The prospective Vax-On study was conducted at our institution as part of introducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA-BNT162b2 (tozinameran) vaccination in actively treated cancer patients. Our preliminary findings confirmed a favorable safety profile and suggested that proximity to treatment hampers immune response to the first vaccine dose (timepoint-2). The second dose induced an exponential rise in anti-Spike protein immunoglobulin G (IgG) titer and seroconversion rates up to >90%, abrogating the disparity between the cohorts (timepoint-3).1 Herein, we report on antibody response assessment scheduled 6 months after the first tozinameran dose (timepoint-4).
The Vax-On study has already been described in its design and eligibility criteria. We carried out the same statistical analysis using SPSS software (Version 23, Armonk, NY), with all tests run two-sided and a P value <0.05 considered significant. The SARS-CoV-2 IgG II Quant assay on ARCHITECT i2000sr automated platform (Abbott Laboratories, Diagnostics Division, Sligo, Ireland) was used for quantitative detection of anti-Spike protein IgG antibodies in human serum or plasma. The study received ethics committee approval (protocol N.595/CE Lazio1) and registration (EudraCT number 2021-002611-54).1 Following the original experimental design, patients on active treatment within 28 days of timepoint-4 represented the exposed cohort (ExC) compared with the control cohort (CC) of those who had discontinued by at least 28 days.
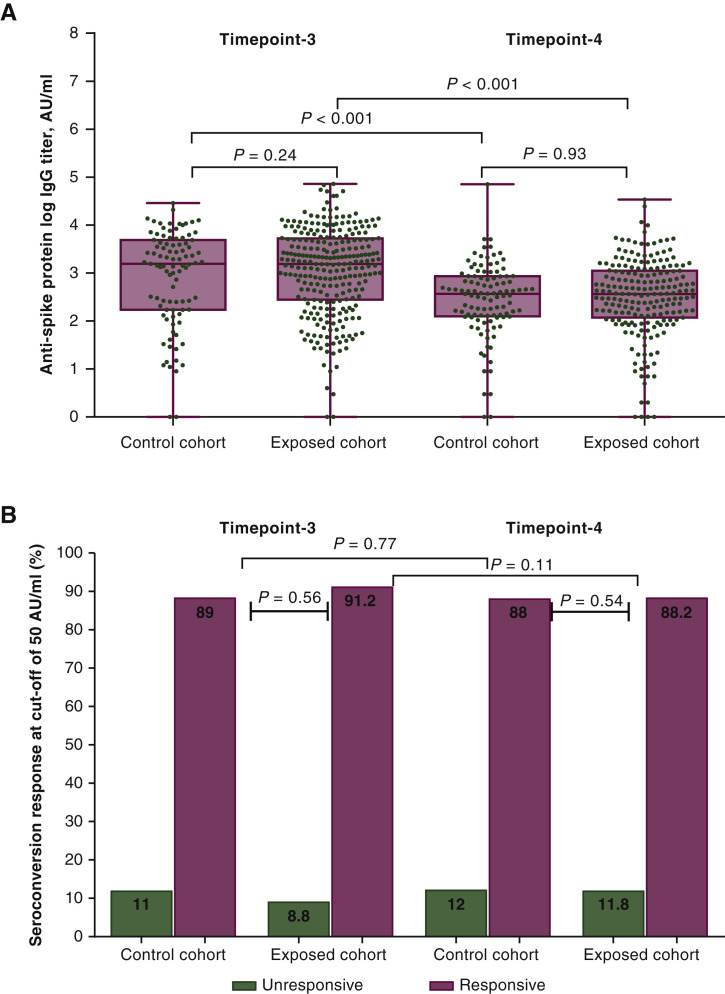
The present analysis involved 311 patients, including 203 in the ExC, all of whom remained on active treatment from timepoint-3, and 108 in the CC. Supplementary Table S1 and Figure S1, available at https://doi.org/10.1016/j.annonc.2021.12.001, show the baseline characteristics and reasons for missing assessments at each timepoint. The median IgG titer [CC 52 BAU/ml; 95% confidence interval (CI), 30.5-62.8 BAU/ml (370 AU/ml; 95% CI, 218-449 AU/ml) versus ExC 51 BAU/ml; 95% CI, 39.3-68.2 BAU/ml (367 AU/ml; 95% CI, 281-487 AU/ml), P = 0.50], median log IgG titer (P = 0.93, Figure 1 A), and seroconversion rates (CC 88% versus ExC 88.2%, P = 0.54, Figure 1B) did not differ at timepoint-4. Compared with timepoint-3, paired assessment at timepoint-4 revealed a significant four- to sixfold decrease in median IgG titer within the same cohort (Figure 1A, P < 0.001), with no difference for seroconversion rates (CC, P = 0.77; ExC, P = 0.11; Figure 1B; Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.12.001). Univariate comparison with the CC at timepoint-4 showed a significantly higher IgG titer for targeted therapy subgroup (P = 0.039), with a lower estimate for chemotherapy and biological agent subgroup (P = 0.035, Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.12.001). Multivariate analysis was carried out by fitting a generalized linear model on log IgG titer and seroconversion response as a function of covariates significantly associated with immunogenicity after previous evaluation. Antibody response did not differ according to antineoplastic treatment subgroup (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.12.001). Male sex (P = 0.002) and Eastern Cooperative Oncology Group performance status 2 (ECOG-PS2) (P = 0.02) were both significantly associated with lower log IgG titer, but only initial corticosteroid therapy was also related to lack of seroconversion (P = 0.005). Of note, only one case of mild coronavirus disease 2019 (COVID-19) infection was documented in the entire patient group after the second tozinameran dose.
