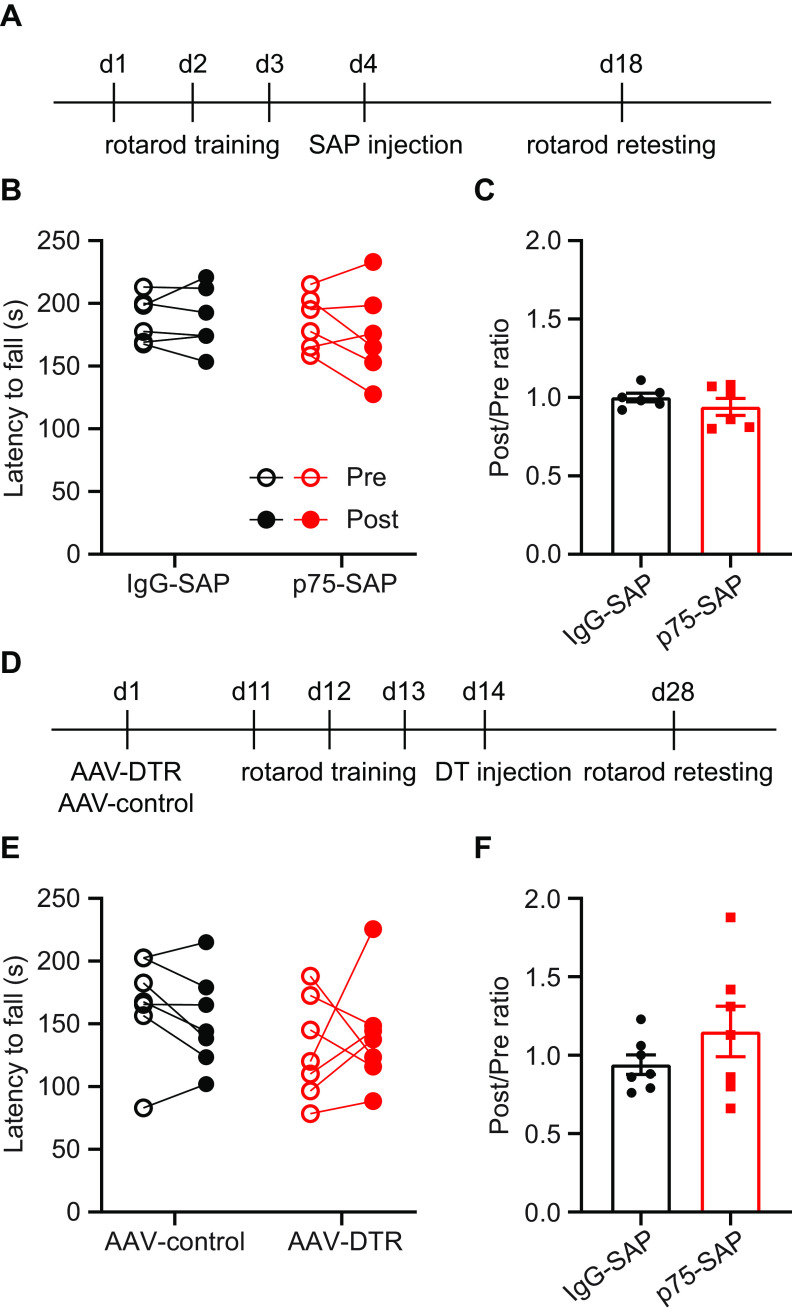
To determine whether basal forebrain cholinergic neurons are simply required for the execution rotarod behavior, rather than for the learning of the coordinated motor task, we trained intact mice before NBM/SI cholinergic neuron ablation. Mice were first trained on the accelerating rotarod for nine trials and then randomly assigned to treatment with either p75-SAP or control IgG-SAP injection into NBM/SI. Two weeks later, the retention of trained rotarod behavior was tested (Fig. 8A). Execution of the previously trained rotarod behavior was unaffected in both groups, with comparable fall latencies before and after SAP injection (Fig. 8B,C). Next, we used a genetic approach to test the effects of cholinergic ablation on coordinated motor task retention. ChAT-Cre transgenic mice were injected with AAV-DIO-DTR-EYFP or control virus. Ten days later, mice were trained through nine trials on the accelerating rotarod before intraperitoneal injection of DT to ablate cholinergic neurons (Fig. 8D). DTR and EYFP control expressing mice exhibited similar rotarod performance both before and after DT injection (Fig. 8E,F). These results demonstrate that basal forebrain cholinergic signaling is not needed for the execution of previously learned coordinated motor behavior.