Figure 1.
Six-month follow-up of antibody responses to full schedule of tozinameran vaccine.
(A) Comparison of violin plot distributions and medians of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Spike protein immunoglobulin G (IgG) titers (logarithmic values). Bars represent median values with 95% confidence interval. (B) Comparison of seroconversion response rates at a cut-off of 50 AU/ml. Timepoint-3: antibody response assessment at 8 weeks after the second tozinameran dose; timepoint-4: antibody response assessment at 6 months after the first tozinameran dose; control cohort: patients with discontinuation of active treatment at least 28 days before assessment of antibody titer at 6 months after the first tozinameran dose; exposed cohort: patients on active treatment within 28 days of antibody titer assessment at 6 months after the first tozinameran dose.
The current study is an extensive, longitudinal follow-up study of tozinameran immunogenicity in patients with actively treated solid malignancies. Our results suggest that proximity to cancer treatment does not affect seroconversion response, which remains adequate even 5 months after the second vaccine dose. In contrast to healthy adults given full mRNA vaccine schedule,2 antibody titer decreased markedly over time. Present data on antibody response and seroconversion are broadly consistent with the results of two comparable studies.3, 4 Multivariate analysis ruled out the predictive value of a specific type of cancer treatment but suggested a potential detrimental effect of corticosteroid therapy, male sex, and ECOG-PS2 on humoral response. Given the ongoing debate about the protective role of antibody titer,5 these findings, along with the deployment of reliable assays for cellular immunity, may provide additional evidence in favor of the third dose of vaccine already approved for actively treated cancer patients.
Acknowledgements
This study is dedicated to all cancer patients who have died due to COVID-19, whose indelible memory strengthens scientific research.
Funding
None declared.
Disclorure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Nelli F., Fabbri A., Onorato A., et al. Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2 vaccine: preliminary results from the prospective observational Vax-On study. Ann Oncol. 2022;33 doi: 10.1016/j.annonc.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goel R.R., Painter M.M., Apostolidis S.A., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374 doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliakim-Raz N., Massarweh A., Stemmer A., Stemmer S.M. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 2021;7:1716–1718. doi: 10.1001/jamaoncol.2021.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldhorn I., Holland R., Goshen-Lago T., et al. Six-month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov. 2021;11:2430–2435. doi: 10.1158/2159-8290.CD-21-1072. [DOI] [PubMed] [Google Scholar]
- 5.Harvey R.A., Rassen J.A., Kabelac C.A., et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021;181:672–679. doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.