Figure 8.
Basal forebrain cholinergic neurons are not required for execution of previously learned coordinated motor behavior. A, Timeline outlining experimental details of cholinergic ablation by p75-SAP following rotarod training. Retention testing was performed two weeks after saporin injection. B, Pharmacological ablation of cholinergic neurons did not affect the performance on the previously learned rotarod behavior (paired t tests, p > 0.05 for both groups). C, The ratio of latencies to fall before and after cholinergic ablation (post/pre; two-tailed t test, p = 0.34). D, Timeline outlining experimental details of cholinergic ablation by AAV-DTR following rotarod training. Rotarod retention testing was performed two weeks after DT injection. E, Genetic ablation of cholinergic neurons did not affect the performance on the previously learned rotarod behavior (paired t tests, p > 0.05 for both groups). F, The ratio of latencies to fall before and after DT-induced cholinergic ablation (post/pre; two-tailed t test, p = 0.24). Data presented as mean ± SEM.
Discussion
Our findings in this study demonstrate that basal forebrain cholinergic neurons exert control over coordinated motor learning. We used both traditional and genetic deletion methods to spatially target and ablate these neurons as well as chemogenetic approaches to specifically inhibit activity during behavior, without the potential inflammatory effects of ablation. Ablation or inhibition of these cholinergic neurons impairs coordinated task acquisition, dramatically reducing performance and shifting the motor learning curve. This down-shift in learning may represent an attenuated process of strengthening motor circuits in the absence of refinement of motor networks by cholinergic input, or it may owe to compensatory mechanisms in the absence of cholinergic input. Importantly, the loss of cholinergic input does not affect execution of previously learned coordinated behavior, demonstrating that depletion of cholinergic neurons does not affect the function of the underlying motor circuits. This is consistent with the role for cholinergic neuromodulation in the training of forelimb motor behaviors, which have demonstrated a dissociation between M1 and movement execution. On simple tasks, M1 appears to be dispensable as complete ablation does not alter trained movements (Kawai et al., 2015); however, more dexterous motor tasks require M1 activity for the appropriate execution of fine motor control (Lemke et al., 2019). Modulated motor control arises during skilled training with M1 and dorsolateral striatum (DLS) contributing to gross movement, while only M1 inactivation disrupts fine movements (Lemke et al., 2019). Neuromodulation of M1, but not mPFC, via cholinergic input from basal forebrain has been shown to be a key mechanism that supports the cortical plasticity that occurs during skilled motor learning in the rat (Conner et al., 2010). Acetylcholine release rapidly modulates neuronal excitability, circuit dynamics, and cortical coding; all processes required for processing complex sensory information, cognition, and attention (Metherate et al., 1992; Goard and Dan, 2009; Ballinger et al., 2016; Villano et al., 2017). Our study raises the possibility that baso-cortical cholinergic input could be a mechanism to rapidly modulate the active state of motor networks during coordinated motor learning.
The learning of coordinated motor behavior requires recruitment of distinct motor centers throughout the central nervous system. Coordinated task acquisition drives dynamic patterns of neuronal activity and synaptic plasticity in the cortex and striatum. Within the striatum, there is a progressive shift in regional influence, with dorsomedial striatum (DMS) critical in early stages, followed by a shift to DLS later in task acquisition (Yin et al., 2009). Coherence develops within corticostriatal motor networks with precise timing of M1 and DLS activity in support of motor performance across learning (Koralek et al., 2012, 2013; Lemke et al., 2019). During accelerating rotarod training, associative mPFC connections to DMS are recruited concurrently alongside motor circuits projecting from M1 to DLS (Kupferschmidt et al., 2017). Early in learning, mPFC-DMS and M1-DLS circuits show parallel activation, while these patterns diverge as animals master the motor skill (Kupferschmidt et al., 2017). Cholinergic neuromodulation of cortical networks shape circuit plasticity and the widespread projections of the basal forebrain cholinergic system may provide a circuit for modulating distant motor centers during coordinated motor learning.
In addition to mPFC and sensorimotor cortex, learned motor movements rely on the coordinated output of thalamus, basal ganglia, cerebellum, and brainstem motor centers. Training drives a shift in the contributions of cortical and subcortical motor circuits, with an early instructional role for motor cortex supporting the development of independent movement initiation by the basal ganglia (Kawai et al., 2015). Indeed, the contribution of the motor cortex to learned movements appears to decline over time with continued training (Hwang et al., 2019). We demonstrate clearly that basal forebrain cholinergic signaling is critical for the early instructional phase of coordinated motor learning. As we found that cholinergic inputs to both mPFC and motor cortex are required for coordinated motor acquisition, it is likely that cholinergic neuromodulation plays a critical role in the coactivation of mPFC-DMS and M1-DLS circuits during early stage rotarod learning (Kupferschmidt et al., 2017). As training progresses and instructional input from cortical projections is no longer driving experience-dependent refinement of striatal motor circuits (Wolff et al., 2019), cholinergic neuromodulation of cortical motor centers may no longer be required. This could be why ablation of cholinergic neurons after rotarod training elicited no effect on performance of the previously trained task.
Basal forebrain cholinergic input to M1 has been shown to be a key modulator of the cortical plasticity mechanisms that underlie skilled forelimb reach training in rats (Conner et al., 2003). Several lines of evidence implicate cholinergic neuromodulation in the large-scale cortical changes that occur following injury or motor learning in other species (Juliano et al., 1991; Conner et al., 2005). While we did not assess cortical reorganization in our studies, our data showed that skilled motor learning on a recessed version of the single pellet reach task was unaffected following cholinergic ablation in mice. These results may owe to species differences in the refinement of cortical mechanisms, or perhaps to differences in the acetylcholine-dependent development of cortical motor representations (Ramanathan et al., 2015). In fact, mice often fail to demonstrate the learning curves exhibited by rats in the standard single pellet reach task used in earlier studies. Typically a large percentage of mice will exhibit a high proficiency on the task on the first days of training and show no subsequent improvement, or even a decline in performance over time (Fig. 2; Chen et al., 2014). The modified, recessed forelimb reach task appears to be more difficult for mice, and reliably results in performance improvements with training. Another critical difference observed was that our studies showed a role for mPFC cholinergic inputs in coordinated motor task acquisition, while p75-SAP targeting of mPFC had no effect on forelimb reach training in rats (Conner et al., 2010). It may be that cholinergic input is required for the refinement of activity across mPFC and M1 that others have shown to occur during rotarod training (Kupferschmidt et al., 2017) and that rotarod behavior requiring forelimb-hindlimb coordination and postural control requires a distinct set of motor circuits than forelimb reach training, which involves the use of an isolated forelimb during learning of the stereotyped forelimb reach movement requires. Whether there are specific effects of basal forebrain circuits on distinct motor circuits that differentially regulate the acquisition of coordinated and skilled tasks in mice is a critical question that requires further study.
Beyond the effects on cortical plasticity, acetylcholine signaling plays a key role in cognitive processes by mediating attention (Thiele and Bellgrove, 2018; Sarter and Lustig, 2019). Within the visual cortex, attention drives increased firing rates via acetylcholine signaling through muscarinic receptors in nonhuman primates (Herrero et al., 2008). The central role of attention in the acquisition of motor skills supports the idea that cognitive components are indispensable in the adjustment of motor output and training-induced improvements in motor performance (McDougle et al., 2016; Gallivan et al., 2018). The current behavioral readouts are relatively simple and cannot isolate contributions of cognition and attention. Further study of the circuit responses to cholinergic modulation in mPFC and M1 during coordinated motor learning may allow for discrimination of cognitive components when combined with nonmotor assays sensitive to attentional deficits. As we observed a critical role for cholinergic input to mPFC in coordinated motor learning, it may be that disruption of circuit level mediators of attention impair motor learning. PFC in primates mediates top-down attentional control over sensory systems through direct input to inferotemporal cortex (Petrides, 2000; Monosov et al., 2010; Baluch and Itti, 2011). Within mPFC, attention drives increases in synchronous activity of fast-spiking parvalbumin inhibitory interneurons and increased γ oscillations (Kim et al., 2016). Acetylcholine signaling through muscarinic receptors in mPFC is required for cue-mediated increases in γ oscillations (Parikh et al., 2007; Howe et al., 2017). On a cued discrimination task, optogenetic activation of basal forebrain cholinergic neurons has been shown to enhance sensitivity to short duration cues but also to increase incorrect attempts in the absence of cues (Gritton et al., 2016). In contrast, silencing of cholinergic inputs impairs cue detection (Gritton et al., 2016). A role for cholinergic modulation of attention during coordinated motor learning may, in part, explain motor deficits in neurologic disorders otherwise characterized by cognitive dysfunction.
Footnotes
This work was supported by the Burke Foundation and the National Institutes of Health Common Fund DP2 NS106663 (to E.H.) and the New York State Department of Health SCIRB Postdoctoral Fellowship C32633GG (to Y.L.). We thank Amanda Bernstein for assisting in behavioral tests and data analysis, Anne Marchildon for animal breeding and genotyping, and Sydney Agger for illustrations. We also thank Rachel Garn for early contributions to the manuscript. We thank the Burke Neurological Institute Structural and Functional Imaging Core, IDDRC Neuroconnectivity Core at Baylor College of Medicine, and the viral core at Boston Children's Hospital for producing DTR virus. pAAV-hSyn-DIO-hM4Di-mCherry was generated by Bryan Roth (Addgene plasmid #44362).
The authors declare no competing financial interests.
References
- Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM (2013) Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp (82):50978. doi: 10.3791/50978 10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM (1996) Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA 93:11219–11224. 10.1073/pnas.93.20.11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, Role LW (2016) Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91:1199–1218. 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluch F, Itti L (2011) Mechanisms of top-down attention. Trends Neurosci 34:210–224. 10.1016/j.tins.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Boskovic Z, Meier S, Wang Y, Milne MR, Onraet T, Tedoldi A, Coulson EJ (2019) Regulation of cholinergic basal forebrain development, connectivity, and function by neurotrophin receptors. Neuronal Signal 3:NS20180066. 10.1042/NS20180066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova A, Kernodle K, Mulligan K, Leventhal D (2019) Automated rat single-pellet reaching with 3-dimensional reconstruction of paw and digit trajectories. J Vis Exp (149):10.3791/59979. doi: 10.3791/59979. 10.3791/59979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Gilmore A, Zuo Y (2014) Study motor skill learning by single-pellet reaching tasks in mice. J Vis Exp (85):51238. doi: 10.3791/51238. 10.3791/51238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiviacowsky S, Wulf G, Avila LT (2013) An external focus of attention enhances motor learning in children with intellectual disabilities. J Intellect Disabil Res 57:627–634. 10.1111/j.1365-2788.2012.01569.x [DOI] [PubMed] [Google Scholar]
- Chua LK, Dimapilis MK, Iwatsuki T, Abdollahipour R, Lewthwaite R, Wulf G (2019) Practice variability promotes an external focus of attention and enhances motor skill learning. Hum Mov Sci 64:307–319. 10.1016/j.humov.2019.02.015 [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Roach EB, Bear MF, Shuler MG (2013) A cholinergic mechanism for reward timing within primary visual cortex. Neuron 77:723–735. 10.1016/j.neuron.2012.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH (2003) Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron 38:819–829. 10.1016/s0896-6273(03)00288-5 [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH (2005) The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46:173–179. 10.1016/j.neuron.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Conner JM, Kulczycki M, Tuszynski MH (2010) Unique contributions of distinct cholinergic projections to motor cortical plasticity and learning. Cereb Cortex 20:2739–2748. 10.1093/cercor/bhq022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RMJ (2013) Measuring motor coordination in mice. J Vis Exp (75):e2609. doi: 10.3791/2609. 10.3791/2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Kremer Y, Crochet S, Petersen CCH (2014) Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep 9:1654–1660. 10.1016/j.celrep.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Falk EN, Norman KJ, Garkun Y, Demars MP, Im S, Taccheri G, Short J, Caro K, McCraney SE, Cho C, Smith MR, Lin HM, Koike H, Bateh J, Maccario P, Waltrip L, Janis M, Morishita H (2021) Nicotinic regulation of local and long-range input balance drives top-down attentional circuit maturation. Sci Adv 7:eabe1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS, Wolpert DM, Flanagan JR (2018) Decision-making in sensorimotor control. Nat Rev Neurosci 19:519–534. 10.1038/s41583-018-0045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y (1997) Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19:679–686. 10.1016/S0896-6273(00)80380-3 [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y (2009) Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci 12:1444–1449. 10.1038/nn.2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE (2003) Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol 458:11–31. 10.1002/cne.10505 [DOI] [PubMed] [Google Scholar]
- Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M (2016) Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci USA 113:E1089–E1097. 10.1073/pnas.1516134113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M, Kepecs A (2015) Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162:1155–1168. 10.1016/j.cell.2015.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Brandel-Ankrapp KL, Arenkiel BR (2021) Dynamic cholinergic tone in the basal forebrain reflects reward-seeking and reinforcement during olfactory behavior. Front Cell Neurosci 15:635837. 10.3389/fncel.2021.635837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TC, Pinto L, Brock JR, Dan Y (2016) Calcium imaging of basal forebrain activity during innate and learned behaviors. Front Neural Circuits 10:36. 10.3389/fncir.2016.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE (2008) Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci 27:654–670. 10.1111/j.1460-9568.2008.06029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, Tepe B, Carlson JC, Ung K, Selever J, Tong Q, Arenkiel BR (2016) A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 538:253–256. 10.1038/nature19789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A (2008) Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454:1110–1114. 10.1038/nature07141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Gritton HJ, Lusk NA, Roberts EA, Hetrick VL, Berke JD, Sarter M (2017) Acetylcholine release in prefrontal cortex promotes gamma oscillations and theta–gamma coupling during cue detection. J Neurosci 37:3215–3230. 10.1523/JNEUROSCI.2737-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EJ, Dahlen JE, Hu YY, Aguilar K, Yu B, Mukundan M, Mitani A, Komiyama T (2019) Disengagement of motor cortex from movement control during long-term learning. Sci Adv 5:eaay0001. 10.1126/sciadv.aay0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SL, Ma W, Eslin D (1991) Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci USA 88:780–784. 10.1073/pnas.88.3.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, Kampff AR, Ölveczky BP (2015) Motor cortex is required for learning but not for executing a motor skill. Neuron 86:800–812. 10.1016/j.neuron.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM (1998) Cortical map reorganization enabled by nucleus basalis activity. Science 279:1714–1718. 10.1126/science.279.5357.1714 [DOI] [PubMed] [Google Scholar]
- Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M (2016) Prefrontal parvalbumin neurons in control of attention. Cell 164:208–218. 10.1016/j.cell.2015.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW (2015) Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci USA 112:3535–3540. 10.1073/pnas.1413625112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek AC, Jin X, Long JD 2nd, Costa RM, Carmena JM (2012) Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature 483:331–335. 10.1038/nature10845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek AC, Costa RM, Carmena JM (2013) Temporally precise cell-specific coherence develops in corticostriatal networks during learning. Neuron 79:865–872. 10.1016/j.neuron.2013.06.047 [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB (2011) Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121:1424–1428. 10.1172/JCI46229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B (2008) Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron 58:911–924. 10.1016/j.neuron.2008.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Juczewski K, Cui G, Johnson KA, Lovinger DM (2017) Parallel, but dissociable, processing in discrete corticostriatal inputs encodes skill learning. Neuron 96:476–489.e5. 10.1016/j.neuron.2017.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke SM, Ramanathan DS, Guo L, Won SJ, Ganguly K (2019) Emergent modular neural control drives coordinated motor actions. Nat Neurosci 22:1122–1131. 10.1038/s41593-019-0407-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Lüthi A (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480:331–335. 10.1038/nature10674 [DOI] [PubMed] [Google Scholar]
- Lewthwaite R, Wulf G (2017) Optimizing motivation and attention for motor performance and learning. Curr Opin Psychol 16:38–42. 10.1016/j.copsyc.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Liu CH, Coleman JE, Davoudi H, Zhang K, Hussain Shuler MG (2015) Selective activation of a putative reinforcement signal conditions cued interval timing in primary visual cortex. Curr Biol 25:1551–1561. 10.1016/j.cub.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse KR, Jones M, Healy AF, Sherwood DE (2014) The role of attention in motor control. J Exp Psychol Gen 143:930–948. 10.1037/a0032817 [DOI] [PubMed] [Google Scholar]
- Ma M, Luo M (2012) Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci 32:10105–10116. 10.1523/JNEUROSCI.0058-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Ivry RB, Taylor JA (2016) Taking aim at the cognitive side of learning in sensorimotor adaptation tasks. Trends Cogn Sci 20:535–544. 10.1016/j.tics.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH (1992) Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12:4701–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov IE, Sheinberg DL, Thompson KG (2010) Paired neuron recordings in the prefrontal and inferotemporal cortices reveal that spatial selection precedes object identification during visual search. Proc Natl Acad Sci USA 107:13105–13110. 10.1073/pnas.1002870107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M (2007) Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56:141–154. 10.1016/j.neuron.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TP, Gullotti DM, Hernandez P, O'Brien WT, Capehart BP, Morrison B 3rd, Bass C, Eberwine JE, Abel T, Meaney DF (2014) An open-source toolbox for automated phenotyping of mice in behavioral tasks. Front Behav Neurosci 8:349. 10.3389/fnbeh.2014.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (2000) Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. J Neurosci 20:7496–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y (2013) Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci 16:1857–1863. 10.1038/nn.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan DS, Conner JM, Anilkumar AA, Tuszynski MH (2015) Cholinergic systems are essential for late-stage maturation and refinement of motor cortical circuits. J Neurophysiol 113:1585–1597. 10.1152/jn.00408.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson DD (2000) The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res 115:205–218. 10.1016/s0166-4328(00)00259-x [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C (2019) Cholinergic double duty: cue detection and attentional control. Curr Opin Psychol 29:102–107. 10.1016/j.copsyc.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH (2019) The role of attention in motor control and learning. Curr Opin Psychol 29:261–265. 10.1016/j.copsyc.2019.08.002 [DOI] [PubMed] [Google Scholar]
- Thiele A, Bellgrove MA (2018) Neuromodulation of attention. Neuron 97:769–785. 10.1016/j.neuron.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villano I, Messina A, Valenzano A, Moscatelli F, Esposito T, Monda V, Esposito M, Precenzano F, Carotenuto M, Viggiano A, Chieffi S, Cibelli G, Monda M, Messina G (2017) Basal forebrain cholinergic system and orexin neurons: effects on attention. Front Behav Neurosci 11:10. 10.3389/fnbeh.2017.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Conner JM, Rickert J, Tuszynski MH (2011) Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci USA 108:2545–2550. 10.1073/pnas.1014335108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM (1990) The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res 41:49–59. 10.1016/0166-4328(90)90053-h [DOI] [PubMed] [Google Scholar]
- Wolff SBE, Ko R, Ölveczky BP (2019) Distinct roles for motor cortical and thalamic inputs to striatum during motor learning and execution. bioRxiv 825810. 10.1101/825810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G (2013) Attentional focus and motor learning: a review of 15 years. Int Rev Sport Exer P 6:77–104. 10.1080/1750984X.2012.723728 [DOI] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM (2009) Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12:333–341. 10.1038/nn.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Gombkoto P, Varsanyi P, Gielow MR, Poe G, Role LW, Ananth M, Rajebhosale P, Talmage DA, Hasselmo ME, Dannenberg H, Minces VH, Chiba AA (2018) Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J Neurosci 38:9446–9458. 10.1523/JNEUROSCI.1676-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmar A, Kast B, Lussi K, Luft AR, Schwab ME (2015) Acquisition of a high-precision skilled forelimb reaching task in rats. J Vis Exp (100):e53010. doi: 10.3791/53010. 10.3791/53010 [DOI] [PMC free article] [PubMed] [Google Scholar]